Abstract
Background
COVID-19 has placed unprecedented demands on hospitals. A clinical service, COVID-19 Oximetry @home (CO@h) was launched in November 2020 to support remote monitoring of COVID-19 patients in the community. Remote monitoring through CO@h aims to identify early patient deterioration and provide timely escalation for cases of silent hypoxia, while reducing the burden on secondary care.
Methods
We conducted a retrospective service evaluation of COVID-19 patients onboarded to CO@h from November 2020 to March 2021 in the North Hampshire (UK) community led service (a collaboration of 15 General Practitioner (GP) practices covering 230 000 people). We have compared outcomes for patients admitted to Basingstoke and North Hampshire Hospital who were CO@h patients (COVID-19 patients with home monitoring of oxygen saturation (SpO2; n=115), with non-CO@h patients (those directly admitted without being monitored by CO@h (n=633)). Crude and adjusted OR analysis was performed to evaluate the effects of CO@h on patient outcomes of 30-day mortality, Intensive care unit (ICU) admission and hospital length of stay greater than 3, 7, 14 and 28 days.
Results
Adjusted ORs for CO@h show an association with a reduction for several adverse patient outcome: 30-day hospital mortality (p<0.001, OR 0.21, 95% CI 0.08 to 0.47), hospital length of stay larger than 3 days (p<0.05, OR 0.62, 95% CI 0.39 to 1.00), 7 days (p<0.001, OR 0.35, 95% CI 0.22 to 0.54), 14 days (p<0.001, OR 0.22 95% CI, 0.11 to 0.41), and 28 days (p<0.05, OR 0.21, 95% CI 0.05 to 0.59). No significant reduction ICU admission was observed (p>0.05, OR 0.43, 95% CI 0.15 to 1.04). Within 30 days of hospital admission, there were no hospital readmissions for those on the CO@h service as opposed to 8.7% readmissions for those not on the service.
Conclusions
We have demonstrated a significant association between CO@h and better patient outcomes; most notably a reduction in the odds of hospital lengths of stays longer than 7, 14 and 28 days and 30-day hospital mortality.
Keywords: COVID-19, Quality improvement, Evidence-Based Practice
Problem
The rapidly evolving COVID-19 pandemic has been responsible for 3.4 million deaths worldwide1 and has placed unprecedented strain on healthcare systems. A significant proportion of patients hospitalised with acute COVID-19 have severe hypoxia (very low blood oxygen saturation) frequently presenting ‘silently’ (ie, without breathlessness).2 Silent hypoxia is an independent indicator of worse patient outcomes,3 4 and delayed presentations of severe COVID-19; often leading to extended hospital stays, higher risk of ICU admission and higher mortality rates.5
Background
UK guidelines recommend that patient acuity should therefore be assessed with the use of pulse oximetry (ie, monitoring oxygen saturation) when diagnosed with COVID-19.6 7 A clinical service COVID-19 Oximetry @home (CO@h) was launched November 2020 within a COVID-19 Integrated Care Pathway to support remote monitoring of COVID-19 patients by primary care and timely escalation to secondary care. Remote home monitoring through CO@h have been implemented to (1) maintain National Health Service (NHS) capacity, (2) decrease nosocomial COVID-19 transmission and (3) identify early patient deterioration and provide timely escalation to reduce hospital length of stay, and mortality from silent hypoxia.8 9
A CO@h Service consists of two fundamental components: (1) using a predictive model to identify individual patients in a population who are at high risk of future unplanned hospital admission; and (2) offering these people a period of intensive, multidisciplinary, case management at home using the systems, staffing and daily routine.10 11 Patients are referred by clinical services responsible for operating CO@h services then triaged prior to onboarding for remote monitoring to ensure that the CO@h offers an appropriate level of care.
