Abstract
There are significant differences between men and women in the efficacy and tolerability of antipsychotic drugs. Here, we provide a comprehensive overview of what is currently known about the pharmacokinetics and pharmacodynamics of antipsychotics in women with schizophrenia spectrum disorders (SSDs) and translate these insights into considerations for clinical practice. Slower drug absorption, metabolism and excretion in women all lead to higher plasma levels, which increase the risk for side-effects. Moreover, women reach higher dopamine receptor occupancy compared to men at similar serum levels, since oestrogens increase dopamine sensitivity. As current treatment guidelines are based on studies predominantly conducted in men, women are likely to be overmedicated by default. The risk of overmedicating generally increases when sex hormone levels are high (e.g. during ovulation and gestation), whereas higher doses may be required during low-hormonal phases (e.g. during menstruation and menopause). For premenopausal women, with the exceptions of quetiapine and lurasidone, doses of antipsychotics should be lower with largest adjustments required for olanzapine. Clinicians should be wary of side-effects that are particularly harmful in women, such as hyperprolactinaemia which can cause oestrogen deficiency and metabolic symptoms that may cause cardiovascular diseases. Given the protective effects of oestrogens on the course of SSD, oestrogen replacement therapy should be considered for postmenopausal patients, who are more vulnerable to side-effects and yet require higher dosages of most antipsychotics to reach similar efficacy. In conclusion, there is a need for tailored, female-specific prescription guidelines, which take into account adjustments required across different phases of life.
Key words: Antipsychotic treatment, dopamine sensitivity, pharmacodynamics, pharmacokinetics, psychosis, schizophrenia, sex differences
Introduction
It was long thought that women with a schizophrenia spectrum disorder (SSD) had a more favourable course compared to male patients, but that idea should be reconsidered. Female patients have the same number of readmissions during their lifetime (Ceskova, Prikryl, Libiger, Svancara, & Jarkovsky, 2015; Sommer, Tiihonen, van Mourik, Tanskanen, & Taipale, 2020), have similar recovery rates (12.9% for women and 12.1% for men) (Jaaskelainen et al., 2013) and similar functional outcomes 10 years after a first psychotic episode (Ayesa-Arriola et al., 2020; Mayston et al., 2020). Although male patients experience more negative symptoms, females have more affective symptoms (Ochoa, Usall, Cobo, Labad, & Kulkarni, 2012). Aggression towards others is more common in males, whereas self-harm and suicide attempts are more common among female patients (Dama et al., 2019; Dubreucq et al., 2021; Jongsma, Turner, Kirkbride, & Jones, 2019; Sommer et al., 2020). Although the later age of onset and lower comorbidity with substance abuse in women may lead to better functioning in the early stages of the illness (Usall, Ochoa, Araya, & Márquez, 2003), this benefit is not maintained in the more chronic phase and advantages for women even seem to reverse after the age of 50 (Mayston et al., 2020; Shlomi Polachek et al., 2017; Thorup, Waltoft, Pedersen, Mortensen, & Nordentoft, 2007). Hence, the idea that women have a better overall course of SSD compared to men is not correct. However, the vast majority of the literature and guidelines on antipsychotic treatment neglects differences between male and female patients and base their conclusions on studies with predominantly male participants (Huhn et al., 2019; Lally & MacCabe, 2015; Lange, Mueller, Leweke, & Bumb, 2017; Phillips & Hamberg, 2016; Santos-Casado & García-Avello, 2019; Zakiniaeiz, Cosgrove, Potenza, & Mazure, 2016).
Psychosocial differences in diet, smoking and substance abuse between men and women (i.e. gender differences), can influence the efficacy and tolerability of antipsychotics (Seeman, 2004). In addition, biologically determined differences (i.e. sex differences), such as body composition and hormonal transitions affect the drug pharmacokinetics, determined by the absorption, distribution, metabolism and excretion, and drug pharmacodynamics which involves receptor binding, receptor sensitivity and the receptor binding profile of a drug (Iversen et al., 2018; Lange et al., 2017; Zucker & Prendergast, 2020). The female sex hormones in general, and oestrogens in particular play a major role in these sex differences (Brand, de Boer, & Sommer, 2021; González-Rodríguez & Seeman, 2019). However, current guidelines on the prescription of antipsychotics do not take these differences in account (Keepers et al., 2020; Ventriglio et al., 2020). This review serves as a comprehensive overview of currently available literature on the differences in the action of antipsychotic medication in female v. male patients with SSD, with special considerations for female-specific pharmacotherapy regimens during different (hormonal) stages of life (i.e. menarche, pregnancy, lactation and menopause).
Gender differences in prescription
Although some patients prefer to be treated without medication, most psychotic episodes require pharmacological treatment with antipsychotics. Although classical antipsychotics such as haloperidol are still being used as second generation and other newer antipsychotics such as aripiprazole, risperidone, olanzapine, amisulpride and quetiapine are often preferred by psychiatrists given their better efficacy–tolerability balance (Kahn et al., 2008). Clozapine is usually provided as a second or third line of treatment, when two other antipsychotics did not yield remission from psychosis. Although clozapine is superior in efficacy for these individuals, it does have some bothersome side-effects, such as being diabetogenic, inducing weight gain, severe constipation and (rarely) agranulocytosis (Nielsen, Damkier, Lublin, & Taylor, 2011).
Although no sex-specific guidelines currently exist for prescribing antipsychotics, notable differences are observed in prescription patterns between men and women, caused by preferences of both the physician and the patient. Women may experience some side-effects as more severe than men and may, for example, encounter more difficulties with antipsychotic-induced weight gain (Achtyes et al., 2018; Connors & Casey, 2006). The risk of weight gain is, in particular, of great influence in the decision to take medication in women, causing non-adherence to prescribed medications specifically in this gender (Achtyes et al., 2018; Lambert et al., 2004).
Based on a Finnish nation-wide cohort study, women are more often prescribed quetiapine and aripiprazole, whereas men are more often prescribed clozapine and olanzapine (Sommer et al., 2020). Also, the use of additional psychotropic medication (e.g. antidepressants, mood stabilisers and benzodiazepines) is more common in women compared to in men (Ceskova & Prikryl, 2012; Sommer et al., 2020). In the USA and European countries, prescription rates of long-acting injectable (LAI) antipsychotics are lower in women (Arnold et al., 2004; Mahadun & Marshall, 2008; Shi et al., 2007; Sommer et al., 2020), although the compliance to medication is similar in men and women (Caqueo-Urízar, Fond, Urzúa, & Boyer, 2018; Castberg, Westin, & Spigset, 2009; Leijala, Kampman, Suvisaari, & Eskelinen, 2021).
Sex differences in the pharmacokinetics of antipsychotics
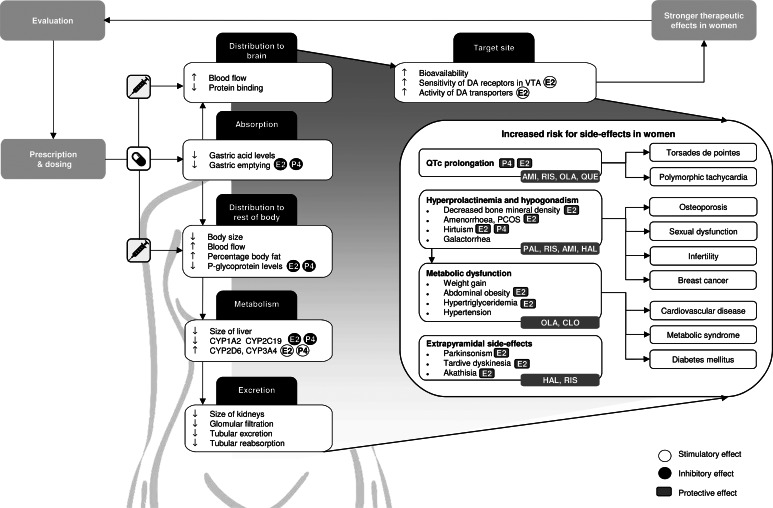
Body composition differs significantly between the sexes: women have smaller organs, more fatty tissue and less muscle tissue, which changes the volume of distribution, especially for lipophilic drugs (Seeman, 2018). In addition, women have some 10–15% greater blood flow to the brain, which makes it easier for a drug to reach their target receptor (Fig. 1) (Gur & Gur, 1990). Castberg, Westin, Skogvoll, and Spigset (2017) included 43 079 blood samples of patients between 18 and 100 years old using olanzapine, risperidone or quetiapine and concluded that women generally have 20–30% higher dose-adjusted concentrations, which is a proxy for bioavailability, compared to men. Another study, including 26 388 patients of all ages, reported higher dose-adjusted concentrations in women for 11 out of 12 antipsychotics (Jönsson, Spigset, & Reis, 2019), with the largest differences for olanzapine and clozapine (59.0% and 40.4% higher in women, respectively), whereas for quetiapine, dose-adjusted concentrations were 6.4% lower in women. Similarly, Eugene and Masiak (2017) reported dopamine receptor occupancy rates of 70% for male and female patients (mean age: 25.6 ± 7.9 for men; 28.9 ± 9.1 for women), whereas men were taking a higher dose (20 v. 10 mg/day). Since women are often treated with multiple psychotropic medications (Sommer et al., 2020), drug interactions are especially relevant for this sex and become even more crucial when women are overmedicated. For example, clinically relevant effects on serum levels of several antipsychotics have been reported for the selective serotonin reuptake inhibitors (SSRIs) fluoxetine, fluvoxamine and paroxetine (Spina & De Leon, 2014). In addition, sex differences in the pharmacokinetics of antidepressants should be taken into account in women with SSD who are also being treated for depression, which are discussed in detail in a review by Damoiseaux, Proost, Jiawan, and Melgert (2014).
Fig. 1.
Graphical overview of relevant differences in pharmacokinetics, pharmacodynamics and therapeutic effects of antipsychotics in women compared to men. Side-effects that should be obtaining additional attention in women are defined, together with commonly used antipsychotics that carry the highest risks for these side-effects. In addition, the stimulatory and inhibitory effects of oestradiol (E2), which is the predominant form of oestrogen, and progesterone (P4) on pharmacokinetic processes are shown, as well as the protective effects of E2 and P4 on specific side-effects. CYP, cytochrome P450; AMI, amisulpride; CLO, clozapine; HAL, haloperidol; OLA, olanzapine; PAL, paliperidone; QUE, quetiapine; RIS, risperidone; E2, oestradiol; P4, progesterone; DA, dopamine; VTA, ventral tegmental area.
Absorption and distribution
When administered orally, the absorption of a drug largely depends on gut physiology. Women's stomachs are on average less acidic compared to men's and this increases the absorption of antipsychotic drugs (Soldin & Mattison, 2009; Walters & Levitan, 2020). Additionally, female sex hormones reduce gastric emptying and intestinal motility (Freire, Basit, Choudhary, Piong, & Merchant, 2011), resulting in lower gastrointestinal transit rates in women (Hutson, Roehrkasse, & Wald, 1989; Jiang, Greenwood-Van Meerveld, Johnson, & Travagli, 2019). This enhances drug absorption and increases the bioavailability of oral antipsychotics (Stillhart et al., 2020). After menopause, transit rates increase to a level similar to that of age-matched men (Camilleri, 2020; Gonzalez, Loganathan, Sarosiek, & McCallum, 2020).
The absorption and distribution of antipsychotic drugs is influenced by P-glycoprotein (P-gp), an efflux transporter that is located on the cell membrane that limits the systemic exposure of its substrates (Elmeliegy, Vourvahis, Guo, & Wang, 2020). P-gp levels are some two-fold lower in women compared to that in men, being decreased by oestrogens and progesterone (Bebawy & Chetty, 2009; Nicolas, Espie, & Molimard, 2009). Although lower P-gp levels thus enable a drug to enter the brain more easily, non-target organs also become more accessible for the drug, potentially causing more side-effects (Fig. 1) (Hiemke et al., 2018). Sex differences in bioavailability of antipsychotics are expected to be more pronounced for drugs that bind P-gp more tightly (e.g. risperidone and aripiprazole) (Table 1) (Bebawy & Chetty, 2009; Doran et al., 2005; Linnet & Ejsing, 2008; Moons, De Roo, Claes, & Dom, 2011; Nagasaka, Oda, Iwatsubo, Kawamura, & Usui, 2012).
Table 1.
Summary of drug-specific pharmacokinetic properties, side-effects and overdosing risks in women
| Pharmacokinetics | Risk for side-effects | Risk of overmedicating women as compared to men | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Metabolism1 | CYP-activity in females as compared to males2,3,4,5,6 | P-gp binding7,8,9 | QTc prolongation | Prolactin elevation | EPS and akathisia10 | Metabolic dysfunction | |||
| Weight gain10 | Lipid/glucose abnormalities12 | ||||||||
| Amisulpride | >90% renal excretion | ++/+++ | +++10 | +++10,12 | +10 | + | ++12 | ++ | |
| Aripiprazole | CYP2D6, CYP3A4 | (+), (+ +) | ++ | −10 | −10,12 | −/+10 | +10 | −12 | + |
| Chlorpromazine | CYP1A2a, CYP2D6 | (− −), (+) | + | ++11ψ | +10ψ | ++10ψ | +++10 | +++12ψ | ++ |
| Clozapine | CYP1A2a, CYP2C19a, CYP3A4 | (− −), (−), (++) | + | ++11ψ | −10,12 | −10 | +++10 | +++13 | +++ |
| Flupentixol | CYP2D6a | (+) | ? | +11ψ | −10 | +++10 | ++10 | ? | + |
| Haloperidol | CYP2D6a, CYP3A4 | (+), (+ +) | + | +10ψ | ++10.12 | +++10 | +10 | −13 | + |
| Lurasidone | CYP3A4 | (+ +) | ? | −10 | +/++10,12 | ++10 | +10 | −13 | +/− |
| Olanzapine | CYP1A2a | (− −) | ++/+++ | ++10 | +10,12 | −10 | +++10 | +++13 | +++ |
| Paliperidone | CYP3A4, UGT1A1, 60% renal excretion |
(++), (+) | ++/+++ | +10 | +++10.12 | +10 | +10 | ++13 | ++ |
| Quetiapine | CYP3A4, CYP2D6a | (++), (+) | −/+ | ++10 | −10.12 | −10 | +++10 | ++13 | − |
| Risperidone | CYP2D6a, CYP3A4 | (+), (++) | +++* | ++10 | +++10.12 | ++10 | ++10 | +13 | ++ |
| Sulpiride | Renal excretion only | ++/+++ | ++ | +++12ψ | ++10ψ | ++10 | ? | ++ | |
| Zuclopenthixol | CYP2D6 | (+) | ? | ? | ? | +++10 | +10 | ? | + |
CYP, cytochrome P450; EPS, extrapyramidal symptoms; P-gp, P-glycoprotein; UGT, UDP glucuronosyl transferase.
