Abstract
Background & Aims
HDV affects 4.5–13% of chronic hepatitis B (CHB) patients globally, yet the prevalence of HDV infection in Canada is unknown. To investigate the prevalence, genotype, demographics, and clinical characteristics of HDV in Canada, we conducted a retrospective analysis of (1) HDV antibody and RNA positivity among referred specimens, and (2) a cross-sectional subset study of 135 HDV seropositive +/-RNA (HDV+) patients compared with 5,132 HBV mono-infected patients in the Canadian HBV Network.
Methods
Anti-HDV IgG-positive specimens collected between 2012 and 2019 were RNA tested and the genotype determined. Patients enrolled in the Canadian HBV Network were >18 years of age and HBsAg-positive. Clinical data collected included risk factors, demographics, comorbidities, treatment, fibrosis assessment, and hepatic complications.
Results
Of the referred patients, 338/7,080 (4.8%, 95% CI 4.3–5.3) were HDV seropositive, with 219/338 RNA-positive (64.8%, 95% CI 59.6–69.7). The HDV+ cohort were more likely to be born in Canada, to be White or Black/African/Caribbean than Asian, and reporting high-risk behaviours, compared with HBV mono-infected patients. Cirrhosis, complications of end-stage liver disease, and liver transplantation were significantly more frequent in the HDV+ cohort. HDV viraemia was significantly associated with elevated liver transaminases and cirrhosis. Five HDV genotypes were observed among referred patients but no association between genotype and clinical outcome was detected within the HDV+ cohort.
Conclusions
Nearly 5% of the Canadian HBV referral population is HDV seropositive. HDV infection is highly associated with risk behaviours and both domestic and foreign-born patients with CHB. HDV was significantly associated with progressive liver disease highlighting the need for increased screening and surveillance of HDV in Canada.
Lay summary
Evidence of HDV infection was observed in approximately 5% of Canadians who were infected with HBV referred to medical specialists. HDV-positive patients were more likely to be male, born in Canada, or White or Black/African/Caribbean compared to Asian, and to have reported high-risk activities such as injection or intranasal drug use or high-risk sexual contact compared with patients infected with only HBV. Patients infected with HDV were also more likely to suffer severe liver disease, including liver cancer, compared with HBV mono-infected patients.
Keywords: Hepatitis B virus, Hepatitis D virus, Genotype, Epidemiology, Cirrhosis
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; bp, base pairs; CanHepB Network, Canadian HBV network; CHB, chronic HBV infection; gt, genotype; HCC, hepatocellular carcinoma; IFNα, pegylated interferon-alpha; INR, international normalised ratio (prothrombin time of blood clotting); n, count; NAs, nucleos(t)ide analogue inhibitors; NML, National Microbiology Laboratory; qHBsAg, quantitative HBsAg; TE, transient elastography
Graphical abstract
Highlights
-
•
There was ∼5% HDV seroprevalence in Canadian HBsAg-positive referred patients.
-
•
HDV genotypes 1, 2, and 5–7 were observed in referred patients.
-
•
HDV+ patients were more likely to be born in Canada, White or Black/African/Caribbean.
-
•
HDV was highly associated with high-risk activities (sexual and intravenous drug use).
-
•
HDV+ patients were more likely diagnosed with cirrhosis or liver cancer.
Introduction
Chronic infection with HDV results in the highest rate of severe liver disease, including the development of cirrhosis in nearly 80% of patients within 2–10 years of infection,1,2 with a significantly increased risk of hepatocellular carcinoma (HCC) compared with HBV infection alone.3 HDV requires HBV to complete its replication cycle and produce enveloped viral particles providing receptor-binding function. HDV superinfection of HBsAg-positive individuals most often results in persistent, chronic infection whereas concurrent co-infection in adults with HBV and HDV usually resolves spontaneously.4 Although the HBV vaccine reduces the risk of HDV infection by preventing the helper virus infection, aside from pegylated interferon-alpha (IFNα), there are no specific antiviral drugs available to treat HDV in Canada. However, new therapies, such as lonafarnib (a prenylation inhibitor) and bulevirtide (a receptor blocker or viral entry inhibitor), demonstrating improved effectiveness in comparison to IFNα,5 are currently in clinical trials or have received conditional approval.6 Screening for HDV infection is recommended in HBsAg-positive individuals, particularly in patients from HDV endemic regions,7,8 those having a history of high-risk (injection drug use, sexual contact) behaviours,9 or who display abnormal liver biochemistry in the presence of low or undetectable HBV DNA.10
There are 8 HDV genotypes (gt1–gt8), all having distinct geographic distribution other than gt1.11 The true prevalence and distribution of HDV seroprevalence and active infection is uncertain, with estimates that vary significantly.12,13 One study established a global HDV prevalence estimate at 12 million infected individuals,14 or approximately 4.5% of HBsAg-positive individuals. Canada has an estimated HBV infection rate of approximately 13.4 per 100,000 (2017 data15) with >250,000 chronically infected Canadians living with HBV.16 Information on HDV prevalence in Canada is lacking, although small studies have described anti-HDV positivity rates ranging from 0 to 1.6% among HBsAg-positive Indigenous populations17,18 to 2.6% in blood donors and clinic patients,19 with evidence of HDV infection primarily observed in non-Canadian-born individuals.
The National Microbiology Laboratory (NML) of the Public Health Agency of Canada serves as the reference laboratory for viral hepatitis diagnostic testing, including the provision of HDV serology and molecular testing services for Canada. This allowed a centralised investigation to estimate the prevalence, molecular epidemiology, and clinical associations of HDV infection in a population of referred patients, in collaboration with the Canadian HBV Network (CanHepB Network).
Patients and methods
Patient population and study design
To estimate the prevalence of HDV antibody and RNA positivity within a referred population, specimens received at the NML for HDV antibody testing from January 2012 to December 2019 were considered. Submission guidelines require specimens to be HBsAg-positive, thus all patients were tested for HBV under the care of a healthcare professional. Patient replicate requests were removed and the first occurrence of HDV antibody positivity was included to create the total study population of unique referred individuals. Antibody-positive specimens were tested for HDV RNA to estimate the prevalence of active HDV infection among the referred population and to characterise HDV genotypes.
A cross-sectional, retrospective study of clinical and demographic factors among patients of physicians participating in the CanHepB Network, an interprovincial network of 21 tertiary care-based clinical cohorts, was also conducted. Retrospective data were analysed from 9 infectious disease or hepatology clinics in 6 provinces across Canada (British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, and Quebec).16 Each of these sites provide care for both HBV mono-infected and HDV-HBV co-infected individuals. Each site conducted data collection by reviewing electronic and paper patient charts. De-identified information was entered into a registry database (i.e. RedCap®) housed at the University of Calgary.
All participants >18 years of age with confirmed HBV infection status (HBsAg-positive for >6 months), and who were seen by a physician affiliated with the CanHepB Network after 1 January, 2012 were included. Patients were classified as HDV-HBV co-infected if HBsAg-positive and/or by physician report based on local standard of care testing procedures, with positive anti-HDV and/or HDV RNA confirmation. Patients were included in an HBV mono-infected comparator arm if not co-infected with HCV, HIV, and presumably HDV. Available retrospective data elements included age at most recent laboratory testing, sex, ethnicity, country of birth, and risk factor history. Most recent values for laboratory tests were used including liver enzymes, viral serology and HBV viral load. Non-invasive tests for fibrosis included liver stiffness measurement/transient elastography (TE, FibroScan®).
Clinical outcomes including co-morbid medical conditions and complications of liver disease were captured from physician medical record reports and based on standard diagnostic criteria. Treatment in both cohorts was defined as ‘treatment at any time’, including those who received multiple treatment courses or prior treatments that were since discontinued. ‘High-risk activities’ were defined as patients who had documented injection/intranasal drug use and/or high-risk sexual contact. Countries with ≥5% prevalence of HBV were considered endemic.7,8
Ethical approval
The study protocol follows the ethical guidelines of the 1975 Declaration of Helsinki as determined through approvals by individual clinic or site Research Ethics Boards, as detailed in the Supplementary data.
