Abstract
Dexamethasone and tocilizumab have been associated with reduction in mortality, however, the beneficial effect is not for all patients and the impact on viral replication is not well defined. We hypostatized that C-reactive protein (CRP) could help in the identification of patients requiring anti-inflammatory therapy. Patients admitted for > 48 h in our hospital for a confirmed or suspected infection by SARS-CoV-2 from February 2020 to February 2021 were retrospectively evaluated. The primary outcome was mortality at 30 days. Demographics and the most relevant variables related with the outcome were included. CRP was stratified by percentiles. Univariate and multivariate analysis were performed. A total of 3218 patients were included with a median (IQR) age of 66 (74–78) years and 58.9% were males. The rate of intensive care unit admission was 24.4% and the 30-day mortality rate was 11.8%. Within the first 5 days from admission, 1018 (31.7%) patients received dexamethasone and 549 tocilizumab (17.1%). The crude analysis showed a mortality reduction in patients receiving dexamethasone when CRP was > 13.75 mg/dL and > 3.5 mg/dL for those receiving tocilizumab. Multivariate analysis identified the interaction of CRP > 13.75 mg/dL with dexamethasone (OR 0.57; CI 95% 0.37–0.89, P = 0014) and CRP > 3.5 mg/dL with tocilizumab (0.65; CI95%:0.44–0.95, P = 0.029) as independent predictors of mortality. Our results suggest that dexamethasone and tocilizumab are associated with a reduction in mortality when prescribed to patients with a certain inflammatory activity assessed by C-reactive protein.
Subject terms: Immunology, Microbiology, Biomarkers, Diseases, Health care, Medical research
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has been detected around the world with more than 4 million related deaths1. A recent analysis of 44.415 confirmed cases in China described that 81% were asymptomatic or have a mild disease, 14% have a severe disease and 5% a critical disease with an overall mortality of 2.3%2. However, among patients that require hospitalization, the mortality rate is around 20%3. The activation of the immune system includes the production of type-I interferon (IFN) mainly by plasmacytoid dendritic cells (pDC), however, coronaviruses, and particularly SARS-CoV-2, can suppress type-I INF response by different pathways4. Patients with mild to moderate COVID-19 are characterized by adequate type-I INF response while severe cases have low serum levels of type I INF, high viral load, and a dysregulated immune response with persistent hypercytokinemia and dysfunctional T cell response leading to acute distress respiratory syndrome (ARDS)5. In this scenario, anti-inflammatory therapies are considered a cornerstone of the management of COVID-19, but its impact on viral replication control particularly during the first days of infection is still a matter of debate.
The most widely evaluated anti-inflammatory drugs are corticosteroids6,7 and tocilizumab8. It is reasonable to hypothesize that CRP, that have demonstrated a potent capacity to predict the outcome of COVID-19 patients9, could be a good parameter to decide when to start an anti-inflammatory therapy. Indeed, some preliminary results showed that IL-6 or CRP serum levels are useful to identify patients that benefit the most from tocilizumab or dexamethasone treatment10–13. The correct selection of patients requiring anti-inflammatory therapy is important since not all patients had a beneficial effect7, these drugs have adverse effects, and could potentially favour viral replication.
The aim of the present study was to evaluate the outcome of patients receiving dexamethasone or tocilizumab within the first 5 days from hospital admission in a large cohort of COVID-19 patients treated during the first year of pandemic in a tertiary hospital in Barcelona, and to identify potential cut-off points of CRP that predict the response to both anti-inflammatory therapies.
Methods
Study design and patients
This observational cohort study was performed at Hospital Clinic in Barcelona (Spain), a 700-bed university centre that provides care for an urban population of 500,000 adults. All patients admitted for ≥ 48 h with confirmed acute SARS-Cov-2 infection by rRT- PCR performed on nasopharyngeal and throat swabs or with a clinical picture highly suggestive of COVID-19 between February 18th, 2020 and February 24th, 2021 were included. Deaths occurring within the first 48 h were included in the analysis. The Institutional Ethics Committee of Hospital Clinic of Barcelona approved the study and due to the nature of the retrospective data review, waived the need for informed consent from individual patients (HCB/2020/0273).
