Abstract
Rheumatic heart disease (RHD) is often considered as a disease of developing countries and India is the home of about 40% of RHD patients. Environment seems to play a major role in its causation. Since gene environment interactions can lead to alterations of various metabolic pathways, identification of altered metabolites can help in understanding the various pathways leading to RHD. Blood plasma samples from 51 RHD and 49 healthy controls were collected for the study. Untargeted metabolomics approach was used to identify the metabolites that are altered in RHD patients. Data showed 25 altered metabolites among RHD patients. These altered metabolites were those involved in Purine, Glutamine, Glutamate, Pyrimidine, Arginine, Proline and Linoleic metabolism. Thus, the present study illuminates metabolic alterations among RHD patients which can help in determining the potential therapeutic targets.
Subject terms: Biological techniques, Cardiology
Introduction
Rheumatic heart disease (RHD) is one of the commonest causes of cardiac disease below 25 years of age1. Recurrences of Rheumatic fever leads to valvular damage and RHD. The global burden of this disease is estimated to be about 33 million worldwide, causing 2,75,000 deaths annually1. Rheumatic fever (RF) and RHD have almost disappeared from high income countries during late twentieth centuries, but are still prevalent in low-income countries. African, South Asian and Pacific Islands are the worst affected regions contributing to almost 84% of the total RHD cases2. In the South-East Asian region, India accounts for highest global prevalence of almost 27% (13.17 million) and 1,19,100 deaths approximately2. RHD is pathologically heterogenous where gene environment interaction plays a very important role3. The pathological mechanism of RHD has not been elucidated in detail till date.
Metabolomics is a powerful technique which has the potential to provide minute details of biological pathways, driven genes or mutations thereby shedding light in understanding the mechanism of disease progression and also discovering diagnostic biomarkers. It is helpful in identifying drug targets for the development of therapeutic agents. Metabolomics approach has helped in detecting biomarkers in diseases like cancer and neurological disorders but to the best of our knowledge there is paucity of metabolomic studies among RHD patients. Metabolomics analysis can be done using untargeted or targeted approach. Untargeted metabolite study is a hypothesis free approach where novel metabolites can be discovered whereas targeted metabolite approach is a hypothesis-based approach where metabolites are known. Thus, metabolomics approach will not only provide insights into the pathogenesis of disease progression but will also help in identifying new therapeutic targets.
In the present study we aim to identify the putative metabolic biomarkers for RHD using high throughput non targeted ultra-high performance liquid chromatography tandem mass spectrometry (UHPLC-MS/MS) which can be useful in early diagnosis and monitoring of RHD, further helping in understanding the disease pathology.
Results
In this study, a total of 51 RHD patients and 49 age sex matched healthy controls were enrolled. There was no significant difference between the mean age and sex among both the groups. The number of females was higher among RHD patients. The mean body mass index (BMI) was significantly different in both the groups (P = 0.01) (Table 1). The diastolic pressure was significantly high among the RHD patients (P = 0.01) (Table 1). All the patients belonged to New York Heart Association (NYHA) class II and III (Table 1). Only 3.92% were smokers among RHD patients and were not significantly different from healthy controls. Diet pattern was almost similar in both the groups (Table 1).
Table 1.
Demographic and clinical characteristics of the patients.
| RHD patients (N = 51) |
Healthy controls (N = 49) |
p value | |
|---|---|---|---|
| Age (years) | 32.63 ± 8.75 | 29.93 ± 7.60 | 0.10 |
| Male n (%) | 17 (33.33) | 22 (44.89) | 0.23 |
| BMI (kg/m2) | 21.83 ± 3.91 | 25.71 ± 6.41 | 0.01 |
| Heart rate (beats/min) | 78 ± 19.03 | 79.89 ± 7.79 | 0.61 |
| Systolic pressure (mmHg) | 111.47 ± 14.52 | 112.17 ± 11.28 | 0.82 |
| Diastolic pressure (mm Hg) | 76.5 ± 11.50 | 70.28 ± 9.09 | 0.01 |
| EF | 57.76 ± 4.86 | ||
| MVA (cm2) | 1.04 ± 0.32 | ||
| Severe MS N (%) | 28 (54.90) | ||
| NYHA class | |||
| NYHA II | 34 (66.67) | ||
| NYHA III | 17 (33.33) | ||
| Diet veg N (%) | 18 (35.29) | 24 (48.97) | 0.16 |
| Alcohol N (%) | 07 (13.72) | 15 (30.61) | 0.04 |
| Smoking N (%) | 02 (3.92) | 07 (14.28) | 0.07 |
Significant values are in bold.
