Abstract
The MIC and MSC (minimum spirocheticidal concentration) and killing rate for Borrelia burgdorferi, the etiological agent of Lyme disease, were assessed for cefodizime in comparison with ceftriaxone, minocycline, azithromycin, roxithromycin, and ciprofloxacin. The range of cefodizime MICs was greater than those of azithromycin and roxithromycin but comparable to those of ceftriaxone and minocycline. The MSCs were 1 to 2 dilutions higher than the MICs of all of the tested compounds. The killing curves of cefodizime and ceftriaxone showed parallel courses. In conclusion, cefodizime exerted an activity comparable to that of ceftriaxone against B. burgdorferi.
Borellia afzelii, B. garinii, and B. burgdorferi sensu stricto are the etiological agents of Lyme disease, causing long-term tissue infections frequently leading to a chronic disease course. Borreliae are primarily transmitted by Ixodes ricinus ticks. In the early stage, the disease is limited to the skin and later may progress toward a multisystem disorder. Recent findings suggest that the three genospecies are associated with different clinical manifestations (i.e., arthritis and neurological symptoms) (9, 14). Although Lyme disease seems to be increasing in the United States and Europe, little information is available on the prevalence of Lyme disease in Italy, although a high number of case reports have been observed in Veneto (3).
Good in vitro activity against borreliae has been reported for penicillins, tetracyclines, macrolides, and some cephalosporins, such as cefotaxime or ceftriaxone (7, 8, 10). Cefodizime is a bactericidal parenteral expanded-spectrum cephalosporin possessing an antibacterial spectrum covering a number of gram-positive and gram-negative bacteria, including most β-lactamase-producing strains (15). Like ceftriaxone, cefodizime can be administered once a day due to its pharmacokinetic and pharmacodynamic properties (2). Clinical experience with cefodizime in the treatment of lower respiratory tract infections and other indications showed good clinical and bacteriological results, with a low incidence of side effects (11). A previous study reported on the in vitro activity of cefodizime against borreliae (12), suggesting that cefodizime may represent an alternative to ceftriaxone in the treatment of Lyme disease. The present investigation provides further information on the in vitro activity of cefodizime against borreliae in comparison to those of ceftriaxone and other reference compounds, including an evaluation of the time course of bacterial killing.
Six isolates of B. burgdorferi sensu lato were used for the experiments. These included three high-passage strains, i.e., B31 (ATCC 35219), belonging to B. burgdorferi sensu stricto; BITS, belonging to B. garinii; and BL3, belonging to B. afzelii (4), and three low-passage isolates, named myo1, a myocardium isolate identified as B. burgdorferi sensu stricto (5), and BL21 and BL31, isolated from erythema migrans skin lesions and belonging to B. garinii and B. afzelii, respectively (13). As these three isolates were subcultured no more than three times, they were considered low-passage strains. Borreliae were grown in BSK II medium at 34°C as previously reported (6).
The antimicrobial agents tested were roxithromycin, cefodizime (Hoechst Marion Roussel, Romainville, France), ceftriaxone, minocycline (Sigma Chemical Co., St. Louis, Mo.), ciprofloxacin, and azithromycin (Mast Diagnostics, Merseyside, United Kingdom). Stock solutions (1 mg/ml) were prepared and stored as recommended by Anhalt and Washington (1). The concentrations tested ranged from 4 to 0.003 μg/ml for azithromycin and roxithromycin and from 16 to 0.007 μg/ml for the other compounds. The MIC was defined as the lowest concentration of antibiotic showing no visible growth, while the MSC (minimal spirocheticidal concentration) was defined as the lowest concentration of antibiotic at which no spirochetes were subcultured. A broth microdilution method was used to determine MICs and MSCs as previously described (6). Briefly, in order to determine MICs, 10 μl of each culture was inoculated into each well, yielding a final concentration of 106 borreliae per ml. MSCs were determined by transferring 50 μl from all of the wells with no visible growth (and from two wells containing actively growing spirochetes as positive controls) into a 24-well culture plate containing 2 ml of antibiotic-free, fresh BSK II medium. The plates were taped and sealed in an airtight nylon bag and incubated for 2 weeks at 34°C. Each determination was performed in duplicate on different days. In order to evaluate the influence of the bacterial inoculum size on cefodizime activity, a culture of strain B31 was adjusted to yield final inocula of 5 × 104, 5 × 105, and 5 × 106 motile cells/ml and the MICs and MSCs were determined as already described.
As reported in Table 1, cefodizime exerted good bacteriostatic activity against all of the different genospecies of borreliae: the cefodizine MIC was 0.06 μg/ml for five of the six strains and for only one B. afzelii isolate was the cefodizime MIC 0.12 μg/ml. Ceftriaxone showed similar activity with MICs of 0.03 (n = 3 strains) and 0.06 (n = 3 strains) μg/ml. The macrolides azithromycin and roxithromycin had MIC ranges of ≤0.003 to 0.007 and ≤0.003 to 0.03 μg/ml, respectively, without noticeable differences among the genospecies. Minocycline had decreased activity (MIC range, 0.03 to 0.25 μg/ml), and ciprofloxacin was the least active agent against borreliae: the MICs ranged from 0.03 to 4 μg/ml.
