ABSTRACT
Tuberous sclerosis complex (TSC) is a rare multisystem, autosomal dominant neurocutaneous syndrome in which epilepsy is the most common of several neurological and psychiatric manifestations. Around two thirds of patients develop drug‐resistant epilepsy for whom surgical resection of epileptogenic foci is indicated when seizures remain inadequately controlled following trial of two antiseizure medications. The challenge with presurgical and surgical approaches with patients with TSC is overcoming the complexity from the number of tubers and the multiplex epileptogenic network forming the epileptogenic zone. Data suggest that seizure freedom is achieved by 55%–60% of patients, but predictive factors for success have remained elusive, which makes for unconfident selection of surgical candidates. This article presents three different cases as illustrations of the potential challenges faced when assessing the suitability of TSC patients for epilepsy surgery.
Keywords: Epilepsy, Everolimus, Imaging, Prognostics, Surgery, Tuberous sclerosis complex (TSC)
Tuberous sclerosis complex (TSC) is a rare multisystem autosomal dominant genetic disease that causes non‐cancerous tumors to grow in the brain and on other vital organs such as the kidneys, heart, liver, eyes, lungs and skin. A combination of symptoms may include seizures, intellectual disability, developmental delay, behavioral problems, skin abnormalities, lung disease, and kidney disease. TSC is caused by a mutation of either of two genes, TSC1 and TSC2, which code for the proteins hamartin and tuberin, respectively, with TSC2 mutations accounting for the majority and tending to cause more severe symptoms. Drug resistant epilepsy is very common in TSC patients. In some cases, an appropriate study with high field brain MR, scalp EEG and in selected patients Stereo‐EEG might be useful to detect. The epileptogenic tuber and propose a surgical approach to patients. Surgery in TSC patients might give a better long‐term outcome in term of seizure control and cognitive development.
INTRODUCTION
Tuberous sclerosis complex (TSC) is a rare neurocutaneous syndrome (one in 6000–22 000 live births) 1 , 2 for which the major morbidity is usually neurologic, though virtually any organ system can be affected. 3 Approximately 85% of patients carry a pathogenic variant of the TSC1 or TSC2 genes, which cause excessive activation of the “mammalian target of rapamycin” (mTOR) signaling pathway through elevated activity of mTOR. 4 A common signaling node of the mTOR pathway mutations seems to be mTOR complex 1 (mTOR and raptor, its binding partner), 5 which has strong association with the characteristic hamartomas, tuberous sclerosis‐associated neuropsychiatric disorders (TAND), 6 and epilepsy. 4
Epilepsy is the most common (80%–90% of patients) 7 of a large spectrum of neurological and psychiatric manifestations of patients with TSC, which also includes intellectual disability, behavioral abnormalities, and autism spectrum disorder. 8 Deaths are most commonly also from status epilepticus and sudden unexpected death in epilepsy. The type of seizures experienced by patients with TSC are diverse (e.g., focal and generalized motor seizures, epileptic spasms [ES], tonic, atonic, and tonic‐clonic seizures), but 62.5%–73% of patients experience seizures during the first year of life. 9 , 10 The most common seizure‐type during this first year is ES and 75% of these patients become drug resistant—proportionally greater than patients without a history of ES of whom a still sizeable 40% develop drug‐resistant epilepsy. 10 Notably, patients with early‐onset ES also experience a higher degree of intellectual disability than patients experiencing either late‐onset ES or other seizure types. 11 , 12 , 13
Although around two thirds of patients eventually develop drug‐resistant epilepsy, 10 early treatment of epilepsy with antiseizure medications (ASMs) is reported to improve longer term outcomes, 14 and controlled epilepsy is associated with reduced symptoms of autism. The recommended first‐line treatment in early‐onset seizures is vigabatrin, which stops TSC‐related infantile spasms in up to 95% of cases. 8 Combination of vigabatrin with hormonal therapy has been reported to provide even better long‐term outcomes. 1
In general, between 52% and 100% patients with TSC are treated with a combination of two or more ASMs. Combining multiple mechanisms of action (valproic acid, carbamazepine, topiramate, lamotrigine, and vigabatrin) is prudent in most cases to cover the multitude of seizure types. 14 Nevertheless, an overly aggressive approach with ASMs should be avoided as cognitive and behavioral side effects can worsen TAND, which is challenging for most parents. Despite the introduction of targeted drugs for TSC, such as vigabatrin and mTOR inhibitors, we are still unable to predict the patients who can benefit from these treatments, and more than half of patients still present seizures. 8 Cannabidiol is an option as it has been associated with halving seizure frequency compared with placebo in a double‐blind randomized clinical trial. 15
An effective nondrug treatment for epilepsy recommended during the early stages is ketogenic diet (KD), 16 the mechanism for which has been suggested to be mTOR pathway inhibition due to carbohydrate depletion. 17 Vagal nerve stimulation is also a consideration in patients unsuited to epilepsy surgery, with about half reducing their seizure frequency by at least 50%. 18
Resective surgery is an important treatment option that is currently underused. 19 Indication for considering surgery are seizures remaining inadequately controlled following trial of two ASMs. Surgery (resective or palliative) was performed in only 10.7% of patients with focal seizures and in 6.4% of patients with infantile spasms of the 1852 patients with epilepsy in the international “TuberOus SClerosis registry to increase disease Awareness” (TOSCA). 20 In comparison, mTOR inhibitors were prescribed in 7.7% of patients with focal seizures and 5.5% of patients with infantile spasms.
