Abstract
Osteoarthritis is a prevalent degenerative disease affecting a large portion of the world’s aging population. Currently, nonsteroidal anti-inflammatory drugs and acetaminophen are first-line medications for treating osteoarthritis patients’ pain. However, several studies have noted that while these medications control pain they do not halt progressive degeneration and tend to have an unfavorable side-effect profile with prolonged use. Recently, due to their more favorable side-effect profiles, herbal alternatives for controlling osteoarthritis symptoms and for alleviating the progression of the disease are being increasingly studied. Synogesic is a newly developed herbal supplement blend by renowned orthopedic surgeons and physiatrists consisting of turmeric, rutin, ginger root, vitamin C, vitamin D, and boswellia extracts. A study by Sharkey et al. has commented on the efficacy of the blend on the patients with knee osteoarthritis. So far, a review on the ingredients of the blend has not yet carried outbeen. By exploring prominent literature databases including PubMed and ScienceDirect, our aim is to write a narrative review to explore the individual ingredients of this blend and delve into their characteristics, as well as the most recent literature on their mechanism and efficacy in patients with osteoarthritis. Through this, we hope to inform clinicians and patients alike on relevant up-to-date research on the supplement and provide insight on the potential for this supplement for alleviating the disease course of patients with osteoarthritis.
Keywords: Osteoarthritis, pain, NSAID alternatives, turmeric, rutin, vitamin C, vitamin D, boswellia, ginger
Introduction
Osteoarthritis (OA) is one of the most common types of arthritis and is a degenerative disease of the articular joints with progressive worsening of the subchondral bone, articular cartilage, and the synovium. 1 It is a predominant cause of pain and disability and is anticipated to place a significant burden on health care systems around the world as our population ages. 2 Osteoarthritis classically involves the entire joint and involves the breakdown of cartilage, ligaments, joint lining, and even, in severe cases, the entire bone. 3 The most commonly affected joints include the hip, knee, spine, and hands. Osteoarthritis involves synovial inflammation and the irreversible destruction of the extracellular matrix of the articular cartilage. 4 It also leads to the formation of osteophytes, subchondral cysts, subchondral sclerosis, and synovial hyperplasia, 5 which can lead to further degeneration of the bone. The primary risk factors for OA include age, gender, genetics, obesity, and chronic joint injury or instability. 6 Knee OA is supposed to be the leading cause of disability and pain in the adult and old age population with an estimated 10% to 15% of all adults aged above 60 having a certain degree of OA. According to the World Health Organization (WHO), it is projected that approximately more than 130 million will be affected by OA, and more than 40 million people will be severely disabled by the disease. 7 This is also because of the medical advances that are increasing the average life expectancy. 8 Women have a higher prevalence of OA than men. 7 Osteoarthritis, especially knee OA, has been proven to impair the physical functions and worsens the overall quality of life.
Paracetamol, also known as acetaminophen, is another drug that is used in the treatment of OA. It is purported mechanism of action is not completely understood but is said to inhibit prostaglandin production in the brain. Per the American College of Rheumatology, acetaminophen is said to be the first line in the treatment of OA. Furthermore, the American Pain Society recommends paracetamol as a first line treatment for OA. 9 A large meta-analysis by Zhang et al 10 has shown that paracetamol is an effective agent for pain relief and for improving function in OA. If the patient does fail acetaminophen, topical and/or oral nonsteroidal anti-inflammatory drugs (NSAIDs) are recommended. This is classically followed by tramadol or intra-articular steroid injections. If patients continue to have pain, opioids are indicated as a second-line therapy. 7 At this point, there are no definitive cures established for the disease, 11 and the aforementioned medications such as NSAIDs and paracetamol help primarily with symptomatic relief and do not have a clear effect on disease prevention. 7 In addition, long-term use of these medications can lead to hepatic, gastrointestinal (GI), renal, and cardiovascular effects, 7 which makes them less than ideal to use in the long term for patients with chronic pain. Therefore, there is a clear and immediate need for other alternative treatments that are safer, can reduce inflammation, and minimize side effects for patients who require long-term pain relief. This is the reason that, in this narrative review, we explore the ingredients of a recently formulated nutritional supplement known as Synogesic, a proprietary blend of turmeric extract, ginger root extract, Boswellia serrata extract, vitamin C, vitamin D, and rutin. 12 This supplement has been developed as an alternative and/or an addition to existing medications used for OA with the purpose of improving the quality of life of patients with OA and reducing progression of the disease and worsening of pain.
