Abstract
Developing convenient and accurate SARS-CoV-2 antigen test and serology test is crucial in curbing the global COVID-19 pandemic. In this work, we report an improved indium oxide (In2O3) nanoribbon field-effect transistor (FET) biosensor platform detecting both SARS-CoV-2 antigen and antibody. Our FET biosensors, which were fabricated using a scalable and cost-efficient lithography-free process utilizing shadow masks, consist of an In2O3 channel and a newly developed stable enzyme reporter. During the biosensing process, the phosphatase enzymatic reaction generated pH change of the solution, which was then detected and converted to electrical signal by our In2O3 FETs. The biosensors applied phosphatase as enzyme reporter, which has a much better stability than the widely used urease in FET based biosensors. As proof-of-principle studies, we demonstrate the detection of SARS-CoV-2 spike protein in both phosphate-buffered saline (PBS) buffer and universal transport medium (UTM) (limit of detection [LoD]: 100 fg/mL). Following the SARS-CoV-2 antigen tests, we developed and characterized additional sensors aimed at SARS-CoV-2 IgG antibodies, which is important to trace past infection and vaccination. Our spike protein IgG antibody tests exhibit excellent detection limits in both PBS and human whole blood ((LoD): 1 pg/mL). Our biosensors display similar detection performance in different mediums, demonstrating that our biosensor approach is not limited by Debye screening from salts and can selectively detect biomarkers in physiological fluids. The newly selected enzyme for our platform performs much better performance and longer shelf life which will lead our biosensor platform to be capable for real clinical diagnosis usage.
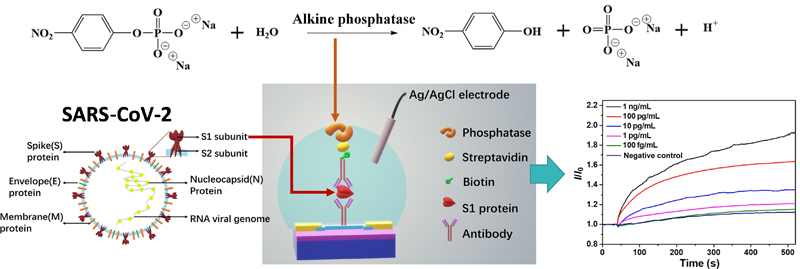
Electronic Supplementary Material
Supplementary material (materials and methods for device fabrication, functionalization of In2O3 devices, photographs of the liquid gate measurement setup, mobilities of the nine devices labeled in Fig. 1(b), family curves of IDS-VDS with the liquid gate setup and current change after bubbling the substrate solution (current vs. time curve for S1 antigen detection)) is available in the online version of this article at 10.1007/s12274-022-4190-0.
Keywords: biosensor, indium oxide transistor, phosphatase, SARS-CoV-2 spike protein, SARS-CoV-2 spike IgG antibody
Electronic Supplementary Material
Highly sensitive, scalable, and rapid SARS-CoV-2 biosensor based on In2O3 nanoribbon transistors and phosphatase
Acknowledgements
We would like to acknowledge the financial support of this research by King Abdul-Aziz City for Science and Technology (KACST) through The Center of Excellence for Nanotechnologies (CEGN).
Footnotes
Mingrui Chen and Dingzhou Cui contributed equally to this work.
Contributor Information
Moh. R. Amer, Email: mamer@seas.ucla.edu
Chongwu Zhou, Email: chongwuz@usc.edu.
