Abstract
Introduction
African swine fever virus (ASFV) causes one of the most dangerous diseases of pigs and wild boar – African swine fever (ASF). Since its second introduction into Europe (in 2007), the disease has been spreading consistently, and now ASF-free European countries are at risk. Complex interactions between the host’s immune system and the virus have long prevented the development of a safe vaccine against ASF. This study analysed the possibility of neutralisation of the ASFV in vitro by sera collected from ASF-survivor animals.
Material and Methods
Two pig and three wild boar serum samples were collected from previously selected potential ASF survivors. All sera presented high antibody titres (>5 log10/mL). Primary alveolar macrophages were cultured in growth medium containing 10% and 20% concentrations of selected sera and infected with a haemadsorbing ASFV strain (Pol18_28298_O111, genotype II). The progress of infection was investigated under a light microscope by observing the cytopathic effect (CPE) and the haemadsorption phenomenon. Growth kinetics were investigated using a real-time PCR assay.
Results
Haemadsorption inhibition was detected in the presence of almost all selected sera; however, the inhibition of virus replication in vitro was excluded. In all samples, a CPE and decreasing quantification cycle values of the viral DNA were found.
Conclusion
Anti-ASFV antibodies alone are not able to inhibit virus replication. Interactions between the humoral and cellular immune response which effectively combat the disease are implicated in an ASF-survivor’s organism.
Keywords: African swine fever, antibodies, ASFV, immune response, survivors
Introduction
ASFV is a member of the Asfarviridae family and the causative agent of ASF, one of the most dangerous and devastating diseases of the Suidae family (13). The large ASFV genome (170 to 193 kbp) consists of double-stranded DNA that may encode more than 150 proteins (9). The virus enters the target cells (mainly monocytes and macrophages) via constitutive macropinocytosis and clathrin-mediated endocytosis pathway (15, 25) and causes massive, devastating inflammation in the host (31, 33). Moreover, the virus is equipped with genes responsible for evading the host’s immune response (8), which enhance its virulence. For this reason, the disease leads to the death of the majority of affected animals; however, a small percentage of them may survive (30).
For years, complex interactions between the virus and the host’s organism, as well as a wide range of ASFV genes, have been preventing the development of an effective vaccine against the disease (26). Several studies have shown promising results regarding vaccination, but the process of developing an effective and safe vaccine to the stage of being ready for the market seems to be excessively long (4, 7, 14).
In Poland, ASF has been spreading consistently since 2014 (19). In active surveillance during epizootics in Poland, wild boar which are PCR negative but seropositive with no visible clinical symptoms or gross lesions may be found (12). These animals probably belong to a convalescent group (30), which is of special interest, since they were able to survive ASFV infection. However, the mechanism for effective combat of ASFV by an ASF-survivor’s immune system is not clearly understood. The importance of cellular and humoral immunity in protection against ASF has been previously indicated by several authors (24, 28, 37), but the role of anti-ASFV antibodies in the neutralisation of the virus remains the subject of discussion (11).
In this study, we analysed five selected sera belonging to ASF survivors to investigate their ability to neutralise the virus in vitro.
Material and Methods
Animals. All serum donors were considered potential ASF survivors. Different clinical symptoms but a constant and low virus load in the blood had been recorded in the selected pigs during animal experiments conducted previously (31, 32). The evolution of the viraemia of survivor pigs is shown in Fig. 1.
Fig. 1.
