This randomized clinical trial compares the effectiveness of 3 minimally invasive posterior decompression techniques for lumbar spinal stenosis at 16 public hospitals in Norway.
Key Points
Question
Are any of the 3 most common minimally invasive procedures for surgical treatment of lumbar spinal stenosis superior to the others?
Findings
In this randomized clinical trial of 437 patients at 16 public hospitals in Norway, there was no difference in the clinical results from the 3 most commonly performed minimally invasive decompression techniques investigated after 2 years.
Meaning
These findings suggest that surgeons can choose decompression techniques according to their skills and preferences.
Abstract
Importance
Operations for lumbar spinal stenosis is the most often performed surgical procedure in the adult lumbar spine. This study reports the clinical outcome of the 3 most commonly used minimally invasive posterior decompression techniques.
Objective
To compare the effectiveness of 3 minimally invasive posterior decompression techniques for lumbar spinal stenosis.
Design, Setting, and Participants
This randomized clinical trial used a parallel group design and included patients with symptomatic and radiologically verified lumbar spinal stenosis without degenerative spondylolisthesis. Patients were enrolled between February 2014 and October 2018 at the orthopedic and neurosurgical departments of 16 Norwegian public hospitals. Statistical analysis was performed in the period from May to June 2021.
Interventions
Patients were randomized to undergo 1 of the 3 minimally invasive posterior decompression techniques: unilateral laminotomy with crossover, bilateral laminotomy, and spinous process osteotomy.
Main Outcomes and Measures
Primary outcome was change in disability measured with Oswestry Disability Index (ODI; range 0-100), presented as mean change from baseline to 2-year follow-up and proportions of patients classified as success (>30% reduction in ODI). Secondary outcomes were mean change in quality of life, disease-specific symptom severity measured with Zurich Claudication Questionnaire (ZCQ), back pain and leg pain on a 10-point numeric rating score (NRS), patient perceived benefit of the surgical procedure, duration of the surgical procedure, blood loss, perioperative complications, number of reoperations, and length of hospital stay.
Results
In total, 437 patients were included with a median (IQR) age of 68 (62-73) years and 230 men (53%). Of the included patients, 146 were randomized to unilateral laminotomy with crossover, 142 to bilateral laminotomy, and 149 to spinous process osteotomy. The unilateral laminotomy with crossover group had a mean change of −17.9 ODI points (95% CI, −20.8 to −14.9), the bilateral laminotomy group had a mean change of −19.7 ODI points (95% CI, −22.7 to −16.8), and the spinous process osteotomy group had a mean change of –19.9 ODI points (95% CI, −22.8 to –17.0). There were no significant differences in primary or secondary outcomes among the 3 surgical procedures, except a longer duration of the surgical procedure in the bilateral laminotomy group.
Conclusions and Relevance
No differences in clinical outcomes or complication rates were found among the 3 minimally invasive posterior decompression techniques used to treat patients with lumbar spinal stenosis.
Trial Registration
ClinicalTrials.gov Identifier: NCT02007083
Introduction
Symptomatic lumbar spinal stenosis (LSS) is characterized by pain and discomfort in the lower back and the lower extremities, impaired walking ability, and functional disability. Imaging shows a narrowing of the lumbar spinal canal.1 LSS is a common condition involving a large patient group who are treated by several medical specialties involved in different aspects of the diagnosis and treatments. Several studies2,3,4,5 have shown superior clinical results after surgical treatment compared with nonsurgical treatment. The surgical procedure for LSS is the most frequently performed procedure in the adult lumbar spine.6,7
A posterior decompression at the level of the stenosis is usually performed, and an open laminectomy has been considered the reference standard.8,9 Less invasive, midline retaining, posterior decompression techniques have been introduced in the last decades. They have shown similar effectiveness as traditional laminectomies, but the duration of the surgical procedure and length of hospital stay is usually shorter because of the less invasive nature of the procedure.10
Various midline retaining techniques have been introduced with scarce scientific evidence regarding the possible advantages and disadvantages. Therefore an effectiveness study of different minimally invasive techniques would be of interest for the medical community and health care planning and allocation of resources. This trial investigates the outcome after 3 commonly used methods that differ in spinal canal access and may differ in surgical radicality.
Some former trials11,12,13,14,15,16,17 show comparable clinical results after different posterior decompression techniques. However, 2 Cochrane reviews, Overdevest et al8 and Machado et al,18 and an umbrella review by Jacobs et al19 concluded that the scientific evidence is of low quality and that high-quality research is required before a scientific conclusion can be reached. This trial aims to investigate whether 1 of the 3 most commonly used minimally invasive posterior decompression techniques is superior in the treatment of LSS with respect to clinical outcomes.
Methods
The Norwegian Degenerative Spondylolisthesis and Spinal Stenosis study (NORDSTEN study) consists of 2 randomized clinical trials, including the NORDSTEN Spinal Stenosis Trial (SST). The SST is a multicenter trial where orthopedic and neurosurgical departments of 16 hospitals participated. The study protocol was prepared according to the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) reporting guideline.20 The protocol is also attached in Supplement 1. The trial was registered at ClinicalTrials.gov (NCT02007083). Ethics approval was given by the Regional Committee for Medical and Health Research Ethics of Central Norway. This randomized clinical trial is reported according to the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.21 The trial was also monitored according to a modified version of the International Conference on Harmonization Guideline for Good Clinical Practice (ICH-GCP),22 and a monitor-report is provided in eAppendix 1 in Supplement 2. A patient representative from the Norwegian Back Association has been a permanent member of both the Scientific Steering Committee and the Working Committee of the NORDSTEN-study (eAppendix 2 in Supplement 2). Informed consent was collected in a paper-based form and was stored in a fire proof safe at each of the study centers according to Norwegian rules for conducting clinical trials.
