Abstract
Endometriosis, a common chronic inflammatory disease in female of reproductive age, is closely related to patient symptoms and fertility. Because of its high contrast resolution and objectivity, MRI can contribute to the early and accurate diagnosis of ovarian endometriotic cysts and deeply infiltrating endometriosis without the need for any invasive procedure or radiation exposure. The ovaries, which are the most frequent site of endometriosis, can be afflicted by multiple related conditions and diseases. For the diagnosis of deeply infiltrating endometriosis and secondary adhesions among pelvic organs, fibrosis around the ectopic endometrial gland is usually found as a T2 hypointense lesion. This review summarizes the MRI findings obtained for ovarian endometriotic cysts and their physiologically and pathologically related conditions. This article also includes the key imaging findings of deeply infiltrating endometriosis.
Keywords: Deeply infiltrating endometriosis, Endometriosis, MRI
INTRODUCTION
Endometriosis, a chronic inflammatory disease involving the growth of endometrium-like epithelium and stromal cells outside the uterus [1], is a common gynecological disease found in 2%–8% of the general population [2]. More than 80% of affected patients are of reproductive age [3]. Female with this disease usually present with pelvic pain, dysmenorrhea, and dyspareunia, all of which can strongly affect quality of life [3,4]. It is also closely related to infertility [3]. Early diagnosis and precise evaluation of the spread of lesions and severity of adhesions before and after treatment are important for formulating a treatment strategy.
Three main entities of pelvic endometriosis have been identified: ovarian endometriotic cysts, superficial peritoneal endometriosis, and deep infiltrating endometriosis (DIE) [5]. Among them, magnetic resonance imaging (MRI), which plays an important role in the diagnosis of ovarian endometriotic cysts and DIE, is also useful for detecting characteristic appearances of various conditions or diseases associated with ovarian endometriotic cysts: decidual change induced by pregnancy, changes after hormonal treatment, and endometriosis-related tumors [5,6,7,8,9]. Regarding DIE, the limited diagnostic ability of physical examinations, such as digital vaginal examination by gynecologists, has been reported (36% accuracy, 46%–54% specificity) [5]. Moreover, MRI can assist gynecologists in evaluating the precise location of the endometriosis and severity of adhesions, which are strongly related to clinical symptoms such as dysmenorrhea, dyspareunia, pelvic pain, and infertility [5].
This review article presents a summary of the MRI findings of ovarian endometriotic cysts and their physiologically and pathologically related conditions. We also reviewed the key points of MRI in the diagnosis of DIE at different anatomical sites (Table 1).
Table 1. Key MRI Findings of Ovarian Endometriotic Cyst, DIE, or Other Related Diseases.
| MR/CT Sign | MRI/CT Findings | References | ||
|---|---|---|---|---|
| Ovary | Ovarian endometrioma | T1-high signal multiplicity | Multiple high signal cysts on T1WI | [31] |
| T2-Shading | Marked T2 shortening or gradations on T2WI | [31,33] | ||
| T2 dark spot sign | Discrete well-defined markedly hypointense foci within the cyst on T2WI | [32] | ||
| Decidualization | Linear, broad-based nodular, or polypoid structures with high SI similar to placental tissue on T2WI | [43,44] | ||
| Polypoid endometriosis | Polypoid mass, slightly high SI similar to endometrium on T2WI | [46,47,48] | ||
| Punctate high signal foci on T1WI | ||||
| Peripheral rim by fibrous tissue on T2WI | ||||
| Strong enhancement on contrast enhanced-T1WI | ||||
| Small cysts on T2WI | ||||
| EAOC | Emergence of enhanced mural nodules within the ovarian endometrioma | [7,57,58] | ||
| Increased tumor size (> 9 cm) | ||||
| (suggestive) Dissapearance of shading on T2WI | [59] | |||
| DIE | Hypointense nodular lesions (T1/T2WI) | [5,60,61,62] | ||
| Soft tissue thickening with irregular, indistinct, or stellate margins (T1/T2WI) | ||||
| Posterior cul-de-sac obliteration (pouch of Douglas, rectouterine/ retrovaginal space) | Retroflexed uterus | [69] | ||
| Elevated posterior vaginal fornix on T1/T2WI | ||||
| Intestinal tethering or tethered appearance of rectum in the direction of the uterus on T2WI | [69] | |||
| Faint strands between the uterus and intestine on T2WI | [69] | |||
| Fibrotic plaques or nodules covering the serosal surface of the uterus on T2WI | [69,70] | |||
| Retrouterine fibrous mass on T2WI | [69,70] | |||
| Intraperitoneal fluid displacement | [70] | |||
| Torus uterinus, USL | T2-low intensity thickening or mass with regular or irregular margins on MRI | [63] | ||
| Ovary | Kissing ovary | Bilateral ovaries are located in the posterior cul-de-sac or at the cornua of the uterus on MRI | [75] | |
| Round ligament | Thickened and irregular with a nodular appearance on MRI | [77] | ||
| Rectosigmoid colon | ‘Fan shaped’/‘mushroom cap’ | Focal thickening of the rectal wall as an umbrella-like head of a mushroom on MRI | [64,81] | |
| Bladder endometriosis and vesicouterine space | Hypointense nodular formation on T1/T2WI | [60,85] | ||
| Extra-pelvic endomtriosis | Abdominal wall endometriosis | On CT, homogeneous density | [93] | |
| Gorgon’ sign | Linear infiltration irradiating peripherally from a central soft tissue nodule on CT | [93] | ||
| On MRI, hypointense solid mass on T2WI with/without hyperintense hemorrhagic cysts on T1WI | [97] | |||
| Diaphragmatic endometriosis | Hyperintense nodule on T1WI, mostly right side | [100] |
DIE = deeply infiltrating endometriosis, EAOC = endometriosis associated ovarian carcinoma, SI = signal intensity, T1WI = T1-weighted image, T2WI = T2-weighted image, US = ultrasonography, USL = uterosacral ligament
Etiologies
The exact etiology of endometriosis remains unclear. The most prevalent theory is the retrograde menstruation phenomenon [3,10]. Sampson [11] proposed that the reflux of endometrial tissue through the fallopian tube by retrograde menstruation results in implantation and growth on the peritoneal surface. Many supporting observations are presented below. Endometriotic lesions are most commonly found in the area of the tubal ostia and the deepest portion of the Douglas pouch. Endometriosis is more common in female with early menarche and heavy or long menstrual flow [3]. Other theories include endometrial stem cell implantation, Mullerian remnant abnormalities that explain cul-de-sac endometriosis, and coelomic metaplasia. Mullerian remnant abnormalities of aberrant migration of the Mullerian duct into the posterior pelvic floor and coelomic metaplasia explain ovarian endometriosis by metaplastic changes of the coelomic epithelium into the endometrium [10]. Recent reports have implicated the ampullary portion of the fallopian tube as the origin of endometriosis, which is similar to that of high-grade serous carcinoma [12]. Other factors, such as female hormones and inflammation, are also considered to be deeply associated with the pathogenesis of endometriosis [10]. Estradiol promotes the growth of ectopic endometrial tissues. The presence of ectopic tissue causes inflammation with overproduction of prostaglandins, cytokines, and chemokines, consequently causing pain, fibrosis, and adhesion formation [10]. Research elucidating the condition’s putative association with genetic factors has also progressed, with the WNT4, CDKN2B-AS1, and GREB1 genes being strong targets for additional studies investigating endometriosis [10]. These genetic factors contribute to approximately half of all conditions carrying the risk of endometriosis [13].
Classifications
Endometriosis is divided into three types: ovarian endometriotic cysts, superficial peritoneal endometriosis, and DIE. Among these, superficial peritoneal endometriosis is the least severe. In this type, endometrium-like tissue attaches to the peritoneum. Most cases are occult on imaging. The ovaries are the most frequent site of ectopic endometrial tissue, and ovarian endometriotic cysts occur. DIE is defined histologically as endometriosis infiltrating the peritoneum to a depth > 5 mm. The consensus of the Society of Abdominal Radiology Disease Focused Panel on endometriosis is that most endometriotic lesions visible on MRI are DIE [14,15]. Several classification and staging systems are used clinically, depending on the purpose, such as the revised American Society for Reproductive Medicine (rASRM) classification, ENZIAN classification, endometriosis fertility index, and American Association of Gynecological Laparoscopists classification [16]. Among them, rASRM classification is the most widely used. It is useful for physicians to report the severity of endometriosis (stages I–IV) in patients in simple terms [16,17]. This scoring system is based on the summation of values assigned to the following items: the size of the endometriosis lesions in the peritoneum and ovaries, adhesion on the ovaries and fallopian tubes, and partial or complete posterior cul-de-sac obliteration. The rASRM classification does not incorporate DIE infiltration into the retroperitoneal structures. As a supplement to the rASRM, the ENZIAN classification was developed to classify DIE [17]. Di Paola et al. [18] reported an excellent correlation between MRI-based ENZIAN scores and histopathological ENZIAN scores, particularly for rectovaginal septum, uterosacral ligament (USL), and rectosigmoid locations.
Clinical Symptoms
Typical symptoms attributed to endometriosis are dysmenorrhea, dyspareunia (which can be related to the menstrual cycle), and infertility [3,4]. Although DIE is known to be closely related to these symptoms, ovarian and superficial peritoneal endometriosis are often asymptomatic [19]. Deeper endometriosis (greater than 10 mm) is associated with pain [3,19,20]. Dysmenorrhea and dyspareunia are the two most frequent symptoms of pain caused by endometriosis [10]. Dysmenorrhea is related to excessive intraperitoneal prostaglandin production by the ectopic endometrium, causing myometrial hypertonus and secondary ischemia [21]. Treatments were considered for patients with the symptoms presented above. Surgery is unnecessary if clear therapeutic benefits of the intervention cannot be found [10]. Overall, medical therapy with oral contraceptives (OCs) and progestins enables satisfactory long-term pain control in approximately two-thirds of symptomatic female [22]. In addition, OCs dramatically reduce the rate of recurrence of endometriotic cysts without damaging future fertility [22]. Regarding its relationship with infertility treatment, the benefit of surgery is limited, even in cases of advanced endometriosis [22].
Role of MRI and Optimal MRI Protocol
Laparoscopy with histological confirmation of ectopic endometrial tissue is the gold standard for the diagnosis of endometriosis [2]. Ultrasonography (US) is the first-line imaging modality for the assessment of pelvic endometriosis because of its easy accessibility, low degree of invasiveness, and cost-effectiveness [23]. Nevertheless, it is constrained by limitations such as limited field of view and operator dependence. Today, MRI is recognized as a valuable tool for diagnosis and presurgical planning. Although it is more expensive and time consuming, MRI has advantages that US does not: it is more objective, and the images can cover a large field-of-view with multiple directions [24]. Due to its excellent contrast resolution, the combination of several MR sequences can provide detailed information about the locations and histological characteristics of endometriosis.
The European Society of Urogenital Radiology provides recommendations on the optimal MRI protocol and guidelines for the diagnosis of pelvic endometriosis based on evidence from the literature and the consensus of expert opinion (Table 2) [9]. For patient preparation, 3–6 hours of fasting and bladder emptying one-hour before the examination are recommended [9]. A moderately distended bladder is preferable because an overly distended bladder might lead to oversight in detecting small endometriosis of the bladder [9]. Regarding the timing of the menstrual cycle, Botterill et al. [25] found no significant difference between menstruating and non-menstruating MRI scans for the evaluation of the extent or severity of endometriosis. An anti-peristaltic agent is recommended to prevent bowel motion artifacts, unless contraindicated [9]. Vaginal or rectal opacification by a gel is regarded as ‘optional’ [9]. Distention of the vagina or rectum by a gel might make pelvic endometriosis easier to find, especially for Douglas pouch or rectosigmoid colon endometriosis. Fiaschetti et al. [26] reported improved sensitivity for the detection of DIE with vaginal opacification, although Bazot et al. [27] found no significant difference. The invasiveness of the procedure and additional time necessary for patient preparation are also hurdles to be overcome.
