Abstract
Background
Cancer pain is one of the most important symptoms for patients. Pharmacological control is central for clinical management and to ensure well-being. In cancer patients, the management of breakthrough cancer pain (BTcP) is also crucial. This study aims to identify factors that can predict patients’ satisfaction with pain relief for BTcP.
Methods
This was a secondary analysis of the IOPS-MS study, a large, observational, multicenter, national study where thirty-two Italian centers were involved to explore BTcP management. Clinical and pathologic features were recorded, as well as the patients’ degree of satisfaction with BTcP medications classified as dissatisfied (not or indifferent satisfied) versus satisfied (or very satisfied). Frequency distributions and the chi-squared test of independence were performed. A multivariate model was carried out by selecting significant variables upon univariate analysis using logistic regression.
Results
From the original 4016 patients enrolled, 3840 were available for the study purpose. Seventy-one per cent of patients declared satisfaction with BTcP medications. Young age [odds ratio (OR) 1.29 (95% confidence interval, CI: 1.12–1.50)], non-metastatic cancer stage [OR 1.53 (95% CI: 1.22–1.91)], high Karnofsky performance status [OR 1.63 (95% CI:1.33–1.99)], the absence of anticancer treatment [OR 1.42 (95% CI: 1.19–1.69)], the NSAIDs/paracetamol use for background pain [OR 1.56 (95% CI: 1.34–1.82)] and a high BTcP interference in activities of daily living [OR 2.34 (95% CI: 1.81–3.01)] resulted positively correlated with dissatisfaction in the multivariate analyses. Also, the setting of care was related to difference in BTcP therapy satisfaction.
Conclusion
This study proposes several key points to be considered in the pharmacological management of BTcP, useful to ensure patients’ satisfaction and optimal quality of life.
Keywords: breakthrough cancer pain, opioids, pain control, patient satisfaction, quality of life
Plain Language Summary
Successful control of cancer pain is a cornerstone in the clinical management of patients, as well as the pharmacological approach to Breakthrough Cancer Pain. Many studied focused to the best antalgic strategies, but features related to patients’ satisfaction with Breakthrough Cancer Pain therapy are vague. This large study identified several clinical and demographic features correlated to patients’ satisfaction or dissatisfaction with Breakthrough Cancer Pain therapy: young age, non-metastatic cancer stage, high Karnofsky performance status, the absence of anticancer treatment, the NSAIDs/paracetamol use for background pain and a high BTcP interference in activities of daily living. These data can help physicians to attention key points to be considered in the global management of the patient with BTcP.
Introduction
Pain of any origin comprises an individual’s life. Cancer pain (CP) is one of the most common and debilitating symptoms, affecting about half of cancer patients.1 Pain can be caused by cancer itself, cancer treatment, or a combination of factors, including metastases, surgery, chemotherapy, immunotherapy, targeted therapy, radiation therapy, supportive therapies such as bisphosphonates or granulocyte stimulating factors and diagnostic procedures. CP has significant implications for quality of life (QOL), psychosocial well-being, and daily functioning.2,3
Even with relatively stable and controlled background pain, patients may often experience transient exacerbation of pain that occurs either spontaneously or in relation to a specific predictable or unpredictable trigger. This phenomenon is commonly named breakthrough cancer pain (BTcP).4
BTcP interferes with QOL and is associated with a relevant morbidity.5 The severity of BTcP impacts patients’ activities of daily living, increases the risk of anxiety and depression, lowers functional capacities, and may lead to poor compliance with cancer treatments.6–8
Despite effective analgesic therapy, patients and caregivers may often perceive inadequate pain control. The aim of our study was to assess patient satisfaction with BTcP management and to describe possible variables related to dissatisfaction, underlining the key aspects of this phenomenon.