Inclusion Process and Patient Recruitment
Patients with symptoms of LSS and corresponding magnetic resonance imaging findings were eligible for inclusion in the trial. An orthopedic or neurosurgical surgeon assessed the participants at 1 of the 16 participating hospitals between February 2014 and October 2018. Patients with degenerative spondylolisthesis were excluded. Eligibility criteria are presented in the eTable 1 in Supplement 2. Initially, patients were excluded from February 2014 to October 2015 if their Oswestry Disability Index (ODI) at baseline was less than 25 points. The removal of this exclusion criterion was done to increase the external pragmatism and validity of the study. An amendment was sent to the ethics authorities, and the amendment was also registered in ClinicalTrials.gov and in the published protocol.20
Randomization and Masking
Patients who provided informed consent were randomized to 1 of the 3 different posterior decompression techniques. The randomization (ie, 1:1:1 allocation) was performed within 6 weeks before the surgical procedure. We used a block randomization design, stratified by 16 hospitals, with the blocks being made as small as possible (randomly selected block size 3 and 6) to ensure that every hospital performed similar amount of all 3 procedures. The randomization procedure was concealed (computer-generated) and administered by the NORDSTEN-study coordination center located at the Communication and Research Unit for Musculoskeletal Health, Oslo University Hospital, Oslo, Norway. Output information regarding allocation was emailed to the local study coordinator, who was not involved in the recruitment or treatment of patients and registered in the patient record. The patients were not blinded to the treatment group; they were informed that none of the treatment options were documented as superior to the other.
Surgical Techniques
All surgeons were familiar with the 3 techniques through previous experience, the surgical protocol, and joint demonstration operations were performed before initiating the study (Figure 1). The surgical target level was confirmed by intraoperative fluoroscopic guidance. When performing unilateral laminotomy with crossover (UL), loupe magnification, or surgical microscope was mandatory, while in bilateral laminotomy (BL) and spinous process osteotomy (SPO) the use of loupes or microscope was optional depending on the surgeon’s preference. The surgeons were instructed to visualize the respective medial borders of the pedicles and the nerve roots from the beginning of the thecal sac passing the pedicle.
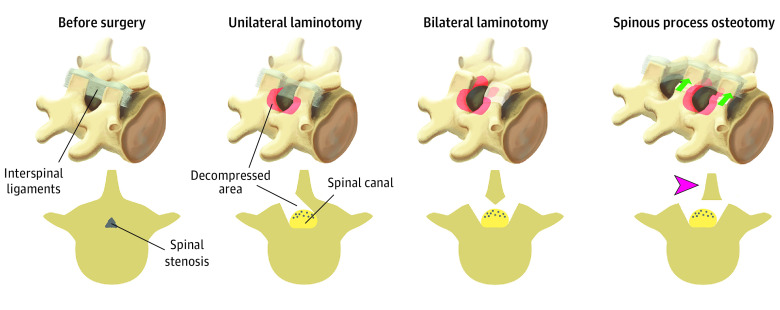
Figure 1. Before and After the Surgical Procedure for Lumbar Spinal Stenosis With the 3 Different Minimally Invasive Decompression Techniques Used in the Study.
UL With Crossover
An ipsilateral flavectomy was performed followed by a laminotomy of the lower part of the superior lamina and the upper part of the inferior lamina.23 Laterally, a medial facetectomy was performed, and the patient was then slightly rotated to visualize the contralateral side. The dura was retracted, and the decompression was performed contralaterally.
BL
A bilateral flavectomy was performed followed by a bilateral laminotomy of the lower part of the superior lamina and the upper part of the inferior lamina. Laterally, a medial facetectomy was performed.24
SPO and Decompression
An osteotomy was performed at the base of the spinous process above and sometimes below the affected level.25 The spinous process was retracted to the contralateral side with intact supraspinal and interspinal ligaments to access and decompress the spinal canal in the midline. A laminotomy of the lower part of the superior lamina and the upper part of the inferior lamina was performed followed by a medial facetectomy. Both nerve roots were visualized, and the lateral recesses were decompressed. Special attention was warranted when a multilevel decompression was performed to preserve at least one-third of the lamina.
Primary Outcome
The primary outcome measure was change in disability measured with Oswestry Disability Index (ODI, version 2.0) from baseline to 24 months after the surgical procedure. ODI is a widely used and validated pain and function score, where 0 is considered asymptomatic and 100 is considered completely disabled.26,27 The patients completed the questionnaires, including ODI, before the surgical procedure and at 3, 12, and 24 months after the surgical procedure. The mean score change from baseline to 2-year follow-up was compared between the 3 groups. Additionally, patient outcomes were classified as a success if they had a 30% reduction of baseline ODI, and the proportion of patients classified as a success in each group were determined at the different follow-up time points.28,29
Secondary Outcomes
Secondary patient-reported outcomes were changes from baseline to follow-up in the EuroQol 5-dimensional questionnaire utility index (EQ-5D), the Zurich Claudication Questionnaire (ZCQ-score), a 10-point numeric rating scale (NRS) for low back pain and leg pain, and a global perceived effect (GPE)-scale.
The EQ-5D is a generic quality-of-life questionnaire, ranging from −0.59 (ie, worst possible) to 1.00 (ie, best possible). This questionnaire was validated for the Norwegian population.30 The 3-level version of EQ-5D and the corresponding UK value set to calculate scores was used.
The ZCQ is a disease-specific questionnaire for LSS and includes symptom severity, physical activity, and patient satisfaction during follow-up. Answers range from 1.0 to 5.0 in the symptom severity scale.31 In the physical activity scale, the range is from 1.0 to 4.0. The patient satisfaction scale was answered postoperatively and ranged from 1.0 to 4.0. For all scales, 1.0 is the best option.
