Abstract
Background
Galantamine is a specific, competitive, and reversible acetylcholinesterase inhibitor.
Objectives
To assess the clinical effects of galantamine in patients with mild cognitive impairment (MCI), probable or possible Alzheimer's disease (AD), and potential moderators of effect.
Search methods
The trials were identified from a search of the Specialized Register of the Cochrane Dementia and Cognitive Improvement Group, last updated on 25 April 2005 using the terms galanthamin*, galantamin* and Reminyl. Published reviews were inspected for further sources. Additional information was collected from unpublished clinical research reports for galantamine obtained from Janssen and from http://www.clinicalstudyresults.org/.
Selection criteria
Trials selected were randomised, double‐blind, parallel‐group comparisons of galantamine with placebo for a treatment duration of greater than 4 weeks in subjects with MCI or AD.
Data collection and analysis
Data were extracted independently by the reviewers and pooled where appropriate and possible. Outcomes of interest include the clinical global impression of change (CIBIC‐plus or CGIC), Alzheimer's Disease Assessment Scale‐cognitive sub scale (ADAS‐cog), Alzheimer's Disease Cooperative Study/Activities of Daily Living (ADCS‐ADL), Disability Assessment for Dementia scale (DAD) and Neuropsychiatric Inventory (NPI). Potential moderating variables of treatment effect assessed included trial duration, dose, and diagnosis of possible versus probable Alzheimer's disease.
Main results
Ten trials with a total 6805 subjects were included in the analysis. Treatment with galantamine led to a significantly greater proportion of subjects with improved or unchanged global rating scale rating (k = 8 studies), at all dosing levels except for 8 mg/d . Confidence intervals for the ORs overlapped across the dose range of 16 mg to 36 mg per day, with point estimates of 1.6 ‐ 1.8 when analysed with the intention‐to‐treat sample. Treatment with galantamine also led to significantly greater reduction in ADAS‐cog score at all dosing levels (k = 8), with greater effect over six months compared to three months. Confidence intervals again overlapped. Point estimate of effect was lower for 8 mg/d but similar for 16 mg to 36 mg per day. For example, treatment effect for 24 mg/d over six months was 3.1 point reduction in ADAS‐cog (95%CI 2.6‐3.7, k = 4, ITT). ADCS‐ADL, DAD and NPI were reported only in a small proportion of trials: all showed significant treatment effect in some individual trials at least. Confidence interval of treatment effect for the one trial recruiting patients with possible AD overlapped with the other seven recruiting patients with probable AD. Galantamine's adverse effects appeared similar to those of other cholinesterase inhibitors and to be dose related. Prolong release / once daily formulation of galantamine at 16 ‐ 24mg/d was found to have similar efficacy and side‐effect profile as the equivalent twice‐daily regime. Data from the two MCI trials suggest marginal clinical benefit, but a yet unexplained excess in death rate.
Authors' conclusions
Subjects in these trials were similar to those seen in earlier anti dementia AD trials, consisting primarily of mildly to moderately impaired outpatients. Galantamine's effect on more severely impaired subjects has not yet been assessed. Nevertheless, this review shows consistent positive effects for galantamine for trials of three to six months' duration. Although there was not a statistically significant dose‐response effect, doses above 8 mg/d were, for the most part, consistently statistically significant. Galantamine's safety profile in AD is similar to that of other cholinesterase inhibitors with respect to cholinergically mediated gastrointestinal symptoms. It appears that doses of 16 mg/d were best tolerated in the single trial where medication was titrated over a four week period, and because this dose showed statistically indistinguishable efficacy with higher doses, it is probably most preferable initially. Longer term use of galantamine has not been assessed in a controlled fashion. Galantamine use in MCI is not recommended due to its association with an excess death rate.
Plain language summary
Galantamine improves global and cognitive symptoms at doses of 16 mg/day or greater, in people with mild to moderate Alzheimer's disease, for at least 6 months
Alzheimer's disease is a progressive neurodegenerative illness, affecting thinking and memory. Galantamine is a reversible cholinesterase inhibitor that inhibits the degradation of the neurotransmitter acetylcholine, and may have other actions on nicotinic receptors as well. The review finds that galantamine was more effective than placebo in improving cognitive function. A greater proportion of people taking galantamine than of those taking placebo was rated as improved or not changed after three to six months. There was evidence of improvement on measures of activities of daily living and behavioral symptoms. Longer‐term controlled studies have yet to be performed or published.
Data from the two MCI trials suggest marginal clinical benefit, but a yet unexplained excess in death rate.
Background
Galantamine (also called galanthamine, marketed as Reminyl by Janssen), an alkaloid extracted from Amaryllidaceae (Galanthus woronowi, the Caucasian snowdrop) and daffodil bulbs, but now synthesized, is a reversible, competitive inhibitor of acetylcholinesterase with very little butyrylcholinesterase inhibitory activity (Harvey 1995; Pacheco 1995). It has received regulatory approval in at least 29 counties, including Argentina, Australia, Canada, Czechia, the European Union (except for The Netherlands), Iceland, Korea, Mexico, Norway, Poland, Singapore, Sweden, South Africa, Switzerland, Thailand, and the United States.
Galantamine is 10 to 50‐fold more selective for acetyl compared to butyryl cholinesterase (Thomsen 1990). Competitive inhibitors compete with acetylcholine at the acetylcholinesterase binding sites, while non‐competitive inhibitors bind to the sites independent of acetylcholine concentration. Because competitive acetylcholinesterase inhibitors are dependent on acetylcholine concentration, they may be less likely to bind to the enzymatic site in areas that have high acetylcholine levels. While in brain areas where acetylcholine is low, there may be a greater amount of binding to acetylcholinesterase. Theoretically, competitive inhibitors will have more effect in areas with low levels of acetylcholine and less effect in areas with higher acetylcholine. This may provide a selective effect in the brain areas affected in AD that have lower acetylcholine levels. In addition, galantamine is an allosteric modulator at nicotinic cholinergic receptor sites, enhancing the effect of acetylcholine at these receptors, and thus may enhance cholinergic transmission (Sweeney 1988; Maelicke 1997).
Early open labeled uncontrolled studies have shown mild benefit for patients with Alzheimer's disease, but for the most part were not statistically significant (Kewitz 1994; Rainer 1993; Thomsen 1990a; Thomsen 1990b; Wilcock 1993). Since then, a number of large scale double‐blind randomised controlled trials have been carried out, providing data needed for this review.
Objectives
The aim of this review is to assess the clinical effect of galantamine in patients with mild cognitive impairment (MCI), probable or possible Alzheimer's disease (AD) and to investigate potential moderators of effect. Additional reasons to undertake this review are to test for significant consensus among trials that may have contradictory findings; to gather potential information about efficacy which can be revealed only by assessing systematic variations in study design, data characteristics, and methodology; and to assess the development of a particular research domain.
Methods
Criteria for considering studies for this review
Types of studies
Studies were selected for this review if they fulfilled the following criteria: (1) it comprised of a clinical trial in MCI or AD (2) the trial was double‐blind, parallel‐group, placebo‐controlled, with randomised and unconfounded treatment assignment to placebo or galantamine (3) sample selection criteria were specified (4) outcome instruments were specified (5) duration was specified
Types of participants
Patients who met criteria for clinical criteria for MCI, NINCDS‐ADRDA 'probable AD' or 'possible AD' (McKhann 1984), for DSM‐III‐R criteria for primary degenerative dementia of the Alzheimer's type (APA 1987), or for DSM ‐IV, 'dementia of the Alzheimer's type'.
Types of interventions
1) Any oral dose of galantamine 2) Placebo
Types of outcome measures
1. Alzheimer's Disease Assessment Scale‐Cognitive Subscale (Rosen 1984). This 70 point scale encompasses a broad range of cognitive function typically affected by AD. Higher scores indicate worsening. Positive change scores indicate worsening. The European ADAS and French ADAS (EURO‐ADAS and Greco‐ADAS, respectively) were also included. 2. Global Rating/ Clinician's Interview‐Based Impression of Change plus Caregiver Input (CIBIC‐plus). These were typically assessed using the process of the Alzheimer's Disease Cooperative Study‐Clinical Global Impression of Change (ADCS‐CGIC; Schneider 1997). Scores range from 1 to 7, with 4 indicating no change, scores below 4 indicating improvement, and scores above 4 indicating worsening. 3. Alzheimer's Disease Cooperative Study‐Activities of Daily Living (ADCS‐ADL; Galasko 1997). This ADL scale was specifically designed for Alzheimer's disease and the version used here was scored from 0 to 78; negative change scores normally indicate worsening, but were re coded to be consistent with MetaView. 4. Disability Assessment for Dementia scale (DAD; Gelinas 1999). This 46‐item scale assesses both basic and instrumental ADLs, leisure activities, initiation, planning and organization, and effective performance. It is administered to an informant and has a total score ranging from 0 to 100. 5. Neuropsychiatric Inventory (NPI; Cummings 1994). This scale assesses the following ten items: delusions, hallucinations, agitation/aggression, depression, anxiety, elation/euphoria, apathy/indifference, disinhibition, irritability/liability, aberrant motor behavior. The total score ranges from 0 to 120; positive change scores indicate worsening.
Search methods for identification of studies
The trials were identified from a search of the Specialized Register of the Cochrane Dementia and Cognitive Improvement Group on 25 April 2005 using the terms galanthamin* galantamin* and reminyl.
The Specialized Register at that time contained records from the following databases: CENTRAL: January 2005 (issue 1); MEDLINE: 1966 to 2005/02; EMBASE: 1980 to 2005/01; PsycINFO: 1887 to 2005/01; CINAHL: 1982 to 2004/12; SIGLE (Grey Literature in Europe): 1980 to 2004/06; ISTP (Index to Scientific and Technical Proceedings): to May 2000; INSIDE (BL database of Conference Proceedings and Journals): to June 2000; Aslib Index to Theses (UK and Ireland theses): 1970 to March 2003; Dissertation Abstract (USA): 1861 to March 2003; ADEAR (Alzheimer's Disease Clinical Trials Database): to 25 March 2005; National Research Register: issue 1/2005; Current Controlled trials (last searched April 2005) which includes: Alzheimer Society GlaxoSmithKline HongKong Health Services Research Fund Medical Research Council (MRC) NHS R&D Health Technology Assessment Programme Schering Health Care Ltd South Australian Network for Research on Ageing US Dept of Veterans Affairs Cooperative Studies National Institutes of Health (NIH) ClinicalTrials.gov: last searched March 2005; LILACS:Latin American and Caribbean Health Science Literature: last searched April 2003
The search strategies used to identify relevant records in MEDLINE, EMBASE, PsycINFO, CINAHL and LILACS can be found in the Group's module.
Published reports were inspected for additional sources. Additional information was collected from unpublished "clinical research reports" obtained from Janssen and the website www.clinicalstudyresults.org.
Data collection and analysis
Selection of studies A single reviewer (JO) discarded irrelevant citations, based on the title of the publication and its abstract. Any suggestion that an article could possibly be relevant, caused it to be retrieved for further assessment. This was repeated by another reviewer (CL) for the 2004 and 2005 updates A single reviewer independently selected the trials for initial inclusion in the review from the culled citation list. These trials were reviewed by a second reviewer (LS).
Quality Assessment The reviewers assessed the methodological quality of each trial using the Cochrane Collaboration guidelines (Mulrow 1997).
Category A (adequate) is where the report describes allocation of treatment by: (i) some form of centralized randomised scheme, such as having to provide details of an enrolled participant to an office by phone to receive the treatment group allocation; (ii) some form of randomizations scheme controlled by a pharmacy; (iii) numbered or coded containers, such as in a pharmaceutical trial in which capsules from identical‐looking numbered bottles are administrated sequentially to enrolled participants; (iv) an on‐site or coded computer system, given that the allocations were in a locked, unreadable file that could be accessed only after inputting the characteristics of an enrolled participant; or (v) if assignment envelopes were used, the report should at least specify that they were sequentially numbered, sealed, opaque envelopes; (vi) other combinations of described elements of the process that provides assurance of adequate concealment.
Category B (unclear) is where the report describes the study as 'randomised' but no further detail is available.
Category C (inadequate) is where the report describes allocation of treatment by: (i) alternation; (ii) reference to case record numbers, dates of birth, day of week, or any other such approach; (iii) any allocation procedure that is entirely transparent before assignment, such as an open list of random numbers or assignments.
Category D (not used) is where randomisation is not used.
Empirical research has shown that lack of adequate allocation concealment is associated with bias. Trials with unclear concealment measures have been shown to yield more pronounced estimates of treatment effects than trials that have taken adequate measures to conceal allocation schedules, but less pronounced than inadequately concealed trials (Chalmers 1983, Schulz 1995). Thus trials will be included if they conform to categories A or B, and those falling into category C were excluded.
Other aspects of trial quality were not assessed by a scoring system although details were noted of blinding, whether intention‐to‐treat analyses were extractable from the published data, and the number of patients lost to follow‐up. Data extraction Data were independently extracted by JO, subsequently by the update reviewer (CL), and cross‐checked by a second reviewer (LS). Any discrepancies were discussed.
Data were required to provide either: (a) means and standard deviations (or standard error) for pre‐ and post‐tests, (b) means and standard deviations (or standard error) for change scores, (c) individual data for each patient in the trial. For categorical ratings, data were required to provide either: (a) the percentage of improvers for both drug and placebo groups, (b) frequencies of improvers for both drug and placebo groups, or (c) individual data for each patient in the trial. Data analysis For continuous or ordinal variables, the main outcome of interest is change in score from baseline (i.e. pre‐randomisation or at randomisation) to the final assessment. If ordinal scale data appear to be approximately normally distributed or if the analysis that the investigators performed suggested parametric tests were appropriate, then the outcome measures were treated as continuous data.
For binary outcomes or ordinal data that could be treated dichotomously, such as global ratings under some instances, the endpoint itself is of interest and the Peto method of the 'typical odds ratio' was used.
A test for heterogeneity of treatment effect among trials was made using a standard chi‐squared statistic or the I‐squared statistic. If a test of heterogeneity is negative then a weighted estimate of the typical treatment effect across trials, the 'typical odds ratio' (e.g., the odds of an unfavourable outcome among treatment‐allocated patients to the corresponding odds among controls) is calculated using Peto's log‐rank test adapted for ordinal data (EBCTCG 1990). If, however, there was evidence of heterogeneity of the treatment effect between trials then either only homogeneous results are pooled, or a random effects model used (in which case the confidence intervals would be broader than a fixed effects model).
Additional hypotheses tested were that galantamine has no differential effect when compared with placebo, based upon daily dose.
Results
Description of studies
Eleven trials were identified that met inclusion criteria for the review (GAL‐95‐05; GAL‐INT‐10 Brodaty; GAL‐INT‐11 DeKosky; GAL‐INT‐6Erkinjuntti; Kewitz 1994b; GAL‐USA‐1 Raskind; GAL‐INT‐2 Rockwood; GAL‐USA‐10 Tariot; GAL‐INT‐1 Wilcock; GAL‐93‐01 Wilkinson; GAL‐INT‐18 Winblad). One of these did not provide sufficient outcome data for analysis (Kewitz 1994b). Of the remaining ten, seven were published in peer‐reviewed journals (GAL‐INT‐10 Brodaty; GAL‐INT‐6Erkinjuntti; GAL‐USA‐1 Raskind; GAL‐INT‐2 Rockwood; GAL‐USA‐10 Tariot; GAL‐INT‐1 Wilcock; GAL‐93‐01 Wilkinson). Unpublished clinical research reports were obtained from Janssen and the website www.clinicalstudyresults.org for the remainder (GAL‐95‐05; GAL‐INT‐11 DeKosky; GAL‐INT‐18 Winblad), and for the breakdown of subgroup data in GAL‐INT‐6Erkinjuntti. Methods All eleven trials were parallel group designs. Treatment lasted from 12 weeks to 2 years with 26 weeks being most common. Trials of 5 months to 29 weeks were aggregated as '6 month' trials for the purposes of analyses.
Participants The number of participants randomised in the trials ranged from 95 to 1062 (k=11). Those trials reporting the number excluded after randomizations (k=10) reported 204 to 1019 completers, with a completion rate ranging from 68.9‐80.4%. Two trials enrolled subjects with MCI and mild impairment / Clinical Dementia Rating= 0.5 (GAL‐INT‐11 DeKosky; GAL‐INT‐18 Winblad). Most trials enrolled mildly to moderately impaired subjects with AD, using the MMSE to set high and low cutoff scores (GAL‐95‐05:12‐24; GAL‐INT‐10 Brodaty: 10‐24; GAL‐INT‐6Erkinjuntti: 10‐25; GAL‐USA‐1 Raskind: 11‐24; GAL‐INT‐2 Rockwood: 11‐24; GAL‐USA‐10 Tariot: 10‐22; GAL‐INT‐1 Wilcock: 11‐24; GAL‐93‐01 Wilkinson: 13‐24), so that the highest MMSE score allowed ranged from 22 to 25, and the lowest from 10 to 12. Interventions All eleven trials compared galantamine against placebo.
The exact dosing regime in Kewitz 1994b was unclear. Four trials tested a single dosing regime against placebo. Two studied subjects with AD (GAL‐95‐05: 32mg/d; GAL‐INT‐6Erkinjuntti: 24mg/d), and two subjects with MCI (GAL‐INT‐11 DeKosky & GAL‐INT‐18 Winblad, both with flexible regimes of 16‐24mg/d). Three trials tested two different dosing regimes against placebo: 24mg/d or 32mg/d (GAL‐USA‐1 Raskind; GAL‐INT‐2 Rockwood; GAL‐INT‐1 Wilcock). GAL‐INT‐2 Rockwood permitted subjects to remain on either 24 or 32mg/d.
Two trials tested three different dosing regimes against placebo. GAL‐USA‐10 Tariot tested doses of 8mg/d, 16mg/d and 24mg/d, and GAL‐93‐01 Wilkinson tested doses of 18mg/d, 24mg/d and 36mg/d. It should be noted that the Wilkinson trial included an interim analysis that resulted in the remaining subjects being allocated solely to the placebo or 18mg/d. Although this was done in a blinded fashion, the integrity of the data may have been compromised.
One trial tested the prolonged release / once daily formulation and twice daily dosing (both with flexible regime of 16‐24mg/d) against placebo (GAL‐INT‐10 Brodaty).
The most frequent doses tested were 24 mg/d (in nine trials) and 32 mg/d (in five trials). Six trials reported 4‐week placebo run‐ins prior to treatment randomisation.
