Abstract
While a time-kill methodology noted no appreciable improvement in bactericidal activity with the addition of azithromycin (AZM) to a ceftazidime (CAZ) regimen, data from the murine pneumonia model showed that the addition of AZM significantly improved survival compared to treatment with CAZ alone. These data suggest that AZM might be a useful adjunctive therapy in the management of pneumonia resulting from mucoid isolates of Pseudomonas aeruginosa.
The ability of Pseudomonas aeruginosa to synthesize extracellular products and biofilms contributes to its overall virulence, while providing protection from the effects of antibacterials and host defenses (1, 4, 5, 7). Recent studies have demonstrated that the macrolides and azalides, not only can inhibit the expression of these virulence factors, but also can reduce the production of glycocalyx or mucoid alginate in biofilm-producing strains of Pseudomonas (2, 5–7, 10, 11). The purpose of this study was to investigate the in vitro and in vivo influence of adjunctive azithromycin (AZM) in the management of mucoid P. aeruginosa.
In this study, the mucoid P. aeruginosa strain 13 was obtained from a hospitalized patient with a urinary tract infection. Analytical-grade AZM (Pfizer, Inc., New York, N.Y.) and ceftazidime (CAZ; Glaxo Wellcome, Research Triangle Park, N.C.) powders were obtained for use during the in vitro portion of this study. Commercially available intravenous formulations of CAZ (Ceptaz; Glaxo Wellcome) and AZM (Zithromax; Pfizer, Inc.) were used for the in vivo portion of the study. The MICs of AZM and CAZ were 8 and 1 μg/ml, respectively.
The in vitro interactions of AZM and CAZ alone or in combination were evaluated by using the time-kill methodology with a starting inoculum of 106 CFU/ml. Antibiotics were added to produce concentrations of 0.25× MIC, 0.5× MIC, MIC, 2× MIC, 8× MIC, and 16× MIC for AZM and CAZ. Synergy was defined as an ≥2-log-unit decrease in CFU per milliliter for the combination compared with the most active single agent, while anything less was considered additive.
Swiss Webster mice (Taconic Farms, Germantown, N.Y.) were rendered transiently neutropenic by injecting cyclophosphamide (Bristol-Myers Squibb Co., Princeton, N.J.) intraperitoneally at a dose of 150 mg/kg of body weight at −4, −1, and +1 days in relation to bacterial inoculation (day 0). Infection was induced by the intranasal administration of 5 × 109 CFU/ml. Experiments to assess bacterial growth revealed consistent growth at 24 h postinoculation in the absence of mortality. For these reasons, all antibiotic therapies were initiated 24 h postinoculation.
Animals were randomized into various treatment regimens: CAZ, 1,500 mg/kg every 24 h (q24h) for two doses alone; AZM, 20 mg/kg q24h for three doses alone; CAZ plus AZM, q24h for three doses; CAZ plus AZM, q24h for five doses; or no treatment (control). The CAZ dose was chosen because it represented the 50% protective dose. The AZM dose was selected because it has previously resulted in beneficial adjunctive effects while producing sub-MICs (0.25- and 6-h postdose serum drug concentrations of 1.47 and 0.14 μg/ml, respectively) for the isolate under study (9, 12). All antimicrobials were administered subcutaneously and initiated 24 h after inoculation. AZM was initiated at the 25th h to allow time for the administration of the first injections (i.e., CAZ or saline). Cumulative mortality was recorded over 7 days. Quantitative cultures were done on the lungs from each treatment group to assess bacterial density before antimicrobial administration (at 24 h) and at 48, 72, and 96 h.
For the purposes of this study, whether an animal died due to the natural infection process or was euthanized (guidelines adapted from Hamm [3]), both were considered the same end point for experimental and statistical purposes. The sample size for the study was calculated with data from a previous study (12) in which CAZ was used alone or in combination with AZM. From these data, 64 animals per group would have 80% power to detect a 25% difference between groups. Time to death was estimated by the Kaplan-Meier method, and the estimates of mortality were compared among the treatment groups by using the log rank test. The log CFU per gram of lung for the groups were compared by a one-way analysis of variance method followed by Scheffe’s test for multiple comparisons.
