Abstract
Objective
Many patients are assessed for chronic symptoms including: dysphonia, ‘globus’, throat clearing, postnasal secretions and cough; commonly grouped together and attributed to ‘laryngopharyngeal reflux’. This study aimed to explore a clinical trial’s baseline dataset for patterns of presenting symptoms, which might provide a more rational basis for treatment.
Design
Baseline data were analysed for participants entering the Trial Of Proton-Pump Inhibitors in Throat Symptoms: age, body mass index, Reflux Symptom Index, Comprehensive Reflux Symptom Score, Laryngopharyngeal Reflux-Health-related Quality of Life questionnaire and Reflux Finding Score (RFS-endoscopic examination). The relationships between the questionnaires and demographic factors were assessed. Exploratory factor analysis (EFA) was conducted on individual symptom items in the combined questionnaires. The EFA factors were applied to a Cluster Analysis of participants, to explore the presence of identifiable patient.
Results
Throat clearing and globus were the highest ranked scores in the 344 participants. Increasing age was inversely associated with symptom severity (p<0.01). There was no relationship between the RFS and any of the three questionnaires. EFA resulted in a seven-factor model with clinically meaningful labels: voice, cough, gastrointestinal symptoms, airway symptoms and dysphagia, throat clearing, lump in throat, and life events. Cluster analysis failed to demonstrate any clinically meaningful clusters of patients.
Conclusion
This study offers a framework for future research and demonstrates that individual symptoms cannot be used to group patients. The analysis supports the use of a broad ‘umbrella’ term such as persistent throat symptoms.
Trial registration number
Keywords: FUNCTIONAL BOWEL DISORDER, PROTON PUMP INHIBITION, DIAGNOSTIC AND THERAPEUTIC ENDOSCOPY
Summary box.
What is already known about this subject?
Chronic throat and voice symptoms are common.
They are frequently grouped together and attributed to ‘laryngopharyngeal reflux’.
Patients often receive empirical treatment for reflux.
Recent evidence demonstrated Lansoprazole offered no benefit over placebo for these symptoms.
What are the new findings?
Patients could not be clustered based on presenting individual throat and voice symptoms, implying that the many symptoms overlap in their nature.
Body mass index and laryngeal endoscopic appearances were not related to reported throat symptoms.
These findings further question the role of reflux in the aetiology of these symptoms.
How might it impact on clinical practice in the foreseeable future?
A broad term to cover the range of symptoms is preferable to individual symptom labels.
‘Persistent throat symptoms’ could replace laryngopharyngeal reflux and would promote research into alternative treatments.
Introduction
Patients are commonly referred to secondary care for assessment of a variety of laryngeal and pharyngeal symptoms. These include: dysphonia, ‘globus’ sensation, throat clearing, excessive mucus, postnasal secretions, cough and throat discomfort. A conservative estimate of 60 000 such patients are seen annually by specialists in England.1 The diagnosis of ‘laryngopharyngeal reflux’ remains a popular moniker for these symptoms, accompanying the vogue for gastric acid-suppression treatment.2 A recent large UK multicentre randomised controlled trial (Trial Of Proton-Pump Inhibitors in Throat Symptoms; TOPPITS) in 346 patients with chronic pharyngeal and laryngeal symptoms found that Lansoprazole 30 mg two times per day conferred no benefit over placebo.3 These findings should lead specialists to re-explore other potential causes of chronic throat symptoms that have received little press in the face of the reflux aetiology theory. The link between chronic throat symptoms and psychological distress is well documented.4 Raised body mass index (BMI), life events, snoring, upper airway dryness and hormonal changes have all been associated with chronic throat and voice symptoms.5–8
The individual symptoms that patients present with, such as catarrh, throat clearing, intermittent hoarseness or globus frequently coexist. The repeated need to clear the throat is the most common symptom in most cohorts. Throat clearing may culminate in voice change and is often associated with globus sensation, but equally may occur with the sensation of mucus coming into the throat from the nose. Predating the popularisation of the global term ‘laryngopharyngeal reflux’, individual symptoms such as globus, catarrh and functional dysphonia were approached discretely, both from clinical and research perspectives.
This study aimed to explore the rich baseline psychometric dataset from recruits to the TOPPITS trial, for any identifiable symptom clusters, which might provide, in the longer term, a more rational basis for treatment.