Table 2. Optimal MRI Protocols for the Diagnosis of Endometriosis.
| Patiens’ preparation | - 3–6 hours of fasting |
| - Bladder emptying one-hour before tde examination | |
| - Use of anti-peristaltic agent (unless contraindicated) | |
| - (optional) vaginal/rectal opacification by gel | |
| T2WI | Axial, sagittal and oblique axial images (optional) 3D-T2WI |
| T1WI | Witd and witdout fat saturation images |
| DWI, contrast enhanced T1WI | Not recommended for ovarian ednometrioma/DIE, necessary in case of suspected ovarian malignancy |
The table shows the suggested protocols for the diagnosis of endometriosis and related diseases based on the European Society of Urogenital Radiology guideline [9]. DIE = deeply infiltrating endometriosis, DWI = diffusion weighted image, T1WI = T1-weighted image, T2WI = T2-weighted image, 3D = three-dimenational
As a standard MR protocol, T2-weighted images (WIs) (axial, sagittal, and oblique axial) and T1WI (with and without fat suppression), as well as single-shot-fast-spin-echo images were considered. Oblique axial T2WI is useful for assessing USL endometriosis [28]. Contrast-enhanced T1WI, diffusion-weighted imaging (DWI), and susceptibility-weighted imaging are not recommended for the evaluation of ovarian endometriotic cysts and DIE [9]. Apparent diffusion coefficient (ADC) values were also not regarded as useful because no significant difference was found among various pelvic lesions, either with or without endometriosis [29]. However, contrast enhancement and DWI are recommended for patients with suspected ovarian malignancy [29]. Relevant details are presented below.
MRI Findings of Ovarian Endometriotic Cyst
Ovarian Endometriotic Cyst
The most frequent site of ectopic endometrial implantation is the ovary, where repeated internal hemorrhage leaves a large hemorrhagic cavity [4]. The presence of ovarian endometriotic cysts is closely related to an increased number of DIE lesions. It is thought to be a marker for DIE severity [30].
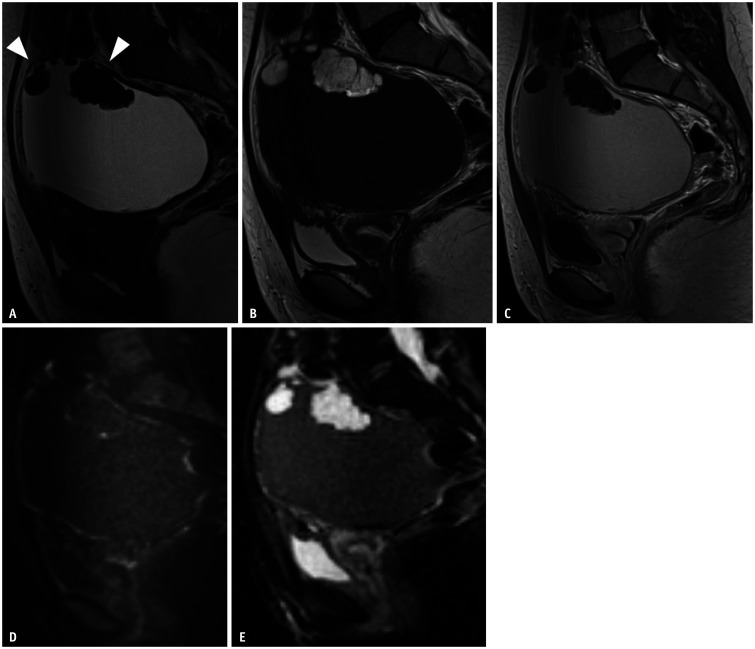
On MRI, the key imaging findings for diagnosing ovarian endometriotic cysts are T1-high signal multiplicity, T2-shading, and the T2 dark spot sign (Fig. 1) [31,32]. Adhesion to the surrounding anatomical structures is also a key finding.
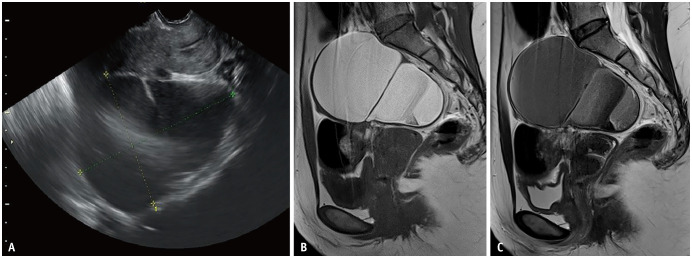
Fig. 1. Ovarian endometriotic cyst in a 42-year-old female with abdominal pain.
A. Transvaginal ultrasound showing a low-echoic cystic lesion with a mixed hyperechoic area posterior to the uterus. A small septum is observed in the middle of the lesion. B, C. Sagittal T1WI (B) and sagittal T2WI (C). On MRI, an ovarian endometriotic cyst is shown as a hyperintensity on T1WI (B), and heterogeneous low signal intensity in the form of shading on T2WI (C). WI = weighted image
Multiple hyperintense cysts on T1WI called ‘multiplicity’, which result from repeated bleeding and formation of new blood locules, is a characteristic finding [31]. Defined as decreased signal intensity (SI) on T2WI that might vary from faint, layered signal loss to complete signal loss, ‘T2-shading’ reflects chronic repeated bleeding with high concentrations of iron and protein within the cyst [31,33]. The presence of ‘shading’ is useful in detecting endometriotic cysts because of its high sensitivity [32]. Magnetism-susceptible inhomogeneity based on iron concentration and blood viscosity causes marked T2 shortening or gradations on T2WI [34,35]. Signal intensities on T2WI vary depending on blood age and content. The overall diagnostic value obtained using the two image findings described above is a sensitivity of 90%, specificity of 98%, and accuracy of 96% [31]. It is noteworthy that the T2WI low SI is visible [32,36] for other cystic lesions such as hemorrhagic functional cysts or ovarian neoplasms (e.g., mucinous tumor, serous/seromucinous borderline tumor, and cancer) [32,36]. In addition, the highly specific ‘T2 dark spot sign’ (93%) is an important MR finding to diagnose ovarian endometriotic cysts, especially for differentiation of functional ovarian hemorrhages [32]. T2 dark spots were defined as discrete well-defined markedly hypointense foci within the cyst on T2WI, which are often linear, punctate, or oval in shape [32]. It is speculated that this sign represents the presence of chronic retracted blood clots with high concentrations of protein and hemosiderin [32].
The Rupture of Endometriotic Cyst
Ruptures can occur in approximately 3% of endometriotic cysts in female of reproductive age [37]. The cause of acute rupture remains unknown, but the relationship between pregnancy, trauma, and infection is inferred [37]. Patients’ symptoms include sudden-onset abdominal pain, nausea, and severe peritonitis, which are similar to those of ovarian hemorrhage or tubo-ovarian abscess (TOA). The extreme increase in serum CA-125 levels might be a clue for the differentiation of ruptured endometriotic cysts from TOA [38]. Regarding MRI findings, a thickened and enhanced cyst wall with hyperintense ascites and strong peritoneal enhancement were observed on contrast-enhanced T1WI with fat saturation [38,39]. However, these imaging findings overlap with those of TOA or ruptured corpus luteal cysts. Moreover, rASRM of stage III–IV endometriosis entails an increased risk of TOA [40]. Therefore, comprehensive decisions based on clinical and imaging findings are necessary for the proper diagnosis of the disease (Fig. 2).
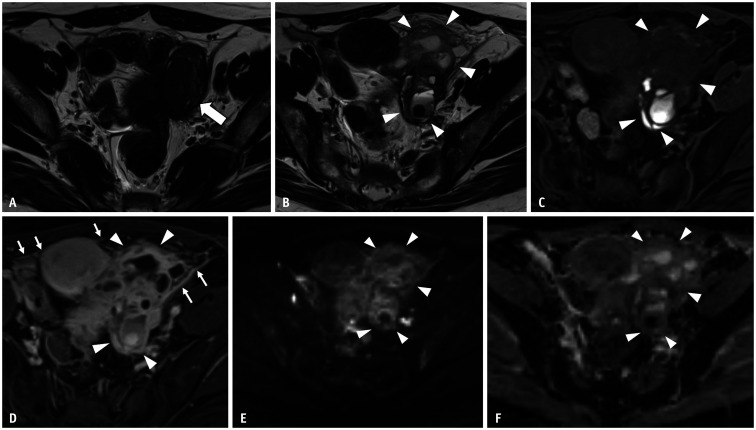
Fig. 2. Rupture of an ovarian endometriotic cyst in a 39-year-old female with abdominal pain and fever.
A. Axial T2WI 2 months before the rupture of the ovarian endometriotic cyst. A lesion with low signal intensity (arrow) is observed just beside the uterus. This is an ovarian endometriotic cyst with shading. B, C. MRI images obtained 2 months after (A) (B, axial T2WI; C, axial T1WI with fat saturation). On axial T2WI, the left ovarian endometriotic cyst (arrowheads) is now visualized as multiple loculated cysts with fluid–fluid levels and mixed signal intensities on T1 and T2WI, signifying a recent hemorrhage. The anterior wall of the cyst is irregular, and hyperintense fluid is observed around the cyst (arrowheads). These findings suggest rupture of an ovarian endometriotic cyst. D. Axial contrast-enhanced T1WI with fat saturation, irregularly thickened and enhanced cysts (arrowheads), and strong peritoneal enhancement (arrows) are observed. Tubo-ovarian abscess caused by infection after endometriotic cyst rupture was suggested and confirmed postoperatively. E, F. Axial DWI (b = 1000 s/mm2) (E); Axial apparent diffusion coefficient map (F). No area (arrowheads) shows restricted diffusion within the tumor, probably because the MRI image was obtained shortly after the rupture of the tumor. WI = weighted image
Change in Hormonal Therapy
Hormonal therapy, such as gonadotropin-releasing hormone (GnRH) agonists, is a choice of treatment for endometriosis. The effects of hormonal therapy on ovarian endometriotic cysts are not uniform. Sugimura et al. [41] reported a difference in effectiveness depending on MRI findings. In their results, a good treatment response was observed in patients without T2-shading and multiplicity (Fig. 3) [41]. Results of multiple logistic analyses indicated that T2-shading was the most important factor related to poor treatment response. A probable reason for the lower effectiveness in cases with T2-shading is that an endometrial tissue lining might be almost absent because of the prolonged pressure of retained nonstromal blood within the cyst [41].
