Abstract
Bio-fertilization is a sustainable agricultural practice that includes using bio-fertilizers to increase soil nutrient content resulting in higher productivity. Soil micro-flora has been exposed to improve soil fertility and increase biomass productivity and identified as a correct environmentally friendly bio-based fertilizer for pollution-free agricultural applies. The majority of cyanobacteria can fix nitrogen from the atmosphere and several species including Anabaena sp., Nostoc sp., and Oscillatoria angustissima is known to be effective cyanobacterial based bio fertilizers. Acutodesmus dimorphus, Spirulina platensis Chlorella vulgaris, Scenedesmus dimorphus, Anabaena azolla, and Nostoc sp. are some of the green microalgae and cyanobacteria species that have been successfully used as bio fertilizers to boost crop growth. Also, Chlorella vulgaris is one of the most commonly used microalgae in bio fertilizer studies. The addition of seaweed species that are Sargassum sp. and Gracilaria verrucosa leads to chemical changes as a soil fertility indicator on clay and sandy soils, and the addition of seaweed conditioner to soil can improve its organic content, return pH to normal, and reduce C/N ratio in both sandy and clay soil. This review provides an effective approach to increase soil fertility via environmentally friendly bio-based fertilizer using micro and macro algae. Instead of the usage of inorganic and organic fertilizers that have polluted impacts to soil as aggregation of heavy metals, in addition to there their human carcinogenic effects.
Keywords: Bio fertilization, Microalgae, Macro algae, Soil Fertility, Plant growth
1. Introduction
The success of agriculture greatly depends on the fertility level of the soil. Soil health is the foundation of organic farming systems. Plants require critical nutrients from fertile soil which also supports a diversified and dynamic biotic population that helps the soil resist environmental degradation. Heavy metals are one of many elements found in soil, but their concentration in the environment has risen in recent years as a result of human activities such as mining, energy generation, fuel manufacturing, electroplating, wastewater sludge treatment, and agriculture. These are conservative pollutants which means they are not degraded by bacteria or other organisms and thus remain in the environment indefinitely. As a result, their concentrations frequently exceed the allowable levels in soil, waterways, and sediments. Heavy metals such as arsenic, mercury, chromium, nickel, lead, cadmium, zinc, and iron are toxic to life when present in excess (Pereira and Da Gama 2008). Nitrogen deficiency reduces plant productivity in a variety of ways, including stunted growth and development, dwarf plant production, leaf yellowing, and lower yield. The recovery efficiency of most cropping systems is less than 50%, Furthermore, a significant amount of applied nitrogen is lost through volatilization, leaching, denitrification, and soil erosion (Chapman 2013). So, the world has become in bad need of effective and correct environmentally friendly bio-based fertilizer for pollution-free agricultural applies to increase soil production.
Bio fertilization is a sustainable agricultural practice that includes using bio fertilizers to upsurge the nutrient content of the soil, resulting in higher productivity (Suleiman et al. 2020). Algae, which are found in almost all terrestrial environments are the most distinctive organisms on the planet with potential applications such as agricultural applications as bio fertilizers and soil conditioning agents for the improvement of soil fertility and plant productivity (Chapman, 2013, Duarteet al., 2018). Soil algae are modest photosynthetic microorganisms that originate in the soil they also stay alive inches under the soil surface, soil is a substantial habitation for algal evolution (Duarte et al. 2018). It can help the soil to develop its features such as carbon content, aeration, texture, and nitrogen fixation. The addition of algae to other living organisms which are found in different soil types in various states could indicate the healthy range of the soil environment. The algal growth also reduces soil erosion by managing water flow. Similarly, they perform a role in soil reclamation, soil fertility, formation of microbiological crust, bio-controlling of agricultural pests, and agricultural wastewater treatment (Abdel-Raouf et al. 2016).
This review provides an effective and correct environmentally friendly bio-based fertilizer for pollution-free agricultural applies to increase soil production using micro and macro algae. It also provides the various types of algae that can be used to increase soil fertility and remove various heavy metals from the soil.
2. Bio-fertilizers
Bio fertilizers are live microorganisms that improve the chemical and biological aspects of soils, restore soil fertility, and encourage plant growth. Plants require nitrogen to flourish, and a deficiency in this nutrient can be corrected by applying enough fertilizer. Excessive and long-term usage of chemical or synthetic fertilizers, on the other hand, has resulted in contamination, which would eventually lead to ecosystem imbalance (Ritika and Utpal 2014).
2.1. Preparation of bio-fertilizers
On the slant, a pure culture of a potent strain of nitrogen-fixing cyanobacteria is grown on the required agar medium, a loopful of inoculum is placed in a conical flask with a capacity of 250 ml and a liquid medium, and a loopful of inoculum is placed in a conical flask with a capacity of 250 ml and a liquid medium, depending on whether the conical flask is quick or slow growing, place it on a rotary shaker or in an incubator for 3–7 days. Depending on whether the conical flask is quick or slow growing, place it on a rotary shaker or in an incubator for 3–7 days. The contents of these flasks normally contain 105–106 cells/mL, which is known as mother culture or starter culture. These mother cultures are reproduced in bigger flasks, which are then shaken on a rotary shaker for 96–120 h until the viable count per ml reaches 109–1010 cells. This broth culture with a population of 109–1010 cells/ml should not be stored for more than 24 h or at below 4 °C since the broth thickens and becomes inconsistent. Fermenters are then utilized to create microbial products such as bio-fertilizers and bio-pesticides on a big scale. The fermenter broth is immediately transferred to automatic filling equipment and packed into 250 ml, 500 ml, or 1 L bottles with a 0.5 mm thickness, leaving a 2/3rd space accessible for M.O. aeration (Ritika and Utpal 2014) Fig. 1.
Fig. 1.
Eleven steps for preparation of bio-fertilizers (Ritika and Utpal 2014).
3. Types of algae that can be used to increase soil fertility
In greenhouse-grown garden pea and field plots of spring wheat, researchers tested the impact of algae addition on plant development, nutrition, and soil physical and chemical features, using five species of algae that contrasted with the basic makeup. The cyanobacterium Arthrospira platensis (Spirulina), unicellular green algae Chlorella sp., red seaweed Palmaria palmate, and brown seaweeds were among them (Laminaria digitata and Ascophyllum nodosum). Chlorella and Spirulina enhanced total nitrogen and accessible phosphorus in the soil, and Spirulina improved soil nitrate levels. The inorganic (NH + 4 and NO3) concentrations in the soil were significantly improved by Palmaria palmata and Laminaria digitata. Chlorella sp increased total P, N, and C in the soil, as well as accessible P, NH + 4, N, and pea production. Algae additions had little effect on the water-stable aggregates in the soil. Chlorella sp., Spirulina, P. palmata, and L. digitata all enhanced soil inorganic nitrogen concentrations in the field (Mahapatra et al. 2018).
4. Algae as bio-fertilizers
Soil microflora has been found to promote biomass productivity and improve soil fertility. However, algal microflora has also been found as a true bio-based fertilizer for agricultural techniques that are both environmentally favorable and pollution-free. Although photosynthetic, these algae are adapted to extremes of environments and have been observed to thrive under extreme light settings with limited nutrients such as C and N levels and a low water need. They are extremely independent of alerts in terrestrial N pools that generate primary necessary amino acids, growth promoters, and hormones that are required for good crop growth in cropping systems because they are excellent N-fixing agents. The agricultural production in the industrialized world is largely determined by nature and the kind of soil, as most farmers in the lower sections of society cannot afford synthetic agricultural fertilizer unless it is subsidized (Mahapatra et al. 2018). Using improved crop varieties and efficient nutrient management strategies, the lost fertility of agricultural soils can be restored. This essentially advises that plentiful natural bio resources be used to improve crop nutrient status and allow for additional potential biological nutrient transfer mechanisms. Bio-fertilizers are important components of the regulated mineralization and fertilization process in these situations Table 1, Table 2. This can replace or supplement the currently utilized cost and energy-intensive chemical fertilizers because it is cost-effective, environmentally friendly, and renewable. Live and/or dead cells containing beneficial microbes are applied to plant and soil systems. They invade the rhizosphere quickly, promoting plant growth and development by converting inaccessible mineral forms into needed nutrients through mechanisms such as nitrogen fixation, mineralization, and rock phosphate solubility (Priya et al. 2014).
Table 1.
Effects of algal bio-fertilizer on plants growth and soil quality (Nosheen et al. 2021).