Virological testing
The methods and materials used for testing HDV and HBV for serological and/or molecular markers and genotype determination are provided in the Supplementary data.
Statistical analysis
Methods of statistical analysis for study results are provided in the Supplementary data.
Results
Patient cohorts
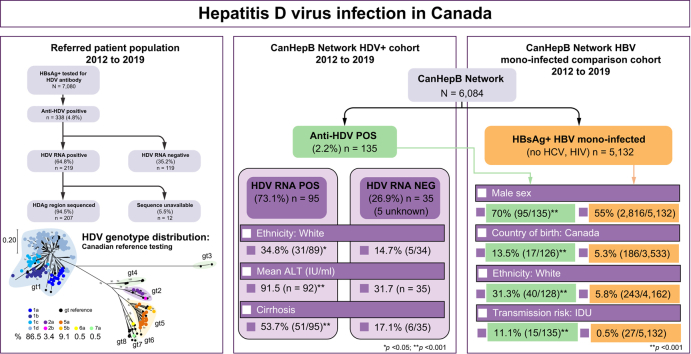
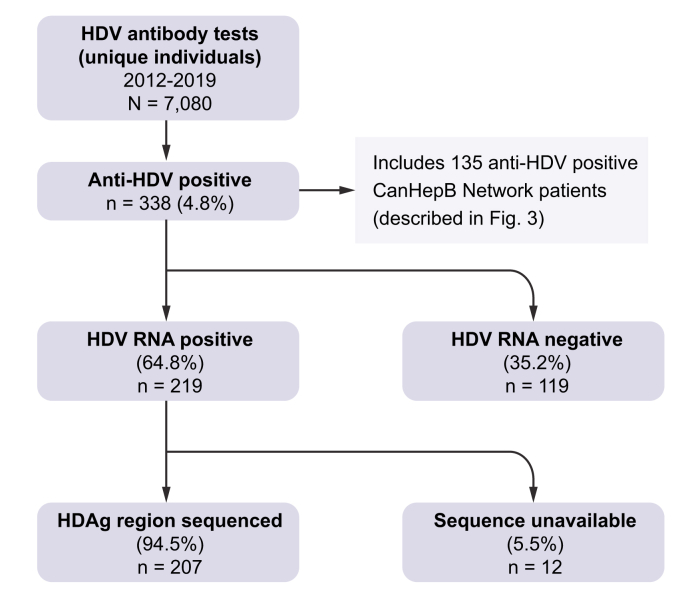
Referral testing for HDV seropositivity was conducted on 7,080 unique patients from 2012 to 2019 (Fig. 1). A total of 338 individuals (4.8%, 95% CI 4.3–5.3) were HDV IgG seropositive, 6,736 were seronegative and 6 were repeatedly borderline. Further investigation focused on the 338 seropositive specimens. HDV RNA positivity was determined in 219/338 and 119 were RNA-negative, indicating an active infection rate of 64.8% (95% CI 59.6–69.7) among HBsAg-positive, HDV-seropositive individuals receiving healthcare in Canada, or 3.09% of the total population referred for HDV serological testing. Age and sex information was available for 99.7% (7059/7080) and 98.7% (6991/7080) of patients, respectively. There was no difference between the mean age or sex distribution of seropositive and seronegative individuals (Table S1). The majority of referred patients were >40 years; however, those 31–40 years of age comprised the highest percentage among the entire cohort (27.3% HDV seropositive; 26.3% HDV seronegative). Males were more frequently represented among those tested for anti-HDV antibody (62.0%) and those seropositive (69.3%; p = 0.0055).
Fig. 1.
Results of HDV reference diagnostic testing, 2012–2019.
Referred patient HDV genotypes
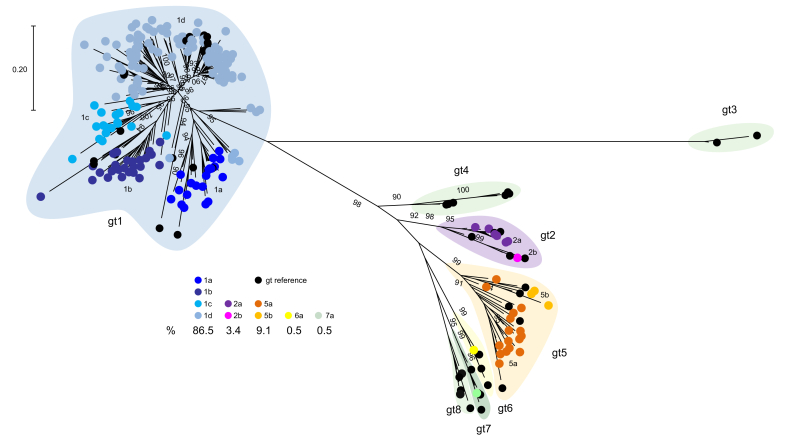
Twelve of the 219 HDV RNA-positive samples could not be sequenced (insufficient specimen or viral load too low for sequence analysis), thus 207 (94.5%) HDV RNA-positive samples were genotyped (Fig. 1; GenBank accession numbers MZ712366–MZ712572). Five HDV genotypes were observed; gt1, 2, and 5–7 (Fig. 2). Genotype 1 predominated at 86.5%, followed by gt5 (9.1%), gt2 (3.4%), and gts6 and 7 (0.5% each). Phylogenetic analysis of RNA-positive specimens, including HDV subgenotype reference sequences,11 allowed the discernment of several subgenotypes within the Canadian referral population, with gt1d observed in over half of all RNA-positive specimens; gt1a 9.7%, gt1b 12.1%, gt1c 8.7%, gt1d 56.0%, gt2a 2.9%, gt2b 0.5%, gt5a 7.7%, gt5b 1.4%, gt6a 0.5%, and gt7a 0.5%.
Fig. 2.
Phylogenetic analysis of HDV sequences, 2012–2019.
Branch support ≥70% is shown and the ruler indicates a pairwise distance equal to 0.2. The proportion of each genotype is indicated.
Demographic, ethnic, and risk factor association with HDV infection
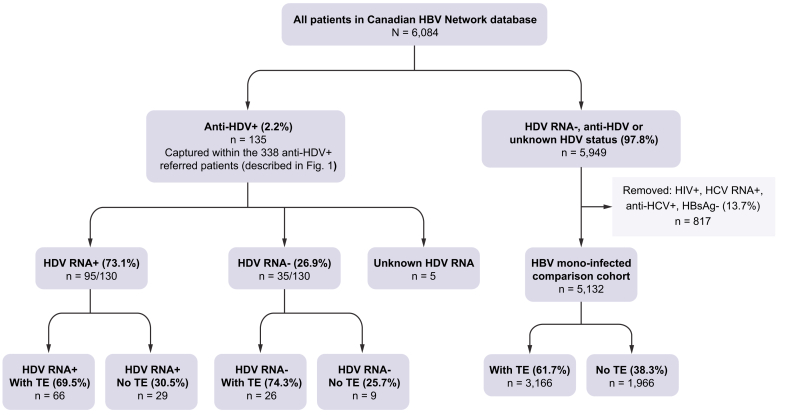
Patients within the CanHepB Network were referred to specialist clinics for management of HBV infection, with HDV antibody screening at intake based on individual physician practices and Canadian guidelines.20 Demographic, risk factor, and medical history, including treatment and hepatic complication information was available for 135 HDV-seropositive Network participants, forming a subset within the total referred HDV-seropositive population (Fig. 3), with 35 HDV RNA-negative, 5 without RNA data, and 95 positive for HDV RNA (95/130; 73.1%). As patients and their data were retrospectively identified and included in the HDV–HBV co-infected study, the seropositive cases without RNA data could not be matched with a laboratory specimen at the NML as a result of disparate laboratory coding.
Fig. 3.
Canadian HBV Network HDV–HBV co-infected and HBV mono-infected participants included in the study.
HDV antibody, HDV RNA and transient elastography (FibroScan®; TE) results for the co-infected cohort is shown, as is the inclusion/exclusion criteria and TE results of the mono-infected comparator cohort.