Dietary supplements, which includes herbal supplements, have been examined for treatment and pain management in OA for several years now. Some dietary supplements including glucosamine and chondroitin have been shown to have efficacy compared with placebo, whereas others such as methylsulfonylmethane have not been as effective. 13 Currently, there is one study by Sharkey et al 12 on the efficacy of the Synogesic supplement that uses 43 patients with diagnosed knee OA with Kellgren-Lawrence scores of grade II or grade III disease. In total, 22 patients were assigned to the Synogesic group (group A) and 21 patients were assigned to the placebo group (PG; group B). Patients were instructed to take the nutritional supplement’s tablets 4 times a day for 12 weeks. It was found that the visual analog scale (VAS) for pain improved by 46.8%, WOMAC (Western Ontario and McMaster Universities Arthritis Index) by 23.4%, KOOS (Knee injury and Osteoarthritis Outcome Score) for pain, and Activities of Daily Living (ADL) by 19.8% and 14.9%, respectively. However, in group B, there was no statistically significant improvement for any of the patient outcome scores assessed. 12 To judge patient’s perception of pain in OA, the WOMAC index was created, a self-administered questionnaire covering 3 main domains: pain, function, and stiffness. It is composed of 24 questions: 5 on pain, 2 on stiffness, and 17 on function. 14 The questions regarding pain include experiencing pain when the patient is walking, using stairs, in bed, sitting or lying, and standing upright. The questions regarding stiffness include after first waking and later in the day. The questions regarding physical function issues include while using stairs, rising from sitting, standing, bending, walking, getting in/out of a car, shopping, putting on or taking off socks, rising from bed, lying in bed, getting in/out of bath, sitting, getting on or off the toilet, heavy domestic duties, or light domestic duties.
At the moment, there is only one paper on the efficacy of the nutritional supplement; however, a narrative review on each of the individual ingredients and their efficacies has not been done yet. The purpose of our study is to perform a review on each of the ingredients involved in this nutritional blend. Through this, we hope to perform an in-depth analysis on each of these ingredients, inform clinicians and patients on relevant up-to-date research on the supplement, and provide insight on the potential for this supplement for alleviating the disease course of patients with OA.
Methods
Four databases (PubMed, ScienceDirect, Google Scholar, and MedLINE) were searched from 1995 to July of 2021 inclusive. We limited our resources to peer-reviewed journal articles, trusted sources including NCBI and PubChem websites, and predominant phytomedical books in the field. In addition, hand texts and authoritative texts were also part of the included literature. Some of the keywords used during our searches included the name of the ingredient plus OA (eg, “Rutin”+“Osteoarthritis” for rutin). Furthermore, we performed searches for studies that investigated the effect of these supplements on patients’ VAS and WOMAC scores (eg, “Rutin” + “Osteoarthritis” + “WOMAC” or “Rutin” + “Osteoarthritis” + “VAS”). We also adapted images from studies or PubChem and NCBI websites detailing the structure and/or a synthetic process of the ingredient analyzed. Overall, we used 110 sources for this narrative review.