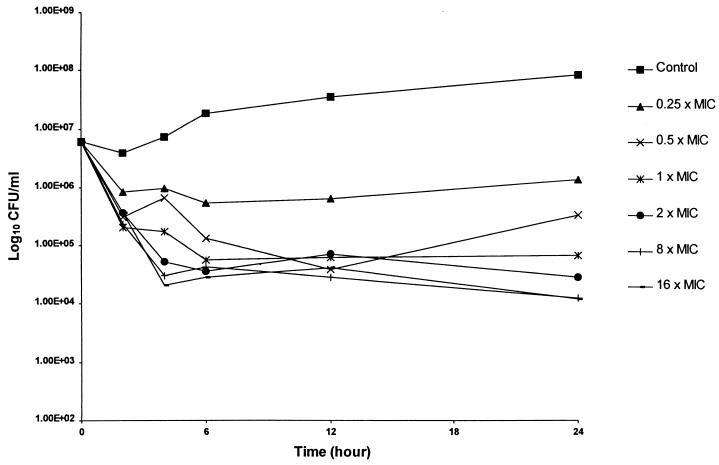
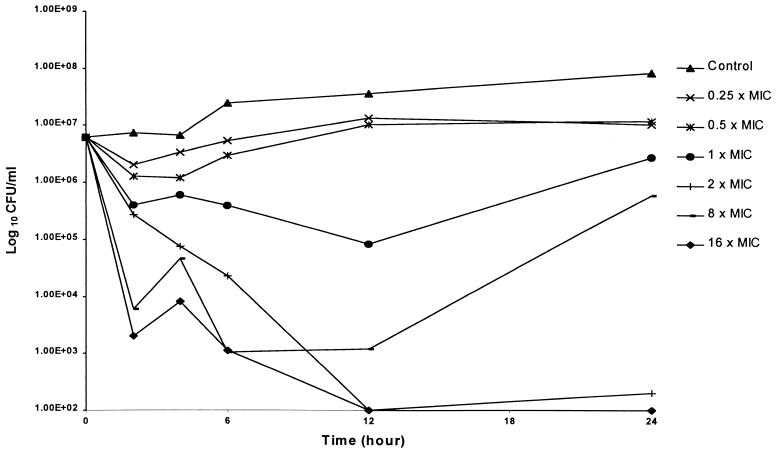
The results of duplicate time-kill studies are displayed in Fig. 1 and 2. The bactericidal activity data for CAZ (Fig. 1) revealed concentration-independent killing, which is consistent with the β-lactams. In contrast, AZM bactericidal activity appeared to increase with increasing multiples of the MIC (Fig. 2). Although combination studies were performed over the MIC ranges described, no appreciable improvement in bactericidal activity was noted.
FIG. 1.
Mean antibacterial activity of CAZ for P. aeruginosa 13 as assessed by the time-kill methodology.
FIG. 2.
Mean antibacterial activity of AZM for P. aeruginosa 13 as assessed by the time-kill methodology.
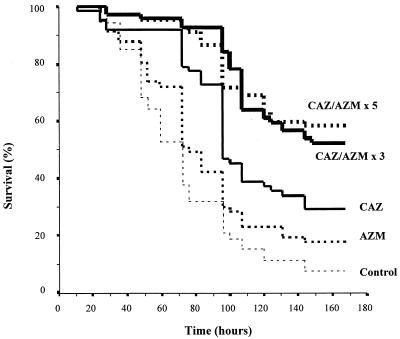
The survival data for all groups (CAZ alone [n = 62]; AZM alone [n = 57]; CAZ plus AZM, three doses [n = 69]; CAZ plus AZM, five doses [n = 67]; or untreated controls [n = 53]) are displayed in Fig. 3. The bacterial inoculum resulted in 92% mortality in untreated controls. AZM-treated mice had a survival rate of 18%, which was not significantly different (P > 0.05) from that of untreated controls (8%). CAZ treatment resulted in a significantly higher rate of survival than either that of the untreated control (P < 0.00001) or that of mice receiving AZM only (P = 0.0102). The addition of AZM to CAZ significantly improved survival for both the 3-day (52%; P = 0.0008) and 5-day (58%; P = 0.0006) regimens compared to CAZ alone (29%). No difference was observed between the survival rates achieved with the 3- and 5-day AZM regimens.
