Abstract
The tumour microenvironment (TME) presents a major block to anti-tumour immune responses and to effective cancer immunotherapy. The inflammatory mediators such as cytokines, chemokines, growth factors and prostaglandins generated in the TME alter the phenotype and function of dendritic cells (DCs) that are critical for a successful adaptive immune response against the growing tumour. In this mini review we discuss how tumour cells and the surrounding stroma modulate DC maturation and trafficking to impact T cell function. Fibroblastic stroma and the associated extracellular matrix around tumours can also provide physical restrictions to infiltrating DCs and other leukocytes. We discuss interactions between the inflammatory TME and infiltrating immune cell function, exploring how the inflammatory TME affects generation of T cell-driven anti-tumour immunity. We discuss the open question of the relative importance of antigen-presentation site; locally within the TME versus tumour-draining lymph nodes. Addressing these questions will potentially increase immune surveillance and enhance anti-tumour immunity.
Keywords: tumour microenvironment (TME), inflammatory cytokines, dendritic cells, anti-tumour immunity, draining lymph nodes, Tertiary Lymphoid Structures (TLS), immune infiltration
Introduction
Anti-tumour immunity is the ability of the body’s immune system to recognise and eliminate tumour cells. This phenomenon has the potential to cure cancer even if cells are widely disseminated through multiple metastatic sites and has been harnessed to develop different immunotherapy drugs. With increased understanding of immune surveillance process by innate immune cells and discovery of T cell immune checkpoints, such as PD-1, PD-L1, and CTLA-4; cancer immunotherapy has significantly improved patient survival and quality of life (1–5). Treatments aim to promote successful infiltration and activation of antigen presenting cells and boost T-cells cytotoxic activity to promote anti-tumour immunity. However, despite promising results, not all tumour types or patients respond equally to immunotherapy (6–8). The major reasons for failure of immunotherapy are (1) reduced antigenicity (9–11) and (2) immunosuppressive tumour microenvironment (TME) (12–15). The TME is highly heterogeneous; consisting of tumour cells, stromal cells, extracellular matrix (ECM) and immune cell types including macrophages, dendritic cells, T and B lymphocytes, Natural killer (NK) cells, mast cells, myeloid derived suppressor cells (MDSCs) and neutrophils (16–20). The anti-tumour immune response relies on the antigen presenting cells (APCs) to prime naïve T cells. Tissue resident macrophages can activate T cells locally in the tumour; whereas dendritic cells (DCs), the professional APCs, are thought to migrate into the tumour draining lymph nodes (TDLNs) to prime T cells (21). However, immune surveillance by APCs and T-cell infiltration can be impaired by dynamic changes within the tumour microenvironment such as induction of chemokines, cytokines, growth factors, inflammation, ECM modulators and immune checkpoint proteins (22–27). This review focuses on the immunosuppressive properties of the TME and how these mechanisms alter activation, maturation and trafficking of dendritic cells to enable immune escape and tumour progression.
DC Maturation and DC Gene Signatures in Tumours
DCs are the professional APCs responsible for activation and maintenance of tumour-specific cytotoxicity by T cells (28, 29). Tumour infiltrating conventional DCs (cDC1 and cDC2) scan and phagocytose tumour antigens (30–32); and subsequently migrate to secondary lymphoid tissues to prime naïve CD8+ and CD4+ T cells (33–39). The phenotype and function of highly motile DCs is influenced by co-stimulatory molecules (CD80, CD86), chemokine receptors such as CCR7 and cell adhesion molecules (integrins, ICAM-1 and VCAM-1) (40–43). It has been well established that the interaction between CC chemokine receptor 7 (CCR7) upregulated on activated DCs and its ligand CC chemokine ligand 21 (CCL21) expressed by lymphatic endothelial cells (LECs) is essential for directional DC migration towards the lymph nodes (44–46). Upon entry to the LN, DCs use the C-type lectin CLEC-2 to migrate through the fibroblastic reticular network to reach the paracortex to stimulate the T cells (47–50). Secondary lymphoid tissues are structurally specialised to facilitate effective adaptive immune responses; however, the microenvironment of the tumour-draining lymph nodes (TDLNs) can be immune-suppressed in cancer patients and can display low DC count, defects in DC development, low levels of costimulatory molecules or accumulation of immature T cells (51, 52). DCs evaluated from TDLNs of an immunized B16F10 melanoma-bearing mice showed decreased functionality and expressed higher CD86 and lower CD206 levels (53). Similarly, in a study by Caronni et al., LNs draining lung tumours exhibited DCs with reduced antigen presentation due to downregulation of the SNARE VAMP3 and failed cytokine (IL12 and IFN-I) secretion. They reported lactic acid formation in the TME to be the main cause of DC function impairment (54). In addition, damage-associated molecular patterns (DAMPs) released from dying cells in the TME can also influence dendritic cells and other immune cells by interacting with toll-like receptors (TLRs) contributing to immunosuppressive phenotype (55). Lack of mature, migratory DCs in tumours correlates with poor prognosis in cancer patients and failure of immunotherapies (56–58). Recent development of single cell transcriptome profiling of tumour infiltrating DCs has proven to be a very powerful tool to map tumour-driven immune changes and to design future immune therapies leveraging DC biology. scRNA-seq studies on various human tumours, including non–small cell lung cancer (NSCLC) (59–62), head and neck squamous cell carcinoma (63), hepatocellular carcinoma (64), melanoma (65, 66), cutaneous squamous cell carcinoma (67), colorectal cancer (61, 68), ovarian cancer (61), and breast cancer (61) have identified tissue-specific DC subsets as well as those conserved across cancer types. By comparing tumour infiltrating DC states across various tumour studies, five major DC subsets have been defined that are conserved in most tumour types (69, 70) ( Table 1 ). Four major ones are cDC1, cDC2, migratory DC3 (mDC3) and plasmacytoid DC (pDC); and the DC subset (DC5) that were less conserved, mostly contained cDC2 state (CD1C+) but additionally either expressed Langerhans cell-specific markers (CD201, CD1A) or monocyte markers (CD14, CD11b) such as in case of NSCLC (61, 62, 69, 70). DC5 were also referred as inflammatory DCs as these have phenotypic similarities to monocytes but are functionally different due to their cDC2-specific antigen presentation properties (71). On the other hand, classical monocytes (CD14+ CD16-) play a key role in tissue homeostasis and inflammation (72). Like monocytes, inflammatory DCs are also capable of releasing TNF-α and inducible nitric oxide synthase (iNOS) upon pathogen recognition. In addition, there is a subset of cDCs that induce antigen-specific tolerance in dLNs; known as regulatory DCs (DCregs) (73, 74). These are characterized by low MHC expression and therefore weaker antigen presentation capability to effector T cells. Instead, they can induce proliferation of regulatory T cells (Tregs) resulting in immune tolerance. These properties have led the use of DCregs in organ transplantations (75).
Table 1.
Tumour infiltrating DC subsets detected in various human solid tumours – Liver, Ovarian, Lung, Breast and Colorectal (69, 70).
| DC subsets | Markers |
|---|---|
| cDC1 | XCR1, CLEC9A, CADM1, CD141, CD103 |
| cDC2 | CD11b, SIRPa, CLEC10A, FCER1A, CD1c |
| mDC3 | MARCKS, CCL19, LAMP3, BATF3, CCR7, CD40 |
| pDC | TCf4, CXCR3, LILRA4, CLCEC4C, IRF7 |
| DC5 or inflammatory DCs | CD1c, CD201, CD1A, CD14 |
Overall cDC2 phenotype is the most abundant, while the other DC subtypes vary in each cancer type (61, 76). Single cell sequencing and clustering analysis have identified transcription factors underlying each DC phenotype, including BATF3 for cDC1s, CEBPB for cDC2s, NFKB2 for migratory cDCs and TCF4 for pDCs (61, 77). Another study reports differential expression of costimulatory molecules and immune checkpoints on different DC subsets present in the TME (78). Although these phenomena are tightly regulated, heterogeneity of TME can influence the transcriptional factor activity, expression of costimulatory molecules and hence DC maturation and/or migration (78–82). This new in-depth knowledge of DC gene signatures can facilitate the design of a favourable antitumour response or identification of response biomarkers for targeted therapies (83).
TME Factors Affecting DC Development in Tumours
Pro- and Anti-Inflammatory Factors
The immunosuppression of tumour-infiltrating DCs can be facilitated by various soluble factors secreted in the TME such as IL-6, IL-10, IDO, M-CSF, transforming growth factor-β1 (TGF-β1), PGE2, VEGF ( Figure 1 ) (84–91); although promisingly some of these defects in DC development or function have been proven to be reversible in pre-clinical models and clinical trials (27, 91–94). Mature DC numbers or functions were improved leading to better immune control of the tumour in several mouse models: IL-6 KO mice (95); tumours treated with anti-VEGF antibody (96, 97); and treatment with anti-IL-8 monoclonal antibody (98, 99). On the other hand, pro-inflammatory cytokines such as IFN-α, IL-2, IL-15, IL-21 and GM-CSF are also present in the TME ( Figure 1 ) that contribute to enhanced antigen priming, improved DC maturation and increased immune infiltration in tumours (100–103). Therefore, the complex balance of inflammatory signals in the TME is an area of intense research interest but is not trivial to target currently. One of the recent studies on human melanoma reported the correlation of pro-inflammatory cytokine FLT3L production (by NK cells) with abundant intratumoral stimulatory DCs, improved patient responsiveness to anti-PD-1 therapies and better overall survival (104).
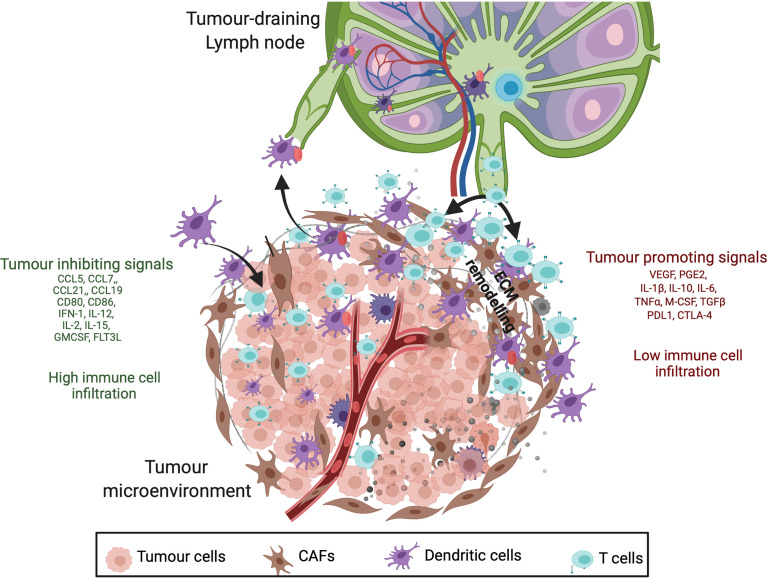
Figure 1.
