Abstract
Background & Aims
Patients with hepatocellular carcinoma (HCC) are selected for liver transplantation (LT) based on pre-LT imaging ± alpha-foetoprotein (AFP) level, but discrepancies between pre-LT tumour assessment and explant are frequent. Our aim was to design an explant-based recurrence risk reassessment score to refine prediction of recurrence after LT and provide a framework to guide post-LT management.
Methods
Adult patients who underwent transplantation between 2000 and 2018 for HCC in 47 centres were included. A prediction model for recurrence was developed using competing-risk regression analysis in a European training cohort (TC; n = 1,359) and tested in a Latin American validation cohort (VC; n=1,085).
Results
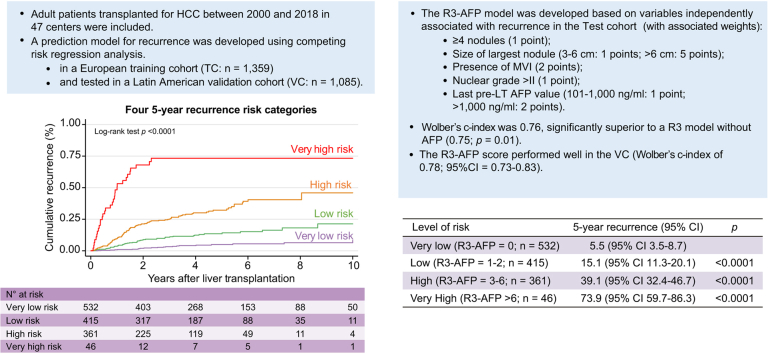
In the TC, 76.4% of patients with HCC met the Milan criteria, and 89.9% had an AFP score of ≤2 points. The recurrence risk reassessment (R3)-AFP model was designed based on variables independently associated with recurrence in the TC (with associated weights): ≥4 nodules (sub-distribution of hazard ratio [SHR] = 1.88, 1 point), size of largest nodule (3–6 cm: SHR = 1.83, 1 point; >6 cm: SHR = 5.82, 5 points), presence of microvascular invasion (MVI; SHR = 2.69, 2 points), nuclear grade >II (SHR = 1.20, 1 point), and last pre-LT AFP value (101–1,000 ng/ml: SHR = 1.57, 1 point; >1,000 ng/ml: SHR = 2.83, 2 points). Wolber’s c-index was 0.76 (95% CI 0.72–0.80), significantly superior to an R3 model without AFP (0.75; 95% CI 0.72–0.79; p = 0.01). Four 5-year recurrence risk categories were identified: very low (score = 0; 5.5%), low (1–2 points; 15.1%), high (3–6 points; 39.1%), and very high (>6 points; 73.9%). The R3-AFP score performed well in the VC (Wolber’s c-index of 0.78; 95% CI 0.73–0.83).
Conclusions
The R3 score including the last pre-LT AFP value (R3-AFP score) provides a user-friendly, standardised framework to design post-LT surveillance strategies, protocols, or adjuvant therapy trials for HCC not limited to the Milan criteria.
Clinical Trials Registration
Lay summary
Considering discrepancies between pre-LT tumour assessment and explant are frequent, reassessing the risk of recurrence after LT is critical to further refine the management of patients with HCC. In a large and international cohort of patients who underwent transplantation for HCC, we designed and validated the R3-AFP model based on variables independently associated with recurrence post-LT (number of nodules, size of largest nodule, presence of MVI, nuclear grade, and last pre-LT AFP value). The R3-AFP model including last available pre-LT AFP value outperformed the original R3 model only based on explant features. The final R3-AFP scoring system provides a robust framework to design post-LT surveillance strategies, protocols, or adjuvant therapy trials, irrespective of criteria used to select patients with HCC for LT.
Keywords: Liver transplantation, Liver cancer, Recurrence, Explants pathology, Prediction
Abbreviations: AFP, alpha-foetoprotein; HCC, hepatocellular carcinoma; LT, liver transplantation; MVI, microvascular invasion; R3, recurrence risk reassessment; RETREAT, Risk Estimation of Tumour Recurrence After Transplant; SHR, sub-distribution of hazard ratio; TC, test cohort; TTR, time to recurrence; VC, validation cohort; HBV, hepatitis B virus; HCV, hepatitis C virus
Graphical abstract
Highlights
-
•
Discrepancies between pretransplant tumour assessment and liver explant are frequent.
-
•
The R3-AFP predictive model of recurrence was designed and validated in a large and international cohort of patients transplanted for HCC.
-
•
The components of the final model are the following: number of nodules, size of the largest nodule, presence of MVI, nuclear grade, and last pre-LT AFP value.
-
•
The R3-AFP model including the last available pre-LT AFP value outperformed the original R3 model only based on explant features.
-
•
The final R3-AFP scoring system provides a standardised framework to refine post-LT management of patients, irrespective of criteria used to select patients with HCC for LT.
Introduction
Hepatocellular carcinoma (HCC) is a major public health issue worldwide.1 Among curative options available at early stages, liver transplantation (LT) has been considered the optimal treatment for HCC because of the removal of the tumour and the underlying cirrhotic liver.2 Because of the shortage of organs, guidelines have restricted LT to patients with expected post-LT survival comparable with non-malignant indication (>70% after 5 years). This goal is achieved with the most commonly used selection criteria, with HCC recurrence rates ranging from 8 to 15%.[3], [4], [5], [6], [7], [8] However, the pre-LT evaluation underestimates tumour burden in 20–40% of cases.9,10 This discrepancy between pre-LT imaging and explant pathology analysis is a result of tumour progression while patients are on the waiting list and/or the presence of additional nodules not fully characterised by conventional radiology assessment, whereas explant analysis is the gold standard for tumour burden assessment, allowing for thorough evaluation.
Consequently, reassessing risk of recurrence after LT based on explant features is of particular interest, firstly, because identification of patients with very low or high risk of recurrence could impact post-LT policies on screening for recurrence11 and, secondly, to help enable development and validation of standardised and reproducible risk stratification tools necessary to evaluate candidate strategies suggested to minimise risk of HCC recurrence.[12], [13], [14], [15]
Several predictive models of HCC recurrence based on pathological findings have been published, identifying risks for 5-year recurrence of HCC ranging from rates 0 to 95%.5,10,[16], [17], [18], [19], [20], [21] However, these models have not been externally validated, use uncommon features, or provide no more than 2 levels of risk, limiting their applicability in clinical practice. More recently, Mehta et al.11,22 reported a scoring model identifying 6 levels of risk of recurrence at 5 years using the pre-LT alpha-foetoprotein (AFP) value and 3 pathological features retrieved from explant (Risk Estimation of Tumour Recurrence After Transplant [RETREAT]). However, this score was designed in a cohort of patients who remained strictly within the Milan criteria during the waiting period, limiting its relevance when other selection criteria are used.[6], [7], [8],23,24 Indeed, a growing body of evidence suggests that the Milan criteria are too restrictive, and some countries, such as France, have already adopted alternate, composite criteria.25,26 For example, up to 10% of patients who underwent transplantation for HCC in the United States have pre-LT tumour features exceeding the Milan criteria.11 Importantly, the RETREAT scoring system suggested that combining pre-LT serum AFP and explant variables may improve predictive accuracy of pathology-based models. This leads to the hypothesis that serum AFP may reflect components of tumour behaviour not taken into account by routinely collected pathological variables and merits further investigation.