FIG. 3.
Survival rates after infection with mucoid P. aeruginosa and no treatment (control), AZM, CAZ, or an antimicrobial combination. The numbers of AZM doses (three or five) are shown.
The bacterial density in lungs was assessed at 24, 48, 72, and 96 h postinoculation. The starting bacterial density was 2.5 log10 CFU/g at 24 h. Statistical differences over the three subsequent sampling times were not observed with the addition of AZM to the CAZ regimen.
Investigation of adjunctive therapies, which enhance host defenses and/or downregulate P. aeruginosa virulence, is one option for future management of invasive P. aeruginosa infections. Several authors have reported the inhibition of P. aeruginosa-associated virulence factors after exposure to subinhibitory antibiotic concentrations of macrolides or azalides (2, 6, 10, 11). The potential utility of adjunctive therapy with these agents may in part be due to the anti-inflammatory and immunomodulating properties or the reduction of alginate production (5, 7, 10, 14).
Previously our group has reported that the addition of AZM sub-MICs significantly reduced the mortality rate in the murine peritonitis-sepsis model with a nonmucoid strain of P. aeruginosa (9, 12). A subsequent study was undertaken to evaluate the adjunctive potential of AZM when combined with trovafloxacin in the murine pneumonia model (13). In that study, using a nonmucoid P. aeruginosa strain, AZM to the baseline antimicrobial regimen did not result in a significant alteration in either the rate or extent of mortality.
The current investigation was initiated with CAZ and a mucoid strain of P. aeruginosa, since AZM can reduce the production of mucoid alginate (5, 7, 8). Previous investigators have reported that cystic fibrosis patients given 500 mg of azithromycin every third day had sputum concentrations that exceed the in vitro concentrations required to inhibit the production of pseudomonal exoproducts (15). Our current data show that the addition of AZM significantly improved survival compared to the use of β-lactam alone. The lack of a significant difference between the AZM regimens was likely due to the prolonged half-life of AZM and the relatively short (2-day) difference in treatment duration.
Despite improvements in survival with adjunctive AZM, we were unable to determine similar statistical correlates related to bacterial density within the lung. This was in part due to selection bias introduced by earlier mortality observed in the CAZ group (Fig. 3), which not only reduced the sample size, but also selected for animals with reduced bacterial densities. While our in vitro data did not reveal enhanced activity with the combination of AZM and CAZ, exposure to AZM alone resulted in impressive bactericidal activity for this isolate (Fig. 2) at concentrations which may be achievable at the site of infection (15).
In conclusion, this study shows that in vivo AZM augmented the antibacterial activity of CAZ and improved outcome as assessed by survival rate. Ultimately, these data may in part serve as the basis for clinical interventions directed at improving the care of patients with chronic conditions, such as cystic fibrosis or diffuse panbronchiolitis, who are often infected with mucoid strains of P. aeruginosa.
Acknowledgments
We thank Jeff Mather for statistical consultation and guidance during the study design and analysis of these investigative data.
This study was supported by a grant from the Society of Infectious Diseases Pharmacists.
