Abstract
Long noncoding RNA (lncRNA) are involved in regulating physiological behaviors for various malignant tumors, including non-small-cell lung cancer (NSCLC). However, few studies comprehensively evaluated both lncRNA–lncRNA interaction effects and main effects of lncRNA on overall survival of NSCLC. Hence, we performed a two-phase designed study of lncRNA expression in tumor tissues using 604 NSCLC patients from The Cancer Genome Atlas as the discovery phase and 839 patients from Gene Expression Omnibus as the validation phase. In the discovery phase, we adopted a two-step strategy, Screening before Testing, for dimension reduction and signal detection. These candidate lncRNAs first screened out by the weighted random forest (Ranger), were then tested through the Cox proportional hazards model adjusted for covariates. Significant lncRNAs with either type of effects aforementioned were carried forward into the validation phase to confirm their significances again. As a result, in the discovery phase, 19 lncRNAs were identified by Ranger, among which five lncRNAs and one pair of lncRNA–lncRNA interaction exhibited significant effects (FDR-q ≤ 0.05) main and interaction effects on NSCLC survival, respectively, through Cox model. After the independent validation, we finally observed that one lncRNA (ENSG00000227403.1) with main effect was robustly associated with NSCLC prognosis (HRdiscovery = 0.90, P = 1.20 × 10–3; HRvalidation = 0.94, P = 4.11 × 10–3) and one pair of lncRNAs (ENSG00000267121.4 and ENSG00000272369.1) had significant interaction effect on NSCLC survival (HRdiscovery = 1.12, P = 3.07 × 10–4; HRvalidation = 1.11, P = 0.0397). Our comprehensive NSCLC prognostic study of lncRNA provided population-level evidence for further functional study.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00438-022-01869-3.
Keywords: lncRNA, Main effect, Interaction, Non-small-cell lung cancer, Overall survival
Introduction
Lung cancer is the leading cause of cancer death worldwide (Sung et al. 2021). It is well known that majority of lung cancers are non-small cell lung cancer (NSCLC) in histological classification, including lung adenocarcinoma (LUAD) and lung squamous cell carcinoma (LUSC) (Travis 2020). In the half past century, great efforts have been made in the treatments of lung cancer, including surgery, chemotherapy, radiotherapy, targeted therapy, anti-angiogenic therapy, immunotherapy, etc. (Mok et al. 2009, 2019; Auperin et al. 2010). Therefore, the survival rate and quality of life of patients has been significantly improved (Wu et al. 2014; Mok et al. 2019; Sung et al. 2021). However, pathological mechanism of lung cancer progression still largely remains unclear (e.g. tumor cell invasion and drug resistance) (Herbst et al. 2018).
Long noncoding RNAs (lncRNAs) are a type of non-protein-coding transcript longer than 200 nucleotides and are one of the emerging regulators which are involved in diverse biological processes (Boon et al. 2016; Jandura and Krause 2017). Many studies have demonstrated that lncRNA plays an important role (e.g. oncogenes or tumor suppressors) in regulating the physiological behaviors of malignant tumors, including lung cancer (Schmitt and Chang 2016; Braicu et al. 2019; Xu et al. 2019). In recent years, accumulating evidences indicate that the interaction effects of lncRNAs are essential for the initiation and progression of cancers, such as lncRNA-protein interaction and lncRNA-miRNA-mRNA network (Ferrè et al. 2016; Wang et al. 2019). Especially, lncRNA-AC020978/PKM2/HIF-1α is a new perspective in the prevention or treatment of NSCLC (Hua et al. 2020). However, few studies ever focused on lncRNA–lncRNA interaction effects on NSCLC overall survival, which may provide pivotal clues for the biologic mechanisms of complex diseases (Zhang et al. 2019b) and enhance prediction accuracy (Chatterjee et al. 2016; Li et al. 2019).
Hence, we performed a comprehensive analysis of lncRNAs to evaluate their main effects and interaction effects on NSCLC survival through a two-phase designed study, using 604 NSCLC patients from The Cancer Genome Atlas (TCGA) as the discovery phase and 839 patients from Gene Expression Omnibus (GEO) as the validation phase.
Materials and methods
Data collection and study population
LncRNA expression data in tumor tissue, as well as demographic and clinical data, were retrieved from TCGA and GEO. (1) TCGA: The fragments per kilobase of per million (FPKM) value of lncRNA expression, as well as corresponding demographic and clinical information of 986 NSCLC patients, were downloaded from TCGA (https://portal.gdc.cancer.gov). (2) GEO: Totally, 6 available datasets consisting of 1096 NSCLC patients, profiled by the Affymetrix Human Genome U133A Plus 2.0 Array, were obtained from GEO (https://www.ncbi.nlm.nih.gov/geo/), which had the maximum overlapped lncRNAs to TCGA data, adequate sample sizes after quality control (e.g., N > 50) and, meanwhile raw data instead of standardized data, including GSE3141, GSE37745, GSE30219, GSE50081, GSE29013 and GSE31210.
