Abstract
Increased expression and activation of tumor necrosis factor-α (TNF-α) could lead to recurrent implantation failure (RIF). Therefore, TNF-α inhibition may be a strategic way to enhance the implantation rate in women with RIF. Nowadays, monoclonal antibodies are considered an effective therapeutic method for TNF-α inhibition. Unfortunately, monoclonal antibody treatments have several disadvantages. Thus, the design of small molecules capable of inhibiting TNF-α has become critical in recent years. In silico drug repurposing of FDA-approved drugs for TNF-α inhibition was used in this study. PyRx tools were employed for virtual screening. Additionally, the free energy of binding, the number of hydrogen bonds, and the number of drug contacts with the protein were calculated using the molecular dynamics (MD) simulation method. Virtual screening results reveal that 17 of 2471 FDA-approved drugs benefited from favorable binding energy with TNF-α (delta G < − 10 kcal/mol). Two of the 17 drugs, progesterone and prednisone, were the most frequently used without adverse effects during pregnancy. As a result, MD simulation was used to investigate these two drugs further. According to the MD simulation results, prednisone appears to have a higher affinity for TNF-α than progesterone, and consequently, the prednisone complex stability is higher. For the first time, this study examined the possible role of prednisone and progesterone in inhibiting TNF-α using in silico methods.
Graphical abstract

Supplementary Information
The online version contains supplementary material available at 10.1007/s00894-022-05093-z.
Keywords: TNF-α, Prednisone, RIF, Progesterone, In silico, Drug repurposing
Introduction
Despite significant advancements in assisted reproductive techniques (ARTs), the rate of embryo implantation following in vitro fertilization (IVF) has not increased nearly as much as expected. Recurrent implantation failure (RIF) is defined as the inability to conceive after a minimum of three embryo transfers using high-quality embryos [1]. TNF-α is a pro-inflammatory cytokine produced in various types of malignant, hematopoietic, and non-hematopoietic cells. These cells include macrophages, B lymphocytes, T lymphocytes, neutrophils, endothelial cells, astrocytes, and smooth muscle cells. Nonetheless, it is produced primarily by activated macrophages. TNF-α engages in the proliferation, differentiation, inflammation, apoptosis, and cellular immune regulation of cells in general. While normal levels of TNF-α are necessary for immune response regulation, abnormal levels of TNF-α may contribute to the development of certain autoimmune or inflammatory diseases [2]. RIF is one of the diseases associated with this abnormality.
TNF-α leads to implantation failure by decreasing the endometrial acceptance rate. A previous study shows that the ratios of inflammatory cytokines such as IFN-γ, IL-6, and TNF-α were higher in plasma of patients with RIF [3]. A balance between the cytokines produced by Th1 and Th2 cells is a key factor in a successful pregnancy. Immune system changes occur during implantation and the early stages of pregnancy, affecting both fetal implantation and pregnancy progression [4]. It has been determined that elevated Th1 cytokines like TNF-α and IFN-γ negatively affect pregnancy, and increased Th1/Th2 cytokine ratios may prevent implantation. Therefore, inhibiting TNF-α through inhibitors may be an effective therapeutic strategy for controlling and curing RIF. For inhibiting TNF-α, several methods are used, such as the production of monoclonal antibodies and the use of small molecules blocking TNF-α mRNA synthesis.
Recently, new studies have been conducted to inhibit the activity of TNF-α using small molecules. In this study, in silico drug repurposing of FDA-approved drugs for inhibiting TNF-α was employed.
Methods
Virtual screening of TNF-α with FDA-approved drugs
The Protein Data Bank (www.pdb.org) provided the starting coordinates of TNF-α (PDB code: 1TNF). Before cutting, the protein crystal structure was established, detaching the water molecules from the protein and then adding hydrogen atoms and improving hydrogen bonds. The GROMACS 5.1.4 package was used to reduce energy consumption during the protein optimization process. The Drug Bank database’s small molecule sector (https://www.drugbank.ca/about) was used to obtain all FDA-approved drugs [5]. The process eliminated non-unique structures such as those containing rare atoms (selenium, platinum, gold, and silicon) and organometallic compounds. Conclusively, the screening database included 2471 compounds.
