Abstract
The polycystic ovary syndrome (PCOS) is the disease featured by elevated levels of androgens, ovulatory dysfunction, and morphological abnormalities. At reproductive stage of women, the rate of PCOS occurrence is measured as 6–10% and the prevalence rate may be double. There are different pathophysiological factors involved in PCOS, and they play a major role in various abnormalities in individual patient. It is clear that there is noteworthy elevation of androgen in PCOS, causing substantial misery and infertility problems. The overexposure of androgen is directly linked with insulin resistance and hyperinsulinaemia. It has been reported previously that PCOS is related to cardiac metabolic miseries and potently increases the risk of heart diseases. Endometrial cancer is also a serious concern which is reported with exceedingly high incidence in women with PCOS. However, the overexposure of androgen has direct and specific influence on the development of insulin resistance. Although many factors are involved, resistance to the insulin and enhanced level of androgen are considered the major causes of PCOS. In the present review, we have focused on the pathophysiology and major revolutions of insulin resistance and excessive levels of androgen in females with PCOS.
1. Introduction
The polycystic ovary syndrome (PCOS) is the disease featured by elevated levels of androgens, ovulatory dysfunction, and morphological abnormalities. According to NIH (National Institutes of Health), it can be defined as “hyperandrogenism with ovulatory dysfunction.” At reproductive stage of women, the rate of PCOS occurrence is measured as 6–10% and the prevalence rate may be double [1–4]. It is clear that there is noteworthy elevation of androgen in PCOS, causing substantial misery and infertility problems. Somehow, PCOS has also some environmental influences like obesity, as well as factors contributing to obesity. Most of the previous reports concluded that deformities associated steroidogenesis and follicular development are crucially involved in PCOS progression [4, 5]. PCOS generally exhibits constantly secreted levels of the gonadotropin-releasing hormone (GnRH), increased levels of the luteinizing hormone (LH), and insufficient follicle-stimulating hormone (FSH) secretion contributing to elevated secretions of the androgens and ovulatory dysfunction. Moreover, majority of the PCOS-suffering population develops insulin resistance, which in turn leads to the amplified secretion levels of the androgen, thereby decreasing the sex hormone-binding globulin secretions [6–11]. Genome research has been discovered numerous genes linked investigations such genes for the beta subunit of FSH, gonadotropin receptors, insulin receptor, neoplastic cells domain-containing protein 1A (DENND1A), differentially expressed in normal, and thyroid adenoma-associated protein (THADA). “Developmental programming” by environmental or hormonal influences may also add to the development of the PCOS. There are different pathophysiological factors involved in PCOS and they play a major role in various abnormalities in individual patient. It has been found that PCOS is related to cardiac metabolic miseries and potently increases the risk of heart diseases [11–16]. The mechanism of androgen biosynthesis in ovaries and adrenal gland is explained in Figure 1.
Figure 1.
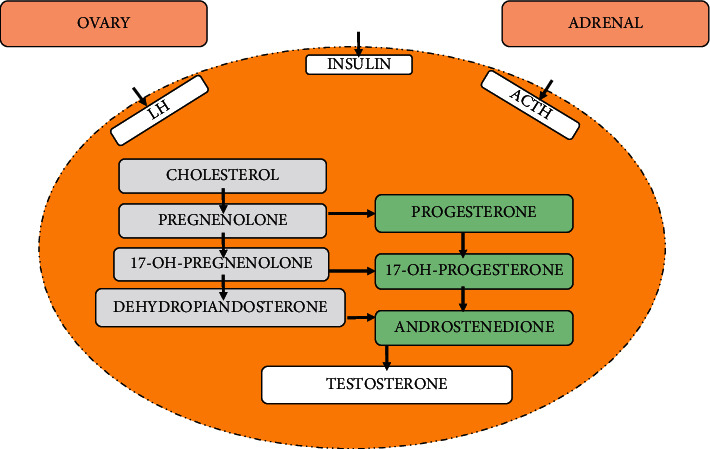
Production of androgen in ovaries and adrenal gland. Biosynthesis of the androgens associated with the ovary and the adrenal gland.
PCOS is found to be the major concern of women health and puts serious life-threatening conditions. Among the ratio of women suffering from PCOS, 50–80% are the obese women, 30–35% are those reported with impaired glucose tolerance, and 8–10% are found to be diabetic or having family diabetes history. The risk of PCOS is related to the history and severity of these influences [5, 16–20]. Generally, women with PCOS exhibit reduced levels of high-density lipoprotein (HDL) and amplified concentrations of triglycerides and low-density lipoproteins (LDL) cholesterols compared with normal women. LDL cholesterol differences appeared to play a significant role in PCOS, and it remains a major concern in most of women. However, sometimes LDL levels in normal range may lead to misconception as the activities of the LDL and HDL in PCOS women are sparse [21, 22]. The elevation of coronary artery calcium scores has been also described in PCOS women and high incidence is reported for the postmenopausal women with PCOS. Many reports provide evidence on the cardiovascular abnormalities in patients with history of PCOS. Endometrial cancer is also a serious concern which is reported with exceedingly high incidence in females with PCOS [21, 22]. The risk factors of the endometrial cancer in menopausal PCOS women include anovulation, heavy fats, and insulin resistance. The fear of chronic anovulation results in prolonged estrogen-mediated mitogenic activation of the endometrium with inadequate progesterone access to cause endometrial differentiation. Females with PCOS also face severe problems in pregnancy like risk of gestational diabetes mellitus, obstructive sleep apnea, and emotional distress [23–29]. Although many factors are involved, resistance to the insulin and high androgen are considered the major causes of PCOS. In the present review, we focus on the pathophysiology and major revolutions of insulin resistance and excessive levels of androgen in females with PCOS. The discussions are focused on insulin resistance, mechanism of insulin resistance, hyperandrogenism in PCOS, PCOS phenotypes, impacts of PCOS on physiological functions of the PCOS woman, and various treatments approaches for the treatment of PCOS, insulin resistance, and high secretions of androgens.
2. Insulin Resistance in PCOS
The actual understanding of insulin resistance can be explained by the requirement of excessive insulin for the metabolic activities, while, besides metabolic activities, insulin is also required for mitogenic and reproductive actions [24]. The rapid and fast glucose analysis make the researchers able to analyse insulin resistance. For this purpose, homeostatic model evaluation, quantitative insulin sensitivity check index, and fasting glucose and insulin levels have been established and utilized in clinical research and also metabolic investigations in PCOS [24–27].
In general, the obesity of abdomen in PCOS is the reason of insulin resistance possibly induced via subclinical swelling but whether the metabolically active intra-abdominal adipose tissues are augmented or not is unclear. However, it was previously investigated that the lower circulating adiponectin detection in PCOS justified subcutaneous adipose tissue as a dysfunctional adipose tissue compartment and having correlation with insulin resistance. Although different methods have been used for prevalence of insulin resistance, where it was found to be varied in PCOS women with respect to the detection method [28–31], more recent findings concluded that obesity is the main risk factor of insulin resistance in individuals suffering from PCOS. The investigations revealed that diagnostic criteria of insulin resistance have limited impact on the insulin resistance detection in PCOS women. Insulin resistance in PCOS patients is the main concern, and its prevalence and mechanism need to be investigated. In previous reports, it was found that large population of PCOS women is suffering from compromised glucose tolerance and type 2 diabetes mellitus (T2DM) [28, 29]. The statistically higher prevalence rate of the compromised glucose tolerance and T2DM serious threatening bell for healthy life. Few clinical studies have reported the glucose tolerance in PCOS women and T2DM risk in PCOS individuals. According to the findings of various studies, it was found that all those PCOS females who are obese and overweight are at greater risk of the disturbances in glucose metabolism and they required to check their glucose regularly with proper metabolic profiling [29–35].