References
- [1].WHO. WHO Coronavirus Disease (COVID-19) Dashboard [Online]. https://covid19.who.int/. (Accessed 2021-11-1)
- [2].WHO. Guidance for Surveillance of SARS-CoV-2 Variants: Interim Guidance, 9 August 2021 [Online]. https://www.who.int/publications/i/item/WHO_2019-nCoV_surveillance_variants. (Accessed 2021-11-1)
- [3].WHO. Weekly Epidemiological Update on COVID-19-10 August 2021 [Online], https://www.who.int/publitations/m/item/weekly-epidemiological-update-on-covid-19—10-august-2021. (Accessed 2021-11-1)
- [4].Goodell J W. COVID-19 and finance: Agendas for future research. Finance Res. Lett. 2020;35:101512. doi: 10.1016/j.frl.2020.101512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chen N S, Zhou M, Dong X, Qu J M, Gong F Y, Han Y, Qiu Y, Wang J L, Liu Y, Wei Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wang D W, Hu B, Hu C, Zhu F F, Liu X, Zhang J, Wang B B, Xiang H, Cheng Z S, Xiong Y, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhu N, Zhang D Y, Wang W L, Li X W, Yang B, Song J D, Zhao X, Huang B Y, Shi W F, Lu R J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lu R J, Zhao X, Li J, Niu P H, Yang B, Wu H L, Wang W L, Song H, Huang B Y, Zhu N, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bastos M L, Tavaziva G, Abidi S K, Campbell J R, Haraoui L P, Johnston J C, Lan Z Y, Law S, MacLean E, Trajman A, et al. Diagnostic accuracy of serological tests for Covid-19: Systematic review and meta-analysis. BMJ. 2020;370:m2516. doi: 10.1136/bmj.m2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Weissleder R, Lee H, Ko J, Pittet M J. COVID-19 diagnostics in context. Sci. Transl. Med. 2020;12:eabc1931. doi: 10.1126/scitranslmed.abc1931. [DOI] [PubMed] [Google Scholar]
- [11].Liu G Q, Rusling J F. COVID-19 antibody tests and their limitations. ACS Sens. 2021;6:593–612. doi: 10.1021/acssensors.0c02621. [DOI] [PubMed] [Google Scholar]
- [12].World Health Organization . Laboratory testing for coronavirus disease (COVID-19) in suspected human cases: Interim guidance, 19 March 2020. Geneva: World Health Organization; 2020. [Google Scholar]
- [13].Noh J Y, Yoon S W, Kim D J, Lee M S, Kim J H, Na W, Song D, Jeong D G, Kim H K. Simultaneous detection of severe acute respiratory syndrome, Middle East respiratory syndrome, and related bat coronaviruses by real-time reverse transcription PCR. Arch. Virol. 2017;162:1617–1623. doi: 10.1007/s00705-017-3281-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chan, J. F. W.; Yip, C. C. Y.; To, K. K. W.; Tang, T. H. C.; Wong, S. C. Y.; Leung, K. H.; Fung, A. Y. F.; Ng, A. C. K.; Zou, Z. J.; Tsoi, H. W. et al. Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/Hel real-time reverse transcription-PCR assay validated in vitro and with clinical specimens. J. Clin. Microbiol.2020, 58, e00310–20. [DOI] [PMC free article] [PubMed]
- [15].Fathi-Hafshejani P, Azam N, Wang L, Kuroda MA, Hamilton MC, Hasim S, Mahjouri-Samani M. Two-dimensional-material-based field-effect transistor biosensor for detecting COVID-19 virus (SARS-CoV-2) ACS Nano. 2021;157:11461–11469. doi: 10.1021/acsnano.1c01188. [DOI] [PubMed] [Google Scholar]
- [16].Stern E, Klemic J F, Routenberg D A, Wyrembak P N, Turner-Evans D B, Hamilton A D, LaVan D A, Fahmy T M, Reed M A. Label-free immunodetection with CMOS-compatible semiconducting nanowires. Nature. 2007;445:519–522. doi: 10.1038/nature05498. [DOI] [PubMed] [Google Scholar]
- [17].Ishikawa F N, Chang H K, Curreli M, Liao H I, Olson C A, Chen P C, Zhang R, Roberts R W, Sun R, Cote R J, et al. Label-free, electrical detection of the SARS virus N-protein with nanowire biosensors utilizing antibody mimics as capture probes. ACS Nano. 2009;3:1219–1224. doi: 10.1021/nn900086c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang B, Zhao C, Wang Z, Yang K A, Cheng X, Liu W, Yu W, Lin S, Zhao Y, Cheung K M, et al. Wearable aptamer-field-effect transistor sensing system for noninvasive cortisol monitoring. Sci. Adv. 2022;8(1):eabk0967. doi: 10.1126/sciadv.abk0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chen Y T, Ren R, Pu H H, Guo X R, Chang J B, Zhou G H, Mao S, Kron M, Chen J H. Field-effect transistor biosensor for rapid detection of Ebola antigen. Sci. Rep. 2017;7:10974. doi: 10.1038/s41598-017-11387-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Afsahi S, Lerner M B, Goldstein J M, Lee J, Tang X L, Bagarozzi D A, Jr, Pan D, Locascio L, Walker A, Barron F, et al. Novel graphene-based biosensor for early detection of Zika virus infection. Biosens. Bioelectron. 2018;100:85–88. doi: 10.1016/j.bios.2017.08.051. [DOI] [PubMed] [Google Scholar]
