Summary
Background
Wasting reflects infections and poor nutrition and affects almost 50 million children at any given time. Wasting comes with immediate risk of mortality and increased risks for long-term negative consequences for development. Children under two are particularly sensitive to undernutrition and infections. We estimated the age patterning in wasting prevalence.
Methods
We calculated wasting prevalence and used Poisson regression models to estimate prevalence ratios comparing prevalence in children under and over two years using data from Demographic and Health Surveys and Multiple Indicator Cluster Surveys from 94 mostly low- and middle-income countries, including 804,172 children under five, born to a nationally representative sample of women 15–49 years old. Wasting prevalence was defined as the percentage of children with weight-for-height below –2 z-score from the median of the WHO 2006 growth standard.
Findings
Wasting prevalence for children under two was 14% (95% CI: 13, 14) while it was 9% (95% CI: 9, 9) for children 2–4 years old—leading to a prevalence ratio of 0·66 (95% CI: 0·64, 0·67) in our pooled sample. Prevalence ratios were less than one, indicating lower prevalence in children over two, in 87 countries and statistically significantly lower than one at a 5% level (non-adjusted) in 68 countries. Wasting prevalence was generally lower in children under two for males and females and the wealthiest and poorest households.
Interpretation
Since wasting prevalence was observed to be greater among children 0–2 years, and adverse exposure to undernutrition and infections are particularly harmful and interventions are more effective during the 1000 days from conception until age two, nutrition interventions should ensure coverage of children under two through programmatic measures to increase detection and enrollment in wasting programs.
Funding
UNICEF, Nutrition Section, Programme Division in New York.
Keywords: Wasting, Children under five, Children under two, First 1000 days
Research in context.
Evidence before this study
Many nutrition interventions to combat child wasting are targeted at children under five with far from complete coverage, while children under two are more vulnerable to undernutrition with particularly severe short- and long-term consequences. A MEDLINE search was conducted to assess the scope of the previous literature reporting age stratified wasting prevalence using the search terms wasting AND (“age distribution” OR “age difference” OR “under two” OR “stratified by age”) for studies published up to December 1, 2021, and identified a few articles showing higher prevalence at younger ages. Most of these studies were from specific settings with main aims other than studying age distribution of wasting.
Added value of this study
This study compared wasting prevalence for children under two and children 2–4 years old in a more comprehensive way using data from 94 primarily low- and middle-income countries. We show that the prevalence of wasting was 34% lower among children 2–4 years old than children under two overall. Prevalence of wasting was considerably lower among children 2–4 years old in most countries.
Implications of all the available evidence
Since undernutrition has especially severe short- and long-term consequences during the first 1,000 days, and our finding that a substantially greater share of children under two years suffers from wasting than children over two, it may in some cases be beneficial for overall child health to prioritize children under two in important health and nutrition interventions aimed at reducing wasting.
Alt-text: Unlabelled box
Introduction
Wasting refers to children who are too thin for their height due to recent weight loss from lack of nutritional intake and illness. Wasting, and especially severe acute malnutrition, in young children is a medical emergency with high risk of mortality and negative consequences for human development. Child wasting remains a problem in low- and middle-income countries, affecting almost 50 million (7·3%) children under five at any given time.1 Over 800 thousand under-5 deaths (or 12–13% of all under-5 deaths) were attributed to wasting in 2011.2 Wasting can also have long-term consequences for human development, with recovering children potentially suffering from cognitive and health impairments, especially if their growth is stunted.1
Nutrient interventions and complementary feeding programs have been shown to be effective in improving child nutrition, particularly at young ages.3, 4, 5, 6 However, evidence-based essential nutrition interventions often fail to reach all children in low- and middle-income countries due to resource scarcity and weak delivery systems. Sub-optimal coverage call for optimizing nutrition interventions, especially for the most vulnerable. Many nutrition interventions, such as vitamin A supplementation, are targeted at children under five. However, the 1000 days from conception until age two is an important yet precarious period for human development, when adverse exposure to infections and undernutrition are particularly determinantal, with greater immediate negative consequences for survival1 and long-term consequences for physical growth, cognitive development, productivity, wages, and health.7,8 These 1000 days have also been suggested as an important window for effective interventions for undernutrition.9
Although most previous work has focused on age patterning of other forms of undernutrition, such as child stunting, previous studies have also indicated that wasting is more common in younger children.10,11 In order to assess the relative burden among children under two years old, the most vulnerable children, this article comprehensively analyzed the age patterning of wasting in children under five years in 94 countries using cross-sectional household surveys. We estimated the wasting prevalence for children under two years old and 2–4 years old and the prevalence ratio, showing the relative difference in wasting prevalence before and after age two. We also investigated differences in the age patterning of wasting by sex and living standards. We present results for countries, UNICEF regions, and World Bank income groups, as well as overall.
Studying the age patterning of wasting helps demonstrate the situation of the most vulnerable children, during the first 1000 days, who need preventative interventions and curative treatments to reduce wasting and improve long-term health and well-being. Studying the age patterning of wasting may also give better indications of sensitivity to and adverse consequences of undernutrition and infections at specific ages, since wasting is linked more strongly to acute events, while stunting, to a greater extent, reflects accumulation of adverse exposures over a longer period. Understanding where and to which extent children are more sensitive to adverse exposures at younger ages can help tailor nutrition and health interventions to those in most need.
Data
We used data from nationally representative cross-sectional household surveys—the Multiple Indicator Cluster Surveys (MICS) and Demographic and Health Surveys (DHS)—which are collected regularly in multiple countries, mostly low- and middle-income.12, 13, 14, 15 We used the most recent survey available for each country but excluded countries without any survey conducted after 2010. We only considered nationally representative surveys and excluded surveys focusing on subnational regions or subpopulations. We obtained data from 94 countries: 44 were collected in the DHS and 50 in the MICS.
The DHS and MICS use stratified multi-stage sampling. Stratification is done by administrative or geographic subnational regions and further by urban and rural areas. Census enumeration areas (usually villages in rural areas and neighborhoods in urban areas) are generally used as primary sampling units, sampled with a probability proportional to size. From the selected primary sampling units, around 20–30 households are sampled using systematic random sampling. An interview is conducted in the selected households where several questionnaires are administered. Information from all women 15–49 years old is recorded in a women's questionnaires. Basic information on the household and its members is recorded in a household questionnaire. In most DHS and MICS, a biomarker questionnaire records biomarker data, such as height and weight for children under five years old. (In some surveys, a subsample of households was selected for the collection of biomarker data.)