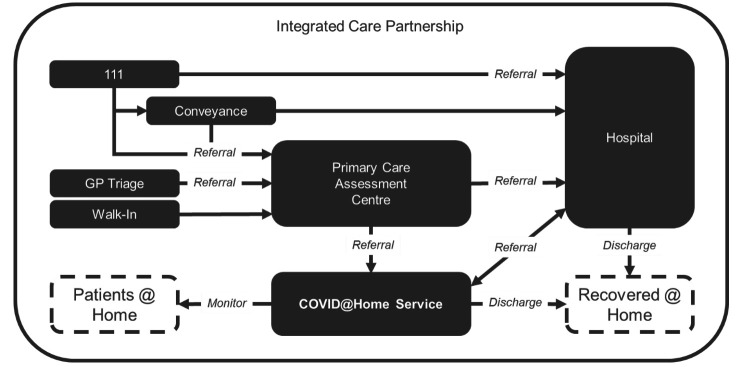
Even though the CO@h service is virtual, it is integrated into a care partnership with physical care for patient assessment (eg, COVID-19 testing and initial observations) when deemed clinically appropriate. A schematic of the Integrated Care Partnership for North Hampshire is shown in figure 1, highlighting the important relationship between physical and virtual services in the overall delivery of care.
Figure 1.
Primary care assessment centre and COVID Oximetry @home service deployed supporting community patient monitoring as defined by North Hampshire Integrated Care System. GP, General Practitioner.
Measurement
This report describes a quality improvement (QI) initiative to implement CO@h service in North Hampshire and retrospectively evaluate their efficacy. Through adoption of the Plan-Do-Study-Act (PDSA) framework and using evidence-based practice, the CO@h services were continually improved as a rapid response to the evolving pandemic. Evidence-based practice was enabled by close communication between professional analysts, who provided data insight, and healthcare professionals, who provided operational insight.
North Hampshire covers 230 000 patients across a single Clinical Commissioning Group and six Primary Care Networks served by one acute hospital trust. The North Hampshire CO@h Standard Operating Procedure V.7.0 was approved October 2020 with service going live November 2020. Patients accessing CO@h were assessed face to face to determine those at high risk of deterioration. The assessment included a COVID-19 swab test, baseline measurements (Pulse, SpO2 and the National Early Warning Score; NEWS2) and risk factors (ethnicity, age, body mass index, comorbidities, mental health, shielding and high-risk professions). Only patients with a SARS-CoV-2 positive test were considered for subsequent pathways categorised by severity of red, amber and green. Red patients with SpO2 ≤92% or any of respiration rate ≥25, heart rate ≥131, new confusion and NEWS2 ≥5 were admitted directly to hospital. Amber patients with 93% ≤ SpO2≤94%, or any of 21≤respiration rate ≤24, 91≤heart rate≤130, 3≤NEWS2≤4 had further tests (chest X-ray, blood and desaturation) to determine if hospital admission is needed for drug therapeutics (remdesivir or dexamethasone) or if not then admitted to CO@h. Green patients identified with risk factors and SpO2 ≥95%, or any of respiration rate ≤20, heart rate ≤90, 0≤NEWS2≤2 were admitted to CO@h.
All patients admitted to CO@h were issued with a standard pulse oximeter and information sheet describing how to use a pulse oximeter and the protocol for reporting SpO2 measurements three times a day using either a remote monitoring mobile application or paper diaries. Patients were remotely monitored for up to 14 days, and escalation decisions made in accordance with red, amber and green criteria described in the categories above.
It should be noted that pilot services were operational in North Hampshire during the first wave of the pandemic (April 2020 to June 2020) to iteratively explore the definition of operating procedures. Data relating to CO@h pilots during the first wave period was not available for analysis. In this report we explore the effectiveness of the CO@h service implemented in the Integrated Care Partnership of North Hampshire. To our knowledge, this is the first QI initiative directly reporting outcomes for COVID-19 patients treated virtually for an NHS Trust. Our findings will be of interest to healthcare organisations looking to implement further CO@h services as a response to the ongoing pandemic.
Methods
Patient population
All patients with suspected COVID-19 admitted to North Hants CO@h or Basingstoke and North Hants Hospital (BNHH) between 1 November 2020 and 31 March 2021 were eligible for inclusion. CO@h patients were then linked to their subsequent hospital admissions. Confirmed COVID-19 cases were then identified from these suspected cases by requiring at least one SARS-CoV-2 positive test associated with the admission.