Data collection
Data were retrospectively collected for all patients included in the study from the electronic health records (EHR). An intelligent system was used to retrieve the high- quality data from EHRs (SILDv1.0 system, S34M@) as previously described14. Variables included were age, sex, co-morbidities (hypertension, chronic heart disease, diabetes mellitus, chronic liver disease, chronic kidney disease, chronic obstructive pulmonary disease, haematological neoplasia, and solid neoplasia), respiratory rate and ambient air arterial Oxygen saturation (SaO2) measured with a pulse oximeter at admission, creatinine, lymphocyte count, C-reactive protein (CRP), and lactate dehydrogenase (LDH) within the first 24 h from hospital admission, the need of intensive care (ICU) admission and invasive mechanical ventilation (IMV). Treatment with dexamethasone or tocilizumab within the first 5 days from admission was gathered. This timeframe was selected to mimic the prescription of both drugs in the RECOVERY trials (IQR from 1 to 5 days for both)7,15. The dose of dexamethasone was 8 mg/24 h intravenously for 10 days and for tocilizumab a single dose of 600 mg intravenously (400 mg for < 65 kg and 800 mg for > 90 kg). Remdesivir administration was included in the analysis as the only antiviral agent approved for the treatment of COVID-19. The primary outcome was mortality at 30 days.
Statistical analysis
Categorical variables were described using the absolute number and percentage and continuous variables were dichotomized according to their medians. Categorical variables were compared using a Chi-squared test or Fisher exact test when necessary. For multivariable analysis, variables with a P value ≤ 0.05 in the univariable analysis were subjected to further selection by using a backwards logistic regression method. Interactions between dexamethasone or tocilizumab treatment and CRP and other variables were explored. The calibration of the model was assessed by means of the Hosmer–Lemeshow goodness-of-fit test and the area under the receiver operating characteristic (ROC) curve was used to measured predictive ability of the model. Statistical significance was defined as a two-tailed P value < 0.05. For interactions included in the final multivariate model, the adjusted OR (95%CI) of the covariate of interest (i.e., dexamethasone or tocilizumab exposure) in each strata of the corresponding risk factor (i.e., CRP group) was calculated according to the formula described by Kleinbaum et al16. The analysis was performed by using SPSS version 26 (SPSS Inc., Chicago, IL).
Results
Study population
The population evaluated consisted of 3218 patients who were admitted to our hospital during the pandemic. The median (IQR) age was 66 (54–78) years and 58.9% were males. The most common co-morbidities were hypertension (46.3%), chronic heart disease (26.4%), chronic pulmonary disease (24.3%), diabetes mellitus (20.1%), solid neoplasia (15.6%), and chronic renal failure (12.3%). A total of 784 patients were admitted to the ICU (24.4%) and 330 required IMV (10.3%). Regarding therapeutic strategies, 549 (17.1%) patients received remdesivir, and within the first 5 days from admission 1018 (31.7%) received dexamethasone and 549 (17.1%) tocilizumab. The global 30-day mortality rate was 11.8%, and the characteristics associated with mortality are shown in Table 1.
Table 1.