To analyse variables in the context of HDV infection, 5,132 HBV-mono-infected CanHepB Network participants with available information were included in the analysis, following exclusion of 817 patients as a result of HIV or HCV RNA or antibody positivity, or because of having spontaneous or treatment-induced HBsAg loss. TE results were available for the majority of HDV RNA-positive and -negative individuals and the HBV mono-infected cohort (Fig. 3). The 135 HDV-seropositive participants are referred to as the HDV+ cohort.
Within the CanHepB cohort there was no age difference between the HDV+ cohort and HBV mono-infected patients, although a significant association with male sex was observed (p <0.001; Table 1). There were a significantly higher number of Canadian-born persons within this cohort compared with those HBV mono-infected (p <0.001). No significant difference was observed between both cohorts based on birth in countries endemic for HBV (Table S2), although birth in an HBV non-endemic country was associated with HBV mono-infection compared to HDV-HBV co-infection (Table 1). Black/African/Caribbean or White patients showed significantly increased representation among the HDV+ cohort, whereas Asian patients were more likely to be HBV mono-infected. High-risk behaviours, such as injection or intranasal drug use and high-risk sexual contact were significantly observed within the co-infected group compared with the mono-infected group (p <0.001; Table 1).
Table 1.
Comparison of demographics and risk factors for HBV and HDV exposure in HBV mono-infected individuals (n = 5,132) compared with HDV-HBV co-infected∗individuals (n = 135) followed in the Canadian HBV Network.
| HDV-HBV co-infected (n = 135) | HBV mono-infected (n = 5,132) | p value | |
|---|---|---|---|
| Age | 46.2 (44.0–48.3, 135) | 47.8 (47.5–48.2, 5,132) | 0.163 |
| Male sex | 70% (95/135) | 55% (2816/5,132) | <0.001 |
| Country of birth | |||
| Canada | 13.5% (17/126) | 5.3% (186/3,533) | <0.001 |
| Country endemic for HBV† | 62.7% (79/126) | 54.7% (1,855/3,393) | 0.075 |
| Country non-endemic for HBV | 23.8% (30/126) | 39.8% (1,352/3,393) | <0.001 |
| Ethnicity | |||
| Asian | 25.0% (32/128) | 72.5% (3,019/4,162) | <0.001 |
| White | 31.3% (40/128) | 5.8% (243/4,162) | <0.001 |
| Black/African/Caribbean | 37.5% (48/128) | 17.8% (740/4,162) | <0.001 |
| Indigenous | 0% (0/115) | 0.3% (11/4,162) | 0.581 |
| Other ethnicity‡ | 6.3% (8/128) | 3.6% (148/4,162) | 0.109 |
| Other sociodemographic factors | |||
| Alcohol use | 33.3% (45/135) | 14.2% (726/5,132) | <0.001 |
| Smoking | 26.7% (36/135) | 7.5% (386/5,132) | <0.001 |
| Injection/intranasal drug use | 11.1% (15/135) | 0.5% (27/5,132) | <0.001 |
| High-risk sexual contact | 5.9% (8/135) | 2.2% (112/5,132) | <0.001 |
Continuous data are shown as mean (95% CI, n known). Categorical data are shown as mean % (n/n known). T test was used, and chi-square tests were done for categorical data. Values of p <0.05 considered significant. For both continuous and categorical variables missing data are excluded (n known is shown in table).
HDV–HBV = anti-HDV+ (and HDV RNA+ or HDV RNA-).
Countries with ≥5% prevalence of HBV were considered endemic (see Table S2).
Hispanic and Middle Eastern.
Co-morbidities, hepatic complications, and treatment experience associated with HDV infection
The HDV+ cohort demonstrated a significantly higher prevalence of severe hepatic outcomes compared with HBV mono-infected patients (p <0.001; Table 2), although comorbidity associations were limited to hypertension, chronic kidney disease, and cancer (excluding HCC; p ≤0.030; Table S3). Mean TE values among the HDV+ cohort were more than twice the mean of the HBV mono-infected group (12.9 kPa, range 10.6–15.2 vs. 6.2 kPa, range 6.0–6.4). The HDV+ cohort were more likely to have undergone liver transplantation and to have been diagnosed with cirrhosis or HCC. Variceal bleeding and hepatic encephalopathy were also more frequent among HDV–HBV co-infected patients. In keeping with these observations, biochemical markers of liver damage and liver dysfunction were significantly higher among the HDV+ cohort (ALT, AST, bilirubin, p <0.001; INR p = 0.012). Not surprisingly, the presence and quantity of HBV DNA were significantly higher among the HBV mono-infected cohort, likely caused by the recognised suppressive effect of HDV on HBV infection.21 A small percentage of HDV+ cohort patients also infected with HIV (11.5%) or HCV (16.8%), were included in the study. Significantly more of the HDV+ cohort in comparison with HBV mono-infected patients had HBV antiviral treatment experience, involving the nucleos(t)ide analogue inhibitors (NAs) entecavir (p = 0.004) or tenofovir (p = 0.002), but not lamivudine. Treatment with IFNα was also significantly more common among the HDV+ cohort (p <0.001; Table 2).
Table 2.
Comparison of hepatic complications, laboratory values, and antiviral treatment experience in HBV mono-infected individuals (n = 5,132) compared with HDV–HBV co-infected∗individuals (n = 135) followed by the Canadian HBV Network.
| HDV–HBV co-infected (n = 135) | HBV mono-infected (n = 5,132) | p value | |
|---|---|---|---|
| Hepatic outcomes | |||
| Liver transplant | 6.7% (9/135) | 0.1% (5/5,132) | <0.001 |
| Cirrhosis | 45.2% (61/135) | 3.2% (165/5,132) | <0.001 |
| Hepatocellular carcinoma | 8.2% (11/135) | 1.0% (51/5,132) | <0.001 |
| Variceal bleeding | 5.2% (7/135) | 0.1% (3/5,132) | <0.001 |
| Hepatic encephalopathy | 2.2% (3/135) | 0.1% (5/5,132) | <0.001 |
| Transient elastography (TE; kPa) | 12.9 kPa (10.6–15.2, 94) | 6.2 kPa (6.0–6.4, 3,166) | <0.001 |
| >F2 fibrosis (TE >7.3 kPa) | 64.9% (61/94) | 16.8% (532/3,166) | <0.001 |
| >F3 fibrosis (TE >10.7 kPa) | 39.4% (37/94) | 6.6% (209/3,166) | <0.001 |
| Laboratory | |||
| ALT (IU/ml) | 75.1 (60.2–89.9, 132) | 39.4 (37.1–41.7, 4,707) | <0.001 |
| ALT % above ULN† | 71.2% (94/132) | 26.8% (1261/4,707) | <0.001 |
| AST (U/L) | 59.9 (50.2–69.7, 109) | 31.4 (29.7–33.0, 4,014) | <0.001 |
| Total bilirubin (μmol/L) | 16.6 (13.1–20.1, 96) | 11.0 (10.6–11.5, 3,297) | <0.001 |
| Creatinine (μmol/L) | 83.3 (72.3–94.3, 90) | 80.1 (77.6–82.5, 2,655) | 0.632 |
| INR | 1.19 (1.13–1.25, 86) | 1.07 (1.05–1.09, 2,634) | 0.012 |
| HBeAg+ | 13.7% (17/124) | 17.6% (617/3,501) | 0.260 |
| HBV DNA, log IU/ml | 1.1 (0.8–1.4, 124) | 2.7 (2.7–2.8, 4,447) | <0.001 |
| HBV DNA detectable | 48.1% (64/133) | 80.6% (3595/4,460) | <0.001 |
| HDV RNA-positive | 73.1% (95/130) | N/A | N/A |
| HIV-positive | 11.5% (11/96) | (Excluded) | N/A |
| HCV-seropositive | 16.8% (18/107) | (Excluded) | N/A |
| Treatment (at any time) | |||
| Antiviral therapy active against HBV | 61.5% (83/135) | 29.5% (1515/5,132) | <0.001 |
| Interferon | 24.4% (33/135) | 2.3% (120/5,132) | <0.001 |
| Lamivudine | 16.3% (22/135) | 12.8% (656/5,132) | 0.229 |
| Tenofovir-based regimen‡ | 28.2% (38/135) | 17.9% (920/5,132) | 0.002 |
| Entecavir | 10.4% (14/135) | 4.8% (248/5,132) | 0.004 |
| Nucleos(t)ide inhibitor | 53.3% (72/135) | 28.6% (1,470/5,132) | <0.001 |
T test was used for continuous data, chi-square tests were used for categorical data. Values of p <0.05 considered significant. For both continuous and categorical variables missing data are excluded (n known is shown in table).