LncRNA was annotated by gencode.v22 (https://www.gencodegenes.org/), and we obtained expression of 15,900 lncRNAs in TCGA. Before association analysis, we performed quality control procedures to acquire reliable lncRNA expression. Briefly, lncRNAs were excluded if they met any of the below criteria: all gene expression values equal to 0 or proportion of missing values is greater than 10%. Further, samples with missing values of any clinical variables were also excluded. Finally, 604 samples (294 LUAD and 310 LUSC) with 4313 lncRNAs in TCGA were retained in subsequent association analysis. In GEO, there were 839 patients (634 LUAD and 205 LUSC) remained after removing patients without complete clinical information. We performed a two-phase designed study of lncRNAs using subjects in TCGA as the discovery phase and subjects in GEO as the validation phase. The demographic and clinical information of subjects from two phases were described in Table 1.
Table 1.
Demographic and clinical descriptions of NSCLC patients in the discovery and validation phases
| Variable | Discovery phase (N = 604) | Validation phase (N = 839) |
|---|---|---|
| Age (years) | 66.02 ± 9.55 | 63.51 ± 9.34 |
| Gender, N (%) | ||
| Male | 355 (58.77%) | 411 (56.38%) |
| Female | 249 (41.23%) | 318 (43.62%) |
| Unknown | 0 | 110 |
| Race, N (%) | ||
| Asian | 8 (1.32%) | 0 |
| Black | 55 (9.11%) | 0 |
| White | 541 (89.57%) | 0 |
| Unknown | 0 | 839 |
| Smoke status, N (%) | ||
| Never | 0 | 26 (6%) |
| Former | 416 (68.87%) | 243 (56.12%) |
| Current | 188 (31.13%) | 164 (37.88%) |
| Unknown | 0 | 406 |
| Smoking packs every year | 47.38 ± 29.96 | – |
| Clinical stage, N (%) | ||
| I | 328 (54.30%) | 496 (68.04%) |
| II | 171 (28.31%) | 172 (23.59%) |
| III | 88 (14.57%) | 53 (7.27%) |
| IV | 17 (2.81%) | 8 (1.10%) |
| Unknown | 0 | 110 |
| Histology, N (%) | ||
| LUAD | 294 (48.68%) | 634 (75.57%) |
| LUSC | 310 (51.32%) | 205 (24.43%) |
| Survival year | ||
| Median (95% CI) | 3.91 (3.33–4.93) | 7.53 (6.30–8.92) |
| Censoring rate | 59.60% | 55.66% |
Statistical analysis
The entire statistical analysis workflow was given in Fig. 1.
Fig. 1.
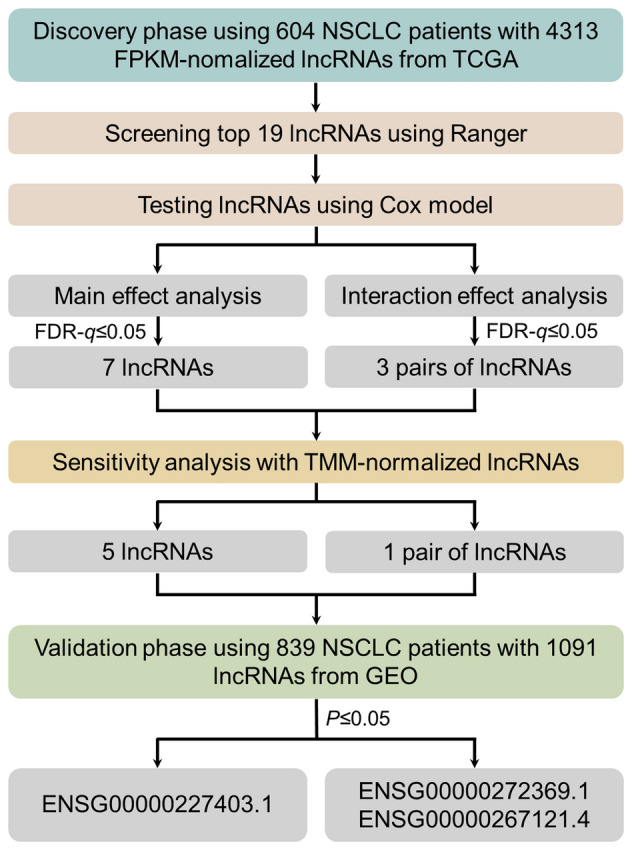
Statistical analysis work flow
Screening before Testing strategy in the discovery phase
In the discovery phase, we adopted a two-step strategy, named Screening before Testing, for dimension reduction and signal detection. In the screening step, Ranger, a weighted version of random forest for analyzing time-to-event data, while adjusted for covariates, was employed to evaluate the importance of each individual lncRNA (Breiman 2001), using R package ranger. A weight of 100% was assigned to each covariate to ensure all covariates were adjusted in each classification tree, including age, gender, race, clinical stage, smoking status and pack-year of smoking. Variable importance score (VIS) of each lncRNA was estimated and ranked in a descending order. The sliding windows sequential forward feature selection (SWSFS) algorithm was then applied to identify the top important lncRNAs (Jiang et al. 2009). The SWSFS algorithm incorporated these lncRNAs one by one into the Ranger model by the order of VIS. Then, we plotted the out of bagging (OOB) error rate, which measured the performance of each model consisting of top k lncRNAs. The top candidate lncRNAs were screened out for further analysis when the Ranger model reaching the lowest OOB error rate.