3. Pathways of Insulin Resistivity in Patients with PCOS
Insulin is receptor binding hormone that binds to its membrane glycoprotein. This consists of two subunits α and β, associated with disulphide bonds. Subunit α is extracellular region responsible for the binding site, while subunit β is the intracellular region responsible for provoking intrinsic tyrosine kinase activity [36]. Ligand bindings lead to generating intrinsic tyrosine kinase activity in subunit β and initiate tyrosine phosphorylation. That further leads to the metabolic activities of insulin upon substrate binding, for example, glucose transport and glycogen synthesis [36, 37]. PCOS is a health issue for women and insulin resistance is one of the crucial issues that need to be emphasized. Insulin is an essential hormone for glucose metabolism and its sensitization is necessary for proper glucose uptake and metabolism [8, 38]. The cell surface receptor is homologous with the insulin-like growth factor 1 (IGF-1) receptor, so there is specific interaction for the binding of insulin to surface. The uptake of glucose is stimulated [19, 28, 39, 40]. The MAPK-ERK pathway initiation takes place, which initiates stimulation of a series of enzymes cascades. In previous studies on PCOS women, cellular and molecular mechanism of insulin was highlighted and glucose uptake in insulin target tissues like adipose and skeletal muscles was evaluated in both lean and obese women [20, 25]. When observed in PCOS patients, it was concluded that although the receptors affinities of insulin are similar in both PCOS women and normal females, decreased insulin binding was recorded at pancreatic β-cell in adipose tissues resulting in low glucose uptake and insulin sensitivity in PCOSs females compared to normal females. The fact might be due the reduced abundance of GLUT4 in subcutaneous adipose tissues in PCOS patients, which leads to insulin insensitivity [19, 20, 25, 28, 41, 42]. Besides the reduction in sensitivity of insulin, β-cell dysfunction is also one of the reasons for low disposition. However, still it is unclear whether the subunit β defected function is the primary cause of insulin resistance or it is secondarily involved in insulin resistance [43, 44]. To analyse insulin resistance in PCOS patients, proinsulin and insulin ratio can be a marker. In PCOS women, the ratio of proinsulin and insulin can explain the insulin resistance and activity of β-cell. It was found that, in obese and overweight PCOS patients, there was increased secretion of insulin followed by the excessive levels of proinsulin, which results in insulin resistance and hyperinsulinaemia [19, 20, 25, 28, 39, 40]. These studies explained the dysfunction of β-cell in PCOS women and insulin resistance mechanism. PCOS is a genetically contributed disorder in its pathogenesis and considered a hereditary condition. It has been shown that first relatives of PCOS history will have reproductive and metabolic issues and more investigations revealed that hyperinsulineaemia will be developed in the early stages of life and remain persistent throughout the puberty of girls with hereditary history of PCOS. In such cases, the subjects are at high risk for hyperinsulinaemia and insulin resistance, even if they are not diagnosed with PCOS. The follow-up study of peripubertal adolescent girls whose mothers were suffering from PCOS presented reduced disposition index persistently which proposes the dysfunction of pancreatic β-cell and this might be one of the genetic causes in first-degree relatives of the PCOS population. Another probable hereditary reason for insulin resistance in PCOS is a significant rate of SH2 domain-containing adaptor protein (Lnk) activity in PCOS female's ovarian cell lines, which suppresses the MAPK-ERK and phosphatidylinositol 3-kinase-AKT signaling responses to insulin. Previously, skin fibroblasts were for the intrinsic problems in in insulin function in PCOS because both hyperandrogenism and hyperinsulinaemia affect insulin sensitivity. When PCOS fibroblasts were assessed for insulin stimulated receptors autophosphorylation, there was reduced receptors stimulation as well as minimal insulin sensitivity. More importantly, immunopurification studies revealed that there was no mutation in receptor gene of insulin in PCOS patients [19, 36, 38, 40, 41].
4. Hyperandrogenism and PCOS
The genetically determined excessive secretions of androgens from ovary are the major concern in clinical evaluation of PCOS [45, 46]. The secretions of androgen at early stages are generally considered premature in PCOS and though to develop insulin resistance in prior stages. In visceral adipocytes, the disturbances in lipid metabolism result in insulin resistance. Nevertheless, the overexposure of androgen has direct and specific influence on onset of insulin resistance [47–52]. The increased secretion of androgen is associated with malfunctioning of islets of Langerhans, thereby compromising the pancreatic metabolic functions and causing hyperinsulinaemia. In preclinical studies, in PCOS women, it was found to be a major cause of T2DM. These facts revealed the direct relationship of overexposure of androgens with hyperinsulinaemia, insulin resistance, and T2DM in PCOS population [45–59]. The impact of insulin on hypersecretion of LH and androgen and its correlation with ovary, pituitary gland, and adrenal gland are illustrated in Figure 2.
Figure 2.
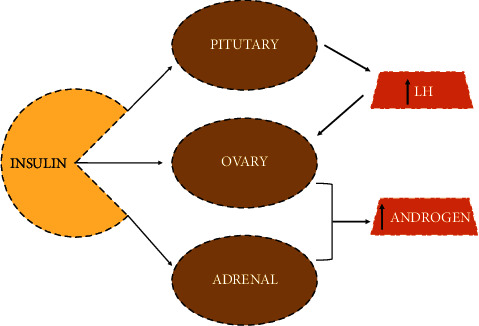
Impact of insulin on hypersecretion of LH and androgen.
Androgens belong to the family of steroid hormones and oversecretion of androgens is considered the main clinical manifesto of the PCOS. So, how androgens are biologically manufactured and regulated should be understood. Since androgens are very important for women's reproductive hormonal system, their normal synthesis and secretion are of prime importance. Androgens are critical female reproductive endocrine system hormones. Androgens include androstenedione (A4), dihydrotestosterone (DHT), dehydroepiandrosterone (DHEA), testosterone (T), and dehydroepiandrosterone sulfate (DHEAS). A4, DHEA, and DHEAS are regarded as precursors of T and DHT. Among these, only DHT and T have a direction with androgenic receptors. Androgens are majorly prepared ovaries and adrenal glands, while steroidogenic enzymes regulate their synthesis [60–64].
Besides this, in PCOS gonadotropin releasing was seen to have much more secretions of luteinizing hormone (LH) with normal abundance of the follicle-stimulating hormone (FSH). The increase in LH secretions in PCOS women may be due to pulsatile increase in the secretions of gonadotropin-releasing hormone (GnRH). Previous analysis raises the point that the change in GRH secretion might be due to defects in hypothalamus in PCOS populations. However, these complications are not only concerned with PCOS, but such changes have been also observed with hyperandrogenism in other cases like ovarian cancer, where there are increased secretions of androgens. The discussion based on previous literature declares that GRH releasing behaviour in correlation with hyperandrogenism in PCOS may be the secondary issue not involved primarily [65, 66]. Women suffering from PCOS generally exhibit hyperandrogenism with increased ovarian androgen as illustrated in Figure 3. The theca cells are the cells of ovary which are responsible for the production of androgens; these cells secrete amplified levels of androgen (androstenedione) and 17-hydroxyprogesterone. 17-Hydroxyprogesterone is a steroidal intermediate for the biosynthesis steps of androgens and glucocorticoids to retort the LH. The questions arise that such abnormalities originate from the theca cells from the ovary of PCOS patients and chronic anovulation-PCOS or from theca cells of the normal ovaries [67–69]. Insulin can have mimicking influences on the LH in women with PCOS. When theca cells from women with PCOS were passaged in tissue culture, they demonstrated elevated activity of numerous steroidogenic enzymes: 3-hydroxysteroid dehydrogenase, 17-hydroxylase/17-20 lyase, and 17-hydroxysteroid hydrogenase. The previously published reports revealed the fact that the amplification of the steroidogenic activity is intrinsic and presumably genetic and leads to blemishes in PCOS. The PCOS might be a morphological associate of these steroidogenic abnormalities [56–62, 70].
Figure 3.
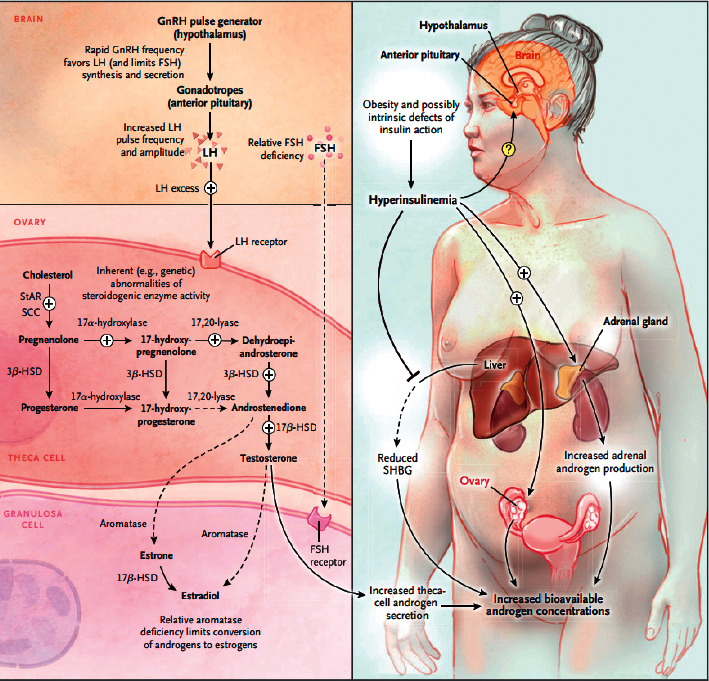
The basic mechanism of androgen overexposure in PCOS women [69]. The figure is cited with permission granted.
5. PCOS Phenotypes
There are 4 various phenotypes of the PCOS identified up till now which are as follows: Type A: polycystic ovaries [PCO], chronic anovulation [CA], and Hyperandrogenism [H]; Type B: chronic anovulation [CA] and hyperandrogenism [H]; Type C: polycystic ovaries [PCO] and hyperandrogenism [H]; and Type D: polycystic ovaries [PCO] and chronic anovulation [CA] [70, 71]. The type of PCOS is related to metabolism and cardiovascular health, as it has been observed that most of the PCOS women are obese with severe or mild metabolic deformities [19, 70, 71] and often face problems of dyslipidaemia, hyperinsulinaemia, insulin resistance, and other metabolic disorders [20]. The major discussion is on fabricating how PCOS phenotypes can be related to aging. Patients with phenotype A are investigated to have high insulin resistance and overexposure of androgens as compared to phenotype B. Phenotype D is generally characterized by the insulin resistance in obese condition even if there is no overexposure of androgens. Meanwhile, in case of type C, the scenario is different where cardiovascular risk is high which may be due to lack of insulin resistance in PCOS. Dyslipidaemia is a serious metabolic issue in PCOS correlated with the HDL and LDL cholesterol levels. According to a research on the PCOS population, both lean and obese PCOS women exhibit aberrant phosphatidylcholine and polyunsaturated fatty acids (PUFAs) levels as well as free fatty acids [71–73].