HDV–HBV = anti-HDV+ (and HDV RNA+ or HDV RNA-).
ALT ULN >35 for males, >25 for females.
Tenofovir-based regimen refers to treatment regimen that contains tenofovir disoproxil fumarate (TDF) or tenofovir alafenamide (TAF).
Quantitative HBsAg (qHBsAg) analysis within the HDV+ cohort
In 112 anti-HDV+ patients, qHBsAg results were available with 15/112 (13.4%) having undetectable HBsAg, 13/112 (11.6%) having qHBsAg <100 IU/ml, and 84/112 (75%) having qHBsAg >100 IU/ml. Results were stratified by undetectable, <100 and >100 IU/ml HBsAg in keeping with 100 IU/ml as a therapeutic and predictive endpoint in HBV infection, and to differentiate low HBsAg levels from undetectable. Stratified results were analysed in association with IFNα treatment, presence of cirrhosis or HCC, TE (kPa), and absence of HDV RNA, and to describe the breakdown of HDV genotypes (Table S4). No significant associations among qHBsAg levels and the outcomes investigated were observed.
Associations with RNA-positive HDV infection
Within the HDV+ cohort, patients were stratified into 2 groups: HDV RNA-positive at last test (N = 95) and HDV RNA-negative (N = 35) at last test (Fig. 3), to investigate associations with demographic, clinical, and treatment indicators (Table 3). There were no significant demographic or treatment experience differences between HDV RNA-positive and -negative patients, although there was a significantly higher proportion of HDV RNA positivity among White patients (p = 0.029). White patients were on average older (56 years, 95% CI 52.5–59.6) compared with non-White HDV+ patients (44.9 years, 95% CI 42.4–47.5; p <0.001). HDV RNA positivity was also significantly associated with the presence of cirrhosis, elevated ALT (mean 91.5 IU/ml vs. 31.7 IU/ml), AST (69.6 U/L vs. 35.6 U/L), TE (mean 14.5 kPa vs. 9.4 kPa), and a Fibrosis-4 score >F2 (p = 0.007), but <F3 (>F3, p = 0.058; Table 3).
Table 3.
Summary of demographics, hepatic complications, laboratory values, and antiviral treatment in anti-HDV-positive individuals living with HDV RNA-negative vs. HDV RNA-positive infections.
| Variable | HDV RNA-negative (n = 35) | HDV RNA-positive (n = 95) | p value |
|---|---|---|---|
| Age | 44.6 (40.1–49.1, 35) | 45.8 (43.4–48.4, 95) | 0.604 |
| Male sex | 65.7% (23/35) | 75.3% (70/95) | 0.388 |
| Canada born | 6.3% (2/32) | 15.7% (14/89) | 0.232 |
| Born in country endemic for HBV | 71.9% (23/32) | 59.6% (53/89) | 0.287 |
| Ethnicity | |||
| Asian | 32.4% (11/34) | 22.5% (20/89) | 0.353 |
| White | 14.7% (5/34) | 34.8% (31/89) | 0.029 |
| Black/African/Caribbean | 47.1% (16/34) | 34.8% (31/89) | 0.221 |
| Other ethnicity | 5.9% (2/34) | 7.9% (7/89) | >0.999 |
| Hepatic outcomes | |||
| Liver transplant | 8.6% (3/35) | 4.2% (4/95) | 0.386 |
| Cirrhosis | 17.1% (6/35) | 53.7% (51/95) | <0.001 |
| Hepatocellular carcinoma | 5.7% (2/35) | 8.4% (8/95) | >0.999 |
| Variceal bleeding | 0% (0/35) | 7.4% (7/95) | 0.189 |
| Hepatic encephalopathy | 0% (0/35) | 3.2% (3/95) | 0.563 |
| Transient elastography (TE; kPa) | 9.4 kPa (6.4–12.3, 26) | 14.5 kPa (11.5–17.5, 66) | 0.003 |
| >F2 fibrosis (TE >7.3 kPa) | 42.3% (11/26) | 74.2% (49/66) | 0.007 |
| >F3 fibrosis (TE >10.7 kPa) | 23.1% (6/26) | 47.0% (31/66) | 0.058 |
| Laboratory | |||
| ALT (IU/ml) | 31.7 (27.2–36.1, 35) | 91.5 (71.2–111.7, 92) | <0.001 |
| ALT % above ULN∗ | 48.6% (17/35) | 80.4% (74/92) | <0.001 |
| AST (U/L) | 35.6 (28.2–42.9, 27) | 69.6 (56.6–82.6, 77) | <0.001 |
| Total bilirubin (μmol/L) | 19.8 (9.5–30.0, 21) | 15.7 (12.1–19.3, 75) | 0.313 |
| Creatinine (μmol/L) | 81.5 (68.5–94.5, 20) | 83.8 (70.0–97.7, 70) | 0.642 |
| INR | 1.3 (1.1–1.4, 22) | 1.2 (1.1–1.2, 64) | 0.857 |
| HBeAg+ | 6.1% (2/33) | 16.1% (14/87) | 0.230 |
| HBV DNA, Log IU/ml | 1.29 (0.67–1.92, 31) | 1.10 (0.76–1.45, 90) | 0.613 |
| HBV DNA detectable | 52.9% (18/34) | 46.8% (44/94) | 0.555 |
| HIV positive | 0% (0/20) | 15.5% (11/71) | 0.113 |
| HCV seropositive | 8.7% (2/23) | 19.8% (16/81) | 0.349 |
| Treatment (at any time) | |||
| Antiviral therapy active against HBV | 51.4% (18/35) | 65.3% (62/95) | 0.161 |
| Interferon | 17.1% (6/35) | 26.3% (25/95) | 0.356 |
| Lamivudine | 8.6% (3/35) | 20.0% (19/95) | 0.187 |
| Tenofovir-based regimen† | 20.0% (7/35) | 31.6% (30/95) | 0.273 |
| Entecavir | 11.4% (4/35) | 9.5% (9/95) | 0.747 |
| Nucleos(t)ide inhibitor | 48.6% (17/35) | 56.8% (54/95) | 0.432 |
Mann-Whitney U tests were used for continuous data, Fisher’s exact tests were used for categorical data. Values of p <0.05 considered significant. For both continuous and categorical variables missing data are excluded (n known is shown in table).
ALT ULN >35 for males, >25 for females.
Tenofovir-based regimen refers to treatment regimen that contains tenofovir disoproxil fumarate (TDF) or tenofovir alafenamide (TAF).
Demographics, risk, and clinical outcome indicators associated with HDV–HBV treatment
The HDV+ cohort was further stratified into 3 groups; (i) IFNα treatment at any time (also includes patients that had been on other forms of HBV treatment), (ii) NA treatment at any time, (iii) no recorded treatment at any time. Individuals having cirrhosis were significantly more likely to have been treated with either NA or IFNα (p <0.001) although other hepatic complications or biochemical indicators of liver disease were not associated with treatment experience (Table 4). A small percentage of the cohort (7/112, 6.3%) showed undetectable HDV RNA >24 weeks (and >48 weeks if data were available) over the study period, with undetectable viraemia significantly associated with IFNα treatment at any time (5/7, 71.4%) compared with patients that remained HDV RNA-positive (20/105, 19.0%; p <0.01). With respect to demographics and HDV treatment, Asian and Black/African/Caribbean patients were significantly more likely to have treatment experience (p = 0.035 and 0.030, respectively), whereas there was no significant difference in the likelihood of treatment among White or other ethnic groups within the cohort. Interestingly, Canadian-born patients were significantly more likely to be treated with either IFNα or NA (p = 0.041) compared with patients born in an HBV-endemic country (p = 0.500). HIV co-infected patients were significantly more likely to be treated with antiviral therapies other than IFNα (p <0.001), whereas patients with higher levels of HBV DNA were more likely to be treatment-naive (p <0.001). High-risk sexual contact (p = 0.037) was also significantly associated with the likelihood of treatment (Table 4).