In the testing step, we further evaluated both main effects and lncRNA–lncRNA interaction effects of these top candidate lncRNAs, through Cox proportional hazards model adjusted for the same covariates aforementioned, using the R package survival. For main effect analysis and interaction effect analysis, we adopted the model 1 and 2 below, respectively.
The association results were described as hazard ratio (HR) and 95% confidence interval (CI). Multiple comparisons were adjusted using false discovery rate method (FDR; measured by FDR-q value) (Klipper-Aurbach et al. 1995). LncRNAs with significant (FDR-q ≤ 0.05) main effects or interaction effects on NSCLC survival were reserved for subsequent analysis.
To retain the robustly significant association between lncRNA and NSCLC survival, we additionally downloaded raw counts of lncRNA expression from TCGA and calculated a trimmed mean of M-values (TMM) between each pair of samples to adjust the library sizes (Robinson and Oshlack 2010), by R package DGEobj.utils. Then, we performed sensitivity analysis of TMM-normalized expression data for top candidate lncRNAs and significant (FDR-q ≤ 0.05) lncRNAs with either type of effects aforementioned were preserved. Finally, only these lncRNAs simultaneously significant in both FPKM and TMM data were carried forward into the validation phase to confirm their significances once again.
Trans-platform pseudo-validation strategy in the validation phase
Due to the difference of gene expression platforms between TCGA and GEO, some significant lncRNAs identified in TCGA were not profiled in GEO. Therefore, we performed trans-platform pseudo-validation using their surrogate lncRNAs, which had significant (FDR-q ≤ 0.05) and maximum correlation with the targets. The main effects and interaction effects of these surrogate lncRNAs were again tested by Cox proportional hazards model adjusted for covariates. Finally, only these lncRNAs were preserved if they met both criteria below: (1) P ≤ 0.05 in the validation phase, and (2) consistent effects across both discovery and validation phases.
Furthermore, the significances of these lncRNAs were evaluated in LUAD and LUSC subgroup populations. Besides, we compared two models to highlight the contribution of lncRNAs to the prognostic prediction of NSCLC survival: (1) a basic model with merely demographic and clinical information, and (2) an optimized model added significant lncRNAs with either main effects or interaction effects. We predicted 3- and 5-year overall survival of NSCLC patients using the Kaplan–Meier method for time-to-event data (Heagerty et al. 2000). The accuracy of the prediction was presented using a receiver operating characteristic (ROC) curve and was measured by time-dependent area under the ROC curve (AUC) by the R package survivalROC. The 95% CI and P value of the AUC improvement were calculated on the basis of 1,000-time bootstrap resampling.
Continuous variables were summarized as mean ± standard deviation (SD), and categorized variables were described by frequency (n) and proportion (%). Statistical analyses were performed using R version 3.6.3 (The R Foundation of Statistical Computing, Vienna, Austria).
Result
The two-phase study identified one lncRNA with significant main effect and one pair of lncRNAs with significant interactions
In the discovery phase, we identified top 19 lncRNAs which together had the lowest OOB error in the screening step by the SWSFS algorithm (Figure S1, S2). In the testing step, we observed five lncRNAs (ENSG00000227403.1, ENSG00000273038.2, ENSG00000269609.4, ENSG00000273230.1 and ENSG00000204949.7) and one pair of lncRNAs (ENSG00000272369.1 and ENSG00000267121.4) had robust and significant (FDR-q ≤ 0.05) main effects and interaction effects on NSCLC survival, respectively (Table S1). The annotation information was presented in Table S2. Compared to the basic model, the optimized model added these lncRNAs with either type of effects had significant improved prediction accuracy (3-year survival: AUCoptimized = 0.72 vs AUCbasic = 0.65, 12.0% increase, P < 2.2 × 10–16; 5-year survival: AUCoptimized = 0.74 vs AUCbasic = 0.63, 16.9% increase, P < 2.2 × 10–16) (Figure S3).
In the validation phase (Table S3), we finally observed one lncRNA, ENSG00000227403.1, significantly associated with NSCLC survival (HRdiscovery = 0.90, P = 1.20 × 10–3; HRvalidation = 0.94, P = 4.11 × 10–3) and one pair of lncRNAs (ENSG00000267121.4 and ENSG00000272369.1) had significant interaction effect on NSCLC survival (HRdiscovery = 1.12, P = 3.07 × 10–4; HRvalidation = 1.11, P = 0.0397) (Table 2).
Table 2.