Sample sizes are guided by the need to construct precise nationally and regionally representative estimates.16 Non-responses are not replaced, but response rates typically exceed 90%.12,14 Sampling weights provided with the data were calculated as the inverse of the probability of being included in the survey and adjust for oversampling and non-response and improve precision.14,17
The total sample consisted of 871,735 children under five, born to interviewed women, alive, present in households, and selected for biomarker measures. After excluding observation with missing or implausible measurements, the final sample used for analysis was 804,172 children under five (see Table S1 in the Supplement for details). Our final sample included ten countries in East Asia & Pacific, 19 in Eastern & Southern Africa, 14 in Europe & Central Asia, 16 in Latin America & Caribbean, 7 in Middle East & North Africa, 6 in South Asia, and 22 in West & Central Africa. The sample included 27 countries classified (at the time of the survey) as Low Income Countries by the World Bank, 37 Lower Middle Income Countries, 28 Upper Middle Income Countries, and two High Income Countries. The two High Income Countries were Trinidad & Tobago and Barbados, which are not a good representation of High Income Countries: For example, they are both small island states, and most countries with similar Human Development Index Score are classified as Upper Middle Income Countries.18 We, therefore, grouped Trinidad & Tobago and Barbados with Upper Middle Income Countries. Due to their small size (0.2% of the population of children under five in Upper Middle Countries in our sample) this classification will not impact the aggregate estimates for Upper Middle Income Countries in any substantial way.
Measures
Wasting
Standing height of children 2–4 years old was measured in millimeters, while the length was measured for children under two years old while laying down. Weight was measured in kilograms with one decimal. Weight-for-height was then calculated as the z-score deviation in weight for a given height, according to the WHO 2006 growth standard, which was constructed based on a sample of healthy children from diverse settings, growing up in optimal conditions.19 Implausible weight-for-height z-scores, below −5 or above 5, were excluded.17 Wasting prevalence was then defined as the percentage of children with weight-for-height below −2 z-score from the WHO 2006 median. For healthy children in optimal conditions—specifically, children defining the WHO 2006 standard— ∼2·3% of children are expected to fall below −2 z-scores in weight-for-height and excess of that indicates population-level shortcomings in nutrition and exposures to infections causing thinness. Wasting is primarily interpreted as indicating acute undernutrition.20
Household living standards
Both the DHS and MICS provide a household wealth index with all surveys. Information on household's ownership of assets (e.g., car, television, refrigerator) and amenities (e.g., type of toilet facility, electricity access, source of water), obtained in the household questionnaire, was used to construct a household wealth index, using principal component analysis to obtain weights for each asset and amenity. (The DHS provides details from the principal component analysis from most surveys on their website.21) Some claim that using a wealth index is more reliable than using data on income due to less concerns about reporting bias.22
Five groups containing 20% of surveyed households (quintiles) were constructed based on living standards indicated by the household wealth index. We show results comparing the top 20% of households with the best living standards (referred to as wealthiest) to the bottom 20% of households with the worst living standards (referred to as poorest). The wealth index was constructed separately for each country, so the categories indicate the top and bottom 20% of households within each country.
Additionally, we show the association between the household wealth index and wasting across the whole distribution of household wealth, by age. We then convert the wealth index factor score for each survey into a wealth index z-score by subtracting its survey-specific mean and dividing by its survey-specific standard deviation for children included in the analysis.
Methods
We first estimated the wasting prevalence for children under two years old and 2–4 years old, separately, as the percentage of children falling below –2 z-scores from the WHO 2006 median weight-for-height. We estimated pooled and regional estimates directly without random or fixed effects. We then calculated prevalence ratios: the wasting prevalence for children over two divided by the wasting prevalence for children under two. The prevalence ratio indicates the relative difference in prevalence between children under and over two: For example, a prevalence ratio of 0·8 means that the wasting prevalence for children over two was 0·8 times the wasting prevalence for children under two (i.e., 20% lower) and a prevalence ratio of 1·3 indicates that the prevalence for children over two was 1·3 times the prevalence for children under two (i.e., 30% greater). The prevalence ratios were estimated using bivariate Poisson models using a binary indicator for wasting as an outcome variable and a binary indicator for being two years or older (as opposed to under two) as an independent variable. The exponent of the obtained coefficient for being over two gives the prevalence ratio. We used prevalence ratios, rather than prevalence differences (i.e., prevalence for children over two minus prevalence for children under two), since countries and regions vary greatly in their underlying prevalence, and prevalence ratios make it easier to assess variation in the relative burden of wasting between age groups. (We show prevalence differences in the Supplement)
We then estimated separately for males and females the wasting prevalence for children under and over two years as well as the prevalence ratio. Relative sex differences in the prevalence ratios were then estimated using a Poisson model with a binary indicator for wasting as an outcome and a binary indicator for being over two years, a binary indicator for being female, and an interaction term for being over two and female, as independent variables. We show the relative sex differences in the prevalence ratios (i.e., the exponent of the interaction terms for being female and over two) in the Supplement. We used the same approach when comparing the poorest children to the wealthiest children.
Finally, we show average marginal effects of a single z-score increase in the household wealth index, across the whole distribution of household wealth, on percentage wasting. The average marginal effect was estimated from a logit model of wasting on a binary indicator for age and an interaction between age and the household wealth index z-score (also including a squared term for household wealth index z-score, interacted with age, to allow for diminishing effect at higher levels of wealth). These models were done separately for each country, region, and the pooled sample. We also show differences in the average marginal effects between children under and over two in the Supplement.
All estimates were weighted using sampling weights, rescaled such that valid observations (i.e., including only children used for analysis) summed up to the under-five population in each country in the survey year. Therefore, the pooled estimates are representative of all countries included in the study and regional estimates representative of all countries included from each region. Population estimates were obtained from the Population Division of the United Nations (provided for five year increments, with population in intervening years then estimated using linear interpolation).23 All 95% confidence intervals (CI) were adjusted for clustering at the level of primary sampling units using robust clustered sandwich estimator. We refer to relative differences as statistically significant if the 95% confidence interval does not contain one in case of prevalence ratios and zero in case of differences. We do not adjust for multiple comparisons.
Supplementary analyses
We show prevalence differences (prevalence for children over two minus prevalence for children under two).
We also explored patterns in the age prevalence ratios and show correlation coefficients for the relationship between the relative difference in wasting between children under and over two and other aggregate level measures: PPP adjusted real GDP per capita, under-5 mortality rate, and the overall wasting prevalence for children under five. Prevalence of wasting for children under five was estimated from our data while GDP and under-5 mortality rate were obtained from the World Bank's World Development Indicators and were linked to the survey year.24 We show both Pearson's correlation coefficients—which shows the extent to which there was a linear relationship between variables—and Spearman's rank correlations—which shows the extent to which there was a monotonic relationship between variables.
