Abstract
Patients with uncontrolled epilepsy have a high risk of sudden unexpected death in epilepsy (SUDEP). Seizure-induced respiratory arrest (S-IRA) is thought to be the determining cause of death in many cases of SUDEP. The goal of the present study was to use Scn1aR1407X/+ (Dravet Syndrome, DS) and DBA/1 mice to determine: 1) the effect of a ketogenic diet (KD) on S-IRA and 2) the relationship between serum ketones and the protective effect of a KD. KD treatment significantly decreased spontaneous seizure-induced mortality in DS mice compared to control (8% vs 39%, p = 0.0021). This protective effect was not abolished when ketosis was prevented by supplementing the KD with glucose (10% mortality, p = 0.0007). In DBA/1 mice, the latency to onset of S-IRA due to audiogenic seizures was delayed from 7.6 to 20.8 seconds by a KD on treatment day (TD) 7 compared to control (p < 0.0001), an effect that was reversed on TD14 when mice were crossed over to a control diet on TD7. β-hydroxybutyrate (BHB) levels were significantly decreased in DBA/1 mice on a KD supplemented with glucose (p = 0.0038), but the protective effect was maintained. Our findings show that a KD decreases SUDEP in DS mice and increases the latency to audiogenic S-IRA in DBA/1 mice. In both mouse models, a KD was protective against S-IRA. This effect may be due in part to specific dietary components rather than generation of ketone bodies.
Keywords: epilepsy, apnea, SUDEP, dietary therapies
1. Introduction
Approximately 30–50% of epilepsy patients fail to achieve adequate seizure control with anticonvulsant medications, placing them at increased risk for sudden unexpected death in epilepsy (SUDEP) [1]. SUDEP is estimated to account for approximately 27% of deaths in epilepsy patients [2] and up to 50% when seizures are refractory to pharmacotherapy [3]. SUDEP ranks second only to stroke in potential years of life lost among all neurological disorders in the USA [4]. Although the mechanisms underlying SUDEP are unclear, growing clinical and animal evidence indicates seizure-induced respiratory dysfunction precedes death in most cases [5–9].
Ketogenic diets (KDs) are a therapeutic approach that are recommended when epilepsy patients fail trials of three anticonvulsant medications [10]. KDs are high-fat, moderate-protein, very low-carbohydrate diets that mimic the metabolic state of fasting by inducing high levels of circulating ketone bodies, or ketosis, due to an increase in fatty acid oxidation in the liver [11]. There has been an increase in clinical usage of KDs in recent decades to treat refractory epilepsy. Up to 55% of pediatric patients become seizure-free and seizures are reduced by up to 85% after three months of treatment with a KD [12]. Because administration and adherence to KDs can be difficult for patients and caregivers, elucidating the anticonvulsant mechanism of KDs has increasingly become a pursuit of great interest for clinical and basic research. A direct effect of ketone bodies is often hypothesized to be the anticonvulsant mechanism of KDs [13]. However, a correlation between serum ketone levels and seizure control has not been consistently found [14, 15], suggesting that KDs may involve mechanisms independent of ketosis.
We recently reported that a KD is protective against SUDEP in Scn1aR1407X/+ mice, a model of Dravet Syndrome (DS) [16]. DS is a devastating epileptic encephalopathy that is most often caused by de novo mutations in the SCN1A gene [17]. DS manifests with febrile and/or spontaneous seizures in early childhood with an incidence of premature mortality of ~21%, half of which is attributed to SUDEP [18, 19]. DS mice recapitulate many aspects of the clinical condition: seizures occur spontaneously and have a high incidence of SUDEP [9, 18, 20]. Additionally, postictal death in DS mice is due to seizure-induced respiratory arrest (S-IRA) [9]. Interestingly, the protective effect of a KD against SUDEP in DS mice is not due to a decrease in seizure frequency [16], suggesting that treatment with a KD specifically prevents S-IRA in DS mice.
DBA/1 mice have proven to be a useful model to study the mechanisms of S-IRA and SUDEP as they can be primed to develop generalized seizures in response to an auditory stimulus. This reliably leads to S-IRA and death [5, 21], but death can be prevented by mechanical ventilation so that different experimental manipulations can be applied in the same cohort of mice. In the present study we assessed the effect of a KD on spontaneous seizure-induced death in DS mice and audiogenic seizures in DBA/1 mice. We found that a KD decreased the risk of spontaneous S-IRA in DS mice and increased the latency to audiogenic S-IRA in DBA/1 mice. In both cases the protective effect of a KD was independent of serum β-hydroxybutyrate (BHB) levels.
2. Materials and methods
2.1. Mouse Husbandry and Genotyping.
Experiments were performed in male and female Scn1aR1407X/+ mice on a C3HFeB/HeJ background (kindly provided by Miriam Meisler, University of Michigan, Ann Arbor, Michigan, USA) and DBA/1 mice (Envigo Bioproducts, Madison WI). Breeding and genotyping of these mice have been previously described [22]. Briefly, male Scn1aR1407X/+ mice were bred with WT (Scn1a+/+) female mice. Offspring were genotyped by PCR amplification with the primers DS-F (5′ CAATGATTCCTAGGGGGATGTC 3′) and DS-R (5′ GTTCTGTGCACTTATCTGGATTCAC 3′). Genomic DNA was PCR amplified, digested with HpaII, and separated on 2% agarose gels containing 0.15 μg/ml ethidium bromide. Digestion of the PCR product with HpaII generated 2 fragments (295 bp and 223 bp) from the WT allele and an uncut fragment (518 bp) from the mutant allele. Scn1aR1407X/+ mice are referred to as “DS mice” for the entirety of this manuscript.
All mice were housed in the animal facility at the University of Iowa Carver College of Medicine in a temperature- and humidity-controlled environment in a 12-h light/dark cycle in standard cages with designated diet and water available ad libitum. All procedures and experiments involving mice were carried out with approval of the University of Iowa Institutional Animal Care and Use Committee, and in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals, 8th edition [23].
