Abstract
Background and Purpose:
Continued smoking after stroke is associated with a high risk of stroke recurrence and other cardiovascular disease. We sought to comprehensively understand the epidemiology of smoking cessation in stroke survivors in the United States. Further, we compared smoking cessation in stroke and cancer survivors because cancer is another smoking-related condition in which smoking cessation is prioritized.
Methods:
We performed a cross-sectional analysis of data from the Centers for Disease Control and Prevention Behavioral Risk Factor Surveillance System, an annual, nationally representative health survey. Using pooled data from 2013-2019, we identified stroke and cancer survivors with a history of smoking. We used survey procedures to estimate frequencies and summarize quit ratios with attention to demographic and geographic (state-wise and rural-urban) factors for stroke survivors. The quit ratio is conventionally defined as the proportion of ever-smokers who have quit. Then, we used multivariable logistic regression to compare quit ratios in stroke and cancer survivors, while adjusting for demographics and smoking-related comorbidities.
Results:
Among 4,434,604 Americans with a history of stroke and smoking, the median age was 68 years (IQR, 59-76), and 45.4% were women. The overall quit ratio was 60.8% (95% CI, 60.1-61.6%). Quit ratios varied by age group, gender, race/ethnicity, and several geographic factors. There was marked geographic variation in quit ratios, ranging from 48.3% in Kentucky to 71.5% in California. Further, compared to cancer survivors, stroke survivors were less likely to have quit smoking (OR, 0.72; 95% CI, 0.67-0.79) after accounting for differences in demographics and smoking-related comorbidities.
Conclusion:
There were considerable demographic and geographic disparities in smoking quit ratios in stroke survivors, who were less likely to have quit smoking than cancer survivors. A targeted initiative is needed to improve smoking cessation for stroke survivors.
Keywords: epidemiology, smoking, secondary prevention, cerebrovascular disease/stroke, risk factors
One in four strokes in the United States is a recurrent stroke, and approximately 13% of patients with minor stroke/TIA face recurrent stroke, heart attack, or cardiovascular death within 5 years.1, 2 Recurrent strokes are generally more disabling than initial strokes.3 These considerations highlight the need to improve secondary prevention by targeting stroke risk factors. Tobacco smoking is a behavioral risk factor for stroke, and there is amassing evidence that people who quit smoking after a stroke/TIA have a lower risk of recurrent stroke and cardiovascular disease.4, 5 The importance of smoking cessation after stroke is widely acknowledged.6 The importance of smoking cessation is also understood for patients with cancer, another major smoking-related illness.7 In contrast to stroke survivors, cancer survivors receive targeted interventions through a dedicated, nationwide initiative to promote smoking cessation.8 There are few population-based data regarding smoking cessation after stroke in the United States to inform similar public health interventions. Therefore, our first objective was to comprehensively describe the epidemiology of smoking cessation in stroke survivors in the United States. Second, given the substantial investment in smoking cessation for patients with cancer,8 we hypothesized that smoking-cessation rates are higher in cancer survivors than stroke survivors.
Materials and Methods
Design
We performed a retrospective cross-sectional analysis of prospectively-collected data from the Centers for Disease Control and Prevention’s Behavioral Risk Factor Surveillance System (BRFSS).9 We used data from 2013-2019. The BRFSS is an annual survey that collects information regarding chronic health conditions and health-related behaviors. The Centers for Disease Control and Prevention, in partnership with individual state governments, administers the standardized health survey by landline and cellular telephone while taking measures10 to mitigate bias. The BRFSS system randomly selects telephone numbers for dialing, and a scripted Computer-Assisted Telephone Interview system is used for data collection by trained interviewers. Calls are made 7 days per week during both daytime and evening hours to avoid selection bias. The response rate in the most recent year (2019) was 50%, and sample weights provided by the BRFSS account for nonresponse rates while ensuring that resulting estimates are representative for the United States population.11 Weights account for demographics and telephone ownership, in addition to nonresponse. To reduce misclassification error, quality assurance protocols include direct monitoring of live interviews and calling back respondents to verify responses. We adhered to Strengthening the Reporting of Observational Studies in Epidemiology guidelines for cross-sectional analyses.12 The data that support the findings of this study are publicly available at https://www.cdc.gov/brfss/annual_data/annual_data.htm. Analytic methods will be made available upon reasonable request from the corresponding author. The Weill Cornell Medicine institutional review board certified these analyses of deidentified data as exempt from review.