Data on surgical series revealed that seizures freedom can be reached in 55%–60% of patients, with early interventions and accurate localization of the epileptogenic region. 21 , 22 Even if with surgery seizure freedom is not reached, tailored surgical resection of epileptogenic foci was still reported to improve seizure frequency by >90% in 18% of patients. 21 , 23 Stereoencephalography‐directed magnetic resonance‐guided laser interstitial thermal therapy (SEEG‐directed MRgLITT) is a minimally invasive technique in development that shows promise. 24
Planning epilepsy surgery for TSC is challenging due to the presence of multiple lesions (tubers). Furthermore, debate continues on whether the “epileptogenic tuber” includes the surrounding altered cortex. Accurate localization of the epileptogenic network should be achieved to the limit permitted from using standard procedures complicated by multiple tubers. Understandably, the approach varies considerably between centers, depending on the clinical focus, scalp or invasive electroencephalography (EEG), and functional neuroimaging. 21 The current recommendation is to identify the target tuber with consideration to avoiding multifocal and even bilateral resection. 8
The aim of this article is to present three different cases as illustrations of the potential challenges faced when assessing the suitability of TSC patients for epilepsy surgery.
RESULTS
Case 1
A 6‐year‐old boy, born at term with prenatal diagnosis of left ventricular rhabdomyosarcoma and subsequent diagnosis of TSC (TSC1 mutation), was referred for consideration of surgery. During the neonatal period, depigmented macules were noted on his left leg and right side. His overall development was delayed and he showed signs of behavior disorder. Seizures began at 3 months of age with infantile spasms characterized by flexing of both upper arms and trunk and stiffening of lower limbs.
The first brain magnetic resonance imaging (MRI) was suggestive of bilateral cortical tubers and multiple subependymal nodules (Figure 1A,B). Seizures were partially responsive to vigabatrin, adrenocorticotropic hormone (ACTH), and carbamazepine. At 11 months of age, frequency and semiology of seizures had worsened, with daily seizures then characterized by stiffening of bilateral upper and lower limbs and head deviation toward the right. The first video‐EEG monitoring showed an interictal EEG characterized by numerous multifocal abnormalities in the right hemisphere (Figure 1D) and a right focal ictal pattern. These correlated with episodes of generalized hypertonia followed by a cluster of spasms (Figure 1D). MRI confirmed multiple cortical tubers in the left hemisphere. Mild improvement of seizure frequency was observed with valproic acid and steroids, and he was successfully reduced to vigabatrin as monotherapy.
FIGURE 1.