Finally, we explored the age patterning of wasting prevalence within the under two age group by comparing children 0–5 months and children 6–23 months.
Sensitivity analyses
We explored the sensitivity of our results to age patterning of measurement errors—which are suggested to be greater for younger children.25 First, we analyzed the relationship between age and having an implausible weight-for-height measure (i.e., beyond 5 z-scores above or below the reference median). Second, we compared wasting prevalence between children 12–23 months and 24–36 months (since we found no differences in the likelihood of having implausible weight-for-height values between these age groups). We also compared the differences in mean weight-for-height z-score between children under and over two years, since measurement error may mainly cause wider tails in the weight-for-height distribution (and hence greater wasting) to a greater extent than changing the mean weight-for-height.
Since season of interview may influence prevalence of wasting,26 we present adjusted prevalence ratios and prevalence of wasting by age predicted from logit models of wasting on interaction terms for region and age and season of interview and age: with the term for season set at summer for the predictions (see27 for details on estimating adjusted prevalence ratios and prevalence from logit models). (Countries on the equator were categorized as being in the southern hemisphere when defining seasons.)
Also, since the data used in this paper was collected over a span of 10 years and wasting prevalence has generally declined globally, we used the same approach to estimate adjusted prevalence ratios and prevalence of wasting by age predicted from logit models of wasting on interaction terms for region and age and year of interview (linear term) and age: with the term for year of interview set at 2019 for the predictions.
Compliance with ethical standards
This project used publicly accessible secondary data obtained from the DHS website and MICS website. The data were not collected specifically for this study and no one on the study team had access to identifiers linked to the data. These activities do not meet the regulatory definition of human subject research. As such, an Institutional Review Board (IRB) review was not required. The Harvard Longwood Campus IRB allows researchers to self-determine when their research does not meet the requirements for IRB oversight via an IRB Decision Tool.
Role of the funding source
The funding source played no role in the data collection and analysis, reporting and interpretation of results, or the decision to submit the manuscript for publication. Karlsson had full access to all data used in the study. Kim and Subramanian took the decision to submit for publication.
Results
Aggregate level analysis
In our pooled sample, the wasting prevalence was 14% (95% CI: 13, 14) for children under two years old and 9% (95% CI: 9, 9) for children 2–4 years old, resulting in a prevalence ratio of 0·66 (95% CI: 0·64, 0·67)—meaning children over two years old had 0·66 times the wasting prevalence as children under two, or 34% lower prevalence (Figure 1 and Supplementary Table S2).
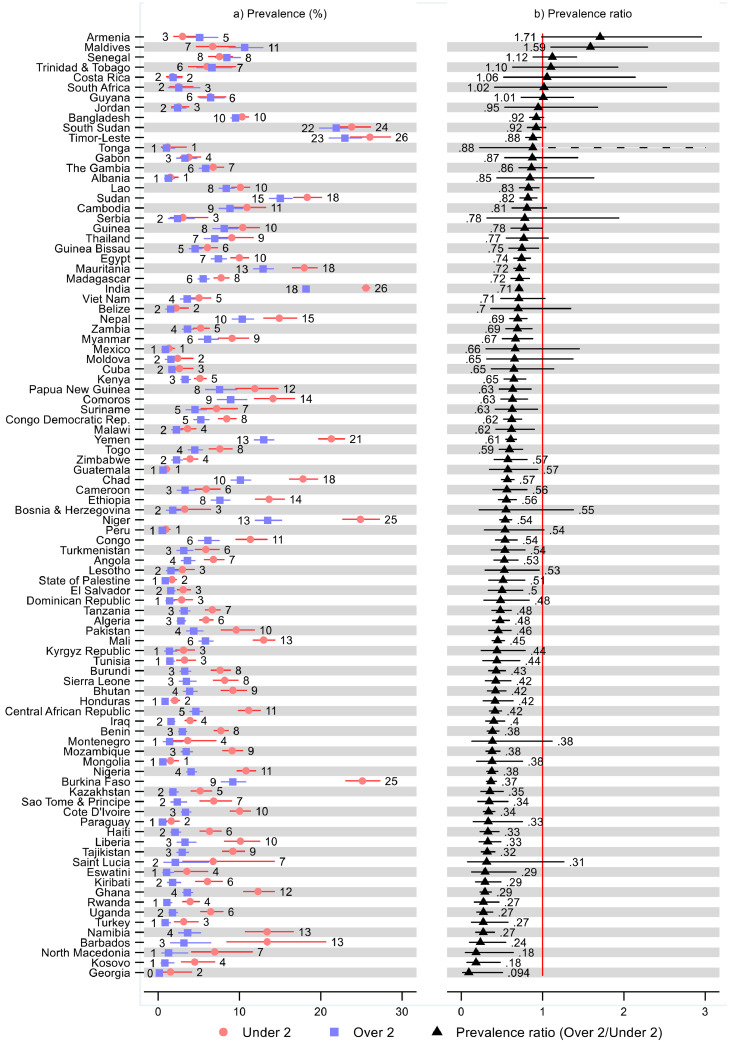
Figure 1.
Prevalence of wasting and prevalence ratios for children under and over two years old by region and World Bank income group. Notes: 95% confidence intervals are shown. See Table S2 in the Supplement for tabulated estimates and confidence intervals. Prevalence ratios show prevalence of wasting for children two and older divided by prevalence of wasting for children less than two years old.
South Asia had the greatest wasting prevalence, or 21% (95% CI: 21, 22) for children under two years and 15% (95% CI: 15, 16) for children over two. Latin America & Caribbean had the lowest wasting prevalence, or 2% for children under two (95% CI: 2, 2) and 1% for children over two (95% CI: 1, 1). The greatest relative difference in wasting prevalence between children under and over two was in Europe & Central Asia, 0·36 (95% CI: 0·27, 0·47) followed by West & Central Africa, 0·47 (95% CI: 0·44, 0·51). The smallest difference in prevalence across age was observed in the East Asia & Pacific region, which had a prevalence ratio of 0·72 (95% CI: 0·63, 0·83).