Population
The BRFSS survey included adult respondents 18 years of age and older. For our analyses, we excluded respondents who were pregnant (3.9%) and those who did not provide information about stroke (0.26%), cancer (0.23%), and smoking history (5.0%) (Figure I; please see https://www.ahajournals.org/journal/str); we performed complete case analysis using data for 3,029,122 respondents (representative of a weighted frequency13 of 236,118,585 adults). Then, we identified stroke and cancer survivors based on self-report of prior stroke and cancer, respectively. Validation studies provide evidence of the validity and reliability of self-reported data gathered in BRFSS.14 Prevalence estimates derived from BRFSS are reliable; prevalence estimates for common conditions like diabetes, heart disease, and stroke are very similar to data derived from in-person examinations, with absolute differences between datasets ranging from 0.0% to 0.8%.15 Prior stroke is a validated measure that has been used to identify stroke survivors in large epidemiological studies.16-18 In a United States cohort, self-reported stroke had substantial agreement with detailed medical record data (kappa: 0.71).16 Similarly, self-reported cancer had >90% sensitivity and specificity for true diagnosis of cancer confirmed in a cancer registry.19
Measurements
Smoking status was self-reported in BRFSS. A prior validation study of United States nationally representative health survey data found that self-reported smoking correlated well with serum cotinine levels (biomarker for smoking), with low misclassification rates (2-5%).20 An analysis of another North American health survey found that self-report only marginally underestimated true smoking prevalence.21
In the present analysis, the outcome of interest was smoking cessation. This was assessed using the quit ratio, which is conventionally defined as the proportion of “ever smokers” who have quit smoking.22 In this definition, “ever smoker” is defined as having smoked at least 100 cigarettes in a person’s lifetime.23 The quit ratio measure and this definition of “ever smoker” are commonly used to assess smoking cessation and persistent smoking at the population level.23-25 The inverse of the quit ratio is the proportion of ever smokers who continue to smoke; we report these data as well. Because the quit ratio is calculated only for ever-smokers, we compare quit ratios across groups who may have varying ever-smoking rates.
We tabulated quit ratios stratified across several demographic and geographic factors. These demographic variables included age (<60 years versus ≥60 years), gender, race/ethnicity, and insurance status (insured versus uninsured). Race/ethnicity was self-reported by respondents and categorized based on BRFSS indicators as Non-Hispanic White, Non-Hispanic Black, Hispanic, Alaskan Native or American Indian, Asian or Hawaiian or other Pacific Islander, and multiracial or other. Geographic factors were state, Stroke Belt residence, and rurality. Stroke Belt states were those defined by the Reasons for Geographic and Racial Differences in Stroke study.26 We evaluated Stroke Belt states as a category because of historically worse stroke outcomes in these states.26 Rurality was determined by applying United States Office of Management and Budget definitions27 to Metropolitan Status Code data recorded in BRFSS: respondents residing in a metropolitan statistical area (urban area and adjacent integrated areas) were categorized as non-rural, and respondents outside a metropolitan statistical area were categorized as rural.
We tabulated additional demographics and comorbidities to characterize the study population. Income was categorized by BRFSS as <$15,000/year, $15,000-24,999/year, $25,000-34,999/year, $35,000-49,999/year, and >$50,000/year. Educational attainment was categorized as less than high school completion, high school completion, some college or technical school, and college or technical school completion. Comorbidities included self-reported hypertension, hyperlipidemia, and diabetes, in addition to two smoking-related conditions: pulmonary disease (asthma and chronic obstructive pulmonary disease) and cardiovascular disease (history of myocardial infarction, angina, or coronary heart disease).
Statistical Analyses
We merged data from the 2013-2019 BRFSS annual surveys into a pooled dataset. BRFSS is a population-based survey that uses nonrandom sampling design to ensure representation of population subgroups and assign sample weights to respondents to account for nonresponse and stratification. We used survey-specific SAS functions to account for survey design, survey strata, and sampling weights when calculating population-weighted frequencies and proportions. We used the Rao-Scott chi-squared test, a chi-squared test for survey data, to assess differences in quit ratios among strata (e.g., age <60 years versus ≥60 years). Then, we used multivariable logistic regression models to compare the odds of having quit smoking in stroke versus cancer survivors. Models were incrementally adjusted for demographics (age, gender, race/ethnicity, income level, educational attainment, health insurance, and rurality) and then smoking-related health conditions (pulmonary and cardiovascular disease). In a sensitivity analysis, we additionally adjusted for hypertension, hyperlipidemia, and diabetes; this analysis was restricted to 2013, 2015, 2017, and 2019 as full comorbidity data were collected by BRFSS only in these years. In a post hoc sensitivity analysis, we further adjusted this sensitivity analysis model for Stroke Belt residence. Last, a formal Wald test of interaction was performed to assess if the relationship of smoking cessation by disease type (stroke versus cancer) changed over time, and trends in quit ratios were separately evaluated for each condition while adjusting for demographics and smoking-related health conditions.28 Statistical analyses were performed using SAS software, version 9.4 (SAS Institute Inc., Cary, NC). State-wise quit ratios were visualized using R 4.0.5 (R Core Team, Vienna, Austria). The threshold of statistical significance was set at α = 0.05.