2.2. Audiogenic Seizure Induction in DBA/1 mice.
DBA/1 mice were primed to have audiogenic seizures as previously described [5, 21]. Briefly, mice were exposed to an acoustic stimulus elicited with an alarm bell (twin metal bell analog quartz alarm clock, La Crosse Technology, La Crosse, WI) once per day starting at postnatal day 21 (P21) until S-IRA occurred. The maximum duration of audiogenic stimulus exposure was 60 seconds. This was repeated once per day until mice had full seizures, defined as generalized convulsive seizures (GCS) progressing to tonic hindlimb extension and respiratory arrest, for at least three consecutive days. If S-IRA occurred, mice were resuscitated using a rodent ventilator (Harvard Apparatus, Germany) set at a stroke volume of 150 μL at 150 strokes per minute. The amount of time, or latency, it took for mice to reach S-IRA from the onset of the audiogenic stimulus was measured.
2.3. Dietary treatments.
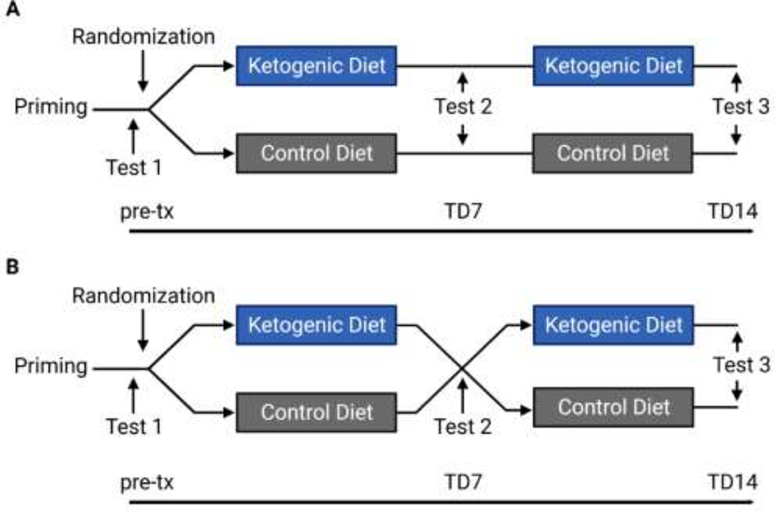
DS mice were randomly weaned onto either a control diet (64.8% carbohydrates, 20% protein, 8.2% fat) consisting of standard chow (7013, Teklad Diets, Madison, WI, USA) or a KD (0.72% carbohydrates, 18.37% protein, and 80.91% fat) (Bio-Serv F3666, Frenchtown, NJ, USA). After priming, DBA/1 mice were randomly placed on a control diet of standard mouse chow or a KD. One cohort of mice was maintained on either a control diet or a KD for 14 days and the latency to S-IRA was assessed before diet initiation, on treatment day (TD) 7 and TD14 (Figure 1A). A second cohort was placed on either a control diet or a KD and tested at TD7, after which mice were crossed over to the other diet group for an additional 7 days and reassessed (Figure 1B). A third cohort was randomized for 7 days to a control diet, a KD or a KD supplemented with either 5% glucose (D-(+)-glucose anhydrous, Research Products International, Mt. Prospect, IL, USA) in the drinking water (KD+GW; wt/vol) or 10% glucose by weight added to food (KD+GF). Based on the average daily consumption of food and water for adult mice [24], 5% glucose in water and 10% glucose added to solid KD food supplied approximately 20% of the daily carbohydrate intake of standard mouse chow. The KD+GW and KD+GF were both high fat but were predicted to provide enough carbohydrates to prevent ketone formation.
Figure 1. Experimental Design.
DBA/1 mice were primed for 3–4 days and then randomized to a KD or control diet. On day 7 they were either placed on the same diet (A) or crossed over to the other diet (B). pre-tx = pre-treatment, TD = treatment day.
2.4. Quantification of spontaneous seizure-induced death in Scn1aR1407X/+ mice.
DS mice were housed in standard cages under continuous video surveillance to monitor for spontaneous seizure-induced deaths from P16-P60 as previously described [9]. Briefly, video recordings were made at 30 frames per second using web cameras with night vision (FL8910W; Foscam Digital Technologies). Video recordings were saved in 8-hr segments and stored on an external hard drive using video webcam software (Blue Iris 4; Blue Iris Software). When a mouse was found dead in a cage, the video was reviewed to determine whether death was preceded by a behavioral seizure.
2.5. β-hydroxybutyrate Measurements.
Serum BHB levels were measured in DS mice as previously described [16]. Briefly, blood samples were collected from randomly selected P35–40 DS mice from each diet group. Animals were anesthetized with a ketamine/xylazine cocktail (87.5 mg/kg ketamine / 12.5 mg/kg xylazine, IP). Blood was sampled via cardiac puncture into an EDTA pre-coated syringe to prevent coagulation, and centrifuged at 3,500 rpm, 4°C, for 5 minutes to obtain plasma. BHB levels were determined in duplicate using a commercially available enzyme colorimetric BHB Assay kit (BioVision, Mountain View, CA). OD450 readings were determined using plate spectrophotometry (BioTek Synergy 4, Winooski, VT). Serum BHB levels were measured in DBA/1 mice using a test strip system and reader (Precision Xtra; Abbott Diabetes Care Inc., Alameda, CA, U.S.A.) from tail vein blood samples.
2.6. Statistical Analysis.
Statistical analysis was performed with Prism 9 (GraphPad Software, Inc., La Jolla, CA, U.S.A.). Survival curves were constructed using the Kaplan-Meier method and comparisons made with the Log-rank test with post hoc Bonferroni correction for multiple comparisons as appropriate. Serum BHB levels were analyzed with a Kruskal-Wallis nonparametric test followed by Dunn’s multiple comparisons test. The latency to S-IRA in DBA/1 mice recorded pre-treatment, at TD7 and TD14 (cohort one) was analyzed using a mixed-effects model with repeated measures followed by Tukey’s multiple comparison test since our experiment manipulated two different variables: diet and treatment duration. The latency to S-IRA at individual treatment days (pre-treatment, TD7 and TD14) was compared between diets with nonparametric Mann-Whitney U tests. Crossover experiment data (cohort two) were analyzed with Wilcoxon matched-pairs signed rank tests to determine if there was a difference in latency within the KD to Control group and the Control to KD group. The differences in latency between the two crossover groups were compared with Mann-Whitney U tests. The latency to S-IRA in DBA/1 mice recorded pre-treatment and at TD7 (cohort three) was analyzed using a repeated measures two-way ANOVA followed by Tukey’s multiple comparison test since our experiment manipulated two different variables: different diets and treatment duration. The incidence of S-IRA was compared between diet groups at TD7 and TD14 with a Fisher’s exact test. The correlation between serum BHB levels and latency to S-IRA in DBA/1 mice was determined with a nonparametric Spearman correlation analysis. All data points presented in the text as X ± Y are mean ± standard deviation, and error bars on data plots represent SEM. Significance was set at p < 0.05.