Table 4.
Demographic, sociodemographic risk factors and clinical outcomes based on treatment types in anti-HDV+ (and HDV RNA+ or HDV RNA-) individuals; comparison between patients treated with interferon, vs. any other treatment active against HBV, vs. no treatment.
| Variable | HDV with IFNα Rx (n = 33) | HDV with other Rx (n = 50) | HDV with no Rx (n = 52) | p value |
|---|---|---|---|---|
| Age | 45.9 (41.5–50.4, 33) | 47.7 (44.3–51.2, 50) | 44.8 (41.1–48.5, 52) | 0.506 |
| Male sex | 78.8% (26/33) | 74.0% (37/50) | 61.5% (32/52) | 0.184 |
| Canada born | 15.6% (5/32) | 21.7% (10/46) | 4.2% (2/48) | 0.041 |
| Born in country endemic for HBV | 56.3% (18/32) | 60.9% (28/46) | 68.8% (33/48) | 0.500 |
| Ethnicity | ||||
| Asian | 40.6% (13/32) | 21.7% (10/46) | 16.0% (8/50) | 0.035 |
| White | 28.1% (9/32) | 30.4% (14/46) | 34.0% (17/50) | 0.845 |
| Black/African/Caribbean | 18.8% (6/32) | 47.8% (22/46) | 40.0% (20/50) | 0.030 |
| Other ethnicity | 12.5% (4/32) | 0% (0/46) | 10.0% (5/50) | 0.060 |
| Other sociodemographic factors | ||||
| Alcohol use | 45.5% (15/33) | 32.0% (16/50) | 26.9% (14/52) | 0.204 |
| Smoking | 27.3% (9/33) | 32.0% (16/50) | 21.2% (11/52) | 0.463 |
| Injection/intranasal drug use | 12.1% (4/33) | 18.0% (9/50) | 3.8% (2/52) | 0.074 |
| High-risk sexual contact | 6.1% (2/33) | 12.0% (6/50) | 0% (0/52) | 0.037 |
| Hepatic outcomes | ||||
| Liver transplant | 6.1% (2/33) | 10.0% (5/50) | 3.8% (2/52) | 0.455 |
| Cirrhosis | 69.7% (23/33) | 50.0% (25/50) | 25.0% (13/52) | <0.001 |
| Hepatocellular carcinoma | 9.1% (3/33) | 10.0% (5/50) | 5.8% (3/52) | 0.718 |
| Variceal bleeding | 6.1% (2/33) | 10.0% (5/50) | 0% (0/52) | 0.072 |
| Hepatic encephalopathy | 0% (0/33) | 6.0% (3/50) | 0% (0/52) | 0.074 |
| Transient elastography (TE; kPa) | 12.9 (9.8-16.0, 25) | 15.1 (10.0-20.2, 35) | 10.6 (7.5–13.8, 34) | 0.260 |
| >F2 fibrosis (TE >7.3 kPa) | 68.0% (17/25) | 68.6% (24/35) | 58.8% (20/34) | 0.649 |
| >F3 fibrosis (TE >10.7 kPa) | 48.0% (12/25) | 45.7% (16/35) | 26.5% (9/34) | 0.154 |
| Laboratory | ||||
| ALT (IU/ml) | 94.4 (58.7–130.2, 32) | 65.1 (48.1–82.2, 49) | 72.4 (44.6–100.2, 51) | 0.319 |
| ALT % above ULN∗ | 81.3% (26/32) | 67.3% (33/49) | 68.6% (35/51) | 0.351 |
| AST (U/L) | 65.1 (46.0–84.1, 25) | 68.1 (48.0–88.1, 42) | 48.7 (37.3–60.0, 42) | 0.190 |
| Total bilirubin (μmol/L) | 19.1 (11.1–27.0, 21) | 18.7 (11.6–25.7, 40) | 12.8 (9.7–16.0, 35) | 0.270 |
| Creatinine (μmol/L) | 75.7 (69.1–82.2, 20) | 82.2 (73.4–90.9, 43) | 90.8 (55.5–126.0, 27) | 0.616 |
| INR | 1.13 (1.04–1.21, 20) | 1.26 (1.13–1.39, 35) | 1.15 (1.08–1.21, 31) | 0.124 |
| HBeAg+ | 6.7% (2/30) | 21.7% (10/46) | 10.4% (5/48) | 0.122 |
| HBV DNA, Log IU/ml | 0.37 (0.05–0.69, 29) | 0.95 (0.51–1.38, 50) | 1.81 (1.24–2.38, 45) | <0.001 |
| HBV DNA detectable | 25.0% (8/32) | 40.0% (20/50) | 70.6% (36/51) | <0.001 |
| HDV RNA-positive | 83.9% (26/31) | 75.5% (37/49) | 66.0% (33/50) | 0.194 |
| HIV-positive | 0% (0/26) | 30.3% (10/33) | 2.7% (1/37) | <0.001 |
| HCV-seropositive | 15.4% (4/26) | 25.0% (9/36) | 11.1% (5/45) | 0.246 |
Continuous data are shown as mean (SD) (n). Categorical data are shown as mean % (n). For continuous variables, where there is missing data n is shown as n/n known. Statistics comparing continuous data (1-way ANOVA) or categorical data (Chi-square). Values of p <0.05 considered significant.
ALT ULN >35 for males, >25 for females.
CanHepB Network HDV and HBV genotypes
Within the CanHepB Network cohort, the HDV genotype could be determined in 89 patients. HDV genotypes were consistent with that observed among the overall referred patient cohort; 83.2% gt1 (including 49.4% gt1d), 1.1% gt2, and 15.7% gt5. Unlike the referred patient population, country of birth information was available for 117 CanHepB patients, which was analysed in association with 83 patients having HDV genotype data (Table S5). Severe outcomes of HDV infection were observed among all genotypes represented in the HDV+ cohort (Table S6), although patient numbers were too few to determine significance. HBV genotypes were also analysed in 54 of the 135 (40.0%) HDV+ cohort patients (Table S7). HBV genotype D was the most predominant (29/54, 53.7%). HDV gt1 cases were observed to be associated with HBV genotypes A, B, D, and E, whereas HDV gt5 cases were only detected in association with HBV genotype E.
Discussion
In Canada, HDV screening of HBsAg-positive individuals is recommended, including within high-risk groups, such as immigrants from regions of high HDV prevalence.20 The current study presented a unique opportunity to investigate HDV prevalence, molecular epidemiology, and clinical outcomes among specialist clinics throughout Canada, through centralised reference laboratory testing of serological and molecular markers of HDV infection.
The observation of 4.8% anti-HDV positivity among HBsAg-positive patients attending specialist clinics is similar to the Stockdale et al.14 HDV seroprevalence estimate of 4.5% among the global HBsAg-positive population. However, meta-analyses conducted by Stockdale et al.14 and Miao et al.22 both describe considerably higher prevalence among hepatology clinic attendees, at 16.4% and 13.02%, respectively. This discrepancy may be a result of meta-analyses study selection12 or screening bias among the Canadian clinic population, but the publicly funded healthcare system in Canada may also influence the prevalence findings. It is plausible that access to universal healthcare may result in a relatively lower ratio of HBV-referred patients having severe liver disease at the time of referral. The percentage of referred HBsAg-positive patients tested for HDV in Canada is speculative as a result of different systems used to capture laboratory screening and reference testing results, but reports23 and personal communication (CanHepB Network members) in combination with reference laboratory data allows an estimate of the HDV testing prevalence. Towards the beginning of the decade (2012 onwards), approximately 4–25% of clinic HBV patient referrals were likely tested for HDV, with the rate increasing by the end of the decade (to 2019), to 50% of referrals on average being tested.
