Abstract
Although posttraumatic stress disorder (PTSD) is on the rise, traumatic events and their consequences are often hidden or minimized by patients for reasons linked to PTSD itself. Traumatic experiences can be broadly classified into mental stress (MS) and traumatic brain injury (TBI), but the cellular mechanisms of MS- or TBI-induced PTSD remain unknown. Recent evidence has shown that the morphological remodeling of astrocytes accompanies and arguably contributes to fearful memories and stress-related disorders. In this review, we summarize the roles of astrocytes in the pathogenesis of MS-PTSD and TBI-PTSD. Astrocytes synthesize and secrete neurotrophic, pro- and anti-inflammatory factors and regulate the microenvironment of the nervous tissue through metabolic pathways, ionostatic control, and homeostatic clearance of neurotransmitters. Stress or trauma-associated impairment of these vital astrocytic functions contribute to the pathophysiological evolution of PTSD and may present therapeutic targets.
Keywords: Astrocytes, Traumatic brain injury, Traumatic events, Neurotrophic factors, Serotonin
Introduction
Exposure to trauma, physical or mental, often acts as an etiological factor in various psychiatric disorders, including depression, anxiety, bipolar disorder, personality disorders, psychotic disorders, and post-traumatic stress disorder (PTSD) [1]. Diagnosis of PTSD as a nosological form is based on the presence of trauma exposure in the anamnesis with a minimum of one month of persistent symptoms and with at least one symptom representing one of the following four clusters: (i) intrusion, (ii) avoidance, (iii) negative mood and cognitive alterations, and (iv) arousal and reactivity [2]. The onset of the illness is triggered by traumatic life events including combat, injury, violence, or natural disasters. According to the different kinds of trauma, PTSD generally emerges consequent to mental stress and/or traumatic brain injury (TBI) with distinct pathophysiology. It is, however, difficult to distinguish between these etiologies; to avoid conflicting semantics, we classify PTSD into mental stress-induced (MS-PTSD) and to TBI-triggered (TBI-PTSD), although both types may (and often do) overlap.
PTSD is associated with substantial medical and economic burdens. Recently, it has been suggested that MS-PTSD should be prioritized as a public mental health focus [3]. As exposure to potentially traumatic events occurs frequently across the world, epidemiological research has drawn a clear link between exposure to traumatic stress and PTSD [4]. It is widely accepted that emotional stimulation involves acute and chronic stressors, both of which strongly impact the central nervous system (CNS), causing behavioral deficits [5–8] and dysregulating multiple physiological systems [9, 10]. Acute stress rapidly alters the activity of the CNS as well as the endocrine system [11]. Chronic stress leads to sustained changes in neural activity and gene expression that pathologically affect various molecular pathways [12–14]. Astrocytes play multiple roles in regulating neuronal activity and synaptic plasticity, with growing evidence implicating the contribution of astrocytes to acute and chronic stress in animal models, as well as in mood disorders in humans [15, 16].
Survivors of coronavirus disease-2019 (COVID-19) may be at high risk of PTSD [17]. Controlling the pandemic and taking care of patients with COVID-19 are major tasks worldwide. Epidemiological studies have demonstrated the substantial prevalence of mental health problems among survivors, victims’ families, medical professionals, and the general public after epidemics of infectious disease such as the severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), Ebola, flu, HIV/AIDS epidemics [18]. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the pathogen of COVID-19, can damage brain endothelial cells by invading the host serine protease, the renin-angiotensin system, and mitochondria, and increase the susceptibility to PTSD [19]. Damaged endothelial cells contribute to the destruction of the blood-brain barrier (BBB), while increased BBB permeability allows a variety of stress-related molecules, such as angiotensin II, endothelin-1, and plasminogen activator-1, to enter the amygdala, hippocampus and medial prefrontal cortex (PFC) and activate their receptors, causing downstream reactions [20–22]. The symptoms of PTSD may last long and result in distress and disability. Considering the already large and constantly increasing number of people being exposed to infection, emotional health services targeted at the prevention of PTSD among survivors need to be prioritized. Possible strategies may involve health education, psychosocial support, and counseling services for the general population as well as early therapeutic intervention including psychosocial support, psychotherapy, and pharmacological treatments for vulnerable and high-risk groups.
Astrocytes: Homeostatic and Protective Arm of the Central Nervous System
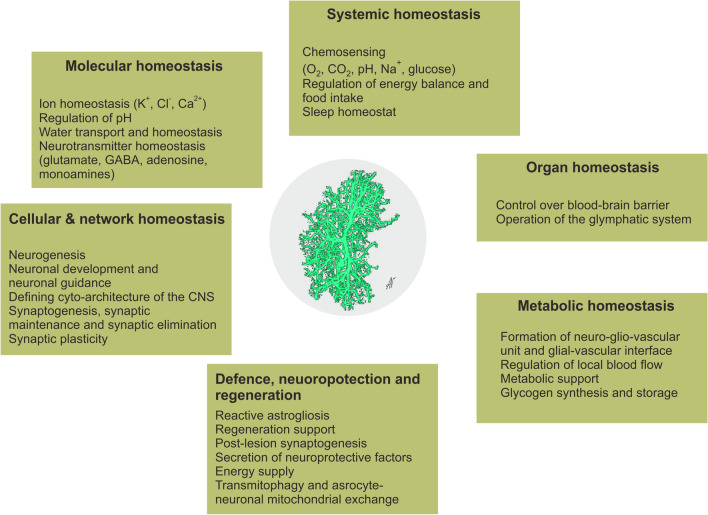
Astrocytes are homeostatic and defensive cells of the central nervous system (CNS); they are fundamental to the homeostasis of nervous tissue, performing extended and diversified supportive, metabolic, and protective roles (Fig. 1) [23]. Astrocytes contribute to the formation of the active milieu of nervous tissue [24] and provide, through multiple mechanisms, for the maintenance and regulation of synaptic transmission and plasticity [25, 26]. Astrocytes respond to neurochemical stimulation associated with neuronal activity with a transient increase in cytosolic ion concentrations, which are the basis for astrocytic excitability [27]. In particular, intracellular signals mediated by Ca2+ and Na+ regulate homeostatic responses of astrocytes aimed at supporting neuronal function [28, 29]. Astrocytic contribution to the pathophysiology of all neurological diseases may be primary, when changes in astrocytes drive neuropathology; examples may include Alexander disease or psychiatric disorders associated with substantial astrocytic atrophy. Astrocytic changes may also be secondary to the pathological lesion; this class of astrocytopathies is mainly represented by reactive astrogliosis [30, 31]. Mounting recent evidence indicates that astrocytes are involved in the formation and pathological remodeling of fear memories and stress-related disorders [32, 33].
Fig. 1.
Functions of astrocytes.
Astrocytes in TBI-PTSD
Mild TBI is a significant predictor of PTSD [34]. The amygdala, a genetically conserved limbic structure [35], is involved in processing emotional and stressful stimulation and is implicated in anxiety and PTSD [36, 37]. After diffuse TBI, neurons, localized in the region of the basolateral amygdala, exhibit increased dendritic intersections in the vicinity to the soma at 1, 7, and 28 days after injury, whereas glial fibrillary acidic protein (GFAP) immunoreactivity increases at 1 and 7 days, indicating the role of reactive astrogliosis in post-traumatic sequelae [38].
Astrocytes control CNS glutamate clearance and support the glutamate (GABA)-glutamine (Glu-Gln) shuttle [23]. Astrocytic glutamate transporters are fundamental for neuronal protection against glutamate excitotoxicity. Brain injury leads to the rapid increase in glutamate in the interstitium [39–41], reflecting massive neuronal release of glutamate following the lesion-induced loss of ionic homeostasis [42]. In recent years, imaging techniques such as magnetic resonance spectroscopy have found that under the pathological state of TBI, the changes of neural metabolites show a complex trend involving many factors, and the changes of various neural metabolites after TBI do not follow the same or similar paths [43]. In general, N-acetylaspartate (NAA) is reduced in the acute and subacute phases of injury and recovers over time. A continuous decrease of NAA reflects the progressive loss of ATP with these neurometabolic alterations modifying the gliotic response [44, 45]. Increases in glutamate and myoinositol, the latter applied as a magnetic resonance imaging (MRI) astrocyte marker because its concentration in astrocytes is several times higher than that in neurons, occur in the chronic stage of injury [46–48]. Changes in choline, a membrane marker, elevation of which may indicate astrogliosis [49], are more variable and depend on the types and regions of TBI [46, 47, 50]. All these changes in metabolites may reflect altered neuronal viability, integrity, excitability, and astrogliosis [43]. Several magnetic resonance spectroscopy studies support the idea that the increase in myoinositol reflects glial hypertrophy and/or glial proliferation, while myoinositol elevation is also associated with an increase in GFAP expression and immunoreactivity [48, 51, 52]. Increased myoinositol content has found been to be associated with a TBI-related risk of suicide [48, 53]. An elevated myoinositol/H2O ratio has been reported in the anterior cingulate cortex impaired by TBI during its chronic stage, which again may be reflective of astrogliosis [48].
The Role of Astrocytes in TBI-induced Secondary Injury
TBI with severe focal tissue damage triggers neuroinflammation essential for the clearance of waste, formation of fibrotic scar, nervous tissue protection, and regeneration, with astrocytes playing key roles in all these processes [54]. Astrocytes can respond to and secrete many immunomodulatory molecules, including cytokines, chemokines, and inflammatory mediators; in addition stressed, injured, or dying astrocytes, similar to other neural cells, may produce and release danger-associated molecular patterns (DAMPs) and alarmins. Prototypical DAMPs, including high-mobility group Box 1, heat shock proteins, and S100 proteins, signal through pattern recognition receptors on phagocytic immune cells to promote the clearance of cytotoxic cellular debris and resolve inflammation [54]. Another archetypal DAMP is ATP, which is massively released from dying cells; ATP action is mediated, at least in part, by the activation of ionotropic P2X7 receptors [55, 56]. Activation of P2X7 receptors promotes cerebral edema and neurological injury following TBI in mice [57]. TBI activates Cx43 connexins in hippocampal astrocytes, which results in ATP release, stimulation of P2X7 receptors, and down-regulation of expression of the glutamate transporter excitatory amino acid transporter EAAT2 [58]. Stimulation of astrocyte pattern recognition receptors by DAMPs activates nuclear factor-κB signaling and the production of proinflammatory cytokines such as tumor necrosis factor α (TNF-α) and α-chemokines as well as the inflammatory mediators cyclooxygenase-2 and matrix metalloproteinase 9 [59–61]. Pathogen-associated molecular patterns, known as PAMPs, such as lipopolysaccharide (LPS), bind to astrocytic pattern recognition receptors and elicit the expression of immunomodulatory and proinflammatory molecules [62, 63].