All trials had patients begin at a lower dose and increase over time to the daily maximum. Exact dose escalation regime for Kewitz 1994b was unclear. One trial escalated daily dose by 4mg each week until maximum assigned dose (GAL‐INT‐6Erkinjuntti). Four trials escalated daily dose by 8mg each week until maximum assigned dose (GAL‐95‐05: GAL‐USA‐1 Raskind; GAL‐INT‐2 Rockwood; GAL‐INT‐1 Wilcock). One trial escalated daily dose by 8mg every 4 weeks (GAL‐USA‐10 Tariot). One trial used a variable escalation regime bringing the dose from 8mg/d to 18‐36mg/d within 5‐14 days(GAL‐93‐01 Wilkinson). Three trials escalated daily dose by 8mg every 4 weeks, with an option to reduce dosage from 24 mg/d back to 16 mg/d (GAL‐INT‐10 Brodaty; GAL‐INT‐11 DeKosky; GAL‐INT‐18 Winblad).
Outcome measures The following range of outcome measures were used among trials:
1. Cognitive Tests ADAS‐cog or Euro‐ADAS‐cog (k=11, 1 of which did not provide sufficient data for analysis) Expanded ADAS‐cog (k=3)
2. Global rating scales CIBIC‐plus (k=4) ADCS‐CGIC (k=4) Unspecified physician's global rating (k=1, which did not provide sufficient data for analysis)
3. Activities of Daily Living ADCS‐ADL (Galasko 1997; k=2)
4.Disability Assessment Disability Assessment for Dementia (Gelinas 1999; k=4)
5. Behavioral measures NPI (Cummings 1994; k=4)
Risk of bias in included studies
Most of the trials had sufficient methodological quality, having been designed as Phase II and III clinical trials. All trials had quality ratings of A except for three trials where the randomizations scheme was not reported. These were all given quality ratings of B, and include: Kewitz 1994b (abstract only); GAL‐INT‐11 DeKosky & GAL‐INT‐18 Winblad (both unpublished clinical research reports).
Effects of interventions
Global Rating scales Out of the eight AD trials that provided sufficient outcome data for analysis, all provided global rating data using observed cases (OC) analyses and ITT. Rating scale data were dichotomized into those who had no change or improvement versus those who worsened. Analyses revealed statistically significant results in favour of treatment in OC and ITT analyses at both 3 months and 6 months.
For trials of 3 months' duration, by OC analysis, dosing at 18 mg/d and 24 mg/d failed to show a statistically significant effect. Dosing at 24‐32 mg/d (Odds Ratio (OR) 2.3; 95%CI 1.3 ‐ 3.9) and 36 mg/d (OR 3.4; 95%CI 1.2 ‐ 9.5) were both statistically significant in favour of treatment.
For trials of 3 months' duration, by ITT analysis, dosing at 24 mg/d and 24‐32 mg/d failed to show a statistically significant effect. Dosing at 18 mg/d (OR 2.4, 95%CI 1.2 ‐5.0) and 36 mg/d (OR 2.7; 95%CI 1.2 ‐6.2) were all statistically significant in favour of treatment.
For trials of 6 months duration (5‐months to 29 weeks), by OC analysis, dosing at 8 mg/d, 16‐24 mg (bid or prolonged release) and 32mg/d (tds dosing) failed to show a statistically significant effect. Dosing at 16 mg (OR 2.3; 95% CI 1.6 ‐ 3.3), 24 mg (OR 2.1; 95%CI 1.6 ‐2.6), 32 mg (bd dosing) (OR 1.9; 95%CI 1.4 ‐ 2.7) were all statistically significant in favour of treatment.
For trials of 6 months' duration, by ITT analyses, doses of 8 mg/d, 16‐24 mg (bid or prolonged release) and 32 mg/d (tds dosing) failed to show a statistically significant effect. Dosing at 16 mg (OR 2.0; 95% CI 1.4 ‐ 2.9), 24 mg (OR 1.9; 95%CI 1.6 ‐2.3), 32 mg (OR 1.8; 95%CI 1.3 ‐ 2.4) were all statistically significant in favour of treatment.
When the global rating results (OC) were aggregated by dose, irrespective of duration, all dosages showed statistically significant effects with overlapping confidence intervals, except for 8mg/d.
OR for 16‐24 mg/d was 1.63 (95%CI 1.3‐2.1, k=3)
OR for 24 mg/d to 24‐32 mg/d was 2.1 (95%CI 1.7‐2.5, k=6)
OR for 32‐36 mg/d was 1.7 (95%CI 1.4‐2.2, k=4)
For ITT analyses, the results were similar with significant and overlapping confidence intervals:
OR for 16‐24 mg/d was 1.7 (95%CI 1.3‐2.1 k=3)
OR for 24 mg/d to 24‐32 mg/d was 1.8 (95%CI 1.5‐2.2, k=6)
OR for 32‐36 mg/d was 1.6 (95%CI 1.3‐2.0)
ADAS‐cog Scores Out of the eight AD trials that provided sufficient outcome data for analysis, all provided ADAS‐cog change from baseline data using observed cases (OC) analyses and ITT. The analyses revealed statistically significant results in favour of treatment in OC and ITT analyses at both 3 months and 6 months.
For trials of 3 months' duration, statistically significant effects were found at all dosing levels by both OC and ITT analysis. As expected, OC analysis yielded slightly more favourable estimates. There was only one trial at each dosing level. Weighted mean difference in ADAS‐cog score by ITT for 18mg/d was 1.7 (95%CI 0.2‐3.6), 24mg 3.0 (95%CI 0.8‐5.2), 24‐32mg 1.7 (95%CI 0.6‐2.8), and 36mg 2.3 (95%CI 0.4‐4.2).
For trials of 6 months' duration, statistically significant effects were found at all dosing levels by both OC and ITT analysis. As expected, OC analysis yielded slightly more favourable estimates. Weighted mean difference in ADAS‐cog score by ITT for 8 mg/d was 1.3 (95%CI 0.03‐2.6), 16 mg 3.1 (95%CI 2.1‐4.1), 16‐24 mg bid 2.8 (95%CI 1.8‐3.8), 16‐24 mg prolonged release 2.5 (95%CI 1.6‐3.4), 24 mg 3.1 (95%CI 2.6‐3.7), 32 mg bd dosing 3.3 (95%CI 2.4‐4.1) and 32 mg tds dosing 2.9 (95%CI 1.8‐4.0).
Proportion of patients with 4 or more point improvement in ADAS‐cog was reported at 3 months in 1 trial and 6 months in 3. The single trial reporting at 3 months showed a statistically significant result by OC but not ITT. Trials reporting at 6 months (all with OC data only) did not find a statistically significant effect for 8 mg/d. Statistically significant effect was found for 16 mg/d (OR 2.2, 95%CI 1.5‐3.4), 24 mg (OR 2.4, 95%CI 1.8‐3.2), and 32 mg (OR 2.7, 95%CI 1.9‐4.0). ADCS‐ADL Scores Treatment effect on ADCS‐ADL scale was reported in one trial only for the dosing levels of 8 mg/d, 16 mg/d, 16‐24 mg/d and 24mg/d. As expected, OC analysis yielded slightly more favourable estimates. Significantly smaller decrease in the ADCS‐ADL score by ITT was reported for 16mg/d (3.1points, 95%CI 1.6‐4.6) and 24mg/d (2.3points, 95%CI 0.6‐4.0). Data was only available in OC form for 16‐24 mg/d, finding a significantly smaller decrease in ADCS‐ADL in the prolonged release group (2.4 points, 95%CI 0.8‐4.0) but not the bid group. DAD Scores Treatment effect on DAD was reported at 3 months in 1 trial and at 6 months in 2. Statistically significant treatment effects were found at 3 months for 24‐32 mg/d by OC and ITT. Change from baseline by ITT was 4.8 points (95%CI 2.1‐7.6). Statistically significant treatment effects were also found at 6 months by OC and ITT. Change from baseline by ITT for 24 mg/d was 3.7 (95%CI 1.4‐6.9), 32 mg/d 3.5 (95%CI 0.5‐6.5). NPI Scores Treatment effect on NPI was reported at 3 months in 1 trial and at 6 months in 3. No statistically significant treatment effect was found at 3 months for 24‐32 mg/d by OC and ITT. At six months, for 16 mg/d, treatment effect was statistically significant by OC and ITT, with a 2.1 point reduction by ITT (95%CI 0.2‐4.0). For 16‐24mg/d, no statistically significant treatment effect was found for the prolong release or bid group. For 24 mg/d, treatment effect was statistically significant by OC but not ITT.
Probable versus possible AD One of the eight AD trials recruited patients with possible AD while the other seven recruited patients with probable AD. Treatment effects found in the former had overlapping confidence intervals with the rest of the trials. Prolonged release/ once daily formulation vs. twice daily dosing The 16‐24mg/d prolonged release formation was found to have overlapping confidence intervals with the 16‐24mg/d twice daily dosing (GAL‐INT‐10 Brodaty)‐ in terms of treatment effects, adverse effects and proportion of treatment withdrawal. MCI trials Neither of the MCI trials (GAL‐INT‐11 DeKosky, GAL‐INT‐18 Winblad) found significant treatment effect in terms of ADAS‐cog at twelve months or twenty‐four months. One of the trials GAL‐INT‐11 DeKosky) reached marginal significance in terms of dementia conversion rate to dementia (change of CDR score from 0.5 to >=1.0) at twenty‐four months. Combining data from both trials, 12‐24 mg/d bid galantamine confers an OR of 0.74 (95%CI 0.58‐0.94) in dementia conversion at twenty‐four months. MRI brain atrophy rate Dekosky et al. (GAL‐INT‐11 DeKosky) also used volumetric MRI brain imaging as an outcome measure. They found significantly lower whole brain but not hippocampal atrophy rate in MCI subjects treated with 12‐24 mg/d galantamine (bid) compared to the placebo group. The clinical significance of this is unclear.
Other comments The majority of ITT data reported in these trials were calculated using the Last Observation Carried Forward (LOCF) method. As a rule, the ITT treatment group size reported in these analyses were also smaller than the randomisation group size. Thus these ITT estimates are less conservative than traditional ITT. Safety In general, galantamine appeared well tolerated. As expected, gastrointestinal side effects were significantly more common in the treatment groups and in a dose‐related fashion. As an example, the OR for nausea ranged from 2.9 (95%CI 1.7‐5.3) for 16mg/d to 4.6 (95%CI 3.0‐7.0) for 32mg/d.
Comparison of adverse effects between trials was limited by different methods of reporting. Adverse events appearing at least 5% more often in the treatment groups were reported in four published trials (GAL‐INT‐10 Brodaty; GAL‐USA‐10 Tariot; GAL‐USA‐1 Raskind; GAL‐INT‐1 Wilcock). The proportion of subjects with these adverse events was also analysed. The adverse events recorded include tremor; anorexia; vomiting; nausea; weight loss; headache; abdominal pain; diarrhea; dizziness; and agitation. For 8mg/d, none of the adverse events was statistically significantly more frequent than placebo. Nausea, vomiting and diarrhea were statistically significant at 16mg/d. Nausea, vomiting, dizziness, weight loss, anorexia, tremor and headache were statistically significant at 24mg/d. Nausea, vomiting, dizziness, weight loss, anorexia, abdominal pain, tremor and headache were statistically significant at 32mg/d.
The data for 3‐month trials were similar to that of 6‐month, with nausea, vomiting, headache, somnolence, and agitation being those of greatest magnitude.
Four trials reported more galantamine‐treated subjects than placebo‐treated subjects discontinuing (GAL‐USA‐1 Raskind; GAL‐INT‐1 Wilcock; GAL‐95‐05; GAL‐93‐01 Wilkinson). Overall, galantamine‐treated subjects were more likely than placebo‐treated subjects to discontinue for any reason from trials of 6 months in length at daily doses of 24 mg (OR 1.7; 95%CI 1.3‐2.2), 32 mg‐bd dosing (OR 2.6; 95%CI 1.9‐3.5), and 32 mg‐tds dosing (OR 2.4; 95%CI 1.6‐3.5) . For 3‐month trials, subjects were more likely to discontinue at doses of 24mg and higher.
Overall, galantamine‐treated subjects were more likely to discontinue due to adverse events from trials of 6 months in length compared to placebo‐treated subjects, for those subjects treated with daily doses of 16‐24 mg (prolonged release: OR 1.9; 95%CI 1.0‐3.6),24 mg (OR 2.1; 95%CI 1.5‐2.9), 32 mg‐bd dosing (OR 3.6; 95%CI 2.6 ‐5.2) and 32 mg‐tds dosing (OR 2.8; 95%CI 1.8‐4.3). Note that ORs for 8 mg/d and 16 mg/d doses were not significantly greater than placebo. For 3‐month trials, subjects were more likely to discontinue at doses of 24mg and higher.
All but 3 (Kewitz 1994b; GAL‐93‐01 Wilkinson; GAL‐INT‐1 Wilcock) of the 9 AD trials reported proportion of subjects deceased during the trial period. None found excess death in the treatment groups compared to the placebo group. On the other hand, pooled data from the MCI trials found significantly higher death rate in the galantamine groups. Causes of death in the galantamine group was detailed in one of the clinical research reports (GAL‐INT‐11 DeKosky), which included: bronchial carcinoma/sudden death, cerebrovascular disorder/syncope, myocardial infarct and suicide. An interim study from the manufacturer (GAL‐COG‐3002 2005) reported a hazard ratio of 4.9 (95%CI 1.8‐13.40) during the double blind phase, and an interim adjusted hazard ratio of 3.0 (95%CI 1.3‐7.3) after taking into account of some of the retrieved dropout subjects. Further data collection from these two MCI trials and another open label MCI trial is in progress (GAL‐COG‐3002 2005).
Discussion
A clear picture is forming regarding the use of galantamine in AD now that the database available for review consists of seven published peer‐reviewed trials, one unpublished multicenter trial, and one meeting abstract. This review shows overall positive effects for galantamine for trials of 3 months, 5 months and 6 months duration. In addition, although there was not a statistically significant dose effect, doses above 8mg/d were, for the most part, consistently statistically significant. Thus, there is evidence demonstrating efficacy for galantamine on global ratings, cognitive tests, assessments of ADLs and behavior. The one trial recruiting patients with possible AD shows similar treatment effect to the other trials recruiting patients with probable AD. The prolong release / once daily formulation of galantamine was found to have similar efficacy and side‐effect profile as the equivalent twice‐daily regime. Magnitude of treatment effect also appears to be similar to other cholinesterase inhibitors including donepezil, rivastigmine, and tacrine. A comparatively narrow range of cognitive impairment ratings were used as entry criteria to these trials, leading to inclusion of mostly mildly or moderately impaired patients. A post‐hoc analysis (Wilkinson 2002) pooling subgroup data among patients with a MMSE score of 10‐12 from four of these trials (GAL‐INT‐1 Wilcock, GAL‐INT‐2 Rockwood, GAL‐USA‐1 Raskind,GAL‐USA‐10 Tariot), found galantamine to have a statistically significant effect on cognitive, functional and behavioral measures. However a proper systematic review and analysis was not completed, and the resulting effect size and significance was not compared to the subgroup with MMSE scores above 12‐ so no conclusions can be drawn. Post‐hoc analysis of data from GAL‐USA‐10 Tariot also found statistically significant drug efficacy among patients with previous exposure to acetylcholinesterase inhibitors (Mintzer 2003), but the same criticism applies.
Galantamine's adverse event profile is similar to other cholinesterase inhibitors with respect to cholinergically mediated gastrointestinal symptoms. No information is available on adverse events that occurred less than 5% more frequently in the treatment groups. There appears to be a dose response relationship for these adverse events with doses of 32mg/day associated with greater incidence of withdrawals. GAL‐USA‐5 randomised 6‐week galantamine withdrawal versus continuing galantamine or placebo. Adverse event rates were similar across the groups though sample size for this trial was small (N=118). It appears that doses of 16 mg/d were best tolerated in the single trial where medication was titrated over 4 week periods, and because this dose showed statistically indistinguishable efficacy with higher doses, it is probably most preferable initially.
Longer term use of galantamine has not been assessed in a controlled fashion. Data have been collected only from participants in open‐label extensions to these published clinical trials, and some of this data has been compared against placebo data from an Alzheimer's Disease trial for another drug, i.e., historical controls (Blesa 2003). Comparisons to historical controls are bias‐prone and a more valid study such as a randomised controlled trial is needed. Economic assessments based on some of the trials included in this review, using Canadian (Getsios 2001) and US (Migliaccio‐Walle2003) cost data have also been published.
Data from the two MCI trials suggests marginal clinical benefit, but a yet unexplained excess in death rate. Galantamine use in MCI is therefore not recommended.
Authors' conclusions
Implications for practice.
The results of this review suggest that doses of 16 mg/d and above improve cognitive function and either improve or maintain global function for at least 6 months. These findings apply to subjects with mildly to moderately severe cognitive impairment. There is limited information on efficacy for improving activities of daily living or overall behavior. The duration of efficacy is unknown as is the length of time patients should be treated. Adverse event data is available for only 6 months as well.
Galantamine use in MCI is not recommended due to excess death rate in the treatment group.
Implications for research.
Future trials are needed in more heterogeneous and typical clinical populations, involving people with more severely impaired cognitive functioning and with more mildly impaired cognitive functioning than the subjects included in these trials, and over durations longer than 6 months. Trials that contrast galantamine with other cholinesterase inhibitors or other medications such as memantine are desirable. Given that adverse events are dose related, an optimal dose is needed that provides sufficient improvement while minimizing adverse events. This dose may be 16 mg/d but further research is needed. It will be important to assess more accurately the effects of galantamine on ADLs, aspects of problematic behavior and caregiver burden, and the implications for health economics.
What's new
| Date | Event | Description |
|---|---|---|
| 4 November 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 2, 1999 Review first published: Issue 1, 2001
| Date | Event | Description |
|---|---|---|
| 16 November 2005 | New citation required and conclusions have changed | November 2005 Update: additional trials and data have been added to the analyses and two MCI trials have been included for the first time, so altering the title of the review. |
Notes
August 2004:
A trial studying the efficacy of Galantamine in mixed Alzheimer's Disease/ vascular dementia was added.
Analyses for GAL‐INT‐2 and GAL‐93‐01 were amended based on press reports, slightly changing existing analyses, and adding additional data that was previously unavailable. Added references for publication of GAL‐INT‐1 and GAL‐93‐01.
Notes: ‐Tariot global rating formerly was stated as cibic‐plus. The citation for the was the ADCS‐CGIC. This has been corrected. ‐Raskind global rating formerly was stated as cibic‐plus. The citation for the was the ADCS‐CGIC. This has been corrected. ‐Rockwood global rating was formerly stated as cibic‐plus. The citation for the was the ADCS‐CGIC. This has been corrected. ‐Wilcock global rating was formerly stated as cibic‐plus. The citation for the was the ADCS‐CGIC. This has been corrected.
New data tables were added for 3‐month adverse events and discontinuations.
November 2005
Trials were added, studying the efficacy of Galantamine in Mild Cognitive Impairment, and the efficacy of Prolonged Release formulation of Galantamine in Alzheimer's Disease.
Death rates for the Galantamine trials were also recorded.
Acknowledgements
We gratefully acknowledge the contributions of Jason Olin who was the original main reviewer of this review and of Enid Light, the consumer editor.
Data and analyses
Comparison 1. Global Rating OC.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global Rating (no change or improvement at 3 months) OC | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 galantamine (18mg/d bid or tid) vs placebo | 1 | 135 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.96 [0.88, 4.37] |
| 1.2 galantamine (24mg/d bid or tid) vs placebo | 1 | 118 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.73 [0.73, 4.09] |
| 1.3 galantamine (24‐32 mg/d bid or tid) vs placebo | 1 | 281 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.28 [1.33, 3.91] |
| 1.4 galantamine (36mg/d bid or tid) vs placebo | 1 | 103 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.41 [1.22, 9.51] |
| 2 Global Rating (no change or improvement at 6 months) OC | 6 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 2.1 galantamine (8mg/d bid) vs placebo | 1 | 340 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.13 [0.71, 1.79] |
| 2.2 galantamine (16mg/d bid) vs placebo | 1 | 445 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.25 [1.55, 3.28] |
| 2.3 galantamine (16‐24mg/d bid) vs placebo | 1 | 499 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.18 [0.83, 1.69] |
| 2.4 galantamine (16‐24mg/d Prolonged Release) vs placebo | 1 | 505 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.19 [0.83, 1.70] |
| 2.5 galantamine (24mg/d bid) vs placebo | 4 | 1314 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.05 [1.64, 2.56] |
| 2.6 galantamine (32mg/d bid) vs placebo | 2 | 606 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.94 [1.40, 2.69] |
| 2.7 galantamine (32mg/d tds) vs placebo | 1 | 414 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.30 [0.87, 1.94] |
1.1. Analysis.