The risk of overmedicating women as compared to men is estimated based on drug-specific metabolism, P-gp binding and CYP-activity in women compared to men. In addition, the risk of QTc prolongation, prolactin elevation, EPS and akathisia, weight gain and lipid/glucose abnormalities are also defined.
1, Hiemke et al. (2018); 2, Scandlyn et al. (2008); 3, Choi et al. (2013); 4, Piccinato et al. (2017); 5, Hagg et al. (2001); 6, Tamminga et al. (1999); 7, Doran et al. (2005); 8, Linnet & Ejsing (2008); 9, Nagasaka et al. (2012); 10, Huhn et al. (2019); 11, Wenzel-Seifert et al. (2011); 12, Peuskens et al. (2014); 13, De Hert et al. (2012).
(++), activity strongly higher in females; (+), activity higher in females; only of relevance during pregnancy; (−), activity lower in females; (− −), activity strongly lower in females; a, inhibited by oral oestrogenic contraceptives; +++, high incidence/high severity/strong; ++, moderate incidence/moderate severity/moderate; +, light incidence/mild severity/mild; −, low/very low/small; ?, unknown; *, main metabolite of risperidone 9-OH-risperidone. ψ, level of evidence is limited. When enzymes are indicated in bold, drug plasma concentrations will significantly increase or decrease when combined with strong to moderate inducers or inhibitors (see Hiemke et al., 2018).
Women naturally have more subcutaneous fat compared to men and this slows the absorption and perfusion of LAI antipsychotics (Soldin, Chung, & Mattison, 2011; Yonkers, Kando, Cole, & Blumenthal, 1992). The accumulation of LAI antipsychotics in adipose tissue increases their half-life time, resulting in extended release to the blood (Soldin et al., 2011; Yonkers et al., 1992). Short dosing intervals could lead to higher serum concentrations over time (Seeman, 2020). Female patients may, thus, benefit from longer dosage intervals for LAI compared to males.
Metabolism
Drug metabolism is generally lower in the female sex, as women have a smaller liver and differential functioning of the hepatic cytochrome P450 (CYP) system compared to men (Soldin et al., 2011). Most antipsychotics are metabolised by CYP1A2, CYP2C19, CYP2D6 and/or CYP3A4 (Table 1) (Hiemke et al., 2018; Ravyn, Ravyn, Lowney, & Nasrallah, 2013). Of note, fluvoxamine has an inhibitory effect on all CYP enzymes, especially on CYP1A2 and CYP2C19, whereas paroxetine and fluoxetine are both potent inhibitors of CYP2D6 metabolism. Co-medication with these SSRIs may, thus, require downward dosage adjustments (Spina & De Leon, 2014).
CYP1A2
CYP1A2 activity is lower in females compared to that in males (Scandlyn, Stuart, & Rosengren, 2008). Clozapine and olanzapine are both mainly metabolised by this enzyme and with similar dosing, these antipsychotics reach 25–60% higher dose-adjusted concentrations in women (Bigos et al., 2008; Castberg et al., 2017; Jönsson et al., 2019; Tang et al., 2007). Since both oestrogen and progesterone are substrates of CYP1A2, they have an inhibitory effect on this enzyme (Lu et al., 2020). Drug plasma concentrations are, therefore, specifically higher in the female sex when hormone levels are high (e.g. before menopause) whereas they decrease in relative terms when hormone levels fall (e.g. after menopause) (Soldin et al., 2011).
CYP3A4
In contrast to CYP1A2, CYP3A4 activity is about 20–30% higher in premenopausal women as compared to the other sex (Greenblatt & von Moltke, 2008; Scandlyn et al., 2008; Wolbold, 2003; Yang & Li, 2012) and its activity is stimulated by oestrogens and progesterone (Choi, Koh, & Jeong, 2013; Piccinato et al., 2017). CYP3A4 metabolism is especially relevant for female patients taking quetiapine and lurasidone since these drugs are only metabolised by this enzyme (Table 1). For CYP3A4 substrates, the previously discussed plasma concentration elevating processes are neutralised by this higher CYP3A4 activity, some of which was demonstrated in a large sample of adult patients (Jönsson et al., 2019). Jönsson et al. (2019) found smaller sex differences in dose-adjusted concentrations for partial CYP3A4 substrates aripiprazole (n = 1610) and haloperidol (n = 390) than for CYP1A2 substrate olanzapine (n = 10 286), and even lower dose-adjusted concentrations for quetiapine (n = 5853). Moreover, Castberg et al. (2017) showed that the blood levels of quetiapine (n = 4316) are similar in males and females before menopause; whereas after menopause, quetiapine concentrations are higher in females compared to that in males.
CYP2D6
Aripiprazole, haloperidol and risperidone are some of the antipsychotics that are primarily metabolised by CYP2D6 (Table 1). According to a population-based study (n = 1376, 23% females, 18–82 years), CYP2D6 activity is 20% higher in women as compared to men (Tamminga et al., 1999). The clinical relevance of this sex difference in CYP2D6 activity is probably small (Aichhorn et al., 2005), as individual differences largely depend on highly variable genetic variations in the CYP2D6 gene (Labbé et al., 2000). Sex differences may, therefore, only become apparent when female sex hormones reach significant heights (Gaedigk, Dinh, Jeong, Prasad, & Leeder, 2018; Konstandi, Andriopoulou, Cheng, & Gonzalez, 2020), for example in pregnancy.
CYP219C
CYP219C is responsible for the metabolism of clozapine and other less commonly used antipsychotics (e.g. cyamemazine) (Hiemke et al., 2018). The population-based study by Tamminga et al. (1999) (n = 2638, 30% females, 18–82 years) reported a 40% lower enzyme activity in females compared to that in males, which was most pronounced in the age range from 18 to 40 years. Yet, these findings were not replicated in a sample of 330 healthy individuals (n = 611, 18–49 years) (Hagg, Spigset, & Dahlqvist, 2001).
Excretion
The three major processes that determine renal clearance [i.e. glomerular filtration rate (GFR), tubular absorption and tubular excretion] are all lower in women compared to that in men (Soldin et al., 2011). Since P-gp facilitates renal drug efflux, lower P-gp levels lead to slower renal and hepatic clearance in women as compared to men (Elmeliegy et al., 2020; Nicolas et al., 2009). After adjusting for body size, GFR is 10–25% lower in females (Whitley & Lindsey, 2009). These differences in renal clearance are particularly relevant for amisulpride, sulpiride and paliperidone, which are cleared mainly by the kidney, leading to much higher plasma levels of these drugs in female patients of all ages (Hoekstra et al., 2021; Li, Li, Shang, Wen, & Ning, 2020).
Differences in pharmacodynamics between men and women
Oestrogens can modulate the effect of dopamine (Gogos et al., 2015; Shams, Sanio, Quinlan, & Brake, 2016; Yoest, Cummings, & Becker, 2019). According to preclinical evidence, oestrogens influence the levels of dopamine transporters (DATs) and receptors in cortical and striatal regions and by increasing D2 receptor sensitivity in the ventral tegmentum (Vandegrift, You, Satta, Brodie, & Lasek, 2017). A single photon emission computerised tomography DAT study showed an effect of sex (effect size: 0.25), whereby female patients with schizophrenia had a higher ratio of specific striatal binding than male patients (17–53 years) (Chen et al., 2013). These sex differences in the organisation of the striatal dopamine system result in higher D2 receptor occupancy in premenopausal women compared to that in men, even when plasma concentrations are similar, whereas postmenopausal women need higher dosages to reach the same efficacy (Fig. 1).
Differences in treatment response between men and women
Meta-analyses on the efficacy of antipsychotic drugs often do not take sex into account (Huhn et al., 2019; Leucht et al., 2020). Yet, based on the limited number of studies that did account for sex, a better response in women has been reported for most antipsychotic drugs (Ceskova et al., 2015; Lange et al., 2017; Usall, Suarez, & Haro, 2007), except for amisulpride (Ceskova et al., 2015; Kahn et al., 2018; Müller et al., 2006), risperidone (Ceskova et al., 2015; Labelle, Light, & Dunbar, 2001; Pu et al., 2020; Segarra et al., 2012; Usall et al., 2007) and perhaps clozapine (Alberich et al., 2019). As premenopausal women overall show better treatment response and fewer hospitalisations compared to postmenopausal women (Ayesa-Arriola et al., 2020; Goldstein et al., 2002; Seeman, 2019; Shlomi Polachek et al., 2017), inconsistent findings may be a result of heterogeneity in age and in duration and severity of illness between study samples. However, all of the abovementioned studies on antipsychotic efficacy fail to account for differences in dose-adjusted plasma concentrations, which may have confounded their results. Specifically, the pronounced sex differences in drug pharmacokinetics and D2 binding rates result in much higher plasma levels in women and especially premenopausal women compared to that in men, even when dose adjustments for body weight are performed (Jönsson et al., 2019).
The augmentative effect of oestrogens on treatment efficacy is also evident in other patient populations. For example, female patients with anorexia nervosa receiving antipsychotics may benefit from oestrogen augmentation therapy, as these women also have low oestrogen levels (Keating, 2010). In addition, when women are more sensitive to antipsychotics, they would also be more sensitive to other forms of dopaminergic medications. Levodopa is a precursor of dopamine and is the most potent medication for Parkinson's disease (PD). Indeed, levodopa appears to be more effective at reducing symptoms in female compared to that in male patients with PD (Lyons, Hubble, Tröster, Pahwa, & Koller, 1998), whereas female sex is also indicated as a risk factor for levodopa-induced side-effects, such as dyskinaesia and hallucinations (Hassin-Baer et al., 2011; Zhu, van Hilten, Putter, & Marinus, 2013).
Side-effects and tolerability
Although higher plasma levels increase bioavailability and efficacy, they also increase the risk for adverse events (Fig. 1) (Castberg et al., 2017; Jönsson et al., 2019; Lange et al., 2017). Indeed, a large study of 1087 patients between 18 and 65 years with psychotic disorders (48% female) shows that female gender was one of the two main risk factors for severe side-effects, together with polypharmacy, which is also more common in women (Iversen et al., 2018).
QTc-prolongation
Prolongation of the QTc interval is a side effect of several antipsychotics and may result in life threatening cardiac ventricular arrhythmia such as torsades de pointes (TdP) (Beach, Celano, Noseworthy, Januzzi, & Huffman, 2013). QTc intervals are typically longer in women compared to that in men and female sex is an independent risk factor for developing drug-related TdP (De Yang et al., 2011; Makkar, 1993). Although testosterone appears to shorten the QTc interval in men, there seems to be a more complex interaction between progesterone and oestrogen in women (Vink, Clur, Wilde, & Blom, 2018). De Yang et al. (2011) evaluated electrocardiograms of 1006 schizophrenia patients (32% female, 25–75 years) on typical and atypical antipsychotic medication and reported that QTc prolongation was more than twice as common in females (7.3%) compared to that in males (3.2%). Although most antipsychotics can cause QTc prolongation, amisulpride, risperidone, olanzapine and quetiapine have the highest risk (Table 1) (Huhn et al., 2019). Elderly women are more vulnerable for developing TdP as a consequence of QTc prolongation, since sex hormone levels decline whereas additional risk factors for TdP, such as heart disease and electrolyte changes, become more common with increasing age (Danielsson et al., 2016; Wenzel-Seifert, Wittmann, & Haen, 2011). Clinicians should, thus, be wary of the increased risk of QTc prolongation in (older) women, especially those with a (family) history of heart disease.
Extrapyramidal side-effects
Antipsychotics such as amisulpride, risperidone, paliperidone and aripiprazole are associated with a high risk of extrapyramidal symptoms (EPSs) (Huhn et al., 2019). Although acute dystonia appears to be less common in premenopausal female patients (Mas et al., 2016), women seem to have a greater risk of developing parkinsonism and akathisia compared to males (Divac, Prostran, Jakovcevski, & Cerovac, 2014), which is likely due to the higher dopamine receptor binding at lower dosages in women (Di Paolo, 1994; Seeman & Lang, 1990; Thanvi & Treadwell, 2009). The higher risk for women of developing parkinsonism has also been reported in other patient populations that are prescribed antipsychotic medications, for example in patients with dementia (Marras et al., 2012). Multiple reviews indicate that an increased prevalence of EPS in women is associated with a postmenopausal oestrogen decline (da Silva & Ravindran, 2015; Leung & Chue, 2000; Seeman & Lang, 1990; Thompson, Kulkarni, & Sergejew, 2000). Long-term exposure to dopamine receptor-blocking agents can cause tardive dyskinaesia (TD), which is also more common in women compared to that in men of all ages (Divac et al., 2014; Yassa & Jeste, 1992). The incidence of antipsychotic-induced TD is relatively low in premenopausal women (3–5%), possibly due to the protective antioxidant effects of oestrogens (Cho & Lee, 2013; Wu, Kosten, & Zhang, 2013), but can reach an incidence rate of 30% in postmenopausal women after 1 year of cumulative exposure to antipsychotics (Waln & Jankovic, 2013).
