Abstract
Purpose:
Despite recommendations from the World Health Organization and the American Academy of Pediatrics to exclusively breastfeed infants for their first 6 months of life, 75% of women do not meet exclusive breastfeeding guidelines and 60% do not meet their own breastfeeding goals. Numerous observational studies have linked maternal psychological distress (e.g., perceived stress, anxiety, depression) with non-optimal breastfeeding outcomes, such as decreased proportion and duration of exclusive breastfeeding. The physiological mechanisms underlying these associations, however, remain unclear.
Methods:
For this narrative review, we evaluated the evidence for relationships between maternal psychological distress and lactation and breastfeeding outcomes in pregnancy and postpartum and the possible physiological mechanisms that facilitate these relationships. We searched PubMed using the following terms: “stress”, “anxiety”, “depression”, “breastfeeding”, and “lactation”. A further hand-search was conducted to ensure a thorough review of the literature.
Findings:
Among the studies examined, methods used to assess maternal psychological distress were not uniform, with some studies examining perceived distress via a variety of validated tools and others measuring biological measures of distress, such as cortisol. Evidence supports a role for psychological distress in multiple breastfeeding outcomes, including delayed secretory activation and decreased duration of exclusive breastfeeding. One physiological mechanism proposed to explain these relationships is that psychological distress may impair the release of oxytocin, a hormone that plays a critical role in milk ejection during lactation. Continued impairment of milk ejection may lead to decreased milk production due to incomplete emptying of the breast during each feed. Maternal distress may also yield elevated levels of serum cortisol and decreased insulin sensitivity, which are associated with decreased milk production. The relationship between psychological distress and breastfeeding is likely to be bidirectional, however, in that breastfeeding appears to reduce maternal distress, again possibly via their effects on the pleasure/reward and calming effects of oxytocin on the mother. This suggests interventions to support lactation and breastfeeding goals in women who score high on measures of psychological distress would be beneficial for both maternal and infant well-being.
Implications:
Evidence to date suggests that maternal psychological distress may impair lactation and breastfeeding outcomes, but stronger study designs and rigorous assessment methods are needed. A better understanding of the physiological mechanisms leading to impaired lactation may assist in the development of early interventions for mothers experiencing distress. In addition, stress-reducing programs and policies should be investigated for their potential to improve breastfeeding outcomes.
Keywords: lactation, breastfeeding, maternal psychological distress
INTRODUCTION
Both the World Health Organization and American Academy of Pediatrics recommend exclusive breastfeeding until an infant reaches 6 months of age.1,2 Breastfeeding is associated with many health benefits, including decreased risk of type 1 diabetes3,4, asthma and respiratory illness5,6, gastrointestinal infections7, and sudden infant death syndrome for the infant8 and decreased risk of ovarian cancer9, cardiovascular disease10, and type 2 diabetes10 for the mother. Despite the known benefits of human milk for infants, only 25% of infants are exclusively breastfed at 6 months of age, and 60% of women do not meet their own breastfeeding goals.11,12United States breastfeeding data for infants born in 2017 reveals that 85% of mothers initiated breastfeeding, but nearly 20% provided formula before their infants were two days old, and less than 50% were exclusively breastfeeding when their infants were 3 months old.12,13 In the US, it is estimated that low rates of breastfeeding add more than $3 billion annually to medical care costs for mothers and infants.13
Several studies have examined reasons for non-optimal breastfeeding outcomes (non-exclusive breastfeeding and/or breastfeeding cessation), which include barriers such as unsupportive employment and hospital policies, lactation difficulties, and maternal or health care provider concerns about infant growth and nutrition.11,14 Additionally, large disparities in breastfeeding rates exist across racial and ethnic groups with non-Hispanic Black mothers and Native American/American Indian mothers less likely to ever breastfeed compared to other groups.15,16 Maternal psychological distress (e.g., perceived stress, anxiety, depression) has also been linked to lactation difficulty and breastfeeding cessation.17–19 Despite being potentially modifiable, the associations between maternal distress and breastfeeding outcomes are unclear. Thus, we will focus our attention on these relationships and the potential physiological processes which drive themfor this narrative review.
Lactation and the mother-infant dyad
Successful lactation begins long before the infant is born. The first stage is controlled primarily via increases in estrogen and progesterone secreted from the placenta20,21 and involves rapid differentiation of the mammary epithelium within the highly branched lobular and ductal network of the developing mammary gland. The basal layer of the ductal epithelium becomes the myoepithelium, responsible for milk ejection. The luminal layer of the mammary epithelium is composed of epithelial secretory cells called lactocytes, which synthesize and secrete milk components into the lumen of the alveoli.22–24 High progesterone concentrations during pregnancy inhibit milk secretion, leading to accumulation of small amounts of colostrum and no milk production.25 Within 48–72 hours after parturition, maternal progesterone levels decline 10-fold, initiating secretory activation in the mammary gland, with copious production of milk23,26. Prolactin facilitates sustained lactation as progesterone falls and can regulate many aspects of milk synthesis and secretion, although its primacy as a driver of lactation has come under scrutiny of late.20,27,28 During early lactation, glucocorticoids assist in the closure of tight junctions between lactocytes, which prevents the leakage of milk components, while insulin is observed to facilitate the expression of genes involved in milk protein synthesis.29,30 Many other hormones play a role including cortisol, thyroid hormone, and possibly serotonin.20 Disruption of these hormonally controlled processes may lead to delayed onset of milk production and insufficient milk volume.18
Sustained adequate milk production depends first on efficient nutrient partitioning and uptake by the mammary gland, and second on frequent and complete emptying of milk from the breast, and thus rests on the bio-behavioral interaction within the mother-infant dyad.31 Infant suckling stimulates neurons in the areola to trigger the release of oxytocin from maternal hypothalamic neurons in the pleasure/reward pathway, leading to calming effects for the nursing mother.32 Infant suckling also prompts oxytocin release from the posterior pituitary into maternal circulation, which causes contraction of the myoepithelial cells surrounding the alveoli to allow milk to be ejected into the ducts for passage to the infant.32,33 If emptying of the breast is partial or infrequent, milk accumulates in the ducts, distending them and exposing the mammary gland to increased concentrations of a small whey protein called feedback inhibition of lactation (FIL). FIL provides autocrine feedback control on milk production to match maternal milk supply with infant milk demand.25 Successful establishment and continuation of lactation therefore relies upon a complex hormonally driven orchestration of glandular development during pregnancy and then secretory activation in the early post-partum period, which must be accompanied by prompt initiation of breastfeeding after delivery, proper infant positioning on the breast and efficient latching to achieve efficient milk removal, positive maternal-infant interaction during feeding, and continued frequent nursing.34 Given the deeply bio-behavioral and interactive nature of lactation, it is reasonable to consider both maternal and infant psychological health and well-being as potentially important drivers of breastfeeding success.