- [21].Seo G, Lee G, Kim M J, Baek S H, Choi M, Ku K B, Lee C S, Jun S, Park D, Kim H G, et al. Rapid detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano. 2020;14:5135–5142. doi: 10.1021/acsnano.0c02823. [DOI] [PubMed] [Google Scholar]
- [22].Shao W T, Shurin M R, Wheeler S E, He X Y, Star A. Rapid detection of SARS-CoV-2 antigens using high-purity semiconducting single-walled carbon nanotube-based field-effect transistors. ACS Appl. Mater. Interfaces. 2021;13:10321–10327. doi: 10.1021/acsami.0c22589. [DOI] [PubMed] [Google Scholar]
- [23].Li C, Curreli M, Lin H, Lei B, Ishikawa F N, Datar R, Cote R J, Thompson M E, Zhou C W. Complementary detection of prostate-specific antigen using In2O3 nanowires and carbon nanotubes. J. Am. Chem. Soc. 2005;127:12484–12485. doi: 10.1021/ja053761g. [DOI] [PubMed] [Google Scholar]
- [24].Kim J, Rim Y S, Chen H, Cao H H, Nakatsuka N, Hinton H L, Zhao C Z, Andrews A M, Yang Y, Weiss P S. Fabrication of high-performance ultrathin In2O3 film field-effect transistors and biosensors using chemical lift-off lithography. ACS Nano. 2015;9:4572–4582. doi: 10.1021/acsnano.5b01211. [DOI] [PubMed] [Google Scholar]
- [25].Liu Q Z, Liu Y H, Wu F Q, Cao X, Li Z, Alharbi M, Abbas A N, Amer M R, Zhou C W. Highly sensitive and wearable In2O3 nanoribbon transistor biosensors with integrated on-chip gate for glucose monitoring in body fluids. ACS Nano. 2018;12:1170–1178. doi: 10.1021/acsnano.7b06823. [DOI] [PubMed] [Google Scholar]
- [26].Chang H K, Ishikawa F N, Zhang R, Datar R, Cote R J, Thompson M E, Zhou C W. Rapid, label-free, electrical whole blood bioassay based on nanobiosensor systems. ACS Nano. 2011;5:9883–9891. doi: 10.1021/nn2035796. [DOI] [PubMed] [Google Scholar]
- [27].Zhao C Z, Liu Q Z, Cheung K M, Liu W F, Yang Q, Xu X B, Man T X, Weiss P S, Zhou C W, Andrews A M. Narrower nanoribbon biosensors fabricated by chemical lift-off lithography show higher sensitivity. ACS Nano. 2021;15:904–915. doi: 10.1021/acsnano.0c07503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Liu Q Z, Zhao C Z, Chen M R, Liu Y H, Zhao Z Y, Wu F Q, Li Z, Weiss P S, Andrews A M, Zhou C W. Flexible multiplexed In2O3 nanoribbon aptamer-field-effect transistors for biosensing. iScience. 2020;23:101469. doi: 10.1016/j.isci.2020.101469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Nakatsuka N, Yang K A, Abendroth J M, Cheung K M, Xu X B, Yang H Y, Zhao C Z, Zhu B W, Rim Y S, Yang Y, et al. Aptamer-field-effect transistors overcome Debye length limitations for small-molecule sensing. Science. 2018;362:319–324. doi: 10.1126/science.aao6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Schomburg, I.; Chang, A.; Placzek, S.; Söhngen, C.; Rother, M.; Lang, M.; Munaretto, C.; Ulas, S.; Stelzer, M.; Grote, A. et al. BRENDA in 2013: Integrated reactions, kinetic data, enzyme function data, improved disease classification: New options and contents in BRENDA. Nucleic Acids Res.2013, 41, D764–D772. [DOI] [PMC free article] [PubMed]
- [31].Danielsson B, Lundström I, Mosbach K, Stiblert L. On a new enzyme transducer combination: The enzyme transistor. Anal. Lett. 1979;12:1189–1199. doi: 10.1080/00032717908067909. [DOI] [Google Scholar]
- [32].Alegret S, Bartrolí J, Jiménez C, Martínez-Fàbregas E, Martorell D, Valdés-Perezgasga F. ISFET-based urea biosensor. Sens. Actuators B:Chem. 1993;16:453–457. doi: 10.1016/0925-4005(93)85227-2. [DOI] [Google Scholar]
- [33].Boubriak O A, Soldatkin A P, Starodub N F, Sandrovsky A K, El’skaya A K. Determination of urea in blood serum by a urease biosensor based on an ion-sensitive field-effect transistor. Sens. Actuators B:Chem. 1995;27:429–431. doi: 10.1016/0925-4005(94)01633-S. [DOI] [Google Scholar]
