Abstract
Mitochondria are considered to be the powerhouse of the cell. Normal functioning of the mitochondria is not only essential for cellular energy production but also for several immunomodulatory processes. Macrophages operate in metabolic niches and rely on rapid adaptation to specific metabolic conditions such as hypoxia, nutrient limitations, or reactive oxygen species to neutralize pathogens. In this regard, the fast reprogramming of mitochondrial metabolism is indispensable to provide the cells with the necessary energy and intermediates to efficiently mount the inflammatory response. Moreover, mitochondria act as a physical scaffold for several proteins involved in immune signaling cascades and their dysfunction is immediately associated with a dampened immune response. In this review, we put special focus on mitochondrial function in macrophages and highlight how mitochondrial metabolism is involved in macrophage activation.
Keywords: itaconic acid, mitochondria, macrophages, metabolism, TCA cycle
INTRODUCTION
Mitochondria are double membrane organelles that are assumed to be of bacterial origin according to the endosymbiotic theory. This theory suggests that mitochondria evolved from an aerobic prokaryote which was engulfed by a host nucleated cell and from then on started to take advantage, depending on the prokaryotic cell for energy production; this led to the development of modern mitochondria over time (1). Although mitochondria have dramatically developed since then, their prokaryotic origin is still evident and includes the presence of their own circular genome, mitochondrial DNA (mtDNA), which encodes some parts of the respiratory chain as well as mitochondrial tRNAs and rRNAs (2). Moreover, mitochondria synthesize their own membrane and reproduce by partitioning and pinching in the middle, a process used by bacteria and is known as fission (3, 4).
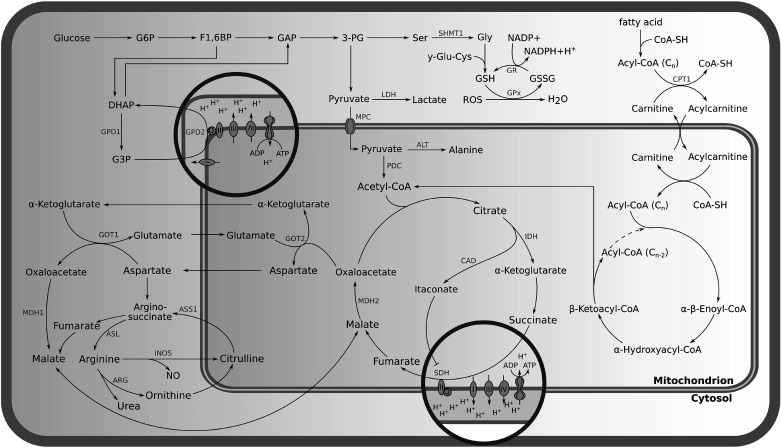
After establishment of the mitochondrion within the early eukaryotic cell, this organelle has become responsible for several cellular key functions such as energy generation, reactive oxygen species (ROS) production, calcium homeostasis, iron sulfur cluster biogenesis, and the regulation of apoptosis (5, 6). Mitochondria supply the major portion of cellular adenosine triphosphate (ATP) through oxidative phosphorylation (OXPHOS) (6, 7) and are a central hub for several metabolic activities such as the tricarboxylic acid cycle (TCA), β-oxidation of fatty acids, parts of the urea cycle, and amino acid synthesis (Fig. 1; 8, 9). Many intermediates of these pathways, including ROS are in`volved in the control of cellular gene expression (10).
Figure 1.
Metabolic pathways associated with mitochondria. 3-PG, 3-phosphoglyceric acid; ADP, adenosine diphosphate; ALT, alanine aminotransferase; Arg, arginase; ASL, argininosuccinate lyase; ASS1, argininosuccinate synthetase 1; ATP, adenosine triphosphate; CAD, cis-aconitate decarboxylase; CoA-SH, coenzyme A; CPT-1, carnitine palmitoyl transferase-1; DHAP, dihydroxyacetone phosphate; F1,6BP, fructose 1,6-bisphosphate; G3P, glycerol 3-phosphate; G6P, glucose 6-phosphate; γ-GLU-Cys, γ-glutamyl-cysteine; GAP, glyceraldehyde 3-phosphate; Gly, glycine; GOT1, cytoplasmic aspartate aminotransferase; GOT2, mitochondrial aspartate aminotransferase; GPD1, cytosolic glycerol 3-phosphate dehydrogenase; GPD2, mitochondrial glycerol 3-phosphate dehydrogenase; GPx, glutathione peroxidase; GR, glutathione reductase; GSH, glutathione; GSSH, glutathione disulfide; IDH, isocitrate dehydrogenase; iNOS, inducible nitric oxide synthase; LDH, lactate dehydrogenase; MDH1, cytosolic malate dehydrogenase; MDH2, mitochondrial malate dehydrogenase; MPC, mitochondrial pyruvate carrier; NADP+, nicotinamide adenine dinucleotide phosphate; NADPH, reduced nicotinamide adenine dinucleotide phosphate; PDC, pyruvate dehydrogenase complex; Ser, serine; ROS, reactive oxygen species; SHMT1, serine hydroxymethyltransferase-1; SDH, succinate dehydrogenase.
Because of their central cellular role, it is not surprising that mitochondrial function is crucial for the normal activity of mammalian cells, and dysfunction of these organelles has been linked to several neurological and metabolic disorders, aging, and cancer (8). Mitochondrial dysfunction might arise from ROS-induced mutations of the mtDNA due to its close proximity to the mitochondrial electron flow (8, 11). Moreover, mtDNA has a nucleotide imbalance or asymmetry that decreases the fidelity of the DNA polymerase, POLG, which is responsible for replicating the mitochondrial genome (12). Depending on the tissue type, a single mammalian cell contains up to 1,000 mitochondria, each of which maintains several copies (2–10) of its circular mtDNA (13, 14). As a consequence, every cell contains up to several thousand mtDNA copies, rather than just one copy of nuclear DNA in postmitotic cells. This makes the mtDNA more susceptible to heterogeneity as this could lead to heterogenous copies of mtDNA within the same cell or even within the same mitochondrion (15). Although mitochondria still maintain their own DNA, they rely heavily on nuclear genes for the production of mitochondrial proteins (16). This heterogeneity and increased mutation rate may be one of the reasons why mitochondria have lost most of their genomic information in favor of the nuclear genome (16).
The fundamental contribution of nuclear DNA and cellular protein biosynthesis to basic mitochondrial function renders a precise cross talk between nuclear and mtDNA essential for regular functioning of the cell (17). To convey the bidirectional cross talk between nuclear and mtDNA, the cells rely on epigenetic and post-transcriptional mechanisms to modulate gene expression (17). These mechanisms include DNA methylation, the regulation of gene expression by microRNA (miRNA), and post-translational histone modifications (17). There are many miRNAs which have been reported to regulate mitochondrial transcripts (17, 18). Of particular interest in the context of this review are miRNAs involved in the regulation of mitochondrial metabolism in macrophages. For example, miR-15a/16 has been shown to play a role in inflammatory macrophages, as Moon et al. (19) demonstrated that the deletion of miR-15a/16 increased phagocytosis and the production of mitochondrial reactive oxygen species (mROS). Moreover, a deficiency of miR-15a/16 resulted in an increase of proinflammatory cytokines (19). Another example is miR-33; this miRNA targets PGC-1α in macrophages, resulting in the inhibition of mitochondrial respiration and ATP production (20, 21). Taken together, miRNAs represent a novel therapeutic target that can be exploited for the treatment of immune disorders (21).
Among the diseases associated with mitochondrial dysfunction are neurological disorders such as neurodegenerative diseases, ischemia, and hypoxia-induced brain injury (22). Dysfunction of mitochondrial biogenesis, fusion and fission have been considered responsible for these pathogeneses (22). Mitochondrial dysfunction has also been implicated in the pathogenesis of immune cells in immunological diseases such as systemic lupus erythematosus (SLE), rheumatoid arthritis, and type I diabetes (23). Furthermore, several classes of pharmaceutical drugs have been reported to exert mitochondrial toxicity, such as pain medications [nonsteroidal anti-inflammatory drugs (NSAIDs)], cholesterol lowering drugs (statins), anticancer drugs (kinase inhibitors and anthracyclines), antidiabetic drugs (thiazolidinediones, fibrates, and biguanides), and antibiotics such as fluroquinolones and macrolide (24).
Mitochondria are known to be involved in the immune response against pathogens as they supply the necessary energy and required metabolic intermediates for proper immune cell function and are even involved in sensing danger or stress signals (25, 26). This has been shown to be essential for host defense and tissue homeostasis (26). Hence, mitochondrial dysfunction has been reported to be associated with defective macrophage phagocytosis in chronic obstructive pulmonary disease (COPD) (27). Furthermore, it was demonstrated to prevent the repolarization of inflammatory macrophages (28).
Macrophages are the effector cells of the innate immune system (29). They are responsible for phagocytosing pathogens and releasing proinflammatory and antimicrobial mediators. Moreover, macrophages are also responsible for maintaining tissue homeostasis by eliminating dead cells, debris, and foreign materials and exerting regulatory and repair functions (29). In this review, we discuss the details of mitochondrial metabolism and how it is involved in the inflammatory response of macrophages. In addition, we will briefly touch upon the role of mitochondria in immunological signal transduction processes.
ROLE OF MITOCHONDRIAL METABOLISM IN IMMUNE SIGNALING
Upon activation, proinflammatory macrophages undergo considerable metabolic transformations to meet the energetic and biochemical requirements of pathogen defense. Due to their central metabolic role, mitochondria are obviously an integral part in the governance of this procedure. In the following, we cover metabolic changes in proinflammatory macrophages with specific focus on mitochondria (Table 1).
Table 1.