Cancer inhibitory and cancer-promoting signals within the tumour microenvironment (TME). Anti-tumour response is initiated by antigen recognition and trafficking by mature DCs to the tumour draining lymph node (TDLN) which involves upregulation of chemokine receptors (CCR7), MHC class II, co-stimulatory molecules (CD80 and CD86), inflammatory molecules (IL-12, INF-1) and adhesion molecules (ICAM-1) (listed in green). Having said that, immunosuppressive nature of TME secretes tumour promoting inflammatory mediators (listed in red) such as prostaglandin E2, cytokines (IL-10, IL-6, TGFß), chemokines (CXCL1) and growth factors (VEGF) that impede anti-tumour response by altering DC phenotype, T-cell infiltration and ECM remodelling. These differences result in poor surveillance by DCs and lower infiltration of T cells in tumours with immunosuppressive molecules (red).
The inflammatory factors described above can be derived from tumour cells, immune cells or stromal cells such as fibroblasts surrounding tumour (61, 88, 105, 106). Various subtypes of fibroblasts based on different tissue specific identity, localization, function, transcription factor expression, collagen factors, cancer hallmark genes etc. make up the total tumour mass. CAFs or cancer associated fibroblasts represent a major population in the TME of many solid tumours, however their origin and role in tumour progression is complex and they can generate pro-tumourigenic and anti-tumourigenic secretory factors. Phenotypically and functionally different CAF subtypes based on cell-surface markers such as podoplanin (PDPN), α-smooth muscle actin (αSMA), fibroblast-activated protein (FAP), fibroblast-specific protein-1 (FSP-1/S100A4), THY1 (also known as CD90), and platelet-derived growth factor receptor-α, and β (PDGFRα and PDGFRβ) have been associated with different tumour types, stages and patient survival (107–111). Recently, the ability of CAFs to modulate the immune responses has been discovered and is being explored to improve cancer therapies. CAFs also share some properties with fibroblasts in lymph nodes that already have a well-established role in DC migration (47, 112, 113); and therefore, parallels can be drawn between the two to better understand the DC trafficking in the TME. For example, PDPN present in fibroblasts interacts with CCL21 and promotes CCL21/CCR7 axis mediated DC migration in lymph node. This knowledge was exploited to study the role of PDPN+ CAFs under the influence of hypoxia in tumour progression (114). The study reported PDPN overexpression due to hypoxia in fact favoured invasion of CCR7+ tumour cell into CCL21+ peripheral lymph nodes leading to metastasis (114, 115). Tumours associated with hypoxia are immunosuppressive and lack high expression of CCL21 and therefore therapeutic use of recombinant chemokines (such as CCL21) to stimulate immune cell recognition in tumours is being considered as a novel treatment approach (116, 117). Also, more research is required to understand the transition of a ‘normal’ fibroblast into an immunosuppressive phenotype such as S100A4+ PDPN+ CAFs as reported in breast cancer patients (109) or into an inflammatory CAF (iCAF) phenotype producing IL-6, IL-10, and IDO (118, 119) linked to poor patient survival. Authors of Fang et al. (118) have shown the role of the urokinase-type plasminogen activator, PLAU in conversion of fibroblasts to iCAFs in esophgeal cancer (118), but much is still unknown about fibroblast differentiation in TME.
Tertiary Lymphoid Structures (TLS)
TLS are established at sites of chronic inflammation and can structurally and functionally resemble secondary lymphoid organs (120–122). Recent studies on murine models of TLS have shown the role of PDPN+ FAP+ immunofibroblasts in driving the development and expansion of TLSs (123, 124). These form part of the TME and can benefit from quick surveillance and locally primed immune response against tumour antigens ( Figure 2 ). Occurrence of TLS correlated with high number of mature DCs, strong T-cell infiltration and long-term survival in human primary lung, breast, colorectal, melanoma and other tumours (120, 125–128). However, factors such as TLS location, tumour stage, tumour mutations, treatment history can affect immune cell infiltration and anti-tumour response (128, 129). The cells residing in TLS in tumours are known to express Th1, CD4, CD8, CD31, CD23, FOXP3, chemokines (CCL19, CCL21) and clusters of DC-Lamp+ mature dendritic cells (120, 130, 131) providing an immune-supportive niche (132–134). Typically, TLS at the periphery of the tumour have more organised and distinct DC/T-cell and B-cell zones than intratumoral TLS which contain mostly B cells (133). Future research understanding the immunological features of extratumoral versus intratumoral TLS will be useful to predict responsiveness to immunotherapy and overall survival.
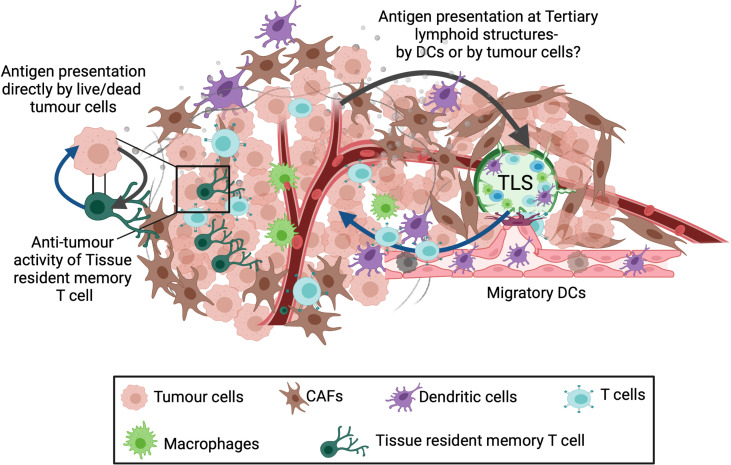
Figure 2.
Alternate sites of antigen presentation and T-cell priming. Three different sites for presentation of tumour associated antigens have been described: Tumour draining lymph node (TDLN), Tertiary lymphoid structures (TLS) and Tissue resident memory T cells. A population of memory precursor cells are believed to differentiate into CD103+ tissue resident memory T cells. These cells reside in the tumour and can recognize tumour antigens followed by killing the target tumour cell. In addition, tertiary lymphoid structures (TLS) also present a potential site for T cell priming. TLSs are organised cell aggregates formed within or at tumour margins in response to local inflammation and numerous cell-cell interactions occurring within the TME. Since these contain various immune cell types, TLSs can activate local immune response against the tumour, however the mechanism for T-cell priming within the TLSs is unknown.
Immune Checkpoint Genes
The other group of molecules responsible for causing dysfunction in tumour-infiltrating DCs are immune checkpoint proteins PD-L1, PD-1, ILT2, CTLA4, TIM3 expressed by tumour cells or other immune cells (135–141). As mentioned before, expression of these inhibitory molecules is variable among DC subsets. For example, PD-1 and TIM-3 are mostly expressed on cDC1s; PD-1 expression specifically has been shown to inhibit NF-kB activation which is critical for DC functions including costimulatory molecule expression, antigen presentation and cytokine release leading to T cell inactivation (78, 135, 137, 139, 140). On contrary, ILT2 is expressed on pDCs and cDC2s, but not on cDC1s (78). The central goal of immunotherapies is inhibition of immune checkpoint genes and the expansion of mature cDCs and cytotoxic CD8+ T cells within tumours. It is associated with positive patient outcomes in multiple cancer types when combined with chemotherapy or radiotherapy treatments (28, 135, 142, 143). Despite this, many patients still fail to respond to immune checkpoint blockade. A better understanding of the role of inflammatory mediators in determining tumour progression will also provide therapeutic avenues to improve immunotherapy outcomes (144–147).
Different labs have reported direct inhibition of pro-tumourigenic inflammation in combination with immune checkpoint blockade as a powerful strategy to improve the patient survival rates (27, 148–150). One such example is the use of aspirin that blocks the COX-2/PGE2 pathway and has shown promising results in preclinical melanoma models (27, 149). Prostaglandin E2 (PGE2), catalysed by the enzyme COX-2 is elevated in many tumours (151) and plays a role in tumour evasion by directly inhibiting cytotoxic immune responses and subsequently mediates expression of other inflammatory molecules such as CXCL9, CXCL10, CXCR4, CXCL12, IDO1 and interferon (IFN)-γ (27, 144, 148, 150, 152–154). Induction of CXCL12, CXCR4 and IDO1 in tumours have been associated with accumulation of myeloid derived suppressor cells (90, 155). Moreover, direct interaction of EP2/EP4 receptors (present on DCs) with the available PGE2 can modulate DC maturation, metalloprotease-driven DC motility, and immune response in tumours (27, 149, 152, 156–158). Thus, targeting the inflammatory environment of the tumour is important to restore DC function to harvest the full potential of immunotherapy.
Leveraging DC Biology in Cancer Therapies
Anti-tumour immunity relies on cross-presentation of tumour antigens by DCs to elicit a CD8+ T cell response. Among various DC subsets, cDC1s (XCR1+, CD103+) play a critical role in anti-tumour immunity. CLEC9A, (also known as DNGR1) is highly expressed on cDC1s and binds necrotic cell debris and promotes antigen processing in tumours (159–161). One of the reasons for checkpoint blockade failure is poor antigen presentation due to absence of co-stimulatory molecules and therefore modulation of DC function could increase responses to these therapies. One method to address this issue is the development of DC vaccines for cancer treatment, bypassing the need to activate and mature DCs within the tumour. DC-based cancer vaccines work by recruiting ex-vivo generated dendritic cells (or monocyte derived patient DCs) that are genetically engineered, matured, and loaded with tumour-specific antigens (162–164) or by reprogramming endogenous DCs by injecting biomaterial-based scaffolds providing favourable microenvironment for the recruitment of activated DCs (165, 166). An ideal DC vaccine must be able to increase cross-presentation by DCs, express high levels of co-stimulatory molecules, induce tumour-specific T cells with high migratory and cytolytic capabilities. Furthermore, the use of dendritic growth factor Flt3L in combination with checkpoint inhibitors or DC vaccines has improved number of activated intratumoural cDC1s and enhanced anti-tumour immunity to BRAF and checkpoint blockade in preclinical models (167–170).