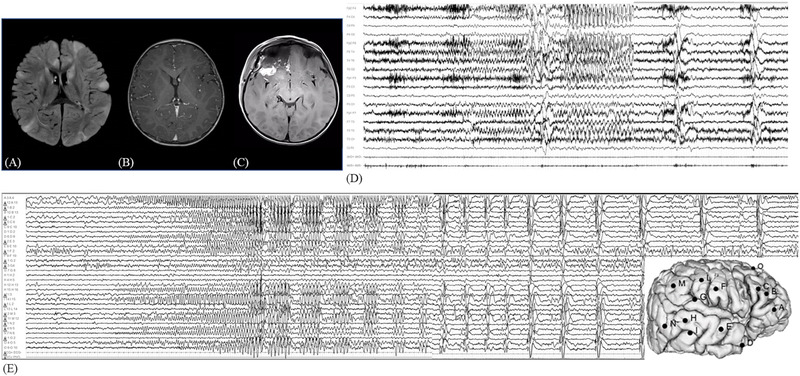
(A) Preoperative brain MRI showing multiple bilateral tubers with hyperintensity on FLAIR‐weighted sequences and subependymal nodules in T1‐sequence (B). (C) Postoperative brain MRI showing the resection area over the right fronto‐basal region. (D) Preoperative video‐EEG recording of right fronto‐temporal seizure with contralateral diffusion and followed by bilateral epileptic spasms. (E) SEEG recording of a focal seizure starting with a low‐voltage fast activity over electrodes B, C, and O, followed by rhythmic theta activity over the same electrodes and over electrode A. Then the ictal discharge involved temporal electrodes (D, E, H, M, and N). This discharge was followed by a cluster of clinically subtle epileptic spasms characterized by pseudorhythmic complexes of spikes, followed by a high‐voltage slow wave, mixed with fast activity evident at electrodes A3–4. EEG, electroencephalography; FLAIR, fluid attenuated inversion recovery; MRI, magnetic resonance imaging; SEEG, stereoelectroencephalography.
Presurgical evaluation with SEEG monitoring at age 2 years showed that the epileptogenic zone (EZ) was in the right fronto‐temporal lobe with early involvement of the orbital region (electrode A) (Figure 1E). Based on these clinical, neurophysiological, and radiological data, a right anterior frontal lobectomy was performed.
Post surgery, the boy remained seizure‐free after 18 months of follow‐up while still undergoing therapy with vigabatrin. He improved both intellectually and behaviorally, and an interictal EEG showed only rare right abnormalities.
Case 2
A 4‐year‐old girl with prenatal diagnosis of cardiac rhabdomyoma, ipomelanotic macules, and angiofibromas has been followed since surgery. Despite a negative family history of epilepsy, genetic testing found deletion of TSC2.
At 1 month of age, she presented with daily right myclonic seizures. Multiple cortical tubers and subependymal nodules were detected in MRI (Figure 2A,B). At first neurological evaluation, the video‐EEG showed a focal seizure—characterized by grimace, palpebral blinking, and oral automatism—that correlated to a left centro‐temporal discharge (Figure 2D). Seizures were partially responsive to levetiracetam, vigabatrin, carbamazepine, and topiramate. At psychological evaluation, she was diagnosed with a psychomotor development delay and deficiency in acquired functional language.
FIGURE 2.
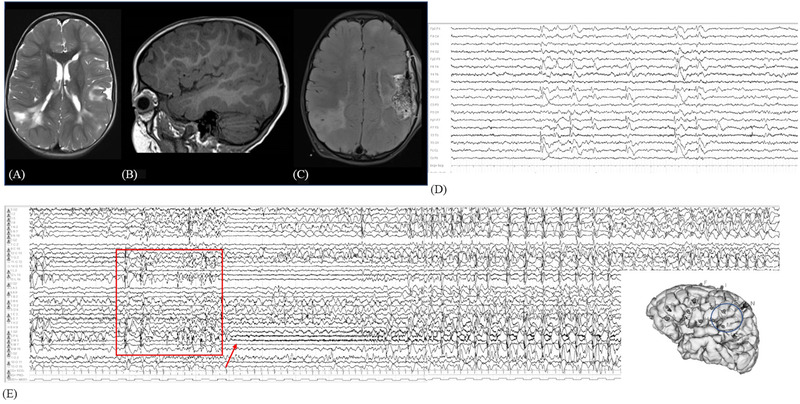
(A) Preoperative axial T2‐weighted brain MRI showing multiple bilateral tubers with hyperintense signal over the fronto‐temporo‐parietal regions. (B) Left T1‐weighted sagittal sequence, showing large hypointense tuber over the posterior suprasylvian region. (C) Postsurgical FLAIR sequence, showing the focal resected area in the suprasylvian posterior operculum, excluding the insula. (D) Interictal video‐EEG with left fronto‐temporal epileptiform abnormalities, with contralateral synchronous diffusion. (E) SEEG seizure recording: rhythmic spike and wave complexes over electrodes P, M, and L (shown in the square) with clinical correlate of right eyelid clonic jerks, then evolving in low‐voltage fast activity over the same electrodes (hypomotor phase, pointed out with the arrow), then again more diffused spike and wave complexes with right face and arm clonic jerks. EEG, electroencephalography; FLAIR, fluid attenuated inversion recovery; MRI, magnetic resonance imaging; SEEG, stereoelectroencephalography.