Summary of the most important metabolic pathways in macrophages
| Metabolic Pathway | Specific Features | References |
|---|---|---|
| Glycolysis |
|
(30, 31) |
| Glycerol 3-phosphate shuttle |
|
(32) |
| Malate aspartate shuttle |
|
(33) |
| One carbon metabolism |
|
(34, 35) |
| TCA cycle |
|
(36) |
| Itaconate |
|
(37) |
| The urea cycle and the aspartate-argininosuccinate shunt |
|
(5) |
ACOD1, cis-aconitate decarboxylase; GPS, glycerol-3-phosphate shuttle; LPS, lipopolysaccharides; MAS, malate-aspartate shuttle; NO, nitric oxide; NOS2, nitric oxide synthase II; OXPHOS, oxidative phosphorylation; TCA, tricarboxylic acid cycle; 1 C, one carbon.
Glycolysis
Although glycolysis is a cytosolic process, it relies on mitochondrial function for NADH/NAD+ cofactor balancing through the malate-aspartate shuttle (MAS) or the glycerol-3-phosphate shuttle (GPS). Moreover, a significant fraction of glycolytic pyruvate is transported into mitochondria for oxidation and ATP production (Fig. 1; 31). Induced glycolysis is one of the hallmarks of classically activated macrophages and several factors are involved in its regulation, including hexokinase (HK), phosphofructokinase (PFK), hypoxia-inducible factor (HIF)-1α, glucose transporter 1 (GLUT1), carbohydrate kinase-like protein (CARKL), and pyruvate kinase (PKM; 31, 38). All of these factors operate in a well-concerted manner to achieve the rapid supply of energy and metabolic precursors for proper macrophage function. Although glycolysis is not economic in terms of energy production per glucose molecule, it provides cells with energy at a much higher rate than OXPHOS (39). Indeed, glycolysis is considered to be 100 times faster in energy production than OXPHOS and a high glycolytic flux ensures a sufficient supply with intermediates of branching pathways required for nucleotide, amino acid, and lipid biosynthesis (30). All of these are essential for the metabolic adaptation of the cell (39).
Glycerol 3-phoshphate Shuttle
The glycerol 3-phosphate shuttle (GPS) is one of the transport mechanisms for glucose-derived electrons to the electron transport chain (ETC) as glycolytic NADH itself cannot pass through the mitochondrial membrane. The GPS starts in the cytosol with a reduction of dihydroxyacetone phosphate (DHAP) to glycerol 3-phosphate (G3P) by cytosolic glycerol 3-phosphate dehydrogenase (GPD1) (Fig. 1). G3P is further transferred to the inner mitochondrial membrane, where it is oxidized back to DHAP by glycerol 3-phosphate dehydrogenase (GPD2) while the electrons are passed to the ETC by the reduction of FAD to FADH2, eventually resulting in the reduction of ubiquinone (Q) to ubiquinol (QH2; 32). The G3P shuttle was found to be important in proinflammatory macrophages and regulates their inflammatory response (32). GPD2 has been demonstrated to increase glucose oxidation, to induce expression of inflammatory mediators, and to fuel the production of acetyl coenzyme A (acetyl-CoA) required for the subsequent acetylation of histones in proinflammatory macrophages (32).
Malate-Aspartate Shuttle
The malate-aspartate shuttle (MAS) is a second mechanism for transferring electrons produced during glycolysis across the inner mitochondrial membrane to the ETC. The pathway starts in the cytosol with the reduction of oxaloacetate to malate by cytosolic malate dehydrogenase (MDH1). Malate is then transported into the mitochondria via the malate-α-ketoglutarate antiporter. Once inside, malate is oxidized back to oxaloacetate by mitochondrial malate dehydrogenase (MDH2). During this reaction, electrons are transferred to mitochondrial NADH to fuel the ETC via complex 1; it is thus more efficient than the GPS. To close the cycle, oxaloacetate and glutamate are transaminated to aspartate and α-ketoglutarate by the mitochondrial aspartate aminotransferase (GOT2). Aspartate is then transported to the cytosol by the glutamate-aspartate antiporter. Finally, it gets converted to oxaloacetate by the cytoplasmic aspartate aminotransferase (GOT1) to allow for the next electron transport process (Fig. 1; 40). Like the GPS, the MAS is required to maintain redox balance between mitochondria and cytoplasm and to support a high glycolytic flux in activated macrophages (33).
One Carbon Metabolism in Activated Macrophages
Folate-mediated one carbon (1 C) metabolism refers to connected metabolic pathways which include the folate and methionine cycles (34). 1 C pathways are located in the cytosol, the mitochondria and the nucleus (41). This pathway is essential to provide 1 C or methyl groups for the synthesis of DNA, amino acids, polyamines, creatine, and phospholipids (41). Moreover, 1 C metabolism plays a role in epigenetic regulations and redox defense (42). 1 C metabolism is particularly vital in highly metabolic active cells such as neuronal and immune cells (42, 43). In this regard, there is evidence which suggests that 1 C metabolism contributes to the energy demand of highly metabolically active cells. This can be demonstrated by reactions of 1 C metabolism which produce ATP and NADPH. For example, serine catabolism has been found to generate ATP with the activity of reverse 10-CHO-THF synthase (FTHFS; 44). Furthermore, serine catabolism is associated with NADPH generation through the activity of cytosolic and mitochondrial methylenetetrahydrofolate dehydrogenase (MTHFD) and 10-CHO-THF dehydrogenase (FTHFD; 44).
Besides their role in energy production, lipopolysaccharides (LPS) were found to induce 1 C metabolism in proinflammatory macrophages (35). Furthermore, 1 C metabolism was found to work synergistically with the induced pentose phosphate pathway and serine synthesis pathway to achieve epigenetic regulation and to induce interleukin (IL)-1β expression (35). S-adenosylmethionine (SAM), an end product of 1 C metabolism, has been demonstrated to be the driver for histone H3 lysine 36 trimethylation and IL-1β induction (35). The inhibition of SAM generation through impairment of the three aforementioned metabolic pathways leads to an anti-inflammatory effect, confirming the role of SAM in driving the inflammatory response (35).
Pyruvate Import into Mitochondria and Its Oxidative Decarboxylation
Mitochondrial pyruvate carrier 1 (MPC1) transfers pyruvate from the cytosol into the mitochondrial matrix where it can be oxidized and decarboxylated by the pyruvate dehydrogenase complex (PDC) to acetyl-CoA, the starting metabolite of the TCA or Krebs cycle (Fig. 1; 45). MPC1 plays a critical role in activated macrophages as inhibition of MPC1 resulted in reduced levels of the immune metabolite itaconate and eventually decreased the expression of immunoresponsive gene 1 (Irg1), inducible nitric oxide synthase (iNOS), and tumor necrosis factor α (Tnfα; 38, 46).
βOxidation and Carnitine Palmitoyl Transferase-1 System
β Oxidation of long chain fatty acids has been proposed to be important for IL-4-induced alternative polarization of macrophages which feature enhanced fatty acid oxidation (47). Carnitine palmitoyl transferase-1 (CPT-1), a mitochondrial outer membrane protein that facilitates long chain fatty acid uptake into the mitochondrial matrix for oxidation, was assumed to play an important role in macrophage polarization (Fig. 1; 47). Experiments with the CPT-1 inhibitor etomoxir highlighted an inhibiting effect on anti-inflammatory macrophage polarization (47–49). However, a recent study by Divakaruni et al. (47) has shown that long chain fatty acid oxidation was not a critical part of this process. In this context, they showed that previously applied etomoxir levels were high enough to deplete the intracellular pool of free coenzyme A, possibly via the conversion of prodrug etomoxir into active etomoxiryl CoA (47).
TCA Cycle
The Krebs or TCA cycle is central to mitochondrial metabolism and fuels the respiratory chain with reduced co-factors in the form of NADH and FADH2. It is a cycle of nine reactions that starts with the irreversible condensation of acetyl-CoA and oxaloacetate to citrate and ends with the synthesis of the next round’s precursor oxaloacetate (Fig. 1). Due to the two oxidative decarboxylation reactions catalyzed by isocitrate dehydrogenase (IDH) and 2-oxoglutarate dehydrogenase (2OGDH) in mammalian metabolism, mammalian cells cannot gain net biomass from acetyl-CoA through this cycle and the main role of the mitochondrial TCA cycle activity is the supply of energy. Nevertheless, TCA cycle activity is also a provider of critical intermediates for cell and macrophage function, such as aspartate and itaconate (36). This is the reason why TCA cycle fluxes are rerouted in pro-inflammatory macrophages to support macrophage function. The first major modification is the reduction of IDH activity. IDH is an enzyme which converts isocitrate to α-ketoglutarate and the downregulation of IDH in proinflammatory macrophages results in citrate accumulation. The accumulated citrate serves as a precursor for the synthesis of itaconate and cytosolic acetyl-CoA (Fig. 1; 37, 50). The second adjustment of TCA cycle activity in activated macrophages is the inhibition of succinate dehydrogenase (SDH) by accumulating itaconate (46, 51). SDH oxidizes succinate to fumarate and its inhibition ultimately yields to succinate accumulation. Tannahill et al. (52, 53) suggested that accumulated succinate induces IL-1β expression through HIF-1α stabilization (Fig. 2).