The NRS scores for leg and low back pain are validated parameters for clinical trials.32 The range is from 0 to 10, where 0 is no pain, and 10 is the worst pain imaginable.
The global perceived effect (GPE) scale is a 7-point score, which is recommended for clinical trials of chronic pain conditions.33 It has 7 response categories: 1, completely recovered; 2, much improved; 3, slightly improved; 4, no change; 5, slightly worse; 6, much worse; 7, worse than ever.
Surgical data defined as secondary outcomes were duration of the procedure, perioperative bleeding volume, complications, number of reoperations, and length of hospital stay. All primary and secondary outcomes data were administered by paper-based patient-reported questionnaires and case report form. The data were obtained from the patient and an independent study coordinator at each hospital, and the data was registered electronically by the study coordinating center.
Statistical Analysis
The trial was conducted with a superiority design to detect a difference in mean change of ODI from baseline to 2-year follow-up of 7 points between any groups. Because this requires 3 tests, the significance level was lowered from the standard P = .05 to P = .02. With an assumed SD of 18, 80% power, and a dropout rate of 15%, the sample size estimation recommended 155 patients in each group and 465 total. However, inclusion was stopped after reaching 437 patients because of the low rate of inclusion in the last months of the inclusion period. At that time, we had reports that the dropout rate was lower than anticipated, ensuring a sufficient number of participants in the statistical analysis.
Standard descriptive statistics were presented as absolute and relative frequencies for categorical variables, as mean (SD) for continuous variables, and as median (IQR) if skewed. Normal distribution was determined by visual inspection of histograms and qq-plots. Outcomes were analyzed by estimating multilevel linear models, including a random intercept for operating hospitals to account for the multicenter design. Continuous outcomes were analyzed using multilevel linear regressions, adjusting for baseline measurement if the outcome was a change score, and proportions were analyzed using multilevel Poisson regressions and adjusted for baseline measurement where appropriate. Predicted marginal effects, with corresponding 95% CIs were presented for all outcomes for all study arms. Actual means with corresponding 95% CIs were also presented graphically, along with standard t tests, as outlined in a published study protocol. Potential interaction effects were analyzed by including interaction terms between study arms and the variable of interest, tested using likelihood ratio tests, and comapred models with and without interaction effects. Complete case analyses were prefered because the proportion of missing observations rarely exceeded 10%. To assess the robustness of the results, we also analyzed the primary outcome after imputing missing data using multiple imputations with chained equations, including study arm, patients’ age, sex, body mass index (BMI; calculated as weight in kilograms divided by height in meters squared), and smoking status as factors in the imputation models.
Analyses were performed following the intention-to-treat principle (ITT). As 386 of 393 (98%) of the study participants that were eligible for analyses (ie, had necessary measurements of ODI at baseline and 2-year follow-up), were treated according to randomization, per-protocol analyses were deemed unnecessary. All analyses were executed by a statistician (T.Å.M.) blinded to the treatment given. Statistical analyses were performed using Stata Statistical Software version 17 (StataCorp). Two-sided t tests were used for the calculation of P values, and statistical significance was set at P = .02 for the primary outcome. Statistical analyses were performed in the period from May to June 2021.
Results
Baseline Data
Baseline characteristics are given in Table 1. Median (IQR) age in the total cohort was 68 (62-73) years with a mean (SD) BMI of 27.8 (4.2) and included 230 men (53%) and 87 individuals (21%) who smoked. The mean (SD) pain and function scores at baseline for the whole cohort were ODI, 38.4 (14.5); EQ-5D, 0.38 (0.32); ZCQ symptoms, 3.4 (0.6); ZCQ function, 2.5 (0.5); NRS leg pain, 6.5 (2.0); and NRS low back pain, 6.3 (2.2).
Table 1. Postrandomization Baseline Characteristics, Patient-Reported Outcome Measures, and Number of Levels of the Patients Included in the 3 Study Groups.
| Characteristic | No./total no. (%) | ||
|---|---|---|---|
| UL (n = 146) | BL (n = 142) | SPO (n = 149) | |
| Age, median (IQR), y | 69 (64-74) | 67 (60-74) | 68 (61-72) |
| Sex | |||
| Female | 73/146 (50.0) | 78/142 (54.9) | 55/149 (36.9) |
| Male | 73/146 (50.0) | 63/142 (45.1) | 94/149 (63.1) |
| Higher level of educationa | 45/138 (32.6) | 35/138 (25.4) | 39/141 (27.7) |
| Smoking | 23/138 (16.7) | 34/139 (24.5) | 30/141 (21.3) |
| BMI, mean (SD) | 28.1 (4.2) | 27.7 (3.9) | 27.5 (4.4) |
| Former surgical procedure | 11/133 (8.3) | 10/133 (7.5) | 8/134 (6.0) |
| Duration of leg pain >1 y | 92/135 (68.2) | 94/131 (71.8) | 88/130 (67.7) |
| Duration of back pain >1 y | 109/134 (81.3) | 107/136 (78.7) | 105/139 (75.5) |
| Use of analgesics | 24/139 (17.3) | 36/138 (26.1) | 48/137 (35.0) |
| ASA score | |||
| 1 | 11/137 (8.0) | 26/137 (19.0) | 12/137 (8.8) |
| 2 | 96/137 (70.1) | 86/137 (62.8) | 98/137 (71.5) |
| 3 | 30/137 (21.9) | 25/137 (18.3) | 27/137 (19.7) |
| HSCL-25, median (IQR) | 1.5 (1.2-1.9) | 1.6 (1.3-1.9) | 1.5 (1.3-1.8) |
| ODI, mean (SD)b | 38.5 (14.9) | 40.2 (14.1) | 36.6 (14.3) |
| ZCQ, mean (SD) | |||
| Symptom severity | 3.4 (0.5) | 3.4 (0.6) | 3.3 (0.5) |
| Physical activity | 2.5 (0.5) | 2.6 (0.5) | 2.5 (0.5) |
| NRS, median (IQR)c | |||
| Leg pain | 7 (5-8) | 7 (5-8) | 7 (5-8) |
| Back pain | 7 (5-8) | 7 (5-8) | 7 (5-8) |
| EQ-5D, mean (SD) | 0.37 (0.33) | 0.35 (0.31) | 0.40 (0.30) |
| Level of surgical procedure | |||
| 1 | 80/135 (59.3) | 82/136 (60.3) | 82/134 (61.2) |
| 2 | 53/135 (39.3) | 49/136 (36.0) | 46/134 (34.3) |
| 3 | 2/135 (1.5) | 5/136 (3.7) | 6/134 (4.5) |
Abbrevations: ASA, American Society of Anesthesiologists; BL, bilateral laminotomy; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); EQ-5D, EuroQol 5-dimensional questionnaire utility index; HSCL: number of patients operated in a different level; HSCL-25, Hopkins Symptom Checklist-25; NRS, Numerical Rating Scale, which ranges from 0 (no pain) to 10 (worst pain imaginable); ODI, Oswestry Disability Index; SPO, spinous process ostetomy; UL, unilateral laminotomy with crossover; ZCQ, Zurich Claudication Questionnaire.