Characteristics of patients according to the primary outcome (mortality at 30 days).
| Variable | Alive (N = 2835) | Dead (N = 381) | P value |
|---|---|---|---|
| Demographics and co-morbidity (%) | |||
| Age > 66 years | 1236 (43.8) | 331 (86.9) | < 0.001 |
| Male sex | 1642 (58.2) | 242 (63.5) | 0.049 |
| Chronic heart disease | 638 (22.5) | 210 (55.1) | < 0.001 |
| Diabetes mellitus | 529 (18.7) | 117 (30.7) | < 0.001 |
| Haematological disease | 165 (5.8) | 41 (10.8) | < 0.001 |
| Chronic kidney disease | 266 (9.4) | 130 (34.1) | < 0.001 |
| Chronic liver disease | 203 (7.2) | 32 (8.4) | 0.383 |
| Hypertension | 1228 (43.3) | 262 (68.8) | < 0.001 |
| Solid tumour | 395 (13.9) | 108 (28.3) | < 0.001 |
| Solid organ transplantation | 62 (2.2) | 13 (3.4) | 0.137 |
| HIV infection | 35 (1.2) | 6 (1.6) | 0.623 |
| Chronic lung disease | 651 (23) | 130 (34.1) | < 0.001 |
| Clinical characteristics and biomarkers at admission (%) | |||
| Temperature > 37ºC | 1356 (48.6) | 130 (28.2) | < 0.001 |
| Oxygen saturation > 94% | 1346 (48.4) | 98 (28.7) | < 0.001 |
| LDH > 305 U/mL | 1283 (48) | 210 (62.7) | < 0.001 |
| Creatinine > 0.92 mg/dL | 1280 (45.5) | 284 (76.5) | < 0.001 |
| Lymphocyte count > 800 cells/mm3 | 1444 (51.3) | 134 (36.1) | < 0.001 |
| C-reactive protein > 7.52 mg/dL | 1323 (47.5) | 248 (68.3) | < 0.001 |
| Critical support and treatment (%) | |||
| Intensive Care Unit admission | 661 (23.3) | 123 (32.3) | < 0.001 |
| Invasive mechanical ventilation | 267 (9.4) | 63 (16.5) | < 0.001 |
| Remdesivir | 519 (18.3) | 30 (7.9) | < 0.001 |
| Dexamethasone the first 5 days | 882 (31.1) | 136 (35.7) | 0.071 |
| Tocilizumab the first 5 days | 495 (17.5) | 54 (14.2) | 0.109 |
HIV: Human Immunodeficiency Virus.
Outcome
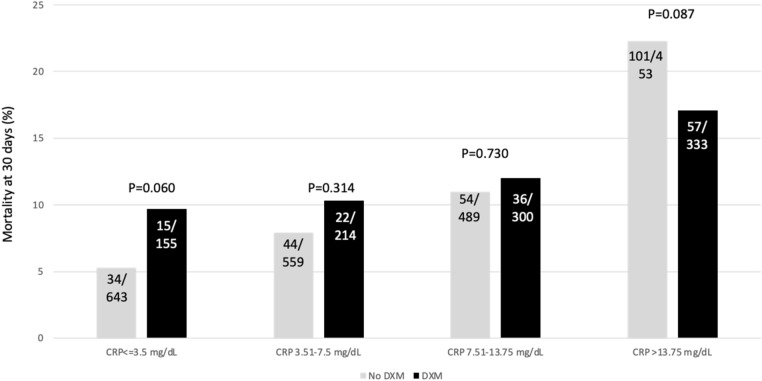
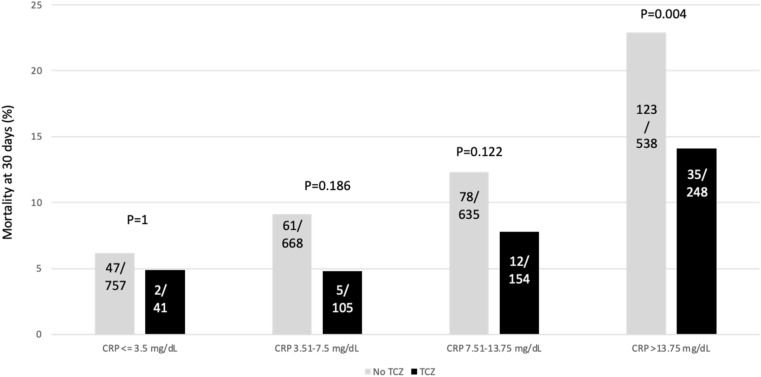
We explored the mortality among patients that received dexamethasone or tocilizumab according to the CRP percentiles at admission. Figure 1 shows the results for dexamethasone and illustrates that the mortality rate was numerically higher in patients with a CRP ≤ 3.50 mg/dL (percentile 25) if treated with dexamethasone (P = 0.06, Chi-square test), similar in those with CRP between 3.51 and 13.75 mg/dL, and numerically lower in patients treated with dexamethasone among those with a CRP > 13.75 mg/dL (P = 0.08, Chi-square test). Regarding tocilizumab (Fig. 2), no difference in mortality was observed in patients with a CRP ≤ 3.50 mg/dL; however, in those with a CRP > 3.50 mg/dL there was a numerically lower mortality rate associated with the receipt of tocilizumab, and this difference achieved statistical significance when CRP was > 13.75 mg/dL (P = 0.004, Chi-square test).