Methods
This was a secondary analysis of a large, multicenter, observational, national study of BTcP: the IOPS-MS study. Thirty-two Italian centers were involved.9 The research was approved by the independent ethic committee of Fondazione PTV Policlinico Tor Vergata Hospital of the University of Rome “Tor Vergata” (Ethical Approval Letter No. 21/13, 20 Feb 2013). The study was carried out in compliance with the Declaration of Helsinki. Written informed consent was obtained from each patient. Patients were visited as outpatient, inpatient, at home or day-hospital and were recruited in different settings, including oncology, palliative care, radiotherapy, and pain clinic. Inclusion criteria were age ≥18 years, a diagnosis of cancer, stable and well-controlled background pain (pain intensity ≤4 on a 0–10 numeric rating scale), the presence of BTcP episodes clearly distinguished from background pain, with moderate-severe intensity, according to a pre-defined algorithm.10 Exclusion criteria were a condition of an unstable or uncontrolled background pain (> 4/10); peaks of low pain intensity (< 5/10), and poor collaboration. Patients who were unable to provide information about the data required for the study because of either cognitive failure or terminal disease were excluded.
For the proposed analysis, the following clinical and pathologic features were recorded: age, gender, setting and place of the visit, primary tumor diagnosis, extent of the disease, the presence of ongoing anticancer treatments, Karnofsky Performance Status (KPS). Furthermore, for each patient, background and BTcP characteristics were collected at enrolling timepoint: type, average pain intensity, site and mechanism of pain, mean daily number and intensity of BTcP episodes (range 0–10) in the last week, predictability and triggering factors, and time to maximum pain intensity. For both background and BTcP, analgesic drugs and related adverse reactions were recorded.
Finally, we analyzed the degree of satisfaction with BTcP medication, expressed by patients and reported by clinician in the database, at the time of enrollment in the study. It was classified as categoric variable: dissatisfied (not or indifferent satisfied) versus satisfied (or very satisfied).
Descriptive analyses were provided both for outcomes and variables available. Frequency distributions as well as the chi-squared test of independence were performed to explore association and correlation for categorical variables. Logistic regression was used to carry out a multivariate analysis using stepwise regression (forward selection) by selecting significant variables upon univariate analysis. Enter limit and remove limit were p = 0.05 and p = 0.10, respectively. The univariate examination guided the models’ covariates choice. The independent samples t-test was used to assess statistical differences between the means of two groups. The Mann–Whitney test was used to identify differences between two group. The level of significance was defined as p < 0.05. Statistical analyses were carried out using SPSS Software (SPSS IBM vs 23).
Results
A total of 4016 patients were enrolled in the study during a period of 24 months; 3840 presented full data available for the study purpose and were evaluated for therapy satisfaction. The flow diagram of the study is reported in Supplementary Figure 1. The majority was male, and the mean age was 64.6 years (range 18–97). Gastrointestinal (upper and lower) cancers patients were the most represented population, followed by lung and breast ones. Nearly 80% of patients (79.3%) had a KPS higher than 40% and 75.4% were receiving active cancer treatments at the time of the enrollment. Medical oncologists were the most common enrolling physicians. Furthermore, inpatient service was the most common setting of visit. The mean background pain was 2.98 (SD ± 1.08) and the mean number of daily BTcP episodes was 2.38 (SD ± 1.45).