Turmeric
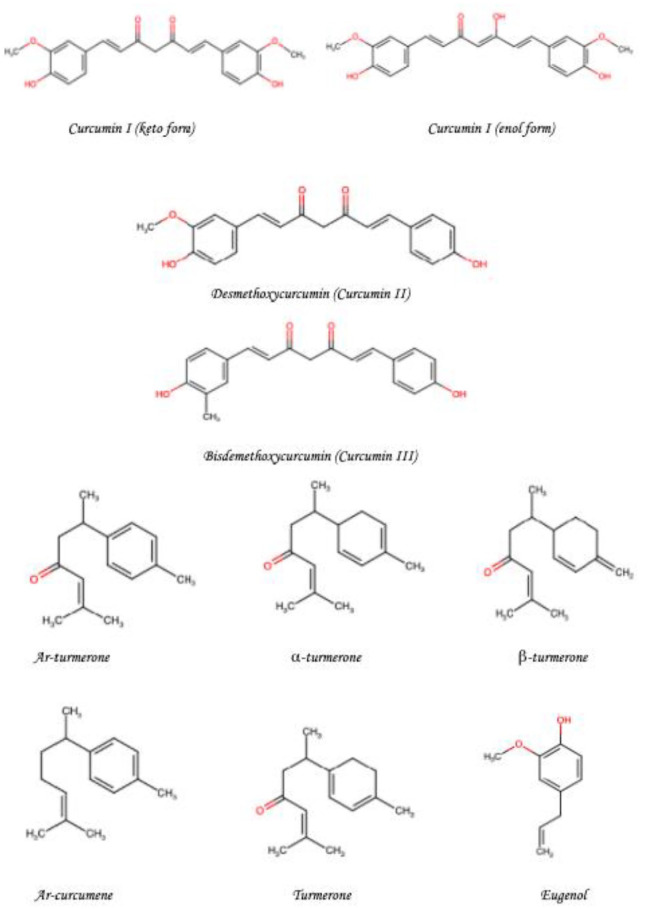
Turmeric is a member of the ginger family and is classically found as a bright yellow aromatic powder obtained from the rhizome of a plant, Curcuma longa of the ginger family, Zingiberaceae. 15 Turmeric has been used in Asian countries for centuries with notable uses in Traditional Chinese Medicine (TCM), Ayurveda, and Siddha medicine. 16 It was historically initially used as a dye and later used for its medicinal properties. 17 Turmeric’s initial uses are traced back to India after which it spread to Southeast Asia through Hinduism and Buddhism. There is also evidence that it has been used by Austronesian people for both food and dye prior to their contact with Europeans. 16 Nowadays, turmeric is principally used as a coloring agent and flavoring agent in many Asian cuisines, especially in curries. It is also known for its use as a dye and has also been approved by the Food and Drug Administration, WHO, and European parliament as a food additive. 18 Curcumin, or diferuloylmethane (82%), is the principal curcuminoid in turmeric. Other components including demethoxycurcumin (15%) and bisdemethoxycurcumin (3%) are together known as the curcuminoids. The chemical structures of these components can be seen in Figure 1. Turmeric has long been used in Ayurvedic medicine for thousands of years to treat various diseases including jaundice, arthritis, GI infections, wound, and skin infections. 8 Turmeric, more recently in preclinical and clinical studies, has been shown to have therapeutic value against chronic diseases including cardiovascular, neoplastic, pulmonary, and metabolic diseases.
Figure 1.
Chemical structures of the important components of turmeric.a,b
aThis is an original figure produced using the ChemSPACE 19 website (https://chem-space.com/search) drawing tool.
bElements from a study by Amalraj et al 20 were used to produce this figure.
More recently, turmeric has been investigated for its anti-inflammatory properties due to its active ingredient, curcumin. Curcumin makes up 3% to 10% of the turmeric powder that can be extracted, 21 and the extract itself has been shown to have similar effect to NSAIDs through affecting the signaling of pro-inflammatory cytokines including interleukins, phospholipase A2, 5-lipoxygenase (5-LOX), and cyclooxygenase-2 (COX-2) by affecting nuclear factor kappa light-chain enhancer of activated B cells (NF-κB) activity. 22 In a study by Plummer et al 22 in 1999, it was shown, using NF-κB transfection assays, that curcumin decreases NF-κB transactivation. It was also shown that curcumin has little to no effects at the tumor necrosis factor-α (TNF-α) level. Its anti-inflammatory properties have also been shown to work at a chondrocyte level in some studies, in which it has been shown to have an inhibitory effect on macrophage inhibitory factor–induced upregulation of matrix metalloproteinase 1 (MMP-1) and matrix metalloproteinase 3 (MMP-3) enzymes. These enzymes catabolize articular cartilage and subsequently lead to development of OA. 8 In a study by Onodera et al, 23 0, 1, and 10 µM concentrations of curcumin were mixed with macrophage migration inhibitory factor (MIF) and a northern blot analysis conducted. The levels of tissue inhibitor matrix metalloproteinase 1, MMP-1, and MMP-3 messenger RNA were measured. The blots were hybridized with 32P-labeled human cDNA probes of MMP-1, MMP-3, and glyceraldehyde 3-phosphate dehydrogenase and visualized by autoradiography. It was noted that MIF upregulation of these factors was inhibited at increasing amounts. Curcumin compounds were also shown to cause a 48% to 99% suppression of MMP-3 and 45% to 97% downregulation of matrix metalloproteinase 13 (MMP-13) in human chondrocytes and 8% to 100% (MMP-3) and 32% to 100% (MMP-13) in bovine chondrocytes. 24