| Main conclusion | Parameters | Plant or crop | Algal species |
|---|---|---|---|
| The use of digested Chlorella sp. at 5 t ha−1 promotes plant growth and increases contents of metals in the corn plant. | Plant: dry weight, metal (Fe, Zn, Mn & Cu) content, uptake of macro-elements (N, P, K, Ca & Mg) | Corn plant |
Chlorella sp., Neochloris conjuncta Botryococcusbraunii |
| Algal fertilizer enhances plant growth and floral production. | Plant: numbers of the lateral root, flower bud, and branch; total fresh plant weight |
Roma tomato plant | Acutodesmusdimorphus |
| (1) Concerning plant growth, algal biomass can replace conventional fertilizer. (2) Algal fertilizer improves fruit quality by increasing the contents of sugars an carotenoids in tomato fruits. |
Plant: leaf length and weight, metal (K, Ca, Mg, Zn, Mn, Fe, & Cu) content Fruit: fruit yield, contents of sugars and carotenoids |
Tomato plant and fruit | Nannochloropsis oculata |
| (1)Algal bio-fertilizer increases the germination rate of seeds and shortens germination period. (2) Algal fertilizer promotes the growth of roots and leaves and enhances photosynthesis. |
Seed: germination rate and germination period Plant: length of roots and leaves, the weight of roots and leaves, photosynthesis activity |
Corn seed and plant | Chlorella sp. |
| Algal biomass is a viable option for delivering nutrients to support agriculture on marginal soils. |
Plant: weights of shoot and root, root density, length, and diameter; Plant: plant height, number of leaves per plant, plant weight, leaves area per plant, seed yield characters |
Wheat Plant |
Chlorella vulgaris |
| (1) Chlorella vulgaris and Spirulina platensis can be used as bio-fertilizer to enhance rice yield. (2) Algal bio-fertilizer improves the biological and chemical properties of the soil. |
Soil: soil biological activity (CO2 evolution, dehydrogenase activity, nitrogenase activity, etc.), soil chemical properties (pH, available-N, available-P, available-K) |
Rice plant |
Chlorella vulgaris Spirulina platensis |
| Table 1. cont.1. | |||
| Main conclusion | Parameters | Plant or crop | Algal species |
| The addition of an appropriate amount of algal biomass in the soil promotes plant growth, improves the elemental composition of the soil, and maintains a safe low level of heavy metals in soil. | Plant: numbers of roots and leaves, shoot length, stem thickness chlorophyll concentration Soil: total N, total P, total K |
Date palm | Tetraselmis sp. |
| (1) Algal biomass after lipid extraction can be used as a soil amendment for agricultural production. (2) At high addition rates, problems with excess soil salinity and sodicity may occur. | Soil: the content of organic carbon, microbial biomass carbon, total N, extractable inorganic N, etc. |
NA * | Nannochloropsi s salina |
| (1) P release from algal biomass increases the concentrations of labile and moderately labile P fractions in soil. (2) Algal fertilizer releases P when incorporated into the soil to support or even sustain plant nutrition |
Soil: P content in the soil | Wheat plant | Chlorella vulgaris |
| Cyanobacterial biomass and exopolysaccharide result in an increase of enzymatic activities. |
Soil: activities of enzymes (β-glucosidase, urease, arylsulphatase, protease, etc.) |
NA |
Nostoc muscorum Tolypothrix tenuis |
| Bio-fertilizer increases nodulation, plant growth, and production of the common bean. |
Plant: plant height, number of nodules, nodule dry matter, shoot dry matter, accumulated shoot nitrogen, number of pods per plant, number of grains per pod, hundred-grain weight, grain plant weight |
Common bean |
Anabaena cylindrica |
| (1) Cyanobacteria perform well in bioameliorating salt-affected semi-arid soils. (2) Grain yield and leaf area are improved. | Soil: nutrient dynamics in soil, microbial activities, physical characteristics (bulk density, water holding capacity, etc.) Plant: leaf area, spike length, grain yield, protein content |
Pearl millet & wheat |
Consortia of Nostoc ellipsosporum and Nostoc punctiforme |
| Table 1. cont.2. | |||
| Main conclusion | Parameters | Plant or crop | Algal species |
| Cyanobacteria promote the formation of soil surface consortia and improve surface stabilization of agricultural soil. |
Soil: formation of soil surface consortia, biomass adherence to the soil under water flush treatment |
NA |
Nostoc sp. and Anabaena sp. |
| Cyanobacteria improve crust formation, favor the proliferation of other microorganisms, and restore microbial populations in soil | Soil: soil microbiota, contents of available nutrients (P, K, Na, Ca & Mg) |
NA |
Oscillatoria sp.,Nostoc sp. and Scytonema sp. |
| (1) Cyanobacteria can colonize soils from arid and semi-arid areas. (2) Extracellular polymeric substances secreted by cyanobacteria blind soil particles together, increasing surface stability and reducing clay dispersion. | Soil: soil physicochemical properties and soil stability parameters |
NA |
Leptolyngbya sp., Oscillatoria sp., Microcoleus vaginatus, Nostoc commune, etc. |
| (1) Cyanobacteria promote plant growth and increase the weight of essential oil. (2) Wollea vaginicola dramatically increases the P content in the soil. | Plant: weights of shoot and root, lengths of shoot and root, flower head diameter and weight, the weight of essential oil Soil: contents of nutrients (Ca, P, & N) |
Chamomile |
Nostoc carneum, Wollea vaginicola,and Nostoc punctiforme |
| Inoculation of cyanobacteria leads to biological soil crust formation and prevent soil loss. | Soil: biological soil crust quality indicators, soil loss |
NA | Nostoc sp. and Oscilatoria sp. |
Table 2.
Algae Used as Bio fertilizer in Different Parts of the World (Chatterjee et al. 2017).
| Contribution | Species Name | Major Class of Algal Bio fertilizer |
|---|---|---|
| 1. Rich in nitrogen, potassium, and phosphorus 2. Carbohydrates (improve aeration and soil structure, especially in clay soils and have good moisture retention properties) 3. Used as a source of naturally occurring plant growth regulators 4. Enhance plant growth, freezing, drought and salt tolerance; photosynthetic activity; and resistance to fungi, bacteria, and virus |
Laminaria digitata (Oarweed), Saccharina latissima (Sugar Kelp), Fucus vesiculosus (Bladder wrack), Ascophyllum nodosum (Knotted wrack), Ecklonia maxima, Stoechospermum marginatum |
Brown macro algae |
| Trace elements |
Phymatolithon calcareum. Lithothamnion corallioides |
Red macro algae |
| 1. Fix 18e45 kg N/ha in submerged rice field 2. Produce growth-promoting substances |
Nostoc, Anabaena, Aulosira, Tolypothrix, Nodularia, Cylindrospermum, Scytonema, Aphanothece, Calothrix, Anabaenopsis, Mastigocladus, Fischerella, Stigonema, Haplosiphon, Chlorogloeopsis, Camptylonema, Gloeotrichia, Nostochopsis, Rivularia, Schytonematopsis, Westiella, Westiellopsis, Wollea, Plectonema and Chlorogloea |
Blue-green algae |
| 1. Fixes 40e80 kg N/ha 2. Used as green manure because of large biomass |
Anabaena azollae | Anabaena Azolla association |
4.1. Cultivation
For the generation or cultivation of algal biomass, open Pond and closed photo-bioreactor (PBR) technologies have been developed. The production of open ponds is divided into two systems 1. Natural waters (ponds, lakes, and lagoons) and 2. Artificial ponds (circular and raceway). The open pond is a less expensive method of producing large-scale algal biomass than the PBR; but, the PBR provides a superior and controlled closed culture system for growing, which prevents contamination from molds, bacteria, protozoa, and competition from other microalgae. It's frequently set up outside to take use of the free energy that sunshine provides (Ozcan Konur 2015).
4.2. Harvesting
Biomass aggregation (flocculation and ultrasonic), flotation, centrifugation, and filtration are four ways for separating algal biomass from the growth media or harvesting it. In some circumstances, a combination of two or more strategies is utilized to boost effectiveness. Algae are chosen depending on a number of factors, including density, size, and desired end products. With the addition of flocculants to the media, such as multivalent captions and cationic polymers, algal cells are aggregated together to form a bigger particle known as a floc in this approach, which helps neutralize the cells' surface charge. Flotation is a technology that uses a micro-air bubble disperser to float algal cells on the water's surface without the use of chemicals; it is cost-effective due to its cheap operational expenses, simple operating procedure, and high biomass yield. Using a centrifuge and gravitational force, centrifugation is a method of extracting algal biomass from culture media. This technology is quick, easy, and effective, but because to the large energy input and maintenance requirements, the cost can soon climb. Another problem of this method is that it damages interior cells, resulting in the loss of delicate nutrients, whereas filtration is a way of extracting alga biomass from the liquid culture media by utilizing a porous membrane with a range of particle sizes. There are three types of filtration: ordinary, microfiltration, and ultrafiltration (isolation of metabolites) (Barros et al. 2015).
4.3. Biomass dehydration
Algae biomass is quickly processed to the next stage after being isolated from the growth medium to prevent spoiling or extend its shelf life. The three most popular types of drying or dehydration procedures are sun drying, spray drying, and freeze drying. The method used is largely influenced by the intended end results; when compared to the other two; sun-drying is the least expensive option. Because this method relies only on solar energy, it is limited by weather conditions, long drying durations, and the size of the drying area required. Overheating, as well as changes in texture, color, and taste of the microalgae, may occur as a result of the unregulated process of drying with sunlight. Spray-drying is a method for making a dry powder from a thin spray of suspension droplets in a big vessel that is constantly in touch with heated air. This method has many advantages, including the ability to operate continuously, the fine powder produced, and the rapid drying ability to maintain a high-quality product because of its efficiency. However, some algae components, such as pigments, can deteriorate significantly, and the operation cost is high. Because large-scale production is prohibitively expensive, freeze-drying, also known as lyophilisation, is frequently employed in laboratories to dry microalgae. Freeze-drying is a dehydration method that uses the sublimation mechanism to dehydrate frozen items. The microalgae are frozen before freeze-drying to solidify the material at low temperatures. As the moisture content of the microalgae declines, the product's solid structure and quality are preserved (Balasubramaniam et al. 2021).
5. Microalgae as bio-fertilizers
Eukaryotic green algae and prokaryotic blue algae are examples of photosynthetic organisms known as microalgae. They have a lot of promise as biological resources in fields including medicine, health care, feed, and fuel. Because of their potential to increase soil nutrients, these intriguing organisms can also be utilized in modern agriculture. They might be unicellular, multicellular, filamentous, or saponaceous in nature. They are also the world's largest primary producers, with over 200,000 species. Microalgae production entails mass cultivation, biomass recovery, and downstream operations to ensure a consistent yield for food, chemicals, feed, biofuel, and bio fertilizers (Balasubramaniam et al. 2021). In addition to enhancing soil fertility and quality, microalgae can create plant growth hormones, polysaccharides, antibacterial chemicals, and other metabolites (e.g., Spirulina sp., Chlorella sp., Cyanobacteria (blue-green algae) (Ronga et al. 2019). Because they participate directly in the assimilation of atmospheric carbon dioxide into organic algal biomass via photosynthesis, cyanobacteria and green microalgae are major organic matter sources in the agro-ecosystem (Guo et al. 2020a). Algae are responsible for half of all photosynthesis on the globe. Algae may greatly enhance soil organic carbon content by assimilating carbon dioxide. Heterocyst cells in cyanobacteria may fix atmospheric nitrogen and thereby meet the needs of soil micro and macrofauna, flora, and plants. Crops injected with cyanobacteria or cyanobacteria consortia boosted soil nitrogen concentration significantly, according to several studies. Cyanobacteria inoculation can economize 25–40% of chemical nitrogen fertilizer in the soil. Various microalgae and cyanobacteria that can be used as bio fertilizers were summarized (Ritika and Utpal 2014) Table 3, Table 4.