The main characteristics of study population are summarized in Table 1.
Table 1.
Descriptive Data of Enrolled Patients
| n (%) | |
|---|---|
| Age | |
| Mean (range) | 64.6 (18–97) |
| Sex | |
| Male | 2103 (54.8) |
| Female | 1737 (45.2) |
| Cancer type | |
| Gastrointestinal (all) | 1081 (28.2) |
| Thoracic | 1024 (26.7) |
| Breast | 428 (11.1) |
| Genitourinary | 412 (10.7) |
| Head and neck | 170 (4.4) |
| Gynecologic | 166 (4.3) |
| Other | 559 (14.6) |
| Cancer stage | |
| Metastatic | 3252 (84.7) |
| Locoregional | 582 (15.2) |
| Unknown | 6 (0.2) |
| Active oncological treatment | |
| Yes | 2895 (75.4) |
| No | 818 (21.3) |
| Unknown | 127 (3.3) |
| Karnofsky performance status | |
| > 40 | 3046 (79.3) |
| ≤ 40 | 794 (20.7) |
| Clinical setting | |
| Oncologist | 2012 (52.4) |
| Pain therapist | 1120 (29.2) |
| Palliative cares | 683 (17.8) |
| Radiotherapist | 25 (0.7) |
| Service setting | |
| Inpatient | 1472 (38.3) |
| Outpatient | 1298 (33.8) |
| Home cares | 544 (14.2) |
| Day hospital | 429 (11.2) |
| Hospice | 97 (2.5) |
| Background pain NRS | |
| Mean (range) | 2.98 (0–4) |
| Number of daily BTcP | |
| Mean (range) | 2.38 (1–10) |
| BTcP therapy satisfaction | |
| Dissatisfaction | 1115 (29.0) |
| Satisfaction | 2725 (71.0) |
| Total patients | 3840 |
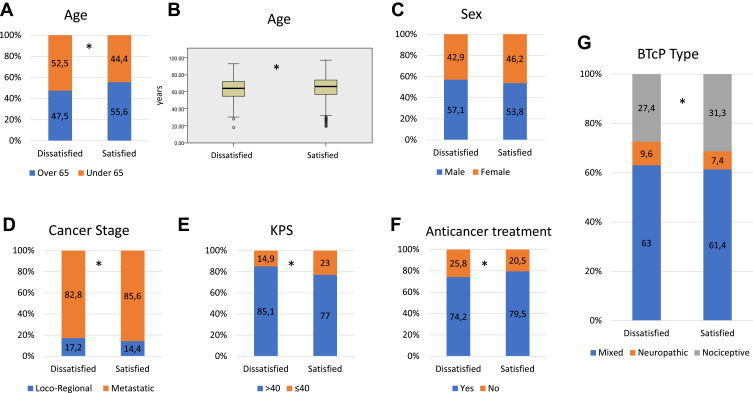
Seventy-one per cent of all evaluable patients reported their satisfaction with BTcP medications. Older patients (over 65 vs under 65 years or per continuous variable) were significantly more satisfied (p<0.001) (Figure 1A and B). Patients affected by non-advanced cancer (loco-regional disease) were more likely to be dissatisfied (p=0.03) compared to metastatic one, as well as patients with high KPS (> 40, p<0.001) (Figure 1D–E). Of interest, patients who were receiving active oncological treatments were significantly more satisfied with BTcP therapy (p<0.001) (Figure 1F). High background pain intensity, number of BTcP episodes, and BTcP intensity (NRS) reported at the enrolling visit were correlated to more dissatisfaction (t-test p<0.001).
Figure 1.
(A) Stacked bar charts exploring distribution in percentage of patients under vs over 65 years among the two groups of interests (dissatisfied vs satisfied). (B) Box plot for age as continuous variable. Stacked bar charts exploring distribution in percentage of patients’ sex (C), cancer stage (D), KPS (E), active anticancer treatment (F) and different BTcP type (G) among the two groups of interests (dissatisfied vs satisfied). Asterisks indicate statistically significant differences (p<0.05).
Abbreviations: KPS, Karnofsky performance status; BTcP, breakthrough cancer pain.
Outpatients enrolled by pain clinicians were more likely to be dissatisfied, compared to other specialists or setting of cares, especially among palliative care specialists and home-care, respectively.
Furthermore, patients with neuropathic/mixed pain, both background pain (p=0.007) or BTcP (Figure 1G, p=0.01), were more likely to be dissatisfied in comparison with patients with nociceptive pain.
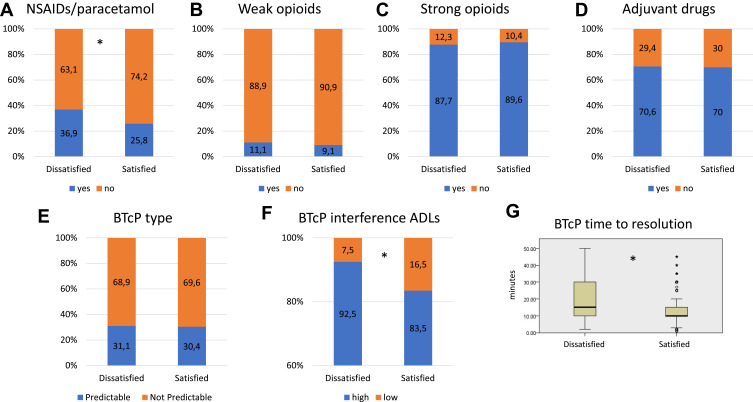
Regarding the distribution of satisfaction groups, according to the type of analgesic treatments used for the background pain at enrolling visit time, no differences were found in patients who were prescribed opioids (weak or strong) or not, as well as adjuvant drugs. However, more dissatisfaction was detected in patients who were receiving NSAIDs/paracetamol (p<0.001) (Figure 2A–D).
