Abstract
We herein summarize currently available and clinically relevant information regarding the human immune responses to SARS-CoV-2 infection and vaccination, in relation to COVID-19 outcomes with a focus on acute respiratory distress syndrome (ARDS) and myocarditis.
Keywords: COVID-19, Vaccination, Infection, Humoral immunity, Cellular immunity, Cardiovascular
Key points
-
•
The severity of COVID-19 illness is directly correlated with early humoral immune response and inversely related with early cellular immune response.
-
•
Innate immune response in pulmonary tissue may be partially evaded by SARS-CoV-2.
-
•
Humoral and cellular immune responses elicited by vaccination wane approximately 6 months after initial vaccination, particularly among men and those over the age of 65.
-
•
An additional vaccine dose can enhance immune protection following waning initial vaccine protection.
-
•
Dysregulated immune response is associated with the severity of COVID-19 disease, including the development of acute respiratory distress syndrome (ARDS) and organ-specific sequelae such as myocarditis.
Significance
In March 2020 the World Health Organization declared coronavirus disease 2019 (COVID-19) a global pandemic. As of January 28, 2022, there have been 364 million confirmed cases of COVID-19 and 5.6 million deaths around the globe.1 In addition to causing overwhelming amounts of human suffering, COVID-19 has devastated health care systems, economies, and the social structures of daily life. Understanding how the human immune system responds to and overcomes SARS-CoV-2 infection is paramount to the discovery of effective treatments and prevention of severe disease.
Virology of SARS-CoV-2
Understanding the immunologic response to SARS-CoV-2 requires understanding the virus’ structure, transmissibility, and fatality. SARS-CoV-2 is an enveloped virus with a single-stranded positive sense RNA genome.2 Like most coronaviruses, SARS-CoV-2 has 4 structural proteins: the nucleocapsid (N) protein which forms a helical structure around the viral genomic RNA and the spike (S), membrane (M), and envelope (E) proteins that are embedded in the outer lipid bilayer.2 Each of these structural factors plays an important role in the infectivity and immunogenicity of the virus. Phylogenic analysis of SARS-CoV-2 revealed it to be in the same subgenus as the severe acute respiratory syndrome (SARS) coronavirus (SARS-CoV) and several other bat coronaviruses.3 Out of the 7 coronaviruses infecting humans, 3 are known to be capable of replicating in the lower respiratory tract and causing severe disease: SARS-CoV, SARS-CoV-2, and Middle East respiratory syndrome coronavirus (MERS-CoV).4
The 2003 SARS and 2012 MERS outbreaks had case fatality rates of 10% and 34%, respectively, while most of the COVID-19 cases have remained relatively mild in comparison.4 Specifically, the reported case fatality rate of COVID-19 peaked at 7.2% in April of 2020, declining to 2.2% by December 2020.5 In the context of its lower mortality rate, SARS-CoV-2 is substantially more contagious than SARS-CoV and MERS-CoV.6, 7, 8 This remarkable transmissibility, paired with its lower case fatality rate, has served as a double-edged sword given that milder illness affecting most of the cases has hampered public compliance with masking and social distancing recommendations.9 , 10
SARS-CoV-2 is spread through respiratory droplets and binds to the angiotensin-converting enzyme 2 (ACE2) receptor in nasal and bronchial epithelial cells and pneumocytes via viral S-protein.11 , 12 The S-protein has 2 subunits, S1 and S2; S1 mediates binding to the host cell ACE2 receptor through the receptor-binding domain (RBD) while S2 directs viral cell membrane fusion. By comparison, the RBD of SARS-CoV-2 binds ACE2 with 5 to 10 times greater affinity than the RBD of SARS-CoV.6 , 7 The recognition of the S-protein’s importance in the cellular entry has made it a key target for the immune response, as well as vaccine-mediated protection.
Immune responses to SARS-CoV-2 infection
Innate Immune Response in the Respiratory Tract
Human expression of ACE2 occurs on epithelial cells in both the upper and lower respiratory tract, with nasal epithelial cells expressing slightly greater amounts, making it an ideal environment for SARS-CoV-2 to enter the body.13 As such, early immune activation often begins in these locations. A study of ex vivo SARS-CoV-2 infected turbinate tissue identified a broad and robust innate immune response in nasal tissue, with increased upregulation of interferon-stimulated genes (ISGs) and cytokine release, vital components in inhibiting viral infection of nucleated cells.13 , 14 Comparatively, this response to SARS-CoV-2 was larger than that initiated by the influenza virus, characterized by significant upregulation of genes that specifically enhanced antigen presentation and immune cell signaling, indicating a robust immune recognition and response to the virus in the nose. SARS-CoV-2 infected lung tissue, on the other hand, failed to upregulate INF types I, II, and III. Upregulation of ISGs in infected lung tissue was observed, but cellular immune pathways such as antigen presentation, immune cell maturation, TNF receptor signaling, and inflammasome pathways were not activated.13 In effect, an early innate immune response is initiated in both the upper and lower respiratory tracts; however, the downstream effect is blunted in the pulmonary tissue.