Our primary aim was therefore to design and externally validate a new explant-based model to reassess risk of recurrence after LT, to refine and standardise prediction of HCC recurrence after LT in a population including a significant proportion of patients selected for LT with expanded criteria. Our secondary objective was to explore the independent effect of pre-LT AFP values when using explant pathology variables.
Patients and methods
Patients
This was a retrospective, multicentre, multinational cohort study of adult patients with HCC who underwent LT in 47 different centres from Europe and Latin America. Four regional databases of patients who underwent transplantation for HCC in France, Italy, and Belgium between 2000 and 2018 and from Argentina, Uruguay, Chile, Brazil, Ecuador, Colombia, and Mexico between 2005 and 2018 were harmonised and merged (by FP and FR) and hosted on a central server, after agreement of all participating centres. The merged database had previously served as a basis to design and test prognostic models.6,23,24 All procedures were followed in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) and Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK) guidelines.27,28 The study complied with ethical standards and the Helsinki Declaration of 1975, as revised in 2008. Each investigator was subject to a confidentiality agreement. This study was registered as part of an open public registry (NCT03775863; www.clinicaltrials.gov).
We included adult patients (>17 years of age) who underwent a first LT for HCC and were followed up after transplantation. We excluded patients if (1) incidental HCC was found at explant pathology, (2) tumours other than HCC were found in the explant, and/or (3) they were included in the TC for AFP score development.6 The European cohort served as the test cohort (TC) for design of the predictive model, which was subsequently validated in the Latin American cohort (VC). There is no consensus on post-LT surveillance algorithms; however, in most centres post-transplant HCC recurrence monitoring consisted of CT or MRI and serum AFP assay every 6 months. Recurrence was determined based on imaging criteria and serum AFP or by biopsy. All patients were followed up until death or last outpatient visit.
Exposure variables during the waiting list period and after transplantation
The following variables were collected at each transplant site: recipient characteristics, tumour burden, and α-foetoprotein (AFP) serum levels, at listing and at the last evaluation before LT. The Milan criteria or AFP score were the common standardised patient-selection criteria in all centres according to local practices and allocation policies. All patients were categorised according to the Milan criteria4 and AFP score6 at the time of listing and at the last tumour re-assessment. In patients receiving bridging therapies during the waiting-list period, the last available radiologic tumour staging and AFP values following these procedures were also registered. Tumour treatment and type of bridging therapies before LT were decided at each transplant centre according to local practice on a case-by-case basis. Immunosuppression regimens were not included for their effect on recurrence because of significant heterogeneity across LT centres and lack of a standardised and evidenced-based recommendation on immunosuppression in patients with HCC.
Pathological tumour features noted at explant analysis included the presence of microvascular invasion (MVI), tumour differentiation, and the number and sizes of nodules, as assessed by experienced pathologists specialised in liver pathology at all centres. Tumours were characterised as well (nuclear grade 1), moderately (nuclear grade 2), or poorly (nuclear grade 3) differentiated according to the Edmondson and Steiner criteria.29 In explants with heterogeneous grades of differentiation, the higher grade was recorded. Necrotic nodules were measured, including both necrotic and viable tumour diameters. In those patients in which complete tumour necrosis was observed, explant risk variables, including MVI or tumour differentiation, were registered as absent for each modelling approach.
Statistical analysis
The primary endpoint, post-LT HCC recurrence, was chosen because it is the most important event specifically affecting post-LT survival in these patients. The secondary endpoints were overall post-LT survival and post-recurrence survival. Early recurrences, defined as recurrences occurring during the first 12 months after transplant, were previously reported to be associated with worse post-recurrence survival.30
We followed the REMARK guidelines for designing and validating the prognostic models.28 We first explored the association between explant features and the primary and secondary endpoints. Secondly, we explored whether AFP values before transplant, either at listing or at the last pre-LT evaluation, had an independent association with HCC recurrence in both cohorts. Finally, we tested if adding AFP values to the explant model changed the discrimination power for HCC recurrence. We conducted multivariable competing-risk regression models for the primary outcome (HCC recurrence), estimating sub-distribution of hazard ratios (SHRs) and 95% CIs using the Fine and Gray method31 (see the Supplementary information). For overall post-LT survival, we used the Kaplan–Meier method (log-rank test) and Cox regression analysis, estimating hazard ratios. The proportional hazard assumption was evaluated through graphic inspection and the Schoenfeld residual test. All statistical procedures were repeated in the validation cohort (VC). Collected data were analysed with STATA 13.0 (StataCorp, College Station, TX, USA).
Results
Study cohort characteristics
A total of 2,444 patients were included: 1,359 (55.6%) in the TC (European centres) and 1,085 (44.4%) in the VC (Latin America centres). Table 1 summarises the main features of the TC and VC. The majority of European patients underwent transplantation between 2000 and 2011, whereas most patients in the VC underwent transplantation between 2012 and 2018 (Table S1). At explant pathology analysis, 62.3 and 66.4% of the TC and VC were within the Milan criteria (p = 0.034), 61.2 and 67.9% were within the up-to-7 criteria and without MVI (p = 0.001), and 72.3 and 71.6% (p = 0.28) presented a RETREAT score ≤3 points, respectively (Table S2). Staging based on last pre-LT imaging underestimated tumour burden as evaluated at explant pathology in 31.6% (95% CI 25.7–38.0) and 28.1% (95% CI 25.3–31.1) of the patients in the TC and VC, respectively. In the TC and VC, there were 94 (6.9%) and 11 (1.0%) patients with complete major nodule necrosis, respectively. Overall 5-year recurrence rates were 19.6% (95% CI 17.1–22.4) in the TC and 16.9% (95% CI 13.7–20.9) in the VC (p = 0.026). The median time to recurrence (TTR) was 16.4 months (IQR 9.0–30.9) in the TC and 13.2 months (IQR 6.0–25.7) in the VC (p = 0.051). Survival rates were 67.0% (95% CI 63.9–69.9) in the TC and 62.1% (95% CI 58.1–65.8) in the VC, respectively (p <0.0001).
Table 1.
Test and validation cohort characteristics.
| Variable | Test cohort (n = 1,359) |
Validation cohort (n = 1,085) |
p value |
|---|---|---|---|
| Age, years (±SD) | 58 ± 8 | 58 ± 8 | 0.99 |
| Male sex, n (%) | 1,124 (82.7) | 844 (77.8) | 0.002 |
| Median waiting list (IQR), months | 6.1 (3.0–11.0) | 4.9 (1.7–10.0) | <0.0001 |
| Aetiology of liver disease, n (%) | |||
| Viral | 786 (57.8) | 610 (56.2) | <0.0001 |
| Alcohol | 426 (31.3) | 183 (16.9) | |
| Other | 147 (10.8) | 292 (26.9) | |
| HBV, n (%) | 94 (6.9) | 149 (13.7) | <0.0001 |
| HCV, n (%) |
696 (51.2) |
466 (42.9) |
<0.0001 |
| Data at listing | |||
| AFP (ng/ml) | |||
| ≤100 ng/ml, n (%) | 1,212 (89.2) | 877 (81.1) | <0.0001 |
| 101–1,000 ng/ml, n (%) | 129 (9.5) | 165 (15.3) | |
| >1,000 ng/ml, n (%) | 18 (1.3) | 39 (3.6) | |
| Within the Milan criteria, n (%) | 1,039 (76.4) | 939 (86.5) | <0.0001 |
| AFP score | |||
| ≤2 points, n (%) | 1,221 (89.9) | 942 (87.1) | 0.032 |
| >2 points, n (%) | 137 (10.1) | 139 (12.9) | |
| Bridging therapy before LT∗, n (%) |
931 (68.5) |
782 (72.1) |
0.055 |
| Data at last tumour reassessment | |||
| AFP (ng/ml) | |||
| ≤100 ng/ml, n (%) | 1,191 (88.0) | 893 (82.8) | 0.001 |
| 101–1,000 ng/ml, n (%) | 136 (10.0) | 147 (13.6) | |
| >1,000 ng/ml, n (%) | 27 (2.0) | 39 (3.6) | |
| Within the Milan criteria, n (%) | 234 (80.1) | 931 (85.8) | 0.020 |
| AFP score | |||
| ≤2 points, n (%) | 250 (87.1) | 953 (88.1) | 0.66 |
| >2 points, n (%) | 37 (12.9) | 129 (11.9) | |
Test cohort (European cohort). Validation cohort (Latin American cohort).