Endocrine side-effects
By diminishing the inhibitory effect of dopamine on prolactin secretion in the pituitary gland, antipsychotics often cause hyperprolactinaemia (González-Rodríguez, Labad, & Seeman, 2020), which can result in galactorrhoea, cessation of normal cyclic ovarian function and hirsutism (Malik et al., 2011; Peuskens, Pani, Detraux, & De Hert, 2014). Premenopausal women have physiologically higher levels of prolactin compared to men and are therefore closer to the threshold for hyperprolactinaemia (Kaar, Natesan, McCutcheon, & Howes, 2020; Riecher-Rössler, 2017). Consequently, they are more than twice as likely to develop antipsychotic-induced hyperprolactinaemia compared to postmenopausal women and men (González-Rodríguez et al., 2020; Kinon, Gilmore, Liu, & Halbreich, 2003). Moreover, prolactin secretion suppresses the production of sex hormones and induces oestrogen deficiency, which is already more frequent in female SSD patients compared to healthy females before menopausal age (Brand et al., 2021; Gogos et al., 2015; Lindamer et al., 2000; Riecher-Rössler, 2005). Oestrogen deficiency can lead to polycystic ovarian syndrome, infertility, osteoporosis, sexual dysfunction and an increased risk of breast cancer (De Hert, Detraux, & Peuskens, 2014; Haring et al., 2014; Pottegård, Lash, Cronin-Fenton, Ahern, & Damkier, 2018; Yum, Kim, & Hwang, 2014). For example, up to 48% of women receiving antipsychotic treatment report irregularities in their menstrual cycle (O'Keane, 2008) and reduced bone mineral density is present in 32% of women treated with prolactin-raising antipsychotics for >10 years (Meaney et al., 2004). Prolactin-sparing antipsychotics (e.g. aripiprazole) should, therefore, be preferred over prolactin-raising antipsychotics (e.g. risperidone) for female patients of all ages (Table 1).
Metabolic side-effects and risk for cardiovascular disease
Women treated with antipsychotics are 1.7 times more likely to develop metabolic syndrome compared to men and are therefore at a higher risk for cardiovascular disease (CVD) (Bener, Al-Hamaq, & Dafeeah, 2014; Carliner et al., 2014; Huang et al., 2009; Ingimarsson, MacCabe, Haraldsson, Jónsdóttir, & Sigurdsson, 2017; McEvoy et al., 2005). When taking antipsychotics, women are specifically more vulnerable to weight gain and abdominal adiposity (up to 73.4% in women v. 36.6% in men) (Alberich et al., 2019; Kraal, Ward, & Ellingrod, 2017; McEvoy et al., 2005; Panariello, De Luca, & de Bartolomeis, 2011; Verma, Liew, Subramaniam, & Poon, 2009), which is worrisome as abdominal obesity is a strong predictor of diabetes (de Vegt et al., 2001). Increased appetite and food intake can be a result of the antagonistic effect of antipsychotics on several neurotransmitter systems (i.e. serotonergic, histaminergic and dopaminergic) (Bak, Fransen, Janssen, Van Os, & Drukker, 2014). Since most antipsychotics are given at doses higher than required in women (Jönsson et al., 2019), they are more vulnerable to gain weight from overeating, as high drug plasma levels lead to higher receptor occupancy and thus induce more appetite. Strong associations are particularly found between weight gain and histamine H1 antagonism (Vehof et al., 2011). Since oestrogens modulate histamine neurotransmission (Provensi, Blandina, & Passani, 2016), the antagonistic action of antipsychotics on this receptor may also be enhanced by oestrogens, which could explain why the effects on appetite and food intake are stronger in premenopausal women compared to that in postmenopausal women and men. Special attention should be paid to clozapine and olanzapine, which both have the highest affinity for the histamine H1 receptor and the highest risk of being prescribed at doses higher than the required. Unsurprisingly, these antipsychotics are also the ones most strongly associated with weight gain (Kaar et al., 2020; Smith, Leucht, & Davis, 2019). Adequate dosing of all antipsychotics is, therefore, crucial (Huhn et al., 2019; Kraal et al., 2017), and medications that induce minimal weight gain like aripiprazole, may be preferred (Table 1).
Oestrogen deficiency in women can induce several metabolic symptoms, including insulin resistance and hypertriglyceridaemia (Fitzgerald, Janorkar, Barnes, & Maranon, 2018; Valencak, Osterrieder, & Schulz, 2017). The risk of metabolic dysfunction and CVD is, thus, increased by all factors that cause oestrogen deficiency (e.g. hyperprolactinaemia and menopause) (Regitz-Zagrosek, Lehmkuhl, & Mahmoodzadeh, 2007). Moreover, low oestrogen levels cause abdominal fat accumulation, since oestrogens augment fat accumulation around the hips and thighs rather than upper abdominal areas (Fitzgerald et al., 2018). This again highlights the importance of avoiding prolactin-raising antipsychotics in women to prevent hyperprolactinaemia.
Special considerations regarding pharmacotherapy in premenopausal women
Since pharmacokinetic processes are generally lower in women whereas D2 occupancy rates are higher during high oestrogen phases, dosing of most antipsychotics should start lower in premenopausal women than in men, except for quetiapine and lurasidone. At times when oestrogen levels are high (i.e. during the ovulatory phase), premenopausal women are especially vulnerable to overdosing of drugs metabolised by the CYP1A2 enzyme (olanzapine and clozapine) (Table 1). Although further research is required, it is possible that premenopausal women may, on average, have enough antipsychotic protection from a dose of clozapine and olanzapine that is half of an average male dose (Eugene & Masiak, 2017). The interaction between antipsychotics and the menstrual cycle is subject to individual differences and requires further investigation for each type of antipsychotic. In general, women with regular cycles who suffer from psychotic exacerbations during the menstrual phase may benefit from slight dose increments shortly before and around menstruation, although this is rarely done in practice (González-Rodríguez & Seeman, 2019; Lange et al., 2017; Seeman, 2020; Yum, Yum, & Kim, 2019). Side-effects of antipsychotic drugs that could be particularly relevant to younger female patients include weight gain, as obesity largely decreases self-esteem in women (Connors & Casey, 2006; Lieberman, Tybur, & Latner, 2012) and hyperprolactinaemia, which reduces oestrogen levels. These low oestrogen levels not only increase the risk for somatic complications but also potentially have also a negative impact on psychotic symptoms (Brand et al., 2021). Aripiprazole and lurasidone may, therefore, be drugs of first choice in this phase and options of second choice are olanzapine, quetiapine and clozapine (Table 2).
Table 2.
Summary of clinically relevant treatment considerations across different hormonal phases
| Premenopausal women | Pregnant women | Postpartum/breastfeeding women | Postmenopausal women | ||
|---|---|---|---|---|---|
| First choice | Aripiprazole, lurasidone | Quetiapine, haloperidol, olanzapine | Olanzapine | Aripiprazole, lurasidone | |
| Second choice | Olanzapine, quetiapine, clozapine | Aripiprazole, lurasidone | Amisulpride, haloperidol (when breastfeeding) | Olanzapine, quetiapine, clozapine | |
| Avoid | Prolactin-raising antipsychotics | Polypharmacy; risperidone, although switching is not recommended | Clozapine (when breastfeeding) | Sertindole, amisulpride, prolactin-raising antipsychotics | |
| Starting dose | Should be generally lower than in men, but similar for quetiapine and lurasidone | Minimum effective dose | Similar dose as before pregnancy | Dose increase after menopause | |
| Monitor | Metabolic complications | Gestational diabetes, (oral) glucose tolerance testing, cholesterol and triglycerides | Neurodevelopment and motor abnormalities in baby | Metabolic complications | |
| Sex hormone/prolactin levels, especially when using prolactin-raising antipsychotics | Drug plasma levels, particularly in third trimester | Maternal clinical symptoms | Sex hormone/prolactin levels, especially when using prolactin-raising antipsychotics | ||
| Special considerations | General | Be wary of different menstrual phases and associated fluctuations in plasma concentrations | Switching from usual prescription is not recommended | Continue antipsychotic treatment postpartum | Consider augmentation with SERM raloxifene |
| Oestrogenic contraceptives strongly increase clozapine levels: progesterone-only contraceptives or frequent monitoring of plasma levels is recommended | The potential benefits of antipsychotics may outweigh their potential risks of discontinuation | Limited amount of evidence on the effects on the baby. Possible antipsychotic use during breastfeeding should be considered in discussion with patient, partner or carer, and paediatrician as required | |||
| Dosing | Consider slight increases (e.g. 10%) in dosing shortly before and during menstruation in women with regular cycles. These dose adjustments are dependent of drug-specific P-gp affinity and metabolism | Plasma levels may change significantly in third trimester and plasma levels of renally excreted drugs decrease | Dose increments may be required to prevent postpartum psychotic exacerbations | Dose adjustments are dependent on drug-specific P-gp affinity and metabolism Doses should be higher for most antipsychotics compared to the premenopausal period, except maybe for quetiapine and lurasidone, which may even be lowered |
|
| Side-effects | Be wary of risk of weight gain and hyperprolactinaemia | Increased risk of gestational diabetes mellitus, except for aripiprazole | Limited amount of evidence on the effects of breastfeeding on the baby | Be wary of increased risk of QTc prolongation, motor symptoms (parkinsonism, akathisia and TD), CVD and osteoporosis | |
Interaction between antipsychotics and contraceptives
Women with SSD are usually prescribed contraceptives to decrease the probability of psychotic exacerbations during (pre)menstrual phases of the cycle. Considering the protective effect of oestrogens on psychosis, oestrogenic contraceptives may be preferred over progesterone-only contraception (Brand et al., 2021). Yet, oestrogenic contraceptives also increase the risk of CVD (McCloskey et al., 2021; Seeman & Ross, 2011), making progesterone-only contraceptives (e.g. long-acting reversible contraceptives or intrauterine devices) preferred methods, especially in women over the age of 35 or in those who smoke or who suffer from hypertension (Curtis et al., 2016). Oestrogenic contraceptives inhibit CYP1A2 and CYP2C19 activity, increasing plasma concentrations of their substrates (Table 1) (Granfors, Backman, Lattila, & Neuvonen, 2005; Hagg et al., 2001; Hiemke et al., 2018; Ramsjö et al., 2010; Tamminga et al., 1999). For clozapine, plasma concentrations may increase two- to three-fold in the active hormone phase compared with the no-hormone phase, which may result in significant side-effects such as hypotension, sedation and sialorrhoea (Table 2) (Bookholt & Bogers, 2014; McCloskey et al., 2021). Progesterone-only contraceptives may be preferable for women treated with clozapine (McCloskey et al., 2021). Alternatively, frequent monitoring of plasma levels is recommended when using oestrogenic contraceptives, since oestrogenic birth control may have beneficial effects on the symptoms of SSD (Brand et al., 2021).
It should be noted that there is paucity in studies on this subject; therefore, the effects of hormonal contraceptives on antipsychotic plasma concentration are not clear for other antipsychotic drugs besides clozapine. Based on research so far, it seems there are no clinically relevant changes in the other antipsychotics when combined with contraceptives, although some studies suggest small changes in olanzapine plasma levels (Berry-Bibee et al., 2016).
Special considerations regarding pharmacotherapy in pregnant women
Women who take antipsychotics are generally at a higher risk of adverse maternal and infant outcomes including congenital malformations, preterm birth, foetal growth abnormalities and poor neonatal adaptation. Compared to healthy, unexposed pregnant women, these women are also more likely to have poor living conditions, poor life-style habits (e.g. smoking, drinking and bad eating habits) and metabolic dysfunctions (McAllister-Williams et al., 2017; Terrana, Koren, Pivovarov, Etwel, & Nulman, 2015). According to studies that control for these factors, antipsychotics themselves are not significantly associated with an increased risk of stillbirth, small/large-for-gestational-age births, preterm delivery or spontaneous miscarriage (Beex-Oosterhuis et al., 2020; Boden, Fergusson, & Horwood, 2011; Cuomo, Goracci, & Fagiolini, 2018; Damkier & Videbech, 2018; Ennis & Damkier, 2015; Huybrechts et al., 2016; Petersen et al., 2016; Reinstein, Cosgrove, Malekshahi, & Deligiannidis, 2020; Tosato et al., 2017; Vigod, Gomes, Wilton, Taylor, & Ray, 2015). Of note, risperidone has been associated with a higher risk for congenital malformations (relative risk of 1.26) (Huybrechts et al., 2016). Quetiapine, haloperidol and olanzapine are the most frequently investigated antipsychotics in pregnancy, which may be the reason why they are most commonly prescribed during this period (Gentile, 2010; Hasan et al., 2015; McAllister-Williams et al., 2017). However, current knowledge on the foetal risks of individual antipsychotics is limited and based on non-randomised studies or case series reports, making it challenging to draw firm conclusions (Damkier & Videbech, 2018; McAllister-Williams et al., 2017). In general, the potential benefits of antipsychotics may outweigh their potential risks of discontinuation. Switching a woman before or during pregnancy from their usual prescription may negatively affect clinical course and well-being. Careful consideration should, thus, guide clinical decisions in women with a diagnosis of SSD at this vulnerable time. In addition, polypharmacy, especially with SSRIs or mood stabilisers should be avoided, as it has been associated with more complications during pregnancy (Table 2) (Sadowski, Todorow, Yazdani Brojeni, Koren, & Nulman, 2013). Although lithium is considered to be relatively safe, valproate and carbamazepine are associated with major congenital malformations (Grover & Avasthi, 2015).
Pregnant women taking antipsychotics have been reported to have up to a 20.9% higher risk of developing gestational diabetes mellitus, especially when using olanzapine, risperidone, clozapine or high doses of quetiapine (>300 mg/day) (Galbally, Frayne, Watson, Morgan, & Snellen, 2020). Two meta-analyses on this subject confirm this higher risk, although evidence remains insufficient due to significant heterogeneity across studies (Kucukgoncu et al., 2019; Wang et al., 2021). Pregnant female patients should, thus, be monitored to prevent metabolic complications, with glucose tolerance testing and assessment of cholesterol and triglycerides (Table 2) (Breadon & Kulkarni, 2019).