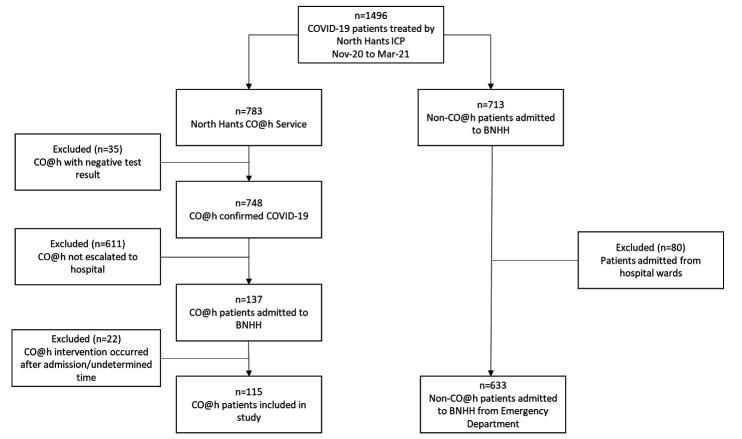
We separated our cohort into an intervention group, where patients had at least one referral to the CO@h service, and a control group, where patients have not had such a referral. There was a total of 1496 patients, with 783 patients in the intervention group and 713 patients in the control group, as shown in figure 2. To evaluate the outcomes between comparable groups, we required that each patient in the intervention group had at least one hospital admission via the emergency department. We excluded patients in the intervention group who returned a negative COVID-19 test result following referral to CO@h (n=35) and those whose outcomes did not result in escalation to hospital (n=611). We also excluded patients who did not engage or had a CO@h admission date after hospital admission (n=22), leaving 115 CO@h patients that were escalated to hospital in our intervention group.
Figure 2.
Patient cohort selection showing CO@h intervention group and non-CO@h control group (those patients not being treated by the CO@h service). BNHH, Basingstoke and North Hants Hospital; CO@h, COVID-19 Oximetry @home. ICP; Integrated Care Partnership.
For the control group, we excluded COVID-19 patients (n=80) admitted from hospital locations other than the emergency department to reduce confounding factors between the intervention and control groups: patients in this group may have already been admitted to hospital with complex ongoing acute care needs in addition to COVID-19; resulting in an increased likelihood for negative outcomes, such as longer length of stay and hospital mortality. This left 633 patients in the Non-CO@h control group, of which 55 patients were readmitted to hospital within 30 days of first admission. For readmissions an episode of care was created by aggregating the length of stay over all admissions. Patient outcome was then deemed to be the outcome from the last admission event.
Target outcome
We evaluated the CO@h service using a comparison of increasingly acute outcomes associated with patient trajectory for those with a positive COVID-19 test who required admission to hospital. We considered the following outcomes: 30-day hospital mortality, ICU admission and hospital length of stay above 3 days, 7 days, 14 days or 28 days. We identified mortality through hospital medical records. We identified ICU admissions through a specific electronic patient record flag. The length of stay was computed from the point of hospital admission to discharge.
Data collection
We linked data from Primary Care systems operated by CO@h with Secondary Care systems operated by Hampshire Hospitals NHS Foundation Trust to create a database supporting analysis of the full trajectory of COVID-19 episodes. The linking included CO@h service and hospital admission records.
To ensure data quality, we subsequently excluded admission records in the overall patient population that did not meet the following criteria: (1) admission date must be equal to or before the discharge date; (2) the discharge date and patient outcome must be recorded. There were 2 hospital admission records with a discharge date before the corresponding admission date, and 11 admissions without a discharge date or outcome. There were two instances of duplicates: one duplicate set of CO@h referral records, and one duplicate set of hospital admission records that were removed.
The dataset also contains some hospital admission records for which the date of admission is prior to discharge from a CO@h service. In some cases, patients admitted to hospital were not discharged from CO@h immediately. If the hospital admission was likely to only be less than 24 hours (ie, same day emergency care), patients would continue intervention by CO@h seamlessly as they left hospital. If hospital admission was longer than 24 hours, patients were discharged from CO@h, and in some cases referred back to CO@h following hospital discharge. Patients were also given advice to contact 999, or another service based on their self-submitted readings. In some cases, the patient would then be conveyed and admitted to hospital ahead of discharge from the CO@h service. In all cases, we include those patients within our intervention group as they have received the CO@h intervention.