Presence of co-inhibitory signals (e.g., IL-10, IL-6, PGE2, TGF-β) or absence of co-stimulatory molecules (e.g. CD80 and CD86) can result in inefficient antigen presentation by DCs and poor induction of antigen-specific CD8+T cells. Therefore, inflammatory cytokines secreted by tumour cells and tumour-associated stroma have been identified as promising candidates to potentiate current immunotherapies including immune checkpoint blockade and CAR-T therapy (149, 171–173). Stroma present around most tumours can also magnify inflammation and impede DC phenotype (174–177) and hence manipulating stroma/DC crosstalk in the TME could help improve DC function.
Discussion
It is now established that tumours can exploit their surroundings to create an immunosuppressive microenvironment to control DC function within both the TME and TDLNs (178, 179). These signals including cytokines, chemokines, prostaglandins, growth factors, immune checkpoint genes, etc., may target different DC subsets infiltrating tumours and influence DC maturation, antigen uptake and DC migration (53, 180). Although the success of immunotherapy relies on enhanced T cell activity, activation of tumour-specific T cells cannot be achieved without prior antigen presentation by professional DCs. To overcome immunosuppressive signals, personalized vaccines loaded with patient-derived engineered DCs or delivery of innate stimulus such as TLR3 ligand or a STING agonist to DCs at the tumour site are being developed and have shown promising results (181, 182). Repurposing of existing anti-inflammatory drugs such as aspirin along with DC vaccines or immunotherapies has also been successfully tested in pre-clinical models (149).
This review also addresses the importance of local versus TDLN priming of anti-tumoural T cell responses. Tissue resident memory CD103+ CD8+ T cells residing in the non-lymphoid tissues have shown to provide local immunosurveillance and enhanced immune responses in melanoma, lung and breast tumours (183–187). Moreover, melanoma patients with higher resident T cell population responded better to anti-PD-1 immunotherapy with improved survival (188, 189). However, what is still unclear is how are tissue resident memory CD8+ T cells primed ( Figure 2 ) and whether there is a distinct population of DCs required to activate them. Although the exact regulatory mechanisms remain to be explored further, it is hypothesized that crosstalk between tissue resident memory T cells, tumour cells, stromal cells and DCs within the TME potentiate secondary T-cell responses against tumours ( Figure 2 ). This also opens discussion on the role of tumour associated tertiary lymphoid structures (TLSs) in intra-tumoural DC maturation; and sourcing T cells and B cells to the tumour (190). Although TLS has been positively correlated with anti-tumour responses, there are still many questions remain to be answered such as TLS composition and TLS induction at tumour site before TLS can be adopted as a predictive tool or as a therapeutic option. Our discussion demonstrates the importance of site of antigen presentation in DC maturation and trafficking which must be exploited therapeutically to enhance immune response against cancer.
Author Contributions
YG and SA planned the concept and design of the review. YG and AK collected previous literature on the topic and drafted the article. YG made the figures. SA performed critical revision of the article. YG and SA edited the final version of the article.
Funding
This work is funded by Cancer Research UK (Career development fellowship CRUK-A19763 to SA) and Medical Research Council (MC-U12266B).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
APC, Antigen Presenting cells; LNs, Lymph nodes; TDLN, Tumour draining lymph node; TME, Tumour microenvironment; DCs, Dendritic cells; PGE2, Prostaglandin E2; ECM, Extracellular matrix; CAF, Cancer associated fibroblasts; TLS, Tertiary lymphoid structures.
References
- 1. Esfahani K, Roudaia L, Buhlaiga N, del Rincon SV, Papneja N, Miller WH. A Review of Cancer Immunotherapy: From the Past, to the Present, to the Future. Curr Oncol (2020) 27:87–97. doi: 10.3747/co.27.5223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Koury J, Lucero M, Cato C, Chang L, Geiger J, Henry D, et al. Immunotherapies: Exploiting the Immune System for Cancer Treatment. J Immunol Res (2018) 2018:1–16. doi: 10.1155/2018/9585614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob J-J, Rutkowski P, Lao CD, et al. Five-Year Survival With Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med (2019) 381:1535–46. doi: 10.1056/NEJMoa1910836 [DOI] [PubMed] [Google Scholar]
- 4. Nogrady B. Game-Changing Class of Immunotherapy Drugs Lengthens Melanoma Survival Rates. Nature (2020) 580:S14–6. doi: 10.1038/d41586-020-01038-9 [DOI] [Google Scholar]
- 5. Zhao B, Zhao H, Zhao J. Efficacy of PD-1/PD-L1 Blockade Monotherapy in Clinical Trials. Ther Adv Med Oncol (2020) 12:1–22. doi: 10.1177/1758835920937612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brahmer JR, Tykodi SS, Chow LQM, Hwu W-J, Topalian SL, Hwu P, et al. Safety and Activity of Anti–PD-L1 Antibody in Patients With Advanced Cancer. N Engl J Med (2012) 366:2455–65. doi: 10.1056/NEJMoa1200694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Haslam A, Prasad V. Estimation of the Percentage of US Patients With Cancer Who Are Eligible for and Respond to Checkpoint Inhibitor Immunotherapy Drugs. JAMA Network Open (2019) 2:1–9. doi: 10.1001/jamanetworkopen.2019.2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, et al. Phase 2 Trial of Single Agent Ipilimumab (Anti-CTLA-4) for Locally Advanced or Metastatic Pancreatic Adenocarcinoma. J Immunother (2010) 33:828–33. doi: 10.1097/CJI.0b013e3181eec14c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Almand B, Resser JR, Lindman B, Nadaf S, Clark JI, Kwon ED, et al. Clinical Significance of Defective Dendritic Cell Differentiation in Cancer. Clin Cancer Res: An Off J Am Assoc Cancer Res (2000) 6:1755–66. [PubMed] [Google Scholar]
- 10. Balachandran VP, Łuksza M, Zhao JN, Makarov V, Moral JA, Remark R, et al. Identification of Unique Neoantigen Qualities in Long-Term Survivors of Pancreatic Cancer. Nature (2017) 551:512–6. doi: 10.1038/nature24462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Delp K, Momburg F, Hilmes C, Huber C, Seliger B. Functional Deficiencies of Components of the MHC Class I Antigen Pathway in Human Tumors of Epithelial Origin. Bone Marrow Transplant (2000) 25:S88–95. doi: 10.1038/sj.bmt.1702363 [DOI] [PubMed] [Google Scholar]
- 12. Bergmann C, Strauss L, Zeidler R, Lang S, Whiteside TL. Expansion of Human T Regulatory Type 1 Cells in the Microenvironment of Cyclooxygenase 2 Overexpressing Head and Neck Squamous Cell Carcinoma. Cancer Res (2007) 67:8865–73. doi: 10.1158/0008-5472.CAN-07-0767 [DOI] [PubMed] [Google Scholar]
- 13. Ene–Obong A, Clear AJ, Watt J, Wang J, Fatah R, Riches JC, et al. Activated Pancreatic Stellate Cells Sequester CD8+ T Cells to Reduce Their Infiltration of the Juxtatumoral Compartment of Pancreatic Ductal Adenocarcinoma. Gastroenterology (2013) 145:1121–32. doi: 10.1053/j.gastro.2013.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Feig C, Jones JO, Kraman M, Wells RJB, Deonarine A, Chan DS, et al. Targeting CXCL12 From FAP-Expressing Carcinoma-Associated Fibroblasts Synergizes With Anti-PD-L1 Immunotherapy in Pancreatic Cancer. Proc Natl Acad Sci (2013) 110:20212–7. doi: 10.1073/pnas.1320318110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kabacaoglu D, Ciecielski KJ, Ruess DA, Algül H. Immune Checkpoint Inhibition for Pancreatic Ductal Adenocarcinoma: Current Limitations and Future Options. Front Immunol (2018) 9:1878. doi: 10.3389/fimmu.2018.01878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chung W, Eum HH, Lee H-O, Lee K-M, Lee H-B, Kim K-T, et al. Single-Cell RNA-Seq Enables Comprehensive Tumour and Immune Cell Profiling in Primary Breast Cancer. Nat Commun (2017) 8:1–12. doi: 10.1038/ncomms15081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kojima Y, Acar A, Eaton EN, Mellody KT, Scheel C, Ben-Porath I, et al. Autocrine TGF- and Stromal Cell-Derived Factor-1 (SDF-1) Signaling Drives the Evolution of Tumor-Promoting Mammary Stromal Myofibroblasts. Proc Natl Acad Sci (2010) 107:20009–14. doi: 10.1073/pnas.1013805107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Medrek C, Pontén F, Jirström K, Leandersson K. The Presence of Tumor Associated Macrophages in Tumor Stroma as a Prognostic Marker for Breast Cancer Patients. BMC Cancer (2012) 12:1–9. doi: 10.1186/1471-2407-12-306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Smith HA, Kang Y. The Metastasis-Promoting Roles of Tumor-Associated Immune Cells. J Mol Med (2013) 91:411–29. doi: 10.1007/s00109-013-1021-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spaeth EL, Dembinski JL, Sasser AK, Watson K, Klopp A, Hall B, et al. Mesenchymal Stem Cell Transition to Tumor-Associated Fibroblasts Contributes to Fibrovascular Network Expansion and Tumor Progression. PloS One (2009) 4:1–11. doi: 10.1371/journal.pone.0004992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ruhland MK, Roberts EW, Cai E, Mujal AM, Marchuk K, Beppler C, et al. Visualizing Synaptic Transfer of Tumor Antigens Among Dendritic Cells. Cancer Cell (2020) 37:789–99. doi: 10.1016/j.ccell.2020.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aiello NM, Bajor DL, Norgard RJ, Sahmoud A, Bhagwat N, Pham MN, et al. Metastatic Progression Is Associated With Dynamic Changes in the Local Microenvironment. Nat Commun (2016) 7:1–9. doi: 10.1038/ncomms12819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Di Blasio S, van Wigcheren GF, Becker A, van Duffelen A, Gorris M, Verrijp K, et al. The Tumour Microenvironment Shapes Dendritic Cell Plasticity in a Human Organotypic Melanoma Culture. Nat Commun (2020) 11:1–17. doi: 10.1038/s41467-020-16583-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oba T, Long MD, Keler T, Marsh HC, Minderman H, Abrams SI, et al. Overcoming Primary and Acquired Resistance to Anti-PD-L1 Therapy by Induction and Activation of Tumor-Residing Cdc1s. Nat Commun (2020) 11:1–20. doi: 10.1038/s41467-020-19192-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Poloso NJ, Urquhart P, Nicolaou A, Wang J, Woodward DF. PGE2 Differentially Regulates Monocyte-Derived Dendritic Cell Cytokine Responses Depending on Receptor Usage (EP2/EP4). Mol Immunol (2013) 54:284–95. doi: 10.1016/j.molimm.2012.12.010 [DOI] [PubMed] [Google Scholar]