Dosing during pregnancy
Physiological changes during pregnancy include increases in plasma volume, drug elimination rates and sex hormone levels (Payne, 2019). The activity of albumin and P-gp decrease by 31% and 19%, respectively, in late pregnancy (Abduljalil, Furness, Johnson, Rostami-Hodjegan, & Soltani, 2012; Ke, Rostami-Hodjegan, Zhao, & Unadkat, 2014), whereas the blood–brain barrier permeability increases, together leading to higher drug bioavailability. Moreover, renal excretion increases (Segarra et al., 2020), which results in lower plasma concentrations of amisulpride, sulpiride and paliperidone (Ke et al., 2014). CYP2D6 and CYP3A4 activity increases up to 50–100% in the third trimester while CYP1A2 and CYP2C19 activity decreases to up to 40–50% (Choi et al., 2013; Hiemke et al., 2018; Payne, 2019) (Table 1). Although research on this topic is limited, these metabolic changes appear to have significant consequences on the plasma levels of CYP3A4/CYP2D6 substrates quetiapine (in 35 pregnancies) and aripiprazole (in 14 pregnancies), which were found to be reduced by more than 50% in the third trimester whereas plasma levels of olanzapine (in 29 pregnancies) and clozapine (in 4 pregnancies) were found to be unchanged (Westin et al., 2018). For clozapine, there is some evidence for increased serum levels in the third trimester (Nguyen, Mordecai, Watt, & Frayne, 2020). Summarising, drug monitoring and blood level assessment is required throughout pregnancy and becomes increasingly important in the third trimester (Table 2) (Dazzan, 2021).
Special considerations regarding pharmacotherapy in postpartum women
After birth and especially in the first few weeks after delivery, women with bipolar disorder, schizoaffective disorder, or a history of postpartum psychosis are at an increased risk of postpartum psychosis due to hormonal alterations, neuro-immune changes and psychosocial factors (Hazelgrove et al., 2021; Meltzer-Brody et al., 2018). In these women, it is important to closely monitor clinical symptoms in this period and to consider continuation of antipsychotic treatment (Jones, Chandra, Dazzan, & Howard, 2014). Of note, pharmacological agents that suppress lactation are usually dopamine D2 agonists and are suggested to increase the risk of postpartum psychosis. This type of medication should, therefore, not be prescribed in postpartum women with SSD (Snellen, Power, Blankley, & Galbally, 2016).
Breastfeeding
Although breastfeeding has clear benefits for bonding and immunity (Chandra, Babu, & Desai, 2015), women with SSD are currently encouraged to breastfeed, unless they are taking clozapine (NICE, 2014). Although all antipsychotics are excreted into the breastmilk, the amount of active ingredients that is transferred to the infant is relatively low (Pacchiarotti et al., 2016). Based on a systematic review of 49 studies, the highest penetration ratio has been found for amisulpride, followed by clozapine and haloperidol (Schoretsanitis, Westin, Deligiannidis, Spigset, & Paulzen, 2020). Anecdotal evidence on clozapine has reported considerable concentrations in breast milk, risk of agranulocytosis in the infant and potentially delayed speech development low (Pacchiarotti et al., 2016). Based on the current literature, positive safety data are most consistent for olanzapine (Zincir, 2019). Apart from clozapine, other common antipsychotics are rarely associated with adverse events (Uguz, 2016), but monitoring of neurodevelopment and motor abnormalities is warranted due to the limited amount of evidence (McAllister-Williams et al., 2017; NICE, 2014). Taken together, the choice of feeding method and about which antipsychotic is best taken is difficult and should be part of a shared decision process involving the partner and paediatrician (Table 2).
Special considerations regarding pharmacotherapy in postmenopausal women
Most women require a higher dose of antipsychotic medication after menopause, when oestrogen levels decline and the sensitivity to dopamine reduces. In this phase of life, symptoms often increase and menopausal complaints such as sleep disturbances and mood swings can increase the risk of psychotic relapse (Brand et al., 2021; Riecher-Rössler, 2017). These dose increments are dependent on drug metabolism. Moreover, dose increments may be smaller or even redundant for lurasidone and quetiapine, since plasma levels of these antipsychotics increase significantly when oestrogen levels decrease.
Amisulpride and sertindole are not recommended as they induce QTc prolongation and typical antipsychotics or risperidone are not the best choice either, as they induce motor symptoms for which older women are more vulnerable (Table 1) (Lange et al., 2017; Leung & Chue, 2000). Clinicians should also be wary of drugs more likely to induce metabolic dysfunction and hyperprolactinaemia (Seeman, 2012). This leaves aripiprazole and lurasidone as potential drugs of first choice for postmenopausal women. Quetiapine has a higher risk for certain side-effects and can therefore be drug of second choice. Olanzapine or clozapine are other possible alternatives, as they are associated with higher risks for side-effects as well (Table 2), but may also be more effective than aripiprazole, quetiapine and lurasidone (Huhn et al., 2019).
Oestrogen augmentation should be considered at an early stage (i.e. at the beginning of menopause, or even earlier) as this can improve antipsychotic response, reduce psychotic symptoms and relief menopausal complaints (e.g. mood and sleep disturbances and osteoporosis) (de Boer, Prikken, Lei, Begemann, & Sommer, 2018; Heringa, Begemann, Goverde, & Sommer, 2015). As oestrogen replacement also increases bone mineral density, the prevention of antipsychotic-induced osteoporosis is a secondary benefit. Selective oestrogen receptor modulators (SERMs) such as raloxifene are more suitable for long-term use compared to oestrogens, since SERMs have oestrogenic effects on the brain and bone tissue but anti-oestrogenic effects on other tissues (such as breast and uterus) (Arevalo, Azcoitia, & Garcia-Segura, 2015). As hormone replacement therapy with either oestrogens or SERMs is associated with increased risk of thromboembolic events (Ellis, Hendrick, Williams, & Komm, 2015), potential benefits and disadvantages should be balanced individually. For example, a (family) history of thrombo-embolic events may be a reason not to opt for oestrogen replacement therapy.
Conclusion
Women tend to have slower drug absorption, distribution, metabolism and elimination rates, resulting in higher plasma levels and bioavailability of most antipsychotic medications. Since oestrogens induce dopamine sensitivity in the brain, the efficacy of antipsychotics is enhanced in premenopausal women, compared to men. Considering that current treatment guidelines are mostly based on data from men, women are likely to be overmedicated and it is therefore not surprising that adverse events are much more common in women. Additionally, women are more vulnerable to many side-effects, such as weight gain, EPS and hyperprolactinaemia, independently of dose. Defining the minimum effective dose in women is of utmost importance, as the risk of overmedicating increases when female sex hormone levels are high, specifically during ovulation and in the later stages of pregnancy. Conversely, effective doses of most antipsychotics (quetiapine and lurasidone are exceptions) may need to be increased during low-hormonal phases in life, specifically post-natal, during menstruation and during and after menopause. Optimal antipsychotic treatment for women is highly dependent on the different life phases. Premenopausal women should be prescribed lower dosages for most antipsychotics (except for quetiapine and lurasidone). During pregnancy, the most reasonable and less harmful choice appears to be maintaining future mothers with SSD at the minimum effective dosage of the drug they were already using before pregnancy. If a medication-free pregnancy is feasible, this is of course the best option. Clozapine should probably be avoided during breastfeeding and while further safety data on other antipsychotics are limited, the inherent benefits of breastfeeding for mother and baby should be balanced carefully against the potential risks for the baby. Postmenopausal women represent a group of patients that is especially vulnerable to side-effects associated with ageing and declining hormone levels such as osteoporosis and CVD, and for these women, oestrogen replacement therapy should be considered to ameliorate both somatic and mental health problems, although the increased risk for thrombosis also needs to be taken into account.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
None.
References
- Abduljalil, K., Furness, P., Johnson, T. N., Rostami-Hodjegan, A., & Soltani, H. (2012). Anatomical, physiological and metabolic changes with gestational age during normal pregnancy. Clinical Pharmacokinetics, 51(6), 365–396. doi: 10.2165/11597440-000000000-00000 [DOI] [PubMed] [Google Scholar]
- Achtyes, E., Simmons, A., Skabeev, A., Levy, N., Jiang, Y., Marcy, P., & Weiden, P. J. (2018). Patient preferences concerning the efficacy and side-effect profile of schizophrenia medications: A survey of patients living with schizophrenia. BMC Psychiatry, 18(1), 292. doi: 10.1186/s12888-018-1856-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aichhorn, W., Weiss, U., Marksteiner, J., Kemmler, G., Walch, T., Zernig, G., … Geretsegger, C. (2005). Influence of age and gender on risperidone plasma concentrations. Journal of Psychopharmacology, 19(4), 395–401. doi: 10.1177/0269881105053306 [DOI] [PubMed] [Google Scholar]
- Alberich, S., Fernández-Sevillano, J., González-Ortega, I., Usall, J., Sáenz, M., González-Fraile, E., & González-Pinto, A. (2019). A systematic review of sex-based differences in effectiveness and adverse effects of clozapine. Psychiatry Research, 280, 112506. doi: 10.1016/j.psychres.2019.112506 [DOI] [PubMed] [Google Scholar]
- Arevalo, M.-A., Azcoitia, I., & Garcia-Segura, L. M. (2015). The neuroprotective actions of oestradiol and oestrogen receptors. Nature Reviews Neuroscience, 16(1), 17–29. doi: 10.1038/nrn3856 [DOI] [PubMed] [Google Scholar]
- Arnold, L. M., Strakowski, S. M., Schwiers, M. L., Amicone, J., Fleck, D. E., Corey, K. B., & Farrow, J. E. (2004). Sex, ethnicity, and antipsychotic medication use in patients with psychosis. Schizophrenia Research, 66(2–3), 169–175. doi: 10.1016/S0920-9964(03)00102-6 [DOI] [PubMed] [Google Scholar]
- Ayesa-Arriola, R., de la Foz, V. O.-G., Setién-Suero, E., Ramírez-Bonilla, M. L., Suárez-Pinilla, P., Son, J. M., … Crespo-Facorro, B. (2020). Understanding sex differences in long-term outcomes after a first episode of psychosis. Npj Schizophrenia, 6(1), 33. doi: 10.1038/s41537-020-00120-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak, M., Fransen, A., Janssen, J., Van Os, J., & Drukker, M. (2014). Almost all antipsychotics result in weight gain: A meta-analysis. PLoS ONE, 9(4), e94112. doi: 10.1371/journal.pone.0094112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach, S. R., Celano, C. M., Noseworthy, P. A., Januzzi, J. L., & Huffman, J. C. (2013). QTC prolongation, torsades de pointes, and psychotropic medications. Psychosomatics, 54, 1–13. doi: 10.1016/j.psym.2012.11.001 [DOI] [PubMed] [Google Scholar]
- Bebawy, M., & Chetty, M. (2009). Gender differences in P-glycoprotein expression and function: Effects on drug disposition and outcome. Current Drug Metabolism, 10(4), 322–328. doi: 10.2174/138920009788498996 [DOI] [PubMed] [Google Scholar]
- Beex-Oosterhuis, M. M., Samb, A., Heerdink, E. R., Souverein, P. C., Van Gool, A. R., Meyboom, R. H. B., & Marum, R. J. (2020). Safety of clozapine use during pregnancy: Analysis of international pharmacovigilance data. Pharmacoepidemiology and Drug Safety, 29(6), 725–735. doi: 10.1002/pds.5016 [DOI] [PubMed] [Google Scholar]
- Bener, A., Al-Hamaq, A. O. A. A., & Dafeeah, E. E. (2014). A two fold risk of metabolic syndrome in a sample of patients with schizophrenia: Do consanguinity and family history increase risk? Diabetes & Metabolic Syndrome: Clinical Research & Reviews, 8(1), 24–29. doi: 10.1016/j.dsx.2013.10.003 [DOI] [PubMed] [Google Scholar]
- Berry-Bibee, E. N., Kim, M. J., Simmons, K. B., Tepper, N. K., Riley, H. E. M., Pagano, H. P., & Curtis, K. M. (2016). Drug interactions between hormonal contraceptives and psychotropic drugs: A systematic review. Contraception, 94(6), 650–667. doi: 10.1016/j.contraception.2016.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigos, K. L., Pollock, B. G., Coley, K. C., Miller, D. D., Marder, S. R., Aravagiri, M., … Bies, R. R. (2008). Sex, race, and smoking impact olanzapine exposure. The Journal of Clinical Pharmacology, 48(2), 157–165. doi: 10.1177/0091270007310385 [DOI] [PubMed] [Google Scholar]