Confounding comorbidity risks were included for patient conditions that increased the chance of a negative outcome from COVID-19.12–15 These conditions include: cardiac disease, diabetes, pulmonary disease, asthma, obesity, respiratory disease, chronic heart disease, liver disease, stroke, dementia, autoimmune disease, malignancy, dialysis, renal failure, cardiac disease, kidney disease, kidney failure, cardiovascular disease, hypertension, cancer, hyperlipidaemia, coronary artery disease, renal disease, Chronic Obstructive Pulmonary Disease, atrial fibrillation and heart failure.
Data analysis
A time series was produced to show the monthly CO@h referrals with number of hospital admissions from November 2020 to March 2021. An empirical cumulative distribution function was computed for length of stay in the intervention and control groups, in addition to their smoothed distribution functions (calculated by kernel density estimate with a Gaussian kernel restricted to positive values).
Pearson’s χ2 test was performed to check for a significant difference in distributions of patient characteristics in the intervention and control group, where p<0.05 is considered significant. We used logistic regression to account for possible confounders for each patient outcome and calculated the crude OR of CO@h and adjusted ORs for patient’s age group, gender and comorbidities. We use the Wald test to evaluate whether logistic regression coefficients are significant with respect to the null hypothesis that the resulting ORs are 1. Analysis was performed in Python V.3.9.4 (using pandas, seaborn, statsmodels) and R V.3.6.1.
Data governance
This service evaluation did not require ethics approval. The study was however evaluated by the University of Southampton Ethics Committee (REF ERGO/61242) and approved as a service evaluation following Data Protection Impact Assessment and establishment of Data Sharing Agreements. NHS England and NHS Improvement have been given legal notice by the Secretary of State for Health and Social Care to support the processing and sharing of information to help the COVID-19 response under Health Service Control of Patient Information Regulations 2002 (COPI). This is to ensure that confidential patient information can be used and shared appropriately and lawfully for purposes related to the COVID-19 response. Data were extracted from medical records by clinicians providing care for the patients and an anonymised extract of the data were provided to the team at the University of Southampton.
Data accessibility
Due to information governance concerns, the data will not be made public. However, it may be made accessible via reasonable request to the corresponding author.
Results
The North Hants CO@h service treated 783 patients of which 115 patients were subsequently escalated and admitted to hospital. The BNNH had a further 692 admissions (633 individual patients) directly via the emergency department who were not treated by the CO@h service.
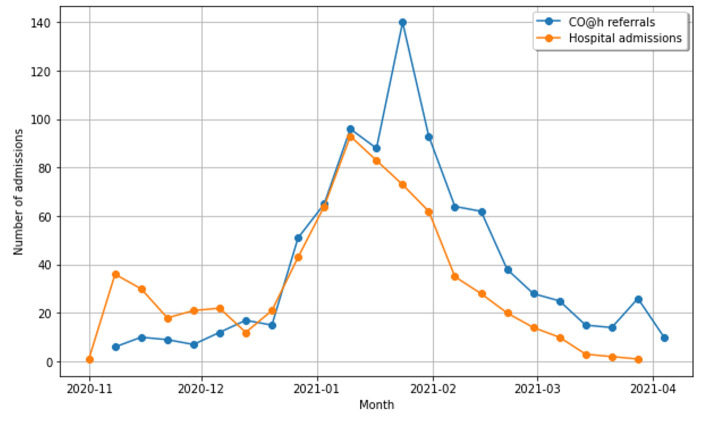
The uptake of the CO@h service is shown in figure 3. The ratio of CO@h referrals to the number of hospital admissions increased consistently throughout the period, with a ratio of 30/113 (1:4) during November 2020, to 85/21 (4:1) during March 2021. The uptake reached a maximum of 363 referrals during January 2021 coinciding with the increase in COVID-19 prevalence during that period. The mean length of stay for CO@h patients who were then subsequently escalated to hospital was 11.62 days (95% CI 10.76 to 12.48 days).
Figure 3.
Monthly COVID-19 referrals for monitoring by CO@h service (blue) and total hospital admissions (orange) from November 2020 to March 2021. Hospital admissions include CO@h referrals escalated and admitted to hospital. CO@h, COVID-19 Oximetry @home.