The PyRx tool was applied for decreasing energy and preparing ligands in PDBQT format, and AutoDock Vina was employed for virtual screening. AutoDock Vina was also used for drug discovery to monitor libraries of compounds against targets [6, 7]. The box was centered on the entire TNF-α, and afterward, TNF-α was used for virtual screening with all FDA-approved drugs. In the first step, we used blind docking for the virtual screening of all the FDA-approved drugs. Then, for drugs with the best binding energy, we used a web server, named CB-Dock (http://cao.labshare.cn/cb-dock/), which predicts binding sites of a given protein and calculates the centers and sizes with a novel curvature-based cavity detection approach. Five binding sites were identified using CB-Dock web server, and six binding poses were predicted for each binding site. The results of CB-Dock web server are presented in the supplementary data. The most suitable binding site was selected based on the most appropriate binding free energy and the best RMSD value (zero or close to zero). The process of evaluating a particular pose was done by counting the number of hydrogen bonds and hydrophobic contacts.
Molecular dynamics simulation
After docking all FDA-approved drugs, drugs with a desirable delta G (∆G < − 10 kcal/mol) with TNF-α that could be used during pregnancy were chosen for further evaluation. At this point, the goal was to identify drugs with an increased ability to bind with the protein. Therefore, the molecular dynamics (MD) simulation method was utilized to calculate the free energy of binding, the number of hydrogen bonds, and the number of drug contacts with the protein.
Install of MD systems
All MD simulations in this study were conducted using the GROMACS 5.1.4 package [8]. Additionally, the GROMOS 54a7 force fields [9] were utilized. The appropriate amounts of chloride ions and sodium were added to all simulation boxes to neutralize the system. In each simulation system, the periodic boundary condition (PBC) was applied along each simulation box axis, and the SP3 water model was also used for system solvation [10]. The LINCS algorithms constrained all covalent bonds. The stimulations are caused by a short-range electrostatic interaction combined with a 1.2-nm-distance cutoff for the van der Waals interaction [11].
The Particle Mesh Ewald (PME) algorithm calculated the long-range electrostatic interaction.
The steepest descent algorithm was used to minimize the energy of all systems, and then the NVT ensemble was used to equilibrate all systems for 500 ps. Afterward, the NPT ensemble gradually directed each system’s equilibration while maintaining the Nose–Hoover algorithm temperature [12, 13] and the temperature at 310 K. During the NPT equilibration, the Parrinello-Rahman barostat [14] maintained the pressures at 1 bar. MD simulations were completed in 200 ns for the complexes.
Analyses
Following MD simulations, GROMACS utilities examined and evaluated each trajectory’s results. The nonpolar and polar interactions between the TNF-α and drugs can be explained via binding the free energy calculation. By exercising the MM-PBSA method, the binding free energy was calculated with the g_mmpbsa tool [15].
The total binding free energy () is calculated by adding the nonpolar interaction free energy () and the polar interaction free energy (), as follows:
where , , , and are the electrostatic energy, polar solvation energy, van der Waals energy, and nonpolar solvation energy, respectively.
Results
In the present study, a drug repurposing strategy for the inhibition of TNF-α was used via virtual screening and MD simulation.
Virtual screening results
Table 1 shows 17 FDA-approved drugs (out of 2471) in which TNF-α has the most favorable free-binding energy. Then, based on a guide to drug safety in pregnancy, we selected the drugs that were safe during pregnancy (https://www.drugs.com/pregnancy/). Of the 17 drugs, progesterone and prednisone are the most frequently used drugs during pregnancy without causing adverse effects. Thus, the molecular simulation technique was used to select these two drugs for further investigation.
Table 1.