Results
Among 7,538,044 stroke survivors in the United States, 58.8% (95% confidence interval [CI], 58.2-59.1%) had any history of smoking cigarettes. The median age was 68 years (interquartile range [IQR], 55-76), 45.4% were women, and 69.9% identified as Non-Hispanic White, 15.0% as Non-Hispanic Black, 8.9% as Hispanic, 1.4% as Asian or Hawaiian or other Pacific Islander, 2.2% as Alaskan native or American Indian, and 2.5% as multiracial or other. Approximately 19.1% were Stroke Belt residents, and 22.9% lived in rural locations. In addition, we identified 16,030,219 cancer survivors, among whom 52.9% had any history of smoking cigarettes. Stroke survivors were more often men and had more cardiovascular risk factors and disease than cancer survivors (Table 1).
Table 1.
Study Population Characteristics* of Stroke and Cancer Survivors in the United States with a History of Cigarette Smoking†
| Stroke Survivors | Cancer Survivors | |
|---|---|---|
| Respondents, raw unweighted frequency | 74,400 | 155,693 |
| Respondents, US weighted frequency | 4,434,604 | 8,488,386 |
| Age, years (median, interquartile range) | 68 (59-76) | 69 (60-76) |
| Women | 45 (45-56) | 56 (55-56) |
| Race/ethnicity§ | ||
| Non-Hispanic White | 70 (70-71) | 81 (80-81) |
| Non-Hispanic Black | 15 (14-16) | 9 (8-9) |
| Hispanic | 9 (8-10) | 6 (6-6) |
| Asian or Hawaiian or other Pacific Islander | 1 (1-2) | 1 (1-1) |
| Alaskan Native or American Indian | 2 (2-2) | 1 1-1) |
| Multiracial or other | 3 (2-3) | 2 (2-2) |
| Education | ||
| Did not finish high school | 26 (25-27) | 16 (16-17) |
| Graduated high school | 32 (32-33) | 31 (30-31) |
| Some college/technical school | 29 (29-30) | 33 (33-34) |
| Completed college/technical school | 12 (12-12) | 20 (20-21) |
| Annual household income category | ||
| <$15,000 | 25 (24-26) | 14 (14-15) |
| $15,000-24,999 | 28 (27-29) | 20 (20-21) |
| $25,000-34,999 | 13 (12-13) | 12 (12-13) |
| $35,000-49,999 | 12 (12-13) | 15 (14-15) |
| ≥$50,000 | 22 (21-22) | 38 (37-38) |
| Rural residence∥ | 23 (22-24) | 20 (19-20) |
| Stroke Belt Residence∥ | 19 (19-20) | 16 (15-16) |
| Lacking health insurance# | 8 (8-9) | 6 (6-6) |
| Hypertension** | 73 (72-74) | 56 (55-56) |
| Hyperlipidemia** | 64 (63-65) | 53 (52-54) |
| Diabetes | 32 (31-32) | 21 (20-21) |
| Heart disease | 39 (38-40) | 19 (18-19) |
| Pulmonary disease | 41 (40-41) | 33 (32-33) |
Data are presented as percentage (95% confidence interval of percentage) unless otherwise specified. Percentages for any given characteristic may not sum to 100% because figures reflect weighted estimates.
A history of cigarette smoking is defined as self-report of having smoked at least 100 cigarettes.
Race/ethnicity is self-reported in the Behavioral Risk Factor Surveillance System survey.
Respondents were assigned Metropolitan Status Codes based on their landline data; respondents residing in a metropolitan statistical area (urban area and adjacent integrated areas) were categorized as non-rural, and respondents not in a metropolitan statistical area were categorized as rural. Stroke Belt residence was conventionally defined.
Patients without public or private health insurance were categorized as uninsured.
In BRFSS, respondents were asked of hypertension or hyperlipidemia in 2013, 2015, 2017, 2019; reported percentages are for years in which those data were collected.
