Abstract
A 25-year-old man presented with a 2-month history of progressively worsening left eye pain and an atypical corneal ring infiltrate. His condition deteriorated despite topical antibiotic therapy. Cultures for bacteria, fungus and acanthamoeba, repeated twice, all demonstrated no growth. On third corneal scraping, culture on Middlebrook agar grew colonies after 3 weeks of incubation. Sixteen-second deep sequencing identified Nocardia sienata as the pathogen. This species of Nocardia has not previously been described as a causative pathogen for infectious keratitis. Sloughing and loose epithelium with recurrent filament formation are unusual in infectious keratitis and could be associated with this species. In culture-negative cases, clinicians should consider Nocardia as a cause of keratitis despite its rarity outside of south Asia and use steroids cautiously. Next generation sequencing technology may facilitate identification of the causate of keratitis and can be especially useful in culture-negative cases and with unexpected pathogens.
Keywords: ophthalmology, anterior chamber, infectious diseases
Background
Nocardia is a genus of weakly staining gram-positive, partially acid-fast branching bacilli, and is ubiquitous in soil and decaying vegetation. Nocardia as a causative organism for keratitis was initially described in and is more commonly diagnosed in south Asia.1–3 Outside of this region, however, it has rarely been reported to cause keratitis without a recent history of travel. Other predisposing factors are surgery, corticosteroids, trauma and contact lens wear.2 The initial clinical findings of Nocardia may be atypical, and resemble fungal and mycobacterial keratitis, which combined with its uncommonness, makes the diagnosis challenging.
An increasing number of Nocardia species have been identified with the emergence of advanced molecular technologies. Most cases of Nocardia keratitis, however, are caused by a limited number of these species, such as cyriageorgica and asteroides.1 4 Many species of Nocardia do not have any clinical significance and have not been associated with ocular diseases.1 Herein, we report the first case of keratitis by Nocardia sienata and describe its unique clinical findings in a US patient with no travel history.
Case presentation
A 25-year-old man presented with a 2-month history of progressively worsening left eye pain. He was treated with combination topical tobramycin/dexamethasone drops four times a day for 2 weeks. Therapy was then switched to prednisolone acetate drops three time daily and then tapered. On worsening, he was noted to have an atypical corneal ring infiltrate and was referred to our institution. Ocular history was notable for soft contact lens use without freshwater exposure. The patient worked in a warehouse that packages marijuana products and had no history of recent travel. Right eye acuity was 20/20 with an unremarkable examination. The left eye acuity was 20/40 and eye pressure was 18 mm Hg. Slit-lamp examination revealed 2+ conjunctival injection. The cornea demonstrated irregular ovoid patchy infiltrates surrounding an indistinct anterior stromal haze. The epithelium was intact but stained in a pseudodendritic pattern over the patchy infiltrates.
Investigations
Confocal microscopy demonstrated no filaments, some hyper-reflective round cells only mildly suspicious for cysts and was ultimately determined non-diagnostic. Repeat cultures for bacteria, fungus and acanthamoeba all demonstrated no growth. PCR testing for VZV and HSV were negative. Cultures, confocal microscopy and PCR were repeated and remained negative. With each culture, the entire epithelium was noted to be loose and sloughed easily. A superficial keratectomy with therapeutic bandage contact lens was performed. The epithelium was sent for histopathologic evaluation and was read negative for corneal dystrophies or organisms. Recurrent corneal filaments developed (figure 1B). Acuity declined to 20/200 with worsening epithelial breakdown (figure 1C). Another round of corneal cultures was performed, this time including testing for atypical mycobacteria and microsporidia. A large new filament emerged, and vision improved to 20/30 (figure 1D). After 3 weeks, colonies of growth occurred on the atypical media (Remel Middlebrook 7H11 Agar).
Figure 1.
Slit-lamp examination of Nocardia sienata keratitis. (A) Conjunctival injection and an irregular patchy ovoid infiltrate on presentation. White arrows annotate the ‘wreath-like’ patchy infiltrate. (B) Post corneal scraping with central corneal filaments (white arrow). (C) Worsening symptoms with epithelial breakdown. (D) A large peripheral filament after the second keratectomy (arrow) associated with prominent nasal limbal inflammation.