A possible explanation for this finding is the action of SARS-CoV-2 nonstructural protein 1 (NSP1) which inactivates the translational activity of the human 40S ribosomal subunit, a key link in the chain in the activation of the immune response following antigen detection.8 , 15 Failure to translate the upregulation of ISGs could potentially explain the restricted innate response observed in the lung tissue. How nasal, but not lung, epithelial tissue overcomes the translation inhibiting effects of NSP1 is not yet understood. Other studies support this finding, demonstrating that in vitro SARS-CoV-2 infected primary human airway epithelial cultures are unable to produce type I or III IFN, yet the addition of exogenous IFN was able to significantly reduce viral burden in the same cultures.16 This suggests that the SARS-CoV-2 inhibition of IFN production, not reduced susceptibility to INF’s antiviral inducing mechanisms, may play an important role in the virus’s ability to modulate and evade the immune response.
Immune Response and Severity of Illness
Growing evidence links the type and temper of the host immune response to SARS-CoV-2 infection with the severity of illness experienced by those infected.17 , 18 A study by Almendro-Vasquez et al. compared the humoral and cellular responses to COVID-19 infection among patients who experienced a range of symptoms from mild to severe, both in the acute setting, as well as 4 to 7 months following recovery.18 Patients with mild infection were well enough to convalesce at home and experienced minimal symptoms, while those with moderate disease were hospitalized, required oxygen, or had nonsevere acute respiratory distress syndrome (ARDS). Comparatively, severe infection was characterized by admission to the ICU, a P:F ratio of less than 200, or death from COVID-19. The researchers found that among patients with mild disease, the development of anti-S1 IgG antibodies was delayed nearly 2 weeks from symptom onset and that once formed, these antibodies demonstrated only modest neutralizing capacity.18 Most patients in the moderate cohort also lacked detectable anti-S1 IgG for several weeks following symptom onset, however, once present, antibody levels were higher and consistent with robust neutralizing capacity. Comparatively, patients with severe illness had detectable anti-S1 IgG within 2 weeks of symptoms, with some as soon as 1 week after initially feeling ill.18 Further, antibodies from patients with severe illness had the strongest correlation between antibody levels and neutralizing viral response. This pattern, with a vigorous early antibody response, demonstrating stronger neutralization capacity among patients with severe COVID-19, has been duplicated by others and clearly demonstrates an association between severity of illness and early humoral immune response to native infection.19
Given this pattern, there has been an appropriate focus on IgG and IgM production over the course of COVID-19 infection; however, antibody levels may not tell the whole story. As noted, higher binding antibody levels have been found among patients suffering from severe, rather than mild, COVID-19 disease.20 Over the course of one to 6 months, anti-RBD IgG and IgM levels decrease, with an associated 5-fold reduction in plasma neutralizing activity. Importantly, memory B-cells, capable of rapidly producing RBD-specific antibodies, remain unchanged 6 months after infection, indicating the potential for the body to rapidly increase antibody levels on repeat antigen exposure.21 Further, while IgG and IgM are important neutralizing antibodies (help clear virus by binding to viral receptors and inhibiting viral particles’ ability to bind host cells, as well as preventing the uncoating of viral genome in the endosome), IgA predominates in serum, saliva, and bronchoalveolar lavage fluid of patients with SARS-CoV-2. Given the described limitations of the innate immune response in the lower respiratory tract, IgA neutralizing antibodies may play an important role in early response to infection.22 Less is known about differential IgA response by disease severity, a knowledge gap that, once filled, may help us better understand variations in host response to infection.