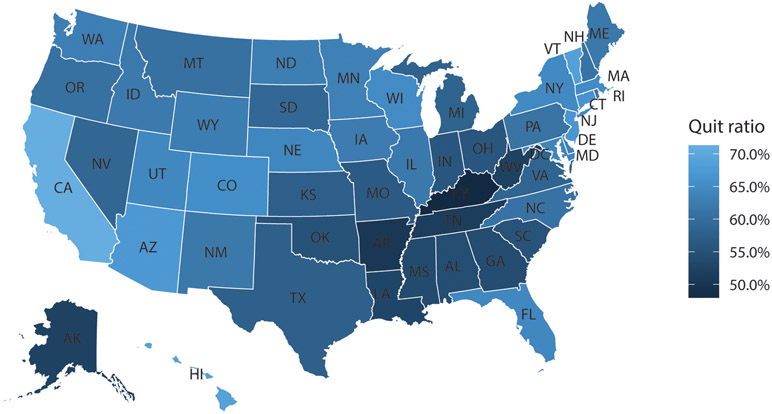
The quit ratio in the overall population of people with a smoking history was 59.5% (95% CI, 59.3-59.7%). The quit ratio for stroke survivors was 60.8% (95% CI, 60.1-61.6%); 39.2% of stroke survivors continue to be active smokers. Quit ratios varied across demographic and geographic factors (Table 2). Stroke survivors <60 years old were less likely to have quit than those ≥60 years old (43.3% vs 74.6%; P<0.0001). Men were more likely to have quit smoking than women (63.4% vs 57.8%; P<0.0001), and variation by race/ethnicity was observed (P<0.0001), with higher quit ratios among Non-Hispanic White, Hispanic, and Asian or Hawaiian or other Pacific Islander stroke survivors than Non-Hispanic Black stroke survivors. Considerable geographic variability was also noted. First, the quit ratio varied between states, ranging from 48.3% in Kentucky to 71.5% in California (Figure; Table I - please see https://www.ahajournals.org/journal/str). Second, the quit ratio was lower in the Stroke Belt than in other states (55.7% vs 62.0%, P<0.0001). Last, the quit ratio was also lower for rural than non-rural stroke survivors (62.7% vs 69.5%, P<0.0001).
Table 2.
Demographic/Geographic Factors and Cigarette Smoking Quit Ratios among Stroke Survivors with a History of Cigarette Smoking
| Factor | Quit Ratio* (95% CI) | P value |
|---|---|---|
| Age | <0.0001 | |
| <60 years old | 43.3 (42.0-44.6) | |
| ≥60 years old | 74.6 (73.8-75.4) | |
| Gender | <0.0001 | |
| Women | 57.8 (56.7-58.8) | |
| Men | 63.4 (62.3-64.4) | |
| Race/ethnicity† | <0.0001 | |
| Non-Hispanic White | 63.4 (62.6-62.4) | |
| Non-Hispanic Black | 51.8 (49.5-54.0) | |
| Hispanic | 61.0 (57.3-64.7) | |
| Asian or Hawaiian or other Pacific Islander | 72.5 (62.4-80.9) | |
| Alaskan Native or American Indian | 45.7 (41.4-50.0) | |
| Multiracial or other | 50.4 (46.1-54.7) | |
| Health insurance§ | <0.0001 | |
| Insured | 62.9 (62.1-63.6) | |
| Uninsured | 38.6 (35.7-41.5) | |
| Stroke Belt∥ | <0.0001 | |
| Stroke Belt residence | 55.7 (54.2-57.1) | |
| Non-Stroke Belt residence | 62.0 (61.2-62.9) | |
| Rurality∥ | <0.0001 | |
| Rural | 62.7 (61.0-64.4) | |
| Non-rural | 69.5 (68.4-70.5) |
Abbreviations: CI, confidence interval.
Quit ratios (proportion of former smokers among ever-smokers) compared across strata using the Rao-Scott chi-squared test of proportions.
Race/ethnicity is self-reported in the Behavioral Risk Factor Surveillance System survey.
Patients without public or private health insurance were categorized as uninsured.
Stroke Belt residence was conventionally defined. Respondents were assigned Metropolitan Status Codes based on their landline data; respondents residing in a metropolitan statistical area (urban area and adjacent integrated areas) were categorized as non-rural, and respondents not in a metropolitan statistical area were categorized as rural.
Figure. Quit ratios in stroke survivors in the United States.
This map shows the variability in cigarette smoking quit ratios among stroke survivors in the United States, based on data from the Behavioral Risk Factor Surveillance System. The quit ratio is defined by convention as the proportion of people with a history of smoking who have quit.
