Patrick Caswell previews work from Duncan et al. which characterizes the mechanism by which Rab40b/Cul5 ubiquitylation of Rap2 controls its activity and correct localization during cell migration.
Abstract
Ubiquitin modification controls protein stability and cargo trafficking, and in this issue Duncan et al. (2022. J. Cell Biol. https://doi.org/10.1083/jcb.202107114) reveal a unique mechanism through which Rab40b/Cul5-mediated ubiquitylation of Rap2 regulates its activity and recycling to the leading edge to control cell migration and invasion.
Cell migration is a fundamental biological process that underpins critical aspects of development, homeostasis, and disease. Decades of research have led to an in-depth knowledge of specific aspects of the migration cycle, but the molecular mechanisms that coordinate polarity and motility are still incompletely understood (1). Vesicle trafficking pathways have been implicated in generating and maintaining polarity in migrating cells, as well as in controlling signaling and adhesion to coordinate the process of motility in vitro and in vivo (2). In this issue, Duncan et al. demonstrate that endocytic trafficking mediates the polarised delivery of Rap2, a GTPase implicated in cytoskeletal regulation of cell migration and adhesion (3).
Rab40b and Cullin 5 (Cul5) are key components of the Cullin-Ring Ligase 5 (CRL5) complex, an E3 ligase system that has recently been shown to contribute to regulation of adhesion, actin dynamics and cell migration by mediating the degradation of the actin bunding protein EPLIN (4). In this new study, the authors elegantly combine microscopy and biochemistry to demonstrate that Rab40b/Cul5 mediates the non-canonical ubiquitylation of Rap2, resulting in endocytic recycling of Rap2 and polarized delivery to lamellipodia for efficient cell migration and invasion (3).
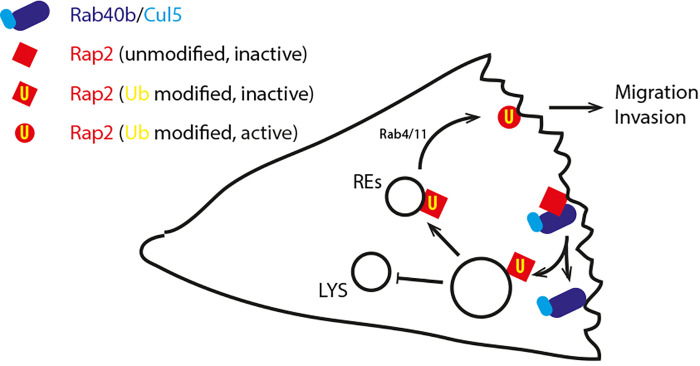
Rap2 plays key roles in cell adhesion, polarity, and migration in a variety of contexts, including gastrulation (5), but the regulation of Rap2 (and related GTPases) is poorly understood. Duncan et al. demonstrate that breast cancer cells that lack all three Rap2 isoforms (Rap2a/b/c) show defective cell migration and chemotaxis, and their ability to invade is similarly impaired. Interestingly, the authors find that Rap2 is distributed between endosomes/lysosomes and the plasma membrane, where Rap2 is enriched at lamellipodial protrusions. Rap2 is constitutively internalized via large macropinosome-like structures that fuse with early endosomes to allow Rab4- and Rab11-dependent recycling of Rap2 vesicles back to the leading edge, where active GTP-Rap2 is localized. These findings indicate that Rap2 is a trafficking cargo and suggest its trafficking is intrinsically linked with activity (Fig. 1).
Figure 1.
Rab40b/Cul5 mediate ubiquitylation of Rap2 to promote cell migration. Rap2 is mono-ubiquitylated at multiple sites by Rab40b/Cul5 to promote the sorting of Rap2 through early endosomes and recycling endosomes (REs) for Rab4- and Rab11-dependent recycling. In the absence of ubiquitylation Rap2 is redistributed to lysosomes (LYS). Rap2 is recycled to the leading edge of migrating cells, where activated Rap2 influences polarity, actin dynamics, and adhesion to promote migration and invasion in breast cancer cells.
Because the Xenopus homologues of Rab40/Cul5 have previously been linked to ubiquitylation of Rap2 (6), the authors next investigated potential links to this E3 ligase complex. Surprisingly, whilst Rap2 was a substrate for Rab40b/Cul5, this did not impact directly on Rap2 stability. Indeed, the pattern of ubiquitylation suggests that at least three mono-ubiquitin modifications are induced by Rab40b/Cul5, rather than the canonical polyubiquitylation linked to proteasomal degradation. Multi-mono-ubiquitylation has been implicated in receptor trafficking, although in this context in receptor endocytosis and lysosomal degradation (7). For Rap2, abrogating ubiquitylation using small molecule inhibitors of CRLs, depletion of Rab40b, or mutation of potential ubiquitylation sites, all increased delivery of Rap2 to lysosomes whilst suppressing Rap2 activation and recruitment to lamellipodia. The Rab40b/Cul5 complex is therefore able to impact on breast cancer cell invasion by controlling non-canonical ubiquitylation of Rap2, which in turn is critical for sorting events that lead to endocytic recycling and activation of this small GTPase such that it can modify actin dynamics, influence polarity, and control motile behavior.
Interestingly, Rab40b/Cul5 mediates both polyubiquitylation of EPLIN (4) and multi-mono-ubiquitylation of Rap2 (3). This suggest that the core E3 ligase may form part of larger multi-protein complexes that could impart specificity to substrates and ubiquitin modifications. Whether this modification occurs at the plasma membrane or on endosomes, and the nature of such monoubiquitin modification of Rap2 is yet to be fully determined. Furthermore, the sorting machinery that directs multi-mono-ubiquitinylated Rap2 for recycling is likely to be distinct from the ESCRT machinery that sorts mono-ubiquitinated cargos for degradation, and this work raises a potential role for deubiquitylating enzymes (DUBs) in Rap2 trafficking/activity regulation. This opens new possibilities into mechanistic crosstalk within small GTPase networks and is likely to have broad implications for the regulation of trafficking, actin dynamics, adhesion, and cell migration.
Acknowledgments
The author declares no competing financial interests.
References
- 1.SenGupta, S., et al. 2021. Nat. Rev. Mol. Cell. Biol. 10.1038/s41580-021-00366-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson, B.J., et al. 2018. Traffic. 10.1111/tra.12605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duncan, E.D., et al. 2022. J. Cell Biol. 10.1083/jcb.202107114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linklater, E.S., et al. 2021. J. Cell Biol. 10.1083/jcb.202008060 [DOI] [Google Scholar]
- 5.Choi, S.-C., and Han J.-K.. 2005. EMBO J. 10.1038/sj.emboj.7600571 [DOI] [Google Scholar]
- 6.Lee, R.H.K., et al. 2007. EMBO J. 10.1038/sj.emboj.7601781 [DOI] [Google Scholar]
- 7.Haglund, K., et al. 2003. Nat. Cell Biol. 10.1038/ncb983 [DOI] [PubMed] [Google Scholar]