3 years or more in college or university.
ODI ranges from 0 (no impairment) to 100 (the greatest impairment).
NRS, Numerical Rating Scale ranges from 0 (no pain) to 10 (worst pain imaginable).
Of 2227 patients assessed for eligibility in the NORDSTEN-study, 1387 were eligible for inclusion in the SST, 950 did not fulfill all eligibility criteria and were excluded, and 437 underwent randomization. Figure 2 presents the study.34
Figure 2. Study Flowchart.

ASA indicates American Society of Anesthesiologists; CRF, case report form; NORDSTEN, Norwegian Degenerative Spondylolisthesis and Spinal Stenosis study; ODI, Oswestry Disability Index; SST, Spinal Stenosis Trial.
aMore than 1 exclusion criteria were noted.
Primary Outcome
The actual mean change in ODI from baseline to 2-year follow-up for the whole cohort was −19.1 (95% CI, −20.8 to −17.5). The overall proportion of patients whose outcomes were classified as a success was 273 (69.5%). When adjusting for baseline ODI and operating hospital, the estimated mean change in ODI after 2 years was −17.8 (95% CI, −20.3 to −15.3) for the UL group, −18.7 (95% CI, −21.3 to −16.0) for the BL group and −21.0 (95% CI, −23.5 to −18.4) for the SPO group (Table 2). There were no statistically significant differences between the 3 surgical method groups in the primary outcome (P = .214). The mean ODI-score in each of the 3 surgical groups from baseline to 3, 12, and 24 months of follow-up appear in Figure 3 and eTable 2 in Supplement 2. Imputing missing data, the estimated mean (SD) changes in ODI for UL, BL, and SPO were −18.1 (95% CI, −20.7 to −15.6) points, −18.6 (95% CI, −21.1 to −15.9) points, and −21.2 (95% CI, −23.7 to −18.6) points, respectively. Analyzing interaction effects between study arms and period of inclusion, level of the surgical procedure, and number of levels operated did not show any evidence of differential effects of surgical technique in these subgroups of patients (eTable 7 in Supplement 2).
Table 2. Primary and Secondary Outcomesa.
| Outcomes | Mean (95% CI) | P value | No. eligible for analyses | ||
|---|---|---|---|---|---|
| Unilateral laminotomy with crossover | Bilateral laminotomy | Spinous process osteotomy | |||
| Primary outcome | |||||
| Change in ODI after 24 mo | −17.8 (−20.3 to −15.3) | −18.7 (−21.3 to −16.0) | −21.0 (−23.5 to −18.4) | .21 | 393 |
| Secondary outcomes | |||||
| Proportion success after 24 mo, % | 67.4 (53.6 to 81.3) | 67.5 (53.1 to 81.8) | 73.5 (58.9 to 88.1) | .80 | 393 |
| Change in global EQ-5D score | 0.31 (0.26 to 0.37) | 0.31 (0.26 to 0.36) | 0.35 (0.30 to 0.40) | .54 | 358 |
| Change in ZCQ symptom score | −0.96 (−1.10 to −0.83) | −1.02 (−1.16 to −0.88) | −1.09 (−1.23 to −0.96) | .41 | 389 |
| Change in ZCQ physical function score | −0.79 (−0.89 to −0.69) | −0.85 (−0.95 to −0.74) | −0.91 (−1.01 to −0.80) | .30 | 390 |
| Change in NRS leg pain score | −3.29 (−3.77 to −2.82) | −3.61 (−4.10 to −3.13) | −3.62 (−4.10 to −3.15) | .55 | 377 |
| Change in NRS low back pain score | −2.59 (−3.05 to −2.13) | −2.42 (−2.89 to −1.94) | −2.96 (−3.43 to −2.50) | .25 | 380 |
| Global perceived effect score after 24 mo | 2.55 (2.32 to 2.78) | 2.55 (2.31 to 2.79) | 2.29 (2.06 to 2.52) | .21 | 398 |
| Duration of procedure, min | 95.7 (81.1 to 110.3) | 123.9 (109.0 to 138.7) | 92.9 (78.2 to 107.7) | <.001 | 416 |
| Length of hospital stay, d | 2.84 (2.18 to 3.50) | 3.17 (2.51 to 3.84) | 3.09 (2.43 to 3.75) | .38 | 363 |
| Blood loss, mL | 139.0 (96.9 to 181.1) | 173.1 (130.8 to 215.4) | 150.7 (107.7 to 193.6) | .15 | 373 |
| Proportion reoperated, % | 7.9 (2.2 to 13.7) | 4.6 (4.3 to 8.8) | 8.2 (2.2 to 14.2) | .44 | 416 |
| Proportion incidental dural tear, % | 5.8 (1.6 to 10.0) | 7.5 (2.6 to 12.5) | 7.8 (2.9 to 12.6) | .79 | 402 |
| Proportion wound infection, % | 0 (NA) | 0.8 (0 to 2.3) | 0.0 (NA) | .32 | 396 |
| Proportion hematoma requiring reoperation, % | 1.0 (0 to 3.4) | 1.0 (0 to 3.5) | 1.9 (0 to 5.8) | .81 | 397 |
| Proportion other complications, %b | 1.5 (0 to 3.5) | 5.5 (1.4 to 9.5) | 4.5 (0.9 to 8.1) | .18 | 397 |
| Proportion neurological deterioration, % | 2.2 (0 to 4.8) | 1.6 (0 to 3.8) | 0.7 (0 to 2.2) | .59 | 395 |
Abbreviations: EQ-5D, EuroQuol 5-dimension questionnaire utility index; NRS, numeric rating score; ODI, Oswestry Disability Index; ZCQ, Zurich Claudication Questionnaire.