In addition to pathogens directly entering the brain through penetrating wounds, the peripheral infection may occur due to peripheral immunosuppression after TBI [64], which may cause the extravasation of PAMPs into the CNS across the damaged BBB. PAMP signals to reactive astrocytes and innate immune cells potentiate inflammation by recruiting blood-borne monocytes, neutrophils, and lymphocytes into the injured brain [65]. Although this pathway is less important than that through the wound, it may occur in the case of secondary meningeal infection.
After TBI, the expression and cellular distribution of the astrocytic water channel aquaporin 4 (AQP4) are affected. These channels lose their polarization to end-feet and glia limitans with a consequent impact on tissue fluid homeostasis and edema formation [66]. Astrogliosis also affects the operation of the glymphatic clearance system [67, 68]. Recent evidence suggests a critical role of the glymphatic system in the clearance of various proteins, including GFAP and S100B, into the blood following TBI [69]. In our previous reports, a decreased presence of AQP4 in astrocytes was triggered in chronic mild stress-induced mice. In this stress-depression model, the function of the glymphatic system was also suppressed, which correlated with anxiety- and depressive-like behaviors [70]. Together, TBI-induced mental malfunction may be partly attributed to the impairment of the glymphatic system by altering the expression of astrocytic receptors and channels, such as AQP4.
TBI induces a significant increase in GFAP immunoreactivity in the basal amygdala 1 and 7 days after trauma [38]. In addition, after TBI, most cortical and subcortical regions show acute increases in glucose metabolism [71, 72] and long-lasting depression of global oxidative metabolism [73]. In contrast, the amygdala shows increased and persistent oxidative metabolic demand after experimental TBI as shown by fluid percussion injury (FPI) [73]. Preclinical models have even shown that FPI enhances fear learning and basolateral amygdala excitatory processing with evidence for reduced GABAergic inhibition [74, 75]. These studies suggest that neurons in the amygdala show different stress responses to TBI than other brain regions, suggesting that astrocytes, which are mainly responsible for neuronal metabolism, may also show specific metabolic patterns that respond to TBI, thus still needing deep studies.
Astrocytes in MS-PTSD
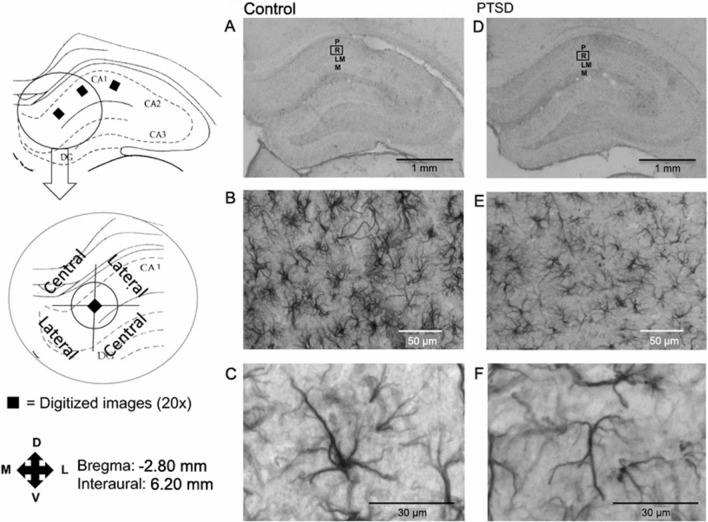
The density of GFAP-positive astrocytes in the hippocampus and frontal cortex decreases after exposure of animals to an inescapable footshock (1 mA at 60 Hz for 20 s) or restraint stress (immobilized for 6 h/day for 21 days); these decreases correlate with mood and cognitive impairments (Fig. 2) [15, 76, 77]. Autopsy of suicides with depression shows a general decrease in the density of GFAP-positive astrocytes and vimentin-positive astrocytes in postmortem specimens from the dorsomedial prefrontal cortex, dorsal caudate nucleus, and medial thalamus, and a significant increase in the cluster of differentiation 31 (CD31)-positive vascularization in the white matter of the prefrontal cortex [78]. A detailed analysis of changes in the fine morphology of astrocytes and especially in leaflets contacting synapses is needed, as changes in these astrocytic structures are involved in the regulation of synaptic transmission [27]. Stress-enhanced fear learning (SEFL) is associated with an increase in hippocampal interleukin-1β (IL-1β), while inhibition of central IL-1 receptors after episodes of severe stress can prevent the development of SEFL. Astrocytes are a predominant source of stress-induced IL-1β [79], and thus are directly involved in the described alterations.
Fig. 2.
Digitized images of the hippocampus after GFAP immunohistochemistry showing the CA1 region. A–C Control; D–F PTSD. A, D Digitized images at 1×; B, E digitized images at 20×; C, F digitized images at 40×. Square areas denote regions of interest at 20×. P, stratum pyramidale; R, stratum radiatum; LM, stratum lacunosum moleculare; M, stratum moleculare. The central and lateral quadrants are defined in relation to the stratum pyramidale.
Reproduced from Saur et al. 2016 [15] with permission from Springer-Nature.
Gene expression profiles of astrocytes are sensitive to stress disorders [80–82]. Dysregulation of astrocyte gap-junction channel protein expression in the prefrontal cortex of individuals with chronic stress may reflect epigenetic mechanisms [83]. The major astrocytic gap-junction channel-forming proteins connexin (Cx) 30 and 43 are down-regulated in chronic depression and suicidal individuals [80, 81]. These connexins are responsible for intercellular communication, including shuttling of energy substrates and metabolites within astrocytic syncytia [84, 85]. In addition, Cx30 and 43 have an impact on neuronal signaling and plasticity [86–88], while Cx30 is involved in the regulation of astrocytic morphology by limiting synapse invasion [86]. Thus astrocyte hypertrophy is associated with reduced gap-junction channel expression or function in stress disorders [80, 81, 89]. Chronic unpredictable stress, which is sufficient to drive behavioral abnormalities, is correlated with a down-regulation in Cx43 [90], while treatment with selective serotonin reuptake inhibitors (SSRIs) or the glucocorticoid receptor antagonist mifepristone ameliorate the effects of chronic stress on astrocytic Cx43 expression and reverse depressive-like behavior [90].
Sleep abnormalities are known to be associated with PTSD and depression [91–93], Astrocytes control sleep homeostasis, contributing to the accumulation of adenosine acting on neuronal adenosine A1 receptors [94, 95]. The clinical antidepressive effects of deep brain stimulation depend on astrocyte function, specifically relating to adenosine release and the activation of neuronal A1 adenosine receptors [96]. The sleep-wake cycle is also affected by the astrocytic metabolic network regulated by connexin Cx43 gap junctions [97]. Transcription factor Sox-9 has been reported to be involved in the Wingless and Int-1 (Wnt)/β-catenin signaling pathway, which regulates the protein expression of Cx43 [98]. Astrocytes specifically express Sox-9 [99], which is decreased in chronic depression and in suicidal individuals [80]. Sox-9 is also involved in the ATP-stimulated proliferation of cultured spinal cord astrocytes induced by extracellular matrix [100].
Chronic stress also affects the astroglia-specific Ca2+-binding protein S100B [101]. This protein is released by astrocytes to control neuronal firing and rhythm generation [102]. The mRNA expression of S100β in postmortem brain tissue of patients with major depressive disorder (MDD) is significantly decreased [101]. Conversely, the serum concentration of S100β has been found to be increased during an episode of mood disorder [103]. Our previous studies demonstrated that chronic stress reduces the astrocytic kainate GluK2 receptor, 5-hydroxytryptamine 2B (5-HT2B) receptor, and water channel AQP4, while the antidepressant fluoxetine rectifies those deficits [70, 104–107]. In addition, a recent study used positron emission tomography of the monoamine oxidase (MAO)-B probe [11C] SL25.1188 in patients with PTSD to verify the levels of MAO-B in six brain regions. It was found that the astrocytic enzyme MAO-B is down-regulated in PTSD, this decrease being greater in PTSD patients with MDD. This indicates that there is a possible loss of astrocytes or independent down-regulation of MAO-B in patients with PTSD, suggesting that PTSD with different phenotypes may be associated with different biological changes [108].
Astrocytes in the Pathophysiology of PTSD
Hippocampal atrophy is one of the most common morphological changes reported in patients with MS-PTSD [109–112]. Hippocampal atrophy, at least in part, may be caused by the loss of astrocytes reported in a PTSD animal model [15, 76]. A decrease in the astrocytic density may induce hippocampal functional impairments. Astrocytes are responsible for synthesizing and releasing many of the neurotrophic factors vital for neuronal health, such as brain-derived neurotrophic factor (BDNF), glial-derived neurotrophic factor, fibroblast growth factor-2, and nerve growth factor [113–115]. These neurotrophic factors regulate neuronal growth and are essential for neural plasticity. Reduced availability of neurotrophic agents may increase cellular vulnerability to stress or even contribute to cell death [116]. Reduction in the number and length of dendrites in the cornu ammonis 1 (CA1) region and dentate gyrus of the hippocampus has been reported in an animal model of PTSD [117]. In another animal model of restraint stress, reductions in synaptic spine density and dendritic length in CA1 neurons and atrophy of apical dendrites in CA3 neurons have also been identified [110, 118]. Chronic stress also reduces the length of astrocytic processes by 40%, volume by 56%, and the number of branches in the prefrontal cortex by 58% [119]. Astrocytic processes are critical for neuronal-glial interactions in the active milieu of nervous tissue [24]. In addition, PTSD changes the area of the astrocytic soma, and the single prolonged stress (SPS) model can cause astrocyte cell body atrophy and process thinning [115]. In rat models of heroin-induced fear learning with PTSD-like symptoms, IL-1β secretion from astrocytes of the dorsal hippocampus is significantly increased, while heroin withdrawal induces a time-dependent, region-specific increase in astrocytic IL-1β; thus, subsequent fear learning is blocked by IL-1 receptor antagonism [120].
The noradrenergic (NE) stimulation of α1-adrenoceptors (α1-ARs) is implicated in MS-PTSD and other mental disorders that involve dysfunctions of the prefrontal cortex, a region that provides top-down control [121]. The effects of α1-AR are specifically evident under stressful conditions of high NE release when they strengthen the effective functioning of the amygdala [122, 123] but weaken the cognitive abilities of the prefrontal cortex (PFC) [124]. In the monkey dorsolateral PFC (dlPFC), in which α1-ARs are prominently expressed in dlPFC layer III astrocytes, increased α1-AR stimulation contributes to the treatment of under-aroused subjects, whereas α1-AR blockade is central to treating stress-related disorders such as PTSD [121]. The excitatory effects of α1-AR arise from the presynaptic excitation of glutamate release, whereas postsynaptic actions suppress firing through Ca2+-protein kinase C opening K+ channels on spines. The latter may predominate under stressful conditions, leading to a loss of dlPFC regulation under uncontrollable stress. In the clinic, α1-AR antagonists are widely used for disorders associated with stress and excessive NE signaling. Stress worsens or causes a variety of disorders associated with PFC dysfunction, including depression, bipolar disorder, schizophrenia, and PTSD. Animal studies also indicate that TBI may involve increased catecholamine release and α1-AR stimulation in the PFC as a key etiological event [125, 126]. There is some evidence of increased α1-ARs in the dorsal PFC of suicide patients [127] and extensive evidence that PTSD involves excessive NE signaling [128–130].