Figure 2.
Stacked bar charts exploring distribution in percentage of different type of patients’ therapy for background pain, eg, NSAIDs/paracetamol (A), weak opioids (B), strong opioids (C), and adjuvant drugs (D), among the two groups of interests (dissatisfied vs satisfied). Stacked bar charts of distribution in percentage of predictable vs not predictable BTcP (E) and high or low interference of BTcP in ADLs (F) among the two groups of interests (dissatisfied vs satisfied). (G) Box plot for time of BTP resolution among the two groups. Asterisks indicate statistically significant differences (p<0.05).
Abbreviations: BTcP, breakthrough cancer pain; ADLs, activities of daily living.
The duration of BTcP did not influence the satisfaction, as well as the presence of unpredictable or predictable BTcP (Figure 2E). The presence of a high level of interference of BTcP on activities of daily living (ADLs) was correlated to dissatisfaction with BTcP medication (p<0.001) (Figure 2F). A meaningful pain relief of BTcP was also correlated to satisfaction with BTcP medication (Mann–Whitney test, p<0.001) (Figure 2G).
Regarding the drugs used for BTcP, the use of opioid drugs was correlated to more satisfaction (vs none or other therapies, p<0.001). No statistical differences between morphine (all routes of administration) or fentanyl-based compounds were found (p=0.765).
In conclusion, the multivariate analysis shown in Table 2 confirmed the following factors as related to a dissatisfaction: young age, non-metastatic cancer stage, high Karnofsky performance status, the absence of anticancer treatment, the NSAIDs/paracetamol use for background pain and a high BTcP interference in activities of daily living. The type background and BTcP pain, resulted significative at univariate analyses, were not confirmed as significant in the multivariate one.
Table 2.
Univariate and Multivariate Analyses for Dissatisfaction. OR > 1 Means a Positive Correlation with Dissatisfaction for the First Indicated Category of the Variables Included
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Age | ||||
| <65 vs ≥65 ys | 1.38 (1.20–1.59) | < 0.01 | 1.29 (1.12–1.50) | < 0.01 |
| Cancer stage | ||||
| Locoregional vs metastatic | 1.45 (1.19–1.78) | < 0.01 | 1.53 (1.22–1.91) | < 0.01 |
| KPS | ||||
| >40 vs ≤40 | 1.71 (1.42–2.07) | < 0.01 | 1.63 (1.33–1.99) | < 0.01 |
| Anticancer treatment | ||||
| No vs yes | 1.35 (1.14–1.59) | < 0.01 | 1.42 (1.19–1.69) | < 0.01 |
| Type background pain | ||||
| Neuropathic/mixed vs nociceptive | 1.23 (1.05–1.44) | 0.01 | 1.17 (0.81–1.67) | 0.41 |
| Type BTcP | ||||
| Neuropathic/mixed vs nociceptive | 1.21 (1.04–1.41) | 0.02 | 1.03 (0.72–1.48) | 0.86 |
| NSAIDs/paracetamol use | ||||
| Yes vs no | 1.68 (1.44–1.95) | < 0.01 | 1.56 (1.34–1.82) | < 0.01 |
| BTcP interference ADLs | ||||
| High vs low | 2.46 (1.92–3.14) | < 0.01 | 2.34 (1.81–3.01) | < 0.01 |
According with our records, in the large majority of dissatisfied patients, analgesic therapy was modified or not confirmed (69.1%) at the time of enrolling visit.
Discussion
Patient satisfaction with treatment is an increasingly outcome measure that modulates the pain experience, while assessing the quality and effectiveness of symptom management, especially for BTcP.11 Although several ancillary analyses of the IOPS-MS study reported descriptive data about patients and pain treatment relationship,12,13 there are some important data worth noting. The findings of this study provided key information regarding the factors influencing the level of satisfaction with BTcP medication.