Means and corresponding 95% CI calculated by estimating marginal effects after fitting multilevel linear models with random intercepts for operating hospital and adjusting for baseline measure when analyzing change scores. Proportions and corresponding 95% CI calculated by estimating marginal effects after fitting multilevel Poisson models with random intercepts for operating hospital.
Other complications include cardiovascular, venous thromboembolism, urological, and respiratory complications.
Figure 3. Oswestry Disability Index After 3 Posterior Decompression Techniques for Lumbar Spinal Stenosis Given as Mean Score and Proportion of Patients Classified as Success.
Patients classified as successs had a reduction in baseline scores 30% or more. BL indicates bilateral laminotomy; SPO, spinous process osteotomy; UL, unilateral laminotomy with crossover.
Secondary Outcome
There were no statistically significant differences in change score for the secondary outcomes between the 3 surgical groups. Results from analyses on secondary outcomes are given in Table 2 and eFigure 1 and eTable 3 in Supplement 2. In the whole cohort, there was a mean improvement in EQ-5D of 0.32 (95% CI, 0.28-0.36). The mean change in ZCQ was 1.02 (95% CI, 0.94-1.11) for symptom severity and 0.85 (95% CI, 0.78-0.92) for physical function. Likewise, the mean improvement from baseline in NRS was 3.5 (95% CI, 3.2-3.8) for leg pain and 2.7 (95% CI, 2.4-3.0) for back pain.
The BL group had a longer mean duration of the surgical procedure, 123.9 (109.0-138.7) minutes compared with 95.7 (81.1-110.3) minutes and 92.9 (78.2-107.7) minutes for UL and SPO, respectively, (P < .001). For other relevant outcomes, there were no differences between the 3 surgical methods. All results related to the surgical procedures are given in Table 2. The mean duration of the surgical procedure was 101 (95% CI, 96-108) minutes for the total cohort, and the length of hospital stay was 3.1 (95% CI, 2.9-3.4) days. The total rate of reoperations in the whole cohort during the 2-year follow up period were 6.4% (95% CI, 4.3%-9.1%), the number of reoperations was 11 of 146 (7.5%) in the UL group, 6 of 142 (4.2%) in the BL group, and 11 of 149 (7.4%) in the SPO group. Overview of the reoperations during the hospital stay, until 3 months follow up, and finally until 2 years of follow up (eTable 4 to eTable 6 in Supplement 2).
Discussion
The results of this study found no association in favor of any of the 3 most commonly used minimally invasive decompression techniques for LSS in terms of effectiveness. We found no clinically relevant or statistically significant association in mean improvement regarding pain and disability or the proportion of patients reporting clinically important changes for the 3 treatment groups after 2 years (eFigure 2 and eFigure 3 in the Supplement). We also found no association that suggested effectiveness varied by level of the surgical procedure or number of levels operated. The results of the secondary patient-reported outcomes were in line with the primary outcome. We found no significant differences in outcomes related to the surgical procedure, such as length of hospital stay, perioperative blood loss, and perioperative complications. The SPO and UL procedures required approximately 30 minutes less than BL. These findings correspond with those of previous trials with smaller numbers of patients,11,12,13,14,15,16,17 both regarding improvement of patient-reported outcome measures and complication rates.
The main strength of the current study is the randomized design and high number of patients (eFigure 4 in the Supplement). Furthermore, the high rate of follow-up improves the internal validity. The external validity would be robust because of the pragmatic inclusion criteria and a large number of highly specialized and smaller orthopedic and neurosurgical centers from all over the country, participating in the inclusion and treatment of the patients. The baseline characteristics and the improvement in disability at 12 months were similar to a previous prospective cohort study from the Norwegian Registry for Spine Surgery,13 further indicating a strong external validity. Other strengths are the public financing of the study, blinding of the person who performed the statistical analyses, and the use of an independent study monitor according to ICH-GCP.
There was no statistical difference between the 3 groups regarding the proportion of reoperations during the primary hospital stay, after 3 months, or after 2 years. A 2-year follow-up period is probably insufficient for a complete evaluation or conclusion to be drawn. The study group plans to follow this cohort for 10 years to evaluate this topic more thoroughly. The number of reoperations will also reflect the durability of the various procedures, an important aspect of the effectiveness evaluation.