The cellular immune response to SARS-CoV-2 also correlates with the severity of COVID-19 illness, however, in an opposite direction than that of humoral immunity. Analysis of blood samples from patients infected with SARS-CoV-2 found that those with mild disease had a robust SARS-CoV-2-specific INF-γ-producing T-cell response against S1, M, and N viral proteins within the first 2 weeks of symptom onset while patients with moderate disease developed cellular responses to these epitopes at a slower rate, reaching equivocal numbers only 3-weeks postsymptom onset. Patients with severe disease had still not started developing high numbers of SARS-CoV-2 specific T-cells by the end of the acute infection follow-up.18 Fortunately, among those who recover, including recovery from severe disease, 97% maintained detectable SARS-CoV-2-specific memory T-cells out to at least 28 weeks, suggesting that even a delayed cellular response may produce longer lasting immunity across the spectrum of disease severity.18
Interestingly, significant peripheral T-cell lymphopenia has been identified as a marker of severe COVID-19 infection. A recent systematic review of the T-cell response to SARS-CoV-2 infection found significantly lower CD3+, CD4+, and CD8+ cell counts in critically ill patients and those who ended up dying of COVID-19 as compared with survivors and moderately ill patients.23 Further, in a multivariable-adjusted analysis, CD8+ T-cell counts of less than 165 cells/μL were independently associated with COVID-19 related mortality.24 Similarly, lower helper T-cell levels and higher CD4:CD8 ratios have also been identified as strong predictors of mortality.25 Dysregulation of the T-regulatory cell (Treg)/Th17 balance has further been implicated in severity of infection, with increased Th17 and decreased Treg cells in patients with severe COVID-19.26 Treg’s play a critical role in dampening an overreactive immune response for the maintenance of immune homeostasis and self-tolerance during viral infections. Th17 cells produce IL-17 which can promote the production of downstream proinflammatory molecules, including IL-1, TNF, IL6, and neutrophil chemoattractants. In the murine model of ARDS, IL-17 has previously been shown to augment lung parenchyma destruction by amplifying proinflammatory mediators and neutrophil recruitment.27
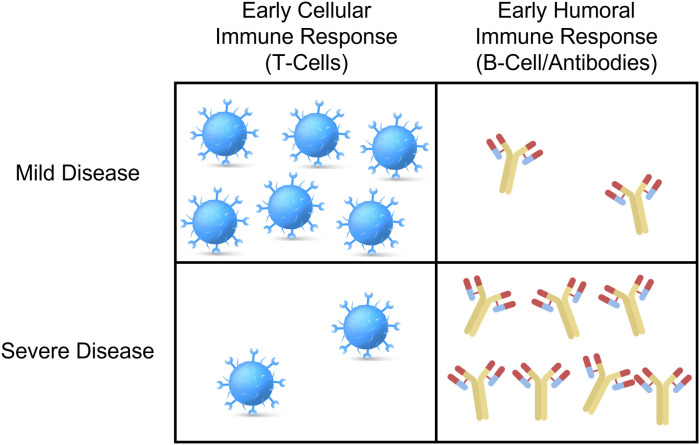
In effect, COVID-19 severity of illness demonstrates a dichotomous immune response, with early humoral activation and delayed cellular immune response associated with more severe disease, while early T-cell activation predominates in milder cases (Fig. 1 ).28 Fortunately, SARS-CoV-2 specific memory B- and T-cells are present following recovery in patients across the spectrum of disease, indicating the potential for a more rapid immune response on future antigenic exposures.
Fig. 1.
Graphical representation of differential humoral and cellular immune response to SARS-CoV-2 infection by disease severity.
Vaccination against SARS-CoV-2
SARS-CoV-2 vaccination is a safe and effective tool for preventing severe illness and death from COVID-19.29 , 30 There are 4 types of vaccines currently approved by the World Health Organization Emergency Use Listing process for the treatment of COVID-19: nucleic acid (BNT162b2/Pfizer, mRNA 1273/Moderna), nonreplicating viral vector (ChAdOx1 nCoV019/AstraZeneca/Covishield, Ad26.COV2.S/Janssen), inactivated whole virus (BBIBP-CorV/Sinopharm, CoronaVac/Sinovac, BBV152/Covaxin), and protein subunit (NVX-CoV2373/Novavax).31 Pfizer, Moderna, and Janssen remain to be the only vaccines with either emergency use or full authorization by the FDA in the United States.32
Pfizer and Moderna vaccines carry mRNA, encapsulated in a lipid nanoparticle that encodes a modified version of the SARS-CoV-2 S-protein.33 This modified mRNA has intentional mutations so translated, the S-protein is in its prefusion conformation, critical for the preservation of neutralization-sensitive epitopes and therefore effective vaccine-induced immunity. In contrast, Janssen’s vaccine delivers DNA that codes for the SARS-CoV-2 S-protein on a recombinant adenovirus vector. The DNA is transcribed, producing S-protein, stimulating strong humoral and cellular immune responses.34 , 35 Study of the mRNA vaccine platforms to demonstrate successful production of neutralizing antibodies, active SARS-CoV-2 specific CD4+ and CD8+ T-cells, and robust levels of cytokines such as IFNγ.36 A study of the Pfizer vaccine demonstrated a strong SARS-CoV-2 spike-specific memory B-cell response, peaking 1 week after the second dose, which gradually declined over time but maintained strong ACE2/RBD binding inhibiting activity for at least 6 months.37 Such circulating vaccine-induced spike-specific memory B-cells were restimulated in vitro and demonstrated ability to differentiate into anti-spike IgG or IgM-secreting cells.