- 26. Valenti R, Huber V, Iero M, Filipazzi P, Parmiani G, Rivoltini L. Tumor-Released Microvesicles as Vehicles of Immunosuppression: Figure 1. Cancer Res (2007) 67:2912–5. doi: 10.1158/0008-5472.CAN-07-0520 [DOI] [PubMed] [Google Scholar]
- 27. Zelenay S, van der Veen AG, Böttcher JP, Snelgrove KJ, Rogers N, Acton SE, et al. Cyclooxygenase-Dependent Tumor Growth Through Evasion of Immunity. Cell (2015) 162:1257–70. doi: 10.1016/j.cell.2015.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Broz ML, Binnewies M, Boldajipour B, Nelson AE, Pollack JL, Erle DJ, et al. Dissecting the Tumor Myeloid Compartment Reveals Rare Activating Antigen-Presenting Cells Critical for T Cell Immunity. Cancer Cell (2014) 26:638–52. doi: 10.1016/j.ccell.2014.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Engelhardt JJ, Boldajipour B, Beemiller P, Pandurangi P, Sorensen C, Werb Z, et al. Marginating Dendritic Cells of the Tumor Microenvironment Cross-Present Tumor Antigens and Stably Engage Tumor-Specific T Cells. Cancer Cell (2012) 21:402–17. doi: 10.1016/j.ccr.2012.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, et al. Batf3 Deficiency Reveals a Critical Role for CD8 + Dendritic Cells in Cytotoxic T Cell Immunity. Science (2008) 322:1097–100. doi: 10.1126/science.1164206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nakahara T, Oba J, Shimomura C, Kido-Nakahara M, Furue M. Early Tumor-Infiltrating Dendritic Cells Change Their Characteristics Drastically in Association With Murine Melanoma Progression. J Invest Dermatol (2016) 136:146–53. doi: 10.1038/JID.2015.359 [DOI] [PubMed] [Google Scholar]
- 32. Theisen DJ, Davidson JT, Briseño CG, Gargaro M, Lauron EJ, Wang Q, et al. WDFY4 Is Required for Cross-Presentation in Response to Viral and Tumor Antigens. Science (2018) 362:694–9. doi: 10.1126/science.aat5030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, Zhan Y, et al. Migratory Dendritic Cells Transfer Antigen to a Lymph Node-Resident Dendritic Cell Population for Efficient CTL Priming. Immunity (2006) 25:153–62. doi: 10.1016/j.immuni.2006.04.017 [DOI] [PubMed] [Google Scholar]
- 34. Binnewies M, Mujal AM, Pollack JL, Combes AJ, Hardison EA, Barry KC, et al. Unleashing Type-2 Dendritic Cells to Drive Protective Antitumor CD4+ T Cell Immunity. Cell (2019) 177:556–71. doi: 10.1016/j.cell.2019.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hirano N, Butler MO, Xia Z, Ansén S, von Bergwelt-Baildon MS, Neuberg D, et al. Engagement of CD83 Ligand Induces Prolonged Expansion of CD8+ T Cells and Preferential Enrichment for Antigen Specificity. Blood (2006) 107:1528–36. doi: 10.1182/blood-2005-05-2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Plantinga M, Guilliams M, Vanheerswynghels M, Deswarte K, Branco-Madeira F, Toussaint W, et al. Conventional and Monocyte-Derived CD11b+ Dendritic Cells Initiate and Maintain T Helper 2 Cell-Mediated Immunity to House Dust Mite Allergen. Immunity (2013) 38:322–35. doi: 10.1016/j.immuni.2012.10.016 [DOI] [PubMed] [Google Scholar]
- 37. Pooley JL, Heath WR, Shortman K. Cutting Edge: Intravenous Soluble Antigen Is Presented to CD4 T Cells by CD8 – Dendritic Cells, But Cross-Presented to CD8 T Cells by CD8 + Dendritic Cells. J Immunol (2001) 166:5327–30. doi: 10.4049/jimmunol.166.9.5327 [DOI] [PubMed] [Google Scholar]
- 38. Schlitzer A, McGovern N, Teo P, Zelante T, Atarashi K, Low D, et al. IRF4 Transcription Factor-Dependent CD11b+ Dendritic Cells in Human and Mouse Control Mucosal IL-17 Cytokine Responses. Immunity (2013) 38:970–83. doi: 10.1016/j.immuni.2013.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Steinman RM. Decisions About Dendritic Cells: Past, Present, and Future. Annu Rev Immunol (2012) 30:1–22. doi: 10.1146/annurev-immunol-100311-102839 [DOI] [PubMed] [Google Scholar]
- 40. Harjunpää H, Llort Asens M, Guenther C, Fagerholm SC. Cell Adhesion Molecules and Their Roles and Regulation in the Immune and Tumor Microenvironment. Front Immunol (2019) 10. doi: 10.3389/fimmu.2019.01078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kobayashi D, Endo M, Ochi H, Hojo H, Miyasaka M, Hayasaka H. Regulation of CCR7-Dependent Cell Migration Through CCR7 Homodimer Formation. Sci Rep (2017) 7:1–14. doi: 10.1038/s41598-017-09113-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Morrison VL, James MJ, Grzes K, Cook P, Glass DG, Savinko T. Loss of Beta2-Integrin-Mediated Cytoskeletal Linkage Reprogrammes Dendritic Cells to a Mature Migratory Phenotype. Nat Commun (2014) 5:1–26. doi: 10.1038/ncomms6359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Roberts EW, Broz ML, Binnewies M, Headley MB, Nelson AE, Wolf DM, et al. Critical Role for CD103 +/CD141 + Dendritic Cells Bearing CCR7 for Tumor Antigen Trafficking and Priming of T Cell Immunity in Melanoma. Cancer Cell (2016) 30:324–36. doi: 10.1016/j.ccell.2016.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jang MH, Sougawa N, Tanaka T, Hirata T, Hiroi T, Tohya K, et al. CCR7 Is Critically Important for Migration of Dendritic Cells in Intestinal Lamina Propria to Mesenteric Lymph Nodes. J Immunol (2006) 176:803–10. doi: 10.4049/jimmunol.176.2.803 [DOI] [PubMed] [Google Scholar]
- 45. Russo E, Teijeira A, Vaahtomeri K, Willrodt A-H, Bloch JS, Nitschké M, et al. Intralymphatic CCL21 Promotes Tissue Egress of Dendritic Cells Through Afferent Lymphatic Vessels. Cell Rep (2016) 14:1723–34. doi: 10.1016/j.celrep.2016.01.048 [DOI] [PubMed] [Google Scholar]
- 46. Vaahtomeri K, Brown M, Hauschild R, de Vries I, Leithner AF, Mehling M, et al. Locally Triggered Release of the Chemokine CCL21 Promotes Dendritic Cell Transmigration Across Lymphatic Endothelia. Cell Rep (2017) 19:902–9. doi: 10.1016/j.celrep.2017.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Acton SE, Astarita JL, Malhotra D, Lukacs-Kornek V, Franz B, Hess PR, et al. Podoplanin-Rich Stromal Networks Induce Dendritic Cell Motility via Activation of the C-Type Lectin Receptor CLEC-2. Immunity (2012) 37:276–89. doi: 10.1016/j.immuni.2012.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Braun A, Worbs T, Moschovakis GL, Halle S, Hoffmann K, Bölter J, et al. Afferent Lymph–Derived T Cells and DCs Use Different Chemokine Receptor CCR7–dependent Routes for Entry Into the Lymph Node and Intranodal Migration. Nat Immunol (2011) 12:879–87. doi: 10.1038/ni.2085 [DOI] [PubMed] [Google Scholar]
- 49. Link A, Vogt TK, Favre S, Britschgi MR, Acha-Orbea H, Hinz B, et al. Fibroblastic Reticular Cells in Lymph Nodes Regulate the Homeostasis of Naive T Cells. Nat Immunol (2007) 8:1255–65. doi: 10.1038/ni1513 [DOI] [PubMed] [Google Scholar]
- 50. Peduto L, Dulauroy S, Lochner M, Späth GF, Morales MA, Cumano A, et al. Inflammation Recapitulates the Ontogeny of Lymphoid Stromal Cells. J Immunol (2009) 182:5789–99. doi: 10.4049/jimmunol.0803974 [DOI] [PubMed] [Google Scholar]
- 51. Núñez NG, Tosello Boari J, Ramos RN, Richer W, Cagnard N, Anderfuhren CD, et al. Tumor Invasion in Draining Lymph Nodes Is Associated With Treg Accumulation in Breast Cancer Patients. Nat Commun (2020) 11:1–15. doi: 10.1038/s41467-020-17046-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shinde P, Fernandes S, Melinkeri S, Kale V, Limaye L. Compromised Functionality of Monocyte-Derived Dendritic Cells in Multiple Myeloma Patients may Limit Their Use in Cancer Immunotherapy. Sci Rep (2018) 8:1–11. doi: 10.1038/s41598-018-23943-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. O’Melia MJ, Rohner NA, Manspeaker MP, Francis DM, Kissick HT, Thomas SN. Quality of CD8+ T Cell Immunity Evoked in Lymph Nodes Is Compartmentalized by Route of Antigen Transport and Functional in Tumor Context. Sci Adv (2020) 6:1–16. doi: 10.1126/sciadv.abd7134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Caronni N, Simoncello F, Stafetta F, Guarnaccia C, Ruiz-Moreno JS, Opitz B, et al. Downregulation of Membrane Trafficking Proteins and Lactate Conditioning Determine Loss of Dendritic Cell Function in Lung Cancer. Cancer Res (2018) 78:1685–99. doi: 10.1158/0008-5472.CAN-17-1307 [DOI] [PubMed] [Google Scholar]