At 2 years old, she underwent presurgical SEEG monitoring with a left‐side implantation that showed an EZ in opercular (electrodes L, P, and M) and parietal (electrode N) regions (Figure 2E) during seizures characterized by right eye clonic jerks with involvement of the right arm.
At 3 years old, she underwent resection of the right parietal lobe, including the epileptogenic tuber (Figure 2C).
At the most recent follow‐up at 16 months after surgery, she was in Engel II and her EEG showed left central abnormalities with ASMs reduced but not fully withdrawn.
Case 3
An 11‐year‐old girl with prenatal diagnosis of cardiac rhabdomyoma revealed by echocardiography at second trimester (7 months of pregnancy) has been followed post surgery. MRI revealed multiple tubers, primarily in the left parietal lobe (Figure 3A). Genetic analysis found a de novo mutation in TSC1, which confirmed the diagnosis of tuberous sclerosis with neurologic, cardiac, and renal involvement. The neurological symptoms had appeared early as daily focal right hemiclonic seizures and asymmetric spasms (Figure 3C,D). Seizures were treated with vigabatrin, carbamazepine, and ACTH with partial response.
FIGURE 3.
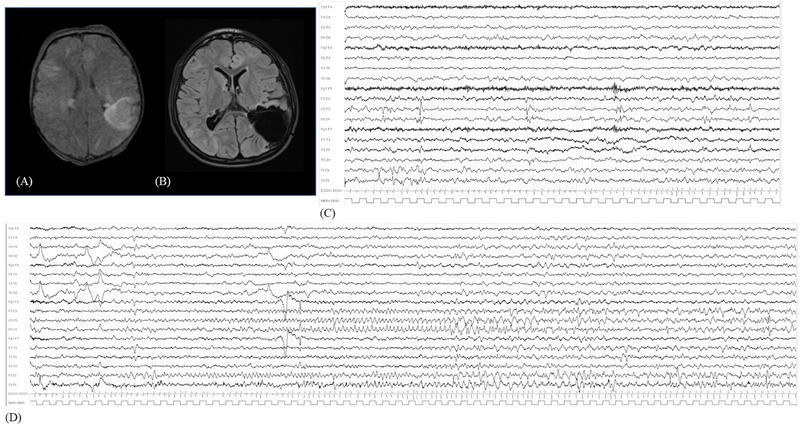
(A) FLAIR‐weighted MRI with bilateral tubers, among which the most prominent over the left parietal lobe. (B) Postsurgical FLAIR sequence showing the parietal resection. (C) Wakefulness interictal video‐EEG with left parietal and vertex epileptiform abnormalities. (D) Video‐EEG recording of left parietal focal seizure, with rhythmic theta activity over C3–P3 and anterior vertex, evolving in focal spike and sharp waves discharge. EEG, electroencephalography; FLAIR, fluid attenuated inversion recovery; MRI, magnetic resonance imaging.
When she was 8 months old, refractory epilepsy meant she underwent a left parietal tuber resection at the Rothschild Hospital in Paris (Figure 3B). After surgery, she was in Engel class I for several years and so drug cessation was attempted when she was 9 years old; however, this was stopped due to considerable interictal abnormalities appearing on the EEG.
At 7 years old, she had a loop recorder implanted after a syncopal event. Cardiac follow‐up revealed the presence of bilateral ventricular rhabdomyoma and confirmed the absence of asystole.
The radiological follow‐up remained invariant. At last neurophysiological follow‐up, the EEG showed an asymmetric activity for the presence of lower activity in the left hemisphere, but she was still seizure‐free. She was in the range of moderate intellectual impairment.
DISCUSSION
Meta‐analysis of the growing published evidence suggest that 55%–60% of postsurgery patients with TSC achieve freedom from seizures. However, the practical development of policy from this statistic is limited, because clear agreement on who are the best candidates for surgical treatment remains unclear, even after assessment with invasive as well as noninvasive tools.