Methods
TOPPITS was an investigator-initiated multicentre, randomised, double-blind, placebo controlled trial conducted in eight hospitals in the UK.9 The full trial methodology has been published previously.10 This study was authorised by the TOPPITS Trials Steering Committee (TSC).
Participants and presenting characteristics
Participants were adult patients, newly referred to secondary care otolaryngology clinics between April 2014 and February 2017, with over 6 weeks unexplained throat or voice symptoms, principally: hoarseness, throat pain, globus sensation, throat clearing, post nasal secretions or mucus excess, cough or choking. All participants underwent an endoscopic laryngopharyngeal assessment to exclude significant pathology. Baseline severity was assessed using the well-established Reflux Symptom Index (RSI),11 which is widely used in voice and general otolaryngology clinics. This study analysed the baseline TOPPITS data of all participants, irrespective of allocated treatment group, and was performed independent to the follow-up data or final TOPPITS results. Patients’ demographics included age, gender and BMI.
Patient and public involvement
Patients were involved in the design and oversight of the TOPPITS trial. Patients were not directly involved in the current study.
Symptom assessment measures
At baseline, all participants completed three symptom questionnaires:
Reflux symptom index
The RSI is a nine-item self-administered questionnaire.11 Each item is scored on a Likert scale zero to five, giving a total score range of 0–45. Higher scores represent increasing severity of patient reported symptoms. In order to ensure that patients had a qualifying level of severity of the non-dyspepsia items, that is, the throat symptoms in question, all participants were required to score at least 10 points on items 1 to 8 of the RSI, irrespective of their score on the ninth item relating to dyspepsia symptoms.
Comprehensive Reflux Symptom Score
The Comprehensive Reflux Symptom Score (CReSS) was originally described in 200912 by an amalgamation of the key components of the RSI items and the Gastro-oesophageal reflux disease Symptom Assessment Scale.13 The CReSS 34 items are scored on a zero to five Likert scale, giving a range of total scores 0–170. The CReSS has three defined factors which map to oesophageal, upper airway and pharyngeal symptoms.14
Laryngopharyngeal Reflux Health Related Quality of Life Questionnaire
The Laryngopharyngeal Reflux Health Related Quality of Life Questionnaire (LPR-HRQL) is a 43 item self-administered questionnaire.15 It is composed of four domains (voice—12 items, cough—6 items, throat clearing—6 items and swallow—5 items). Each of these items is scored on a 0–6 Likert scale. The reliability, validity and responsiveness of this instrument were assessed in 2005,15 but it has not been widely reported. Following each set of domain questions, there is a ‘thermometer’ question for that domain. The last 10 questions cover the domain ‘overall impact of acid-reflux’. An ‘overall score’ is calculated by adding the four thermometer scores to the 10 overall impact of acid reflux questions, to give a score of 14–140.
Endoscopic assessment of the larynx and pharynx
Following consent to enter TOPPITS, participants underwent a further endoscopic examination. The Reflux Finding Score (RFS)16 is a clinician assessed rating of the appearance of the larynx and pharynx. The RFS comprises eight items, with each item scored using varying categories. The total score range is between 0 and 29. Endoscopic images were captured and later assessed, according to the RFS, by an experienced speech and language therapist who was blind to the patient-reported symptom scores.
Statistical analyses
All statistical analyses were conducted using SPSS V.24. Descriptive statistics for the RSI, CReSS, LPR-HRQL and RFS have been described in full in the TOPPITS trial report.10 In this analysis, the relationships among the questionnaires and demographic factors (age and BMI) were assessed using the Pearson correlation coefficients—r (total questionnaire scores were normally distributed).
Exploratory factor analysis of symptom items
Data from the RSI, CReSS and LPR-HRQL were combined for the Exploratory Factor Analysis (EFA). EFA is used to identify latent constructs, or factors, within a set of measured variables, which group correlated variables together. It is a recognised technique to reduce a large number of items (in this case, symptoms from the questionnaires) to a smaller number of related groups of items—factors. These factors can then be used to define the variables included in cluster analysis.17 All nine items and all 34 items from the RSI and CReSS were included. The voice, cough, throat and swallow domain items (29 in total) were included from the LPR-HRQL. The ‘overall impact of acid’ items and ‘thermometer question’ were omitted as these were considered too varied in their nature to reasonably comprise one set of related questions.