The Pfizer, Moderna, and Janssen vaccines have each shown to be safe and effective at reducing severe COVID-19 illness by 88%, 93%, and 71%, respectively, following 2 doses of the mRNA or one dose of the adenovirus vector vaccine.29 The Washington State Department of Health has been compiling and releasing data on COVID-19 cases, hospitalizations, and deaths by vaccination status since February 2021. They report that, among 12 to 34 year olds, unvaccinated individuals were 5-times more likely to be hospitalized with COVID-19 compared with their fully vaccinated age-matched counterparts. For those over the age of 65, the risk of COVID-19 hospitalization is 7-fold higher among the unvaccinated, with a risk of death 13-times greater than fully vaccinated individuals in the same age group.38 Comparably, significant adverse events associated with vaccine were exceedingly rare (between 0.2% and 0.3%).39 Patients do report symptoms following vaccination, most frequently injection site pain and fatigue or malaise; however, these are overwhelmingly mild and short lasting, with greater than 80% resolving within 2 days.40
Dual antigen exposure from both vaccine and infection may also augment the host immune response. Early work comparing serial IgG levels between previously infected and COVID-19 naïve individuals found that IgG levels were similar after 1 mRNA vaccine dose in those with a history of COVID-19 to those following 2 doses in participants without prior infection.41 Expanding on this work, researchers found that while COVID-19 naïve individuals developed lower overall IgG levels, the 2 groups had comparable neutralizing antibodies and S-specific B-cell levels 8 months after vaccination. Interestingly, the COVID-19 naïve subjects also exhibited higher SARS-CoV-2-specific cytokine production, CD4+ T-cell activation, and proliferation than those who recovered from COVID-19.42 While the exact mechanism behind the elevated T-cell response following vaccination in COVID-19 individuals remains uncertain, this finding is reassuring, particularly in the setting of the previously noted association of a robust cellular response to infection and the development of only mild symptoms.
As the pandemic has stretched over multiple years, appropriate concern has been directed to the potential of waning immunity following initial vaccination. Marot and colleagues found that neutralizing antibodies against SARS-CoV-2 declined 38.5% among health care workers as soon as 2 months after primary COVID-19 infection and 3 months after the second mRNA vaccine dose.43 Such declines varied by age and sex, with those over age 65 experiencing a larger decrease in antibody levels following vaccination compared with those between the ages of 18 to 44. Further, vaccine-mediated antibodies among men in this older cohort were found to decrease 46% more than in similarly aged women.44 Such declines in antibody levels have led the Centers for Disease Control and Prevention, to recommend vaccine boosters and continued use of masks in high-risk areas to limit viral transmission.