First, younger patients (<65 years), having with higher KPS, and without an advanced disease, resulted to be the least satisfied population with ongoing BTcP treatment at the enrolling visit. This effect appears particularly noticeable in those who are not receiving active cancer treatment. This is an important aspect to underline, because literature on the prevalence, quality, and relief of pain in young adolescents and adults (AYA) patients is lacking. After having completed medical treatment, cancer patients often suffer from pain that can impair the QOL and a substantial proportion of AYA patients reported the need for support. This arises the question whether medical pain management is sufficient. Poor pain control is frequently associated with impaired health-related satisfaction or other social aspects.14 However, these data need to be better clarified with confirmatory studies. A systematic review on the quality of cancer pain management has shown a 25% decrease in under-treatment of pain among cancer patients based on the Pain Management Index.15 Regardless of the meaning of this score, debated in literature one-third of patients still did not receive pain medication proportional to their pain intensity level.16–19
Other considerations regard the setting of care. Outpatient patients evaluated by pain clinicians or patients in the home care setting were more likely to be dissatisfied, compared to other specialists, or setting of cares.
In a study involving hospitalized patients receiving opioid therapy, 89% of patients reported having pain during the preceding 24 h, and 42% reported pain scores >5.20 However, 85.5% of patients reported being “satisfied” or “very satisfied” with their pain medication, and there was no correlation between pain intensity and patient satisfaction. This finding could be explained by the fact that the patient in the hospital or in a specialized care environment can feel more later for his condition and have a greater sense of well-being, regardless of the level of pain. A second possible explanation is that the typology of the patients observed in these settings can experience difficult pain conditions or may have concurrent symptoms that are perceived by the patient as a “total pain”, which includes physical, psychological, and spiritual pain.21 In a previous analysis, it has been reported that variables associated with fast-onset BTcP are represented by day-hospital and in-hospital places of visit with higher level of interference in comparison with other settings.22 This phenomenon is easily explained because Time to maximum BTP intensity has obvious clinical implications for a timely therapeutic intervention and possible psychological input in asking for a medication.
About pain characteristics, we found that patients with neuropathic cancer pain (NCP) or mixed pain were less satisfied with background pain or BTcP management in comparison with patients reporting nociceptive pain. These results are in line with other reported data in literature.23 The prevalence of NCP has been reported to be as high as 40% among cancer patients. The cited meta-analysis explored NCP etiologies. Sixty-four percent of NCP was caused by cancer per se, and 20% by treatments such as chemotherapy, radiotherapy, and cancer surgery.24 NCP is chronic and often manifests itself as persistent background pain with acute exacerbations of BTcP several times a day. BTcP is often spontaneous, but can also be triggered by movement, touch, cold and heat. Moreover, opioids alone are often ineffective, and the addition of adjuvant analgesics is essential.25 The American Society of Clinical Oncology guideline suggests that serotonin-norepinephrine reuptake inhibitors (SNRIs like duloxetine) should be the first drugs of choice for NCP.26
The fourth key point about drug treatment was that most patients were satisfied or very satisfied with opioids medications given for BTcP. The use of morphine and fentanyl preparation was significantly associated with a higher level of satisfaction (satisfied and very satisfied) in comparison with other opioids or non-opioid analgesics. Moreover, we also previously demonstrate that level of satisfaction was significantly associated with the use of fastest onset opioids, especially fentanyl pectin nasal spray (FPNS).2,6,7,9
The last point is represented by the interference of BTcP with Activities of daily living (ADLs). Limiting BTcP will allow patients to maintain their QOL as well as daily functioning. This data is strongly confirmed in literature.27 Tagami et al analyzed the BTcP influence on general activities and pain management, demonstrating that BTcP has a negative impact on general activities and pain management, as well as reported also in other papers.28–30
There are several limitations of this study. A limitation of demographic data analysis is represented by the fact that study protocol did not include important variables like race, income, occupation, and education of enrolled patients. According to the finding of the present study, current curative treatment, nonadvanced disease, and better performance status may be at risk of under-treatment, also for BTcP.
Moreover, this is an unplanned with not pre-specified subgroup analysis of IOPS-MS study. Furthermore, we underline that the study was carry out as observational research, with no control arm or randomized design. Several patient selections or methodological biases can be considered (geographic, economic, attitudes of doctors towards BTcP). However, the large number of patients enrolled and analyzed ensure the quality of the results displayed, that clearly require other confirmatory data. In addition, patients’ satisfaction was reported by clinician based on a scale with 4 satisfaction variables (not satisfied, indifferent satisfied, satisfied, very satisfied). Discrepancies between patient and staff evaluations related to the quality of care highlight the need to consider patient-reported outcomes in pain management.