In addition, K+ inward-rectifying Kir4.1 channels, exclusively expressed in astrocytes [131], are thought to be a potential therapeutic target for mental disorders [132, 133]. Astroglial Kir4.1 channels in the lateral habenula drive neuronal bursts in depression (Cui et al. 2018), whereas specific inhibition of Kir4.1 rapidly eliminates depression-like behaviors [132, 133]. In particular, Kir4.1 plays critical roles in modulating astrocyte-neuron interactions [134]. In LPS- or SPS-treated mouse models, the expression of astrocytic Kir4.1 is significantly increased in the hippocampus, while reducing hippocampal Kir4.1 promotes fear extinction [135]. Treatment with ginsenoside Rg1 has protective effects by suppressing an increase in hippocampal Kir4.1 induced by LPS or SPS; thus, the intracerebroventricular injection of TNF-α causes impairment of fear extinction by increasing Kir4.1 expression in the hippocampus [135].
Astrocytes and Neurological Complications of PTSD
The prevalence of depressive symptoms in individuals with PTSD suggests that pathophysiological mechanisms contributing to MDDs may be relevant to PTSD, especially for individuals with comorbid PTSD/MDD; however, potential mechanisms of this overlap are poorly understood [136]. A twin study found a significant genetic correlation between the pro-inflammatory cytokine marker IL-6 and depressive symptoms, indicating that the genetics of inflammation and mental health outcomes may be partially shared [137].
Sleep disturbances are the principal symptoms of PTSD [138, 139]. Approximately 70% of patients with PTSD have sleep problems [140]. Disrupted sleep, common to both depression and PTSD, has been linked to an altered hypothalamic-pituitary-adrenal axis (HPA) axis and IL-6 dynamics and to alterations in gene transcription, including that of circadian clock genes in the brain, including astrocytes, radial astrocyte stem cells responsible for adult neurogenesis, and neurons [95, 141–145]. While PTSD is associated with a decrease in hippocampal subfield volume, this is even more strongly associated with insomnia in the population studied [146, 147].
As reported by us previously, astrocytic NLR family pyrin domain containing 3 (NLRP3) inflammation is activated and associated with depressive-like behaviors in mice under sleep deprivation [148, 149]. Sleep deprivation promotes a gradual elevation in extracellular ATP, which activates astroglial P2X7R purinoceptors. This, in turn, selectively down-regulates the expression of 5-HT2B receptors in astrocytes (Fig. 3; [148]). In sleep-deprived mice, the antidepressant fluoxetine alleviates the activation of NLRP3 inflammation, the reduced release of IL-1β/18, and depressive-like behaviors by stimulating 5-HT2BR [148, 149]. Hence, astrocytes play key roles in the relationship between sleep disorders and depression and likely also PTSD.
Fig. 3.
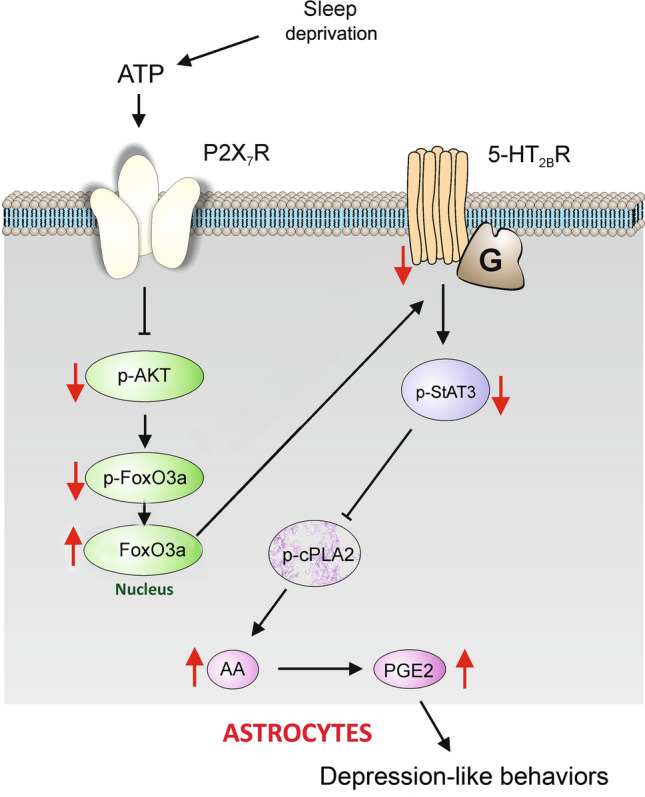
The expression and function of 5-HT2B receptors are selectively decreased by sleep deprivation (SD) through P2X7 receptors in astrocytes. Prolonged SD stimulates P2X7Rs by ATP, activated P2X7Rs suppress the phosphorylation of AKT and forkhead box O3 (FoxO3a) in the cytoplasm, and the dephosphorylated FoxO3a accumulates in the nucleus of astrocytes. The increased FoxO3a down-regulates the expression of 5-HT2BRs, and the phosphorylation of signal transducer and activator of transcription 3 (STAT3) is also decreased, which relieves the inhibition of the phosphorylation of cPLA2. The activated cPLA2 promotes the release of arachidonic acid (AA) and prostaglandin E2 (PGE2), eventually causing depression-like behaviors. Red arrows indicate the increase of function; black arrows show stimulatory pathways, while black lines with the bar denote the inhibitory pathway.
Reproduced from Xia et al. 2020 [148] with permission from Springer-Nature.
Pharmacological Treatments of PTSD
Psychopharmacological treatment is commonly used to treat PTSD despite limited evidence of its effectiveness; only SSRIs sertraline and paroxetine are approved by the US Food and Drug Administration [150, 151]. The main therapeutic focus is on treating the consequences of PTSD, as the disease impacts an individual’s ability to engage in daily activities, and on enabling those living with PTSD to promote wellness, role competence, and satisfaction while improving their quality of life [152, 153].
Serotonergic modulation of mood, anxiety, and impulse control, and empirical demonstrations of abnormalities are strongly manifested in PTSD patients [154, 155]. SSRIs are used as therapeutics for PTSD. Relatively high doses of sertraline or paroxetine are sometimes prescribed to patients with PTSD as off-label treatments for non-responders [151]. SSRIs may help to treat PTSD by reducing amygdala hyperactivity: chronic daily administration of fluoxetine, paroxetine, and sertraline has been shown to be beneficial [37]. SSRIs act as emotional stabilizers, but the underlying pharmacological mechanisms are unclear. Generally, therapeutic strategies are aimed at neurons (monoamine hypothesis [156], hypercortisolism hypothesis [157], BDNF hypothesis [158], and myo-inositol hypothesis [159]). None of the antidepressants developed based on these theories can alleviate all the symptoms of mood disorders in all patients. More attention has therefore been paid to the role of astrocytes in the pathogenesis of depression and the pharmacological mechanism of antidepressants.
Five classic SSRIs, fluoxetine, fluvoxamine, sertraline, paroxetine, and citalopram, are widely used as antidepressants and anxiolytics in the clinic. Chronic treatment of cultured astrocytes with SSRIs increases the mRNA expression of Ca2+-dependent phospholipase A2 (cPLA2) [160, 161]. Chronic treatment of mice with fluoxetine increases the expression of cPLA2-specific mRNA solely in astrocytes, as has been demonstrated in experiments with fluorescence-activated sorting of neurons and astrocytes in transgenic animals [105]. When administered acutely, fluoxetine at 10 µmol/L triggers cellular Ca2+ signaling, transactivates the phosphorylation of epidermal growth factor receptor (EGFR), and phosphorylates downstream extracellular signal-regulated protein kinase 1/2 (ERK1/2). The latter enters cell nuclei and regulates the mRNA and protein expression of cFos and FosB, consequently changing the expression of several key proteins [162]. Chronic treatment with 10 µmol/L fluoxetine, which acts as an agonist of astrocytic 5-HT2B receptors increases the protein expression of cPLA2, kainate receptor GluK2, subtype 2 of adenosine deaminases acting on RNAs, transient receptor potential canonical 1 channel, L-type Ca2+ channels Cav1.2, caveolin-1 and BDNF [104, 107, 149, 163–165]. Finally, at low concentrations (< 1 µmol/L), fluoxetine decreases the mRNA expression of c-Fos by the phosphoinositide 3-kinases/protein kinase B (PI3K/AKT) signaling pathway, whereas at higher concentrations (> 5 µmol/L), fluoxetine elevates c-Fos through the mitogen-activated protein kinase (MAPK)/ERK signaling pathway (Fig. 4; [166]).
Fig. 4.
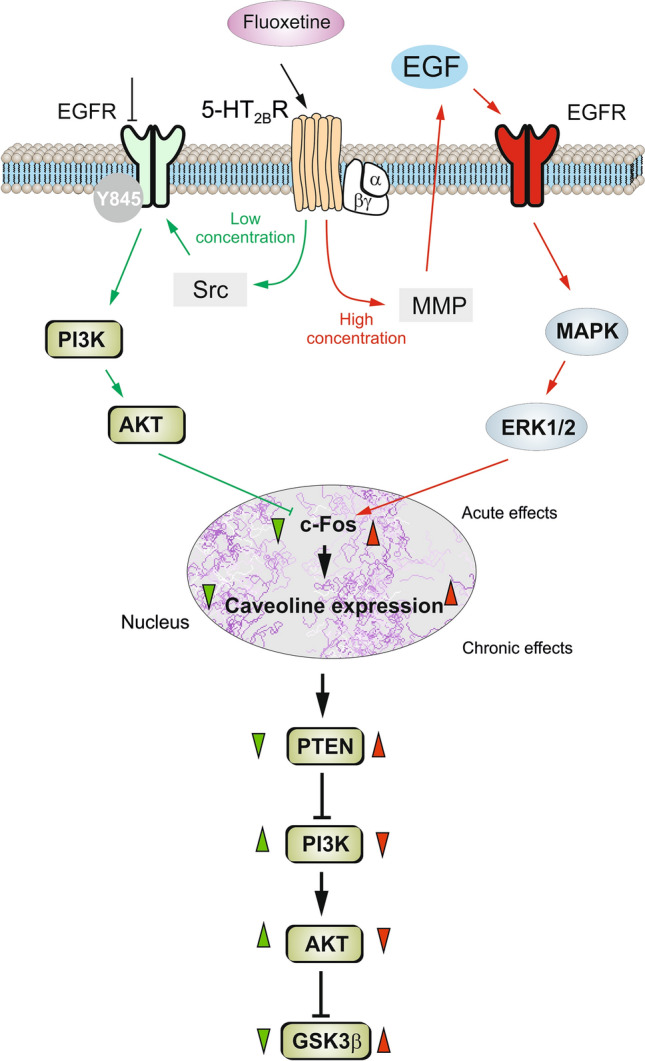
Schematic of biphasic concentration-dependent regulation of caveolin-1 (Cav-1) gene expression and glycogen synthase kinase 3β (GSK-3β) activity by fluoxetine in astrocytes. Acute treatment with fluoxetine at low concentrations (green arrows) stimulates the Src protein tyrosine kinase which phosphorylates EGFRs and activates the PI3K/AKT signal pathway. The AKT phosphorylation by fluoxetine at low concentrations inhibits cFos gene expression, and subsequently decreases Cav-1 gene expression (chronic effects) that in turn, decreases the membrane content of phosphatase and tensin homolog (PTEN), induces phosphorylation and stimulation of PI3K, and elevates GSK-3β phosphorylation thus suppressing its activity. At higher concentrations, fluoxetine (red arrows) stimulates metalloproteinase and induces shedding of growth factor which stimulates the EGFR and activates the MAPK/ERK1/2 signal pathway. The ERK1/2 phosphorylation by fluoxetine at high concentrations stimulates cFos gene expression, and subsequently increases Cav-1 gene expression (chronic effects), that acts on PTEN/PI3K/AKT/GSK-3β in an inverse fashion. Green arrowheads show effects of low concentration, whereas red arrowheads show effects of high concentrations of fluoxetine.