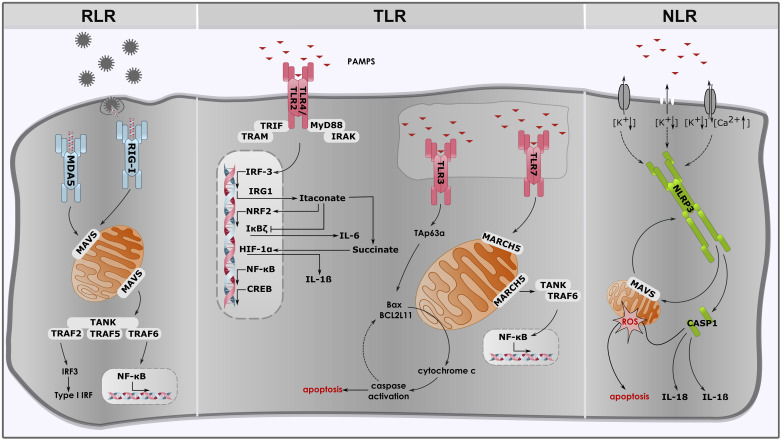
Figure 2.
Immune signaling of pattern recognition receptors (PRRs) and how mitochondria contribute to immune cell activation. RIG-I, retinoic acid inducible gene-I; MDA5, melanoma differentiation associated gene 5; MAVS, mitochondrial-antiviral-signaling-protein; TANK, TRAF family member-associated NF-κB activator; TRAF, tumor necrosis factor receptor (TNFR) associated factor; IRF3, interferon regulatory factor 3; IFNs, interferons; NF-κB, inducing nuclear factor κ-light-chain-enhancer of activated B cells; TLR, toll-like receptor; TRIF, TIR domain containing adapter-inducing interferon-β; TRAM, TRIF-related adapter molecule; MyD88, myeloid differentiation factor 88; IRAK, IL-1 receptor-associated kinase; IRG1, immunoresponsive gene 1; NRF2, nuclear factor erythroid 2-related factor 2; IκBζ, nuclear factor κ B zeta; HIF-1α, hypoxia-inducible factor; CREB, cAMP response element-binding protein; IL-6, interleukin-6; IL-1β, interleukin-1β; BAX, Bcl-2-associated X protein; BCL2L11, BCL2 like 11; MARCH5, protein E3 ubiquitin protein ligase; NLR, nuclear oligomerization domain (NOD)-like receptor; NLRP3, NLR family pyrin domain containing 3; ROS, reactive oxygen species; CASP1, caspase 1; IL-18, interleukin-18.
Contrary to proinflammatory macrophages, TCA cycle activity is induced in anti-inflammatory macrophages (5). Upon the induction of anti-inflammatory macrophages with IL-4, signal transducer activator of transcription 6 (STAT6), and peroxisome proliferator activator receptor γ (PPARγ)-coactivator-1β (PGC-1β) are induced, contributing to the development of the anti-inflammatory macrophage phenotype, metabolically including the upregulation of fatty acid oxidation, mitochondrial respiration, and the stimulation of mitochondrial biogenesis (5, 54).
Itaconate
Besides energy supply, TCA cycle metabolism is directly involved in antimicrobial defense mechanisms and immune modulation through the compound itaconic acid. Itaconic acid is an organic unsaturated dicarboxylic acid also known as methylene succinic acid (50). Michelucci et al. (37) demonstrated that this compound is produced in proinflammatory macrophages from cis-aconitate. Irg1 has been revealed as the gene encoding cis-aconitate decarboxylase (ACOD1), the enzyme producing itaconic acid (Fig. 1; 37). Irg1 is one of the most upregulated genes during proinflammatory conditions in murine macrophages and microglial cells (Fig. 2; 37, 55–57).
Itaconate acts as an antimicrobial compound because it is a potent inhibitor of isocitrate lyase (ICL), a key enzyme of the glyoxylate shunt, which is a biosynthetic pathway present in many microorganisms but absent in animals (58, 59). ICL in pathogens catalyzes the important conversion of isocitrate to glyoxylate and succinate, thereby circumventing the two decarboxylation reactions of the TCA cycle. The glyoxylate shunt and ICL activity are essential for microbial biomass production when growing on acetate or fatty acids as carbon sources (60). For example, the growth of Mycobacterium avium and M. tuberculosis depends on ICL activity when surviving inside macrophages, because they mainly employ host cell cholesterol as a carbon source, which is degraded to acetyl-CoA (58, 61). Another enzyme that is inhibited by itaconate is propionyl-CoA carboxylase. This enzyme catalyzes the conversion of propionyl-CoA to methylmalonyl-CoA, an important step for the detoxification of propionate via the citramalate cycle (62).
Many pathogens such as Yersinia pestis or Pseudomonas aeruginosa have been associated with developing countermeasures against itaconate intoxication (63). Three enzymes have been identified to degrade itaconate to pyruvate and acetyl-CoA: itaconate CoA transferase (Ict), itaconyl CoA hydratase (Ich) and (S)-citramalyl-CoA lyase (Ccl) (64). The genes encoding for these enzymes cluster in a genomic region called “required for intracellular proliferation” (ripABC) and represent a bacterial barrier against the antimicrobial effects of itaconate, even exploiting this anti-microbial metabolite as a carbon source (65). Inhibiting the itaconate degradation pathway in bacteria might represent a promising target for future anti-bacterial treatments (50, 65).
Apart from its antimicrobial properties, itaconate has also been demonstrated to possess anti-inflammatory properties. Part of the anti-inflammatory effect of itaconate has been suggested to be rooted in its inhibition of SDH (64). That is because of the role of SDH in driving the inflammatory response by reverse electron flow to complex I and ROS-mediated HIF-1α stabilization in activated macrophages (66). Mills et al. (67) have investigated the effect of the itaconate ester 4-octyl itaconate (4-OI) on mouse and human macrophages and revealed that this itaconate derivative increases levels of the transcription factor nuclear factor erythroid 2-related factor 2 (NRF2) via the inhibition of Kelch-like ECH-associated protein 1 (KEAP1). In turn, this results in an increased expression of NRF2 downstream target genes such as the anti-inflammatory protein heme oxygenase 1 (HMOX1). The increase of NRF2 expression was explained by an alkylation of a cysteine residue in KEAP1 through 4-OI. Under normal conditions, KEAP1 promotes degradation of NRF2, however, alkylation of cysteine residues in KEAP1 protein resulted in accumulation and activation of newly synthesized NRF2 (67).
In addition to the anti-inflammatory effect mediated by NRF2, itaconate has also been found to impair the glycolytic flux by inhibiting fructose-bisphosphate aldolase A (ALDOA) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), subsequently attenuating the inflammatory response in activated macrophages (68, 69). Furthermore, Bambouskova et al. (70) investigated the impact of the itaconate derivative dimethyl itaconate (DI) on mouse macrophages. DI was found to induce electrophilic stress and regulate the NF-kappa-B inhibitor zeta (IκBζ) protein via the key mediator activating transcription factor 3 (ATF3). Moreover, DI was found to inhibit IL-17 mediated-IκBζ induction in keratinocytes and ameliorate the pathology of psoriasis in a psoriasis mouse model which underlines the importance of this pathway as a new target for the treatment of autoimmune disease (70). However, recently ElAzzouny et al. (71) and Swain et al. (72) have demonstrated that none of these derivatives were converted to itaconate intracellularly, putting the proposed anti-inflammatory mechanisms of itaconate into question.
Finally, itaconate has been proposed to work synergistically with iNOS to achieve NLR Family Pyrin Domain Containing 3 (NLRP3) tolerance and prevent full caspase-1 activation (73). In another study, 4-OI was found to block NLRP3 activation by blocking NLRP3-NEK7 interaction, whereas Irg1−/− BMDMs exhibited increased NLRP3 inflammasome activation (74). This indicates a potential role of itaconate as a regulator of the NLRP3 inflammasome. Even though many studies have addressed itaconate’s function in immunomodulation, several aspects of related mechanisms are still not fully understood. Unraveling the details of the itaconate mode of action in mammals and bacteria could help to identify new promising antibacterial targets (65).
UREA CYCLE AND THE ASPARTATE-ARGININOSUCCINATE SHUNT
The aspartate-argininosuccinate shunt interconnects the urea and the TCA cycle. Argininosuccinate synthetase catalyzes the conversion of citrulline and aspartate to arginosuccinate which is then hydrolyzed to arginine and the TCA intermediate fumarate by argininosuccinate lyase (5). After hydration to malate by fumarase activity, malate can then pass the mitochondrial membrane. Malate is further involved in the TCA cycle, where it can be converted into either oxaloacetate or fumarate (Fig. 1). The action of the arginosuccinate shunt is twofold: First, it is an alternative source for fumarate synthesis and can replenish reduced fumarate production in proinflammatory macrophages in which SDH is inhibited by endogenous itaconate (75). Second, it is crucial for arginine synthesis which is critical for antimicrobial nitric oxide (NO) production through nitric oxide synthase II (NOS2; 5).
ATP-CITRATE LYASE AND HISTONE ACETYLATION
Upon Toll-like receptor (TLR)4 activation with LPS, glucose uptake and mitochondrial citrate synthesis are induced, as highlighted earlier. The accumulated citrate is partially exported into the cytosol via the citrate transporter protein (CTP) and then further converted to acetyl-CoA and oxaloacetate by ATP-dependent citrate lyase (ACLY), an enzyme which is upregulated under pro-inflammatory conditions. Lauterbach et al. (76) recently demonstrated that ACLY inhibition in activated macrophages leads to the downregulation of inflammatory genes such as Il6, Il12β, Il18, Il27, Cxcl9, and Cxcl10. The authors showed that reduced cytosolic acetyl-CoA caused by the inhibition of ACLY directly attenuates histone acetylation which is a prerequisite for the induction of specific LPS-inducible gene sets (76).
Even though ACLY is a major regulator of cytosolic acetyl-CoA production, this metabolite can also be produced from acetate through acetyl-CoA synthetase short-chain family member 2 (ACSS2) (77). This could explain why ACLY inhibition only reduces histone acetylation to a basal level and does not completely abolish it (76).