Country-level analysis
The prevalence ratios showed a lower wasting prevalence in children over two than under two in 87 countries: statistically significantly in 68 countries (Figure 2 and Supplementary Table S2). The prevalence ratios were the lowest (indicating the largest difference in wasting prevalence between children under and over two) in Georgia (0·09; 95% CI: 0·02, 0·51), Kosovo (0·18; 95% CI: 0·07, 0·48), and North Macedonia (0·18; 95% CI: 0·05, 0·64). Armenia and Maldives were the only countries where children over two had a substantially greater wasting prevalence than children under two, with a prevalence ratio indicating a 1·71- (95% CI: 0·99, 2·95) and 1·59-times (95% CI: 1·10, 2·29) greater prevalence for children over two (i.e., 71% and 59% greater) than under two, respectively. These countries, with the highest and lowest prevalence ratios, had a very low prevalence of wasting overall or small sample sizes, so these prevalence ratios should be interpreted with caution.
Figure 2.
Prevalence of wasting and prevalence ratios for children under and over two years old by country. Notes: 95% confidence intervals are shown. See Table S2 in the Supplement for tabulated estimates and confidence intervals. Prevalence ratios show prevalence of wasting for children two and older divided by prevalence of wasting for children less than two years old.
Focusing on the largest countries in our sample from each region; Viet Nam, in East Asia & Pacific, had a 5% (95% CI: 4, 7) wasting prevalence in children younger than age two and 4% (95% CI: 3, 5) for children aged two and older, leading to a non-statistically significant prevalence ratio of 0·71 (95% CI: 0·49, 1·03). India had a 26% (95% CI: 25, 26) wasting prevalence for children under two and 18% (95% CI: 18, 19) for those older than two, with a statistically significant prevalence ratio of 0·71 (95% CI: 0·69, 0·73). Turkey had a wasting prevalence of 3% (95% CI: 2, 5) before age two and 1% (95% CI: 1, 2) after age two, leading to a statistically significant prevalence ratio of 0·27 (95% CI: 0·13, 0·58). Egypt had a 10% (95% CI: 9, 11) wasting prevalence for children less than two years old and 7% (95% CI: 7, 8) for children two and older, with the ratio of these two estimates being 0·74 (95% CI: 0·65, 0·85) and statistically significant. Ethiopia had a 14% (95% CI: 12, 16) wasting prevalence in children under two and 8% (95% CI: 7, 9) over two, and a statistically significant prevalence ratio of 0·56 (95% CI: 0·46, 0·68). Nigeria had an 11% (95% CI: 10, 12) and 4% (95% CI: 4, 5) wasting prevalence for children under and over two, respectively, and a statistically significant prevalence ratio of 0·38 (95% CI: 0·31, 0·45). The wasting prevalence in Mexico was 1·3% (95% CI: 0·9, 2·0) for children under two and 0·9% (95% CI: 0·4, 1·7) for children over two, leading to a non-statistically significant prevalence ratio of 0·66 (95% CI: 0·30, 1·45).
Results by sex
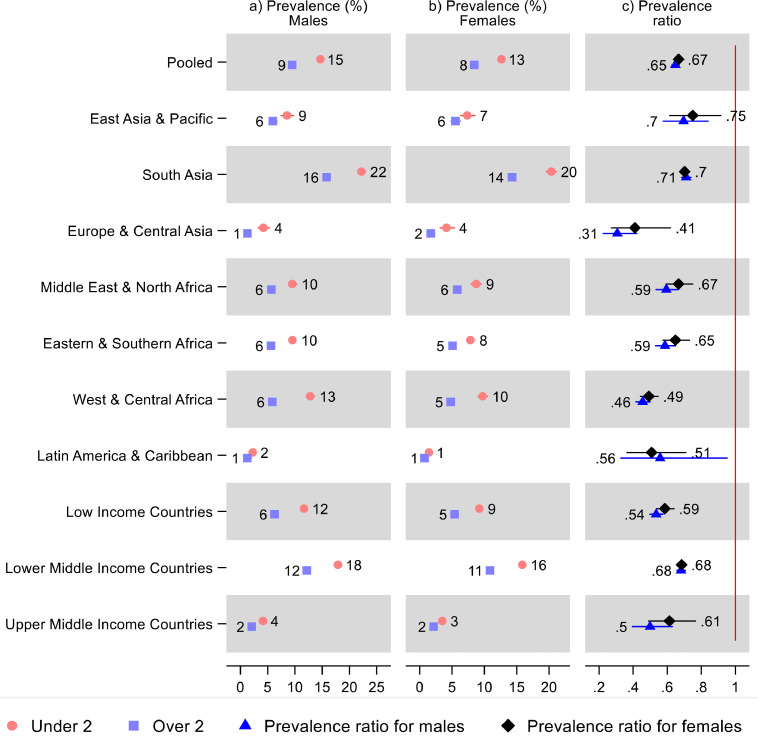
Both males and females in all regions had a statistically significant lower wasting prevalence for children over two years old compared to under two (Figure 3 and Supplementary Table S3). In the pooled sample, males less than two years old had a 15% (95% CI: 14, 15) wasting prevalence and males over two 9% (95% CI: 9, 10): indicating that males over two had 0·65 times (95% CI: 0·63, 0·67) the prevalence of those under two—or 35% lower prevalence. Females under two had 13% (95% CI: 12, 13) prevalence and females over two had 8% (95% CI: 8, 9), leading to a prevalence ratio of 0·67 (95% CI: 0·64, 0·70). There were no statistically significant sex-differences in the prevalence ratios in any region or overall. In most countries, both males and females had a lower wasting prevalence after age two (Figure S1 and Supplementary Table S3): The exceptions were mostly imprecise estimates from small countries or countries with low prevalence.
Figure 3.
Prevalence of wasting and prevalence ratios for children under and over two years old by sex, region, and World Bank income group. Notes: 95% confidence intervals are shown. Upper confidence bounds above 3 for prevalence ratios were cut, indicated by a broken line. See Table S3 in the Supplement for tabulated estimates and confidence intervals, and additional estimates showing relative sex differences in the prevalence ratios.