Association results of one lncRNA and one pair of lncRNAs derived from Cox proportional hazards model adjusted for covariates in main effect and interaction effect analyses
| Type of analysis | LncRNA | Discovery phase | Validation phase | ||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Main effect | ENSG00000227403.1 | 0.90 (0.84, 0.96) | 1.20 × 10–3 | 0.94 (0.90, 0.98) | 4.11 × 10–3 |
| Interaction effect | ENSG00000272369.1 | 0.69 (0.57, 0.83) | 8.28 × 10–5 | 0.44 (0.24, 0.79) | 6.41 × 10–3 |
| ENSG00000267121.4 | 1.30 (1.12, 1.51) | 5.61 × 10–4 | 0.38 (0.17, 0.88) | 0.0228 | |
| Interaction term | 1.12 (1.05, 1.19) | 3.07 × 10–4 | 1.11 (1.01, 1.23) | 0.0397 | |
In the validation phase, the surrogates of ENSG00000227403.1, ENSG00000272369.1 and ENSG00000267121.4 were ENSG00000253738.1, ENSG00000265666.1 and ENSG00000227039.5, respectively
The lncRNA with significant main effect substantially discriminated subjects at high risk of mortality from NSCLC patients
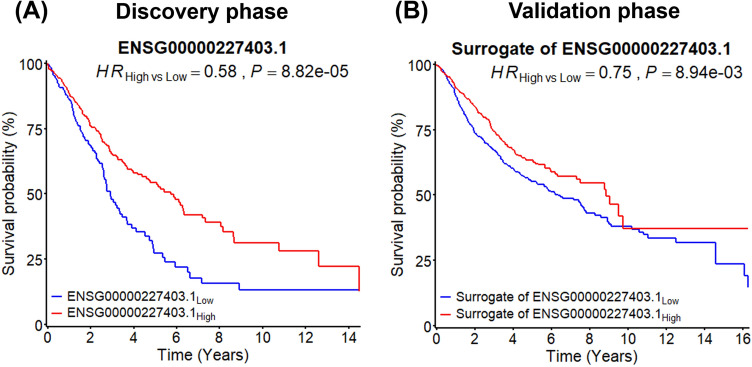
NSCLC patients were divided into low and high expression groups based on median value of ENSG00000227403.1. By comparison of the Kaplan–Meier survival curves between two groups (Fig. 2), these patients in high expression group had significant better overall survival in the discovery phase (HRhigh vs low = 0.58, P = 8.82 × 10–5) and the validation phase (HRhigh vs low = 0.75, P = 8.94 × 10–3), indicating that subjects with low expression of ENSG00000227403.1 were at high risk of mortality.
Fig. 2.
Kaplan–Meier survival curves for patients with low and high expression of ENSG00000227403.1 in the discovery and validation phases
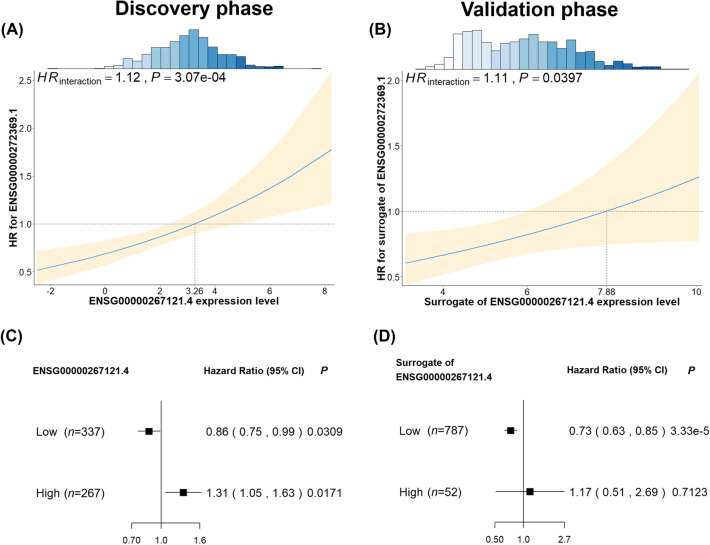
The effect of one lncRNA on NSCLC survival was modified by another lncRNA
For the interaction between ENSG00000272369.1 and ENSG00000267121.4, we observed that, with increased expression level of ENSG00000267121.4, there was an elevated effect of ENSG00000272369.1 on NSCLC survival in the discovery phase (Fig. 3A) and the validation phase (Fig. 3B). Therefore, ENSG00000267121.4 was a modifier of the association between ENSG00000272369.1 and NSCLC survival. Besides, to better understand this interaction, patients were divided into low and high expression groups based on their ENSG00000267121.4 values, using cutoff value 3.26 and 7.88 in the discovery and validation phase, respectively. We observed varied effects of ENSG00000272369.1 between different ENSG00000267121.4 expression groups. High expression of ENSG00000272369.1 exhibited a significantly protective effect on NSCLC for these patients in low expression group of ENSG00000267121.4 in the discovery phase (HR = 0.86, P = 0.0309) and the validation phase (HR = 0.73, P = 3.33 × 10–5) (Fig. 3C, D). However, the effect of ENSG00000272369.1 was reversed for these patients in high expression group of ENSG00000267121.4 in the discovery phase (HR = 1.31, P = 0.0171) and the validation phase (HR = 1.17, P = 0.7123).