PROFILING MITOCHONDRIAL METABOLISM
In the previous sections, we highlighted the essential role of mitochondrial metabolism to mount the inflammatory response. In this regard, profiling mitochondrial metabolism is of high relevance for the understanding of immunometabolic mechanisms. However, the isolation of mitochondrial metabolites can be challenging as mitochondria are heavily tied to and intermingled with their cell. Several techniques have been developed to address this issue and aim to fractionate mitochondria from their cellular context. In 2007, Frezza et al. (78) reported a protocol for the isolation of mitochondria and performed a subsequent metabolic analysis. While this procedure is very efficient in terms of purity, it requires 1–2 h for the isolation, which drastically impacts the stability of the mitochondrial metabolome as metabolic reactions operate in the range of seconds. Later on, Chen et al. introduced a protocol to access mitochondrial metabolites based on high-affinity magnetic immunocapture and successfully profiled mitochondrial metabolites after a period of only 12 min (79). More recently, Nonnenmacher et al. developed a protocol to enrich mitochondrial metabolites via the selective permeabilization of the cytosolic membrane using the detergent digitonin (80, 81). This protocol has been demonstrated to be the fastest since selective permeabilization requires only one minute for removing the cytosolic contents of the cell while maintaining functional mitochondria for either direct metabolomic measurements or for further stable isotope-based targeted and non-targeted metabolic flux analyses (82, 83).
ROLE OF MITOCHONDRIA IN MOUNTING AN INFLAMMATION
Macrophages are activated by sensing sets of molecular targets known as pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) by pattern recognition receptors (PRRs) (84). PRRs include receptors such as retinoic acid inducible gene (RIG-I)-like receptors (RLRs), TLRs and nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) (25). In the next paragraphs, we will highlight how mitochondria support the signal transmission of PRRs after their activation.
RIG-I-LIKE RECEPTORS
RLR receptors such as RIG-I and melanoma differentiation associated gene 5 (MDA-5) are responsible for recognizing viral double stranded (ds) RNA. Activation of these receptors results in the induction of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and interferon regulatory factor 3 (IRF-3). This, in turn, causes the upregulation of type I interferons (IFNs) and other pro-inflammatory cytokines which promote antiviral immunity (85–88). Mitochondria have been demonstrated to be an integral part of this mechanism as they express the outer mitochondrial membrane (OMM) protein mitochondrial-antiviral-signaling-protein (MAVS). MAVS is also known as IFNβ promoter stimulator 1 (IPS1) or CARD adapter inducing IFNβ (CARDIF) or virus-induced signaling adaptor (VISA) (89–93). Upon activation with viral dsRNA, MAVS interacts with RIG-1 which results in the recruitment of tumor necrosis factor receptor (TNFR) associated factor (TRAF) 2, 5 and 6 (92). This promotes the antiviral response, including the activation of NF-κB and IRF signaling pathways (Fig. 2; 25, 86, 87). In this regard, localization of MAVS to the mitochondrial membrane has been demonstrated to be critical for its function (91). Seth et al. demonstrated that the mislocalization of MAVS either to the plasma membrane or the endoplasmic reticulum (ER) significantly reduced its activity (91). Additionally, an intact mitochondrial membrane potential as well as mitochondrial fusion and fission are a prerequisite for the RLR-induced antiviral response (25, 94).
TOLL-LIKE RECEPTORS
Another set of receptors involved in immune cell activation are TLRs. TLRs are transmembrane receptors expressed on the plasma membrane, the endosome or ER (25, 95). There are 13 members of the TLR family that have been identified in mammals so far, which are activated by PAMPs from bacteria, fungi, parasites or viruses (96–98). The activation of TLRs results in the upregulation and processing of several inflammatory cytokines such as IL-1β, IL-6 and TNFα (95). In this regard, mitochondria were found to be involved in TLR7 signaling (99). This receptor senses viral single stranded (ss)RNA and its activation induces increased expression of pro-inflammatory cytokines and type I interferons. The OMM protein E3 ubiquitin-protein ligase MARCH5 is important for TLR7 signal transmission to NF-κB (99). For this purpose, it interacts with TRAF family member-associated NF-κB-activator (TANK) and impairs its ability to inhibit TRAF6 (99). This is required for the induction of pro-inflammatory cytokines and immune cell activation (Fig. 2) (99). Furthermore, MARCH5 localization to the outer membrane of the mitochondrion is critical for its function as mislocalization has been shown to diminish its function (99). Another receptor thought to be associated with mitochondria is TLR3. TLR3 is activated by both, viral dsRNA and host mRNA released from damaged cells (100). Triggering TLR3 has been reported to induce apoptosis via a downstream regulator known as TAp63α (101). TAp63α induces the mitochondrial apoptosis pathway through an upregulation of B cell lymphoma 2 (Bcl-2) family proteins such as Bcl-2-associated X protein (Bax) and BCL2-like 11 (BCL2L11) (Fig. 2; 102).
Lastly, TLR2/4 also have an impact on mitochondria as they control mitochondrial biogenesis through several transcription factors such as cAMP response element-binding protein (CREB), NF-κB, IRF3 and NRF2 (Fig. 2; 25, 103). TLR4 is possibly the most studied TLR receptor in nucleated cells and recognizes LPS, part of the outer membrane of gram-negative bacteria (104). Upon LPS recognition, two major signaling pathways are activated. The first pathway is activated by myeloid differentiation factor 88 (Myd88) and IL-1 receptor-associated kinase 4 (IRAK4), which leads to NF-κB activation and the upregulation of inflammatory cytokines such as TNFα and IL-1β (104). The second pathway is activated via TIR domain-containing adapter-inducing interferon-β (TRIF) and TRIF-related adapter molecule (TRAM), which leads to IRF3 activation and the induction of type I interferons (104). LPS activation of macrophages leads to several modifications of mitochondrial metabolism, finally resulting in the polarization of macrophages into a pro-inflammatory subtype.
NOD-LIKE RECEPTORS
NLRs are the third family of receptors which are involved in innate immune cell activation. In addition to other intracellular danger signals, NLRs sense microbial products (105). Upon triggering NLRs, the innate immune system is activated through the induction of NF-κB and inflammatory caspases (105, 106). There are currently 22 known members of the NLR family (105), with NLRP3 possibly being the most studied among those (107, 108). It is an integral component of the inflammasome, a multiprotein complex acting as a danger signaling platform (25, 109), whereby it regulates the activity of caspase 1 and thus controls the processing and secretion of IL-1β and IL-18 (109–111).
The NLRP3 inflammasome is activated by a two-step procedure which includes several signals triggered by infection and metabolic dysregulation (108). To activate the NLRP3 Inflammasome, macrophages have to be primed with microbial molecules or endogenous cytokines to first induce NLRP3 and pro-IL-1β expression via NF-κB activation (112, 113). The second step is not fully understood and involves the sensing of either of ATP, viral RNA or pore-forming toxins, eventually inducing inflammasome assembly and the activation of pro-caspase 1 which then catalyzes the proteolytic cleavage of IL-1ß and IL-18 (112, 114).
Upon inflammasome activation, NLRP3 translocates from its resting location at the ER to mitochondria and mitochondria-associated ER membranes (25, 115). MAVS promotes NLRP3 recruitment to mitochondria and the induction of IL-1β processing (Fig. 2; 108). NLRP3 inflammasome activation is highly dependent on mitochondrial function, hence mitochondrial dysfunction was found to impair NLRP3 inflammasome activation (25, 115).
Independent of DAMPs and PAMPs, there are other factors which play a role in modulating mitochondrial metabolism and macrophage response (116). Other factors include oxygen and nutrient availability and metabolic cargo from cell debris (116). For example, decreased oxygen supply in hypoxic conditions lead to a shift in macrophage metabolism to anerobic glycolysis (46). Moreover, it was reported that hypoxic and inflammatory signals share certain transcriptional events such as the activation of HIF-1α and NF-κB families (117). This demonstrates that the microenvironment could modulate the metabolic profile of macrophages (117). This is especially important in cases such as ischemia and cancer, where the microenvironment changes, imposing metabolic alterations on macrophages (117). Studying such metabolic changes may help to identify novel targets which could have therapeutic applications in the future.
MITOCHONDRIAL REACTIVE OXYGEN SPECIES
Mitochondrial reactive oxygen species are produced as a byproduct during OXPHOS or in response to external stimuli (118–120). Too high levels of ROS are considered to be toxic as they can cause severe damage to DNA, lipids and proteins (120, 121). To a certain extent, cells can counteract this toxicity with the production of several ROS scavenging enzymes such as superoxide dismutase, catalase, and glutathione peroxidase (120).
Despite the harmful effects of ROS, several studies have revealed that moderate levels of ROS are essential for proper cell signaling (119, 122–124). As an example, H2O2 is produced when cells are stimulated by cytokines such as TNF-α, transforming growth factor beta-1 (TGF-β1) or interleukin-1 (119, 125–127). Upon induction, H2O2 functions as a second messenger in NF-κB activation (128). Furthermore, mROS produced by complex I is involved in NF-κB induction through the stabilization of HIF-1α and c-Src mediated phosphorylation of IκB-α (129). This activation could be prevented by treating cells with an antioxidant such as N-acetyl-l-cysteine (128). ROS also functions as an activator of the mitogen-activated protein kinase (MAPK) pathway and triggers its activation either by the inactivation of MAPK phosphatases (MKPs) (a group of protein phosphatases, responsible for dephosphorylation and inactivation of MAPKs) or by the oxidative modification of MAPK signaling proteins such as receptor tyrosine kinase (RTK) and MAP3Ks (130, 131). The activation of MAPKs and NF-κB pathways by ROS highlights their role during inflammation.