Table 3.
Microalgal and cyanobacterial metabolites with potential interest for agriculture (Gonçalves 2021).
|
Role in Agriculture |
Biological Activity |
Microalgal/Cyanobacterial Sources | Examples | Metabolites |
|---|---|---|---|---|
| Crops’ protection against pathogens or other biotic and abiotic stress conditions | Antibacterial; antioxidant; antifungal |
Botryococcus braunii; Chaetoceros calcitrans; Chlorella vulgaris; Isochrysis galbana; Isochrysis sp.;Neochloris oleoabundans; Odontella sinensis; Phaeodactylum tricornutum; Saccharina japonica; Skeletonema costatum; Tetraselmis suecica |
Polyphenols; phenolic acids; flavonoids; phenylpropanoids |
Phenolic compounds |
| Crops’ protection against bacteria, insects, and other organisms Stimulation of preliminary growth and development of plants Attraction of pollinators | Antibacterial; anticarcinogenic; antioxidant |
Chondrococcus hornemanni; Hypnea pannosa; Oscillatoria perornata; Planktothricoids raciborskii; Plocamium cornutum; Plocamium leptophyllum; Portieria hornemann; Pseudanabaena articulate; Pseudanabaena sp.; Sphaerococcus coronopifolius; Synechocystis sp.; Thermosynechococcus elongate. |
Hemiterpenes; monoterpenes; sesquiterpenes; diterpenes; triterpenes; polyterpenes |
Terpenoids |
| Crops’ protection against pathogens or other biotic and abiotic stress conditions | Antibiotic; anticarcinogenic; antifungal; antioxidant; antiviral |
Anabaena; Chlorella; Dunaliella; Nannochloropsis; Porphyridium; Scenedesmus; Spirulina |
Saturated and unsaturated fatty acids |
Free fatty acids |
| Improvement of soil Quality Plant growth stimulation Crops’ protection against biotic and abiotic stress conditions |
Antibacterial; anticancer; anticoagulant; anti-inflammatory; antioxidant |
Aphanothece; Arthrospira; Chlamydomonas; Chlorella; Cylindrotheca; Dunaliella; Navicula; Nostoc; Phaeodactylum; Porphyridium; Rhodella; Scytonema |
Extracellular polysaccharides; structural polysaccharides; energy-storage polysaccharides |
Polysaccharides |
| Table 3. cont.1. | ||||
|
Role in Agriculture |
Biological Activity |
Microalgal/Cyanobacterial Sources | Examples | Metabolites |
| Soil bioremediation and fertilization Crops’ protection against bacteria, insects, and other biotic and abiotic stress conditions Crops’ fortification | Anticancer; anti-inflammatory; antioxidant |
Chlorella protothecoides; Chlorella pyrenoidosa; Chlorella zofingiensis; Dunaliella salina; Haematococcus pluvialis; Muriellopsis sp.; Phaeodactylum tricornutum; Spirulina sp. |
Alpha-carotene; beta-carotene; lutein; lycopene; astaxanthin; zeaxanthin |
Carotenoids |
| Plant growth stimulation Regulation of cellular activities in crops’ response to stress conditions | Chemical messengers |
Arthrospira; Chlamydomonas; Chlorella; Phormidium; Protococcus; Scenedesmus |
Auxins; abscisic acid; cytokinins; ethylene; gibberellins |
Phytohormones |
Table 4.
Impacts of microalgae and cyanobacteria (and their metabolites) on soils’ improvement (Gonçalves 2021).
| Observed Improvements | Mode of Action | Target Crop/Soil | Algae/Algal Extracts |
|---|---|---|---|
| Increase in nitrogen availability in the soil. Increase in grain and straw yield |
Nitrogen fixation | Rice |
Cyanobacterial inoculum composed by Aulosira fertilissima, Anabaena sphaerica, Nostoc hatei, Cylindrospermum majus and Westiellopsis prolifica |
| Increase in grain, straw and seeds yields Increase in plant height and leaf length |
Nitrogen fixation | Rice |
Wild type and herbicide-resistant strains of Anabaena variabilis |
| Increase in grain yields comparable to those obtained with a chemical fertilizer |
Nitrogen fixation | Rice | Nostoc sp. vegetative cells |
| Increase in nitrogen fixation Increase in growth parameters, germination percentage and photosynthetic pigments Increase in the nutritional value of pea seeds |
Nitrogen fixation | Pea | Nostoc entophytum and Oscillatoria angustissima |
| Increase in nitrogen availability in the soil | Nitrogen fixation | Wheat | Anabaena torulosa biofilm |
| Increase in nitrogen availability in the soil Increase in plant fresh weight |
Nitrogen fixation | Soybean and mungbean |
Cyanobacterial-bacterial biofilms including the species: Calothrix sp., Anabaena laxa, Anabaena torulosa, Anabaena doliolum, Nostoc carneum, Nostoc piscinale, Trichoderma viride, Pseudomonasfluorescens and Azotobacter chroococcum |
| Increase in organic carbon content in the soil Increase in grain yield |
Nutrients’ availability in soils |
Wheat |
Calothrix ghosei, Hapalosiphon intricatus and Nostoc sp. |
| Increase in nitrogen and phosphorus availability in the soil Decrease in soil density Improvement of water retention capacity Increase in grain yields Improvement of nutritional properties (increase in protein content of grain and leaves). |
Nutrients’ availability in soils |
Wheat and millet |
Cyanobacterial consortia including the species: Anabaena doliolum, Cylindrospermum sphaerica and Nostoc calcicola |
| Table 4. cont.1. | |||
| Observed Improvements | Mode of Action | Target Crop/Soil | Algae/Algal Extracts |
| Increase in ammonium, phosphorus and potassium availability in the soil Improvement of fruit quality (increase in sugar and carotenoids contents) |
Nutrients’ availability in soils |
Tomato |
Microalgal-bacterial flocs and Nannochloropsis oculata |
| Increase in zinc and iron availability in the soil Beneficial changes in the microbiome Increase in root yield and weight |
Nutrients’ availability in soils |
Okra |
Consortia and biofilms including the species: Azotobacter sp., Anabaena sp., Providencia sp. and Calothrix sp. |
| Increase in nitrogen, phosphorus, and potassium availability in the soil Increase in organic carbon content in the soil Improvement of product quality (increase in nitrogen, phosphorus, and potassium contents in roots, shoots, and grains) |
Nutrients’ availability in soils |
Wheat |
Microalgal-cyanobacterial unicellular and filamentous consortia including species of: Chlorella, Scenedesmus, Chlorococcum, Chroococcus, Phormidium, Anabaena, Fischerella and Spirogyra |
5.1. Extraction of bioactive compound
Carbohydrates, fatty components, proteins, minerals, and a variety of other substances make up microalgae. The algal biomass will be processed to release the bioactive compounds that have been contained in the cells, allowing the compounds to be used in a variety of applications including biofuels/energy and agricultural use (Guo et al., 2020a, Tyoker Kukwa and Chetty, 2021). The cell walls will be lysed in a variety of ways, including physical, mechanical (bead milling, homogenization, microwave, ultrasonic, and pulsed electric field), chemical (solvent, acid, and alkali), and biological (enzymes). The desired end products define the pretreatment method utilized (Salinas-Salazar et al. 2019). For 10 min, one kilogram of freeze-dried biomass was suspended in 150 g/ L of distilled (DI) water and swirled on a stirring plate to allow the biomass to dissociate. To rupture the cell wall and get intracellular extracts, the suspension was run through a Micro fluidizer, a mobile high-shear fluid processor, at a flow rate of 450 ml/min at 172 mPa. To decrease the likelihood of deterioration, the extract was centrifuged at 8983 rpm for 10 min at 22 °C to separate the cell extracts from the biomass residue. To decrease the likelihood of deterioration, the extract was centrifuged at 8983 rpm for 10 min at 22 °C to separate the cell extracts from the biomass residue. The extract supernatant was collected in an aluminum-foiled flask and stored in a cold room at 4 °C; the biomass residue was also maintained in the cold room for future use. Two commonly employed microalgae species in wastewater treatment, Chlorella sp. and Spirulina (Arthrospira platensis and Arthrospira maxima), have been demonstrated to have strong nutrient (N and P) removal capacities from effluents, making them suitable candidates for soil conditioners. Biomass from Spirulina platensis has been demonstrated to improve soil macronutrients (nitrogen, phosphorus, and potassium), function as a bio fortifier, raise plant protein content, and boost crop development (5 g Spirulina in 500 g soil). When compared to the control group, the height of Bayam red (Ameranthus gangeticus) increased by 58.3 percent, and the fresh and dry weights increased by 110.1 percent and 155.8 percent, respectively (Alobwede et al. 2019). Microalgae, as an alternative, have been intensively examined to assess their potential as plant bio-fertilizers and bio-stimulants by adding 2–3 g dried Chlorella vulgaris kg−1 soil. The plurality of cyanobacteria can belay nitrogen from the atmosphere, and certain species, such as Anabaena sp., Nostoc sp., and Oscillatoria angustissima, have been used as cyanobacteria-based bio fertilizers. Some of the green microalgae and cyanobacteria species that have been successfully used as bio fertilizers to boost crop growth including Chlorella vulgaris, Scenedesmus dimorphus, Anabaena azolla, and Nostoc sp., with Chlorella vulgaris being one of the most generally used microalgae in bio fertilizer studies. Germination of Hibiscus esculentus was enhanced by utilizing a combination of seed and soil treated with Chlorella.Vulgaris. Before applying the bio fertilizers, the composition and number of microorganisms were measured. Plants and soils have reaped several benefits from green microalgae/cyanobacteria treatments. Furthermore, the excretion of carbon (exopolysaccharides) by green microalgae/cyanobacteria into the soil improved soil fertility, seed germination, plant growth, yield, and nutritional value of crops, as well as the carbon and organic content of the soil, biomass degradation, and grazing activity. These factors have an impact on microbial activity and biomass of other microflora and fauna in the soil which eventually will stimulate the growth of the crops. Chlorella vulgaris has the potential to be used as a bio fertilizer for the germination of tomato and cucumber seeds. To improve the length of tomato and cucumber roots and shoots, 0.17 and 0.25 g/L algae solutions were utilized. When compared to the control group and the treatment group administered during transplant, plants treated with Acutodesmus dimorphus bio-fertilizers before seedling transplant demonstrated better germination, branch, and flower development (Alobwede et al. 2019).