Reproduced from Li et al. 2017 [166] with permission from Springer-Nature.
Astrocytic dysfunction is potentially the main pathological basis for the co-morbidity of PTSD and sleep disturbances [106]. In particular, astrocytes support the glymphatic system [167] responsible for the clearance of brain metabolic waste by paravascular pathways. Polarized expression of astrocytic AQP4 to the endfeet is critical for glymphatic operation [168]. In our previous report, chronic treatment with chronic mild stress or blockade of the glymphatic pathway via the AQP4 antagonist TGN-020 also increases the level of Aβ42 in the frontal cortex and hippocampus [70]. Fluoxetine up-regulates the expression of AQP4 in astrocytes and promotes the clearance of Aβ42 via accelerating the circulation of the glymphatic system, and the associated depressive-like behaviors are also improved [70]. Some special inducers, such as overloaded iron, worsen the dysfunction of the glymphatic system by triggered reactive astrogliosis in the cortex and hippocampus, as indicated by an increase in GFAP expression, and exacerbates the depressive-like and anxiety-like behaviors induced by chronic mild stress, associated with the more serious neuronal apoptosis [169].
Besides SSRIs, other agents have also been reported to have therapeutic effects on PTSD. Prazosin, an α1 adrenoceptor antagonist, is effective for the reduction of nightmares and sleep-related hyperarousal in PTSD [170–173]. Cognitive deficits involving working memory and executive function in PTSD are associated with alterations in prefrontal dopamine function, so dopamine projections from the ventral tegmental area to the amygdala are directly involved in the processing of fear signals [174]. Roitman et al. (2014) [175] showed that treatment of 10 PTSD patients with δ-9 tetrahydrocannabinol, a naturally-occurring cannabinol receptor agonist found in the marijuana plant, results in a nonsignificant improvement in intrusive and recurring PTSD symptoms but significantly improves sleep disorders.
Conclusion and Future Directions for Research
We summarized the latest research advances in the etiology and therapeutics of PTSD as well as its peripheral complications. The pathophysiological and psychological mechanisms of PTSD are still unclear and as a result, treatments remain symptomatic. Astrocytes play important roles in the pathogenesis of PTSD. Astrocytes secrete numerous neuromodulatory, neurotrophic and inflammatory factors and sustain neuronal networks by providing metabolic support and removal of waste. Research on astrocytes in PTSD needs more rigor and effort. According to our previous studies, astrocytic 5-HT2B receptors play key roles in mood disorders, including MDD and bipolar disorders, and sleep disorder-related emotional changes [106, 148, 149]. Meanwhile, five SSRIs can regulate the same targets, such as cPLA2 in astrocytes [176]. Activation of cPLA2 specifically releases arachidonic acid from the sn-2 position of membrane-bound phospholipid [177]. It has repeatedly been reported that selective serotonin reuptake inhibitor (SSRI) treatment can normalize regional decreases in brain metabolism occurring during the major depression, decreases that are more pronounced with the lower the plasma concentrations of arachidonic acid [178]; similarly, emotional stabilizers (lithium salt, carbamazepine, and valproic acid salt) have the same sites (cPLA2, inositol, GluK2, and others) in astrocytes [179]. Moreover, research on the effects of astrocytes on the pharmacological mechanisms of PTSD medicines is limited.
Acknowledgements
This review was supported by the National Natural Science Foundation of China, (81871852); Shenyang Science and Technology Innovation Talents Project (RC210251); Liaoning Revitalization Talents Program (XLYC1807137), the Scientific Research Foundation for Returned Scholars of Education Ministry of China (20151098), Liaoning Thousand Talents Program (202078), and the “Chunhui” Program of Education Ministry (2020703).
Conflict of interests
The authors declare no competing interests.
References
- 1.Schäfer I, Fisher HL. Childhood trauma and psychosis—What is the evidence? Dialogues Clin Neurosci. 2011;13:360–365. doi: 10.31887/DCNS.2011.13.2/ischaefer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gayle MC, Raskin JD. DSM-5: Do counselors really want an alternative? J Humanist Psychol. 2017;57:650–666. doi: 10.1177/0022167817696839. [DOI] [Google Scholar]
- 3.Watson P. PTSD as a public mental health priority. Curr Psychiatry Rep. 2019;21:61. doi: 10.1007/s11920-019-1032-1. [DOI] [PubMed] [Google Scholar]
- 4.Vujanovic AA, Schnurr PP. Editorial overview: Advances in science and practice in traumatic stress. Curr Opin Psychol. 2017;14:iv–viii. doi: 10.1016/j.copsyc.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Carlson EB, Rosser-Hogan R. Trauma experiences, posttraumatic stress, dissociation, and depression in Cambodian refugees. Am J Psychiatry. 1991;148:1548–1551. doi: 10.1176/ajp.148.11.1548. [DOI] [PubMed] [Google Scholar]
- 6.Resnick SG, Bond GR, Mueser KT. Trauma and posttraumatic stress disorder in people with schizophrenia. J Abnorm Psychol. 2003;112:415–423. doi: 10.1037/0021-843X.112.3.415. [DOI] [PubMed] [Google Scholar]
- 7.Johansson L, Guo XX, Waern M, Ostling S, Gustafson D, Bengtsson C, et al. Midlife psychological stress and risk of dementia: A 35-year longitudinal population study. Brain. 2010;133:2217–2224. doi: 10.1093/brain/awq116. [DOI] [PubMed] [Google Scholar]
- 8.Lee SP, Sung IK, Kim JH, Lee SY, Park HS, Shim CS. The effect of emotional stress and depression on the prevalence of digestive diseases. J Neurogastroenterol Motil. 2015;21:273–282. doi: 10.5056/jnm14116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conrad CD. Chronic stress-induced hippocampal vulnerability: The glucocorticoid vulnerability hypothesis. Rev Neurosci. 2008;19:395–411. doi: 10.1515/REVNEURO.2008.19.6.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGonigle P. Animal models of CNS disorders. Biochem Pharmacol. 2014;87:140–149. doi: 10.1016/j.bcp.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 11.Joëls M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10:459–466. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McEwen BS. Neurobiological and systemic effects of chronic stress. Chronic Stress (Thousand Oaks) 2017, 1: 2470547017692328. [DOI] [PMC free article] [PubMed]
- 13.Bisht K, Sharma K, Tremblay MÈ. Chronic stress as a risk factor for Alzheimer's disease: Roles of microglia-mediated synaptic remodeling, inflammation, and oxidative stress. Neurobiol Stress. 2018;9:9–21. doi: 10.1016/j.ynstr.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaletel I, Filipović D, Puškaš N. Chronic stress, hippocampus and parvalbumin-positive interneurons: What do we know so far? Rev Neurosci. 2016;27:397–409. doi: 10.1515/revneuro-2015-0042. [DOI] [PubMed] [Google Scholar]
- 15.Saur L, Baptista PPA, Bagatini PB, Neves LT, de Oliveira RM, Vaz SP, et al. Experimental post-traumatic stress disorder decreases astrocyte density and changes astrocytic polarity in the CA1 hippocampus of male rats. Neurochem Res. 2016;41:892–904. doi: 10.1007/s11064-015-1770-3. [DOI] [PubMed] [Google Scholar]
- 16.Ongür D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao SY, Luo D, Xiao Y. Survivors of COVID-19 are at high risk of posttraumatic stress disorder. Glob Health Res Policy. 2020;5:29. doi: 10.1186/s41256-020-00155-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mak IWC, Chu CM, Pan PC, Yiu MGC, Chan VL. Long-term psychiatric morbidities among SARS survivors. Gen Hosp Psychiatry. 2009;31:318–326. doi: 10.1016/j.genhosppsych.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sfera A, Osorio C, Rahman L, Zapata-Martín del Campo CM, Maldonado JC, Jafri N, et al. PTSD as an endothelial disease: Insights from COVID-19. Front Cell Neurosci. 2021;15:770387. doi: 10.3389/fncel.2021.770387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang HT, Li XL, Chen SZ, Lu N, Yue YY, Liang JF, et al. Plasminogen activator inhibitor-1 in depression: Results from animal and clinical studies. Sci Rep. 2016;6:30464. doi: 10.1038/srep30464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen M, Shu S, Yan HH, Pei L, Wang ZF, Wan Q, et al. Hippocampal Endothelin-1 decreases excitability of pyramidal neurons and produces anxiolytic effects. Neuropharmacology. 2017;118:242–250. doi: 10.1016/j.neuropharm.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 22.Bouarab C, Roullot-Lacarrière V, Vallée M, le Roux A, Guette C, Mennesson M, et al. PAI-1 protein is a key molecular effector in the transition from normal to PTSD-like fear memory. Mol Psychiatry. 2021;26:4968–4981. doi: 10.1038/s41380-021-01024-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verkhratsky A, Nedergaard M. Physiology of astroglia. Physiol Rev. 2018;98:239–389. doi: 10.1152/physrev.00042.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Semyanov A, Verkhratsky A. Astrocytic processes: From tripartite synapses to the active milieu. Trends Neurosci. 2021;44:781–792. doi: 10.1016/j.tins.2021.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Verkhratsky A, Nedergaard M. Astroglial cradle in the life of the synapse. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130595. doi: 10.1098/rstb.2013.0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Augusto-Oliveira M, Arrifano GP, Takeda PY, Lopes-Araújo A, Santos-Sacramento L, Anthony DC, et al. Astroglia-specific contributions to the regulation of synapses, cognition and behaviour. Neurosci Biobehav Rev. 2020;118:331–357. doi: 10.1016/j.neubiorev.2020.07.039. [DOI] [PubMed] [Google Scholar]