On the other hand, quality of data entry was guaranteed by sharing the definitions regarding BTcP characteristics in an investigator meeting and continuous data monitoring by a web platform for which each center received a specific investigator manual. This should guarantee about the quality of data gathered from the primary study. The large number of patients recruited in different settings, however, reproduced exactly what is happening in the real world.
At today, we know that patients’ perspectives on living with their health condition and its treatment or management are most useful in medical evaluation when they are relevant to the regulatory decision and reliably measured. Patient-reported outcomes (PROs) instruments facilitate the systematic collection of how patients feel, function, and survive as valid scientific evidence to support the regulatory and healthcare decision-making process.31 When patient-reported outcomes were used, pain management criteria were met for 93% of patients, compared with only 23% when medical records were used, showing a discrepancy of perception between care teams and patients.32
Conclusions
Based on the findings of this study, we have identified five key points to be considered in the global pain management that can impact on patients’ satisfaction to BTcP therapy: demographic data, setting of care, BTcP characteristics, BTcP medication and interference of BTcP with ADLs.
A pilot study focused on the analysis of these five variables in relation to the level of patient satisfaction evaluated according to reproducible standards could lead to the creation of a therapeutic diagnostic algorithm focused on the recognition of the importance of the quality of life of our patients. In addition, PROs assessment in this type of studies is challenging and prone to biases. On other hands, they certainly make clinical trial results more reliable and valuables, adding patients’ perspective.
Acknowledgments
The authors wish to thank the members of the Italian IOPS-MS study group for their contribution in the original study. The abstract of this paper was presented at the 21th National Congress of Italian Association of Medical Oncology (AIOM) as a poster presentation with interim findings. The poster’s abstract was published in AIOM abstracts. Tumori Journal. 2021;107(2_suppl):1–188. doi:10.1177/03008916211041664.
Funding Statement
The study was sponsored by Molteni Farmaceutici, Italy. The publication fee was unconditionally supported by Molteni Farmaceutici, Italy. Data were independently analyzed by the authors.
Disclosure
RG received speaker fees and grant consultancies by Takeda, AstraZeneca and Roche. PM received honoraria from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eisai, Incyte, Merck Sharp & Dohme, Novartis, Pierre Fabre, Pfizer, Roche; Consulting or Advisory Role: AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Merck Sharp & Dohme, Novartis, Pierre Fabre, Roche; Research Funding: AstraZeneca, Bristol Myers Squibb, Incyte, Eli Lilly, Merck Serono, Merck Sharp & Dohme, Novartis, NanoString Technologies, Pierre Fabre, Pfizer, Roche, Takeda; Travel, Accommodations, Expenses: Boehringer Ingelheim, Bristol Myers Squibb, Incyte, Merck Sharp & Dohme, Roche. MM, MF, MP and SM declare no conflicts of interest in this work.