The most widely evaluated tool for identifying epileptogenic tubers has been the concordance between EEG and MRI (both ictal and interictal). 25 , 26 , 27 The potential of EEG–MRI concordance as a predictive feature of postsurgical seizure freedom has been supported by meta‐analysis. 28 Nevertheless, these hypothesis‐generating data were only partially corroborated by subsequent reports and no clear‐cut correlations have yet been found with long‐term follow‐up. 23 , 29
Presurgical evaluation in patients with TSC has commonly also included positron emission tomography (PET) co‐registered with MRI. More complex cases often necessitate invasive monitoring with intracranial electrodes, and bilateral explorations are frequently required to define the EZ and the area to resect. Locating the EZ is now aided by source‐localization techniques, which is expected to improve outcomes in terms of seizure freedom and cognitive performances.
Age at onset on seizure has been evaluated as a predictor of postsurgical outcome in four studies—two retrospective single‐center studies 30 , 31 and two multicenter studies 27 , 30 —and assessed by meta‐analysis. 33 In general, these studies suggested that onset of seizures after the first year of life was associated with a greater proportion of seizure freedom, but support was weak by meta‐analysis. 33 Many studies have also suggested that shorter duration of epilepsy is a determinant of postsurgical seizure freedom, 27 , 30 , 31 , 32 , 34 , 35 though these have not yet been assessed by meta‐analysis. Similarly, better overall postsurgical outcome has been associated with higher IQ prior to surgery. 27 , 35 , 36 , 37
The origin of seizure onset being within the tuber area (rather than perituberal cortex) has received support from recent studies combining strip, grid, and tuber depth electrodes. Consequently, this evidence favors a tuber‐oriented surgical approach, though diverse surgical techniques remain with no consensus on the relative merits for each. Intuitively, resections beyond tuber borders (tuberectomy plus and lobectomy) are likely to result in better seizure control on average.
In the majority of published surgical series, assessments were at only one follow‐up time point, ranging from a few months to several years post surgery. Longitudinal data were available from seven studies, which collectively suggest seizure freedom is achieved by 65%–75% at 1 year, which reduces to 48%–51% after 10 years of follow‐up. In 21 of 28 studies, data regarding the outcome are expressed as average 21 : seizure freedom ranges from 70% at 1 year to 57% at 5 years, which is the longest follow‐up duration reported. 21
The postsurgical follow‐up was quite favorable in our three reported cases, being two of them in Engel class I (Case 1 and Case 3) and one in Engel class II (Case 2). Despite rare, isolated, and short focal seizures in Case 2, we decided to partially decrease ASMs to reduce the burden of treatment: her seizure frequency remained stable.
Brain MRI is challenging in patients with TSC, and individual studies have focused on diverse features (size or localization, 38 presence of calcification and/or cyst‐like appearance, 39 tuber‐center characteristics 40 ), with each potentially associated with epileptogenicity. The strongest predictive factor of seizure freedom after surgery is the co‐occurrence within a single tuber of both bigger size and calcifications, 23 , 29 or without invasive recordings, a single, clear‐cut lesion. 41 As TSC is almost always associated with multiple brain lesions, invasive recordings are more frequently used than with other etiologies in patients with drug‐resistant epilepsies.
The challenge with the presurgical and surgical approaches with patients with TSC and drug‐resistant epilepsy is overcoming the complexity from the number of tubers and the multiplex epileptogenic network forming the EZ. Despite the use of targeted antiepileptogenic drugs, which has reduced the overall number of drug‐resistant patients, more than half of patients with TSC still present persistent seizures and we are still unable to predict reliably the patients who will respond best post surgery.
CONSENT FOR PUBLICATION
Consent for publication was obtained from patients/caregivers.
CONFLICT OF INTEREST
Nicola Specchio has received support from Livanova and Biomarin, and has served as a paid consultant for Livanova. Paolo Curatolo has served as a paid consultant for Novartis. The remaining authors report no conflict of interest.
ACKNOWLEDGMENTS
We thank Dr. David Macari for English text editing and Prof. Olivier Delalande for the support given in the surgical treatment of Case 3.