- 27.Verkhratsky A, Semyanov A, Zorec R. Physiology of astroglial excitability. Function. 2020;1:zqaa016. doi: 10.1093/function/zqaa016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim D, Semyanov A, Genazzani A, Verkhratsky A. Calcium signaling in neuroglia. Int Rev Cell Mol Biol. 2021;362:1–53. doi: 10.1016/bs.ircmb.2021.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Rose CR, Verkhratsky A. Principles of sodium homeostasis and sodium signalling in astroglia. Glia. 2016;64:1611–1627. doi: 10.1002/glia.22964. [DOI] [PubMed] [Google Scholar]
- 30.Escartin C, Galea E, Lakatos A, O'Callaghan JP, Petzold GC, Serrano-Pozo A, et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat Neurosci. 2021;24:312–325. doi: 10.1038/s41593-020-00783-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verkhratsky A, Zorec R, Parpura V. Stratification of astrocytes in healthy and diseased brain. Brain Pathol. 2017;27:629–644. doi: 10.1111/bpa.12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li YL, Li LX, Wu JT, Zhu ZG, Feng X, Qin LM, et al. Activation of astrocytes in hippocampus decreases fear memory through adenosine A1 receptors. Elife. 2020;9:e57155. doi: 10.7554/eLife.57155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Izquierdo I, Furini CR, Myskiw JC. Fear memory. Physiol Rev. 2016;96:695–750. doi: 10.1152/physrev.00018.2015. [DOI] [PubMed] [Google Scholar]
- 34.Yurgil KA, Barkauskas DA, Vasterling JJ, Nievergelt CM, Larson GE, Schork NJ, et al. Association between traumatic brain injury and risk of posttraumatic stress disorder in active-duty Marines. JAMA Psychiatry. 2014;71:149–157. doi: 10.1001/jamapsychiatry.2013.3080. [DOI] [PubMed] [Google Scholar]
- 35.Moreno N, González A. Evolution of the amygdaloid complex in vertebrates, with special reference to the anamnio-amniotic transition. J Anat. 2007;211:151–163. doi: 10.1111/j.1469-7580.2007.00780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23:727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- 38.Hoffman AN, Paode PR, May HG, Ortiz JB, Kemmou S, Lifshitz J, et al. Early and persistent dendritic hypertrophy in the basolateral amygdala following experimental diffuse traumatic brain injury. J Neurotrauma. 2017;34:213–219. doi: 10.1089/neu.2015.4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harris JL, Yeh HW, Choi IY, Lee P, Berman NE, Swerdlow RH, et al. Altered neurochemical profile after traumatic brain injury: 1H-MRS biomarkers of pathological mechanisms. J Cereb Blood Flow Metab. 2012;32:2122–2134. doi: 10.1038/jcbfm.2012.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lei HX, Berthet C, Hirt L, Gruetter R. Evolution of the neurochemical profile after transient focal cerebral ischemia in the mouse brain. J Cereb Blood Flow Metab. 2009;29:811–819. doi: 10.1038/jcbfm.2009.8. [DOI] [PubMed] [Google Scholar]
- 41.Xu S, Zhuo JC, Racz J, Shi D, Roys S, Fiskum G, et al. Early microstructural and metabolic changes following controlled cortical impact injury in rat: A magnetic resonance imaging and spectroscopy study. J Neurotrauma. 2011;28:2091–2102. doi: 10.1089/neu.2010.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bartnik-Olson BL, Harris NG, Shijo K, Sutton RL. Insights into the metabolic response to traumatic brain injury as revealed by 13C NMR spectroscopy. Front Neuroenergetics. 2013;5:8. doi: 10.3389/fnene.2013.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilde EA, Bouix S, Tate DF, Lin AP, Newsome MR, Taylor BA, et al. Advanced neuroimaging applied to veterans and service personnel with traumatic brain injury: State of the art and potential benefits. Brain Imaging Behav. 2015;9:367–402. doi: 10.1007/s11682-015-9444-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Henry LC, Tremblay S, Leclerc S, Khiat A, Boulanger Y, Ellemberg D, et al. Metabolic changes in concussed American football players during the acute and chronic post-injury phases. BMC Neurol. 2011;11:105. doi: 10.1186/1471-2377-11-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vagnozzi R, Signoretti S, Tavazzi B, Floris R, Ludovici A, Marziali S, et al. Temporal window of metabolic brain vulnerability to concussion: A pilot 1H-magnetic resonance spectroscopic study in concussed athletes—Part III. Neurosurgery. 2008;62:1286–1295. doi: 10.1227/01.neu.0000333300.34189.74. [DOI] [PubMed] [Google Scholar]
- 46.Lin AP, Ramadan S, Stern RA, Box HC, Nowinski CJ, Ross BD, et al. Changes in the neurochemistry of athletes with repetitive brain trauma: Preliminary results using localized correlated spectroscopy. Alzheimers Res Ther. 2015;7:13. doi: 10.1186/s13195-015-0094-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koerte IK, Lin AP, Muehlmann M, Merugumala S, Liao HJ, Starr T, et al. Altered neurochemistry in former professional soccer players without a history of concussion. J Neurotrauma. 2015;32:1287–1293. doi: 10.1089/neu.2014.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sheth C, Prescot AP, Legarreta M, Renshaw PF, McGlade E, Yurgelun-Todd D. Increased myoinositol in the anterior cingulate cortex of veterans with a history of traumatic brain injury: A proton magnetic resonance spectroscopy study. J Neurophysiol. 2020;123:1619–1629. doi: 10.1152/jn.00765.2019. [DOI] [PubMed] [Google Scholar]
- 49.Garnett MR, Corkill RG, Blamire AM, Rajagopalan B, Manners DN, Young JD, et al. Altered cellular metabolism following traumatic brain injury: A magnetic resonance spectroscopy study. J Neurotrauma. 2001;18:231–240. doi: 10.1089/08977150151070838. [DOI] [PubMed] [Google Scholar]
- 50.Garnett MR, Blamire AM, Corkill RG, Cadoux-Hudson TA, Rajagopalan B, Styles P. Early proton magnetic resonance spectroscopy in normal-appearing brain correlates with outcome in patients following traumatic brain injury. Brain. 2000;123(Pt 10):2046–2054. doi: 10.1093/brain/123.10.2046. [DOI] [PubMed] [Google Scholar]
- 51.Chen CS, Chiang IC, Li CW, Lin WC, Lu CY, Hsieh TJ, et al. Proton magnetic resonance spectroscopy of late-life major depressive disorder. Psychiatry Res. 2009;172:210–214. doi: 10.1016/j.pscychresns.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 52.Ashwal S, Holshouser B, Tong KR, Serna T, Osterdock R, Gross M, et al. Proton spectroscopy detected myoinositol in children with traumatic brain injury. Pediatr Res. 2004;56:630–638. doi: 10.1203/01.PDR.0000139928.60530.7D. [DOI] [PubMed] [Google Scholar]
- 53.Jollant F, Near J, Turecki G, Richard-Devantoy S. Spectroscopy markers of suicidal risk and mental pain in depressed patients. Prog Neuropsychopharmacol Biol Psychiatry 2016: S0278–S5846(16)30167–1. [DOI] [PubMed]
- 54.Burda JE, Sofroniew MV. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron. 2014;81:229–248. doi: 10.1016/j.neuron.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.di Virgilio F, dal Ben D, Sarti AC, Giuliani AL, Falzoni S. The P2X7 receptor in infection and inflammation. Immunity. 2017;47:15–31. doi: 10.1016/j.immuni.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 56.Bernier LP. Purinergic regulation of inflammasome activation after central nervous system injury. J Gen Physiol. 2012;140:571–575. doi: 10.1085/jgp.201210875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kimbler DE, Shields J, Yanasak N, Vender JR, Dhandapani KM. Activation of P2X7 promotes cerebral edema and neurological injury after traumatic brain injury in mice. PLoS One. 2012;7:e41229. doi: 10.1371/journal.pone.0041229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun LQ, Gao JL, Zhao MM, Cui JZ, Li YX, Yang XJ, et al. A novel cognitive impairment mechanism that astrocytic p-connexin 43 promotes neuronic autophagy via activation of P2X7R and down-regulation of GLT-1 expression in the hippocampus following traumatic brain injury in rats. Behav Brain Res. 2015;291:315–324. doi: 10.1016/j.bbr.2015.05.049. [DOI] [PubMed] [Google Scholar]
- 59.Pedrazzi M, Patrone M, Passalacqua M, Ranzato E, Colamassaro D, Sparatore B, et al. Selective proinflammatory activation of astrocytes by high-mobility group box 1 protein signaling. J Immunol. 2007;179:8525–8532. doi: 10.4049/jimmunol.179.12.8525. [DOI] [PubMed] [Google Scholar]
- 60.Ponath G, Schettler C, Kaestner F, Voigt B, Wentker D, Arolt V, et al. Autocrine S100B effects on astrocytes are mediated via RAGE. J Neuroimmunol. 2007;184:214–222. doi: 10.1016/j.jneuroim.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 61.Gorina R, Font-Nieves M, Márquez-Kisinousky L, Santalucia T, Planas AM. Astrocyte TLR4 activation induces a proinflammatory environment through the interplay between MyD88-dependent NFκB signaling, MAPK, and Jak1/Stat1 pathways. Glia. 2011;59:242–255. doi: 10.1002/glia.21094. [DOI] [PubMed] [Google Scholar]
- 62.Hoarau JJ, Krejbich-Trotot P, Jaffar-Bandjee MC, Das T, Thon-Hon GV, Kumar S, et al. Activation and control of CNS innate immune responses in health and diseases: A balancing act finely tuned by neuroimmune regulators (NIReg) CNS Neurol Disord Drug Targets. 2011;10:25–43. doi: 10.2174/187152711794488601. [DOI] [PubMed] [Google Scholar]
- 63.Hamby ME, Coppola G, Ao Y, Geschwind DH, Khakh BS, Sofroniew MV. Inflammatory mediators alter the astrocyte transcriptome and calcium signaling elicited by multiple G-protein-coupled receptors. J Neurosci. 2012;32:14489–14510. doi: 10.1523/JNEUROSCI.1256-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lenz A, Franklin GA, Cheadle WG. Systemic inflammation after trauma. Injury. 2007;38:1336–1345. doi: 10.1016/j.injury.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 65.Burda JE, Bernstein AM, Sofroniew MV. Astrocyte roles in traumatic brain injury. Exp Neurol. 2016;275(Pt 3):305–315. doi: 10.1016/j.expneurol.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Papadopoulos MC, Verkman AS. Aquaporin water channels in the nervous system. Nat Rev Neurosci. 2013;14:265–277. doi: 10.1038/nrn3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Iliff JJ, Wang MH, Liao YH, Plogg BA, Peng WG, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid Β. Sci Transl Med. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thrane AS, Rangroo Thrane V, Nedergaard M. Drowning stars: Reassessing the role of astrocytes in brain edema. Trends Neurosci. 2014;37:620–628. doi: 10.1016/j.tins.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Plog BA, Dashnaw ML, Hitomi E, Peng WG, Liao YH, Lou NH, et al. Biomarkers of traumatic injury are transported from brain to blood via the glymphatic system. J Neurosci. 2015;35:518–526. doi: 10.1523/JNEUROSCI.3742-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xia MS, Yang L, Sun GF, Qi S, Li BM. Mechanism of depression as a risk factor in the development of Alzheimer’s disease: The function of AQP4 and the glymphatic system. Psychopharmacology. 2017;234:365–379. doi: 10.1007/s00213-016-4473-9. [DOI] [PubMed] [Google Scholar]