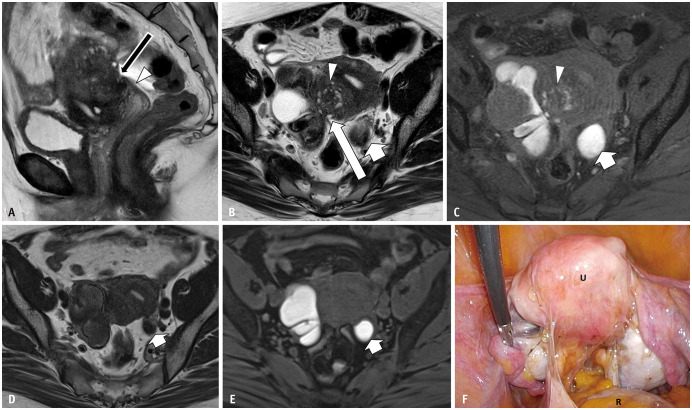
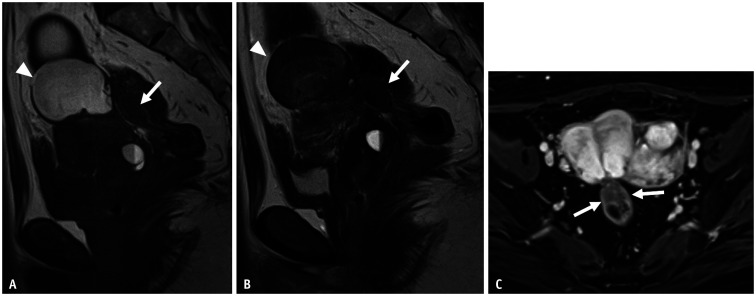
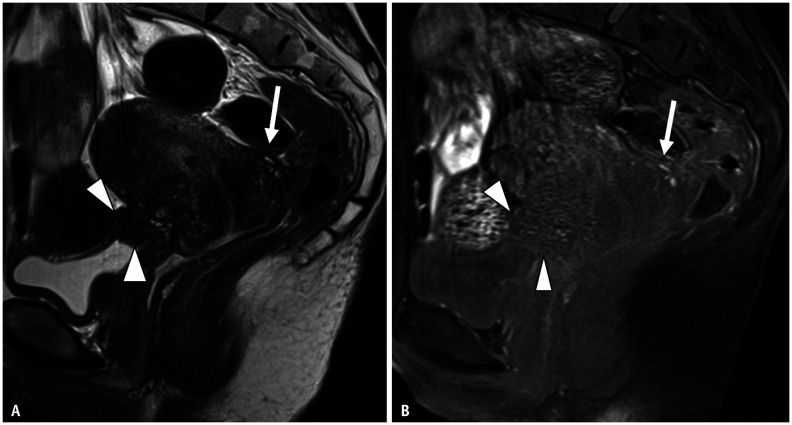
Fig. 3. Bilateral endometriotic cysts with Douglas obliteration in a 46-year-old female with dysmenorrhea and lower abdominal pain.
A-C. Sagittal T2WI (A); axial T2WI (B); axial T1WI (C) with fat saturation T2WI imaging shows an elevated posterior vaginal fornix (A, arrowhead), fibrotic plaque on the serosal surface of the uterus (A, arrow), and tethered appearance of the rectum to the uterus (B, long arrow), indicating Douglas obliteration. Bilateral ovarian endometriotic cysts and adenomyosis on the posterior wall (arrowheads in B and C) of the uterine surface are observed. In the right ovary, a functional cyst showing a high signal intensity on T2WI is also visualized. An endometriotic cyst with shading and multiplicity is observed. In the left ovary, a solitary endometriotic cyst (short arrows in B and C) with shading is observed. D-F. Axial T2WI (D); Axial T1WI (E) with fat saturation. (D) and (E) were obtained 4 months after gonadotropin-releasing hormone (GnRH) treatment. The left solitary ovarian endometriotic cyst (arrows) and adenomyosis have decreased in volume, but not for the right endometriotic cyst with multiplicity. The right functional cyst has disappeared (E). During the operation (F), strong adhesion and strands are observed in the uterine (U) serosal surface, rectum (R), and bilateral adnexa.
Decidualization
During pregnancy, hormonal changes, mainly progesterone, induce the uterine endometrium to thicken and transform to decidua with hypertrophy of the endometrial stromal cells, which is known as decidualization [3]. Similar histological changes can occur in the ectopic endometrial tissue. Reportedly, its prevalence is approximately 12% in pregnant patients with endometriotic cysts [42]. Endometriotic cysts with decidualization can show mural nodules on US and MRI, mimicking ovarian tumors derived from ovarian endometriotic cysts. In clinical situations, obstetricians find mural nodules on US during screening. A detailed examination by MRI is requested. Although decidualization is a transient condition that disappears during follow-up, it can be removed surgically when misdiagnosed as a tumor with a solid portion [43]. Consequently, recognition of this entity is clinically important. Similar pathological changes might also be observed in premenopausal patients treated with OCs, danazol, and anti-progesterone steroids [3]. Several characteristic MRI findings have been reported. On T2WI, decidualization is recognized as linear, broad-based nodular, or polypoid structures with high SI similar to that of placental tissue (Fig. 4) [43,44]. It also shows high SI on both DWI and ADC maps [43]. Several differential points of decidual tissue from ovarian tumors derived from endometriotic cysts have been reported. The heights of the mural nodules measured from the cystic wall or the septum were less than 11 mm in endometriotic cysts with decidualization (no overlap with carcinomas) [43]. This finding might represent the difference between the hormonal reaction of stromal cells and the autonomous growth of cancers. Other differential points are higher ADC values and higher SI of intra-cystic fluid on T1WI [43].
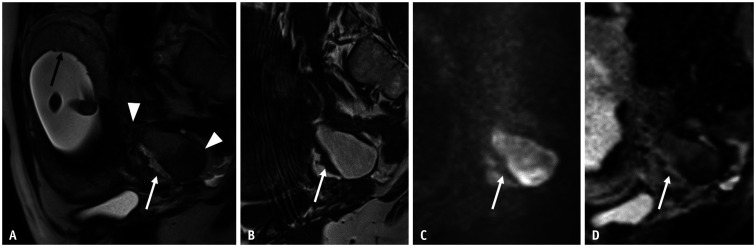
Fig. 4. Decidual change of an ovarian endometriotic cyst at 16 weeks of gestation in a 33-year-old female.
A, B. Sagittal HASTE (A); sagittal T1WI (B). Small ovarian endometriotic cyst (arrowheads), hyperintense on T1WI (B), and shading on HASTE (A) are observed just behind the pregnant uterus. In the middle of the endometriotic cyst, decidual changes are observed as irregularly thickened soft tissue (white arrows). It shows hyperintensity on T2WI, similar to the placenta (black arrow) and low signal intensity on T1WI. C, D. Sagittal DWI (C); Sagittal ADC map soft tissues (D) showed hypointensity on DWI (arrow) and hyperintensity on the ADC map (arrow), indicating no diffusion restriction. Linear and broad-based appearances are also typical findings of decidual change. ADC = apparent diffusion coefficient, DWI = diffusion-weighted imaging, HASTE = half-Fourier single-shot turbo spin echo, WI = weighted image
Polypoid Endometriosis
Polypoid endometriosis, a distinct form of endometriosis, is rare but important because it might be mistaken for a neoplasm on imaging as well as on intraoperative and pathologic examinations [3,45,46]. As a clinical characteristic, patients with polypoid endometriosis are over 50 years of age. This is in contrast to usual endometriosis, which typically affects female of reproductive age [45]. In addition, hormonal factors such as hormonal replacement therapy may play a role in its pathogenesis in many cases [45]. The lesion form is that of polypoid masses that project from the serosal surface or from the mucosa of the ovarian endometriotic cyst, bowel, or bladder (Fig. 5) [3]. Although multiple sites tend to be involved in the same patient, the rectum and sigmoid colon are the most frequent sites, followed by the ovary [45]. Macroscopically, it shows exophytic or polypoid tumor-like masses and is sometimes accompanied by variable degrees of cystic change and hemorrhage [45]. Microscopically, the polypoid masses comprise an admixture of endometriotic glands and stroma. Reflecting their macroscopic and microscopic appearance, MRI shows a high SI mass with a peripheral hypointense rim on T2WI and prominent enhancement similar to that of the endometrium [46,47,48]. Because this is benign endometrial tissue, DWI shows high SI. Moreover, the ADC values are high, without diffusion restrictions [46,48].
Fig. 5. Polypoid endometriosis in a 24-year-old female with dysmenorrhea and lower abdominal pain.
The patient received cervical cancer screening. An ovarian tumor is found.
A-E. Sagittal T1WI (A); sagittal T2WI (B); sagittal contrast-enhanced T1WI (C); diffusion weighted image (D); apparent diffusion coefficient map (E). Tumor contents showed high signal intensity on T1WI and strong low signal intensity on T2, which indicates a hemorrhagic cyst. The cyst wall is thick. These findings indicate an endometriotic cyst. Multiple mural nodules (arrowheads) show a high signal intensity on T2WI. Each nodule includes many septations on T2WI. Contrast-enhanced T1WI shows no solid lesions within the mural nodules. No area has restricted diffusion. WI = weighted image
Endometriosis-Associated Ovarian Carcinoma
Endometriotic cysts are associated with a risk of malignant transformation. Although rare (approximately 1%), the relative risk of developing ovarian cancer is up to 4.2 times greater in female with endometriosis than in the general population [49]. Patients with endometriosis-associated ovarian carcinoma (EAOC) tend to be younger (10–20 years old) and tend to have better prognoses and outcomes than female with primary epithelial ovarian cancers [50]. Scott [51] and Sampson [52] proposed the following histological criteria for EAOC: 1) coexistence of carcinoma and endometriosis within the same ovary, 2) a similar histological pattern, 3) exclusion of a second malignant tumor elsewhere, and 4) demonstration of a histology-proven transition from benign endometriosis to cancer [51,52]. The most frequent histological types of EAOC are endometrioid adenocarcinoma and clear cell carcinoma [4]. Less frequently, benign and borderline seromucinous tumors have been observed [4]. In Asian countries, especially in Japan, clear cell carcinoma is more frequent than endometrioid adenocarcinoma, but the opposite is true in Western countries, although the cause of this difference remains unknown [10].
Early detection of malignant transformation is important because clear cell carcinoma is chemotherapy resistant [53,54,55,56]. Of the three key MRI findings for EAOC diagnosis, the most sensitive finding was the emergence of enhanced mural nodules within the ovarian endometriotic cyst (Figs. 6, 7) [7,57]. Although 97% EAOC accompanies enhanced mural nodules, it is noteworthy that a certain number of benign lesions also show enhanced mural nodules [7]. The second key finding was increased tumor size. Nishio et al. [57] reported that tumor size increased considerably to almost double in the tumor group during follow-up, although it did not increase in control subjects. Kobayashi et al. [58] reported that an endometriotic cyst diameter of ≥ ‘9 cm’ was an independent predictive factor for the future development of ovarian cancer. The last finding is a lack of T2-shading, which might be induced by the dilution of hemorrhagic fluid by secretion from a malignant tumor [59]. T2-Shading was present in 81.3% of patients with benign conditions, which is in contrast to 33.3% in EAOC [7]. Based on investigations of longitudinal changes in the same endometriotic cyst by Nishio et al. [57], T2-shading disappeared in two of ten EAOC cases but continued to be observed in seven of ten EAOC cases. In that study, 20% of benign endometriotic cysts also showed a change in SI from low SI to high SI on T2WI during follow-up [57]. The lack of shading might be an alarming finding, although it is not specific to the EAOC.
Fig. 6. Rectal endometriosis in a 33-year-old female with dysmenorrhea.