References
- 1.Cancer pain (PDQ®) – patient Version. National Cancer Institute. Available from: https://www.cancer.gov/about-cancer/treatment/side-effects/pain/pain-pdq. Accessed April 24, 2021. [Google Scholar]
- 2.Fallon M, Giusti R, Aielli F, et al. Management of cancer pain in adult patients: ESMO clinical practice guidelines. Ann Oncol. 2018;29(Suppl4):iv166–iv191. doi: 10.1093/annonc/mdy152 [DOI] [PubMed] [Google Scholar]
- 3.Breivik H, Cherny N, Collett B, et al. Cancer-related pain: a pan-European survey of prevalence, treatment, and patient attitudes. Ann Oncol. 2009;20(8):1420–1433. doi: 10.1093/annonc/mdp001 [DOI] [PubMed] [Google Scholar]
- 4.Davies AN, Dickman A, Reid C, Stevens AM, Zeppetella G; Science Committee of the Association for Palliative Medicine of Great Britain and Ireland. The management of cancer-related breakthrough pain: recommendations of a task group of the Science Committee of the Association for Palliative Medicine of Great Britain and Ireland. Eur J Pain. 2009;13(4):331–338. doi: 10.1016/j.ejpain.2008.06.014 [DOI] [PubMed] [Google Scholar]
- 5.Portenoy RK, Payne D, Jacobsen P. Breakthrough pain: characteristics and impact in patients with cancer pain. Pain. 1999;81(1–2):129–134. doi: 10.1016/s0304-3959(99)00006-8 [DOI] [PubMed] [Google Scholar]
- 6.Pointreau Y, Bensadoun RJ, Bera G, et al. Patient satisfaction with fentanyl pectin nasal spray in breakthrough cancer pain management during radiotherapy for head and neck cancer. Patient Prefer Adherence. 2020;14:859–868. doi: 10.2147/PPA.S246757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giusti R, Bossi P, Mazzotta M, Filetti M, Iacono D, Marchetti P. The use of fentanyl in pain management in head and neck cancer patients: a narrative review. Br J Pain. 2018;12(3):155–162. doi: 10.1177/2049463717736787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reid CM, Gooberman-Hill R, Hanks GW. Opioid analgesics for cancer pain: symptom control for the living or comfort for the dying? A qualitative study to investigate the factors influencing the decision to accept morphine for pain caused by cancer. Ann Oncol. 2008;19(1):44–48. doi: 10.1093/annonc/mdm462 [DOI] [PubMed] [Google Scholar]
- 9.Mercadante S, Marchetti P, Cuomo A, et al. Breakthrough cancer pain: preliminary data of the Italian Oncologic Pain Multisetting Multicentric Survey (IOPS-MS). Adv Ther. 2017;34(1):120–135. doi: 10.1007/s12325-016-0440-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Portenoy RK, Bruns D, Shoemaker B, Shoemaker SA. Breakthrough pain in community-dwelling patients with cancer pain and noncancer pain, part 2: impact on function, mood, and quality of life. J Opioid Manag. 2010;6(2):109–116. doi: 10.5055/jom.2010.0010 [DOI] [PubMed] [Google Scholar]
- 11.Antón A, Montalar J, Carulla J, et al. Pain in clinical oncology: patient satisfaction with management of cancer pain. Eur J Pain. 2012;16(3):381–389. doi: 10.1002/j.1532-2149.2011.00036.x [DOI] [PubMed] [Google Scholar]
- 12.Mercadante S, Adile C, Masedu F, Marchetti P, Costanzi A, Aielli F. Factors influencing the use of opioids for breakthrough cancer pain: a secondary analysis of the IOPS-MS study. Eur J Pain. 2019;23(4):719–726. doi: 10.1002/ejp.1339 [DOI] [PubMed] [Google Scholar]
- 13.Pantano F, Manca P, Armento G, et al. Breakthrough cancer pain clinical features and differential opioids response: a machine learning approach in patients with cancer from the IOPS-MS study. JCO Precis Oncol. 2020:PO.20.00158. doi: 10.1200/PO.20.00158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geue K, Schmidt R, Sender A, Friedrich M. Junge Erwachsene mit Krebs – schmerzerleben und Lebenszufriedenheit [Pain experiences and life satisfaction of young adult cancer patients]. Schmerz. 2017;31(1):23–30. doi: 10.1007/s00482-016-0125-6 [DOI] [PubMed] [Google Scholar]
- 15.Greco MT, Roberto A, Corli O, et al. Quality of cancer pain management: an update of a systematic review of undertreatment of patients with cancer. J Clin Oncol. 2014;32(36):4149–4154. doi: 10.1200/JCO.2014.56.0383 [DOI] [PubMed] [Google Scholar]