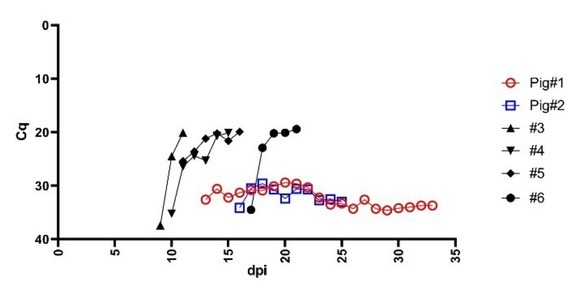
Evolution of viraemia during a previously conducted experiment showing the comparison between survivor pigs (coloured and hollow symbol) and selected pigs with acute or subacute form of ASF (black solid symbol) (31). Cq - quantification cycle; dpi – days post inoculation
At the end of that experiment, pig#1 recovered partially, presenting a better feed intake, lower fever and no pathological lesions during necropsy. No clinical findings except a moderate fever and enlargement of submandibular lymph nodes noted during necropsy, were observed in pig#2. Pig#1 and pig#2 survived the infection and were euthanised on the 32nd and 25th day post infection, respectively (32). No pathological lesions were observed among the selected wild boar. The characteristics of the animals are summarised in Table 1.
Table 1.
Characteristics of the animals from which the sera used in the study were obtained
| Serum/Sample | Species | Sex | Age | Clinical symptoms | Gross lesions |
|---|---|---|---|---|---|
| Pig#1 | Domestic pig | Male | 9 weeks | Fever, dyspnoea joint swelling, | n/d |
| Pig#2 | Domestic pig | Male | 9 weeks | Moderate fever | Enlargement/hyperaemia of submandibular lymph nodes |
| WB#1 | Wild boar | Male | 18 months | n/a | n/d |
| WB#2 | Wild boar | Female | 18 months | n/a | n/d |
| WB#3 | Wild boar | Male | 24 months | n/a | n/d |
n/d – not detected; n/a – not applicable
Blood. Pig blood was collected during thepreceding experiment at −7, 0, 1 and 4 days post inoculation (dpi), then twice a week or daily, whenever clinical signs (i.e. fever) were recorded. Blood was collected into MLVacuCol tubes (Medlab, Raszyn,
Poland) containing an anticoagulant (K2‐EDTA). Wild boar blood was collected during hunting into 4 mL plastic tubes and retained for further analyses. An aliquot of 200 μL of each diluted (1:10 PBS, v/v) wild boar and pig blood sample was submitted for DNA extraction.
Sera. Three sera originated from wild boar, sampled during active surveillance coinciding with hunt activity. Two sera were collected from domestic pigs experimentally infected with a virulent genotype II ASFV field isolate (Pol18_28298_O111) during the previously conducted animal experiments (32). Pigs’ sera were collected on the day of euthanasia. Wild boar sera were separated from the blood collected during hunting. Whole blood from pigs and wild boar was collected into MLVacuCol tubes (Medlab) containing a coagulation accelerator as well as a separating gel. The tubes containing blood were centrifuged (1,800 × g for 10 min, 20℃) and the obtained sera were frozen at −20°C until further analysis. For ASFV DNA detection, an amount of 200 μL of each serum was used for DNA extraction. The serum obtained from pig#2 was heat-inactivated (25 min, 65℃).
DNA extraction and real-time PCR. DNA extraction was carried out in accordance with the Qiagen DNA Mini Kit protocol (Qiagen, Hilden, Germany). A Virotype ASFV PCR Kit (Qiagen) was used to conduct a real-time PCR reaction in a Rotor-GeneQ thermocycler (Qiagen) according to the manufacturer’s instructions.
Virus. A virulent ASFV genotype II Pol18_28298_O111 field isolate was used as the inoculum for the infection of the cells containing the sera in a growth medium. The virus was homologous to the sera obtained from the pigs.
Cells and experimental settings. Porcine primary pulmonary alveolar macrophages (PPAM) were purchased from the Technical University of Denmark (DTU, Lindholm, Denmark). Cells (1 × 106/mL) were cultured in RPMI 1640 growth medium (PAN Biotech, Aidenbach, Germany) supplemented with 1% Antibiotic-Antimycotic (A/A) solution (Sigma-Aldrich, St. Louis, MO, USA), and 10% and 20% of foetal bovine serum (FBS) (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) as the positive control (FBS 10%, FBS 20%) or 10% and 20% of a selected serum of survivor pigs and wild boars (pig#1, pig#2, wild boar (WB)#1, WB#2 and WB#3).