Figure 1.
Mortality rate at 30 days in patients receiving or not dexamethasone (DXM) within the first 5 days from admission and stratified by the C-reactive protein (CRP) percentiles (fractions within the bars represents the number of dead patients/total number of patients in this category).
Figure 2.
Mortality rate at 30 days in patients receiving or not tocilizumab (TCZ) within the first 5 days from admission and stratified by the C-reactive protein (CRP) percentiles (fractions within the bars represents the number of dead patients/total number of patients in this category).
Risk factors for mortality
We assessed risk factors associated with 30-days mortality by a multivariate analysis. In this analysis, we included the interaction between dexamethasone or tocilizumab and CRP dichotomized by percentiles. Age > 66 years, co-morbidity, clinical condition (oxygen saturation and fever), creatinine levels, lymphocyte count, invasive mechanical ventilation, treatment with remdesivir, and the interactions between dexamethasone and CRP (cut-off 13.75 mg/dL) and between tocilizumab and CRP (cut-off of 3.5 mg/dL) were retained in the model as independent predictors of mortality (Table 2). The p value of the Hosmer–Lemeshow goodness of fit test was > 0.05, and the area under the ROC curve was 0.873 (95% CI 0.851–0.896, P = 0.0001) showing a good ability to predict mortality at 30 days. The separate adjusted OR (95% CI) of the association of tocilizumab and dexamethasone with 30-day mortality within each stratum of the corresponding cut-off-defined CRP variables are shown in Table 3. In both cases, over the proposed CRP cut-off points, treatment with either tocilizumab or dexamethasone was significantly associated with a reduction in the mortality rate. Below the proposed cut-offs, the ORs for mortality were not significant.
Table 2.
Independent variables associated with mortality at 30 days.
| Variable | OR (95%CI) | P value |
|---|---|---|
| Age > 66 years | 4.961 (3.367–7.307) | 0.001 |
| Chronic heart disease | 1.629 (1.213–2.188) | 0.001 |
| Haematological disease | 1.868 (1.197–2.915) | 0.006 |
| Chronic kidney disease | 2.392 (1.706–3.354) | 0.001 |
| Solid tumour | 1.459 (1.067–1.996) | 0.018 |
| Temperature > 37 °C | 0.716 (0.541–0.947) | 0.019 |
| Oxygen saturation > 94% | 0.490 (0.360–0.667) | 0.001 |
| Creatinine > 0.92 mg/dL | 1.569 (1.134–2.172) | 0.007 |
| CRP > 3.5 mg/dL (p25) | 1.683 (1.118–2.534) | 0.013 |
| CRP > 13.75 mg/dL (p75) | 2.487 (1.689–3.661) | 0.001 |
| Lymphocyte count > 800 cells/mm3 | 0.711 (0.536–0.944) | 0.018 |
| Invasive mechanical ventilation | 1.668 (1.046–2.659) | 0.032 |
| Remdesivir | 0.531 (0.336–0.836) | 0.006 |
| CRP > 3.5 mg/dL (p25) by tocilizumab the first 5 days | 0.682 (0.464–1.002) | 0.052 |
| CRP > 13.75 mg/dL (p25) by dexamethasone the first 5 days | 0.435 (0.247–0.766) | 0.004 |
Variables included in the model: age, sex, co-morbidity (Chronic heart diseases, Diabetes mellitus, Haematological disease, Chronic kidney disease, hypertension, Solid tumour and Chronic respiratory disease); LDH, creatinine, C-reactive protein, and lymphocyte count at admission [C-reactive protein was introduced by percentiles as well as the interactions between each percentile and tocilizumab or dexamethasone administration within the first 5 days (both variables were also individually included)]; Temperature, and Oxygen saturation at admission; And the need of intensive care admission and invasive mechanical ventilation.