Curcumin has also been shown to inhibit early degenerative changes induced by IL-1β and antagonizes the suppression of collagen type II and β1-integrin synthesis. 25 Thus, it is demonstrated to exert anti-apoptotic and anticatabolic effects on chondrocytes, preventing the articular degeneration induced by OA. A study by Shakibaei et al 25 showed that curcumin inhibits the effect of interleukin 18 (IL-18) 26 on vascular endothelial growth factor, which typically stimulates angiogenesis, in a dose-dependent manner. Several trials have analyzed the effects of turmeric on OA, and these trials typically analyze a patient’s pain scale using the scale previously described in this study known as the WOMAC scale. We describe 5 studies in this article that have investigated the effects of curcumin on WOMAC scores. In the first study by Singhal et al, 7 193 people with diagnosed knee OA were analyzed, with 97 of them receiving turmeric extract and 96 of them receiving paracetamol. In all, 24 people were lost in the turmeric group and 25 people were lost to follow-up in the paracetamol group. The 2 groups’ WOMAC scores were compared after 6 weeks of taking each of the medications. In the paracetamol group, three 650 mg tablets were taken daily, and in the turmeric group, two 500 mg capsules were taken by the participants daily. It was found that patients with knee OA in the paracetamol group and turmeric extract group both very similarly responded to curcumin with a >20% reduction in WOMAC pain score as well as WOMAC pain and function/stiffness score (80% vs 77% in paracetamol vs turmeric extract group, and 61% vs 58%, respectively). It was also found that 18% of patients with knee OA in the turmeric extract group showed >50% improvement in pain and function/stiffness score and another 3% of patients showed a >70% improvement. However, none of the patients in the paracetamol group had a >50% improvement. 7 Furthermore, TNF-α levels were also found to have a statistically significant decrease in the turmeric group versus the paracetamol group.
In the second study by Ross, 27 a randomized, double-blind, controlled multicenter clinical trial was conducted at 8 tertiary hospitals across Thailand. Of the 367 patients diagnosed with knee OA recruited for the study, there were 160 patients in the ibuprofen group (IBP) and 171 patients in the curcumin group remaining. At baseline it was found that WOMAC scores were more than 5 out of 10, and the patients were followed up at 2 and 4 weeks with the WOMAC scores assessed and calculated. A noninferiority test revealed that the mean difference in the 95% confidence interval of the WOMAC total (P = .010), WOMAC pain (P = .018), and function (P = .010) subscale scores at week 4 with respect to values at week 0 was within 0.5 points. This indicated similar improvements in pain between the ibuprofen and curcumin groups. In addition, there was found to be similar adverse effects in both groups, but there was a lower incidence of abdominal pain and distension in the curcumin group. In the third study by Panahi et al, 28 27 patients were provided curcuminoids, and 26 patients were provided a placebo for 6 weeks. Curcuminoids were administered in 500 mg capsules containing 5 mg of BioPerine, which were the same size as the placebos. This was a double-blind placebo-controlled parallel group trial and included subjects who were diagnosed with knee OA who referred to the Baqiyatallah University Clinic in 2011 to 2012. Statistically significant decreases in the WOMAC score between the turmeric group versus PG were observed in the total score (25.0 vs 40.6), physical function (18.7 vs 30.4), and pain index (6.1 vs 9.4); however, the difference between the stiffness score was not statistically significant. Reductions in the VAS and Lequesne Pain and Function Index (LPFI) scores were also found in the curcuminoid group.