Untargeted LC–MS analysis
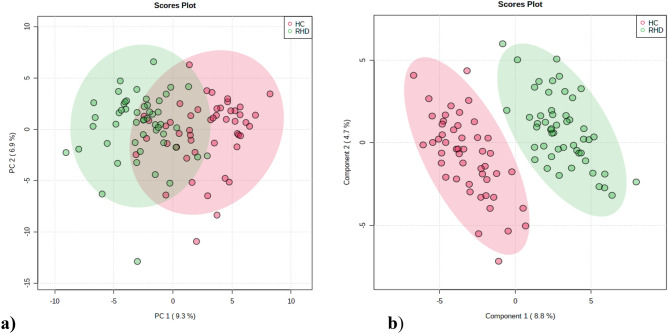
351 metabolites were identified in blood plasma through UHPLC-MS/MS analysis (Supplementary Table 1 and Supplementary Table 2). Metabolites with more than 20% missing values were removed from the study rest were replaced by LoDs (1/5 of the minimum positive value of each variable). The peak area data matrix was sum normalized, log transformed and pareto scaled. PCA analysis was performed to understand the aggregation and description of the samples (Fig. 1a.). Whereas PLS-DA score plot helped us to clearly discriminate between the two groups (R2 = 0.92 and Q2 = 0.76) (Fig. 1b). Cross validation analysis using 100 random permutations were done to prevent overfitting of the PLS-DA model. The R2 and Q2 values of the originally obtained model were better than the 100 randomly permutated models indicating good predictive capacity of the obtained PLS-DA model.
Figure 1.
Chemometric analysis of metabolites among RHD and healthy controls. (a) Principal Component Analysis (PCA) score plot from RHD and healthy control. The green dots represent RHD patients and the red dots represent healthy controls in the 2D PCA score plots. (b) Partial least squares discriminant analysis (PLS-DA) score plot from RHD and healthy control. The two groups are well separated in the PLS-DA score plot, indicating that they had markedly different metabolic characteristics.
Identification of significantly altered metabolites
Identification of altered metabolites was based on univariant and multivariant analysis. Consideration of metabolites was based on VIP score > 1.2 from PLS-DA analysis, p < 0.05 selected upon student’s t test analysis. 25 metabolites were significantly differentially expressed in the blood plasma samples in RHD patients. Among these 25 metabolites 17 metabolites (N-acetylneuraminate, Arachidonic acid, D-Sphingosine, 16(R)-HETE, orotate, inosine, Hypoxanthine, linoleate, Prostaglandin B, d-( +)-Pyroglutamic Acid, l-5-Hydroxytryptophan, Adenosine monophosphate, l-glutamic acid, 5-Methoxysalicylic acid, Prostaglandin A1, d-pantothenic acid, xanthine) were up regulated among RHD patients (Table 2). Remaining 8 metabolites (Caprolactam, trans-4-Hydroxy-l-proline, dihydroxymandelic acid, alpha-Aspartylphenylalanine, 2'-Deoxyuridine, alpha-Lactose, 4-Nitrophenol, 4-Anisic acid) were down regulated (Table 2). Caprolactam, N-acetylneuraminate, trans-4-Hydroxy-l-proline, Dihydroxymandelic acid had VIP score > 2 indicating strong difference between RHD patients from the control group (Table 2).
Table 2.