A, B. Sagittal T1WI (A); sagittal T2WI (B). Ovarian endometriotic cyst (arrowheads), which shows high signal intensity on T1WI (A); shading on T2WI (B) is apparent on the uterine body. The findings of an elevated posterior vaginal fornix, tethered appearance of the rectum to the uterus, and a fibrotic plaque covering the serosal surface of the uterus indicates Douglas obliteration. Thickening of the rectal anterior wall of the rectum showing a mushroom cap sign (arrows) is observed at a height of 16 mm. This finding indicates rectal endometriosis. C. On axial contrast-enhanced T1WI, rectal endometriosis is enhanced. It involves more than half of the circumference (arrows). WI = weighted image
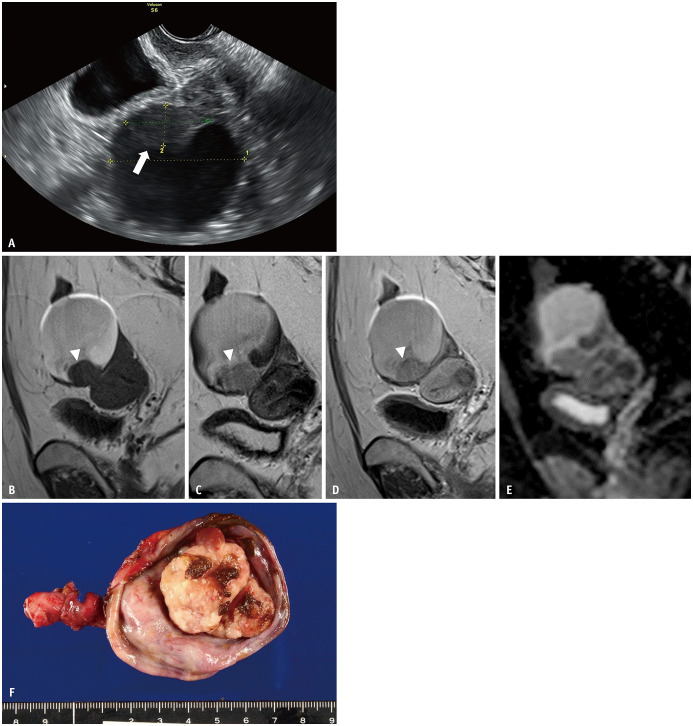
Fig. 7. Four years after Figure 6; the patient had taken progestins to treat her endometriosis.
A. Upon follow-up examination of the ovarian endometriotic cyst (depicted in Fig. 6) by transvaginal ultrasonography, the mural nodule (arrow) has emerged within the cyst. B-E. Sagittal T1WI (B); sagittal T2WI (C); sagittal contrast-enhanced T1WI (D); ADC map (E). The endometriotic cyst size is similar to that shown in Figure 6, even after hormonal therapy. On T2WI (C), the fluid intensity increased, although T2 dark spot signs are still visible. Mural nodules (arrowheads) are observed at the bottom of the cyst. The nodule (arrowhead) is well-enhanced on contrastenhanced T1WI (D) and there is low signal intensity on the ADC map (E), indicating restricted diffusion. F. Surgery has been performed. An irregularly shaped mural nodule is observed within the cyst. The mass has beem pathologically diagnosed as clear cell carcinoma (FIGO stage Ia). ADC = apparent diffusion coefficient, WI = weighted image
Malignant transformation is related to several factors, including molecular genomic alterations, oxidative stress, inflammation, and hormonal influences [53]. Hyperestrogenic status, which is affected by factors such as obesity and hormonal replacement therapy, is known to be a significant factor [54]. Recent genomic studies have suggested a relationship between several genome sequences and EAOC. Grandi et al. [55] reported the activation of oncogenic KRAS and PI3K pathways. Inactivation of the tumor suppressor genes PTEN and ARID1A might be pathogenic mechanisms for clear cell carcinoma and endometrioid carcinoma. Free iron in the contents of endometriotic cysts is also considered to be a strong oxidative stressor, which might be a cause of DNA mutations and malignant changes in endometriotic cysts [56].
MRI Findings of Deeply Infiltrating Endometriosis
Histologically, DIE has been defined as endometriosis infiltrating the peritoneum to a depth > 5 mm [14]. As described above, most endometriotic lesions visible on MRI are DIE, because superficial peritoneal implants are usually not visible [15]. DIE is frequently found in organs and structures surrounding an ovarian endometriotic cyst, where it affects their function and causes chronic symptoms and infertility. Several types of pain are related to DIE, including severe dysmenorrhea, intense dyspareunia, noncyclic chronic pelvic pain, painful defecation during menstruation, urinary tract symptoms, and gastrointestinal symptoms [19]. On microscopic examination, a characteristic finding of DIE is fibromuscular hyperplasia around the ectopic endometrial glands. The endometrial glands and stroma infiltrate the adjacent tissue and induce smooth muscle proliferation as well as a fibrous reaction, resulting in solid nodule formation, fibromuscular tissue thickening, and anatomical structure distortion [60]. Reflecting these microscopic features of fibrotic tissue, MR images show hypointense nodular lesions or soft tissue thickening with irregular, indistinct, or stellate margins on both T1WI and T2WI [5,60,61,62]. Sometimes, bright lesions on T1WI can be found, representing hemorrhagic foci. Diagnostic values of MRI for DIE are quite high: sensitivity, 90%; specificity, 91%; positive predictive value (PPV), 92%; negative predictive value (NPV), 89%; and accuracy, 90% [63]. Accurate diagnosis of DIE and evaluation of its spread and severity support appropriate treatment selection for a diversity of patients. In fact, MRI findings might vary from institution to institution. Thus, it is important to obtain the characteristic findings of DIE from several institutions [5,15,42,64].
This section presents a review of MRI findings of DIE in representative pelvic organs and structures, along with the diagnostic ability of each imaging method.
Posterior Cul-De-Sac (Pouch of Douglas, Rectouterine and Retrovaginal Spaces)
The posterior cul-de-sac is a frequently involved site of endometriosis. Complete posterior cul-de-sac obliteration was assigned the highest score in the rASRM classification [65]. In cases of posterior cul-de-sac obliteration during laparotomy, advanced laparoscopic skills are required for the separation of adhesions and for increased risk of bowel endometriosis, wherein segmental resection and anastomosis might be necessary [66,67]. Therefore, preoperative diagnosis may help gynecologists in surgical planning.
On transvaginal US, the ‘sliding sign’ is known to be useful for diagnosing posterior cul-de-sac obliteration, providing a sensitivity of 92.9%–100%, specificity of 90.9%–100%, and accuracy of 93.1%–100% [68]. The sliding sign uses gentle pressure by the transvaginal US probe to assess whether the anterior rectum glides freely across the posterior aspect of the cervix and posterior vaginal wall, and whether the rectosigmoid glides freely over the posterior aspect of the posterior upper uterus [67]. On MRI, five related findings were obtained: retroflexed uterus, elevated posterior vaginal fornix, intestinal tethering or a tethered appearance of the rectum in the direction of the uterus, faint strands between the uterus and intestine, and fibrotic plaques or nodules covering the serosal surface of the uterus (Figs. 3, 8) [69]. Among these findings, the retroflexed uterus had the highest sensitivity (mean sensitivity, 60.8%). Fibrotic plaques on the uterine serosal surface showed the highest specificity, PPV, and accuracy (84.3%, 76.8%, and 64.5%, respectively) [69]. The most accurate combination was intestinal tethering in the direction of the uterus and fibrotic plaque on the uterine serosal surface [69]. Macario et al. [70] reported that a retrouterine fibrous mass, intraperitoneal fluid displacement, and adherence of bowel loops showed the best performance (92.8%, 93.1%, and 86.1% accuracy, respectively). The cul-de-sac can be difficult to evaluate using laparoscopy in cases of strong adhesions. These MRI findings are useful for identifying DIE in the obliterated posterior cul-de-sac and to avoid underestimation.
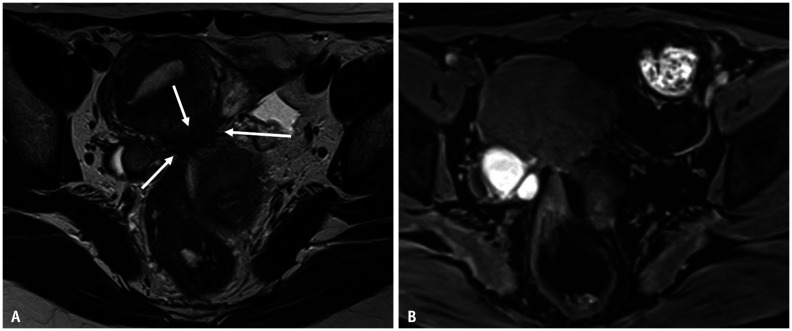
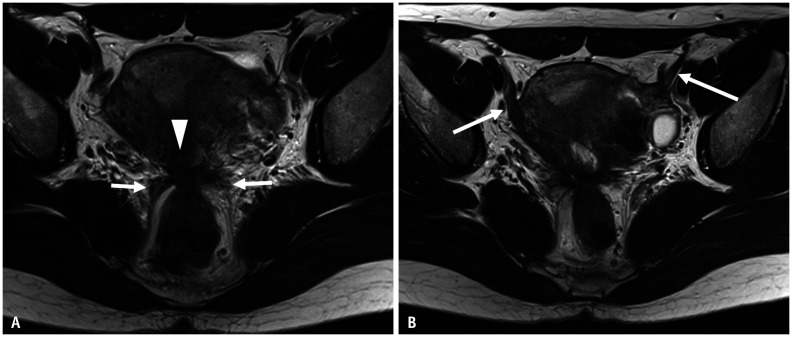
Fig. 8. Pelvic endometriosis in a 42-year-old female with abdominal pain.
A, B. Axial T2WI (A); Axial T1WI (B) with fat saturation. Bilateral endometriotic cysts are observed posterior to the uterus, appearing as a ‘kissing ovary’. At the posterior surface of the uterus, fibrotic irregular thickening (arrows) is observed as a low signal intensity on T2WI (A). Small high-signal foci are included within the lesion on T1WI (B). The bilateral ovaries and rectum converge at this point. This point corresponds to the insertion area of the uterosacral ligament, called the torus uterinus. During laparoscopic cystectomy, the pouch of Douglas is closed by adhesion. WI = weighted image
Torus Uterinus and Uterosacral Ligament
Torus uterinus is a small transverse thickening where the USL is attached. Originating from the torus uterinus, the USL courses dorsocranially toward the sacrum. Torus uterinus and USL (86%) are the most frequent sites of DIE and the second most frequent site of endometriosis (46%) after ovarian endometriosis (67%) [63,71]. The involvement of the USL is asymmetrical. For example, 40% of patients have unilateral involvement, while 60% have bilateral involvement [63]. Endometriosis of the USL can be more often observed close to the torus uterinus [42].
On US, endometriosis of the USL appears hypoechoic or with a mixed echotexture [24]. On MRI, the involvement of the USL is found to have low SI on T2WI, representing fibrous tissues (Fig. 9) [63]. T2-low intensity thickening or a mass with regular or irregular margins is observed at the torus uterinus (Figs. 8, 9, 10) [63]. Observation of the USL might be difficult because of the retroflexed uterus or endometriotic cyst at the posterior uterus by laparoscopy [63]. The diagnostic capability of MRI for detecting endometriosis in USL is superior to that of transvaginal US. A meta-analysis showed that the pooled sensitivity of MRI was 0.70, specificity was 0.93, US sensitivity was 0.67, and specificity was 0.86 [72]. A recent study also found significant superiority of MRI for the detection of DIE of USL, with an accuracy of 71% in US and 84% [73]. For better visualization of the total USL, Bazot et al. [28] reported the utility of taking thin-section oblique axial and perpendicular views to the cervix. Reportedly, the addition of oblique axial T2WI improves the accuracy of USL endometriosis on MRI, especially for left USL endometriosis, with good agreement between readers (Kappa = 0.51) [28].