- 55. Wang X, Ji J, Zhang H, Fan Z, Zhang L, Shi L, et al. Stimulation of Dendritic Cells by DAMPs in ALA-PDT Treated SCC Tumor Cells. Oncotarget (2015) 6:44688–702. doi: 10.18632/oncotarget.5975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bol KF, Schreibelt G, Rabold K, Wculek SK, Schwarze JK, Dzionek A, et al. The Clinical Application of Cancer Immunotherapy Based on Naturally Circulating Dendritic Cells. J ImmunoTher Cancer (2019) 7:1–13. doi: 10.1186/s40425-019-0580-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Spranger S, Luke JJ, Bao R, Zha Y, Hernandez KM, Li Y, et al. Density of Immunogenic Antigens Does Not Explain the Presence or Absence of the T-Cell-Inflamed Tumor Microenvironment in Melanoma. Proc Natl Acad Sci USA (2016) 113:7759–68. doi: 10.1073/pnas.1609376113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Spranger S, Bao R, Gajewski TF. Melanoma-Intrinsic β-Catenin Signalling Prevents Anti-Tumour Immunity. Nature (2015) 523:231–5. doi: 10.1038/nature14404 [DOI] [PubMed] [Google Scholar]
- 59. Kim N, Kim HK, Lee K, Hong Y, Cho JH, Choi JW, et al. Single-Cell RNA Sequencing Demonstrates the Molecular and Cellular Reprogramming of Metastatic Lung Adenocarcinoma. Nat Commun (2020) 11:1–15. doi: 10.1038/s41467-020-16164-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Maier B, Leader AM, Chen ST, Tung N, Chang C, LeBerichel J, et al. A Conserved Dendritic-Cell Regulatory Program Limits Antitumour Immunity. Nature (2020) 580:257–62. doi: 10.1038/s41586-020-2134-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Qian J, Olbrecht S, Boeckx B, Vos H, Laoui D, Etlioglu E, et al. A Pan-Cancer Blueprint of the Heterogeneous Tumor Microenvironment Revealed by Single-Cell Profiling. Cell Res (2020) 30:745–62. doi: 10.1038/s41422-020-0355-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zilionis R, Engblom C, Pfirschke C, Savova V, Zemmour D, Saatcioglu HD, et al. Single-Cell Transcriptomics of Human and Mouse Lung Cancers Reveals Conserved Myeloid Populations Across Individuals and Species. Immunity (2019) 50:1317–34. doi: 10.1016/j.immuni.2019.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cillo AR, Kürten CHL, Tabib T, Qi Z, Onkar S, Wang T, et al. Immune Landscape of Viral- and Carcinogen-Driven Head and Neck Cancer. Immunity (2020) 52:183–99. doi: 10.1016/j.immuni.2019.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhang Q, He Y, Luo N, Patel SJ, Han Y, Gao R, et al. Landscape and Dynamics of Single Immune Cells in Hepatocellular Carcinoma. Cell (2019) 179:829–45. doi: 10.1016/j.cell.2019.10.003 [DOI] [PubMed] [Google Scholar]
- 65. Brown CC, Gudjonson H, Pritykin Y, Deep D, Lavallée V-P, Mendoza A, et al. Transcriptional Basis of Mouse and Human Dendritic Cell Heterogeneity. Cell (2019) 179:846–63. doi: 10.1016/j.cell.2019.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nirschl CJ, Suárez-Fariñas M, Izar B, Prakadan S, Dannenfelser R, Tirosh I, et al. Ifnγ-Dependent Tissue-Immune Homeostasis Is Co-Opted in the Tumor Microenvironment. Cell (2017) 170:127–41. doi: 10.1016/j.cell.2017.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ji AL, Rubin AJ, Thrane K, Jiang S, Reynolds DL, Meyers RM, et al. Multimodal Analysis of Composition and Spatial Architecture in Human Squamous Cell Carcinoma. Cell (2020) 182:497–514. doi: 10.1016/j.cell.2020.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhang L, Li Z, Skrzypczynska KM, Fang Q, Zhang W, O’Brien SA, et al. Single-Cell Analyses Inform Mechanisms of Myeloid-Targeted Therapies in Colon Cancer. Cell (2020) 181. doi: 10.1016/j.cell.2020.03.048 [DOI] [PubMed] [Google Scholar]
- 69. Cheng S, Li Z, Gao R, Xing B, Gao Y, Yang Y, et al. A Pan-Cancer Single-Cell Transcriptional Atlas of Tumor Infiltrating Myeloid Cells. Cell (2021) 184:792–809. doi: 10.1016/j.cell.2021.01.010 [DOI] [PubMed] [Google Scholar]
- 70. Gerhard GM, Bill R, Messemaker M, Klein AM, Pittet MJ. Tumor-Infiltrating Dendritic Cell States Are Conserved Across Solid Human Cancers. J Exp Med (2021) 218:1–13. doi: 10.1084/jem.20200264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Dutertre C-A, Becht E, Irac SE, Khalilnezhad A, Narang V, Khalilnezhad S, et al. Single-Cell Analysis of Human Mononuclear Phagocytes Reveals Subset-Defining Markers and Identifies Circulating Inflammatory Dendritic Cells. Immunity (2019) 51:573–89.e8. doi: 10.1016/j.immuni.2019.08.008 [DOI] [PubMed] [Google Scholar]
- 72. Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, et al. Monitoring of Blood Vessels and Tissues by a Population of Monocytes With Patrolling Behavior. Science (2007) 317:666–70. doi: 10.1126/science.1142883 [DOI] [PubMed] [Google Scholar]
- 73. Boldison J, da Rosa LC, Davies J, Wen L, Wong FS. Dendritic Cells License Regulatory B Cells to Produce IL-10 and Mediate Suppression of Antigen-Specific CD8 T Cells. Cell Mol Immunol (2020) 17:843–55. doi: 10.1038/s41423-019-0324-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhang Q, Fujino M, Iwasaki S, Hirano H, Cai S, Kitajima Y, et al. Generation and Characterization of Regulatory Dendritic Cells Derived From Murine Induced Pluripotent Stem Cells. Sci Rep (2015) 4:3979. doi: 10.1038/srep03979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Audiger C, Rahman MJ, Yun TJ, Tarbell K, Lesage S. The Importance of Dendritic Cells in Maintaining Immune Tolerance. J Immunol (2017) 198:2223–31. doi: 10.4049/jimmunol.1601629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. del Prete A, Sozio F, Barbazza I, Salvi V, Tiberio L, Laffranchi M, et al. Functional Role of Dendritic Cell Subsets in Cancer Progression and Clinical Implications. Int J Mol Sci (2020) 21:1–24. doi: 10.3390/ijms21113930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Aibar S, González-Blas CB, Moerman T, Huynh-Thu VA, Imrichova H, Hulselmans G, et al. SCENIC: Single-Cell Regulatory Network Inference and Clustering. Nat Methods (2017) 14:7083–6. doi: 10.1038/nmeth.4463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Carenza C, Calcaterra F, Oriolo F, di Vito C, Ubezio M, della Porta MG, et al. Costimulatory Molecules and Immune Checkpoints Are Differentially Expressed on Different Subsets of Dendritic Cells. Front Immunol (2019) 10. doi: 10.3389/fimmu.2019.01325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hernandez A, Burger M, Blomberg BB, Ross WA, Gaynor JJ, Lindner I, et al. Inhibition of NF-κb During Human Dendritic Cell Differentiation Generates Anergy and Regulatory T-Cell Activity for One But Not Two Human Leukocyte Antigen DR Mismatches. Hum Immunol (2007) 68:715–29. doi: 10.1016/j.humimm.2007.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Medina BD, Liu M, Vitiello GA, Seifert AM, Zeng S, Bowler T, et al. Oncogenic Kinase Inhibition Limits Batf3-Dependent Dendritic Cell Development and Antitumor Immunity. J Exp Med (2019) 216:1359–76. doi: 10.1084/jem.20180660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Scholz F, Grau M, Menzel L, Graband A, Zapukhlyak M, Leutz A, et al. The Transcription Factor C/Ebpβ Orchestrates Dendritic Cell Maturation and Functionality Under Homeostatic and Malignant Conditions. Proc Natl Acad Sci (2020) 117:26328–39. doi: 10.1073/pnas.2008883117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Xiao X, Yang G, Bai P, Gui S, Nyuyen TMB, Mercado-Uribe I, et al. Inhibition of Nuclear Factor-Kappa B Enhances the Tumor Growth of Ovarian Cancer Cell Line Derived From a Low-Grade Papillary Serous Carcinoma in P53-Independent Pathway. BMC Cancer (2016) 16:1–13. doi: 10.1186/s12885-016-2617-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Melaiu O, Chierici M, Lucarini V, Jurman G, Conti LA, de Vito R, et al. Cellular and Gene Signatures of Tumor-Infiltrating Dendritic Cells and Natural-Killer Cells Predict Prognosis of Neuroblastoma. Nat Commun (2020) 11:1–15. doi: 10.1038/s41467-020-19781-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Brencicova E, Jagger AL, Evans HG, Georgouli M, Laios A, Attard Montalto S, et al. Interleukin-10 and Prostaglandin E2 Have Complementary But Distinct Suppressive Effects on Toll-Like Receptor-Mediated Dendritic Cell Activation in Ovarian Carcinoma. PloS One (2017) 12:1–24. doi: 10.1371/journal.pone.0175712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Imai K, Minamiya Y, Koyota S, Ito M, Saito H, Sato Y, et al. Inhibition of Dendritic Cell Migration by Transforming Growth Factor-β1 Increases Tumor-Draining Lymph Node Metastasis. J Exp Clin Cancer Res (2012) 31:1–9. doi: 10.1186/1756-9966-31-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Llopiz D, Ruiz M, Infante S, Villanueva L, Silva L, Hervas-Stubbs S, et al. IL-10 Expression Defines an Immunosuppressive Dendritic Cell Population Induced by Antitumor Therapeutic Vaccination. Oncotarget (2017) 8:2659–71. doi: 10.18632/oncotarget.13736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Long J, Hu Z, Xue H, Wang Y, Chen J, Tang F, et al. Vascular Endothelial Growth Factor (VEGF) Impairs the Motility and Immune Function of Human Mature Dendritic Cells Through the VEGF Receptor 2-RhoA-Cofilin1 Pathway. Cancer Sci (2019) 110:2357–67. doi: 10.1111/cas.14091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ohno Y, Kitamura H, Takahashi N, Ohtake J, Kaneumi S, Sumida K, et al. IL-6 Down-Regulates HLA Class II Expression and IL-12 Production of Human Dendritic Cells to Impair Activation of Antigen-Specific CD4+ T Cells. Cancer Immunol Immunother (2016) 65:193–204. doi: 10.1007/s00262-015-1791-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Tauriello DVF, Palomo-Ponce S, Stork D, Berenguer-Llergo A, Badia-Ramentol J, Iglesias M, et al. Tgfβ Drives Immune Evasion in Genetically Reconstituted Colon Cancer Metastasis. Nature (2018) 554:538–43. doi: 10.1038/nature25492 [DOI] [PubMed] [Google Scholar]