Plasma metabolites differentiating RHD patients from control group.
| VIP | P value | Log2(FC) | FDR | AUC | |
|---|---|---|---|---|---|
| Caprolactam | 2.3412 | 3.31E−24 | −1.2333 | 1.16E−21 | 0.967 |
| N-Acetylneuraminate | 2.1912 | 1.27E−05 | 2.0395 | 0.000149 | 0.824 |
| Trans-4-hydroxy-l-proline | 2.0438 | 8.19E−07 | −1.6103 | 2.05E−05 | 0.779 |
| Dihydroxymandelic acid | 2.04 | 4.05E−06 | −2.3744 | 5.92E−05 | 0.736 |
| Arachidonic acid | 1.8545 | 2.82E−08 | 1.4512 | 1.24E−06 | 0.805 |
| d-Sphingosine | 1.7667 | 1.99E−05 | 0.88739 | 0.000208 | 0.768 |
| 16(R)-HETE | 1.7518 | 2.40E−06 | 0.71539 | 4.44E−05 | 0.733 |
| Orotate | 1.7127 | 0.00309 | 1.3078 | 0.013226 | 0.58 |
| Inosine | 1.6591 | 0.001455 | 2.44 | 0.006809 | 0.719 |
| Hypoxanthine | 1.6467 | 0.005737 | 1.3465 | 0.022375 | 0.747 |
| Linoleate | 1.595 | 0.006022 | 2.1162 | 0.023227 | 0.76 |
| Prostaglandin B1 | 1.5482 | 5.03E−06 | 1.1173 | 6.61E−05 | 0.758 |
| d-( +)-pyroglutamic acid | 1.4993 | 3.12E−06 | 0.8164 | 5.01E−05 | 0.773 |
| Alpha-aspartylphenylalanine | 1.4817 | 0.016667 | −1.7362 | 0.047952 | 0.667 |
| l-5-Hydroxytryptophan | 1.4711 | 3.33E−10 | 0.70395 | 3.89E−08 | 0.876 |
| Adenosine monophosphate | 1.4458 | 0.000781 | 0.80453 | 0.004153 | 0.721 |
| l-Glutamic acid | 1.4397 | 3.27E−06 | 0.75709 | 5.01E−05 | 0.751 |
| 5-Methoxysalicylic acid | 1.4297 | 5.09E−06 | 0.9186 | 6.61E−05 | 0.747 |
| Prostaglandin A1 | 1.4266 | 6.22E−05 | 1.1885 | 0.000555 | 0.71 |
| 2'-Deoxyuridine | 1.3609 | 0.000346 | −1.1365 | 0.002131 | 0.692 |
| d-Pantothenic acid | 1.3421 | 0.001059 | 0.83355 | 0.005236 | 0.808 |
| Alpha-lactose | 1.2985 | 8.95E−05 | −0.73137 | 0.000748 | 0.742 |
| Xanthine | 1.2797 | 0.000363 | 1.3465 | 0.002158 | 0.731 |
| 4-Nitrophenol | 1.2617 | 9.41E−08 | −0.65097 | 3.30E−06 | 0.803 |
| 4-Anisic acid | 1.2602 | 0.00021 | −1.4004 | 0.001502 | 0.717 |
VIP score variable of importance score obtained from PLS-DA analysis (VIP > 1.2), p value p value of the Wilcoxon signed-rank test, Log2(FC) log2 value of fold change, FC fold change (FC > 1.5), FDR false discovery rate, AUC area under the curve calculated from ROC analysis.
Binary logistic regression analysis was also performed to assess the association between altered metabolites and RHD after controlling the effect of BMI and alcohol consumption. Findings of logistic regression analysis are provided in Supplementary table 3.
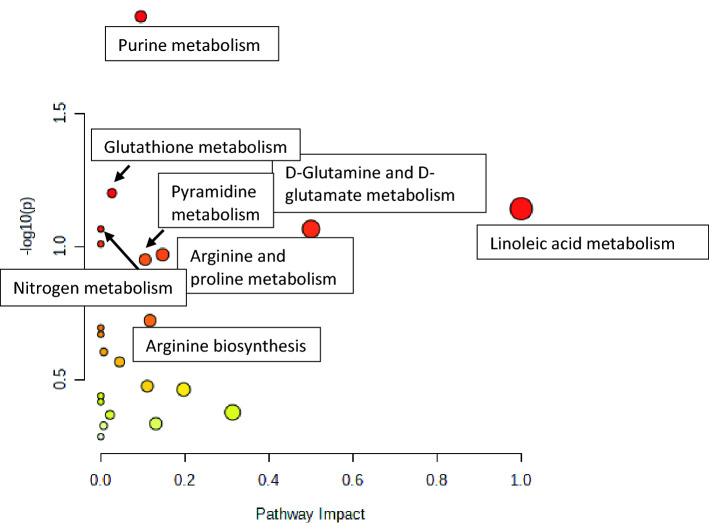
Statistically significant metabolites were subjected to KEGG pathway analysis (https://www.kegg.jp/kegg/kegg1.html). Purine metabolism pathway was significantly altered (p = 0.01) with more than 2 hits. The results obtained from MetPA analysis is illustrated in Fig. 2.