There has also been concern that mutations in the S-protein, such as those appreciated in the Omicron variant, may allow the virus to evade neutralization by vaccine-elicited antibodies. To assess this possibility, researchers evaluated the neutralizing capacity of sera collected from individuals recently (<3 months) and remotely (6–12 months) vaccinated with any of the 3 major vaccines available in the United States against wild-type, Delta, and Omicron SARS-CoV-2 variants.45 They further tested the sera of subjects recently boosted with either mRNA vaccine. While the neutralization of the Omicron variant was not appreciated among those who received only the initial vaccine regimen, neutralization was appreciated using sera from those who received a booster dose. Taken together, and particularly in the setting of recurrent COVID-19 surges, boosters remain a recommended mechanism to increase protection from severe COVID-19 illness.45
Clinical outcomes of an imbalanced immune response
While the immune response to COVID-19 facilitates the viral clearance and imparts at least some protection against future infections, an excessively robust response has been implicated as a potential driver of severe disease. Specifically, high levels of cytokines including increases in circulating T-cell chemoattractants, natural killer (NK) cells, macrophages, and classic neutrophils, with an absence of IFN types I and III are found in individuals suffering from severe COVID-19.46 Such response, paired with elevated IL-6 and IL1RA cytokines suggest a link between COVID-19 and cytokine release syndrome, characterized by vigorous activation of vast numbers of white blood cells resulting in exuberant release of proinflammatory cytokines that in turn activate more white blood cells in a cascading fashion.23 , 46 , 47 High concentrations of IL-6, a proinflammatory cytokine, have also been correlated with lymphopenia, increased severity of disease and mortality.23 , 48 Together, local and systemic inflammation, particularly when paired with risk for secondary bacterial infections, increase the risk of progression of disease to ARDS.49 A review of 72 publications reported that 30% of patients admitted to the ICU with COVID-19 went on to develop lung edema, dyspnea, hypoxemia, or ARDS and 65% of those who developed ARDS died.49 , 50 Infection is one of the main inducers of lung edema in ARDS, traditionally known to be caused by neutrophil activation in other illnesses such as influenza. However, given the high rate of ARDS noted, it is uncertain whether SARS-CoV-2 has a unique pathophysiologic immune response that may further predispose to lung injury.49
Among the more feared complications of COVID-19 infection are the development of myocarditis and pericarditis. Importantly, while cardiac involvement from COVID-19 infection does occur, clinically significant myocarditis remains a rare complication, with cases typically mild and self-limited.51 , 52 In a study of 100 COVID-19 recovered individuals 78% demonstrated myocardial inflammation on cardiac MRI, directly associated with severity of illness, the presence of cardiac symptoms, and time from infection to imaging.53 Fortunately, most cases are asymptomatic, with an analysis of nearly 1600 COVID-19 positive college athletes finding only 5 who developed cardiac symptoms, although cardiac MRI identified more than 7-times greater number of individuals with evidence of cardiac involvement. Fortunately, all individuals with abnormal cardiac MRIs saw some degree of improvement on serial imaging, again indicating a largely self-limited disease course.54 In comparison, vaccine-associated myocarditis remains rare. Data from Israel found only 54 cases out of 2.5 million vaccinated individuals, with data from the European Medicines Agency identifying even lower rates of 1.6 per million Pfizer vaccines and 3.0 per million doses of Moderna.55 , 56 Cases were predominantly among young or adolescent males, with 76% described as mild. In fact, only 14 individuals in the Israeli data were found to have reduced left ventricular systolic function, with most having recovery of function at follow-up. As opposed to myocarditis, pericarditis has been observed more commonly among older patients and at a later time point with a median of 20 days after the most recent vaccine dose.51
Multiple potential immunologic mechanisms have been proposed as drivers of infection and vaccine-associated myocarditis. Specifically following infection, the two-leading hypothesis for the mechanism of myocarditis include direct viral infection of cardiac myocytes and a maladaptive immune response, as described above. Interestingly, histologic analysis of biopsy tissue from patients with SARS-CoV-2 infected myocarditis did not display the classic lymphocytic pattern associated with viral myocarditis. This has led many to believe that overactivation of the immune response, particularly in the setting of severe COVID-19, likely contributes to cardiac dysfunction through thromboinflammation and endothelial dysfunction.57 Work has also specifically implicated CD68+ T-cells found in high numbers in the myocardium of COVID-19 hearts, in the development of myocarditis following infection.58 Vaccine-associated myocarditis is thought to result from parallel, though importantly different mechanisms. The previously noted nucleoside modifications to the mRNA vaccines are designed in part to reduce the innate immune system’s response to foreign RNA molecules. At least some vaccine-associated myocarditis cases may represent a breakthrough of this innate immune response, a mechanism associated with certain genetic predispositions.59 Another proposed mechanism evokes molecular mimicry of the S-protein transcribed from the delivered mRNA resulting in cross-reactivity with alpha-myosin.60 Eosinophilic hypersensitivity has further been implicated as a mechanism particularly given the predilection for second or third dose and been described with the smallpox vaccine, as well.61 Finally, sex hormones may play a role in the noted male predominance of COVID-19 and vaccine-associated myocarditis. Specifically, estrogen may work to mitigate proinflammatory T-cells, while testosterone may block the function of certain anti-inflammatory cells, allowing for excess immune activation and subsequent myocardial damage.57
Summary
The immune response to the novel SARS-CoV-2 virus is pivotal in both regaining health and potentially attenuating the development of severe disease. Early robust humoral response coupled with a delayed cellular immune response is associated with severe disease. Further, dysregulated immune response, including cytokine release syndrome, can lead to worse outcomes, particularly via the development of ARDS. Finally, while rare, infection and vaccine-associated myocarditis can occur, though typically in mild and self-limited forms. Importantly, our understanding of the immune mechanisms underlying health and disease in COVID-19 have been essential to the rapid development of safe and effective vaccines, which remain among our best tools for combating this pandemic.