6. Impact of PCOS on Physiologic Functions
PCOS is a heterogeneous disease of endocrine system which is followed by various clinical and physiological abnormalities. It exerts harmful and pervasive effects on physiological as well as metabolic system, and these characteristics categorise PCOS as a disorder associated with metabolism. Various dysfunctions like insulin resistance, hyperinsulinaemia, obesity, dyslipidaemia, hypertension, elevating risk of developing T2DM, endometrial hyperplasia, and coronary artery diseases. Here, we discussed the impact of the PCOS on various physiological functions of the body.
6.1. Liver Function
The excessive aggregation of fats in liver is called nonalcoholic fatty liver disease (NAFLD) and presents high risk of T2DM and CVS in PCOS. NAFLD is characterized by the presentation of insulin resistance and obesity. These issues are specifically related to abnormalities in liver metabolism. In PCOS population, there is always a high risk of NAFLD because PCOS women usually have insulin resistance with metabolic dysfunctions and unconditional obesity. So, PCOS consequences are found to be associated with NAFLD [74, 75].
6.2. Cardiac Functions
It was concluded that all the PCOS phenotypes have serious cardiovascular risks in PCOS patients. Phenotypes, insulin resistance, hyperinsulinaemia, overexposure of androgens, and ovaries function are reported to display increased cardiovascular health risk for PCOS women [76–78]. The insulin resistance is associated with the inactivation of NO after release from endothelial cells and decreased production of nitric oxide (NO) and synthesis of vasoconstricting agents in excessive amounts; all these defects lead to impaired vasodilatation and cardiac muscles stiffness [79, 80]. Insulin resistance and hyperinsulinaemia also display hypertrophic effect directly and proceed with the endothelial dysfunction. Clinical reports revealed increased risk of cardiac dysfunctions in PCOS population [77–81].
6.3. Reproductive Functions
PCOS is a primary disorder of the ovaries and will directly affect the reproductive system and functions. The excessive secretions of insulin have been observed to cause amplified levels of estrogens and progesterone secretions in women with PCOS. Insulin receptors are responsible for mediating these effects. It was reported that the insulin activity in in vitro granulosa cells can be treated with troglitazone, where IGF-1 mitogenic pathways are increased with the therapy. Besides this, there was an amplification in the IGF-1 receptor in the follicle cells of PCOS patient. This occurred in all stages of development in PCOS women [82]. In the recently reported data, it was concluded that the activity of cortisol is defective in follicular fluid and granulosa cells in PCOS population, where insulin resistance can further lead to tissue-specific insulin resistance. Endometrium cancer is a health-depriving deadly disorder and PCOS women are at high risk to develop endometrium cancer because of the high incidence of insulin resistance and hyperinsulinaemia together with overexposure of androgens. In PCOS, there is always increased upregulation of insulin receptors that have the direct implication of insulin signaling, thereby leading to cardiogenesis and development of endometrium cancer [83]. It might be due to metabolic defects, proteins expression in endometrium with insulin activity, and faulty glucose metabolism. Furthermore, the insulin receptor expression and IGF-1 signaling synergistically contribute to the development of endometrium cancer in PCOS population. So, PCOS is one of the major reasons for establishing endometrium cancer [82–84]. In Figure 4, the mechanism of PCOS associated infertility is illustrated, which displays the reproductive defects in PCOS women. The PCOS leads to increased production of androgens and decreased sensitivity of the follicle-stimulating hormone receptors (FSHR) as illustrated in Figure 4. The increased production of the androgens leads to failure in dominant follicles development and corpus luteum. It is responsible for decreased production of aromatase and progesterone. On the other hand, decreased sensitivity of the FSHR also leads to decreased aromatase production that further decreases the production of estrogens. So, overall, the syndrome leads to decreased production of the estrogens and progesterone followed by infertility.
Figure 4.
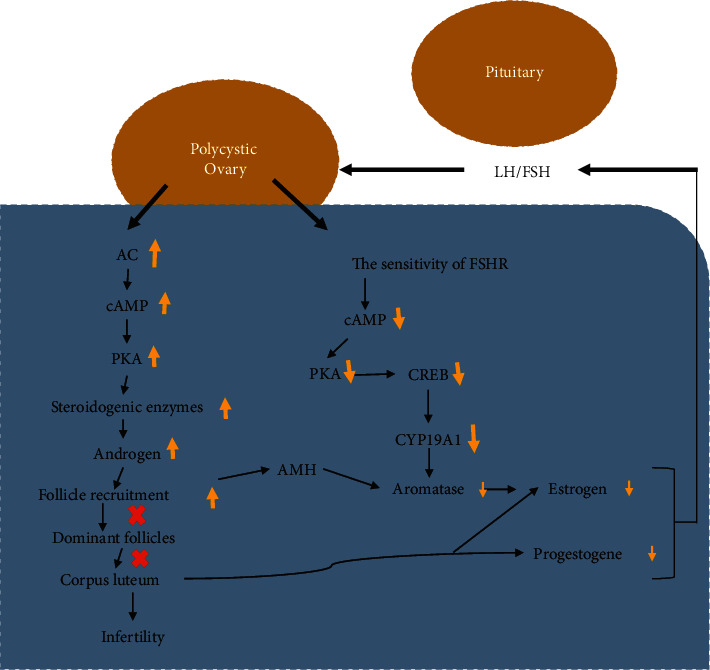
Schematic representation of PCOS associated infertility. The increased production of the androgens leads to failure in dominant follicles development and corpus luteum that is responsible for decreased production of aromatase and progesterone. Decreased sensitivity of the FSHR also leads to decreased aromatase production that further decreases the production of estrogens. So, overall the syndrome leads to decreased production of the estrogens and progesterone leading to infertility. H: anti-Mullerian hormone; PKA: protein kinase A; AC: adenylate cyclase; cAMP-response element binding protein:CREB.
6.4. PCOS and Hypertension
Hypertension is a persistently elevated blood pressure that affects a large human population and leads to serious health issues [84]. In PCOS population, the existence of systemic arterial hypertension (SAH) is more usual and has high incidence. A survey conducted on SAH in PCOS women demonstrated that there are 40% more chances of SAH occurrence in PCOS women than in the normal women. SAH has specific and central contribution in the development of PCOS and secondary cardiovascular disorders [84–88]. According to clinical investigational studies, there is a major and specific relationship between hypertension and endocrine system, so any abnormality in endocrine system will be definitely associated with the hypertension. In PCOS, the defective endocrine system is commonly observed leading to hypertension [85]. Besides this, insulin resistance and metabolic defects have also more commonly occurring issues in PCOS women, resulting in increased incidence of hypertension [84, 85]. The insulin resistance results in hyperinsulinaemia and increased production of LH, consequently increasing the androgen secretions causing persistently high blood pressure in PCOS women [89, 90]. The critical clinical studies show that there is a high occurrence of hypertension in females with PCOS. Moreover, it should be clearly understood that women suffering from PCOS should always monitor their blood pressure regularly in order to avoid delayed management [89–91].
6.5. Inflammation in PCOS
PCOS also promotes basis for chronic low-grade inflammation and inflammation pathways including interleukin-6 (IL-6), TNF-a, and type 2 TNF receptors. The circulating C-reactive protein (CRP) observation also leads to the point that PCOS is concerned with low-grade chronic inflammation [81, 91]. The basis can also be displayed from excessive adipose tissues which is direct producer of the CRP [92, 93]. Additionally, CRP has a physiological purpose by increasing lipid absorption into foamy macrophages inside atherosclerotic plaques. CRP is a direct and specific biomarker of abnormally low inflammation, according to a study [94, 95]. In general, PCOS patients have mild risk of chronic inflammation but it is not reflective at molecular levels.
7. Treatment Options for Hyperandrogenism, Insulin Resistance, and PCOS
7.1. Exercise and Weight Loss
Exercise is the best treatment modality for all the embolic manifestations and is recognized as necessary as food for human health. In PCOS, exercise and weight losing activities have supreme importance because they will help in lowering the adipose tissues having a major contribution to insulin resistance and androgenism [89, 96, 97]. The fats deposits are the key factors for insulin resistance, hyperinsulinaemia, hyperglycemia, T2DM, and oversecretion of androgen, so losing fats, specifically abdomen fats, has a direct and positive impact on all the major issues in PCOS women. It is also investigated that exercise not only diminishes fats but also promotes the normal endocrine and adrenal functions [97]. Physical exercises and weight losing activities can result in complete recovery from the clinical characteristics of PCOS.