Comparison 1 Global Rating OC, Outcome 1 Global Rating (no change or improvement at 3 months) OC.
1.2. Analysis.

Comparison 1 Global Rating OC, Outcome 2 Global Rating (no change or improvement at 6 months) OC.
Comparison 2. ADAS‐cog (Change from baseline) OC.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ADAS‐cog (Change from baseline at 3 months) OC | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 galantamine (18mg/d bid) vs placebo | 1 | 115 | Mean Difference (IV, Fixed, 95% CI) | ‐3.10 [‐5.45, ‐0.75] |
| 1.2 galantamine (16‐24mg/d bid) vs placebo | 1 | 543 | Mean Difference (IV, Fixed, 95% CI) | ‐2.6 [‐3.47, ‐1.73] |
| 1.3 galantamine (16‐24mg/d Prolonged Release) vs placebo | 1 | 544 | Mean Difference (IV, Fixed, 95% CI) | ‐2.2 [‐3.09, ‐1.31] |
| 1.4 galantamine (24mg/d bid) vs placebo | 1 | 97 | Mean Difference (IV, Fixed, 95% CI) | ‐4.20 [‐6.84, ‐1.56] |
| 1.5 galantamine (24‐32 mg/d bid) vs placebo | 1 | 278 | Mean Difference (IV, Fixed, 95% CI) | ‐1.90 [‐3.04, ‐0.76] |
| 1.6 galantamine (32mg/d tds) vs placebo | 1 | 398 | Mean Difference (IV, Fixed, 95% CI) | ‐2.4 [‐3.65, ‐1.15] |
| 1.7 galantamine (36mg/d bid) vs placebo | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐4.1 [‐6.60, ‐1.60] |
| 2 ADAS‐cog (Change from baseline at 6 months) OC | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 galantamine (8mg/d bid) vs placebo | 1 | 351 | Mean Difference (IV, Fixed, 95% CI) | ‐1.7 [‐3.02, ‐0.38] |
| 2.2 galantamine (16mg/d bid) vs placebo | 1 | 433 | Mean Difference (IV, Fixed, 95% CI) | ‐3.3 [‐4.45, ‐2.15] |
| 2.3 galantamine (16‐24mg/d bid) vs placebo | 1 | 475 | Mean Difference (IV, Fixed, 95% CI) | ‐3.10 [‐4.18, ‐2.02] |
| 2.4 galantamine (16‐24mg/d Prolonged Release) vs placebo | 1 | 488 | Mean Difference (IV, Fixed, 95% CI) | ‐2.7 [‐3.67, ‐1.73] |
| 2.5 galantamine (24mg/d bid) vs placebo | 4 | 1290 | Mean Difference (IV, Fixed, 95% CI) | ‐3.38 [‐4.04, ‐2.72] |
| 2.6 galantamine (32mg/d bid or tds) vs placebo | 2 | 599 | Mean Difference (IV, Fixed, 95% CI) | ‐3.99 [‐4.99, ‐2.99] |
| 2.7 galantamine (32mg/d tds) vs placebo | 1 | 394 | Mean Difference (IV, Fixed, 95% CI) | ‐2.9 [‐4.29, ‐1.51] |
2.1. Analysis.

Comparison 2 ADAS‐cog (Change from baseline) OC, Outcome 1 ADAS‐cog (Change from baseline at 3 months) OC.
2.2. Analysis.
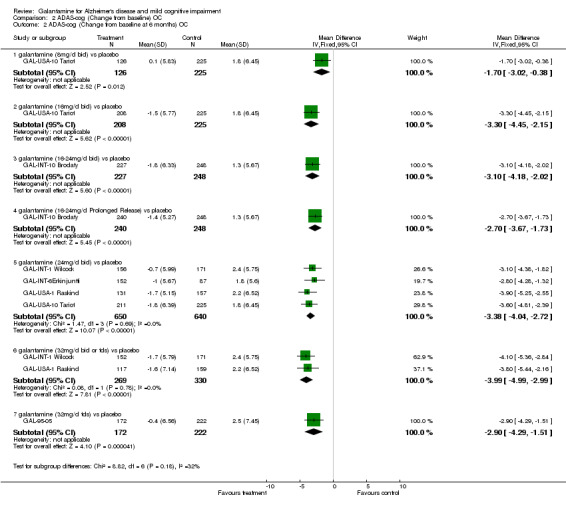
Comparison 2 ADAS‐cog (Change from baseline) OC, Outcome 2 ADAS‐cog (Change from baseline at 6 months) OC.
Comparison 3. ADAS‐cog (4 points or more improvement) OC.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ADAS‐cog (4 points or more improvement at 3 months) OC | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.3 galantamine (24‐32 mg/d bid or tid) vs placebo | 1 | 270 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.79 [1.04, 3.09] |
| 2 ADAS‐cog (4 points or more improvement at 6 months) OC | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 2.1 galantamine (8mg/d bid) vs placebo | 1 | 326 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.44 [0.81, 2.55] |
| 2.2 galantamine (16mg/d bid) vs placebo | 1 | 433 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.24 [1.47, 3.42] |
| 2.3 galantamine (24mg/d bid) vs placebo | 3 | 1051 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.42 [1.83, 3.20] |
| 2.4 galantamine (32mg/d bid) vs placebo | 2 | 597 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.72 [1.86, 3.96] |
3.1. Analysis.

Comparison 3 ADAS‐cog (4 points or more improvement) OC, Outcome 1 ADAS‐cog (4 points or more improvement at 3 months) OC.
3.2. Analysis.

Comparison 3 ADAS‐cog (4 points or more improvement) OC, Outcome 2 ADAS‐cog (4 points or more improvement at 6 months) OC.
Comparison 4. ADCS‐ADL (Change from baseline) OC.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ADCS/ADL (Change from baseline at 6 months) OC | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 galantamine (8mg/d bid) vs placebo | 1 | 341 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐1.22, 3.02] |
| 1.2 galantamine (16mg/d bid) vs placebo | 1 | 447 | Mean Difference (IV, Fixed, 95% CI) | 3.50 [1.84, 5.16] |
| 1.3 galantamine (16‐24mg/d bid) vs placebo | 1 | 500 | Mean Difference (IV, Fixed, 95% CI) | 1.4 [‐1.09, 3.89] |
| 1.4 galantamine (16‐24mg/d Prolonged Release ) vs placebo | 1 | 503 | Mean Difference (IV, Fixed, 95% CI) | 2.4 [0.80, 4.00] |
| 1.5 galantamine (24mg/d bid) vs placebo | 1 | 447 | Mean Difference (IV, Fixed, 95% CI) | 2.4 [0.74, 4.06] |
4.1. Analysis.

Comparison 4 ADCS‐ADL (Change from baseline) OC, Outcome 1 ADCS/ADL (Change from baseline at 6 months) OC.
Comparison 5. NPI (Change from baseline) OC.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 NPI (Change from baseline at 3 months) OC | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 galantamine (24‐32 mg/d bid or tid) vs placebo | 1 | 282 | Mean Difference (IV, Fixed, 95% CI) | ‐0.7 [‐4.07, 2.67] |
| 2 NPI (Change from baseline at 6 months) OC | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 galantamine (8mg/d bid) vs placebo | 1 | 340 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐2.55, 2.55] |
| 2.2 galantamine (16mg/d bid) vs placebo | 1 | 445 | Mean Difference (IV, Fixed, 95% CI) | ‐2.4 [‐4.48, ‐0.32] |
| 2.3 galantamine (16‐24mg/d bid) vs placebo | 1 | 500 | Mean Difference (IV, Fixed, 95% CI) | ‐1.3 [‐3.59, 0.99] |
| 2.4 galantamine (16‐24mg/d Prolonged Release ) vs placebo | 1 | 503 | Mean Difference (IV, Fixed, 95% CI) | ‐0.7 [‐2.80, 1.40] |
| 2.5 galantamine (24mg/d bid) vs placebo | 2 | 677 | Mean Difference (IV, Fixed, 95% CI) | ‐2.09 [‐3.84, ‐0.34] |
5.1. Analysis.

Comparison 5 NPI (Change from baseline) OC, Outcome 1 NPI (Change from baseline at 3 months) OC.
5.2. Analysis.

Comparison 5 NPI (Change from baseline) OC, Outcome 2 NPI (Change from baseline at 6 months) OC.
Comparison 6. DAD (Change from baseline) OC.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 DAD (Change from baseline at 3 months) OC | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 galantamine (24‐32 mg/d bid or tid) vs placebo | 1 | 282 | Mean Difference (IV, Fixed, 95% CI) | 4.3 [1.46, 7.14] |
| 2 DAD (Change from baseline at 6 months) OC | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 galantamine (24mg/d bid) vs placebo | 2 | 575 | Mean Difference (IV, Fixed, 95% CI) | 3.87 [1.34, 6.39] |
| 2.2 galantamine (32mg/d bid) vs placebo | 1 | 334 | Mean Difference (IV, Fixed, 95% CI) | 3.80 [0.29, 7.31] |
6.1. Analysis.

Comparison 6 DAD (Change from baseline) OC, Outcome 1 DAD (Change from baseline at 3 months) OC.
6.2. Analysis.

Comparison 6 DAD (Change from baseline) OC, Outcome 2 DAD (Change from baseline at 6 months) OC.
Comparison 7. Global Rating ITT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global Rating (no change or improvement at 3 months) ITT | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 galantamine (18mg/d bid) vs placebo | 1 | 162 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.44 [1.18, 5.04] |
| 1.2 galantamine (24mg/d bid) vs placebo | 1 | 136 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.11 [0.96, 4.62] |
| 1.3 galantamine (24‐32 mg/d bid or tid) vs placebo | 1 | 363 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.48 [0.92, 2.37] |
| 1.4 galantamine (36mg/d bid) vs placebo | 1 | 130 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.70 [1.18, 6.17] |
| 2 Global Rating (no change or improvement at 6 months) ITT | 6 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 2.1 galantamine (8mg/d bid) vs placebo | 1 | 391 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.17 [0.77, 1.78] |
| 2.2 galantamine (16mg/d bid) vs placebo | 1 | 517 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.04 [1.44, 2.89] |
| 2.3 galantamine (16‐24mg/d bid) vs placebo | 1 | 603 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.27 [0.92, 1.76] |
| 2.4 galantamine (16‐24mg/d Prolonged Release ) vs placebo | 1 | 592 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.20 [0.86, 1.66] |
| 2.5 galantamine (24mg/d bid) vs placebo | 4 | 1570 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.90 [1.55, 2.33] |
| 2.6 galantamine (32mg/d bid) vs placebo | 2 | 768 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.79 [1.34, 2.38] |
| 2.7 galantamine (32mg/d tds) vs placebo | 1 | 525 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.28 [0.89, 1.83] |
7.1. Analysis.

Comparison 7 Global Rating ITT, Outcome 1 Global Rating (no change or improvement at 3 months) ITT.
7.2. Analysis.
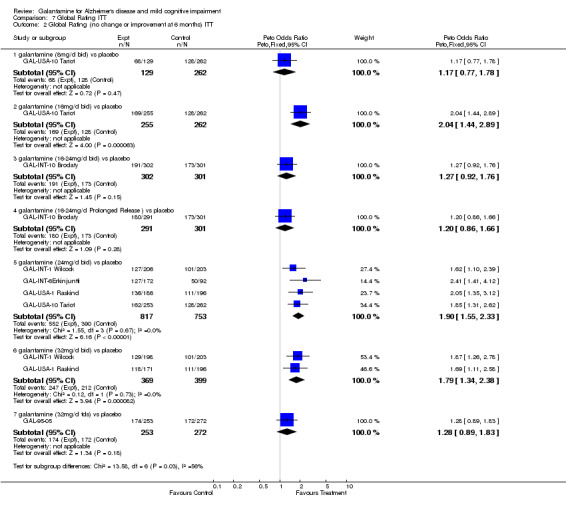
Comparison 7 Global Rating ITT, Outcome 2 Global Rating (no change or improvement at 6 months) ITT.
Comparison 8. ADAS‐cog (Change from baseline) ITT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ADAS‐cog (Change from baseline at 3 months) ITT | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 galantamine (18mg/d bid) vs placebo | 1 | 163 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐3.64, 0.24] |
| 1.2 galantamine (16‐24mg/d bid) vs placebo | 1 | 592 | Mean Difference (IV, Fixed, 95% CI) | ‐2.7 [‐3.55, ‐1.85] |
| 1.3 galantamine (16‐24mg/d Prolonged Release ) vs placebo | 1 | 586 | Mean Difference (IV, Fixed, 95% CI) | 1.8 [0.94, 2.66] |
| 1.4 galantamine (24mg/d bid) vs placebo | 1 | 137 | Mean Difference (IV, Fixed, 95% CI) | ‐3.0 [‐5.23, ‐0.77] |
| 1.5 galantamine (24‐32 mg/d bid or tid) vs placebo | 1 | 359 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐2.79, ‐0.61] |
| 1.6 galantamine (36mg/d bid) vs placebo | 1 | 133 | Mean Difference (IV, Fixed, 95% CI) | ‐2.3 [‐4.24, ‐0.36] |
| 2 ADAS‐cog (Change from baseline at 6 months) ITT | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 galantamine (8mg/d bid) vs placebo | 1 | 381 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐2.57, ‐0.03] |
| 2.2 galantamine (16mg/d bid ) vs placebo | 1 | 508 | Mean Difference (IV, Fixed, 95% CI) | ‐3.10 [‐4.13, ‐2.07] |
| 2.3 galantamine (16‐24mg/d bid) vs placebo | 1 | 592 | Mean Difference (IV, Fixed, 95% CI) | ‐2.8 [‐3.76, ‐1.84] |
| 2.4 galantamine (16‐24mg/d Prolonged Release ) vs placebo | 1 | 587 | Mean Difference (IV, Fixed, 95% CI) | ‐2.5 [‐3.39, ‐1.61] |
| 2.5 galantamine (24mg/d bid) vs placebo | 4 | 1630 | Mean Difference (IV, Fixed, 95% CI) | ‐3.13 [‐3.70, ‐2.55] |
| 2.6 galantamine (32mg/d bid) vs placebo | 2 | 825 | Mean Difference (IV, Fixed, 95% CI) | ‐3.29 [‐4.14, ‐2.44] |
| 2.7 galantamine (32mg/d tds) vs placebo | 1 | 553 | Mean Difference (IV, Fixed, 95% CI) | ‐2.9 [‐4.01, ‐1.79] |
| 3 ADAS‐cog (Change from baseline at 12 months in MCI) ITT | 2 | 1903 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.62, 0.40] |
| 3.1 galantamine (16‐24mg/d bid) vs placebo in MCI | 2 | 1903 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.62, 0.40] |
| 4 ADAS‐cog (Change from baseline at 24 months in MCI) ITT | 2 | 1903 | Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.78, 0.37] |
| 4.1 galantamine (16‐24mg/d bid) vs placebo in MCI | 2 | 1903 | Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.78, 0.37] |
8.1. Analysis.

Comparison 8 ADAS‐cog (Change from baseline) ITT, Outcome 1 ADAS‐cog (Change from baseline at 3 months) ITT.
8.2. Analysis.

Comparison 8 ADAS‐cog (Change from baseline) ITT, Outcome 2 ADAS‐cog (Change from baseline at 6 months) ITT.
8.3. Analysis.

Comparison 8 ADAS‐cog (Change from baseline) ITT, Outcome 3 ADAS‐cog (Change from baseline at 12 months in MCI) ITT.
8.4. Analysis.

Comparison 8 ADAS‐cog (Change from baseline) ITT, Outcome 4 ADAS‐cog (Change from baseline at 24 months in MCI) ITT.
Comparison 9. ADAS‐cog (4 point or more improvement) ITT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ADAS‐cog (4 points or more improvement at 3 months) OC | 1 | 359 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.46 [0.90, 2.39] |
9.1. Analysis.

Comparison 9 ADAS‐cog (4 point or more improvement) ITT, Outcome 1 ADAS‐cog (4 points or more improvement at 3 months) OC.
Comparison 10. ADCS‐ADL (Change from baseline) ITT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ADCS/ADL (Change from baseline at 6 months) ITT | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 galantamine (8mg/d bid) vs placebo | 1 | 391 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐1.36, 2.56] |
| 1.2 galantamine (16mg/d bid) vs placebo | 1 | 517 | Mean Difference (IV, Fixed, 95% CI) | 3.10 [1.57, 4.63] |
| 1.3 galantamine (24mg/d bid) vs placebo | 1 | 515 | Mean Difference (IV, Fixed, 95% CI) | 2.3 [0.64, 3.96] |
10.1. Analysis.