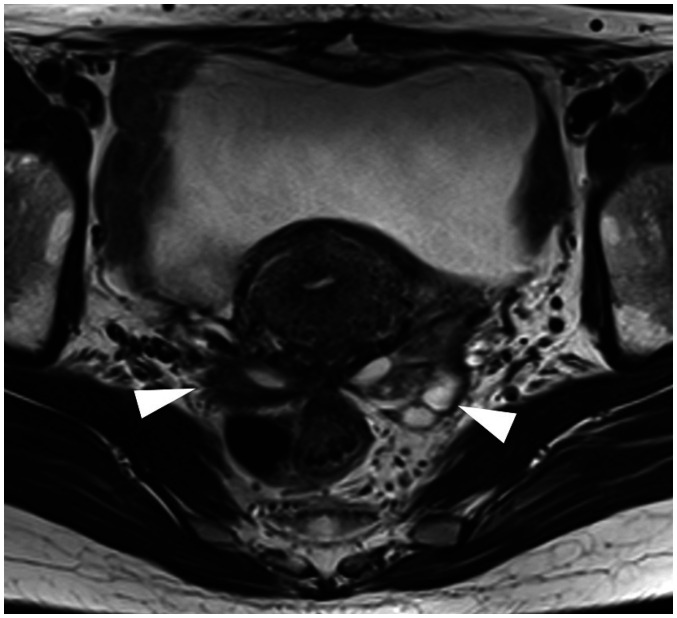
Fig. 9. Pelvic endometriosis in a 45-year-old female with severe dysmenorrhea and hypermenorrhea.
A. Axial T2 weighted images shows fibrous thickening of the torus uterinus (arrowhead) and continuous irregular thickening of the bilateral uterosacral ligament (arrows). The rectal wall is stretched strongly to the torus uterinus, suggesting severe adhesions. B. Upper slice of (A). Bilateral round ligaments (arrows) are also thickened from the origin of the uterine attachment.
Fig. 10. A 43-year-old female with dysmenorrhea.
Axial T2 weighted imaging shows a typical image finding of a ‘kissing ovary’. The bilateral ovaries (arrowheads) and the rectum are concentrated at the point of the torus uterinus, suggesting strong adhesion among these structures.
Kissing Ovaries
When bilateral ovaries are located as mutually adjacent in the posterior cul-de-sac or at the cornua of the uterus because of adhesion by endometriosis, the condition is termed ‘kissing ovaries’ (Figs. 8, 11) [15]. US examination has revealed that this finding is strongly related to disease severity, frequency of infertility, and operation time [74]. Kissing ovaries and retropositioned ovaries (ovaries located behind the uterus) on MRI show a significant correlation with DIE (sensitivity, 67%; specificity, 68%; PPV, 55% in the detection of DIE) [75]. As Williams et al. [75] described, ovaries are anchored in position by three structures: the mesovarium, utero-ovarian ligaments, and suspensory ligaments. Kissing ovaries result from the distortion of these anchoring structures following intense inflammation and fibrotic changes stimulated by endometriosis [75].
Fig. 11. Pelvic and bladder endometriosis in a 39-year-old female after hospital admission because of infertility.
A, B. Sagittal T2WI (A); Sagittal T1WI (B). Irregularly shaped nodules are observed at the level of the torus uterinus (arrows). Low signal intensity is observed on T2WI (A) and include hyperintense cysts on fat-suppressed T1WI (B). These findings indicate fibrosis caused by deep infiltrating endometriosis. The anterior surface of the rectum is strongly stretched to the torus uterinus. The anterior wall of the uterine cervix and lower uterine body show low signal intensity, probably reflecting fibrosis caused by adenomyosis. Continuing from uterine adenomyosis, soft tissue (arrowheads) with low signal intensity on T2WI continues to the bladder wall. Hyperintense foci on T1WI are visible at the mass periphery. Although focal bladder thickening resembles a bladder tumor, a hyperintense cyst on T1WI and the presence of adenomyosis and fibrous tissue around the lesion led to a diagnosis of endometriosis. WI = weighted image
Round Ligament of the Uterus
The prevalence of round ligament of the uterus (RLU) involvement is reported to be 3%–5%, with left-side predominance [76]. Anatomically, the round ligament originates anterolateral of the uterine horn, coursing under the broad ligament toward the inguinal canal. Frequently involved sites have also been reported. As reported by Gui et al. [77], RLU is divisible into intra-pelvic and extra-pelvic parts. Most cases of endometriosis involving the RLU are located extrapelvically, at the distal part of the ligament near the inguinal region. Therefore, the clinical symptom is the presence of a painful, palpable inguinal mass, with or without menstrual variation in the size or severity of symptoms [77]. Another report described the most frequent site as the proximal third of the ligament (approximately 14%) [15]. Predominance of right side involvement is also known to occur, probably because of clockwise intra-peritoneal fluid circulation from the left paracolic gutter to the right side through the pelvic floor [77]. However, questions regarding side predominance (right or left) persist [77]. Laparoscopically, endometriosis is diagnosed by the presence of shortening, thickening, and deviation of the RLU [78]. Similarly, a thickened and shortened RLU was observed on MRI. It can be irregular with a nodular appearance (Fig. 9) [77]. The SI depends on the degree of hemorrhagic foci and fibrous tissue, but most cases tend to show hypointense lesions on both T1WI and T2WI.
Rectosigmoid Colon
The rectosigmoid colon, which is the most frequent site of gastrointestinal endometriosis, accounts for up to 65.7% of disease incidence, followed by the ileocecal junction (20%) and rectum (15%) [42,79]. Depending on the disease severity, such as the percentage of circumference involved, surgical procedures including segmental resection, discoid resection, and rectal shaving can be considered [80]. The characteristic MRI findings of rectal endometriosis are the ‘fan-shaped’ and ‘mushroom cap’ appearances (Fig. 6) [64,81]. The ‘fan-shaped’ appearance is defined as fan-shaped bowel wall thickening with hypointense T2WI (similar signal to muscle) and slightly high SI at the mucosal side [64]. Occasionally, intense signal spots are visible on T1WI. The ‘mushroom cap’ appearance refers to focal thickening of the rectal wall to resemble an umbrella-like head of a mushroom [81]. Both signs correspond to hypertrophy of the muscularis propria and smooth muscle hyperplasia, which might be induced by stimulation of invading endometrial tissue located within the muscle layer or submucosal layer of the bowel [64,81]. Consequently, when these are observed on MR images, muscular invasion of endometrial tissue is expected [64]. Reportedly, the sensitivity, specificity, PPV, and NPV, as well as accuracy of fan-shaped appearance for infiltration of the muscular layer of the bowel wall are 100%, 75%, 96%, 100%, and 96% [64]. Distention of the rectal lumen by US gel was proposed for better visualization of the disease, because of better contrast between the rectal lumen and the wall [82]. However, the diagnostic capability of colon wall infiltration was not significantly different before and after US gel application [82]. Motion artifacts due to the distended rectum and lack of US gel at the upper part of the rectum are regarded as causes of ineffectiveness [82]. Recently, Rousset et al. [83] demonstrated that greater than 135° circumference involvement and greater than 14 mm lesion thickness are specific MRI findings predicting the need for segmental resection for rectal endometriosis. Thus, MRI can provide important information that supports decisions made in formulating a treatment strategy.
Urinary Bladder and Vesicouterine Space
Urinary tract involvement occurs in 1.0%–6.4% of endometriosis patients, depending on the series [84]. The most frequently involved area is the posterior bladder wall [42]. Symptoms may be very subtle or be as concerning, such as dysuria or hematuria [85]. Endometrial cells that seed on the anterior uterine wall or bladder wall often during earlier surgery, such as caesarean section, are thought to infiltrate into the muscular layer and manifest as mural masses [85]. The lesion may be clinically mistaken for cancer. On MRI, bladder endometriosis is depicted as hypointense nodular formation on both T1 and T2WI, sometimes accompanying high signal foci reflecting ectopic endometrial glands and hemorrhage (Fig. 10) [60,85]. In correlation with surgical and pathologic findings, MRI showed a sensitivity of 88%, specificity of 98%, PPV of 88%, NPV of 98%, and an accuracy of 98% [63]. In 90% of cases with bladder endometriosis, several other sites with endometriosis have been reported, including those in the ovaries and retrocervical areas, as well as adenomyosis [85]. Bladder endometriosis is sometimes found as a continuous lesion with uterine adenomyosis occurring via the vesicouterine space [85].
Pelvic Nerve
Although not usual, the sciatic nerve can also be involved in endometriosis in patients with DIE. Peripheral nerve involvement or compression by endometriosis is regarded as a cause of chronic pelvic pain symptoms, such as dysmenorrhea, non-cyclical pain, and deep dyspareunia [86]. Although some aspects of the pathogenesis of pain in endometriosis remain unclear, pathological intraneural and perineural invasion are observed more often in endometriosis patients with severe pelvic pain [86]. Additionally, presacral neurectomy is known to relieve these symptoms, although it presents with some risk of adverse effects [87]. Recognizing nerve involvement by endometriosis using conventional MR sequences might be difficult, but diffusion tensor images (DTIs) and magnetic resonance neurography might facilitate its visualization [88,89,90]. Manganaro et al. [89] reported an irregular and disorganized appearance of the sacral nerve root (S1, S2, and S3) on DTI in most patients with endometriosis. Furthermore, fractional anisotropy values calculated from DTI in the S1, S2, and S3 roots were significantly lower in patients than in control subjects [89]. Zhang et al. [90] used contrast-enhanced 3D short time inversion recovery sampling perfection with application-optimized contrasts using different flip angle evolution sequences to visualize compression and adhesion of the sacral plexus nerve as a result of ectopic endometriotic lesions. These new techniques may provide additional information for future clinical applications.
Extra-Pelvic Endometriosis
Extra-pelvic endometriosis is a rare condition of endometriosis found at sites distant from the pelvic organs [91]. Although the ‘extra-pelvic’ area is large and the exact frequency of this disease remains unclear, abdominal wall endometriosis and thoracic endometriosis are frequent [91,92].
As for abdominal wall endometriosis, the frequent sites are the inguinal and umbilical regions [91,92]. A study of 105 patients with ‘abdominal/pelvic wall mass’ by Yarmish et al. [93] revealed that 24.8% (26/105) of them had histologically proven abdominal wall endometriosis. Such patients are often admitted to the hospital with cyclic abdominal pain, and are therefore referred to dermatology and not to gynecology [29,93]. In addition, the influence of factors such as prior surgery, including cesarean section and hysterectomy, are well known. A systematic review article noted that an earlier surgical history was recognized in approximately 80% of patients, but not in 20% of patients [94]. Spontaneous inguinal endometriosis is, in most cases, an extension of pelvic endometriosis along the round ligament [95]. Regarding imaging diagnosis, US is the first modality to be used because it is a superficial lesion. On US, the lesion shows hypoechoic round or oval nodules with ill-defined borders and a hyperechoic rim [96]. In fact, CT showed a sensitivity of 69% and specificity of 97% by a combination of the following three imaging features: location below the umbilicus, homogeneous density, and the presence of linear infiltration irradiating peripherally from a central soft tissue nodule, designated as the ‘gorgon’ sign [93]. The ‘gorgon’ sign reflects histological features of histiocytic infiltration and fibrosis because of chronic hemorrhage, as well as a few glands found in pelvic endometriosis [93]. Based on these histological findings, MRI showed a hypointense solid mass on T2WI with hyperintense hemorrhagic cysts or hyperintense hemorrhagic cysts on T1WI (Fig. 12) [97]. Fat-saturated T1WI is recommended for the diagnosis of abdominal wall endometriosis [97].