In the fourth study by Bharti et al, 29 the efficacies of tocotrienol and curcumin were compared. A total of 72 patients with diagnosed grade II or grade III OA were divided into 1 of 4 groups, group I—Diclofenac 50 mg (twice a day), group II—Diclofenac 50 mg + CL 500 mg (twice a day), group III—Diclofenac 50 mg + Tocotrienol 200 mg (twice a day), and group IV—Diclofenac 50 mg + CL 500 mg + Tocotrienol 200 mg (twice a day), where CL was the turmeric/curcumin group. The WOMAC scores at 60 and 120 days were measured for each of the groups. It was found that the reduction in the WOMAC scores for the blend group of Diclofenac, Curcumin, and Tocotrienol (133.83 → 30.67) was the most pronounced, whereas it was found that there was a slight difference in mean WOMAC reductions between the Curcumin + Diclofenac (134.28 → 67.44) and Tocotrienol + Diclofenac (134.06 → 64.33) groups. This suggests that a blend of herbs and medications is likely more effective than an individual herb or medication alone in treating the inflammation and reducing pain. In the fifth study by Calderón-Pérez et al, 30 35 patients were assigned to a group provided B-turmactive, constituted by Turmacin and curcuminoid Betasorb, and 33 patients were assigned to the group provided brewer’s yeast as the placebo. The WOMAC scores for each patient was calculated after 3 days and after 1 week. It was found that both treatments reduced pain with statistically significant P values when walking on a flat surface, going up or down stairs, and sitting or lying; however, it was found that only B-Turmactive reduced pain at night while in bed and in an upright standing position. B-Turmactive also caused a statistically significant reduction in C-reactive protein levels with a reduction of 0.104 in the B-Turmactive compared with the placebo. From our analysis and review of turmeric extract, it can be seen that turmeric has significant effects on reducing the pain indices of patients with OA, and there is literature evidence for the effects on a cellular level through inhibition of the NF-κB pathway and other cytokines including IL-1β and IL-18.
Rutin
Rutin is a flavonoid, a ubiquitous group of naturally occurring polyphenolic compounds that are classically found in vegetables, fruits, and plant-based beverages, 31 and is classically found in plants including buckwheat, citrus fruits, apples, tea, onions, and red wine. 32 The interest in rutin derived from buckwheat can date back to 1940s, when buckwheat was cultivated as a source of rutin for medicinal use in the United States. 33 Rutin is a flavone glycoside and is also known as vitamin P. 34 It is also one of the phenolic compounds found in the plant species, Carpobrotus edulis, and has been shown to contribute to the antibacterial properties of the plant. 35 The etymology of the name, rutin, is derived from the plant, Ruta graveolens, a plant that contains a significant amount of rutin. Citrus fruit peels contain 32 to 49 mg/g of rutin equivalents, and citrus leaves contain rutin at concentrations of 11 g and 7 g/kg in orange and lime trees, respectively. 36 It is also known as rutoside, quercetin-3-O-rutinoside, and sophorin, and rutin is primarily a glycoside that combines quercetin with the disaccharide rutinose. The main chemical structures of quercetin and its rutinoside, rutin, are depicted in Figure 2. Quercetin is the circulating aglycone form of rutin that is considered a strong antioxidant as studies have shown it can scavenge free radicals.37,38
Figure 2.
Chemical structure of quercetin and its rutinoside, rutin.a,b
aThis is an original figure produced using the ChemSPACE 19 website (https://chem-space.com/search) drawing tool.
bElements from a study by Djelili et al 39 were used to produce this figure.
Rutin, in the form of quercetin, is already known for its anti-inflammatory, antimutagenic, antioxidant, anticancer, gastroprotective, and neuroinflammatory effects.40,41(p2) In terms of anti-inflammatory effects, a study by Picot et al, 42 it was shown that rutin can block the COX pathway and the LOX pathway at high concentrations. However, the compounds primarily block the LOX pathway at lower concentrations. Most NSAIDs primarily target COX-1 inhibition; however, selective inhibition of COX-2 might reduce the side effects of synthetic drugs. 