Abstract
Objectives:
To assess the association between all-cause mortality and hs-CRP, based mainly on the cumulative burden approach.
Methods:
Cohort study with adults ≥35 years from general population, using hs-CRP at two timepoints: at baseline and 30 months later to establish different exposures: change over time, cumulative, and weighted cumulative hs-CRP. The outcome was all-cause mortality assessed 7 years later. Cox models were generated to quantify the association.
Results:
Data from 3,119 participants (mean age 55.6 years, and 51.2% females), were analyzed. During follow-up, 164 (5.6%) deaths occurred over 20,314.5 person-years, indicating an overall mortality rate of 8.1 per 1,000 person-years. In multivariable model, hs-CRP at baseline was associated with high risk of mortality (HR = 1.77; 95%CI: 1.28–2.46). Similarly, hs-CRP change over time (HR = 2.50; 95%CI: 1.46–4.29), as well as cumulative and weighted cumulative hs-CRP (HR = 2.05; 95%CI: 1.31–3.20) were associated with greater risk of all-cause mortality. The weighted cumulative hs-CRP had the best goodness-of-fit for mortality prediction.
Conclusions:
In this cohort across diverse geographical low-resource settings, high levels of hs-CRP were strongly associated with all-cause mortality. Two measurements of HS-CRP are better than one to predict mortality, and the weighted cumulative approach had the best prognostic fit.
Keywords: Mortality, inflammation, C-reactive protein, cohort, Peru
INTRODUCTION
The C-reactive protein (CRP) is a well-known measure of inflammation [1] and can be detected with a simple test. There are two types of tests used to determine CRP levels: conventional and high-sensitive CRP. Of these, high-sensitive CRP (hs-CRP) is used for the assessment of conditions thought to be associated with inflammation in otherwise healthy individuals [2]. Different literature has determined that hs-CRP is a predictor of different conditions [3–6]. Nevertheless, many of these studies have been conducted using clinical trials or hospital-based studies, unlikely to reflect the underlying health profile of the general population.
hs-CRP measured at a single time point has been widely studied and associated with different non-communicable conditions, including cardiovascular disease (CVD) (i.e., coronary heart disease, heart failure, and stroke) [7, 8] and incident diabetes [9]. In addition, the studies have also assessed the prediction performance of hs-CRP on cardiovascular and all-cause mortality [9–11]. However, there are sparse data about the potential role of the cumulative effect of hs-CRP with outcomes in the general population, especially from low- and middle-income countries [12, 13], where the inflammation profile may be different to high income countries from where most evidence derives. Moreover, the cumulative effect of hs-CRP has been evaluated following diverse definitions. For example, a study with the Atherosclerosis Risk in Communities (ARIC) data used two different hs-CRP measurements separated by six years to evaluate the risk of diabetes, CVD and all-cause mortality [9]. In another study, hs-CRP levels were measured three times, and participants were classified according to the cumulative exposure, from 0 to 3, depending on the number of positive hs-CRP tests (≥3 mg/L) over time to determine the association with incident cardiovascular events [7]. Another study used a weighted sum of different measurements and its association with cardiovascular risk [8].
As a result, whether any of the longitudinal approaches to assess the cumulative effect of hs-CRP are equivalent or not, requires further assessment, especially in resource-constrained settings. In this vein, using a prospective design and including subjects with different socioeconomic, cultural, and health backgrounds, this study aimed to assess the association between all-cause mortality and hs-CRP levels, using different definitions especially focused on the cumulative burden of hs-CRP.
MATERIALS AND METHODS
Study design
The methodology of the CRONICAS Cohort Study has been detailed elsewhere [14]. Subjects were recruited from Pampas de San Juan de Miraflores, an area located at the sea level and in highly-urbanized Lima; Tumbes, a semiurban area in a coastal region in the north of Peru; and Puno, a high-altitude region, contributing with similar number of participants from urban and rural settings. For this analysis, information of the baseline assessment, conducted between 2010 and 2012; the follow-up evaluation carried out in 2013–2014; and the last evaluation conducted in 2018, was utilized.
Study participants
An age- and sex-stratified (35–44, 45–54, 55–64, and 65+ years) random sampling approach was used to enroll participants using the most updated census in each study site. Only one participant per household was enrolled to avoid clustering of risk factors. Individuals aged ≥35 years and with full-time residence in the study area were invited to participate. Pregnant women, those incapable of providing informed consent, those bedridden or with physical disability preventing measurements of blood pressure or anthropometrics, or those with active tuberculosis, were excluded.
Definition of variables
Outcome:
All cause-mortality, defined by the occurrence of any fatal event between baseline enrollment and any point during follow-up. The time between baseline assessment and the date of death or censorship was then estimated in years. In 2018, the vital status of participants (dead or alive) was retrieved from national vital records. For this assessment, only vital status and date of death or censoring was used. If a participant had died, the date of death was used for analysis purposes; but if participant was alive, then the date when the search in the National Registry of Identification and Civil Status (RENIEC in Spanish) database was conducted was considered as the censoring date. The RENIEC, using the National Identification Number (DNI), identifies Peruvian individuals, and for instance, the risk of misclassification of the vital status is very low. [15].