- 90. Trabanelli S, Lecciso M, Salvestrini V, Cavo M, Očadlíková D, Lemoli RM, et al. PGE 2 -Induced IDO1 Inhibits the Capacity of Fully Mature DCs to Elicit an In Vitro Antileukemic Immune Response. J Immunol Res (2015) 2015:1–10. doi: 10.1155/2015/253191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Xu X, Liu X, Long J, Hu Z, Zheng Q, Zhang C, et al. Interleukin-10 Reorganizes the Cytoskeleton of Mature Dendritic Cells Leading to Their Impaired Biophysical Properties and Motilities. PloS One (2017) 12:1–15. doi: 10.1371/journal.pone.0172523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, et al. Heterozygous Embryonic Lethality Induced by Targeted Inactivation of the VEGF Gene. Nature (1996) 380:439–42. doi: 10.1038/380439a0 [DOI] [PubMed] [Google Scholar]
- 93. Wakabayashi H, Hamaguchi T, Nagao N, Kato S, Iino T, Nakamura T, et al. Interleukin-6 Receptor Inhibitor Suppresses Bone Metastases in a Breast Cancer Cell Line. Breast Cancer (2018) 25:566–74. doi: 10.1007/s12282-018-0853-9 [DOI] [PubMed] [Google Scholar]
- 94. Yang J, Yan J, Liu B. Targeting VEGF/VEGFR to Modulate Antitumor Immunity. Front Immunol (2018) 9:978. doi: 10.3389/fimmu.2018.00978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Park S-J, Nakagawa T, Kitamura H, Atsumi T, Kamon H, Sawa S, et al. IL-6 Regulates In Vivo Dendritic Cell Differentiation Through STAT3 Activation. J Immunol (2004) 173:3844–54. doi: 10.4049/jimmunol.173.6.3844 [DOI] [PubMed] [Google Scholar]
- 96. Gabrilovich DI, Ishida T, Nadaf S, Ohm JE, Carbone DP. Antibodies to Vascular Endothelial Growth Factor Enhance the Efficacy of Cancer Immunotherapy by Improving Endogenous Dendritic Cell Function. Clin Cancer research : An Off J Am Assoc Cancer Res (1999) 5:2963–70. [PubMed] [Google Scholar]
- 97. Mashima T, Wakatsuki T, Kawata N, Jang M-K, Nagamori A, Yoshida H, et al. Neutralization of the Induced VEGF-A Potentiates the Therapeutic Effect of an Anti-VEGFR2 Antibody on Gastric Cancer In Vivo . Sci Rep (2021) 11:1–12. doi: 10.1038/s41598-021-94584-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Bilusic M, Heery CR, Collins JM, Donahue RN, Palena C, Madan RA, et al. Phase I Trial of HuMax-IL8 (BMS-986253), an Anti-IL-8 Monoclonal Antibody, in Patients With Metastatic or Unresectable Solid Tumors. J ImmunoTher Cancer (2019) 7:1–8. doi: 10.1186/s40425-019-0706-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Feijoó E, Alfaro C, Mazzolini G, Serra P, Peñuelas I, Arina A, et al. Dendritic Cells Delivered Inside Human Carcinomas Are Sequestered by Interleukin-8. Int J Cancer (2005) 116:275–81. doi: 10.1002/ijc.21046 [DOI] [PubMed] [Google Scholar]
- 100. Cauwels A, van Lint S, Paul F, Garcin G, de Koker S, van Parys A, et al. Delivering Type I Interferon to Dendritic Cells Empowers Tumor Eradication and Immune Combination Treatments. Cancer Res (2018) 78:463–74. doi: 10.1158/0008-5472.CAN-17-1980 [DOI] [PubMed] [Google Scholar]
- 101. Le DT, Lutz E, Uram JN, Sugar EA, Onners B, Solt S, et al. Evaluation of Ipilimumab in Combination With Allogeneic Pancreatic Tumor Cells Transfected With a GM-CSF Gene in Previously Treated Pancreatic Cancer. J Immunother (2013) 36:382–9. doi: 10.1097/CJI.0b013e31829fb7a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Li Y, Bleakley M, Yee C. IL-21 Influences the Frequency, Phenotype, and Affinity of the Antigen-Specific CD8 T Cell Response. J Immunol (2005) 175:2261–9. doi: 10.4049/jimmunol.175.4.2261 [DOI] [PubMed] [Google Scholar]
- 103. Rhode PR, Egan JO, Xu W, Hong H, Webb GM, Chen X, et al. Comparison of the Superagonist Complex, ALT-803, to IL15 as Cancer Immunotherapeutics in Animal Models. Cancer Immunol Res (2016) 4:49–60. doi: 10.1158/2326-6066.CIR-15-0093-T [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Barry KC, Hsu J, Broz ML, Cueto FJ, Binnewies M, Combes AJ, et al. A Natural Killer–Dendritic Cell Axis Defines Checkpoint Therapy–Responsive Tumor Microenvironments. Nat Med (2018) 24:1178–91. doi: 10.1038/s41591-018-0085-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Bai W, Zhang W, Hu B. Vascular Endothelial Growth Factor Suppresses Dendritic Cells Function of Human Prostate Cancer. OncoTargets Ther (2018) 11:1267–74. doi: 10.2147/OTT.S161302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Cheng J, Deng Y, Yi H, Wang G, Fu B, Chen W, et al. Hepatic Carcinoma-Associated Fibroblasts Induce IDO-Producing Regulatory Dendritic Cells Through IL-6-Mediated STAT3 Activation. Oncogenesis (2016) 5:1–8. doi: 10.1038/oncsis.2016.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Costa A, Kieffer Y, Scholer-Dahirel A, Pelon F, Bourachot B, Cardon M, et al. Fibroblast Heterogeneity and Immunosuppressive Environment in Human Breast Cancer. Cancer Cell (2018) 33:463–79. doi: 10.1016/j.ccell.2018.01.011 [DOI] [PubMed] [Google Scholar]
- 108. Elyada E, Bolisetty M, Laise P, Flynn WF, Courtois ET, Burkhart RA, et al. Cross-Species Single-Cell Analysis of Pancreatic Ductal Adenocarcinoma Reveals Antigen-Presenting Cancer-Associated Fibroblasts. Cancer Discov (2019) 9:1102–23. doi: 10.1158/2159-8290.CD-19-0094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Friedman G, Levi-Galibov O, David E, Bornstein C, Giladi A, Dadiani M, et al. Cancer-Associated Fibroblast Compositions Change With Breast Cancer Progression Linking the Ratio of S100A4+ and PDPN+ CAFs to Clinical Outcome. Nat Cancer (2020) 1:692–708. doi: 10.1101/2020.01.12.903039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Rodda LB, Lu E, Bennett ML, Sokol CL, Wang X, Luther SA, et al. Single-Cell RNA Sequencing of Lymph Node Stromal Cells Reveals Niche-Associated Heterogeneity. Immunity (2018) 48:1014–28. doi: 10.1016/j.immuni.2018.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Song Y-J, Xu Y, Deng C, Zhu X, Fu J, Chen H, et al. Gene Expression Classifier Reveals Prognostic Osteosarcoma Microenvironment Molecular Subtypes. Front Immunol (2021) 12:62376. doi: 10.3389/fimmu.2021.623762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Acton SE, Farrugia AJ, Astarita JL, Mourão-Sá D, Jenkins RP, Nye E, et al. Dendritic Cells Control Fibroblastic Reticular Network Tension and Lymph Node Expansion. Nature (2014) 514:498–502. doi: 10.1038/nature13814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Prados A, Onder L, Cheng H-W, Mörbe U, Lütge M, Gil-Cruz C, et al. Fibroblastic Reticular Cell Lineage Convergence in Peyer’s Patches Governs Intestinal Immunity. Nat Immunol (2021) 22:510–9. doi: 10.1038/s41590-021-00894-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Tejchman A, Lamerant-Fayel N, Jacquinet J-C, Bielawska-Pohl A, Mleczko-Sanecka K, Grillon C, et al. Tumor Hypoxia Modulates Podoplanin/CCL21 Interactions in CCR7+ NK Cell Recruitment and CCR7+ Tumor Cell Mobilization. Oncotarget (2017) 8:31876–87. doi: 10.18632/oncotarget.16311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Hwang T-L, Lee L-Y, Wang C-C, Liang Y, Huang S-F, Wu C-M. CCL7 and CCL21 Overexpression in Gastric Cancer Is Associated With Lymph Node Metastasis and Poor Prognosis. World J Gastroenterol (2012) 18:1249–56. doi: 10.3748/wjg.v18.i11.1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Crola Da Silva C, Lamerant-Fayel N, Paprocka M, Mitterrand M, Gosset D, Dus D, et al. Selective Human Endothelial Cell Activation by Chemokines as a Guide to Cell Homing. Immunology (2009) 126:394–404. doi: 10.1111/j.1365-2567.2008.02906.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Lin Y, Sharma S, John M. CCL21 Cancer Immunotherapy. Cancers (2014) 6:1098–110. doi: 10.3390/cancers6021098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Fang L, Che Y, Zhang C, Huang J, Lei Y, Lu Z, et al. PLAU Directs Conversion of Fibroblasts to Inflammatory Cancer-Associated Fibroblasts, Promoting Esophageal Squamous Cell Carcinoma Progression via uPAR/Akt/NF-κb/IL8 Pathway. Cell Death Discov (2021) 7:1–14. doi: 10.1038/s41420-021-00410-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Öhlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, Ponz-Sarvise M, et al. Distinct Populations of Inflammatory Fibroblasts and Myofibroblasts in Pancreatic Cancer. J Exp Med (2017) 214:579–96. doi: 10.1084/jem.20162024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Goc J, Germain C, Vo-Bourgais TKD, Lupo A, Klein C, Knockaert S, et al. Dendritic Cells in Tumor-Associated Tertiary Lymphoid Structures Signal a Th1 Cytotoxic Immune Contexture and License the Positive Prognostic Value of Infiltrating CD8+ T Cells. Cancer Res (2014) 74:705–15. doi: 10.1158/0008-5472.CAN-13-1342 [DOI] [PubMed] [Google Scholar]