Cells with the medium supplemented with a serum were placed into 24-well plates, in three replicates, infected at the multiplicity of infection (MOI) ~1.0, and incubated at 37℃ in 5% CO2. The cells and medium were collected at 0, 2, 4 and 7 dpi for real-time PCR analysis. In parallel, for each tested serum, a negative control was prepared to exclude the serum’s ability to infect. Negative controls contained a growth medium, A/A and selected serum.
Antibody detection and titration. Anti-ASF antibodies were detected by an ELISA using an ID Screen African Swine Fever Indirect Kit (IDVet, Grabels, France) according to the manufacturer’s instructions. Specific anti-ASFV antibodies were titrated in two-fold serial dilutions, using an indirect immunoperoxidase assay (IPT) described by the EU Reference Laboratory for ASF (CISA-INIA, Valdeolmos, Spain) in the standard operating procedure (SOP/CISA/ASF/IPT/1).
Haemadsorption assay. The culturing procedure of PPAM was held in 24-well plates containing a growth medium and serum, as specified in the “Cells and experimental settings” section. In addition, the medium was supplemented with washed pig erythrocytes 1:300 (v/v). The cell culture was examined daily under a light microscope from 1 to 7 dpi to observe the status of the infection by the Pol18_28298_O111 strain. The positive control contained FBS instead of the selected pig and wild boar sera. The negative control was not infected with the chosen ASFV strain, to exclude the possibility of the infectiousness of the sera.
Statistical analysis. Growth kinetics and mean differences in Cq values are presented as means with standard deviation. Statistical analysis of Cq differences between samples and control was performed using the unpaired Student’s t-test in GraphPad Prism 8.4.2 (GraphPad Software, San Diego, CA, USA).
Results
Viral DNA and antibody detection
The pigs’ blood contained detectable ASFV DNA. The mean Cq value (from the first day of detected viraemia to the day of euthanasia) was estimated at 32.3(±1.7) for pig#1 and 31.7(±1.4) for pig#2. Viral DNA was not detected in blood collected from hunted wild boar. In serum, viral DNA was detected only in the case of pig#2. All sera presented a high antibody titre (>5 log10/mL). The highest titres were observed in sera of WB#1 and WB#2. The results are summarised in Table 2.
Table 2.
ASFV DNA detection and anti-ASFV antibody detection and titre in selected samples
| Serum/Sample | Blood qPCR Cq (±SD) | Serum qPCR Cq | Antibodies (ELISA) | Antibody titre (log10/mL) |
|---|---|---|---|---|
| Pig#1 | 32.3 (±1.7) | NEG | POS | 5.01 |
| Pig#2 | 31.7 (±1.4) | 34.7* | POS | 5.31 |
| WB#1 | NEG | NEG | POS | 5.51 |
| WB#2 | NEG | NEG | POS | 5.51 |
| WB#3 | NEG | NEG | POS | 5.21 |
qPCR – quantitative (real-time) PCR; WB – wild boar; POS– positive; NEG –negative; * – serum was inactivated for further analysis
Haemadsorption. In almost all selected sera (n = 4), regardless of serum concentration (10% or 20%), inhibition of haemadsorption (HAI) was recorded on all the 7 days of observation. In the case of the inactivated serum from pig#2, HAI was observed only on the first dpi. In the positive control (FBS), haemadsorption was found from the first day of the experiment. The results for 20% serum concentration are summarised in Table 3.
Table 3.