Specchio N, Pavia GC, de Palma L, De Benedictis A, Pepi C, Conti M, et al. Current role of surgery for tuberous sclerosis complex‐associated epilepsy. Pediatr Investig. 2022;6:16–22. 10.1002/ped4.12312
REFERENCES
- 1. Curatolo P, Moavero R, de Vries PJ. Neurological and neuropsychiatric aspects of tuberous sclerosis complex. Lancet Neurol. 2015;14:733‐745. DOI: 10.1016/s1474-4422(15)00069-1 [DOI] [PubMed] [Google Scholar]
- 2. Ebrahimi‐Fakhari D, Mann LL, Poryo M, Graf N, von Kries R, Heinrich B, et al. Incidence of tuberous sclerosis and age at first diagnosis: new data and emerging trends from a national, prospective surveillance study. Orphanet J Rare Dis. 2018;13:117. DOI: 10.1186/s13023-018-0870-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Amin S, Lux A, Calder N, Laugharne M, Osborne J, O'callaghan F. Causes of mortality in individuals with tuberous sclerosis complex. Dev Med Child Neurol. 2017;59:612‐617. DOI: 10.1111/dmcn.13352 [DOI] [PubMed] [Google Scholar]
- 4. Curatolo P. Mechanistic target of rapamycin (mTOR) in tuberous sclerosis complex‐associated epilepsy. Pediatr Neurol. 2015;52:281‐289. DOI: 10.1016/j.pediatrneurol.2014.10.028 [DOI] [PubMed] [Google Scholar]
- 5. Curatolo P, Moavero R, van Scheppingen J, Aronica E. mTOR dysregulation and tuberous sclerosis‐related epilepsy. Expert Rev Neurother. 2018;18:185‐201. DOI: 10.1080/14737175.2018.1428562 [DOI] [PubMed] [Google Scholar]
- 6. de Vries PJ, Belousova E, Benedik MP, Carter T, Cottin V, Curatolo P, et al. TSC‐associated neuropsychiatric disorders (TAND): findings from the TOSCA natural history study. Orphanet J Rare Dis. 2018;13:157. DOI: 10.1186/s13023-018-0901-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Northrup H, Krueger DA. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013;49:243‐254. DOI: 10.1016/j.pediatrneurol.2013.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Curatolo P, Nabbout R, Lagae L, Aronica E, Ferreira JC, Feucht M, et al. Management of epilepsy associated with tuberous sclerosis complex: updated clinical recommendations. Eur J Paediatr Neurol. 2018;22:738‐748. DOI: 10.1016/j.ejpn.2018.05.006 [DOI] [PubMed] [Google Scholar]
- 9. Davis PE, Filip‐Dhima R, Sideridis G, Peters JM, Au KS, Northrup H, et al. Presentation and diagnosis of tuberous sclerosis complex in infants. Pediatrics. 2017;140:e2016040. DOI: 10.1542/peds.2016-4040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chu‐Shore CJ, Major P, Camposano S, Muzykewicz D, Thiele EA. The natural history of epilepsy in tuberous sclerosis complex. Epilepsia. 2010;51:1236‐1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. French JA, Lawson JA, Yapici Z, Ikeda H, Polster T, Nabbout R, et al. Adjunctive everolimus therapy for treatment‐resistant focal‐onset seizures associated with tuberous sclerosis (EXIST‐3): a phase 3, randomised, double‐blind, placebo‐controlled study. Lancet. 2016;388:2153‐2163. DOI: 10.1016/S0140-6736(16)31419-2 [DOI] [PubMed] [Google Scholar]
- 12. Curatolo P, Franz DN, Lawson JA, Yapici Z, Ikeda H, Polster T, et al. Adjunctive everolimus for children and adolescents with treatment‐refractory seizures associated with tuberous sclerosis complex: post‐hoc analysis of the phase 3 EXIST‐3 trial. Lancet Child Adolesc Health. 2018;2:495‐504. DOI: 10.1016/S2352-4642(18)30099-3 [DOI] [PubMed] [Google Scholar]
- 13. Capal JK, Bernardino‐Cuesta B, Horn PS, Murray D, Byars AW, Bing NM, et al. Influence of seizures on early development in tuberous sclerosis complex. Epilepsy Behav. 2017;70:245‐252. DOI: 10.1016/j.yebeh.2017.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cusmai R, Moavero R, Bombardieri R, Vigevano F, Curatolo P. Long‐term neurological outcome in children with early‐onset epilepsy associated with tuberous sclerosis. Epilepsy Behav. 2011;22:735‐739. DOI: 10.1016/j.yebeh.2011.08.037 [DOI] [PubMed] [Google Scholar]