SUMMARY AND PERSPECTIVE
During evolution, mitochondria have developed to an integral player of macrophage function. This is not only because of their role in energy supply but also due to their metabolic support during inflammatory conditions. Moreover, these organelles provide macrophages with anti-microbial intermediates such as NO and itaconate, which are needed to combat pathogens. Our understanding of mitochondrial metabolism provides a key aspect for our knowledge of innate immune system signaling.
Proinflammatory and anti-inflammatory macrophages have distinct metabolic needs. Whereas pro-inflammatory macrophages depend more on glycolysis to achieve rapid energy supply at the inflammatory site, anti-inflammatory macrophages depend on OXPHOS to achieve sustainability for long-term processes (5, 26, 132).
Moreover, mitochondria are an important source of ROS which play a regulatory function during inflammation. Besides the stabilization of HIF-1α, ROS triggers the activation of NF-κB, MAPKs pathways and the NLRP3 inflammasome.
A key metabolite produced in activated macrophages is itaconate. In addition to its anti-microbial function, it is involved in immunomodulatory processes. Although its anti-inflammatory function is under debate, the specific mechanism is still not clear. Moreover, neither the subcellular location of its synthesis nor its degradation pathway in macrophages is completely explained.
With this review, we highlight the importance of mitochondria in macrophages. Furthermore, we want to motivate to study these organelles in more depth to gain a better understanding of their contribution to inflammatory processes and thus to the pathogenesis of infectious, inflammatory and autoimmune diseases.
GRANTS
This work was supported by Federal State of Lower Saxony, Niedersaechsisches Vorab CDInfect project, the University of Bonn and integrative data analytics for respiratory syncytial virus risk assessment (INDIRA).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.E.H. prepared figures; M.N. drafted manuscript; M.Z.N. and K.H. edited and revised manuscript; M.Z.N., J.E.H., and K.H. approved final version of manuscript.
REFERENCES
- 1.Gray MW. Mitochondrial evolution. Cold Spring Harb Perspect Biol 4: a011403, 2012. doi: 10.1101/cshperspect.a011403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fernández-Silva P, Enriquez JA, Montoya J. Replication and transcription of mammalian mitochondrial DNA. Exp Physiol 88: 41–56, 2003. doi: 10.1113/eph8802514. [DOI] [PubMed] [Google Scholar]
- 3.Detmer SA, Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol 8: 870–879, 2007. doi: 10.1038/nrm2275. [DOI] [PubMed] [Google Scholar]
- 4.Tandler B, Hoppel CL, Mears JA. Morphological pathways of mitochondrial division. Antioxidants (Basel) 7: 30, 2018. doi: 10.3390/antiox7020030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tur J, Vico T, Lloberas J, Zorzano A, Celada A. Macrophages and mitochondria: a critical interplay between metabolism, signaling, and the functional activity. In: Advances in Immunology, edited by Frederick WA. Amsterdam: Academic Press, 2017, vol. 133, p. 1–36. doi: 10.1016/bs.ai.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 6.van der Giezen M, Tovar J. Degenerate mitochondria. EMBO Rep 6: 525–530, 2005. doi: 10.1038/sj.embor.7400440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan NA, Govindaraj P, Meena AK, Thangaraj K. Mitochondrial disorders: challenges in diagnosis & treatment. Indian J Med Res Suppl 141: 13–26, 2015. doi: 10.4103/0971-5916.154489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greaves LC, Reeve AK, Taylor RW, Turnbull DM. Mitochondrial DNA and disease. J Pathol 226: 274–286, 2012. doi: 10.1002/path.3028. [DOI] [PubMed] [Google Scholar]
- 9.Newmeyer DD, Ferguson-Miller S. Mitochondria: releasing power for life and unleashing the machineries of death. Cell 112: 481–490, 2003. [Erratum in Cell 6: 873]. doi: 10.1016/S0092-8674(03)00116-8. [DOI] [PubMed] [Google Scholar]
- 10.McElroy GS, Chandel NS. Probing mitochondrial metabolism in vivo. Proc Natl Acad Sci USA 116: 20–22, 2019. doi: 10.1073/PNAS.1819614116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown WM, George M Jr, Wilson AC. Rapid evolution of animal mitochondrial DNA. Proc Natl Acad Sci USA 76: 1967–1971, 1979. doi: 10.1073/pnas.76.4.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song S, Pursell ZF, Copeland WC, Longley MJ, Kunkel TA, Mathews CK. DNA precursor asymmetries in mammalian tissue mitochondria and possible contribution to mutagenesis through reduced replication fidelity. Proc Natl Acad Sci USA 102: 4990–4995, 2005. doi: 10.1073/pnas.0500253102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fazzini F, Schöpf B, Blatzer M, Coassin S, Hicks AA, Kronenberg F, Fendt L. Plasmid-normalized quantification of relative mitochondrial DNA copy number. Sci Rep 8: 15347, 2018. doi: 10.1038/s41598-018-33684-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robin ED, Wong R. Mitochondrial DNA molecules and virtual number of mitochondria per cell in mammalian cells. J Cell Physiol 136: 507–513, 1988. doi: 10.1002/jcp.1041360316. [DOI] [PubMed] [Google Scholar]
- 15.Aryaman J, Johnston IG, Jones NS. . Mitochondrial heterogeneity. Front Genet 9: 718, 2019. doi: 10.3389/fgene.2018.00718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berg OG, Kurland CG. Why mitochondrial genes are most often found in nuclei. Mol Biol Evol 17: 951–961, 2000. doi: 10.1093/oxfordjournals.molbev.a026376. [DOI] [PubMed] [Google Scholar]
- 17.Castegna A, Iacobazzi V, Infantino V. The mitochondrial side of epigenetics. Physiol Genomics 47: 299–307, 2015. doi: 10.1152/physiolgenomics.00096.2014. [DOI] [PubMed] [Google Scholar]
- 18.Sripada L, Tomar D, Prajapati P, Singh R, Singh AK, Singh R. Systematic analysis of small RNAs associated with human mitochondria by deep sequencing: detailed analysis of mitochondrial associated miRNA. PLoS One 7: e44873, 2012. doi: 10.1371/journal.pone.0044873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moon H-G, Yang J, Zheng Y, Jin Y. miR-15a/16 regulates macrophage phagocytosis after bacterial infection. J Immunol 193: 4558–4567, 2014. doi: 10.4049/jimmunol.1401372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karunakaran D, Thrush AB, Nguyen MA, Richards L, Geoffrion M, Singaravelu R, Ramphos E, Shangari P, Ouimet M, Pezacki JP, Moore KJ, Perisic L, Maegdefessel L, Hedin U, Harper ME, Rayner KJ. Macrophage mitochondrial energy status regulates cholesterol efflux and is enhanced by anti-miR33 in atherosclerosis. Circ Res 117: 266–278, 2015. doi: 10.1161/CIRCRESAHA.117.305624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei Y, Zhu M, Schober A. Macrophage MicroRNAs as therapeutic targets for atherosclerosis, metabolic syndrome, and cancer. IJMS 19: 1756, 2018. doi: 10.3390/ijms19061756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao X-Y, Lu M-H, Yuan D-J, Xu D-E, Yao P-P, Ji W-L, Chen H, Liu W-L, Yan C-X, Xia Y-Y, Li S, Tao J, Ma Q-H. Mitochondrial dysfunction in neural injury. Front Neurosci 13: 30, 2019. doi: 10.3389/fnins.2019.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faas MM, de Vos P. Mitochondrial function in immune cells in health and disease. Biochim Biophys Acta Mol Basis Dis 1866: 165845, 2020. doi: 10.1016/j.bbadis.2020.165845. [DOI] [PubMed] [Google Scholar]
- 24.Will Y, Shields JE, Wallace KB. Drug-induced mitochondrial toxicity in the geriatric population: challenges and future directions. Biology (Basel) 8: 32, 2019. doi: 10.3390/biology8020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cloonan SM, Choi AMK. Mitochondria: sensors and mediators of innate immune receptor signaling. Curr Opin Microbiol 16: 327–338, 2013. doi: 10.1016/j.mib.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ganeshan K, Chawla A. Metabolic regulation of immune responses. Annu Rev Immunol 32: 609–634, 2014. doi: 10.1146/annurev-immunol-032713-120236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eapen MS, Sharma P, Sohal SS. Mitochondrial dysfunction in macrophages: a key to defective bacterial phagocytosis in COPD. Eur Respir J 54: 1901641, 2019. doi: 10.1183/13993003.01641-2019. [DOI] [PubMed] [Google Scholar]