Haemadsorption assay results in the presence of selected sera at 20% concentration
| dpi | |||||||
|---|---|---|---|---|---|---|---|
| Serum/sample | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| FBS | + | + | ++ | ++ | ++ | ++ | ++ |
| Pig#1 | − | − | − | − | − | − | − |
| Pig#2 * | − | + | + | ++ | ++ | ++ | ++ |
| WB#1 | − | − | − | − | − | − | − |
| WB#2 | − | − | − | − | − | − | − |
| WB#3 | − | − | − | − | − | − | − |
dpi – day post inoculation of cell culture; − – lack of haemadsorption; + – single cell haemadsorption; ++ – multiple cell haemadsorption; * – inactivated
Growth kinetics
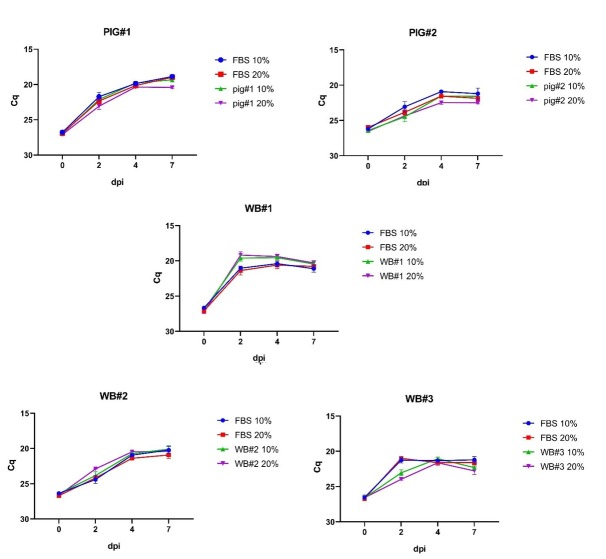
Despite slight differences observed in the growth kinetics of the virus, it was clearly visible that the virus was able to replicate (Fig. 2). Replication of ASFV could be observed both from a decrease of the Cq value in real-time PCR on successive days and by recording the cytopathic effect (CPE) under a light microscope. No clearly visible differences were observed in the virus growth rate between samples containing 10% of a serum and those containing 20%.
Fig. 2.
ASFV growth kinetics in the presence of selected sera at 10% and 20% concentrations. Error bars indicate standard deviation. dpi – days post inoculation
ASFV DNA was not detectable during any experiments in the negative control samples, except as denoted by the stable Cq value (~Cq 37.00) of a sample containing the inactivated serum of pig#2. Neither CPE nor haemadsorption were observed in any negative controls.
For each serum sample and control sample (FBS), the difference in the Cq value was recorded between 0 dpi and 7 dpi (Fig. 3). In most cases, no statistically significant differences were noted in the decrease of this value between the samples and the respective control, which indicated similar growth rates of the virus in the presence of FBS or the survivor’s serum. Statistically significant differences were recorded in the case of the 10% concentration of WB#3 serum and the 20% concentration of pig#1 serum set against their respective controls. Surprisingly, enhanced growth rates of ASFV could be observed in the presence of the 10% and 20% concentrations of WB#1 serum, but they were not significantly different from the growth rate in the relevant controls (Figs 2 and 3).
Fig. 3.
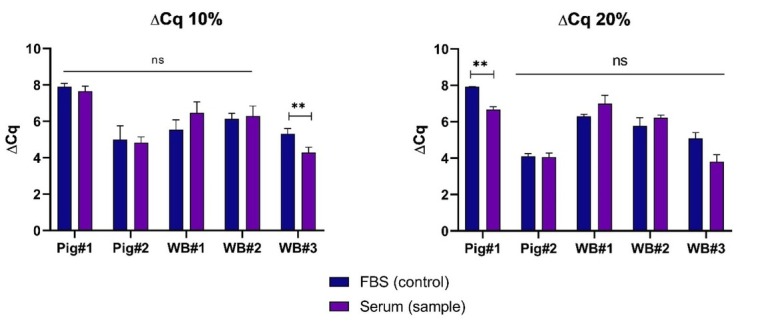
Mean differences in Cq values between 0 dpi and 7 dpi recorded in selected sera and controls (FBS) at 10% and 20% concentrations. Error bars indicate standard deviation. ** – statistically significant (P < 0.01); ns – not significant
Discussion
The role of anti-ASF antibodies in effectively combating the disease is not clearly revealed and still remains the subject of discussion (11). Antibody-mediated neutralisation of ASFV has been reported previously (3, 10, 35), but our study showed that anti-ASFV antibodies alone cannot inhibit the replication of ASFV in vitro. Our findings are supported by studies by Neilan et al. (20), where antibodies against major structural proteins such as p30, p54 and p72 were not sufficient for antibody-mediated protection. Moreover, recent studies reporting an antibody-producing vaccination strategy showed that immunised, seropositive animals were unable to resist succumbing to ASFV (2, 28). Such results confirmed that classic inactivated vaccines are mostly ineffective (irrespective of the inactivation method and the adjuvant), despite their antibody production potential (6). More often, an operative defence against ASF involves cellular immunity. Several previous studies have described the possible role of T lymphocytes in counteracting ASF (23, 29); however, others have indicated an impaired T-cell response insufficient to protect animals against the disease (16). Inducing cellular immunity has great potential against ASFV infection. Netherton et al. (21) identified more than thirty ASFV proteins inducing the cellular immune response and reducing viraemia. Recent advances