AFP, alpha-foetoprotein; LT, liver transplantation.
Refer to Table S8.
Explant-based R3 model in the test cohort
In multivariable analysis, the following were independently associated with HCC recurrence: ≥4 nodules (SHR = 1.81, 95% CI 1.30–2.53), size of the largest nodule 3–6 cm (SHR = 1.91, 95% CI 1.35–2.70), size of the largest nodule >6 cm (SHR = 5.82, 95% CI 3.60–9.39), presence of MVI (SHR = 2.70, 95% CI 1.94–3.76), and nuclear grade >II (SHR = 1.22, 95% CI 1.02–1.46; Table 2).
Table 2.
Explant liver variables associated with HCC recurrence after liver transplantation in the test cohort.
| Variable | 5-year recurrence rate (95% CI) | Unadjusted sub-hazard ratio (95% CI) | p value | Adjusted sub-hazard ratio (95% CI) | p value |
|---|---|---|---|---|---|
| Number of nodules | 1.03 (1.01–1.04) | <0.0001 | |||
| 1–3 nodules (n = 1,005) | 14.2 (11.7–17.1) | ||||
| ≥4 nodules (n = 354) | 35.7 (29.4–42.9) | 2.73 (2.07–3.59) | <0.0001 | 1.81 (1.30–2.53) | <0.0001 |
| Major nodule diameter | 1.37 (1.31–1.44) | <0.0001 | |||
| ≤3 cm (n = 849) | 13.8 (11.1–17.1) | ||||
| 3–6 cm (n = 361) | 30.4 (24.5.37.7) | 2.32 (1.72–3.14) | <0.0001 | 1.91 (1.35–2.70) | <0.0001 |
| >6 cm (n = 54) | 74.5 (58.7–87.9) | 9.21 (5.98–14.17) | <0.0001 | 5.82 (3.60–9.39) | <0.0001 |
| Microvascular invasion | |||||
| Absence (n = 369) | 11.4 (9.2–14.0) | ||||
| Presence (n = 990) | 39.6 (32.9–46.3) | 4.04 (3.06–5.32) | <0.0001 | 2.70 (1.94–3.76) | <0.0001 |
| Nuclear grade >II | |||||
| Absence (n = 1,003) | 15.9 (13.3–19.0) | ||||
| Presence (n = 173) | 28.2 (21.2–36.9) | 1.47 (1.23–1.74) | <0.0001 | 1.22 (1.02–1.46) | 0.02 |
Competing-risk regression analysis. Calibration between observed/predictive events was adequate, and c-statistic (Wolber’s c-index) was 0.75 (95% CI 0.72–0.79).
HCC, hepatocellular carcinoma.
From this final explant-based model, we used the adjusted SHRs to propose a clinical recurrence risk reassessment (R3) score (range 0–9 points; Table S3). The R3 score as a continuous variable was associated with an incremental SHR of 1.51 (95% CI 1.43–1.60) for every additional point (Fig. S1). The discriminatory power of the R3 model for prediction of HCC recurrence, as evaluated using Wolber’s c-index, was 0.75 (95% CI 0.72–0.79).
The R3 model including AFP values
In the TC, we further explored the relevance of adding the last available pre-LT AFP value into the model. In multivariable competing-risk regression analysis, the last available AFP value was independently associated with recurrence (AFP 101–1,000 ng/ml, SHR = 1.57 [95% CI 1.03–2.39; p = 0.035]; AFP >1,000 ng/ml, SHR = 2.83 [95% CI 1.01–7.96; p = 0.049]; vs. AFP ≤100], adjusted for the other independent variables included in the model (Table S4). The discriminatory power of the R3 model with AFP was 0.76 (95% CI 0.72–0.80), which was significantly superior to the original R3 model only including explant features (p = 0.01), as evaluated using Wolber’s c-index.
Consequently, we built a refined version of the R3 scoring model, adjusting the independent effect of explant features. Points were allocated to each variable in a similar way to the original score (R3-AFP score, Table 3). The median R3-AFP score value in the TC was 1 point (IQR 0–3 points). This refined version of the scoring model was associated with an incremental SHR of 1.58 (95% CI 1.40–1.56) for every additional point (Fig. S2).
Table 3.
Development of the R3-AFP score. Points assigned from the multivariable competing-risk regression analysis in the test cohort.
| Variable | Adjusted SHR (95% CI) | p value | Points |
|---|---|---|---|
| Number of nodules | |||
| 1–3 nodules (n = 1,005) | 0 | ||
| ≥4 nodules (n = 354) | 1.88 (1.34–2.64) | <0.0001 | 1 |
| Major nodule diameter | |||
| ≤3 cm (n = 849) | 0 | ||
| 3–6 cm (n = 361) | 1.83 (1.29–2.59) | 0.001 | 1 |
| >6 cm (n = 54) | 5.82 (2.97–8.20) | <0.0001 | 5 |
| Microvascular invasion | |||
| Absence (n = 990) | 0 | ||
| Presence (n = 369) | 2.69 (1.93–3.75) | <0.0001 | 2 |
| Nuclear grade >II | |||
| Absence (n = 1,003) | 0 | ||
| Presence (n = 173) | 1.20 (1.01–1.43) | 0.048 | 1 |
| AFP (ng/ml)∗ | |||
| ≤100 (n = 1,191) | 0 | ||
| 101–1,000 (n = 136) | 1.57 (1.03–2.39) | 0.035 | 1 |
| >1,000 (n = 27) | 2.83 (1.01–7.96) | 0.049 | 2 |
Scoring model was done by dividing each SHR with the lowest SHR observed, rounding SHR estimates to construct a continuous score (total of 11 points). Median 1 point (IQR 0–3); SHR 1.48 (95% CI 1.40–1.56).
AFP, alpha-foetoprotein; LT, liver transplantation; R3, recurrence risk reassessment; SHR, sub-distribution of hazard ratio.
Last available AFP values before LT. Median time from last AFP values to transplantation was 2.2 months (IQR 0.9–4.0 months).