- 28.Van den Bossche J, Baardman J, Otto NA, van der Velden S, Neele AE, van den Berg SM, Luque-Martin R, Chen H-J, Boshuizen MCS, Ahmed M, Hoeksema MA, de Vos AF, de Winther MPJ. Mitochondrial dysfunction prevents repolarization of inflammatory macrophages. Cell Rep 17: 684–696, 2016. doi: 10.1016/j.celrep.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Hirayama D, Iida T, Nakase H. The phagocytic function of macrophage-enforcing innate immunity and tissue homeostasis. Int J Mol Sci 19: 92, 2017. doi: 10.3390/ijms19010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Epstein T, Gatenby RA, Brown JS. The Warburg effect as an adaptation of cancer cells to rapid fluctuations in energy demand. PLoS One 12: e0185085–e0185085, 2017. doi: 10.1371/journal.pone.0185085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soto-Heredero G, Gómez de las Heras MM, Gabandé-Rodríguez E, Oller J, Mittelbrunn M. Glycolysis–a key player in the inflammatory response. FEBS J 287: 3350–3369, 2020. doi: 10.1111/febs.15327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langston PK, Nambu A, Jung J, Shibata M, Aksoylar HI, Lei J, Xu P, Doan MT, Jiang H, MacArthur MR, Gao X, Kong Y, Chouchani ET, Locasale JW, Snyder NW, Horng T. Glycerol phosphate shuttle enzyme GPD2 regulates macrophage inflammatory responses. Nat Immunol 20: 1186–1195, 2019. [Erratum in Nat Immunol 20: 1555, 2019]. doi: 10.1038/s41590-019-0453-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spinelli JB, Haigis MC. The multifaceted contributions of mitochondria to cellular metabolism. Nat Cell Biol 20: 745–754, 2018. doi: 10.1038/s41556-018-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clare CE, Brassington AH, Kwong WY, Sinclair KD. One-carbon metabolism: linking nutritional biochemistry to epigenetic programming of long-term development. Annu Rev Anim Biosci 7: 263–287, 2019. doi: 10.1146/annurev-animal-020518-115206. [DOI] [PubMed] [Google Scholar]
- 35.Yu W, Wang Z, Zhang K, Chi Z, Xu T, Jiang D, Chen S, Li W, Yang X, Zhang X, Wu Y, Wang D. One-carbon metabolism supports S-adenosylmethionine and histone methylation to drive inflammatory macrophages. Mol Cell 75: 1147–1160.e5, 2019. doi: 10.1016/j.molcel.2019.06.039. [DOI] [PubMed] [Google Scholar]
- 36.Sullivan LB, Gui DY, Hosios AM, Bush LN, Freinkman E, Vander Heiden MG. Supporting aspartate biosynthesis is an essential function of respiration in proliferating cells. Cell 162: 552–563, 2015. doi: 10.1016/j.cell.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Michelucci A, Cordes T, Ghelfi J, Pailot A, Reiling N, Goldmann O, Binz T, Wegner A, Tallam A, Rausell A, Buttini M, Linster CL, Medina E, Balling R, Hiller K. Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proc Natl Acad Sci USA 110: 7820–7825, 2013. doi: 10.1073/pnas.1218599110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nonnenmacher Y, Hiller K. Biochemistry of proinflammatory macrophage activation. Cell Mol Life Sci 75: 2093–2109, 2018. doi: 10.1007/s00018-018-2784-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Viola A, Munari F, Sánchez-Rodríguez R, Scolaro T, Castegna A. The metabolic signature of macrophage responses. Front Immunol 10: 1462, 2019. doi: 10.3389/fimmu.2019.01462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borst P. The malate-aspartate shuttle (Borst cycle): how it started and developed into a major metabolic pathway. IUBMB Life 72: 2241–2259, 2020. doi: 10.1002/iub.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dekhne AS, Hou Z, Gangjee A, Matherly LH. Therapeutic targeting of mitochondrial one-carbon metabolism in cancer. Mol Cancer Ther 19: 2245–2255, 2020. doi: 10.1158/1535-7163.MCT-20-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ducker GS, Rabinowitz JD. One-carbon metabolism in health and disease. Cell Metab 25: 27–42, 2017. doi: 10.1016/j.cmet.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kurniawan H, Kobayashi T, Brenner D. The emerging role of one-carbon metabolism in T cells. Curr Opin Biotechnol 68: 193–201, 2021. doi: 10.1016/j.copbio.2020.12.001. [DOI] [PubMed] [Google Scholar]
- 44.Meiser J, Vazquez A. Give it or take it: the flux of one‐carbon in cancer cells. FEBS J 283: 3695–3704, 2016. doi: 10.1111/febs.13731. [DOI] [PubMed] [Google Scholar]
- 45.Bender T, Martinou J-C. The mitochondrial pyruvate carrier in health and disease: to carry or not to carry? Biochim Biophys Acta 1863: 2436–2442, 2016. doi: 10.1016/j.bbamcr.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 46.Meiser J, Krämer L, Sapcariu SC, Battello N, Ghelfi J, D'Herouel AF, Skupin A, Hiller K. Pro-inflammatory macrophages sustain pyruvate oxidation through pyruvate dehydrogenase for the synthesis of itaconate and to enable cytokine expression. J Biol Chem 291: 3932–3946, 2016. doi: 10.1074/jbc.M115.676817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Divakaruni AS, Hsieh WY, Minarrieta L, Duong TN, Kim KKO, Desousa BR, Andreyev AY, Bowman CE, Caradonna K, Dranka BP, Ferrick DA, Liesa M, Stiles L, Rogers GW, Braas D, Ciaraldi TP, Wolfgang MJ, Sparwasser T, Berod L, Bensinger SJ, Murphy AN. Etomoxir inhibits macrophage polarization by disrupting CoA homeostasis. Cell Metab 28: 490–503.e7, 2018. doi: 10.1016/j.cmet.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang SC-C, Smith AM, Everts B, Colonna M, Pearce EL, Schilling JD, Pearce EJ. Metabolic reprogramming mediated by the mTORC2-IRF4 signaling axis is essential for macrophage alternative activation. Immunity 45: 817–830, 2016. doi: 10.1016/j.immuni.2016.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang SC-C, Everts B, Ivanova Y, O'Sullivan D, Nascimento M, Smith AM, Beatty W, Love-Gregory L, Lam WY, O'Neill CM, Yan C, Du H, Abumrad NA, Urban JF, Artyomov MN, Pearce EL, Pearce EJ. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat Immunol 15: 846–855, 2014. doi: 10.1038/ni.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cordes T, Michelucci A, Hiller K. Itaconic acid: the surprising role of an industrial compound as a mammalian antimicrobial metabolite. Annu Rev Nutr 35: 451–473, 2015. doi: 10.1146/annurev-nutr-071714-034243. [DOI] [PubMed] [Google Scholar]
- 51.Cordes T, Wallace M, Michelucci A, Divakaruni AS, Sapcariu SC, Sousa C, Koseki H, Cabrales P, Murphy AN, Hiller K, Metallo CM. Immunoresponsive gene 1 and itaconate inhibit succinate dehydrogenase to modulate intracellular succinate levels. J Biol Chem 291: 14274–14284, 2016. doi: 10.1074/jbc.M115.685792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, Pan Y, Simon MC, Thompson CB, Gottlieb E. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-α prolyl hydroxylase. Cancer Cell 7: 77–85, 2005. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 53.Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH, Zheng L, Gardet A, Tong Z, Jany SS, Corr SC, Haneklaus M, Caffrey BE, Pierce K, Walmsley S, Beasley FC, Cummins E, Nizet V, Whyte M, Taylor CT, Lin H, Masters SL, Gottlieb E, Kelly VP, Clish C, Auron PE, Xavier RJ, O'Neill LA. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 496: 238–242, 2013. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lelliott CJ, Medina-Gomez G, Petrovic N, Kis A, Feldmann HM, Bjursell M, Parker N, Curtis K, Campbell M, Hu P, Zhang D, Litwin SE, Zaha VG, Fountain KT, Boudina S, Jimenez-Linan M, Blount M, Lopez M, Meirhaeghe A, Bohlooly-Y M, Storlien L, Strömstedt M, Snaith M, Orešič M, Abel ED, Cannon B, Vidal-Puig A. Ablation of PGC-1β results in defective mitochondrial activity, thermogenesis, hepatic function, and cardiac performance. PLoS Biol 4: e369, 2006. doi: 10.1371/journal.pbio.0040369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Basler T, Jeckstadt S, Valentin-Weigand P, Goethe R. Mycobacterium paratuberculosis, Mycobacterium smegmatis, and lipopolysaccharide induce different transcriptional and post-transcriptional regulation of the IRG1 gene in murine macrophages. J Leukoc Biol 79: 628–638, 2006. doi: 10.1189/jlb.0905520. [DOI] [PubMed] [Google Scholar]
- 56.Degrandi D, Hoffmann R, Beuter-Gunia C, Pfeffer K. The proinflammatory cytokine-induced IRG1 protein associates with mitochondria. J Interf Cytokine Res 29: 55–67, 2009. [Erratum in J Interferon Cytokine Res 29: 643, 2009]. doi: 10.1089/jir.2008.0013. [DOI] [PubMed] [Google Scholar]
- 57.Thomas DM, Francescutti-Verbeem DM, Kuhn DM. Gene expression profile of activated microglia under conditions associated with dopamine neuronal damage. FASEB J 20: 515–517, 2006. doi: 10.1096/fj.05-4873fje. [DOI] [PubMed] [Google Scholar]
- 58.McFadden BA, Purohit S. Itaconate, an isocitrate lyase-directed inhibitor in Pseudomonas indigofera. J Bacteriol 131: 136–144, 1977. doi: 10.1128/jb.131.1.136-144.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Williams JO, Roche TE, Mcfadden BA. Mechanism of action of isocitrate lyase from pseudomonas indigoferd. Biochemistry 10: 1384–1390, 1971. doi: 10.1021/bi00784a017. [DOI] [PubMed] [Google Scholar]
- 60.Park C, Shin B, Park W. Alternative fate of glyoxylate during acetate and hexadecane metabolism in Acinetobacter oleivorans DR1. Sci Rep 9: 14402, 2019. doi: 10.1038/s41598-019-50852-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Patel TR, McFadden BA. Caenorhabditis elegans and Ascaris suum: inhibition of isocitrate lyase by itaconate. Exp Parasitol 44: 262–268, 1978. doi: 10.1016/0014-4894(78)90107-8. [DOI] [PubMed] [Google Scholar]