7.2. Pharmacological Mediation
Drug therapy is always considered prime requirement for PCOS, where drugs like pioglitazone, inositol, and metformin isoforms have been recognized as therapeutic regimens for reproductive abnormalities and metabolic disorders in PCOS. Metformin is an insulin-sensitizing hormone that is used in PCOS even without diabetes and exerts actions on adipose tissue, skeletal muscles, ovary, and endothelium, impacted by insulin resistance. Prolonged use of metformin in PCOS treatment can augment ovulation rate, regulate menstrual cycle, and decrease the androgens secretions [87, 98–100]. The combinatorial regimen of clomiphene and metformin is considered more beneficial than single therapy of clomiphene or metformin for ovulation and pregnancy in PCOS women [101]. Metformin had no impact on fasting glucose, serum lipids, or anthropometric characteristics in women with PCOS, although it may postpone the advancement of glucose intolerance. Other insulin-sensitizing agents have also been shown to be effective in the treatment of PCOS [102]. Pioglitazone and rosiglitazone are also considered effective in eliminating insulin resistance, abnormal glucose tolerance, hyperandrogenaemia, ovulation rate, and menstrual regularity in PCOS patients. The combination of metformin and pioglitazone is also reported to have synergistic clinical profile in PCOS women's treatment; however, it should be avoided if pregnancy is desired due to teratogenic effects [103]. Inositol is another novel insulin-sensitizing agent that withholds superb insulin-sensitizing efficiency in PCOS women. Insulin resistance is troublesome along with hyperinsulinaemia for PCOS population and needs further research for the radical treatments. Hypersecretion of androgen in PCOS is considered fatal and hereditary. Therefore, there is an unmet need to evaluate the pathogenic and molecular network of this syndrome, insulin resistance, and excessive secretion of androgen [102, 103]. The metabolic irregularities and malfunctioning lead to complications like obesity, excessive lipids aggregations, impaired glucose tolerance, hypertension, endometrium cancer, and hyperinsulinaemia [104, 105]. There is intended need for randomised clinical control trials for effective therapeutic approach in order to treat PCOS and its related complications.
7.3. Assisted Reproductive Technology (ART)
Women infertility is also a major concern in PCOS, where assisted reproductive technology (ART) is employed for the fertility purposes. In ART, ovary is hyperstimulated in order to regulate the growth of multiple follicles, but it is ineffective in most cases due to augmented response to gonadotropins [106, 107]. In vitro maturation (IVM) methods have been employed for the fertilization of women with PCOS. IVF and IVM-IVF methods are also used for the embryo implantation to PCOS women [106–108]. However, further clinical research is aimed at yielding specified model for the success of ART in PCOS women to get fertilized.
7.4. Laparoscopic Ovarian Drilling (LOD)
In 1984, laparoscopic ovarian drilling (LOD) was established to replace the invasive ovarian wedge resection surgery [109]. Currently, this technique is highly recommended and is developing pregnancy in 84% of the PCOS women who are facing infertility problems. LOD also augments insulin resistance and androgen production from ovaries. The improvements achieved with LOD have been observed to remain for a long time in 54% of the PCOS population. LOD is also a beneficial approach, as it results in low incidence of miscarriages in PCOS patients. LOD is also thought to be the first-line treatment when the CC treatment fails [26, 110–116]. These findings should be further evaluated for the confined use of LOD and its clinical benefits.
7.5. Oral Contraceptive Pills (OCPs)
Oral contraceptives pills (OCPs) are regarded first-line therapy for people with PCOS who are not pursuing pregnancy. Not only are OCPs helpful in regulating the menstrual cycle, but also they reduce the secretion of androgens and regulate other physical activities [117]. OCPs have been reported which can significantly decrease the risk of endometrium cancer. OCPs are a combination of estrogen and progestogen, where estrogen is intended to decrease the levels of LH and FSH; the reduction in LH and FSH levels leads to suppression of T secretions and reduces the secretion of androgens. Progestogen is generally recommended for low androgenic activity in women with PCOS [118, 119]. Three commonly used progestogens are desogestrel, cyproterone acetate, and drospirenone. As discussed earlier in this review, the PCOS population has high risk to develop disorders like insulin resistance, T2DM, hyperglycemia, abnormal glucose tolerance, enhanced levels of HDL and LDL cholesterols, hyperinsulinaemia, and so forth. Investigations in clinics revealed that OCPs can cause serious cardiac disorders like thrombosis, hypertension, insulin resistance, and myocardial infarction. These anomalies are thought to be having high risk in PCOS women compared to normal women and those who are on OCPs therapy. However, up till now, no clinical examination has been reported, to the best of our knowledge, regarding the metabolic effects of OCPs in PCOS population [118–122]. So, there is a great demand of clinical research in this area of interest because OCPs are the first-line treatment modality in females with PCOS. The recent interventions declared a new regimen with OCPs by adding metformin along with OCP, and the results found were satisfactory in reducing insulin resistance as well as decreasing the androgen production in PCOS population.
7.6. Dietary Therapy
Dietary therapy to reduce the weight of women with PCOS has a significant impact on metabolic conditions and is recognized to improve many PCOS issues like regulating androgen secretions, reducing insulin resistance, regularity of endocrine secretions, and menstrual cycle regulation [123, 124]. Weight loss can surely meet the goals of obtaining improved symptoms of PCOS and metabolic issues can intently resolve without medications. Moreover, the reproductive consequences in PCOS women can be improved with weight loss for those PCOS women who are interested in getting pregnant [123]. The weight loss results in high rates of pregnancy as well as decrease of the chances of miscarriages, suggesting weight loss significance for PCOS population. Dietary plan for weight loss is important and should be considered as first clinical regimen in order to live a healthy life with PCOS [125–128]. There is a need for clinical research and trials on the significance and effectiveness of dietary therapy for PCOS women.
8. Conclusion
Polycystic ovarian syndrome (PCOS) is considered as a major health issue. Women with PCOS face insulin resistance and overexposure of androgen, leading to a number of metabolic and reproductive abnormalities. These are considered the major causes of PCOS and other PCOS related manifestations. Herein, we have discussed the mechanism and treatment modalities of PCOS based on hypersecretion of androgen and insulin resistance. The current study gives concise and comprehensive outlook for the understanding of insulin resistance and androgen overexposure. We for the first time reported detailed review on the mechanism, pathophysiology, and treatment interventions for the insulin resistance and hypersecretion of insulin. The current study provides better understanding of the PCOS and provides a base for further exploration.
Acknowledgments
This study was supported financially by the National Natural Science Foundation of China (no. 81730038) and CAMS Innovation Fund for Medical Sciences (no. 2019-I2M-5-001).
Data Availability
All the data can be requested from the corresponding author.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Authors' Contributions
YX and JQ collected the data and wrote the manuscript. JQ supervised the whole study.
References
- 1.Livadas S., Diamanti-Kandarakis E. Polycystic ovary syndrome: definitions, phenotypes and diagnostic approach. Frontiers of Hormone Research . 2013;40:1–21. doi: 10.1159/000341673. [DOI] [PubMed] [Google Scholar]
- 2.Conway G., Dewailly D., Diamanti-Kandarakis E., et al. European survey of diagnosis and management of the polycystic ovary syndrome: results of the ESE PCOS Special Interest Group’s Questionnaire. European Journal of Endocrinology . 2014;171(4):489–498. doi: 10.1530/eje-14-0252. [DOI] [PubMed] [Google Scholar]
- 3.Pasquali R., Gambineri A. Glucose intolerance states in women with the polycystic ovary syndrome. Journal of Endocrinological Investigation . 2013;36(8):648–653. doi: 10.1007/bf03346757. [DOI] [PubMed] [Google Scholar]
- 4.Zawadski J. K. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach; Polycystic ovary syndrome . Boston: Blackwell Scientific Publications; 1992. pp. 377–384. [Google Scholar]