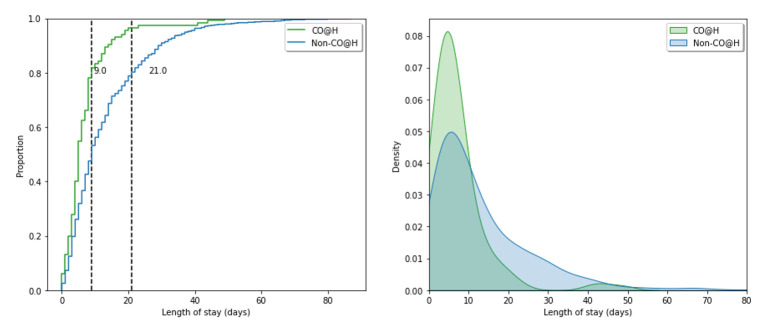
Distributions representing the hospital length of stay for both the intervention and control groups are shown in figure 4. The 80th percentile hospital length of stay for the intervention group was 9 days, 12 days shorter than the 21 days for the control group. The mean hospital length of stay was 7.1 days (95% CI 5.7 to 8.5 days) in the intervention group, and 13.2 days (95% CI 12.2 to 14.1 days) in the control group.
Figure 4.
Hospital length of stay distributions for our intervention (CO@h; green) and control cohorts (non-CO@h; blue). (Left) cumulative distribution for length of stay with 80th percentile length of stay shown as vertical lines for each period. (Right) kernel density estimate for hospital length of stay. CO@h, COVID-19 Oximetry @home.
Table 1 gives a contingency table showing the age group, gender, and comorbidity risk for patients in the intervention and control groups ascertained from medical records. Pearson’s χ2 test identified that there were no significant differences between the distributions of the age group, gender and comorbidity risk.
Table 1.
Contingency table of patients' age group, gender and comorbidity risk with positive SARS-CoV-2 test result. Brackets give characteristic group percentages
| Characteristic | Co@h | Non-CO@h |
| No. | 115 | 633 |
| Age group | ||
| 0–19 | 0 (0) | 9 (1.42) |
| 20–45 | 12 (10.43) | 93 (14.69) |
| 46–65 | 43 (37.39) | 167 (26.38) |
| 66–85 | 47 (40.87) | 263 (41.54) |
| 85+ | 13 (11.30) | 101 (15.96) |
| Gender | ||
| Female | 61 (53.04) | 289 (45.66) |
| Male | 54 (46.96) | 344 (54.34) |
| Comorbidity risk | ||
| Yes | 82 (71.30) | 456 (72.04) |
| No | 33 (28.70) | 177 (27.96) |
CO@h, COVID-19 Oximetry @home.
In online supplemental table 1, we summarise the ORs and 95% CI for each outcome. Crude ORs for CO@h are calculated along with adjusted ORs.
bmjoq-2021-001584supp001.pdf (62.1KB, pdf)
Adjusted ORs for CO@h show an association with a reduction for several adverse patient outcomes: 30-day hospital mortality (p<0.001, OR 0.21, 95% CI 0.08 to 0.47), hospital length of stay larger than 3 days (p<0.05, OR 0.62, 95% CI 0.39 to 1.00), 7 days (p<0.001, OR 0.35, 95% CI 0.22 to 0.54), 14 days (p<0.001, OR 0.22, 95% CI 0.11 to 0.41) and 28 days (p<0.05, OR 0.21, 95% CI 0.05 to 0.59). No significant reduction ICU admission was observed (p>0.05 OR 0.43 95% CI 0.15 to 1.04).
Within the CO@h cohort 6/115 (5.22%) had a 30-day mortality outcome compared with 130/633 (20.54%) for Non-CO@h demonstrating a 25.40% reduction. The CO@h cohort included 5/115 (4.35%) that were admitted to ICU in comparison to 52/633 (8.21%) for Non-CO@h giving a 52.93% reduction. No patients were readmitted to hospital within 30 days of hospital admission for those admitted from CO@h, compared with 55/633 (8.7%) for Non-CO@h.
Discussion
The CO@h initiative was implemented nationally to protect patients by improving early recognition of deterioration in COVID-19 and to protect the healthcare system from being overwhelmed with inappropriate admissions. The CO@h service was universally implemented across England (by Feb 2021) over a period of 3 months.