The principal axis factoring extraction method was used, as it is appropriate for non-normal data (relating to the distribution of the individual questionnaire items rather than the total questionnaire scores, which were normally distributed). Factors were retained with an eigenvalue greater than one, or using scree plots to assess the factors’ eigenvalues graphically. Oblique promax rotation of the data was used, given the large dataset and the expected relationship of the factors. The suppression of small coefficients was explored where appropriate, to remove items with low loading values.
Cluster analysis of patients
Cluster analysis techniques were used to explore whether groups of patients (as opposed to questionnaire items) could be reliably defined according to their presenting symptoms. Cluster analysis was initially performed using a two-step approach. However, other cluster analysis methods (K-means and hierarchical) were also considered to ensure a robust solution was obtained. The factor score variables, produced from the EFA, were included in the cluster analysis as standardised values. Cluster analysis was performed for the three methods initially using all seven factors and was performed then with a reduced number of factors to establish if a reproducible model could be defined over the three methods.
Missing data
Where appropriate, imputation of missing data was considered. Participants were excluded from the analysis if imputation was not possible where entire questionnaires were not completed.
Results
Data were available for 344 participants. Missing data occurred for the RSI (two participants), CReSS (nine participants), LPR-HRQL (six participants). These participants were excluded from the analysis. Imputation of missing data was appropriate for six participants’ LPR-HRQL scores. Missing data occurred for the RFS scores in 90 patients. This was due to blocks of consecutive participants’ images in certain institutions not being saved appropriately. The demographics and presenting symptom scores are presented in table 1. The highest ranked items from the RSI were lump in the throat, throat clearing and excess throat mucus (table 2). The highest ranked items from the CReSS were ‘throat clearing’, ‘feeling things stuck in throat’ and ‘lump in throat’. The LPR-HRQL domain score means (SD) were: voice 14.9 (15.2), cough 8.6 (9.1), throat clearing 9.3 (7.4), swallowing 7.1 (6.7). When accounting for the differing number of items in each domain of the LPR-HRQL, the ranking from highest to lowest scores was throat clearing, cough, swallowing and voice.
Table 1.
Demographics and presenting symptom scores
| Variable | ||
| Gender | Female | 195 (57%) |
| Male | 149 (43%) | |
| Age | Mean (SD) | 52.2 (13.7) |
| Range | 20–84 | |
| Body mass index | Mean (SD) | 28.1 (5.6) |
| Range | 11.3–56.9 | |
| RSI | Mean (SD) | 21.9 (7.2) |
| Range | 10–43 | |
| CReSS | Mean (SD) | 51.2 (27.2) |
| Range | 2–142 | |
| LPR-HRQL—Overall Score | Mean (SD) | 50.8 (28.0) |
| Range | 14–134 | |
| RFS | Mean (SD) | 8.8 (4.1) |
| Range | 0–24 | |
CReSS, Comprehensive Reflux Symptom Score; LPR-HRQL, Laryngopharyngeal Reflux – Health-Related Quality of Life; RFS, Reflux Finding Score; RSI, Reflux Symptom Index.
Table 2.
Ranked individual items from the RSI
| Rank mean score | RSI item | Mean score |
| 1 | Lump in throat | 3.51 |
| 2 | Throat clearing | 3.44 |
| 3 | Excess throat mucus | 2.85 |
| 4 | Troublesome cough | 2.49 |
| 5 | Hoarseness | 2.39 |
| 6 | Coughing lying down | 2.12 |
| 7 | Heartburn, chest pain, indigestion or stomach acid coming up | 1.79 |
| 8 | Difficulty swallowing | 1.68 |
| 9 | Breathing difficulties | 1.58 |
RSI, Reflux Symptom Index.
Increasing age was significantly negatively correlated with both the CReSS (r=−0.24, p<0.01) and LPR-HRQL (r=−0.29, p<0.01), but not with the RSI (r=−0.08, p=0.15). Patients’ BMI was not related to age or any of the three questionnaires. The RSI was strongly positively correlated with the CReSS (r=0.73, p<0.01) and moderately positively correlated with the LPR-HRQL (r=0.58, p<0.01). The CReSS and LPR-HRQL were strongly positively correlated (r=0.71, p<0.01). The RFS was weakly positively correlated with BMI (r=0.25, p<0.01). There was no relationship between the RFS and any of the three symptoms questionnaires: RSI r=0.06 (p=0.40), CReSS r=0.04 (p=0.57), LPR-HRQL r=−0.02 (p=0.72).