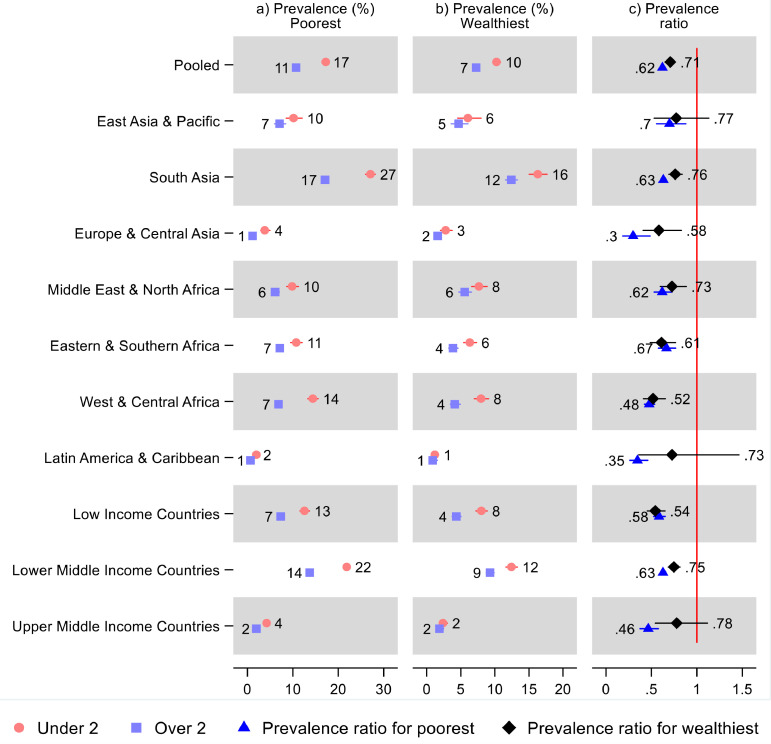
Results by living standards
Both the poorest and the wealthiest had a lower wasting prevalence after age two than before age two in all regions as well as overall (Figure 4 and Supplementary Table S4). However, the prevalence ratios indicating lower prevalence for children over two were not statistically significant for the wealthiest in East Asia & Pacific (0·77; 95% CI: 0·53, 1·13) and Latin America & Caribbean (0·73; 95% CI: 0·36, 1·47). In our pooled sample, children under two years old in the poorest 20% of households had a 17% (95% CI: 17, 18) wasting prevalence, while children over two had 11% (95% CI: 10, 11), leading to a prevalence ratio of 0·62 (95% CI: 0·60, 0·65); while for the wealthiest 20% of households, the wasting prevalence was 10% (95% CI: 10, 11) for children under two and 7% (95% CI: 7, 8) for children over two, leading to a prevalence ratio of 0·71 (95% CI: 0·66, 0·76). The relative difference in wasting prevalence between children under and over two was smaller in the wealthiest households overall and in all regions except Eastern & Southern Africa (0.92; 95% CI: 0·70, 1·20), with a statistically significant difference observed in Europe & Central Asia (95% CI: 1·07, 3·58), South Asia (95% CI: 1·08, 1·35), as well as in the pooled sample (95% CI: 1·05, 1·24). Most countries had a lower wasting prevalence after age two than before age two for both the wealthiest and poorest 20% of households (Figure S2 and Supplementary Table S4): The exceptions were imprecise, and none showed a statistically significant difference.
Figure 4.
Prevalence of wasting and prevalence ratios for children under and over two years old by living standards, region, and World Bank income group. Notes: 95% confidence intervals are shown. Upper confidence bounds above 3 were cut for readability, indicated by a broken line. See Table S4 in the Supplement for tabulated estimates and confidence intervals, and additional estimates showing relative differences in the prevalence ratios across living standards.
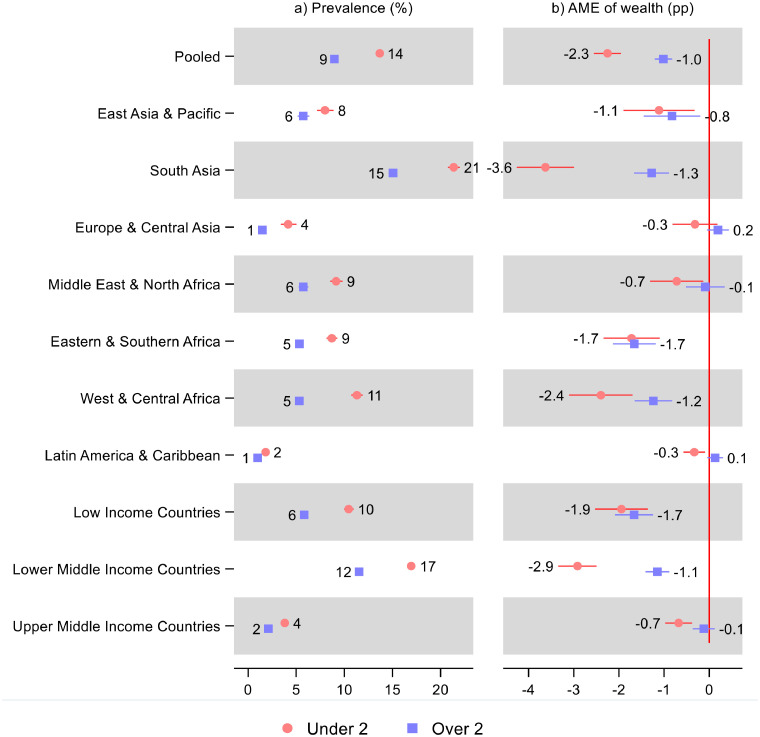
In the pooled sample, a single z-score increase in household wealth was associated with 2·3 (95% CI: –2·5, –2·00) percentage point lower prevalence of wasting for children under two, while the association for children over two showed 1·0 (95% CI: –1·2, –0·8) percentage point lower prevalence of wasting for a single z-score increase in household wealth (Figure 5 and Supplementary Table S5). The association was statistically significant and negative in all regions except for children over two in Europe & Central Asia (0·19; 95% CI: −0·04, 0·4), Middle East & North Africa (−0·09; 95% CI: −0·5, 0.3), and Latin America & Caribbean (0·13; 95% CI: −0·03, 0.3), and children under two in Europe & Central Asia (-0·32; 95% CI: -0·80, 0·17). For children over two, there was an apparent gradient in the AME across country income groups.
Figure 5.
Prevalence of wasting and average marginal effect (AME) of a single z-score increase in the household wealth index for children under and over two. Notes: 95% confidence intervals are shown. Average marginal effects (AME) were estimated from logit models. AME show average percentage point (pp) change in wasting prevalence for a one z-score increase in the household wealth index. The prevalence was predicted from the same model at mean household wealth. See Table S5 in the Supplement for tabulated estimates and confidence intervals and estimated difference in AME across age.
The association between prevalence of wasting and household wealth was stronger for children under two in all regions, although the difference in association across age was only statistically significant in South Asia (95% CI: 1·7, 3·0), Middle East & North Africa (95% CI: 0·02, 1·25), West & Central Africa (95% CI: 0·37, 1·96), and Latin America & Caribbean (95% CI: 0·19, 0·73). When considering the underlying differences in prevalence of wasting by age, it was only in South Asia where the relative difference in association was considerably greater for children under two.
Majority of countries also show greater average marginal effect for wealth for children under two than children over two, although there is large variance between countries (Figure S3 and Supplementary Table S5).