34 In several cases, NSAIDs are unable to halt the progression of OA, leading to irreversible joint erosion and deformity. In a study by Guardia et al, 43 a treatment of rutin at 80 µM produced an obvious inhibitory effect of LOX-induced nitric oxide (NO) production in peritoneal macrophages. In a different study by Shen et al as noted in the study by Guardia et al, 43 6 mg of rutin per kilogram of body weight was injected in mice and a similar observation of inhibition of LOX-induced NO production was noticed. Han 44 in 2009 showed an inhibition of NO at the dose of 20 µg of rutin per milliliter without killing the macrophages, whereas most other anti-arthritis drugs tend to reduce the inflammation with the destruction of macrophages. In this same study, the anti-arthritis properties of rutin were demonstrated based on its effects on the articular elastase activity, and its activity is proportional to the accumulation and activation of polymorphonuclear leukocytes. It was also found that the detection of NO relates to the inflammation response from activated macrophages and T cells. Nitric oxide induction in excess has been shown to cause chondrocyte apoptosis. 45 Furthermore, rutin has been shown to inhibit the transcription of pro-inflammatory genes including ones encoding interleukin 1 (IL-1), interleukin 8 (IL-8), and TNF-α. 46
There are a few studies showing the effects of rutin on the progression of OA. For the purpose of this review, 3 studies will be explored. In one such study by Horcajada et al, 47 60 four-week-old Dunkin-Hartley guinea pigs were randomized into 4 groups and were fed 1 of 4 diets for 31 weeks. These diets included standard guinea pig diet (control group) or a standard guinea pig diet enriched with oleuropein (0.025%), rutin (0.5%), or rutin/curcumin (0.5%/0.25%) association. Biomarkers of OA including type II collagen degradation markers (Coll2-1 and Coll2-1NO2), fibulin 3 peptides (Fib3-1 and Fib3-2), and a fragment of aggrecan (ARGS) were quantified at 4 weeks and at 35 weeks as well as a quantification of prostaglandin E2 (PGE2) in the serum. Histological assessments of knee cartilage during these 2 time points were also performed. The results of this experiment showed a statistically significant reduction in the cartilage degradation score for all the treatments; however, only oleuropein caused significant decreases in serum PGE2 levels, and Coll2-1 was seen to be decreased by the rutin and rutin/curcumin combination treatments. Furthermore, Fib3-1 and Fib3-2 (fibrillin) were only reduced by the rutin/curcumin combination, whereas Coll2-1NO2 was decreased by all treatments. In a different study by Na et al,48(p1) they found that rutin protects rat articular chondrocytes against oxidative stress induced by hydrogen peroxide through sirtuin 1 (SIRT1) activation. SIRT1 is involved in the inhibition of the NF-κB/MAPK (mitogen-activated protein kinase) signaling pathway in hydrogen peroxide–induced oxidative stress in rat chondrocytes. Rat chondrocytes were treated with rutin in concentrations of 1, 5, 10, and 20 μg/mL, and it was found that rutin increases expression of SIRT1 in a dose-dependent manner. In addition, treatment with hydrogen peroxide (500 μM) resulted in downregulation of SIRT1 protein expression, which was reversed by treatment with rutin, showing that rutin induces SIRT1 expression significantly in normal chondrocytes as well as hydrogen peroxide–treated chondrocytes. At this time, there are no specific studies that isolate rutin and analyze it individually in terms of WOMAC scores of patients with OA. However, there is a study by Klein et al 49 that analyzes a proteolytic enzyme blend known as Phlogenzym (PE) which contains bromelain, trypsin, and rutin. After a 6-week follow-up, with one group taking Diclofenac (DC) 100 mg/d and the other group taking Phlogenzym (bromelain 90 mg, trypsin 48 mg, and rutosid 100 mg) 4 times a day, the results of the study were as follows: WOMAC subscale pain (PE −10.3 ± 1.2, DC −9.5 ± 1.2), WOMAC subscale joint stiffness (PE −3.9 ± 0.5, DC −3.6 ± 0.5), and WOMAC subscale physical function (PE −31.7 ± 3.5, DC − 29.7 ± 3.5). There was no significant difference in pain relief between Diclofenac and Phlogenzym; however, it was noted that Phlogenzym was slightly better tolerated than Diclofenac.
From our analysis and review of studies detailing rutin, we can see that there is sufficient evidence backing the physiology behind rutin’s therapeutic effects in OA; however, there is a paucity of clinical trials on humans detailing these effects of isolated rutin, even though combination blends have been explored.