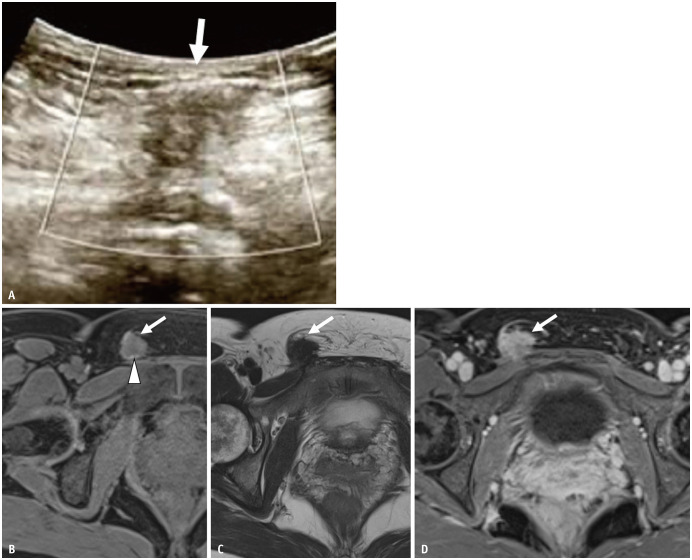
Fig. 12. A 46-year-old female with inguinal lesion.
The patient had undergone laparoscopic cystectomy for a left endometriotic cyst 10 years previously.
A. On ultrasound, the lesion (arrow) is visualized as a hypoechoic round nodule with an ill-defined border. B-D. Axial FS T1WI (B); axial T2WI (C); axial contrast-enhanced FS T1WI (D). The lesion (arrows) shows low signal intensity on both T1 and T2WI with an ill-defined border. There is a small high-intensity spot on FS T1WI (arrowhead). The mass is almost homogeneously enhanced. The patient received hormonal therapy, which reduced the lesion. FS = fat saturated, WI = weighted image
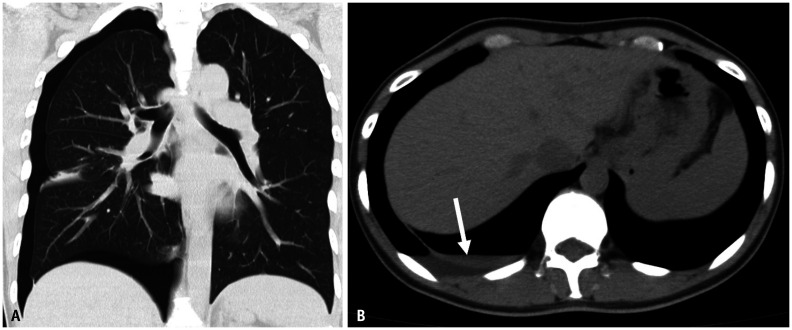
Thoracic endometriosis is characterized by the presence of endometriotic lesions in the thoracic cavity [98]. The most common clinical presentation of thoracic endometriosis is catamenial pneumothorax (70%), followed by catamenial hemothorax and lung nodules [99]. Because CT is unable to detect lesions easily, especially in cases of small lesions, the gold standard for the diagnosis of thoracic endometriosis is video-assisted thoracoscopic surgery (Fig. 13) [92]. For the evaluation of diaphragmatic endometriosis using MRI, the lesions were detected as hyperintense nodules on T1WI, which were mostly located at the right side and posterior with respect to the vena cava [100]. The MRI sensitivity was 83%, and the kappa value of the two readers was 0.86 [100].
Fig. 13. A 45-year-old female with regularly recurring pneumothorax.
A, B. CT without contrast. Pneumothorax is found on the right side. A small amount of pleural effusion (arrow) is also observed. The fluid density is somewhat high. Hemothorax is suspected. During the operation, a small defect is observed in the diaphragm. It was thought to be the migration path of the endometrial tissue from the peritoneal cavity to the pleural cavity. On the basis of a pathological examination, endometriosis was diagnosed from the tissue around a small defect on the diaphragm.
Summary
Endometriosis, a common chronic inflammatory disease affecting female of reproductive age, can severely affect patients’ quality of life as well as their fertility. Because of its high contrast and objectivity, MRI can contribute to the early and accurate diagnosis of ovarian endometriotic cysts and DIE while obviating the need for invasive procedures and radiation exposure. Furthermore, MRI plays a role in evaluating severity, leading to optimal treatment selection and preoperative planning. It is noteworthy that ovarian endometriotic cysts sometimes present with an atypical appearance, such as decidual change or polypoid endometriosis. Complications such as rupture, abscess, and endometriosis-related tumors should also be noted. For the diagnosis of DIE and secondary adhesion among pelvic organs, we look for ‘fibrosis’ with T2 hypointense nodules or thickened structures. Hemorrhagic foci on T1WI can be helpful, but they are infrequent. Indirect imaging findings of adhesion, such as kissing ovaries or intestinal tethering, are also important. Radiologists should be familiar with both common and uncommon locations of endometriosis, their characteristic imaging findings, and their relationship to disease severity and treatment selection.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Aki Kido.
- Investigation: Aki Kido.
- Writing—original draft: Aki Kido.
- Writing—review & editing: Yuki Himoto, Yusaku Moribata, Yasuhisa Kurata, Yuji Nakamoto.
Funding Statement: None
Availability of Data and Material
Data sharing does not apply to this article as no datasets were generated or analyzed during the current study.
References
- 1.Johnson NP, Hummelshoj L World Endometriosis Society Montpellier Consortium. Consensus on current management of endometriosis. Hum Reprod. 2013;28:1552–1568. doi: 10.1093/humrep/det050. [DOI] [PubMed] [Google Scholar]
- 2.Ozkan S, Murk W, Arici A. Endometriosis and infertility: epidemiology and evidence-based treatments. Ann N Y Acad Sci. 2008;1127:92–100. doi: 10.1196/annals.1434.007. [DOI] [PubMed] [Google Scholar]
- 3.Clement PB. In: Blaustein’s pathology of the female genital tract. 5th ed. Kurman RJ, editor. New York: Springer-Verlag; 2002. Diseases of the peritoneum; pp. 729–790. [Google Scholar]
- 4.Stewart CJR, Ayhan A, Fukunaga M, Huntsman DG. WHO Classification of Tumours Editorial Board, editors. WHO classification of tumours female genital tumours. 5th ed. Lyon: IARC Library Cataloguing-in-Publication Data; 2020. Endometriosis and related conditions; pp. 169–174. [Google Scholar]
- 5.Bazot M, Daraï E. Diagnosis of deep endometriosis: clinical examination, ultrasonography, magnetic resonance imaging, and other techniques. Fertil Steril. 2017;108:886–894. doi: 10.1016/j.fertnstert.2017.10.026. [DOI] [PubMed] [Google Scholar]
- 6.Bazot M, Lafont C, Rouzier R, Roseau G, Thomassin-Naggara I, Daraï E. Diagnostic accuracy of physical examination, transvaginal sonography, rectal endoscopic sonography, and magnetic resonance imaging to diagnose deep infiltrating endometriosis. Fertil Steril. 2009;92:1825–1833. doi: 10.1016/j.fertnstert.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka YO, Okada S, Yagi T, Satoh T, Oki A, Tsunoda H, et al. MRI of endometriotic cysts in association with ovarian carcinoma. AJR Am J Roentgenol. 2010;194:355–361. doi: 10.2214/AJR.09.2985. [DOI] [PubMed] [Google Scholar]
- 8.Vimercati A, Achilarre MT, Scardapane A, Lorusso F, Ceci O, Mangiatordi G, et al. Accuracy of transvaginal sonography and contrast-enhanced magnetic resonance-colonography for the presurgical staging of deep infiltrating endometriosis. Ultrasound Obstet Gynecol. 2012;40:592–603. doi: 10.1002/uog.11179. [DOI] [PubMed] [Google Scholar]
- 9.Bazot M, Bharwani N, Huchon C, Kinkel K, Cunha TM, Guerra A, et al. European society of urogenital radiology (ESUR) guidelines: MR imaging of pelvic endometriosis. Eur Radiol. 2017;27:2765–2775. doi: 10.1007/s00330-016-4673-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vercellini P, Viganò P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014;10:261–275. doi: 10.1038/nrendo.2013.255. [DOI] [PubMed] [Google Scholar]
- 11.Sampson JA. Peritoneal endometriosis due to menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:422–469. [Google Scholar]
- 12.Nisolle M, Donnez J. Reprint of: peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil Steril. 2019;112:e125–e136. doi: 10.1016/j.fertnstert.2019.08.081. [DOI] [PubMed] [Google Scholar]
- 13.Nyholt DR, Low SK, Anderson CA, Painter JN, Uno S, Morris AP, et al. Genome-wide association meta-analysis identifies new endometriosis risk loci. Nat Genet. 2012;44:1355–1359. doi: 10.1038/ng.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koninckx PR, Martin DC. Deep endometriosis: a consequence of infiltration or retraction or possibly adenomyosis externa? Fertil Steril. 1992;58:924–928. doi: 10.1016/s0015-0282(16)55436-3. [DOI] [PubMed] [Google Scholar]
- 15.Jha P, Sakala M, Chamie LP, Feldman M, Hindman N, Huang C, et al. Endometriosis MRI lexicon: consensus statement from the society of abdominal radiology endometriosis disease-focused panel. Abdom Radiol (NY) 2020;45:1552–1568. doi: 10.1007/s00261-019-02291-x. [DOI] [PubMed] [Google Scholar]
- 16.Lee SY, Koo YJ, Lee DH. Classification of endometriosis. Yeungnam Univ J Med. 2021;38:10–18. doi: 10.12701/yujm.2020.00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson NP, Hummelshoj L, Adamson GD, Keckstein J, Taylor HS, Abrao MS, et al. World Endometriosis Society consensus on the classification of endometriosis. Hum Reprod. 2017;32:315–324. doi: 10.1093/humrep/dew293. [DOI] [PubMed] [Google Scholar]
- 18.Di Paola V, Manfredi R, Castelli F, Negrelli R, Mehrabi S, Pozzi Mucelli R. Detection and localization of deep endometriosis by means of MRI and correlation with the ENZIAN score. Eur J Radiol. 2015;84:568–574. doi: 10.1016/j.ejrad.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 19.Fauconnier A, Chapron C, Dubuisson JB, Vieira M, Dousset B, Bréart G. Relation between pain symptoms and the anatomic location of deep infiltrating endometriosis. Fertil Steril. 2002;78:719–726. doi: 10.1016/s0015-0282(02)03331-9. [DOI] [PubMed] [Google Scholar]
- 20.Cornillie FJ, Oosterlynck D, Lauweryns JM, Koninckx PR. Deeply infiltrating pelvic endometriosis: histology and clinical significance. Fertil Steril. 1990;53:978–983. doi: 10.1016/s0015-0282(16)53570-5. [DOI] [PubMed] [Google Scholar]
- 21.Howard FM. Endometriosis and mechanisms of pelvic pain. J Minim Invasive Gynecol. 2009;16:540–550. doi: 10.1016/j.jmig.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 22.Vercellini P, Crosignani P, Somigliana E, Viganò P, Frattaruolo MP, Fedele L. ‘Waiting for Godot’: a commonsense approach to the medical treatment of endometriosis. Hum Reprod. 2011;26:3–13. doi: 10.1093/humrep/deq302. [DOI] [PubMed] [Google Scholar]
- 23.Piketty M, Chopin N, Dousset B, Millischer-Bellaische AE, Roseau G, Leconte M, et al. Preoperative work-up for patients with deeply infiltrating endometriosis: transvaginal ultrasonography must definitely be the first-line imaging examination. Hum Reprod. 2009;24:602–607. doi: 10.1093/humrep/den405. [DOI] [PubMed] [Google Scholar]
- 24.Chamié LP, Blasbalg R, Pereira RM, Warmbrand G, Serafini PC. Findings of pelvic endometriosis at transvaginal US, MR imaging, and laparoscopy. Radiographics. 2011;31:E77–E100. doi: 10.1148/rg.314105193. [DOI] [PubMed] [Google Scholar]