Fig. 3.
Line and forest plots illustrating lncRNA–lncRNA interaction effect between ENSG00000267121.4 and ENSG0000027236.9 on NSCLC survival
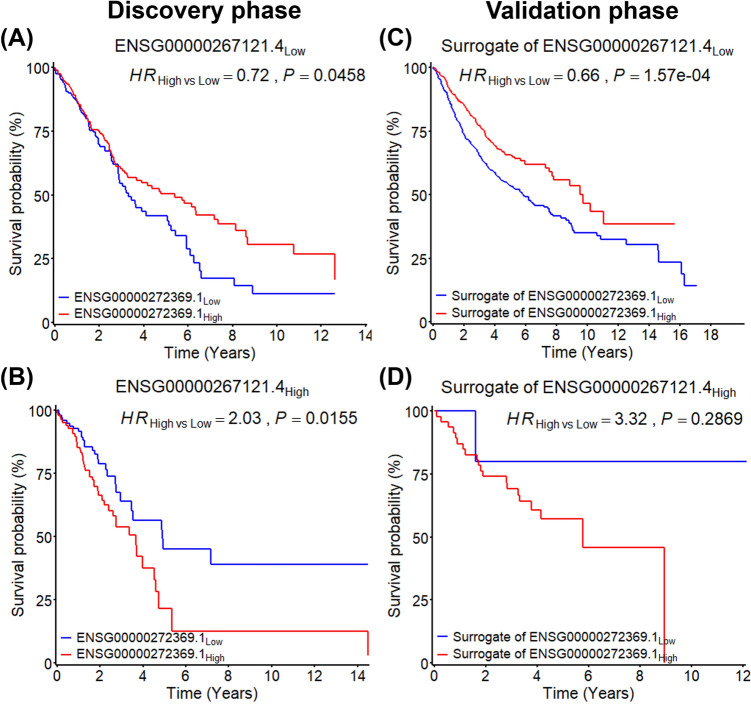
To visualize the heterogeneous effect of ENSG00000272369.1 between two expression groups of ENSG00000267121.4, we further categorized ENSG00000272369.1 into dichotomous variable by its median value. In low expression group of ENSG00000267121.4, subjects with high expression of ENSG00000272369.1 had significantly better survival compared to these with low ENSG00000272369.1 in the discovery phase (HRhigh vs low = 0.72, P = 0.0458) (Fig. 4A) and the validation phase (HRhigh vs low = 0.66, P = 1.57 × 10–4) (Fig. 4C). On the contrary, in high expression group of ENSG00000267121.4, subjects with high expression of ENSG00000272369.1 had worse survival in two phases (Fig. 4B, D).
Fig. 4.
Kaplan–Meier survival curves for patients having different expression levels of two lncRNAs
The stratified analysis by histology confirmed the significance of main effect of ENSG00000227403.1 on NSCLC survival (Table S4), except for LUSC subgroup with small sample size in the validation phase. Besides, the interaction effect between ENSG00000272369.1 and ENSG00000267121.4 maintained significance only in LUAD subgroup in the discovery phase.
Discussion
Non-coding RNAs (ncRNAs), accounting for 98% of the human genome, are classified into several types including: lncRNAs, micro RNAs (miRNAs), circular RNA (circRNAs) and so on (Zhang et al. 2020a; b). Human lncRNAs are abundant and diverse, and nearly 53,000 different lncRNAs are known but only about 1000 are present in sufficiently high copy number to authentically justify their functional importance (Djebali et al. 2012). In recent years, extensive evidence has suggested that lncRNAs are involved in the occurrence of many diseases, including cancer (Liang et al. 2018). LncRNAs have been shown to participate in the development, progression, proliferation, and invasion of NSCLC used variety of ways (Zhang et al. 2019c; Chen et al. 2020a, b).
Emerging evidence indicates that there exist many types of interactions associated with NSCLC survival, including gene–gene (Zhang et al. 2019b), gene-smoking (Zhang et al. 2019a), gene-age (Chen et al. 2020a; b) and gene-histology interactions (Ji et al. 2020). As is well known, interactions provide important clues for the biologic mechanisms and heritability of complex diseases (Trerotola et al. 2015). Thus, biomarkers with main effects only explained a small proportion of the phenotypic variations and the GxG interactions may be one of the important reasons accounting for the missing heritability (Trerotola et al. 2015). The studies of lncRNAs account for 13% of the total studies of ncRNAs in lung cancer until year 2019 (Braicu et al. 2019). Anyway, a mass of studies only focused on the association between lncRNAs and lung cancer, by testing their main effects. GxG interaction analysis provides a different way of identifying new biomarkers (Cordell 2009), which can further improve the predictive accuracy of statistical models (Cordell 2009; Zhang et al. 2020a, b), or offer statistical evidence for functional studies. To our knowledge, this perhaps was the first attempt to explore the association between lncRNA–lncRNA interaction and NSCLC survival in population level. But functional experiments are still warranted to elaborate underlying mechanism.