- 121. Li Q, Liu X, Wang D, Wang Y, Lu H, Wen S, et al. Prognostic Value of Tertiary Lymphoid Structure and Tumour Infiltrating Lymphocytes in Oral Squamous Cell Carcinoma. Int J Oral Sci (2020) 12:1–8. doi: 10.1038/s41368-020-00092-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Salem D, Chelvanambi M, Storkus WJ, Fecek RJ. Cutaneous Melanoma: Mutational Status and Potential Links to Tertiary Lymphoid Structure Formation. Front Immunol (2021) 12:629519. doi: 10.3389/fimmu.2021.629519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Nayar S, Campos J, Smith CG, Iannizzotto V, Gardner DH, Mourcin F, et al. Immunofibroblasts Are Pivotal Drivers of Tertiary Lymphoid Structure Formation and Local Pathology. Proc Natl Acad Sci (2019) 116:13490–7. doi: 10.1073/pnas.1905301116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Rodriguez AB, Peske JD, Woods AN, Leick KM, Mauldin IS, Meneveau MO, et al. Immune Mechanisms Orchestrate Tertiary Lymphoid Structures in Tumors via Cancer-Associated Fibroblasts. Cell Rep (2021) 36:109422. doi: 10.1016/j.celrep.2021.109422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Dieu-Nosjean M-C, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, et al. Long-Term Survival for Patients With Non–Small-Cell Lung Cancer With Intratumoral Lymphoid Structures. J Clin Oncol (2008) 26:4410–7. doi: 10.1200/JCO.2007.15.0284 [DOI] [PubMed] [Google Scholar]
- 126. Figenschau SL, Fismen S, Fenton KA, Fenton C, Mortensen ES. Tertiary Lymphoid Structures Are Associated With Higher Tumor Grade in Primary Operable Breast Cancer Patients. BMC Cancer (2015) 15:1–11. doi: 10.1186/s12885-015-1116-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. He W, Zhang D, Liu H, Chen T, Xie J, Peng L, et al. The High Level of Tertiary Lymphoid Structure Is Correlated With Superior Survival in Patients With Advanced Gastric Cancer. Front Oncol (2020) 10. doi: 10.3389/fonc.2020.00980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Jacquelot N, Tellier J, , Nutt SL, Belz GT. Tertiary Lymphoid Structures and B Lymphocytes in Cancer Prognosis and Response to Immunotherapies. OncoImmunology (2021) 10(1):1900508. doi: 10.1080/2162402X.2021.1900508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Lin L, Hu X, Zhang H, Hu H. Tertiary Lymphoid Organs in Cancer Immunology: Mechanisms and the New Strategy for Immunotherapy. Front Immunol (2019) 10:1. doi: 10.3389/fimmu.2019.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Barone F, Nayar S, Campos J, Cloake T, Withers DR, Toellner K-M, et al. IL-22 Regulates Lymphoid Chemokine Production and Assembly of Tertiary Lymphoid Organs. Proc Natl Acad Sci (2015) 112:11024–9. doi: 10.1073/pnas.1503315112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Fleige H, Ravens S, Moschovakis GL, Bölter J, Willenzon S, Sutter G, et al. IL-17–Induced CXCL12 Recruits B Cells and Induces Follicle Formation in BALT in the Absence of Differentiated FDCs. J Exp Med (2014) 211:643–51. doi: 10.1084/jem.20131737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Dieu-Nosjean M-C, Goc J, Giraldo NA, Sautès-Fridman C, Fridman WH. Tertiary Lymphoid Structures in Cancer and Beyond. Trends Immunol (2014) 35:571–780. doi: 10.1016/j.it.2014.09.006 [DOI] [PubMed] [Google Scholar]
- 133. Engelhard VH, Rodriguez AB, Mauldin IS, Woods AN, Peske JD, Slingluff CL. Immune Cell Infiltration and Tertiary Lymphoid Structures as Determinants of Antitumor Immunity. J Immunol (2018) 200:432–42. doi: 10.4049/jimmunol.1701269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Sofopoulos M, Fortis SP, Vaxevanis CK, Sotiriadou NN, Arnogiannaki N, Ardavanis A, et al. The Prognostic Significance of Peritumoral Tertiary Lymphoid Structures in Breast Cancer. Cancer Immunol Immunother (2019) 68:1733–45. doi: 10.1007/s00262-019-02407-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. de Mingo Pulido Á., Gardner A, Hiebler S, Soliman H, Rugo HS, Krummel MF, et al. TIM-3 Regulates CD103+ Dendritic Cell Function and Response to Chemotherapy in Breast Cancer. Cancer Cell (2018) 33:60–74. doi: 10.1016/j.ccell.2017.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Dixon KO, Tabaka M, Schramm MA, Xiao S, Tang R, Dionne D, et al. TIM-3 Restrains Anti-Tumour Immunity by Regulating Inflammasome Activation. Nature (2021) 595:101–6. doi: 10.1038/s41586-021-03626-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Karyampudi L, Lamichhane P, Krempski J, Kalli KR, Behrens MD, Vargas DM, et al. PD-1 Blunts the Function of Ovarian Tumor–Infiltrating Dendritic Cells by Inactivating NF-κb. Cancer Res (2016) 76:239–50. doi: 10.1158/0008-5472.CAN-15-0748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Oh SA, Wu D-C, Cheung J, Navarro A, Xiong H, Cubas R, et al. PD-L1 Expression by Dendritic Cells Is a Key Regulator of T-Cell Immunity in Cancer. Nat Cancer (2020) 1:681–91. doi: 10.1038/s43018-020-0075-x [DOI] [PubMed] [Google Scholar]
- 139. Ouaaz F, Arron J, Zheng Y, Choi Y, Beg AA. Dendritic Cell Development and Survival Require Distinct NF-κb Subunits. Immunity (2002) 16:257–70. doi: 10.1016/s1074-7613(02)00272-8 [DOI] [PubMed] [Google Scholar]
- 140.8 Peng Q, Qiu X, Zhang Z, Zhang S, Zhang Y, Liang Y, et al. PD-L1 on Dendritic Cells Attenuates T Cell Activation and Regulates Response to Immune Checkpoint Blockade. Nat Commun (2020) 11:1–. doi: 10.1038/s41467-020-18570-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Wang XB, Fan ZZ, Anton D, Vollenhoven A, Ni ZH, Chen XF, et al. CTLA4 Is Expressed on Mature Dendritic Cells Derived From Human Monocytes and Influences Their Maturation and Antigen Presentation. BMC Immunol (2011) 12:1–8. doi: 10.1186/1471-2172-12-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Ruffell B, Chang-Strachan D, Chan V, Rosenbusch A, Ho CMT, Pryer N, et al. Macrophage IL-10 Blocks CD8+ T Cell-Dependent Responses to Chemotherapy by Suppressing IL-12 Expression in Intratumoral Dendritic Cells. Cancer Cell (2014) 26:623–37. doi: 10.1016/j.ccell.2014.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Sánchez-Paulete AR, Cueto FJ, Martínez-López M, Labiano S, Morales-Kastresana A, Rodríguez-Ruiz ME, et al. Cancer Immunotherapy With Immunomodulatory Anti-CD137 and Anti–PD-1 Monoclonal Antibodies Requires BATF3-Dependent Dendritic Cells. Cancer Discov (2016) 6:71–9. doi: 10.1158/1538-7445.AM2016-4908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Bonavita E, Bromley CP, Jonsson G, Pelly VS, Sahoo S, Walwyn-Brown K, et al. Antagonistic Inflammatory Phenotypes Dictate Tumor Fate and Response to Immune Checkpoint Blockade. Immunity (2020) 53:1215–29. doi: 10.1016/j.immuni.2020.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, et al. Adenoma-Linked Barrier Defects and Microbial Products Drive IL-23/IL-17-Mediated Tumour Growth. Nature (2012) 491:254–8. doi: 10.1038/nature11465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Hu Z, Yang Y, Zhao Y, Huang Y. The Prognostic Value of Cyclooxygenase-2 Expression in Patients With Esophageal Cancer: Evidence From a Meta-Analysis. OncoTargets Ther (2017) 10:2893–901. doi: 10.2147/OTT.S134599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Shalapour S, Karin M. Pas De Deux: Control of Anti-Tumor Immunity by Cancer-Associated Inflammation. Immunity (2019) 51:15–26. doi: 10.1016/j.immuni.2019.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Grasso CS, Tsoi J, Onyshchenko M, Abril-Rodriguez G, Ross-Macdonald P, Wind-Rotolo M, et al. Conserved Interferon-γ Signaling Drives Clinical Response to Immune Checkpoint Blockade Therapy in Melanoma. Cancer Cell (2020) 38:500–15. doi: 10.1016/j.ccell.2020.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Pelly VS, Moeini A, Roelofsen LM, Bonavita E, Bell CR, Hutton C, et al. Anti-Inflammatory Drugs Remodel the Tumor Immune Environment to Enhance Immune Checkpoint Blockade Efficacy. Cancer Discov (2021) 11(10):2602–19. doi: 10.1158/2159-8290.CD-20-1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Zelenay S, Reis e Sousa C. Reducing Prostaglandin E 2 Production to Raise Cancer Immunogenicity. OncoImmunology (2016) 5:1–3. doi: 10.1080/2162402X.2015.1123370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Zhang Y, Tighe S, Zhu Y-T. COX-2 Signaling in the Tumor Microenvironment. Adv Exp Med Biol (2020) 1277:87–104. doi: 10.1007/978-3-030-50224-9_6 [DOI] [PubMed] [Google Scholar]
- 152. Böttcher JP, Bonavita E, Chakravarty P, Blees H, Cabeza-Cabrerizo M, Sammicheli S, et al. NK Cells Stimulate Recruitment of Cdc1 Into the Tumor Microenvironment Promoting Cancer Immune Control. Cell (2018) 172:1022–37. doi: 10.1016/j.cell.2018.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Wang D, DuBois RN. The Role of COX-2 in Intestinal Inflammation and Colorectal Cancer. Oncogene (2010) 29:781–8. doi: 10.1038/onc.2009.421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Zhu Y, Shi C, Zeng L, Liu G, Jiang W, Zhang X, et al. High COX-2 Expression in Cancer-Associated Fibiroblasts Contributes to Poor Survival and Promotes Migration and Invasiveness in Nasopharyngeal Carcinoma. Mol Carcinogenesis (2020) 59:265–80. doi: 10.1002/mc.23150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Obermajer N, Muthuswamy R, Odunsi K, Edwards RP, Kalinski P. PGE 2 -Induced CXCL12 Production and CXCR4 Expression Controls the Accumulation of Human MDSCs in Ovarian Cancer Environment. Cancer Res (2011) 71:7463–70. doi: 10.1158/0008-5472.CAN-11-2449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Baratelli FE, Heuzé-Vourc’h N, Krysan K, Dohadwala M, Riedl K, Sharma S, et al. Prostaglandin E 2 -Dependent Enhancement of Tissue Inhibitors of Metalloproteinases-1 Production Limits Dendritic Cell Migration Through Extracellular Matrix. J Immunol (2004) 173:5458–66. doi: 10.4049/jimmunol.173.9.5458 [DOI] [PubMed] [Google Scholar]
