Abstract
Antibiotics release inflammatory fragments, such as lipoteichoic acid (LTA) and peptidoglycan (PG), from the cell wall of Staphylococcus aureus. In this study, we exposed S. aureus cultures to a number of β-lactam antibiotics (imipenem, flucloxacillin, and cefamandole) and protein synthesis-inhibiting antibiotics (erythromycin, clindamycin, and gentamicin) and investigated whether supernatants of these cultures differ in their capacity to stimulate endothelial cells (EC). After 24 h of incubation, endothelial adhesiveness for leukocytes, surface expression of various adhesion molecules, and secretion of the chemokines interleukin-8 (IL-8) and monocyte chemotactic protein-1 (MCP-1) were measured. Supernatants of β-lactam-exposed cultures (designated β-lactam supernatants) enhanced the adhesiveness of EC for granulocytes, whereas those of protein synthesis-inhibiting antibiotic-exposed cultures (designated protein synthesis-inhibitor supernatants) did not. This hyperadhesiveness coincided with a higher intercellular adhesion molecule-1 expression on the surface of the stimulated EC. In addition, EC stimulated with β-lactam supernatants secreted significantly higher concentrations of the chemokines IL-8 and MCP-1 than those stimulated with protein synthesis-inhibitor supernatants. The finding that the concentrations of LTA and PG in β-lactam supernatants were much higher than those in protein synthesis-inhibitor supernatants suggests that the observed differences in stimulatory effect between these supernatants are a result of differences in the release of cell wall fragments, although the presence of other stimulatory factors in the supernatants cannot be excluded. In conclusion, our results argue for a release of LTA and PG from S. aureus after exposure to β-lactam antibiotics that enhances the development of a systemic inflammatory response by stimulating EC such that adhesiveness for granulocytes is increased and large amounts of IL-8 and MCP-1 are secreted.
Endothelial cells (EC) play an important role in the development of an inflammatory response after infection. Upon activation by stimuli such as tumor necrosis factor alpha, interleukin-1β (IL-1β), and lipopolysaccharide (LPS), EC increase their surface expression of adhesion molecules and their secretion of chemokines such as IL-8 and monocyte chemotactic protein-1 (MCP-1) (15, 22, 36). These chemokines attract granulocytes and monocytes (10, 17, 23, 38) which then adhere to EC and subsequently migrate to the site of inflammation. However, systemic activation of the endothelium as may occur during severe sepsis can result in diffuse damage to the EC and loss of their integrity as well as aggregation of leukocytes in various organs. These effects may contribute to the onset of adult respiratory distress syndrome and multiple organ failure (28, 32, 37). The stimulatory effects of LPS on EC have been studied extensively in vitro (15, 22). However, little is known about the effects of gram-positive cell wall fragments on EC. Exposure of gram-positive bacteria to antibiotics can lead to the release of stimulatory cell wall fragments such as lipoteichoic acid (LTA) and peptidoglycan (PG) (33, 39). In a previous study members of our group showed that for Staphylococcus aureus the release due to exposure to β-lactam antibiotics was greater than that due to exposure to protein synthesis-inhibiting antibiotics (34). The aim of the present study was to investigate whether supernatants of S. aureus cultures, exposed to different classes of antibiotics, have a stimulatory effect on EC. To this end, endothelial adhesiveness for leukocytes, surface expression of a number of adhesion molecules, and secretion of the chemokines IL-8 and MCP-1 were measured.
MATERIALS AND METHODS
Reagents.
M199 medium and fetal calf serum (FCS) were purchased from Gibco BRL Life Technologies (Grand Island, N.Y.). Human serum (HuS) prepared from healthy volunteers (n = 3) and FCS were heat-inactivated for 30 min at 56°C. LTA (S. aureus), collagenase type I, and EDTA were obtained from Sigma Chemical Co. (St. Louis, Mo.). Gelatin and trypsin were purchased from Difco Laboratories (Detroit, Mich.). Glutaraldehyde was obtained from Polyscience (Northampton, United Kingdom), l-glutamine was obtained from Flow Laboratories (Irvine, United Kingdom), and amphotericin B was obtained from Squibb B.V. (Rijswijk, The Netherlands). EC growth factor was isolated from bovine hypothalamus as described previously (16).
Benzyl-penicillin and streptomycin were purchased from Brocades Pharma B.V. (Leiderdorp, The Netherlands) and Gist-Brocades N.V. (Delft, The Netherlands), respectively. Imipenem, flucloxacillin, and cefamandole were obtained from Merck Sharp & Dohme B.V. (Haarlem, The Netherlands), SmithKline Beecham (Hertfordshire, United Kingdom), and Eli Lilly (Nieuwegein, The Netherlands), respectively. Erythromycin, clindamycin, and gentamicin were purchased from Abbott B.V. (Amstelveen, The Netherlands), Pharmacia-Upjohn (Puurs, Belgium), and Sigma-Aldrich Chemie B.V. (Zwijndrecht, The Netherlands), respectively.
MAbs.
A mouse immunoglobulin G3 (IgG3) monoclonal antibody (MAb) against the glycerol phosphate moiety of LTA was a kind gift from B. J. Appelmelk (Department of Medical Microbiology, Free University, Amsterdam, The Netherlands). The MAbs against adhesion molecules on EC used in this study were as follows: anti-E-selectin MAb ENA-1 (IgG1; Monosan, Uden, The Netherlands), anti-intercellular adhesion molecule-1 (anti-ICAM-1) MAb RR1/1 (IgG1; kindly donated by T. A. Springer, Dana-Farber Cancer Institute, Boston, Mass.), and anti-VCAM-1 MAb E1/6 (IgG1; Becton Dickinson, Leiden, The Netherlands). Anti-CD18 (β2-integrin) MAb IB4 (IgG2a) was obtained as a supernatant of the IB4 hybridoma cell line (American Type Culture Collection, Rockville, Md.). An aspecific isotype-matched control antibody and a phycoerythrin (PE)-conjugated goat anti-mouse Ig MAb were purchased from Southern Biotechnology Associates, Inc. (Birmingham, Ala.). Peroxidase-conjugated goat anti-mouse IgG (Fcγ-specific) was obtained from Jackson Immunoresearch Laboratories Inc. (West Grove, Pa.).