For the three lncRNAs (ENSG00000227403.1, ENSG00000267121.4 and ENSG00000272369.1) successfully validated in an independent population, previous study indicated that AC009299.3, to which ENSG00000227403.1 mapped, was involved in the control of autophagy (Zhu et al. 2018; Wu et al. 2021). Meanwhile, high expression of this gene was also associated with better LUAD prognosis (Wu et al. 2021). Besides, CTD-2020K17.1, where ENSG00000267121.4 located, has been identified to promote migration, invasion, and proliferation of ovarian cancer (Zhu et al. 2018). Although these three lncRNAs lack explicit functional level evidence relevant to NSCLC survival, we provided robust and significant population-level evidence for further mechanistic study.
For other four lncRNAs (ENSG00000204949.7, ENSG00000273038.2, ENSG00000269609.4 and ENSG00000273230.1) failed in the independent validation, FAM83A-AS1 (ENSG00000204949.7) and RP11-479G22.8 (ENSG00000273038.2) play essential roles in the development and progression of several cancers, including lung cancer, esophageal cancer and hepatocellular carcinoma (Wei and Zhang 2016; Wu et al. 2018; He and Yu 2019; Shi et al. 2019; Huang et al. 2020; Jia et al. 2021). Besides, RPARP-AS1 (ENSG00000269609.4) could promote the proliferation, migration and invasion of tumor cells through sponging mir-125a-5p, which had been proved functional in the growth, invasion and metastasis of lung cancer and other cancers (Naidu et al. 2017; Ren et al. 2021).
Our study has several strengths. First, our study simultaneously evaluated both main effects of lncRNAs and lncRNA–lncRNA interaction effects on NSCLC survival, which was a comprehensive prognostic study of lncRNAs. And, to our knowledge, this is perhaps the first lncRNA–lncRNA interaction study of NSCLC survival. Besides one lncRNA with significant main effect, we additionally identified one pair of lncRNAs which exhibited significant interaction effect, providing potential evidence that complex disease (e.g., lung cancer) was driven by complex association pattern. Second, we utilized a two-phase study design to control the false positives, where statistical significance was corrected using FDR method in the discovery phase and again confirmed in the validation phase. Third, we adopted a two-step strategy, Screening before Testing, to improve the calculation speed of analysis and boost the statistical power of analysis in high dimensional scenario. Meanwhile, covariates were adjusted in both screening and testing steps to obtain more roust association results.
We also acknowledge some limitations. First, the significant lncRNAs identified in TCGA happen to be not profiled in GEO. Thus, we compromised by a trans-platform pseudo-validation using surrogate lncRNAs of these target lncRNAs. Even though one lncRNA and one pair of lncRNAs were successfully validated, additional available public database and further studies of target lncRNAs are still warranted. Second, the censoring rate of time-to-event data is high in TCGA and GEO, which may result in low statistical power in analysis. Thus, we only focused on two-way interaction between pair of lncRNAs. Third, further functional experiments of lncRNAs are warranted to provide biological evidence, beyond our statistical evidence. Thus, the association still should be interpreted with caution.
Conclusion
Our two-phase comprehensive NSCLC prognostic study of lncRNAs identified one lncRNA (ENSG00000227403.1) with significant main effects and one pair of lncRNAs (ENSG00000267121.4 and ENSG00000272369.1) with significant interaction effects on overall survival, providing population-level evidence for further functional study.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank TCGA and GEO for contributing demographic, clinical, and gene expression data, as well as all subjects who participated in these study cohorts.
Author contributions
RZ, RG, RW, QZ and JZ contributed to the study design. RZ, JZ, JG and JX contributed to data collection. JZ, JG, JX, RW and YS performed statistical analysis and interpretation. RZ, JZ, JG, JX, XX and QW drafted and revised the manuscript. All authors contributed to critical revision of the manuscript and approved its final version. Financial support and study supervision were provided by RZ and RG.
Funding
This study was funded by the Natural Science Foundation of Jiangsu Province (BK20191354 to R. Z.), China Postdoctoral Science Foundation (2018M633767 to R. Z.), National Natural Science Foundation of China (81772995 to R. W. and 81972188 to R. G.), and Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). R. Z. was partially supported by the Qing Lan Project of the Higher Education Institutions of Jiangsu Province and the Outstanding Young Teachers Training Program of Nanjing Medical University.
Availability of data and material
The datasets used in the current study are available from the corresponding author on reasonable request.
Code availability
The datasets used in the current study are available from the corresponding author on reasonable request.
Declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval
NA.
Consent to participate
NA.
Consent for publication
All authors have reviewed the manuscript and consented for publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jing Zhu, Jinxing Guan and Xinyu Ji contributed equally to this work.