3. Results
3.1. A ketogenic diet increased survival in DS mice even when ketosis was prevented.
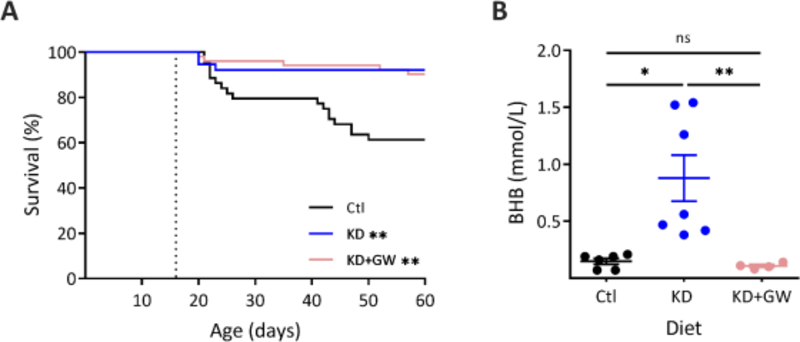
To test whether the effect of KDs on survival in DS mice correlated with serum BHB levels, DS mice were randomly placed on a control diet, a KD, or a KD+GW. During long-term, continuous video surveillance, 39% of DS mice on a control diet died of SUDEP between P16 & P60 (n = 17 of 44) (Figure 2A). KD treatment significantly decreased mortality to approximately 8% (n = 3 of 38 died, p = 0.006) (Figure 2A). DS mice in the KD+GW group also had a significant decrease in mortality when compared to control to 10% (n = 5 out of 52 p = 0.002), but when compared to a KD they were not significantly different (p > 0.999). In line with what we previously reported, there was no difference in mortality between male and female mice [16]. As expected, KD-treated DS mice had significantly higher circulating levels of BHB compared to control (0.88 ± 0.54 vs 0.15 ± 0.06 mmol/L, p = 0.016) (Figure 2B). In contrast, DS mice treated with a KD+GW displayed serum BHB levels that were not significantly different from control (0.11 ± 0.03 mmol/L, p > 0.999).
Figure 2. Ketosis is not necessary for a ketogenic diet to prevent mortality in DS mice.
(A) Kaplan-Meir survival curves of DS mice placed on either a KD (n = 38), KD supplement with 5% glucose in drinking water (KD+GW, n = 51), or control diet (Ctl, n = 44). DS mice placed on either a KD or a KD+GW showed significantly increased survival when compared to control (**p = 0.006, **p = 0.002, Log-rank test with Bonferroni’s correction). There were no differences in survival rates between KD and KD+GW groups (p > 0.999). (B) BHB levels were increased in DS mice on a KD (n = 7) compared to control (n = 6) (*p = 0.016, Kruskal-Wallis test with Dunn’s multiple comparisons). Supplementing a KD with glucose prevented ketosis from occurring (KD+GW, n = 4, **p = 0.008). There were no differences in BHB levels between control and KD+GW (p > 0.999).
3.2. DBA/1 mice on a ketogenic diet showed a longer latency to S-IRA.
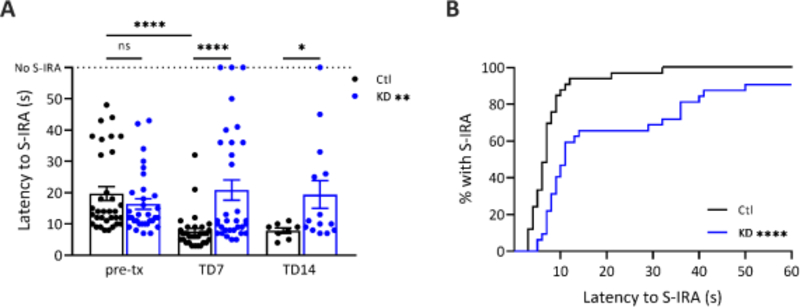
To test whether a KD influenced S-IRA in DBA/1 mice, primed mice in cohort one were randomly placed on a control diet or a KD and the latency to S-IRA was measured in response to an audiogenic stimulus. There were no differences in the latency to S-IRA between groups prior to randomization (16.4 ± 9.6 in KD vs 19.7 ± 12.6 s in control, p = 0.315). There was a decrease in latency to S-IRA from pre-treatment to TD7 in control mice (n = 33, p < 0.001), presumably due to plasticity induced by the priming process. In contrast, mice placed on a KD (n = 32) showed increased latency to S-IRA compared to the control diet group (F(1, 63) = 8.406, p = 0.005). At TD7, KD-treated DBA/1 mice had a latency to S-IRA 2.7-fold longer than mice on a control diet (20.8 ± 18.2 vs 7.6 ± 5.6 s) (p < 0.001) (Figure 3A). All mice on a control diet reached S-IRA within 32 s of audiogenic stimulus onset at TD7, a time at which only 65% of mice on a KD reached S-IRA (p < 0.001) (Figure 3B). By 60 seconds, 91% of KD-treated mice exhibited S-IRA (n = 29 of 32) compared to 100% of mice on a control diet (n = 33 of 33, p = 0.1136). From the control group, we were able to revive 8 out of 33 mice on TD7 and 14 out of 32 mice in the KD group. KD-treated mice that survived (n = 14) were tested again at TD14 and had a latency to S-IRA that was 2.5-fold longer than control (n = 8) mice (19.4 ± 16.5 vs 7.9 ± 2.4 s, p = 0.043). At TD14, 93% of KD-treated mice had S-IRA by 60 seconds (n = 13 of 14) compared to 100% of mice on a control diet (n = 8 of 8, p > 0.999). Because all audiogenic S-IRA in DBA/1 results in death if no mechanical ventilation is performed, we determined the number of mice that had S-IRA whether or not they were rescued with mechanical ventilation as representative of mortality.