Isolation and culture of human EC.
EC were isolated from human umbilical cord veins, as described previously (6, 13). In short, the umbilical cord was cannulated with a trumpet tip glass cannula at both ends, rinsed, and filled with warm (37°C) 0.2% collagenase type I. The EC were collected in medium containing 10% heat-inactivated HuS (HuSi); after washing they were cultured to confluent monolayers in culture medium consisting of M199 enriched with 10% HuSi, 2 mM glutamine, 0.1 mg of EC growth factor per ml, 5 U of heparin per ml, 0.1 mg of streptomycin per ml, 100 U of penicillin G per ml, and 100 U of amphotericin B per ml in plastic tissue culture dishes (Becton Dickinson, Lincoln Park, N.J.). EC were harvested with 0.05% trypsin and 0.01% EDTA in phosphate-buffered saline (PBS) and subcultured onto 0.75% gelatin-coated glass coverslips (6) in 24-well tissue-culture plates (first passage). First-passage EC grown to confluence were used in the leukocyte adherence assay; for the remaining experiments monolayers of second-passage EC were used. For flow cytometric analysis the EC were cultured on gelatin-coated 12-well tissue-culture plates.
Release of bacterial fragments after exposure to antibiotics.
S. aureus ATCC 25923 was cultured in M199 enriched with 1% d-(+)-glucose and 2 mM l-glutamine, a medium that supports logarithmic bacterial growth but does not stimulate EC (34). In this medium the minimal inhibitory concentrations (MIC) of various antibiotics, determined by standard microdilution techniques (1), were as follows: 0.016 mg/liter (imipenem), 0.125 mg/liter (clindamycin, cefamandole, and gentamicin), and 0.25 mg/liter (flucloxacillin and erythromycin). The bacteria were diluted to an inoculum of 6.2 × 106 ± 0.8 × 106 bacteria per ml (i.e., a log10 of 6.89 ± 0.11; values are means ± standard errors of the means) and cultured for 2 h at 37°C to obtain logarithmic growth. Next, the bacteria were incubated in the absence (control) or presence of the various antibiotics (each at a final concentration of 20 times the MIC). Four hours after addition of the antibiotics, the numbers of viable bacteria were determined by plating serial 10-fold dilutions of 0.1-ml samples on blood agar plates. The number of viable bacteria was expressed in CFU per milliliter. Supernatants were collected by centrifugation and filtration (pore size, 0.45 μm), and stored at −20°C until assessment of LTA and PG. Supernatants of nonantibiotic-exposed cultures were designated control supernatants. Similarly, supernatants of cultures exposed to β-lactam or protein synthesis-inhibiting antibiotics were referred to as β-lactam supernatants or protein synthesis-inhibitor supernatants, respectively. Before stimulation of the EC, heat-inactivated FCS was added to the bacterial supernatants to a final concentration of 10% to ensure the viability of the EC.
LTA ELISA.
An LTA enzyme-linked immunosorbent assay (ELISA) was used, as described previously (34). In short, different dilutions of purified LTA (0 to 500 ng/ml) and of the bacterial supernatants in M199 medium were incubated overnight on 96-well plates. Next, 1.2 μg of mouse anti-LTA IgG3 MAb per ml was added. After being washed the plates were incubated with 2 μg of goat anti-mouse IgG (Fcγ-specific)-peroxidase conjugate per ml. A color reaction was obtained with a substrate containing 3,3′,5,5′-tetramethylbenzidine and H2O2. After stopping the reaction with H2SO4, the optical density (OD) was measured at 450 nm. The limit of detection of the ELISA was 30 ng/ml.
PG measurement.
A silkworm larva plasma (SLP) test (Wako Pure Chemical Industries Ltd., Osaka, Japan) was used to measure the concentration of PG in the bacterial supernatants, as described previously (31, 34). Plasma of the silkworm Bombyx mori contains multiple serine proteases which can become activated by bacterial PG or (1→3)-β-d-glucan, a component of the cell wall of yeast and fungi. This activation initiates a cascade of reactions eventually leading to the formation of melanin, which can be measured optically. Bacterial supernatants (in 10-fold dilutions) and a PG standard of Micrococcus luteus (Wako Pure Chemical Industries Ltd.) (in twofold dilutions) in distilled water were added to 96-well plates. An equal volume of reconstituted SLP reagent was added to the dilutions in the wells, and after 30 min of incubation at 30°C the OD was measured at 690 nm. The sensitivity of the test was 0.3 ng/ml, and no cross-reactivity for LTA from S. aureus (Sigma Chemical Co.) was observed.
Human monocytes and granulocytes.
Human granulocytes as well as monocytes were isolated from fresh buffy coats of peripheral venous blood from healthy volunteers as described previously (4, 5). In short, after differential centrifugation on a Ficoll-amidotrizoate gradient, purified suspensions of granulocytes were obtained from the pellet fraction by lysis of the erythrocytes with an isotonic ammonium chloride solution. Monocyte- and lymphocyte-rich interphases were collected and, after washing, were further purified by centrifugal elutriation. Monocyte-rich fractions were pooled and analyzed by means of FACScan for purity. Granulocyte suspensions were approximately 100% pure, and monocyte suspensions were more than 88% pure with fewer than 8% lymphocytes, 4% granulocytes, and negligible amounts of platelets. In each experiment freshly isolated granulocytes and monocytes with a viability of more than 97%, as determined by trypan blue exclusion, were used. The cells were diluted in M199 plus 10% HuSi to a concentration of 0.8 × 106 to 1.2 × 106 cells/ml. In some experiments, granulocytes were pretreated with 8 μg of either anti-CD18 MAb or an isotype-matched aspecific control antibody per ml for 30 min at 4°C to determine the role of ICAM-1 and its ligands in the adherence of granulocytes to stimulated EC.