- 62.Berg IA, Filatova LV, Ivanovsky RN. Inhibition of acetate and propionate assimilation by itaconate via propionyl-CoA carboxylase in isocitrate lyase-negative purple bacterium Rhodospirillum rubrum. FEMS Microbiol Lett 216: 49–54, 2002. doi: 10.1111/j.1574-6968.2002.tb11413.x. [DOI] [PubMed] [Google Scholar]
- 63.Sasikaran J, Ziemski M, Zadora PK, Fleig A, Berg IA. Bacterial itaconate degradation promotes pathogenicity. Nat Chem Biol 10: 371–377, 2014. doi: 10.1038/nchembio.1482. [DOI] [PubMed] [Google Scholar]
- 64.Cordes T, Lucas A, Divakaruni AS, Murphy AN, Cabrales P, Metallo CM. Itaconate modulates tricarboxylic acid and redox metabolism to mitigate reperfusion injury. Mol Metab 32: 122–135, 2020. doi: 10.1016/j.molmet.2019.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ménage S, Attrée I. Pathogens love the poison. Nat Chem Biol 10: 326–327, 2014. doi: 10.1038/nchembio.1505. [DOI] [PubMed] [Google Scholar]
- 66.Mills EL, Kelly B, Logan A, Costa ASH, Varma M, Bryant CE, Tourlomousis P, Däbritz JHM, Gottlieb E, Latorre I, Corr SC, McManus G, Ryan D, Jacobs HT, Szibor M, Xavier RJ, Braun T, Frezza C, Murphy MP, O’Neill LA. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell 167: 457–470.e13, 2016. doi: 10.1016/j.cell.2016.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mills EL, Ryan DG, Prag HA, Dikovskaya D, Menon D, Zaslona Z, Jedrychowski MP, Costa ASH, Higgins M, Hams E, Szpyt J, Runtsch MC, King MS, McGouran JF, Fischer R, Kessler BM, McGettrick AF, Hughes MM, Carroll RG, Booty LM, Knatko EV, Meakin PJ, Ashford MLJ, Modis LK, Brunori G, Sévin DC, Fallon PG, Caldwell ST, Kunji ERS, Chouchani ET, Frezza C, Dinkova-Kostova AT, Hartley RC, Murphy MP, O'Neill LA. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature 556: 113–117, 2018. doi: 10.1038/nature25986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qin W, Qin K, Zhang Y, Jia W, Chen Y, Cheng B, Peng L, Chen N, Liu Y, Zhou W, Wang YL, Chen X, Wang C. S-glycosylation-based cysteine profiling reveals regulation of glycolysis by itaconate. Nat Chem Biol 15: 983–991, 2019. doi: 10.1038/s41589-019-0323-5. [DOI] [PubMed] [Google Scholar]
- 69.Liao S-T, Han C, Xu D-Q, Fu X-W, Wang J-S, Kong L-Y. 4-Octyl itaconate inhibits aerobic glycolysis by targeting GAPDH to exert anti-inflammatory effects. Nat Commun 10: 5091, 2019. doi: 10.1038/s41467-019-13078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bambouskova M, Gorvel L, Lampropoulou V, Sergushichev A, Loginicheva E, Johnson K, Korenfeld D, Mathyer ME, Kim H, Huang LH, Duncan D, Bregman H, Keskin A, Santeford A, Apte RS, Sehgal R, Johnson B, Amarasinghe GK, Soares MP, Satoh T, Akira S, Hai T, de Guzman Strong C, Auclair K, Roddy TP, Biller SA, Jovanovic M, Klechevsky E, Stewart KM, Randolph GJ, Artyomov MN. Electrophilic properties of itaconate and derivatives regulate the IκBζ-ATF3 inflammatory axis. Nature 556: 501–504, 2018. doi: 10.1038/s41586-018-0052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.ElAzzouny M, Tom CTMB, Evans CR, Olson LL, Tanga MJ, Gallagher KA, Martin BR, Burant CF. Dimethyl itaconate is not metabolized into itaconate intracellularly. J Biol Chem 292: 4766–4769, 2017. doi: 10.1074/jbc.C117.775270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Swain A, Bambouskova M, Kim H, Andhey PS, Duncan D, Auclair K, Chubukov V, Simons DM, Roddy TP, Stewart KM, Artyomov MN. Comparative evaluation of itaconate and its derivatives reveals divergent inflammasome and type I interferon regulation in macrophages. Nat Metab 2: 594–602, 2020. doi: 10.1038/s42255-020-0210-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bambouskova M, Potuckova L, Paulenda T, Kerndl M, Mogilenko DA, Lizotte K, Swain A, Hayes S, Sheldon RD, Kim H, Kapadnis U, Ellis AE, Isaguirre C, Burdess S, Laha A, Amarasinghe GK, Chubukov V, Roddy TP, Diamond MS, Jones RG, Simons DM, Artyomov MN. Itaconate confers tolerance to late NLRP3 inflammasome activation. Cell Rep 34: 108756, 2021. doi: 10.1016/j.celrep.2021.108756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hooftman A, Angiari S, Hester S, Corcoran SE, Runtsch MC, Ling C, Ruzek MC, Slivka PF, McGettrick AF, Banahan K, Hughes MM, Irvine AD, Fischer R, O’Neill LAJ. The immunomodulatory metabolite itaconate modifies NLRP3 and inhibits inflammasome activation. Cell Metab 32: 468–478.e7, 2020. doi: 10.1016/j.cmet.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu Y, Xu R, Gu H, Zhang E, Qu J, Cao W, Huang X, Yan H, He J, Cai Z. Metabolic reprogramming in macrophage responses. Biomark Res 9: 1, 2021. doi: 10.1186/s40364-020-00251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lauterbach MA, Hanke JE, Serefidou M, Mangan MSJ, Kolbe C-C, Hess T, Rothe M, Kaiser R, Hoss F, Gehlen J, Engels G, Kreutzenbeck M, Schmidt SV, Christ A, Imhof A, Hiller K, Latz E. Toll-like receptor signaling rewires macrophage metabolism and promotes histone acetylation via ATP-citrate lyase. Immunity 51: 997–1011.e7, 2019. doi: 10.1016/j.immuni.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 77.Vysochan A, Sengupta A, Weljie AM, Alwine JC, Yu Y. ACSS2-mediated acetyl-CoA synthesis from acetate is necessary for human cytomegalovirus infection. Proc Natl Acad Sci USA 114: E1528–E1535, 2017. doi: 10.1073/PNAS.1614268114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Frezza C, Cipolat S, Scorrano L. Organelle isolation: functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nat Protoc 2: 287–295, 2007. doi: 10.1038/nprot.2006.478. [DOI] [PubMed] [Google Scholar]
- 79.Chen WW, Freinkman E, Sabatini DM. Rapid immunopurification of mitochondria for metabolite profiling and absolute quantification of matrix metabolites. Nat Protoc 12: 2215–2231, 2017. doi: 10.1038/nprot.2017.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nonnenmacher Y, Palorini R, d'Herouël AF, Krämer L, Neumann-Schaal M, Chiaradonna F, Skupin A, Wegner A, Hiller K. Analysis of mitochondrial metabolism in situ: combining stable isotope labeling with selective permeabilization. Metab Eng 43: 147–155, 2017. doi: 10.1016/j.ymben.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 81.Nonnenmacher Y, Palorini R, Hiller K. Determining compartment-specific metabolic fluxes. In: Methods in Molecular Biology, edited by Fendt SM, Lunt S.. New York, NY: Humana Press Inc. 2019, p. 137–149. [DOI] [PubMed] [Google Scholar]
- 82.Weindl D, Cordes T, Battello N, Sapcariu SC, Dong X, Wegner A, Hiller K. Bridging the gap between non-targeted stable isotope labeling and metabolic flux analysis. Cancer Metab 4: 10, 2016. doi: 10.1186/s40170-016-0150-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Weindl D, Wegner A, Hiller K. MIA: non-targeted mass isotopolome analysis. Bioinformatics 32: 2875–2876, 2016. doi: 10.1093/bioinformatics/btw317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schroder K, Tschopp J. The Inflammasomes. Cell 140: 821–832, 2010. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 85.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science 327: 291–295, 2010. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Koshiba T. Mitochondrial-mediated antiviral immunity. Biochim Biophys Acta 1833: 225–232, 2013. doi: 10.1016/j.bbamcr.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 87.Le Bon A, Tough DF. Links between innate and adaptive immunity via type I interferon. Curr Opin Immunol 14: 432–436, 2002. doi: 10.1016/S0952-7915(02)00354-0. [DOI] [PubMed] [Google Scholar]
- 88.Takeuchi O, Akira S. Innate immunity to virus infection. Immunol Rev 227: 75–86, 2009. doi: 10.1111/j.1600-065X.2008.00737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kawai T, Takahashi K, Sato S, Coban C, Kumar H, Kato H, Ishii KJ, Takeuchi O, Akira S. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol 6: 981–988, 2005. doi: 10.1038/ni1243. [DOI] [PubMed] [Google Scholar]
- 90.Meylan E, Curran J, Hofmann K, Moradpour D, Binder M, Bartenschlager R, Tschopp J. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 437: 1167–1172, 2005. doi: 10.1038/nature04193. [DOI] [PubMed] [Google Scholar]
- 91.Seth RB, Sun L, Ea C-K, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-κB and IRF3. Cell 122: 669–682, 2005. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 92.Wu B, Hur S. How RIG-I like receptors activate MAVS. Curr Opin Virol 12: 91–98, 2015. doi: 10.1016/j.coviro.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xu L-G, Wang Y-Y, Han K-J, Li L-Y, Zhai Z, Shu H-B. VISA is an adapter protein required for virus-triggered IFN-β signaling. Mol Cell 19: 727–740, 2005. doi: 10.1016/j.molcel.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 94.Koshiba T, Yasukawa K, Yanagi Y, Kawabata SI. Mitochondrial membrane potential is required for MAVS-mediated antiviral signaling. Sci Signal 4: ra7, 2011. doi: 10.1126/scisignal.2001147. [DOI] [PubMed] [Google Scholar]