The overall quit ratio in cancer survivors was 71.3% (95% CI, 70.9-71.8%); 28.7% of cancer survivors remain active smokers. Compared to cancer survivors, stroke survivors were less likely to have quit (odds ratio [OR], 0.59; 95% CI, 0.56-0.61) (Table 3). This remained the case after accounting for differences in demographics, rurality, and smoking-related comorbidities (OR, 0.72; 95% CI, 0.67-0.79). Results were consistent in a sensitivity analysis adjusted for additional comorbidities. Trends analyses adjusted for demographics and comorbidities suggested that the gap between stroke and cancer survivors’ quit ratios worsened over time (P=0.006 for interaction by time). The odds of having quit among cancer survivors decreased each year (OR, 0.95; 95% CI, 0.93-0.97) but not among stroke survivors (OR, 1.00; 95% CI, 0.97-1.03) in adjusted models. However, raw quit ratios appeared stable over time for stroke and cancer survivors (Table II; please see https://www.ahajournals.org/journal/str).
Table 3.
Odds* of Having Quit Smoking among Stroke Survivors versus Cancer Survivors in the United States.
| Model | Odds Ratio (95% CI) |
|---|---|
| Unadjusted | 0.59 (0.56-0.61) |
| Adjusted for demographics† | 0.72 (0.67-0.77) |
| Adjusted for demographics, comorbidities‡ | 0.72 (0.67-0.79) |
| Sensitivity analysis§ | 0.68 (0.61-0.75) |
| Post hoc sensitivity analysis¶ | 0.68 (0.61-0.75) |
Abbreviations: CI, confidence interval.
Association reported as odds ratio (95% confidence interval) of having quit smoking in respondents with a history of smoking, comparing stroke versus cancer survivors.
Adjusted for age, gender, race/ethnicity, income level, educational attainment, health insurance, and rurality.
Additionally adjusted for smoking-related comorbidities: pulmonary disease (asthma and chronic obstructive pulmonary disease) and cardiovascular disease (myocardial infarction and coronary heart disease).
Additionally adjusted for hypertension, hyperlipidemia, and diabetes using data from only 2013, 2015, 2017, and 2019, based on data availability in these years.
The post hoc sensitivity analysis represents the most adjusted model, adjusted for demographics, smoking-related comorbidities, geographic factors (Stroke Belt residence in addition to rurality), and hypertension, hyperlipidemia, and diabetes.
Discussion
In this analysis of nationally representative U.S. health survey data, we estimated the smoking quit ratio among stroke survivors to be approximately 61%; approximately two out of five stroke survivors with a history of smoking remain active smokers. The quit ratio varied considerably with respect to demographic and geographic factors, and the quit ratio was lower among stroke survivors than cancer survivors.
Our analysis provides a contemporary benchmark quit ratio estimate based on a large, nationally representative sample of stroke survivors. These self-reported data are consistent with a prior estimate based on biochemical measures of cigarette smoking from the National Health and Nutrition Examination Survey;29 however, the small sample size of stroke survivors (n=658) precluded analyses of demographic and geographic factors. Other prior data focused on active smoking rates as opposed to quit ratios.30, 31 Active smoking prevalence rates are influenced by smoking initiation rates. In contrast, quit ratios are calculated only for persons with a history of smoking and thus reflect smoking cessation and its alternative, persistent smoking. Accordingly, quit ratios are better suited for evaluating smoking cessation in the secondary prevention realm.
Prior studies have investigated factors associated with smoking cessation in stroke survivors. However, apart from neuroanatomical considerations, such as lesion location,32-34 data regarding the impact of sociodemographic factors are conflicting in part because of small sample sizes and use of outdated data from cohorts that predate the introduction of smoking-cessation pharmacotherapies.29, 31, 34-39 Our analysis of a large, recent dataset spanning 7 years revealed that quit ratios were lower among stroke survivors who were under 60 years old, women, uninsured, and those identifying as Non-Hispanic Black. Race/ethnic disparities in quit ratios have also been observed in the general population, with lower quit ratios in Non-Hispanic Black individuals attributed to menthol cigarette use, lower access to cessation treatment, psychosocial stressors, and marketing exposure.23, 25, 40 Additionally, we noted lower quit ratios in rural areas and in the Stroke Belt, consistent with observations of disparities in stroke care and outcomes in these areas.26, 41 These data may inform the development and dissemination of smoking-cessation interventions for stroke survivors.
Smoking cessation in cancer has become a priority for oncologists, as reflected in the National Cancer Institute Cancer Moonshot Program’s establishment of the Cancer Center Cessation Initiative.8 This commitment is consistent with our observation that quit ratios are higher in cancer survivors than stroke survivors. Indeed, smoking cessation has increased in cancer survivors since 200042 whereas the prevalence of active smoking in stroke survivors has not decreased in this time period.30 In our data, the quit ratio among stroke survivors was similar to that of the general population, while cancer survivors had a significantly higher quit rate. Improving smoking cessation in individuals with medical comorbidities requires attention to unique aspects of each condition.43 Efforts akin to the Cancer Center Cessation Initiative may be needed to identify and implement treatment strategies specifically for stroke survivors.