Leukocyte-EC adherence assay.
Monolayers of EC on glass coverslips were washed once with PBS before incubation with the various bacterial supernatants containing 10% heat-inactivated FCS for 24 h in a CO2 incubator at 37°C. After washing the EC carefully with warm PBS, 4 × 105 to 6 × 105 granulocytes (either untreated or pretreated with an anti-CD18 MAb or an isotype-matched control antibody) or an equal number of monocytes were added to each well (i.e., approximately three leukocytes for each EC). These cells were allowed to adhere to the EC during 30 min of incubation at 37°C under static conditions. Then, the coverslips were removed from the wells, washed carefully to remove nonadherent cells, and fixed in methanol for 15 min. Next, the adherence of leukocytes was assessed as described previously (4) by Giemsa staining and counting of the cells under light microscopy. The numbers of granulocytes or monocytes that adhered to EC are expressed as percentages of the total number of cells added. The mean level of adherence of monocytes to unstimulated EC was 31.7% ± 6.7% (mean ± standard error of the mean), and that of granulocytes amounted to 3.2%. Data of supernatant-induced granulocyte adherence were corrected for this specific background value. Control experiments were performed to investigate the role of the antibiotics in the stimulation of granulocyte adherence to EC. Therefore EC were stimulated with IL-1 alone (5 ng/ml) or IL-1 spiked with the antibiotics (at concentrations of 20 times their respective MIC). Subsequent granulocyte adherence was determined as described above and was not influenced by addition of any of the antibiotics.
Flow cytometric analysis of the expression of adhesion molecules on stimulated EC.
Confluent monolayers of EC were incubated with medium, control supernatants, or different antibiotic-exposed supernatants for 4 or 24 h in a CO2 incubator at 37°C. After stimulation, the EC incubation medium was collected for measurement of chemokine concentrations. Then the EC were harvested by mild trypsinization and prepared for flow cytometry, as previously described (5). In short, after incubation with 1% goat serum to block aspecific binding of the MAbs, the EC were incubated for 30 min with optimal concentrations (1 to 5 μg/ml) of MAb against the various endothelial adhesion molecules. Next, HuSi-preadsorbed PE-conjugated goat anti-mouse Ig (1.5 μg/ml) was added and, after washing, the EC were analyzed by flow cytometry. Of each sample 5,000 cells were analyzed, and EC incubated with PE-conjugated MAb were used to determine background fluorescence. ICAM-1 expression on unstimulated EC amounted to a median fluorescence intensity (MFI) of 117 ± 9 (mean ± standard error of the mean). Data for ICAM-1 expression induced by bacterial supernatants were corrected for this background value.
IL-8 ELISA.
The concentration of IL-8 in the EC incubation medium of unstimulated EC or EC stimulated with bacterial fragments was measured by ELISA (Pelikine Compact human IL-8 kit; Central Laboratory for Blood Transfusion Service, Amsterdam, The Netherlands) according to the instructions of the manufacturers. The limit of detection of the ELISA was 1 to 3 pg/ml. Unstimulated EC secreted 0.34 ± 0.12 ng of IL-8 per ml after 4 h and 3.20 ± 0.29 ng of IL-8 per ml after 24 h of culture (values are means ± standard errors of the means). No correction for these background levels was performed.
MCP-1 ELISA.
To test for MCP-1, an ELISA was performed as described previously (29). In short, 96-well plates were coated overnight with a monoclonal mouse anti-human MCP-1 antibody. Next, the plates were incubated for 1 h with serial dilutions of the samples and a standard of recombinant human MCP-1. Plates were washed and then incubated with a polyclonal goat anti-human MCP-1 antibody. Next, a peroxidase-conjugated swine anti-goat Ig was added. After washing, a substrate solution of 3,3′,5,5′-tetramethylbenzidine and H2O2 in NaAc buffer was added. The color reaction was stopped by addition of H2SO4 and the OD was measured at 450 nm. The detection limit of the ELISA was 30 pg/ml. Unstimulated EC secreted 1.81 ± 1.54 ng of MCP-1 per ml after 4 h and 13.98 ± 5.3 ng of MCP-1 per ml after 24 h. No correction for these background levels was performed.
Statistical analysis.
Data expressed are the means ± the standard errors of the means of the results from three to four separate experiments. Differences in stimulatory effect between antibiotics were analyzed with Student’s t test for independent samples, and the differences between groups of antibiotics were analyzed by analysis of variance; P values of ≤0.05 were considered significant.
RESULTS
Inhibition of bacterial growth by antibiotics.
During logarithmic growth of S. aureus ATCC 25923 in enriched M199 medium in the absence of any antibiotic (control culture), the log number of viable bacteria increased from 7.08 ± 0.06 at the start of the experiment to 8.55 ± 0.14 after 4 h of culture. Incubation of S. aureus with erythromycin or clindamycin stopped bacterial growth quickly; a bacteriostatic effect was observed after culturing for 4 h (data not shown). Gentamicin had a bactericidal effect: after 4 h of incubation the number of bacteria had decreased by 3.18 ± 0.25 log units. For the β-lactam antibiotics imipenem, flucloxacillin, and cefamandole, a bactericidal effect resulted in a maximum decrease of the number of bacteria, relative to the growth in the control culture at 4 h, of 2.91 ± 0.42, 2.42 ± 0.39, and 2.59 ± 0.27 log units, respectively.
Release of cell wall fragments of S. aureus.