Figure 2.
The MetPA analysis based on KEGG Analysis. The darker the red colour of the metabolic pathway, the greater its-log (p) value, indicating a more significant difference.
Impact value more than 0.10 directs that the altered pathway evidently affects RHD patients therefore we consider Purine metabolism, Linoleic acid metabolism, d-Glutamine and D-glutamate metabolism, Arginine and proline metabolism, Pyrimidine metabolism, Arginine biosynthesis, Galactose metabolism, Alanine, aspartate and glutamate metabolism, Arachidonic acid metabolism and Tryptophan metabolism.
Discussion
Till date there is scarcity of studies to understand the metabolomic changes in blood plasma of RHD patients. Metabolomics is a new approach after genomics and proteomics, which is being used extensively to identify disease biomarkers and biological systems4. Metabolomics approach has been used in detecting biomarkers in diseases like cancer, neurological disorders, infectious diseases, inflammation and also in cardiovascular diseases5. It is also used to understand the regulation of pathways of various biological processes.
In the present study untargeted LCMS based metabolomics approach has been used to identify potential metabolites for RHD. To the best of our available findings the present study is the first to understand the potential difference between RHD patients and Healthy controls. The main finding of the present study includes identification of 25 significantly altered metabolites, 17 upregulated and 8 down regulated in RHD patients compared to healthy controls.
The 25 significantly altered metabolites were mapped for different pathways. The most important altered pathways were Purine metabolism, Linoleic acid metabolism, D-Glutamine and D-glutamate metabolism, Arginine and proline metabolism, Pyrimidine metabolism, Arginine biosynthesis, Galactose metabolism, Alanine, aspartate and glutamate metabolism, Arachidonic acid metabolism and Tryptophan metabolism (Fig. 2). Notably, purine metabolism comprises increase in inosine, adenosine monophosphate, hypoxanthine and xanthine (Table 2). In ischemic pig myocardium hypoxanthine accumulation has been reported earlier6. Xanthine oxidase metabolizes hypoxanthine to xanthine and uric acid. Increased level of hypoxanthine is mainly due to deficiency in hypoxanthine guanine phosphoribosyl transferase (HGPRT). It has been reported in earlier studies that hypoxanthine can lead to endothelial dysfunction by oxidative stress induced apoptosis7. Thus, the present study may suggest that hypoxanthine imbalance may lead to RHD.
Other significant altered pathways discovered in RHD patients were d-Glutamine and d-glutamate metabolism and Linoleic acid metabolism (Fig. 2). Glutamate and glutamine are nonessential amino acids that are transformed into each other by glutamine synthase and glutaminase. Framingham heart study reported that the circulating glutamate levels lead to cardiometabolic risk factors whereas circulating level of glutamine and the glutamine:glutamate ratio exhibits opposite association with the cardiometabolic risk factors8. Yan Zheng et al., 2016 has reported association of CVD and especially stroke with metabolites in the Glutamate pathway9. Since patients of RHD with AF have a high risk of stroke, thus the present study gives evidence for considering glutamate as an early marker for RHD.
Linoleic acid is predominant n-6 polyunsaturated fatty acid (PUFA) which is commonly obtained from vegetable oils and nuts. It has been previously reported that linoleic acid reduces LDL cholesterol thus lowers the risk of chronic heart disease10. Therefore, polyunsaturated fatty acid (PUFA) has been recommended for prevention of chronic heart disease. Higher concentration of Linoleic acid shows a proinflammatory and thrombogenic effect11 but this result has not been confirmed by randomised controlled trials. Chowdhury et al., 2014 performed meta-analysis and reported no significant association between n-6 polyunsaturated fatty acids (PUFA) and chronic heart disease12. Since inflammation and thrombogenic effect are common among RHD patients therefore high consumption of n-6 polyunsaturated fatty acids (PUFA) should be discouraged.