- [34].Mu L Y, Droujinine I A, Rajan N K, Sawtelle S D, Reed M A. Direct, rapid, and label-free detection of enzyme-substrate interactions in physiological buffers using CMOS-compatible nanoribbon sensors. Nano Lett. 2014;14:5315–5322. doi: 10.1021/nl502366e. [DOI] [PubMed] [Google Scholar]
- [35].Pijanowska D G, Torbicz W. pH-ISFET based urea biosensor. Sens. Actuators B:Chem. 1997;44:370–376. doi: 10.1016/S0925-4005(97)00194-9. [DOI] [Google Scholar]
- [36].Liu Q Z, Aroonyadet N, Song Y, Wang X L, Cao X, Liu Y H, Cong S, Wu F Q, Thompson M E, Zhou C W. Highly sensitive and quick detection of acute myocardial infarction biomarkers using In2O3 nanoribbon biosensors fabricated using shadow masks. ACS Nano. 2016;10:10117–10125. doi: 10.1021/acsnano.6b05171. [DOI] [PubMed] [Google Scholar]
- [37].Aroonyadet N, Wang X L, Song Y, Chen H T, Cote R J, Thompson M E, Datar R H, Zhou C W. Highly scalable, uniform, and sensitive biosensors based on top-down indium oxide nanoribbons and electronic enzyme-linked immunosorbent assay. Nano Lett. 2015;15:1943–1951. doi: 10.1021/nl5047889. [DOI] [PubMed] [Google Scholar]
- [38].Nannipieri P, Ceccanti B, Cervelli S, Sequi P. Stability and kinetic properties of humus-urease complexes. Soil Biol. Biochem. 1978;10:143–147. doi: 10.1016/0038-0717(78)90085-8. [DOI] [Google Scholar]
- [39].Poźniak G, Krajewska B, Trochimczuk W. Urease immobilized on modified polysulphone membrane: Preparation and properties. Biomaterials. 1995;16:129–134. doi: 10.1016/0142-9612(95)98275-J. [DOI] [PubMed] [Google Scholar]
- [40].Reddy K R C, Kayastha A M. Improved stability of urease upon coupling to alkylamine and arylamine glass and its analytical use. J. Mol. Catal. B:Enzym. 2006;38:104–112. doi: 10.1016/j.molcatb.2005.12.001. [DOI] [Google Scholar]
- [41].Yang Z P, Si S H, Zhang C J. Study on the activity and stability of urease immobilized onto nanoporous alumina membranes. Micropor. Mesopor. Mater. 2008;111:359–366. doi: 10.1016/j.micromeso.2007.08.009. [DOI] [Google Scholar]
- [42].Yang D, Fan J H, Cao F Y, Deng Z J, Pojman J A, Ji L. Immobilization adjusted clock reaction in the urea-urease-H+ reaction system. RSC Adv. 2019;9:3514–3519. doi: 10.1039/C8RA09244C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ko Y C, Mukaida N, Panyutich A, Voitenok N N, Matsushima K, Kawai T, Kasahara T. A sensitive enzyme-linked immunosorbent assay for human interleukin-8. J. Immunol. Methods. 1992;149:227–235. doi: 10.1016/0022-1759(92)90254-Q. [DOI] [PubMed] [Google Scholar]
- [44].Daniilidou M, Tsolaki M, Giannakouros T, Nikolakaki E. Detection of elevated antibodies against SR protein kinase 1 in the serum of Alzheimer’s disease patients. J. Neuroimmunol. 2011;238:67–72. doi: 10.1016/j.jneuroim.2011.06.013. [DOI] [PubMed] [Google Scholar]
- [45].Cui Y, Wei Q Q, Park H, Lieber C M. Nanowire nanosensors for highly sensitive and selective detection of biological and chemical species. Science. 2001;293:1289–1292. doi: 10.1126/science.1062711. [DOI] [PubMed] [Google Scholar]
- [46].Pan Z W, Dai Z R, Wang Z L. Nanobelts of semiconducting oxides. Science. 2001;291:1947–1949. doi: 10.1126/science.1058120. [DOI] [PubMed] [Google Scholar]
- [47].Chen R J, Zhang Y G, Wang D W, Dai H J. Noncovalent sidewall functionalization of single-walled carbon nanotubes for protein immobilization. J. Am. Chem. Soc. 2001;123:3838–3839. doi: 10.1021/ja010172b. [DOI] [PubMed] [Google Scholar]
- [48].Shao Y Y, Wang J, Wu H, Liu J, Aksay I A, Lin Y H. Graphene based electrochemical sensors and biosensors: A review. Electroanalysis. 2010;22:1027–1036. doi: 10.1002/elan.200900571. [DOI] [Google Scholar]
- [49].Wang Y H, Huang K J, Wu X. Recent advances in transition-metal dichalcogenides based electrochemical biosensors: A review. Biosens. Bioelectron. 2017;97:305–316. doi: 10.1016/j.bios.2017.06.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Highly sensitive, scalable, and rapid SARS-CoV-2 biosensor based on In2O3 nanoribbon transistors and phosphatase