- Boden, J. M., Fergusson, D. M., & Horwood, L. J. (2011). Age of menarche and psychosocial outcomes in a New Zealand birth cohort. Journal of the American Academy of Child & Adolescent Psychiatry, 50(2), 132–140.e5. doi: 10.1016/j.jaac.2010.11.007 [DOI] [PubMed] [Google Scholar]
- Bookholt, D. E., & Bogers, J. P. A. M. (2014). Oral contraceptives raise plasma clozapine concentrations. Journal of Clinical Psychopharmacology, 34(3), 389–390. doi: 10.1097/JCP.0000000000000074 [DOI] [PubMed] [Google Scholar]
- Brand, B. A., de Boer, J. N., & Sommer, I. E. C. (2021). Estrogens in schizophrenia: Progress, current challenges and opportunities. Current Opinion in Psychiatry, 34(3), 228–237. doi: 10.1097/YCO.0000000000000699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breadon, C., & Kulkarni, J. (2019). An update on medication management of women with schizophrenia in pregnancy. Expert Opinion on Pharmacotherapy, 20(11), 1365–1376. doi: 10.1080/14656566.2019.1612876 [DOI] [PubMed] [Google Scholar]
- Camilleri, M. (2020). Sex as a biological variable in irritable bowel syndrome. Neurogastroenterology & Motility, 32(7), e13802. doi: 10.1111/nmo.13802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caqueo-Urízar, A., Fond, G., Urzúa, A., & Boyer, L. (2018). Gender differences in schizophrenia: A multicentric study from three Latin-America countries. Psychiatry Research, 266, 65–71. doi: 10.1016/j.psychres.2018.05.032 [DOI] [PubMed] [Google Scholar]
- Carliner, H., Collins, P. Y., Cabassa, L. J., McNallen, A., Joestl, S. S., & Lewis-Fernández, R. (2014). Prevalence of cardiovascular risk factors among racial and ethnic minorities with schizophrenia spectrum and bipolar disorders: A critical literature review. Comprehensive Psychiatry, 55(2), 233–247. doi: 10.1016/j.comppsych.2013.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castberg, I., Westin, A. A., Skogvoll, E., & Spigset, O. (2017). Effects of age and gender on the serum levels of clozapine, olanzapine, risperidone, and quetiapine. Acta Psychiatrica Scandinavica, 136(5), 455–464. doi: 10.1111/acps.12794 [DOI] [PubMed] [Google Scholar]
- Castberg, I., Westin, A. A., & Spigset, O. (2009). Does level of care, sex, age, or choice of drug influence adherence to treatment with antipsychotics? Journal of Clinical Psychopharmacology, 29(5), 415–420. doi: 10.1097/JCP.0b013e3181b2fced [DOI] [PubMed] [Google Scholar]
- Ceskova, E., & Prikryl, R. (2012). Importance of gender in the treatment of schizophrenia. The Primary Care Companion for CNS Disorders. doi: 10.4088/PCC.12m01407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceskova, E., Prikryl, R., Libiger, J., Svancara, J., & Jarkovsky, J. (2015). Gender differences in the treatment of first-episode schizophrenia: Results from the European first episode schizophrenia trial. Schizophrenia Research, 169(1–3), 303–307. doi: 10.1016/j.schres.2015.10.013 [DOI] [PubMed] [Google Scholar]
- Chandra, P., Babu, G., & Desai, G. (2015). Antipsychotics in pregnancy and lactation. Indian Journal of Psychiatry, 57(6), 303. doi: 10.4103/0019-5545.161497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, K. C., Yang, Y. K., Howes, O., Lee, I. H., Landau, S., Yeh, T. L., … Bramon, E. (2013). Striatal dopamine transporter availability in drug-naive patients with schizophrenia: A case-control SPECT study with [99mTc]-TRODAT-1 and a meta-analysis. Schizophrenia Bulletin, 39(2), 378–386. doi: 10.1093/schbul/sbr163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, C. H., & Lee, H. J. (2013). Oxidative stress and tardive dyskinesia: Pharmacogenetic evidence. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 46, 207–213. doi: 10.1016/j.pnpbp.2012.10.018 [DOI] [PubMed] [Google Scholar]
- Choi, S.-Y., Koh, K. H., & Jeong, H. (2013). Isoform-specific regulation of cytochromes P450 expression by estradiol and progesterone. Drug Metabolism and Disposition, 41(2), 263–269. doi: 10.1124/dmd.112.046276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors, J., & Casey, P. (2006). Sex, body-esteem and self-esteem. Psychological Reports, 98(3), 699–704. doi: 10.2466/PR0.98.3.699-704 [DOI] [PubMed] [Google Scholar]
- Cuomo, A., Goracci, A., & Fagiolini, A. (2018). Aripiprazole use during pregnancy, peripartum and lactation. A systematic literature search and review to inform clinical practice. Journal of Affective Disorders, 228, 229–237. doi: 10.1016/j.jad.2017.12.021 [DOI] [PubMed] [Google Scholar]
- Curtis, K. M., Tepper, N. K., Jatlaoui, T. C., Berry-Bibee, E., Horton, L. G., Zapata, L. B., … Whiteman, M. K. (2016). U.S. Medical eligibility criteria for contraceptive use, 2016. MMWR. Recommendations and Reports, 65(3), 1–103. doi: 10.15585/mmwr.rr6503a1 [DOI] [PubMed] [Google Scholar]
- Dama, M., Veru, F., Schmitz, N., Shah, J., Iyer, S., Joober, R., & Malla, A. (2019). Sex differences in clinical and functional outcomes among patients treated in an early intervention service for psychotic disorders: An observational study. Canadian Journal of Psychiatry. Revue Canadienne de Psychiatrie, 64(10), 708–717. doi: 10.1177/0706743719854069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damkier, P., & Videbech, P. (2018). The safety of second-generation antipsychotics during pregnancy: A clinically focused review. CNS Drugs, 32(4), 351–366. doi: 10.1007/s40263-018-0517-5 [DOI] [PubMed] [Google Scholar]
- Damoiseaux, V. A., Proost, J. H., Jiawan, V. C. R., & Melgert, B. N. (2014). Sex differences in the pharmacokinetics of antidepressants: Influence of female sex hormones and oral contraceptives. Clinical Pharmacokinetics, 53(6), 509–519. doi: 10.1007/s40262-014-0145-2 [DOI] [PubMed] [Google Scholar]
- Danielsson, B., Collin, J., Jonasdottir Bergman, G., Borg, N., Salmi, P., & Fastbom, J. (2016). Antidepressants and antipsychotics classified with torsades de pointes arrhythmia risk and mortality in older adults – A Swedish nationwide study. British Journal of Clinical Pharmacology, 81(4), 773–783. doi: 10.1111/bcp.12829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva, T. L., & Ravindran, A. V. (2015). Contribution of sex hormones to gender differences in schizophrenia: A review. Asian Journal of Psychiatry, 18, 2–14. doi: 10.1016/j.ajp.2015.07.016 [DOI] [PubMed] [Google Scholar]
- Dazzan, P. (2021). Schizophrenia during pregnancy. Current Opinion in Psychiatry, 34(3), 238–244. doi: 10.1097/YCO.0000000000000706 [DOI] [PubMed] [Google Scholar]
- de Boer, J., Prikken, M., Lei, W. U., Begemann, M., & Sommer, I. (2018). The effect of raloxifene augmentation in men and women with a schizophrenia spectrum disorder: A systematic review and meta-analysis. Npj Schizophrenia, 4(1), 1. doi: 10.1038/s41537-017-0043-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Hert, M., Detraux, J., & Peuskens, J. (2014). Second-generation and newly approved antipsychotics, serum prolactin levels and sexual dysfunctions: A critical literature review. Expert Opinion on Drug Safety, 13(5), 605–624. doi: 10.1517/14740338.2014.906579 [DOI] [PubMed] [Google Scholar]
- De Hert, M., Detraux, J., van Winkel, R., Yu, W., & Correll, C. U. (2012). Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nature Reviews Endocrinology, 8(2), 114–126. doi: 10.1038/nrendo.2011.156 [DOI] [PubMed] [Google Scholar]
- de Vegt, F., Dekker, J. M., Jager, A., Hienkens, E., Kostense, P. J., Stehouwer, C. D. A., … Heine, R. J. (2001). Relation of impaired fasting and postload glucose with incident type 2 diabetes in a Dutch population. JAMA, 285(16), 2109. doi: 10.1001/jama.285.16.2109 [DOI] [PubMed] [Google Scholar]
- De Yang, F., Wang, X. Q., Liu, X. P., Zhao, K. X., Fu, W. H., Hao, X. R., … Zhang, X. Y. (2011). Sex difference in QTc prolongation in chronic institutionalized patients with schizophrenia on long-term treatment with typical and atypical antipsychotics. Psychopharmacology, 216(1), 9–16. doi: 10.1007/s00213-011-2188-5 [DOI] [PubMed] [Google Scholar]
- Di Paolo, T. (1994). Modulation of brain dopamine transmission by sex steroids. Reviews in the Neurosciences, 5(1), 27–41. doi: 10.1515/revneuro.1994.5.1.27 [DOI] [PubMed] [Google Scholar]
- Divac, N., Prostran, M., Jakovcevski, I., & Cerovac, N. (2014). Second-generation antipsychotics and extrapyramidal adverse effects. BioMed Research International, 2014, 1–6. doi: 10.1155/2014/656370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran, A., Obach, R. S., Smith, B. J., Hosea, N. A., Becker, S., Callegari, E., … Zhang, C. (2005). The impact of P-glycoprotein on the disposition of drugs targeted for indications of the central nervous system: Evaluation using the MDR1A/1B knockout mouse model. Drug Metabolism and Disposition, 33(1), 165–174. doi: 10.1124/dmd.104.001230 [DOI] [PubMed] [Google Scholar]
- Dubreucq, M., Plasse, J., Gabayet, F., Blanc, O., Chereau, I., Cervello, S., … Dubreucq, J. (2021). Sex differences in recovery-related outcomes and needs for psychiatric rehabilitation in people with schizophrenia spectrum disorder. The Journal of Clinical Psychiatry, 82(4), 20m13732. doi: 10.4088/jcp.20m13732 [DOI] [PubMed] [Google Scholar]
- Ellis, A. J., Hendrick, V. M., Williams, R., & Komm, B. S. (2015). Selective estrogen receptor modulators in clinical practice: A safety overview. Expert Opinion on Drug Safety, 14(6), 921–934. doi: 10.1517/14740338.2015.1014799 [DOI] [PubMed] [Google Scholar]
- Elmeliegy, M., Vourvahis, M., Guo, C., & Wang, D. D. (2020). Effect of P-glycoprotein (P-gp) inducers on exposure of P-gp substrates: Review of clinical drug–drug interaction studies. Clinical Pharmacokinetics, 59(6), 699–714. doi: 10.1007/s40262-020-00867-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis, Z. N., & Damkier, P. (2015). Pregnancy exposure to olanzapine, quetiapine, risperidone, aripiprazole and risk of congenital malformations. A systematic review. Basic & Clinical Pharmacology & Toxicology, 116(4), 315–320. doi: 10.1111/bcpt.12372 [DOI] [PubMed] [Google Scholar]
- Eugene, A. R., & Masiak, J. (2017). A pharmacodynamic modelling and simulation study identifying gender differences of daily olanzapine dose and dopamine D2-receptor occupancy. Nordic Journal of Psychiatry, 71(6), 417–424. doi: 10.1080/08039488.2017.1314011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald, S. J., Janorkar, A. V., Barnes, A., & Maranon, R. O. (2018). A new approach to study the sex differences in adipose tissue. Journal of Biomedical Science, 25, 89. doi: 10.1186/s12929-018-0488-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire, A. C., Basit, A. W., Choudhary, R., Piong, C. W., & Merchant, H. A. (2011). Does sex matter? The influence of gender on gastrointestinal physiology and drug delivery. International Journal of Pharmaceutics, 415(1–2), 15–28. doi: 10.1016/j.ijpharm.2011.04.069 [DOI] [PubMed] [Google Scholar]
- Gaedigk, A., Dinh, J. C., Jeong, H., Prasad, B., & Leeder, J. S. (2018). Ten years’ experience with the CYP2D6 activity score: A perspective on future investigations to improve clinical predictions for precision therapeutics. Journal of Personalized Medicine, 8, 15. doi: 10.3390/jpm8020015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbally, M., Frayne, J., Watson, S. J., Morgan, V., & Snellen, M. (2020). The association between gestational diabetes mellitus, antipsychotics and severe mental illness in pregnancy: A multicentre study. Australian and New Zealand Journal of Obstetrics and Gynaecology, 60(1), 63–69. doi: 10.1111/ajo.12986 [DOI] [PubMed] [Google Scholar]
- Gentile, S. (2010). Antipsychotic therapy during early and late pregnancy. A systematic review. Schizophrenia Bulletin, 36(3), 518–544. doi: 10.1093/schbul/sbn107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogos, A., Sbisa, A. M., Sun, J., Gibbons, A., Udawela, M., & Dean, B. (2015). A role for estrogen in schizophrenia: Clinical and preclinical findings. International Journal of Endocrinology, 2015, 1–16. doi: 10.1155/2015/615356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein, J. M., Cohen, L. S., Horton, N. J., Lee, H., Andersen, S., Tohen, M., … Tollefson, G. (2002). Sex differences in clinical response to olanzapine compared with haloperidol. Psychiatry Research, 110(1), 27–37. doi: 10.1016/S0165-1781(02)00028-8 [DOI] [PubMed] [Google Scholar]
- Gonzalez, Z., Loganathan, P., Sarosiek, I., & McCallum, R. W. (2020). Gender-related differences in gastroparesis. American Journal of the Medical Sciences, 360, 474–483. doi: 10.1016/j.amjms.2020.04.018 [DOI] [PubMed] [Google Scholar]
- González-Rodríguez, A., Labad, J., & Seeman, M. V. (2020). Antipsychotic-induced hyperprolactinemia in aging populations: Prevalence, implications, prevention and management. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 101, 109941. doi: 10.1016/j.pnpbp.2020.109941 [DOI] [PubMed] [Google Scholar]
- González-Rodríguez, A., & Seeman, M. V. (2019). The association between hormones and antipsychotic use: A focus on postpartum and menopausal women. Therapeutic Advances in Psychopharmacology, 9, 204512531985997. doi: 10.1177/2045125319859973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granfors, M., Backman, J., Lattila, J., & Neuvonen, P. (2005). Oral contraceptives containing ethinyl estradiol and gestodene markedly increase plasma concentrations and effects of tizanidine by inhibiting cytochrome P450 1A2. Clinical Pharmacology & Therapeutics, 78(4), 400–411. doi: 10.1016/j.clpt.2005.06.009 [DOI] [PubMed] [Google Scholar]