Figure 3. DBA/1 mice on a KD showed a higher latency to S-IRA than a control diet.
(A) DBA/1 mice placed on a KD showed increased latency to S-IRA compared to the control diet group [F(1, 63) = 8.406, **p = 0.005, mixed-effects analysis with Tukey’s multiple comparisons test]. There was a decrease in latency from pre-treatment (pre-tx) to TD7 in control mice (n = 33, ****p < 0.001), but mice treated with a KD displayed a higher latency to S-IRA on TD7 compared to control (n = 32, ****p < 0.001, Mann-Whitney U test). From the control group, 25 mice died from S-IRA after inducing audiogenic seizures on TD7 compared to 18 in the KD group. Among the mice that survived, the latency to S-IRA on TD14 in KD-treated mice (n = 14) was higher compared to control (n = 8) (*p = 0.043, Mann-Whitney U test). (B) A KD decreased the percentage of DBA/1 mice reaching S-IRA within the same time compared to control at TD7. Kaplan-Meir survival curves of DBA/1 mice placed on either a KD or control diet for 7 days (****p < 0.001, Log-rank test).
3.3. The protective effect of a ketogenic diet in DBA/1 mice required ongoing exposure.
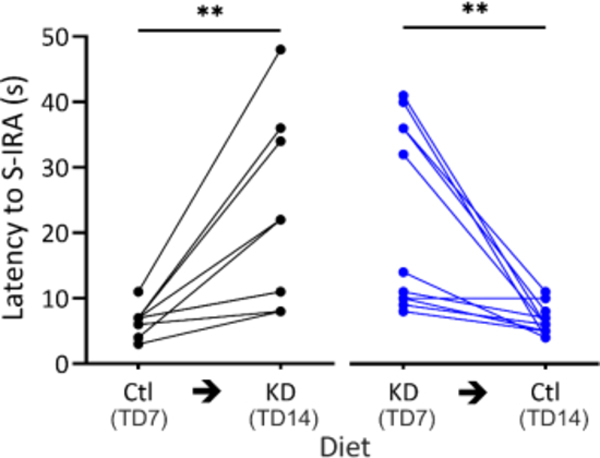
To determine whether the effect of a KD on S-IRA had long term effects on brain excitability, DBA/1 mice in cohort two were placed on either a control diet (n = 9) or a KD (n = 11) for 7 days and the latency to S-IRA was assessed at TD7. Animals were then crossed over to the other diet for another 7 days, after which the latency to S-IRA was reassessed. There was a significant increase in latency when mice started on a control diet and were switched to a KD (6.6 ± 2.2 vs 22.2 ± 14.4 s, p = 0.004). There was a significant reduction in latency to S-IRA when switched from KD to control (22.5 ± 14.2 vs 6.5 ± 2.3 s, p = 0.002) (Figure 4). The difference in total change in latency to S-IRA between the control to KD cohort and the KD to control cohort was not significant (p = 0.837). These results indicate that the latency to S-IRA is prolonged by a KD only if continuous treatment is maintained.
Figure 4. DBA/1 mice crossed over to a control diet after a KD had decreased latency to S-IRA.
DBA/1 mice on TD7 and after being crossed over to the other diet for 7 days. A KD increased latency to S-IRA after being switched from a control diet (n = 9, black, control to KD) (**p = 0.004, Wilcoxon matched-pairs signed rank test). The latency to S-IRA decreased on TD14 when mice were crossed over on TD7 from a KD to control (n = 11, blue, KD to control) (**p = 0.002, Wilcoxon matched-pairs signed rank test). The difference in total change in latency to S-IRA between the control to KD group and the KD to control group was not significant (p = 0.838, Mann-Whitney U test).
3.4. A ketogenic diet protects DBA/1 mice from S-IRA independent of ketosis
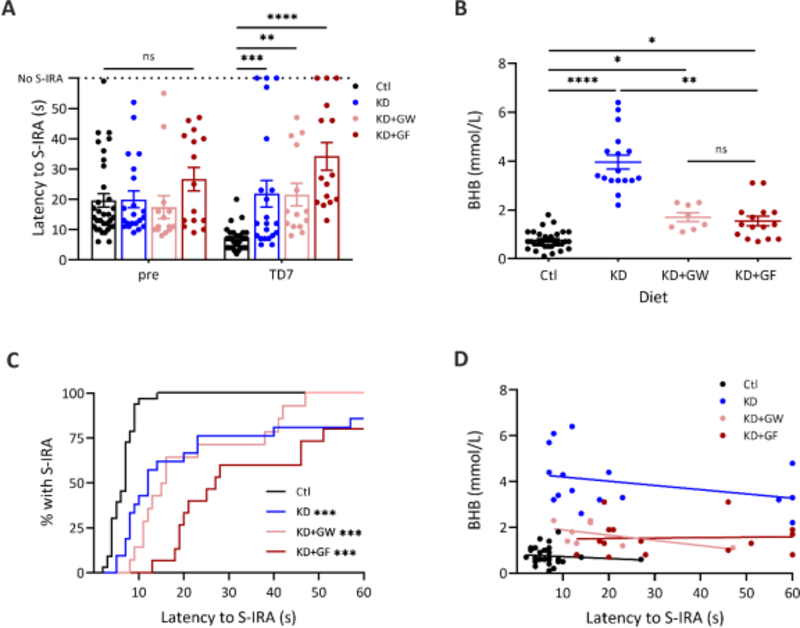
To determine whether the latency to S-IRA in DBA/1 mice correlated with ketone levels, cohort three was placed on a control diet (n = 33), a KD (n = 21), a KD+GW (n = 14) or a KD+GF (n = 15). DBA/1 mice placed on KDs showed increased latency to S-IRA compared to the control diet group on TD7 (F(3, 79) = 9.244, p < 0.001). The latency to S-IRA was 3.2-fold higher in mice treated with a KD compared to a control diet on TD7 (21.9 ± 20.3 s vs 6.9 ± 3.6 s, p < 0.001) (Figure 5A). Mice on KD+GW and KD+GF also showed a higher latency to S-IRA (21.6 ± 14.0 and 34.2 ± 17.5 s, respectively) when compared to control (p = 0.006 and p < 0.001, respectively). There were no significant differences in latency to S-IRA between any of the groups prior to dietary treatment. Three KD mice and three KD+GF mice on TD7 did not have S-IRA within 60 s of audiogenic stimulation.