- 25.Botterill EM, Esler SJ, McIlwaine KT, Jagasia N, Ellett L, Maher PJ, et al. Endometriosis: does the menstrual cycle affect magnetic resonance (MR) imaging evaluation? Eur J Radiol. 2015;84:2071–2079. doi: 10.1016/j.ejrad.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Fiaschetti V, Crusco S, Meschini A, Cama V, Di Vito L, Marziali M, et al. Deeply infiltrating endometriosis: evaluation of retro-cervical space on MRI after vaginal opacification. Eur J Radiol. 2012;81:3638–3645. doi: 10.1016/j.ejrad.2011.06.058. [DOI] [PubMed] [Google Scholar]
- 27.Bazot M, Gasner A, Lafont C, Ballester M, Daraï E. Deep pelvic endometriosis: limited additional diagnostic value of postcontrast in comparison with conventional MR images. Eur J Radiol. 2011;80:e331–e339. doi: 10.1016/j.ejrad.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 28.Bazot M, Gasner A, Ballester M, Daraï E. Value of thin-section oblique axial T2-weighted magnetic resonance images to assess uterosacral ligament endometriosis. Hum Reprod. 2011;26:346–353. doi: 10.1093/humrep/deq336. [DOI] [PubMed] [Google Scholar]
- 29.Busard MP, Mijatovic V, van Kuijk C, Hompes PG, van Waesberghe JH. Appearance of abdominal wall endometriosis on MR imaging. Eur Radiol. 2010;20:1267–1276. doi: 10.1007/s00330-009-1658-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chapron C, Pietin-Vialle C, Borghese B, Davy C, Foulot H, Chopin N. Associated ovarian endometrioma is a marker for greater severity of deeply infiltrating endometriosis. Fertil Steril. 2009;92:453–457. doi: 10.1016/j.fertnstert.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Togashi K, Nishimura K, Kimura I, Tsuda Y, Yamashita K, Shibata T, et al. Endometrial cysts: diagnosis with MR imaging. Radiology. 1991;180:73–78. doi: 10.1148/radiology.180.1.2052726. [DOI] [PubMed] [Google Scholar]
- 32.Corwin MT, Gerscovich EO, Lamba R, Wilson M, McGahan JP. Differentiation of ovarian endometriomas from hemorrhagic cysts at MR imaging: utility of the T2 dark spot sign. Radiology. 2014;271:126–132. doi: 10.1148/radiol.13131394. [DOI] [PubMed] [Google Scholar]
- 33.Glastonbury CM. The shading sign. Radiology. 2002;224:199–201. doi: 10.1148/radiol.2241010361. [DOI] [PubMed] [Google Scholar]
- 34.Dillon WP, Som PM, Fullerton GD. Hypointense MR signal in chronically inspissated sinonasal secretions. Radiology. 1990;174:73–78. doi: 10.1148/radiology.174.1.2294574. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi K, Okada S, Okada M, Kitao M, Kaji Y, Sugimura K. Magnetic resonance relaxation time in evaluating the cyst fluid characteristics of endometrioma. Hum Reprod. 1996;11:857–860. doi: 10.1093/oxfordjournals.humrep.a019266. [DOI] [PubMed] [Google Scholar]
- 36.Outwater E, Schiebler ML, Owen RS, Schnall MD. Characterization of hemorrhagic adnexal lesions with MR imaging: blinded reader study. Radiology. 1993;186:489–494. doi: 10.1148/radiology.186.2.8421756. [DOI] [PubMed] [Google Scholar]
- 37.Evangelinakis N, Grammatikakis I, Salamalekis G, Tziortzioti V, Samaras C, Chrelias C, et al. Prevalence of acute hemoperitoneum in patients with endometriotic ovarian cysts: a 7-year retrospective study. Clin Exp Obstet Gynecol. 2009;36:254–255. [PubMed] [Google Scholar]
- 38.Suzuki S, Yasumoto M, Matsumoto R, Andoh A. MR findings of ruptured endometrial cyst: comparison with tubo-ovarian abscess. Eur J Radiol. 2012;81:3631–3637. doi: 10.1016/j.ejrad.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 39.Choi NJ, Rha SE, Jung SE, Choi BG, Oh SN, Byun JY, et al. Ruptured endometrial cysts as a rare cause of acute pelvic pain: can we differentiate them from ruptured corpus luteal cysts on CT scan? J Comput Assist Tomogr. 2011;35:454–458. doi: 10.1097/RCT.0b013e31821f4bd2. [DOI] [PubMed] [Google Scholar]
- 40.Chen MJ, Yang JH, Yang YS, Ho HN. Increased occurrence of tubo-ovarian abscesses in women with stage III and IV endometriosis. Fertil Steril. 2004;82:498–499. doi: 10.1016/j.fertnstert.2004.01.032. [DOI] [PubMed] [Google Scholar]
- 41.Sugimura K, Okizuka H, Kaji Y, Imaoka I, Shiotani S, Mukumoto H, et al. MRI in predicting the response of ovarian endometriomas to hormone therapy. J Comput Assist Tomogr. 1996;20:145–150. doi: 10.1097/00004728-199601000-00026. [DOI] [PubMed] [Google Scholar]
- 42.Bourgioti C, Preza O, Panourgias E, Chatoupis K, Antoniou A, Nikolaidou ME, et al. MR imaging of endometriosis: spectrum of disease. Diagn Interv Imaging. 2017;98:751–767. doi: 10.1016/j.diii.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 43.Morisawa N, Kido A, Kataoka M, Minamiguchi S, Konishi I, Togashi K. Magnetic resonance imaging manifestations of decidualized endometriotic cysts: comparative study with ovarian cancers associated with endometriotic cysts. J Comput Assist Tomogr. 2014;38:879–884. doi: 10.1097/RCT.0000000000000136. [DOI] [PubMed] [Google Scholar]
- 44.Takeuchi M, Matsuzaki K, Nishitani H. Magnetic resonance manifestations of decidualized endometriomas during pregnancy. J Comput Assist Tomogr. 2008;32:353–355. doi: 10.1097/RCT.0b013e3181238362. [DOI] [PubMed] [Google Scholar]
- 45.Parker RL, Dadmanesh F, Young RH, Clement PB. Polypoid endometriosis: a clinicopathologic analysis of 24 cases and a review of the literature. Am J Surg Pathol. 2004;28:285–297. doi: 10.1097/00000478-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 46.Ghafoor S, Lakhman Y, Park KJ, Petkovska I. Polypoid endometriosis: a mimic of malignancy. Abdom Radiol (NY) 2020;45:1776–1782. doi: 10.1007/s00261-019-02143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takeuchi M, Matsuzaki K, Furumoto H, Nishitani H. Case report: a case of polypoid endometriosis: MR pathological correlation. Br J Radiol. 2008;81:e118–e119. doi: 10.1259/bjr/23847518. [DOI] [PubMed] [Google Scholar]
- 48.Kozawa E, Inoue K, Iwasa N, Fujiwara K, Yasuda M, Tanaka J, et al. MR imaging of polypoid endometriosis of the ovary. Magn Reson Med Sci. 2012;11:201–204. doi: 10.2463/mrms.11.201. [DOI] [PubMed] [Google Scholar]
- 49.Brinton LA, Gridley G, Persson I, Baron J, Bergqvist A. Cancer risk after a hospital discharge diagnosis of endometriosis. Am J Obstet Gynecol. 1997;176:572–579. doi: 10.1016/s0002-9378(97)70550-7. [DOI] [PubMed] [Google Scholar]
- 50.Li Q, Sun Y, Zhang X, Wang L, Wu W, Wu M, et al. Endometriosis-associated ovarian cancer is a single entity with distinct clinicopathological characteristics. Cancer Biol Ther. 2019;20:1029–1034. doi: 10.1080/15384047.2019.1595278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scott RB. Malignant changes in endometriosis. Obstet Gynecol. 1953;2:283–289. [PubMed] [Google Scholar]
- 52.Sampson JA. Endometrial carcinoma of the ovary, arising in endometrial tissue in that organ. Arch Surg. 1925;10:1–72. [Google Scholar]
- 53.Robinson KA, Menias CO, Chen L, Schiappacasse G, Shaaban AM, Caserta MP, et al. Understanding malignant transformation of endometriosis: imaging features with pathologic correlation. Abdom Radiol (NY) 2020;45:1762–1775. doi: 10.1007/s00261-019-01914-7. [DOI] [PubMed] [Google Scholar]
- 54.Zanetta GM, Webb MJ, Li H, Keeney GL. Hyperestrogenism: a relevant risk factor for the development of cancer from endometriosis. Gynecol Oncol. 2000;79:18–22. doi: 10.1006/gyno.2000.5905. [DOI] [PubMed] [Google Scholar]
- 55.Grandi G, Toss A, Cortesi L, Botticelli L, Volpe A, Cagnacci A. The association between endometriomas and ovarian cancer: preventive effect of inhibiting ovulation and menstruation during reproductive life. Biomed Res Int. 2015;2015:751571. doi: 10.1155/2015/751571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamaguchi K, Mandai M, Toyokuni S, Hamanishi J, Higuchi T, Takakura K, et al. Contents of endometriotic cysts, especially the high concentration of free iron, are a possible cause of carcinogenesis in the cysts through the iron-induced persistent oxidative stress. Clin Cancer Res. 2008;14:32–40. doi: 10.1158/1078-0432.CCR-07-1614. [DOI] [PubMed] [Google Scholar]
- 57.Nishio N, Kido A, Kataoka M, Kuwahara R, Nakao K, Kurata Y, et al. Longitudinal changes in magnetic resonance imaging of malignant and borderline tumors associated with ovarian endometriotic cyst comparing with endometriotic cysts without arising malignancy. Eur J Radiol. 2018;105:175–181. doi: 10.1016/j.ejrad.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 58.Kobayashi H, Ooi H, Yamada Y, Sakata M, Kawaguchi R, Kanayama S, et al. Serum CA125 level before the development of ovarian cancer. Int J Gynaecol Obstet. 2007;99:95–99. doi: 10.1016/j.ijgo.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 59.Tanaka YO, Yoshizako T, Nishida M, Yamaguchi M, Sugimura K, Itai Y. Ovarian carcinoma in patients with endometriosis: MR imaging findings. AJR Am J Roentgenol. 2000;175:1423–1430. doi: 10.2214/ajr.175.5.1751423. [DOI] [PubMed] [Google Scholar]
- 60.Coutinho A, Jr, Bittencourt LK, Pires CE, Junqueira F, Lima CM, Coutinho E, et al. MR imaging in deep pelvic endometriosis: a pictorial essay. Radiographics. 2011;31:549–567. doi: 10.1148/rg.312105144. [DOI] [PubMed] [Google Scholar]
- 61.Siegelman ES, Outwater EK. Tissue characterization in the female pelvis by means of MR imaging. Radiology. 1999;212:5–18. doi: 10.1148/radiology.212.1.r99jl455. [DOI] [PubMed] [Google Scholar]
- 62.Loubeyre P, Petignat P, Jacob S, Egger JF, Dubuisson JB, Wenger JM. Anatomic distribution of posterior deeply infiltrating endometriosis on MRI after vaginal and rectal gel opacification. AJR Am J Roentgenol. 2009;192:1625–1631. doi: 10.2214/AJR.08.1856. [DOI] [PubMed] [Google Scholar]
- 63.Bazot M, Darai E, Hourani R, Thomassin I, Cortez A, Uzan S, et al. Deep pelvic endometriosis: MR imaging for diagnosis and prediction of extension of disease. Radiology. 2004;232:379–389. doi: 10.1148/radiol.2322030762. [DOI] [PubMed] [Google Scholar]