CRP, C-reactive protein. LDH, Lactate Dehydrogenase. P25-75, percentile 25–75.
Table 3.
Odds ratios for the 2 strata of C-reactive protein (lower or equal or higher than the cut- off) from the significant interactions identified in the final model (calculated according to reference 16).
| C-reactive protein (mg/dL) | OR (95%CI) of mortality for patients receiving tocilizumab versus not receiving it | P value | C-reactive protein (mg/dL) | OR (95%CI) of mortality for patients receiving dexamethasone versus not receiving it | P value |
|---|---|---|---|---|---|
| ≤ 3.5 | 1.42 (0.32–6.74) | 0.640 | ≤ 13.75 | 1.30 (0.92–1.85) | 0.13 |
| > 3.5 | 0.65 (0.44–0.95) | 0.029 | > 13.75 | 0.57 (0.37–0.89) | 0.014 |
Discussion
The current cornerstone of COVID-19 treatment is the anti-inflammatory therapy to halt the inflammatory response triggered by SARS-CoV-2. However, we are far from understanding the exact role of persistent viral replication in the maintenance of immune stimulation and even its responsibility in the immune dysregulation leading to severe COVID-19. Therefore, immunosuppressants could be deleterious and the concept of “one size fits for all” probably is not valid for COVID-19 management.
Our results, suggest that dexamethasone significantly reduces the mortality when the patient has an intense systemic inflammatory response measured as a CRP > 13.75 mg/dL, but at the same time, there was a hint of a possible higher mortality when it was administered in patients with low systemic inflammatory response after adjusting for the major risk factors already described in the literature. This is in line with the results obtained by Keller et al.12 showing that glucocorticoid treatment of patients with initial CRP ≥ 20 mg/dL was associated with significantly reduced risk of mortality or mechanical ventilation (odds ratio, 0.23; 95% CI, 0.080–0.70), while glucocorticoid treatment of patients with CRP < 10 mg/dL was associated with significantly increased risk of mortality or mechanical ventilation (OR, 2.64; 95% CI, 1.39–5.03). A similar retrospective study also identified a CRP ≥ 10 mg/dL as the cut-off point that predicts the beneficial effect of steroids17. Since high viral load18, prolonged viral shedding19 and the presence of RNAemia20 have been associated with worse outcomes in COVID-19, it seems prudent to adequately select the patients and the timing for using corticosteroids. Indeed, previous experience in viral pneumonia (Influenza virus, SARS- CoV and MERS) showed prolonged viral shedding and worse outcome among those patients receiving corticosteroids21,22. Data in SARS-CoV-2 is contradictory, while some authors reported longer viral shedding in corticosteroid group23,24, others did not25.