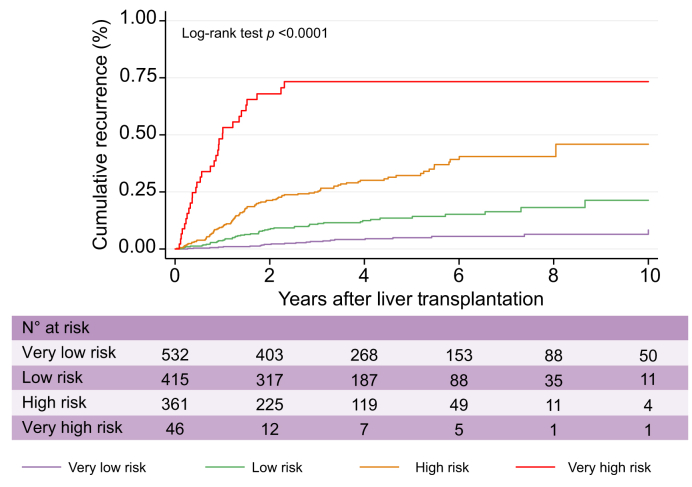
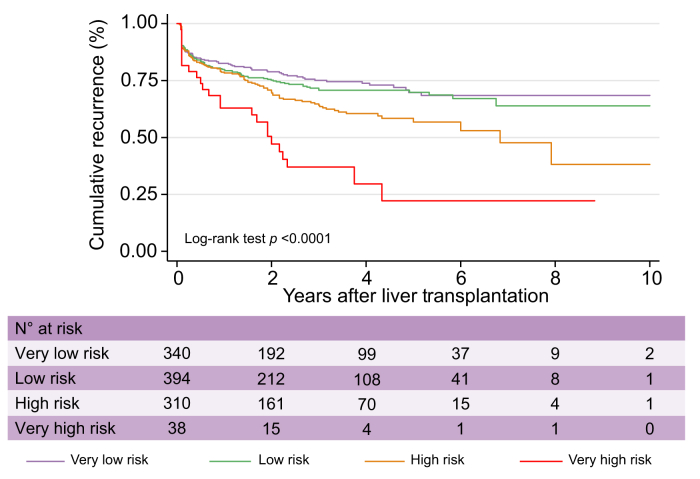
Given the distribution of the observed cumulative recurrence curves using competing-risk assessment, we subsequently stratified these levels of risk into 4 groups (Fig. 1): R3-AFP = 0 points (n = 532; very low risk of recurrence at 5 years: 5.5%; 95% CI 3.5–8.7), R3-AFP = 1–2 points (n = 415; low risk: 15.1%; 95% CI 11.3–20.1), R3-AFP = 3–6 points (n = 361; high risk: 39.1%; 95% CI 32.4–46.7), and R3-AFP score >6 points (n = 46; very high risk: 73.9%; 95% CI 59.7–86.3). When compared with the reference category (R3-AFP = 0), patients within the low-risk category presented an SHR for HCC recurrence of 3.09 (95% CI 1.88–5.08; p <0.0001), the high-risk group an SHR of 8.11 (95% CI 4.14–12.80; p <0.0001), and the very high-risk group an SHR of 33.0 (95% CI 18.18–59.82; p <0.0001).
Fig. 1.
Cumulative recurrence curves in the test cohort according to R3-AFP score categories.
R3-AFP, recurrence risk reassessment–alpha-foetoprotein.
The median TTR for each risk group showed that patients in the very low-risk group had a longer median TTR (29.2 months [IQR 19.0–40.5]) compared with other groups, including low risk (20.1 months [IQR 10.7–34.1]), high risk (16.4 months [IQR 9.8–32.6]), and very high risk (8.9 months [IQR 3.5–14.6]; p <0.0001).
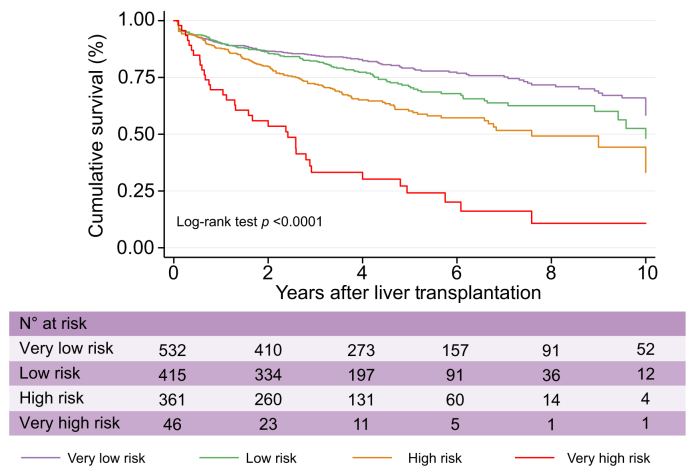
Also, the R3-AFP categorisation showed different overall post-LT survival curves (Fig. 2), with 5-year survival rates of 77.2% (95% CI 72.7–81.1) for the very low-risk group, 67.8% (95% CI 61.8–73.0) for the low-risk group, 57.2% (95% CI 50.6–63.2) for the high-risk group, and 19.8% (95% CI 8.7–34.3) for the very high-risk group. Hazard ratio estimates for post-LT survival were 1.35 (95% CI 1.04–1.74; p = 0.023) for the low-risk group, 2.01 (95% CI 1.57–2.59; p <0.0001) for the high-risk group, and 5.36 (95% CI 3.66–7.83; p <0.0001) for the very high-risk group, respectively, when compared with the reference group (very low risk).
Fig. 2.
Cumulative survival curves in the test cohort according to R3-AFP score categories.
R3-AFP, recurrence risk reassessment–alpha-foetoprotein.
Median post-recurrence survival in patients with recurrent HCC was 12.2 months (IQR 4.4–23.0 months). Early recurrences occurred in 34.8% (n = 71) of all recurrent HCC and were associated with lower median post-recurrence survival (6.5 months [IQR 2.9–18.5] vs. non-early recurrences 17.2 months [IQR 5.7–26.2]; p <0.0001). The proportion of early recurrence by R3-AFP risk category was 18.2% in the very low-risk group, 28.0% in the low-risk group, 32.0% in the high-risk group, and 67.7% in the very high-risk group (p <0.0001).
External validation of the R3 scores
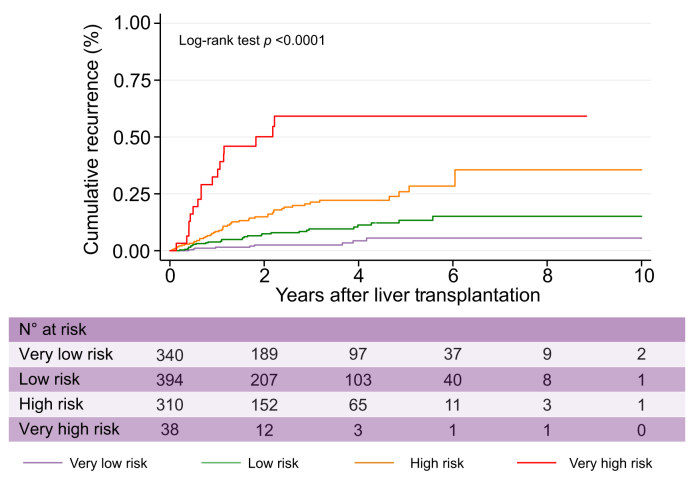
Despite differences in patients, centres’ policies, periods of LT procedure, and HCC characteristics between the TC and the VC (Table 1), calibration between expected and observed events according to the R3-AFP risk categorisation was not statistically significant (Fig. S3). The R3-AFP scoring model performed well in the VC with an increasing SHR of 1.50 (95% CI 1.39–1.63) for every point of the R3-AFP score, and it also identified 4 levels of risk of recurrence (Fig. 3) and cumulative post-transplant survival (Fig. 4). The R3-AFP model in the VC had a Wolber’s c-index of 0.78 (95% CI 0.73–0.83) and also outperformed the original R3 model without AFP (0.73; 95% CI 0.67–0.79; p = 0.018) in this cohort Tables S5 & S6.