- 5.Rotterdam E. S. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Human Reproduction . 2004;19:41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 6.Azziz R., Carmina E., Dewailly D., et al. The androgen excess and PCOS society criteria for the polycystic ovary syndrome: the complete task force report. Fertility and Sterility . 2009;91(2):456–488. doi: 10.1016/j.fertnstert.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 7.Diamanti-Kandarakis E., Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocrine Reviews . 2012;33(6):981–1030. doi: 10.1210/er.2011-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunaif A., Segal K. R., Shelley D. R., Green G., Dobrjansky A., Licholai T. Evidence for distinctive and intrinsic defects in insulin action in polycystic ovary syndrome. Diabetes . 1992;41(10):1257–1266. doi: 10.2337/diabetes.41.10.1257. [DOI] [PubMed] [Google Scholar]
- 9.Carmina E., Bucchieri S., Esposito A., et al. Abdominal fat quantity and distribution in women with polycystic ovary syndrome and extent of its relation to insulin resistance. Journal of Clinical Endocrinology & Metabolism . 2007;92(7):2500–2505. doi: 10.1210/jc.2006-2725. [DOI] [PubMed] [Google Scholar]
- 10.Vgontzas A. N., Trakada G., Bixler E. O., et al. Plasma interleukin 6 levels are elevated in polycystic ovary syndrome independently of obesity or sleep apnea. Metabolism . 2006;55(8):1076–1082. doi: 10.1016/j.metabol.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Mannerås-Holm L., Leonhardt H., Kullberg J., et al. Adipose tissue has aberrant morphology and function in PCOS: enlarged adipocytes and low serum adiponectin, but not circulating sex steroids, are strongly associated with insulin resistance. Journal of Clinical Endocrinology & Metabolism . 2011;96(2):E304–E311. doi: 10.1210/jc.2010-1290. [DOI] [PubMed] [Google Scholar]
- 12.Shulman G. I. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. New England Journal of Medicine . 2014;371(12):1131–1141. doi: 10.1056/nejmra1011035. [DOI] [PubMed] [Google Scholar]
- 13.Morrison S. A., Goss A. M., Azziz R., Raju D. A., Gower B. A. Peri-muscular adipose tissue may play a unique role in determining insulin sensitivity/resistance in women with polycystic ovary syndrome. Human Reproduction (Oxford) . 2017;32:185–192. doi: 10.1093/humrep/dew279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ducluzeau P.-H., Cousin P., Malvoisin E., et al. Glucose-to-insulin ratio rather than sex hormone-binding globulin and adiponectin levels is the best predictor of insulin resistance in nonobese women with polycystic ovary syndrome. Journal of Clinical Endocrinology & Metabolism . 2003;88(8):3626–3631. doi: 10.1210/jc.2003-030219. [DOI] [PubMed] [Google Scholar]
- 15.Vigil P., Contreras P., Alvarado J. L., Godoy A., Salgado A. M., Cortes M. E. Evidence of subpopulations with different levels of insulin resistance in women with polycystic ovary syndrome. Human Reproduction . 2007;22(11):2974–2980. doi: 10.1093/humrep/dem302. [DOI] [PubMed] [Google Scholar]
- 16.Dunaif A., Segal K. R., Futterweit W., Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes . 1989;38(9):1165–1174. doi: 10.2337/diabetes.38.9.1165. [DOI] [PubMed] [Google Scholar]
- 17.Barber T. M., Wass J. A., McCarthy M. I., Franks S. Metabolic characteristics of women with polycystic ovaries and oligo-amenorrhoea but normal androgen levels: implications for the management of polycystic ovary syndrome. Clinical Endocrinology . 2007;66:513–517. doi: 10.1111/j.1365-2265.2007.02764.x. [DOI] [PubMed] [Google Scholar]
- 18.Cassar S., Misso M. L., Hopkins W. G., Shaw C. S., Teede H. J., Stepto N. K. Insulin resistance in polycystic ovary syndrome: a systematic review and meta-analysis of euglycaemic-hyperinsulinaemic clamp studies. Human Reproduction . 2016;31(11):2619–2631. doi: 10.1093/humrep/dew243. [DOI] [PubMed] [Google Scholar]
- 19.Legro R. S., Kunselman A. R., Dodson W. C., Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected Women1. Journal of Clinical Endocrinology & Metabolism . 1999;84(1):165–169. doi: 10.1210/jcem.84.1.5393. [DOI] [PubMed] [Google Scholar]
- 20.Ehrmann D. A., Barnes R. B., Rosenfield R. L., Cavaghan M. K., Imperial J. Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care . 1999;22(1):141–146. doi: 10.2337/diacare.22.1.141. [DOI] [PubMed] [Google Scholar]
- 21.Ehrmann D. A., Kasza K., Azziz R., Legro R. S., Ghazzi M. N. Effects of race and family history of type 2 diabetes on metabolic status of women with polycystic ovary syndrome. Journal of Clinical Endocrinology & Metabolism . 2005;90(1):66–71. doi: 10.1210/jc.2004-0229. [DOI] [PubMed] [Google Scholar]
- 22.Gambineri A., Patton L., Altieri P., et al. Polycystic ovary syndrome is a risk factor for type 2 diabetes. Diabetes . 2012;61(9):2369–2374. doi: 10.2337/db11-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Norman R. J., Masters L., Milner C. R. Relative risk of conversion from normoglycaemia to impaired glucose tolerance or non-insulin dependent diabetes mellitus in polycystic ovarian syndrome. Human Reproduction . 2001;16:1995–1998. doi: 10.1093/humrep/16.9.1995. [DOI] [PubMed] [Google Scholar]
- 24.Saltiel A. R., Kahn C. R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature . 2001;414(6865):799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 25.Cheatham B., Kahn C. R. Insulin action and the insulin signaling network∗. Endocrine Reviews . 1995;16(2):117–142. doi: 10.1210/edrv-16-2-117. [DOI] [PubMed] [Google Scholar]
- 26.Seow K.-M., Juan C.-C., Hsu Y.-P., Hwang J.-L., Huang L.-W., Ho L.-T. Amelioration of insulin resistance in women with PCOS via reduced insulin receptor substrate-1 Ser312 phosphorylation following laparoscopic ovarian electrocautery. Human Reproduction . 2007;22(4):1003–1010. doi: 10.1093/humrep/del466. [DOI] [PubMed] [Google Scholar]
- 27.Ciaraldi T. P., Aroda V., Mudaliar S., Chang R. J., Henry R. R. Polycystic ovary syndrome is associated with tissue-specific differences in insulin resistance. Journal of Clinical Endocrinology & Metabolism . 2009;94(1):157–163. doi: 10.1210/jc.2008-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dunaif A., Finegood D. T. Beta-cell dysfunction independent of obesity and glucose intolerance in the polycystic ovary syndrome. Journal of Clinical Endocrinology & Metabolism . 1996;81(3):942–947. doi: 10.1210/jcem.81.3.8772555. [DOI] [PubMed] [Google Scholar]
- 29.Wareham N. J., Byrne C. D., Williams R., Day N. E., Hales C. N. Fasting proinsulin concentrations predict the development of type 2 diabetes. Diabetes Care . 1999;22(2):262–270. doi: 10.2337/diacare.22.2.262. [DOI] [PubMed] [Google Scholar]
- 30.Panidis D., Macut D., Farmakiotis D., et al. Indices of insulin sensitivity, beta cell function and serum proinsulin levels in the polycystic ovary syndrome. European Journal of Obstetrics & Gynecology and Reproductive Biology . 2006;127(1):99–105. doi: 10.1016/j.ejogrb.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 31.Sir-Petermann T., Maliqueo M., Codner E., et al. Early metabolic derangements in daughters of women with polycystic ovary syndrome. Journal of Clinical Endocrinology & Metabolism . 2007;92(12):4637–4642. doi: 10.1210/jc.2007-1036. [DOI] [PubMed] [Google Scholar]
- 32.Raissouni N., Kolesnikov A., Purushothaman R. Altered glucose disposition and insulin sensitivity in peri-pubertal first-degree relatives of women with polycystic ovary syndrome. International Journal of Pediatric Endocrinology . 2012;2012:p. 14. doi: 10.1186/1687-9856-2012-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Torchen L. C., Fogel N. R., Brickman W. J., Paparodis R., Dunaif A. Persistent apparent pancreatic β-cell defects in premenarchal PCOS relatives. Journal of Clinical Endocrinology & Metabolism . 2014;99(10):3855–3862. doi: 10.1210/jc.2014-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Biyasheva A., Legro R. S., Dunaif A., Urbanek M. Evidence for association between polycystic ovary syndrome (PCOS) andTCF7L2and glucose intolerance in women with PCOS andTCF7L2. Journal of Clinical Endocrinology & Metabolism . 2009;94(7):2617–2625. doi: 10.1210/jc.2008-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hao M., Yuan F., Jin C., et al. Overexpression of Lnk in the ovaries is involved in insulin resistance in women with polycystic ovary syndrome. Endocrinology . 2016;157(10):3709–3718. doi: 10.1210/en.2016-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burghen G. A., Givens J. R., Kitabchi A. E. Correlation of hyperandrogenism with hyperinsulinism in polycystic ovarian disease∗. Journal of Clinical Endocrinology & Metabolism . 1980;50(1):113–116. doi: 10.1210/jcem-50-1-113. [DOI] [PubMed] [Google Scholar]