Maternal psychological distress
For this narrative review, we use the term ‘psychological distress’ to refer to different psychological or physiological responses to stressful challenges, such as cognitive appraisals of stress (i.e., perceived stress), symptoms of anxiety or depression, and cortisol levels.35,36 Depression, anxiety, and perceived stress are commonly grouped together to characterize maternal distress in perinatal research, and these symptoms are highly comorbid in the perinatal period.37–39 Psychological distress is commonly measured by assessing maternal perception via psychometric instruments40 or using biological markers such as cortisol (serum, saliva, or hair).41,42 In response to stress, neurobiological systems activate to protect the body and promote adaptation, facilitated by the sympathetic adrenomedullary system (SAM) and the hypothalamic-pituitary-adrenocortical (HPA) axis.43–45 As part of the sympathetic nervous system, the SAM releases epinephrine which signals the fight/flight response46, while the HPA axis is a component of the central nervous system and produces cortisol in response to stress.43 Repeated or chronic exposure to stress creates a cumulative burden on the body, increasing its “allostatic load” and increasing the risk for disease.44
Maternal psychological distress during pregnancy has been associated with negative birth outcomes such as low birth weight, preterm birth, and later risk for neurodevelopmental, psychiatric, cardiovascular, and metabolic disease.47–52 Activation of the HPA axis leading to increased maternal cortisol concentrations is the most commonly studied physiological mechanism for assessing the effects of maternal psychological distress on infant outcomes.53 Maternal cortisol can be directly transported across the placenta to enter fetal circulation and has been observed to account for approximately 40% of the variance in fetal cortisol concentrations.54 While the majority of maternal cortisol is metabolized during passage through the placenta, increased maternal cortisol concentrations may still have a significant effect on fetal concentrations.55 Further, a growing body of evidence suggests that maternal HPA axis dysregulation is not the only physiological mechanism responsible for adverse infant outcomes, and indirect mechanisms such as colonization of the infant gut by maternal microbes may be affected by maternal distress.56
The physiological mechanisms explaining the observed relationships between maternal psychological distress and adverse breastfeeding outcomes remain unclear.17–19 Elucidating these mechanisms may assist in better development of early interventions targeted at improving breastfeeding outcomes among mothers at risk of experiencing psychological distress. Thus, for this narrative review, we aimed to evaluate the evidence for relationships between psychological distress and non-optimal breastfeeding outcomes in humans and to identify potential physiological mechanisms that could underlie these associations.
METHODS
For this narrative review, we evaluated the evidence for relationships between maternal psychological distress and lactation and breastfeeding outcomes (i.e. exclusivity and duration) and the possible physiological mechanisms that facilitate these relationships. We reviewed PubMed using the following search terms: “stress”, “anxiety”, “depression”, “breastfeeding”, and “lactation”. A further hand-search was conducted to ensure a thorough review of the literature. Peer-reviewed publications from the United States, Canada, Europe, and Australia were considered for inclusion and selected for their relevance to the review’s objectives.
RESULTS
Maternal psychological distress and lactation and infant feeding outcomes
Multiple studies have examined lactation and infant feeding outcomes in relation to maternal psychological distress with varied results, likely due to differences in stress measurement (type and timing of measures), feeding outcomes, and adjustment for confounding factors. Additionally, attributing feeding outcomes specifically to maternal psychological distress may be difficult because of the numerous interrelated factors which are potentially associated with lactogenesis (e.g. delivery mode, duration of labor, and maternal distress).18,57
We identified 7 systematic reviews and one meta-analysis examining maternal distress and breastfeeding outcomes.58–65 The associations between maternal anxiety, depression, or perceived stress and breastfeeding outcomes were inconclusive, with several meta-analyses citing low quality studies (inadequate adjustment for confounding, lack of uniform definition of breastfeeding, lack of prospective measurements, inadequate statistical power or insufficient analysis to determine relationship between variables, etc.) and heterogenous outcomes. The link between maternal depression and breastfeeding outcomes, however, appears to be stronger, with one meta-analysis reporting associations between elevated maternal depressive symptoms and non-exclusive breastfeeding or shorter breastfeeding duration for over half of the 38 studies examined. In our further review of the literature, 15 studies found positive associations between maternal distress (perceived stress, anxiety, and/or depression) and non-optimal breastfeeding outcomes, while 6 studies found no association, and still 5 studies found that maternal distress was associated with increased breastfeeding or provided potential evidence for a bidirectional relationship (as most studies assessed multiple types of maternal distress (i.e. prenatal and postnatal anxiety and depression), some overlap does exist between the provided numbers).
There are two primary time points when maternal stress may reduce breastfeeding: before breastfeeding begins (e.g., by delaying initial milk production i.e. lactogenesis), or after breastfeeding is initiated. The next sections describe each of these time points.
Delayed onset of lactogenesis
Delayed onset of lactogenesis is defined as insufficient milk production in the first two weeks postpartum.26 This may occur for a multitude of reasons such as poor infant suckling ability and subsequent incomplete emptying of milk from the breast66, but several studies have noted associations with long duration of labor and urgent Cesarean section, which are strongly linked with postpartum stress for both mother and infant at birth.57,67,68 Higher post-delivery stress is also associated with delayed onset of lactation, which may lead to non-exclusive breastfeeding.68–70 Women who experience delayed onset of lactation may introduce formula to their infants shortly after birth, and supplementation of human milk with infant formula during hospitalization is predictive of breastfeeding cessation in early lactation.71,72 However, it is possible that providing a small amount of formula in the hospital setting could decrease acute maternal psychological distress and/or improve the perception of adequate milk supply, benefiting long term exclusivity of maternal breastmilk feeding. A randomized control trial conducted by Flaherman et al. assigned participants to receive small formula volumes (10 mL after each breastfeeding) or exclusive breastfeeding before the onset of mature milk production. They hypothesized that early provision of formula would reduce the discontinuation of exclusive breastfeeding by alleviating maternal concerns about milk supply and found that 79% of the formula group were breastfeeding exclusively at 6 months postpartum compared with 42% of the exclusive breastfeeding group (p = 0.02).
Insufficient milk supply and decreased duration and exclusivity of breastfeeding
Many women discontinue exclusive breastfeeding when infants are between 3 and 6 months of age, and evidence suggests that maternal psychological distress may also affect breastfeeding even after milk production has been fully established (i.e., after one month postpartum).12,73–75 Maternal anxiety and depressive symptoms during pregnancy are predictive of depressive symptoms in the postpartum period, which are associated with shorter duration of exclusive (and/or any) breastfeeding.39,76–79 In a sample of 255 Canadian women, Adedinsewo et al. found that every point increase in State Trait Anxiety Inventory score at 3 months postpartum (when lactation had already been established) was associated with decreased odds of any breastfeeding at 12 months postpartum. Exposure to stressful life events may negatively affect breastfeeding exclusivity. Data from the Pregnancy Risk Assessment and Monitoring System (PRAMS) reveals that among women ≤ 24 years old, the odds of exclusive breastfeeding for 3 months were lower for those who experienced ≥ 2 stressful life events compared with those who experienced no stressful life events.80 Another study documented evidence of bidirectional associations between maternal depressive symptoms and breastfeeding duration in a group of 205 women who were followed prenatally and at 3, 6, 12, and 24 months after birth.81 Women with elevated depressive symptoms during pregnancy weaned their infants approximately 2 months earlier than women who did not experience prenatal depression, and in turn, women who breastfed more frequently at 3 months postpartum showed greater declines in depressive symptoms up to 2 years after birth. Similarly, mothers who were exclusively breastfeeding at 3 months postpartum had significantly lower perceived stress scores compared to those providing mixed feedings (breast milk and formula).82 Exclusive breastfeeding through 4–6 months postpartum is also associated with lower maternal perceived stress compared to formula feeding.83 Other studies have failed to find associations between anxiety or depressive symptoms in the later postpartum period84–86, and others found differing associations with breastfeeding outcomes for prenatal vs postnatal maternal distress.19,76
Inadequate milk supply (perceived or actual) is a commonly cited reason for discontinuation of exclusive breastfeeding both early in lactation and after lactation has been established; approximately 35% of women report early perceived insufficient milk supply as the primary reason for cessation of breastfeeding.87 By 8 days postpartum, the average lactating woman produces 650 mL of breastmilk per 24 hours; from 1 to 6 months of lactation, a woman who is exclusively breastfeeding produces an average of 750–800 mL per 24 hours.88 In a sample of 1323 women, the perception that infants were not satisfied by breastmilk alone was among the top three reasons provided by mothers for breastfeeding cessation regardless of weaning age (assessed from 2–12 months postpartum).89 In this study, inadequate milk supply was reported more often by Hispanic mothers and those with a household income of <350% of the federal poverty level in comparison to white mothers and those with income >350% of the federal poverty level, suggesting that multiple factors (including non-physiological factors) may influence breastfeeding duration.