TABLE 1.
In vitro susceptibilities of borrelia strains to cefodizime and selected agents
| Test agent | MIC (μg/ml) for:
|
|||||
|---|---|---|---|---|---|---|
|
B. burgdorferi sensu stricto
|
B. garinii
|
B. afzelii
|
||||
| B31 | myo1 | BITS | BL21 | BL3 | BL31 | |
| Cefodizime | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 | 0.12 |
| Ceftriaxone | 0.03 | 0.03 | 0.03–0.06 | 0.03 | 0.06 | 0.03–0.06 |
| Ciprofloxacin | 0.125–0.5 | 0.03–0.25 | 1 | 0.06–2 | 2–4 | 2–4 |
| Minocycline | 0.12–0.25 | 0.03–0.06 | 0.03 | 0.03–0.06 | 0.03–0.12 | 0.03–0.06 |
| Azithromycin | ≤0.003 | ≤0.003 | ≤0.003 | ≤0.003–0.007 | ≤0.003 | ≤0.003 |
| Roxithromycin | 0.015 | ≤0.003 | 0.015 | 0.03 | 0.015 | 0.015 |
Cephalosporins exerted high bactericidal activity against borreliae (Table 2). All of the cefodizime MSCs (six of six) were within a onefold dilution higher than the MICs, as was observed with four of the six strains for ceftriaxone. Macrolides also showed bactericidal activity: azithromycin MSCs were equal to or within a onefold dilution of to the MICs, while the MSCs of roxithromycin were twofold higher than the MICs for three strains. Minocycline and ciprofloxacin MSC/MIC ratios were frequently higher than 8, indicating a lower bactericidal activity of these compounds against borreliae. No difference in the MICs or MSCs of any of the antimicrobial agents studied was observed with respect to the number of passages (high- or low-passage strains).
TABLE 2.
Bactericidal activities of cefodizime and selected agents against borrelia strains
| Test agent | MBC (μg/ml) for:
|
|||||
|---|---|---|---|---|---|---|
|
B. burgdorferi sensu stricto
|
B. garinii
|
B. afzelii
|
||||
| B31 | myo1 | BITS | BL21 | BL3 | BL31 | |
| Cefodizime | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.25 |
| Ceftriaxone | 0.06 | 0.03–0.06 | 0.06 | 0.12 | 0.06 | 0.06–0.12 |
| Ciprofloxacin | 4 | 0.06–0.25 | 8–16 | 4–8 | 4–8 | 8 |
| Minocycline | 2–4 | 0.25 | 1–2 | 1–2 | 0.5–2 | 0.25–1 |
| Azithromycin | 0.007 | ≤0.003 | ≤0.003 | 0.015 | ≤0.003 | ≤0.003 |
| Roxithromycin | 0.06–0.12 | 0.015 | 0.03 | 0.25–0.5 | 0.03 | 0.03–0.06 |
Our results parallel the previous report on cefodizime activity against borreliae (12), even if the MICs and MSCs in the present study were twofold lower, and no difference in cefodizime activity was recorded among the three genospecies. As Peter and Bretz (12) used a broth macrodilution method, this feature may partly account for the slightly different values.
There was no effect of bacterial inoculum size on either the MIC or MSC of cefodizime against the reference strain B. burgdorferi B31 at inocula ranging from 5 × 104 to 5 × 106 spirochetes/ml. It might be speculated that cefodizime could retain its bactericidal activity even in the presence of a high in vivo count of borreliae, but further studies on the subject should be conducted before any conclusion can be drawn.
The bactericidal activity of cefodizime compared to that of ceftriaxone was also evaluated in a time course study. B. burgdorferi B31 was used to determine generation times in BSK II medium and rates of killing by ceftriaxone and cefodizime at concentrations equal to two and four times the MIC. Polystyrene tubes filled with 6 ml of antibiotic-containing BSK II medium and one antibiotic-free control tube were inoculated with 600 μl of a growing culture adjusted to yield a final inoculum of ca. 106 cells/ml. Tubes were incubated at 34°C.
A broth microdilution method was used to evaluate the number of survivors. A 100-μl of volume of BSK II medium was dispensed into each well of a microtiter tray in duplicate rows. At 0, 12, 24, and 72 h, the borrelia-containing tubes were gently vortexed and 100 μl from each tube was dispensed into the first tray well, carefully mixed by repeated pipetting, and subsequently diluted twofold in each well. Microtiter trays were sealed with sterile polyester adhesive film (Sigma) and incubated at 34°C. After a week, the wells were examined by dark-field microscopy and the number of surviving spirochetes was determined.
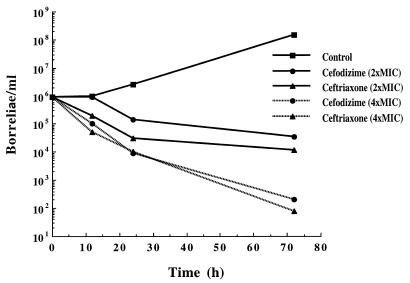
According to our results, a bacterial inoculum of 106 borreliae/ml was selected for the time-kill studies with cefodizime and ceftriaxone at concentrations of two and four times the MIC. Both cephalosporins reached a 2-log10 reduction of the initial inoculum with a concentration of twice the MIC and a more-than-3-log10 reduction with a concentration of four times the MIC after 72 h, with minor differences in the time course (Fig. 1).