Figure 5. A KD increases latency to S-IRA in DBA/1 mice independent of ketone levels.
(A) Latency to S-IRA remained increased when a KD (n = 21, ***p = 0.001) was supplemented with glucose in either water (n = 14, **p = 0.006) or food (n = 15, ****p < 0.001) when compared with control (n = 33) [F(3, 79) = 9.244, p < 0.001, two-way RM ANOVA]. There were no differences pre-treatment (pre) between any of the diets. (B) A KD increased BHB levels in DBA/1 mice (n = 17, ****p < 0.001, Kruskal-Wallis test with Dunn’s multiple comparisons). Mice on a KD+GW (n = 8) had lower serum BHB levels when compared to a KD, but this did not reach statistical significance (p = 0.155). Mice on a KD+GF (n = 15) had significantly lower serum BHB compared to a KD (**p = 0.004). Mice on a KD+GW and KD+GF had higher levels of BHB than those on a control diet (*p < 0.05). (C) Mice treated with a KD supplemented with glucose had a decreased percentage of S-IRA when compared to control. ***p < 0.001, Log-rank survival analysis with Bonferroni’s correction. (D) There was no correlation between latency to S-IRA and serum BHB levels in the control [r (28) = −0.190, p = 0.314], KD [r (15) = −0.336, p = 0.186], KD+GW [r (6) = −0.560, p = 0.153], or KD+GF groups [r (13) = 0.061, p = 0.828, Spearman r].
As expected, serum BHB levels were significantly increased in DBA/1 mice on a KD compared to a control diet (3.96 ± 1.21 vs 0.75 ± 0.39 mmol/L, p < 0.001) (Figure 5B). In contrast, BHB levels in KD+GF mice were significantly lower compared to KD (1.54 ± 0.76 mmol/L, p = 0.004). KD+GW mice also had lower serum BHB levels compared to KD (1.63 ± 0.52), but this did not reach statistical significance (p = 0.087). BHB levels were significantly higher in both KD+GW and KD+GF mice compared to control diet (p < 0.05). BHB levels were similar between KD+GW and KD+GF cohorts (p > 0.999).
KD-treatment significantly increased latency to S-IRA when compared to a control diet (p < 0.001) as did KD+GW and KD+GF (Figure 5C). There were no significant differences in the latency to S-IRA between mice on a KD, KD+GW and KD+GF. When data were grouped by diet, there was no significant correlation between serum BHB and latency to S-IRA within any of the groups (Figure 5D).
4. Discussion
Previous studies have shown that treatment with a KD increases the seizure threshold to flurothyl-induced seizures in a heterozygous knockout (Scn1a+/−) mouse model of DS [25]. We previously reported that chronic treatment with a KD markedly increases survival in DS mice, and this is associated with an increase in serum BHB [16]. The present study expands our previous findings by showing that a KD supplemented with glucose to abolish ketosis was equally effective in reducing seizure-induced mortality in DS mice. This is supported by another study that found a diet combining macronutrients in different ratios is protective against seizures in the absence of ketosis [26]. Furthermore, dietary therapies such as the modified Atkins diet and the low glycemic index diet are effective in DS patients despite not inducing a state of ketosis [27, 28]. Since only levels of BHB were measured in this study, we cannot exclude the possibility that other ketone bodies (i.e. acetone and acetoacetate) played a role in the protective effect of the KD. However, this is unlikely because KDs induce a larger increase in circulating BHB than acetoacetate [29]. Moreover, the latter is less stable than BHB and can spontaneously be converted into acetone, which is difficult to measure since it is exhaled through the lungs [30]. Although exogenous ketones can be beneficial for treatment of seizures associated with metabolic abnormalities [31], our findings challenge the notion that ketosis is necessary for the KD to be anticonvulsive and raise the question of the potential roles of other constituents of the diet.
The anticonvulsant effects of KDs have been documented in the clinical setting and in animal models of epilepsy. Several studies in DS patients have shown an overall positive response rate with KD therapy [32]. Studies in Kcna1-null mice, which display respiratory pathophysiology that parallels that of DS mice and model terminal events associated with SUDEP [33], have demonstrated that KD treatment reduces spontaneous seizures and reduces mortality due to SUDEP [34]. This protective effect was attributed to a delay in disease progression in terms of seizure frequency and severity. Interestingly, seizure semiology in older Kcna1-null mice prior to sudden death was similar between diet groups, suggesting that a KD may also prevent death from a seizure. This paralleled what we previously reported in DS mice, where protection conferred by a KD on mortality was independent of an antiseizure effect [16]. We also showed that the number of convulsive seizures leading up to SUDEP were highly variable between animals. Collectively, these findings challenge the view that uncontrolled convulsive seizures are the strongest risk factor for SUDEP and highlight the need to understand what determines the fatality of a seizure.
The underlying mechanisms of SUDEP remain elusive, but a growing body of evidence implicate a primarily respiratory cause of death [6–9]. In all monitored SUDEP cases in the MORTEMUS study, central apnea preceded terminal asystole [6]. Similarly, we demonstrated that S-IRA precedes SUDEP in DS mice [9]. Since we did not measure EEG activity in DS or DBA/1 mice we were not able to accurately identify seizure onset. However, all documented sudden deaths were preceded by behavioral seizures, similar to our previous report in DS mice [16]. Therefore, our goal was to assess the effect of a KD on the progression of a seizure to S-IRA in DBA/1 mice rather than seizure threshold. Although we found that fewer DBA/1 mice treated with a KD died from S-IRA following an audiogenic stimulus on TD7 compared to control, we were unable to compare mortality of the two groups directly because the procedure used to resuscitate mice by manual ventilation after S-IRA was not standardized. Instead, we used the occurrence of S-IRA as a surrogate of fatal seizures since all S-IRAs were fatal without mechanical ventilation, similar to previous reports [5]. We found that some DBA/1 mice treated with a KD did not reach S-IRA while all control animals did, but the difference was minor and not statistically significant. These findings suggest that a KD increases the latency to a convulsive seizure that results in S-IRA rather preventing S-IRA once a seizure occurs. Further studies with EEG monitoring and plethysmography will be necessary to determine how this and other terminal events are altered by KDs.