Exposure:
hs-CRP levels were the exposure of interest, assessed using latex (Tina-quant CRP-hs Roche/Hitachi analyzer, IN, US) from an 8 to 12-hour fasting serum sample and reported in mg/L. As suggested by literature [2, 9], a cut-off of 3 mg/L was used to split the population in those with high (≥3 mg/L) and low (<3 mg/L) hs- CRP levels, as follows:
First, high or low level of hs-CRP, assessed at baseline, were used and analyzed as potential predictor of all-cause mortality. In addition to that definition, hs-CRP levels at baseline were also split into quartiles to evaluate dose response.
In the second definition, hs-CRP change over time was used instead. For instance, the combination of hs-CRP levels at baseline and 30 months apart (follow-up) was pursued, generating four groups for analysis: low hs-CRP level both at baseline and follow-up (used as reference group), low hs-CRP levels at baseline but high at follow-up, high hs-CRP levels at baseline but low at follow-up, and high hs-CRP levels both at baseline and follow-up.
In the third definition, cumulative hs-CRP was assessed as the number of positive tests (≥3 mg/L) allowing a classification of study subjects from 0 (low in both rounds) to 2 (high in both rounds).
In the fourth definition, the weighted cumulative hs-CRP was defined as the average of the two hs-CRP measurements subtracted from the usual threshold for low/high hs-CRP (3 mg/L), and then multiplied by the time (years) between measurements, using the following formula:
If the values of the cumulative hs-CRP between the two consecutive examinations were less than 0, then this value was considered as 0. Any value greater than 0 was considered as high cumulative hs-CRP as in a previous work [8]. Additionally, weighted cumulative hs-CRP levels were also split into quartiles to assess dose response.
Other variables:
Baseline demographic, behavioral, and cardiovascular variables were included in the analysis as potential confounders of the association of interest. Demographic variables were: sex (female vs. male); age (in years, stratified as the original sampling approach: 35–44, 45–54, 55–64, and 65+ years); education level (in years, <7, 7–11, and 12+ years); socioeconomic position, based on a wealth index using household assets and facilities separately for each study site and then combined into a single variable and presented in tertiles; and study site (highly-urbanized Lima, urban Puno, rural Puno, and semiurban Tumbes).
Behavioral factors included self-reported smoking status (never smoker, former smoker, and current smoker); alcohol drinking, based on the consumption of at least 6 drinks of beer or any equivalent beverage once or more per month (high vs. low); physical activity levels, based on the leisure-time and transport-related domains of the International Physical Activity Questionnaire (IPAQ), and split into low and moderate/high levels [16]; and body mass index, with the traditional cutoffs for normal (BMI<25 kg/m2), overweight (25 kg/m2 ≤ BMI ≤30 kg/m2), and obesity (BMI ≥30 kg/m2).
Finally, well-known cardiovascular risk factors were also included: metabolic syndrome, defined using the 2009 harmonized definition [17]; hypertension, defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or self-report of physician diagnosis or current use of antihypertensive medication (yes vs. no) [18]; total cholesterol levels, defined as normal (total cholesterol <200 mg/dL), high-normal (200 – 239 mg/dL), and high (≥240 mg/dL) [19]; and type 2 diabetes status, defined as fasting glucose ≥126 mg/dL, or self-report of physician diagnosis or currently receiving antidiabetic drugs [20].
Statistical analysis
STATA 16 for Windows (StataCorp, College Station, TX, US) was utilized for statistical analysis. At the beginning, a description of the study participants at baseline was pursued. Mean and standard deviation (SD) or median and interquartile range (IQR) were used for numerical variables based on assessment of normal distribution using the Shapiro-Wilk test, whereas frequencies and proportions were used for categorical variables.
The baseline prevalence of high levels of hs-CRP and 95% confidence intervals (95%CI) were estimated. In addition, the proportion of individuals with the different categories of hs-CRP levels (i.e., taking into account both baseline and follow-up assessment) was also estimated. Distribution of population characteristics by exposures of interest were also carried out and compared using the Chi-squared test.
Mortality rates for the overall sample as well as by hs-CRP definitions were estimated by using person-time and incidence rates. These estimates were reported by 1,000 person-years of follow-up. Crude and adjusted Cox proportional hazard models were generated to assess the association between each hs-CRP exposure (i.e., baseline, change over time, cumulative, and weighted cumulative) and all-cause mortality, reporting hazard ratios (HR) and 95%CI. Proportional hazard assumptions were tested using the Schoenfeld residuals in post-estimation fashion. Study site was the only variable violating this assumption, and as a result, a stratified Cox procedure was used to deal with this violation, and control for study site. The variance inflation factor (VIF) was also estimated to assess collinearity given the number of covariables included in the adjusted models.