Clinics care points
-
•
Humoral and cellular immune responses are induced by both natural and vaccine mediated COVID-19 antigenic exposure.
-
•
The balance of early cellular and humoral immune response is associated with the severity of COVID-19 illness.
-
•
Clinically significant COVID-19 vaccine mediated myocarditis is rare.
Acknowledgments
Disclosure
All others report no conflicts of interest. J.E. Ebinger is supported by grant funding from the NIH/NHLBI (K23-HL153888). B. Weber is supported by grant funding from NHLBI K23 HL15927601, AHA 21CDA851511, and IANC/ASNC 2021 Award.
Footnotes
Funded by: NIH/NHLBI Grant number(s): K23HL 153888-02.
References
- 1.Organization W.H. WHO coronavirus (COVID-19) Dashboard. https://covid19.who.int Available at: Accessed January 19, 2022.
- 2.Huang Y., Yang C., Xu X-f, et al. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol Sin. 2020;41(9):1141–1149. doi: 10.1038/s41401-020-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorbalenya A.E., Baker S.C., Baric R.S., et al. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5(4):536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boechat J.L., Chora I., Morais A., et al. The immune response to SARS-CoV-2 and COVID-19 immunopathology - Current perspectives. Pulmonology. 2021;27(5):423–437. doi: 10.1016/j.pulmoe.2021.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hasan M.N., Haider N., Stigler F.L., et al. The global case-fatality rate of COVID-19 has been declining since may 2020. Am J Trop Med Hyg. 2021;104(6):2176–2184. doi: 10.4269/ajtmh.20-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Q., Zhang Y., Wu L., et al. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020;181(4):894–904.e899. doi: 10.1016/j.cell.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shang J., Ye G., Shi K., et al. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581(7807):221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thoms M., Buschauer R., Ameismeier M., et al. Structural basis for translational shutdown and immune evasion by the Nsp1 protein of SARS-CoV-2. Science. 2020;369(6508):1249–1255. doi: 10.1126/science.abc8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mallinas S.R., Maner J.K., Ashby Plant E. What factors underlie attitudes regarding protective mask use during the COVID-19 pandemic? Pers Individ Dif. 2021;181:111038. doi: 10.1016/j.paid.2021.111038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paul E., Steptoe A., Fancourt D. Attitudes towards vaccines and intention to vaccinate against COVID-19: Implications for public health communications. Lancet Reg Health - Europe. 2021;1:100012. doi: 10.1016/j.lanepe.2020.100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou L., Ayeh S.K., Chidambaram V., et al. Modes of transmission of SARS-CoV-2 and evidence for preventive behavioral interventions. BMC Infect Dis. 2021;21(1):496. doi: 10.1186/s12879-021-06222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sungnak W., Huang N., Bécavin C., et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26(5):681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alfi O., Yakirevitch A., Wald O., et al. Human nasal and lung tissues infected ex vivo with SARS-CoV-2 Provide Insights into differential tissue-specific and virus-specific innate immune responses in the upper and lower respiratory tract. J Virol. 2021;95(14):e0013021. doi: 10.1128/JVI.00130-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacMicking J.D. Interferon-inducible effector mechanisms in cell-autonomous immunity. Nat Rev Immunol. 2012;12(5):367–382. doi: 10.1038/nri3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narayanan K., Huang C., Lokugamage K., et al. Severe acute respiratory syndrome coronavirus nsp1 suppresses host gene expression, including that of type I interferon, in infected cells. J Virol. 2008;82(9):4471–4479. doi: 10.1128/JVI.02472-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vanderheiden A., Ralfs P., Chirkova T., et al. Type I and type III interferons Restrict SARS-CoV-2 infection of human airway epithelial cultures. J Virol. 2020;94(19) doi: 10.1128/JVI.00985-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carsetti R., Zaffina S., Piano Mortari E., et al. Different innate and adaptive immune responses to SARS-CoV-2 infection of asymptomatic, mild, and severe cases. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.610300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Almendro-Vázquez P., Laguna-Goya R., Ruiz-Ruigomez M., et al. Longitudinal dynamics of SARS-CoV-2-specific cellular and humoral immunity after natural infection or BNT162b2 vaccination. Plos Pathog. 