Comparison 10 ADCS‐ADL (Change from baseline) ITT, Outcome 1 ADCS/ADL (Change from baseline at 6 months) ITT.
Comparison 11. NPI (Change from baseline) ITT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 NPI (Change from baseline at 3 months) ITT | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 galantamine (24‐32 mg/d bid or tid) vs placebo | 1 | 364 | Mean Difference (IV, Fixed, 95% CI) | ‐0.8 [‐2.67, 1.07] |
| 2 NPI (Change from baseline at 6 months) ITT | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 galantamine (8mg/d bid) vs placebo | 1 | 391 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐2.09, 2.69] |
| 2.2 galantamine (16mg/d bid) vs placebo | 1 | 517 | Mean Difference (IV, Fixed, 95% CI) | ‐2.1 [‐4.04, ‐0.16] |
| 2.3 galantamine (24mg/d bid) vs placebo | 2 | 788 | Mean Difference (IV, Fixed, 95% CI) | ‐1.49 [‐3.11, 0.13] |
11.1. Analysis.

Comparison 11 NPI (Change from baseline) ITT, Outcome 1 NPI (Change from baseline at 3 months) ITT.
11.2. Analysis.

Comparison 11 NPI (Change from baseline) ITT, Outcome 2 NPI (Change from baseline at 6 months) ITT.
Comparison 12. DAD (Change from baseline) ITT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 DAD (Change from baseline at 3 months) ITT | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 galantamine (24‐32 mg/d bid or tid) vs placebo | 1 | 364 | Mean Difference (IV, Fixed, 95% CI) | 4.8 [2.05, 7.55] |
| 2 DAD (Change from baseline at 6 months) ITT | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 galantamine (24mg/d bid) vs placebo | 2 | 701 | Mean Difference (IV, Fixed, 95% CI) | 3.66 [1.41, 5.90] |
| 2.2 galantamine (32mg/d bid) vs placebo | 1 | 424 | Mean Difference (IV, Fixed, 95% CI) | 3.5 [0.52, 6.48] |
12.1. Analysis.

Comparison 12 DAD (Change from baseline) ITT, Outcome 1 DAD (Change from baseline at 3 months) ITT.
12.2. Analysis.
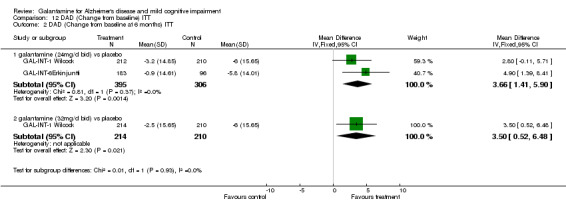
Comparison 12 DAD (Change from baseline) ITT, Outcome 2 DAD (Change from baseline at 6 months) ITT.
Comparison 13. Global Rating dose analyses OC.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global Rating (no change or improvement; 8 mg) OC | 1 | 340 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.13 [0.71, 1.79] |
| 2 Global Rating (no change or improvement 16‐24mg/d) OC | 3 | 1079 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.63 [1.28, 2.09] |
| 3 Global Rating (no change or improvement 24mg/d to 24‐32mg/d) OC | 6 | 1713 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.06 [1.69, 2.52] |
| 4 Global Rating (no change or improvement 32‐36mg/d) OC | 4 | 1123 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.72 [1.35, 2.20] |
13.1. Analysis.

Comparison 13 Global Rating dose analyses OC, Outcome 1 Global Rating (no change or improvement; 8 mg) OC.
13.2. Analysis.

Comparison 13 Global Rating dose analyses OC, Outcome 2 Global Rating (no change or improvement 16‐24mg/d) OC.
13.3. Analysis.

Comparison 13 Global Rating dose analyses OC, Outcome 3 Global Rating (no change or improvement 24mg/d to 24‐32mg/d) OC.
13.4. Analysis.

Comparison 13 Global Rating dose analyses OC, Outcome 4 Global Rating (no change or improvement 32‐36mg/d) OC.
Comparison 14. Global Rating dose analyses ITT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global Rating (no change or improvement; 8 mg) ITT | 1 | 391 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.17 [0.77, 1.78] |
| 2 Global Rating (no change or improvement 16‐24mg/d) ITT | 3 | 1282 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.65 [1.32, 2.07] |
| 3 Global Rating (no change or improvement 24mg/d to 24‐32mg/d) ITT | 6 | 2069 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.84 [1.53, 2.21] |
| 4 Global Rating (no change or improvement 32‐36mg/d) ITT | 4 | 1423 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.63 [1.31, 2.02] |
14.1. Analysis.
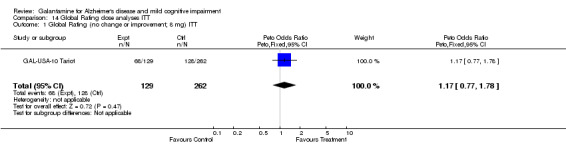
Comparison 14 Global Rating dose analyses ITT, Outcome 1 Global Rating (no change or improvement; 8 mg) ITT.
14.2. Analysis.

Comparison 14 Global Rating dose analyses ITT, Outcome 2 Global Rating (no change or improvement 16‐24mg/d) ITT.
14.3. Analysis.
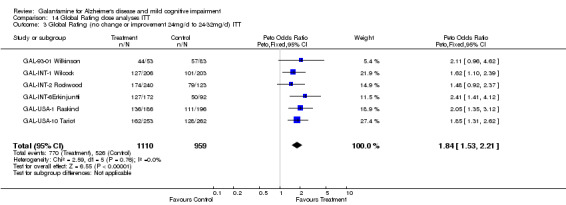
Comparison 14 Global Rating dose analyses ITT, Outcome 3 Global Rating (no change or improvement 24mg/d to 24‐32mg/d) ITT.
14.4. Analysis.
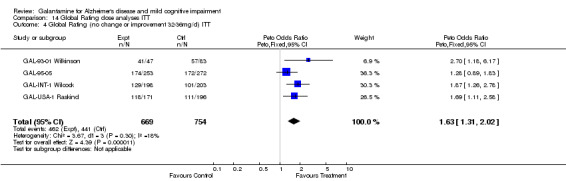
Comparison 14 Global Rating dose analyses ITT, Outcome 4 Global Rating (no change or improvement 32‐36mg/d) ITT.
Comparison 15. Conversion from MCI to dementia (change of CDR‐SB from 0.5 to >=1) ITT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2 Conversion from MCI to dementia at 24 months | 2 | 1903 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.58, 0.94] |
| 2.1 galantamine (16‐24mg/d bid) vs placebo in MCI | 2 | 1903 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.58, 0.94] |
15.2. Analysis.

Comparison 15 Conversion from MCI to dementia (change of CDR‐SB from 0.5 to >=1) ITT, Outcome 2 Conversion from MCI to dementia at 24 months.
Comparison 16. Withdrawals before end of treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of all cause discontinuations (3 months) | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 galantamine (18mg/d bid) vs placebo | 1 | 175 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.03 [1.00, 4.13] |
| 1.2 galantamine (24mg/d bid) vs placebo | 1 | 143 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.75 [0.75, 4.08] |
| 1.3 galantamine (24mg/d to 24‐32mg/d) vs placebo | 1 | 386 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.42 [2.10, 5.58] |
| 1.4 galantamine (36mg/d bid) vs placebo | 1 | 141 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.79 [2.26, 10.14] |
| 2 Proportion of discontinuations due to adverse events (3 months) | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 2.1 galantamine (18mg/d bid) vs placebo | 1 | 175 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.57 [1.13, 5.83] |
| 2.2 galantamine (24mg/d bid) vs placebo | 1 | 143 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.99 [1.03, 8.64] |
| 2.3 galantamine (24mg/d to 24‐32mg/d) vs placebo | 1 | 386 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.11 [2.37, 7.13] |
| 2.4 galantamine (36mg/d bid) vs placebo | 1 | 141 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 9.26 [4.05, 21.17] |
| 3 Proportion of all cause discontinuations (6 months) | 5 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 3.1 galantamine (8mg/d bid) vs placebo | 1 | 426 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.57 [0.93, 2.65] |
| 3.2 galantamine (16mg/d bid) vs placebo | 1 | 565 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.43 [0.94, 2.18] |
| 3.3 galantamine (16‐24mg/d bid) vs placebo | 1 | 651 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.39 [0.95, 2.03] |
| 3.4 galantamine (16‐24mg/d Prolonged Release ) vs placebo | 1 | 640 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.24 [0.84, 1.83] |
| 3.5 galantamine (24mg/d bid) vs placebo | 3 | 1419 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.67 [1.29, 2.17] |
| 3.6 galantamine (32mg/d bid) vs placebo | 2 | 857 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.55 [1.87, 3.48] |
| 3.7 galantamine (32mg/d tds) vs placebo | 1 | 554 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.36 [1.62, 3.45] |
| 4 Proportion of discontinuations due to adverse events (6 months) | 5 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 4.1 galantamine (8mg/d bid) vs placebo | 1 | 426 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.92 [0.41, 2.04] |
| 4.2 galantamine (16mg/d bid) vs placebo | 1 | 565 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.97 [0.51, 1.86] |
| 4.3 galantamine (16‐24mg/d bid) vs placebo | 1 | 651 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.62 [0.85, 3.09] |
| 4.4 galantamine (16‐24mg/d Prolonged Release ) vs placebo | 1 | 644 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.94 [1.04, 3.59] |
| 4.5 galantamine (24mg/d bid) vs placebo | 3 | 1419 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.09 [1.51, 2.91] |
| 4.6 galantamine (32mg/d bid) vs placebo | 2 | 857 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.64 [2.56, 5.18] |
| 4.7 galantamine (32mg/d tds) vs placebo | 1 | 554 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.79 [1.81, 4.28] |
16.1. Analysis.

Comparison 16 Withdrawals before end of treatment, Outcome 1 Proportion of all cause discontinuations (3 months).
16.2. Analysis.

Comparison 16 Withdrawals before end of treatment, Outcome 2 Proportion of discontinuations due to adverse events (3 months).
16.3. Analysis.

Comparison 16 Withdrawals before end of treatment, Outcome 3 Proportion of all cause discontinuations (6 months).
16.4. Analysis.

Comparison 16 Withdrawals before end of treatment, Outcome 4 Proportion of discontinuations due to adverse events (6 months).
Comparison 17. Specific adverse events (3 months).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of subjects experiencing nausea (3 months) | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 galantamine (18mg/d bid) vs placebo | 1 | 175 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.33 [1.64, 11.45] |
| 1.2 galantamine (24‐32mg/d bid) vs placebo | 2 | 529 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.32 [2.11, 5.21] |
| 1.4 galantamine (36mg/d bid) vs placebo | 1 | 141 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 11.51 [4.61, 28.75] |
| 2 Proportion of subjects experiencing vomiting (3 months) | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 2.1 galantamine (18mg/d bid) vs placebo | 1 | 175 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.59 [1.39, 9.29] |
| 2.2 galantamine (24‐32mg/d bid) vs placebo | 2 | 529 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.14 [1.75, 5.63] |
| 2.3 galantamine (36mg/d bid) vs placebo | 1 | 141 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.19 [1.30, 13.48] |
| 3 Proportion of subjects experiencing dizziness (3 months) | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 3.1 galantamine (18mg/d bid) vs placebo | 1 | 175 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.33 [0.29, 6.00] |
| 3.2 galantamine (24‐32mg/d bid) vs placebo | 2 | 529 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.60 [1.39, 4.88] |
| 3.3 galantamine (36mg/d bid) vs placebo | 1 | 141 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.30 [0.48, 10.92] |
| 4 Proportion of subjects experiencing diarrhea (3 months) | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 4.1 galantamine (18mg/d bid) vs placebo | 1 | 175 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.14, 7.14] |
| 4.2 galantamine (24‐32mg/d bid) vs placebo | 1 | 143 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.46 [0.40, 15.20] |
| 4.3 galantamine (36mg/d bid) vs placebo | 1 | 141 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.66 [0.22, 12.73] |
| 5 Proportion of subjects experiencing anorexia (3 months) | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 5.1 galantamine (24‐32mg/d bid) vs placebo | 1 | 386 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.24 [1.53, 6.88] |
| 6 Proportion of subjects experiencing somnolence (3 months) | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 6.1 galantamine (24‐32mg/d bid) vs placebo | 1 | 386 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.78 [1.48, 9.67] |
| 7 Proportion of subjects experiencing abdominal pain (3 months) | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 7.1 galantamine (24‐32mg/d bid) vs placebo | 1 | 386 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.93 [1.12, 7.66] |
| 8 Proportion of subjects experiencing decreased appetite (3 months) | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 8.1 galantamine (18mg/d bid) vs placebo | 1 | 175 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.40 [0.53, 10.85] |
| 8.2 galantamine (24‐32mg/d bid) vs placebo | 1 | 143 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.59 [0.21, 12.11] |
| 8.3 galantamine (36mg/d bid) vs placebo | 1 | 141 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.47 [0.65, 18.56] |
| 9 Proportion of subjects experiencing agitation (3 months) | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 9.1 galantamine (24‐32mg/d bid) vs placebo | 1 | 386 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.54 [1.25, 9.98] |
| 10 Proportion of subjects experiencing headache (3 months) | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 10.1 galantamine (18mg/d bid) vs placebo | 1 | 175 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.25 [0.33, 4.75] |
| 10.2 galantamine (24‐32mg/d bid) vs placebo | 1 | 143 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.54 [0.69, 9.45] |
| 10.3 galantamine (36mg/d bid) vs placebo | 1 | 141 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.80 [1.36, 16.96] |
17.1. Analysis.

Comparison 17 Specific adverse events (3 months), Outcome 1 Proportion of subjects experiencing nausea (3 months).
17.2. Analysis.

Comparison 17 Specific adverse events (3 months), Outcome 2 Proportion of subjects experiencing vomiting (3 months).
17.3. Analysis.

Comparison 17 Specific adverse events (3 months), Outcome 3 Proportion of subjects experiencing dizziness (3 months).
17.4. Analysis.

Comparison 17 Specific adverse events (3 months), Outcome 4 Proportion of subjects experiencing diarrhea (3 months).
17.5. Analysis.

Comparison 17 Specific adverse events (3 months), Outcome 5 Proportion of subjects experiencing anorexia (3 months).
17.6. Analysis.

Comparison 17 Specific adverse events (3 months), Outcome 6 Proportion of subjects experiencing somnolence (3 months).
17.7. Analysis.

Comparison 17 Specific adverse events (3 months), Outcome 7 Proportion of subjects experiencing abdominal pain (3 months).
17.8. Analysis.

Comparison 17 Specific adverse events (3 months), Outcome 8 Proportion of subjects experiencing decreased appetite (3 months).
17.9. Analysis.
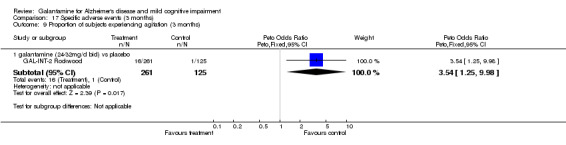
Comparison 17 Specific adverse events (3 months), Outcome 9 Proportion of subjects experiencing agitation (3 months).
17.10. Analysis.