in vaccine development against ASF have proved that live attenuated vaccines are the most promising, as they may induce both cellular and humoral immunity (6). This type of vaccine has great advantages, such as meeting the requirements of the differentiation of infected from vaccinated animals strategy, and has been proved to be suitable for oral vaccination (especially important in the vaccination of wild boars) (1, 5, 6). Our study does not exclude the role of anti-ASF antibodies in the productive immune response to ASFV in vivo, since all examined sera were collected from survivor animals. This and a previously published study (31) underline the importance of possible interactions between humoral and cellular immunity in protection against the disease.
Since we observed HAI with evidence of simultaneous replication of the virus, this study confirmed that the haemadsorption phenomenon is not essential for virus replication – even for haemadsorbing ASFV isolates. This is in line with research presented by Dixon et al. (8), which excluded the EP402R gene (encoding the CD2v protein responsible for the haemadsorption phenomenon) as playing a major role in virus replication. Haemadsorption inhibition may be used for serotyping purposes (18); however, in this study we observed the same reaction among the five selected sera, (except in the serum belonging to pig#2, where HAI could have been disturbed by the inactivation process) – suggesting that all isolates belong to the same serotype.
Inhibition of haemadsorption showed that the amount of sera used in this experiment was sufficient to opsonise target cells, but not to inhibit internalisation of the virus. This suggests that ASFV does not need the target receptor in order to be internalised, and internalisation may occur passively, i.e., in constitutive micropinocytosis as previously indicated (15). Therefore, passive internalisation may be a key to understanding the mechanism by which the virus evades the host’s defences during infection, even in the presence of anti-ASFV antibodies.
Currently, there is no available treatment for ASFV and for epidemiological reasons and legal regulations, an attempt at ASF treatment is not permitted. Nevertheless, a study conducted to identify a potential target for an antiviral anti-ASFV drug has been published before (17). Several authors have previously reported a beneficial effect of passively acquired antibodies against ASFV in in vivo studies. These studies, conducted on historic genotypes of ASFV, proved the possibility of achieving partial protection against the disease (22, 27, 34). Our study, conducted using the recently circulating genotype II of ASFV, also cannot exclude the possibility of using a convalescent’s serum as a potential treatment for ASF, since other mechanisms such as antibody-dependent cell cytotoxicity may be employed in fighting the disease in vivo (34).
Footnotes
Conflict of Interest
Conflict of Interests Statement: The authors declare that there is no conflict of interests regarding the publication of this article.
Financial Disclosure Statement
This study was supported by the Polish Ministry of Agriculture and Rural Development, Project no. S/932: “Improvement of National Veterinary Research Institute scientific potential, in order to develop an ASFV vaccine”.
Animal Rights Statement
Samples of pig blood and sera were collected during the animal experiment with the approval of the Local Ethical Commission for Animal Experiments in Lublin (approval number 145/2018). Samples of wild boar blood and serum were collected during active surveillance hunting. All procedures were carried out according to the Polish Act of the 15th January 2015 on the Protection of Animals Used for Scientific or Educational Purposes (Official Journal of Laws 2015 item 266) based on Directive 2010/63/EU of the European Parliament and of the Council of the 22nd September 2010 on the Protection of Animals Used for Scientific Purposes.