The cardiorespiratory collapse observed in DS mice and in witnessed SUDEP cases implicates involvement of brainstem respiratory and autonomic networks [35]. It is unclear how treatment with a KD prevents SUDEP in DS mice without reducing the incidence of spontaneous seizures, but it is possible that KDs alter the dynamics of seizure propagation from the forebrain to key respiratory centers in the brainstem and prevents fatal apnea by stabilizing respiratory output during seizures. Emerging evidence suggests that the amygdala is a critical node in a pathway to the brainstem that is required for S-IRA to occur in DBA/1 mice [21], as lesions of the amygdala reduce the incidence of S-IRA and death. Further work will be needed to determine the nature of these connections and how KDs alter them.
Although DBA/1 mice do not meet the criteria for SUDEP because they do not have spontaneous seizures, respiratory arrest in these mice also occurs following a GCS, and this can be induced with acoustic stimulation [5]. In the present study we showed that treatment with a KD for seven days significantly delayed the onset of S-IRA in DBA/1 mice and completely prevented S-IRA in three animals compared to mice on a control diet. The protective effect of the KD was reversed when DBA/1 mice were switched from a KD to a control diet. This suggests that ongoing exposure to a KD is necessary to maintain the protective effect in DBA/1 mice rather than remodeling of hyperexcitable circuits. In pediatric epilepsy patients, seizures typically recur in 20% of cases after the KD has been discontinued after two years of seizure freedom [36]. The cause of this recurrence is not clear, but may be due to withdrawal of a KD component that has anticonvulsant properties.
The anticonvulsant mechanism of a KD has been predominantly ascribed to the significant increase in ketones that occurs with the diet [13], but animal studies have yielded inconsistent results [14, 15]. In the current dataset, DBA/1 mice treated with KDs supplemented with glucose (KD+GW and KD+GF) showed BHB levels higher than the control group (Figure 5A). Although they were much lower than with a KD, we cannot entirely exclude the possibility that dietary ketosis contributed to the effect of the KD on S-IRA in DBA/1 mice. However, the lack of correlation between serum BHB levels and latency to S-IRA (Figure 5D) indicates that ketosis was not the driving force in this effect. Taken together, our data and existing evidence do not support a necessary role of ketones in the protective effect of a KD against seizure-induced mortality.
In addition to ketones, KDs also cause an increase in plasma levels of medium-chain fatty acids (MCFAs) [37, 38]. Decanoic acid (C10) is a MCFA present in KDs capable of crossing the blood-brain barrier that has been shown to have antiseizure effects at clinically relevant concentrations both in vivo and in vitro [39–41]. In in vitro models, C10 has been shown to directly and selectively inhibit AMPA receptors [40] – key players in the generation and propagation of seizures and a recognized target for seizure control [42]. Further studies examining the exact role of MCFAs such as C10 in protection against SUDEP in DS and DBA/1 mice are needed.
The KD may also function indirectly by modulating neurotransmitter systems. MCFAs such as C10 have been shown to displace L-tryptophan, the precursor to the monoamine neurotransmitter 5-hydroxytryptamine (5-HT), from its plasma protein-binding site, facilitating CNS entry across the blood-brain barrier [43]. Administration of MCFAs increased the current required to induce after-discharges in the rat hippocampus, an effect that was abolished if the passage of L-tryptophan into the brain was blocked. Many lines of evidence support that 5-HT plays a significant role in epilepsy [44, 45]. Agents that increase extracellular 5-HT such as the precursor 5-hydroxytryptophan (5-HTP) [46] or selective serotonin reuptake inhibitors (SSRI) such as fluoxetine have been reported to inhibit both focal and generalized seizures in rodents [47, 48] and in humans [49]. 5-HT is also well known to stimulate breathing and to be important for the respiratory response to hypercapnia [7]. S-IRA in DBA/1 and DBA/2 mice can be prevented with pretreatment with an SSRI [50–52]. Nevertheless, it remains unknown whether 5-HT modulators can prevent seizure-induced respiratory depression, or if there is a decrease in the incidence of SUDEP in patients on these medications. It is possible that treatment with a KD increases brain 5-HT by increasing the bioavailability of L-tryptophan. Future experiments investigating the effect of KDs on 5-HT and other neurotransmitter levels in DS and DBA/1 mice will be important to determine whether 5-HT neurotransmission plays a role in the protective effect of KDs.
Highlights.
A KD decreased SUDEP incidence in Scn1aR1407X/+ mice independent of ketosis.
A KD delayed the onset of audiogenic S-IRA in DBA/1 mice independent of ketosis.
The protective effect of a KD in DBA/1 mice required continuous treatment.
Mechanisms other than formation of ketones may have a protective effect against SUDEP.
Acknowledgements.
We thank Xiuqiong Zhou for mouse husbandry, and Lori Smith for technical contributions. Research reported in this study was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award Numbers U01 NS090414, U01 NS090407 and F31 NS110333. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest statement: The authors declare no competing financial interests.