Differential diagnosis
Corneal infiltrates in a contact lens wearer associated with inflammation and eye pain are highly suspicious of an infectious cause.5 Epidemiology of contact lens infections varies by location with bacterial aetiologies Staphylococcus spp, Streptococcus spp and Pseudomonas spp accounting for the majority of cases in the US.6 Sterile infiltrates may be seen with contact lenses, more commonly with extended wear, and should be a diagnosis of exclusion.
Filamentary keratitis and loose sloughing corneal epithelium were noted in this case. Filamentary keratitis is commonly seen in keratoconjunctivitis sicca and other inflammatory eye conditions. Poorly adherent corneal epithelium can suggest corneal epithelial dystrophies. This patient had an unaffected contralateral eye and normal aqueous production. Of note, no dystrophic changes were observed on histopathologic evaluation.
This patient was referred for a ring infiltrate which later became clear was more wreath-like than ring-like (figure 1A). Ring infiltrates are commonly associated with Acanthamoeba keratitis. In culture-negative cases, documentation of perineuritis as well as identification of double-walled cyst structures on confocal microscopy are highly suggestive of this organism. However, many other infectious causes can also present with ring-like infiltrates, including herpes simplex virus and fungi.7 8 The agar on which the organism was recovered from is selective for mycobacteria. Atypical microbacterial keratitis is more commonly associated with trauma and corneal refractive surgery, which were absent in this case. Nocardia has been described clinically as patchy, white satellite infiltrates that progress into a wreath-like pattern. Nocardia keratitis can also demonstrate satellite lesions with feathery making differentiation from fungal keratitis further challenging.
Treatment
About 2.5% iodopovidone drops were added after the second round cultures were sent. The organism which grew on atypical agar was identified as the Nocardia genus after 3 weeks. Treatment was switched to amikacin. A send-out CLIA-certified microbiology analysis by 16S sequencing identified N. sienata as the pathogen. Antibiotic sensitivity testing confirmed susceptibility to amikacin.
Outcome and follow-up
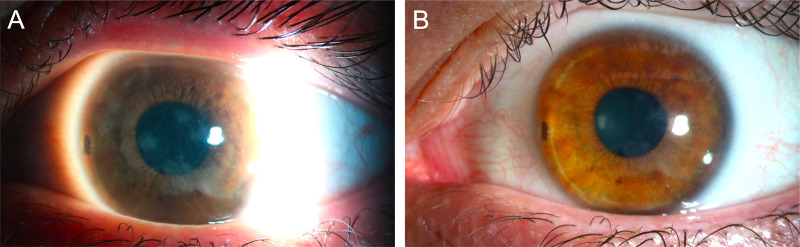
Four weeks after initiating amikacin therapy, there was complete resolution of infection and Visual acuty (VA) recuperated to 20/30 (figure 2A, B). At the most recent follow-up, 6 months after the resolution of infection, cornea remained stable.
Figure 2.
(A) Slit-lamp examination after 4 weeks of topical amikacin. (B) Complete resolution of infection and VA recuperating to 20/30.
Discussion
In this case, 16S rRNA sequencing, presently the standard method of species differentiation in Nocardia, revealed N. sienata. Nocardia has a complicated taxonomic history with over 92 recognised species.4 Only 54 of these species have clinical significance with some previously identified in ocular diseases.4 N. sienata was first isolated in 2004 from a patient’s sputum in Japan and has not been reported since.9 Our findings suggest this unique species may have pathologic sequelae and that Nocardia keratitis may be found in the USA without travel history. Exam findings described here, including sloughing and loose epithelium with recurrent filament formation (figure 1B–D), are not classically described with Nocardia keratitis and could perhaps be suggestive of this newly described species.
The definitive pathogen here did not grow on traditional blood agar and was only identified on atypical pathogen agar after 3 weeks. Since common media are not typically held past 5 days, cultures of Nocardia may be finalised as negative, and the diagnosis may be missed or delayed. Nocardia may be identified by Carbol-Fuchsin or Ziehl-Nelson stains as well.10 However, the agar on which this species of nocardia grew is highly selective for atypical mycobacteria and studies have shown that phenotypic methods are not accurate for Nocardia identification.11 In atypical cases or when cultures are negative, molecular diagnostic sequencing tools are powerful alternatives to identify or confirm causative organisms.12
Amikacin is the first line of therapy once a diagnosis of Nocardia keratitis is established and almost all cases are susceptible to this antibiotic.1 In the meantime, topical steroids should be cautiously used since they are associated with worse visual outcomes in Nocardia keratitis.13 In this case, the patient was initially treated with steroids, which could have contributed to a lower final acuity. Alternatively, a course of iodopovidone treatment helped control the infection while the cause was still being investigated.