REFERENCES
- 1.Bolister N, Basker M, Hodges N, Marriott C. Reduced susceptibility of a mucoid strain of P. aeruginosa to lysis by ticarcillin and piperacillin. J Antimicrob Chemother. 1989;24:619–621. doi: 10.1093/jac/24.4.619. [DOI] [PubMed] [Google Scholar]
- 2.Grimwood K, To M, Rabin H R, Woods D E. Inhibition of Pseudomonas aeruginosa exoenzyme expression by subinhibitory antibiotic concentrations. Antimicrob Agents Chemother. 1989;33:41–47. doi: 10.1128/aac.33.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamm T E. Proposed institutional animal care and use committee guidelines for death as an end point in rodent studies. AALAS Contemp Topics. 1995;34:69–71. [Google Scholar]
- 4.Hodges N A, Gordon C A. Protection of Pseudomonas aeruginosa against ciprofloxacin and β-lactams by homologous alginate. Antimicrob Agents Chemother. 1991;35:2450–2452. doi: 10.1128/aac.35.11.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ichimiya T, Takeoka K, Hiramatsu K, Hirai K, Yamasaki T, Nasu M. The influence of azithromycin on the biofilm formation of Pseudomonas aeruginosa in vitro. Chemotherapy. 1996;42:186–191. doi: 10.1159/000239440. [DOI] [PubMed] [Google Scholar]
- 6.Kita E, Sawaki M, Oku D, Hamuro A, Mikasa K, Konishi M, Emoto M, Takeuchi S, Narita N, Kashiba S. Suppression of virulence factors of Pseudomonas aeruginosa by erythromycin. J Antimicrob Chemother. 1991;27:273–284. doi: 10.1093/jac/27.3.273. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi H. Airway biofilm disease: its clinical manifestation and therapeutic possibilities of macrolides. J Infect Chemother. 1995;1:1–15. doi: 10.1016/s0002-9343(99)80282-4. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi H. Biofilm disease: its clinical manifestation and therapeutic possibilities of macrolides. Am J Med. 1995;99(Suppl. 6A):26S–30S. doi: 10.1016/s0002-9343(99)80282-4. [DOI] [PubMed] [Google Scholar]
- 9.Marangos M N, Klepser M E, Nicolau D P, Nightingale C H, Quintiliani R. In vivo effect of azithromycin subinhibitory concentrations on the mortality of experimental Pseudomonas aeruginosa sepsis. In: Zinner S H, Young L S, Acar J F, Neu H C, editors. Expanding indications for the new macrolides, azalides, and streptogramins. New York, N.Y: Marcel Dekker; 1997. pp. 571–575. [Google Scholar]
- 10.Molinari G, Guzman C A, Pesce A, Schito G C. Inhibition of Pseudomonas aeruginosa virulence factors by subinhibitory concentrations of azithromycin and other macrolide antibiotics. J Antimicrob Chemother. 1993;31:681–688. doi: 10.1093/jac/31.5.681. [DOI] [PubMed] [Google Scholar]
- 11.Molinari G, Paglia P, Schito G C. Inhibition of motility of Pseudomonas aeruginosa and Proteus mirabilis by subinhibitory concentrations of azithromycin. Eur J Clin Microbiol Infect Dis. 1992;11:469–471. doi: 10.1007/BF01961867. [DOI] [PubMed] [Google Scholar]
- 12.Nicolau D P, Banevicius M A, Marangos M N, Klepser M E, Quintiliani R, Nightingale C H. Influence of adjunct azithromycin on the mortality of experimental Pseudomonas aeruginosa sepsis. Int J Antimicrob Agents. 1997;8:239–241. doi: 10.1016/s0924-8579(97)00017-4. [DOI] [PubMed] [Google Scholar]
- 13.Nicolau, D. P., M. A. Banevicius, C. H. Nightingale, and R. Quintiliani. Influence of adjunct azithromycin on the outcome of experimental Pseudomonas aeruginosa pneumonia. J. Infect. Dis. Pharmacother., in press. [DOI] [PubMed]
- 14.Nicolls M R, Weiss S M, Schwartz M I. Recognition and treatment of diffuse panbronchiolitis. Infect Med. 1998;15:657–662. [Google Scholar]
- 15.Rosen J M, Smith T C, Myerholz K A, Hessong S. Program and abstracts of the Fourth International Conference on the Macrolides, Azalides, Streptogramins & Ketolides, Barcelona, Spain, 21 to 23 January 1998. 1998. Azithromycin levels in the sputum of patients with cystic fibrosis, abstr. 6.27; p. 64. [Google Scholar]