In ischemic rat heart, UTP and CTP degrades quickly compared to ATP13. Coronary heart disease patients also show a great disturbance in pyrimidine nucleotides14. There are two main pathways for pyrimidine synthesis: de novo pathway which use amino acids and CO2to synthesize orotate and Salvage pathways uses pyrimidine precursors from the diet or from other tissues. The de novo pathway is not very prominent in cardiac tissue15 but Salvage pathways appears to play a significant role in pyrimidine synthesis in heart tissue. The low efficacy of the de novo pathway could be due to limited availability of phosphoribosyl pyrophosphate (PRPP). The low availability of PRPP is due to inefficient pentose shunt of carbohydrate catabolism in myocardium. Studies have shown that administration of orotate increases the pyrimidine nucleotide content in heart tissue16. Pyrimidine precursor administration can accelerate the reconstitution of glycogen stores17. In cardioplegic arrest orotic acid has provided protection to the heart18. Orotic acid has also prevented changes in contractility and sarcolemmal glycoproteins in hamsters with muscular dystrophy19. Thus, pyrimidine pathway plays an important role in supporting the myocardium20. In the present study Orotate was upregulated which may be a protective mechanism in RHD patients.
Arginine and proline metabolism is also observed to be altered in current study. Arginine is one of the most adaptable amino acid which acts as a precursor for protein, nitric oxide, polyamines, urea, glutamate, proline, agmatine and creatinine21. Lower arginine availability has been earlier reported to be associated with cardiovascular risk22. Proline and hydroxyproline are amino acids which help in maintaining cell structure and functions.
Further, one of the metabolites caprolactam which is a xenobiotic compound was significantly reduced among RHD patients. The direct association of caprolactam with RHD has not been reported previously. However, caprolactam has been reported to be associated with sensory and dermal irritation, dysmenorrhea among humans23. Cardiovascular and respiratory effects have been reported in animals with an increase in blood pressure followed by a decrease and an increased respiratory rate24. Further studies are required to establish the role of caprolactam in RHD.
To summarize, the present study is the first study to comprehend the complete metabolic alterations among RHD patients. The untargeted metabolomics approach leads to finding of a broad range of metabolites which will aid in understanding the complete view of key metabolic pathway alteration in RHD. The results suggest alteration of several metabolic pathways including purine metabolism, d-Glutamine and d-glutamate metabolism, Pyrimidine metabolism, Arginine and Proline metabolism and Linoleic acid metabolism. Thus, the findings from the present study can act as a tool for validation and identification of possible targets for future therapeutic executives.
Methods
Participants and sample collection
5 ml of intravenous blood samples were collected from 51 RHD patients and 49 age sex matched healthy controls. The plasma from the blood samples was separated and stored at −80 °C until analysis. The patients were included in the study after obtaining written informed consent. 12 lead electrocardiogram and two-dimensional echocardiography was done in all the patients. All methods were carried out in accordance with relevant guidelines and regulations. The study has been approved by the Ethics committee of All India Institute of Medical Sciences, New Delhi and National Institute of Pathology, Indian Council of Medical Research, New Delhi.
Sample preparation
Frozen samples were thawed at room temperature. Metabolites were extracted using chilled methanol in ratio of 1:3 (plasma: methanol) followed by vertexing and centrifugation at 10,000 rpm for 10 min. The supernatant containing metabolites was then collected in a microcentrifuge tube and was lyophilized. The lyophilized samples were reconstituted in 15% methanol and 5 µl was injected for LCMS analysis. Samples were run in randomised way and all the acquisition has been done in single batch.