- 37.O’Meara N. M., Blackman J. D., Ehrmann D. A. Defects in beta-cellfunction in functional ovarian hyperandrogenism. Journal of Clinical Endocrinology & Metabolism . 1993;76:1241–1247. doi: 10.1210/jcem.76.5.8496316. [DOI] [PubMed] [Google Scholar]
- 38.Annika K. S., Sascha T., Olaf O. Insulin resistance in patients with polycystic ovary syndrome. Annals of Medicine . 2004;36:426–439. doi: 10.1080/07853890410035296. [DOI] [PubMed] [Google Scholar]
- 39.Bergman R. N., Watanabe R., Rebrin K., Ader M., Steil G. Toward an integrated phenotype in pre-NIDDM. Diabetic Medicine . 1996;13:67–77. doi: 10.1002/dme.1996.13.s6.67. [DOI] [PubMed] [Google Scholar]
- 40.Ehrmann D. A., Sturis J., Byrne M. M., Karrison T., Rosenfield R. L., Polonsky K. S. Insulin secretory defects in polycystic ovary syndrome. Relationship to insulin sensitivity and family history of non-insulin-dependent diabetes mellitus. Journal of Clinical Investigation . 1995;96(1):520–527. doi: 10.1172/jci118064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Virkamäki A., Ueki K., Kahn C. R. Protein-protein interaction in insulin signaling and the molecular mechanisms of insulin resistance. Journal of Clinical Investigation . 1999;103(7):931–943. doi: 10.1172/jci6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shepherd P. R., Kahn B. B. Glucose transporters and insulin action - implications for insulin resistance and diabetes mellitus. New England Journal of Medicine . 1999;341(4):248–257. doi: 10.1056/nejm199907223410406. [DOI] [PubMed] [Google Scholar]
- 43.Ciaraldi T. P., el-Roeiy A., Madar Z., Reichart D., Olefsky J. M., Yen S. S. Cellular mechanisms of insulin resistance in polycystic ovarian syndrome. Journal of Clinical Endocrinology & Metabolism . 1992;75(2):577–583. doi: 10.1210/jcem.75.2.1322430. [DOI] [PubMed] [Google Scholar]
- 44.Marsden P. J., Murdoch A., Taylor R. Severe impairment of insulin action in adipocytes from amenorrheic subjects with polycystic ovary syndrome. Metabolism . 1994;43(12):1536–1542. doi: 10.1016/0026-0495(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 45.Rodrigues J. K., Navarro P. A., Zelinski M. B., Stouffer R. L., Xu J. Direct actions of androgens on the survival, growth and secretion of steroids and anti-Mullerian hormone by individual macaque follicles during three-dimensional culture. Human Reproduction . 2015;30(3):664–674. doi: 10.1093/humrep/deu335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pierre A., Taieb J., Giton F., et al. Dysregulation of the anti-müllerian hormone system by steroids in women with polycystic ovary syndrome. Journal of Clinical Endocrinology & Metabolism . 2017;102(11):3970–3978. doi: 10.1210/jc.2017-00308. [DOI] [PubMed] [Google Scholar]
- 47.Lim J. J., Han C. Y., Lee D. R., Tsang B. K. Ring finger protein 6 mediates androgen-induced granulosa cell proliferation and follicle growth via modulation of androgen receptor signaling. Endocrinology . 2017;158(4):993–1004. doi: 10.1210/en.2016-1866. [DOI] [PubMed] [Google Scholar]
- 48.Nanba A. T., Rege J., Ren J., Auchus R. J., Rainey W. E., Turcu A. F. 11-Oxygenated C19 steroids do not decline with age in women. Journal of Clinical Endocrinology & Metabolism . 2019;104(7):2615–2622. doi: 10.1210/jc.2018-02527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li H., Chen Y., Yan L.-y., Qiao J. Increased expression of P450scc and CYP17 in development of endogenous hyperandrogenism in a rat model of PCOS. Endocrine . 2012;43(1):184–190. doi: 10.1007/s12020-012-9739-3. [DOI] [PubMed] [Google Scholar]
- 50.Bakhshalizadeh S., Amidi F., Shirazi R., Shabani Nashtaei M. Vitamin D3 regulates steroidogenesis in granulosa cells through AMP-activated protein kinase (AMPK) activation in a mouse model of polycystic ovary syndrome. Cell Biochemistry and Function . 2018;36(4):183–193. doi: 10.1002/cbf.3330. [DOI] [PubMed] [Google Scholar]
- 51.Lerner A., Owens L. A., Coates M., et al. Expression of genes controlling steroid metabolism and action in granulosa-lutein cells of women with polycystic ovaries. Molecular and Cellular Endocrinology . 2019;486:47–54. doi: 10.1016/j.mce.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 52.Salilew-Wondim D., Wang Q., Tesfaye D. Polycystic ovarian syndrome is accompanied by repression of gene signatures associated with biosynthesis and metabolism of steroids, cholesterol and lipids. Journal of Ovarian Research . 2015;8:15–24. doi: 10.1186/s13048-015-0151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gonzalez E., Guengerich F. P. Kinetic processivity of the two-step oxidations of progesterone and pregnenolone to androgens by human cytochrome P450 17A1. Journal of Biological Chemistry . 2017;292(32):13168–13185. doi: 10.1074/jbc.m117.794917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kakuta H., Iguchi T., Sato T. The involvement of granulosa cells in the regulation by gonadotropins of Cyp17a1 in theca cells. In Vivo . 2018;32(6):1387–1401. doi: 10.21873/invivo.11391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peng H.-M., Im S.-C., Pearl N. M., et al. Cytochrome b5 activates the 17,20-lyase activity of human cytochrome P450 17A1 by increasing the coupling of NADPH consumption to androgen production. Biochemistry . 2016;55(31):4356–4365. doi: 10.1021/acs.biochem.6b00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakamura Y., Fujishima F., Hui X.-G., et al. 3βHSD and CYB5A double positive adrenocortical cells during adrenal development/aging. Endocrine Research . 2014;40(1):8–13. doi: 10.3109/07435800.2014.895377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qin K., Ehrmann D. A., Cox N., Refetoff S., Rosenfield R. L. Identification of a functional polymorphism of the human type 5 17β-hydroxysteroid dehydrogenase gene associated with polycystic ovary syndrome. Journal of Clinical Endocrinology & Metabolism . 2006;91(1):270–276. doi: 10.1210/jc.2005-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hattori K., Orisaka M., Fukuda S., et al. Luteinizing hormone facilitates antral follicular maturation and survival via thecal paracrine signaling in cattle. Endocrinology . 2018;159(6):2337–2347. doi: 10.1210/en.2018-00123. [DOI] [PubMed] [Google Scholar]
- 59.Xu J.-N., Zeng C., Zhou Y., Peng C., Zhou Y.-F., Xue Q. Metformin InhibitsStARExpression in human endometriotic stromal cells via AMPK-mediated disruption of CREB-CRTC2 complex formation. Journal of Clinical Endocrinology & Metabolism . 2014;99(8):2795–2803. doi: 10.1210/jc.2014-1593. [DOI] [PubMed] [Google Scholar]
- 60.Martinat N., Crépieux P., Reiter E., Guillou F. Extracellular signal-regulated kinases (ERK) 1, 2 are required for luteinizing hormone (LH)-induced steroidogenesis in primary Leydig cells and control steroidogenic acute regulatory (StAR) expression. Reproduction Nutrition Development . 2005;45(1):101–108. doi: 10.1051/rnd:2005007. [DOI] [PubMed] [Google Scholar]
- 61.Chow L. S., Mashek D. G., Wang Q., Shepherd S. O., Goodpaster B. H., Dubé J. J. Effect of acute physiological free fatty acid elevation in the context of hyperinsulinemia on fiber type-specific IMCL accumulation. Journal of Applied Physiology . 2017;123(1):71–78. doi: 10.1152/japplphysiol.00209.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li M., Xue K., Ling J., Diao F.-Y., Cui Y.-G., Liu J.-Y. The orphan nuclear receptor NR4A1 regulates transcription of key steroidogenic enzymes in ovarian theca cells. Molecular and Cellular Endocrinology . 2010;319(1-2):39–46. doi: 10.1016/j.mce.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 63.Ma X., Hayes E., Prizant H., Srivastava R. K., Hammes S. R., Sen A. Leptin-induced CART (cocaine- and amphetamine-regulated transcript) is a novel intraovarian mediator of obesity-related infertility in females. Endocrinology . 2016;157(3):1248–1257. doi: 10.1210/en.2015-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang F., Ruan Y.-C., Yang Y.-j., et al. Follicular hyperandrogenism downregulates aromatase in luteinized granulosa cells in polycystic ovary syndrome women. Reproduction . 2015;150(4):289–296. doi: 10.1530/rep-15-0044. [DOI] [PubMed] [Google Scholar]
- 65.McGee W. K., Bishop C. V., Bahar A., et al. Elevated androgens during puberty in female rhesus monkeys lead to increased neuronal drive to the reproductive axis: a possible component of polycystic ovary syndrome. Human Reproduction . 2011;27(2):531–540. doi: 10.1093/humrep/der393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maas K. H., Chuan S., Harrison E., Cook-Andersen H., Duleba A. J., Chang R. J. Androgen responses to adrenocorticotropic hormone infusion among individual women with polycystic ovary syndrome. Fertility and Sterility . 2016;106(5):1252–1257. doi: 10.1016/j.fertnstert.2016.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.O’Reilly M. W., Kempegowda P., Jenkinson C. 11-oxygenated C19 steroids are the predominant androgens in polycystic ovary syndrome. Journal of Clinical Endocrinology & Metabolism . 2016;104:56–65. doi: 10.1210/jc.2016-3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim S.-H., Moon J.-Y., Sasano H., Choi M. H., Park M.-J. Body fat mass is associated with ratio of steroid metabolites reflecting 17,20-lyase activity in prepubertal girls. Journal of Clinical Endocrinology & Metabolism . 2016;101(12):4653–4660. doi: 10.1210/jc.2016-2515. [DOI] [PubMed] [Google Scholar]