- Greenblatt, D. J., & von Moltke, L. L. (2008). Gender has a small but statistically significant effect on clearance of CYP3A substrate drugs. The Journal of Clinical Pharmacology, 48(11), 1350–1355. doi: 10.1177/0091270008323754 [DOI] [PubMed] [Google Scholar]
- Grover, S., & Avasthi, A. (2015). Mood stabilizers in pregnancy and lactation. Indian Journal of Psychiatry, 57(6), 308. doi: 10.4103/0019-5545.161498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur, R. E., & Gur, R. C. (1990). Gender differences in regional cerebral blood flow. Schizophrenia Bulletin, 16(2), 247–254. doi: 10.1093/schbul/16.2.247 [DOI] [PubMed] [Google Scholar]
- Hagg, S., Spigset, O., & Dahlqvist, R. (2001). Influence of gender and oral contraceptives on CYP2D6 and CYP2C19 activity in healthy volunteers. British Journal of Clinical Pharmacology, 51(2), 169–173. doi: 10.1046/j.1365-2125.2001.01328.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haring, R., Friedrich, N., Volzke, H., Vasan, R. S., Felix, S. B., Dorr, M., … Wallaschofski, H. (2014). Positive association of serum prolactin concentrations with all-cause and cardiovascular mortality. European Heart Journal, 35(18), 1215–1221. doi: 10.1093/eurheartj/ehs233 [DOI] [PubMed] [Google Scholar]
- Hasan, A., Falkai, P., Wobrock, T., Lieberman, J., Glenthøj, B., Gattaz, W. F., … Möller, H.-J. (2015). World federation of societies of biological psychiatry (WFSBP) guidelines for biological treatment of schizophrenia part 3: Update 2015 management of special circumstances: Depression, suicidality, substance use disorders and pregnancy and lactation. The World Journal of Biological Psychiatry, 16(3), 142–170. doi: 10.3109/15622975.2015.1009163 [DOI] [PubMed] [Google Scholar]
- Hassin-Baer, S., Molchadski, I., Cohen, O. S., Nitzan, Z., Efrati, L., Tunkel, O., … Korczyn, A. D. (2011). Gender effect on time to levodopa-induced dyskinesias. Journal of Neurology, 258(11), 2048–2053. doi: 10.1007/s00415-011-6067-0 [DOI] [PubMed] [Google Scholar]
- Hazelgrove, K., Biaggi, A., Waites, F., Fuste, M., Osborne, S., Conroy, S., … Dazzan, P. (2021). Risk factors for postpartum relapse in women at risk of postpartum psychosis: The role of psychosocial stress and the biological stress system. Psychoneuroendocrinology, 128, 105218. doi: 10.1016/j.psyneuen.2021.105218 [DOI] [PubMed] [Google Scholar]
- Heringa, S. M., Begemann, M. J. H., Goverde, A. J., & Sommer, I. E. C. (2015). Sex hormones and oxytocin augmentation strategies in schizophrenia: A quantitative review. Schizophrenia Research, 168(3), 603–613. doi: 10.1016/j.schres.2015.04.002 [DOI] [PubMed] [Google Scholar]
- Hiemke, C., Bergemann, N., Clement, H., Conca, A., Deckert, J., Domschke, K., … Baumann, P. (2018). Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: Update 2017. Pharmacopsychiatry, 51(01/02), 9–62. doi: 10.1055/s-0043-116492 [DOI] [PubMed] [Google Scholar]
- Hoekstra, S., Bartz-Johannessen, C., Sinkeviciute, I., Reitan, S. K., Kroken, R. A., Løberg, E.-M., … Sommer, I. E. (2021). Sex differences in antipsychotic efficacy and side effects in schizophrenia spectrum disorder: Results from the BeSt InTro study. Npj Schizophrenia, 7(1), 39. doi: 10.1038/s41537-021-00170-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, M.-C., Lu, M.-L., Tsai, C.-J., Chen, P.-Y., Chiu, C.-C., Jian, D.-L., … Chen, C.-H. (2009). Prevalence of metabolic syndrome among patients with schizophrenia or schizoaffective disorder in Taiwan. Acta Psychiatrica Scandinavica, 120(4), 274–280. doi: 10.1111/j.1600-0447.2009.01401.x [DOI] [PubMed] [Google Scholar]
- Huhn, M., Nikolakopoulou, A., Schneider-Thoma, J., Krause, M., Samara, M., Peter, N., … Leucht, S. (2019). Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: A systematic review and network meta-analysis. The Lancet, 394(10202), 939–951. doi: 10.1016/S0140-6736(19)31135-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson, W. R., Roehrkasse, R. L., & Wald, A. (1989). Influence of gender and menopause on gastric emptying and motility. Gastroenterology, 96(1), 11–17. doi: 10.1016/0016-5085(89)90758-0 [DOI] [PubMed] [Google Scholar]
- Huybrechts, K. F., Hernández-Díaz, S., Patorno, E., Desai, R. J., Mogun, H., Dejene, S. Z., … Bateman, B. T. (2016). Antipsychotic use in pregnancy and the risk for congenital malformations. JAMA Psychiatry, 73(9), 938. doi: 10.1001/jamapsychiatry.2016.1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingimarsson, O., MacCabe, J. H., Haraldsson, M., Jónsdóttir, H., & Sigurdsson, E. (2017). Risk of diabetes and dyslipidemia during clozapine and other antipsychotic drug treatment of schizophrenia in Iceland. Nordic Journal of Psychiatry, 71(7), 496–502. doi: 10.1080/08039488.2017.1334821 [DOI] [PubMed] [Google Scholar]
- Iversen, T. S. J., Steen, N. E., Dieset, I., Hope, S., Mørch, R., Gardsjord, E. S., … Jönsson, E. G. (2018). Side effect burden of antipsychotic drugs in real life – Impact of gender and polypharmacy. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 82, 263–271. doi: 10.1016/j.pnpbp.2017.11.004 [DOI] [PubMed] [Google Scholar]
- Jaaskelainen, E., Juola, P., Hirvonen, N., McGrath, J. J., Saha, S., Isohanni, M., … Miettunen, J. (2013). A systematic review and meta-analysis of recovery in schizophrenia. Schizophrenia Bulletin, 39(6), 1296–1306. doi: 10.1093/schbul/sbs130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, Y., Greenwood-Van Meerveld, B., Johnson, A. C., & Travagli, R. A. (2019). Role of estrogen and stress on the brain-gut axis. American Journal of Physiology-Gastrointestinal and Liver Physiology, 317(2), G203–G209. doi: 10.1152/ajpgi.00144.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, I., Chandra, P. S., Dazzan, P., & Howard, L. M. (2014). Bipolar disorder, affective psychosis, and schizophrenia in pregnancy and the post-partum period. The Lancet, 384(9956), 1789–1799. doi: 10.1016/S0140-6736(14)61278-2 [DOI] [PubMed] [Google Scholar]
- Jongsma, H. E., Turner, C., Kirkbride, J. B., & Jones, P. B. (2019). International incidence of psychotic disorders, 2002–17: A systematic review and meta-analysis. The Lancet. Public Health, 4(5), e229–e244. doi: 10.1016/S2468-2667(19)30056-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jönsson, A. K., Spigset, O., & Reis, M. (2019). A compilation of serum concentrations of 12 antipsychotic drugs in a therapeutic drug monitoring setting. Therapeutic Drug Monitoring, 41(3), 348–356. doi: 10.1097/FTD.0000000000000585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaar, S. J., Natesan, S., McCutcheon, R., & Howes, O. D. (2020). Antipsychotics: Mechanisms underlying clinical response and side-effects and novel treatment approaches based on pathophysiology. Neuropharmacology, 172, 107704. doi: 10.1016/j.neuropharm.2019.107704 [DOI] [PubMed] [Google Scholar]
- Kahn, R. S., Fleischhacker, W. W., Boter, H., Davidson, M., Vergouwe, Y., Keet, I. P., … Grobbee, D. E. (2008). Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: An open randomised clinical trial. The Lancet, 371(9618), 1085–1097. doi: 10.1016/S0140-6736(08)60486-9 [DOI] [PubMed] [Google Scholar]
- Kahn, R. S., Winter van Rossum, I., Leucht, S., McGuire, P., Lewis, S. W., Leboyer, M., … Eijkemans, M. J. C. (2018). Amisulpride and olanzapine followed by open-label treatment with clozapine in first-episode schizophrenia and schizophreniform disorder (OPTiMiSE): A three-phase switching study. The Lancet. Psychiatry, 5(10), 797–807. doi: 10.1016/S2215-0366(18)30252-9 [DOI] [PubMed] [Google Scholar]
- Ke, A. B., Rostami-Hodjegan, A., Zhao, P., & Unadkat, J. D. (2014). Pharmacometrics in pregnancy: An unmet need. Annual Review of Pharmacology and Toxicology, 54(1), 53–69. doi: 10.1146/annurev-pharmtox-011613-140009 [DOI] [PubMed] [Google Scholar]
- Keating, C. (2010). Sex differences precipitating anorexia Nervosa in females: The estrogen paradox and a novel framework for targeting sex-specific neurocircuits and behavior. In Neill J. & Kulkarni J. (Eds.), Current topics in behavioral neurosciences (pp. 189–207). Berlin, Heidelberg: Springer. doi: 10.1007/7854_2010_99 [DOI] [PubMed] [Google Scholar]
- Keepers, G. A., Fochtmann, L. J., Anzia, J. M., Benjamin, S., Lyness, J. M., Mojtabai, R., … Hong, S. H. (2020). The American psychiatric association practice guideline for the treatment of patients with schizophrenia. American Journal of Psychiatry, 177(9), 868–872. doi: 10.1176/appi.ajp.2020.177901 [DOI] [PubMed] [Google Scholar]
- Kinon, B. J., Gilmore, J. A., Liu, H., & Halbreich, U. M. (2003). Prevalence of hyperprolactinemia in schizophrenic patients treated with conventional antipsychotic medications or risperidone. Psychoneuroendocrinology, 28, 55–68. doi: 10.1016/S0306-4530(02)00127-0 [DOI] [PubMed] [Google Scholar]
- Konstandi, M., Andriopoulou, C. E., Cheng, J., & Gonzalez, F. J. (2020). Sex steroid hormones differentially regulate CYP2D in female wild-type and CYP2D6-humanized mice. Journal of Endocrinology, 245(2), 301–314. doi: 10.1530/JOE-19-0561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraal, A. Z., Ward, K. M., & Ellingrod, V. L. (2017). Sex differences in antipsychotic related metabolic functioning in schizophrenia spectrum disorders. Psychopharmacology Bulletin, 47(2), 8–21. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/28626268. [PMC free article] [PubMed] [Google Scholar]
- Kucukgoncu, S., Guloksuz, S., Celik, K., Bahtiyar, M. O., Luykx, J. J., Rutten, B. P. F., & Tek, C. (2019). Antipsychotic exposure in pregnancy and the risk of gestational diabetes: A systematic review and meta-analysis. Schizophrenia Bulletin, 46(2), 311–318. doi: 10.1093/schbul/sbz058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbé, L., Sirois, C., Pilote, S., Arseneault, M., Robitaille, N. M., Turgeon, J., & Hamelin, B. A. (2000). Effect of gender, sex hormones, time variables and physiological urinary pH on apparent CYP2D6 activity as assessed by metabolic ratios of marker substrates. Pharmacogenetics, 10(5), 425–438. doi: 10.1097/00008571-200007000-00006 [DOI] [PubMed] [Google Scholar]
- Labelle, A., Light, M., & Dunbar, F. (2001). Risperidone treatment of outpatients with schizophrenia: No evidence of Sex differences in treatment response. The Canadian Journal of Psychiatry, 46(6), 534–541. doi: 10.1177/070674370104600608 [DOI] [PubMed] [Google Scholar]
- Lally, J., & MacCabe, J. H. (2015). Antipsychotic medication in schizophrenia: A review. British Medical Bulletin, 114(1), 169–179. doi: 10.1093/bmb/ldv017 [DOI] [PubMed] [Google Scholar]
- Lambert, M., Conus, P., Eide, P., Mass, R., Karow, A., Moritz, S., … Naber, D. (2004). Impact of present and past antipsychotic side effects on attitude toward typical antipsychotic treatment and adherence. European Psychiatry, 19(7), 415–422. doi: 10.1016/j.eurpsy.2004.06.031 [DOI] [PubMed] [Google Scholar]
- Lange, B., Mueller, J. K., Leweke, F. M., & Bumb, J. M. (2017). How gender affects the pharmacotherapeutic approach to treating psychosis – A systematic review. Expert Opinion on Pharmacotherapy, 18(4), 351–362. doi: 10.1080/14656566.2017.1288722 [DOI] [PubMed] [Google Scholar]
- Leijala, J., Kampman, O., Suvisaari, J., & Eskelinen, S. (2021). Daily functioning and symptom factors contributing to attitudes toward antipsychotic treatment and treatment adherence in outpatients with schizophrenia spectrum disorders. BMC Psychiatry, 21(1), 37. doi: 10.1186/s12888-021-03037-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leucht, S., Crippa, A., Siafis, S., Patel, M. X., Orsini, N., & Davis, J. M. (2020). Dose-response meta-analysis of antipsychotic drugs for acute schizophrenia. American Journal of Psychiatry, 177(4), 342–353. doi: 10.1176/appi.ajp.2019.19010034 [DOI] [PubMed] [Google Scholar]
- Leung, A., & Chue, P. (2000). Sex differences in schizophrenia, a review of the literature. Acta Psychiatrica Scandinavica. Supplementum, 401, 3–38. doi: 10.1111/j.0065-1591.2000.0ap25.x [DOI] [PubMed] [Google Scholar]
- Li, L., Li, L., Shang, D., Wen, Y., & Ning, Y. (2020). A systematic review and combined meta-analysis of concentration of oral amisulpride. British Journal of Clinical Pharmacology, 86(4), 668–678. doi: 10.1111/bcp.14246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman, D. L., Tybur, J. M., & Latner, J. D. (2012). Disgust sensitivity, obesity stigma, and gender: Contamination psychology predicts weight bias for women, not men. Obesity, 20(9), 1803–1814. doi: 10.1038/oby.2011.247 [DOI] [PubMed] [Google Scholar]
- Lindamer, L. A., Harris, M. J., Gladsjo, J. A., Heaton, R., Paulsen, J. S., Heaton, S. C., … Jeste, D. V. (2000). Gender and schizophrenia. In Hormones, gender and the aging brain (pp. 223–240). Cambridge, England: Cambridge University Press. doi: 10.1017/CBO9780511544071.011 [DOI] [Google Scholar]