De-oiled microalgae biomass Scenedesmus sp. was successfully employed as a bio fertilizer to boost rice plant development. Cyanobacteria may colonize numerous sections of a plant's tissue, such as the roots and shoots, causing the microbial population to fix nitrogen and solubilize phosphorus. As a result, the plant's development, nutritional condition, and defensive mechanisms, as well as soil fertility, are enhanced and improved. Cyanobacteria create siderophores (organic molecules) to help in the chelation of micronutrients (e.g., Fe and Cu) to make them more accessible for plant development, a process known as biomineralization (Chittora et al. 2020) Table 5. Maize plants treated with Chlorella vulgaris and Spirulina platensis along with cow dung manure for 75 days under greenhouse conditions appeared to improve maize plant growth and yield, possibly by combining different microalgae species or combining microalgae with other organic or chemical fertilizers. The rice plant was bio fertilized using a mixed culture of Chlorella vulgaris (UTEX2714) and Scenedesmus dimorphus (UTEX1237), which showed efficiency by dramatically increasing the rice plant's height. In comparison to the control group, onion plant growth characteristics improved with the application of a mixture of Spirulina platensis and Chlorella vulgaris, with a greater growth rate and yield. Green microalgae and cyanobacteria consortiums also showed encouraging outcomes, such as enhanced soil microbial activity and soil organic carbon (Chittora et al. 2020).
Table 5.
Commercial seaweed products are used in agriculture (Nabti et al. 2016b).
| Application | Seaweed name | Product name |
|---|---|---|
| Plant growth stimulant |
Ascophyllum nodosum Ascophyllum |
Acadian |
| Plant growth stimulant |
Nodosum Macrocystis pyrifera |
Agri-Gro Ultra |
| Plant growth stimulant |
Ascophyllum nodosum | AgroKelp |
| Plant growth stimulant |
Unspecified | Bio-GenesisTM High TideTM |
| Bio fertilizer | Ecklonia maxima | Fartum |
| Plant growth stimulant |
Durvillea antarctica unspecified |
Kelpak |
| Plant biostimulant | Unspecified | Profert |
| Plant biostimulant | Durvillea potatorum | Sea Winner |
6. Macro algae as bio-fertilizers
Seaweeds are macro-algae that have a variety of uses such as fertilizer, soil conditioner, animal feed, cosmetics, biofuel, integrated aquaculture, and waste treatment (Khan et al. 2009). These sea plants are also recognized to be a bioactive compound-rich natural resource (Hashem et al. 2019). Carotenoids, terpenoids, xanthophylls, chlorophylls, phycobilins, polyunsaturated fatty acids, polysaccharides, vitamins, sterols, tocopherol, and phycocyanins are among the physiologically active phytochemicals generated by macro algae. On a worldwide basis, seaweed is still considered an undervalued resource (Osório et al. 2020) (Table 3). Sargassum is a fast-growing macro algae with high levels of antioxidants, carotenoids, and phenols, including the well-known anti-cancer substance fucoxanthin, making it a possible source of a range of pharmaceutically relevant elements (Silva et al. 2019). Over 300 species of Gracilariales rhodophyta have been identified, with 160 of them being taxonomically recognized. Because they can generate significant amounts of commercially useful biomass, the macro algae of this genus are essential for industrial and biotechnological purposes. They are regarded economically valuable resources. Sargassum is a brown macroalga that may be found in temperate and tropical waters all over the world. They're typically found in shallow water and on coral reefs, although there are a few free-floating species as well. It is regularly washed up on the shore and discovered. In certain locations, sargassum is harvested for use as a food source and fertilizer. It is regularly washed up on the shore and discovered. In certain locations, sargassum is harvested for use as a food source and fertilizer. It is also used as a medication source (Silva et al. 2019). Seaweed fertilizer products have been created with the goal of enhancing germination, deeper root penetration, nutrient absorption, and crop output in the treated crop. Seaweeds have been employed as a fertilizer since the eighteenth century, although exclusively in the coastal areas. Drifted seaweeds have long been employed as soil conditioners by coastal communities all over the world (Nabti et al. 2016a). The Jepara seaweeds Gracilaria verrucosa and Sargassum sp. were harvested in Central Java, rinsed properly to eliminate contaminating soil, and dried in the sun to achieve their minimal water content. The dried seaweeds were then immersed in fresh water to remove the salt and lower the salinity (Nabti et al. 2016a). This process was continued until the salinity of the water was zero (0 ppt). To make seaweed powder, a second phase of drying and milling was performed. The dried seaweed powders were then used to treat the soil. To achieve equitable distribution, up to 10% seaweed powder was placed into a 40 cm × 40 cm × 40 cm polyethylene bag previously filled with sandy and clay soil (about 90% of capacity). In this experiment, four treatments were used: 90% sandy soil with 10% Sargassum powder, 90% sandy soil with 10% Gracilaria powder, 90% clay soil with 10% Sargassum powder, 90% clay soil with 10% Gracilaria powder, and 100% sandy soil and 100% clay soil as controls (each treatment was repeated four times). A completely randomized design with two elements was used to construct this. The two types of soil (sandy and clay soil as the principal medium) are one influence, while the two types of soil conditioners are another (Sargassum and Gracilaria powder) (Khanet al., 2009, Chittoraet al., 2020, Zouet al., 2021). Soil organic content, pH, C/N ratio, water holding capacity, and soil infiltration are some of the soil fertility metrics that are examined. The dry combustion technique was used to determine the organic content of the soil. pH meter was used to determine the acidity of the soil. To estimate the soil's C/N ratio, total carbon and total nitrogen in the soil were computed (Kaur 2020).
6.1. Effect of macro algae as bio fertilizers on soil organic content
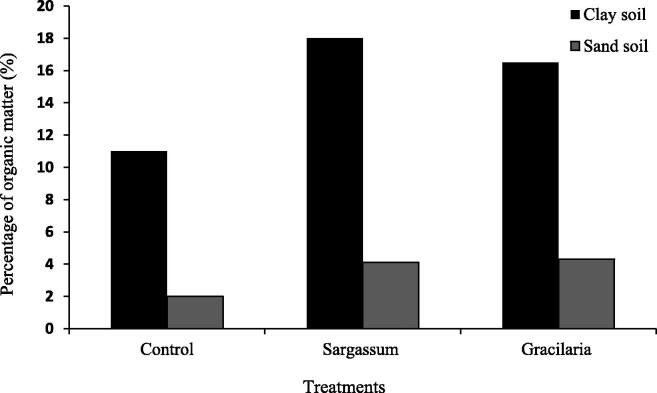
One of the most important determinants of soil fertility is organic content; Soil organic matter is defined by FAO in 2017 as a complex and continuous mixture of partially decomposed organic substances derived from plant litter. It serves an important purpose and the ecosystem acts as a buffer against climate change. As a result, it aids in food production and water availability. the use of plant litter was evaluated derived from seaweed biomass from two species Sargassum sp. and Gracilaria verrucosa (Izzati 2015). The biomass of these two species differs in their ability to support organic content in the soil by the addition of soil conditioner derived from seaweeds significantly increased the organic content of both sandy and clay soil, there is a significant difference in organic material content between sandy and clay soil in the controls (media without the addition of any of these soil conditioners). By comparing with sandy soil; clay soil contains significantly more organic material. The amount of organic matter in the soil is strongly related to the texture of the soil, soil texture and organic matter content have a strong relationship (Feller and Beare 1997). The higher the organic matter content, the finer the texture of the soil because organic matter breaks down into smaller particles more easily than soil particles. Soil fertility will improve as organic matter content increases. The most important determinant of soil fertility is organic matter in the soil. The addition of a soil conditioner will have a significant impact on the total organic matter in the soil, in both sandy and clay soil. The addition of Sargassum powder can increase organic matter more than the addition of Gracilaria regardless of the significance. The difference in organic matter improvement between these two types of soil conditioners is based on the possibility to conclude that adding soil conditioners derived from seaweeds will significantly increase organic matter content in both sandy and clay soil (Feller and Beare, 1997, Izzati, 2015) Fig. 2.
Fig. 2.
Effect of conditioners made from seaweed Sargassum and Gracillaria on soil organic content.
6.2. Effect of macro algae as bio-fertilizers on soil PH
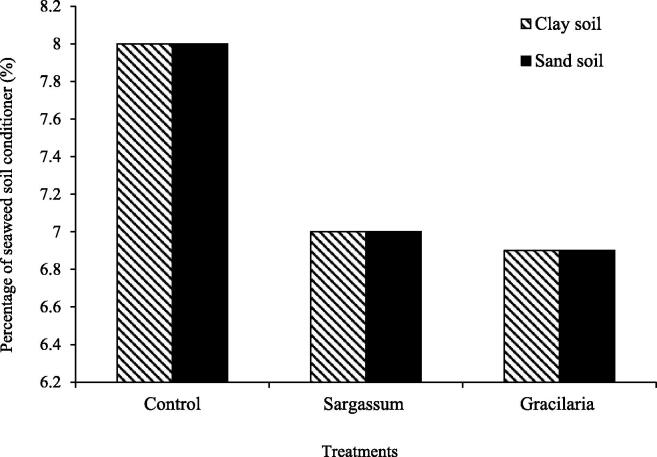
Soil pH is a measure of acidity in the soil and is involved in a number of chemical reactions. It regulates nutrient chemical forms, which affects plant nutrient availability. The optimal pH range for most plants is between 5.5 and7.5; however, the addition of seaweed soil conditioner has a substantial impact on soil pH; the pH of both media was about 8 before the soil conditioner was added. There was a significant suggestion of a pH reduction near to normal, which is7, following the application of seaweeds as a soil conditioner. When compared to Gracillaria sp., the seaweed soil conditioner with Sargassum sp. was substantially greater (Izzati et al. 2019) Fig. 3.