References
- 1.Barasona J.A., Gallardo C., Cadenas-Fernández E., Jurado C., Rivera B., Rodríguez-Bertos A., Arias M., Sánchez-Vizcaíno J.M.. First Oral Vaccination of Eurasian Wild Boar Against African Swine Fever Virus Genotype II. Front Vet Sci. 2019;6:137. doi: 10.3389/fvets.2019.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blome S., Gabriel C., Beer M.. Modern adjuvants do not enhance the efficacy of an inactivated African swine fever virus vaccine preparation. Vaccine. 2014;32:3879–3882. doi: 10.1016/j.vaccine.2014.05.051. [DOI] [PubMed] [Google Scholar]
- 3.Borca M.V., Irusta P., Carrillo C., Afonso C.L., Burrage T., Rock D.L.. African Swine Fever Virus Structural Protein p72 Contains a Conformational Neutralizing Epitope. Virology. 1994;201:413–418. doi: 10.1006/viro.1994.1311. [DOI] [PubMed] [Google Scholar]
- 4.Borca M.V., Ramirez-Medina E., Silva E., Vuono E., Rai A., Pruitt S., Holinka L., Velazquez-Salinas L., Zhu J., Gladue D.P., Shisler J.L.. Development of a Highly Effective African Swine Fever Virus Vaccine by Deletion of the I177L Gene Results in Sterile Immunity against the Current Epidemic Eurasia Strain. J Virol. 2020;94:e02017–19. doi: 10.1128/JVI.02017-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borca M.V., Ramirez-Medina E., Silva E., Vuono E., Rai A., Pruitt S., Espinoza N., Velazquez-Salinas L., Gay C.G., Gladue D.P.. ASFV-G-ΔI177L as an Effective Oral Nasal Vaccine against the Eurasia Strain of Africa Swine Fever. Viruses. 2021;13:765. doi: 10.3390/v13050765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bosch-Camós L., López E., Rodriguez F.. African swine fever vaccines: a promising work still in progress. Porcine Health Manag. 2020;6:17. doi: 10.1186/s40813-020-00154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen W., Zhao D., He X., Liu R., Wang Z., Zhang X., Li F., Shan D., Chen H., Zhang J., Wang L., Wen Z., Wang X., Guan Y., Liu J., Bu Z.. A seven-gene-deleted African swine fever virus is safe and effective as a live attenuated vaccine in pigs. Sci China Life Sci. 2020;63:623–634. doi: 10.1007/s11427-020-1657-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dixon L.K., Abrams C.C., Bowick G., Goatley L.C., Kay-Jackson P.C., Chapman D., Liverani E., Nix R., Silk R., Zhang F.. African swine fever virus proteins involved in evading host defence systems. Immunol Immunopathol. 2004;100:117–134. doi: 10.1016/j.vetimm.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Dixon L.K., Chapman D.A.G., Netherton C.L., Upton C.. African swine fever virus replication and genomics. Virus Res 201. 173:3–14. doi: 10.1016/j.virusres.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 10.Escribano J.M.. Neutralizing antibodies to different proteins of African swine fever virus inhibit both virus attachment and internalization. J Virol. 1996;70:5689–5694. doi: 10.1128/JVI.70.8.5689-5694.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Escribano J.M., Galindo I., Alonso C.. Antibody-mediated neutralization of African swine fever virus: Myths and facts. Virus Res. 2013;173:101–109. doi: 10.1016/j.virusres.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 12.Frant M., Gal A., Bocian Ł., Ziętek-Barszcz A., Niemczuk K., Woźniakowski G.. African Swine Fever Virus (ASFV) in Poland in 2019—Wild Boars: Searching Pattern. Agriculture. 2021;11:45. doi: 10.3390/agriculture11010045. [DOI] [Google Scholar]