Comparison 17 Specific adverse events (3 months), Outcome 10 Proportion of subjects experiencing headache (3 months).
Comparison 18. Specific adverse events (6 months).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of subjects experiencing nausea (6 months) | 5 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 galantamine (8mg/d bid) vs placebo | 1 | 426 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.28 [0.50, 3.26] |
| 1.2 galantamine (16mg/d bid) vs placebo | 1 | 565 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.94 [1.65, 5.25] |
| 1.3 galantamine (16‐24mg/d bid) vs placebo | 1 | 646 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.80 [1.65, 4.73] |
| 1.4 galantamine (16‐24mg/d Prolonged Release ) vs placebo | 1 | 639 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.39 [2.06, 5.57] |
| 1.5 galantamine (24mg/d bid) vs placebo | 3 | 1419 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.69 [2.82, 4.82] |
| 1.6 galantamine (32mg/d bid) vs placebo | 2 | 856 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.21 [1.66, 2.94] |
| 1.7 galantamine (32mg/d tds) vs placebo | 1 | 554 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.58 [3.00, 6.98] |
| 2 Proportion of subjects experiencing vomiting (6 months) | 5 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 2.1 galantamine (8mg/d bid) vs placebo | 1 | 426 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.85 [0.70, 11.62] |
| 2.2 galantamine (16mg/d bid) vs placebo | 1 | 565 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.70 [1.55, 8.85] |
| 2.3 galantamine (16‐24mg/d bid) vs placebo | 1 | 646 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.48 [1.76, 6.88] |
| 2.4 galantamine (16‐24mg/d Prolonged Release ) vs placebo | 1 | 639 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.85 [1.34, 6.08] |
| 2.5 galantamine (24mg/d bid) vs placebo | 3 | 1419 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.01 [2.15, 4.21] |
| 2.6 galantamine (32mg/d bid) vs placebo | 2 | 857 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.07 [2.09, 4.50] |
| 2.7 galantamine (32mg/d tds) vs placebo | 1 | 554 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 6.33 [3.33, 12.03] |
| 3 Proportion of subjects experiencing dizziness (6 months) | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 3.1 galantamine (8mg/d bid) vs placebo | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 galantamine (16mg/d bid) vs placebo | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 galantamine (16‐24mg/d bid) vs placebo | 1 | 646 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.71 [0.89, 3.30] |
| 3.4 galantamine (16‐24mg/d Prolonged Release ) vs placebo | 1 | 639 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.40 [1.32, 4.34] |
| 3.5 galantamine (24mg/d bid) vs placebo | 2 | 860 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.62 [1.04, 2.52] |
| 3.6 galantamine (32mg/d bid) vs placebo | 2 | 857 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.04 [1.34, 3.11] |
| 3.7 galantamine (32mg/d tds) vs placebo | 1 | 554 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.47 [1.03, 5.90] |
| 4 Proportion of subjects experiencing diarrhea (6 months) | 5 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 4.1 galantamine (8mg/d bid) vs placebo | 1 | 426 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.84 [0.35, 2.01] |
| 4.2 galantamine (16mg/d bid) vs placebo | 1 | 565 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.14 [1.20, 3.80] |
| 4.3 galantamine (16‐24mg/d bid) vs placebo | 1 | 646 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.98 [0.53, 1.81] |
| 4.4 galantamine (16‐24mg/d Prolonged Release ) vs placebo | 1 | 639 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.67 [0.35, 1.30] |
| 4.5 galantamine (24mg/d bid) vs placebo | 3 | 1419 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.37 [0.91, 2.05] |
| 4.6 galantamine (32mg/d bid) vs placebo | 2 | 857 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.03 [1.35, 3.04] |
| 4.7 galantamine (32mg/d tds) vs placebo | 1 | 554 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.14 [1.43, 6.89] |
| 5 Proportion of subjects experiencing anorexia (6 months) | 5 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 5.1 galantamine (8mg/d bid) vs placebo | 1 | 426 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.95 [0.70, 5.47] |
| 5.2 galantamine (16mg/d bid) vs placebo | 1 | 565 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.06 [0.95, 4.47] |
| 5.3 galantamine (16‐24mg/d bid) vs placebo | 1 | 646 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.61 [1.25, 5.42] |
| 5.4 galantamine (16‐24mg/d Prolonged Release ) vs placebo | 1 | 639 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.35 [1.09, 5.07] |
| 5.5 galantamine (24mg/d bid) vs placebo | 3 | 1425 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.84 [1.24, 2.73] |
| 5.6 galantamine (32mg/d bid) vs placebo | 2 | 857 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.71 [2.95, 7.53] |
| 5.7 galantamine (32mg/d tds) vs placebo | 1 | 554 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.47 [1.87, 10.68] |
| 6 Proportion of subjects experiencing weight loss (6 months) | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 6.1 galantamine (8mg/d bid) vs placebo | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 galantamine (16mg/d bid) vs placebo | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 galantamine (16‐24mg/d bid) vs placebo | 1 | 646 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.52 [1.48, 8.40] |
| 6.4 galantamine (16‐24mg/d Prolonged Release ) vs placebo | 1 | 639 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.14 [1.23, 8.01] |
| 6.5 galantamine (24mg/d bid) vs placebo | 2 | 860 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.55 [2.04, 6.17] |
| 6.6 galantamine (32mg/d bid) vs placebo | 2 | 857 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.98 [1.63, 5.46] |
| 7 Proportion of subjects experiencing abdominal pain (6 months) | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 7.1 galantamine (8mg/d bid) vs placebo | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 galantamine (16mg/d bid) vs placebo | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 galantamine (24mg/d bid) vs placebo | 1 | 425 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.59 [0.69, 3.68] |
| 7.4 galantamine (32mg/d bid) vs placebo | 1 | 424 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.60 [1.26, 5.33] |
| 7.5 galantamine (32mg/d tds) vs placebo | 1 | 554 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.84 [1.05, 7.66] |
| 8 Proportion of subjects experiencing tremor (6 months) | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 8.1 galantamine (8mg/d bid) vs placebo | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 galantamine (16mg/d bid) vs placebo | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 galantamine (24mg/d bid) vs placebo | 1 | 425 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.56 [1.77, 17.50] |
| 8.4 galantamine (32mg/d bid) vs placebo | 1 | 424 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.64 [1.15, 18.77] |
| 9 Proportion of subjects experiencing agitation (6 months) | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 9.1 galantamine (8mg/d bid) vs placebo | 1 | 426 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.74 [0.92, 3.30] |
| 9.2 galantamine (16mg/d bid) vs placebo | 1 | 565 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.07 [0.61, 1.87] |
| 9.3 galantamine (16‐24mg/d bid) vs placebo | 1 | 646 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.93 [0.49, 1.75] |
| 9.4 galantamine (16‐24mg/d Prolonged Release ) vs placebo | 1 | 639 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.05 [0.57, 1.96] |
| 9.5 galantamine (24mg/d bid) vs placebo | 1 | 559 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.84 [0.47, 1.51] |
| 9.6 galantamine (32mg/d bid) vs placebo | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.7 galantamine (32mg/d tds) vs placebo | 1 | 554 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.33 [0.58, 3.07] |
| 10 Proportion of subjects experiencing headache (6 months) | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 10.1 galantamine (8mg/d bid) vs placebo | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 galantamine (16mg/d bid) vs placebo | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 galantamine (16‐24mg/d bid) vs placebo | 1 | 646 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.98 [0.50, 1.92] |
| 10.4 galantamine (16‐24mg/d Prolonged Release ) vs placebo | 1 | 639 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.54 [0.84, 2.83] |
| 10.5 galantamine (24mg/d bid) vs placebo | 1 | 435 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.83 [1.32, 6.09] |
| 10.6 galantamine (32mg/d bid) vs placebo | 1 | 433 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.31 [1.61, 6.80] |
| 10.7 galantamine (32mg/d tds) vs placebo | 1 | 554 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.02 [0.45, 2.30] |
18.1. Analysis.

Comparison 18 Specific adverse events (6 months), Outcome 1 Proportion of subjects experiencing nausea (6 months).
18.2. Analysis.

Comparison 18 Specific adverse events (6 months), Outcome 2 Proportion of subjects experiencing vomiting (6 months).
18.3. Analysis.

Comparison 18 Specific adverse events (6 months), Outcome 3 Proportion of subjects experiencing dizziness (6 months).
18.4. Analysis.

Comparison 18 Specific adverse events (6 months), Outcome 4 Proportion of subjects experiencing diarrhea (6 months).
18.5. Analysis.

Comparison 18 Specific adverse events (6 months), Outcome 5 Proportion of subjects experiencing anorexia (6 months).
18.6. Analysis.

Comparison 18 Specific adverse events (6 months), Outcome 6 Proportion of subjects experiencing weight loss (6 months).
18.7. Analysis.

Comparison 18 Specific adverse events (6 months), Outcome 7 Proportion of subjects experiencing abdominal pain (6 months).
18.8. Analysis.
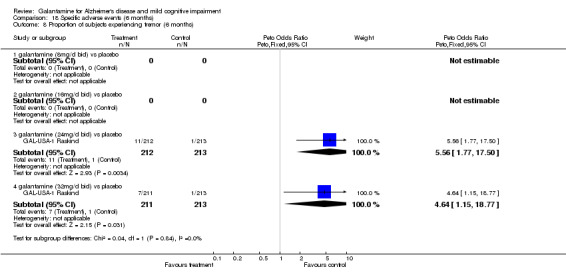
Comparison 18 Specific adverse events (6 months), Outcome 8 Proportion of subjects experiencing tremor (6 months).
18.9. Analysis.

Comparison 18 Specific adverse events (6 months), Outcome 9 Proportion of subjects experiencing agitation (6 months).
18.10. Analysis.
Comparison 18 Specific adverse events (6 months), Outcome 10 Proportion of subjects experiencing headache (6 months).
Comparison 19. Proportion of subjects deceased.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of subjects deceased ( 3 months) | 2 | 940 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.10, 1.95] |
| 1.1 galantamine (24‐32mg/d bid) vs placebo | 1 | 386 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.00, 1.98] |
| 1.2 galantamine (32mg/d tds) vs placebo | 1 | 554 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.14, 7.25] |
| 2 Proportion of subjects deceased ( 6 months) | 4 | 4286 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.50, 1.78] |
| 2.1 galantamine (16‐24mg/d bid ) vs placebo | 1 | 651 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.06, 15.91] |
| 2.2 galantamine (16‐24mg/d Prolonged Release ) vs placebo | 1 | 644 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.06 [0.32, 29.54] |
| 2.3 galantamine (24mg/d bid) vs placebo | 3 | 1576 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.35, 1.98] |
| 2.4 galantamine (32mg/d bid or tds) vs placebo | 1 | 424 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.04 [0.12, 75.12] |
| 2.5 galantamine (8mg/d bid) vs placebo | 1 | 426 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.06, 4.58] |
| 2.6 galantamine (16mg/d bid) vs placebo | 1 | 565 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.17, 3.46] |
| 3 Proportion of subjects deceased ( 24 months in MCI) | 2 | 1903 | Odds Ratio (M‐H, Fixed, 95% CI) | 9.33 [1.72, 50.54] |
| 3.1 galantamine (16‐24mg/d bid ) vs. placebo in MCI | 2 | 1903 | Odds Ratio (M‐H, Fixed, 95% CI) | 9.33 [1.72, 50.54] |
19.1. Analysis.

Comparison 19 Proportion of subjects deceased, Outcome 1 Proportion of subjects deceased ( 3 months).
19.2. Analysis.

Comparison 19 Proportion of subjects deceased, Outcome 2 Proportion of subjects deceased ( 6 months).
19.3. Analysis.