Background, FDA pregnancy category, and docking results of FDA drugs against TNF-α
| FDA drugs | Binding free energy (kcal/mol) against TNF-α | Background | US FDA pregnancy category (The guide to drug safety in pregnancy) |
|---|---|---|---|
| Conivaptan | − 11.9 | Conivaptan is an antidiuretic hormone inhibitor used to raise serum sodium levels | Not assigned |
| Bictegravir | − 10.9 | Bictegravir is an integrase inhibitor used to treat HIV infections | Not assigned |
| Ouabain | − 10.8 | Ouabain is a steroid hormone that inhibits the plasma membrane Na( +)/K( +)-ATPase | Not assigned |
| Doxazosin | − 10.7 | Doxazosin is an alpha-adrenergic (AL-fa ad-ren-ER-jik) blockers | Not assigned |
| Diosmin | − 10.7 | Diosmin is most often used for hemorrhoids and leg sores caused by poor blood flow | Diosmin is not recommended for use in pregnant or breast-feeding women |
| Sorafenib | − 10.6 | Sorafenib is used to treat liver cancer, thyroid cancer, or kidney cancer | Not assigned |
| NADH | − 10.5 | NADH is a coenzyme composed of ribosylnicotinamide 5′-diphosphate coupled to adenosine 5′-phosphate by pyrophosphate linkage. NADH is used in some supplement products | NADH supplements should not be used in children, pregnant women, or nursing mothers |
| Imatinib | − 10.4 | Imatinib is a small molecule kinase inhibitor used to treat certain types of cancer | Not assigned |
| Terconazole | − 10.3 | Terconazole is an anti-fungal drug that is mainly used to treat vaginal yeast infections | Use is not recommended during the first trimester of pregnancy |
| Progesterone | − 10.2 | Progesterone is a hormone that occurs naturally in females, and is essential for endometrial receptivity, embryo implantation, and the successful establishment of pregnancy |
US FDA pregnancy category: B (category B drugs include several medications used routinely and safely during pregnancy.) |
| Domperidone | − 10.2 | Domperidone is a dopamine receptor antagonist and is used as antiemetic and indigestion | Domperidone is not usually recommended in pregnancy |
| Prednisone | − 10.1 | A synthetic anti-inflammatory glucocorticoid derived from cortisone and converted to prednisolone in the liver |
US FDA pregnancy category: C Animal reproduction studies have shown an adverse effect on the fetus and there are no adequate and well-controlled studies in humans, but potential benefits may warrant use of the drug in pregnant women despite potential risks |
| Ziprasidone | − 10.1 | Ziprasidone is an atypical antipsychotic used to manage schizophrenia, bipolar mania, and agitation in patients with schizophrenia | Neonates exposed to antipsychotic drugs during the third trimester of pregnancy are at risk for extrapyramidal and/or withdrawal symptoms following delivery |
| Sitagliptin | − 10.1 | Sitagliptin is an oral dipeptidyl peptidase-4 inhibitor used to improve glycemic control in patients with type 2 diabetes mellitus | Not assigned |
| Levofloxacin | − 10.1 | Levofloxacin is an antibiotic used to treat infections caused by bacteria of the upper respiratory tract, urinary tract and prostate | Not assigned |
| Estazolam | − 10 | Estazolam is a benzodiazepine used for the short-term management of insomnia | US FDA pregnancy category: X (The risk of the use of the drug in pregnant women clearly outweighs any possible benefit) |
| Dasatinib | − 10 | Dasatinib is a tyrosine kinase inhibitor used for the treatment of lymphoblastic | Not assigned |
MD results for TNF-α/progesterone complex and TNF-α/ prednisone complex
RMSD analysis
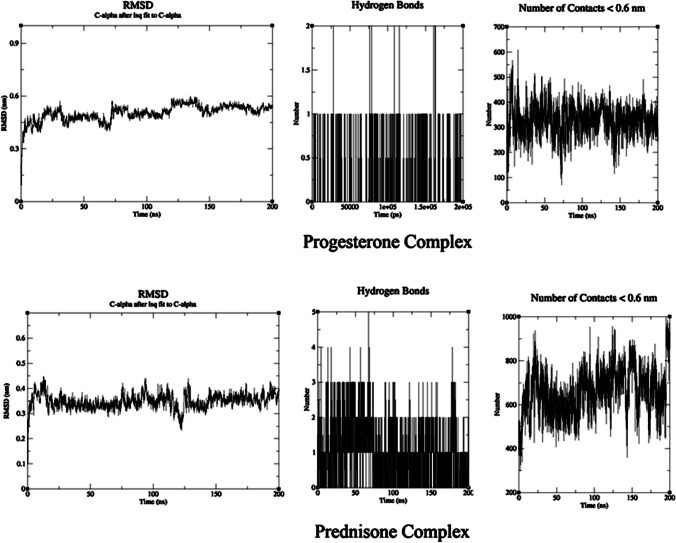
RMSD was used to determine the complexes’ flexibility and stability. RMSD measurement was conducted for progesterone and prednisone carbon alpha 2 complexes. The RMSD of the two complexes is illustrated in Fig. 1. According to the results, the RMSD values for protein in the progesterone complex were significantly greater than those of protein in the prednisone complex (Fig. 1).
Fig. 1.