- 13.Galindo I., Alonso C.. African Swine Fever Virus: A Review. Viruses. 2017;9:103. doi: 10.3390/v9050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goatley L.C., Reis A.L., Portugal R., Goldswain H., Shimmon G.L., Hargreaves Z., Ho C., Montoya M., Sánchez-Cordón P., Taylor G., Dixon L.K., Netherton C.L.. A Pool of Eight Virally Vectored African Swine Fever Antigens Protect Pigs Against Fatal Disease. Vaccines. 2020;8:234. doi: 10.3390/vaccines8020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hernáez B., Guerra M., Salas M.L., Andrés G.. African Swine Fever Virus Undergoes Outer Envelope Disruption, Capsid Disassembly and Inner Envelope Fusion before Core Release from Multivesicular Endosomes. PLOS Pathog. 2016;12:e1005595. doi: 10.1371/journal.ppat.1005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hühr J., Schäfer A., Schwaiger T., Zani L., Sehl J., Mettenleiter T.C., Blome S., Blohm U.. Impaired T‐cell responses in domestic pigs and wild boar upon infection with a highly virulent African swine fever virus strain. Transbound Emerg Dis. 2020;67:3016–3032. doi: 10.1111/tbed.13678. [DOI] [PubMed] [Google Scholar]
- 17.Li G., Liu X., Yang M., Zhang G., Wang Z., Guo K., Gao Y., Jiao P., Sun J., Chen C., Wang H., Deng W., Xiao H., Li S., Wu H., Wang Y., Cao L., Jia Z., Shang L., Yang Ch., Guo Y., Rao Z.. Crystal Structure of African Swine Fever Virus pS273R Protease and Implications for Inhibitor Design. J Virol. 2020;94:e02125–19. doi: 10.1128/JVI.02125-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malogolovkin A., Burmakina G., Tulman E.R., Delhon G., Diel D.G., Salnikov N., Kutish G.F., Kolbasov D., Rock D.L.. African swine fever virus CD2v and C-type lectin gene loci mediate serological specificity. J Gen Virol. 2015;96:866–873. doi: 10.1099/jgv.0.000024. [DOI] [PubMed] [Google Scholar]
- 19.Mazur-Panasiuk N., Walczak M., Juszkiewicz M., Woźniakowski G.. The spillover of African swine fever in Western Poland revealed its estimated origin on the basis of O174L, K145R, MGF 505-5R and IGR I73R/I329L genomic sequences. Viruses. 2020;12:1094. doi: 10.3390/v12101094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neilan J., Zsak L., Lu Z., Burrage T., Kutish G.F., Rock D.L.. Neutralizing antibodies to African swine fever virus proteins p30, p54, and p72 are not sufficient for antibody-mediated protection. Virology. 2004;319:337–342. doi: 10.1016/j.virol.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 21.Netherton C.L., Goatley L.C., Reis A.L., Portugal R., Nash R.H., Morgan S.B., Gault L., Nieto R., Norlin V., Gallardo C., Ho C.S., Sánchez-Cordón P.J., Taylor G., Dixon L.K.. Identification and Immunogenicity of African Swine Fever Virus Antigens. Front Immunol. 2019;10:1318. doi: 10.3389/fimmu.2019.01318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Onisk D.V., Borca M.V., Kutish S., Kramer E., Irusta P., Rock D.L.. Passively Transferred African Swine Fever Virus Antibodies Protect Swine against Lethal Infection. Virology. 1994;198:350–354. doi: 10.1006/viro.1994.1040. [DOI] [PubMed] [Google Scholar]
- 23.Oura C.A.L, Denyer M.S., Takamatsu H., Parkhouse R.M.E.. In vivo depletion of CD8+ T lymphocytes abrogates protective immunity to African swine fever virus. J Gen Virol. 2005;86:2445–2450. doi: 10.1099/vir.0.81038-0. [DOI] [PubMed] [Google Scholar]
- 24.Pérez-Núñez D., Sunwoo S.-Y., Sánchez E.G., Haley N., García-Belmonte R., Nogal M., Morozov I., Madden D., Gaudreault N., Mur L., Shivanna V., Richt J., Revilla Y.. Evaluation of a viral DNA-protein immunization strategy against African swine fever in domestic pigs. Vet Immunol Immunopathol. 2019;208:34–43. doi: 10.1016/j.vetimm.2018.11.018. [DOI] [PubMed] [Google Scholar]