- 69.Cartney M., Ru C., Marshall J. C. Polycystic ovary syndrome. New England Journal of Medicine . 2016;375:11–18. doi: 10.1056/nejmcp1514916. [DOI] [PubMed] [Google Scholar]
- 70.Idkowiak J., Elhassan Y. S., Mannion P., et al. Causes, patterns and severity of androgen excess in 487 consecutively recruited pre- and post-pubertal children. European Journal of Endocrinology . 2019;180(3):213–221. doi: 10.1530/eje-18-0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Azziz R., Woods K. S., Reyna R., Key T. J., Knochenhauer E. S., Yildiz B. O. The prevalence and features of the polycystic ovary syndrome in an unselected population. Journal of Clinical Endocrinology & Metabolism . 2004;89(6):2745–2749. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 72.futterweit W. Polycystic ovary syndrome: a common reproductive and metabolic disorder necessitating early recognition and treatment. Primary Care: Clinics in Office Practice . 2007;34(4):761–789. doi: 10.1016/j.pop.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 73.Cibula D., Cifková R., fanta M. Increased risk of non-insulin dependent diabetes mellitus, arterial hypertension and coronary artery disease in perimenopausal women with a history of the polycystic ovary syndrome. Human Reproduction . 2000;15(4):785–789. doi: 10.1093/humrep/15.4.785. [DOI] [PubMed] [Google Scholar]
- 74.Azziz R., Carmina E., Dewailly D., et al. Criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an androgen excess society guideline. Journal of Clinical Endocrinology & Metabolism . 2006;91(11):4237–4245. doi: 10.1210/jc.2006-0178. [DOI] [PubMed] [Google Scholar]
- 75.Fraser A., Harris R., Sattar N., Ebrahim S., Davey Smith G., Lawlor D. A. Alanine aminotransferase, γ-glutamyltransferase, and incident diabetes. Diabetes Care . 2009;32(4):741–750. doi: 10.2337/dc08-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tziomalos K., Athyros V. G., Karagiannis A. Non-alcoholic fatty liver disease in type 2 diabetes: pathogenesis and treatment options. Current Vascular Pharmacology . 2012;10(2):162–172. doi: 10.2174/157016112799305012. [DOI] [PubMed] [Google Scholar]
- 77.Macut D., Tziomalos K., Božić-Antić I., et al. Non-alcoholic fatty liver disease is associated with insulin resistance and lipid accumulation product in women with polycystic ovary syndrome. Human Reproduction . 2016;31(6):1347–1353. doi: 10.1093/humrep/dew076. [DOI] [PubMed] [Google Scholar]
- 78.O’Reilly M. W., Taylor A. E., Crabtree N. J. Hyperandrogenemia predicts metabolic phenotype in polycystic ovary syndrome: the utility of serum androstenedione. Journal of Clinical Endocrinology & Metabolism . 2014;99:1027–1036. doi: 10.1210/jc.2013-3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kelly C. J. G., Speirs A., Gould G. W., Petrie J. R., Lyall H., Connell J. M. C. Altered vascular function in young women with polycystic ovary syndrome. Journal of Clinical Endocrinology & Metabolism . 2002;87(2):742–746. doi: 10.1210/jcem.87.2.8199. [DOI] [PubMed] [Google Scholar]
- 80.Diamanti-Kandarakis E., Alexandraki K., Piperi C., et al. Inflammatory and endothelial markers in women with polycystic ovary syndrome. European Journal of Clinical Investigation . 2006;36(10):691–697. doi: 10.1111/j.1365-2362.2006.01712.x. [DOI] [PubMed] [Google Scholar]
- 81.Bajuk K. S., Jensterle M. S., Pfeifer M. Cardiovascular risk and subclinical cardiovascular disease in polycystic ovary syndrome. Frontiers of Hormone Research . 2013;40:64–82. doi: 10.1159/000341838. [DOI] [PubMed] [Google Scholar]
- 82.Hurliman A., Keller Brown J., Maille N., Mandala M., Casson P., Osol G. Hyperandrogenism and Insulin resistance, not changes in body weight, mediate the development of endothelial dysfunction in a female rat model of polycystic ovary syndrome (PCOS) Endocrinology . 2015;156(11):4071–4080. doi: 10.1210/en.2015-1159. [DOI] [PubMed] [Google Scholar]
- 83.Franks S., Stark J., Hardy K. Follicle dynamics and anovulation in polycystic ovary syndrome. Human Reproduction Update . 2008;14(4):367–378. doi: 10.1093/humupd/dmn015. [DOI] [PubMed] [Google Scholar]
- 84.Wu X. K., Zhou S. Y., Liu J. X., et al. Selective ovary resistance to insulin signaling in women with polycystic ovary syndrome. Fertility and Sterility . 2003;80(4):954–965. doi: 10.1016/s0015-0282(03)01007-0. [DOI] [PubMed] [Google Scholar]
- 85.Zhu Q., Zuo R., He Y., et al. Local regeneration of cortisol by 11β-HSD1 contributes to insulin resistance of the granulosa cells in PCOS. Journal of Clinical Endocrinology & Metabolism . 2016;101(5):2168–2177. doi: 10.1210/jc.2015-3899. [DOI] [PubMed] [Google Scholar]
- 86.Wild R. A., Carmina E., Diamanti-Kandarakis E., et al. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the androgen excess and polycystic ovary syndrome (AE-PCOS) society. Journal of Clinical Endocrinology & Metabolism . 2010;95(5):2038–2049. doi: 10.1210/jc.2009-2724. [DOI] [PubMed] [Google Scholar]
- 87.Diamanti-Kandarakis E., Christakou C. D., Kandaraki E., Economou F. N. Metformin: an old medication of new fashion: evolving new molecular mechanisms and clinical implications in polycystic ovary syndrome. European Journal of Endocrinology . 2010;162(2):193–212. doi: 10.1530/eje-09-0733. [DOI] [PubMed] [Google Scholar]
- 88.Wang Y.-W., He S.-J., Feng X., et al. Metformin: a review of its potential indications. Drug Design, Development and Therapy . 2017;11:2421–2429. doi: 10.2147/dddt.s141675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Harrison C. L., Lombard C. B., Moran L. J., Teede H. J. Exercise therapy in polycystic ovary syndrome: a systematic review. Human Reproduction Update . 2011;17(2):171–183. doi: 10.1093/humupd/dmq045. [DOI] [PubMed] [Google Scholar]
- 90.Inzucchi S. E., Bergenstal R. M., Buse J. B. American DiabetesAssociation (ADA); European association for the study of diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American diabetes association (ADA) and the European association for the study of diabetes (EASD) Diabetes Care . 2012;35:1364–1379. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Legro R. S., Arslanian S. A., Ehrmann D. A., et al. Diagnosis and treatment of polycystic ovary syndrome: an endocrine society clinical practice guideline. Journal of Clinical Endocrinology & Metabolism . 2013;98(12):4565–4592. doi: 10.1210/jc.2013-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bajuk Studen K., Šebeštjen M., Pfeifer M., Preželj J. Influence of spironolactone treatment on endothelial function in non-obese women with polycystic ovary syndrome. European Journal of Endocrinology . 2011;164(3):389–395. doi: 10.1530/eje-10-0709. [DOI] [PubMed] [Google Scholar]
- 93.Escobar-Morreale H. F., Calvo R. M., Villuendas G., Sancho J., San Millán J. L. Association of polymorphisms in the interleukin 6 receptor complex with obesity and hyperandrogenism. Obesity Research . 2003;11(8):987–996. doi: 10.1038/oby.2003.136. [DOI] [PubMed] [Google Scholar]
- 94.Peral B., San Millán J. L., Castello R., Moghetti P., Escobar-Morreale H. F. The methionine 196 arginine polymorphism in exon 6 of the TNF receptor 2 gene (TNFRSF1B) is associated with the polycystic ovary syndrome and hyperandrogenism. Journal of Clinical Endocrinology & Metabolism . 2002;87(8):3977–3983. doi: 10.1210/jcem.87.8.8715. [DOI] [PubMed] [Google Scholar]
- 95.Villuendas G., San Millán J. L., Sancho J., Escobar-Morreale H. F. The −597 G⟶A and −174 G⟶C polymorphisms in the promoter of the IL-6 gene are associated with hyperandrogenism. Journal of Clinical Endocrinology & Metabolism . 2002;87(3):1134–1141. doi: 10.1210/jcem.87.3.8309. [DOI] [PubMed] [Google Scholar]
- 96.Moshage H. J., Roelofs H. M. J., van Pelt J. F., et al. The effect of interleukin-1, interleukin-6 and its interrelationship on the synthesis of serum amyloid A and C-reactive protein in primary cultures of adult human hepatocytes. Biochemical and Biophysical Research Communications . 1988;155(1):112–117. doi: 10.1016/s0006-291x(88)81056-8. [DOI] [PubMed] [Google Scholar]
- 97.Ouchi N., Kihara S., Funahashi T., et al. Reciprocal association of C-reactive protein with adiponectin in blood stream and adipose tissue. Circulation . 2003;107(5):671–674. doi: 10.1161/01.cir.0000055188.83694.b3. [DOI] [PubMed] [Google Scholar]