2021;17(12):e1010211. doi: 10.1371/journal.ppat.1010211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Padoan A., Sciacovelli L., Basso D., et al. IgA-Ab response to spike glycoprotein of SARS-CoV-2 in patients with COVID-19: a longitudinal study. Clin Chim Acta. 2020;507:164–166. doi: 10.1016/j.cca.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X., Wang J., Xu X., et al. Patterns of IgG and IgM antibody response in COVID-19 patients. Emerg Microbes Infect. 2020;9(1):1269–1274. doi: 10.1080/22221751.2020.1773324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaebler C., Wang Z., Lorenzi J.C.C., et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021;591(7851):639–644. doi: 10.1038/s41586-021-03207-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sterlin D., Mathian A., Miyara M., et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci Transl Med. 2021;13(577) doi: 10.1126/scitranslmed.abd2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shrotri M., van Schalkwyk M.C.I., Post N., et al. T cell response to SARS-CoV-2 infection in humans: a systematic review. PLoS One. 2021;16(1):e0245532. doi: 10.1371/journal.pone.0245532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo M., Liu J., Jiang W., et al. IL-6 and CD8+ T cell counts combined are an early predictor of in-hospital mortality of patients with COVID-19. JCI Insight. 2020;5(13) doi: 10.1172/jci.insight.139024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Q., Fang X., Tokuno S., et al. Prediction of the clinical outcome of COVID-19 patients using T lymphocyte subsets with 340 cases from Wuhan, China: a retrospective cohort study and a web visualization tool. medRxiv. 2020 [Google Scholar]
- 26.Xu Z., Shi L., Wang Y., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muir R., Osbourn M., Dubois A.V., et al. Innate lymphoid cells are the predominant Source of IL-17A during the early Pathogenesis of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2016;193(4):407–416. doi: 10.1164/rccm.201410-1782OC. [DOI] [PubMed] [Google Scholar]
- 28.Gao L., Zhou J., Yang S., et al. The dichotomous and incomplete adaptive immunity in COVID-19 patients with different disease severity. Signal Transduction Targeted Ther. 2021;6(1):113. doi: 10.1038/s41392-021-00525-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Self WH, Tenforde MW, Rhoads JP, et al. Comparative Effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) Vaccines in Preventing COVID-19 Hospitalizations Among Adults Without Immunocompromising Conditions — United States, March—August 2021. MMWR Morb Mortal Wkly Rep 2021;70:1337–1343. [DOI] [PMC free article] [PubMed]
- 30.Baden L.R., El Sahly H.M., Essink B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Organization WH COVID-19 vaccine Tracker. https://covid19.trackvaccines.org/agency/who/ Available at: Accessed January 30, 2022.
- 32.Administration UFaD COVID-19 vaccines. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines Available at: Accessed January 30, 2022.
- 33.Bettini E., Locci M. SARS-CoV-2 mRNA vaccines: Immunological mechanism and beyond. Vaccines (Basel) 2021;9(2) doi: 10.3390/vaccines9020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Custers J., Kim D., Leyssen M., et al. Vaccines based on replication incompetent Ad26 viral vectors: Standardized template with key considerations for a risk/benefit assessment. Vaccine. 2021;39(22):3081–3101. doi: 10.1016/j.vaccine.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barouch D.H., Stephenson K.E., Sadoff J., et al. Durable humoral and cellular immune responses 8 Months after Ad26.COV2.S vaccination. New Engl J Med. 2021;385(10):951–953. doi: 10.1056/NEJMc2108829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sahin U., Muik A., Derhovanessian E., et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586(7830):594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 37.Ciabattini A., Pastore G., Fiorino F., et al. Evidence of SARS-CoV-2-specific memory B cells six Months after vaccination with the BNT162b2 mRNA vaccine. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.740708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Washington State Department of Health Public Health Outbreak Coordination I, and Surveillance; Disease Control and Health Statistics. COVID-19 cases, hospitalizations, and deaths by vaccination status. January 26, 2022.