- 157. Legler DF, Krause P, Scandella E, Singer E, Groettrup M. Prostaglandin E2 Is Generally Required for Human Dendritic Cell Migration and Exerts Its Effect via EP2 and EP4 Receptors. J Immunol (2006) 176:966–73. doi: 10.4049/jimmunol.176.2.966 [DOI] [PubMed] [Google Scholar]
- 158. Yen J-H, Khayrullina T, Ganea D. PGE2-Induced Metalloproteinase-9 is Essential for Dendritic Cell Migration. Blood (2008) 111:260–70. doi: 10.1182/blood-2007-05-090613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Canton J, Blees H, Henry CM, Buck MD, Schulz O, Rogers NC, et al. The Receptor DNGR-1 Signals for Phagosomal Rupture to Promote Cross-Presentation of Dead-Cell-Associated Antigens. Nat Immunol (2021) 22:140–53. doi: 10.1038/s41590-020-00824-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Giampazolias E, Schulz O, Lim KHJ, Rogers NC, Chakravarty P, Srinivasan N, et al. Secreted Gelsolin Inhibits DNGR-1-Dependent Cross-Presentation and Cancer Immunity. Cell (2021) 184:4016–31. doi: 10.1016/j.cell.2021.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Sancho D, Joffre OP, Keller AM, Rogers NC, Martínez D, Hernanz-Falcón P, et al. Identification of a Dendritic Cell Receptor That Couples Sensing of Necrosis to Immunity. Nature (2009) 458:899–903. doi: 10.1038/nature07750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Hsu FJ, Benike C, Fagnoni F, Liles TM, Czerwinski D, Taidi B, et al. Vaccination of Patients With B–cell Lymphoma Using Autologous Antigen–Pulsed Dendritic Cells. Nat Med (1996) 2:52–8. doi: 10.1038/nm0196-52 [DOI] [PubMed] [Google Scholar]
- 163. León B, López-Bravo M, Ardavín C. Monocyte-Derived Dendritic Cells Formed at the Infection Site Control the Induction of Protective T Helper 1 Responses Against Leishmania. Immunity (2007) 26:519–31. 10.1016/j.immuni.2007.01.017 [DOI] [PubMed] [Google Scholar]
- 164. Palucka AK, Ueno H, Connolly J, Kerneis-Norvell F, Blanck J-P, Johnston DA, et al. Dendritic Cells Loaded With Killed Allogeneic Melanoma Cells can Induce Objective Clinical Responses and MART-1 Specific CD8+ T-Cell Immunity. J Immunother (2006) 29:545–57. doi: 10.1097/01.cji.0000211309.90621.8b [DOI] [PubMed] [Google Scholar]
- 165. Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, et al. Vaccination With Irradiated Tumor Cells Engineered to Secrete Murine Granulocyte-Macrophage Colony-Stimulating Factor Stimulates Potent, Specific, and Long-Lasting Anti-Tumor Immunity. Proc Natl Acad Sci (1993) 90:3539–43. doi: 10.1073/pnas.90.8.3539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Mach N, Dranoff G. Cytokine-Secreting Tumor Cell Vaccines. Curr Opin Immunol (2000) 12:571–5. doi: 10.1016/S0952-7915(00)00144-8 [DOI] [PubMed] [Google Scholar]
- 167. Bhardwaj N, Friedlander PA, Pavlick AC, Ernstoff MS, Gastman BR, Hanks BA, et al. Flt3 Ligand Augments Immune Responses to Anti-DEC-205-NY-ESO-1 Vaccine Through Expansion of Dendritic Cell Subsets. Nat Cancer (2020) 1:1204–17. doi: 10.1038/s43018-020-00143-y [DOI] [PubMed] [Google Scholar]
- 168. Hammerich L, Marron TU, Upadhyay R, Svensson-Arvelund J, Dhainaut M, Hussein S, et al. Systemic Clinical Tumor Regressions and Potentiation of PD1 Blockade With in Situ Vaccination. Nat Med (2019) 25:814–24. doi: 10.1038/s41591-019-0410-x [DOI] [PubMed] [Google Scholar]
- 169. Lai J, Mardiana S, House IG, Sek K, Henderson MA, Giuffrida L, et al. Adoptive Cellular Therapy With T Cells Expressing the Dendritic Cell Growth Factor Flt3L Drives Epitope Spreading and Antitumor Immunity. Nat Immunol (2020) 21:914–26. doi: 10.1038/s41590-020-0676-7 [DOI] [PubMed] [Google Scholar]
- 170. Salmon H, Idoyaga J, Rahman A, Leboeuf M, Remark R, Jordan S, et al. Expansion and Activation of CD103 + Dendritic Cell Progenitors at the Tumor Site Enhances Tumor Responses to Therapeutic PD-L1 and BRAF Inhibition. Immunity (2016) 44:924–38. doi: 10.1016/j.immuni.2016.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Hou J, Karin M, Sun B. Targeting Cancer-Promoting Inflammation — Have Anti-Inflammatory Therapies Come of Age? Nat Rev Clin Oncol (2021) 18:261–79. doi: 10.1038/s41571-020-00459-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Ritter B, Greten FR. Modulating Inflammation for Cancer Therapy. J Exp Med (2019) 216. doi: 10.1084/jem.20181739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Zappavigna S, Cossu AM, Grimaldi A, Bocchetti M, Ferraro GA, Nicoletti GF, et al. Anti-Inflammatory Drugs as Anticancer Agents. Int J Mol Sci (2020) 21:1–29. doi: 10.3390/ijms21072605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Chen X, Song E. Turning Foes to Friends: Targeting Cancer-Associated Fibroblasts. Nat Rev Drug Discov (2019) 18:99–115. doi: 10.1038/s41573-018-0004-1 [DOI] [PubMed] [Google Scholar]
- 175. Kobayashi H, Enomoto A, Woods SL, Burt AD, Takahashi M, Worthley DL. Cancer-Associated Fibroblasts in Gastrointestinal Cancer. Nat Rev Gastroenterol Hepatol (2019) 16:282–95. doi: 10.1038/s41575-019-0115-0 [DOI] [PubMed] [Google Scholar]
- 176. Liu T, Han C, Wang S, Fang P, Ma Z, Xu L, et al. Cancer-Associated Fibroblasts: An Emerging Target of Anti-Cancer Immunotherapy. J Hematol Oncol (2019). doi: 10.1186/s13045-019-0770-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Ziani L, Chouaib S, Thiery J. Alteration of the Antitumor Immune Response by Cancer-Associated Fibroblasts. Front Immunol (2018) 9:414. doi: 10.3389/fimmu.2018.00414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Wang J-B, Huang X, Li F-R. Impaired Dendritic Cell Functions in Lung Cancer: A Review of Recent Advances and Future Perspectives. Cancer Commun (2019) 39(1):43. doi: 10.1186/s40880-019-0387-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF, Sancho D. Dendritic Cells in Cancer Immunology and Immunotherapy. Nat Rev Immunol (2020) 20(1):7–24. doi: 10.1038/s41577-019-0210-z [DOI] [PubMed] [Google Scholar]
- 180. Martín-Fontecha A, Sebastiani S, Höpken UE, Uguccioni M, Lipp M, Lanzavecchia A, et al. Regulation of Dendritic Cell Migration to the Draining Lymph Node. J Exp Med (2003) 198:615–21. doi: 10.1084/jem.20030448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Perez CR, de Palma M. Engineering Dendritic Cell Vaccines to Improve Cancer Immunotherapy. Nat Commun (2019) 10:1–10. doi: 10.1038/s41467-019-13368-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Ramanjulu JM, Pesiridis GS, Yang J, Concha N, Singhaus R, Zhang S-Y, et al. Design of Amidobenzimidazole STING Receptor Agonists With Systemic Activity. Nature (2018) 564:439–43. doi: 10.1038/s41586-018-0705-y [DOI] [PubMed] [Google Scholar]
- 183. Ganesan A-P, Clarke J, Wood O, Garrido-Martin EM, Chee SJ, Mellows T, et al. Tissue-Resident Memory Features Are Linked to the Magnitude of Cytotoxic T Cell Responses in Human Lung Cancer. Nat Immunol (2017) 18:940–50. doi: 10.1038/ni.3775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Malik BT, Byrne KT, Vella JL, Zhang P, Shabaneh TB, Steinberg SM, et al. Resident Memory T Cells in the Skin Mediate Durable Immunity to Melanoma. Sci Immunol (2017) 2.:1–24 doi: 10.1126/sciimmunol.aam6346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Park SL, Buzzai A, Rautela J, Hor JL, Hochheiser K, Effern M, et al. Tissue-Resident Memory CD8+ T Cells Promote Melanoma–Immune Equilibrium in Skin. Nature (2019) 565:366–71. doi: 10.1038/s41586-018-0812-9 [DOI] [PubMed] [Google Scholar]
- 186. Savas P, Virassamy B, Ye C, Salim A, Mintoff CP, Caramia F, et al. Single-Cell Profiling of Breast Cancer T Cells Reveals a Tissue-Resident Memory Subset Associated With Improved Prognosis. Nat Med (2018) 24:986–93. doi: 10.1038/s41591-018-0078-7 [DOI] [PubMed] [Google Scholar]
- 187. Schenkel JM, Fraser KA, Beura LK, Pauken KE, Vezys V, Masopust D. Resident Memory CD8 T Cells Trigger Protective Innate and Adaptive Immune Responses. Science (2014) 346:98–101. doi: 10.1126/science.1254536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Edwards J, Wilmott JS, Madore J, Gide TN, Quek C, Tasker A, et al. CD103 + Tumor-Resident CD8 + T Cells Are Associated With Improved Survival in Immunotherapy-Naïve Melanoma Patients and Expand Significantly During Anti–PD-1 Treatment. Clin Cancer Res (2018) 24:3036–45. doi: 10.1158/1078-0432.CCR-17-2257 [DOI] [PubMed] [Google Scholar]
- 189. León-Letelier RA, Castro-Medina DI, Badillo-Godinez O, Tepale-Segura A, Huanosta-Murillo E, Aguilar-Flores C, et al. Induction of Progenitor Exhausted Tissue-Resident Memory CD8+ T Cells Upon Salmonella Typhi Porins Adjuvant Immunization Correlates With Melanoma Control and Anti-PD-1 Immunotherapy Cooperation. Front Immunol (2020) 11:583382. doi: 10.3389/fimmu.2020.583382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Sautès-Fridman C, Petitprez F, Calderaro J, Fridman WH. Tertiary Lymphoid Structures in the Era of Cancer Immunotherapy. Nat Rev Cancer (2019) 19:1–21. doi: 10.1038/s41568-019-0144-6 [DOI] [PubMed] [Google Scholar]