Limitations
This study has limitations. The minimally invasive surgical methods evaluated in this study are not compared with a full laminectomy. All the midline retaining procedures could potentially be important to avoid postlaminectomy spondylolisthesis.9,35,36 The reason for not including the laminectomy method was that most of the centers already had stopped using this technique and used the 3 minimally invasive techniques as standard methods. Moreover, a study from the Norwegian Registry for Spine Surgery has shown similar results after minimally invasive decompression and full laminectomy.10
A decompressive procedure is performed to relieve the dural compression at the affected level of the spine and be comprehensive enough to achieve sufficient symptom relief. A secondary radiological study from this trial reported that UL, BL, and SPO provided a similar increase of the dural sac cross-sectional area (DSCA).37 Hence, both radiological and clinical outcomes seem similar in the surgical techniques compared 2 years postoperatively. The outcome for these patients will be followed up with for 10 years to investigate eventual changes over time.
Mannion et al38 reported that a high degree of stenosis preoperatively was associated with a better outcome after the surgical procedure. However, it is unclear how extensive the increase of DSCA needs to be to obtain long-term symptom relief. One study has shown an association between a large increase of DSCA postoperatively and patient-reported outcome,39 but 2 studies did not confirm these findings.14,40 In our opinion, it has not been established whether a wide decompression yields superior clinical results compared with less extensive decompression. The threshold value for the decompression size will be addressed in a future study. In our opinion, it is important to differentiate the effect of the surgical procedure and to evaluate the impact of the decompression method used in terms of the effect on the surrounding structures, including stability of the spine and muscular damage. This will also be addressed in further studies from the NORDSTEN-SST cohort.
The 3 different surgical techniques vary in how much the surrounding tissue is affected. BL requires a bilateral release of the multifidus muscle, and SPO requires an osteotomy of the spinous process. The degree of surgical trauma can affect postoperative fibrosis of the muscles and nerve innervation. The equivalent clinical result from the present study indicates that the surgical impact of the surrounding tissue is of minor importance and that other factors concerning the surgical outcome must be assessed to improve the results after the surgical procedure for LSS.
The change in inclusion criteria, including patients with a baseline ODI of fewer than 25 points, might be seen as a limitation. To investigate the robustness of our findings, we performed analyses studying the effect of surgical technique by timing of study inclusion (before or after November 1, 2015). No evidence suggesting differential effects by inclusion period were found (eTable 7 in Supplement 2).
The actual sample size was somewhat lower than initially planned, which would reduce the statistical power of the study. Originally, we planned our study with a 15% drop-out, corresponding to an actual sample size of 135 participants per study arm. However, the drop-out rate turned out to be lower so the number of participants eligible for analyzing the primary outcome was 393, corresponding to a sample size of 131 per arm. With the same a priori assumptions, this sample size would give an estimated power of 79%, only marginally lower than the required 80%.
Conclusions
In the present trial of patients treated surgically for lumbar spinal stenosis, there were no differences in the effectiveness between the 3 most commonly used minimally invasive posterior decompression techniques. The complication rates did not differ among the 3 methods, although surgical time differed among them.
eAppendix 1. The NORDSTEN-Study Spinal Stenosis Trial-SST Protocol
eAppendix 2. Norwegian Spinal Stenosis Study Research Protocol
eTable 1. Inclusion and Exclusion Criteria for the Spinal Stenosis Trial (SST) in the NORDSTEN-Study
eTable 2. Primary Outcome
eTable 3. Secondary Outcome
eTable 4. Reoperations During the Hospital Stay
eTable 5. Reoperation Recorded After Discharge and Until 3 Months of Follow Up
eTable 6. Reoperation Recorded After 3 Months and Until 24 Months of Follow Up
eTable 7. Interactions
eFigure 1. Secondary Outcomes After 3 Posterior Decompression Techniques for Lumbar Spinal Stenosis
eFigure 2. Global Perceived Effect
eFigure 3. Distribution of the Primary Outcome (Change in ODI) for Each Study Arm
eFigure 4. Cumulative Number of Included Patients Over Time
eAppendix 1. The NORDSTEN-SST Study Monitor Report
eAppendix 2. The NORDSTEN Collaboration
eReferences.