In this QI initiative we focused on evaluating the efficacy of CO@h by retrospectively evaluating COVID-19 patient outcomes for those admitted to CO@h in North Hampshire Primary Care Network and subsequently being admitted to hospital. The initiative achieved its aim with reductions in length of stay and 30-day mortality rates for patients admitted to hospital via the CO@h pathway relative to direct hospital admission. In the majority of acute COVID-19 cases, severe hypoxia (often presenting ‘silently’ without breathlessness) has been a significant contributor in patient deterioration from pneumonia to acute respiratory distress syndrome.2–4 16 17 Pathologically, hypoxia due to COVID-19 is likely driven by a mixture of intrapulmonary shunting, instability in lung perfusion, intravascular microthrombi and alveolar collapse.16 18 In particular, silent hypoxia from COVID-19 is associated with rapid deterioration (ie, patients immediately being admitted to ICU from home or shorter ward stays) and higher rates of respiratory failure.3 Driving factors include unrecognised lung function decline leading to damage to the brain and central nervous system.19 Early identification and proactive management of severe COVID-19, directly improves patient outcomes.3–5 Prospective outcomes can be improved by prompt prescription of medication such as dexamethasone20 and trials of non-invasive ventilation.21 To our knowledge, this QI service evaluation is the first to demonstrate that CO@h is associated with improved patient outcomes for an NHS trust (ie, mortality, ICU admission and length of stay). This CO@h service was implemented as part of the national framework22 and therefore these findings should be of interest to future CO@h operations in response to the pandemic. CO@h services have now been provisioned internationally, for example, StepOne have applied the CO@h model to support intervention of COVID-19 patients across 16 states in India.23
COVID-19 is endemic worldwide, and hence, there is an urgent need for optimised early identification of patient deterioration for patients at home. As healthcare systems aim to restore elective activity, the backlog of non-COVID patients requiring intervention is stark. In England, the British Medical Association estimates there were 3.37 million fewer elective procedures and 21.4 million fewer outpatient attendances between April 2020 and March 2021.24
The COVID-19 pandemic has generated research into effective and streamlined patient care which has ramifications beyond the context of the pandemic. CO@h is one aspect of the nationally led programme NHS @home; which aims to maximise the use of technology to support more people to better self-manage their health and care at home.7 With access to more timely preventative care, patient burden on both primary and emergency care can be reduced while providing patients more personalised intervention. In particular, home pulse oximetry has long been used in primary care settings as a cost-effective approach to monitor chronic lung conditions and heart disease,25 and there is a growing evidence base for the model’s effectiveness and safety in COVID-19.2 5 6 26 Further prospective studies are required to understand how remote monitoring can be implemented in wider contexts, potentially focused on high-risk patients with significant comorbidities.
These findings must be understood in light of their limitations. CO@h was rapidly developed in response to the pandemic, and as a result, the improvement cycles were conducted at pace. PDSA QI was conducted using evidence-based practice, where insights were provided by data professionals to clinicians. A multidisciplinary team of healthcare professionals, QI personnel and data scientists met frequently to discuss patient care, and CO@h efficacy. Operational improvements were implemented through these discussions to deliver continual improvement especially procedures relating to integrated services between conveyance, CO@h and hospitals. Formally, the distinct improvement cycles were as follows: (1) CO@h service pilots (first wave of the pandemic: March 2020 to July 2020) including community COVID-19 assessment centres implemented without remote monitoring beyond paper diaries and phone, treating n=1600 suspected-COVID patients and escalating n=105 to hospital; and support by hospital Same Day Emergency Care and care homes telemedicine services (2) NHS Trust-wide implementation of CO@h (second wave of the pandemic: November 2020 to March 2021) with efficacy evaluation presented here.
Although we show that patients admitted to hospital after CO@h had better outcomes relative to an unmonitored comparison group, the suggestion that this was driven by CO@h is complicated by other factors. For example, the severity of illness at the time of admission to hospital is not considered in our study. Patient severity from CO@h referrals are likely to be within red and amber categories (in accordance with standard operating procedure), physiological measurement data for all patients at the time of hospital attendance was not available for analysis. This limits inferences that can be made regarding the timely escalation of patients to hospital between each cohort, while many may have stayed at home and survived had their SpO2 not been monitored. A related limitation, but likely to be a minor factor in this study, is that measurements are self-reported by patients rather than taken by a healthcare professional. This may introduce some inaccuracy in measurements used for CO@h escalation decisions. Our study did not have a mode of assessing mortality outside of the hospital and therefore may be under reporting mortality for patients in both cohorts. Although, we have considered objective differences regarding age, gender and comorbidities, there may be other qualitative socioeconomic or demographic differences that may influence the outcomes not considered in our study. Finally, this service evaluation is for an integrated community care pathway and a single hospital trust, therefore generalisation is limited by population size and clinical setting.