Finally, in order to identify which hs-CRP definition has a better fit to predict all-cause mortality, we used the Bayesian Information criterion (BIC); that is, the model with the best BIC (smallest value) would predict all-cause mortality better than the models with other hs-CRP definitions. The decision of using the information criterion relies on the comparison done using the maximum likelihood models and because the BIC penalizes depending on the number of parameters and the number of observations [21].
Ethics
The study was reviewed and approved by the Institutional Review Boards (IRB) at Universidad Peruana Cayetano Heredia (UPCH) and A.B. PRISMA, in Peru, and the Bloomberg school of Public Health at Johns Hopkins University, in US. Participants provided oral informed consent because of high illiteracy rates, mainly in rural areas. Follow-up evaluation to assess participants’ vital status was approved by the IRB at UPCH.
RESULTS
Description of the study population
A total of 3,601 participants were enrolled at the baseline of the cohort, but 482 (13.4%) records were excluded as blood samples were not available for hs-CRP use. Thus, data from 3,119 (86.6%) records were analyzed, with a mean age of 55.6 (SD: 12.7) years, 1,597 (51.2%) females, and 1,429 (45.8%) with <7 years of education.
hs-CRP levels and associated factors
At baseline, the median of hs-CRP level in the population was 1.7 mg/L (IQR: 0.8 – 3.6), and 937 (30.0%; 95%CI: 28.4% - 31.7%) had high levels. Sex (p<0.001), age (p<0.001), education level (p<0.001), study site (p<0.001), physical activity (p<0.001), body mass index (p<0.001), metabolic syndrome (p<0.001), hypertension (p<0.001), total cholesterol (p=0.009), and type 2 diabetes (p<0.001) were associated with high hs-CRP levels at baseline (Table 1). Description of the population characteristics by baseline hs-CRP levels split into quartiles are shown in supplemental file (e-Table 1).
Table 1:
Characteristics of the study population by HS-CRP level at baseline.
| HS-CRP at baseline |
|||
|---|---|---|---|
| Low (n = 2,180) | High (n = 937) | p-value | |
|
| |||
| Sex | <0.001 | ||
| Female | 1,025 (47.0%) | 572 (61.1%) | |
| Age | <0.001 | ||
| 35 – 44 years | 573 (26.3%) | 187 (19.9%) | |
| 45 – 54 years | 574 (26.3%) | 223 (23.8%) | |
| 55 – 64 years | 527 (24.2%) | 263 (28.1%) | |
| 65+ years | 506 (23.2%) | 264 (28.2%) | |
| Education level | <0.001 | ||
| < 7 years | 953 (43.7%) | 476 (50.8%) | |
| 7 – 11 years | 725 (33.3%) | 302 (32.2%) | |
| 12+ years | 502 (23.0%) | 159 (17.0%) | |
| Socioeconomic position | 0.07 | ||
| Low | 716 (32.8%) | 271 (28.9%) | |
| Middle | 719 (33.0%) | 339 (36.2%) | |
| High | 747 (34.2%) | 327 (34.9%) | |
| Study site | <0.001 | ||
| Lima | 672 (30.8%) | 359 (38.3%) | |
| Urban Puno | 397 (18.2%) | 120 (12.8%) | |
| Rural Puno | 466 (21.4%) | 74 (7.9%) | |
| Tumbes | 647 (29.6%) | 384 (41.0%) | |
| Smoking | 0.08 | ||
| Never smoker | 1,196 (54.8%) | 554 (59.2%) | |
| Former smoker | 723 (33.1%) | 282 (30.1%) | |
| Current smoker | 263 (12.1%) | 100 (10.7%) | |
| Alcohol drinking | 0.69 | ||
| High | 117 (5.4%) | 47 (5.0%) | |
| Physical activity level | <0.001 | ||
| Low | 634 (29.1%) | 373 (39.8%) | |
| Body mass index | <0.001 | ||
| Normal | 715 (32.8%) | 196 (21.0%) | |
| Overweight | 1,023 (46.9%) | 343 (36.7%) | |
| Obesity | 442 (20.3%) | 396 (42.4%) | |
| Metabolic syndrome | <0.001 | ||
| Yes | 911 (41.8%) | 553 (59.2%) | |
| Hypertension | <0.001 | ||
| Yes | 494 (22.7%) | 313 (33.5%) | |
| Total cholesterol | 0.009 | ||
| < 200 mg/dL | 1,181 (54.1%) | 474 (50.6%) | |
| 200 – 239 mg/dL | 685 (31.4%) | 287 (30.6%) | |
| ≥240 mg/dL | 316 (14.5%) | 176 (18.8%) | |
| Type 2 diabetes | <0.001 | ||
| Yes | 130 (6.0%) | 129 (13.8%) | |
P-values were estimated using Chi-squared test
During follow-up, on average 30 months later, 2,529 (81.1% of the analyzed sample) subjects had information related to hs-CRP level, with a median of 1.7 (IQR: 0.9 – 3.4) and 728 (28.8%; 95%CI: 27.0% - 30.6%) were categorized as having high hs-CRP levels.