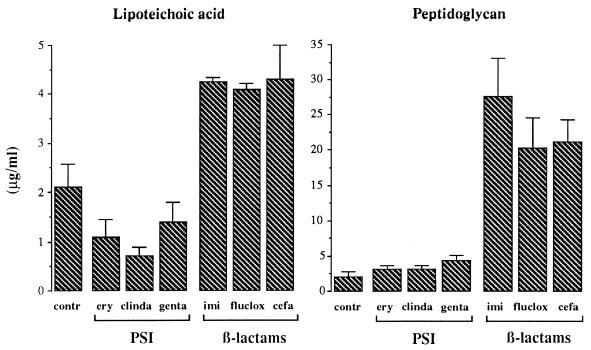
During logarithmic growth of S. aureus in enriched M199 medium (i.e., control culture) the amount of LTA released amounted to 2.10 ± 0.48 μg/ml after 4 h (Fig. 1). Incubation with the protein synthesis-inhibiting macrolides as well as gentamicin led to a twofold decrease (P < 0.05) in the amount of LTA released compared to that in the control cultures. In contrast, the β-lactam antibiotics enhanced the LTA release response significantly (P < 0.01). Compared to those in control and protein synthesis-inhibitor supernatants, the LTA concentrations in these supernatants were increased approximately twofold and fourfold, respectively. No significant differences in LTA release between the three β-lactam antibiotics or between the protein synthesis-inhibiting antibiotics were observed.
FIG. 1.
Release of LTA and PG from S. aureus ATCC 25923 after culture for 4 h in the absence (contr) or presence of various antibiotics at concentrations 20 times the MIC. Antibiotics studied were the protein synthesis-inhibitors (PSI) erythromycin (ery), clindamycin (clinda), and gentamicin (genta) and the β-lactam antibiotics imipenem (imi), flucloxacillin (fluclox), and cefamandole (cefa). After 4 h of incubation with the antibiotics, bacterial supernatants were collected by centrifugation and filtration of the cultures, and the amounts of LTA and PG were measured by means of a specific sandwich ELISA and an SLP assay, respectively. Data are the means + standard errors of the means (error bars) of the results of three to four separate experiments.
The amount of PG released from S. aureus in control cultures was 2.10 ± 0.85 μg/ml after 4 h (Fig. 1). Incubation with the three protein synthesis-inhibiting antibiotics did not significantly influence the amount of PG released compared to that in control cultures. In contrast, β-lactam antibiotics markedly enhanced the PG release response (P < 0.001). Again, within each of the two groups of antibiotics no significant differences in the release of PG were observed.
Adherence of granulocytes and monocytes to EC.
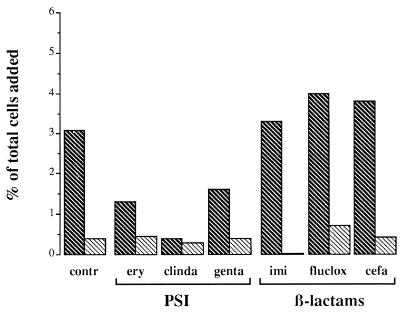
The effect of gram-positive cell wall fragments on the adhesion of human granulocytes to EC was investigated by incubating EC cells for 24 h with supernatants of S. aureus cultures of the different experimental groups. After correction for background, granulocyte adherence to EC that had been exposed to supernatants of control S. aureus cultures (i.e., control supernatants) amounted to 3.1%, which was significantly higher than that to unstimulated EC (P < 0.05) (Fig. 2). After incubation of EC with the protein synthesis-inhibitor supernatants the adherence of granulocytes was not significantly higher than that to unstimulated EC, being 1.3, 0.4, and 1.6% for cultures exposed to erythromycin, clindamycin, and gentamicin, respectively. However, incubation of EC with the β-lactam supernatants significantly increased (P < 0.05) the adhesion of granulocytes, 3.3% for cultures exposed to imipenem, 4.0% for cultures exposed to flucloxacillin, and 3.8% for cultures exposed to cefamandole (Fig. 2). As a group the β-lactam supernatants induced a significantly higher adherence of granulocytes than the protein synthesis-inhibitor supernatants. Control experiments with IL-1-stimulated EC spiked with antibiotics or not showed that this effect was primarily the result of the released bacterial components rather than the antibiotics present in the bacterial supernatants.
FIG. 2.
Adherence of granulocytes to EC that were incubated for 24 h with various supernatants of S. aureus cultures. The supernatants were collected after 4 h of culture in the absence (contr) or presence of different protein synthesis-inhibiting (PSI) and β-lactam antibiotics at concentrations 20 times the MIC. After stimulation, the EC were washed and incubated with 4 × 105 to 6 × 105 granulocytes, with (▧) or without ( ) preincubation with anti-CD18 MAb, for 30 min. After removal of the nonadherent granulocytes by washing, the remaining cells were stained with Giemsa and counted by light microscopy. The number of adherent granulocytes is presented as a percentage of the total number of granulocytes added. The binding of granulocytes to unstimulated EC amounted to 3.2%. Data were corrected for this background adherence and are the means of the results of three to four separate experiments. ery, erythromycin; clinda, clindamycin; genta, gentamicin; imi, imipenem; fluclox, flucloxacillin, cefa, cefamandole.
) preincubation with anti-CD18 MAb, for 30 min. After removal of the nonadherent granulocytes by washing, the remaining cells were stained with Giemsa and counted by light microscopy. The number of adherent granulocytes is presented as a percentage of the total number of granulocytes added. The binding of granulocytes to unstimulated EC amounted to 3.2%. Data were corrected for this background adherence and are the means of the results of three to four separate experiments. ery, erythromycin; clinda, clindamycin; genta, gentamicin; imi, imipenem; fluclox, flucloxacillin, cefa, cefamandole.