The HDV RNA positivity rate among HDV-seropositive individuals in this study (approximately 65–73%) may be influenced by false-positive antibody results, as has been suggested with other anti-HDV serological platforms.24 The wide range of HDV RNA positivity among seropositive patients reported in the literature[25], [26], [27], [28] may be a function of the endemicity of the population28 or the time point during chronic infection when RNA testing is performed.29 However, HDV RNA positivity observed in this study is consistent with other recent clinic or nationwide investigations of HDV seropositive patients (69.1%,30 70%31). It is acknowledged that a certain percentage of HBsAg-positive persons who have resolved HDV superinfection will remain HDV seropositive,1 thus using sensitive and specific methods for detecting HDV RNA are necessary to answer this question and aid in patient management, such as predicting relapse following IFNα treatment.32 The present study used an in-house RNA assay with a sensitivity of approximately 11 copies/ml (Supplementary data).
The subset of 135 HDV+ patients having demographic, epidemiological, clinical and hepatic outcome data allowed an analysis of these factors in comparison with HBV mono-infected patients within the CanHepB Network. The HDV+ cohort were more likely to be male, born in Canada, White or Black/African/Caribbean, and to have reported high risk factor activities such as injection or intranasal drug use or high-risk sexual contact. The significant representation of White and Canadian-born individuals among those HDV+ was a striking observation, as chronic HBV infection prevalence in Canada15 is understood to be associated with immigration from HBV- or HDV-endemic countries,33 with the assumption that this same population would primarily comprise HDV-infected persons in Canada. Indeed, the majority of patients in both cohorts were not born in Canada. Canada has experienced increased immigration over the past 10 years primarily from regions, including endemic countries, throughout Asia (48%), Europe (28%), the Americas (15%), and Africa (8.5%).7,8,34 White study participants were also significantly represented among the HDV cohort, with the majority (51.4%) not born in Canada (birth country; Switzerland, Russia, Romania, Moldova, Portugal, Ukraine, Uzbekistan, Georgia). The global epidemiology of HDV is changing as HBV prevalence is reduced in regions having a robust and long-term vaccination program.13 Furthermore, regions of high HDV prevalence often do not correspond to highly prevalent regions of HBV. Other factors, including high-risk activity, differing transmission routes (early horizontal or perinatal transmission for HBV mono-infected vs. sexual or parenteral transmission for HDV-HBV co-infected), and the broad immigrant population within Canada, also likely contribute to the demographic observations of this study. Canada introduced universal childhood (i.e. infant or adolescent) HBV vaccination in the early 1990s, leading to increased protection against HBV (and HDV) in the younger Canadian-born generation. These findings highlight the importance of consistent HDV screening according to the recommended guidelines.
HDV infection is estimated to result in approximately 16–20% of cirrhosis or HCC among persons chronically infected with HBV,14 although this is likely an underestimation. Our cross-sectional analysis confirmed the significant association between the HDV+ cohort and severe hepatic outcomes, including decompensation, liver transplantation, cirrhosis, and HCC, compared to HBV mono-infected patients. Ten of the eleven co-infected patients having HCC were also diagnosed with cirrhosis, whilst the remaining individual was noted to have a low platelet count, and thus is suspected of having cirrhosis35 despite not meeting the FibroScan criteria for cirrhosis. TE can be impacted by hepatic necroinflammation in CHB and significantly, there is presently no validated TE threshold for HDV co-infection.36 A study of Taiwanese chronic hepatitis B patients treated with NA showed that HDV viraemia contributed significantly to the development of HCC, with a 5-year cumulative incidence of 22.2% compared with HDV RNA-negative patients (7.3%, p = 0.01).37 In the present study, upon stratification by RNA positivity, cirrhosis, elevated ALT, AST, and TE indicating greater than stage 2 fibrosis, were significantly associated with HDV viraemia. The significant association between HDV viraemia and increased ALT levels has also been described in both univariate and multivariate analyses in a cross-sectional study of over 1,500 patients from 15 countries.28 An investigation of qHBsAg (< or >100 IU/ml) in association with HDV RNA, treatment experience, and severe hepatic outcomes did not show any significant associations, likely as a result of the low number of patients in each grouping. To aid in clinical prediction, non-invasive fibrosis scoring variables and specific cut-off values have been created for HDV co-infection, although their validation in larger and more ethnically diverse cohorts is required.[38], [39], [40]
At present, IFNα is the only antiviral drug approved for treatment of HDV in Canada. Within the HDV+ cohort, treatment experience with IFNα, entecavir or tenofovir was significantly more common than among HBV mono-infected patients. Although the number of patients testing HDV RNA-negative over the study period was few, undetectable HDV RNA was significantly associated with IFNα treatment at any time when compared with patients that remained persistently HDV-positive. The typical response rate to a standard course of IFNα treatment is only 20–30%, although post-treatment effects induced by IFNα have been associated with improved outcomes.6 Patients with other HDV-related severe hepatic complications were not more likely to have received antiviral treatment. This observation highlights the fact that although intervention may be indicated, the limited options available for HDV treatment reduces therapeutic initiation.
Asian, Black/African/Caribbean, and Canadian-born patients and those reporting high-risk sexual contact were also more likely to have been treated with either IFNα or NA, whereas HDV patients born in an HBV endemic country did not have a greater likelihood of prior treatment. Markedly, this observation suggests that even with sociodemographic factors consistent with healthcare access disparities,41,42 Canadian-born patients were more likely to be treated for HBV or HDV compared with most foreign-born patients, although admittedly immigrant populations have shown inadequate entry into care.43,44 A possible reason for these findings may be because of healthcare disparities (including language, cultural barriers, or drug reimbursement policies). Further to this, individuals co-infected with HIV (11.5%) were also observed to have a higher likelihood of entry into care, possibly for treatment of HIV and HBV before HDV diagnosis.
HDV gt1d was the most prevalent genotype observed in the study population, thus consistent with the North American and global distribution of gt1.11 The observed association between HDV genotype and country of birth among 83 CanHepB network patients (Table S5) supports and expands previously reported geographic and ethnic associations among HDV genotypes.11 Although there is little clinical evidence associated with outcomes of infection with specific HDV genotypes, several reports have described differences in severity. Compared with gt2, gt1 was significantly associated with a higher incidence of HCC and mortality.45 Genotype 3 has been reported in cases of severe or fulminant hepatitis, familiarly known as Labrea hepatitis, throughout northern regions of South America.46,47 In contrast, gt5 is associated with an improved prognosis in comparison to gt1, including a lower 10-year cumulative incidence of cirrhosis.48 Severe outcomes of HDV infection were observed among all HDV genotypes represented in the HDV+ cohort, with fewer gt5 patients represented compared with gt1 patients, although the significance of this observation could not be determined because of the low numbers among each outcome/genotype.
There were several limitations to the study. The retrospective cross-sectional nature of the study meant that patients were at different stages of HDV infection. Capturing the most recent laboratory test result may introduce bias as it cannot take into account the fluctuation of clinical markers over time. Certain virological markers, such as quantitative HDV RNA, or demographic markers, such as country of birth, were not available for all patients. Similarly, the number of patients within stratified groups were often too low to determine the significance of findings among groups. HDV screening selection bias is an important limitation that will confound calculation of prevalence. Additionally, the inability to match several specimens with data highlights the need for an electronic reporting system between reference and local laboratories.