- Linnet, K., & Ejsing, T. B. (2008). A review on the impact of P-glycoprotein on the penetration of drugs into the brain. Focus on psychotropic drugs. European Neuropsychopharmacology, 18(3), 157–169. doi: 10.1016/j.euroneuro.2007.06.003 [DOI] [PubMed] [Google Scholar]
- Lu, J., Shang, X., Zhong, W., Xu, Y., Shi, R., & Wang, X. (2020). New insights of CYP1A in endogenous metabolism: A focus on single nucleotide polymorphisms and diseases. Acta Pharmaceutica Sinica B, 10, 91–104. doi: 10.1016/j.apsb.2019.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons, K. E., Hubble, J. P., Tröster, A. I., Pahwa, R., & Koller, W. C. (1998). Gender differences in Parkinson's disease. Clinical Neuropharmacology. doi: 10.1007/978-3-030-04245-5_24 [DOI] [PubMed] [Google Scholar]
- Mahadun, P. N., & Marshall, M. (2008). Insight and treatment attitude in schizophrenia: Comparison of patients on depot and atypical antipsychotics. Psychiatric Bulletin, 32(2), 53–56. doi: 10.1192/pb.bp.107.015875 [DOI] [Google Scholar]
- Makkar, R. R. (1993). Female gender as a risk factor for torsades de pointes associated with cardiovascular drugs. JAMA: The Journal of the American Medical Association, 270(21), 2590. doi: 10.1001/jama.1993.03510210076031 [DOI] [PubMed] [Google Scholar]
- Malik, P., Kemmler, G., Hummer, M., Riecher-Roessler, A., Kahn, R. S., & Fleischhacker, W. W. (2011). Sexual dysfunction in first-episode schizophrenia patients. Journal of Clinical Psychopharmacology, 31(3), 274–280. doi: 10.1097/JCP.0b013e3182199bcc [DOI] [PubMed] [Google Scholar]
- Marras, C., Herrmann, N., Anderson, G. M., Fischer, H. D., Wang, X., & Rochon, P. A. (2012). Atypical antipsychotic use and parkinsonism in dementia: Effects of drug, dose, and sex. American Journal of Geriatric Pharmacotherapy, 10(6), 381–389.e3. doi: 10.1016/j.amjopharm.2012.11.001 [DOI] [PubMed] [Google Scholar]
- Mas, S., Gassó, P., Lafuente, A., Bioque, M., Lobo, A., Gonzàlez-Pinto, A., … Bernardo, M. (2016). Pharmacogenetic study of antipsychotic induced acute extrapyramidal symptoms in a first episode psychosis cohort: Role of dopamine, serotonin and glutamate candidate genes. The Pharmacogenomics Journal, 16(5), 439–445. doi: 10.1038/tpj.2016.44 [DOI] [PubMed] [Google Scholar]
- Mayston, R., Kebede, D., Fekadu, A., Medhin, G., Hanlon, C., Alem, A., & Shibre, T. (2020). The effect of gender on the long-term course and outcome of schizophrenia in rural Ethiopia: A population-based cohort. Social Psychiatry and Psychiatric Epidemiology, 55(12), 1581–1591. doi: 10.1007/s00127-020-01865-1 [DOI] [PubMed] [Google Scholar]
- McAllister-Williams, R. H., Baldwin, D. S., Cantwell, R., Easter, A., Gilvarry, E., Glover, V., … Young, A. H. (2017). British Association for psychopharmacology consensus guidance on the use of psychotropic medication preconception, in pregnancy and postpartum 2017. Journal of Psychopharmacology, 31(5), 519–552. doi: 10.1177/0269881117699361 [DOI] [PubMed] [Google Scholar]
- McCloskey, L. R., Wisner, K. L., Cattan, M. K., Betcher, H. K., Stika, C. S., & Kiley, J. W. (2021). Contraception for women with psychiatric disorders. American Journal of Psychiatry, 178(3), 247–255. doi: 10.1176/appi.ajp.2020.20020154 [DOI] [PubMed] [Google Scholar]
- McEvoy, J. P., Meyer, J. M., Goff, D. C., Nasrallah, H. A., Davis, S. M., Sullivan, L., … Lieberman, J. A. (2005). Prevalence of the metabolic syndrome in patients with schizophrenia: Baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophrenia Research, 80(1), 19–32. doi: 10.1016/j.schres.2005.07.014 [DOI] [PubMed] [Google Scholar]
- Meaney, A. M., Smith, S., Howes, O. D., O'Brien, M., Murray, R. M., & O'Keane, V. (2004). Effects of long-term prolactin-raising antipsychotic medication on bone mineral density in patients with schizophrenia. British Journal of Psychiatry, 184(6), 503–508. doi: 10.1192/bjp.184.6.503 [DOI] [PubMed] [Google Scholar]
- Meltzer-Brody, S., Howard, L. M., Bergink, V., Vigod, S., Jones, I., Munk-Olsen, T., … Milgrom, J. (2018). Postpartum psychiatric disorders. Nature Reviews. Disease Primers, 4(1), 1–18. doi: 10.1038/nrdp.2018.22 [DOI] [PubMed] [Google Scholar]
- Moons, T., De Roo, M., Claes, S., & Dom, G. (2011). Relationship between P-glycoprotein and second-generation antipsychotics. Pharmacogenomics, 12, 1193–1211. doi: 10.2217/pgs.11.55 [DOI] [PubMed] [Google Scholar]
- Müller, M. J., Regenbogen, B., Sachse, J., Eich, F. X., Härtter, S., & Hiemke, C. (2006). Gender aspects in the clinical treatment of schizophrenic inpatients with amisulpride: A therapeutic drug monitoring study. Pharmacopsychiatry, 39(2), 41–46. doi: 10.1055/s-2006-931540 [DOI] [PubMed] [Google Scholar]
- Nagasaka, Y., Oda, K., Iwatsubo, T., Kawamura, A., & Usui, T. (2012). Effects of aripiprazole and its active metabolite dehydroaripiprazole on the activities of drug efflux transporters expressed both in the intestine and at the blood-brain barrier. Biopharmaceutics & Drug Disposition, 33(6), 304–315. doi: 10.1002/bdd.1801 [DOI] [PubMed] [Google Scholar]
- Nguyen, T., Mordecai, J., Watt, F., & Frayne, J. (2020). Obstetric and neonatal outcomes of clozapine exposure in pregnancy: A consecutive case series. Archives of Women's Mental Health, 23(3), 441–445. doi: 10.1007/s00737-019-00985-1 [DOI] [PubMed] [Google Scholar]
- NICE (2014). Antenatal and postnatal mental health: Clinical management and service guidance. Essentially MIDIRS. [PubMed]
- Nicolas, J. M., Espie, P., & Molimard, M. (2009). Gender and interindividual variability in pharmacokinetics. Drug Metabolism Reviews, 41(3), 408–421. doi: 10.1080/10837450902891485 [DOI] [PubMed] [Google Scholar]
- Nielsen, J., Damkier, P., Lublin, H., & Taylor, D. (2011). Optimizing clozapine treatment. In Acta Psychiatrica Scandinavica. doi: 10.1111/j.1600-0447.2011.01710.x [DOI] [PubMed] [Google Scholar]
- Ochoa, S., Usall, J., Cobo, J., Labad, X., & Kulkarni, J. (2012). Gender differences in schizophrenia and first-episode psychosis: A comprehensive literature review. Schizophrenia Research and Treatment, 2012, 1–9. doi: 10.1155/2012/916198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keane, V. (2008). Antipsychotic-induced hyperprolactinaemia, hypogonadism and osteoporosis in the treatment of schizophrenia. Journal of Psychopharmacology, 22(suppl), 70–75. doi: 10.1177/0269881107088439 [DOI] [PubMed] [Google Scholar]
- Pacchiarotti, I., León-Caballero, J., Murru, A., Verdolini, N., Furio, M. A., Pancheri, C., … Vieta, E. (2016). Mood stabilizers and antipsychotics during breastfeeding: Focus on bipolar disorder. European Neuropsychopharmacology, 26(10), 1562–1578. doi: 10.1016/j.euroneuro.2016.08.008 [DOI] [PubMed] [Google Scholar]
- Panariello, F., De Luca, V., & de Bartolomeis, A. (2011). Weight gain, schizophrenia and antipsychotics: New findings from animal model and pharmacogenomic studies. Schizophrenia Research and Treatment, 2011, 1–16. doi: 10.1155/2011/459284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne, J. L. (2019). Psychopharmacology in pregnancy and breastfeeding. Medical Clinics of North America, 103(4), 629–650. doi: 10.1016/j.mcna.2019.02.009 [DOI] [PubMed] [Google Scholar]
- Petersen, I., McCrea, R. L., Sammon, C. J., Osborn, D. P. J., Evans, S. J., Cowen, P. J., … Nazareth, I. (2016). Risks and benefits of psychotropic medication in pregnancy: Cohort studies based on UK electronic primary care health records. Health Technology Assessment, 20(23), 1–176. doi: 10.3310/hta20230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peuskens, J., Pani, L., Detraux, J., & De Hert, M. (2014). The effects of novel and newly approved antipsychotics on serum prolactin levels: A comprehensive review. CNS Drugs, 28, 421–453. doi: 10.1007/s40263-014-0157-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips, S. P., & Hamberg, K. (2016). Doubly blind: A systematic review of gender in randomised controlled trials. Global Health Action, 9(1), 29597. doi: 10.3402/gha.v9.29597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccinato, C. A., Neme, R. M., Torres, N., Silvério, R., Pazzini, V. B., Rosa e Silva, J. C., & Ferriani, R. A. (2017). Is cytochrome P450 3A4 regulated by menstrual cycle hormones in control endometrium and endometriosis?. Molecular and Cellular Biochemistry, 427(1-2), 81–89. doi: 10.1007/s11010-016-2899-3 [DOI] [PubMed] [Google Scholar]
- Pottegård, A., Lash, T. L., Cronin-Fenton, D., Ahern, T. P., & Damkier, P. (2018). Use of antipsychotics and risk of breast cancer: A Danish nationwide case-control study. British Journal of Clinical Pharmacology, 84(9), 2152–2161. doi: 10.1111/bcp.13661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provensi, G., Blandina, P., & Passani, M. B. (2016). Histamine and appetite. In Blandina P. & Passani M. (Eds.), Receptors (pp. 341–360). United States: Humana Press. doi: 10.1007/978-3-319-40308-3_15 [DOI] [Google Scholar]
- Pu, C., Huang, B., Zhou, T., Cheng, Z., Wang, Y., Shi, C., & Yu, X. (2020). Gender differences in the first-year antipsychotic treatment for Chinese first-episode schizophrenia. Neuropsychiatric Disease and Treatment, 16, 3145–3152. doi: 10.2147/NDT.S280719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsjö, M., Aklillu, E., Bohman, L., Ingelman-Sundberg, M., Roh, H.-K., & Bertilsson, L. (2010). CYP2C19 activity comparison between Swedes and Koreans: Effect of genotype, sex, oral contraceptive use, and smoking. European Journal of Clinical Pharmacology, 66(9), 871–877. doi: 10.1007/s00228-010-0835-0 [DOI] [PubMed] [Google Scholar]
- Ravyn, D., Ravyn, V., Lowney, R., & Nasrallah, H. A. (2013). CYP450 pharmacogenetic treatment strategies for antipsychotics: A review of the evidence. Schizophrenia Research, 149(1–3), 1–14. doi: 10.1016/j.schres.2013.06.035 [DOI] [PubMed] [Google Scholar]
- Regitz-Zagrosek, V., Lehmkuhl, E., & Mahmoodzadeh, S. (2007). Gender aspects of the role of the metabolic syndrome as a risk factor for cardiovascular disease. Gender Medicine, 4, S162–S177. doi: 10.1016/S1550-8579(07)80056-8 [DOI] [PubMed] [Google Scholar]
- Reinstein, S. A., Cosgrove, J., Malekshahi, T., & Deligiannidis, K. M. (2020). Long-acting injectable antipsychotic use during pregnancy. The Journal of Clinical Psychiatry, 81(6), e13802. doi: 10.4088/JCP.20ac13597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riecher-Rössler, A. (2005). Estrogens and schizophrenia. In Bergemann N. & Riecher-Rössler A. (Eds.), Estrogen effects in psychiatric disorders (pp. 31–52). Vienna: Springer-Verlag. doi: 10.1007/3-211-27063-9_2 [DOI] [Google Scholar]
- Riecher-Rössler, A. (2017). Oestrogens, prolactin, hypothalamic-pituitary-gonadal axis, and schizophrenic psychoses. The Lancet. Psychiatry, 4(1), 63–72. doi: 10.1016/S2215-0366(16)30379-0 [DOI] [PubMed] [Google Scholar]
- Sadowski, A., Todorow, M., Yazdani Brojeni, P., Koren, G., & Nulman, I. (2013). Pregnancy outcomes following maternal exposure to second-generation antipsychotics given with other psychotropic drugs: A cohort study. BMJ Open, 3(7), e003062. doi: 10.1136/bmjopen-2013-003062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Casado, M., & García-Avello, A. (2019). Systematic review of gender bias in the clinical trials of new long-acting antipsychotic drugs. Journal of Clinical Psychopharmacology, 39(3), 264–272. doi: 10.1097/JCP.0000000000001041 [DOI] [PubMed] [Google Scholar]
- Scandlyn, M. J., Stuart, E. C., & Rosengren, R. J. (2008). Sex-specific differences in CYP450 isoforms in humans. Expert Opinion on Drug Metabolism & Toxicology, 4(4), 413–424. doi: 10.1517/17425255.4.4.413 [DOI] [PubMed] [Google Scholar]
- Schoretsanitis, G., Westin, A. A., Deligiannidis, K. M., Spigset, O., & Paulzen, M. (2020). Excretion of antipsychotics into the amniotic fluid, umbilical cord blood, and breast milk: A systematic critical review and combined analysis. Therapeutic Drug Monitoring, 42(2), 245–254. doi: 10.1097/FTD.0000000000000692 [DOI] [PubMed] [Google Scholar]
- Seeman, M. V. (2004). Gender differences in the prescribing of antipsychotic drugs. American Journal of Psychiatry, 161, 1324–1333. doi: 10.1176/appi.ajp.161.8.1324 [DOI] [PubMed] [Google Scholar]
- Seeman, M. V. (2012). Treating schizophrenia at the time of menopause. Maturitas. doi: 10.1016/j.maturitas.2012.03.008 [DOI] [PubMed] [Google Scholar]
- Seeman, M. V. (2018). Women who suffer from schizophrenia: Critical issues. World Journal of Psychiatry, 8(5), 125–136. doi: 10.5498/wjp.v8.i5.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman, M. V. (2019). Does gender influence outcome in schizophrenia? Psychiatric Quarterly, 90(1), 173–184. doi: 10.1007/s11126-018-9619-y [DOI] [PubMed] [Google Scholar]
- Seeman, M. V. (2020). Men and women respond differently to antipsychotic drugs. Neuropharmacology, 163, 107631. doi: 10.1016/j.neuropharm.2019.05.008 [DOI] [PubMed] [Google Scholar]
- Seeman, M. V., & Lang, M. (1990). The role of estrogens in schizophrenia gender differences. Schizophrenia Bulletin, 16(2), 185–194. doi: 10.1093/schbul/16.2.185 [DOI] [PubMed] [Google Scholar]