Potential mechanisms by which psychological distress affects lactation
Physiological mechanisms by which maternal psychological distress may impair lactation are not fully elucidated, and much of the existing support for the association between these variables arises from animal studies or from observational studies in humans. Here, we discuss the potential impact of distress on hormones implicated in lactation and on HPA axis-related mechanisms.
Regulation of hormones implicated in lactation
Lactation is dependent on the intricate coordination of hormones such as progesterone, prolactin, and oxytocin. One hypothesized physiological mechanism for the relationship between maternal distress and lactation is the impaired release of maternal oxytocin, which impedes the contraction of myoepithelial cells involved in milk ejection.18 In a sample of 22 women at 5 days postpartum, Ueda and colleagues found that maternal exposure to stress impaired oxytocin release in response to infant suckling.90 Participants were exposed to stressors via either noise or mental calculation (arithmetic problems), and the number of pulsatile releases of oxytocin in maternal blood was measured. Women in the stressor groups had decreased and delayed responses to suckling and decreased number of oxytocin pulsatile releases. Prolactin levels before and during infant suckling were also measured in relationship to milk secretion volume, and no differences were observed among the groups. The authors concluded that unlike oxytocin, the increase in prolactin level in response to infant suckling was not impaired by maternal psychological distress. These results are confirmed by studies which have found that higher anxiety and depression scores are associated with lower maternal oxytocin levels during breastfeeding91 and that significant interactions between early breastfeeding cessation and depression status on maternal oxytocin levels are present at 8 weeks postpartum.92
Similarly, Doulougheri et al. found that positive maternal emotions were associated with increased infant feeding frequency and greater milk volume. Since positive emotions are correlated with increased maternal oxytocin93, they suggested oxytocin was responsible for the favorable feeding and lactation outcomes.68 Thus, mothers who experience positive emotions and less distress may have better lactation outcomes. However, maternal depression and anxiety are not necessarily predictive of maternal oxytocin levels and may be influenced by other factors. At 2 and 6 months postpartum, Whitley et al. found no significant differences in oxytocin levels across a feed between women with depressive or anxious symptoms versus asymptomatic women.
Maternal distress may affect lactation by interfering with insulin sensitivity and secretion. Insulin and its regulation of glucose are important for optimal lactation. Insulin resistance and impaired glucose tolerance such as that observed in type 2 diabetes mellitus or gestational diabetes have been associated with delayed onset of lactation, low maternal milk supply, and decreased breastfeeding duration.94–96 As reviewed by Nommsen-Rivers, insulin promotes the expression of genes involved in milk protein synthesis.97 Nommsen-Rivers hypothesized that the combined effects of insulin resistance and impaired insulin secretion on insulin-sensitive signaling in the lactocyte (rather than circulating levels of insulin or glucose) are responsible for the decreased rate of milk synthesis. This hypothesis is supported by work from Neville and colleagues, who found that genes involved in mammary differentiation and milk synthesis were significantly downregulated in an insulin receptor knockout mouse model.98 While rarely examined in studies assessing impaired glucose tolerance and lactation, psychological distress is associated with decreased insulin sensitivity. In animal models, early life stress impairs insulin secretion in response to psychological stress in adulthood.99 Animal (mouse) models also reveal that acute psychological stress can alter glucose metabolism by decreasing hepatic sensitivity to insulin, leading to insulin resistance.100 In humans, maternal perception of discrimination, an established psychological stressor, is associated with increased odds of developing gestational diabetes mellitus.101 Further, patients diagnosed with major depression exhibit insulin resistance after administration of oral glucose tolerance tests compared to healthy patients.102 These studies outline a potential physiological mechanism by which stress-induced glucose dysregulation and insulin resistance may lead to lactation difficulty.
Cortisol/HPA axis-related mechanisms
The HPA axis is activated in response to stress and involves a cascade of hormonal events which leads to release of the glucocorticoid steroid hormone cortisol into the bloodstream. The effects of stress-induced alterations in circulating cortisol are numerous, as glucocorticoid receptors are widely distributed throughout the body. Pregnancy and the postpartum period involve normative changes in HPA axis function which play a role in lactation.103 Cortisol is a necessary cofactor for milk production and is involved in the differentiation of mammary gland cells into lactocytes, as well as milk secretion and lactogenesis.28 At the same time, lactation results in a reduction in maternal cortisol levels during feeding and an attenuation of maternal HPA axis reactivity to stress104–108, further highlighting the protective effects of lactation as well as the likelihood that psychological distress-lactation associations are bidirectional. Both maternal psychosocial distress and difficulties with lactation are associated with altered HPA axis functioning, suggesting that maternal HPA axis dysregulation is a pathway by which maternal distress compromises lactation.106 However, the extant literature regarding maternal psychological distress and HPA axis activity associations is equivocal109, potentially due to measurement differences across studies, failure to consider the interacting effects of multiple hormones (e.g., cortisol and oxytocin105), and/or involvement of other mechanisms.
Future Directions
The lack of uniform study results is likely reflective of varying sample sizes, measures of psychological distress, and breastfeeding outcomes, as well as limited evidence from animal studies and observational studies in humans due to ethicality of assigning participants into non-breastfeeding groups. Future exploration of maternal distress and lactation and feeding outcomes should employ adequate adjustment for potential confounding factors (i.e. parity74) and explore additional variables which may mediate or moderate relationships such as those discussed below.18
Milk composition may be altered by maternal psychological distress and have an impact on lactation and breastfeeding success. The macronutrient content, specifically fatty acid concentration, of human milk is negatively associated with stress reactivity (measured via saliva cortisol in response to cold).110 Fatty acids comprise the majority of calories in human milk, and decreased concentrations of fatty acids in milk may lead to decreased infant satiation, affecting maternal perception of adequate milk supply.110 Maternal psychological distress may also affect the immune components present in milk.111 Recent evidence supports the finding that milk bioactives may serve as biomarkers for lactation difficulty. We found that bioactives present at 1 month postpartum in human milk were associated with breastfeeding cessation by 3 months postpartum. Specifically, milk glucose and smaller decreases in glucose from 1–3 months postpartum were associated with increased odds of exclusive breastfeeding, while leptin and increases in insulin and leptin were associated with decreased odds of exclusive breastfeeding (data not yet published). Whether changes in these bioactives are associated with maternal psychological distress is unknown and requires further study.