Results from supplementary analyses
Under five wasting prevalence (Pearson's r = 0·24; p = 0·02) and GDP (Pearson's r = 0·15; p = 0·16) had a small correlation with the magnitude of the over vs. under two prevalence ratios for wasting, and not statistically significant for the latter (Supplementary Table S6): That is, countries where the wasting prevalence was concentrated before age two to a less extent, had, on average, slightly higher GDP and wasting prevalence. Under-5 mortality rate had only a small (Pearson's r = −0·17) and non-statistically significant (p = 0·10) negative correlation with the prevalence ratios.
The prevalence differences were the largest in the regions with the greatest prevalence of wasting: South Asia (−6·2 percentage points; 95% CI: −6·8, −5·7) and West & Central Africa (−6 percentage points; 95% CI: −6·6, −5·4; Supplementary Figures S4, 5 and Table S2).
Prevalence of wasting appears to be greater before age 6 months than at age 6–23 months in most cases (Figures S6, 7 and Tables S7 in the Supplement). West & Central Africa was a notable exception where wasting prevalence was 32% (95% CI: 1·18, 1·48) greater for children 6–23 months than children under six months, as well as Eastern and Southern Africa where there was no difference (1; 95% CI: 0·89, 1·15).
Results from sensitivity analyses
We found clear age patterning in the percentage of children excluded due to having implausible weight-for-height values, where children under two were considerably more likely to be excluded (Supplementary Figures S8, 9 and Table S8). The relationship largely remained strong—although not observed in all regions—when excluding children under 6 months old (Supplementary Figures S10, 11 and Table S9). However, when comparing children 12–23 months old and children 24–35 months old, most regions had no clear difference in the percentage with implausible values between age groups (Supplementary Figures S12, 13 and Table S10). The observed age patterning in the prevalence of wasting between children under and over two holds when excluding children under 6 months (Supplementary Figures S14, 15 and Table S11) and restricting the comparison to children 12–23 months and 24–35 months (Supplementary Figures S16, 17 and Table S12), although the prevalence ratios were somewhat lower overall for the latter. (Note that there was high variability for countries when comparing children 12–23 months and 24–35 months since sample sizes were small in many cases.) Finally, when comparing children under and over two on weight-for-height z-scores, we found that children over two generally have considerably greater weight-for-height, indicating less deficit compared to the growth standard, although this was primarily observed in West & Central Africa (0·22 z-score difference across age; 95% CI: 0·19, 0·25), South Asia (0·05; 95% CI: 0·03, 0·07), and Europe & South Asia (0·08; 95% CI: 0·01, 0·14; Supplementary Figures S18, 19 and Table S13).
Predicting the prevalence of wasting in summer shows greater prevalence but adjusting the prevalence ratios for season did not change the prevalence ratios substantially (Supplementary Figure S20 and Table S14). Predicting the prevalence of wasting in 2019 shows lower prevalence but adjusting the prevalence ratios for year of interview did not change the prevalence ratios substantially (Figure S21 and Table S15 in the Supplement).
Discussion
In our sample of 94 countries, 14% of children under two years old suffered from wasting, while the wasting prevalence was 9% for children 2–4 years—or 34% lower. At the time of survey, most regions were close to or had already achieved the World Health Assembly global nutrition targets of less than 5% prevalence of child wasting, but only for children 2–4 years old20 : although South Asia was an important exception, with 15% and 21% prevalence of wasting for children under and over two, respectively.
Out of 94 countries, 87 had a lower wasting prevalence in children over two, ranging from 5% lower to 81% lower, statistically significant at the 5% level (not adjusted for multiple comparisons) in 68 of these countries. As in other studies, we find greater wasting prevalence in males28 and poorer households.29 However, with some exceptions (mostly imprecise estimates for small countries or countries with low prevalence), the higher prevalence for children under two was observed for both males and females and in the wealthiest and poorest 20% of households. Wasting in children under two appears to be more sensitive to variation in household living standards than for children 2–4 years, particularly in South Asia.
Insufficient nutritional intake and infections contribute to wasting, separately and in combination: infections reduce appetite, restrict nutrient absorption, and increase energy requirements, while undernourished children have depressed immune function, which makes them more susceptible, sick, and likely to die from infections.30 Preventative solutions, such as better water and sanitation and food and health service access during pregnancy and childhood, are needed to address the underlying causes of malnutrition. Dietary diversification, supplementation, and food fortification interventions are used to prevent undernutrition.32 Children small for gestational age have been found to have over two-fold risk of wasting compared to children adequate for gestational age, pointing toward pregnancy related exposures.31
The immune system matures as children age.33,34 Diarrheal episodes—a major cause of morbidity and mortality—are more common in younger children: for example, in studies of several developing countries, children had on average 2·7 diarrheal episodes before age six months, 4·8 at age 6–11 months, and 3.9 at age 12–23, which was reduced to 2.6, 1.5 and 1.4 episodes at age two, three, and four years, respectively.35 Effectiveness of nutrition interventions and complementary feeding to improve child health have been found to be particularly successful at younger ages.36,37 Many nutrition services support only a proportion of children in need, regardless of their relative risk of mortality and morbidity: For example, vitamin A supplementation is targeted at children 6–59 months old and coverage is 62%.1 However, the immediate and long-term risks associated with undernutrition during the first 1000 days, and our results that wasting is more prevalent among children under two, suggest that interventions should ensure coverage of pregnant women and children under two. Further, there is compelling evidence to suggest that the effectiveness of nutrition interventions is more pronounced when delivered in the first 1000 days.9 However, feasibility of prioritizing interventions to younger children also depends factors such marginal costs of delivery and the benefit of specific interventions for older children, as well as context specific factors.38
This study had limitations. We used data from cross-sectional household surveys, which may include inaccuracies. First, since our data were cross-sectional, seasonality may influence the measure for the wasting prevalence.26 Our interest was, however, primarily to estimate age patterning in the wasting prevalence. We further conducted a sensitivity check where we adjusted the prevalence measure and ratios for season of interview, which did not change the age patterning much, although the overall prevalence changed in some regions.
Another disadvantage of using cross-sectional data was not knowing at what age wasting first occurred: wasting observed for children older than two may have first occurred before age two and persisted over time, since the duration of untreated wasting has been suggested to last several months.39 Further, children that first suffered wasting before age two may be more vulnerable to repeated episodes of wasting. Therefore, our estimates may underestimate the extent to which wasting occurrence is concentrated in children under two.