On the other hand, tocilizumab showed a beneficial effect among patients with a CRP cut-off point > 3.50 mg/dL, a significantly lower cut-off value in comparison to the one for dexamethasone (CRP > 13.75 mg/dL). As an inhibitor of the IL-6, tocilizumab is a selective immunosuppressor blocking exclusively one of the multiple pathways of the inflammatory response. This could explain that using this drug the potential to hamper the viral replication control by the host immune system is limited. In line with this hypothesis, Masia et al.26 reported that after adjustment for the baseline viral load, the use of tocilizumab was not associated with a prolonged viral shedding, and it has been suggested that this is because tocilizumab does not reduce the activity of B lymphocytes. Indeed, it was previously documented that tocilizumab did not reduce the efficacy of influenza vaccine in patients with rheumatoid arthritis27. Interestingly, the efficacy of tocilizumab in the RECOVERY study15, was demonstrated including only patients with a CRP ≥ 7.5 mg/dL. More recently, a post-hoc analysis of the CORIMUNO- TOCI-I trial showed that the likelihood of suffering the primary end point (non-invasive or invasive ventilation requirement, or death) was also lower in the tocilizumab group (18% vs. 57%), when patients with CRP levels > 15 mg/dL were selected (HR 0.18; 95% CI 0.06–0.59)28,29.
Our study has several limitations. The major drawback of our study is the retrospective nature. To reduce the potential bias, we have included in the multivariable analysis all the variables potentially implicated in the mortality to adjust for confounding. Secondly, only those treatments already accepted in the majority of the current guidelines or supported by large clinical trials have been evaluated (remdesivir, dexamethasone and tocilizumab), but not other treatments which definitive role is not yet clarified. Thirdly, some patients received both dexamethasone and tocilizumab and the potential effect of combined treatment was not evaluated. Fourthly, days from symptoms onset to admission or to treatment were not available; however, although the timing is important in COVID-19, our results suggest that inflammatory biomarkers by itself are helpful to prescribe dexamethasone or tocilizumab. Finally, viral load or a surrogate marker of the viral load was not available, and this is a critical information since we hypothesise that a potential harmful effect of immunomodulators in patients with low CRP maybe due to the worse control of viral replication as in other respiratory viruses.
Therefore, we propose to start tocilizumab to patients with CRP > 3.5 mg/dL and consider the addition of dexamethasone only to those cases in whom no clinical or biological improvement is observed in the next 48 h. For patients with a CRP > 13 mg/dL we support the initial use of dexamethasone and in line with the recent RECOVERY study, to combine dexamethasone with tocilizumab. In any case, our data also suggest the need of an antiviral agent, and, consequently, we recommend monitoring, in parallel to inflammatory response, the viral load using the cycle threshold of the real time polymerase chain reaction, the RNAemia or any other available marker.
In conclusion, our results suggest that anti-inflammatory therapy with dexamethasone and tocilizumab are associated with a reduction in mortality when prescribed to patients with a certain inflammatory activity as assessed by C-reactive protein, a cheap and widely available biomarker. Personalized treatment following the cut-off points for prescription of tocilizumab (CRP > 3.50 mg/dL) or dexamethasone (CRP > 13.75 mg/dL) within the first 5 days from hospital admission should be considered.
Acknowledgements
“This work was financed by ad hoc patronage funds for research on COVID-19 from donations from citizens and organizations to the Hospital Clínic de Barcelona-Fundació Clínic per a la Recerca Biomèdica”.
Author contributions
A.M.P., R.A. and A.S. wrote the main manuscript text. All authors have seen and approved the final version of the manuscript and have contributed significantly to the work.
Competing interests
CGV has received honoraria for talks on behalf of Gilead Science, MSD, Novartis, Pfizer, Jannsen, and Lilly, as well as a grant from Gilead Science and MSD. PPA has received honoraria for talks on behalf of Gilead Science and MSD. JM has received honoraria for talks on behalf of Merck Sharp and Dohme, Pfizer, Novartis, and Angellini. AS has received honoraria for talks on behalf of Merck Sharp and Dohme, Pfizer, Novartis, Gilead, Menarini, and Angellini, as well as grant support from Pfizer and Gilead. MT has received grants from Janssen, Gilead, ViiV and Merck Sharp and Dohme. LM has received honoraria for talks on behalf of Merck Sharp and Dohme, Pfizer and Angellini. PC has received honoraria for talks on behalf of Merck Sharp and Dohme, has participated in Advisory Boards for Gilead and Alexion, and has received grant support from Pfizer and Gilead. Other authors do not declare conflict of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Rodrigo Alonso.