Ginger Root
Zingiber officinale is derived from the Zingiberacae family, which comprises more than 1300 species of plants, with 90 of them being part of the Zingiber family. 50 Zingiber officinale has been used for thousands of years as both food and medicine in ancient Indian and Chinese cultures. In Indian medicine, it has been classically used in herbal extracts and in food preparation, and externally as a compress or massage with oil. 50 According to Ayurvedic medicine, OA leads to an imbalance in elemental forces or energies known as vata dosha. In TCM, ginger has been used internally and externally, mostly as a compress, patch, or during combustion in moxibustion. 51 Ginger is supposed to activate stagnating Chi, dispeling cold, and strengthening yang. 50 Ginger has also found uses in anthroposophic medicine, a branch of medicine that combines allopathic, TCM, and Ayurvedic medicine; ginger is used externally through ginger compresses over the kidneys to enliven and stimulate the metabolic region. 50 The rhizome is the medicinal part of ginger and has been known to have anti-spasmodic, anti-inflammatory, and carminative effects.52 -54 The rhizome contains about 40% to 60% carbohydrates, 9% to 10% proteins, 6% to 10% lipids containing lecithins, free fatty acids, and phosphatidic acid and contains 4% to 7.5% oleoresin such as gingerols and their dehydration products including shogaol, which sometimes takes place in the human body after being exposed to heat/acid. Conversion to shogaols, depicted in Figure 3, is said to decrease the medicinal value of ginger. 55 Nowadays, the ginger rhizome is cultivated widely for commercial use in several tropical countries including Australia, Africa, Fiji, India, Indonesia, Sri Lanka, and China. 50
Figure 3.

Chemical conversion of gingerol to shogaol.a,b,c
aGingerols and shogaols are biologically active compounds of ginger responsible for its medicinal value. However, conversion to shogaol in the body can lead to a reduction of ginger’s value in the body.
bThis is an original figure produced utilizing the ChemSPACE 19 website (https://chem-space.com/search) drawing tool.
cElements from a study by West and Kim 55 were used to produce this figure.
Ginger has been shown to inhibit the activation of TNF-α, interleukin 1β (IL-1β), and inducible nitric oxide synthase. 56 In a study by Lee et al, using macrophages derived from bone marrows, it was shown using a immunoblot analysis and a kinase assay, D10G (1-dehydro-[10]-gingerdione), a derivative of ginger extract, caused disruption of NF-κB transcriptional activity leading to suppression of iNOS, COX-2, and interleukin 6 (IL-6). In another study by Naderi et al, 57 it was found that ginger extract leads to a reduction in levels of inflammatory markers, NO, and C-reactive protein (CRP). In this study, 120 outpatients with diagnosed OA were split into a PG and a ginger group (GG). The GG was provided 500 mg capsules 2 times a day for 3 months, and by the end of the 3 months, a larger decrease of CRP (11.21 → 9.66 in PG and 11.06 → 8.47 in GG) and NO (29.02 → 26.02 in GG; 29.21 → 27.02 in PG) was found in the GG. In another study by Mozaffari-Khosravi et al, 58 similar to the previous study, 120 patients were split into GG and PG groups. The levels of the cytokines, TNF-α, and IL-1β, at the end of 3 months decreased in GG relative to PG. Ginger has also been shown to suppress levels of inflammatory cytokines in human synoviocytes. 59 In vitro and in vivo animal studies have also shown that ginger can work as a dual inhibitor of both COX and LOX synthesis. 60 In addition, it is also known that ginger inhibits several genes encoding chemokines and cytokines. 61 In a study by Tjendraputra et al, 62 it was shown that gingerols are more potent inhibitors of cyclooxygenase 1 (COX-1) than cyclooxygenase (COX-2). Inhibition of COX-2 is said to lead to more anti-inflammatory effects without many side effects; however, inhibition of COX-1 tends to lead to GI and renal side effects. 63 Surprisingly, ginger is gastroprotective and has anti-ulcer activity. 64
There are several studies investigating the effects of ginger on pain in OA. For the purpose of this review, we will be delving into 3 of such studies. In a study by Rayati et al, 65 120 outpatients were divided into 3 groups, PG, ginger extract group (GE), and IBP. The GE was provided 30 mg ginger tablets in two 500 mg tablets daily, and the IBP received three 400 mg ibuprofen tablets daily. Four outcomes were measured, namely VAS, gelling pain, joint swelling measurements, and joint motion slope measurements. Dunn’s test for multiple comparisons showed significant differences in these parameters between the GE versus PL and the IBP versus PL groups but not significant differences in the GE versus IBP groups. The improvements in the 4 parameters were roughly similar between the 2 groups showing ginger’s equivalence in treating chronic OA pain. In another study by Paramdeep, 61 60 patients with established knee OA were divided into 3 groups, 20 patients each. Group I was provided diclofenac 50 mg and placebo capsule, group II received a capsule of ginger 750 mg and placebo capsule, and group III received 750 mg ginger capsule and 50 mg diclofenac for 12 weeks with WOMAC and VAS scores assessed every 2 weeks. In terms of WOMAC scores, with group I, a 74.83% improvement was found; with group II, a 63.68% improvement was found; and with group III, a 79.43% improvement was found. Furthermore, no major statistically significant differences were found in adverse events between the groups. It was concluded that ginger has beneficial effects for patients with OA, with the study finding that ginger can reduce the symptoms of OA with a favorable side-effect profile. In another study by Tosun et al, 66 improvements in WOMAC scores were found 5 weeks after using daily ginger massages in established patients with knee OA. Improvements in total WOMAC scores were noted in the self-massage group (12.13 → 8.31) compared with the standard treatment group (14.02 → 12.53), each consisting of 34 patients. This study concluded that massage treatments with ginger oil can act as an adjuvant to standard OA treatment.