Fig. 3.
Effect of conditioners made from seaweed Sargassum and Gracillaria on soil pH.
6.3. Effect of macro algae as bio-fertilizers on soil C\N ratio
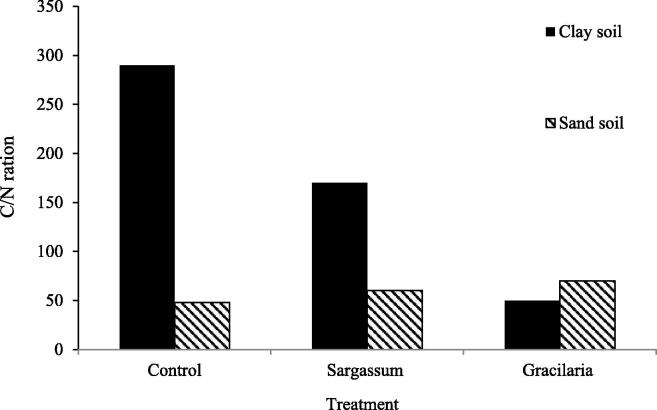
The C/N ratio is the ratio of carbon to nitrogen in a particular material, and it is an essential component in assessing soil fertility since it has a direct influence on residue breakdown and nitrogen cycling in soils. For agricultural residue decomposition, a C/N ratio of 24 is optimum. The greater the C/N ratio, the more carbon and the lower the nitrogen content of the medium. A C/N ratio of less than 24 also implies that the soil is more productive owing to the increased nitrogen concentration. Because it is essential for protein synthesis and enzyme function in metabolism, nitrogen is the most crucial nutrient for plant development. Because soil conditioner is mostly made up of plant litter, it will raise more C/N ratio when added to particular medium. In control medium, a substantial variation in the C/N ratio was identified between sandy and clay soil. Clay soil has a much higher C/N ratio; clay soil's high organic matter concentration is a key element in establishing its high carbon content (Izzati et al. 2019) Fig. 4. In sandy soil, the presence of a soil conditioner generated from seaweeds enhances the C/N ratio, which is larger with Sargassum than with Gracilaira. Because the quantity of organic carbon in sandy soil is substantially smaller, adding a soil conditioner improves its organic carbon content. Clay soil, on the other hand, has a very high C/N ratio due to its high organic carbon content. The use of a soil conditioner lowers the C/N ratio substantially. The increase in nitrogen mass in the soil conditioner appears to be the cause. Seaweed is recognized for having a high protein content, which explains why utilizing it as a soil conditioner would offer additional nitrogen. Consequently, the C/N ratio will be significantly reduced. The quantity of protein in marine algae differs depending on the species (Fleurence et al. 2012), the protein content of brown seaweeds was still lower than that of green and red seaweeds. Because red seaweeds like Gracilaria may sustain higher protein and nitrogen availability in the soil, the C/N ratio is substantially lower than when brown seaweeds like Sargassum are added. The effects of seaweed species Sargassum sp. and Gracilaria verrucosa as soil conditioners on chemical alterations as a soil fertility indicator on sandy and clay soil were investigated. In both sandy and clay soil, the use of a seaweed soil conditioner can enhance soil organic content, bring pH to normal, and lower C/N ratio. Because red seaweeds have greater protein content than brown seaweeds, Gracilaria surpasses Sargassum in terms of decreasing the C/N ratio (Izzati et al. 2019).
Fig. 4.
Ratio of C\N performance on media added with soil conditioner made from seaweed Sargassum and Gracillaria.
7. Heavy metal removal from soil using algae
Heavy metals are just one of many elements found in soil, but their presence in the environment has grown in recent years as a result of human activities such as mining, energy production, fuel production, electroplating, wastewater treatment, and agriculture. These are conservative pollutants, meaning they are not destroyed by bacteria or other organisms and hence persist indefinitely in the environment. As a result, their concentrations in soil, streams, and sediments regularly exceed the permissible limits. When heavy metals such as arsenic, mercury, chromium, nickel, lead, cadmium, zinc, and iron are present in excess, they are poisonous to life (Fleurenceet al., 2012, Hassan et al., 2017). These toxins have the ability to penetrate deep into the subsurface layer, affecting both groundwater and surface water. Heavy metals in the soil are subsequently taken up by plants and enter the human food chain (Izzati et al. 2019). When they enter the food chain, they cause major biological disruptions, posing a substantial health risk to humans. They also bio accumulate, which means they can be passed from one food chain to the next. As a result, their pollution of agricultural soils has become a severe problem in many industrialized and developing countries, threatening both crop output and human health. Heavy metal is divided into two categories: 1. Metals that are necessary as nutrient sources in trace amounts for many organisms become hazardous when present in large numbers (e.g., As, Cr, Co, Cu, Ni, Se, Va, and Zn) Table 6.and 2. Extremely poisonous metals (e.g., Pb, Hg, Cd, Ur, Ag, and Be) are not known to have any nutritional value (Fleurence et al. 2012).
Table 6.
Uptake and accumulation of metals by some algal species (Chekroun and Baghour 2013).
| Metal | Species |
|---|---|
| Gold (Au) Cobalt (Co) Nickel (Ni) Lead (Pb) |
Ascophyllum nodosum |
| Boron (B) | Caulerpa racemosa |
| Arsenic (As) | Daphnia magna, Chlorella pyrenoidosa and Spirogyra hyaline |
| Nickel (Ni) | Oscillatoria sp., Spirogyra sp., Anabaena variabilis, Aulosira sp., Nostoc muscorum, Oscillatoria sp., and Westiellopsis sp. |
| Zinc (Zn) | Laminaria japonica, Fucus vesiculosus |
| Cadmium (Cd) |
Micrasterias denticulate, Scenedesmus acutus, Chlorella vulgaris, Chlorella pyrenoidosa, Spirogyra hyaline, Fischerella sp., Lyngbya spiralis, Tolypothrix tenuis, Stigonema sp., Phormidium molle, Lyngbya heironymusii, Gloeocapsa sp., Oscillatoria jasorvensis, and Nostoc sp. |
| Cromium (Cr) |
Phormedium bohner, Anabaena variabilis, Aulosira sp., Nostoc muscorum, Oscillatoria sp. and Westiellopsis sp., and Chlorella minutissim |
| Strontium (Sr) | Platymonas subcordiformis |
| Copper (Cu) |
Sargassum filipendula, Tetraselmis chuii, Scenedesmus bijuga, Oscillatoria quadripunctulata, Spirulina platensis, Padina sp., Scenedesmus obliquus, Chlorella pyrenoidosa, and Closterium lunula |
| Copper (Cu) Iron (Fe) Zinc (Zn) Nickel (Ni) |
Sargassum fluitans |
| Lead (Pb) |
Sargassum natans, Spirulina (Arthrospira) platensis, Spirogyra hyaline, Dunaliella, Scenedesmus bijuga, Spirulina platensis, Oscillatoria quadripunctulata, Chlorella pyrenoidosa, Chlorococcum sp., Chlorella vulgaris var. vulgaris, Fischerella sp., Lyngbya spiralis, Tolypothrix tenuis, Stigonema sp., Phormidium molle, Lyngbya heironymusii, Gloeocapsa sp., Oscillatoria jasorvensis, Nostoc sp., and Scenedesmus acutus, Sargassum vulgare. |
| Cadmium (Cd), Mercury (Hg), Lead (Pb), Arsenic (As), and Cobalt (Co) | Spirogyra hyalina |
| Arsenic (As) | Tetraselmis chuil |
7.1. Mechanism of removal heavy metal by algae
Algae peptide chains bind to heavy metals to generate organometallic complexes that enter vacuoles and regulate heavy metal concentrations in the cytoplasm. The poisonous effects of heavy metals are checked by algae cells in the following way: phytochelatins and metallothioneins are the names given to the peptide chains. PCs are peptides that are generated enzymatically, whereas MTs are polypeptides that are encoded by genes. Class-III metallothioneins are also known as phytochelatins. Metallothioneins of classes II and III are present in algae, however Class-I metallothioneins are not found in algae. Heavy metals including Cd + 2, Ag+, Zn + 2, Hg + 2, Au + 2, Pb + 2, and Bi + 3 can stimulate Mt III synthesis. Mt III peptide molecules are critical in algae because their presence helps them to survive in high concentrations of heavy metals; Mt III production is related to pollution level (Fleurence et al. 2012).
7.1.1. Heavy metal removal process by microalgae
Reverse osmosis, electrodialysis, ultrafiltration, ion exchange, chemical precipitation, phytoremediation, and other traditional heavy metal removal procedures are used. However, these methods have drawbacks such as the removal of unfinished metal, high reagent, and energy requirements, as well as the creation of hazardous sludge or other waste products that must be carefully disposed of. Microalgal metabolism can convert, cleanse, and volatilize these xenobiotic chemicals and heavy metals, and because microalgae are nonpathogenic, there is no chance of unintended pollutant discharge into the environment. Bio-sorption has also been proposed as a way for removing heavy metals from water. Microalgae absorb waste as a source of nourishment and destroy contaminants enzymatically. Bio-sorption is the ability of biological materials to gather heavy metals on their surfaces, and it has gained favor in recent years because it uses bio-sorbents derived from naturally plentiful microalgae or byproducts of fermentation businesses. Moss, aquatic plants, and leaf-based adsorbents have all been discovered to be bio-sorbents/biomass. Microalgae have been shown to have significant metal-binding capacity, which has been linked to the presence of polysaccharides, proteins, or lipids on the surface of their cell walls, which include functional groups such as amino, hydroxyl, carboxyl, and sulphate, which can act as metal-binding sites. Microalgae cell walls can thus trap heavy metals. As a result, micro-algal biomass is expected to be extremely effective at removing heavy metals from aqueous solutions. This makes them a good source of the complex multifunctional polymers that are used to sequester a range of metals through adsorption or ion exchange. Heavy metals, on the other hand, have an effect on various cellular properties of microalgae, including cell viability, membrane structure, and other functions (Dhaliwal et al. 2020).