To determine whether bacterial fragments could induce changes in the granulocytes during the period of incubation, these cells were incubated with the bacterial supernatants for 30 min. After this period the granulocytes did not show an increased adherence to unstimulated EC. This indicates that the higher adhesion of the granulocytes is the result of changes in the EC rather than in the granulocytes (data not shown). The adherence of monocytes to unstimulated EC amounted to 31.7% ± 6.7% of the total number of monocytes added. Exposure of the EC for 24 h to bacterial supernatants from either control or antibiotic-exposed cultures did not lead to a significant increase in the adherence of monocytes to these cells (data not shown).
Expression of adhesion molecules on EC.
To determine which adhesion molecules on EC accounted for the increased adherence of granulocytes to stimulated EC, surface expression of the endothelial adhesion molecules E-selectin, ICAM-1, and VCAM-1 was determined by flow cytometry. Exposure of EC to control or antibiotic supernatants for 4 or 24 h did not enhance the surface expression of E-selectin or VCAM-1. After 4 h of stimulation of the EC with bacterial supernatants there was almost no increase in ICAM-1 expression compared to that observed for unstimulated EC. However, after 24 h of incubation with either of the bacterial supernatants the expression of ICAM-1 was significantly higher than that of unstimulated EC. ICAM-1 expression induced by control supernatants (Fig. 3) showed an MFI of 232 ± 66. When EC were incubated with protein synthesis-inhibitor supernatants the increase in ICAM-1 expression was less, with MFI levels of 142 ± 47, 95 ± 63 and 149 ± 73 for erythromycin-, clindamycin-, and gentamicin-exposed cultures, respectively. β-Lactam supernatants induced an increase of ICAM-1 expression which was similar to that induced by control supernatants and corresponded with an MFI of 250 ± 49 for cultures exposed to imipenem, 243 ± 81 for cultures exposed to flucloxacillin, and 232 ± 22 for cultures exposed to cefamandole (Fig. 3). As a group, ICAM-1 expression with β-lactam supernatants was significantly higher (P < 0.05) than that with the protein synthesis-inhibitor supernatants.
FIG. 3.
Endothelial surface expression of ICAM-1 after stimulation for 24 h with various supernatants of S. aureus. The bacterial supernatants were collected after 4 h of culture in the absence (contr) or presence of different protein synthesis-inhibiting (PSI) and β-lactam antibiotics at concentrations of 20 times the MIC. Stimulated EC were washed, trypsinized, and incubated with a mouse anti-human ICAM-1 MAb for 30 min. Next, the EC were incubated with peroxidase-conjugated goat anti-mouse Ig MAb for 30 min, and 5,000 cells of each sample were analyzed by flow cytometry. As a measure of ICAM-1 surface expression the MFI, which was 117 ± 9 for unstimulated EC, was used. Data were corrected for this background ICAM-1 expression and are the means + standard errors of the means (error bars) of the results of three to four separate experiments. ery, erythromycin; clinda, clindamycin; genta, gentamicin; imi, imipenem; fluclox, flucloxacillin; cefa, cefamandole.
Blocking of ICAM-1 ligands on granulocytes.
To determine whether ICAM-1 expression plays a role in the increased adhesion of granulocytes to EC stimulated with bacterial supernatants, ICAM-1 ligands (i.e., the CD11a/CD18 or CD11b/CD18 molecules) on the granulocytes were blocked by preincubating these cells with an anti-CD18 MAb. The number of adherent granulocytes decreased to less than 0.75% of the total number of cells added (Fig. 2), which is a reduction of approximately 79% (range, 70.9 to 89.3%). Control experiments with an aspecific isotype-matched antibody showed no inhibitory effect (data not shown).
Secretion of IL-8 by EC.
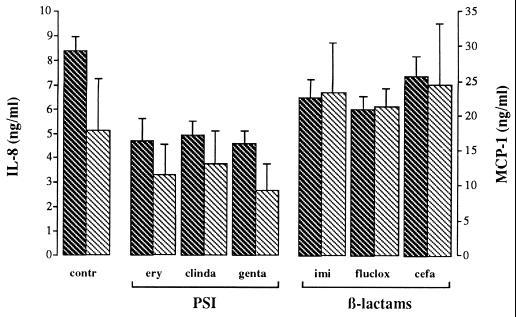
No significant differences in secretion of the granulocyte-activating and chemotactic factor IL-8 were observed between unstimulated EC and EC incubated for 4 h with control supernatants or antibiotic-exposed supernatants. After 24 h, the level of IL-8 secreted was significantly increased (P < 0.05) when EC were incubated with control supernatants compared to that secreted by unstimulated EC (Fig. 4). Incubation of EC with protein synthesis-inhibitor supernatants for 24 h did not significantly change the amount of IL-8 secreted compared to that secreted by unstimulated EC. When analyzed as a group, the β-lactam supernatants induced a significantly higher (P < 0.05) level of IL-8 secretion than the different protein synthesis-inhibitor supernatants (Fig. 4).
FIG. 4.
Secretion of the chemokines IL-8 ( ) and MCP-1 (▧) by EC stimulated with various supernatants of S. aureus. The bacterial supernatants were collected after 4 h of culture in the absence (contr) or presence of different protein synthesis-inhibiting (PSI) and β-lactam antibiotics at concentrations 20 times the MIC. After 24 h of incubation with these supernatants, the culture medium was collected and chemokine concentrations were measured by means of specific sandwich ELISAs. Data are the means + standard errors of the means (error bars) of the results of three to four separate experiments; no correction for background levels was made. ery, erythromycin; clinda, clindamycin; genta, gentamicin; imi, imipenem; fluclox, flucloxacillin; cefa, cefamandole.