- 95.Tannahill GM, O'Neill LAJ. The emerging role of metabolic regulation in the functioning of Toll-like receptors and the NOD-like receptor Nlrp3. FEBS Lett 585: 1568–1572, 2011. doi: 10.1016/j.febslet.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 96.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11: 373–384, 2010. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 97.Lim KH, Staudt LM. Toll-Like receptor signaling. Cold Spring Harb Perspect Biol 5: a011247, 2013. doi: 10.1101/cshperspect.a011247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.O'Neill LAJ, Golenbock D, Bowie AG. The history of toll-like receptors—redefining innate immunity. Nat Rev Immunol 13: 453–460, 2013. doi: 10.1038/nri3446. [DOI] [PubMed] [Google Scholar]
- 99.Shi HX, Liu X, Wang Q, Tang PP, Liu XY, Shan YF, Wang C. Mitochondrial ubiquitin ligase MARCH5 promotes TLR7 signaling by attenuating TANK action. PLoS Pathog 7: e1002057, 2011. doi: 10.1371/journal.ppat.1002057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Djafarzadeh S, Vuda M, Takala J, Ochs M, Jakob SM. Toll-like receptor-3-induced mitochondrial dysfunction in cultured human hepatocytes. Mitochondrion 11: 83–88, 2011. doi: 10.1016/j.mito.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 101.Sun R, Zhang Y, Lv Q, Liu B, Jin M, Zhang W, He Q, Deng M, Liu X, Li G, Li Y, Zhou G, Xie P, Xie X, Hu J, Duan Z. Toll-like receptor 3 (TLR3) induces apoptosis via death receptors and mitochondria by up-regulating the transactivating p63 isoform α (TAP63α). J Biol Chem 286: 15918–15928, 2011. doi: 10.1074/jbc.M110.178798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gressner O, Schilling T, Lorenz K, Schleithoff ES, Koch A, Schulze-Bergkamen H, Lena AM, Candi E, Terrinoni A, Catani MV, Oren M, Melino G, Krammer PH, Stremmel W, Müller MT. induces apoptosis by activating signaling via death receptors and mitochondria. EMBO J 24: 2458–2471, 2005. doi: 10.1038/sj.emboj.7600708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Piantadosi CA, Suliman HB. Transcriptional control of mitochondrial biogenesis and its interface with inflammatory processes. Biochim Biophys Acta 1820: 532–541, 2012. doi: 10.1016/j.bbagen.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vallance TM, Zeuner MT, Williams HF, Widera D, Vaiyapuri S. Toll-like receptor 4 signalling and its impact on platelet function, thrombosis, and haemostasis. Mediators Inflamm 2017: 9605894, 2017. doi: 10.1155/2017/9605894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Proell M, Riedl SJ, Fritz JH, Rojas AM, Schwarzenbacher R. The nod-like receptor (NLR) family: a tale of similarities and differences. PLoS One 3: e2119, 2008. doi: 10.1371/journal.pone.0002119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, Erickson S, Dixit VM. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 430: 213–218, 2004. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 107.Gaidt MM, Hornung V. The NLRP3 inflammasome renders cell death pro-inflammatory. J Mol Biol 430: 133–141, 2018. doi: 10.1016/j.jmb.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 108.Subramanian N, Natarajan K, Clatworthy MR, Wang Z, Germain RN. The adaptor MAVS promotes NLRP3 mitochondrial localization and inflammasome activation. Cell 153: 348–361, 2013. doi: 10.1016/j.cell.2013.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nakahira K, Haspel JA, Rathinam VAK, Lee SJ, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, Fitzgerald KA, Ryter SW, Choi AMK. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol 12: 222–230, 2011. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Próchnicki T, Latz E. Inflammasomes on the crossroads of innate immune recognition and metabolic control. Cell Metab 26: 71–93, 2017. doi: 10.1016/j.cmet.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 111.Rathinam VAK, Jiang Z, Waggoner SN, Sharma S, Cole LE, Waggoner L, Vanaja SK, Monks BG, Ganesan S, Latz E, Hornung V, Vogel SN, Szomolanyi-Tsuda E, Fitzgerald KA. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol 11: 395–402, 2010. doi: 10.1038/ni.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.He Y, Hara H, Núñez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci 41: 1012–1021, 2016. doi: 10.1016/j.tibs.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hornung V, Latz E. Critical functions of priming and lysosomal damage for NLRP3 activation. Eur J Immunol 40: 620–623, 2010. doi: 10.1002/eji.200940185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Neumann K, Ruland J. Kinases conquer the inflammasomes. Nat Immunol 14: 1207–1208, 2013. doi: 10.1038/ni.2763. [DOI] [PubMed] [Google Scholar]
- 115.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature 469: 221–225, 2011. [Erratum in Nature 475: 122, 2011]. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 116.Zhao M, Wang DD-H, Liu X, Tian R. Metabolic modulation of macrophage function post myocardial infarction. Front Physiol 11: 674, 2020. doi: 10.3389/fphys.2020.00674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Riboldi E, Porta C, Morlacchi S, Viola A, Mantovani A, Sica A. Hypoxia-mediated regulation of macrophage functions in pathophysiology. Int Immunol 25: 67–75, 2013. doi: 10.1093/intimm/dxs110. [DOI] [PubMed] [Google Scholar]
- 118.Gyu Choi T, Soo Kim S. Physiological functions of mitochondrial reactive oxygen species. In: Free Radical Medicine and Biology. Amsterdam: IntechOpen, 2019. doi: 10.5772/intechopen.88386. [DOI] [Google Scholar]
- 119.Rhee SG. Redox signaling: hydrogen peroxide as intracellular messenger. Exp Mol Med 31: 53–59, 1999. doi: 10.1038/emm.1999.9. [DOI] [PubMed] [Google Scholar]
- 120.Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol 279: L1005–L1028, 2000. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- 121.Freeman BA, Crapo JD. Biology of disease. Free radicals and tissue injury. Lab Investig 47: 412–426, 1982. [PubMed] [Google Scholar]
- 122.Finkel T. Oxygen radicals and signaling. Curr Opin Cell Biol 10: 248–253, 1998. doi: 10.1016/S0955-0674(98)80147-6. [DOI] [PubMed] [Google Scholar]
- 123.Kurniawan H, Franchina DG, Guerra L, Bonetti L, -Baguet LS, Grusdat M, Schlicker L, Hunewald O, Dostert C, Merz MP, Binsfeld C, Duncan GS, Farinelle S, Nonnenmacher Y, Haight J, Das Gupta D, Ewen A, Taskesen R, Halder R, Chen Y, Jäger C, Ollert M, Wilmes P, Vasiliou V, Harris IS, Knobbe-Thomsen CB, Turner JD, Mak TW, Lohoff M, Meiser J, Hiller K, Brenner D. Glutathione restricts serine metabolism to preserve regulatory T cell function. Cell Metab 31: 920–936.e7, 2020. doi: 10.1016/j.cmet.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mak TW, Grusdat M, Duncan GS, Dostert C, Nonnenmacher Y, Cox M, Binsfeld C, Hao Z, Brüstle A, Itsumi M, Jäger C, Chen Y, Pinkenburg O, Camara B, Ollert M, Bindslev-Jensen C, Vasiliou V, Gorrini C, Lang PA, Lohoff M, Harris IS, Hiller K, Brenner D. Glutathione primes T cell metabolism for inflammation. Immunity 46: 675–689, 2017. doi: 10.1016/j.immuni.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 125.Meier B, Radeke HH, Selle S, Younes M, Sies H, Resch K, Habermehl GG. Human fibroblasts release reactive oxygen species in response to interleukin-1 or tumour necrosis factor-α. Biochem J 263: 539–545, 1989. doi: 10.1042/bj2630539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ohba M, Shibanuma M, Kuroki T, Nose K. Production of hydrogen peroxide by transforming growth factor-β1 and its involvement in induction of egr-1 in mouse osteoblastic cells. J Cell Biol 126: 1079–1088, 1994. doi: 10.1083/jcb.126.4.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Thannickal VJ, Fanburg BL. Activation of an H2O2-generating NADH oxidase in human lung fibroblasts by transforming growth factor β1. J Biol Chem 270: 30334–30338, 1995. doi: 10.1074/jbc.270.51.30334. [DOI] [PubMed] [Google Scholar]
- 128.Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-κB transcription factor and HIV-1. EMBO J 10: 2247–2258, 1991. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lluis JM, Buricchi F, Chiarugi P, Morales A, Fernandez-Checa JC. Dual role of mitochondrial reactive oxygen species in hypoxia signaling: activation of nuclear factor-κB via c-SRC- and oxidant-dependent cell death. Cancer Res 67: 7368–7377, 2007. doi: 10.1158/0008-5472.CAN-07-0515. [DOI] [PubMed] [Google Scholar]
- 130.Son Y, Cheong Y-K, Kim N-H, Chung H-T, Kang DG, Pae H-O. Mitogen-activated protein kinases and reactive oxygen species: how can ROS activate MAPK pathways? J Signal Transduct 2011: 792639, 2011. doi: 10.1155/2011/792639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Son Y, Kim S, Chung HT, Pae HO. Reactive oxygen species in the activation of MAP kinases. In: Methods in Enzymology. Cambridge, MA: Academic Press Inc., 2013, vol. 528, p. 27–48. doi: 10.1016/B978-0-12-405881-1.00002-1. [DOI] [PubMed] [Google Scholar]
- 132.He W, Heinz A, Jahn D, Hiller K. Complexity of macrophage metabolism in infection. Curr Opin Biotechnol 68: 231–239, 2021. doi: 10.1016/j.copbio.2021.01.020. [DOI] [PubMed] [Google Scholar]