When assessing the hs-CRP change over time, 1,470 (58.4%) participants had low levels of hs-CRP at baseline and during follow-up assessment, whereas 269 (10.7%) had low levels at baseline and high levels during follow-up. In addition, 323 (12.8%) participants had high levels of hs-CRP at baseline and low levels at follow-up, and 456 (18.1%) had high levels of hs-CRP at both assessments. Variables associated with hs-CRP change over time are shown in supplemental file (e-Table 2).
When cumulative hs-CRP baseline and follow-up information were used, 1,470 (58.4%) had both assessments in low levels, but 592 (23.5%) and 456 (18.1%) had only one and two assessments over 3 mg/L, respectively.
When weighted cumulative HS-CRP was evaluated, 829 (33.0%) subjects had values compatible with high levels. Variables associated with this hs-CRP definition are shown in supplemental file (e-Table 3). Besides, population characteristics by weighted cumulative hs-CRP levels split into quartiles are shown in supplemental file (e-Table 4).
HS-CRP levels and all-cause mortality
A total of 2,905 (93.1%) out of the 3,119 subjects analyzed at baseline had data of mortality available for analysis, with a mean time of follow-up of 7.0 (SD: 1.0) years, 164 (5.6%) deaths, accruing 20,314.5 person-years, and an overall mortality rate of 8.1 per 1,000 person-years of follow-up
When assessing only hs-CRP level at baseline (n = 2,894), mortality rate among those with high values of hs-CRP almost doubled that of those with low levels (11.6 vs. 6.5 per 1,000 person-years). In multivariable model, high levels of hs-CRP at baseline were associated with 81% increased risk of mortality during follow-up (HR = 1.81; 95%CI: 1.30 – 2.51). In dose-response analysis, and despite of a clear increasing trends in the rate of mortality, only those in the highest quartile had significantly greater risk of mortality compared to those in the lowest quartile (HR = 2.88; 95%CI: 1.78 – 4.66). See Figure 1.
Figure 1:
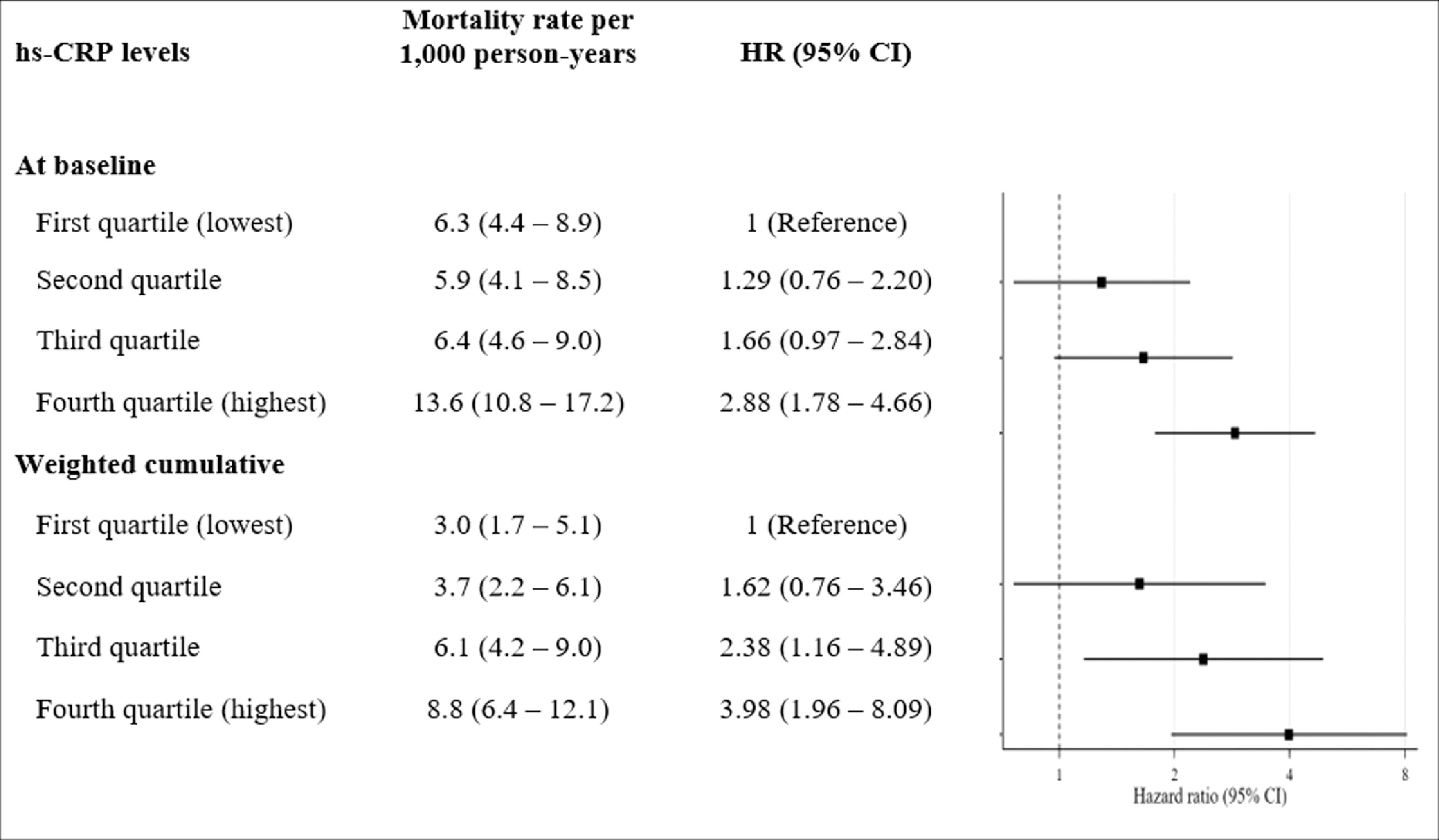
Dose response effect of hs-CRP on all-cause mortality
When evaluating the hs-CRP change over time (n = 2,393), mortality rate was more than double among those with high levels of hs-CRP at both baseline and follow-up when compared to those with low levels of hs-CRP at both assessments (8.9 vs. 4.0 per 1,000 person-years). In multivariable model, those with high levels of hs-CRP at both evaluations had 151% greater risk of death (HR = 2.51; 95%CI: 1.46 – 4.31). Nevertheless, any variation of hs-CRP level during follow-up (i.e., those who changed from low levels at baseline to high level at follow-up, and vice-versa) was not associated with the outcome. Findings were similar when using the cumulative hs-CRP effect.