MD results for TNF-α/progesterone complex and TNF-α/prednisone complex
Calculation of the number of H-bonds, the number of contacts, and the free binding energy (∆G)
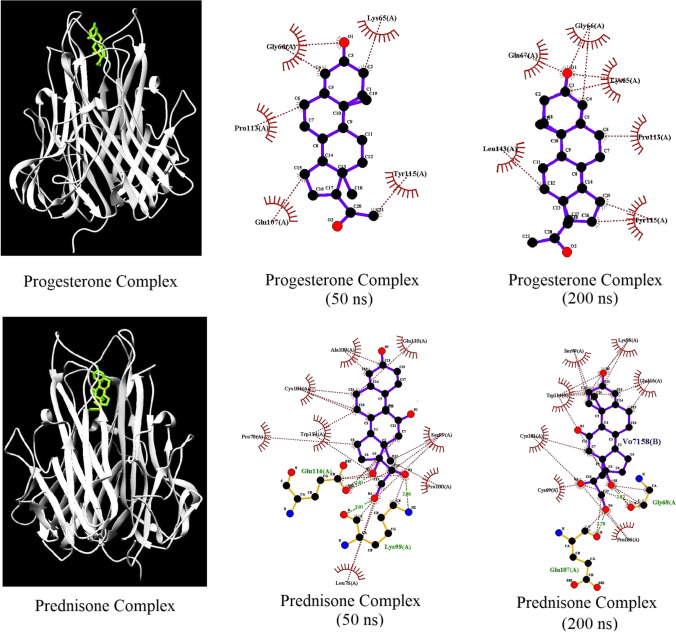
The number of hydrogen bonds, the number of contacts, and the free binding energy between the protein and the drugs play a key role in stabilizing the protein/drug complex. The total number of H-bonds in the progesterone and prednisone complexes with TNF-α at 310 K was calculated and is shown in Fig. 1. The number of H-bonds and the number of contacts in the prednisone complex are more significant than those in the progesterone complex. The number of hydrogen bonds with LIGPLOT calculations is shown in Fig. 2. The calculation of ∆G values for the protein–ligand complexes is shown in Table 2.
Fig. 2.
Display of hydrogen bonds with LIGPLOT
Table 2.
Calculation of binding free energy between TNF-α with progesterone and prednisone
| Energetic analysis of TNF-α/progesterone binding (kcal/mol) | |
|---|---|
| Van der Waals energy | − 156.776 ± 12.094 kJ/mol |
| Electrostatic energy | − 13.571 ± 24.450 kJ/mol |
| Polar solvation energy | 73.370 ± 23.455 kJ/mol |
| SASA energy | − 14.720 ± 0.983 kJ/mol |
| SAV energy | 0.000 ± 0.000 kJ/mol |
| WCA energy | 0.000 ± 0.000 kJ/mol |
| Binding energy | − 111.696 ± 20.076 kJ/mol |
| Energetic analysis of TNF-α/prednisone binding (kcal/mol) | |
| Van der Waals energy | − 157.592 ± 9.136 kJ/mol |
| Electrostatic energy | − 23.277 ± 19.191 kJ/mol |
| Polar solvation energy | 75.918 ± 20.649 kJ/mol |
| SASA energy | − 16.355 ± 1.151 kJ/mol |
| SAV energy | 0.000 ± 0.000 kJ/mol |
| WCA energy | 0.000 ± 0.000 kJ/mol |
| Binding energy | − 121.307 ± 18.841 kJ/mol |
The free binding energy of the prednisone complex is more favorable compared to that of the progesterone complex (the free binding energy of progesterone and prednisone with TNF-α respectively was − 111.696 and − 121.307 (kcal/mol)). Given the results, prednisone has a greater affinity for TNF-α than progesterone, implying that the prednisone complex is more stable than progesterone.
Discussion
The causes of repeated implantation failure (RIF) vary according to the individual. Increased expression and activation of TNF-α could lead to immune cell aggregation and inhibition of embryo implantation. Under these circumstances, the inhibition of TNF-α can be effective. Thus, several theories about TNF-α inhibition and increased fertility in women with RIF may be correct [16]. TNF-α inhibition may be a strategic way to enhance the implantation rate in women with RIF. Nowadays, direct inhibition of TNF-α using monoclonal antibodies is an effective therapeutic method. Unfortunately, monoclonal antibody treatments have several drawbacks, including high manufacturing costs. Thus, the development of small molecules capable of inhibiting TNF-α has become critical in recent years [17]. Nowadays, drugs previously approved are used to treat various diseases, referred to as drug repurposing [18]. In a previous study, in silico drug repurposing was used to identify the most effective drugs in treating antiphospholipid syndrome, another cause of RIF [19]. Several studies indicate that prednisone may be highly beneficial in treating patients with RIF [20]. A 2020 study found that prednisone can affect pregnancy outcomes in patients with RIF [21]. The in vitro studies reveal that progesterone may inhibit TNF-α-induced apoptosis [22]. Additional animal studies suggest that progesterone injections reduce TNF-α expression [23]. Progesterone has been shown in some studies to decrease recurrent miscarriage by balancing the Th1/Th2 cytokines [24]. Several studies have shown that residues Cys101-Glu116 play an essential role at the binding site of TNF-α [25]. As shown in Fig. 2, prednisone interacted with Cys101-Glu116 residues by hydrogen bonds.