) and MCP-1 (▧) by EC stimulated with various supernatants of S. aureus. The bacterial supernatants were collected after 4 h of culture in the absence (contr) or presence of different protein synthesis-inhibiting (PSI) and β-lactam antibiotics at concentrations 20 times the MIC. After 24 h of incubation with these supernatants, the culture medium was collected and chemokine concentrations were measured by means of specific sandwich ELISAs. Data are the means + standard errors of the means (error bars) of the results of three to four separate experiments; no correction for background levels was made. ery, erythromycin; clinda, clindamycin; genta, gentamicin; imi, imipenem; fluclox, flucloxacillin; cefa, cefamandole.
Secretion of MCP-1 by EC.
When EC were incubated for 4 h with control S. aureus supernatants the secretion of the monocyte-activating and chemotactic factor MCP-1 amounted to 3.85 ± 1.22 ng/ml (data not shown, no correction for background). With protein synthesis-inhibitor supernatants, at 4 h the level of MCP-1 secreted was similar to that secreted by unstimulated EC, being 1.10 ± 0.51, 1.42 ± 0.94, and 0.92 ± 0.18 ng/ml for erythromycin-, clindamycin-, and gentamicin-exposed cultures, respectively. In contrast, MCP-1 secretion by all β-lactam supernatants was significantly (P < 0.05) increased compared to that by unstimulated EC after only 4 h, being 4.29 ± 0.85 ng/ml for cultures exposed to imipenem, 4.07 ± 0.19 ng/ml for cultures exposed to flucloxacillin, and 6.30 ± 0.60 ng/ml for cultures exposed to cefamandole, respectively (data not shown).
After EC were incubated with control S. aureus supernatants for 24 h, the secretion of MCP-1 was slightly but not significantly enhanced compared to MCP-1 secretion by unstimulated EC (Fig. 4). Incubation with protein synthesis-inhibitor supernatants for 24 h resulted in a level of MCP-1 secretion that was similar to that of unstimulated EC. When analyzed as a group, the β-lactam supernatants induced significantly (P < 0.05) higher levels of MCP-1 secretion than the different protein synthesis-inhibitor supernatants at 4 h as well as 24 h.
DISCUSSION
This study reports the effects on functional properties of EC by supernatants of cultures of S. aureus exposed to different classes of antibiotics. Two classes of antibiotics with a different mode of action were compared, i.e., β-lactam antibiotics that inhibit cell wall synthesis via binding to penicillin-binding proteins and protein synthesis-inhibiting antibiotics which exert their effect via reversible or irreversible binding to the bacterial ribosome. The main findings were that β-lactam supernatants contain high levels of LTA and PG and that they were able to induce hyperadhesiveness of EC for granulocytes, probably via a higher surface expression of ICAM-1. In addition, these supernatants markedly enhanced the secretion of the endothelial chemokines IL-8 and MCP-1 compared to that observed for non-supernatant-exposed EC. In contrast, supernatants of cultures exposed to protein synthesis-inhibiting antibiotics contained low concentrations of LTA and PG and did not enhance either granulocyte adherence or chemokine secretion. In a previous study members of our group showed that S. aureus supernatants after β-lactam exposure stimulate the secretion of large amounts of tumor necrosis factor alpha and IL-10 by leukocytes in whole blood, whereas after protein synthesis-inhibitor exposure supernatants were much less active in this respect (34). Thus, supernatants of bacteria exposed to β-lactam antibiotics not only activate circulating leukocytes but also stimulate EC lining the blood vessels. This results in an increase in EC adhesiveness for leukocytes as well as in chemokine secretion. At the site of infection such a response may enhance migration of leukocytes into tissues in order to control the infection. However, if such a reaction occurs excessively and uncontrollably in the circulation this could lead to systemic endothelial activation resulting in leukocyte aggregation. This is thought to occur in severe sepsis causing adult respiratory distress syndrome and multiple organ failure (28, 32, 37). Thus, the damaging effects of an enhanced release of stimulatory bacterial components might outweigh the beneficial effect of a rapid killing of bacteria by β-lactam antibiotics. To solve this problem therapeutic studies are required, even though so far there are no clear indications of the clinical relevance of this problem (18, 19, 21).
We have shown that incubation of EC with supernatants of β-lactam-exposed S. aureus cultures results in the secretion of large amounts of chemokines and an enhanced adhesiveness for granulocytes. This hyperadhesiveness is probably mediated by the increased expression of ICAM-1, as was shown by blocking of the ICAM-1 ligands with an anti-CD18 MAb. In contrast to other inflammatory stimuli such as IL-1 and LPS, these supernatants were unable to induce the expression of E-selectin or VCAM-1. It is known that the binding of granulocytes to EC depends on the interaction between ICAM-1 and its ligands CD11a/CD18 and CD11b/CD18 (5, 8, 9, 11). However, for transendothelial migration of leukocytes sequential expression of selectins, ICAM-1, and VCAM-1 is necessary (2, 7, 8, 15). Our data show that the inflammatory response induced by bacterial supernatants yields the appropriate circumstances for transendothelial migration of leukocytes. However, the observed increase in adhesion together with the presence of chemokines in turn could lead to activation of the granulocytes. A release of reactive oxygen metabolites and proteolytic enzymes from these activated granulocytes in close proximity to EC could result in EC injury and vascular damage (24–26, 35).
The correlation between the concentrations of LTA and PG in the β-lactam supernatants and the level of EC stimulation by these supernatants points in the direction of one or a combination of these components as a stimulus for the EC. We found that supernatants of S. aureus cultured in the absence of any antibiotic contained much lower concentrations of LTA and PG, yet they stimulated EC to a level comparable to that of β-lactam supernatants. This could be explained by the release of additional stimulatory bacterial components other than LTA and PG, for instance, exotoxins or capsular polysaccharides (12, 27). Alternatively, differences in the chemical composition of the bacterial cell wall fragments released during normal bacterial growth or after exposure to an antibiotic could be responsible for this discrepancy. Antibiotics can change the form in which LTA (20) and PG molecules (3, 39) are released, which might affect their stimulatory effect. In addition, biochemical alterations induced by sonication, enzymatic degradation (30), and purification (14) might also influence this effect. For this reason we tested the bacterial supernatants without prior in vitro purification and modulation.