Conclusion
Our study has shown that CO@h has a significant association with better patient outcomes. To our knowledge, this is the first QI report concerning the efficacy of CO@h service and evaluation of hospital mortality, ICU admission, and length of stay benefits for an NHS Hospital Trust. COVID-19 patients admitted to the CO@h service have been found to have significantly reduced odds of longer length hospital stays, ICU admission and of hospital mortality. These findings demonstrate that, despite the study limitations, CO@h should be considered nationally and internationally in response to the ongoing pandemic and that larger evaluations of efficacy and quality should be undertaken.
Acknowledgments
We thank Claire Parker from Hampshire, Southampton & Isle of Wight Clinical Commissioning Group for support during the specification, delivery, and evaluation of CO@h. We extend our special thanks to the CO@h Clinical Team who cared for patients and collated data including Alison Hullah (Lead Advanced Nurse Practitioner), Roisin Howell (Lead Advanced Nurse Practitioner) and Giselle Beaumont (Care Coordinator). This report received funding from the NHSX RECOxCARE (Remote oximetry in community care for COVID-19 patients) project and from NHS England to support data collection.
Footnotes
Twitter: @carolineokeeffe
Contributors: Conceptualisation: MB, DB, CJD, MA, HA, NR, JD, CO'K and MI-K. Methodology: MB, DB, FD and MI-K. Writing original draft: MB; DB; CJD and MA. Writing–review and editing: MB, DB, CJD, MA, HA, NR; JD, CO'K and MI-K. All authors approved the final submitted draft. Guarantor: MB.
Funding: NHS England (No Award/Grant Number); NHSX RECOxCARE-Collaboration Agreement 321103 between UoS and HHFT.
Disclaimer: The views expressed in this publication are those of the author(s) and not necessarily those of the funders.
Competing interests: MI-K is National Clinical Lead Deterioration & National Specialist Advisor Sepsis, NHS England and NHS Improvement.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This service evaluation did not require ethics approval. The study was however evaluated by the University of Southampton Ethics Committee (REF ERGO/61242) and approved as a service evaluation following Data Protection Impact Assessment and establishment of Data Sharing Agreements. NHS England and NHS Improvement have been given legal notice by the Secretary of State for Health and Social Care to support the processing and sharing of information to help the COVID-19 response under Health Service Control of Patient Information Regulations 2002 (COPI). This is to ensure that confidential patient information can be used and shared appropriately and lawfully for purposes related to the COVID-19 response. Data were extracted from medical records by clinicians providing care for the patients and an anonymised extract of the data were provided to the team at the University of Southampton.
References
- 1.World Health Organization . WHO coronavirus (COVID-19) Dashboard, 2021. Available: https://covid19.who.int/
- 2.Greenhalgh T, Knight M, Inda-Kim M, et al. Remote management of covid-19 using home pulse oximetry and virtual ward support. BMJ 2021;372, :n677. 10.1136/bmj.n677 [DOI] [PubMed] [Google Scholar]
- 3.Brouqui P, Amrane S, Million M, et al. Asymptomatic hypoxia in COVID-19 is associated with poor outcome. Int J Infect Dis 2021;102:233–8. 10.1016/j.ijid.2020.10.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Carroll O, MacCann R, O'Reilly A, et al. Remote monitoring of oxygen saturation in individuals with COVID-19 pneumonia. Eur Respir J 2020;56. 10.1183/13993003.01492-2020. [Epub ahead of print: 13 08 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vindrola-Padros C, Singh KE, Sidhu MS. Remote home monitoring (virtual wards) during the COVID-19 pandemic: a systematic review. medRxiv 2020. https://www.medrxiv.org/content/early/2020/10/12/2020.10.07.20208587 10.1101/2020.10.07.20208587 [DOI] [Google Scholar]
- 6.Inada-Kim M, Chmiel FP, Boniface MJ. Validation of home oxygen saturations as a marker of clinical deterioration in patients with suspected COVID-19. medRxiv 2020. 10.1101/2020.11.06.20225938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.NHS England, “NHS @home,” 2021. Available: https://www.england.nhs.uk/nhs-at-home/