Untargeted LCMS metabolomic profiling
LC–MS acquisition was done using orbitrap Fusion (Thermo Fischer) coupled with ultimate 3000 UHPLC system. Ion source used for positive and negative data acquisition was heated electrospray ion source. Resolution of MS was set to 120,000 for MS1 and 3000 for MSMS. Mass range of data acquisition was 60–900 Da. Extracted metabolites were separated on reverse phase column HSS T3 column (Waters)25 before infusing to mass spectrometer. Mobile phase A was water with 0.1% formic acid and mobile phase B was methanol with 0.1% formic acid with flow rate of 0.3 mL/min. Total run time was of 14 min with gradient varying from 1% B to 95% B26. Quality control (5ul of all samples) run was used after every five samples to monitor the retention time (RT) shift and signal variations. The RT was in minutes. Data were acquired in data dependent mode with intensity threshold an input. Collision energy was 35 ± 15 for MS/MS. Precursor ion selection was from 100 to 1000 Da and the ion isolation width was 1 Da.
Data processing
Data pre-processing, RT alignment, deconvolution, feature detection, elemental composition prediction and metabolites annotation was done using Progenesis QI software. Metascope plug of Progenesis QI has been used for annotation of the metabolites, the in-house library with accurate mass, fragmentation pattern and RT for database search27. The in-house library compounds were purchased from IROA technology that has ~ 600 commands. Same library with chemical class and other information has been published by Phapale et al. 202128 (https://pubs.acs.org/doi/abs/10.1021/acs.jproteome.0c00930). In Progenesis QI there is metascope plugin which can take .msp file (with fragment mass and their corresponding intensities information). Extract algorithm for spectral similarity match could be found at (https://www.nonlinear.com/progenesis/qi/v2.0/faq/fragment-databases.aspx). Identification of metabolites based MSMS spectral similarity was considered only when the fragmentation pattern was > 30 in Progenesis metascope. MSP file of MSMS was downloaded from MS-DIAL spectral database (http://prime.psc.riken.jp/compms/msdial/main.html#MSP) and same has been used for the identification of metabolites using progenesis metascope. Cut-off for RT match was 0.5 min and spectral similarity was more than 30% fragmentation match in Progenesis QI25. All features that had coefficient of variation (CV) less than 30% in pool QC samples were rejected25. Further, manual verification of each filtered feature has been to done to select the right peaks.
Multivariate statistical analysis
Statistical analysis in data was done using Metaboanalyst 5.0. The data matrix was sum normalized, log transformed and Pareto scaled. Principal Component Analysis (PCA) was done to understand the clustering pattern. Partial least squares discriminant analysis (PLS-DA) was conducted to clarify groups among clusters. Goodness of the fit and predictive ability of PLS-DA models were evaluated by R2 and Q2 values respectively29.
Comparison of socio-demographic and clinical characteristics across the two study groups:
Continuous variables in Table 1 were represented as mean ± SD and between group comparison was made by two sample t test. Categorical outcomes were reported as frequency (percentages) and compared with Chi squared test. Stata ver. 14.2 was used to perform the analysis.
Multivariate logistic regression analysis
Binary logistic regression analysis was performed to assess the association between altered metabolites and RHD after controlling the effect of BMI and alcohol consumption. In logistic regression analysis, RHD and healthy controls were assumed as dependent variables. Metabolite peak area, alcohol consumption and BMI were assumed as independent variables. BMI was categorized as normal (BMI ≤ 22.9), overweight (BMI 23–24.9) & obesity (≥ 25 kg/m2)30 respectively. Stata ver. 14.2 was used to perform logistic regression analysis.
Significant metabolites selection
Variables with VIP (variable importance of projection) score greater than 1.2 were considered for discrimination. Student’s t-test (P < 0.05) were adjusted for multiple hypothesis testing using FDR correction. Metabolites with fold change threshold of 1.5 and above were considered in the study. Metabolites passing fold change, VIP, P value and FDR criteria were considered for the study. AUC was calculated from ROC analysis. All the univariate analysis was performed with Metaboanalyst 5.0.
Pathway analysis
Pathway analysis was done using MetPA in Metaboanalyst 5.0. Information from Kyoto encyclopaedia of genes and genomes (KEGG) and human metabolome database (HMDB) for metabolic pathway analysis was used.