- Seeman, M. V., & Ross, R. (2011). Prescribing contraceptives for women with schizophrenia. Journal of Psychiatric Practice, 17(4), 258–269. doi: 10.1097/01.pra.0000400263.52913.dc [DOI] [PubMed] [Google Scholar]
- Segarra, M., Aburto, M. R., Acker-Palmer, A., Segarra, M., Uni-Frankfurt, A.-P., & De Acker-Palmer, A. (2020). Blood-brain barrier dynamics to maintain brain homeostasis. Trends in Neurosciences, 44(5), 393–405. doi: 10.1016/j.tins.2020.12.002 [DOI] [PubMed] [Google Scholar]
- Segarra, R., Ojeda, N., Zabala, A., García, J., Catalán, A., Eguíluz, J. I., & Gutiérrez, M. (2012). Similarities in early course among men and women with a first episode of schizophrenia and schizophreniform disorder. European Archives of Psychiatry and Clinical Neuroscience, 262(2), 95–105. doi: 10.1007/s00406-011-0218-2 [DOI] [PubMed] [Google Scholar]
- Shams, W. M., Sanio, C., Quinlan, M. G., & Brake, W. G. (2016). 17β-Estradiol infusions into the dorsal striatum rapidly increase dorsal striatal dopamine release in vivo. Neuroscience, 330, 162–170. doi: 10.1016/j.neuroscience.2016.05.049 [DOI] [PubMed] [Google Scholar]
- Shi, L., Ascher-Svanum, H., Zhu, B., Faries, D., Montgomery, W., & Marder, S. R. (2007). Characteristics and use patterns of patients taking first-generation depot antipsychotics or oral antipsychotics for schizophrenia. Psychiatric Services, 58(4), 482–488. doi: 10.1176/ps.2007.58.4.482 [DOI] [PubMed] [Google Scholar]
- Shlomi Polachek, I., Manor, A., Baumfeld, Y., Bagadia, A., Polachek, A., Strous, R. D., & Dolev, Z. (2017). Sex differences in psychiatric hospitalizations of individuals with psychotic disorders. Journal of Nervous & Mental Disease, 205(4), 313–317. doi: 10.1097/NMD.0000000000000645 [DOI] [PubMed] [Google Scholar]
- Smith, R. C., Leucht, S., & Davis, J. M. (2019). Maximizing response to first-line antipsychotics in schizophrenia: A review focused on finding from meta-analysis. Psychopharmacology, 236(2), 545–559. doi: 10.1007/s00213-018-5133-z [DOI] [PubMed] [Google Scholar]
- Snellen, M., Power, J., Blankley, G., & Galbally, M. (2016). Pharmacological lactation suppression with D2 receptor agonists and risk of postpartum psychosis: A systematic review. Australian and New Zealand Journal of Obstetrics and Gynaecology, 56(4), 336–340. doi: 10.1111/ajo.12479 [DOI] [PubMed] [Google Scholar]
- Soldin, O. P., Chung, S. H., & Mattison, D. R. (2011). Sex differences in drug disposition. Journal of Biomedicine and Biotechnology, 2011, 1–14. doi: 10.1155/2011/187103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldin, O. P., & Mattison, D. R. (2009). Sex differences in pharmacokinetics and pharmacodynamics. Clinical Pharmacokinetics, 48(3), 143–157. doi: 10.2165/00003088-200948030-00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer, I. E., Tiihonen, J., van Mourik, A., Tanskanen, A., & Taipale, H. (2020). The clinical course of schizophrenia in women and men – A nation-wide cohort study. Npj Schizophrenia, 6(1), 12. doi: 10.1038/s41537-020-0102-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spina, E., & De Leon, J. (2014). Clinically relevant interactions between newer antidepressants and second-generation antipsychotics. Expert Opinion on Drug Metabolism and Toxicology, 10(5), 721–746. doi: 10.1517/17425255.2014.885504 [DOI] [PubMed] [Google Scholar]
- Stillhart, C., Vučićević, K., Augustijns, P., Basit, A. W., Batchelor, H., Flanagan, T. R., … Müllertz, A. (2020). Impact of gastrointestinal physiology on drug absorption in special populations – An UNGAP review. European Journal of Pharmaceutical Sciences, 147, 105280. doi: 10.1016/j.ejps.2020.105280 [DOI] [PubMed] [Google Scholar]
- Tamminga, W. J., Wemer, J., Oosterhuis, B., Wieling, J., Wilffert, B., de Leij, L. F. M. H., … Jonkman, J. H. G. (1999). CYP2D6 And CYP2C19 activity in a large population of Dutch healthy volunteers: Indications for oral contraceptive-related gender differences. European Journal of Clinical Pharmacology, 55(3), 177–184. doi: 10.1007/s002280050615 [DOI] [PubMed] [Google Scholar]
- Tang, Y. L., Mao, P., Li, F. M., Li, W., Chen, Q., Jiang, F., … Mitchell, P. B. (2007). Gender, age, smoking behaviour and plasma clozapine concentrations in 193 Chinese inpatients with schizophrenia. British Journal of Clinical Pharmacology, 64(1), 49–56. doi: 10.1111/j.1365-2125.2007.02852.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrana, N., Koren, G., Pivovarov, J., Etwel, F., & Nulman, I. (2015). Pregnancy outcomes following in utero exposure to second-generation antipsychotics. Journal of Clinical Psychopharmacology, 35(5), 559–565. doi: 10.1097/JCP.0000000000000391 [DOI] [PubMed] [Google Scholar]
- Thanvi, B., & Treadwell, S. (2009). Drug induced parkinsonism: A common cause of parkinsonism in older people. Postgraduate Medical Journal, 85, 322–326. doi: 10.1136/pgmj.2008.073312 [DOI] [PubMed] [Google Scholar]
- Thompson, K. N., Kulkarni, J., & Sergejew, A. A. (2000). Extrapyramidal symptoms and oestrogen. Acta Psychiatrica Scandinavica, 101(2), 130–134. doi: 10.1034/j.1600-0447.2000.90067.x [DOI] [PubMed] [Google Scholar]
- Thorup, A., Waltoft, B. L., Pedersen, C. B., Mortensen, P. B., & Nordentoft, M. (2007). Young males have a higher risk of developing schizophrenia: A Danish register study. Psychological Medicine, 37(4), 479–484. doi: 10.1017/S0033291707009944 [DOI] [PubMed] [Google Scholar]
- Tosato, S., Albert, U., Tomassi, S., Iasevoli, F., Carmassi, C., Ferrari, S., … Fiorillo, A. (2017). A systematized review of atypical antipsychotics in pregnant women. The Journal of Clinical Psychiatry, 78(5), e477–e489. doi: 10.4088/JCP.15r10483 [DOI] [PubMed] [Google Scholar]
- Uguz, F. (2016). Second-generation antipsychotics during the lactation period. Journal of Clinical Psychopharmacology, 36(3), 244–252. doi: 10.1097/JCP.0000000000000491 [DOI] [PubMed] [Google Scholar]
- Usall, J., Ochoa, S., Araya, S., & Márquez, M. (2003). Gender differences and outcome in schizophrenia: A 2-year follow-up study in a large community sample. European Psychiatry, 18(6), 282–284. doi: 10.1016/j.eurpsy.2003.06.001 [DOI] [PubMed] [Google Scholar]
- Usall, J., Suarez, D., & Haro, J. M. (2007). Gender differences in response to antipsychotic treatment in outpatients with schizophrenia. Psychiatry Research, 153(3), 225–231. doi: 10.1016/j.psychres.2006.09.016 [DOI] [PubMed] [Google Scholar]
- Valencak, T. G., Osterrieder, A., & Schulz, T. J. (2017). Sex matters: The effects of biological sex on adipose tissue biology and energy metabolism. Redox Biology, 12, 806–813. doi: 10.1016/j.redox.2017.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandegrift, B. J., You, C., Satta, R., Brodie, M. S., & Lasek, A. W. (2017). Estradiol increases the sensitivity of ventral tegmental area dopamine neurons to dopamine and ethanol. PLoS ONE, 12(11), e0187698. doi: 10.1371/journal.pone.0187698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vehof, J., Risselada, A. J., Al Hadithy, A. F. Y., Burger, H., Snieder, H., Wilffert, B., … Bruggeman, R. (2011). Association of genetic variants of the histamine H1 and muscarinic M3 receptors with BMI and HbA1c values in patients on antipsychotic medication. Psychopharmacology, 216(2), 257–265. doi: 10.1007/s00213-011-2211-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventriglio, A., Ricci, F., Magnifico, G., Chumakov, E., Torales, J., Watson, C., … Bellomo, A. (2020). Psychosocial interventions in schizophrenia: Focus on guidelines. International Journal of Social Psychiatry, 66, 735–747. doi: 10.1177/0020764020934827 [DOI] [PubMed] [Google Scholar]
- Verma, S., Liew, A., Subramaniam, M., & Poon, L. Y. (2009). Effect of treatment on weight gain and metabolic abnormalities in patients with first-episode psychosis. Australian & New Zealand Journal of Psychiatry, 43(9), 812–817. doi: 10.1080/00048670903107609 [DOI] [PubMed] [Google Scholar]
- Vigod, S. N., Gomes, T., Wilton, A. S., Taylor, V. H., & Ray, J. G. (2015). Antipsychotic drug use in pregnancy: High dimensional, propensity matched, population based cohort study. BMJ, 350(may11 5), h2298–h2298. doi: 10.1136/bmj.h2298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink, A. S., Clur, S.-A. B., Wilde, A. A. M., & Blom, N. A. (2018). Effect of age and gender on the QTc-interval in healthy individuals and patients with long-QT syndrome. Trends in Cardiovascular Medicine, 28(1), 64–75. doi: 10.1016/j.tcm.2017.07.012 [DOI] [PubMed] [Google Scholar]
- Waln, O., & Jankovic, J. (2013). An update on tardive dyskinesia: From phenomenology to treatment. Tremor and Other Hyperkinetic Movements, 3, tre-03-161-4138-1. doi: 10.5334/tohm.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters, S. H., & Levitan, E. S. (2020). Vesicular antipsychotic drug release evokes an extra phase of dopamine transmission. Schizophrenia Bulletin, 46(3), 643–649. doi: 10.1093/schbul/sbz085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z., Wong, I. C. K., Man, K. K. C., Alfageh, B. H., Mongkhon, P., & Brauer, R. (2021). The use of antipsychotic agents during pregnancy and the risk of gestational diabetes mellitus: A systematic review and meta-analysis. Psychological Medicine. doi: 10.1017/S003329171900401X [DOI] [PubMed] [Google Scholar]
- Wenzel-Seifert, K., Wittmann, M., & Haen, E. (2011). QTc prolongation by psychotropic drugs and the risk of Torsade de Pointes. Deutsches Aerzteblatt Online, 108(41), 687–693. doi: 10.3238/arztebl.2011.0687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westin, A. A., Brekke, M., Molden, E., Skogvoll, E., Castberg, I., & Spigset, O. (2018). Treatment with antipsychotics in pregnancy: Changes in drug disposition. Clinical Pharmacology & Therapeutics, 103(3), 477–484. doi: 10.1002/cpt.770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitley, H., & Lindsey, W. (2009). Sex-based differences in drug activity. American Family Physician, 80(11), 1254–1258. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/19961138 [PubMed] [Google Scholar]
- Wolbold, R. (2003). Sex is a major determinant of CYP3A4 expression in human liver. Hepatology, 38(4), 978–988. doi: 10.1053/jhep.2003.50393 [DOI] [PubMed] [Google Scholar]
- Wu, J. Q., Kosten, T. R., & Zhang, X. Y. (2013). Free radicals, antioxidant defense systems, and schizophrenia. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 46, 200–206. doi: 10.1016/j.pnpbp.2013.02.015 [DOI] [PubMed] [Google Scholar]
- Yang, L., & Li, Y. (2012). Sex differences in the expression of drug-metabolizing and transporter genes in human liver. Journal of Drug Metabolism & Toxicology, 3(3). doi: 10.4172/2157-7609.1000119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa, R., & Jeste, D. V. (1992). Gender differences in tardive dyskinesia: A critical review of the literature. Schizophrenia Bulletin, 18(4), 701–715. doi: 10.1093/schbul/18.4.701 [DOI] [PubMed] [Google Scholar]
- Yoest, K. E., Cummings, J. A., & Becker, J. B. (2019). Oestradiol influences on dopamine release from the nucleus accumbens shell: Sex differences and the role of selective oestradiol receptor subtypes. British Journal of Pharmacology, 176(21), 4136–4148. doi: 10.1111/bph.14531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonkers, K. A., Kando, J. C., Cole, J. O., & Blumenthal, S. (1992). Gender differences in pharmacokinetics and pharmacodynamics of psychotropic medication. American Journal of Psychiatry, 149(5), 587–595. doi: 10.1176/ajp.149.5.587 [DOI] [PubMed] [Google Scholar]
- Yum, S. K., Kim, T., & Hwang, M. Y. (2014). Polycystic ovaries is a disproportionate signal in pharmacovigilance data mining of second generation antipsychotics. Schizophrenia Research, 158(1–3), 275–276. doi: 10.1016/j.schres.2014.06.003 [DOI] [PubMed] [Google Scholar]
- Yum, S. K., Yum, S. Y., & Kim, T. (2019). The problem of medicating women like the men: Conceptual discussion of menstrual cycle-dependent psychopharmacology. Translational and Clinical Pharmacology, 27(4), 127. doi: 10.12793/tcp.2019.27.4.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakiniaeiz, Y., Cosgrove, K. P., Potenza, M. N., & Mazure, C. M. (2016). Balance of the sexes: Addressing sex differences in preclinical research. The Yale Journal of Biology and Medicine, 89(2), 255–259. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/27354851. [PMC free article] [PubMed] [Google Scholar]
- Zhu, K., van Hilten, J. J., Putter, H., & Marinus, J. (2013). Risk factors for hallucinations in Parkinson's disease: Results from a large prospective cohort study. Movement Disorders, 28(6), 755–762. doi: 10.1002/mds.25389 [DOI] [PubMed] [Google Scholar]
- Zincir, S. B. (2019). Schizophrenia and related psychoses. In Perinatal psychopharmacology (pp. 259–269). Cham: doi: 10.1007/978-3-319-92919-4_15 [DOI] [Google Scholar]
- Zucker, I., & Prendergast, B. J. (2020). Sex differences in pharmacokinetics predict adverse drug reactions in women. Biology of Sex Differences, 11(1), 32. doi: 10.1186/s13293-020-00308-5 [DOI] [PMC free article] [PubMed] [Google Scholar]