Lactation and breastfeeding outcomes for non-Hispanic Black mothers are less optimal than those of white mothers.15 Racism has been linked with maternal psychological distress112,113 but has not been thoroughly studied in conjunction with breastfeeding outcomes. However, work-related racism has been associated with lower odds of longer breastfeeding duration, while police-related racism has been associated with higher odds of longer breastfeeding duration.114 The intersection between racism and maternal psychological distress should be examined in conjunction with lactation and breastfeeding outcomes to understand policies and interventions which may improve these outcomes.
Future research should include an exploration of the bidirectional benefits of breastfeeding on maternal psychological distress and overall maternal health.18,115 While maternal psychological distress may decrease breastfeeding duration, breastfeeding itself may decrease maternal distress.82,83,93 Additionally, breastfeeding may be used as a coping mechanism for some mothers; higher prenatal maternal marital distress has been associated with longer duration of exclusive breastfeeding.76 This may be because skin-to-skin contact, such as that practiced during breastfeeding, is associated with decreased maternal anxiety.116,117 The intention to breastfeed vs actual practice may also influence the relationship between maternal psychological distress and breastfeeding.118
CONCLUSION
Current evidence suggests a potential role for acute and chronic maternal psychological distress in lactation success and difficulties, including both establishment of lactation and continued duration of breastfeeding. Both physiological and social/behavioral factors may be involved, but additional studies are needed to understand the mechanisms behind these relationships. Comprehensive strategies to decrease maternal psychological distress, as well as policies that focus on dismantling structural barriers to breastfeeding, may improve breastfeeding outcomes.
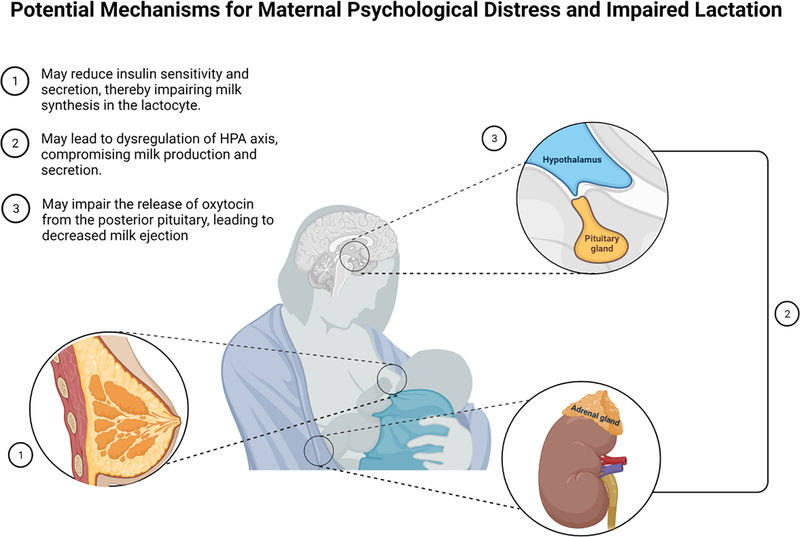
Figure 1.
Maternal psychological distress may impair lactation by (1) interfering with oxytocin release, (2) reducing insulin sensitivity and secretion, or by (3) causing dysregulation of the hypothalamic-pituitary-adrenocortical (HPA) axis. Figure created with BioRender.com.
Acknowledgments
Conflict of Interest and Financial Disclosures EMN is supported by an NIH/NIDDK grant (T32DK083250). MAH is supported by a National Science Foundation Graduate Research Fellowship. CP is supported by an NICHD grant (T32HD095134). EWD and DAF are supported by an NIH/NICHD grant (R01HD080444). No other COI to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eidelman AI, Schanler RJ. Breastfeeding and the use of human milk. Pediatrics. 2012;129(3). doi: 10.1542/peds.2011-3552 [DOI] [Google Scholar]
- 2.World Health Organization. Breastfeeding. Accessed June 3, 2021. https://www.who.int/health-topics/breastfeeding#tab=tab_1
- 3.Lund-Blix NA, Sander SD, Størdal K, Nybo Andersen AM, Rønningen KS, Joner G, Skrivarhaug T, Njølstad PR, Husby S, Stene LC. Infant feeding and risk of type 1 diabetes in two large scandinavian birth cohorts. Diabetes Care. 2017;40(7):920–927. doi: 10.2337/dc17-0016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cardwell CR, Stene LC, Ludvigsson J, Rosenbauer J, Cinek O, Svensson J, Perez-Bravo F, Memon A, Gimeno SG, Wadsworth EJK, et al. Breast-feeding and childhood-onset type 1 diabetes: A pooled analysis of individual participant data from 43 observational studies. Diabetes Care. 2012;35(11):2215–2225. doi: 10.2337/dc12-0438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miliku K, Azad MB. Breastfeeding and the developmental origins of asthma: Current evidence, possible mechanisms, and future research priorities. Nutrients. 2018;10(8). doi: 10.3390/nu10080995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azad MB, Vehling L, Lu Z, Dai D, Subbarao P, Becker AB, Mandhane PJ, Turvey SE, Lefebvre DL, Sears MR, et al. Breastfeeding, maternal asthma and wheezing in the first year of life: A longitudinal birth cohort study. Eur Respir J. 2017;49(5). doi: 10.1183/13993003.02019-2016 [DOI] [PubMed] [Google Scholar]
- 7.Frank NM, Lynch KF, Uusitalo U, Yang J, Lönnrot M, Virtanen SM, Hyöty H, Norris JM. The relationship between breastfeeding and reported respiratory and gastrointestinal infection rates in young children. BMC Pediatr. 2019;19(1):339. doi: 10.1186/s12887-019-1693-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.JMD T, K T, RY M, EA M, C M, D T, PS B, FR H. Duration of Breastfeeding and Risk of SIDS: An Individual Participant Data Meta-analysis. Pediatrics. 2017;140(5). doi: 10.1542/PEDS.2017-1324 [DOI] [PubMed] [Google Scholar]
- 9.Danforth KN, Tworoger SS, Hecht JL, Rosner BA, Colditz GA, Hankinson SE. Breastfeeding and risk of ovarian cancer in two prospective cohorts. Cancer Causes Control. 2007;18(5):517–523. doi: 10.1007/s10552-007-0130-2 [DOI] [PubMed] [Google Scholar]
- 10.Schwarz EB, Ray RM, Stuebe AM, Allison MA, Ness RB, Freiberg MS, Cauley JA. Duration of lactation and risk factors for maternal cardiovascular disease. Obstet Gynecol. 2009;113(5):974–982. doi: 10.1097/01.AOG.0000346884.67796.ca [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Odom EC, Li R, Scanlon KS, Perrine CG, Grummer-Strawn L. Reasons for earlier than desired cessation of breastfeeding. Pediatrics. 2013;131(3):e726. doi: 10.1542/peds.2012-1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Breastfeeding Report Card.; 2020. Accessed October 21, 2020. https://www.cdc.gov/breastfeeding/data/reportcard.htm
- 13.Centers for Disease Control and Prevention. Breastfeeding: Why It Matters. Accessed June 3, 2021. https://www.cdc.gov/breastfeeding/about-breastfeeding/why-it-matters.html
- 14.Sriraman NK, Kellams A. Breastfeeding: What are the barriers? Why women struggle to achieve their goals. J Women’s Heal. 2016;25(7):714–722. doi: 10.1089/jwh.2014.5059 [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Breastfeeding: Facts. Accessed June 3, 2021. https://www.cdc.gov/breastfeeding/data/facts.html#stopearly