Comparison 19 Proportion of subjects deceased, Outcome 3 Proportion of subjects deceased ( 24 months in MCI).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
GAL‐93‐01 Wilkinson.
| Methods | Randomized Double‐blind Parallel‐group Placebo‐controlled Duration: 12 weeks | |
| Participants | Country: UK No. of Centers: 8 Diagnosis: Probable Alzheimer's disease defined by: NINCDS‐ADRDA. Inclusion: MMSE 13‐24, presence of a caregiver. Exclusion: Failure to provide informed consent; presence of any condition likely to interfere with the trial; use of antidepressants, antipsychotic drugs, antiparkinsonian drugs, insulin, anticonvulsants, sedatives, antihypertensive agents (except ACE inhibitors and diuretics), other centrally acting cholinergic or anticholinergic agents (except inhaled drugs for asthma). Total No. of patients: 285 Sex: 42.3% male Age: [placebo 74.2 (+/‐0.9)] [galantamine 24mg 72.9 (+/‐1.1)] [galantamine 24mg 72.9 (+/‐1.1)] [galantamine 36mg 75.4 (+/‐1.0)] | |
| Interventions | Route: oral Treatment: galantamine 6mg t.i.d. galantamine 8mg t.i.d. galantamine 12mg t.i.d. Treatment commenced at 4mg b.i.d. and was progressively increased every several days and then weekly by to assigned maximum dose (5, 8, and 14 days respectively). Control: Placebo t.i.d. | |
| Outcomes | ADAS‐cog (Alzheimer's Disease Assessment Scale, Cognitive subscale/11 item) CIBIC‐Plus (Clinician Interview Based Impression of Change) IADL (Instrumental Activities of Daily Living) PDS‐1 (Progressive Deterioration Scale) | |
| Notes | No. excluded after randomization: 81 No. not included in analysis: 81 excluded from completer analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
GAL‐95‐05.
| Methods | Randomized Double‐blind Parallel‐group Placebo‐controlled Duration: 29 weeks | |
| Participants | Country: UK, Finland, Denmark, France, Belgium, Germany, Netherlands No. of Centers:73 Diagnosis: Probable Alzheimer's Disease defined by: DSMIV and NINCDS‐ADRDA. Inclusion: MMSE 12‐24, age 45 or greater, Hachinski ischaemic score <4, consent, responsible caregiver Exclusion: other neurodegenerative disorders, secondary causes of dementia, co‐exisiting medical conditions, concurrent medications including psychotropic , cognitive enhancers & others Total No. of patients: 554 Sex: 38.3% male Age: 72.9+/‐8.5 | |
| Interventions | Route: oral Treatment: galantamine HBr 40mg (glantamine 32mg/d) Treatment commenced at 8mg/d and was progressively increased weekly by 8mg/d for two weeks (16mg/d, 24mg/d), then raised by 4mg/d at week 4 (28mg/d) then to assigned maximum dose at week 5. Control: Placebo t.i.d. | |
| Outcomes | EURO‐ADAS‐cog CIBIC‐Plus NOSGER (Nurses' Observation Scale for Geriatric Patients) | |
| Notes | No. excluded after randomization: 133 No. not included in analysis: Not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
GAL‐INT‐1 Wilcock.
| Methods | Randomized Double‐blind Parallel‐group Placebo‐controlled, with 4‐week placebo run‐in Duration: 26 weeks | |
| Participants | Country: 8 European No. of Centers: 86 Diagnosis: At least 6 month history of progressive cognitive decline, Senile Dementia Alzheimer's Type defined by: NINCDS‐ADRDA. Inclusion: MMSE score of 11 to 24, ADAS‐cog score > 11; CT or MRI < 12 months previously with no evidence of multi‐infarct dementia or active cerebrovascular disease; responsible caregiver; discontinued from antidementia medications; discontinued where possible form anticholinergic or cholinomimetic agents. Exclusion: Past cholinesterase inhibitor use; uncontrolled hypertension, heart failure, type II diabetes mellitus, hypothyroidism; other neurodegenerative disorders; cardiovascular disease that would affect completion of the trial; clinically significant psychiatric, hepatic, renal, pulmonary, metabolic, endocrine conditions; urinary outflow obstruction; active peptic ulcer; history of epilepsy, significant substance abuse. Total No. of patients: 653 Sex: Not stated. Age: [placebo 72.7 (7.6)] [galantamine 24mg 71.9 (8.3)] [galantamine 32mg 72,1 (8.6)] | |
| Interventions | Route: oral Treatment: galantamine 12mg b.i.d. galantamine 16mg b.i.d. Treatment commenced at 4mg b.i.d. and was progressively increased weekly by 8mg/d to assigned maximum dose. Control: Placebo b.i.d. | |
| Outcomes | ADAS‐cog ADCS‐CGIC Expanded ADAS‐cog DAD (Disability Assessment for Dementia) | |
| Notes | No. excluded after randomization: 128 No. not included in analysis: 128 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
GAL‐INT‐10 Brodaty.
| Methods | Randomized double‐blind parallel, 3‐group, active & placebo‐controlled trial, with 4‐week placebo run‐in Duration: 7 months | |
| Participants | Country: 5, international No. of Centres: 93 Diagnosis: At least 6 month history of progressive cognitive decline, mild to moderate probable Alzheimer's Disease defined by: NINCDS‐ADRDA. Inclusion: MMSE 10‐24 and ADAS‐cog/11>=18, living with or regularly visited daily by respobsible caregiver >=5 days/week Exclusion: other neurodegenerative disorders or cognitive impairment due to other identifiable causes; vascular dementia or clinically active cerebrovascular disease; epilepsy or significant psychiatric disease or a range of other medical illnesses; use of any dementia treatment agents, recently commenced or dose‐adjusted Vitamin E, chronic NSAID or COX‐2 inhibitor use; or use of a range of other medications. Total No. of Patients: 971 Sex: Male 36% Age: [placebo 76.3 (SD8.0)] [galantamine 76.5 (7.8)] [galantamine prolonged‐release 76.6 (7.6)] | |
| Interventions | Route: oral Treatment groups: galantamine & prolonged‐ release galantamine groups were both titrated from an initial dose of 8mg/d for the first 4 weeks to a maximum daily dose of 16 or 24 mg/d, by week 12, depending of safety & tolerability. Galantamine was administered in a twice daily regime, and the controlled‐release group was given placebo doses in the evening to maintain blinding. Placebo control: placebo b.i.d. | |
| Outcomes | Primary outcomes: Change from baseline at 26 weeks in‐ ADAS‐cog/11 (Alzheimer's Disease Assessment Scale, Cognitive subscale/11 item) CIBIC‐Plus (Clinician Interview Based Impression of Change) Key secondary outcomes: Change from baseline at 26 weeks in: ADCS‐ADL NPI | |
| Notes | No. not receiving treatment: 1/320 prolonged release galantamine, 1/327 galantamine, 4/324 placebo No. withdrawn: 68/320 prolonged release galantamine, 75/327 galantamine, 54/324 placebo No. with outcome data at end of trial: ADAS‐cog: 240/320 prolonged release galantamine, 227/327 galantamine, 248/324 placebo CIBIC‐plus: 246/320 prolonged release galantamine, 240/327 galantamine, 259/324 placebo No. with safety data at end of trial: 319/320 prolonged release galantamine, 326/327 galantamine, 320/324 placebo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
GAL‐INT‐11 DeKosky.
| Methods | Randomised double‐blind parallel group placebo‐controlled trial Duration: 24 months | |
| Participants | Country: 10, international No. of centres: not stated Diagnosis: men or women outpatient >= 50 years of age with gradual clinical decline of cognitive ability consistent with Mild Cognitive Impairment (CDR=0.5, memory score >=0.5), impairment of activities of daily living insufficient for diagnosis of dementia, and a New York University Paragarph Recall test with delayed recall score<=10. Exclusion: Not stated Total No. of patients: 995 randomized Sex: Not stated Age: >= 50 years of age | |
| Interventions | Route: oral Treatment: galantamine was titrated from an initial dose of 8mg/d (4mg bd), to a final dose of 16‐24mg/d, at month 3, as a twice‐daily regime (8‐12mg bd) Control: placebo bd | |
| Outcomes | Primary efficacy outcome at 12 months: ADAS‐cog/MCI CDR‐SB Primary efficacy outcome at 24 months: Number and percentage of subjects converting from MCI to dementia (CDR>1.0) at 24 months Rate of brain/ hippocampal atrophy was also asssessed by MRI measurement | |
| Notes | 898/995 subjects analyzed for efficacy, 990/995 for safety A retreived dropout study (GAL‐COG‐3002) in progress | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
GAL‐INT‐18 Winblad.
| Methods | Randomised double‐blind parallel group placebo‐controlled trial Duration: 24 months | |
| Participants | Country: 8, international No. of centres: not stated Diagnosis: men or women outpatient >= 50 years of age with gradual clinical decline of cognitive ability consistent with Mild Cognitive Impairment (CDR=0.5, memory score >=0.5), impairment of activities of daily living insufficient for diagnosis of dementia, and a New York University Paragarph Recall test with delayed recall score<=10. Exclusion: Not stated Total No. of patients: 1062 randomized Sex: Not stated Age: >= 50 years of age | |
| Interventions | Route: oral Treatment: galantamine was titrated from an initial dose of 8mg/d (4mg bd), to a final dose of 16‐24mg/d, at month 3, as a twice‐daily regime (8‐12mg bd) Control: placebo bd | |
| Outcomes | Primary efficacy outcome at 12 months: ADAS‐cog/MCI CDR‐SB Primary efficacy outcome at 24 months: Number and percentage of subjects converting from MCI to dementia (CDR>1.0) at 24 months Rate of brain/ hippocampal atrophy was also asssessed by MRI measurement | |
| Notes | 1019/1062 subjects analyzed for efficacy, 1058/1062 for safety A retreived dropout study (GAL‐COG‐3002) in progress | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
GAL‐INT‐2 Rockwood.
| Methods | Randomized Double‐blind Parallel‐group, with 4‐week placebo run‐in Placebo‐controlled Duration: 12 weeks | |
| Participants | Country: International No. of Centers: 43 Diagnosis: At least 6 month history of progressive cognitive decline, Senile Dementia Alzheimer's Type defined by: NINCDS‐ADRDA. Inclusion: MMSE score of 11 to 24, ADAS‐cog score > 11; CT or MRI < 12 months previously with no evidence of multi‐infarct dementia or active cerebrovascular disease; regular contact with responsible caregiver; discontinued from antidementia medications; discontinued where possible form anticholinergic or cholinomimetic agents. Exclusion: Past cholinesterase inhibitor use; uncontrolled hypertension, congestive heart failure, type II diabetes mellitus, hypothyroidism; other neurodegenerative disorders; cardiovascular disease that would affect completion of the trial; clinically significant psychiatric, hepatic, renal, pulmonary, metabolic, endocrine conditions; urinary outflow obstruction; active peptic ulcer; history of epilepsy, significant substance abuse. Total No. of patients: 386 randomized Sex: [placebo 46.4% male] [galantamine 24‐32mg 43.3% male] Age: [placebo 74.6 (0.7)] [galantamine 24‐32mg 75.2 (0.45)] | |
| Interventions | Route: oral Treatment: galantamine 12mg b.i.d. galantamine 16mg b.i.d. Treatment commenced at 4mg b.i.d. and was progressively increased weekly by 8mg/d to 12 mg/ b.i.d. Investigator could increase dose to 16mg b.i.d. at end of week 3 or maintain at 12mg b.i.d. At week 4, dose could be kept at 16mg b.i.d. or reduced to 12mg b.i.d. Control: Placebo b.i.d. | |
| Outcomes | ADAS‐cog ADCS‐CGIC Expanded ADAS‐cog DAD (Disability Assessment for Dementia) Neuropsychiatric Inventory (NPI) | |
| Notes | No. who failed to complete after randomization: 108 No. not included in OC analysis: 108 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
GAL‐INT‐6Erkinjuntti.
| Methods | Randomized Double‐blind Parallel‐group Placebo‐controlled, with 4‐week placebo run‐in Duration: 7 months | |
| Participants | Country: Canada, Denmark, Finland, France, Germany, Ireland, Israel, Netherlands, Poland, UK; No. of centres: 66 Diagnosis: Probable vascular dementia according to NINDS‐AIREN or possible Alzheimer's disease according to NINCDS‐ADRDA with MMSE 10‐25 and radiological evidence of significant cerebrovascular disease; Exclusion: other neurodegenerative disorders, secondary causes of dementia, co‐exisiting medical conditions, significant cardiovascular disease, concurrent medications including dementia treatment, NSAID & others Total No. of patients: [592 randomized] [possible Alzheimer's disease with cerebrovascular disease‐285 randomized] Sex: [placebo 54% male] [galantamine 24mg 52% male] Age: [placebo 75.2 (7.3)] [galantamine 24mg 75.0 (6.84)]; efficacy data used in analysis represents the possibly Alzheimer's Disease with cerebrovascular disease subgroup | |
| Interventions | Route: oral Treatment: galantamine 12mg b.i.d. Treatment commenced at 4mg daily and was increased weekly by 4mg/d to 12mg b.i.d. Control: Placebo b.i.d. | |
| Outcomes | ADAS‐cog CIBIC‐plus Response rate Expanded ADAS‐cog DAD NPI | |
| Notes | No. lost to follow‐up (post randomization): 135 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
GAL‐USA‐1 Raskind.
| Methods | Randomized Double‐blind Parallel‐group Placebo‐controlled, with 4‐week placebo run‐in Duration: 26 weeks/ 6 months | |
| Participants | Country: USA No. of Centers: 33 Diagnosis: Senile Dementia Alzheimer's Type defined by: NINCDS‐ADRDA. Inclusion: MMSE score of 11 to 24 inclusive, ADAS‐cog score > 11; responsible caregiver; free for 30 days of medications indicated for dementia (3 months for cholinesterase inhibitors); written informed consent by patient or appropriate representative. Exclusion: Uncontrolled hypertension, heart failure, type II diabetes mellitus, hypothyroidism; other neurodegenerative disorders; cardiovascular disease that would affect completion of the trial; clinically significant psychiatric, hepatic, renal, pulmonary, metabolic, endocrine conditions; urinary outflow obstruction; active peptic ulcer; history of epilepsy, significant substance abuse. Total No. of patients: 636 Sex: 242 males. Age: 70.3 +/‐ 1.6 to 71.1 +/‐ 1.5 (broken down by treatment group) | |
| Interventions | Route: oral Treatment: galantamine 12mg b.i.d. galantamine 16mg b.i.d. Treatment commenced at 4mg b.i.d. and was increased weekly by 8mg/d to assigned maximum dose. Control: Placebo b.i.d. | |
| Outcomes | ADAS‐cog ADCS‐CGIC DAD (Disability Assessment for Dementia) | |
| Notes | No. excluded after randomization: 198 No. not included in observed case analysis: 198 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
GAL‐USA‐10 Tariot.
| Methods | Randomized Double‐blind Parallel‐group Placebo‐controlled, with 4‐week placebo run in Duration: 5 months | |
| Participants | Country: United States No. of Centers: Unstated Diagnosis: At least 6 month history of progressive cognitive decline, Senile Dementia Alzheimer's Type defined by: NINCDS‐ADRDA. Inclusion: MMSE score of 10 to 22, ADAS‐cog score > 17; CT or MRI < 12 months previously with no evidence of multi‐infarct dementia or active cerebrovascular disease; responsible caregiver; free for 30 days of medications indicated for dementia; free for 60 days for cholinomimetic agents. Exclusion: Uncontrolled hypertension, heart failure, type II diabetes mellitus, hypothyroidism; other neurodegenerative disorders; cardiovascular disease that would affect completion of the trial; clinically significant psychiatric, hepatic, renal, pulmonary, metabolic, endocrine conditions; urinary outflow obstruction; active peptic ulcer; history of epilepsy, significant substance abuse. Total No. of patients: 978 Sex: 353 males Age: 76.0 +/‐ 0.6 to 77.7 +/‐ 0.4 | |
| Interventions | Route: oral Treatment: galantamine 4mg b.i.d. galantamine 8mg b.i.d. galantamine 12mg b.i.d. Treatment commenced at 8mg/d and was increased 8mg/d every 4 weeks until the target dose had been reached. Control: Placebo b.i.d. | |
| Outcomes | ADAS‐cog ADCS‐CGIC ADCS‐ADL (Alzheimer's Disease Cooperative Study Activities of Daily Living), NPI (Neuropsychiatric Inventory) | |
| Notes | No. excluded after randomization: 199 No. not included in observed cases analysis: 199 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Kewitz 1994b.
| Methods | Double‐blind Parallel‐group Placebo‐controlled, with initial single‐blind galantamine treatment (unstated duration) Duration: 13 weeks | |
| Participants | Country: Unstated No. of Centers: Unstated Diagnosis: mild to moderately severe primary degenerative dementia Inclusion: Unstated Exclusion: Unstated Total No. of patients: 95 Sex: Unstated Age: Range = 60‐87 | |
| Interventions | Route: oral Treatment: galantamine 10mg b.i.d. increased up to 50mg/d during first 3‐weeks. Control: Placebo b.i.d. | |
| Outcomes | ADAS‐cog, CGIC | |
| Notes | No. excluded after randomization: Not stated. No. not included in analysis: Not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anon 2001f | Commentary on GAL‐INT6 |
| Anon 2001g | Commentary on GAL‐INT6 |
| Bickel 1991 | Pharmacokinetics study |
| Bores 1994 | Pharmacokinetics study |
| Brashear 2003 | GAL‐INT‐6 |
| Brodaty 1996 | Review article |
| Bullock 2001 | Conference abstract‐ preliminary data from GAL‐INT‐6 |
| Bullock 2004 | Open label extension to GAL‐INT‐6 |
| Burke 2002 | Conference abstract describing NPI from GAL‐USA‐10 & GAL‐INT‐6 |
| Caro 2002 | Post hoc, subgroup, or selected combined exploratory analyses |
| Clegg 2001 | HTA report covering all three cholinesterase inhibitors |
| Clegg 2002 | Summary of HTA report (Clegg 2001) |
| Corey 2003 | Review only |
| Coyle 2001 | Review only |
| Cummings 2003 | Review only |
| Cummings 2004 | post hoc, subgroup, or selected combined exploratory analyses |
| Dal Bianco 1991 | Not placebo‐controlled |
| Dengiz 2004 | Review only |
| Doran 2003 | Randomised different washout periods before switching from Donepezil to Galantamine; no results on register, not on Medline. |
| Erkinjuntti 2002a | Reply to Van Gool re GAL‐INT‐6 |
| Erkinjuntti 2002b | Letter summarising GAL‐INT‐6 |
| Erkinjuntti 2003 | Open‐labelled 6 month extension to GAL‐INT‐6 |
| Fulton 1996 | Review article |
| GAL‐COG‐3002 2005 | Pooled MCI mortality data from different trials‐ referred to in Discussion/ Other references |
| GAL‐MCI‐301 2004 | Open‐label extension to MCI studies‐ stopped |
| Galasko 2004 | post hoc, subgroup, or selected combined exploratory analyses |
| Gold 2004a | Superceded by Brodaty 05 |
| Gold 2004b | Conference abstract only: GAL MCI trial baseline data |
| Hager 2004 | Conference abstract only: open‐label uncontrolled study |
| Haworth 2003b | Rater‐blinded randomised trial of donepezil vs. galantamine. Likely to have been reported as Wilcock03 in Drugs Aging. |
| Janssen 2005 | Prescription information only. |
| Jones 2004 | GAL vs. Donepezil‐ RCT but open‐labelled |
| Kertesz 2002 | Comment on GAL‐INT‐6 |
| Kewitz 1994 | Not double‐blind |
| Kewitz 1997 | Pharmacokinetics study |
| Kurz 1998 | GAL‐INT‐6 |
| Kurz 2002 | Non‐cognitive outcome of GAL‐INT‐6 |
| Kurz 2003 | Open‐label extension to GAL‐INT‐6 |
| Kurz 2004 | Conference abstract only, two Donepezil to GAL switching studies: randomizing washout period and galantamine dose |
| Lilienfeld 2001 | Conference abstract on additional outcome (carer time requirement) from GAL‐INT‐1 |
| Lyketsos 2002 | Conference abstract reporting serial ADAS‐cog up to 18.5 months from extension studies (from which 2 RCTs: unclear) |
| Lyketsos 2004 | Open‐label extension to Raskind and Tariot |
| MacGowan 1998 | Not placebo‐controlled |
| Marder 2002 | Comment on GAL‐INT‐6 |
| Markowitz 2003 | post hoc, subgroup, or selected combined exploratory analyses |
| Mintzer 2000 | Preliminary conference abstract for Mintzer 2003 (see 'other references: additional references') |
| Mintzer 2001 | Conference abstract comparing uncontrolled open label data at twelve months against historical controls |
| Moretti 2002 | Comment on GAL‐INT‐6 |
| Morris 2002 | Conference abstract: 18.5 month open label extension study of GAAL‐USA‐10 and another unspecified trial |
| Mucke 1997 | Review article. Preclinical studies |
| Nordberg 1998 | Review article. Pharmacokinetics study |
| Novak 2004 | Conference abstract only: effect of MCI clinical subtype on GAL efficacy‐ outcome not stated in abstract |
| Nye 2004 | Conference abstract only: effect of ApoE genotype on GAL efficacy‐ outcome not stated in abstract |
| Orgogozo 2004 | Superceded by subsequent paper in Current Medical Research & Opinion, which will be included under 'Other References' |
| Paskov 1974 | Subjects did not have a diagnosis of dementia. Study was examining sexual function |
| Patterson 2002 | Conference abstract: six month open label study (N=36) |