In conclusion, we have shown that exposure of S. aureus to β-lactam antibiotics releases large amounts of LTA and PG. Supernatants of these cultures induce in EC an enhanced adhesiveness for granulocytes and expression of ICAM-1, as well as secretion of IL-8 and MCP-1, whereas with protein synthesis-inhibitor supernatants such effects were not found. Future studies will have to show whether endothelial activation by gram-positive cell wall fragments, released as a result of antibiotic treatment, plays a role in the induction of the systemic response to infection.
REFERENCES
- 1.Acar J F, Goldstein F W. Dilution in agar. In: Lorian V, editor. Antibiotics in laboratory medicine. 4th ed. Baltimore, Md: The Williams & Wilkins Co.; 1996. pp. 28–32. [Google Scholar]
- 2.Albelda S M, Smith C W, Ward P A. Adhesion molecules and inflammatory injury. FASEB J. 1994;8:504–512. [PubMed] [Google Scholar]
- 3.Barrett J F, Shockmann G D. Isolation and characterization of soluble peptidoglycan from several strains of Streptococcus faecium. J Bacteriol. 1984;159:511–519. doi: 10.1128/jb.159.2.511-519.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beekhuizen H, Corsèl-van Tilburg A J, van Furth R. Characterization of monocyte adherence to human macrovascular and microvascular endothelial cells. J Immunol. 1990;145:510–518. [PubMed] [Google Scholar]
- 5.Beekhuizen H, van de Gevel J S, Olsson B, van Benten J J, van Furth R. Infection of human vascular endothelial cells with Staphylococcus aureus induces hyperadhesiveness for human monocytes and granulocytes. J Immunol. 1997;158:774–782. [PubMed] [Google Scholar]
- 6.Beekhuizen H, van Furth R. Growth characteristics of cultured human macrovascular venous and arterial and microvascular endothelial cells. J Vasc Res. 1994;31:230–239. doi: 10.1159/000159048. [DOI] [PubMed] [Google Scholar]
- 7.Beekhuizen H, van Furth R. Monocyte adherence to human endothelium. J Leukoc Biol. 1993;54:363–378. [PubMed] [Google Scholar]
- 8.Carlos T M, Harlan J M. Leukocyte-endothelial adhesion molecules. Blood. 1994;4:2068–2101. [PubMed] [Google Scholar]
- 9.Detmers P A, Lo S K, Olsen-Egbert E, Walz A, Baggiolini M, Cohn Z A. Neutrophil-activating protein 1/interleukin-8 stimulates the binding activity of the leukocyte adhesion receptor CD11b/CD18 on human neutrophils. J Exp Med. 1990;171:1155–1162. doi: 10.1084/jem.171.4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foster S J, Aked D M, Schroder J M, Christophers E. Acute inflammatory effects of a monocyte-derived neutrophil-activating peptide in rabbit skin. Immunology. 1989;67:181–183. [PMC free article] [PubMed] [Google Scholar]
- 11.Furie M B, Tancinco M C A, Smith C W. Monoclonal antibodies to leukocyte integrins CD11a/CD18 and CD11b/CD18 or intercellular adhesion molecule-1 inhibit chemoattractant-stimulated neutrophil transendothelial migration in vitro. Blood. 1991;78:2089–2097. [PubMed] [Google Scholar]
- 12.Henderson B, Wilson M. Cytokine induction by bacteria: beyond lipopolysaccharide. Cytokine. 1996;8:269–282. doi: 10.1006/cyto.1996.0036. [DOI] [PubMed] [Google Scholar]
- 13.Jaffe E A. Culture and identification of large vessel endothelial cells. In: Jaffe E A, editor. Biology of endothelial cells. Boston, Mass: Martinus Nijhoff Publishers; 1984. pp. 1–13. [Google Scholar]
- 14.Kawamura N, Imanishi N, Koike H, Nakahara H, Philips L, Morooka S. Lipoteichoic acid-induced neutrophil adhesion via E-selectin to human umbilical vein endothelial cells (HUVECs) Biochem Biophys Res Commun. 1995;217:1208–1215. doi: 10.1006/bbrc.1995.2897. [DOI] [PubMed] [Google Scholar]
- 15.Malik A B, Lo S K. Vascular endothelial adhesion molecules and tissue inflammation. Pharmacol Rev. 1996;48:213–229. [PubMed] [Google Scholar]
- 16.Marciag T, Cerundolo J, Isley S, Kelly P R, Forand R. An endothelial cell growth factor from bovine hypothalamus: identification and partial characterization. Proc Natl Acad Sci USA. 1979;76:5674–5678. doi: 10.1073/pnas.76.11.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsushima K, Larsen C G, DuBois G C, Oppenheim J J. Purification and characterization of a novel monocyte chemotactic and activating factor produced by a human myelomonocytic cell line. J Exp Med. 1989;169:1485–1490. doi: 10.1084/jem.169.4.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCabe W R. Antibiotics and endotoxic shock. Bull N Y Acad Med. 1975;15:1084–1095. [PMC free article] [PubMed] [Google Scholar]
- 19.Oppenheim J J, Zachariae C O C, Mukaida N, Matsushima K. Properties of the novel proinflammatory supergene “intercrine” cytokine family. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- 20.Pollack J H, Ntamere A S, Neuhaus F C. d-Alanyl-lipoteichoic acid in Lactobacilus casei: secretion of vesicles in response to benzylpenicillin. J Gen Microbiol. 1992;138:849–859. doi: 10.1099/00221287-138-5-849. [DOI] [PubMed] [Google Scholar]