- 98.Gower B. A., Chandler-Laney P. C., Ovalle F., et al. Favourable metabolic effects of a eucaloric lower-carbohydrate diet in women with PCOS. Clinical Endocrinology . 2013;79(4):550–557. doi: 10.1111/cen.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pasquali R., Gambineri A., Cavazza C., et al. Heterogeneity in the responsiveness to long-term lifestyle intervention and predictability in obese women with polycystic ovary syndrome. European Journal of Endocrinology . 2011;164(1):53–60. doi: 10.1530/eje-10-0692. [DOI] [PubMed] [Google Scholar]
- 100.Siebert T. I., Viola M. I., Steyn D. W., Kruger T. F. Is metformin indicated as primary ovulation induction agent in women with PCOS? A systematic review and meta-analysis. Gynecologic and Obstetric Investigation . 2012;73(4):304–313. doi: 10.1159/000335253. [DOI] [PubMed] [Google Scholar]
- 101.Tan X., Li S., Chang Y., et al. Effect of metformin treatment during pregnancy on women with PCOS: a systematic review and meta-analysis. Clinical and Investigative Medicine . 2016;39(4):120–131. doi: 10.25011/cim.v39i4.27091. [DOI] [PubMed] [Google Scholar]
- 102.Sharma S. T., Wickham E. P., Nestler J. E. Changes in glucose tolerance with metformin treatment in polycystic ovary syndrome: a retrospective analysis. Endocrine Practice . 2007;13(4):373–379. doi: 10.4158/ep.13.4.373. [DOI] [PubMed] [Google Scholar]
- 103.Valsamakis G., Lois K., Kumar S., Mastorakos G. Metabolic and other effects of pioglitazone as an add-on therapy to metformin in the treatment of polycystic ovary syndrome (PCOS) Hormones . 2013;12(3):363–378. doi: 10.1007/bf03401302. [DOI] [PubMed] [Google Scholar]
- 104.Conway G., Dewailly D., Diamanti-Kandarakis E., et al. The polycystic ovary syndrome: a position statement from the European Society of Endocrinology. European Journal of Endocrinology . 2014;171:P1–P29. doi: 10.1530/EJE-14-0253. [DOI] [PubMed] [Google Scholar]
- 105.Unfer V., Nestler J. E., Kamenov Z. A. Effects of inositol(s) in women with PCOS: a systematic review of randomized controlled trials. The Internet Journal of Endocrinology . 2016;2016:1849–1862. doi: 10.1155/2016/1849162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Elkind-Hirsch K., Marrioneaux O., Bhushan M., Vernor D., Bhushan R. Comparison of single and combined treatment with exenatide and metformin on menstrual cyclicity in overweight women with polycystic ovary syndrome. Journal of Clinical Endocrinology & Metabolism . 2008;93(7):2670–2678. doi: 10.1210/jc.2008-0115. [DOI] [PubMed] [Google Scholar]
- 107.Jensterle Sever M., Kocjan T., Pfeifer M., Kravos N. A., Janez A. Short-term combined treatment with liraglutide and metformin leads to significant weight loss in obese women with polycystic ovary syndrome and previous poor response to metformin. European Journal of Endocrinology . 2014;170(3):451–459. doi: 10.1530/eje-13-0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tummon I., Gavrilova-Jordan L., Allemand M. C., Session D. Polycystic ovaries and ovarian hyperstimulation syndrome: a systematic review∗. Acta Obstetricia et Gynecologica Scandinavica . 2005;84(7):611–616. doi: 10.1111/j.0001-6349.2005.00788.x. [DOI] [PubMed] [Google Scholar]
- 109.Choi M. H., Lee S. H., Kim H. O., et al. Comparison of assisted reproductive technology outcomes in infertile women with polycystic ovary syndrome:In vitromaturation, GnRH agonist, and GnRH antagonist cycles. Clinical and Experimental Reproductive Medicine . 2012;39(4):166–171. doi: 10.5653/cerm.2012.39.4.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Son W.-Y., Chung J.-T., Herrero B., et al. Selection of the optimal day for oocyte retrieval based on the diameter of the dominant follicle in hCG-primed in vitro maturation cycles. Human Reproduction . 2008;23(12):2680–2685. doi: 10.1093/humrep/den332. [DOI] [PubMed] [Google Scholar]
- 111.Gjönnaess H. Polycystic ovarian syndrome treated by ovarian electrocautery through the laparoscope. Fertility and Sterility . 1984;41(1):20–25. doi: 10.1016/s0015-0282(16)47534-5. [DOI] [PubMed] [Google Scholar]
- 112.Gjønnaess H. Ovarian electrocautery in the treatment of women with polycystic ovary syndrome (PCOS). Factors affecting the results. Acta obstetricia et gynecologica Scandinavica . 1994;73(5):407–412. doi: 10.3109/00016349409006253. [DOI] [PubMed] [Google Scholar]
- 113.Flyckt R., Goldberg J. Laparoscopic ovarian drilling for clomiphene-resistant polycystic ovary syndrome. Seminars in Reproductive Medicine . 2011;29(02):138–146. doi: 10.1055/s-0031-1272476. [DOI] [PubMed] [Google Scholar]
- 114.Amer S. A. K. S., Banu Z., Li T. C., Cooke I. D. Long-term follow-up of patients with polycystic ovary syndrome after laparoscopic ovarian drilling: endocrine and ultrasonographic outcomes. Human Reproduction . 2002;17(11):2851–2857. doi: 10.1093/humrep/17.11.2851. [DOI] [PubMed] [Google Scholar]
- 115.Nahuis M. J., Kose N., Bayram N., et al. Long-term outcomes in women with polycystic ovary syndrome initially randomized to receive laparoscopic electrocautery of the ovaries or ovulation induction with gonadotrophins. Human Reproduction . 2011;26(7):1899–1904. doi: 10.1093/humrep/der141. [DOI] [PubMed] [Google Scholar]
- 116.Cocksedge K. A., Li T.-C., Saravelos S. H., Metwally M. A reappraisal of the role of polycystic ovary syndrome in recurrent miscarriage. Reproductive BioMedicine Online . 2008;17(1):151–160. doi: 10.1016/s1472-6483(10)60304-5. [DOI] [PubMed] [Google Scholar]
- 117.Farquhar C. M., Williamson K., Brown P. M. An economic evaluation of laparoscopic ovarian diathermy versus gonadotrophin therapy for women with clomiphene citrate resistant polycystic ovary syndrome. Human Reproduction . 2004;19(5):1110–1115. doi: 10.1093/humrep/deh219. [DOI] [PubMed] [Google Scholar]
- 118.Nahuis M. J., Oude Lohuis E., Kose N., et al. Long-term follow-up of laparoscopic electrocautery of the ovaries versus ovulation induction with recombinant FSH in clomiphene citrate-resistant women with polycystic ovary syndrome: an economic evaluation. Human Reproduction . 2012;27(12):3577–3582. doi: 10.1093/humrep/des336. [DOI] [PubMed] [Google Scholar]
- 119.Schlesselman J. J. Risk of endometrial cancer in relation to use of combined oral contraceptives. A practitioner’s guide to meta-analysis. Human Reproduction . 1997;12(9):1851–1863. doi: 10.1093/humrep/12.9.1851. [DOI] [PubMed] [Google Scholar]
- 120.Vessey M., Painter R. Oral contraceptive use and cancer. Findings in a large cohort study, 1968-2004. British Journal of Cancer . 2006;95(3):385–389. doi: 10.1038/sj.bjc.6603260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hannaford P. C., Selvaraj S., Elliott A. M., Angus V., Iversen L., Lee A. J. Cancer risk among users of oral contraceptives: cohort data from the Royal College of General Practitioner’s oral contraception study. BMJ . 2007;335(7621):651–658. doi: 10.1136/bmj.39289.649410.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Murphy A. A., Cropp C. S., Smith B. S., Burkman R. T., Zacur H. A. Effect of low-dose oral contraceptive on gonadotropins, androgens, and sex hormone binding globulin in nonhirsute women∗∗Supported in part by the general research center grant PHS RR-00827 and grant NO1-HD-32816 from the national institutes of health, bethesda, Maryland. ††Presented at the thirty-fifth annual meeting of the society of gynecologic investigations, march 18 to 20, 1988, baltimore, Maryland. Fertility and Sterility . 1990;53(1):35–39. doi: 10.1016/s0015-0282(16)53212-9. [DOI] [PubMed] [Google Scholar]
- 123.Azziz R., Gay F. The treatment of hyperandrogenism with oral contraceptives. Seminars in Reproductive Medicine . 1989;7(03):246–254. doi: 10.1055/s-2007-1021407. [DOI] [Google Scholar]
- 124.Azziz R. The evaluation and management of hirsutism∗1. Obstetrics & Gynecology . 2003;101(5):995–1007. doi: 10.1016/s0029-7844(02)02725-4. [DOI] [PubMed] [Google Scholar]
- 125.Crave J. C., Fimbel S., Lejeune H., Cugnardey N., Déchaud H., Pugeat M. Effects of diet and metformin administration on sex hormone-binding globulin, androgens, and insulin in hirsute and obese women. Journal of Clinical Endocrinology & Metabolism . 1995;80(7):2057–2062. doi: 10.1210/jcem.80.7.7608255. [DOI] [PubMed] [Google Scholar]
- 126.Crosignani P. G., Colombo M., Vegetti W. Overweight and obese anovulatory patients with polycystic ovaries: parallel improvements in anthropometric indices, ovarian physiology and fertility rate induced by diet. Human Reproduction . 2003;18(9):1928–1932. doi: 10.1093/humrep/deg367. [DOI] [PubMed] [Google Scholar]
- 127.Clark A. M., Ledger W., Galletly C., et al. Weight loss results in significant improvement in pregnancy and ovulation rates in anovulatory obese women. Human Reproduction . 1995;10(10):2705–2712. doi: 10.1093/oxfordjournals.humrep.a135772. [DOI] [PubMed] [Google Scholar]
- 128.Yanovski S. Z., Yanovski J. A. Obesity. New England Journal of Medicine . 2002;346(8):591–602. doi: 10.1056/nejmra012586. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data can be requested from the corresponding author.