| Ping 2000 | Randomised against Huperzine |
| Rabheru 2004 | Conference abstract only, pooling behavioral outcome from 3 RCTs (details not stated) |
| Rainer 1993 | Abstract with insufficient data |
| Rainer 1994 | Single blind trial, not placebo controlled |
| Rainer 1997a | Review article |
| Rainer 1997b | Review article |
| Rainer 2001 | Cross sectional study assessing cognition post drug cessation; only 5 patients on galantamine |
| Raskind 2000 | Conference abstract reporting GAL‐USA‐1 |
| Riemann 1994a | Subjects did not have a diagnosis of dementia. Outcome measure was sleep pattern. |
| Riemann 1994b | Review article on sleep and cholinergic function. |
| Sano 2003b | post hoc, subgroup, or selected combined exploratory analyses |
| Scheltens 2004 | Conference abstract only: atrophy as outcome in MCI trial‐ methodology only: outcome not stated in abstract |
| Schwalen 2004a | Conference abstract only: pooled efficacy data for patients with baseline ADAS‐cog>30 pooled from 6 studies (details not in abstract), examining continuous vs. interrupted vs. delayed treatment. |
| Schwalen 2004b | Conference abstract only: post‐hoc analysis of pooled data for patients with baseline MMSE <18 randomised to 24mg or 16mg/d |
| Scott 2000 | Review only |
| Small 2003 | Posthoc analysis of GAL‐INT‐6 plus open‐label extension |
| Snorrason 1996 | Subjects did not have a diagnosis of dementia. Not a clinical trial |
| Steiger‐Baechler 200 | Case description of cognition in 7 patients post cholinesterase inhibitor cessation‐ none on galantamine |
| Tariot 2000 | Preliminary conference abstract for GAL‐USA‐10 Tariot |
| Thomsen 1990 | Not a clinical trial |
| Thomsen 1990a | Not a randomized clinical trial |
| Thomsen 1990b | Not a clinical trial |
| Truyen 2000a | summary presentation of earlier galantamine findings |
| Truyen 2000b | summary presentation of earlier galantamine findings |
| Truyen 2001 | specific data not included in abstract |
| van Gool 2002 | Comment on GAL‐INT‐6 |
| Vellas 2004 | Conference abstract only: Pooled data on changes in DAD score/ADL from 6 RCTs |
| Wasielewski 1997 | Review article |
| Wilcock 1993 | Not placebo‐controlled |
| Wilcock 2000i | Conference abstract on additional outcome (carer time requirement) from RCTs, referenced to Blesa 2000. |
| Wilcock 2000j | Conference abstract comparing uncontrolled open label data at twelve months against historical controls. |
| Wilkinson 2000 | Conference abstract reporting GAL‐INT‐2 |
| Zarotsky 2003 | Review only |
Characteristics of ongoing studies [ordered by study ID]
Galantamine CFIDS.
| Trial name or title | Not reported |
| Methods | |
| Participants | Adults with Chronic Fatigue Syndrome |
| Interventions | Galantamine‐‐dose not reportedPlaceboDuration: 4 monthsSites: 12Total N sought: 140 |
| Outcomes | Not Reported |
| Starting date | Jan 29 1999 |
| Contact information | |
| Notes | When completed, study will be excluded due to no AD subjects |
Wilcock.
| Trial name or title | The safety and efficacy of Galanthamine in the treatment of vascular and mixed dementia |
| Methods | |
| Participants | Patients with vascular and mixed vascular/alzheimer's dementia |
| Interventions | Galanthamine‐‐dosage not reportedPlacebo |
| Outcomes | Not reported |
| Starting date | 1 Oct 1998 |
| Contact information | Professor G WilcockDay HospitalFrenchay HospitalBeckspool RdFrenchayBristolBS16 1NDUKTel: 0117 970 1212 |
| Notes | When completed, study will be excluded due to no AD patients enrolled. |
Contributions of authors
Clement Loy took over from Jason Olin as main reviewer for the updates of this review from November 2003. He dealt with article collection, in‐ and exclusion of new references, updated the data‐ analyses and rewrote the manuscript where necessary
Lon Schneider co‐wrote, reviewed article coding, and assisted with editing the manuscript, for the original review as well as all subsequent updates.
Consumer Editors: Enid Light and Mervyn Richardson Contact Editor: Leon Flicker
Sources of support
Internal sources
No sources of support supplied
External sources
National Institute of Mental Health (MH01368 (OLIN), MH19074, MH48759), USA.
National Institute on Aging (AG05142), USA.
NIMH Clinical Antipsychotic Trials of Interventions Effectiveness, USA.
Declarations of interest
Dr. Schneider and the University of Southern California have received clinical trials‐and other contracts from Janssen Pharmaceutica and Johnson and Johnson, Inc. Dr. Schneider has served as a consultant to Janssen, Pfizer, and Novartis, all manufacturers of cholinesterase inhibitors used to treat AD, and to Forest Pharmaceuticals, manufacturer of memantine used to treat AD, for which he received payments. Dr. Loy has received a Wellcome Trust Travelling Award in relation to his work with the Cochrane Collaboration.
Edited (no change to conclusions)
References
References to studies included in this review
GAL‐93‐01 Wilkinson {published and unpublished data}
- Wilcock G, Wilkinson D. Galanthamine hydrobromide: Interim results of a group comparative, placebo‐controlled study of efficacy and safety in patients with a diagnosis of senile dementia of the Alzheimer Type. In: Iqbal K, Winblad B, Nishimura T, Takeda M, Wisniewski HM editor(s). Alzheimer's Disease: Biology, Diagnosis and Therapeutics. John Wiley, 1997:661‐664. [Google Scholar]
- Wilkinson D, Murray J. Galantamine: a randomized, double‐blind, dose comparison in patients with Alzheimer's disease. International Journal of Geriatric Psychiatry 2001;16(9):852‐857. [DOI] [PubMed] [Google Scholar]
GAL‐95‐05 {unpublished data only}
- Kristensen M, Richardson A, Osselaer N, Vangeneugden T, Morrison S, Fleet D, Lilienfeld S, Truyen L, Parys W. A Europan multicentre placebo‐controlled trial to determine the safety and efficacy of galantamine hydrobromide 40mg/d (32mg/d GAL bas, tid dose regimen) in patients diagnosed with Alzheimer‐type dementia (GAL 95‐05). Janssen Research Foundation 1997.
GAL‐INT‐1 Wilcock {published data only}
- Wilcock GK. Erratum: Efficacy and safety of galantamine in patients with mild to moderate Alzheimer's disease: Multicentre randomised controlled trial (British Medical Journal (December 9)(1445‐1449)). BR‐MED‐J: British‐Medical‐Journal 2001;322(7278):90. [Google Scholar]
- Wilcock GK. GALANTAMINE ALLEVIATES CAREGIVER BURDEN IN ALZHEIMER'S DISEASE: A 6‐MONTH PLACEBO‐CONTROLLED STUDY. Conference Proceedings World Alzheimer Congress; 9‐13 July, 2000, Washington. 2000.
- Wilcock GK, Lilienfeld S, Gaens E on behalf of the Galantamine International‐1 Study Group. Efficacy and safety of galantamine in patients with mild to moderate Alzheimer's disease: a multicentre randomised controlled trial. British Medical Journal 2000;321:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
GAL‐INT‐10 Brodaty {published and unpublished data}
- Brodaty H, Corey‐Bloom J, Potocnik FCV, Truyen L, Gold M, Damaraju CRV. Galantamine Prolonged‐Release Formulation in the Treatment of Mild to Moderate Alzheimer's Disease. Dement Geriatr Cogn Disord 2005;20(2‐3):120‐132. [DOI] [PubMed] [Google Scholar]
GAL‐INT‐11 DeKosky {unpublished data only}
- GAl‐INT‐11. A Randomized Double‐Blind, Placebo‐Controlled Trial to Evaluate the Efficacy and Safety of Galantamine in Subjects With Mild Cognitive Impairment (MCI) Clinically at Risk for Development of Clinically Probable Alzheimer’s Disease. http://www.clinicalstudyresults.org/documents/ 2004.
GAL‐INT‐18 Winblad {unpublished data only}
- GAL‐INT‐18. A Randomized Double‐Blind, Placebo‐Controlled Trial to Evaluate the Efficacy and Safety of Galantamine in Subjects With Mild Cognitive Impairment (MCI) Clinically at Risk for Development of Clinically Probable Alzheimer’s Disease. http://www.clinicalstudyresults.org/documents/ 2004.
GAL‐INT‐2 Rockwood {unpublished data only}
- Rockwood K, Mintzer J, Truyen L, Wessel T, Wilkinson D, on behalf of the Galantamine International‐2 Study Group. Effects of a flexible galantamine dose in Alzheimer's disease: a randomized, controlled trial. Journal of Neurology, Neurosurgery and Psychiatry 2001;71(5):589‐595. [DOI] [PMC free article] [PubMed] [Google Scholar]
GAL‐INT‐6Erkinjuntti {published and unpublished data}
- Erkinjuntti T, Kurz A, Gauthier S, Bullock R, Lilienfeld S, Damaraju CV. Efficacy of galantamine in probable vascular dementia and Alzheimer's disease combined with cerebrovascular disease: a randomised trial. Lancet 2002;359:1283‐1290.. [DOI] [PubMed] [Google Scholar]
- Erkinjuntti T, Kurz A, Gauthier S, Bullock R, Lilienfeld S, Damaraju CV. The safety and efficacy of Galantamine in the treatment of vascular and mixed dementia (Double‐blind part only). (GAL‐INT‐6). Johnson and Johnson Pharmaceutical Research & Development. January 2004.
GAL‐USA‐1 Raskind {published data only}
- Raskind MA, Peskind ER, Wessel T, Yuan W, Galantamine USA‐1 Study Group. Galantamine in AD: A 6‐month randomized placebo‐controlled trial with a 6‐month extension. Neurology 2000;54(12):2261‐2268. [DOI] [PubMed] [Google Scholar]
GAL‐USA‐10 Tariot {published data only}
- Tariot PN, Solomon PR, Morris JC, Kershaw P, Lilienfeld S, Ding C, Galantamine USA‐10 Study Group. A 5‐month, randomized, placebo‐controlled trial of galamtine in AD. Neurology 2000;54(12):2269‐2276. [DOI] [PubMed] [Google Scholar]
Kewitz 1994b {published data only}
- Kewitz H, Berzewski H, Rainer M, Dal‐Bianco P, Friedl E, Deisenhammer J, Urbelhack R, Engel R, Lee C, Hartung U. Galantamine, a selective non‐toxic acetylcholinesterase inhibitor is significantly superior over placebo in treatment of SDAT. Neuropsychopharmacology 1994;10(3S):130S. [Google Scholar]
References to studies excluded from this review
Anon 2001f {published data only}
- Anon. New Alzheimer's drug is first therapy to show efficacy in vascular dementia. Formulary 2001f;36(8):569. [Google Scholar]
Anon 2001g {published data only}
- Anon. Galantamine effective in treating dementia in patients with cerebrovascular disease. Pharmaceutical Journal 2001g;266(7153):842. [Google Scholar]
Bickel 1991 {published data only}
- Bickel U, Thomsen T, Weber W, Fischer JP, Bachus R, Nitz M, Kewitz H. Pharmacokinetics of galanthamine in humans and corresponding cholinesterase inhibition. Clin Pharmacol Ther 1991;50:420‐8. [DOI] [PubMed] [Google Scholar]
Bores 1994 {published data only}
- Bores GM, Huger F, Petko W, Mutlib A, Camacho F, Selk D, Taberna V, Kosley R, Davis L, Vargas H. Evaluation of P11012 and P11149 for the treatment of Alzheimer's disease. Third International Springfield Symposium on Advances in Alzheimer's Therapy. Springfield IL, May 11‐15, 1994:114.
Brashear 2003 {published data only}
- Brashear HR. Galantamine in the treatment of vascular dementia. International Psychogeriatrics 2003;15(Suppl 1):187‐93. [DOI] [PubMed] [Google Scholar]
Brodaty 1996 {published data only}
- Brodaty H. Galanthamine. Drugs and Aging 1996;9(1):66‐67. [DOI] [PubMed] [Google Scholar]
Bullock 2001 {published data only}
- Bullock R, Lillenfeld S. Galantamine shows promising results in alzheimer's disease with cerebrovascular components and probable vascular dementia (preliminary results). Journal of Neuroscience 2001;187(Suppl 1):S59. [Google Scholar]
Bullock 2004 {published data only}
- Bullock R, Erkinjuntti T, Lilienfeld S, GAL INT 6 Study Group. Management of patients with Alzheimer's disease plus cerebrovascular disease: 12‐month treatment with galantamine. Dementia and Geriatric Cognitive Disorders 2004;17(1‐2):29‐34. [DOI] [PubMed] [Google Scholar]
Burke 2002 {published data only}
- Burke W, Lilienfeld S. Galantamine improves behaviour and relieves caregiver distress in Alzheimer's disease (AD), vascular dementia and AD with cerebrovascular disease. Proceedings of the 8th International Conference on Alzheimer's Disease and Related Disorders; 2002 July 20‐25, Stockholm, Sweden. 2002:Abstract No 428.
Caro 2002 {published data only}
- Caro J, Ward A, Ishak K, Migliaccio Walle K, Getsios D, Papadopoulos G, Torfs K. To what degree does cognitive impairment in Alzheimer's disease predict dependence of patients on caregivers?. BMC neurology [electronic resource] 2002;2(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clegg 2001 {published data only}
- Clegg A, Bryant J, Nicholson T, McIntyre L, Broe S, Gerard K, Waugh N. Clinical and cost effectiveness of donepezil rivastigmine and galantamine for Alzheimer's disease a rapid and systematic review. Health Technology Assessment 2001;5(1):i‐iv+1‐128. [DOI] [PubMed] [Google Scholar]
Clegg 2002 {published data only}
- Clegg A, Bryant J, Nicholson T, McIntyre L, Broe S, Gerard K, Waugh N. Clinical and cost‐effectiveness of donepezil, rivastigmine, and galantamine for Alzheimer's disease: a systematic review. International Journal of Technology Assessment in Health Care 2002;18(3):497‐507. [DOI] [PubMed] [Google Scholar]
Corey 2003 {published data only}
- Corey Bloom J. Galantamine: a review of its use in Alzheimer's disease and vascular dementia. International Journal of Clinical Practice 2003;57(3):219‐23. [PubMed] [Google Scholar]
Coyle 2001 {published data only}
- Coyle J, Kershaw P. Galantamine, a cholinesterase inhibitor that allosterically modulates nicotinic receptors: Effects on the course of Alzheimer's disease. Biological Psychiatry 2001;49(3):289‐99. [DOI] [PubMed] [Google Scholar]
Cummings 2003 {published data only}
- Cummings JL. Use of cholinesterase inhibitors in clinical practice: evidence‐based recommendations. American journal of geriatric psychiatry official journal of the American Association for Geriatric Psychiatry, The 2003;11(2):131‐45. [PubMed] [Google Scholar]
Cummings 2004 {published data only}
- Cummings JL, Schneider L, Tariot PN, Kershaw PR, Yuan W. Reduction of behavioral disturbances and caregiver distress by galantamine in patients with Alzheimer's disease. American Journal of Psychiatry 2004;161(3):532‐8. [DOI] [PubMed] [Google Scholar]
Dal Bianco 1991 {published data only}
- Dal Bianco P, Maly J, Woeber C, Lind C, Koch G, Hufgard J, Marsehall I, Mraz M, Deecke L. Galanthamine treatment in Alzheimer's disease. J Neurol Transm Suppl 1991;33:59‐63. [DOI] [PubMed] [Google Scholar]
Dengiz 2004 {published data only}
- Dengiz AN, Kershaw P. The clinical and safety of galantamine in the treatment of alzheimer's disease. CNS Spectrums 2004;9(5):377‐92. [DOI] [PubMed] [Google Scholar]
Doran 2003 {published data only}
- Doran MD. A randomised 26 week double‐blind placebo controlled trial to evaluate the safety and efficacy of galantamine in the treatment of dementia secondary to cerebrovascular disease. National Research Register 2003.
Erkinjuntti 2002a {published data only}
- Erkinjuntti T, Kurz A, Gauthier S, Bullock R, Lilienfeld S, Damaraju CV. "Efficacy of galantamine in probable vascular dementia and Alzheimer's disease combined with cerebrovascular disease: A randomised trial": Reply. Lancet 2002;360(9344):1513. [DOI] [PubMed] [Google Scholar]
Erkinjuntti 2002b {published data only}
- Erkinjuntti T. Broad therapeutic benefits in patients with probable vascular dementia or Alzheimer's disease with cerebrovascular disease after treatment with galantamine. European Journal of Neurology 2002;9(5):545. [DOI] [PubMed] [Google Scholar]
Erkinjuntti 2003 {published data only}
- Erkinjuntti T, Kurz A, Small GW, Bullock R, Lilienfeld S, Damaraju CV. An open‐label extension trial of galantamine in patients with probable vascular dementia and mixed dementia. Clinical Therapeutics 2003;25(6):1765‐82. [DOI] [PubMed] [Google Scholar]
Fulton 1996 {published data only}
- Fulton B, Benfield P. Galanthamine. Drugs Aging 1996;9:60‐5. [DOI] [PubMed] [Google Scholar]
GAL‐COG‐3002 2005 {published data only}
- GAL‐COG‐3002. An Analysis of Mortality in Subjects Who Participated in Three Studies of Galantamine in Mild Cognitive Impairment (MCI). http://www.clinicalstudyresults.org/ 2005.
GAL‐MCI‐301 2004 {published data only}
- GAL‐MCI‐301. An Open‐Label Extension Study to Assess the Long‐Term Safety and Tolerability of Galantamine HBr in the Treatment of Mild Cognitive Impairment. http://www.clinicalstudyresults.org/drugdetails/ 2004.
Galasko 2004 {published data only}
- Galasko D, Kershaw PR, Schneider L, Zhu Y, Tariot PN. Galantamine maintains ability to perform activities of daily living in patients with Alzheimer's disease.. Journal of the American Geriatrics Society 2004;52(7):1070‐6. [DOI] [PubMed] [Google Scholar]
Gold 2004a {published data only}
- Gold M, Corey‐Bloom J, Yan B, Truyen L, Johnson & Johnson Pharmaceutical Research & Development, Titusville, NJ, USA. The Safety and Efficacy of an Extended‐Release Formulation of Galantamine (Reminyl ER) in the Treatment of Alzheimer's Disease. NeuroBiology of Aging 2004;25(S2):18. [Google Scholar]
Gold 2004b {published data only}
- Gold M, Goldstein HR, Johnson & Johnson Pharmaceutical Research and Development, LLC, Titusville, NJ, USA. Galantamine In The Treatment of Patients With Mild Cognitive Impairment: Baseline Demographics and Psychometric Testing Results. NeuroBiology of Aging 2004;25(S2):472. [Google Scholar]
Hager 2004 {published data only}
- Hager K, Schreiner A, Schmitt A. Long‐term treatment with galantamine slows the disease progression of patients with alzheimer's disease. The Journal of Nutrition, Health and Aging 2004;8(4):263. [Google Scholar]
Haworth 2003b {published data only}
- Haworth J. GAL‐INT‐11: a randomised placebo‐controlled trial to evaluate the efficacy and safety of galantamine in patients with minimal cognitive impairment (MCI) clinically at risk for development of probable Alzheimer's disease. National Research Register 2003b.
Janssen 2005 {published data only}
- Janssen Pharmaeuticals. Reminyl: tablets and oral solution. http://www.us.reminyl.com/ 2005.
Jones 2004 {published data only}
- Jones RW, Soininen H, Hager K, Aarsland D, Passmore P, Murthy A, Zhang R, Bahra R, DONGAL Study Group. A multinational, randomised, 12‐week study comparing the effects of donepezil and galantamine in patients with mild to moderate Alzheimer's disease. International Journal of Geriatric Psychiatry 2004;19(1):58‐67. [DOI] [PubMed] [Google Scholar]
Kertesz 2002 {published data only}
- Kertesz A. Efficacy of galantamine in probable vascular dementia and Alzheimer's disease combined with cerebrovascular disease: a randomized trial. Current Neurology & Neuroscience Reports 2002;2(6):503‐4. [DOI] [PubMed] [Google Scholar]
Kewitz 1994 {published data only}
- Kewitz H, Wilcock G, Davis B. Galanthamine in Alzheimer's disease. In: Giacobini E, Becker R editor(s). Alzheimer's disease: Therapeutic strategies. Boston: Birkhauser, 1994:140‐144. [Google Scholar]
Kewitz 1997 {published data only}
- Kewtiz H. Pharmacokinetics and metabolism of galanthamine. Drugs of Today 1997;33(4):265‐272. [Google Scholar]
Kurz 1998 {published data only}
- Kurz A. The safety and efficacy of galantamine in the treatment of vascular and mixed dementia (double‐blind part only) Protocol GAL‐INT‐6; phase 3. Johnson & Johnson Pharmaceutical research & development, L.L.C. 1998.
Kurz 2002 {published data only}
- Kurz A. Non‐cognitive benefits of galantamine (Reminyl(R)) treatment in vascular dementia. Acta Neurologica Scandinavica 2002;106(178 Suppl):19‐24. [DOI] [PubMed] [Google Scholar]
Kurz 2003 {published data only}
- Kurz AF, Erkinjuntti T, Small GW, Lilienfeld S, Damaraju CR. Long‐term safety and cognitive effects of galantamine in the treatment of probable vascular dementia or Alzheimer's disease with cerebrovascular disease. European journal of neurology the official journal of the European Federation of Neurological Societies 2003;10(6):633‐40. [DOI] [PubMed] [Google Scholar]
Kurz 2004 {published data only}
- Kurz AF, Gold M, Technische Universitaet Munchen, Munich, Germany. Tolerability, Safety and Efficacy In Patients With Alzheimer's Disease Who Are Switched From Donepezil to Galantamine. NeuroBiology of Aging 2004;25(S2):202. [Google Scholar]
Lilienfeld 2001 {published data only}
- Lilienfeld S, Papadopoulos G. Galantamine alleviates caregiver burden in Alzheimer's disease. Proceedings of the 14th Annual Meeting of the American Association for Geriatric Psychiatry; 2001 Feb 23‐26, San Francisco. 2001.
Lyketsos 2002 {published data only}