A series of exploratory factor analyses including all three questionnaire items, with low loading items removed, resulted in a seven-factor model (table 3). The following clinically meaningful labels were assigned to factors: factor 1—voice factor, factor 2—cough factor, factor 3—gastrointestinal symptoms (GI) factor, factor 4—airway symptoms and dysphagia factor, factor 5—throat clearing factor, factor 6—lump in throat factor, factor 7—life events factor.
Table 3.
Exploratory Factor Analysis: Pattern Matrix for Three Factor Model
| Symptom (Questionnaire) | Factor | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Straining to talk is tiring (LPR-HRQL) | 0.90 | ||||||
| Being hoarse makes it hard for me to communicate my true self (LPR-HRQL) | 0.88 | ||||||
| I feel embarrassed about the sound of my voice (LPR-HRQL) | 0.85 | ||||||
| My voice makes others feel uncomfortable to listen to me (LPR-HRQL) | 0.81 | ||||||
| I avoid talking because of the effort (LPR-HRQL) | 0.78 | ||||||
| Hoarseness (CReSS) | 0.76 | ||||||
| My voice problems make it difficult for me to work (LPR-HRQL) | 0.75 | ||||||
| Hoarseness (RSI) | 0.75 | ||||||
| I find it hard to meet new people because of what they will think (LPR-HRQL) | 0.68 | ||||||
| I am afraid I might lose my voice forever (LPR-HRQL) | 0.65 | ||||||
| I can’t sing as much as I would like to because of my voice (LPR-HRQL) | 0.60 | ||||||
| The sound of my voice makes people think I’m angry or upset (LPR-HRQL) | 0.60 | ||||||
| Troublesome cough (RSI) | 0.85 | ||||||
| Coughing when upright (CReSS) | 0.85 | ||||||
| Coughing after eating or lying down (RSI) | 0.83 | ||||||
| Coughing when lying down (CReSS) | 0.78 | ||||||
| Cough after eating (CReSS) | 0.71 | ||||||
| People think I am sick because of my coughing (LPR-HRQL) | 0.69 | ||||||
| I worry about having a coughing spell at a bad time (LPR-HRQL) | 0.67 | ||||||
| I have to leave the room because of my coughing (LPR-HRQL) | 0.62 | ||||||
| My co-workers can hear me coming because of my coughing (LPR-HRQL) | 0.53 | ||||||
| Indigestion (CReSS) | 0.87 | ||||||
| Heartburn, chest pain, indigestion or stomach acid coming up(RSI) | 0.87 | ||||||
| Stomach acid coming up (CReSS) | 0.86 | ||||||
| Heartburn (CReSS) | 0.86 | ||||||
| Acid/ sour taste in mouth (CReSS) | 0.63 | ||||||
| Regurgitation (CReSS) | 0.54 | ||||||
| Belching (CReSS) | 0.53 | ||||||
| Bloating (CReSS) | 0.46 | ||||||
| Excess mucus (CReSS) | 0.77 | ||||||
| Throat clearing (CReSS) | 0.74 | ||||||
| Excess throat mucus or post-nasal drip (RSI) | 0.71 | ||||||
| Throat clearing (RSI) | 0.70 | ||||||
| Mucus dripping down back of throat (CReSS) | 0.67 | ||||||
| I feel frustrated about having to clear my throat so often (LPR-HRQL) | 0.56 | ||||||
| People notice how much I have to clear my throat (LPR-HRQL) | 0.50 | ||||||
| Difficulty swallowing food (CReSS) | 0.70 | ||||||
| I awaken from sleep gasping for breath (LPR-HRQL) | 0.64 | ||||||
| Choking (CReSS) | 0.60 | ||||||
| I am afraid of choking in my sleep (LPR-HRQL) | 0.58 | ||||||
| Difficulty swallowing food liquids or tablets (RSI) | 0.57 | ||||||
| Difficulty swallowing liquids (CReSS) | 0.57 | ||||||
| Difficulty Breathing (CReSS) | 0.53 | ||||||
| Breathing difficulties or choking episodes (RSI) | 0.46 | ||||||
| Something caught or lump in throat (RSI) | 0.68 | ||||||
| Feeling things stuck throat (CReSS) | 0.62 | ||||||
| Lump in Throat (CReSS) | 0.62 | ||||||
| Clearing my throat has a negative effect on friendships (LPR-HRQL) | 0.70 | ||||||
| Clearing my throat has a negative effect on sex (LPR-HRQL) | 0.58 | ||||||
| I avoid social events because of the need to clear my throat (LPR-HRQL) | 0.53 | ||||||
CReSS, Comprehensive Reflux Symptom Score; LPR-HRQL, Laryngopharyngeal Reflux – Health-Related Quality of Life; RSI, Reflux Symptom Index.