- 8.Stockly S. RCGP paper on virtual wards, silent hypoxia and improving COVID, 2020. Available: https://elearning.rcgp.org.uk/pluginfile.php/149506/mod_page/content/88/Virtual%20wards%2C%20silent%20hypoxia%20and%20improving%20COVID%20outcomes_formatted_28.10.20.pdf
- 9.Boniface M, Zlatev Z, Guerrero-Luduena R, et al. An Evidence-Based Approach to Quality Improvement for COVIDoximetry@home,” NIHR Applied Research Collaboration (ARC) Wessex, 2020. Available: https://eprints.soton.ac.uk/445388/1/RECOxCARE_Evidence_Based_QI_CWD_v1.0_final.pdf
- 10.Lewis G, Vaithianathan R, Wright L, et al. Integrating care for high-risk patients in England using the virtual ward model: lessons in the process of care integration from three case sites. Int J Integr Care 2013;13:e046. 10.5334/ijic.1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis G. Case study: virtual wards at Croydon primary care trust. London. UK: The King’s Fund, 2006. [Google Scholar]
- 12.Deng G, Yin M, Chen X, et al. Clinical determinants for fatality of 44,672 patients with COVID-19. Crit Care 2020;24:1–2. 10.1186/s13054-020-02902-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020;584:430–6. 10.1038/s41586-020-2521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Docherty A, Harrison E, Green C. Features of 16,749 hospitalised UK patients with COVID-19 using the ISARIC who clinical characterisation protocol. Medrxiv 2020;5. 10.1101/2020.04.23.20076042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanyaolu A, Okorie C, Marinkovic A, et al. Comorbidity and its impact on patients with COVID-19. SN Compr Clin Med 2020;2:1069–76. 10.1007/s42399-020-00363-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Couzin-Frankel J. The mystery of the pandemic’s 'happy hypoxia', Science AAAS, 2020: 455–6. [DOI] [PubMed] [Google Scholar]
- 17.Knight SR, Ho A, Pius R, al e, et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC who clinical characterisation protocol: development and validation of the 4C mortality score. BMJ 2020;370, :m3339. 10.1136/bmj.m3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levitan RM. Pulse oximetry as a biomarker for early identification and hospitalization of COVID-19 pneumonia. Acad Emerg Med 2020;27:785–6. 10.1111/acem.14052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rahman A, Tabassum T, Araf Y, et al. Silent hypoxia in COVID-19: pathomechanism and possible management strategy. Mol Biol Rep 2021;48:3863–9. 10.1007/s11033-021-06358-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021;384:693–704. 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menzella F, Barbieri C, Fontana M, et al. Effectiveness of noninvasive ventilation in COVID-19 related-acute respiratory distress syndrome. Clin Respir J 2021;15:779–87. 10.1111/crj.13361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.NHS England . Pulse oximetry to detect early deterioration of patients with COVID-19 in primary and community care settings, 2020. [Google Scholar]
- 23.George Institute . Home-Based COVID care through teleconsultation services can prevent overcrowding in hospitals, 2021. Available: https://www.georgeinstitute.org.in/news/home-based-covid-care-through-teleconsultation-services-can-prevent-overcrowding-in-hospitals
- 24.B. M. Association . Pressure points in the NHS, 2021. Available: https://www.bma.org.uk/advice-and-support/nhs-delivery-and-workforce/pressures/pressure-points-in-the-nhs
- 25.Plüddemann A, Thompson M, Heneghan C, et al. Pulse oximetry in primary care: primary care diagnostic technology update. Br J Gen Pract 2011;61:358–9. 10.3399/bjgp11X572553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nunan J, Clarke D, Malakouti A, et al. Triage Into the Community for COVID-19 (TICC-19) Patients Pathway - Service evaluation of the virtual monitoring of patients with COVID pneumonia. Acute Med 2020;19:183–91. 10.52964/AMJA.0826 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjoq-2021-001584supp001.pdf (62.1KB, pdf)
Data Availability Statement
Due to information governance concerns, the data will not be made public. However, it may be made accessible via reasonable request to the corresponding author.
Data are available upon request.