- 71.Yoshino A, Hovda DA, Kawamata T, Katayama Y, Becker DP. Dynamic changes in local cerebral glucose utilization following cerebral conclusion in rats: Evidence of a hyper- and subsequent hypometabolic state. Brain Res. 1991;561:106–119. doi: 10.1016/0006-8993(91)90755-K. [DOI] [PubMed] [Google Scholar]
- 72.Kawamata T, Katayama Y, Hovda DA, Yoshino A, Becker DP. Administration of excitatory amino acid antagonists via microdialysis attenuates the increase in glucose utilization seen following concussive brain injury. J Cereb Blood Flow Metab. 1992;12:12–24. doi: 10.1038/jcbfm.1992.3. [DOI] [PubMed] [Google Scholar]
- 73.Hovda DA, Yoshino A, Kawamata T, Katayama Y, Becker DP. Diffuse prolonged depression of cerebral oxidative metabolism following concussive brain injury in the rat: A cytochrome oxidase histochemistry study. Brain Res. 1991;567:1–10. doi: 10.1016/0006-8993(91)91429-5. [DOI] [PubMed] [Google Scholar]
- 74.Almeida-Suhett CP, Prager EM, Pidoplichko V, Figueiredo TH, Marini AM, Li Z, et al. Reduced GABAergic inhibition in the basolateral amygdala and the development of anxiety-like behaviors after mild traumatic brain injury. PLoS One. 2014;9:e102627. doi: 10.1371/journal.pone.0102627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reger ML, Poulos AM, Buen F, Giza CC, Hovda DA, Fanselow MS. Concussive brain injury enhances fear learning and excitatory processes in the amygdala. Biol Psychiatry. 2012;71:335–343. doi: 10.1016/j.biopsych.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Perez-Urrutia N, Mendoza C, Alvarez-Ricartes N, Oliveros-Matus P, Echeverria F, Grizzell JA, et al. Intranasal cotinine improves memory, and reduces depressive-like behavior, and GFAP+ cells loss induced by restraint stress in mice. Exp Neurol. 2017;295:211–221. doi: 10.1016/j.expneurol.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 77.Han F, Xiao B, Wen LL. Loss of glial cells of the hippocampus in a rat model of post-traumatic stress disorder. Neurochem Res. 2015;40:942–951. doi: 10.1007/s11064-015-1549-6. [DOI] [PubMed] [Google Scholar]
- 78.O’Leary LA, Belliveau C, Davoli MA, Ma JC, Tanti A, Turecki G, et al. Widespread decrease of cerebral vimentin-immunoreactive astrocytes in depressed suicides. Front Psychiatry. 2021;12:640963. doi: 10.3389/fpsyt.2021.640963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jones ME, Lebonville CL, Paniccia JE, Balentine ME, Reissner KJ, Lysle DT. Hippocampal interleukin-1 mediates stress-enhanced fear learning: A potential role for astrocyte-derived interleukin-1β. Brain Behav Immun. 2018;67:355–363. doi: 10.1016/j.bbi.2017.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ernst C, Nagy C, Kim S, Yang JP, Deng XM, Hellstrom IC, et al. Dysfunction of astrocyte connexins 30 and 43 in dorsal lateral prefrontal cortex of suicide completers. Biol Psychiatry. 2011;70:312–319. doi: 10.1016/j.biopsych.2011.03.038. [DOI] [PubMed] [Google Scholar]
- 81.Nagy C, Torres-Platas SG, Mechawar N, Turecki G. Repression of astrocytic connexins in cortical and subcortical brain regions and prefrontal enrichment of H3K9me3 in depression and suicide. Int J Neuropsychopharmacol. 2017;20:50–57. doi: 10.1093/ijnp/pyw071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Torres-Platas SG, Nagy C, Wakid M, Turecki G, Mechawar N. Glial fibrillary acidic protein is differentially expressed across cortical and subcortical regions in healthy brains and downregulated in the thalamus and caudate nucleus of depressed suicides. Mol Psychiatry. 2016;21:509–515. doi: 10.1038/mp.2015.65. [DOI] [PubMed] [Google Scholar]
- 83.Nagy C, Suderman M, Yang J, Szyf M, Mechawar N, Ernst C, et al. Astrocytic abnormalities and global DNA methylation patterns in depression and suicide. Mol Psychiatry. 2015;20:320–328. doi: 10.1038/mp.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Giaume C, Koulakoff A, Roux L, Holcman D, Rouach N. Astroglial networks: A step further in neuroglial and gliovascular interactions. Nat Rev Neurosci. 2010;11:87–99. doi: 10.1038/nrn2757. [DOI] [PubMed] [Google Scholar]
- 85.Charvériat M, Mouthon F, Rein W, Verkhratsky A. Connexins as therapeutic targets in neurological and neuropsychiatric disorders. Biochim Biophys Acta Mol Basis Dis. 2021;1867:166098. doi: 10.1016/j.bbadis.2021.166098. [DOI] [PubMed] [Google Scholar]
- 86.Pannasch U, Freche D, Dallérac G, Ghézali G, Escartin C, Ezan P, et al. Connexin 30 sets synaptic strength by controlling astroglial synapse invasion. Nat Neurosci. 2014;17:549–558. doi: 10.1038/nn.3662. [DOI] [PubMed] [Google Scholar]
- 87.Rouach N, Koulakoff A, Abudara V, Willecke K, Giaume C. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science. 2008;322:1551–1555. doi: 10.1126/science.1164022. [DOI] [PubMed] [Google Scholar]
- 88.Sibille J, Pannasch U, Rouach N. Astroglial potassium clearance contributes to short-term plasticity of synaptically evoked currents at the tripartite synapse. J Physiol. 2014;592:87–102. doi: 10.1113/jphysiol.2013.261735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Torres-Platas SG, Hercher C, Davoli MA, Maussion G, Labonté B, Turecki G, et al. Astrocytic hypertrophy in anterior cingulate white matter of depressed suicides. Neuropsychopharmacology. 2011;36:2650–2658. doi: 10.1038/npp.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sun JD, Liu Y, Yuan YH, Li J, Chen NH. Gap junction dysfunction in the prefrontal cortex induces depressive-like behaviors in rats. Neuropsychopharmacology. 2012;37:1305–1320. doi: 10.1038/npp.2011.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kalmbach DA, Pillai V, Cheng P, Arnedt JT, Drake CL. Shift work disorder, depression, and anxiety in the transition to rotating shifts: The role of sleep reactivity. Sleep Med. 2015;16:1532–1538. doi: 10.1016/j.sleep.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Park H, Lee HK, Lee K. Chronotype and suicide: The mediating effect of depressive symptoms. Psychiatry Res. 2018;269:316–320. doi: 10.1016/j.psychres.2018.08.046. [DOI] [PubMed] [Google Scholar]
- 93.Scarpelli S, Alfonsi V, Gorgoni M, Musetti A, Filosa M, Quattropani MC, et al. Dreams and nightmares during the first and second wave of the COVID-19 infection: A longitudinal study. Brain Sci. 2021;11:1375. doi: 10.3390/brainsci11111375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Florian C, Vecsey CG, Halassa MM, Haydon PG, Abel T. Astrocyte-derived adenosine and A1 receptor activity contribute to sleep loss-induced deficits in hippocampal synaptic plasticity and memory in mice. J Neurosci. 2011;31:6956–6962. doi: 10.1523/JNEUROSCI.5761-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Halassa MM, Florian C, Fellin T, Munoz JR, Lee SY, Abel T, et al. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61:213–219. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Etiévant A, Oosterhof C, Bétry C, Abrial E, Novo-Perez M, Rovera R, et al. Astroglial control of the antidepressant-like effects of prefrontal cortex deep brain stimulation. EBioMedicine. 2015;2:898–908. doi: 10.1016/j.ebiom.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Clasadonte J, Scemes E, Wang ZY, Boison D, Haydon PG. Connexin 43-mediated astroglial metabolic networks contribute to the regulation of the sleep-wake cycle. Neuron. 2017;95:1365–1380.e1365. doi: 10.1016/j.neuron.2017.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ai Z, Fischer A, Spray DC, Brown AM, Fishman GI. Wnt-1 regulation of connexin43 in cardiac myocytes. J Clin Invest. 2000;105:161–171. doi: 10.1172/JCI7798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sun W, Cornwell A, Li JS, Peng SS, Osorio MJ, Aalling N, et al. SOX9 is an astrocyte-specific nuclear marker in the adult brain outside the neurogenic regions. J Neurosci. 2017;37:4493–4507. doi: 10.1523/JNEUROSCI.3199-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xia MS, Zhu Y. The regulation of Sox2 and Sox9 stimulated by ATP in spinal cord astrocytes. J Mol Neurosci. 2015;55:131–140. doi: 10.1007/s12031-014-0393-5. [DOI] [PubMed] [Google Scholar]
- 101.Bernard R, Kerman IA, Thompson RC, Jones EG, Bunney WE, Barchas JD, et al. Altered expression of glutamate signaling, growth factor, and glia genes in the locus coeruleus of patients with major depression. Mol Psychiatry. 2011;16:634–646. doi: 10.1038/mp.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Morquette P, Verdier D, Kadala A, Féthière J, Philippe AG, Robitaille R, et al. An astrocyte-dependent mechanism for neuronal rhythmogenesis. Nat Neurosci. 2015;18:844–854. doi: 10.1038/nn.4013. [DOI] [PubMed] [Google Scholar]
- 103.Schroeter ML, Abdul-Khaliq H, Diefenbacher A, Blasig IE. S100B is increased in mood disorders and may be reduced by antidepressive treatment. Neuroreport. 2002;13:1675–1678. doi: 10.1097/00001756-200209160-00021. [DOI] [PubMed] [Google Scholar]