The EFA on the RSI items alone failed to produce a clean factor structure. For the CReSS items alone, a three-factor model emerged (table 4). For the LPR-HRQL domain items alone, the EFA showed a single factor for each domain, other than the throat domain which split into two factors.
Table 4.
Pattern matrix for the cress exploratory factor analysis
| Questionnaire item | Factor | ||
| 1 | 2 | 3 | |
| Indigestion | 0.90 | ||
| Stomach acid up | 0.87 | ||
| Heartburn | 0.82 | ||
| Acid or sour taste in mouth | 0.67 | ||
| Regurgitation | 0.61 | ||
| Belching | 0.59 | ||
| Bloating | 0.55 | ||
| Gurgling stomach | 0.51 | ||
| Flatulence | 0.40 | ||
| Nausea | 0.38 | ||
| Rush of saliva in mouth | 0.34 | ||
| Coughing when upright | 0.86 | ||
| Cough when lying | 0.75 | ||
| Coughing after eating | 0.72 | ||
| Wheezing | 0.65 | ||
| Mucus dripping in throat | 0.51 | ||
| Excess mucus | 0.51 | ||
| Difficulty breathing | 0.47 | ||
| Throat clearing | 0.44 | ||
| Hoarseness | 0.34 | ||
| Difficulty swallowing food | 0.86 | ||
| Difficulty swallowing liquids | 0.74 | ||
| Lump in throat | 0.59 | ||
| Feeling things stuck throat | 0.55 | ||
| Pain in throat | 0.47 | ||
| Choking | 0.34 | 0.46 | |
| Decreased appetite | 0.45 | ||
| Headache | 0.33 | ||
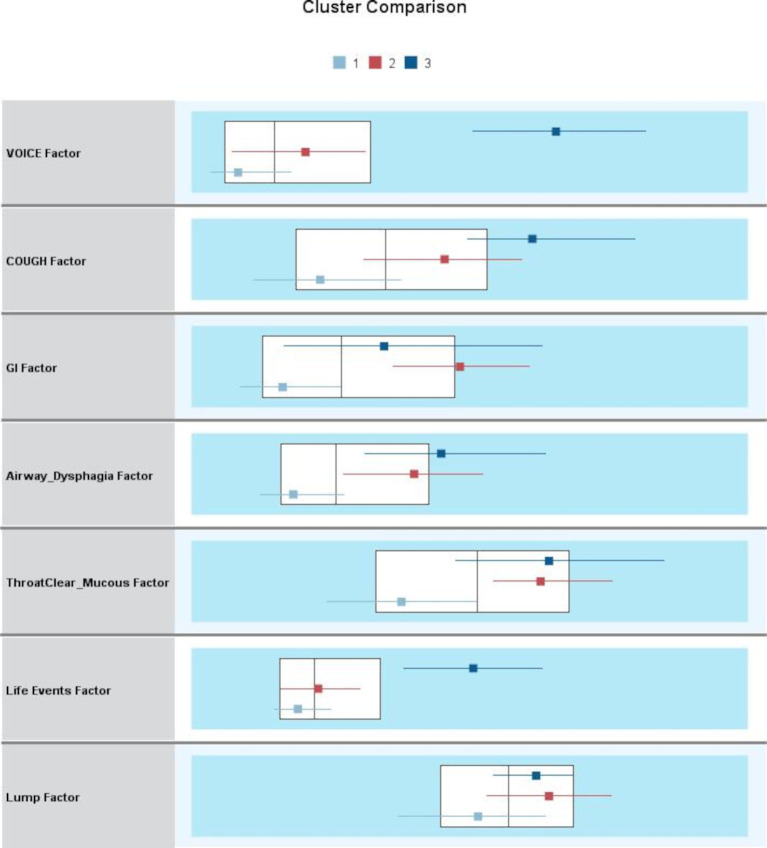
Cluster analysis failed to demonstrate any clear or clinically meaningful clusters of patients, neither when all seven factors from the three questionnaire EFA were included nor when the factors were reduced in number. Figure 1 is a demonstration of the two-step cluster analysis outputs, showing no clinically meaningful clusters of patients when the seven factors were included.