Learning points.
Nocardia should be considered in patients with atypical ring-like, ‘wreath-like’, infiltrates and clinical worsening after topical steroid therapy.
Nocardia sienata may have pathologic sequelae and can be found in ocular diseases.
Sloughing and loose epithelium with recurrent filament formation is an unusual finding in infectious keratitis and was present in this case.
In culture-negative cases, next generation sequencing technology may facilitate the identification of the causative organism.
Footnotes
Contributors: GDS conceived of the report and was the primary doctor caring for the patient described. AM wrote the manuscript and was supervised by GDS. AN and MM participated in the care of the patient described and edited the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1.Lalitha P, Tiwari M, Prajna NV, et al. Nocardia keratitis: species, drug sensitivities, and clinical correlation. Cornea 2007;26:255–9. 10.1097/ICO.0b013e318033d853 [DOI] [PubMed] [Google Scholar]
- 2.Soleimani M, Masoumi A, Khodavaisy S, et al. Current diagnostic tools and management modalities of Nocardia keratitis. J Ophthalmic Inflamm Infect 2020;10:36. 10.1186/s12348-020-00228-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Upadhyay MP, Karmacharya PC, Koirala S, et al. Epidemiologic characteristics, predisposing factors, and etiologic diagnosis of corneal ulceration in Nepal. Am J Ophthalmol 1991;111:92–9. 10.1016/S0002-9394(14)76903-X [DOI] [PubMed] [Google Scholar]
- 4.Conville PS, Brown-Elliott BA, Smith T, et al. The complexities of Nocardia taxonomy and identification. J Clin Microbiol 2018;56:e01419–17. 10.1128/JCM.01419-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleiszig SMJ, Evans DJ. Pathogenesis of contact lens-associated microbial keratitis. Optom Vis Sci 2010;87:225–32. 10.1097/OPX.0b013e3181d408ee [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ormerod LD, Hertzmark E, Gomez DS, et al. Epidemiology of microbial keratitis in southern California. A multivariate analysis. Ophthalmology 1987;94:1322–33. 10.1016/s0161-6420(87)80019-2 [DOI] [PubMed] [Google Scholar]
- 7.Singh RB, Batta P. Herpes simplex virus keratitis mimicking Acanthamoeba keratitis: a clinicopathological correlation. BMJ Case Rep 2018;2018:bcr-2018-226100. 10.1136/bcr-2018-226100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broadway DC, Kerr-Muir MG, Eykyn SJ, et al. Mycobacterium chelonei keratitis: a case report and review of previously reported cases. Eye 1994;8 (Pt 1):134–42. 10.1038/eye.1994.27 [DOI] [PubMed] [Google Scholar]
- 9.Kageyama A, Yazawa K, Nishimura K, et al. Nocardia testaceus sp. nov. and Nocardia senatus sp. nov., isolated from patients in Japan. Microbiol Immunol 2004;48:271–6. 10.1111/j.1348-0421.2004.tb03523.x [DOI] [PubMed] [Google Scholar]
- 10.Chang EL, Chu RL, Wittpenn JR, et al. Nocardia keratitis mimicking superior limbic keratoconjunctivitis and herpes simplex virus. Am J Ophthalmol Case Rep 2021;22:101030. 10.1016/j.ajoc.2021.101030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muricy ECM, Lemes RA, Bombarda S, et al. Differentiation between Nocardia spp. and Mycobacterium spp.: critical aspects for bacteriological diagnosis. Rev Inst Med Trop Sao Paulo 2014;56:397–401. 10.1590/S0036-46652014000500005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weng S-S, Zhang H-Y, Ai J-W, et al. Rapid Detection of Nocardia by Next-Generation Sequencing. Front Cell Infect Microbiol 2020;10:13. 10.3389/fcimb.2020.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Srinivasan M, Mascarenhas J, Rajaraman R, et al. The steroids for corneal ulcers trial (SCUT): secondary 12-month clinical outcomes of a randomized controlled trial. Am J Ophthalmol 2014;157:327–33. 10.1016/j.ajo.2013.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]