Declarations of interest: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Chen Z, Brodie MJ, Liew D, Kwan P. Treatment Outcomes in Patients With Newly Diagnosed Epilepsy Treated With Established and New Antiepileptic Drugs: A 30-Year Longitudinal Cohort Study. JAMA Neurol 2018;75: 279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sveinsson O, Andersson T, Carlsson S, Tomson T. The incidence of SUDEP: A nationwide population-based cohort study. Neurology 2017;89: 170–177. [DOI] [PubMed] [Google Scholar]
- [3].Shorvon S, Tomson T. Sudden unexpected death in epilepsy. Lancet 2011;378: 2028–2038. [DOI] [PubMed] [Google Scholar]
- [4].Thurman DJ, Hesdorffer DC, French JA. Sudden unexpected death in epilepsy: assessing the public health burden. Epilepsia 2014;55: 1479–85. [DOI] [PubMed] [Google Scholar]
- [5].Faingold CL, Randall M, Tupal S. DBA/1 mice exhibit chronic susceptibility to audiogenic seizures followed by sudden death associated with respiratory arrest. Epilepsy Behav 2010;17: 436–40. [DOI] [PubMed] [Google Scholar]
- [6].Ryvlin P, Nashef L, Lhatoo SD, Bateman LM, Bird J, Bleasel A, Boon P, Crespel A, Dworetzky BA, Hogenhaven H, Lerche H, Maillard L, Malter MP, Marchal C, Murthy JM, Nitsche M, Pataraia E, Rabben T, Rheims S, Sadzot B, Schulze-Bonhage A, Seyal M, So EL, Spitz M, Szucs A, Tan M, Tao JX, Tomson T. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol 2013;12: 966–77. [DOI] [PubMed] [Google Scholar]
- [7].Massey CA, Sowers LP, Dlouhy BJ, Richerson GB. Mechanisms of sudden unexpected death in epilepsy: the pathway to prevention. Nat Rev Neurol 2014;10: 271–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dlouhy BJ, Gehlbach BK, Richerson GB. Sudden unexpected death in epilepsy: basic mechanisms and clinical implications for prevention. J Neurol Neurosurg Psychiatry 2016;87: 402–13. [DOI] [PubMed] [Google Scholar]
- [9].Kim Y, Bravo E, Thirnbeck CK, Smith-Mellecker LA, Kim SH, Gehlbach BK, Laux LC, Zhou X, Nordli DR Jr., Richerson GB. Severe peri-ictal respiratory dysfunction is common in Dravet syndrome. J Clin Invest 2018;128: 1141–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kossoff EH, Zupec-Kania BA, Amark PE, Ballaban-Gil KR, Christina Bergqvist AG, Blackford R, Buchhalter JR, Caraballo RH, Helen Cross J, Dahlin MG, Donner EJ, Klepper J, Jehle RS, Kim HD, Christiana Liu YM, Nation J, Nordli DR Jr., Pfeifer HH, Rho JM, Stafstrom CE, Thiele EA, Turner Z, Wirrell EC, Wheless JW, Veggiotti P, Vining EP, Charlie Foundation PCotCNS, Practice Committee of the Child Neurology S, International Ketogenic Diet Study G. Optimal clinical management of children receiving the ketogenic diet: recommendations of the International Ketogenic Diet Study Group. Epilepsia 2009;50: 304–17. [DOI] [PubMed] [Google Scholar]
- [11].Vidali S, Aminzadeh S, Lambert B, Rutherford T, Sperl W, Kofler B, Feichtinger RG. Mitochondria: The ketogenic diet--A metabolism-based therapy. Int J Biochem Cell Biol 2015;63: 55–9. [DOI] [PubMed] [Google Scholar]
- [12].Zarnowska IM. Therapeutic Use of the Ketogenic Diet in Refractory Epilepsy: What We Know and What Still Needs to Be Learned. Nutrients 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Simeone TA, Simeone KA, Stafstrom CE, Rho JM. Do ketone bodies mediate the antiseizure effects of the ketogenic diet? Neuropharmacology 2018;133: 233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Likhodii SS, Musa K, Mendonca A, Dell C, Burnham WM, Cunnane SC. Dietary fat, ketosis, and seizure resistance in rats on the ketogenic diet. Epilepsia 2000;41: 1400–10. [DOI] [PubMed] [Google Scholar]
- [15].Thavendiranathan P, Chow C, Cunnane S, Burnham WM. The effect of the ‘classic’ ketogenic diet on animal seizure models. Brain Research 2003;959: 206–213. [DOI] [PubMed] [Google Scholar]
- [16].Teran FA, Kim Y, Crotts MS, Bravo E, Emaus KJ, Richerson GB. Time of day and a ketogenic diet influence susceptibility to SUDEP in Scn1a R1407X/+ mice. Front Neurol 2019;10: 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Claes L, Del-Favero J, Ceulemans B, Lagae L, Van Broeckhoven C, De Jonghe P. De novo mutations in the sodium-channel gene SCN1A cause severe myoclonic epilepsy of infancy. Am J Hum Genet 2001;68: 1327–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kalume F, Westenbroek RE, Cheah CS, Yu FH, Oakley JC, Scheuer T, Catterall WA. Sudden unexpected death in a mouse model of Dravet syndrome. J Clin Invest 2013;123: 1798–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Shmuely S, Sisodiya SM, Gunning WB, Sander JW, Thijs RD. Mortality in Dravet syndrome: A review. Epilepsy Behav 2016;64: 69–74. [DOI] [PubMed] [Google Scholar]
- [20].Ogiwara I, Miyamoto H, Morita N, Atapour N, Mazaki E, Inoue I, Takeuchi T, Itohara S, Yanagawa Y, Obata K, Furuichi T, Hensch TK, Yamakawa K. Nav1.1 localizes to axons of parvalbumin-positive inhibitory interneurons: a circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation. J Neurosci 2007;27: 5903–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Marincovich A, Bravo E, Dlouhy B, Richerson GB. Amygdala lesions reduce seizure-induced respiratory arrest in DBA/1 mice. Epilepsy Behav 2019: 106440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Auerbach DS, Jones J, Clawson BC, Offord J, Lenk GM, Ogiwara I, Yamakawa K, Meisler MH, Parent JM, Isom LL. Altered cardiac electrophysiology and SUDEP in a model of Dravet syndrome. PLoS One 2013;8: e77843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Albus U Guide for the Care and Use of Laboratory Animals (8th edn). Laboratory Animals 2012;46: 267–268. [Google Scholar]