- 16.Chiang KV Racial and Ethnic Disparities in Breastfeeding Initiation - United States, 2019. MMWR Morb Mortal Wkly Rep. 2021;70(21):769–774. doi: 10.15585/MMWR.MM7021A1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dozier AM, Nelson A, Brownell E. The Relationship between Life Stress and Breastfeeding Outcomes among Low-Income Mothers. Adv Prev Med. 2012;2012:1–10. doi: 10.1155/2012/902487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dewey KG. Maternal and fetal stress are associated with impaired lactogenesis in humans. J Nutr. 2001;131(11):3012S–3015S. doi: 10.1093/jn/131.11.3012s [DOI] [PubMed] [Google Scholar]
- 19.Riedstra JP, Aubuchon-Endsley NL. A moderated mediation model of maternal perinatal stress, anxiety, infant perceptions and breastfeeding. Nutrients. 2019;11(12). doi: 10.3390/nu11122981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.IA F, MC N. Introduction: hormonal regulation of mammary development and milk protein gene expression at the whole animal and molecular levels. J Mammary Gland Biol Neoplasia. 2009;14(3):317–319. doi: 10.1007/S10911-009-9146-4 [DOI] [PubMed] [Google Scholar]
- 21.MA C. The endocrine function of human placenta: an overview. Reprod Biomed Online. 2016;32(1):14–43. doi: 10.1016/J.RBMO.2015.10.005 [DOI] [PubMed] [Google Scholar]
- 22.Geddes DT. The anatomy of the lactating breast. Infant. 2007;3(2):59–63. [Google Scholar]
- 23.Neville MC, Morton J, Umemura S. Lactogenesis: The transition from pregnancy to lactation. Pediatr Clin North Am. 2001;48(1):35–52. doi: 10.1016/S0031-3955(05)70284-4 [DOI] [PubMed] [Google Scholar]
- 24.MC N. Classic studies of mammary development and milk secretion: 1945 – 1980. J Mammary Gland Biol Neoplasia. 2009;14(3):193–197. doi: 10.1007/S10911-009-9151-7 [DOI] [PubMed] [Google Scholar]
- 25.Linzell JL, Peaker M. Mechanism of milk secretion. https://doi.org/101152/physrev1971513564. 1971;51(3):564–597. doi: 10.1152/PHYSREV.1971.51.3.564 [DOI] [PubMed] [Google Scholar]
- 26.Neville MC, Keller R, Seacat J, Lutes V, Neifert M, Casey C, Allen J, Archer P. Studies in human lactation: Milk volumes in lactating women during the onset of lactation and full lactation. Am J Clin Nutr. 1988;48(6):1375–1386. doi: 10.1093/ajcn/48.6.1375 [DOI] [PubMed] [Google Scholar]
- 27.GLASIER A, MCNEILLY AS, HOWIE PW. The prolactin response to suckling. Clin Endocrinol (Oxf). 1984;21(2):109–116. doi: 10.1111/j.1365-2265.1984.tb03449.x [DOI] [PubMed] [Google Scholar]
- 28.Pang WW, Hartmann PE. Initiation of Human Lactation: Secretory Differentiation and Secretory Activation. Published online 2007. doi: 10.1007/s10911-007-9054-4 [DOI] [PubMed] [Google Scholar]
- 29.Neville MC, Morton J. Physiology and endocrine changes underlying human lactogenesis II. J Nutr. 2001;131(11):3005S–3008S. doi: 10.1093/jn/131.11.3005s [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi K, Tsugami Y, Matsunaga K, Oyama S, Kuki C, Kumura H. Prolactin and glucocorticoid signaling induces lactation-specific tight junctions concurrent with β-casein expression in mammary epithelial cells. Biochim Biophys Acta - Mol Cell Res. 2016;1863(8):2006–2016. doi: 10.1016/j.bbamcr.2016.04.023 [DOI] [PubMed] [Google Scholar]
- 31.ER N, Steube A. Lactation and breastfeeding. In: Landon M, Galan H, Jauniaux E, Driscoll D, Berghella V, Grobman W, Kilpatrick S, Cahill A, Cahill A, eds. Gabbe’s Obstetrics: Normal and Problem Pregnancies. Saunders; 2020:475–502. [Google Scholar]
- 32.Moberg K, Prime D. Oxytocin effects in mothers and infants during breastfeeding. Infant. 2013;9(6):201–206. [Google Scholar]
- 33.Ramsay DT, Kent JC, Owens RA, Hartmann PE. Ultrasound Imaging of Milk Ejection in the Breast of Lactating Women. Pediatrics. 2004;113(2):361–367. doi: 10.1542/peds.113.2.361 [DOI] [PubMed] [Google Scholar]
- 34.Institute of Medicine (US) Committee on Nutritional Status During Pregnancy and Lactation. Milk Volume. National Academies Press (US); 1991. Accessed June 3, 2021. https://www.ncbi.nlm.nih.gov/books/NBK235589/ [Google Scholar]
- 35.Gunnar M, Quevedo K. The Neurobiology of Stress and Development. Published online 2006. doi: 10.1146/annurev.psych.58.110405.085605 [DOI] [PubMed] [Google Scholar]
- 36.Folkman S Stress: Appraisal and Coping. In: Gellman MD, Turner JR, eds. Encyclopedia of Behavioral Medicine. Springer, New York, NY; 2013:1913–1915. doi: 10.1007/978-1-4419-1005-9_215 [DOI] [Google Scholar]
- 37.JA D. Maternal stress in pregnancy: considerations for fetal development. J Adolesc Health. 2012;51(2 Suppl). doi: 10.1016/J.JADOHEALTH.2012.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.MT K, C M. Impact of maternal stress, depression and anxiety on fetal neurobehavioral development. Clin Obstet Gynecol. 2009;52(3):425–440. doi: 10.1097/GRF.0B013E3181B52DF1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ystrom E Breastfeeding cessation and symptoms of anxiety and depression: A longitudinal cohort study. BMC Pregnancy Childbirth. 2012;12(1):36. doi: 10.1186/1471-2393-12-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nast I, Bolten M, Meinlschmidt G, Hellhammer DH. How to measure prenatal stress? A systematic review of psychometric instruments to assess psychosocial stress during pregnancy. Paediatr Perinat Epidemiol. 2013;27(4):313–322. doi: 10.1111/ppe.12051 [DOI] [PubMed] [Google Scholar]
- 41.Kim MY, Kim GU, Son HK. Hair cortisol concentrations as a biological marker of maternal prenatal stress: A systematic review. Int J Environ Res Public Health. 2020;17(11). doi: 10.3390/ijerph17114002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levine A, Zagoory-Sharon O, Feldman R, Lewis JG, Weller A. Measuring cortisol in human psychobiological studies. Published online 2006. doi: 10.1016/j.physbeh.2006.08.025 [DOI] [PubMed] [Google Scholar]
- 43.STRATAKIS CA, CHROUSOS GP. Neuroendocrinology and Pathophysiology of the Stress System. Ann N Y Acad Sci. 1995;771(1):1–18. doi: 10.1111/j.1749-6632.1995.tb44666.x [DOI] [PubMed] [Google Scholar]
- 44.McEwen BS, Seeman T. Protective and damaging effects of mediators of stress. Elaborating and testing the concepts of allostasis and allostatic load. In: Annals of the New York Academy of Sciences. Vol 896. New York Academy of Sciences; 1999:30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x [DOI] [PubMed] [Google Scholar]