Figure 1.
The Cluster Comparison output from the two-step cluster analysis plots the median and interquartile range for each cluster for each variable, overlying the overall population median and interquartile range—displayed as box plots. The x-axis is a standardised value (mean = 0, SD = 1), produced from the exploratory factor analysis of the three questionnaires. GI, gastrointestinal.
Discussion
This study has explored the wealth of presenting throat and voice symptoms within a population of patients recruited to a large randomised clinical trial and adds clarity to the clinical condition. The most troublesome symptoms reported across the three questionnaires were consistently those of a lump in the throat (globus sensation) and throat clearing. Patients could not be separated into definable clusters based on presenting symptoms, implying that patients’ perceptions of individual symptoms overlap. This study is unique in suggesting that the wide variety of subjective, patient-reported throat symptoms (or the labels that clinicians have assigned to these symptoms) may represent a single underlying condition.
The term ‘laryngopharyngeal reflux’ has become a very popular label to encompass a range of upper aerodigestive tract symptoms. Given the present study’s findings, laryngopharyngeal reflux may be clinically useful in grouping together individual symptoms. In light of the TOPPITS results, the inclusion of ‘reflux’ within such a label must now be questioned. Further research is required to improve the diagnostic precision of reflux as a cause of throat symptoms. The continued use of a reflux label could detract from researching more appropriate management strategies for this group of patients. ‘Persistent throat symptoms’ would be an alternative term to laryngopharyngeal reflux. Patients with symptoms that lack a demonstrable underlying cause find the term ‘persistent’ more acceptable than other possibilities, such as ‘unexplained’18
This work identifies further evidence which may question the popularised mechanistic link between reflux and chronic throat and voice symptoms: The RFS data from this study offers by far the largest published analysis of pharyngeal and laryngeal images to date. In contrast to the most popular cited study of 40 patients, which showed high concordance between symptoms (RSI) and throat signs (RFS),19 this study found no association between any of the three questionnaires and the available RFS scores from 254 patients. Gastro-oesophageal reflux is associated with increasing BMI.20 This work found no relationship between chronic pharyngeal and laryngeal symptom reporting and BMI. It did find that increasing BMI might be related to higher RFS scores. However, this was a weak relationship and the lack of coherence otherwise between symptoms, signs and BMI means drawing conclusions from this would be inappropriate. Obesity may affect the appearance of the pharynx and larynx through physical compression.
Laryngopharyngeal reflux is often referred to as a ‘diagnosis’, yet it remains an undefined condition. Ilgen et al state that ‘diagnosis can align with assignment of a ‘label,’ where a constellation of signs, symptoms, and test results is unified into a solution…’.21 As demonstrated from the present research and other published observations,22 there is insufficient evidence of causality to refer to laryngopharyngeal reflux as a diagnosis. We would recommend persistent throat symptoms, not as a diagnosis, but as a term to describe a group of related symptoms.
EFA techniques have been used previously to analyse patient-reported outcome measures in pharyngeal and laryngeal symptoms. EFA demonstrated seven factors of variables (symptoms) using the combined data from the RSI, CReSS and LPR-HRQL. This offers a potential clinically meaningful and simplified classification of symptoms: voice, cough, GI symptoms, airway symptoms and dysphagia, throat clearing, lump in the throat sensation and life events. The dimension reduction produced through this methodology could be useful in future research, such as defining an optimal patient-reported outcome tool. While this study did not aim to perform questionnaire validation analysis, it did demonstrate a factor structure within the CReSS alone which was a close fit to the previously published oesophageal, upper airway and pharyngeal factors.14 The reproducible nature of these factors, or subgroups, adds further evidence in favour of the openly available CReSS as an appropriate tool to measure symptoms in this patient population. In contrast, no definable factors were identified within the RSI to support previously published analyses.23 24 The LPR-HRQL has not been reported frequently. While the individual sets of domain questionnaire do seem appropriate in terms of factor structure, the layout of the questionnaire, with the separate thermometer questions and overall impact of acid items, complicates the interpretation of the overall results.