- 21.Prins J M, van Agtmael M A, Kuijper E J, van Deventer S J H, Speelman P. Antibiotic-induced endotoxin release in patients with gram-negative urosepsis: a double-blind study comparing imipenem and ceftazidime. J Infect Dis. 1995;172:886–891. doi: 10.1093/infdis/172.3.886. [DOI] [PubMed] [Google Scholar]
- 22.Pugin J, Schürer-Maly C-C, Leturcq D, Moriarty A, Ulevitch R J, Tobias P S. Lipopolysaccharide activation of human endothelial and epithelial cells is mediated by lipopolysaccharide-binding protein and soluble CD14. Proc Natl Acad Sci USA. 1993;90:2744–2748. doi: 10.1073/pnas.90.7.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rampart M, Van Damme J, Zonnekeyn L, Herman A G. Granulocyte chemotactic protein/interleukin-8 induces plasma leakage and neutrophil accumulation in rabbit skin. Am J Pathol. 1989;135:21–25. [PMC free article] [PubMed] [Google Scholar]
- 24.Rot G. Endothelial cell binding of NAP-1/IL-8: role in neutrophil emigration. Immunol Today. 1992;13:291–294. doi: 10.1016/0167-5699(92)90039-A. [DOI] [PubMed] [Google Scholar]
- 25.Sachs T, Moldow C F, Craddock P R, Bowers T K, Jacob H S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. J Clin Investig. 1978;61:1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneeberger P M, van Langevelde P, van Kessel K P M, Vandenbroucke-Grauls C M J E, Verhoef J. Lipopolysaccharide induces hyperadhesion of endothelial cells for neutrophils leading to damage. Shock. 1994;2:296–300. doi: 10.1097/00024382-199410000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Soell M, Diab M, Haas-Archipoff G, Beretz A, Herbelin C, Poutrel B, Klein J P. Capsular polysaccharide type 5 and 8 of Staphylococcus aureus bind specifically to human epithelial (KB) cells, endothelial cells, and monocytes and induce release of cytokines. Infect Immun. 1995;63:1380–1386. doi: 10.1128/iai.63.4.1380-1386.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strieter R M, Kunkel S L. Acute lung injury: role of cytokines in the elicitation of neutrophils. J Investig Med. 1994;42:640–651. [PubMed] [Google Scholar]
- 29.Tekstra J, Visser C E, Tuk C W, Brouwer-Steenbergen J J, Burger C W, Krediet R T, Beelen R H. Identification of the major chemokines that regulate cell influxes in peritoneal dialysis patients. J Am Soc Nephrol. 1996;7:2379–2384. doi: 10.1681/ASN.V7112379. [DOI] [PubMed] [Google Scholar]
- 30.Timmerman C P, Mattsson E, Martinez-Martinez L, de Graaf L, van Strijp J A, Verbrugh H A, Verhoef J, Fleer A. Induction of release of tumor necrosis factor from human monocytes by staphylococci and staphylococcal peptidoglycans. Infect Immun. 1993;61:4167–4172. doi: 10.1128/iai.61.10.4167-4172.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsuchiya M, Asahi N, Suzuoki F, Ashida M, Matsuura S. Detection of peptidoglycan and β-glucan with silkworm larvae plasma test. FEMS Immunol Med Microbiol. 1996;15:129–134. doi: 10.1111/j.1574-695X.1996.tb00063.x. [DOI] [PubMed] [Google Scholar]
- 32.Turnage R H, Guice K S, Oldham K T. Pulmonary microvascular injury following intestinal reperfusion. New Horiz. 1994;2:463–475. [PubMed] [Google Scholar]
- 33.Utsui Y, Ohya S, Takenouchi Y, Tajima M, Sugawara S, Deguchi K, Suginaka H. Release of lipoteichoic acid from Staphylococcus aureus by treatment with cefmetazole and other beta-lactam antibiotics. J Antibiot. 1983;36:1380–1386. doi: 10.7164/antibiotics.36.1380. [DOI] [PubMed] [Google Scholar]
- 34.van Langevelde P, van Dissel J T, Ravensbergen E, Appelmelk B J, Schrijver I A, Groeneveld P H P. Antibiotic-induced release of lipoteichoic acid and peptidoglycan from Staphylococcus aureus: quantitative measurements and biological reactivity. Antimicrob Agents Chemother. 1998;42:3073–3078. doi: 10.1128/aac.42.12.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weiss S J, Young J, Lobuglio A F, Slivka A. Role of hydrogen peroxide in neutrophil-mediated destruction of cultured endothelial cells. J Clin Investig. 1981;68:714–721. doi: 10.1172/JCI110307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wellicome S M, Thornhill M H, Pitzalis C, Thomas D S, Lanchbury J S, Panayi G S, Haskard D O. A monoclonal antibody that detects a novel antigen on endothelial cells that is induced by tumor necrosis factor, IL-1, or lipopolysaccharide. J Immunol. 1990;144:2558–2565. [PubMed] [Google Scholar]
- 37.Wortel C H, Doerschuk C M. Neutrophils and neutrophil-endothelial cell adhesion in adult respiratory distress syndrome. New Horiz. 1993;1:631–637. [PubMed] [Google Scholar]
- 38.Zachariae C O, Anderson A O, Thompson H L, Appella E, Mantovani A, Oppenheim J J, Matsushima K. Properties of monocyte chemotactic and activating factor (MCAF) purified from a human fibrosarcoma cell line. J Exp Med. 1990;171:2177–2182. doi: 10.1084/jem.171.6.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeiger A R, Wong W, Chatterjee A N, Young F E, Tuazon C U. Evidence for the secretion of soluble peptidoglycans by clinical isolates of Staphylococcus aureus. Infect Immun. 1982;37:1112–1118. doi: 10.1128/iai.37.3.1112-1118.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]