- 45.Frankenhaeuser M A Psychobiological Framework for Research on Human Stress and Coping. In: Dynamics of Stress. Springer US; 1986:101–116. doi: 10.1007/978-1-4684-5122-1_6 [DOI] [Google Scholar]
- 46.Cannon WB. Bodily Changes in Pain, Hunger, Fear and Rage. Branford; 1929. doi: 10.1080/08856559.1930.10532290 [DOI] [Google Scholar]
- 47.Rondó PHC, Ferreira RF, Nogueira F, Ribeiro MCN, Lobert H, Artes R. Maternal psychological stress and distress as predictors of low birth weight, prematurity and intrauterine growth retardation. Eur J Clin Nutr. 2003;57(2):266–272. doi: 10.1038/sj.ejcn.1601526 [DOI] [PubMed] [Google Scholar]
- 48.Van den Bergh BRH, van den Heuvel MI, Lahti M, Braeken M, de Rooij SR, Entringer S, Hoyer D, Roseboom T, Räikkönen K, King S, et al. Prenatal developmental origins of behavior and mental health: The influence of maternal stress in pregnancy. Neurosci Biobehav Rev. 2020;117:26–64. doi: 10.1016/j.neubiorev.2017.07.003 [DOI] [PubMed] [Google Scholar]
- 49.Kingston D, McDonald S, Austin M-P, Tough S. Association between Prenatal and Postnatal Psychological Distress and Toddler Cognitive Development: A Systematic Review. PLoS One. 2015;10(5):e0126929. doi: 10.1371/JOURNAL.PONE.0126929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.S M, H O, N R, RMP F, L S, E A, JE C, GM T. A Meta-Analysis of Maternal Prenatal Depression and Anxiety on Child Socioemotional Development. J Am Acad Child Adolesc Psychiatry. 2018;57(9):645–657.e8. doi: 10.1016/J.JAAC.2018.06.012 [DOI] [PubMed] [Google Scholar]
- 51.TG O, C M, AS B. Maternal Affective Illness in the Perinatal Period and Child Development: Findings on Developmental Timing, Mechanisms, and Intervention. Curr Psychiatry Rep. 2016;18(3):1–5. doi: 10.1007/S11920-016-0660-Y [DOI] [PubMed] [Google Scholar]
- 52.KJ O, V G, ED B, TG O. The persisting effect of maternal mood in pregnancy on childhood psychopathology. Dev Psychopathol. 2014;26(2):393–403. doi: 10.1017/S0954579414000029 [DOI] [PubMed] [Google Scholar]
- 53.Zijlmans MAC, Riksen-Walraven JM, de Weerth C. Associations between maternal prenatal cortisol concentrations and child outcomes: A systematic review. Neurosci Biobehav Rev. 2015;53:1–24. doi: 10.1016/j.neubiorev.2015.02.015 [DOI] [PubMed] [Google Scholar]
- 54.Gitau R, Cameron A, Fisk NM, Glover V. Fetal exposure to maternal cortisol. Lancet. 1998;352(9129):707–708. doi: 10.1016/S0140-6736(05)60824-0 [DOI] [PubMed] [Google Scholar]
- 55.Murphy BE, Clark SJ, Donald IR, Pinsky M, Vedady D. Conversion of maternal cortisol to cortisone during placental transfer to the human fetus. Am J Obstet Gynecol. 1974;118(4):538–541. doi: 10.1016/S0002-9378(16)33697-3 [DOI] [PubMed] [Google Scholar]
- 56.Zijlmans MAC, Korpela K, Riksen-Walraven JM, de Vos WM, de Weerth C. Maternal prenatal stress is associated with the infant intestinal microbiota. Psychoneuroendocrinology. 2015;53:233–245. doi: 10.1016/j.psyneuen.2015.01.006 [DOI] [PubMed] [Google Scholar]
- 57.Dewey KG, Nommsen-Rivers LA, Heinig MJ, Cohen RJ. Lactogenesis and infant weight change in the first weeks of life. In: Integrating Population Outcomes, Biological Mechanisms and Research Methods in the Study of Human Milk and Lactation. Vol 503. Kluwer Academic/Plenum Publishers; 2002:159–166. doi: 10.1007/978-1-4615-0559-4_18 [DOI] [PubMed] [Google Scholar]
- 58.MS B, SL Y, EL T. Perinatal depressive symptoms and breastfeeding behaviors: A systematic literature review and biosocial research agenda. J Affect Disord. 2021;283:441–471. doi: 10.1016/J.JAD.2020.11.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.E de J, H S, J B, L A, K M. Psychosocial correlates of exclusive breastfeeding: a systematic review. Midwifery. 2013;29(5):506–518. doi: 10.1016/J.MIDW.2012.04.009 [DOI] [PubMed] [Google Scholar]
- 60.CC D, B F. Breastfeeding and depression: a systematic review of the literature. J Affect Disord. 2015;171:142–154. doi: 10.1016/J.JAD.2014.09.022 [DOI] [PubMed] [Google Scholar]
- 61.Fallon V, Bennett KM, Harrold JA. Prenatal Anxiety and Infant Feeding Outcomes: A Systematic Review. J Hum Lact. 2016;32(1):53–66. doi: 10.1177/0890334415604129 [DOI] [PubMed] [Google Scholar]
- 62.V F, R G, JC H, KM B, JA H. Postpartum Anxiety and Infant-Feeding Outcomes. J Hum Lact. 2016;32(4):740–758. doi: 10.1177/0890334416662241 [DOI] [PubMed] [Google Scholar]
- 63.Grigoriadis S, Graves L, Peer M, Mamisashvili L, Tomlinson G, Vigod SN, Dennis C-L, Steiner M, Brown C, Cheung A, et al. A systematic review and meta-analysis of the effects of antenatal anxiety on postpartum outcomes. Arch Women’s Ment Heal 2018 225. 2018;22(5):543–556. doi: 10.1007/S00737-018-0930-2 [DOI] [PubMed] [Google Scholar]
- 64.CE H, N M, AK RV, R P-E. Impact of Maternal Anxiety on Breastfeeding Outcomes: A Systematic Review. Adv Nutr. 2019;10(5):816–826. doi: 10.1093/ADVANCES/NMY132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mangrio E, Persson K, Bramhagen A-C. Sociodemographic, physical, mental and social factors in the cessation of breastfeeding before 6 months: a systematic review. Scand J Caring Sci. 2018;32:451–465. doi: 10.1111/scs.12489 [DOI] [PubMed] [Google Scholar]
- 66.Daly SEJ, Kent JC, Owens RA, Hartmann PE. Frequency and degree of milk removal and the short-term control of human milk synthesis. Exp Physiol. 1996;81(5):861–875. doi: 10.1113/expphysiol.1996.sp003982 [DOI] [PubMed] [Google Scholar]
- 67.Chapman DJ, Pérez-Escamilla R. Identification of risk factors for delayed onset of lactation. J Am Diet Assoc. 1999;99(4):450–454. doi: 10.1016/S0002-8223(99)00109-1 [DOI] [PubMed] [Google Scholar]
- 68.Doulougeri K, Panagopoulou E, Montgomery A. The impact of maternal stress on initiation andestablishment of breastfeeding. J Neonatal Nurs. 2013;19(4):162–167. doi: 10.1016/j.jnn.2013.02.003 [DOI] [Google Scholar]
- 69.Chapman DJ, Pérez-Escamilla R. Does Delayed Perception of the Onset of Lactation Shorten Breastfeeding Duration? J Hum Lact. 1999;15(2):107–111. doi: 10.1177/089033449901500207 [DOI] [PubMed] [Google Scholar]