Finally, when weighted cumulative hs-CRP was used (n = 2,389), multivariable model showed that those with high weighted cumulative hs-CRP had double risk of mortality compared to those with low risk (HR = 2.09; 95%CI: 1.33 – 3.28). In dose-response analysis, a clear increasing trend in the rate of mortality was observed at high levels of weighted cumulative hs-CRP (Figure 1). Thus, those in the high and highest quartiles were at greater risk of mortality (HR = 2.38; 95%CI: 1.16 – 4.89 and HR = 3.98; 1.96 – 8.09, respectively). There was no collinearity in the multivariable models as all the VIF were <5.
When BIC was used to compare models, hs-CRP at baseline had the highest value, and differences between hs-CRP change over time and cumulative hs-CRP were small. The BIC of the model using the weighted cumulative hs-CRP had the best predictive fit (lowest BIC). See details in Table 2.
Table 2:
All-cause mortality by HS-CRP definition: crude and adjusted Cox regression models
| Mortality rate (per 1,000 person-years) | Cox regression model | BIC of adjusted model | |||
|---|---|---|---|---|---|
| Crude model | Adjusted model* | Adjusted model** | |||
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |||
| HS-CRP at baseline | 2073.7 | ||||
| Low | 6.5 (5.3 – 8.0) | 1 (Reference) | 1 (Reference) | 1 (Reference) | |
| High | 11.6 (9.3 – 14.6) | 1.81 (1.33 – 2.46) | 1.84 (1.34 – 2.54) | 1.81 (1.30 – 2.51) | |
| HS-CRP change over time | 1216.0 | ||||
| Low (baseline) and low (follow-up) | 4.0 (2.9 – 5.4) | 1 (Reference) | 1 (Reference) | 1 (Reference) | |
| Low (baseline) and high (follow-up) | 6.6 (3.7 – 11.6) | 1.67 (0.88 – 3.20) | 1.54 (0.81 – 2.96) | 1.62 (0.84 – 3.13) | |
| High (baseline) and low (follow-up) | 6.3 (3.7 – 10.7) | 1.61 (0.87 – 2.96) | 1.53 (0.82 – 2.83) | 1.57 (0.85 – 2.93) | |
| High (baseline) and high (follow-up) | 8.9 (6.2 – 12.9) | 2.28 (1.41 – 3.71) | 2.50 (1.50 – 4.17) | 2.51 (1.46 – 4.31) | |
| Cumulative HS-CRP | 1208.3 | ||||
| Low in both assessments | 4.0 (2.9 – 5.4) | 1 (Reference) | 1 (Reference) | 1 (Reference) | |
| High in one of the assessments | 6.4 (4.4 – 9.5) | 1.64 (1.00 – 2.70) | 1.54 (0.93 – 2.53) | 1.60 (0.96 – 2.64) | |
| High in both assessments | 8.9 (6.2 – 12.9) | 2.28 (1.41 – 3.71) | 2.50 (1.50 – 4.17) | 2.51 (1.46 – 4.31) | |
| Weighted cumulative HS-CRP | 1190.3 | ||||
| Low | 4.2 (3.2 – 5.6) | 1 (Reference) | 1 (Reference) | 1 (Reference) | |
| High | 7.7 (5.8 – 10.4) | 1.84 (1.22 – 2.78) | 1.92 (1.25 – 2.95) | 2.09 (1.33 – 3.28) | |
BIC = Bayesian information criterion. Bold estimates are significant.