- 39.Beatty A.L., Peyser N.D., Butcher X.E., et al. Analysis of COVID-19 vaccine type and adverse effects following vaccination. JAMA Netw Open. 2021;4(12):e2140364. doi: 10.1001/jamanetworkopen.2021.40364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ebinger J.E., Lan R., Sun N., et al. Symptomology following mRNA vaccination against SARS-CoV-2. Prev Med. 2021;153:106860. doi: 10.1016/j.ypmed.2021.106860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ebinger J.E., Fert-Bober J., Printsev I., et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat Med. 2021;27(6):981–984. doi: 10.1038/s41591-021-01325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lozano-Rodríguez R., Valentín-Quiroga J., Avendaño-Ortiz J., et al. Cellular and humoral functional responses after BNT162b2 mRNA vaccination differ longitudinally between naive and subjects recovered from COVID-19. Cell Rep. 2022;38(2):110235. doi: 10.1016/j.celrep.2021.110235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marot S., Malet I., Leducq V., et al. Rapid decline of neutralizing antibodies against SARS-CoV-2 among infected healthcare workers. Nat Commun. 2021;12(1):844. doi: 10.1038/s41467-021-21111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levin E.G., Lustig Y., Cohen C., et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 Months. N Engl J Med. 2021;385(24):e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garcia-Beltran WF, St Denis KJ, Hoelzemer A, et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell 2022;185(3):457–466.e4. [DOI] [PMC free article] [PubMed]
- 46.Blanco-Melo D., Nilsson-Payant B.E., Liu W.C., et al. Imbalanced host response to SARS-CoV-2 Drives development of COVID-19. Cell. 2020;181(5):1036–1045.e1039. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woodruff M.C., Ramonell R.P., Nguyen D.C., et al. Extrafollicular B cell responses correlate with neutralizing antibodies and morbidity in COVID-19. Nat Immunol. 2020;21(12):1506–1516. doi: 10.1038/s41590-020-00814-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hadjadj J., Yatim N., Barnabei L., et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369(6504):718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li L., Huang Q., Wang D.C., et al. Acute lung injury in patients with COVID-19 infection. Clin Transl Med. 2020;10(1):20–27. doi: 10.1002/ctm2.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boehmer T.K., Kompaniyets L., Lavery A.M., et al. Association between COVID-19 and myocarditis using hospital-based Administrative data - United States, March 2020-January 2021. MMWR Morb Mortal Wkly Rep. 2021;70(35):1228–1232. doi: 10.15585/mmwr.mm7035e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Diaz G.A., Parsons G.T., Gering S.K., et al. Myocarditis and pericarditis after vaccination for COVID-19. JAMA. 2021;326(12):1210–1212. doi: 10.1001/jama.2021.13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mevorach D, Anis E, Cedar N, et al. Myocarditis after BNT162b2 Vaccination in Israeli Adolescents. New England Journal of Medicine 2020;386(10):998–999. [DOI] [PMC free article] [PubMed]
- 53.Puntmann V.O., Carerj M.L., Wieters I., et al. Outcomes of Cardiovascular Magnetic Resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(11):1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Daniels C.J., Rajpal S., Greenshields J.T., et al. Prevalence of clinical and Subclinical myocarditis in Competitive athletes with recent SARS-CoV-2 infection: Results from the Big ten COVID-19 cardiac Registry. JAMA Cardiol. 2021;6(9):1078–1087. doi: 10.1001/jamacardio.2021.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Witberg G., Barda N., Hoss S., et al. Myocarditis after Covid-19 vaccination in a Large health Care Organization. New Engl J Med. 2021;385(23):2132–2139. doi: 10.1056/NEJMoa2110737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lazaros G., Klein A.L., Hatziantoniou S., et al. The novel platform of mRNA COVID-19 vaccines and myocarditis: Clues into the potential underlying mechanism. Vaccine. 2021;39(35):4925–4927. doi: 10.1016/j.vaccine.2021.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bozkurt B., Kamat I., Hotez P.J. Myocarditis with COVID-19 mRNA vaccines. Circulation. 2021;144(6):471–484. doi: 10.1161/CIRCULATIONAHA.121.056135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fox S.E., Falgout L., Vander Heide R.S. COVID-19 myocarditis: quantitative analysis of the inflammatory infiltrate and a proposed mechanism. Cardiovasc Pathol. 2021;54:107361. doi: 10.1016/j.carpath.2021.107361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Caso F., Costa L., Ruscitti P., et al. Could Sars-coronavirus-2 trigger autoimmune and/or autoinflammatory mechanisms in genetically predisposed subjects? Autoimmun Rev. 2020;19(5):102524. doi: 10.1016/j.autrev.2020.102524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vojdani A., Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2020;217:108480. doi: 10.1016/j.clim.2020.108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Murphy J.G., Wright R.S., Bruce G.K., et al. Eosinophilic-lymphocytic myocarditis after smallpox vaccination. Lancet. 2003;362(9393):1378–1380. doi: 10.1016/S0140-6736(03)14635-1. [DOI] [PubMed] [Google Scholar]