A list of authors and their affiliations appears at the end of the paper.
Contributor Information
Alex Soriano, Email: viladomiu@me.com.
Hospital Clinic of Barcelona COVID-19 Research Group:
J. L. Blanco, J. Mallolas, E. Martínez, M. Martínez, J. M. Miró, A. Moreno, M. Solá, A. Ugarte, Ana Gonzalez-Cordón, Montse Laguno, Lorna Leal, John Rojas, Berta Torres, S. Fernandez, A. Tellez, F. Fuentes, M. Ayala, E. Sancho, D. Campubri, M. T. de Alba, M. Fernandez, E. Ferrer, B. Grau, H. Marti, M. Muelas, M. J. Pinazo, N. Rodriguez, M. Roldan, C. Subira, I. Vera, N. Williams, A. Almuedo-Riera, A. Aldea, M. Camafort, J. Calvo, A. Capdevila, F. Cardellach, I. Carbonell, E. Coloma, A. Foncillas, R. Estruch, M. Feliu, J. Fernández-Solá, I. Fuertes, C. Gabara, I. Grafia, A. Ladino, R. López-Alfaro, A. López-Soto, I. Macaya, F. Masanés, A. Matas, M. Navarro, J. Marco- Hernández, L. Miguel, J. C. Milisenda, P. Moreno, J. Naval, D. Nicolás, H. Oberoi, J. Padrosa, M. Pellicé, J. Ribot, O. Rodríguez-Núnez, E. Sacanella, F. Seguí, C. Sierra, A. Tomé, M. Torres, H. Ventosa, C. Zamora-Martínez, M. Almela, M. Alvarez, J. Bosch, J. Costa, G. Cuesta, B. Fidalgo, J. Gonzàlez, F. Marco, S. Narvaez, C. Pitart, E. Rubio, A. Vergara, M. E. Valls, Y. Zboromyrska, and E. López
References
- 1.Hopkins, J. COVID-19 dashboard: By the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). https://coronavirus.jhu.edu/map.html.
- 2.Wu Z, McGoogan J. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in china summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1240–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Cummings MJ, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: A prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramasamy S, Subbian S. Critical determinants of cytokine storm and type I interferon response in COVID-19 pathogenesis. Clin. Microbiol. Rev. 2021;34:e00299–20. doi: 10.1128/CMR.00299-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hadjadj J, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johns M, George S, Taburyanskaya M, Poon YK. A Review of the Evidence for Corticosteroids in COVID-19. J. Pharm. Pract. 2021 doi: 10.1177/0897190021998502. [DOI] [PubMed] [Google Scholar]
- 7.Group RC, et al. Dexamethasone in hospitalized patients with covid-19. New Engl. J. Med. 2020;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta S, Leaf DE. Tocilizumab in COVID-19: Some clarity amid controversy. Lancet. 2021;397:1599–1601. doi: 10.1016/S0140-6736(21)00712-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knight, S. R. et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: development and validation of the 4C Mortality Score. BMJ 370, (2020). [DOI] [PMC free article] [PubMed]
- 10.Galván-Román JM, et al. IL-6 serum levels predict severity and response to tocilizumab in COVID-19: An observational study. J. Allergy Clin. Immunol. 2021;147:72–80.e8. doi: 10.1016/j.jaci.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martínez-Sanz J, et al. Effects of tocilizumab on mortality in hospitalized patients with COVID-19: A multicentre cohort study. Clin. Microbiol. Infect. 2021;27:238–243. doi: 10.1016/j.cmi.2020.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keller MJ, et al. Effect of Systemic Glucocorticoids on Mortality or Mechanical Ventilation in Patients With COVID-19. J Hosp Med. 2020;15:489–493. doi: 10.12788/jhm.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pagnesi M, et al. Determinants of the protective effect of glucocorticoids on mortality in hospitalized patients with COVID-19: Insights from the Cardio-COVID-Italy multicenter study. Int. J. Infect. Dis. 2021 doi: 10.1016/j.ijid.2021.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Vidal C, Sanjuan G, Puerta-Alcalde P, Moreno-García E, Soriano A. Artificial intelligence to support clinical decision-making processes. EBioMedicine. 2019;46:27–29. doi: 10.1016/j.ebiom.2019.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abani O, et al. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kleinbaum, D., Kupper, L., Muller, K. & Nizam, A. Logistic regression analysis (chapter 23). In Applied Regression Analysis and Other Multivariable Methods (Brooks/Cole Publishing Company, 511 Forest Lodge Road, Pacific Grove, CA 93950, USA, 2013).