Adjusted by sex, age, education level, and socioeconomic position. Study site was included as a stratified variable in the Cox regression model.
Adjusted by sex, age, education level, socioeconomic position, smoking, alcohol drinking, physical activity level, metabolic syndrome, hypertension, total cholesterol, and type 2 diabetes. Study site was included as a stratified variable in the Cox regression model.
DISCUSSION
Main findings
Using a population-based cohort study from resource-constrained settings in Peru, this analysis documented a strong association between hs-CRP levels and all-cause mortality using several definitions afforded by repeated hs-CRP measures over time and ranging from a 81% increase to trebling the risk. This association hold for all the hs-CRP definitions we used, including one-off measurement (i.e., at baseline) and two hs-CRP measures over time. The BIC suggested the weighted cumulative hs-CRP definition fit best the association with all-cause mortality. Of note, almost a third of individuals from general population presented high levels of HS-CRP at the cohort baseline.
Comparison with previous studies
Our results are similar to those reported by two systematic reviews of cohort studies [12, 13], which only included hs-CRP at baseline. In the first systematic review [12], Li et al analyzed 12 population-based cohorts from high-income countries (Japan, Germany, USA, UK, Finland, Denmark, and Norway) and reported a similar estimate (pooled relative risk = 1.75) compared to our results using hs-CRP assessed at baseline. In the second systematic review [13], 27 studies, none of them from Latin America, were included and the pooled estimate was identical to the previous review. The latter systematic review also reported an increase in the risk of all-cause mortality of 15% per 1 mg/L increment of hs-CRP [13]. Thus, these two systematic reviews highlight the dearth of evidence from Latin America, where the cardio-metabolic and inflammation profile may be different to that of other world regions, especially because exposure to chronic infections diseases. Similarly, these reviews evidenced the lack of research in which multiple hs-CRP measurements were analyzed. Our work overcame both limitations.
A limited number of studies have worked with two or more hs-CRP assessments. The ARIC cohort study including 14 years of follow-up with two hs-CRP measurement 6 years apart, showed that increased hs-CRP (i.e., from low to high hs-CRP levels) and sustained elevated hs-CRP (i.e., consistent measures at high level) were associated with higher all-cause mortality (34% and 52%, respectively) after controlling for several confounders [9]. The magnitude of the association by the ARIC study was lower compared to our findings. This difference may be due to the larger lapse between hs-CRP measurements in the ARIC cohort as the proportion of hs-CRP change over time was similar between studies. Another large, community-based, prospective study involved repeated hs-CRP and LDL-cholesterol measurements, using up to three different assessments over time and the weighted cumulative approach, found a higher risk of all-cause mortality among participants with high hs-CRP, indifferent of low LDL-cholesterol levels [7]. Another study assessed the effect of hs-CRP on cardiovascular events and not mortality, using the Kailuan Cohort Study (China) and the cumulative hs-CRP definitions we used in this study (i.e., cumulative and weighted cumulative) [7]. They reported association between cumulative and weighted cumulative hs-CRP and CVD and myocardial infarction, but not with stroke. Finally, with a median follow-up of six years, a strong dose-response was seen between CRP levels and mortality risk in men using data if the National Health and Nutrition Survey Examination linked to the National Death Index in the United States [22]. Our study expands literature by using more than these two definitions and finding association with all-cause mortality. Moreover, our analysis also reported that the weighted cumulative approach may be better than other HS-CRP categories.
Implications of results
There are several mechanisms to explain the role and prognostic value of hs-CRP levels on all-cause mortality. Prior evidence support that high levels of inflammation may be relevant in the long-term development of atherosclerosis [23, 24], endothelial dysfunction [25], and cardiovascular disease [7, 11]. Thus, CRP seems to be not only an inflammatory marker but also an important predictor of ageing-related diseases.
A better understanding of the role of inflammatory markers, including hs-CRP, in the general population and in resource-constrained settings may help clarify their utility for guiding screening and prevention of cardiovascular outcomes and mortality. The existing evidence highlights the inclusion of hs-CRP in the assessment of patients with cardiovascular risk and, as a result, the need of a more intensive preventative therapy may be needed if this inflammatory marker is elevated [26]. For example, physical activity may have effects on the inflammatory pathways of atherosclerosis, specifically C-reactive protein, besides its beneficial effects of reducing levels of adiposity [27]. In addition, some reports suggest the potential benefit of nutraceuticals on selected inflammatory parameters, and might be useful given the currently limited therapeutic options available [28].