In this study, and for the first time, using in silico methods, it is shown that prednisone and progesterone tend to bind well with TNF-α. The number of hydrogen bonds, the number of contacts, and the free binding energy of prednisone and TNF-α are greater than those in the progesterone complex, as determined by MD simulations. Given the results, it seems that the binding tendency of prednisone to TNF is more than that of progesterone; hence, the prednisone complex stability is higher. The results of this study which used in silico methods reveal that the role of prednisone and progesterone in TNF-α inhibition is consistent with the results of clinical studies. Prednisone is a potent immunomodulatory drug that helps to prevent or attenuate the hyper-inflammation state of COVID-19. According to the results of this study, one of the possible molecular pathways could be the inhibition of TNF-α with prednisone [26]. Therefore, the computational results in this study could provide new insight into the mechanism of action of the two drugs of progesterone and prednisone. Future studies recommend conducting in vitro and in vivo studies to determine the efficacy of these two drugs in inhibiting TNF-α.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
Soodeh Mahdian contributed to the design and prepared the manuscript. Mahboobeh Zarrabi participated in the data analysis. Ashraf Moini helped analyze the data. Monireh Movahedi revised the manuscript. Maryam Shahhoseini contributed to the design the manuscript.
Funding
The project was funded by the first author.
Data availability
Data and material are available.
Code availability
The software used was available for free.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this article was revised due to a minor changes in the second affiliation.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
5/23/2023
A Correction to this paper has been published: 10.1007/s00894-023-05576-7
Contributor Information
Maryam Shahhoseini, Email: m.shahhoseini@royaninstitute.org.
Monireh Movahedi, Email: mon_movahedi@yahoo.com.
References
- 1.Bashiri A, Halper KI, Orvieto R. Recurrent implantation failure-update overview on etiology, diagnosis, treatment. and future directions. Reprod Biol Endocrinol. 2018;16(1):121. doi: 10.1186/s12958-018-0414-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farajzadeh D, Karimi-Gharigh S, Dastmalchi S. Tumor necrosis factor-alpha and its inhibition strategies. Tehran University Medical Journal. 2017;75(3):159–171. [Google Scholar]
- 3.Liang PY, Dia LH, Huang CY, et al. The pro-inflammatory and anti-inflammatory cytokine profile in peripheral blood of women with recurrent implantation failure. Reprod Biomed Online. 2015;31(6):823–826. doi: 10.1016/j.rbmo.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Ghasemnejad-Berenji H, Novin MG, Hajshafiha M, et al. Immunomodulatory effects of hydroxychloroquine on Th1/Th2 balance in women with repeated implantation failure. Biomed Pharmacother. 2018;107:1277–1285. doi: 10.1016/j.biopha.2018.08.027. [DOI] [PubMed] [Google Scholar]
- 5.Wishart DS, Feunang YD, Guo AC, et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic acids res. 2018;46(1):1074–1082. doi: 10.1093/nar/gkx1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dallakyan S, Olson AJ. Small-molecule library screening by docking with PyRx. Chem Biol. 2015;1263:243–250. doi: 10.1007/978-1-4939-2269-7_19. [DOI] [PubMed] [Google Scholar]
- 7.Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J Comput Chem. 2010;2010(31):455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berendsen HJC, van der Spoel D. Gromacs: a message-passing parallel molecular dynamics implementation. Comput Phys Commun. 1995;1995(91):43–56. doi: 10.1016/0010-4655(95)00042-E. [DOI] [Google Scholar]