Supplementary Information
Author contributions
S.D.: Conceptualized the study, recruited the patients, collected the clinical data, analysed the data, drafted and written the manuscript Y.K.: Conducted the experiment S.S.: Conceptualized the study, supervised, revised and edited the final manuscript R.R.: supervised, revised and edited the final manuscript S.A.: Supervised, revised and edited the final manuscript S.S.: Supervised, revised and edited the final manuscript A.A.: analysed the data statistically G.S.: Supervised, revised and edited the final manuscript.
Funding
This study was funded by Indian Council of Medical Research (Grant No. 3/1/3/PDF(19)/2019-HRD).
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-09191-z.
References
- 1.Dougherty S, Khorsandi M, Herbst P. Rheumatic heart disease screening: Current concepts and challenges. Ann. Pediatr. Cardiol. 2017;10(1):39. doi: 10.4103/0974-2069.197051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watkins DA, et al. Global, regional, and national burden of rheumatic heart disease, 1990–2015. N. Engl. J. Med. 2017;377(8):713–722. doi: 10.1056/NEJMoa1603693. [DOI] [PubMed] [Google Scholar]
- 3.de Oliveira Martins C, et al. Distinct mitral valve proteomic profiles in rheumatic heart disease and myxomatous degeneration: Clinical medicine insights. Cardiology. 2014;8:CMC-S17622. doi: 10.4137/CMC.S17622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lai Q, et al. Metabolomic profiling of metoprolol-induced cardioprotection in a murine model of acute myocardial ischemia. Biomed. Pharmacother. 2020;124:109820. doi: 10.1016/j.biopha.2020.109820. [DOI] [PubMed] [Google Scholar]
- 5.Khaji A, Kowey PR. Update on atrial fibrillation. Trends Cardiovasc. Med. 2017;27:14–25. doi: 10.1016/j.tcm.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 6.Podzuweit T, Braun W, Müller A, Schaper W. Arrhythmias and infarction in the ischemic pig heart are not mediated by xanthine oxidase-derived free oxygen radicals. Basic Res. Cardiol. 1987;82(5):493–505. doi: 10.1007/BF01907097. [DOI] [PubMed] [Google Scholar]
- 7.Kim YJ, et al. Hypoxanthine causes endothelial dysfunction through oxidative stress-induced apoptosis. Biochem. Biophys. Res. Commun. 2017;482(4):821–827. doi: 10.1016/j.bbrc.2016.11.119. [DOI] [PubMed] [Google Scholar]
- 8.Cheng S, et al. Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation. 2012;125(18):2222–2231. doi: 10.1161/CIRCULATIONAHA.111.067827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng Y, et al. Metabolites of glutamate metabolism are associated with incident cardiovascular events in the PREDIMED PREvencion con DIetaMEDiterranea (PREDIMED) Trial. J. Am. Heart Assoc. 2016;5(9):e003755. doi: 10.1161/JAHA.116.003755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bjermo H, et al. Effects of n-6 PUFAs compared with SFAs on liver fat, lipoproteins, and inflammation in abdominal obesity: A randomized controlled trial. Am. J. Clin. Nutr. 2012;95(5):1003–1012. doi: 10.3945/ajcn.111.030114. [DOI] [PubMed] [Google Scholar]
- 11.Farvid MS, et al. Dietary linoleic acid and risk of coronary heart disease: A systematic review and meta-analysis of prospective cohort studies. Circulation. 2014;130(18):1568–1578. doi: 10.1161/CIRCULATIONAHA.114.010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Souza, R. J. & Anand, S. S. Saturated fat and heart disease (2016). [DOI] [PubMed]
- 13.Aussedat J. Effect of uridine supply on glycogen resynthesis after ischaemia in the isolated perfused rat heart. Cardiovasc. Res. 1983;17:145–151. doi: 10.1093/cvr/17.3.145. [DOI] [PubMed] [Google Scholar]
- 14.Swain JL, Sabina RL, McHale PA, Greeneld JC, Holmes EW. Prolonged myocardial nucleotide depletion after brief ischemia in open-chest dog. Am. J. Physiol. 1982;242:H818–H826. doi: 10.1152/ajpheart.1982.242.5.H818. [DOI] [PubMed] [Google Scholar]
- 15.Rossi A, Olivares J. Basis of pyrimidine nucleotide metabolism in the myocardium. Cardiovasc. Drugs Ther. 1998;12(2):171–177. doi: 10.1023/A:1007752700936. [DOI] [PubMed] [Google Scholar]
- 16.Williams, J. F., Donohoe, J. A., Rosenfeldt, F. L., & Munsch, C. M. Biochemistry and functional roles of orotic acid for support of the infarcted heart during open heart surgery. in Orotic Acid in Cardiology. International Symposium on Orotic Acid and Magnesium Orotate. 1–24. (1991).