Fig. 3.
Cumulative recurrence curves in the validation cohort according to R3-AFP score categories.
R3-AFP, recurrence risk reassessment–alpha-foetoprotein.
Fig. 4.
Cumulative survival curves in the validation cohort according to R3-AFP score categories.
R3-AFP, recurrence risk reassessment–alpha-foetoprotein.
Comparison with existing models
Finally, we compared both scoring systems (R3 and R3-AFP) with other explant-based tools. In both the VC and the TC, we showed composite R3-AFP had more discriminatory power than R3. The R3-AFP was compared with the RETREAT, also including AFP and pathological variables. R3-AFP had similar performance in the TC and in the VC (Table 4). In addition, purely explant-based R3 had better discrimination power than the Milan and up-to-7 criteria (Table S7).
Table 4.
Comparison regarding discrimination power of each model in the test and validation cohorts.
| Variable | Wolber’s c-index (95% CI) | p value |
|---|---|---|
| Test cohort (comparison against composite models including AFP) | ||
| RETREAT | 0.75 (0.72–0.79) | 0.40 |
| R3-AFP | 0.76 (0.72–0.80) | |
| Validation cohort (comparison against composite models including AFP) | ||
| RETREAT | 0.77 (0.72–0.82) | 0.59 |
| R3-AFP | 0.78 (0.73–0.83) | |
AFP, alpha-foetoprotein; R3, recurrence risk reassessment; RETREAT, Risk Estimation of Tumour Recurrence After Transplant.
Discussion
Reassessment of the risk of recurrence based on explant pathological features is a critical step in the management of patients who underwent transplantation for HCC as tumour burden is underestimated before LT in 20–30% of cases, as observed in our study as well as in previous reports.9,10 Recent, disruptive advances in treatment of HCC make reassessment of the risk of recurrence even more critical, because early diagnosis of recurrence based on individualised screening strategies may be the optimal way to select LT recipients for next-generation therapeutic approaches intended to delay recurrence after LT and optimise post-LT outcomes.
With this in mind, we generated and validated a new composite predictive scoring system, the R3-AFP score, to reassess risk of HCC recurrence after LT. This predictive tool combines typical pathological features of HCC noted from the explant and the last available pre-LT AFP value.
The most striking result of our study is that the composite R3-AFP scoring system outperformed the purely explant-driven R3, despite the latter model having been designed to reflect the prevailing hypothesis that explant features are the gold-standard variables associated with HCC recurrence. Although R3 performed quite well and significantly improved discrimination of HCC recurrence risk compared with other scores assessed on explants such as the Milan and up-to-7, we show that combining the last available pre-LT AFP with explant pathological variables further improved predictive accuracy.
Following the study by Mehta et al.,11,22 who recently proposed a predictive model based on explant pathological variables and AFP in a population meeting all the Milan criteria, we tested this composite approach for the first time in a population not restricted to the Milan criteria and therefore more relevant to clinical practice worldwide. Indeed, a number of countries across Asia and Europe have implemented expanded selection criteria, and in the United States up to 10% of patients receiving a liver graft are beyond the Milan criteria at listing.11,25 Using a 2-step approach, we built our predictive model first solely with explant features and subsequently introduced pre-LT AFP in order to demonstrate the impact of this feature. Indeed, pre-LT AFP adds to pathological variables in predicting HCC recurrence, and utility of the composite approach is not only relevant before LT6,7,26 but also extends to the reassessment of the risk of recurrence after LT, once pathological features are available.
The reason AFP adds predictive value to pathological variables has not been fully elucidated. This finding suggests that AFP may reflect tumour cells’ signalling pathways and behaviour not reflected by routine pathology features. Supporting this hypothesis, AFP, MVI, and nuclear grade each remained independently associated with HCC recurrence in multivariate analysis in the TC. It also could be that AFP levels are more closely related to specific molecular subclasses and signalling pathways involved in tumour progression or response to therapy than is tumour differentiation.32 Supportive of this hypothesis, AFP >400 ng/ml has recently been shown to be independently associated with HCC molecular subclass S2,33,34 enrichment for genes corresponding to MYC target activation, high cell proliferation, and poor clinical prognosis.33
Interestingly, the composite R3-AFP model displayed similar discriminatory power compared with the aforementioned composite RETREAT,11 in both the TC and the VC. This is an original and important finding as the TC and VC were not restricted to patients with HCC meeting the Milan criteria on pre-LT imaging, consistent with real-world practice, whereas RETREAT was originally designed and validated in a population limited by the Milan criteria. Moreover, ‘last AFP available’ seems to be as relevant as ‘AFP measured the day of transplant’, which might enhance the score implementation in clinical practice as all sites might not check AFP level at transplant in routine practice.
Another important finding of our study was that median TTR varied across the risk groups defined by R3-AFP score, ranging from 8.9 months in the very high-risk group to 16.4, 20.1, and 29.2 months in the high-, low-, and very low-risk groups, respectively. The survival time after recurrence also varied across the groups; the higher the risk of recurrence, the shorter the post-recurrence survival time. These results strongly support the need for personalised post-LT HCC screening procedures based on individual risk of HCC recurrence. There is currently no consensus on post-LT surveillance strategies, and a recent study from the United States showed surveillance strategies remain highly heterogeneous across US centres.35 Also, some centres do not screen for HCC recurrence owing to concerns on cost-effectiveness. A recent working group report from the ILTS Transplant Oncology Consensus Conference emphasised that post-LT surveillance strategies should be based on prediction tools, with a view to tailor surveillance schedules to patients’ individual risk of recurrence, and, relatedly, noted the identification of patients at minimal risk of HCC recurrence as an important unmet need, given surveillance may not be necessary for such patients.36 Although predictive tools are available, the working group could not reach consensus on a choice of one to best guide post-LT surveillance strategies, although it acknowledged that surveillance in patients who underwent transplantation for HCC prolongs survival.37
Therefore, there is an urgent need among transplant programmes for evidence-based surveillance schedules optimised according to patients’ individual level of risk. RETREAT provides guidance for patients meeting the Milan criteria all along the waiting time period: patients with RETREAT = 0 could be relieved from HCC screening. The R3-AFP scoring system, designed and validated in large international cohorts defined by expanded selection criteria, provides an ideal framework for research in this regard. Indeed, it has previously been demonstrated that a patient with HCC exceeding the Milan criteria but within the French AFP criteria can have a low risk of recurrence.6 Therefore, it would be valuable to identify within this group of patients (exceeding Milan but within AFP) a subgroup with no or minimal risk of recurrence who could avoid surveillance. This would spare cost and patient radiation exposure. R3-AFP is easy to compute from readily available variables, including last available pre-LT AFP value and variables available in all explant pathological reports. However, surveillance strategies should be tested and validated before routine implementation. Therefore R3-AFP paves the way for development of a surveillance algorithm and its prospective evaluation.