- 17.Committee W, et al. Corticosteroids in patients hospitalized for COVID-19 pneumonia who require oxygen: Observational comparative study using routine care data. Clin. Microbiol. Infect. 2021;27:603–610. doi: 10.1016/j.cmi.2020.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magleby, R. et al. Impact of SARS-CoV-2 viral load on risk of intubation and mortality among hospitalized patients with coronavirus disease 2019. Clin. Infect. Dis. 10.1093/cid/ciaa851 (2020). [DOI] [PMC free article] [PubMed]
- 19.Munker D, et al. Dynamics of SARS-CoV-2 shedding in the respiratory tract depends on the severity of disease in COVID-19 patients. Eur. Respir. J. 2021 doi: 10.1183/13993003.02724-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veyer D, et al. Highly sensitive quantification of plasma SARS-CoV-2 RNA shelds light on its potential clinical value. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moreno G, et al. Corticosteroid treatment in critically ill patients with severe influenza pneumonia: A propensity score matching study. Intensive Care Med. 2018;2:395. doi: 10.1007/s00134-018-5332-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brun-Buisson C, et al. Early corticosteroids in severe influenza A/H1N1 pneumonia and acute respiratory distress syndrome. Am. J. Resp. Crit. Care. 2011;183:1200–1206. doi: 10.1164/rccm.201101-0135OC. [DOI] [PubMed] [Google Scholar]
- 23.Huang R, et al. Corticosteroid therapy is associated with the delay of SARS-CoV-2 clearance in COVID-19 patients. Eur. J. Pharmacol. 2020;889:173556. doi: 10.1016/j.ejphar.2020.173556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang CL, et al. A novel prediction model for long-term SARS-CoV-2 RNA shedding in non-severe adult hospitalized patients with COVID-19: A Retrospective Cohort Study. Infect. Dis. Ther. 2021;10:897–909. doi: 10.1007/s40121-021-00437-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ji J, et al. Glucocorticoid therapy does not delay viral clearance in COVID-19 patients. Crit Care. 2020;24:565. doi: 10.1186/s13054-020-03287-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masiá M, et al. Impact of interleukin-6 blockade with tocilizumab on SARS-CoV-2 viral kinetics and antibody responses in patients with COVID-19: A prospective cohort study. EBioMedicine. 2020;60:102999. doi: 10.1016/j.ebiom.2020.102999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mori S, et al. Impact of tocilizumab therapy on antibody response to influenza vaccine in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2012;71:2006–2010. doi: 10.1136/annrheumdis-2012-201950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mariette X, et al. Effectiveness of tocilizumab in patients hospitalized with COVID-19. JAMA Intern Med. 2021;181:1241–1243. doi: 10.1001/jamainternmed.2021.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hermine O, et al. Effect of tocilizumab vs usual care in adults hospitalized with Covid-19 and moderate or severe pneumonia. JAMA Intern Med. 2021;181:32–40. doi: 10.1001/jamainternmed.2020.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]