- 9.Best RB, Zhu X, Shim J, et al. Optimization of the additive charmm all-atom protein force field targeting improved sampling of the backbone Φ, Ψ and side-chain Χ (1) and Χ (2) dihedral angles. J Chem Theory Comput. 2012;8:3257–3273. doi: 10.1021/ct300400x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hess B. P-Lincs: a parallel linear constraint solver for molecular simulation. J Chem Theory Comput. 2008;4:116–122. doi: 10.1021/ct700200b. [DOI] [PubMed] [Google Scholar]
- 11.Darden T, York D, Pedersen L. Particle mesh Ewald: an N⋅ Log (N) method for Ewald sums in large systems. J Chem Phys. 1993;98:10089–10092. doi: 10.1063/1.464397. [DOI] [Google Scholar]
- 12.Hoover WG. Canonical dynamics: equilibrium phase-space distributions. Phys Rev A. 1985;31(3):1695–1697. doi: 10.1103/PhysRevA.31.1695. [DOI] [PubMed] [Google Scholar]
- 13.Nose S. A unified formulation of the constant temperature molecular dynamics methods. J Chem Phys. 1984;81:511–519. doi: 10.1063/1.447334. [DOI] [Google Scholar]
- 14.Parrinello M, Rahman A. Polymorphic transitions in single crystals: a new molecular dynamics method. J Appl Phys. 1981;52:7182. doi: 10.1063/1.328693. [DOI] [Google Scholar]
- 15.Kumari R, Kumar R, Lynn A, et al. g_mmpbsa A GROMACS tool for high-throughput MM-PBSA calculations. J Chem Inf Model. 2014;54:1951–1962. doi: 10.1021/ci500020m. [DOI] [PubMed] [Google Scholar]
- 16.Winger E, Reed JL, Ashoush S, et al. Degree of TNF-α/IL-10 cytokine elevation correlates with IVF success rates in women undergoing treatment with adalimumab (Humira) and IVIG. Am J Reprod Immunol. 2011;65(6):610–618. doi: 10.1111/j.1600-0897.2010.00946.x. [DOI] [PubMed] [Google Scholar]
- 17.He MM, Smith AS, Oslob JD, et al. Small-molecule inhibition of TNF-α. Science. 2005;310(5750):1022–1025. doi: 10.1126/science.1116304. [DOI] [PubMed] [Google Scholar]
- 18.Pushpakom S, Iorio F, Eyers PA, et al. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discovery. 2019;18(1):41–58. doi: 10.1038/nrd.2018.168. [DOI] [PubMed] [Google Scholar]
- 19.Mahdian S, Zarrabi M, Moini A, et al. In silico identification of new inhibitors for βeta-2-glycoprotein I as a major antigen in antiphospholipid antibody syndrome. J Mol Model. 2020;26(6):156–156. doi: 10.1007/s00894-020-04406-4. [DOI] [PubMed] [Google Scholar]
- 20.Ledee N, Prat-Ellenberg L, Petitbarat M, et al. Impact of prednisone in patients with repeated embryo implantation failures: beneficial or deleterious? J Reprod Immunol. 2018;127:11–15. doi: 10.1016/j.jri.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Lu Y, Yan J, Liu J, et al. Prednisone for patients with recurrent implantation failure: study protocol for a double-blind, multicenter, randomized, placebo-controlled trial. Trials. 2020;21(1):1–7. doi: 10.1186/s13063-020-04630-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo G, Abrahams VM, Tadesse S, et al. Progesterone inhibits basal and tnf-α-induced apoptosis in fetal membranes: a novel mechanism to explain progesterone-mediated prevention of preterm birth. Reprod Sci. 2010;17(6):532–539. doi: 10.1177/1933719110363618. [DOI] [PubMed] [Google Scholar]
- 23.- Farahabadi A, Akbari M, Pishva A, A.; et al. Effect of progesterone therapy on TNF-α and iNOS gene expression in spinal cord injury model. Acta medica Iranica. 2016; 345–351. [PubMed]
- 24.Nardo LG, Sallam HN. Progesterone supplementation to prevent recurrent miscarriage and to reduce implantation failure in assisted reproduction cycles. Reprod Biomed Online. 2006;13(1):47–57. doi: 10.1016/S1472-6483(10)62015-9. [DOI] [PubMed] [Google Scholar]
- 25.Willam A, Aufy M, Tzotzos S, et al. TnF lectin-like Domain restores epithelial sodium channel Function in Frameshift Mutants associated with Pseudohypoaldosteronism Type 1B. Front Immunol. 2017;8:601. doi: 10.3389/fimmu.2017.00601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.- Annane, D. (2021). Corticosteroids for COVID-19. Journal of Intensive Medicine. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and material are available.
The software used was available for free.