Data Sharing Statement
References
- 1.Watters WC III, Baisden J, Gilbert TJ, et al. Degenerative lumbar spinal stenosis: an evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spinal stenosis. Spine J. 2008;8(2):305-310. doi: 10.1016/j.spinee.2007.10.033 [DOI] [PubMed] [Google Scholar]
- 2.Amundsen T, Weber H, Nordal HJ, Magnaes B, Abdelnoor M, Lilleas F. Lumbar spinal stenosis: conservative or surgical management: a prospective 10-year study. Spine (Phila Pa 1976). 2000;25(11):1424-1435. doi: 10.1097/00007632-200006010-00016 [DOI] [PubMed] [Google Scholar]
- 3.Malmivaara A, Slatis P, Heliovaara M, et al. Surgical or nonoperative treatment for lumbar spinal stenosis: a randomized controlled trial. Spine (Phila Pa 1976). 2007;32(1):1-8. doi: 10.1097/01.brs.0000251014.81875.6d [DOI] [PubMed] [Google Scholar]
- 4.Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical versus nonoperative treatment for lumbar spinal stenosis four-year results of the Spine Patient Outcomes Research Trial. Spine (Phila Pa 1976). 2010;35(14):1329-1338. doi: 10.1097/BRS.0b013e3181e0f04d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaina F, Tomkins-Lane C, Carragee E, Negrini S. Surgical versus nonsurgical treatment for lumbar spinal stenosis. Spine (Phila Pa 1976). 2016;41(14):E857-E868. doi: 10.1097/BRS.0000000000001635 [DOI] [PubMed] [Google Scholar]
- 6.Deyo RA, Mirza SK, Martin BI, Kreuter W, Goodman DC, Jarvik JG. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA. 2010;303(13):1259-1265. doi: 10.1001/jama.2010.338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grotle M, Småstuen MC, Fjeld O, et al. Lumbar spine surgery across 15 years: trends, complications and reoperations in a longitudinal observational study from Norway. BMJ Open. 2019;9(8):e028743. doi: 10.1136/bmjopen-2018-028743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Overdevest GM, Jacobs W, Vleggeert-Lankamp C, Thome C, Gunzburg R, Peul W. Effectiveness of posterior decompression techniques compared with conventional laminectomy for lumbar stenosis. Cochrane Database Syst Rev. 2015;3:CD010036. doi: 10.1002/14651858.CD010036.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guha D, Heary RF, Shamji MF. Iatrogenic spondylolisthesis following laminectomy for degenerative lumbar stenosis: systematic review and current concepts. Neurosurg Focus. 2015;39(4):E9. doi: 10.3171/2015.7.FOCUS15259 [DOI] [PubMed] [Google Scholar]
- 10.Nerland US, Jakola AS, Solheim O, et al. Minimally invasive decompression versus open laminectomy for central stenosis of the lumbar spine: pragmatic comparative effectiveness study. BMJ. 2015;350:h1603. doi: 10.1136/bmj.h1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalgic A, Uckun O, Ergungor MF, et al. Comparison of unilateral hemilaminotomy and bilateral hemilaminotomy according to dural sac area in lumbar spinal stenosis. Minim Invasive Neurosurg. 2010;53(2):60-64. doi: 10.1055/s-0029-1246147 [DOI] [PubMed] [Google Scholar]
- 12.Fu YS, Zeng BF, Xu JG. Long-term outcomes of two different decompressive techniques for lumbar spinal stenosis. Spine (Phila Pa 1976). 2008;33(5):514-518. [DOI] [PubMed] [Google Scholar]
- 13.Hermansen E, Romild UK, Austevoll IM, et al. Does surgical technique influence clinical outcome after lumbar spinal stenosis decompression: a comparative effectiveness study from the Norwegian Registry for Spine Surgery. Eur Spine J. 2017;26:420-427. doi: 10.1007/s00586-016-4643-9 [DOI] [PubMed] [Google Scholar]
- 14.Hong SW, Choi KY, Ahn Y, et al. A comparison of unilateral and bilateral laminotomies for decompression of L4-L5 spinal stenosis. Spine (Phila Pa 1976). 2011;36(3):E172-E178. doi: 10.1097/BRS.0b013e3181db998c [DOI] [PubMed] [Google Scholar]
- 15.Munting E, Roder C, Sobottke R, Dietrich D, Aghayev E. Patient outcomes after laminotomy, hemilaminectomy, laminectomy and laminectomy with instrumented fusion for spinal canal stenosis: a propensity score-based study from the Spine Tango registry. EurSpine J. 2014;24:358-368. doi: 10.1007/s00586-014-3349-0 [DOI] [PubMed] [Google Scholar]
- 16.Postacchini F, Cinotti G, Perugia D, Gumina S. The surgical treatment of central lumbar stenosis: multiple laminotomy compared with total laminectomy. J Bone Joint Surg Br. 1993;75(3):386-392. doi: 10.1302/0301-620X.75B3.8496205 [DOI] [PubMed] [Google Scholar]
- 17.Thome C, Zevgaridis D, Leheta O, et al. Outcome after less-invasive decompression of lumbar spinal stenosis: a randomized comparison of unilateral laminotomy, bilateral laminotomy, and laminectomy. J Neurosurg Spine. 2005;3(2):129-141. [DOI] [PubMed] [Google Scholar]
- 18.Machado GC, Ferreira PH, Yoo RI, et al. Surgical options for lumbar spinal stenosis. Cochrane Database Syst Rev. 2016;11(11):CD012421. doi: 10.1002/14651858.CD012421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobs WC, Rubinstein SM, Willems PC, et al. The evidence on surgical interventions for low back disorders, an overview of systematic reviews. Eur Spine J. 2013;22(9):1936-1949. doi: 10.1007/s00586-013-2823-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hermansen E, Austevoll IM, Romild UK, et al. Study-protocol for a randomized controlled trial comparing clinical and radiological results after three different posterior decompression techniques for lumbar spinal stenosis: the Spinal Stenosis Trial (SST) (part of the NORDSTEN Study). BMC Musculoskelet Disord. 2017;18(1):121. doi: 10.1186/s12891-017-1491-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hopewell S, Clarke M, Moher D, et al. CONSORT for reporting randomized controlled trials in journal and conference abstracts: explanation and elaboration. PLoSMed. 2008;5(1):e20. doi: 10.1371/journal.pmed.0050020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh J. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. J Pharmacol Pharmacother. 2015;6(3)185-187. doi: 10.4103/0976-500X.162004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mariconda M, Fava R, Gatto A, Longo C, Milano C. Unilateral laminectomy for bilateral decompression of lumbar spinal stenosis: a prospective comparative study with conservatively treated patients. J Spinal Disord Tech. 2002;15(1):39-46. doi: 10.1097/00024720-200202000-00006 [DOI] [PubMed] [Google Scholar]