Second, mothers reported the child's age or date of birth from memory which may therefore include recall bias. Further, incomplete dates of birth were imputed. The measure for wasting does not require information on age (since it is based on weight-for-height): However, children may be misclassified when comparing wasting between age groups. This is unlikely to bias our results to a great extent, except if age misclassification around age two happens to a very different extent for children suffering from wasting and those not suffering from wasting. Displacement of births by interviewers in the DHS birth histories have been primarily observed at ages 12 as well as 59 months—the latter importantly is the age limit of anthropometric measurement and other more detailed health measures. However, displacement has been found to be less of a problem in more recent surveys—e.g., 1·2% in phase 6 (2008–2013) of the DHS—and mainly observed for children that died.40
Third, weight-for-height, the measure which prevalence of wasting is based on, has been reported to be prone to measurement errors, particularly the measure of height for younger children.10,25,41 These measurement errors can result in wider tails in the weight-for-height distribution and hence higher wasting prevalence. Canalization of growth at older ages and larger shifts in relative growth percentiles before age two may also lead to greater variance before age two.10 Indeed, we do find greater likelihood of having implausible weight-for-height measure for children under two compared to children 2–4 years, indicating that measurement error may be a greater problem for the younger age group. However, we generally do not find major difference in likelihood of having implausible weight-for-height between children 12–23 months and children 24–35 months: while the age patterning in prevalence of wasting for this age group corroborates the paper's main results, although relative differences across age were somewhat lower overall. Measurement errors before age two should, however, be kept in mind when interpreting the results from this study.
Finally, this study does not cover other measures and forms of acute malnutrition, such as mid-upper arm circumference42 and edema,43 which were not recorded in our data. Studies have suggested that mid-upper arm circumference is a more sensitive measure of acute malnutrition for younger children.44
To conclude, since the wasting prevalence was observed to be greater among children 0–2 years old than 2–4 years, and since adverse exposure to undernutrition and infections are particularly harmful and interventions are particularly effective during the 1000 days from conception until age two, nutrition interventions should ensure full coverage among children under two and pregnant women. Prioritization through programmatic measures to increase detection and enrollment of younger children in wasting programs could be considered.
Funding
This paper was funded by UNICEF, Nutrition Section, Programme Division in New York.
Contributions
Karlsson did data management, analysis, and reporting and led the writing of the manuscript.
Kim, Guerrero, Hasman, and Subramanian contributed to the manuscript's writing and provided a critical review of the data analysis and reporting of results. Kim and Subramanian provided overall supervision.
Data availability
DHS data are available at https://dhsprogram.com and MICS at https://mics.unicef.org/ (requiring a simple application).
Declaration of interests
None.
Acknowledgements
None.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101353.
Contributor Information
Rockli Kim, Email: rocklikim@korea.ac.kr.
S.V. Subramanian, Email: svsubram@hsph.harvard.edu.
Supplementary materials
References
- 1.UNICEF . UNICEF; New York: 2019. The State of the World's Children 2019. Children, Food and Nutrition: Growing well in a Changing World.https://www.unicef.org/media/63016/file/SOWC-2019.pdf [Google Scholar]
- 2.Black R.E., Victora C.G., Walker S.P., et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382:427–451. doi: 10.1016/S0140-6736(13)60937-X. [DOI] [PubMed] [Google Scholar]
- 3.Panjwani A., Heidkamp R. Complementary feeding interventions have a small but significant impact on linear and ponderal growth of children in low- and middle-income countries: a systematic review and meta-analysis. J Nutr. 2017;147:2169S–2178S. doi: 10.3945/jn.116.243857. [DOI] [PubMed] [Google Scholar]
- 4.UNICEF . UNICEF; 2021. 10 Proven Nutrition Interventions: Providing Children the Best Chance to Grow and Develop to Their Full Potential.https://www.unicef.org/rosa/stories/10-proven-nutrition-interventions South Asia. Accessed 15 Feb. [Google Scholar]
- 5.Kristjansson E., Francis D.K., Liberato S., et al. Food supplementation for improving the physical and psychosocial health of socio-economically disadvantaged children aged three months to five years: a systematic review. Campbell Syst Rev. 2015;11:1–226. doi: 10.1002/14651858.CD009924.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Victora C.G., Bahl R., Barros A.J.D., et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387:475–490. doi: 10.1016/S0140-6736(15)01024-7. [DOI] [PubMed] [Google Scholar]
- 7.McGovern M.E., Krishna A., Aguayo V.M., Subramanian S. A review of the evidence linking child stunting to economic outcomes. Int J Epidemiol. 2017;46:1171–1191. doi: 10.1093/ije/dyx017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perkins J.M., Subramanian S.V., Smith G.D., Özaltin E. Adult height, nutrition, and population health. Nutr Rev. 2016;74:149–165. doi: 10.1093/nutrit/nuv105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martorell R. Improved nutrition in the first 1000 days and adult human capital and health. Am J Hum Biol. 2017;29:e22952. doi: 10.1002/ajhb.22952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ricardo L.I.C., Gatica-Domínguez G., Crochemore-Silva I., et al. Age patterns in overweight and wasting prevalence of under 5-year-old children from low- and middle-income countries. Int J Obes. 2021;45:2419–2424. doi: 10.1038/s41366-021-00911-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martorell R., Young M.F. Patterns of stunting and wasting: potential explanatory factors. Adv Nutr. 2012;3:227–233. doi: 10.3945/an.111.001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corsi D.J., Neuman M., Finlay J.E., Subramanian S.V. Demographic and health surveys: a profile. Int J Epidemiol. 2012;41:1602–1613. doi: 10.1093/ije/dys184. [DOI] [PubMed] [Google Scholar]
- 13.DHS . DHS; 2020. The Demographic and Health Surveys. DHS Program. https://dhsprogram.com/(accessed Jan 9, 2020) [Google Scholar]
- 14.Khan S., Hancioglu A. Multiple indicator cluster surveys: delivering robust data on children and women across the globe. Stud Fam Plan. 2019;50:279–286. doi: 10.1111/sifp.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.UNICEF . UNICEF; 2021. Multiple Indicator Cluster Surveys (MICS) https://mics.unicef.org/(accessed Feb 16, 2021) [Google Scholar]
- 16.The DHS Program. Sample Size. DHS Survey Design: Frequently Asked Questions. https://dhsprogram.com/pubs/pdf/DHSM16/DHSM16.pdf. Accessed 12 March 2022.