Contributor Information
Quanan Zhang, Email: zhang_quan_an_njmu@163.com.
Renhua Guo, Email: rhguo@njmu.edu.cn.
Rui Wang, Email: wangrui218@163.com.
Ruyang Zhang, Email: zhangruyang@njmu.edu.cn.
References
- Auperin A, Le Pechoux C, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28(13):2181–2190. doi: 10.1200/JCO.2009.26.2543. [DOI] [PubMed] [Google Scholar]
- Boon RA, Jaé N, et al. Long noncoding RNAs: from clinical genetics to therapeutic targets? J Am Coll Cardiol. 2016;67(10):1214–1226. doi: 10.1016/j.jacc.2015.12.051. [DOI] [PubMed] [Google Scholar]
- Braicu C, Zimta AA, et al. The function of non-coding RNAs in lung cancer tumorigenesis. Cancers. 2019 doi: 10.3390/cancers11050605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiman L. Random forests. Mach Learn. 2001;45(1):5–32. doi: 10.1023/A:1010933404324. [DOI] [Google Scholar]
- Chatterjee N, Shi J, et al. Developing and evaluating polygenic risk prediction models for stratified disease prevention. Nat Rev Genet. 2016;17(7):392–406. doi: 10.1038/nrg.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Wei Y, et al. Epigenome-wide gene-age interaction analysis reveals reversed effects of PRODH DNA methylation on survival between young and elderly early-stage NSCLC patients. Aging. 2020;12(11):10642–10662. doi: 10.18632/aging.103284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Chen X, et al. Integrative analysis of NSCLC identifies LINC01234 as an oncogenic lncRNA that interacts with HNRNPA2B1 and regulates miR-106b biogenesis. Mol Ther. 2020;28(6):1479–1493. doi: 10.1016/j.ymthe.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell HJ. Detecting gene-gene interactions that underlie human diseases. Nat Rev Genet. 2009;10(6):392–404. doi: 10.1038/nrg2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djebali S, Davis CA, et al. Landscape of transcription in human cells. Nature. 2012;489(7414):101–108. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrè F, Colantoni A, et al. Revealing protein-lncRNA interaction. Brief Bioinform. 2016;17(1):106–116. doi: 10.1093/bib/bbv031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Yu J. 2019. Long noncoding RNA FAM83A-AS1 facilitates hepatocellular carcinoma progression by binding with NOP58 to enhance the mRNA stability of FAM83A. Biosci Rep. [DOI] [PMC free article] [PubMed]
- Heagerty PJ, Lumley T, et al. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000;56(2):337–344. doi: 10.1111/j.0006-341x.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- Herbst RS, Morgensztern D, et al. The biology and management of non-small cell lung cancer. Nature. 2018;553(7689):446–454. doi: 10.1038/nature25183. [DOI] [PubMed] [Google Scholar]
- Hua Q, Mi B, et al. Hypoxia-induced lncRNA-AC020978 promotes proliferation and glycolytic metabolism of non-small cell lung cancer by regulating PKM2/HIF-1α axis. Theranostics. 2020;10(11):4762–4778. doi: 10.7150/thno.43839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GM, Zang HL, et al. LncRNA FAM83A-AS1 aggravates the malignant development of esophageal cancer by binding to miR-495-3p. Eur Rev Med Pharmacol Sci. 2020;24(18):9408–9415. doi: 10.26355/eurrev_202009_23024. [DOI] [PubMed] [Google Scholar]
- Jandura A, Krause HM. The new RNA world: growing evidence for long noncoding RNA functionality. TIG. 2017;33(10):665–676. doi: 10.1016/j.tig.2017.08.002. [DOI] [PubMed] [Google Scholar]
- Ji X, Lin L, et al. Epigenetic-smoking interaction reveals histologically heterogeneous effects of TRIM27 DNA methylation on overall survival among early-stage NSCLC patients. Mol Oncol. 2020;14(11):2759–2774. doi: 10.1002/1878-0261.12785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J, Li H, et al. LncRNA FAM83A-AS1 promotes ESCC progression by regulating miR-214/CDC25B axis. J Cancer. 2021;12(4):1200–1211. doi: 10.7150/jca.54007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang R, Tang W, et al. A random forest approach to the detection of epistatic interactions in case-control studies. BMC Bioinform. 2009;10(Suppl 1):S65. doi: 10.1186/1471-2105-10-s1-s65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klipper-Aurbach Y, Wasserman M, et al. Mathematical formulae for the prediction of the residual beta cell function during the first two years of disease in children and adolescents with insulin-dependent diabetes mellitus. Med Hypothes. 1995;45(5):486–490. doi: 10.1016/0306-9877(95)90228-7. [DOI] [PubMed] [Google Scholar]
- Li J, Li X, et al. Gene-environment interaction in the era of precision medicine. Cell. 2019;177(1):38–44. doi: 10.1016/j.cell.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C, Qi Z, et al. Long non-coding RNA PCAT-1 in human cancers: a meta-analysis. Clin Chim Acta. 2018;480:47–55. doi: 10.1016/j.cca.2018.01.043. [DOI] [PubMed] [Google Scholar]