- 17.Verdetti J, Aussedat J, Rossi A. Effets de l’administration “in vivo” de divers précurseurs sur les teneursen nucleotides adényliques du coeur de rat. J. Physiol. 1980;76:693–698. [PubMed] [Google Scholar]
- 18.Munsch CM, Williams JF, Rosenfeldt FL. The impaired tolerance of the recently infarcted rat heart to cardioplegic arrest. The protective effect of orotic acid. J. Mol. Cell Cardiol. 1989;21:751–754. doi: 10.1016/0022-2828(89)90713-X. [DOI] [PubMed] [Google Scholar]
- 19.Bailey LE. Orotic acid prevents changes in cardiac sarcolemmal glycoproteins on contractility associated with muscular dystrophy in hamster. Experimentia. 1980;36:94–95. doi: 10.1007/BF02003995. [DOI] [PubMed] [Google Scholar]
- 20.Smolenski RT, et al. Formation and breakdown of uridine in ischemic hearts of rats and human. J. Mol. Cell Cardiol. 1993;25:67–74. doi: 10.1006/jmcc.1993.1008. [DOI] [PubMed] [Google Scholar]
- 21.Wu G, Morris SM., Jr Arginine metabolism: Nitric oxide and beyond. Biochem. J. 1998;336(1):1–7. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molek P, Zmudzki P, Wlodarczyk A, Nessler J, Zalewski J. The shifted balance of arginine metabolites in acute myocardial infarction patients and its clinical relevance. Sci. Rep. 2021;11(1):1–13. doi: 10.1038/s41598-020-80230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Y, et al. Effects of low concentration caprolactam on worker health [in Chinese] Chin. J. Ind. Med. 1997;10(5):290–292. [Google Scholar]
- 24.Gross P, Zapp JA. Biologic activity of ε-caprolactam. CRC Crit. Rev. Toxicol. 1984;13(3):205–216. doi: 10.3109/10408448409003372. [DOI] [PubMed] [Google Scholar]
- 25.Rizvi, Z. A. et al. Immunological and cardio-vascular pathologies associated with SARS-CoV-2 infection in golden Syrian hamster. bioRxiv. (2021).
- 26.Naz S, et al. Development of a liquid chromatography–high resolution mass spectrometry metabolomics method with high specificity for metabolite identification using all ion fragmentation acquisition. Anal. Chem. 2017;89(15):7933–7942. doi: 10.1021/acs.analchem.7b00925. [DOI] [PubMed] [Google Scholar]
- 27.Sadhu S, et al. Gefitinib results in robust host-directed immunity against Salmonella infection through proteo-metabolomic reprogramming. Front. Immunol. 2021;12:888. doi: 10.3389/fimmu.2021.648710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phapale P, Palmer A, Gathungu RM, Kale D, Brügger B, Alexandrov T. Public LC-orbitrap tandem mass spectral library for metabolite identification. J. Proteome Res. 2021;20(4):2089–2097. doi: 10.1021/acs.jproteome.0c00930. [DOI] [PubMed] [Google Scholar]
- 29.Mi S, Yu W, Li J, Liu M, Sang Y, Wang X. Characterization and discrimination of chilli peppers based on multi-element and non-targeted metabolomics analysis. LWT. 2020;131:109742. doi: 10.1016/j.lwt.2020.109742. [DOI] [Google Scholar]
- 30.Mahajan K, Batra A. Obesity in adult Asian Indians-the ideal BMI cut-off. Indian Heart J. 2018;70(1):195. doi: 10.1016/j.ihj.2017.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.