- 17.Croft T.N., Marshall A.M., Allen C.K. ICF; Rockv, MD USA: 2018. Guide to DHS statistics. others. [Google Scholar]
- 18.UNDP . UNDP; 2018. Human Development Index (HDI) | Human Development Reports. http://hdr.undp.org/en/content/human-development-index-hdi (accessed July 26, 2018) [Google Scholar]
- 19.WHO . World Health Organization; 2006. WHO Child Growth Standards: Length/Height for Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age, Methods and Development. https://www-cabdirect-org.ludwig.lub.lu.se/cabdirect/abstract/20063123347 (accessed June 20, 2017) [Google Scholar]
- 20.WHO/UNICEF/WFP . World Health Organization; Geneva: 2014. Global Nutrition Targets 2025: Wasting Policy Brief (WHO/NMH/NHD/14.8) https://apps.who.int/iris/rest/bitstreams/665593/retrieve (accessed April 4, 2021) [Google Scholar]
- 21.DHS . DHS; 2021. Wealth Index Construction. DHS Program. https://dhsprogram.com/topics/wealth-index/Wealth-Index-Construction.cfm (accessed Jan 10, 2022) [Google Scholar]
- 22.Sahn D.E., Stifel D. Exploring alternative measures of welfare in the absence of expenditure data. Rev Income Wealth. 2003;49:463–489. [Google Scholar]
- 23.United Nations, Department of Economic and Social Affairs, Population Division. World population prospects 2019, Online Edition. Rev. 1. 2019. https://esa.un.org/unpd/wpp/Download/Standard/Population/. Accessed 12 March 2022.
- 24.World Bank . World Bank; 2019. World Development Indicators. DataBank. http://databank.worldbank.org/data/reports.aspx?source=world-development-indicators. Accessed 19 February 2019. [Google Scholar]
- 25.Bilukha O., Couture A., McCain K., Leidman E. Comparison of anthropometric data quality in children aged 6-23 and 24-59 months: lessons from population-representative surveys from humanitarian settings. BMC Nutr. 2020;6:60. doi: 10.1186/s40795-020-00385-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baye K., Hirvonen K. Seasonality: a missing link in preventing undernutrition. Lancet Child Adolesc Health. 2020;4:e3. doi: 10.1016/S2352-4642(19)30343-8. [DOI] [PubMed] [Google Scholar]
- 27.Cummings P. Estimating adjusted risk ratios for matched and unmatched data: an update. Stata J. 2011;11:290–298. [Google Scholar]
- 28.Thurstans S., Opondo C., Seal A., et al. Boys are more likely to be undernourished than girls: a systematic review and meta-analysis of sex differences in undernutrition. BMJ Glob Health. 2020;5 doi: 10.1136/bmjgh-2020-004030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karlsson O., Kim R., Sarwal R., James K.S., Subramanian S.V. Trends in underweight, stunting, and wasting prevalence and inequality among children under three in Indian states, 1993–2016. Sci Rep. 2021;11:14137. doi: 10.1038/s41598-021-93493-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katona P., Katona-Apte J. The interaction between nutrition and infection. Clin Infect Dis. 2008;46:1582–1588. doi: 10.1086/587658. [DOI] [PubMed] [Google Scholar]
- 31.Christian P., Lee S.E., Donahue Angel M., et al. Risk of childhood undernutrition related to small-for-gestational age and preterm birth in low-and middle-income countries. Int J Epidemiol. 2013;42:1340–1355. doi: 10.1093/ije/dyt109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.United Nations Children's Fund. (UNICEF) UNICEF; 2020. Nutrition, for Every Child: UNICEF Nutrition Strategy 2020–2030.https://www.unicef.org/media/92031/file/UNICEF%20Nutrition%20Strategy%202020-2030.pdf UNICEF. [Google Scholar]
- 33.Maggini S., Pierre A., Calder P.C. Immune function and micronutrient requirements change over the life course. Nutrients. 2018;10:1531. doi: 10.3390/nu10101531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ygberg S., Nilsson A. The developing immune system-from foetus to toddler. Acta Paediatr. 2012;101:120–127. doi: 10.1111/j.1651-2227.2011.02494.x. [DOI] [PubMed] [Google Scholar]
- 35.Kosek M., Bern C., Guerrant R.L. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull World Health Organ. 2003:8. [PMC free article] [PubMed] [Google Scholar]
- 36.Dewey K.G., Adu-Afarwuah S. Systematic review of the efficacy and effectiveness of complementary feeding interventions in developing countries. Matern Child Nutr. 2008;4(Suppl 1):24–85. doi: 10.1111/j.1740-8709.2007.00124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keats E.C., Das J.K., Salam R.A., et al. Effective interventions to address maternal and child malnutrition: an update of the evidence. Lancet Child Adolesc Health. 2021;5:367–384. doi: 10.1016/S2352-4642(20)30274-1. [DOI] [PubMed] [Google Scholar]
- 38.Loevinsohn B.P., Sutter R.W., Otelia Costales M. Using cost-effectiveness analysis to evaluate targeting strategies: the case of vitamin A supplementation. Health Policy Plan. 1997;12:29–37. doi: 10.1093/heapol/12.1.29. [DOI] [PubMed] [Google Scholar]
- 39.Isanaka S., Grais R.F., Briend A., Checchi F. Estimates of the duration of untreated acute malnutrition in children from Niger. Am J Epidemiol. 2011;173:932–940. doi: 10.1093/aje/kwq436. [DOI] [PubMed] [Google Scholar]
- 40.Pullum T.W., Becker S. ICF International; Rockville, Maryland, USA: 2014. Evidence of Omission and Displacement in DHS Birth Histories.http://dhsprogram.com/pubs/pdf/MR11/MR11.pdf [Google Scholar]
- 41.Assaf S., Kothari M.T., Pullum T.W. ICF International; 2015. An Assessment of the Quality of DHS Anthropometric Data, 2005–2014. [Google Scholar]
- 42.Briend A., Maire B., Fontaine O., Garenne M. Mid-upper arm circumference and weight-for-height to identify high-risk malnourished under-five children. Matern Child Nutr. 2012;8:130–133. doi: 10.1111/j.1740-8709.2011.00340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frison S., Checchi F., Kerac M. Omitting edema measurement: how much acute malnutrition are we missing? Am J Clin Nutr. 2015;102:1176–1181. doi: 10.3945/ajcn.115.108282. [DOI] [PubMed] [Google Scholar]
- 44.Goossens S., Bekele Y., Yun O., Harczi G., Ouannes M., Shepherd S. Mid-upper arm circumference based nutrition programming: evidence for a new approach in regions with high burden of acute malnutrition. PLoS ONE. 2012;7:e49320. doi: 10.1371/journal.pone.0049320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
DHS data are available at https://dhsprogram.com and MICS at https://mics.unicef.org/ (requiring a simple application).