The key strengths of this analysis are our use of a large, nationally representative health survey dataset spanning 7 years, and our use of standard epidemiological measures of smoking. Our results should be interpreted taking into consideration the following limitations. First, these data are cross-sectional. Second, BRFSS reaches people who can participate; our study does not account for institutionalized stroke survivors, institutionalized cancer survivors, and people who died. As such, temporality between medical events and smoking cessation cannot be assessed, and generalization beyond community-dwelling stroke survivors is unsupported. However, our use of the quit ratio, which by definition is only calculated for people who qualify as ever-smokers, allows us to assess smoking among stroke survivors across demographic and geographic groups despite possible differences in prevalence of ever-smoking between these groups. Thus, these limitations do not detract from the key findings of our descriptive analysis, wherein the proportion of ever-smoker stroke survivors who has quit, and conversely continues to actively smoke, varies across demographic and geographic factors and is higher among stroke survivors than cancer survivors. Third, survey data do not specify whether the prior stroke was ischemic or hemorrhagic, so we cannot determine whether smoking behavior differs by stroke type. Fourth, self-reported quit ratios may modestly overestimate true quit ratios, in which case there may be greater need to address smoking cessation than indicated by these data. Regardless, these data support the need for interventions to address the substantial proportion of stroke survivors with a smoking history who continues to smoke.
Conclusion
Substantial population-level variation in smoking quit ratios in stroke survivors highlights the need to optimize smoking-cessation interventions for this at-risk population.
Supplementary Material
Sources of Funding
Dr. Parikh is supported by the New York State Department of Health Empire Clinical Research Investigator Program and the Florence Gould Endowment for Discovery in Stroke.
Conflicts-of-Interest/Disclosures
Dr. Parikh: has received personal compensation for medicolegal consulting on stroke and research funding from the Leon Levy Foundation and NIH/NIA (K23AG073524) unrelated to this work.
Dr. Parasram: none.
Dr. White: none.
Dr. Merkler has received personal compensation for medicolegal consulting on stroke and research funding from the American Heart Association unrelated to this work.
Dr. Navi: none.
Dr. Kamel serves as co-PI for the NIH-funded ARCADIA trial which receives in-kind study drug from the BMS-Pfizer Alliance and in-kind study assays from Roche Diagnostics, serves as Deputy Editor for JAMA Neurology, serves as a steering committee member of Medtronic’s Stroke AF trial (uncompensated), serves on an endpoint adjudication committee for a trial of empagliflozin for Boehringer-Ingelheim, and has served on an advisory board for Roivant Sciences related to Factor XI inhibition.
Abbreviations
- BRFSS
Behavioral Risk Factor Surveillance System
- TIA
transient ischemic attack
References
- 1.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al. Heart Disease and Stroke Statistics-2020 Update: A report from the American Heart Association. Circulation. 2020;141:e139–e596 [DOI] [PubMed] [Google Scholar]
- 2.Amarenco P, Lavallee PC, Monteiro Tavares L, Labreuche J, Albers GW, Abboud H, Anticoli S, Audebert H, Bornstein NM, Caplan LR, et al. Five-year risk of stroke after TIA or minor ischemic stroke. NEJM. 2018;378:2182–2190 [DOI] [PubMed] [Google Scholar]
- 3.Hankey GJ, Spiesser J, Hakimi Z, Bego G, Carita P, Gabriel S. Rate, degree, and predictors of recovery from disability following ischemic stroke. Neurology. 2007;68:1583–1587 [DOI] [PubMed] [Google Scholar]
- 4.Epstein KA, Viscoli CM, Spence JD, Young LH, Inzucchi SE, Gorman M, Gerstenhaber B, Guarino PD, Dixit A, Furie KL, et al. Smoking cessation and outcome after ischemic stroke or TIA. Neurology. 2017;89:1723–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J, Li S, Zheng K, Wang H, Xie Y, Xu P, Dai Z, Gu M, Xia Y, Zhao M, et al. Impact of smoking status on stroke recurrence. J Am Heart Assoc. 2019;8:e011696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, Fang MC, Fisher M, Furie KL, Heck DV, et al. Guidelines for the Prevention of Stroke in Patients with Stroke and Transient Ischemic Attack: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160–2236 [DOI] [PubMed] [Google Scholar]
- 7.Gritz ER, Toll BA, Warren GW. Tobacco use in the oncology setting: Advancing clinical practice and research. Cancer Epidemiology Biomarkers & Prevention. 2014;23:3–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Croyle RT, Morgan GD, Fiore MC. Addressing a core gap in cancer care - the NCI Moonshot Program to help oncology patients stop smoking. NEJM. 2019;380:512–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention (CDC). Behavioral Risk Factor Surveillance System Survey Data. https://www.cdc.gov/brfss/index.html. Accessed February 25, 2021.