On the other hand, previous reports have widely used a single measure of hs-CRP to measure inflammation, but have not taken into account the inherently time-varying nature and short-term variability of this marker. Our results expand previous research and highlight the robustness of using weighted cumulative hs-CRP in the evaluation of mortality risk.
Strengths and limitations
This analysis was based on an ongoing population-based cohort study with 7 years of follow-up in a low- and middle-income country in Latin America region. The sample was representative from the general population and diverse settings in Peru, and hs-CRP values, associated with inflammation among healthy individuals, were used for analysis. Nevertheless, this study has limitations that merit discussion. First, this is an observational study, and therefore, general causal conclusions cannot be inferred. Second, some bias may arise as a no negligible proportion of subjects were lost to follow-up, albeit with range compared to other cohort studies in high-income settings. In addition, some participants did not accept blood sampling at follow-up, arising potential selection bias. Third, only two hs-CRP measurements 30 months apart were used in the analysis, which may not fully capture trajectories over time. Fourth, validity of death ascertainment could not be confirmed; nevertheless, the RENIEC is the governmental institution checking vital status using the National Identification Number. Thus, we expect the risk of misclassification for this should be very low. Finally, residual confounding may be present because the list of confounders was not exhaustive. For example, fitness data [29, 30], one of the strongest predictors of survival, was not available. Similarly, indigenous, African or Asian ancestry, occupational physical activity, or a detailed list of chronic conditions were not available for analyses.
Conclusions
High levels of hs-CRP were strongly associated with all-cause mortality. Two measurements of hs-CRP are better than one to predict all-cause mortality, and the weighted cumulative hs-CRP approach had the best prognostic fit.
Supplementary Material
Funding
The original CRONICAS Cohort Study was funded in whole with Federal Funds from the United States National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under contract No. HHSN268200900033C. RMC-L is funded by a Wellcome Trust International Training Fellowship (214185/Z/18/Z). The funders had no role in the preparation of the manuscript, or the decision to publish.
Footnotes
Competing interests
The authors declare no conflict of interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
STATEMENT AND DECLARATIONS
Data Statement
Data for analyses (dataset and dictionary) is available at Figshare:
Bernabe-Ortiz, Antonio; Miranda, J. Jaime (2021): CRP and mortality. figshare. Dataset. https://doi.org/10.6084/m9.figshare.17129321.v1
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Casas JP, Shah T, Hingorani AD, Danesh J, Pepys MB. C-reactive protein and coronary heart disease: a critical review. J Intern Med 2008;264(4):295–314. [DOI] [PubMed] [Google Scholar]
- 2.US Department of Health and Human Services. Review Criteria for Assessment of C-Reactive Protein (CRP), high sensitive C-Reactive Protein (hsCRP) and Cardiac C-Reactive Protein (cCRP) assays: Guidance for Industry and FDA Staff. Washington DC, US: US Department of Health and Human Services; 2005. [Google Scholar]
- 3.Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med 2004;350(14):1387–97. [DOI] [PubMed] [Google Scholar]
- 4.Buckley DI, Fu R, Freeman M, Rogers K, Helfand M. C-reactive protein as a risk factor for coronary heart disease: a systematic review and meta-analyses for the U.S. Preventive Services Task Force. Ann Intern Med 2009;151(7):483–95. [DOI] [PubMed] [Google Scholar]
- 5.Araújo JP, Lourenço P, Azevedo A, Friões F, Rocha-Gonçalves F, Ferreira A, et al. Prognostic value of high-sensitivity C-reactive protein in heart failure: a systematic review. J Card Fail 2009;15(3):256–66. [DOI] [PubMed] [Google Scholar]
- 6.Patgiri D, Pathak MS, Sharma P, Kutum T, Mattack N. Serum hsCRP: A Novel Marker for Prediction of Cerebrovascular Accidents (Stroke). J Clin Diagn Res 2014;8(12):Cc08–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang A, Liu J, Li C, Gao J, Li X, Chen S, et al. Cumulative Exposure to High-Sensitivity C-Reactive Protein Predicts the Risk of Cardiovascular Disease. J Am Heart Assoc 2017;6(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mo J, Chen Z, Xu J, Wang A, Meng X, Zhao X, et al. The impact of the cumulative burden of LDL-c and hs-CRP on cardiovascular risk: a prospective, population-based study. Aging (Albany NY) 2020;12(12):11990–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parrinello CM, Lutsey PL, Ballantyne CM, Folsom AR, Pankow JS, Selvin E. Six-year change in high-sensitivity C-reactive protein and risk of diabetes, cardiovascular disease, and mortality. Am Heart J 2015;170(2):380–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marsik C, Kazemi-Shirazi L, Schickbauer T, Winkler S, Joukhadar C, Wagner OF, et al. C-reactive protein and all-cause mortality in a large hospital-based cohort. Clin Chem 2008;54(2):343–9. [DOI] [PubMed] [Google Scholar]