Another important unmet need concerns determination of optimal, evidence-based post-transplant treatment management strategies for patients after LT for HCC. Recent data indicate prognosis of HCC recurrence may have changed over the last decade, with increases in time from LT to recurrence and time from recurrence to death.15 This phenomenon may be related to better post-LT surveillance strategies, aggressive surgical management in case of unique extrahepatic metastases, changes in immunosuppressive policies in high-risk patients or in case of recurrence, or wider use of targeted therapies such as sorafenib or mammalian target of rapamycin inhibitors after recurrence.38 However, the ILTS working group failed to achieve consensus regarding use of a specific immunosuppressive regimen or adjuvant chemotherapy36 owing to the lack of strong data supporting any specific approach.[12], [13], [14], [15],[39], [40], [41] Indeed, most studies have been retrospective and have not featured robust stratification on individual risk of recurrence, which greatly varies by patient, as observed in our study. To tackle this unmet need, R3-AFP again provides a simple framework to design clinical trials adjusted on risk of recurrence and test new adjuvant therapies[42], [43], [44] or immunosuppressive strategies to prevent HCC recurrence after LT, allowing comparison of observed and predicted recurrence rates.
Our study has limitations inherent to multicentre, multinational retrospective databases such as absence of data on variables of interest. For example, AFP at the time of LT was unavailable, and we therefore used the last available pre-LT AFP value. However, the median time elapsed between the last available pre-LT AFP time point and LT was short (<10 weeks) in both the TC and the VC, and therefore, the lack of AFP at the time of LT is unlikely to have had a major impact on our results. In addition, immunosuppression regimens were not captured in the database and so could not be included in the prediction model. We lack information on tumour progression after locoregional treatment, which is known as a risk factor for HCC recurrence.45 Of note, the time from locoregional treatment to LT was only available in the VC (the median time from the last locoregional therapy to LT was 4.6 months [IQR 2.0–8.5]).
In conclusion, we developed and validated a new composite R3-AFP scoring system based on usual explant features and the last available pre-LT AFP value to reassess the risk of HCC recurrence after LT in patients who underwent transplantation for HCC within or exceeding the Milan criteria. We confirmed that inclusion of the last available pre-LT AFP value improves predictive accuracy and AFP should be considered a mandatory component of tools designed to re-assess risk of recurrence, even after LT. We advocate for systematic, routine reassessment of the risk of recurrence once explant pathology is available in order to best guide assessment of post-LT HCC surveillance strategies tailored to individual risk of recurrence. The R3-AFP scoring system also provides a standardised framework to assess the impact of immunosuppression regimens on recurrence and identify the best adjuvant candidates for upcoming clinical trials in this setting.
Financial support
The authors received no financial support to produce this manuscript.
Authors’ contributions
Had full access to all data in the study and take responsibility for the integrity of data and the accuracy of data analysis: CC, FP, CD. Study concept and design: CC, FP, CD. Acquisition of data: FP, HD, AD, IFB, KB, CB, LGP, PB, ME, JP, FM, FD, SHD, ES, UC, SM, CV, SF, FR, PB, HVV, DC, CD. Analysis and interpretation of data: CC, FP, FR, MS, CD. Drafting of the manuscript: CC, FP, CD. Statistical analysis: FP, FR. Study supervision: CC, FP, CD. Critical revision and final approval of the manuscript: CC, FP, HD, AD, IFB, KB, CB, LGP, PB, ME, JP, FM, FD, SHD, ES, UC, SM, CV, SF, FR, PB, HVV, DC, MS, QL, CD.
Data availability statement
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Conflicts of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
This work is dedicated to the memory of Professor Federico Manenti.
We would like to thank all co-authors from this French–Italian–Latin American collaborative group for HCC and liver transplantation.
To every co-author who participated in this cohort study:
France: Karim Boudjema, Philippe Bachellier, Filomena Conti, Olivier Scatton, Fabrice Muscari, Ephrem Salame, Pierre Henri Bernard, Claire Francoz, Francois Durand, Sébastien Dharancy, Marie-lorraine Woehl, Claire Vanlemmens, Alexis Laurent, Sylvie Radenne, Jérôme Dumortier, Armand Abergel, Daniel Cherqui, Louise Barbier, Pauline Houssel-Debry, Georges Philippe Pageaux, Laurence Chiche, Victor Deledinghen, Jean Hardwigsen, J Gugenheim, M Altieri, Marie Noelle Hilleret, Thomas Decaens, and Christophe Duvoux.
Latin America: Federico Piñero, Aline Chagas, Paulo Costa, Elaine Cristina de Ataide, Emilio Quiñones, Sergio Hoyos Duque, Sebastián Marciano, Margarita Anders, Adriana Varón, Alina Zerega, Jaime Poniachik, Alejandro Soza, Martín Padilla Machaca, Diego Arufe, Josemaría Menéndez, Rodrigo Zapata, Mario Vilatoba, Linda Muñoz, Ricardo Chong Menéndez, Martín Maraschio, Luis G Podestá, Lucas McCormack, Juan Mattera, Adrian Gadano, Ilka SF Fatima Boin, Jose Huygens Parente García, Flair Carrilho, and Marcelo Silva.
Italy: Andrea Notarpaolo, Giulia Magini, Lucia Miglioresi, Martina Gambato, Fabrizio Di Benedetto, Cecilia D’Ambrosio, Giuseppe Maria Ettorre, Alessandro Vitale, Patrizia Burra, Stefano Fagiuoli, Umberto Cillo, Michele Colledan, Domenico Pinelli, Paolo Magistri, Giovanni Vennarecci, Marco Colasanti, Valerio Giannelli, Adriano Pellicelli, Cizia Baccaro, and Quirino Lai.
Belgium: Helena Degroote, Hans Van Vlierberghe, Callebout Eduard, Iesari Samuele, Dekervel Jeroen, Schreiber Jonas, Pirenne Jacques, Verslype Chris, Ysebaert Dirk, Michielsen Peter, Lucidi Valerio, Moreno Christophe, Detry Olivier, Delwaide Jean, Troisi Roberto, and Lerut Jan Paul.
We also thank all surgeons, hepatologists, pathologists, and radiologists from all participating French and Italian centres for their contribution, which made this work possible.
To the patients included in this study.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2022.100445.
Contributor Information
Charlotte Costentin, Email: ccostentin@chu-grenoble.fr.