- 15. Thiele EA, Bebin EM, Bhathal H, Jansen FE, Kotulska K, Lawson JA, et al. Add‐on cannabidiol treatment for drug‐resistant seizures in tuberous sclerosis complex: a placebo‐controlled randomized clinical trial. JAMA Neurol. 2021;78:285‐292. DOI: 10.1001/jamaneurol.2020.4607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kossoff EH, Zupec‐Kania BA, Auvin S, Ballaban‐Gil KR, Christina Bergqvist AG, Blackford R, et al. Optimal clinical management of children receiving dietary therapies for epilepsy: updated recommendations of the International Ketogenic Diet Study Group. Epilepsia Open. 2018;3:175‐192. DOI: 10.1002/epi4.12225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boison D. New insights into the mechanisms of the ketogenic diet. Curr Opin Neurol. 2017;30:187‐192. DOI: 10.1097/WCO.0000000000000432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Major P, Thiele EA. Vagus nerve stimulation for intractable epilepsy in tuberous sclerosis complex. Epilepsy Behav. 2008;13:357‐360. DOI: 10.1016/j.yebeh.2008.04.001 [DOI] [PubMed] [Google Scholar]
- 19. Romanelli P, Verdecchia M, Rodas R, Seri S, Curatolo P. Epilepsy surgery for tuberous sclerosis. Pediatr Neurol. 2004;31:239‐247. DOI: 10.1016/j.pediatrneurol.2004.05.012 [DOI] [PubMed] [Google Scholar]
- 20. Nabbout R, Belousova E, Benedik MP, Carter T, Cottin V, Curatolo P, et al. Epilepsy in tuberous sclerosis complex: findings from the TOSCA study. Epilepsia Open. 2019;4:73‐84. DOI: 10.1002/epi4.12286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Specchio N, Pepi C, de Palma L, Moavero R, De Benedictis A, Marras CE, et al. Surgery for drug‐resistant tuberous sclerosis complex‐associated epilepsy: who, when, and what. Epileptic Disord. 2021;23:53‐73. DOI: 10.1684/epd.2021.1253 [DOI] [PubMed] [Google Scholar]
- 22. Vannicola C, Tassi L, Barba C, Boniver C, Cossu M, de Curtis M, et al. Seizure outcome after epilepsy surgery in tuberous sclerosis complex: results and analysis of predictors from a multicenter study. J Neurol Sci. 2021;427:117506. DOI: 10.1016/j.jns.2021.117506 [DOI] [PubMed] [Google Scholar]
- 23. Liu S, Yu T, Guan Y, Zhang K, Ding P, Chen L, et al. Resective epilepsy surgery in tuberous sclerosis complex: a nationwide multicentre retrospective study from China. Brain. 2020;143:570‐581. DOI: 10.1093/brain/awz411 [DOI] [PubMed] [Google Scholar]
- 24. Stellon MA, Cobourn K, Whitehead MT, Elling N, McClintock W, Oluigbo CO. “Laser and the Tuber”: thermal dynamic and volumetric factors influencing seizure outcomes in pediatric subjects with tuberous sclerosis undergoing stereoencephalography‐directed laser ablation of tubers. Childs Nerv Syst. 2019;35:1333‐1340. DOI: 10.1007/s00381-019-04255-4 [DOI] [PubMed] [Google Scholar]
- 25. Krsek P, Jahodova A, Kyncl M, Kudr M, Komarek V, Jezdik P, et al. Predictors of seizure‐free outcome after epilepsy surgery for pediatric tuberous sclerosis complex. Epilepsia. 2013;54:1913‐1921. DOI: 10.1111/epi.12371 [DOI] [PubMed] [Google Scholar]
- 26. Lachhwani DK, Pestana E, Gupta A, Kotagal P, Bingaman W, Wyllie E. Identification of candidates for epilepsy surgery in patients with tuberous sclerosis. Neurology. 2005;64:1651‐1654. DOI: 10.1212/01.WNL.0000160389.93984.53 [DOI] [PubMed] [Google Scholar]
- 27. Liang S, Zhang J, Yang Z, Zhang S, Cui Z, Cui J, et al. Long‐term outcomes of epilepsy surgery in tuberous sclerosis complex. J Neurol. 2017;264:1146‐1154. DOI: 10.1007/s00415-017-8507-y [DOI] [PubMed] [Google Scholar]
- 28. Zhang K, Hu WH, Zhang C, Meng FG, Chen N, Zhang JG. Predictors of seizure freedom after surgical management of tuberous sclerosis complex: a systematic review and meta‐analysis. Epilepsy Res. 2013;105:377‐383. DOI: 10.1016/j.eplepsyres.2013.02.016 [DOI] [PubMed] [Google Scholar]