- 64.Busard MP, van der Houwen LE, Bleeker MC, Pieters van den Bos IC, Cuesta MA, van Kuijk C, et al. Deep infiltrating endometriosis of the bowel: MR imaging as a method to predict muscular invasion. Abdom Imaging. 2012;37:549–557. doi: 10.1007/s00261-011-9790-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schenken RS, Guzick DS. Revised endometriosis classification: 1996. Fertil Steril. 1997;67:815–816. doi: 10.1016/s0015-0282(97)81390-8. [DOI] [PubMed] [Google Scholar]
- 66.Khong SY, Bignardi T, Luscombe G, Lam A. Is pouch of Douglas obliteration a marker of bowel endometriosis? J Minim Invasive Gynecol. 2011;18:333–337. doi: 10.1016/j.jmig.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 67.Reid S, Condous G. Transvaginal sonographic sliding sign: accurate prediction of pouch of Douglas obliteration. Ultrasound Obstet Gynecol. 2013;41:605–607. doi: 10.1002/uog.12469. [DOI] [PubMed] [Google Scholar]
- 68.Reid S, Lu C, Casikar I, Mein B, Magotti R, Ludlow J, et al. The prediction of pouch of Douglas obliteration using offline analysis of the transvaginal ultrasound ‘sliding sign’ technique: inter- and intra-observer reproducibility. Hum Reprod. 2013;28:1237–1246. doi: 10.1093/humrep/det044. [DOI] [PubMed] [Google Scholar]
- 69.Kataoka ML, Togashi K, Yamaoka T, Koyama T, Ueda H, Kobayashi H, et al. Posterior cul-de-sac obliteration associated with endometriosis: MR imaging evaluation. Radiology. 2005;234:815–823. doi: 10.1148/radiol.2343031366. [DOI] [PubMed] [Google Scholar]
- 70.Macario S, Chassang M, Novellas S, Baudin G, Delotte J, Toullalan O, et al. The value of pelvic MRI in the diagnosis of posterior cul-de-sac obliteration in cases of deep pelvic endometriosis. AJR Am J Roentgenol. 2012;199:1410–1415. doi: 10.2214/AJR.11.7898. [DOI] [PubMed] [Google Scholar]
- 71.Audebert A, Petousis S, Margioula-Siarkou C, Ravanos K, Prapas N, Prapas Y. Anatomic distribution of endometriosis: a reappraisal based on series of 1101 patients. Eur J Obstet Gynecol Reprod Biol. 2018;230:36–40. doi: 10.1016/j.ejogrb.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 72.Guerriero S, Saba L, Pascual MA, Ajossa S, Rodriguez I, Mais V, et al. Transvaginal ultrasound vs magnetic resonance imaging for diagnosing deep infiltrating endometriosis: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2018;51:586–595. doi: 10.1002/uog.18961. [DOI] [PubMed] [Google Scholar]
- 73.Indrielle-Kelly T, Frühauf F, Fanta M, Burgetova A, Lavu D, Dundr P, et al. Diagnostic accuracy of ultrasound and MRI in the mapping of deep pelvic endometriosis using the International Deep Endometriosis Analysis (IDEA) consensus. Biomed Res Int. 2020;2020:3583989. doi: 10.1155/2020/3583989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ghezzi F, Raio L, Cromi A, Duwe DG, Beretta P, Buttarelli M, et al. “Kissing ovaries”: a sonographic sign of moderate to severe endometriosis. Fertil Steril. 2005;83:143–147. doi: 10.1016/j.fertnstert.2004.05.094. [DOI] [PubMed] [Google Scholar]
- 75.Williams JC, Burnett TL, Jones T, Venkatesh SK, VanBuren WM. Association between kissing and retropositioned ovaries and severity of endometriosis: MR imaging evaluation. Abdom Radiol (NY) 2020;45:1637–1644. doi: 10.1007/s00261-019-02153-6. [DOI] [PubMed] [Google Scholar]
- 76.Redwine DB. Ovarian endometriosis: a marker for more extensive pelvic and intestinal disease. Fertil Steril. 1999;72:310–315. doi: 10.1016/s0015-0282(99)00211-3. [DOI] [PubMed] [Google Scholar]
- 77.Gui B, Valentini AL, Ninivaggi V, Marino M, Iacobucci M, Bonomo L. Deep pelvic endometriosis: don’t forget round ligaments. Review of anatomy, clinical characteristics, and MR imaging features. Abdom Imaging. 2014;39:622–632. doi: 10.1007/s00261-014-0091-3. [DOI] [PubMed] [Google Scholar]
- 78.Crispi CP, de Souza CA, Oliveira MA, Dibi RP, Cardeman L, Sato H, et al. Endometriosis of the round ligament of the uterus. J Minim Invasive Gynecol. 2012;19:46–51. doi: 10.1016/j.jmig.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 79.Dousset B, Leconte M, Borghese B, Millischer AE, Roseau G, Arkwright S, et al. Complete surgery for low rectal endometriosis: long-term results of a 100-case prospective study. Ann Surg. 2010;251:887–895. doi: 10.1097/SLA.0b013e3181d9722d. [DOI] [PubMed] [Google Scholar]
- 80.De Cicco C, Corona R, Schonman R, Mailova K, Ussia A, Koninckx P. Bowel resection for deep endometriosis: a systematic review. BJOG. 2011;118:285–291. doi: 10.1111/j.1471-0528.2010.02744.x. [DOI] [PubMed] [Google Scholar]
- 81.Yoon JH, Choi D, Jang KT, Kim CK, Kim H, Lee SJ, et al. Deep rectosigmoid endometriosis: “mushroom cap” sign on T2-weighted MR imaging. Abdom Imaging. 2010;35:726–731. doi: 10.1007/s00261-010-9643-3. [DOI] [PubMed] [Google Scholar]
- 82.Hottat N, Larrousse C, Anaf V, Noël JC, Matos C, Absil J, et al. Endometriosis: contribution of 3.0-T pelvic MR imaging in preoperative assessment--initial results. Radiology. 2009;253:126–134. doi: 10.1148/radiol.2531082113. [DOI] [PubMed] [Google Scholar]
- 83.Rousset P, Buisson G, Lega JC, Charlot M, Gallice C, Cotte E, et al. Rectal endometriosis: predictive MRI signs for segmental bowel resection. Eur Radiol. 2021;31:884–894. doi: 10.1007/s00330-020-07170-4. [DOI] [PubMed] [Google Scholar]
- 84.Vercellini P, Meschia M, De Giorgi O, Panazza S, Cortesi I, Crosignani PG. Bladder detrusor endometriosis: clinical and pathogenetic implications. J Urol. 1996;155:84–86. doi: 10.1016/s0022-5347(01)66550-9. [DOI] [PubMed] [Google Scholar]
- 85.Busard MP, Mijatovic V, Lüchinger AB, Bleeker MC, Pieters-van den Bos IC, Schats R, et al. MR imaging of bladder endometriosis and its relationship with the anterior uterine wall: experience in a tertiary referral centre. Eur J Radiol. 2012;81:2106–2111. doi: 10.1016/j.ejrad.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 86.Anaf V, Simon P, El Nakadi I, Fayt I, Buxant F, Simonart T, et al. Relationship between endometriotic foci and nerves in rectovaginal endometriotic nodules. Hum Reprod. 2000;15:1744–1750. doi: 10.1093/humrep/15.8.1744. [DOI] [PubMed] [Google Scholar]
- 87.Latthe PM, Proctor ML, Farquhar CM, Johnson N, Khan KS. Surgical interruption of pelvic nerve pathways in dysmenorrhea: a systematic review of effectiveness. Acta Obstet Gynecol Scand. 2007;86:4–15. doi: 10.1080/00016340600753117. [DOI] [PubMed] [Google Scholar]
- 88.Porpora MG, Vinci V, De Vito C, Migliara G, Anastasi E, Ticino A, et al. The role of magnetic resonance imaging–diffusion tensor imaging in predicting pain related to endometriosis: a preliminary study. J Minim Invasive Gynecol. 2018;25:661–669. doi: 10.1016/j.jmig.2017.10.033. [DOI] [PubMed] [Google Scholar]
- 89.Manganaro L, Porpora MG, Vinci V, Bernardo S, Lodise P, Sollazzo P, et al. Diffusion tensor imaging and tractography to evaluate sacral nerve root abnormalities in endometriosis-related pain: a pilot study. Eur Radiol. 2014;24:95–101. doi: 10.1007/s00330-013-2981-0. [DOI] [PubMed] [Google Scholar]
- 90.Zhang X, Li M, Guan J, Wang H, Li S, Guo Y, et al. Evaluation of the sacral nerve plexus in pelvic endometriosis by three-dimensional MR neurography. J Magn Reson Imaging. 2017;45:1225–1231. doi: 10.1002/jmri.25435. [DOI] [PubMed] [Google Scholar]
- 91.Andres MP, Arcoverde FVL, Souza CCC, Fernandes LFC, Abrão MS, Kho RM. Extrapelvic endometriosis: a systematic review. J Minim Invasive Gynecol. 2020;27:373–389. doi: 10.1016/j.jmig.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 92.Hirata T, Koga K, Osuga Y. Extra-pelvic endometriosis: a review. Reprod Med Biol. 2020;19:323–333. doi: 10.1002/rmb2.12340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yarmish G, Sala E, Goldman DA, Lakhman Y, Soslow RA, Hricak H, et al. Abdominal wall endometriosis: differentiation from other masses using CT features. Abdom Radiol (NY) 2017;42:1517–1523. doi: 10.1007/s00261-016-0998-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Horton JD, Dezee KJ, Ahnfeldt EP, Wagner M. Abdominal wall endometriosis: a surgeon’s perspective and review of 445 cases. Am J Surg. 2008;196:207–212. doi: 10.1016/j.amjsurg.2007.07.035. [DOI] [PubMed] [Google Scholar]
- 95.Tokue H, Tsushima Y, Endo K. Magnetic resonance imaging findings of extrapelvic endometriosis of the round ligament. Jpn J Radiol. 2009;27:45–47. doi: 10.1007/s11604-008-0293-0. [DOI] [PubMed] [Google Scholar]
- 96.Savelli L, Manuzzi L, Di Donato N, Salfi N, Trivella G, Ceccaroni M, et al. Endometriosis of the abdominal wall: ultrasonographic and Doppler characteristics. Ultrasound Obstet Gynecol. 2012;39:336–340. doi: 10.1002/uog.10052. [DOI] [PubMed] [Google Scholar]
- 97.Arakawa T, Hirata T, Koga K, Neriishi K, Fukuda S, Ma S, et al. Clinical aspects and management of inguinal endometriosis: a case series of 20 patients. J Obstet Gynaecol Res. 2019;45:2029–2036. doi: 10.1111/jog.14059. [DOI] [PubMed] [Google Scholar]
- 98.Gil Y, Tulandi T. Diagnosis and treatment of catamenial pneumothorax: a systematic review. J Minim Invasive Gynecol. 2020;27:48–53. doi: 10.1016/j.jmig.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 99.Joseph J, Sahn SA. Thoracic endometriosis syndrome: new observations from an analysis of 110 cases. Am J Med. 1996;100:164–170. doi: 10.1016/s0002-9343(97)89454-5. [DOI] [PubMed] [Google Scholar]
- 100.Rousset P, Gregory J, Rousset-Jablonski C, Hugon-Rodin J, Regnard JF, Chapron C, et al. MR diagnosis of diaphragmatic endometriosis. Eur Radiol. 2016;26:3968–3977. doi: 10.1007/s00330-016-4226-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing does not apply to this article as no datasets were generated or analyzed during the current study.