Microalgae are not typically recommended as food because of their proclivity to absorb toxic metals. However, their efficiency in removing metals from effluent water, impurities in nutrients, or atmospheric deposition into open ponds is so high that the biomass produced may contain amounts of metals that exceed the upper limit of metal content for food use. All species on this planet have evolved methods to maintain equilibrium with heavy metal ions in the surrounding medium, therefore cells have two major goals: 1. To identify which heavy metals are essential for growth and to eliminate those that aren't; 2. Maintain optimal intracellular concentrations of vital ions. Heavy metal removal characteristics have been observed in several micro-algal strains. The vast majority of surveys to date have relied on batch growth of microalgal species. With the use of this approach, a Chlorella strain capable of sustaining growth at 11.24 mg Cd 21/L and 65 percent elimination when exposed to 5.62 mg Cd21/L was identified. In batch cultures with Chlorella and Scenedesmus strains at 20 mg Cr 61/L, clearance percentages of 48 percent and 31 percent, respectively, were achieved. Al, Fe, Mg, and Mn can all be removed by Chlorella sp. Chlorella pyrenoidosa is also useful in this sense because it can remove a wide range of heavy metals, including copper, zinc, lead, mercury, arsenic, chromium, nickel, and cadmium. Chlorella minutissima is capable of removing Cr (VI), and Phaeodactylum tricornutum, which has a high Cd 21 tolerance (CE50 5 22.3 mg/L), is likewise capable of removing Cr (VI). It was categorised according to the Mt III production pattern. Heavy metal removal investigations typically use Scenedesmus, a genus of microalgae. It has been demonstrated that it can be used to remove Several Microalgae, namely, Scenedesmus sp., Chlorococcum sp., Chlorella vulgaris, Fischerella sp., Lyngbya spiralis, Tolypothrix tenuis, Stigonema sp., Phormidium molle, Lyngbya heironymusii, Gloeocapsa sp., Oscillatoria jasorvensis and Nostoc sp. can be used for the bioremediation of heavy metals like lead, mercury, and cadmium. Tetraselmis chuii, Scenedesmus bijuga, Oscillatoria quadripunctulata and Padina sp. can remove copper (Dhaliwal et al. 2020). Various microalgae that can remove heavy metals from the soil and water were summarized in Table 7.
Table 7.
Heavy metal removal by specific microalgae (Hassan et al. 2017).
| Microalgae | Heavy metals | Initial conc. (mg/L) | Final removal conc. (mg/g) |
|---|---|---|---|
| Chlorella vulgaris | Cd | 25–150 | 58.4 |
| Desmodes mupleiomorphus | Zn | 1–30 | 360.2 |
| Desmodes pleiomorphus | Cd | 0.5–5 | 61.2 |
| Chlorella vulgaris | Ni | 100 | 28.6 (immobilized) |
| Chlamydomonas reinhardtii | Cd | 20–400 | 77.62 |
7.1.2. Macro algae used to remove heavy metal
Bioaccumulation and bio-sorption are two methods that can remove heavy metals from seaweeds. Bio-sorption is the process of removing heavy metals from non-living materials through passive binding. Bioaccumulation, on the other hand, refers to biomass generated from an aqueous solution. Metal removal is an active process that needs the metabolic activity of a living organism. The environmental importance of studying organic xenobiotic bio-accumulation / bio-degradation in green algae is crucial since the extensive distribution of these chemicals in agricultural regions has become a major problem in aquatic ecosystems. In heavy metal-polluted environments, some algae and microorganisms have devised a variety of survival methods. Bio sorption, bioaccumulation, biotransformation, and bio mineralization are examples of detoxifying mechanisms developed and adopted by these organisms, which can be exploited for ex situ or in situ bioremediation. The removal of chemicals from a solution by biological material is known as bio-sorption. Organic or inorganic compounds, soluble or insoluble, are examples of such substances. Absorption, adsorption, ion exchange, surface complication, and precipitation processes are all part of the bio-sorption process (Guo et al. 2020a). Table 8 summarizes the various macro algae that can remove heavy metals from soil and water.
Table 8.
Heavy metals removal by specific macro algae (Hassan et al. 2017).
| Macro algae | Heavy metals | Ionic conc. (mg/L) | Final removal conc. (mg/g) |
|---|---|---|---|
| Ulva reticulata | Zn | 1500 | 125.5 |
| Cladophora fascicularis | Cu | 12.7–254.2 | 70.54 |
| Spirogyra insignis | Cd | 10–150 | 87.7 |
| Sargassum wightii | Cu | 100–1000 | 115 |
| Fucus spiralis | Cd/ Zn | 10–150 | 114.9/ 53.2 |
| Asparagopsis armata | Cd/ Zn | 10–150 | 32.3 / 21.6 |
| Chondrus crispus | Cd/ Zn | 10–150 | 75.2/45.7 |
8. Benefits
Natural manures can be used to create additives that help improve organic activities in soils. The expansion of customized nutrients improves plant health support. Food is provided, and bacteria and beneficial soil worms are encouraged to grow. Root development is accelerated as a result of the excellent construction provided to the ground. Natural matter in soil has a higher content than normal levels. Promotes the growth of mycorrhizal associations, which increases the availability of phosphorus (P) in the soil. Aid in the prevention of plantar diseases and provide a continuous supply of micronutrients to the soil. Stable nitrogen (N) and phosphorus (P) fixations are added to the mix. Enhancements for supplement trade in the dirt's limit (Carvajal-Muñoz and Carmona-Garcia 2012).
9. Disadvantages of inorganic and organic fertilizer
9.1. Disadvantages of inorganic fertilizer
According to the EPA's Office of Pesticide Programs, inorganic fertilizers are human carcinogens, hence the majority of pesticides used contain cancer-causing chemicals. Furthermore, nutrient imbalance can emerge as a result of the careless use of inorganic fertilizers, which results in reduced uptake of other necessary nutrients and soil acidity, resulting in decreased crop yields. On the other hand, if the common NPK type is constantly utilized, secondary and micronutrient shortage occurs in the soil and crop. Furthermore, relying on inorganic fertilizers simply reduces soil organic matter, degrades soil physical characteristics and structure, raises soil acidity, and causes erosion. Finally, agricultural chemicals have contaminated surface and ground waters, endangered wildlife and fish, and increased agriculture's reliance on fossil fuel supplies significantly (Sharma 2017). Inorganic fertilizers also need a lot of money; they're hard to get by, especially in rural places; they're risky in low- and high-rainfall locations; and they have to be administered seasonally (Sharma 2017).
9.2. Disadvantages of organic fertilizer
Temperature and soil moisture influence the decomposition of organic material in organic fertilizers, thus nutrients may be released when the plant does not require them. Furthermore, because the nutrient content of organic fertilizers is low and only a limited amount of organic material is accessible in many places, it is difficult to supply crop nutrient demands only through organic fertilizers. Organic fertilizers, on the other hand, are required in big quantities and may not be readily available to small-scale farmers (Guo et al. 2020b).
10. Some aspects of the superiority of bio fertilizer over organic and chemical fertilizers
-
o
When chemical fertilizers are used, greenhouse gases such as NO2 and CO2 are released into the atmosphere. Photosynthesis is directly engaged in the incorporation of atmospheric CO2 into the organic algal biomass by cyanobacteria and green microalgae. As a result, greenhouse gas emissions are reduced (Guo et al. 2020b).
-
o
Cyanobacteria inoculation can save 25–40% of the high-cost chemical nitrogen fertiliser. A study found that inoculating rice plants with filamentous N-fixing cyanobacteria reduced fertilizer use by 50% while maintaining grain yield and quality (Guo et al. 2020b).
-
o
A study found that bio-fertilizers Nostoc entophytum and Oscillatoria augustissima can boost the nutritional value of pea seedlings while reducing chemical fertilizer use by 50%. The use of cyanobacterial bio-fertilizer not only reduced the usage of chemical fertilizers, but it also boosted rice and other crop yields (Guo et al. 2020b).
-
o
Arugula (Eruca sativa), Bayam Red (Ameranthus gangeticus), and Pak Choy leaf vegetables received bio fertilizer from the biomass (Brassica rapa ssp. chinensis). The iron (Fe), magnesium (Mg), calcium (Ca), and zinc (Zn) content of algal biomass was higher than that of chemical fertilizer (TriplePro 15–15–15) (Guo et al. 2020b).
-
o
Microalgae are regarded an organic fertilizer because they can minimize nutrient losses by a progressive release of P, N, and K, but unlike organic fertilizer, they are more temperature and soil moisture tolerant (Mulbryet al., 2008, Coppens et al., 2016).
-
o
Microalga extracts have the ability to substitute micronutrient foliar fertilizers that function as a complement to N and hence boost nutrient uptake, as well as raise the amount of N in shoot and root tissues (Adamczewska-Sowińska and Uklańska 2009).
-
o
A pepper plant study found that using Arthrospira spp. as a bio stimulator as a foliar treatment was superior to compost and the appropriate NPK dose. The yield of pepper treated with bio stimulant was shown to be higher than that of organic fertilizer containing NPK (L et al. 2016).
-
o
In an organic farming system, a study on the red beet (Beta vulgaris L.) found that using the extract of microalgae Arthrospira spp. as a bio stimulator resulted in higher dry and fresh weights compared to the control (Oliveira, J., Mógor, G., Mógor 2013).
11. Promising algae for use as bio fertilizer
-
•
Scenedesmus spp.: Studies have shown its ability to increase the development of plant and the growth, showing a great number of shoots, leaves and flowers in petunias (Petunia × ibrida) (Plaza et al. 2018).
-
•
Aulosira fertilissima: a study reported that the presence of root-promoting hormones that found in algea (auxins, gibberellic acid and cytokinins) increased the growth of rice seedlings (Oryza sativa L.) (Karthikeyan et al. 2007).
-
•
Dunaliella spp. and Phaeodactylum spp.: Studies have shown thier ability to reduce salt stress during the seed germination process of bell pepper (Capsicum annuum L.) (Guzmán-Murillo et al. 2013).