FIG. 1.
Time-kill curves for B. burgdorferi sensu stricto B31. Concentrations of cefodizime and ceftriaxone were two and four times the MIC.
To our knowledge, this is the first report on the bactericidal activity of cefodizime against borreliae including a time course evaluation. Cefodizime proved to be effective in vitro against the reference strains and the clinical isolates of the three different genospecies of borreliae. As far as in vitro activity is concerned, no significant difference from ceftriaxone was observed. In conclusion, our results support the potential role of cefodizime in the treatment of Lyme disease.
REFERENCES
- 1.Anhalt J P, Washington J A., II . Preparation and storage of antimicrobial solutions. In: Lennette E H, Balows A, Hausler W J Jr, Shadomy H J, editors. Manual of clinical microbiology. 4th ed. Washington, D.C.: American Society for Microbiology; 1985. pp. 1019–1020. [Google Scholar]
- 2.Barradell L B, Brogden R N. Cefodizime: a review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs. 1992;44:800–834. doi: 10.2165/00003495-199244050-00008. [DOI] [PubMed] [Google Scholar]
- 3.Caruso G, Zasio C, Monardini V, Benedetti P, Murgia R, Cinco M. Lyme borreliosis in Belluno, North-East Italy. Alpe Adria. Microbiol J. 1995;43:219–220. [Google Scholar]
- 4.Cinco M, Costantini C, Wilske B, Graziosi G, Trevisan G, Florian F. Use of polymerase chain reaction and specific monoclonal antibodies as rapid method to recognize Borrelia burgdorferi sensu stricto, B. garinii and B. afzelii among Italian isolates of B. burgdorferi. Med Microbiol Immunol. 1994;183:307–313. doi: 10.1007/BF00196681. [DOI] [PubMed] [Google Scholar]
- 5.Cinco M, Lardieri G, Salvi A, Camerini F, Trevisan G. Isolation of Borrelia burgdorferi from myocardium. Lancet. 1993;342:490. doi: 10.1016/0140-6736(93)91612-p. [DOI] [PubMed] [Google Scholar]
- 6.Cinco M, Padovan D, Stinco G, Trevisan G. In vitro activity of rokitamycin, a new macrolide, against Borrelia burgdorferi. Antimicrob Agents Chemother. 1995;39:1185–1186. doi: 10.1128/aac.39.5.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson R C, Kodner C, Russel M. In vitro and in vivo susceptibility of the Lyme disease spirochete Borrelia burgdorferi to four antimicrobial agents. Antimicrob Agents Chemother. 1987;31:164–167. doi: 10.1128/aac.31.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson R C, Kodner B C, Jurkovich P J, Collins J J. Comparative in vitro susceptibilities of the Lyme disease spirochete Borrelia burgdorferi to cefuroxime and other antimicrobial agents. Antimicrob Agents Chemother. 1990;34:2133–2136. doi: 10.1128/aac.34.11.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kramer M D, Wallich R, Schaible U E, Zimmer G, Simon M M. Die Borrelia burgdorferi Infektion. Hautarzt. 1990;41:648–657. [PubMed] [Google Scholar]
- 10.Levin J M, Nelson J A, Segreti J, Harrison B, Benson C A, Strle F. In vitro susceptibility of Borrelia burgdorferi to 11 antimicrobial agents. Antimicrob Agents Chemother. 1993;37:1444–1446. doi: 10.1128/aac.37.7.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Periti P. Cefodizima: farmacologia clinica e chemioterapia. Farm Ter. 1995;XII:65–115. [Google Scholar]
- 12.Peter O, Bretz A G. In vitro susceptibility of B. burgdorferi, B. garinii and B. afzelii to 7 antimicrobial agents. Can J Infect Dis. 1995;6(Suppl. C):473C. [Google Scholar]
- 13.Postic D, Assous M V, Grimont P A D, Baranton G. Diversity of Borrelia burgdorferi sensu lato evidenced by restriction fragment length polymorphism of rrf (5S)-rrl (23S) intergenic spacer amplicons. Int J Syst Bacteriol. 1994;44:743–752. doi: 10.1099/00207713-44-4-743. [DOI] [PubMed] [Google Scholar]
- 14.Preac-Mursic V, Weber K, Pfister H W, Wilske B, Gross B, Baumann A, Prokpo J. Survival of Borrelia burgdorferi in antibiotically treated patients with Lyme borreliosis. Infection. 1989;17:355–359. doi: 10.1007/BF01645543. [DOI] [PubMed] [Google Scholar]
- 15.Soussy C J, Chanal M, Kitzis M D. The in vitro activity of cefodizime: a review. J Antimicrob Chemother. 1990;26(Suppl. C):13–21. doi: 10.1093/jac/26.suppl_c.13. [DOI] [PubMed] [Google Scholar]