- 10.Centers for Disease Control and Prevention (CDC). Behavioral Risk Factor Surveillance System Overview: BRFSS 2019. https://www.cdc.gov/brfss/annual_data/2019/pdf/overview-2019-508.pdf Accessed April 1, 2021.
- 11.Centers for Disease Control and Prevention (CDC). Behavioral Risk Factor Suirveillance System. 2019 Summary Data Quality Report. https://www.cdc.gov/brfss/annual_data/2019/pdf/2019-sdqr-508.pdf Accessed April 1, 2021. [Google Scholar]
- 12.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457 [DOI] [PubMed] [Google Scholar]
- 13.Behavioral risk factor surveillance system (brfss). Complex sampling weights and preparing 2019 brfss module data for analysis. 2020 [Google Scholar]
- 14.Pierannunzi C, Hu SS, Balluz L. A systematic review of publications assessing reliability and validity of the Behavioral Risk Factor Surveillance System (BRFSS), 2004–2011. BMC Medical Research Methodology. 2013;13:49. 10.1186/1471-2288-13-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li C, Balluz LS, Ford ES, Okoro CA, Zhao G, Pierannunzi C. A comparison of prevalence estimates for selected health indicators and chronic diseases or conditions from the Behavioral Risk Factor Surveillance System, the National Health Interview Survey, and the National Health and Nutrition Examination Survey, 2007-2008. Prev Med. 2012;54:381–387 [DOI] [PubMed] [Google Scholar]
- 16.Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol. 2004;57:1096–1103 [DOI] [PubMed] [Google Scholar]
- 17.Lin MP, Ovbiagele B, Markovic D, Towfighi A. "Life's simple 7' and long-term mortality after stroke. J Am Heart Assoc. Nov 2015;4(11)doi: 10.1161/JAHA.114.001470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jamrozik E, Hyde Z, Alfonso H, Flicker L, Almeida O, Yeap B, Norman P, Hankey G, Jamrozik K. Validity of self-reported versus hospital-coded diagnosis of stroke: A cross-sectional and longitudinal study. Cerebrovascular diseases (Basel, Switzerland). 2014;37:256–262 [DOI] [PubMed] [Google Scholar]
- 19.Bergmann MM, Calle EE, Mervis CA, Miracle-McMahill HL, Thun MJ, Heath CW. Validity of self-reported cancers in a prospective cohort study in comparison with data from state cancer registries. Am J Epidemiol. 1998;147:556–562 [DOI] [PubMed] [Google Scholar]
- 20.Benowitz NL, Bernert JT, Caraballo RS, Holiday DB, Wang J. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol. 2009;169:236–248 [DOI] [PubMed] [Google Scholar]
- 21.Wong SL, Shields M, Leatherdale S, Malaison E, Hammond D. Assessment of validity of self-reported smoking status. Health Rep. 2012;23:47–53 [PubMed] [Google Scholar]
- 22.Jamal A, King BA, Neff LJ, Whitmill J, Babb SD, Graffunder CM. Current cigarette smoking among adults - United States, 2005-2015. MMWR Morb Mortal Wkly Rep. 2016;65:1205–1211 [DOI] [PubMed] [Google Scholar]
- 23.Jamal A, Phillips E, Gentzke AS, Homa DM, Babb SD, King BA, Neff LJ. Current cigarette smoking among adults — United States, 2016. MMWR. Morbidity and Mortality Weekly Report. 2018;67:53–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Streck JM, Weinberger AH, Pacek LR, Gbedemah M, Goodwin RD. Cigarette smoking quit rates among persons with serious psychological distress in the United States from 2008 to 2016: Are mental health disparities in cigarette use increasing? Nicotine & Tobacco Research. 2020;22:130–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weinberger AH, Giovenco DP, Zhu J, Lee J, Kashan RS, Goodwin RD. Racial/ethnic differences in daily, nondaily, and menthol cigarette use and smoking quit ratios in the United States: 2002 to 2016. Preventive Medicine. 2019;125:32–39 [DOI] [PubMed] [Google Scholar]
- 26.Howard G, Howard VJ. Twenty years of progress toward understanding the Stroke Belt. Stroke. 2020;51:742–750 [DOI] [PubMed] [Google Scholar]