- 70.M D, P T, B M, T G, N K, S Z, G G. Evaluation of the effect of natural and emotional stress of labor on lactation and breast-feeding. Arch Gynecol Obstet. 2016;293(2):317–328. doi: 10.1007/S00404-015-3783-1 [DOI] [PubMed] [Google Scholar]
- 71.DiGirolamo AM, Grummer-Strawn LM, Fein S. Maternity care practices: Implications for breastfeeding. Birth. 2001;28(2):94–100. doi: 10.1046/j.1523-536X.2001.00094.x [DOI] [PubMed] [Google Scholar]
- 72.Bolton TA, Chow T, Benton PA, Olson BH. Characteristics associated with longer breastfeeding duration: An analysis of a peer counseling support program. J Hum Lact. 2009;25(1):18–27. doi: 10.1177/0890334408325985 [DOI] [PubMed] [Google Scholar]
- 73.Kent JC. How Breastfeeding Works. J Midwifery Women’s Heal. 2007;52(6):564–570. doi: 10.1016/j.jmwh.2007.04.007 [DOI] [PubMed] [Google Scholar]
- 74.Paul IM, Downs DS, Schaefer EW, Beiler JS, Weisman CS. Postpartum Anxiety and Maternal-Infant Health Outcomes. Pediatrics. 2013;131(4):e1218–e1224. doi: 10.1542/PEDS.2012-2147 [DOI] [PubMed] [Google Scholar]
- 75.Mehta UJ, Siega-Riz AM, Herring AH, Adair LS, Bentley ME. Pregravid body mass index, psychological factors during pregnancy and breastfeeding duration: Is there a link? Matern Child Nutr. 2012;8(4):423–433. doi: 10.1111/j.1740-8709.2011.00335.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ahlqvist-Björkroth S, Vaarno J, Junttila N, Pajulo M, Räihä H, Niinikoski H, Lagström H. Initiation and exclusivity of breastfeeding: Association with mothers’ and fathers’ prenatal and postnatal depression and marital distress. Acta Obstet Gynecol Scand. 2016;95(4):396–404. doi: 10.1111/aogs.12857 [DOI] [PubMed] [Google Scholar]
- 77.Skouteris H, Wertheim EH, Rallis S, Milgrom J, Paxton SJ. Depression and anxiety through pregnancy and the early postpartum: An examination of prospective relationships. J Affect Disord. 2009;113(3):303–308. doi: 10.1016/j.jad.2008.06.002 [DOI] [PubMed] [Google Scholar]
- 78.B F, CC D, S B, C C, R N-C. Breastfeeding and postpartum depression: state of the art review. J Pediatr (Rio J). 2013;89(4):332–338. doi: 10.1016/J.JPED.2012.12.002 [DOI] [PubMed] [Google Scholar]
- 79.Woolhouse H, James J, Gartland D, McDonald E, Brown SJ. Maternal depressive symptoms at three months postpartum and breastfeeding rates at six months postpartum: Implications for primary care in a prospective cohort study of primiparous women in Australia. Women and Birth. 2016;29(4):381–387. doi: 10.1016/J.WOMBI.2016.05.008 [DOI] [PubMed] [Google Scholar]
- 80.VM D, IRA C, ZT H. Association Between Stressful Life Events and Exclusive Breastfeeding Among Mothers in the United States. Breastfeed Med. 2019;14(7):475–481. doi: 10.1089/BFM.2019.0058 [DOI] [PubMed] [Google Scholar]
- 81.J H-H, MG H, C DS, LM G. Does breastfeeding offer protection against maternal depressive symptomatology?: A prospective study from pregnancy to 2 years after birth. Arch Womens Ment Health. 2013;16(5):411–422. doi: 10.1007/S00737-013-0348-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gila-Díaz A, Carrillo GH, Pablo ÁLL de, Arribas SM, Ramiro-Cortijo D. Association between Maternal Postpartum Depression, Stress, Optimism, and Breastfeeding Pattern in the First Six Months. Int J Environ Res Public Health. 2020;17(19):1–13. doi: 10.3390/IJERPH17197153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.MW G. Differences between exclusive breastfeeders, formula-feeders, and controls: a study of stress, mood, and endocrine variables. Biol Res Nurs. 2005;7(2):106–117. doi: 10.1177/1099800405280936 [DOI] [PubMed] [Google Scholar]
- 84.DC H, J H-H, S C, V D, LB C, DA M. Symptoms of postpartum depression and breastfeeding. J Hum Lact. 2005;21(4):444–449. doi: 10.1177/0890334405280947 [DOI] [PubMed] [Google Scholar]
- 85.Ahn S, Corwin EJ. The association between breastfeeding, the stress response, inflammation, and postpartum depression during the postpartum period: Prospective cohort study Authors. Int J Nurs Stud. 2015;52(10):1582. doi: 10.1016/J.IJNURSTU.2015.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.M G, SJ W, H B, AJ L. Breastfeeding, Antidepressants, and Depression in the Mercy Pregnancy and Emotional Well-Being Study. J Hum Lact. 2019;35(1):127–136. doi: 10.1177/0890334418758658 [DOI] [PubMed] [Google Scholar]
- 87.Gatti L Maternal perceptions of insufficient milk supply in breastfeeding. J Nurs Scholarsh. 2008;40(4):355–363. doi: 10.1111/j.1547-5069.2008.00234.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.JC K, LR M, MD C, DT R, DA D, PE H. Volume and frequency of breastfeedings and fat content of breast milk throughout the day. Pediatrics. 2006;117(3). doi: 10.1542/PEDS.2005-1417 [DOI] [PubMed] [Google Scholar]
- 89.Li R, Fein SB, Chen J, Grummer-Strawn LM. Why mothers stop breastfeeding: Mothers’ self-reported reasons for stopping during the first year. Pediatrics. 2008;122(SUPPL. 2). doi: 10.1542/peds.2008-1315i [DOI] [PubMed] [Google Scholar]
- 90.Ueda T, Yokoyama Y, Irahara M, Aono T. Influence of psychological stress on suckling-induced pulsatile oxytocin release. Obs Gynecol. 1994;84(2):259–262. Accessed June 7, 2021. https://pubmed.ncbi.nlm.nih.gov/8041543/ [PubMed] [Google Scholar]
- 91.Stuebe AM, Grewen K, Meltzer-Brody S. Association between maternal mood and oxytocin response to breastfeeding. J Women’s Heal. 2013;22(4):352–361. doi: 10.1089/jwh.2012.3768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lara-Cinisomo S, McKenney K, Florio A Di, Meltzer-Brody S. Associations Between Postpartum Depression, Breastfeeding, and Oxytocin Levels in Latina Mothers. Breastfeed Med. 2017;12(7):436. doi: 10.1089/BFM.2016.0213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mezzacappa ES, Katkin ES. Breast-feeding is associated with reduced perceived stress and negative mood in mothers. Heal Psychol. 2002;21(2):187–193. doi: 10.1037/0278-6133.21.2.187 [DOI] [PubMed] [Google Scholar]