The authors believe that for most patients with persistent throat symptoms, strategies that employ techniques used in speech and language therapy25 or cognitive behavioural therapy, while addressing underlying anxiety and depression, may prove most beneficial. Hydration techniques that help reduce habitual throat clearing and dry swallowing should be recommended. Weight loss, smoking cessation, avoiding late eating before bed and raising the head of the bed are effective measures that improve gastro-oesophageal reflux.26 27 They are also safe, promote general health and are patient delivered interventions.
Conclusions
In this population, cluster analysis demonstrated no clinically meaningful or reproducible clusters of patients. Based on these results, we cannot conclude that individual throat and voice symptoms can be used to categorise groups of patients reliably. A global term that encompasses the range of interlinked symptoms, such as ‘persistent throat symptoms’, would appear more appropriate than referring to individual symptoms. This work and the TOPPITS results imply that a broad term such as ‘persistent throat symptoms’ may be more appropriate to recommend to clinicians than ‘laryngopharyngeal reflux’. Persistent throat symptoms are common. Their nomenclature is important if clinicians are to avoid unwarranted connotation of causation, medicalised terminology, and over diagnosis of reflux. This study highlights the need for future research into more generic throat symptoms.
Footnotes
Contributors: JO’H is responsible for the overall content as guarantor. JO’H accepts full responsibility for the finished work and the conduct of the study, had access to the data, and controlled the decision to publish. JO’H and JW collected the data. JO’H, LH, HF and JW all contributed to the analysis of the data, interpretation of results and manuscript writing.
Funding: This study received no financial support and was independent to the Trial Of Proton-Pump Inhibitors in Throat Symptoms, funded by the National Institute for Health Research in the UK.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
No data are available. All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The trial protocol was approved by the regional ethics committee (National Research Ethics Service Committee—North East: Tyne and Wear South reference number: 13/NE/0336). A proportionate review was approved for the present study 18/EE/0158.
References
- 1.Powell J, O'Hara J, Wilson JA. Are persistent throat symptoms atypical features of gastric reflux and should they be treated with proton pump inhibitors? BMJ 2014;349:g5813. 10.1136/bmj.g5813 [DOI] [PubMed] [Google Scholar]
- 2.Lechien JR, Saussez S, Schindler A, et al. Clinical outcomes of laryngopharyngeal reflux treatment: a systematic review and meta‐analysis. Laryngoscope 2019;129:1174–87. 10.1002/lary.27591 [DOI] [PubMed] [Google Scholar]
- 3.O'Hara J, Stocken DD, Watson GC, et al. Use of proton pump inhibitors to treat persistent throat symptoms: multicentre, double blind, randomised, placebo controlled trial. BMJ 2021;372:m4903. 10.1136/bmj.m4903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deary IJ, Wilson JA, Harris MB, et al. Globus pharyngis: development of a symptom assessment scale. J Psychosom Res 1995;39:203–13. 10.1016/0022-3999(94)00104-D [DOI] [PubMed] [Google Scholar]
- 5.Bouchoucha M, Fysekidis M, Julia C, et al. Body mass index association with functional gastrointestinal disorders: differences between genders. results from a study in a tertiary center. J Gastroenterol 2016;51:337–45. 10.1007/s00535-015-1111-y [DOI] [PubMed] [Google Scholar]
- 6.Harris MB, Deary IJ, Wilson JA. Life events and difficulties in relation to the onset of globus pharyngis. J Psychosom Res 1996;40:603–15. 10.1016/0022-3999(96)00024-4 [DOI] [PubMed] [Google Scholar]
- 7.Deary IJ, Smart A, Wilson JA. Depression and ‘Hassles’ in Globus Pharyngis. Br J Psychiatry 1992;161:115–7. 10.1192/bjp.161.1.115 [DOI] [PubMed] [Google Scholar]
- 8.Cathcart RA, Wilson JA. Catarrh - the patient experience. Rhin 2011;49:387–91. 10.4193/Rhino11.055 [DOI] [PubMed] [Google Scholar]