- 104.Li BM, Zhang S, Zhang HY, Hertz L, Peng L. Fluoxetine affects GluK2 editing, glutamate-evoked Ca2+ influx and extracellular signal-regulated kinase phosphorylation in mouse astrocytes. J Psychiatry Neurosci. 2011;36:322–338. doi: 10.1503/jpn.100094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li BM, Dong L, Wang B, Cai LP, Jiang N, Peng L. Cell type-specific gene expression and editing responses to chronic fluoxetine treatment in the in vivo mouse brain and their relevance for stress-induced anhedonia. Neurochem Res. 2012;37:2480–2495. doi: 10.1007/s11064-012-0814-1. [DOI] [PubMed] [Google Scholar]
- 106.Xia M, Li X, Yang L, Ren J, Sun G, Qi S, et al. The ameliorative effect of fluoxetine on neuroinflammation induced by sleep deprivation. J Neurochem. 2017;146:63–75. doi: 10.1111/jnc.14272. [DOI] [PubMed] [Google Scholar]
- 107.Hertz L, Rothman DL, Li BM, Peng L. Chronic SSRI stimulation of astrocytic 5-HT2B receptors change multiple gene expressions/editings and metabolism of glutamate, glucose and glycogen: A potential paradigm shift. Front Behav Neurosci. 2015;9:25. doi: 10.3389/fnbeh.2015.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gill T, Watling SE, Richardson JD, McCluskey T, Tong JC, Meyer JH, et al. Imaging of astrocytes in posttraumatic stress disorder: A PET study with the monoamine oxidase B radioligand [11C]SL25.1188. Eur Neuropsychopharmacol. 2022;54:54–61. doi: 10.1016/j.euroneuro.2021.10.006. [DOI] [PubMed] [Google Scholar]
- 109.Kitayama N, Vaccarino V, Kutner M, Weiss P, Bremner JD. Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: A meta-analysis. J Affect Disord. 2005;88:79–86. doi: 10.1016/j.jad.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 110.Lindauer RJL, Olff M, van Meijel EPM, Carlier IVE, Gersons BPR. Cortisol, learning, memory, and attention in relation to smaller hippocampal volume in police officers with posttraumatic stress disorder. Biol Psychiatry. 2006;59:171–177. doi: 10.1016/j.biopsych.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 111.Bremner JD, Elzinga B, Schmahl C, Vermetten E. Structural and functional plasticity of the human brain in posttraumatic stress disorder. Prog Brain Res. 2008;167:171–186. doi: 10.1016/S0079-6123(07)67012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shin LM, Shin PS, Heckers S, Krangel TS, Macklin ML, Orr SP, et al. Hippocampal function in posttraumatic stress disorder. Hippocampus. 2004;14:292–300. doi: 10.1002/hipo.10183. [DOI] [PubMed] [Google Scholar]
- 113.Friedman WJ, Black IB, Kaplan DR. Distribution of the neurotrophins brain-derived neurotrophic factor, neurotrophin-3, and neurotrophin-4/5 in the postnatal rat brain: An immunocytochemical study. Neuroscience. 1998;84:101–114. doi: 10.1016/S0306-4522(97)00526-5. [DOI] [PubMed] [Google Scholar]
- 114.Althaus HH, Richter-Landsberg C. Glial cells as targets and producers of neurotrophins. Int Rev Cytol. 2000;197:203–277. doi: 10.1016/S0074-7696(00)97005-0. [DOI] [PubMed] [Google Scholar]
- 115.Xia L, Zhai MZ, Wang LY, Miao DM, Zhu X, Wang W. FGF2 blocks PTSD symptoms via an astrocyte-based mechanism. Behav Brain Res. 2013;256:472–480. doi: 10.1016/j.bbr.2013.08.048. [DOI] [PubMed] [Google Scholar]
- 116.Czéh B, Simon M, Schmelting B, Hiemke C, Fuchs E. Astroglial plasticity in the hippocampus is affected by chronic psychosocial stress and concomitant fluoxetine treatment. Neuropsychopharmacology. 2006;31:1616–1626. doi: 10.1038/sj.npp.1300982. [DOI] [PubMed] [Google Scholar]
- 117.Cohen H, Kozlovsky N, Matar MA, Zohar J, Kaplan Z. Distinctive hippocampal and amygdalar cytoarchitectural changes underlie specific patterns of behavioral disruption following stress exposure in an animal model of PTSD. Eur Neuropsychopharmacol. 2014;24:1925–1944. doi: 10.1016/j.euroneuro.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 118.Kassem MS, Lagopoulos J, Stait-Gardner T, Price WS, Chohan TW, Arnold JC, et al. Stress-induced grey matter loss determined by MRI is primarily due to loss of dendrites and their synapses. Mol Neurobiol. 2013;47:645–661. doi: 10.1007/s12035-012-8365-7. [DOI] [PubMed] [Google Scholar]
- 119.Tynan RJ, Beynon SB, Hinwood M, Johnson SJ, Nilsson M, Woods JJ, et al. Chronic stress-induced disruption of the astrocyte network is driven by structural atrophy and not loss of astrocytes. Acta Neuropathol. 2013;126:75–91. doi: 10.1007/s00401-013-1102-0. [DOI] [PubMed] [Google Scholar]
- 120.Parekh SV, Paniccia JE, Lebonville CL, Lysle DT. Dorsal hippocampal interleukin-1 signaling mediates heroin withdrawal-enhanced fear learning. Psychopharmacology. 2020;237:3653–3664. doi: 10.1007/s00213-020-05645-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Datta D, Yang ST, Galvin VC, Solder J, Luo F, Morozov YM, et al. Noradrenergic α1-adrenoceptor actions in the primate dorsolateral prefrontal cortex. J Neurosci. 2019;39:2722–2734. doi: 10.1523/JNEUROSCI.2472-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ferry B, Roozendaal B, McGaugh JL. Role of norepinephrine in mediating stress hormone regulation of long-term memory storage: A critical involvement of the amygdala. Biol Psychiatry. 1999;46:1140–1152. doi: 10.1016/S0006-3223(99)00157-2. [DOI] [PubMed] [Google Scholar]
- 123.Rajbhandari AK, Baldo BA, Bakshi VP. Predator stress-induced CRF release causes enduring sensitization of basolateral amygdala norepinephrine systems that promote PTSD-like startle abnormalities. J Neurosci. 2015;35:14270–14285. doi: 10.1523/JNEUROSCI.5080-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Birnbaum S, Gobeske KT, Auerbach J, Taylor JR, Arnsten AF. A role for norepinephrine in stress-induced cognitive deficits: Alpha-1-adrenoceptor mediation in the prefrontal cortex. Biol Psychiatry. 1999;46:1266–1274. doi: 10.1016/S0006-3223(99)00138-9. [DOI] [PubMed] [Google Scholar]
- 125.Kobori N, Clifton GL, Dash PK. Enhanced catecholamine synthesis in the prefrontal cortex after traumatic brain injury: Implications for prefrontal dysfunction. J Neurotrauma. 2006;23:1094–1102. doi: 10.1089/neu.2006.23.1094. [DOI] [PubMed] [Google Scholar]
- 126.Kobori N, Hu B, Dash PK. Altered adrenergic receptor signaling following traumatic brain injury contributes to working memory dysfunction. Neuroscience. 2011;172:293–302. doi: 10.1016/j.neuroscience.2010.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Arango V, Ernsberger P, Sved AF, Mann JJ. Quantitative autoradiography of alpha 1- and alpha 2-adrenergic receptors in the cerebral cortex of controls and suicide victims. Brain Res. 1993;630:271–282. doi: 10.1016/0006-8993(93)90666-B. [DOI] [PubMed] [Google Scholar]
- 128.Southwick SM, Krystal JH, Morgan CA, Johnson D, Nagy LM, Nicolaou A, et al. Abnormal noradrenergic function in posttraumatic stress disorder. Arch Gen Psychiatry. 1993;50:266–274. doi: 10.1001/archpsyc.1993.01820160036003. [DOI] [PubMed] [Google Scholar]
- 129.Southwick SM, Bremner JD, Rasmusson A, Morgan CA, 3rd, Arnsten A, Charney DS. Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biol Psychiatry. 1999;46:1192–1204. doi: 10.1016/S0006-3223(99)00219-X. [DOI] [PubMed] [Google Scholar]
- 130.O'Donnell T, Hegadoren KM, Coupland NC. Noradrenergic mechanisms in the pathophysiology of post-traumatic stress disorder. Neuropsychobiology. 2004;50:273–283. doi: 10.1159/000080952. [DOI] [PubMed] [Google Scholar]
- 131.Nwaobi SE, Cuddapah VA, Patterson KC, Randolph AC, Olsen ML. The role of glial-specific Kir4.1 in normal and pathological states of the CNS. Acta Neuropathol. 2016;132:1–21. doi: 10.1007/s00401-016-1553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ohno Y. Astrocytic Kir4.1 potassium channels as a novel therapeutic target for epilepsy and mood disorders. Neural Regen Res. 2018;13:651–652. doi: 10.4103/1673-5374.230355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tong XP, Ao Y, Faas GC, Nwaobi SE, Xu J, Haustein MD, et al. Astrocyte Kir4.1 ion channel deficits contribute to neuronal dysfunction in Huntington's disease model mice. Nat Neurosci. 2014;17:694–703. doi: 10.1038/nn.3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kelley KW, Ben Haim L, Schirmer L, Tyzack GE, Tolman M, Miller JG, et al. Kir4.1-dependent astrocyte-fast motor neuron interactions are required for peak strength. Neuron. 2018;98:306–319.e7. doi: 10.1016/j.neuron.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang ZR, Song ZJ, Shen FM, Xie P, Wang J, Zhu AS, et al. Ginsenoside Rg1 prevents PTSD-like behaviors in mice through promoting synaptic proteins, reducing Kir4.1 and TNF-α in the hippocampus. Mol Neurobiol. 2021;58:1550–1563. doi: 10.1007/s12035-020-02213-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ginzburg K, Ein-Dor T, Solomon Z. Comorbidity of posttraumatic stress disorder, anxiety and depression: A 20-year longitudinal study of war veterans. J Affect Disord. 2010;123:249–257. doi: 10.1016/j.jad.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 137.Su SY, Miller AH, Snieder H, Bremner JD, Ritchie J, Maisano C, et al. Common genetic contributions to depressive symptoms and inflammatory markers in middle-aged men: The Twins Heart Study. Psychosom Med. 2009;71:152–158. doi: 10.1097/PSY.0b013e31819082ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Calhoun PS, Wiley M, Dennis MF, Means MK, Edinger JD, Beckham JC. Objective evidence of sleep disturbance in women with posttraumatic stress disorder. J Trauma Stress. 2007;20:1009–1018. doi: 10.1002/jts.20255. [DOI] [PubMed] [Google Scholar]
- 139.Dagan Y, Zinger Y, Lavie P. Actigraphic sleep monitoring in posttraumatic stress disorder (PTSD) patients. J Psychosom Res. 1997;42:577–581. doi: 10.1016/S0022-3999(97)00013-5. [DOI] [PubMed] [Google Scholar]