- 25.Razzuoli E., Franzoni G., Carta T., Zinellu S., Amadori M., Modesto P., Oggiano A.. Modulation of Type I Interferon System by African Swine Fever Virus. Pathogens. 2020;9:361. doi: 10.3390/pathogens9050361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sánchez E.G., Pérez-Núñez D., Revilla Y.. Development of vaccines against African swine fever virus. Virus Res. 2019;265:150–155. doi: 10.1016/j.virusres.2019.03.022. [DOI] [PubMed] [Google Scholar]
- 27.Schlafer D.H., McVicar J.W., Mebus C.A.. African swine fever convalescent sows: subsequent pregnancy and the effect of colostral antibody on challenge inoculation of their pigs. Am J Vet Res. 1984;45:1361–1366. [PubMed] [Google Scholar]
- 28.Sunwoo S.-Y., Pérez-Núñez D., Morozov I., Sánchez E., Gaudreault N., Trujillo J., Mur L., Nogal M., Madden D., Urbaniak K., Kim I., Ma W., Revilla Y., Richt J.. DNA-Protein Vaccination Strategy Does Not Protect from Challenge with African Swine Fever Virus Armenia 2007 Strain. Vaccines 2019. 7:12. doi: 10.3390/vaccines7010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takamatsu H.H., Denyer M.S., Lacasta A., Stirling C.M.A., Argilaguet J.M., Netherton C.L., Oura C.A.L., Martins C., Rodríguez F.. Cellular immunity in ASFV responses. Virus Res. 2013;173:110–121. doi: 10.1016/j.virusres.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 30.Walczak M., Frant M., Juszkiewicz M., Szymankiewicz K., Bruczyńska M., Woźniakowski G.. Vertical transmission of anti-ASFV antibodies as one of potential causes of seropositive results among young wild boar population in Poland. Pol J Vet Sci. 2020;23:21–25. doi: 10.24425/pjvs.2019.131415. [DOI] [PubMed] [Google Scholar]
- 31.Walczak M., Wasiak M., Dudek K., Kycko A., Szacawa E., Olech M., Woźniakowski G., Szczotka-Bochniarz A.. Blood Counts, Biochemical Parameters, Inflammatory, and Immune Responses in Pigs Infected Experimentally with the African Swine Fever Virus Isolate Pol18_28298_O111. Viruses. 2021;13:521. doi: 10.3390/v13030521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walczak M., Żmudzki J., Mazur-Panasiuk N., Juszkiewicz M., Woźniakowski G... Analysis of the Clinical Course of Experimental Infection with Highly Pathogenic African Swine Fever Strain, Isolated from an Outbreak in Poland. Aspects Related to the Disease Suspicion at the Farm Level. Pathogens. 2020;9:237. doi: 10.3390/pathogens9030237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang S., Zhang J., Zhang Y., Yang J., Wang L., Qi Y., Han X., Zhou X., Miao F., Chen T., Wang Y., Zhang F., Zhang S., Hu R.. Cytokine Storm in Domestic Pigs Induced by Infection of Virulent African Swine Fever Virus. Front Vet Sci. 2021;7:601641. doi: 10.3389/fvets.2020.601641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wardley R.C., Norley S.G., Wilkinson P.J., Williams S.. The role of antibody in protection against African swine fever virus. Vet Immunol Immunopathol. 1985;9:201–212. doi: 10.1016/01652427(85)90071-6. [DOI] [PubMed] [Google Scholar]
- 35.Zsak L., Onisk D.V., Afonso C.L., Rock D.L.. Virulent African Swine Fever Virus Isolates Are Neutralized by Swine Immune Serum and by Monoclonal Antibodies Recognizing a 72-kDa Viral Protein. Virology. 1993;196:596–602. doi: 10.1006/viro.1993.1515. [DOI] [PubMed] [Google Scholar]