- Lyketsos K, Reichman W, Kershaw P. Long‐term cognitive benefits of galantamine in alzheimer disease using a responder analysis at 18.5 months. Proceedings of the 7th International Geneva/Springfield Symposium on Advances in Alzheimer therapy, 2002 Apr 3‐6, Geneva. 2002:214.
Lyketsos 2004 {published data only}
- Lyketsos CG, Reichman WE, Kershaw P, Zhu Y. Long‐term outcomes of galantamine treatment in patients with Alzheimer disease. American Journal of Geriatric Psychiatry 2004;12(5):473‐82. [DOI] [PubMed] [Google Scholar]
MacGowan 1998 {published data only}
- MacGowan SH, Wilcock GK, Scott M. Effect of gender and apolipoprotein E genotype on response to anticholinesterase therapy in Alzheimer's disease. Int J Geriat Psychiatry 1998;13:625‐30. [DOI] [PubMed] [Google Scholar]
Marder 2002 {published data only}
- Marder K. Efficacy of galantamine in probable vascular dementia and Alzheimer's disease combined with cerebrovascular disease: a randomized trial. Current Neurology and Neuroscience Reports 2002;2(5):389‐90. [DOI] [PubMed] [Google Scholar]
Markowitz 2003 {published data only}
- Markowitz JS, Gutterman EM, Lilienfeld S, Papadopoulos G. Sleep‐related outcomes in persons with mild to moderate Alzheimer disease in a placebo‐controlled trial of galantamine. Sleep 2003;26(5):602‐6. [DOI] [PubMed] [Google Scholar]
Mintzer 2000 {published data only}
- Mintzer JE, Yuan W, Kershaw P. Efficacy of galantamine in patients with alzheimer's disease (AD) with previous exposure to cholinesterase inhibitors. Proceedings of the 39th Annual Meeting of the American College of Neuropsychopharmacology (ACNP); 2000 Dec 10‐14, San Juan, Puerto Rico. 2000.
Mintzer 2001 {published data only}
- Mintzer J, Kershaw P. Galantamine provides cognitive and functional benefits over 12 months in patients with Alzheimer's disease. Proceedings of the 14th Annual Meeting of the American Association for Geriatric Psychiatry; 2001 Feb 23‐26, San Francisco. 2001.
Moretti 2002 {published data only}
- Moretti R, Torre P, Antonello RM, Cazzato G, Bava A. "Efficacy of galantamine in probable vascular dementia and Alzheimer's disease combined with cerebrovascular disease: A randomised trial": Comment. Lancet 2002;360(9344):1512‐3. [DOI] [PubMed] [Google Scholar]
Morris 2002 {published data only}
- Morris JC, Kershaw P. Cognitive benefits of long‐term, continuous galantamine treatment in patients with alzheimer disease. Proceedings of the 7th International Geneva/Springfield Symposium on Advances in Alzheimer therapy, 2002 Apr 3‐6, Geneva. 2002:220.
Mucke 1997 {published data only}
- Mucke HAM. Preclinical studies with galanthamine. Drugs of Today 1997;3(4):259‐264. [Google Scholar]
Nordberg 1998 {published data only}
- Nordberg A, Svensson AL. Cholinesterase inhibitors in the treatment of Alzheimer's disease: a comparison of tolerability and pharmacology. Drug Safety 1998;19:465‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Novak 2004 {published data only}
- Novak G, Johnson & Johnson Pharmaceutical Research and Development LLC, Raritan, NJ, USA. Galantamine inthe Treatment of Patients with MildCognitive Impairment: Effect ofDiagnostic Type at Baseline. NeuroBiology of Aging 2004;25(S2):474. [Google Scholar]
Nye 2004 {published data only}
- Nye JS, Gold M, Francke S, Goldstein HR, Fijal B, Truyen L, Cohen N, Johnson and Johnson Pharmaceutical Research and Development, LLC, Titusville, NJ, USA. Impact of ApoE Genotype on The Efficacy of Galantamine For The Treatment of Mild Cognitive Impairment. NeuroBiology of Aging 2004;25(S2):521. [Google Scholar]
Orgogozo 2004 {published data only}
- Orgogozo J, Small G, Hammond G, Schwalen S. Galantamine improves cognition and function in patients with very mild Alzheimer's disease. 8th Congress of the European Federation of the Neurological Sciences. Paris, France. September 4‐7, 2004. 2004.
Paskov 1974 {published data only}
- Paskov D, Traikov D. Treatment of the psychogenic form of sexual asthenia with Nivalin (Bulgarian). Savrmed 1974;25:30‐4. [Google Scholar]
Patterson 2002 {published data only}
- Patterson CE, Passmore AP, Quinlan M, Browne G, Thompson C. A 6 month open label study of the efficacy and tolerability of galantamine in patients with Alzheimers disease (AD). Proceedings of the 8th International Conference on Alzheimer's Disease and Related Disorders; 2002 July 20‐25, Stockholm, Sweden. 2002:Abstract No 342.
Ping 2000 {published data only}
- Ping G, JQingwen J, Xinsheng D, et al. [Observation of effect on Alzheimer disease treated by Galanthamine hydrobromide capules]. Practical Geriatrics 2000;14(6):307‐8. [Google Scholar]
Rabheru 2004 {published data only}
- Rabheru K, Binder C, Wang J, Herrmann N, Regional Mental Health Care, London, ON, Canada. Does Galantamine Therapy in Mild to Moderate Alzheimer's Disease Improve Behaviour?. NeuroBiology of Aging 2004;25(S2):189. [Google Scholar]
Rainer 1993 {published data only}
- Rainer M, Janoch P, Reiss A, et al. Galanthamine treatment in Alzheimer's Disease: a preliminary evaluation of forty patients of forty patients.. Canadian Journal of Neurological Sciences. 1993; Vol. 20, issue Suppl 4:S177.
Rainer 1994 {published data only}
- Rainer M. Galanthamine treatment in Alzheimer's disease ‐ The identification of responders. Neuropsychopharmacology. 1994; Vol. 10, issue 3S:215S.
Rainer 1997a {published data only}
- Rainer M. Clinical studies with galantamine. Drugs of Today 1997;33:273‐279. [Google Scholar]
Rainer 1997b {published data only}
- Rainer M. Galanthamine in Alzheimer's disease. A new alternative to tacrine?. CNS Drugs 1997;7(2):89‐97. [DOI] [PubMed] [Google Scholar]
Rainer 2001 {published data only}
- Rainer M, Mucke HA, Kruger Rainer C, Kraxberger E, Haushofer M, Jellinger KA. Cognitive relapse after discontinuation of drug therapy in Alzheimer's disease: cholinesterase inhibitors versus nootropics. Journal of neural transmission 2001;108(11):1327‐33. [DOI] [PubMed] [Google Scholar]
Raskind 2000 {published data only}
- Raskind M, Peskind E, Parys W, Wessel T. Galantamine produces long term cognitive and functional in patients with Alzheimer's disease. Proceedings of the 6th International Stockholm/Springfield Symposium on Advances in Alzheimer Therapy; 2000 Apr 5‐8, Stockholm, Sweden. 2000:225.
Riemann 1994a {published data only}
- Riemann D, Gann H, Dressing H, Muller WE, Aldenhoff JB. Influence of the cholinesterase inhibitor galanthamine hydrobromide on normal sleep. Psychiatry Res 1994;51:253‐67. [DOI] [PubMed] [Google Scholar]
Riemann 1994b {published data only}
- Riemann D, Hohagen F, Bahro M, Lis S, Stadmuller G, Gann H, Berger M. Cholinergic neurotransmission, REM sleep and depression. J Psychosom Res 1994;38(suppl 1). [DOI] [PubMed] [Google Scholar]
Sano 2003b {published data only}
- Sano M, Wilcock G K, Baelen B, Kavanagh S. The effects of galantamine treatment on caregiver time in Alzheimer's disease. International journal of geriatric psychiatry 2003b;18(10):942‐50. [DOI] [PubMed] [Google Scholar]
Scheltens 2004 {published data only}
- Scheltens P, Fox NC, Barkhof F, Gold M, VU Medical Center, Amsterdam, Netherlands. Effect of Galantamine Treatment on Brain Atrophy as Assessed by MRI in Patients with Mild Cognitive Impairment. NeuroBiology of Aging 2004;25(S2):270. [Google Scholar]
Schwalen 2004a {published data only}
- Schwalen S, Blesa R, Baelen B, Kavanagh S, Janssen‐Cilag GmBH. Effects of continuous, interrupted and delayed galantamine treatment of long‐term cognitive function in patients with advanced‐moderate alzheimer's disease. The Journal of Nutrition, Health and Aging 2004;8(4):262. [Google Scholar]
Schwalen 2004b {published data only}
- Schwalen S, Baelen B, Kavanagh S. Comparison of the effects of 24mg versus 16mg galantamine in patients with moderate alzheimer's disease. The Journal of Nutrition, Health and Aging 2004;8(4):261. [Google Scholar]
Scott 2000 {published data only}
- Scott LJ, Goa KL. Galantamine: a review of its use in Alzheimer's disease. Drugs 2000;60(5):1095‐122. [DOI] [PubMed] [Google Scholar]
Small 2003 {published data only}
- Small G, Erkinjuntti T, Kurz A, Lilienfeld S. Galantamine in the treatment of cognitive decline in patients with vascular dementia or Alzheimer's disease with cerebrovascular disease. CNS drugs 2003;17(12):905‐14. [DOI] [PubMed] [Google Scholar]
Snorrason 1996 {published data only}
- Snorrason E, Geirsson A, Stefansson K. Trial of a selective acetylcholinesterase inhibitor, galanthamine hydrobromide, in the treatment of chronic fatigue syndrome. J Chronic Fatigue Syndr 1996;2:35‐54. [Google Scholar]
Steiger‐Baechler 200 {published data only}
- Steiger‐Baechler G, Monsch AU, Bertoli S, Staehelin HB. Discontinuation of Acetylcholinesterase‐Inhibitors in Alzheimer's disease and itrs effects on cognitive functioning. unknown. 2002.
Tariot 2000 {published data only}
- Tariot P, Parys W, Kershaw P. The efficacy and tolerability of galantamine in alzheimer's disease a 5 month placebo controlled study with slow dose escalation. Neurology 2000;54(Suppl 3):A415. [Google Scholar]
Thomsen 1990 {published data only}
- Thomsen T, Kewitz H. Selective inhibition of human acetylcholinesterase by galanthamine in vitro and in vivo. Life Sci 1990;46:1553‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Thomsen 1990a {published data only}
- Thomsen T, Bickel U, Fischer JP, Kewitz H. Galantamine hydrobromide in a long‐term treatment of Alzheimer's disease. Dementia 1990;1:46‐51. [Google Scholar]
Thomsen 1990b {published data only}
- Thomsen T, Bickel U, Fischer JP, Kewitz H. Stereoselectivity of cholinesterase inhibition by galanthamine and tolerance in humans. Eur J Clin Pharmacol 1990;39:603‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Truyen 2000a {published data only}
- Truyen L. Galantamine‐New Clinical Studies. Proceedings of the Sixth International Stockholm/Springfield Symposium on Advances in Alzheimer's Disease. 2000:156.
Truyen 2000b {published data only}
- Truyen L. Clinical implications for galantamine's nicotinic receptor modulation. Proceedings of the Sixth International Stockholm/Springfield Symposium on Advances in Alzheimer's Disease. 2000:157.
Truyen 2001 {published data only}
- Truyen L. Galantamine clinical implications for nicotinic receptor modulation.. The second kuopio Alzheimer symposium, Kuopio Music Centre, Kuopio, Finland, March 13‐15, 2001. 2001:42.
van Gool 2002 {published data only}
- Gool WA. Efficacy of galantamine in probable vascular dementia and Alzheimer's disease combined with cerebrovascular disease: a randomised trial: comment. Lancet 2002;360(9344):1512. [DOI] [PubMed] [Google Scholar]
Vellas 2004 {published data only}
- Vellas B, Kavanagh S, Schwalen S. Effects of galantamine on activities of daily living. The Journal of Nutrition, Health and Aging 2004;8(4):262. [Google Scholar]
Wasielewski 1997 {published data only}
- Wasielewski S. Acetylcholinesterase inhibitors: Galanthamine in Alzheimer's Dementia. Deutsche Apotheker Zeitung 1997;137(51‐52):31‐32. [Google Scholar]
Wilcock 1993 {published data only}
- Wilcock GK, Scott M, Pearsall T, Neubauer K, Boyle M, Razay G. Galanthamine and the treatment of Alzheimer's disease. Int J Geriat Psychiatry 1993;8:781‐2. [Google Scholar]
Wilcock 2000i {published data only}
- Wilcock G, Wright T. Reminyl (galantamine) alleviates caregiver burden in alzheimer's disease in two 6 month studies. Clinical Neuropsychological Assessment 2000i;1(6):16. [Google Scholar]
Wilcock 2000j {published data only}
- Wilcock G, Wright T. Long term cognitive and functional benefits of reminyl (galantamine ) in patients with alzheimer's disease. Clinical Neuropsychological Assessment 2000j;1(6):43. [Google Scholar]
Wilkinson 2000 {published data only}
- Wilkinson D, Lilienfeld S, Truyen L. Galantamine improves activities of daily living in patients with alzheimer's disease: A 3 month placebo‐controlled study. Proceedings of the 6th International Stockholm/Springfield Symposium on Advances in Alzheimer Therapy; 2000 Apr 5‐8, Stockholm, Sweden. 2000:233.
Zarotsky 2003 {published data only}
- Zarotsky V, Sramek JJ, Cutler NR. Galantamine hydrobromide: an agent for Alzheimer's disease. American journal of health system pharmacy AJHP official journal of the American Society of Health System Pharmacists 2003;60(5):446‐52. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Galantamine CFIDS {published data only (unpublished sought but not used)}
- Not reported. Ongoing study Jan 29 1999.
Wilcock {published data only (unpublished sought but not used)}
- The safety and efficacy of Galanthamine in the treatment of vascular and mixed dementia. Ongoing study 1 Oct 1998.
Additional references
APA 1987
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd Edition. Washington DC: American Psychiatric Association, 1987. [Google Scholar]
Blesa 2003
- Blesa R, Davidson M, Kurz A, Reichman W, Baelen B, Schwalen S. Galantamine provides sustained benefits in patients with 'advanced moderate' Alzheimer's disease for at least 12 months. Dementia and geriatric cognitive disorders 2003;15(2):79‐87. [DOI] [PubMed] [Google Scholar]
Chalmers 1983
- Chalmers TC, Celano P, Sacks HS, Smith H, Junior. Bias in Treatment Assignment in Controlled Clinical Trials. N Eng J Med, 1983;309:1358‐61. [DOI] [PubMed] [Google Scholar]
Clegg 2001
- Clegg A, Bryant J, Nicholson T, McIntyre L, Broe S, Gerard K, Waugh N. Clinical and cost effectiveness of donepezil rivastigmine and galantamine for Alzheimer's disease a rapid and systematic review. Health Technology Assessment 2001;5(1):i‐iv+1‐128. [DOI] [PubMed] [Google Scholar]
Cummings 1994
- Cummings JL, Mega M, Gray K, Rosenberg‐Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994;44(12):2308‐2314. [DOI] [PubMed] [Google Scholar]
EBCTCG 1990
- Early Breast Cancer Trialists' Collaborative Group. Treatment of early breast cancer: Worldwide evidence 1985‐1990. Oxford University Press, 1990. [Google Scholar]
Erkinjuntti 2002c
- Erkinjuntti T, Kurz A, Gauthier S, Bullock R, Lilienfeld S, Damaraju CV. Efficacy of galantamine in probable vascular dementia and Alzheimer's disease combined with cerebrovascular disease: a randomised trial. The Lancet 2002;359:1283‐1290. [DOI] [PubMed] [Google Scholar]
Folstein 1975
- Folstein M, Folstein S, McHugh P. Mini‐mental State. A practical method for grading the cognitive status of patients for the clinician. Journal of Psychiatry Research 1975;12:189‐198. [DOI] [PubMed] [Google Scholar]
GAL‐COG‐3002
- Johnson, Johnson. GAL‐COG‐3002. www.clinicaltrials.org.
GAL‐USA‐5
- Gutierrez R, Kershaw P, Wessel T, Pontecorvo M, Shnaidman M, Chang S. Safety and efficacy of galantamine during its withdrawal in the treatment of Alzheimer's Disease. Jansseen Research Foundation 1998.
Galasko 1997
- Galasko D, Bennett D, Sano M, Ernesto C, Thomas R, Grundman M, Ferris S. An inventory to assess activities of daily living for clinical trials in Alzheimer's disease. The Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord 1997;11:S33‐S39. [PubMed] [Google Scholar]
Gelinas 1999
- Gelinas I, Gauthier L, McIntyre M, Gauthier S. Development of a functional measure for persons with Alzheimer's disease: the disability assessment for dementia. Am J Occup Ther 1999;53(5):471‐481. [DOI] [PubMed] [Google Scholar]
Getsios 2001
- Getsios D, Caro JJ, Caro G, Ishak K, The AHEAD Study Group. Assessment of health economics in Alzheimer's disease AHEAD galantamine treatment in canada. Neurology 2001;57(6):972‐8. [DOI] [PubMed] [Google Scholar]
Guy 1976
- Guy W. Clinical Global Impressions (CGI). In: Guy W editor(s). ECDEU Assessment Manual for Psychopharmacology. Rockville MD: US Department of Health and Human Services, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, 1976:218‐222. [Google Scholar]
Harvey 1995
- Harvey AL. The pharmacology of galanthamine and its analogues. Pharmac. Ther. 1995;68:113‐128. [DOI] [PubMed] [Google Scholar]
ICD 1992
- World Health Organization. The ICD‐10 classification of mental and behavioural disorders: clinical description and diagnostic guidelines. World Health Organization. World Health Organisation, Division of Mental Health, 1992. [Google Scholar]
Maelicke 1997
- Maelicke A, Coban T, Storch A, Schrattenholz A, Pereira EF, Albuquerque EX. Allosteric modulation of Torpedo nicotinic acetylcholine receptor ion channel activity by noncompetitive agonists.. Journal‐of‐receptor‐and‐signal‐transduction‐research 1997;17(1‐3):11‐28. [DOI] [PubMed] [Google Scholar]
McKhann 1984
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical Diagnosis of Alzheimer’s Disease: Report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984;4:939‐44. [DOI] [PubMed] [Google Scholar]
Migliaccio‐Walle2003
- Migliaccio‐Walle K, Getsios D, Caro JJ, Ishak KJ, O'Brien JA, Papadopoulos G. Economic evaluation of galantamine in the treatment of mild to moderate Alzheimer's disease in the united states. Clinical Therapeutics 2003;25(6):1806‐25. [DOI] [PubMed] [Google Scholar]
Mintzer 2003
- Mintzer JE, Kershaw P. The efficacy of galantamine in the treatment of Alzheimer's disease: comparison of patients previously treated with acetylcholinesterase inhibitors to patients with no prior exposure. International Journal of Geriatric Psychiatry 2003;18(4):292‐7. [DOI] [PubMed] [Google Scholar]
Moretti 2002
- Moretti R, Torre P, Antonello RM, Cazzato G, Bava A. "Efficacy of galantamine in probable vascular dementia and Alzheimer's disease combined with cerebrovascular disease: A randomised trial": Comment. Lancet 2002;360(9344):1512‐3. [DOI] [PubMed] [Google Scholar]
Mulrow 1997
- Mulrow CD, Oxman AD. Cochrane Collaboration Handbook. In: Mulrow CD, Oxman AD editor(s). Cochrane Collaboration Handbook [updated 9 December 1996] Available in The Cochrane Library [database on disk and CDROM], Issue 1. Updated quarterly. Oxford: The Cochrane Collaboration, Update Software, 1997. [Google Scholar]
Pacheco 1995
- Pacheco G, Palacios‐Esquivael R, Moss DE. Cholinesterase inhibitors proposed for treating dementia in Alzheimer's disease: selectivity toward human brain acetycholinesterase. J Pharmacol Exp Ther 1995;274:767‐770. [PubMed] [Google Scholar]
Rosen 1984
- Rosen W, Mohs R, Davis K. A new rating scale for Alzheimer's disease. American Journal of Psychiatry 1984;141:939‐944. [DOI] [PubMed] [Google Scholar]
Schneider 1997
- Schneider LS, Olin JT, Doody RS, Clark CM, Morris JC, Reisberg B, Schmitt FA, Grundman M, Thomas RG, Ferris SH. Validity and reliability of the Alzheimer's Disease Cooperative Study‐Clinical Global Impression of Change (ADCS‐CGIC). Alzheimer's Disease and Associated Disorders 1997;11:S22‐S32. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Stahl 2004
- Stahl SM, Markowitz JS, Papadopoulos G, Sadik K. Examination of nighttime sleep‐related problems during double‐blind, placebo‐controlled trials of galantamine in patients with Alzheimer's disease. Current medical research and opinion 2004;20(4):517‐24. [DOI] [PubMed] [Google Scholar]
Sweeney 1988
- Sweeney JE, Hohmann CF, Moran TH, Coyle JT. A long‐acting cholinesterase inhibitor reverses spatial memory deficits in mice.. Pharmacology Biochemistry and Behavior 1988;31(1):147‐7. [DOI] [PubMed] [Google Scholar]
Tonkopii 1976
- Tonkopii VD, Prozorovskii VB, Suslova IM. Interaction of reversible inhibitors with catalytic centres and allosteric site of cholinesterases. Bull. Exp. Bio. Med 1976;82:1180‐1183. [PubMed] [Google Scholar]
van Gool 2002
- Gool WA. Efficacy of galantamine in probable vascular dementia and Alzheimer's disease combined with cerebrovascular disease: a randomised trial: comment. Lancet 2002;360(9344):1512. [DOI] [PubMed] [Google Scholar]
Wilcock 2000
- Wilcock GK, et al. GALANTAMINE ALLEVIATES CAREGIVER BURDEN IN ALZHEIMER¢S DISEASE: A 6‐MONTH PLACEBO‐CONTROLLED STUDY. Conference Proceedings World Alzheimer Congress; 9‐13 July, 2000; Washington 2000;15:562‐5. [Google Scholar]
Wilcock 2001
- Wilcock GK. Erratum: Efficacy and safety of galantamine in patients with mild to moderate Alzheimer's disease: Multicentre randomised controlled trial (British Medical Journal (December 9)(1445‐1449)). BR‐MED‐J: British‐Medical‐Journal 2001;322(7278):90. [Google Scholar]
Wilkinson 2002
- Wilkinson DG, Hock C, Farlow M, Baelen B, Schwalen S. Galantamine provides broad benefits in patients with 'advanced moderate' Alzheimer's disease (MMSE < or = 12) for up to six months. International Journal of Clinical Practice 2002;56(7):509‐14. [PubMed] [Google Scholar]