- 94.Riddle SW, Nommsen-Rivers LA. A Case Control Study of Diabetes during Pregnancy and Low Milk Supply. Breastfeed Med. 2016;11(2):80–85. doi: 10.1089/bfm.2015.0120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nommsen-Rivers LA, Dolan LM, Huang B. Timing of Stage II Lactogenesis Is Predicted by Antenatal Metabolic Health in a Cohort of Primiparas. Breastfeed Med. 2012;7(1):43–49. doi: 10.1089/bfm.2011.0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Oza-Frank R, Chertok I, Bartley A. Differences in breast-feeding initiation and continuation by maternal diabetes status. Public Health Nutr. 2015;18(4):727–735. doi: 10.1017/S1368980014000792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nommsen-Rivers LA. Does insulin explain the relation between maternal obesity and poor lactation outcomes? An overview of the literature. Adv Nutr. 2016;7(2):407–414. doi: 10.3945/an.115.011007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Neville MC, Webb P, Ramanathan P, Mannino MP, Pecorini C, Monks J, Anderson SM, MacLean P. The insulin receptor plays an important role in secretory differentiation in the mammary gland. Am J Physiol - Endocrinol Metab. 2013;305(9). doi: 10.1152/ajpendo.00337.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zardooz H, Sadeghimahalli F, Khodagholi F. Early postnatal stress impairs insulin secretion in response to psychological stress in adult rats. J Endocrinol Invest. 2021;44(2):277–286. doi: 10.1007/s40618-020-01291-9 [DOI] [PubMed] [Google Scholar]
- 100.Li L, Li X, Zhou W, Messina JL. Acute psychological stress results in the rapid development of insulin resistance. J Endocrinol. 2013;217(2):175–184. doi: 10.1530/JOE-12-0559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.MacGregor C, Freedman A, Keenan-Devlin L, Grobman W, Wadhwa P, Simhan HN, Buss C, Borders A. Maternal perceived discrimination and association with gestational diabetes. Am J Obstet Gynecol MFM. 2020;2(4):100222. doi: 10.1016/j.ajogmf.2020.100222 [DOI] [PubMed] [Google Scholar]
- 102.Winokur A, Maislin G, Phillips JL, Amsterdam JD. Insulin Resistance After Oral Glucose Tolerance Testing in Patients With Major Depression. Vol 145.; 1988. [DOI] [PubMed] [Google Scholar]
- 103.PJ B, JA R, AJ D. Adaptive responses of the maternal hypothalamic-pituitary-adrenal axis during pregnancy and lactation. J Neuroendocrinol. 2008;20(6):764–776. doi: 10.1111/J.1365-2826.2008.01735.X [DOI] [PubMed] [Google Scholar]
- 104.M H, G M, I N, S W, C K, U E, DH H. Effects of suckling on hypothalamic-pituitary-adrenal axis responses to psychosocial stress in postpartum lactating women. J Clin Endocrinol Metab. 2001;86(10):4798–4804. doi: 10.1210/JCEM.86.10.7919 [DOI] [PubMed] [Google Scholar]
- 105.EQ C, A S, B P, K G, D R, S M-B. Oxytocin and HPA stress axis reactivity in postpartum women. Psychoneuroendocrinology. 2015;55:164–172. doi: 10.1016/J.PSYNEUEN.2015.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stuebe AM, Grewen K, Pedersen CA, Propper C, Meltzer-Brody S. Failed Lactation and Perinatal Depression: Common Problems with Shared Neuroendocrine Mechanisms? J Women’s Heal. 2012;21(3):264. doi: 10.1089/JWH.2011.3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.MT T, SJ L, CD W. Multiparity reveals the blunting effect of breastfeeding on physiological reactivity to psychological stress. J Neuroendocrinol. 2006;18(7):494–503. doi: 10.1111/J.1365-2826.2006.01441.X [DOI] [PubMed] [Google Scholar]
- 108.JA A, JM J, AH V. Suckling-induced attenuation of plasma cortisol concentrations in postpartum lactating women. Endocr Res. 1994;20(1):79–87. doi: 10.3109/07435809409035858 [DOI] [PubMed] [Google Scholar]
- 109.Seth S, Lewis AJ, Galbally M. Perinatal maternal depression and cortisol function in pregnancy and the postpartum period: a systematic literature review. BMC Pregnancy Childbirth 2016 161. 2016;16(1):1–19. doi: 10.1186/S12884-016-0915-Y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ziomkiewicz A, Babiszewska M, Apanasewicz A, Piosek M, Wychowaniec P, Cierniak A, Barbarska O, Szołtysik M, Danel D, Wichary S. Psychosocial stress and cortisol stress reactivity predict breast milk composition. Sci Rep. 2021;11(1):11576. doi: 10.1038/s41598-021-90980-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Thibeau S, D’Apolito K, Minnick AF, Dietrich MS, Kane B, Cooley S, Groer M. Relationships of maternal stress with milk immune components in African American mothers of healthy term infants. Breastfeed Med. 2016;11(1):6–14. doi: 10.1089/bfm.2015.0117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bécares L, Nazroo J, Kelly Y. A longitudinal examination of maternal, family, and area-level experiences of racism on children’s socioemotional development: Patterns and possible explanations. Soc Sci Med. 2015;142:128–135. doi: 10.1016/J.SOCSCIMED.2015.08.025 [DOI] [PubMed] [Google Scholar]
- 113.Murry VM, Brown PA, Brody GH, Cutrona CE, Simons RL. Racial Discrimination as a Moderator of the Links Among Stress, Maternal Psychological Functioning, and Family Relationships. J Marriage Fam. 2001;63(4):915–926. doi: 10.1111/J.1741-3737.2001.00915.X [DOI] [Google Scholar]
- 114.Griswold MK, Crawford SL, Perry DJ, Person SD, Rosenberg L, Cozier YC, Palmer JR. Experiences of Racism and Breastfeeding Initiation and Duration Among First-Time Mothers of the Black Women’s Health Study. J racial Ethn Heal disparities. 2018;5(6):1180. doi: 10.1007/S40615-018-0465-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Henshaw EJ, Fried R, Siskind E, Newhouse L, Cooper M. Breastfeeding self-efficacy, mood, and breastfeeding outcomes among primiparous women. J Hum Lact. 2015;31(3):511–518. doi: 10.1177/0890334415579654 [DOI] [PubMed] [Google Scholar]
- 116.Bigelow AE, Power M. Mother–Infant Skin-to-Skin Contact: Short- and Long-Term Effects for Mothers and Their Children Born Full-Term. Front Psychol. 2020;11:1921. doi: 10.3389/fpsyg.2020.01921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.De Alencar AEMA, Arraes LC, De Albuquerque EC, Alves JGB. Effect of kangaroo mother care on postpartum depression. J Trop Pediatr. 2009;55(1):36–38. doi: 10.1093/tropej/fmn083 [DOI] [PubMed] [Google Scholar]
- 118.C B, M I, A S. New evidence on breastfeeding and postpartum depression: the importance of understanding women’s intentions. Matern Child Health J. 2015;19(4):897–907. doi: 10.1007/S10995-014-1591-Z [DOI] [PMC free article] [PubMed] [Google Scholar]