- 11.Singh-Manoux A, Shipley MJ, Bell JA, Canonico M, Elbaz A, Kivimäki M. Association between inflammatory biomarkers and all-cause, cardiovascular and cancer-related mortality. Cmaj 2017;189(10):E384–e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Zhong X, Cheng G, Zhao C, Zhang L, Hong Y, et al. Hs-CRP and all-cause, cardiovascular, and cancer mortality risk: A meta-analysis. Atherosclerosis 2017;259:75–82. [DOI] [PubMed] [Google Scholar]
- 13.Ni P, Yu M, Zhang R, Cheng C, He M, Wang H, et al. Dose-response association between C-reactive protein and risk of all-cause and cause-specific mortality: a systematic review and meta-analysis of cohort studies. Ann Epidemiol 2020;51:20–7.e11. [DOI] [PubMed] [Google Scholar]
- 14.Miranda JJ, Bernabe-Ortiz A, Smeeth L, Gilman RH, Checkley W. Addressing geographical variation in the progression of non-communicable diseases in Peru: the CRONICAS cohort study protocol. BMJ Open 2012;2(1):e000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vargas-Herrera J, Ruiz KP, Nuñez GG, Ohno JM, Pérez-Lu JE, Huarcaya WV, et al. [Preliminary results of the strengthening of the national death registry information system]. Rev Peru Med Exp Salud Publica 2018;35(3):505–14. [DOI] [PubMed] [Google Scholar]
- 16.Miranda JJ, Carrillo-Larco RM, Gilman RH, Avilez JL, Smeeth L, Checkley W, et al. Patterns and Determinants of Physical Inactivity in Rural and Urban Areas in Peru: A Population-Based Study. J Phys Act Health 2016;13(6):654–62. [DOI] [PubMed] [Google Scholar]
- 17.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120(16):1640–5. [DOI] [PubMed] [Google Scholar]
- 18.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr., et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. Jama 2003;289(19):2560–72. [DOI] [PubMed] [Google Scholar]
- 19.Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). Jama 2001;285(19):2486–97. [DOI] [PubMed] [Google Scholar]
- 20.American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021;44(Suppl 1):S15–s33. [DOI] [PubMed] [Google Scholar]
- 21.Vrieze SI. Model selection and psychological theory: a discussion of the differences between the Akaike information criterion (AIC) and the Bayesian information criterion (BIC). Psychol Methods 2012;17(2):228–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J, Zhang Y, Lavie CJ, Tabung FK, Xu J, Hu Q, et al. Associations of C-reactive protein and fibrinogen with mortality from all-causes, cardiovascular disease and cancer among U.S. adults. Prev Med 2020;139:106044. [DOI] [PubMed] [Google Scholar]
- 23.Koenig W High-sensitivity C-reactive protein and atherosclerotic disease: from improved risk prediction to risk-guided therapy. Int J Cardiol 2013;168(6):5126–34. [DOI] [PubMed] [Google Scholar]
- 24.Legein B, Temmerman L, Biessen EA, Lutgens E. Inflammation and immune system interactions in atherosclerosis. Cell Mol Life Sci 2013;70(20):3847–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Low A, Mak E, Rowe JB, Markus HS, O’Brien JT. Inflammation and cerebral small vessel disease: A systematic review. Ageing Res Rev 2019;53:100916. [DOI] [PubMed] [Google Scholar]
- 26.Lavie CJ, Milani RV, Verma A, O’Keefe JH. C-reactive protein and cardiovascular diseases--is it ready for primetime? Am J Med Sci 2009;338(6):486–92. [DOI] [PubMed] [Google Scholar]
- 27.Mendoza MVF, Kachur SM, Lavie CJ. The Effects of Exercise on Lipid Biomarkers. Methods Mol Biol 2022;2343:93–117. [DOI] [PubMed] [Google Scholar]
- 28.Ruscica M, Penson PE, Ferri N, Sirtori CR, Pirro M, Mancini GBJ, et al. Impact of nutraceuticals on markers of systemic inflammation: Potential relevance to cardiovascular diseases - A position paper from the International Lipid Expert Panel (ILEP). Prog Cardiovasc Dis 2021;67:40–52. [DOI] [PubMed] [Google Scholar]
- 29.Kaminsky LA, Arena R, Ellingsen Ø, Harber MP, Myers J, Ozemek C, et al. Cardiorespiratory fitness and cardiovascular disease - The past, present, and future. Prog Cardiovasc Dis 2019;62(2):86–93. [DOI] [PubMed] [Google Scholar]
- 30.Nevill AM, Myers J, Kaminsky LA, Arena R. Improving reference equations for cardiorespiratory fitness using multiplicative allometric rather than additive linear models: Data from the Fitness Registry and the Importance of Exercise National Database Registry. Prog Cardiovasc Dis 2019;62(6):515–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