- 16.Apolone G, Corli O, Caraceni A, et al. Pattern and quality of care of cancer pain management. Results from the Cancer Pain Outcome Research Study Group. Br J Cancer. 2009;100(10):1566–1574. doi: 10.1038/sj.bjc.6605053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisch MJ, Lee JW, Weiss M, et al. Prospective, observational study of pain and analgesic prescribing in medical Oncology outpatients with breast, colorectal, lung, or prostate cancer. J Clin Oncol. 2012;30(16):1980–1988. doi: 10.1200/JCO.2011.39.2381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitera G, Zeiadin N, Kirou-Mauro A, et al. Retrospective assessment of cancer pain management in an outpatient palliative radiotherapy clinic using the Pain Management Index. J Pain Symptom Manage. 2010;39(2):259–267. doi: 10.1016/j.jpainsymman.2009.07.005 [DOI] [PubMed] [Google Scholar]
- 19.Portenoy RK. Treatment of cancer pain. Lancet. 2011;377(9784):2236–2247. doi: 10.1016/S0140-6736(11)60236-5 [DOI] [PubMed] [Google Scholar]
- 20.Phillips S, Gift M, Gelot S, Duong M, Tapp H. Assessing the relationship between the level of pain control and patient satisfaction. J Pain Res. 2013;6:683–689. doi: 10.2147/JPR.S42262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gryschek G, Machado DA, Otuyama LJ, Goodwin C, Lima MCP. Spiritual coping and psychological symptoms as the end approaches: a closer look on ambulatory palliative care patients. Psychol Health Med. 2020;25(4):426–433. doi: 10.1080/13548506.2019.1640887 [DOI] [PubMed] [Google Scholar]
- 22.Mercadante S, Marchetti P, Cuomo A, et al. Factors influencing the clinical presentation of breakthrough pain in cancer patients. Cancers (Basel). 2018;10(6):175. doi: 10.3390/cancers10060175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davies AN, Vriens J, Kennett A, McTaggart M. An observational study of oncology patients’ utilization of breakthrough pain medication. J Pain Symptom Manage. 2008;35(4):406–411. doi: 10.1016/j.jpainsymman.2007.05.010 [DOI] [PubMed] [Google Scholar]
- 24.Bennett MI, Rayment C, Hjermstad M, Aass N, Caraceni A, Kaasa S. Prevalence and aetiology of neuropathic pain in cancer patients: a systematic review. Pain. 2012;153(2):359–365. doi: 10.1016/j.pain.2011.10.028 [DOI] [PubMed] [Google Scholar]
- 25.Oldenmenger WH, Sillevis Smitt PA, van Dooren S, Stoter G, van der Rijt CC. A systematic review on barriers hindering adequate cancer pain management and interventions to reduce them: a critical appraisal. Eur J Cancer. 2009;45(8):1370–1380. doi: 10.1016/j.ejca.2009.01.007 [DOI] [PubMed] [Google Scholar]
- 26.Loprinzi CL, Lacchetti C, Bleeker J, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: ASCO guideline update. J Clin Oncol. 2020;38(28):3325–3348. doi: 10.1200/JCO.20.01399 [DOI] [PubMed] [Google Scholar]
- 27.Bedard G, Davies A, McDonald R, et al. Breakthrough cancer pain: a comparison of surveys with European and Canadian patients. Support Care Cancer. 2015;23(3):791–796. doi: 10.1007/s00520-014-2426-6 [DOI] [PubMed] [Google Scholar]
- 28.Tagami K, Okizaki A, Miura T, et al. Breakthrough cancer pain influences general activities and pain management: a comparison of patients with and without breakthrough cancer pain. J Palliat Med. 2018;21(11):1636–1640. doi: 10.1089/jpm.2017.0675 [DOI] [PubMed] [Google Scholar]
- 29.Hjermstad MJ, Kaasa S, Caraceni A, et al. Characteristics of breakthrough cancer pain and its influence on quality of life in an international cohort of patients with cancer. BMJ Support Palliat Care. 2016;6(3):344–352. doi: 10.1136/bmjspcare-2015-000887 [DOI] [PubMed] [Google Scholar]
- 30.Gonella S, Sperlinga R, Sciannameo V, Dimonte V, Campagna S. Characteristics of breakthrough pain and its impact on quality of life in terminally ill cancer patients. Integr Cancer Ther. 2019;18:1534735419859095. doi: 10.1177/1534735419859095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.FDA’s guidance. Patient-reported outcome measures: use in medical product development to support labeling claims. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-reported-outcome-measures-use-medical-product-development-support-labeling-claims. Accessed March 26, 2021. [DOI] [PMC free article] [PubMed]
- 32.Dy SM, Walling AM, Mack JW, et al. Evaluating the quality of supportive oncology using patient-reported data. J Oncol Pract. 2014;10(4):e223–e230. doi: 10.1200/JOP.2013.001237 [DOI] [PMC free article] [PubMed] [Google Scholar]