French-Italian-Belgium and Latin American collaborative group for HCC and liver transplantation:
Karim Boudjema, Philippe Bachellier, Filomena Conti, Olivier Scatton, Fabrice Muscari, Ephrem Salame, Pierre Henri Bernard, Claire Francoz, Francois Durand, Sébastien Dharancy, Marie-lorraine Woehl, Claire Vanlemmens, Alexis Laurent, Sylvie Radenne, Jérôme Dumortier, Armand Abergel, Daniel Cherqui, Louise Barbier, Pauline Houssel-Debry, Georges Philippe Pageaux, Laurence Chiche, Victor Deledinghen, Jean Hardwigsen, J. Gugenheim, M. Altieri, Marie Noelle Hilleret, Thomas Decaens, Christophe Duvoux, Federico Piñero, Aline Chagas, Paulo Costa, Elaine Cristina de Ataide, Emilio Quiñones, Sergio Hoyos Duque, Sebastián Marciano, Margarita Anders, Adriana Varón, Alina Zerega, Jaime Poniachik, Alejandro Soza, Martín Padilla Machaca, Diego Arufe, Josemaría Menéndez, Rodrigo Zapata, Mario Vilatoba, Linda Muñoz, Ricardo Chong Menéndez, Martín Maraschio, Luis G. Podestá, Lucas McCormack, Juan Mattera, Adrian Gadano, Ilka S.F. Fatima Boin, Jose Huygens Parente García, Flair Carrilho, Marcelo Silva, Andrea Notarpaolo, Giulia Magini, Lucia Miglioresi, Martina Gambato, Fabrizio Di Benedetto, Cecilia D’Ambrosio, Giuseppe Maria Ettorre, Alessandro Vitale, Patrizia Burra, Stefano Fagiuoli, Umberto Cillo, Michele Colledan, Domenico Pinelli, Paolo Magistri, Giovanni Vennarecci, Marco Colasanti, Valerio Giannelli, Adriano Pellicelli, Cizia Baccaro, Quirino Lai, Helena Degroote, Hans Van Vlierberghe, Callebout Eduard, Iesari Samuele, Dekervel Jeroen, Schreiber Jonas, Pirenne Jacques, Verslype Chris, Ysebaert Dirk, Michielsen Peter, Lucidi Valerio, Moreno Christophe, Detry Olivier, Delwaide Jean, Troisi Roberto, and Lerut Jan Paul
Supplementary data
The following are the supplementary data to this article:
References
- 1.Llovet J.M., Kelley R.K., Villanueva A., Singal A.G., Pikarsky E., Roayaie S., et al. Hepatocellular carcinoma. Nat Rev Dis Prim. 2021;7:6. doi: 10.1038/s41572-020-00240-3. [DOI] [PubMed] [Google Scholar]
- 2.Llovet J.M., Fuster J., Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434–1440. doi: 10.1002/hep.510300629. [DOI] [PubMed] [Google Scholar]
- 3.Clavien P.A., Lesurtel M., Bossuyt P.M.M., Gores G.J., Langer B., Perrier A., et al. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13:e11–e22. doi: 10.1016/S1470-2045(11)70175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mazzaferro V., Regalia E., Doci R., Andreola S., Pulvirenti A., Bozzetti F., et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 5.Mazzaferro V., Llovet J.M., Miceli R., Bhoori S., Schiavo M., Mariani L., et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35–43. doi: 10.1016/S1470-2045(08)70284-5. [DOI] [PubMed] [Google Scholar]
- 6.Duvoux C., Roudot-Thoraval F., Decaens T., Pessione F., Badran H., Piardi T., et al. Liver transplantation for hepatocellular carcinoma: a model including alpha-fetoprotein improves the performance of Milan criteria. Gastroenterology. 2012;143:986–994 e3. doi: 10.1053/j.gastro.2012.05.052. quiz e14–5. [DOI] [PubMed] [Google Scholar]
- 7.Mazzaferro V., Sposito C., Zhou J., Pinna A.D., De Carlis L., Fan J., et al. Metroticket 2.0 model for analysis of competing risks of death after liver transplantation for hepatocellular carcinoma. Gastroenterology. 2018;154:128–139. doi: 10.1053/j.gastro.2017.09.025. [DOI] [PubMed] [Google Scholar]
- 8.Yao F.Y., Ferrell L., Bass N.M., Watson J.J., Bacchetti P., Venook A., et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 9.Shah S.A., Tan J.C.C., McGilvray I.D., Cattral M.S., Cleary S.P., Levy G.A., et al. Accuracy of staging as a predictor for recurrence after liver transplantation for hepatocellular carcinoma. Transplantation. 2006;81:1633–1639. doi: 10.1097/01.tp.0000226069.66819.7e. [DOI] [PubMed] [Google Scholar]
- 10.Costentin C.E., Amaddeo G., Decaens T., Boudjema K., Bachellier P., Muscari F., et al. Prediction of hepatocellular carcinoma recurrence after liver transplantation: comparison of four explant-based prognostic models. Liver Int. 2017;37:717–726. doi: 10.1111/liv.13388. [DOI] [PubMed] [Google Scholar]
- 11.Mehta N., Heimbach J., Harnois D.M., Sapisochin G., Dodge J.L., Lee D., et al. Validation of a Risk Estimation of Tumor Recurrence after Transplant (RETREAT) score for hepatocellular carcinoma recurrence after liver transplant. JAMA Oncol. 2017;3:493–500. doi: 10.1001/jamaoncol.2016.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menon K.V., Hakeem A.R., Heaton N.D. Meta-analysis: recurrence and survival following the use of sirolimus in liver transplantation for hepatocellular carcinoma. Aliment Pharmacol Ther. 2013;37:411–419. doi: 10.1111/apt.12185. [DOI] [PubMed] [Google Scholar]
- 13.Toso C., Meeberg G.A., Bigam D.L., Oberholzer J., Shapiro A.M.J., Gutfreund K., et al. De novo sirolimus-based immunosuppression after liver transplantation for hepatocellular carcinoma: long-term outcomes and side effects. Transplantation. 2007;83:1162–1168. doi: 10.1097/01.tp.0000262607.95372.e0. [DOI] [PubMed] [Google Scholar]
- 14.Cholongitas E., Mamou C., Rodríguez-Castro K.I., Burra P. Mammalian target of rapamycin inhibitors are associated with lower rates of hepatocellular carcinoma recurrence after liver transplantation: a systematic review. Transpl Int. 2014;27:1039–1049. doi: 10.1111/tri.12372. [DOI] [PubMed] [Google Scholar]
- 15.Geissler E.K., Schnitzbauer A.A., Zülke C., Lamby P.E., Proneth A., Duvoux C., et al. Sirolimus use in liver transplant recipients with hepatocellular carcinoma: a randomized, multicenter, open-label phase 3 trial. Transplantation. 2016;100:116–125. doi: 10.1097/TP.0000000000000965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan E.Y., Larson A.M., Fix O.K., Yeh M.M., Levy A.E., Bakthavatsalam R., et al. Identifying risk for recurrent hepatocellular carcinoma after liver transplantation: implications for surveillance studies and new adjuvant therapies. Liver Transpl. 2008;14:956–965. doi: 10.1002/lt.21449. [DOI] [PubMed] [Google Scholar]
- 17.Iwatsuki S., Dvorchik I., Marsh J.W., Madariaga J.R., Carr B., Fung J.J., et al. Liver transplantation for hepatocellular carcinoma: a proposal of a prognostic scoring system. J Am Coll Surg. 2000;191:389–394. doi: 10.1016/s1072-7515(00)00688-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parfitt J.R., Marotta P., Alghamdi M., Wall W., Khakhar A., Suskin N.G., et al. Recurrent hepatocellular carcinoma after transplantation: use of a pathological score on explanted livers to predict recurrence. Liver Transpl. 2007;13:543–551. doi: 10.1002/lt.21078. [DOI] [PubMed] [Google Scholar]
- 19.Decaens T., Roudot-Thoraval F., Badran H., Wolf P., Durand F., Adam R., et al. Impact of tumour differentiation to select patients before liver transplantation for hepatocellular carcinoma. Liver Int. 2011;31:792–801. doi: 10.1111/j.1478-3231.2010.02425.x. [DOI] [PubMed] [Google Scholar]