- 27.Office of Management and Budget. 2010 standards for delineating metropolitan and micropolitan statistical areas. Federal Register. https://www.federalregister.gov/documents/2010/06/28/2010-15605/2010-standards-for-delineating-metropolitan-and-micropolitan-statistical-areas. Accessed February 15, 2021. [Google Scholar]
- 28.Ingram D, Malec D, Makuc D, Kruszon-Moran D, Gindi RM, Albert M, Beresovsky V, Hamilton BE, Holmes J, Schiller J, et al. National center for health statistics guidelines for analysis of trends. Vital Health Stat. 2018;179:1–71. [PubMed] [Google Scholar]
- 29.Parikh NS, Salehi Omran S, Kamel H, Elkind MSV, Willey J. Symptoms of depression and active smoking among survivors of stroke and myocardial infarction: An NHANES analysis. Prev Med. 2020;137:106131 doi: 10.1016/j.ypmed.2020.106131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parikh NS, Chatterjee A, Diaz I, Merkler AE, Murthy SB, Iadecola C, Navi BB, Kamel H. Trends in active cigarette smoking among stroke survivors in the United States, 1999 to 2018. Stroke. 2020;51:1656–1661 [DOI] [PubMed] [Google Scholar]
- 31.Tran P, Tran L, Tran L. Smoking levels and associations between sociodemographic factors and smoking continuation in U.S. Stroke survivors. Annals of Epidemiology. 2020;43:66–70 [DOI] [PubMed] [Google Scholar]
- 32.Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suñer-Soler R, Grau A, Gras ME, Font-Mayolas S, Silva Y, Dávalos A, Cruz V, Rodrigo J, Serena J. Smoking cessation 1 year poststroke and damage to the insular cortex. Stroke. 2012;43:131–136 [DOI] [PubMed] [Google Scholar]
- 34.Suñer-Soler R, Grau-Martín A, Terceno M, Silva Y, Davalos A, Sánchez JM, Font-Mayolas S, Gras E, Rodrigo J, Kazimierczak M, et al. Biological and psychological factors associated with smoking abstinence six years post-stroke. Nicotine Tob Res. 2018;20:1182–1188 [DOI] [PubMed] [Google Scholar]
- 35.Bak S, Sindrup SH, Alslev T, Kristensen O, Christensen K, Gaist D. Cessation of smoking after first-ever stroke: A follow-up study. Stroke. 2002;33:2263–2269 [DOI] [PubMed] [Google Scholar]
- 36.Sienkiewicz-Jarosz H, Zatorski P, Baranowska A, Ryglewicz D, Bienkowski P. Predictors of smoking abstinence after first-ever ischemic stroke: A 3-month follow-up. Stroke. 2009;40:2592–2593 [DOI] [PubMed] [Google Scholar]
- 37.Sienkiewicz-Jarosz H, Zatorski P, Ryglewicz D, Bienkowski P. Reasons for quitting smoking in patients with first-ever ischemic stroke. Eur Addict Res. 2012;18:275–278 [DOI] [PubMed] [Google Scholar]
- 38.Ives SP, Heuschmann PU, Wolfe CD, Redfern J. Patterns of smoking cessation in the first 3 years after stroke: The South London stroke register. Eur J Cardiovasc Prev Rehabil. 2008;15:329–335 [DOI] [PubMed] [Google Scholar]
- 39.Lim YK, Shin DW, Kim HS, Yun JM, Shin JH, Lee H, Koo HY, Kim MJ, Yoon JY, Cho MH. Persistent smoking after a cardiovascular event: A nationwide retrospective study in Korea. PLoS One. 2017;12:e0186872 doi: 10.1371/journal.pone.0186872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kulak JA, Cornelius ME, Fong GT, Giovino GA. Differences in Quit Attempts and Cigarette Smoking Abstinence between Whites and African Americans in the United States: Literature Review and Results from the International Tobacco Control US Survey. Nicotine & Tobacco Research. 2016;18:S79–S87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hammond G, Luke AA, Elson L, Towfighi A, Joynt Maddox KE. Urban-rural inequities in acute stroke care and in-hospital mortality. Stroke. 2020;51:2131–2138 [DOI] [PubMed] [Google Scholar]
- 42.Talluri R, Fokom Domgue J, Gritz ER, Shete S. Assessment of Trends in Cigarette Smoking Cessation After Cancer Diagnosis Among US Adults, 2000 to 2017. JAMA Network Open. 2020;3:e2012164. doi: 10.1001/jamanetworkopen.2020.12164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rojewski AM, Baldassarri S, Cooperman NA, Gritz ER, Leone FT, Piper ME, Toll BA, Warren GW. Exploring Issues of Comorbid Conditions in People Who Smoke. Nicotine & Tobacco Research. 2016;18:1684–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.