- Mok TS, Wu YL, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- Mok TSK, Wu YL, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet (london, England) 2019;393(10183):1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- Naidu S, Shi L, et al. PDGFR-modulated miR-23b cluster and miR-125a-5p suppress lung tumorigenesis by targeting multiple components of KRAS and NF-kB pathways. Sci Rep. 2017;7(1):15441. doi: 10.1038/s41598-017-14843-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, Zhao C, et al. RPARP-AS1/miR125a-5p axis promotes cell proliferation, migration and invasion in colon cancer. Onco Targ Ther. 2021;14:5035–5043. doi: 10.2147/ott.S304494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, Oshlack A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 2010;11(3):R25. doi: 10.1186/gb-2010-11-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt AM, Chang HY. Long noncoding RNAs in cancer pathways. Cancer Cell. 2016;29(4):452–463. doi: 10.1016/j.ccell.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi R, Jiao Z, et al. Long noncoding antisense RNA FAM83A-AS1 promotes lung cancer cell progression by increasing FAM83A. J Cell Biochem. 2019;120(6):10505–10512. doi: 10.1002/jcb.28336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung H, Ferlay J, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Travis WD. Lung cancer pathology: current concepts. Clin Chest Med. 2020;41(1):67–85. doi: 10.1016/j.ccm.2019.11.001. [DOI] [PubMed] [Google Scholar]
- Trerotola M, Relli V, et al. Epigenetic inheritance and the missing heritability. Hum Genom. 2015;9(1):17. doi: 10.1186/s40246-015-0041-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Cho KB, et al. Long noncoding RNA (lncRNA)-mediated competing endogenous RNA networks provide novel potential biomarkers and therapeutic targets for colorectal cancer. Int J Mol Sci. 2019 doi: 10.3390/ijms20225758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Zhang X. Transcriptome analysis of distinct long non-coding RNA transcriptional fingerprints in lung adenocarcinoma and squamous cell carcinoma. Tumour Biol. 2016 doi: 10.1007/s13277-016-5422-2. [DOI] [PubMed] [Google Scholar]
- Wu YL, Zhou C, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15(2):213–222. doi: 10.1016/S1470-2045(13)70604-1. [DOI] [PubMed] [Google Scholar]
- Wu Y, Wang PS, et al. Genomewide identification of a novel six-LncRNA signature to improve prognosis prediction in resectable hepatocellular carcinoma. Cancer Med. 2018;7(12):6219–6233. doi: 10.1002/cam4.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Wen Z, et al. A novel autophagy-related lncRNA survival model for lung adenocarcinoma. J Cell Mol Med. 2021;25(12):5681–5690. doi: 10.1111/jcmm.16582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu YH, Deng JL, et al. Long non-coding RNAs in prostate cancer: functional roles and clinical implications. Cancer Lett. 2019;464:37–55. doi: 10.1016/j.canlet.2019.08.010. [DOI] [PubMed] [Google Scholar]
- Zhang R, Lai L, et al. SIPA1L3 methylation modifies the benefit of smoking cessation on lung adenocarcinoma survival: an epigenomic-smoking interaction analysis. Mol Oncol. 2019;13(5):1235–1248. doi: 10.1002/1878-0261.12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Lai L, et al. EGLN2 DNA methylation and expression interact with HIF1A to affect survival of early-stage NSCLC. Epigenetics. 2019;14(2):118–129. doi: 10.1080/15592294.2019.1573066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Wang Q, et al. LncRNA FENDRR suppresses the progression of NSCLC via regulating miR-761/TIMP2 axis. Biomed Pharmacother. 2019;118:109309. doi: 10.1016/j.biopha.2019.109309. [DOI] [PubMed] [Google Scholar]
- Zhang R, Chen C, et al. Independent validation of early-stage NSCLC prognostic scores incorporating epigenetic and transcriptional biomarkers with gene-gene interactions and main effects. Chest. 2020 doi: 10.1016/j.chest.2020.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Xie K, et al. Role of non-coding RNAs and RNA modifiers in cancer therapy resistance. Mol Cancer. 2020;19(1):47. doi: 10.1186/s12943-020-01171-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Guo Q, et al. CTD-2020K17.1, a novel long non-coding rna, promotes migration, invasion, and proliferation of serous ovarian cancer cells in vitro. Med Sci Monit Int Med J Exp Clin Res. 2018;24:1329–1339. doi: 10.12659/msm.908456. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- He J, Yu J. 2019. Long noncoding RNA FAM83A-AS1 facilitates hepatocellular carcinoma progression by binding with NOP58 to enhance the mRNA stability of FAM83A. Biosci Rep. [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The datasets used in the current study are available from the corresponding author on reasonable request.
The datasets used in the current study are available from the corresponding author on reasonable request.