-
•
Spirulina spp. and Chlorella spp.: Water extract of microalgae Spirulina spp. and Chlorella spp. shown their ability to improved wheat tolerance to salinity, and increase the capacity of antioxidant and the content of protein of whole grains (El-Baky et al. 2010).
-
•
C.vulgaris and S.quadricauda: An interesting study assessed morphological and molecular responses induced by microalga in sugar beet (Beta vulgaris L. ssp. vulgaris) production. At the morphological level, given higher values of total root length, the number of root tips, and fine root length, than the untreated plants. At the molecular level, microalgal extracts up-regulated some genes linked to different biological pathways and processes, including primary and secondary metabolisms and intracellular transports, especially involved in root traits related to nutrient acquisition (Barone et al. 2018).
-
•
Dry biomass of Nannochloropsis spp., Ulothrix spp. and Klebsormidium spp.: Studies have shown their ability to increase sugar and carotenoid concentrations in tomato fruits, thus increase the quality and economic value of tomato fruits (Misra and Kaushik 1989).
-
•
Nostoc spp., Hapalosiphon spp., and Aulosira fertilissima: Studies have shown their ability to improve rice seed germination, shoot and root growth, weight of grains and the content of protein (Singh and Trehan, 1973, Misra and Kaushik, 1989).
12. Effective proposals to reduce the cost of bio-fertilizers from algae
There are a number of processes that can be employed to minimize the cost of algae production, including the exploitation of low-cost resources such as nutrient-rich wastewaters and agricultural by-products (Gong and Jiang 2011). Furthermore, recycling greenhouse wastewaters for the production of microalgae may be able to reduce reliance on other fertilizers, such as inorganic fertilizers, while also generating additional income from hydroponic co-productions (Zhanget al., 2017, Baroneet al., 2019). (Barone et al. 2019), for example, proposed employing a hydroponic system to co-produce microalgae (Chlorella infusionum) and tomato (Solanum lycopersicum) and shown that microalgae and tomato could be co-cultivated without the inclusion of other inputs. In addition, (Barone et al. 2019), suggested tomato plants and microalgae co-production (Scenedesmus quadricauda or Chlorella vulgaris). They discovered that S. quadricauda boosted the growth of tomato shoots as well as the biomass of microalgae. With the growing popularity of hydroponic crops around the world, it is possible to propose using tomato and microalgae co-production as a novel way for better, cheaper, and environmentally friendly microalgae and tomato plant production (Barone et al. 2019).
On the other hand, by using empty greenhouse space for indoor microalgae production, artificial lighting and heating could help to lower the relatively high cost of production. Furthermore, wastewater-derived microalgal biomass can be used to convert waste nutrients into sustainable and innovative bio fertilizers with commercial potential in crop production (Coppens et al. 2016).
These suggestions did not take into consideration the additional cost savings that microalgae can provide. Such as the ability to endure salt and temperatures, as well as the ability to recover land, in addition to its ability to aid the plant in the fight against pests and diseases and preserve the soil from erosion, which saves a lot of money for farmers.
13. Limitations
Lack of bio-fertilizer rules and standards, limited transportation and storage space, weak and fragmented labelling of bio-fertilizer goods, lack of advanced organization and exposure among end users, inaccessibility of suitable strain, strain's tendency to vary with age, Non-availability of the proper inoculant at the right time, soil characteristics such as causticity, presence of salts and toxic components in the soil, and extreme climatic circumstances may cause bio-fertilizer aftereffects to be contradictory, a lack of bio-inoculant awareness within the farming community, and a lack of suitable assets and hardware from government and commercial bodies (Coppens et al. 2016). Fertilizers contain a lot of different supplement groupings. Similarly, the costs of implementation are larger than those of some material compost. A large-scale and long-term application could result in a buildup of salts, nutrients, and heavy metals, which could have negative repercussions for plant development, soil life, water quality, and human health. Because of the low content of supplements in comparison to complex manures, large amounts are required for land application. Basic macronutrients may not be available in sufficient amounts for plant development and improvement. There may be nutritional deficiencies as a result of the low exchange of small and full-scale supplements (Coppens et al. 2016).
14. Recommendations
The government should enact significant legislation to screen bio fertilizers. Bio fertilizers should be suitably named (with a class name, a practical tally, and an expiry date, for example) and given widespread exposure through logical preparation, fairs, exhibits, or the media. It's also crucial to identify strains and test their efficacy in different types of soil and agro-climatic settings. It's used to bring bio fertilizer levels back to normal in a given harvest and soil. To fully appreciate strain viability, extensive research in the field of bio fertilizers is required. To reduce impacts and check stated soil and natural situations, it is critical to strengthen the investigation and advancements. To conscious and rouse ranchers, it is recommended to begin a strong preparing and mindfulness programme, and the employment of cutting-edge equipment is indicated for quality things. Subsidies and advances from the government should be given to the development of production units (Nosheen et al. 2021).
15. Conclusion
The soil should be fertile enough to give high production and plants require critical nutrients from fertile soil which also supports a diversified and dynamic biotic population that helps the soil resist environmental degradation. Bio fertilization is a sustainable agricultural practice that includes using bio-fertilizer to upsurge the nutrient content of the soil and organic matters, resulting in higher productivity. Micro and Macro algae are correct environmentally friendly bio-based fertilizers for pollution-free agricultural applies. Microalgae are more effective bio-fertilizers to soil than macroalga, but macro alga gives the best results in mega scaled aquatic media in addition to the availability for the reproduction of huge bulk from microalga rapidly in the laboratory. Microalga recorded the utmost levels of soil fertility with clay soil than a sandy one. Both micro and macro alga can be used for heavy metal removal similarly.
16. Future prospective
Without a doubt, the use of bio-fertilizer is the future of agriculture in the world, where it is expected to replace chemical fertilizer. because it is safer on the soil and also facilitates the process of biodegradation carried out by microorganisms, thus leads to an increase in soil fertility in a safe way without leaving chemical residues. We also expect to add nanomaterial to bio-fertilizer, as nanotechnology would provide green and efficient alternatives for the management of plant diseases and improve of plant resistance to environment stress and increase plant growth, yield productivity, and could increase the quality and quantity of plant.
Author’s contributions
E. Ammar, A. Aioub have conceptualized, interpreted, corrected, and made scientifically sound final versions of the manuscript; while, A. Elesawy, A. Karkour, M. Mouhamed, A. Amer, N. EL-Shershaby have provided technical suggestions and corrections for the final version of the manuscript. All authors critically reviewed and approved the final version of the manuscript for submission.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdel-Raouf N., Al-Homaidan A., Ibraheem I. Agricultural importance of algae. African J. Biotechnol. 2016;11:11648–11658. doi: 10.4314/ajb.v11i54. [DOI] [Google Scholar]
- Adamczewska-Sowińska K., Uklańska C.M. Effect of nitrogen fertilization on yield and quality of endive. Veg Crop Res Bull. 2009;70:193–201. doi: 10.2478/v10032-009-0019-6. [DOI] [Google Scholar]
- Alobwede E., Leake J.R., Pandhal J. Circular economy fertilization: Testing micro and macro algal species as soil improvers and nutrient sources for crop production in greenhouse and field conditions. Geoderma. 2019;334:113–123. doi: 10.1016/J.GEODERMA.2018.07.049. [DOI] [Google Scholar]
- Balasubramaniam V., Gunasegavan R.D.N., Mustar S., et al. Isolation of Industrial Important Bioactive Compounds from Microalgae. Molecules. 2021;26 doi: 10.3390/MOLECULES26040943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone V., Baglieri A., Stevanato P., et al. Root morphological and molecular responses induced by microalgae extracts in sugar beet (Beta vulgaris L.) J. Appl. Phycol. 2018;30:1061–1071. doi: 10.1007/S10811-017-1283-3/FIGURES/6. [DOI] [Google Scholar]
- Barone V., Puglisi I., Fragalà F., et al. Novel bioprocess for the cultivation of microalgae in hydroponic growing system of tomato plants. J. Appl. Phycol. 2019;31:465–470. doi: 10.1007/s10811-018-1518-y. [DOI] [Google Scholar]
- Barros A.I., Gonçalves A.L., Simões M., Pires J.C.M. Harvesting techniques applied to microalgae: A review. Renew. Sustain. Energy Rev. 2015;41:1489–1500. doi: 10.1016/J.RSER.2014.09.037. [DOI] [Google Scholar]
- Carvajal-Muñoz J.S., Carmona-Garcia C.E. Benefits and limitations of biofertilization in agricultural practices. Livest. Res. Rural Dev. 2012;24:1–8. [Google Scholar]
- Chapman R.L. Algae: The world’s most important “plants”-an introduction. Mitig Adapt Strateg. Glob. Chang. 2013;18:5–12. doi: 10.1007/s11027-010-9255-9. [DOI] [Google Scholar]
- Chatterjee A., Singh S., Agrawal C., et al. Role of Algae as a Biofertilizer. Algal Green Chem. Recent Prog. Biotechnol. 2017;189–200 doi: 10.1016/B978-0-444-63784-0.00010-2. [DOI] [Google Scholar]
- Chekroun K Ben, Baghour M (2013) The role of algae in phytoremediation of heavy metals: A review. J. Mater. Environ. Sci. 4:873–880.