- [24].Bachmanov AA, Reed DR, Beauchamp GK, Tordoff MG. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav Genet 2002;32: 435–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dutton SB, Sawyer NT, Kalume F, Jumbo-Lucioni P, Borges K, Catterall WA, Escayg A. Protective effect of the ketogenic diet in Scn1a mutant mice. Epilepsia 2011;52: 2050–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Dallérac G, Moulard J, Benoist JF, Rouach S, Auvin S, Guilbot A, Lenoir L, Rouach N. Non-ketogenic combination of nutritional strategies provides robust protection against seizures. Sci Rep 2017;7: 5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Auvin S Should we routinely use modified Atkins diet instead of regular ketogenic diet to treat children with epilepsy? Seizure 2012;21: 237–40. [DOI] [PubMed] [Google Scholar]
- [28].Muzykewicz DA, Lyczkowski DA, Memon N, Conant KD, Pfeifer HH, Thiele EA. Efficacy, safety, and tolerability of the low glycemic index treatment in pediatric epilepsy. Epilepsia 2009;50: 1118–26. [DOI] [PubMed] [Google Scholar]
- [29].Laffel L Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab Res Rev 1999;15: 412–26. [DOI] [PubMed] [Google Scholar]
- [30].Qiao Y, Gao Z, Liu Y, Cheng Y, Yu M, Zhao L, Duan Y, Liu Y. Breath ketone testing: a new biomarker for diagnosis and therapeutic monitoring of diabetic ketosis. Biomed Res Int 2014;2014: 869186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Poff AM, Rho JM, D’Agostino DP. Ketone Administration for Seizure Disorders: History and Rationale for Ketone Esters and Metabolic Alternatives. Front Neurosci 2019;13: 1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Cross JH, Caraballo RH, Nabbout R, Vigevano F, Guerrini R, Lagae L. Dravet syndrome: Treatment options and management of prolonged seizures. Epilepsia 2019;60 Suppl 3: S39–S48. [DOI] [PubMed] [Google Scholar]
- [33].Simeone KA, Hallgren J, Bockman CS, Aggarwal A, Kansal V, Netzel L, Iyer SH, Matthews SA, Deodhar M, Oldenburg PJ, Abel PW, Simeone TA. Respiratory dysfunction progresses with age in Kcna1-null mice, a model of sudden unexpected death in epilepsy. Epilepsia 2018;59: 345–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Simeone KA, Matthews SA, Rho JM, Simeone TA. Ketogenic diet treatment increases longevity in Kcna1-null mice, a model of sudden unexpected death in epilepsy. Epilepsia 2016;57: e178–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Aiba I, Noebels JL. Spreading depolarization in the brainstem mediates sudden cardiorespiratory arrest in mouse SUDEP models. Sci Transl Med 2015;7: 282ra46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Martinez CC, Pyzik PL, Kossoff EH. Discontinuing the ketogenic diet in seizure-free children: recurrence and risk factors. Epilepsia 2007;48: 187–90. [DOI] [PubMed] [Google Scholar]
- [37].Sills MA, Forsythe WI, Haidukewych D. Role of octanoic and decanoic acids in the control of seizures. Arch Dis Child 1986;61: 1173–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Haidukewych D, Forsythe WI, Sills M. Monitoring octanoic and decanoic acids in plasma from children with intractable epilepsy treated with medium-chain triglyceride diet. Clin Chem 1982;28: 642–5. [PubMed] [Google Scholar]
- [39].Wlaz P, Socala K, Nieoczym D, Zarnowski T, Zarnowska I, Czuczwar SJ, Gasior M. Acute anticonvulsant effects of capric acid in seizure tests in mice. Prog Neuropsychopharmacol Biol Psychiatry 2015;57: 110–6. [DOI] [PubMed] [Google Scholar]
- [40].Chang P, Augustin K, Boddum K, Williams S, Sun M, Terschak JA, Hardege JD, Chen PE, Walker MC, Williams RS. Seizure control by decanoic acid through direct AMPA receptor inhibition. Brain 2016;139: 431–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chang P, Terbach N, Plant N, Chen PE, Walker MC, Williams RS. Seizure control by ketogenic diet-associated medium chain fatty acids. Neuropharmacology 2013;69: 105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rogawski MA. AMPA receptors as a molecular target in epilepsy therapy. Acta Neurol Scand Suppl 2013: 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Maciejak P, Szyndler J, Turzynska D, Sobolewska A, Kolosowska K, Krzascik P, Plaznik A. Is the interaction between fatty acids and tryptophan responsible for the efficacy of a ketogenic diet in epilepsy? The new hypothesis of action. Neuroscience 2016;313: 130–48. [DOI] [PubMed] [Google Scholar]
- [44].Bagdy G, Kecskemeti V, Riba P, Jakus R. Serotonin and epilepsy. J Neurochem 2007;100: 857–73. [DOI] [PubMed] [Google Scholar]
- [45].Petrucci AN, Joyal KG, Purnell BS, Buchanan GF. Serotonin and sudden unexpected death in epilepsy. Exp Neurol 2020;325: 113145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Truscott TC. Effects of phenylalanine and 5-hydroxytryptophan on seizure severity in mice. Pharmacol Biochem Behav 1975;3: 939–41. [DOI] [PubMed] [Google Scholar]
- [47].Wada Y, Shiraishi J, Nakamura M, Hasegawa H. Prolonged but not acute fluoxetine administration produces its inhibitory effect on hippocampal seizures in rats. Psychopharmacology (Berl) 1995;118: 305–9. [DOI] [PubMed] [Google Scholar]
- [48].Pasini A, Tortorella A, Gale K. Anticonvulsant effect of intranigral fluoxetine. Brain Res 1992;593: 287–90. [DOI] [PubMed] [Google Scholar]
- [49].Albano C, Cupello A, Mainardi P, Scarrone S, Favale E. Successful treatment of epilepsy with serotonin reuptake inhibitors: proposed mechanism. Neurochem Res 2006;31: 509–14. [DOI] [PubMed] [Google Scholar]
- [50].Tupal S, Faingold CL. Evidence supporting a role of serotonin in modulation of sudden death induced by seizures in DBA/2 mice. Epilepsia 2006;47: 21–6. [DOI] [PubMed] [Google Scholar]
- [51].Faingold CL, Randall M, Mhaskar Y, Uteshev VV. Differences in serotonin receptor expression in the brainstem may explain the differential ability of a serotonin agonist to block seizure-induced sudden death in DBA/2 vs. DBA/1 mice. Brain Res 2011;1418: 104–10. [DOI] [PubMed] [Google Scholar]
- [52].Zeng C, Long X, Cotten JF, Forman SA, Solt K, Faingold CL, Feng HJ. Fluoxetine prevents respiratory arrest without enhancing ventilation in DBA/1 mice. Epilepsy Behav 2015;45: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]