- 29. Fallah A, Rodgers SD, Weil AG, Vadera S, Mansouri A, Connolly MB, et al. Resective epilepsy surgery for tuberous sclerosis in children: determining predictors of seizure outcomes in a multicenter retrospective cohort study. Neurosurgery. 2015;77:517‐524; discussion 524. DOI: 10.1227/NEU.0000000000000875 [DOI] [PubMed] [Google Scholar]
- 30. Fohlen M, Taussig D, Ferrand‐Sorbets S, Chipaux M, Dorison N, Delalande O, et al. Refractory epilepsy in preschool children with tuberous sclerosis complex: early surgical treatment and outcome. Seizure. 2018;60:71‐79. DOI: 10.1016/j.seizure.2018.06.005 [DOI] [PubMed] [Google Scholar]
- 31. Arya R, Tenney JR, Horn PS, Greiner HM, Holland KD, Leach JL, et al. Long‐term outcomes of resective epilepsy surgery after invasive presurgical evaluation in children with tuberous sclerosis complex and bilateral multiple lesions. J Neurosurg Pediatr. 2015;15:26‐33. DOI: 10.3171/2014.10.PEDS14107 [DOI] [PubMed] [Google Scholar]
- 32. Madhavan D, Schaffer S, Yankovsky A, Arzimanoglou A, Renaldo F, Zaroff CM, et al. Surgical outcome in tuberous sclerosis complex: a multicenter survey. Epilepsia. 2007;48:1625‐1628. DOI: 10.1111/j.1528-1167.2007.01112.x [DOI] [PubMed] [Google Scholar]
- 33. Zhang K, Hu WH, Zhang C, Meng FG, Chen N, Zhang JG. Predictors of seizure freedom after surgical management of tuberous sclerosis complex: a systematic review and meta‐analysis. Epilepsy Res. 2013;105:377‐383. DOI: 10.1016/j.eplepsyres.2013.02.016 [DOI] [PubMed] [Google Scholar]
- 34. Wu JY, Salamon N, Kirsch HE, Mantle MM, Nagarajan SS, Kurelowech L, et al. Noninvasive testing, early surgery, and seizure freedom in tuberous sclerosis complex. Neurology. 2010;74:392‐398. DOI: 10.1212/WNL.0b013e3181ce5d9e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fallah A, Weil AG, Sur S, Miller I, Jayakar P, Morrison G, et al. Epilepsy surgery related to pediatric brain tumors: Miami Children's Hospital experience. J Neurosurg Pediatr. 2015;16:675‐680. DOI: 10.3171/2015.4.PEDS14476 [DOI] [PubMed] [Google Scholar]
- 36. Jarrar RG, Buchhalter JR, Raffel C. Long‐term outcome of epilepsy surgery in patients with tuberous sclerosis. Neurology. 2004;62:479‐481. DOI: 10.1212/01.wnl.0000106947.18643.1d [DOI] [PubMed] [Google Scholar]
- 37. Jansen FE, van Huffelen AC, Algra A, van Nieuwenhuizen O. Epilepsy surgery in tuberous sclerosis: a systematic review. Epilepsia. 2007;48:1477‐1484. DOI: 10.1111/j.1528-1167.2007.01117.x [DOI] [PubMed] [Google Scholar]
- 38. Ellingson BM, Hirata Y, Yogi A, Karavaeva E, Leu K, Woodworth DC, et al. Topographical distribution of epileptogenic tubers in patients with tuberous sclerosis complex. J Child Neurol. 2016;31:636‐645. DOI: 10.1177/0883073815609151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gallagher A, Chu‐Shore CJ, Montenegro MA, Major P, Costello DJ, Lyczkowski DA, et al. Associations between electroencephalographic and magnetic resonance imaging findings in tuberous sclerosis complex. Epilepsy Res. 2009;87:197‐202. DOI: 10.1016/j.eplepsyres.2009.09.001 [DOI] [PubMed] [Google Scholar]
- 40. Kannan L, Vogrin S, Bailey C, Maixner W, Harvey AS. Centre of epileptogenic tubers generate and propagate seizures in tuberous sclerosis. Brain. 2016;139:2653‐2667. DOI: 10.1093/brain/aww192 [DOI] [PubMed] [Google Scholar]
- 41. Martinez‐Lizana E, Fauser S, Brandt A, Schuler E, Wiegand G, Doostkam S, et al. Long‐term seizure outcome in pediatric patients with focal cortical dysplasia undergoing tailored and standard surgical resections. Seizure. 2018;62:66‐73. DOI: 10.1016/j.seizure.2018.09.021 [DOI] [PubMed] [Google Scholar]