- Chittora D., Meena M., Barupal T., Swapnil P. Cyanobacteria as a source of biofertilizers for sustainable agriculture. Biochem Biophys Reports. 2020;22 doi: 10.1016/J.BBREP.2020.100737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppens J., Grunert O., Van Den Hende S., et al. The use of microalgae as a high-value organic slow-release fertilizer results in tomatoes with increased carotenoid and sugar levels. J. Appl. Phycol. 2016;28:2367–2377. doi: 10.1007/S10811-015-0775-2/FIGURES/6. [DOI] [Google Scholar]
- Dhaliwal S.S., Singh J., Taneja P.K., Mandal A. Remediation techniques for removal of heavy metals from the soil contaminated through different sources: a review. Environ. Sci. Pollut. Res. 2020;27:1319–1333. doi: 10.1007/S11356-019-06967-1/TABLES/4. [DOI] [PubMed] [Google Scholar]
- Duarte I., Hernández S., Ibañez A., Canto A. Macroalgae as Soil Conditioners or Growth Promoters of Pisum sativum (L) Annu. Res. Rev. Biol. 2018;27:1–8. doi: 10.9734/arrb/2018/43272. [DOI] [Google Scholar]
- El-Baky H.H.A., El-Baz F.K., Baroty G.S.E. Enhancing antioxidant availability in wheat grains from plants grown under seawater stress in response to microalgae extract treatments. J. Sci. Food Agric. 2010;90:299–303. doi: 10.1002/JSFA.3815. [DOI] [PubMed] [Google Scholar]
- Feller C., Beare M.H. Physical control of soil organic matter dynamics in the tropics. Geoderma. 1997;79:69–116. doi: 10.1016/S0016-7061(97)00039-6. [DOI] [Google Scholar]
- Fleurence J., Morançais M., Dumay J., et al. What are the prospects for using seaweed in human nutrition and for marine animals raised through aquaculture? Trends Food Sci. Technol. 2012;27:57–61. doi: 10.1016/J.TIFS.2012.03.004. [DOI] [Google Scholar]
- Gonçalves AL (2021) The Use of Microalgae and Cyanobacteria in the Improvement of Agricultural Practices: A Review on Their Biofertilising, Biostimulating and Biopesticide Roles. Appl Sci 2021, Vol 11, Page 871 11:871. https://doi.org/10.3390/APP11020871.
- Gong Y., Jiang M. Biodiesel production with microalgae as feedstock: from strains to biodiesel. Biotechnol. Lett. 2011;33:1269–1284. doi: 10.1007/S10529-011-0574-Z. [DOI] [PubMed] [Google Scholar]
- Guo S., Wang P., Wang X., et al. Microalgae as Biofertilizer in Modern Agriculture. Microalgae Biotechnol Food, Heal High Value Prod. 2020;397–411 doi: 10.1007/978-981-15-0169-2_12. [DOI] [Google Scholar]
- Guo S., Wang P., Wang X., et al. Microalgae as biofertilizer in modern agriculture. Microalgae Biotechnol Food, Heal High Value Prod. 2020;397–411 doi: 10.1007/978-981-15-0169-2_12. [DOI] [Google Scholar]
- Guzmán-Murillo M.A., Ascencio F., Larrinaga-Mayoral J.A. Germination and ROS detoxification in bell pepper (Capsicum annuum L.) under NaCl stress and treatment with microalgae extracts. Protoplasma. 2013;250:33–42. doi: 10.1007/S00709-011-0369-Z. [DOI] [PubMed] [Google Scholar]
- Hashem HA, Mansour HA, El-Khawas SA, Hassanein RA (2019) The Potentiality of Marine Macro-Algae as Bio-Fertilizers to Improve the Productivity and Salt Stress Tolerance of Canola (Brassica napus L.) Plants. Agron 2019, Vol 9, Page 146 9:146. https://doi.org/10.3390/AGRONOMY9030146.
- Hassan Z ul, Ali S, Rizwan M, et al (2017) Role of Bioremediation Agents (Bacteria, Fungi, and Algae) in Alleviating Heavy Metal Toxicity. undefined 517–537. https://doi.org/10.1007/978-981-10-4059-7_27.
- Izzati M (2015) The Use of Seaweeds Sargassum Sp and Gracilaria Verrucosa as Soil Conditioneer to Enhance The Growth of Vigna Radiata in Sandy and Clay Soil. Undefined.
- Izzati M., Haryanti S., Setiari N. The use of macroalga sargassum sp. and Gracilaria verrucosa in improving sandy and clay soil fertility. J. Phys. Conf. Ser. 2019;1217 doi: 10.1088/1742-6596/1217/1/012179. [DOI] [Google Scholar]
- Karthikeyan S., Balasubramanian R., Iyer C.S.P. Evaluation of the marine algae Ulva fasciata and Sargassum sp. for the biosorption of Cu(II) from aqueous solutions. Bioresour. Technol. 2007;98:452–455. doi: 10.1016/j.biortech.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Kaur I (2020) Seaweeds: Soil Health Boosters for Sustainable Agriculture. 163–182. https://doi.org/10.1007/978-3-030-44364-1_10.
- Khan W., Rayirath U.P., Subramanian S., et al. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 2009;28:386–399. doi: 10.1007/S00344-009-9103-X/FIGURES/2. [DOI] [Google Scholar]
- L A, P K, G SB (2016) Evaluation of Spirulina platensis as microbial inoculants to enhanced protein levels in Amaranthus gangeticus. African J Agric Res 11:1353–1360. https://doi.org/10.5897/ajar2013.7953.
- Mahapatra D.M., Chanakya H.N., Joshi N.V., et al. Algae-Based Biofertilizers. A Biorefinery Approach. 2018;177–196 doi: 10.1007/978-981-10-7146-1_10. [DOI] [Google Scholar]
- Misra S, Kaushik BD (1989) Growth promoting substances of Cyanobacteria. I: Vitamins and their influence on rice plant. Undefined.
- Mulbry W, Kondrad S, Pizarro C (2008) Biofertilizers from Algal Treatment of Dairy and Swine Manure Effluents. http://dx.doi.org/101300/J484v12n04_08 12:107–125. https://doi.org/10.1300/J484V12N04_08.
- Nabti E., Jha B., Hartmann A. Impact of seaweeds on agricultural crop production as biofertilizer. Int. J Environ. Sci Technol. 2016;145(14):1119–1134. doi: 10.1007/S13762-016-1202-1. [DOI] [Google Scholar]
- Nabti E., Jha B., Hartmann A. Impact of seaweeds on agricultural crop production as biofertilizer. Int. J. Environ. Sci. Technol. 2016;145(14):1119–1134. doi: 10.1007/S13762-016-1202-1. [DOI] [Google Scholar]
- Nosheen S, Ajmal I, Song Y (2021) Microbes as Biofertilizers, a Potential Approach for Sustainable Crop Production. Sustain 2021, Vol 13, Page 1868 13:1868. https://doi.org/10.3390/SU13041868.
- Oliveira, J., Mógor, G., Mógor A (2013) Oliveira, J., Mógor, G., Mógor, A. Cad Agroecol 1–4.
- Osório C., Machado S., Peixoto J., et al. Pigments content (Chlorophylls, fucoxanthin and phycobiliproteins) of different commercial dried algae. Separations. 2020;7:1–14. doi: 10.3390/separations7020033. [DOI] [Google Scholar]
- Konur Ozcan. CRC Press; 2015. Algal Economics and Optimization. [Google Scholar]
- Pereira R.C., Da Gama B.A.P. Macroalgal chemical defenses and their roles in structuring tropical marine communities. Algal. Chem. Ecol. 2008;9783540741:25–55. doi: 10.1007/978-3-540-74181-7_2. [DOI] [Google Scholar]
- Plaza B.M., Gómez-Serrano C., Acién-Fernández F.G., Jimenez-Becker S. Effect of microalgae hydrolysate foliar application (Arthrospira platensis and Scenedesmus sp.) on Petunia x hybrida growth. J. Appl. Phycol. 2018;30:2359–2365. doi: 10.1007/S10811-018-1427-0/FIGURES/9. [DOI] [Google Scholar]
- Priya M., Gurung N., Mukherjee K., Bose S. Microalgae in Removal of Heavy Metal and Organic Pollutants from Soil. Microb. Biodegrad. Bioremediation. 2014;519–537 doi: 10.1016/B978-0-12-800021-2.00023-6. [DOI] [Google Scholar]
- Ritika B, Utpal D (2014) Biofertilizer, a way towards organic agriculture: A review. undefined 8:2332–2343. https://doi.org/10.5897/AJMR2013.6374.
- Ronga D, Biazzi E, Parati K, et al (2019) Microalgal Biostimulants and Biofertilisers in Crop Productions. Agron 2019, Vol 9, Page 192 9:192. https://doi.org/10.3390/AGRONOMY9040192.
- Salinas-Salazar C., Garcia-Perez J.S., Chandra R., et al. Methods for extraction of valuable products from microalgae biomass. Microalgae Biotechnol. Dev. Biofuel Wastewater Treat. 2019;245–263 doi: 10.1007/978-981-13-2264-8_11. [DOI] [Google Scholar]
- Sharma A. A Review on the Effect of Organic and Chemical Fertilizers on Plants. Int. J. Res. Appl. Sci. Eng. Technol. 2017;V:677–680 doi: 10.22214/IJRASET.2017.2103. [DOI] [Google Scholar]
- Silva L.D., Bahcevandziev K. Pereira L (2019) Production of bio-fertilizer from Ascophyllum nodosum and Sargassum muticum (Phaeophyceae) J. Oceanol. Limnol. 2019;373(37):918–927. doi: 10.1007/S00343-019-8109-X. [DOI] [Google Scholar]
- Singh V.P., Trehan K. Effect of extracellular products of Aulosira fertilissima. Plant Soil. 1973;38 [Google Scholar]
- Suleiman A.K.A., Lourenço K.S., Clark C., et al. From toilet to agriculture: Fertilization with microalgal biomass from wastewater impacts the soil and rhizosphere active microbiomes, greenhouse gas emissions and plant growth. Resour. Conserv. Recycl. 2020;161 doi: 10.1016/j.resconrec.2020.104924. [DOI] [Google Scholar]
- Tyoker Kukwa D., Chetty M. Microalgae: The Multifaceted Biomass of the 21st Century. Biotechnol. Appl. Biomass. 2021 doi: 10.5772/INTECHOPEN.94090. [DOI] [Google Scholar]
- Zhang J., Wang X., Zhou Q. Co-cultivation of Chlorella spp and tomato in a hydroponic system. Biomass Bioenergy. 2017;97:132–138. doi: 10.1016/J.BIOMBIOE.2016.12.024. [DOI] [Google Scholar]
- Zou Y., Zeng Q., Li H., et al. Emerging technologies of algae-based wastewater remediation for bio-fertilizer production: a promising pathway to sustainable agriculture. J. Chem. Technol. Biotechnol. 2021;96:551–563. doi: 10.1002/JCTB.6602. [DOI] [Google Scholar]