- 140.Babson KA, Feldner MT. Temporal relations between sleep problems and both traumatic event exposure and PTSD: A critical review of the empirical literature. J Anxiety Disord. 2010;24:1–15. doi: 10.1016/j.janxdis.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166:1756–1762. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 142.Mackiewicz M, Zimmerman JE, Shockley KR, Churchill GA, Pack AI. What are microarrays teaching us about sleep? Trends Mol Med. 2009;15:79–87. doi: 10.1016/j.molmed.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Meerlo P, Mistlberger RE, Jacobs BL, Heller HC, McGinty D. New neurons in the adult brain: The role of sleep and consequences of sleep loss. Sleep Med Rev. 2009;13:187–194. doi: 10.1016/j.smrv.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.O'Hara BF, Mongrain V. Sleepy genes. Front Neurosci. 2010;4:183. doi: 10.3389/fnins.2010.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Prather AA, Marsland AL, Hall M, Neumann SA, Muldoon MF, Manuck SB. Normative variation in self-reported sleep quality and sleep debt is associated with stimulated pro-inflammatory cytokine production. Biol Psychol. 2009;82:12–17. doi: 10.1016/j.biopsycho.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Neylan TC, Mueller SG, Wang Z, Metzler TJ, Lenoci M, Truran D, et al. Insomnia severity is associated with a decreased volume of the CA3/dentate gyrus hippocampal subfield. Biol Psychiatry. 2010;68:494–496. doi: 10.1016/j.biopsych.2010.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang Z, Xiao ZP. Magnetic resonance imaging study of hippocampus structural alterations in post-traumatic stress disorder: A brief review (translated version) East Asian Arch Psychiatry. 2010;20:138–144. [PubMed] [Google Scholar]
- 148.Xia MS, Li ZX, Li S, Liang SS, Li XW, Chen BN, et al. Sleep deprivation selectively down-regulates astrocytic 5-HT2B receptors and triggers depressive-like behaviors via stimulating P2X7 receptors in mice. Neurosci Bull. 2020;36:1259–1270. doi: 10.1007/s12264-020-00524-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li XW, Liang SS, Li ZX, Li S, Xia MS, Verkhratsky A, et al. Leptin increases expression of 5-HT2B receptors in astrocytes thus enhancing action of fluoxetine on the depressive behavior induced by sleep deprivation. Front Psychiatry. 2018;9:734. doi: 10.3389/fpsyt.2018.00734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Stoddard FJ, Jr, Luthra R, Sorrentino EA, Saxe GN, Drake J, Chang Y, et al. A randomized controlled trial of sertraline to prevent posttraumatic stress disorder in burned children. J Child Adolesc Psychopharmacol. 2011;21:469–477. doi: 10.1089/cap.2010.0133. [DOI] [PubMed] [Google Scholar]
- 151.Qi W, Gevonden M, Shalev A. Efficacy and tolerability of high-dose escitalopram in posttraumatic stress disorder. J Clin Psychopharmacol. 2017;37:89–93. doi: 10.1097/JCP.0000000000000626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Edgelow MM, MacPherson MM, Arnaly F, Tam-Seto L, Cramm HA. Occupational therapy and posttraumatic stress disorder: A scoping review. Can J Occup Ther. 2019;86:148–157. doi: 10.1177/0008417419831438. [DOI] [PubMed] [Google Scholar]
- 153.Snedden D. Enhancing practice: Mental health: Trauma-informed practice: An emerging role of occupational therapy. Occupational Therapy Now. 2012;14:26–28. [Google Scholar]
- 154.Kelmendi B, Adams TG, Yarnell S, Southwick S, Abdallah CG, Krystal JH. PTSD: from neurobiology to pharmacological treatments. Eur J Psychotraumatol. 2016;7:31858. doi: 10.3402/ejpt.v7.31858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kelmendi B, Adams TG, Southwick S, Abdallah CG, Krystal JH. Posttraumatic Stress Disorder: An integrated overview and neurobiological rationale for pharmacology. Clin Psychol (New York) 2017;24:281–297. doi: 10.1111/cpsp.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Marathe SV, Virmani G, Bathini P, Alberi L. Effects of monoamines and antidepressants on astrocyte physiology: Implications for monoamine hypothesis of depression. J Exp Neurosci. 2018;12:1179069518789149. doi: 10.1177/1179069518789149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jayan D, deRoon-Cassini TA, Sauber G, Hillard CJ, Fitzgerald JM. A cluster analytic approach to examining the role of cortisol in the development of post-traumatic stress and dysphoria in adult traumatic injury survivors. Psychoneuroendocrinology. 2022;135:105450. doi: 10.1016/j.psyneuen.2021.105450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Milaneschi Y, Simmons WK, van Rossum EFC, Penninx BW. Depression and obesity: Evidence of shared biological mechanisms. Mol Psychiatry. 2019;24:18–33. doi: 10.1038/s41380-018-0017-5. [DOI] [PubMed] [Google Scholar]
- 159.Yu WX, Greenberg ML. Inositol depletion, GSK3 inhibition and bipolar disorder. Future Neurol. 2016;11:135–148. doi: 10.2217/fnl-2016-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Peng L, Huang JY. Astrocytic 5-HT2B receptor as in vitro and in vivo target of SSRIs. Recent Pat CNS Drug Discov. 2012;7:243–253. doi: 10.2174/157488912803252078. [DOI] [PubMed] [Google Scholar]
- 161.Hertz L, Li BM, Song D, Ren JN, Dong L, Chen Y, et al. Astrocytes as a 5-HT2B-mediated SERT-independent SSRI target, slowly altering depression-associated genes and function. Curr Signal Transduct Ther. 2012;7:65–80. doi: 10.2174/1574362799278154. [DOI] [Google Scholar]
- 162.Li BM, Zhang S, Zhang HY, Nu WW, Cai LP, Hertz L, et al. Fluoxetine-mediated 5-HT2B receptor stimulation in astrocytes causes EGF receptor transactivation and ERK phosphorylation. Psychopharmacology. 2008;201:443–458. doi: 10.1007/s00213-008-1306-5. [DOI] [PubMed] [Google Scholar]
- 163.Li BM, Zhang S, Li M, Hertz L, Peng L. Serotonin increases ERK1/2 phosphorylation in astrocytes by stimulation of 5-HT2B and 5-HT2C receptors. Neurochem Int. 2010;57:432–439. doi: 10.1016/j.neuint.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 164.Li BM, Zhang S, Li M, Hertz L, Peng L. Chronic treatment of astrocytes with therapeutically relevant fluoxetine concentrations enhances cPLA2 expression secondary to 5-HT2B-induced, transactivation-mediated ERK1/2 phosphorylation. Psychopharmacology. 2009;207:1–12. doi: 10.1007/s00213-009-1631-3. [DOI] [PubMed] [Google Scholar]
- 165.Peng L, Song D, Li BM, Verkhratsky A. Astroglial 5-HT2B receptor in mood disorders. Expert Rev Neurother. 2018;18:435–442. doi: 10.1080/14737175.2018.1458612. [DOI] [PubMed] [Google Scholar]
- 166.Li BM, Jia S, Yue TT, Yang L, Huang C, Verkhratsky A, et al. Biphasic regulation of caveolin-1 gene expression by fluoxetine in astrocytes: Opposite effects of PI3K/AKT and MAPK/ERK signaling pathways on c-fos. Front Cell Neurosci. 2017;11:335. doi: 10.3389/fncel.2017.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hablitz LM, Nedergaard M. The glymphatic system: A novel component of fundamental neurobiology. J Neurosci. 2021;41:7698–7711. doi: 10.1523/JNEUROSCI.0619-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Xie LL, Kang HY, Xu QW, Chen MJ, Liao YH, Thiyagarajan M, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Liang SS, Lu Y, Li ZX, Li S, Chen BN, Zhang MM, et al. Iron aggravates the depressive phenotype of stressed mice by compromising the glymphatic system. Neurosci Bull. 2020;36:1542–1546. doi: 10.1007/s12264-020-00539-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.George KC, Kebejian L, Ruth LJ, Miller CWT, Himelhoch S. Meta-analysis of the efficacy and safety of prazosin versus placebo for the treatment of nightmares and sleep disturbances in adults with posttraumatic stress disorder. J Trauma Dissociation. 2016;17:494–510. doi: 10.1080/15299732.2016.1141150. [DOI] [PubMed] [Google Scholar]
- 171.de Berardis D, Marini S, Serroni N, Iasevoli F, Tomasetti C, de Bartolomeis A, et al. Targeting the noradrenergic system in posttraumatic stress disorder: A systematic review and meta-analysis of prazosin trials. Curr Drug Targets. 2015;16:1094–1106. doi: 10.2174/1389450116666150506114108. [DOI] [PubMed] [Google Scholar]
- 172.Khachatryan D, Groll D, Booij L, Sepehry AA, Schütz CG. Prazosin for treating sleep disturbances in adults with posttraumatic stress disorder: A systematic review and meta-analysis of randomized controlled trials. Gen Hosp Psychiatry. 2016;39:46–52. doi: 10.1016/j.genhosppsych.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 173.Singh B, Hughes AJ, Mehta G, Erwin PJ, Parsaik AK. Efficacy of prazosin in posttraumatic stress disorder: A systematic review and meta-analysis. Prim Care Companion CNS Disord 2016, 18(4):1–11 [DOI] [PubMed]
- 174.Lee JH, Lee S, Kim JH. Amygdala circuits for fear memory: A key role for dopamine regulation. Neuroscientist. 2017;23:542–553. doi: 10.1177/1073858416679936. [DOI] [PubMed] [Google Scholar]
- 175.Roitman P, Mechoulam R, Cooper-Kazaz R, Shalev A. Preliminary, open-label, pilot study of add-on oral Δ9-tetrahydrocannabinol in chronic post-traumatic stress disorder. Clin Drug Investig. 2014;34:587–591. doi: 10.1007/s40261-014-0212-3. [DOI] [PubMed] [Google Scholar]
- 176.Zhang S, Li BM, Lovatt D, Xu JN, Song D, Goldman SA, et al. 5-HT2B receptors are expressed on astrocytes from brain and in culture and are a chronic target for all five conventional ‘serotonin-specific reuptake inhibitors’. Neuron Glia Biol. 2010;6:113–125. doi: 10.1017/S1740925X10000141. [DOI] [PubMed] [Google Scholar]
- 177.Li BM, Gu L, Zhang HY, Huang JY, Chen Y, Hertz L, et al. Up-regulation of cPLA2 gene expression in astrocytes by all three conventional anti-bipolar drugs is drug-specific and enzyme-specific. Psychopharmacology. 2007;194:333–345. doi: 10.1007/s00213-007-0853-5. [DOI] [PubMed] [Google Scholar]
- 178.Sublette ME, Milak MS, Hibbeln JR, Freed PJ, Oquendo MA, Malone KM, et al. Plasma polyunsaturated fatty acids and regional cerebral glucose metabolism in major depression. Prostaglandins Leukot Essent Fatty Acids. 2009;80:57–64. doi: 10.1016/j.plefa.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Peng L, Li BM, Verkhratsky A. Targeting astrocytes in bipolar disorder. Expert Rev Neurother. 2016;16:649–657. doi: 10.1586/14737175.2016.1171144. [DOI] [PubMed] [Google Scholar]