- 9.Watson G, O’Hara J, Carding P, et al. TOPPITS: trial of proton pump inhibitors in throat symptoms. study protocol for a randomised controlled trial. Trials 2016;17:175. 10.1186/s13063-016-1267-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson JA, Stocken DD, Watson GC, et al. Lansoprazole for persistent throat symptoms in secondary care: the TOPPITS RCT. Health Technol Assess 2021;25:1–118. 10.3310/hta25030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belafsky PC, Postma GN, Koufman JA. Validity and reliability of the reflux symptom index (RSI). Journal of Voice 2002;16:274–7. 10.1016/S0892-1997(02)00097-8 [DOI] [PubMed] [Google Scholar]
- 12.Papakonstantinou L, Leslie P, Gray J, et al. Laryngopharyngeal reflux: a prospective analysis of a 34 item symptom questionnaire. Clin Otolaryngol 2009;34:455–9. 10.1111/j.1749-4486.2009.01998.x [DOI] [PubMed] [Google Scholar]
- 13.Rothman M, Farup C, Stewart W, et al. Symptoms associated with gastroesophageal reflux disease: development of a questionnaire for use in clinical trials. Dig Dis Sci 2001;46:1540–9. 10.1023/A:1010660425522 [DOI] [PubMed] [Google Scholar]
- 14.Drinnan M, Powell J, Nikkar-Esfahani A, et al. Gastroesophageal and extraesophageal reflux symptoms: similarities and differences. Laryngoscope 2015;125:424–30. 10.1002/lary.24950 [DOI] [PubMed] [Google Scholar]
- 15.Carrau RL, Khidr A, Gold KF, et al. Validation of a quality-of-life instrument for laryngopharyngeal reflux. Arch Otolaryngol Head Neck Surg 2005;131:315–20. 10.1001/archotol.131.4.315 [DOI] [PubMed] [Google Scholar]
- 16.Belafsky PC, Postma GN, Koufman JA. The validity and reliability of the reflux finding score (RFS). Laryngoscope 2001;111:1313–7. 10.1097/00005537-200108000-00001 [DOI] [PubMed] [Google Scholar]
- 17.Adnane C, Adouly T, Khallouk A, et al. Using preoperative unsupervised cluster analysis of chronic rhinosinusitis to inform patient decision and endoscopic sinus surgery outcome. Eur Arch Otorhinolaryngol 2017;274:879–85. 10.1007/s00405-016-4315-8 [DOI] [PubMed] [Google Scholar]
- 18.Marks EM, Hunter MS. Medically unexplained symptoms: an acceptable term? Br J Pain 2015;9:109–14. 10.1177/2049463714535372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mesallam TA, Stemple JC, Sobeih TM, et al. Reflux symptom index versus reflux finding score. Ann Otol Rhinol Laryngol 2007;116:436–40. 10.1177/000348940711600608 [DOI] [PubMed] [Google Scholar]
- 20.Jacobson BC, Somers SC, Fuchs CS, et al. Body-mass index and symptoms of gastroesophageal reflux in women. N Engl J Med 2006;354:2340–8. 10.1056/NEJMoa054391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ilgen JS, Eva KW, Regehr G. What’s in a Label? Is diagnosis the start or the end of clinical reasoning? J Gen Intern Med 2016;31:435–7. 10.1007/s11606-016-3592-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneider GT, Vaezi MF, Francis DO. Reflux and voice disorders: have we established causality? Curr Otorhinolaryngol Rep 2016;4:157–67. 10.1007/s40136-016-0121-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cathcart RA, Steen N, Natesh BG, et al. Non-voice-related throat symptoms: comparative analysis of laryngopharyngeal reflux and globus pharyngeus scales. J Laryngol Otol 2011;125:59–64. 10.1017/S0022215110001866 [DOI] [PubMed] [Google Scholar]
- 24.Printza A, Kyrgidis A, Oikonomidou E, et al. Assessing laryngopharyngeal reflux symptoms with the reflux symptom index: validation and prevalence in the Greek population. Otolaryngol Head Neck Surg 2011;145:974–80. 10.1177/0194599811425142 [DOI] [PubMed] [Google Scholar]
- 25.Khalil HS, Bridger MW, Hilton-Pierce M, et al. The use of speech therapy in the treatment of globus pharyngeus patients. A randomised controlled trial. Rev Laryngol Otol Rhinol 2003;124:187–90. [PubMed] [Google Scholar]
- 26.Ness-Jensen E, Hveem K, El-Serag H, et al. Lifestyle intervention in gastroesophageal reflux disease. Clin Gastroenterol Hepatol 2016;14:175–82. e171-173. 10.1016/j.cgh.2015.04.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaltenbach T, Crockett S, Gerson LB. Are lifestyle measures effective in patients with gastroesophageal reflux disease? Arch Intern Med 2006;166:965–71. 10.1001/archinte.166.9.965 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data are available. All data relevant to the study are included in the article or uploaded as online supplemental information.