Despite determining the percentage of HDV RNA positivity within the referral population (64.8%) and the specialist clinic subset of seropositive patients (73.1%), we cannot estimate the true prevalence of HDV infection as not all HBsAg-positive patients are tested. However, the majority of chronic viral hepatitis patients in Canada are referred to hepatologists or infectious diseases specialists for disease management,20 thus the active participation of specialist physicians in HDV screening suggests that results of the current study provide an accurate picture of HDV prevalence in HBsAg-positive Canadians in care. According to Rizzetto and Hamid,1 referred patient surveys from specialist clinics provide the most reliable information on HDV infection, which is ever more pertinent for large and diverse immigrant populations, such as within Canada. Therefore, ongoing accordance with screening recommendations will allow confirmation of the prevalence estimate of HDV infection in referred clinic populations.
Conclusions
The extremely high burden associated with HDV–HBV co-infection, including severe disease, mortality, and attendant healthcare costs49 reinforce the need to improve and increase HDV screening and entry into care and treatment. With the advent of new therapies for HDV, understanding the national epidemiology and prevalence of HDV is necessary to properly target and fund effective interventions. The current study illustrates how targeted surveillance of a referred population can illuminate details to achieve a successful care and management plan for the HDV-infected population.
Financial support
No financial support was involved in completing the study or writing the manuscript.
Authors’ contributions
Study concept and design: CO, SH-J, CSC. Acquisition of data: CO, KS, SH-J, MDS, SF, DW, GYM, KED, PW, ET, CC, AR, MM, CN, KT, CSC. Analysis and interpretation of data: CO, KS, SH-J, CSC. Drafting of the manuscript: CO. Critical revision of the manuscript: CO, SH-J, MDS, SF, DW, GYM, KED, PW, ET, CC, AR, MM, CN, KT, CSC. Technical support: KS. Statistical analysis: SH-J. Administrative support: SH-J.
Data availability statement
HDV sequence data is available from the GenBank database (https://www.ncbi.nlm.nih.gov/nucleotide/) under accession numbers MZ712366–MZ712572. Patient information is unsuitable to post owing to privacy requirements.
Conflicts of interest
CSC within past 36 months: Grant or contract from Gilead Sciences, Janssen Pharmaceuticals, GSK; Consulting fees from Janssen Pharmaceuticals; Payment or honoraria from Gilead Sciences (all made to the University of Calgary). The other authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2022.100461.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Rizzetto M., Hamid S. The medical impact of hepatitis D virus infection in Asia and Africa; time for a reappraisal. Liver Int. 2021;41:16–19. doi: 10.1111/liv.14729. [DOI] [PubMed] [Google Scholar]
- 2.Da B.L., Rahman F., Lai W.C., Kleiner D.E., Heller T., Koh C. Risk factors for Delta hepatitis in a North American cohort: who should be screened? Am J Gastroenterol. 2021;116:206–209. doi: 10.14309/ajg.0000000000000954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alfaiate D., Clément S., Gomes D., Goossens N., Negro F. Chronic hepatitis D and hepatocellular carcinoma: a systematic review and meta-analysis of observational studies. J Hepatol. 2020;73:533–539. doi: 10.1016/j.jhep.2020.02.030. [DOI] [PubMed] [Google Scholar]
- 4.Negro F. Hepatitis D virus coinfection and superinfection. Cold Spring Harb Perspect Med. 2014;4:a021550. doi: 10.1101/cshperspect.a021550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koh C., Da B.L., Glenn J.S. HBV/HDV coinfection: a challenge for therapeutics. Clin Liver Dis. 2019;23:557–572. doi: 10.1016/j.cld.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Urban S., Neumann-Haefelin C., Lampertico P. Hepatitis D virus in 2021: virology, immunology and new treatment approaches for a difficult-to-treat disease. Gut. 2021;70:1782–1794. doi: 10.1136/gutjnl-2020-323888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Razavi H. Global epidemiology of viral hepatitis. Gastroenterol Clin North Am. 2020;49:179–189. doi: 10.1016/j.gtc.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 8.WHO . 2021. Global progress report on HIV, viral hepatitis and sexually transmitted infections.https://www.who.int/publications/i/item/978924002707 [Google Scholar]
- 9.Toy M., Ahishali E., Yurdaydın C. Hepatitis delta virus epidemiology in the industrialized world. AIDS Rev. 2020;22:203–212. doi: 10.24875/AIDSRev.20000056. [DOI] [PubMed] [Google Scholar]
- 10.Hercun J., Koh C., Heller T. Hepatitis delta: prevalence, natural history, and treatment options. Gastroenterol Clin North Am. 2020;49:239–252. doi: 10.1016/j.gtc.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Le Gal F., Brichler S., Drugan T., Alloui C., Roulot D., Pawlotsky J.M., et al. Genetic diversity and worldwide distribution of the deltavirus genus: a study of 2,152 clinical strains. Hepatology. 2017;66:1826–1841. doi: 10.1002/hep.29574. [DOI] [PubMed] [Google Scholar]
- 12.Papatheodoridi M., Papatheodoridis G. Is hepatitis delta underestimated? Liver Int. 2021;41:38–44. doi: 10.1111/liv.14833. [DOI] [PubMed] [Google Scholar]
- 13.Rizzetto M., Hamid S., Negro F. The changing context of hepatitis D. J Hepatol. 2021;74:1200–1211. doi: 10.1016/j.jhep.2021.01.014. [DOI] [PubMed] [Google Scholar]
- 14.Stockdale A.J., Kreuels B., Henrion M.Y., Giorgi E., Kyomuhangi I., de Martel C., et al. The global prevalence of hepatitis D virus infection: systematic review and meta-analysis. J Hepatol. 2020;73:523–532. doi: 10.1016/j.jhep.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Public Health Agency of Canada, (PHAC) 2019. Report on hepatitis B and C in Canada: 2017.https://www.canada.ca/en/services/health/publications/diseases-conditions/reporthepatitis-b-c-canada-2017.html#a3.2.1 [Google Scholar]
- 16.Coffin C.S., Ramji A., Cooper C.L., Miles D., Doucette K.E., Wong P., et al. Epidemiologic and clinical features of chronic hepatitis B virus infection in 8 Canadian provinces: a descriptive study by the Canadian HBV network. CMAJ Open. 2019;7:E610–E617. doi: 10.9778/cmajo.20190103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ratnam S., Head C.B., Butler R.W. Lack of evidence of hepatitis D (delta) infection in Newfoundland and Labrador. CMAJ. 1986;134:905–907. [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng H.H., Wang D.Q., Minuk G.Y., Anand C.M., Stowe T.C., Buchan K.A. The prevalence of antibody to delta virus in western Canada. Clin Invest Med. 1986;9:156–159. [PubMed] [Google Scholar]
- 19.Parker C., Chaudhary R. Delta infection in Canada. N Engl J Med. 1986;314:320–321. doi: 10.1056/nejm198601303140520. [DOI] [PubMed] [Google Scholar]
- 20.Coffin C.S., Fung S.K., Alvarez F., Cooper C.L., Doucette K.E., Fournier C., et al. Management of hepatitis B virus infection: 2018 guidelines from the Canadian Association for the Study of Liver Disease and Association of Medical Microbiology and Infectious Disease Canada. Can Liver J. 2018;1:156–217. doi: 10.3138/canlivj.2018-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giersch K., Dandri M. Hepatitis B and delta virus: advances on studies about interactions between the two viruses and the infected hepatocyte. J Clin Transl Hepatol. 2015;3:220–229. doi: 10.14218/JCTH.2015.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miao Z., Zhang S., Ou X., Li S., Ma Z., Wang W., et al. Estimating the global prevalence, disease progression, and clinical outcome of hepatitis delta virus infection. J Infect Dis. 2020;221 doi: 10.1093/infdis/jiz633. 1677–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grubert Van Iderstine M., Iluz-Freundlich D., Dolovich C., Villarin E., Minuk G.Y. Liver disease referrals to an urban, hospital-based hepatology outpatient clinic over the past 25 years. JGH Open. 2020;4:484–489. doi: 10.1002/jgh3.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soriano V., Gómez-Gallego F., Corral O. Hepatitis delta estimates in the United States revisited. Clin Infect Dis. 2019;69:1833–1834. doi: 10.1093/cid/ciz271. [DOI] [PubMed] [Google Scholar]