- 24.Aryanpur J, Ducker T.. Multilevel lumbar laminotomies: an alternative to laminectomy in the treatment of lumbar stenosis. Neurosurgery. 1990;26(3):429-432. doi: 10.1227/00006123-198807000-00021 [DOI] [PubMed] [Google Scholar]
- 25.Yong-Hing K, Kirkaldy-Willis WH. Osteotomy of lumbar spinous process to increase surgical exposure. Clin Orthop Relat Res. 1978;(134):218-220. [PubMed] [Google Scholar]
- 26.Fairbank JC, Pynsent PB. The oswestry disability index. Spine (Phila Pa 1976). 2000;25(22):2940-2952. doi: 10.1097/00007632-200011150-00017 [DOI] [PubMed] [Google Scholar]
- 27.Grotle M, Brox JI, Vøllestad NK. Functional status and disability questionnaires: what do they assess: a systematic review of back-specific outcome questionnaires. Spine (Phila Pa 1976). 2005;30(1):130-140. doi: 10.1097/01.brs.0000149184.16509.73 [DOI] [PubMed] [Google Scholar]
- 28.Austevoll IM, Gjestad R, Grotle M, et al. Follow-up score, change score or percentage change score for determining clinical important outcome following surgery: an observational study from the Norwegian registry for spine surgery evaluating patient reported outcome measures in lumbar spinal stenosis and lumbar degenerative spondylolisthesis. BMC Musculoskelet Disord. 2019;20(1):31. doi: 10.1186/s12891-018-2386-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parai C, Hägg O, Lind B, Brisby H. The value of patient global assessment in lumbar spine surgery: an evaluation based on more than 90,000 patients. Eur Spine J. 2018;27(3):554-563. doi: 10.1007/s00586-017-5331-0 [DOI] [PubMed] [Google Scholar]
- 30.Solberg TK, Olsen JA, Ingebrigtsen T, Hofoss D, Nygaard OP. Health-related quality of life assessment by the EuroQol-5D can provide cost-utility data in the field of low-back surgery. Eur Spine J. 2005;14(10):1000-1007. doi: 10.1007/s00586-005-0898-2 [DOI] [PubMed] [Google Scholar]
- 31.Stucki G, Daltroy L, Liang MH, Lipson SJ, Fossel AH, Katz JN. Measurement properties of a self-administered outcome measure in lumbar spinal stenosis. Spine (Phila Pa 1976). 1996;21(7):796-803. doi: 10.1097/00007632-199604010-00004 [DOI] [PubMed] [Google Scholar]
- 32.Ferreira-Valente MA, Pais-Ribeiro JL, Jensen MP. Validity of four pain intensity rating scales. Pain. 2011;152(10):2399-2404. doi: 10.1016/j.pain.2011.07.005 [DOI] [PubMed] [Google Scholar]
- 33.Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105-121. doi: 10.1016/j.jpain.2007.09.005 [DOI] [PubMed] [Google Scholar]
- 34.Cuschieri S. The CONSORT statement. Saudi J Anaesth. 2019;13(suppl 1):S27-S30. doi: 10.4103/sja.SJA_559_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hopp E, Tsou PM. Postdecompression lumbar instability. Clin Orthop Relat Res. 1988;227(227):143-151. [PubMed] [Google Scholar]
- 36.Iida Y, Kataoka O, Sho T, et al. Postoperative lumbar spinal instability occurring or progressing secondary to laminectomy. Spine (Phila Pa 1976). 1990;15(11):1186-1189. doi: 10.1097/00007632-199011010-00018 [DOI] [PubMed] [Google Scholar]
- 37.Hermansen E, Austevoll IM, Hellum C, et al. Comparable increases in dural sac area after three different posterior decompression techniques for lumbar spinal stenosis: radiological results from a randomized controlled trial in the NORDSTEN study. Eur Spine J. 2020;29(9):2254-2261. doi: 10.1007/s00586-020-06499-0 [DOI] [PubMed] [Google Scholar]
- 38.Mannion AF, Fekete TF, Pacifico D, et al. Dural sac cross-sectional area and morphological grade show significant associations with patient-rated outcome of surgery for lumbar central spinal stenosis. Eur Spine J. 2017;26(10):2552-2564. doi: 10.1007/s00586-017-5280-7 [DOI] [PubMed] [Google Scholar]
- 39.Hermansen E, Moen G, Barstad J, Birketvedt R, Indrekvam K. Laminarthrectomy as a surgical approach for decompressing the spinal canal: assessment of preoperative versus postoperative dural sac cross-sectional areal (DSCSA). Eur Spine J. 2013;22(8):1913-1919. doi: 10.1007/s00586-013-2737-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chung SW, Kang MS, Shin YH, Baek OK, Lee SH. Postoperative expansion of dural sac cross-sectional area after unilateral laminotomy for bilateral decompression: correlation with clinical symptoms. Korean J Spine. 2014;11(4):227-231. doi: 10.14245/kjs.2014.11.4.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. The NORDSTEN-Study Spinal Stenosis Trial-SST Protocol
eAppendix 2. Norwegian Spinal Stenosis Study Research Protocol
eTable 1. Inclusion and Exclusion Criteria for the Spinal Stenosis Trial (SST) in the NORDSTEN-Study
eTable 2. Primary Outcome
eTable 3. Secondary Outcome
eTable 4. Reoperations During the Hospital Stay
eTable 5. Reoperation Recorded After Discharge and Until 3 Months of Follow Up
eTable 6. Reoperation Recorded After 3 Months and Until 24 Months of Follow Up
eTable 7. Interactions
eFigure 1. Secondary Outcomes After 3 Posterior Decompression Techniques for Lumbar Spinal Stenosis
eFigure 2. Global Perceived Effect
eFigure 3. Distribution of the Primary Outcome (Change in ODI) for Each Study Arm
eFigure 4. Cumulative Number of Included Patients Over Time
eAppendix 1. The NORDSTEN-SST Study Monitor Report
eAppendix 2. The NORDSTEN Collaboration
eReferences.
Data Sharing Statement