- 20.Halazun K.J., Najjar M., Abdelmessih R.M., Samstein B., Griesemer A.D., Guarrera J.V., et al. Recurrence after liver transplantation for hepatocellular carcinoma: a new MORAL to the story. Ann Surg. 2017;265:557–564. doi: 10.1097/SLA.0000000000001966. [DOI] [PubMed] [Google Scholar]
- 21.Lee J.H., Cho Y., Kim H.Y., Cho E.J., Lee D.H., Yu S.J., et al. Serum tumor markers provide refined prognostication in selecting liver transplantation candidate for hepatocellular carcinoma patients beyond the Milan criteria. Ann Surg. 2016;263:842–850. doi: 10.1097/SLA.0000000000001578. [DOI] [PubMed] [Google Scholar]
- 22.Mehta N., Dodge J.L., Roberts J.P., Yao F.Y. Validation of the prognostic power of the RETREAT score for hepatocellular carcinoma recurrence using the UNOS database. Am J Transpl. 2018;18:1206–1213. doi: 10.1111/ajt.14549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Notarpaolo A., Layese R., Magistri P., Gambato M., Colledan M., Magini G., et al. Validation of the AFP model as a predictor of HCC recurrence in patients with viral hepatitis-related cirrhosis who had received a liver transplant for HCC. J Hepatol. 2017;66:552–559. doi: 10.1016/j.jhep.2016.10.038. [DOI] [PubMed] [Google Scholar]
- 24.Piñero F., Tisi Baña M., de Ataide E.C., Hoyos Duque S., Marciano S., Varón A., et al. Liver transplantation for hepatocellular carcinoma: evaluation of the alpha-fetoprotein model in a multicenter cohort from Latin America. Liver Int. 2016;36:1657–1667. doi: 10.1111/liv.13159. [DOI] [PubMed] [Google Scholar]
- 25.Costentin C.E., Bababekov Y.J., Zhu A.X., Yeh H. Is it time to reconsider the Milan criteria for selecting patients with hepatocellular carcinoma for deceased-donor liver transplantation? Hepatology. 2019;69:1324–1336. doi: 10.1002/hep.30278. [DOI] [PubMed] [Google Scholar]
- 26.European Association for the Study of the Liver EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 27.von Elm E., Altman D.G., Egger M., Pocock S.J., Gotzsche P.C., Vandenbroucke J.P., et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 28.McShane L.M., Altman D.G., Sauerbrei W., Taube S.E., Gion M., Clark G.M., et al. Reporting recommendations for tumor marker prognostic studies (REMARK) J Natl Cancer Inst. 2005;97:1180–1184. doi: 10.1093/jnci/dji237. [DOI] [PubMed] [Google Scholar]
- 29.Edmondson H.A., Steiner P.E. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7:462–503. doi: 10.1002/1097-0142(195405)7:3<462::aid-cncr2820070308>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 30.Maccali C., Chagas A.L., Boin I., Quinonez E., Marciano S., Vilatoba M., et al. Recurrence of hepatocellular carcinoma after liver transplantation: prognostic and predictive factors of survival in a Latin American cohort. Liver Int. 2021;41:851–862. doi: 10.1111/liv.14736. [DOI] [PubMed] [Google Scholar]
- 31.Dignam J.J., Zhang Q., Kocherginsky M. The use and interpretation of competing risks regression models. Clin Cancer Res. 2012;18:2301–2308. doi: 10.1158/1078-0432.CCR-11-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montal R., Andreu-Oller C., Bassaganyas L., Esteban-Fabró R., Moran S., Montironi C., et al. Molecular portrait of high alpha-fetoprotein in hepatocellular carcinoma: implications for biomarker-driven clinical trials. Br J Cancer. 2019;121:340–343. doi: 10.1038/s41416-019-0513-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishioka S.T., Sato M.M., Wong L.L., Tiirikainen M., Kwee S.A. Clinical and molecular sub-classification of hepatocellular carcinoma relative to alpha-fetoprotein level in an Asia-Pacific island cohort. Hepatoma Res. 2018;4:1. doi: 10.20517/2394-5079.2017.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan P.S., Nakagawa S., Goossens N., Venkatesh A., Huang T., Ward S.C., et al. Clinicopathological indices to predict hepatocellular carcinoma molecular classification. Liver Int. 2016;36:108–118. doi: 10.1111/liv.12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aggarwal A., Te H.S., Verna E.C., Desai A.P. A national survey of hepatocellular carcinoma surveillance practices following liver transplantation. Transpl Direct. 2021;7:e638. doi: 10.1097/TXD.0000000000001086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berenguer M., Burra P., Ghobrial M., Hibi T., Metselaar H., Sapisochin G., et al. Posttransplant management of recipients undergoing liver transplantation for hepatocellular carcinoma. Working Group Report from the ILTS Transplant Oncology Consensus Conference. Transplantation. 2020;104:1143–1149. doi: 10.1097/TP.0000000000003196. [DOI] [PubMed] [Google Scholar]
- 37.Lee D.D., Sapisochin G., Mehta N., Gorgen A., Musto K.R., Hajda H., et al. Surveillance for HCC after liver transplantation: increased monitoring may yield aggressive treatment options and improved postrecurrence survival. Transplantation. 2020;104:2105–2112. doi: 10.1097/TP.0000000000003117. [DOI] [PubMed] [Google Scholar]
- 38.Verna E.C., Patel Y.A., Aggarwal A., Desai A.P., Frenette C., Pillai A.A., et al. Liver transplantation for hepatocellular carcinoma: management after the transplant. Am J Transpl. 2020;20:333–347. doi: 10.1111/ajt.15697. [DOI] [PubMed] [Google Scholar]
- 39.Zimmerman M.A., Trotter J.F., Wachs M., Bak T., Campsen J., Skibba A., et al. Sirolimus-based immunosuppression following liver transplantation for hepatocellular carcinoma. Liver Transpl. 2008;14:633–638. doi: 10.1002/lt.21420. [DOI] [PubMed] [Google Scholar]
- 40.Vivarelli M., Dazzi A., Zanello M., Cucchetti A., Cescon M., Ravaioli M., et al. Effect of different immunosuppressive schedules on recurrence-free survival after liver transplantation for hepatocellular carcinoma. Transplantation. 2010;89:227–231. doi: 10.1097/TP.0b013e3181c3c540. [DOI] [PubMed] [Google Scholar]
- 41.Zhou J., Wang Z., Wu Z.Q., Qiu S.J., Yu Y., Huang X.W., et al. Sirolimus-based immunosuppression therapy in liver transplantation for patients with hepatocellular carcinoma exceeding the Milan criteria. Transpl Proc. 2008;40:3548–3553. doi: 10.1016/j.transproceed.2008.03.165. [DOI] [PubMed] [Google Scholar]
- 42.Bruix J., Qin S., Merle P., Granito A., Huang Y.H., Bodoky G., et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 43.Abou-Alfa G.K., Meyer T., Cheng A.L., El-Khoueiry A.B., Rimassa L., Ryoo B.Y., et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379:54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kudo M., Finn R.S., Qin S., Han K.H., Ikeda K., Piscaglia F., et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 45.Cucchetti A., Serenari M., Sposito C., Di Sandro S., Mosconi C., Vicentin I., et al. Including mRECIST in the Metroticket 2.0 criteria improves prediction of hepatocellular carcinoma-related death after liver transplant. J Hepatol. 2020;73:342–348. doi: 10.1016/j.jhep.2020.03.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analysed during the current study are available from the corresponding author on reasonable request.