- 25.Servant-Delmas A., Le Gal F., Gallian P., Gordien E., Laperche S. Increasing prevalence of HDV/HBV infection over 15 years in France. J Clin Virol. 2014;59:126–128. doi: 10.1016/j.jcv.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 26.El Bouzidi K., Elamin W., Kranzer K., Irish D.N., Ferns B., Kennedy P., et al. Hepatitis delta virus testing, epidemiology and management: a multicentre cross-sectional study of patients in London. J Clin Virol. 2015;66:33–37. doi: 10.1016/j.jcv.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 27.Shirazi R., Ram D., Rakovsky A., Bucris E., Gozlan Y., Lustig Y., et al. Characterization of hepatitis B and delta coinfection in Israel. BMC Infect Dis. 2018;18:97. doi: 10.1186/s12879-018-3008-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wranke A., Pinheiro Borzacov L.M., Parana R., Lobato C., Hamid S., Ceausu E., et al. Clinical and virological heterogeneity of hepatitis delta in different regions world-wide: the Hepatitis Delta International Network (HDIN) Liver Int. 2018;38:842–850. doi: 10.1111/liv.13604. [DOI] [PubMed] [Google Scholar]
- 29.Ferrante N.D., Lo Re V. Epidemiology, natural history, and treatment of hepatitis delta virus infection in HIV/hepatitis B virus coinfection. Curr HIV/AIDS Rep. 2020;17:405–414. doi: 10.1007/s11904-020-00508-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamal H., Westman G., Falconer K., Duberg A.S., Weiland O., Haverinen S., et al. Long-term study of hepatitis delta virus infection at secondary care centers: the impact of viremia on liver-related outcomes. Hepatology. 2020;72:1177–1190. doi: 10.1002/hep.31214. [DOI] [PubMed] [Google Scholar]
- 31.Barbosa J.V., Sahli R., Aubert V., Chaouch A., Moradpour D., Fraga M. Demographics and outcomes of hepatitis B and D: a 10-year retrospective analysis in a Swiss tertiary referral center. PLoS One. 2021;16 doi: 10.1371/journal.pone.0250347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bremer B., Anastasiou O.E., Hardtke S., Caruntu F.A., Curescu M.G., Yalcin K., et al. Residual low HDV viraemia is associated HDV RNA relapse after PEG-IFNa-based antiviral treatment of hepatitis delta: results from the HIDIT-II study. Liver Int. 2021;41:295–299. doi: 10.1111/liv.14740. [DOI] [PubMed] [Google Scholar]
- 33.Sharma S., Carballo M., Feld J.J., Janssen H. Immigration and viral hepatitis. J Hepatol. 2015;63:515–522. doi: 10.1016/j.jhep.2015.04.026. [DOI] [PubMed] [Google Scholar]
- 34.Statistics Canada . 2017. Immigrant population by selected places of birth, admission category and period of immigration, Canada, provinces and territories, census metropolitan areas and areas outside of census metropolitan areas, 2016 Census.https://www12.statcan.gc.ca/census-recensement/2016/dp-pd/dv-vd/imm/index-eng.cfm [Google Scholar]
- 35.Moore A.H. Thrombocytopenia in cirrhosis: a review of pathophysiology and management options. Clin Liver Dis. 2019;14:183–186. doi: 10.1002/cld.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Couto I., Victoria M., Veloso V.G., Rodrigues L., Grinsztejn B., Lacerda M., et al. Prevalence and predictors for compensated advanced chronic liver disease (c-ACLD) in patients with chronic hepatitis delta virus (HDV) infection. PLoS One. 2017;12 doi: 10.1371/journal.pone.0174453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jang T., Wei Y., Liu T., Yeh M., Liu S., Hsu C., et al. Role of hepatitis D virus infection in development of hepatocellular carcinoma among chronic hepatitis B patients treated with nucleotide/nucleoside analogues. Sci Rep. 2021;11:8184. doi: 10.1038/s41598-021-87679-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alves Vasconcelos M.P., DallÁcqua D.V., Wedemeyer H., Witkin S.S., Mendes-Corrêa M.C., Villalobos-Salcedo J.M. Noninvasive models for predicting liver fibrosis in individuals with hepatitis D virus/hepatitis B virus coinfection in the Brazilian Amazon region. Am J Trop Med Hyg. 2020;103:169–174. doi: 10.4269/ajtmh.19-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lutterkort G.L., Wranke A., Yurdaydin C., Budde E., Westphal M., Lichtinghagen R., et al. Non-invasive fibrosis score for hepatitis delta. Liver Int. 2017;37:196–204. doi: 10.1111/liv.13205. [DOI] [PubMed] [Google Scholar]
- 40.Da B.L., Surana P., Kleiner D.E., Heller T., Koh C. The delta-4 fibrosis score (D4FS): a novel fibrosis score in chronic hepatitis D. Antivir Res. 2020;174:104691. doi: 10.1016/j.antiviral.2019.104691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Socías M.E., Ti L., Wood E., Nosova E., Hull M., Hayashi K., et al. Disparities in uptake of direct-acting antiviral therapy for hepatitis C among people who inject drugs in a Canadian setting. Liver Int. 2019;39:1400–1407. doi: 10.1111/liv.14043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tarasuk J., Zhang J., Lemyre A., Cholette F., Bryson M., Paquette D. National findings from the Tracks survey of people who inject drugs in Canada, phase 4, 2017–2019. CCDR. 2020;46:138–148. doi: 10.14745/ccdr.v46i05a07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lau K., Shaheen A.A., Aspinall A.A., Ricento T., Qureshi K., Congly S.E., et al. Hepatitis B virus testing and linkage to care in a Canadian urban tertiary referral centre: a retrospective cohort study. CMAJ Open. 2020;5:E431–E436. doi: 10.9778/cmajo.20170002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yasseen I A.S., Kwong J.C., Kustra R., Holder L., Chung H., Macdonald L., et al. Validating viral hepatitis B and C diagnosis codes: a retrospective analysis using Ontario’s health administrative data. Can J Public Health. 2021;112:502–512. doi: 10.17269/s41997-020-00435-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Su C., Huang Y., Huo T., Shih H.H., Sheen I., Chen S., et al. Genotypes and viremia of hepatitis B and D viruses are associated with outcomes of chronic hepatitis D patients. Gastroenterology. 2006;130:1625–1635. doi: 10.1053/j.gastro.2006.01.035. [DOI] [PubMed] [Google Scholar]
- 46.Nakano T., Shapiro C.N., Hadler S.C., Casey J.L., Mizokami M., Orito E., et al. Characterization of hepatitis D virus genotype III among Yucpa Indians in Venezuela. J Gen Virol. 2001;82:2183–2189. doi: 10.1099/0022-1317-82-9-2183. [DOI] [PubMed] [Google Scholar]
- 47.Gomes-Gouvea M.S., Soares M.C.P., Bensabath G., de Carvalho-Mello I.M.V.G., Brito E.M.F., Souza O.S.C., et al. Hepatitis B virus and hepatitis delta virus genotypes in outbreaks of fulminant hepatitis (labrea black fever) in the western Brazilian Amazon region. J Gen Virol. 2009;90:2638–2643. doi: 10.1099/vir.0.013615-0. [DOI] [PubMed] [Google Scholar]
- 48.Roulot D., Brichler S., Layese R., BenAbdesselam Z., Zoulim F., Thibault V., et al. Origin, HDV genotype and persistent viremia determine outcome and treatment response in patients with chronic hepatitis delta. J Hepatol. 2020;73:1046–1062. doi: 10.1016/j.jhep.2020.06.038. [DOI] [PubMed] [Google Scholar]
- 49.Elsaid M.I., Li Y., John T., Narayanan N., Catalano C., Rustgi V.K. Economic and health care burdens of hepatitis delta: a study of commercially insured adults in the United States. Hepatology. 2020;72:399–411. doi: 10.1002/hep.31055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
HDV sequence data is available from the GenBank database (https://www.ncbi.nlm.nih.gov/nucleotide/) under accession numbers MZ712366–MZ712572. Patient information is unsuitable to post owing to privacy requirements.