From our analysis and review of ginger root, it can be seen that ginger appears to have significant effects on reducing the pain indices of patients with OA, and there is literature evidence for the effects on a cellular level through inhibition of COX and LOX and other cytokines including TNF-α and IL-1β.
Ascorbic Acid
Ascorbic acid or vitamin C is a water-soluble vitamin that cannot be synthesized by the body and instead is taken up from diet. It is rich in foods such as oranges, broccoli, and potatoes to name a few and is a crucial component for 15 mammalian enzymes. It accomplishes its enzymatic support by being an electron donor, which also accounts for its physiological effects.67,68 When it loses one electron, the product left is an ascorbate radical, an unstable radical species. When another electron is lost, a more stable species called dehydroascorbic acid is formed. Plasma and tissue concentrations are dependent on consumption, excretion (primarily through the kidneys), utilization, and its availability biologically. 69 Deficiencies can leave the body with devastating effects as ascorbic acid is a key player in collagen production. The most well-known pathology associated with significant deficiency is scurvy, a disease defined by bruising and bleeding due to lack of adequate collagen production. Although this disease has been known about since ancient times, scurvy has caused quite a few large-scale deaths in the past 500 years as exploration boomed and people were on ships for long periods of time following poor diets. During the 18th century, James Lind found that citrus fruits could cure scurvy in what may have been the first controlled clinical trial.67,69 However, it would not be until several decades later that vitamin c would be used to remedy scurvy. As far as the scientific community was concerned at that time, the causation of scurvy had no concept of some nutritional deficiency. It was not until 1928 when Albert Szent-Gyorgyi first isolated ascorbic acid and until 1932 when it was shown to be the antiscorbutic factor by Szent-Gyorgyi and King.70,71
As mentioned above, vitamin C is crucial for collagen synthesis by acting as a cofactor for 2 enzymes essential for collagen synthesis: prolyl hydroxylase, which stabilizes the collagen molecule, and lysyl hydroxylase, which gives structural strength cross-linking. By producing collagen, the human body strengthens its tendons and ligaments which provide additional structural support for its joints. 72 More recently, studies have shown that vitamin C plays a key role in reducing inflammation in the body. One study conducted showed that daily supplementation vitamin C 500 mg twice a day could reduce inflammatory profile by reducing high-sensitivity CRP and IL-6 in hypertensive and diabetic obese patients. 73 Interestingly, there is evidence that vitamin c levels are often depressed in individuals with active inflammation.74 -77
Even more recently, some studies have suggested vitamin c may play a role in OA. One study was performed where researchers treated a chondrosarcoma line with monosodium iodoacetate (MIA) which not only inhibited cell growth but also increased oxidative stress, proteoglycan loss, and apoptosis of cells. At the center, they found increased pro-inflammatory markers IL-6, interleukin 17A (IL-17A), and TNF-α along with increased MMPs. 78 All of these changes were prevented with treatment of 100 µM vitamin C. The same researchers took things one step further and experimented in animal models where MIA was injected intra-articularly to mimic degradation seen in OA. They found vitamin C lessened these degradative changes but only at the 100 µM level. 78 Another study implemented an experimental design with 2 groups; one treated with placebo (collagen peptide and hyaluronic acid) and the other treated with the intervention (collagen peptides, hyaluronic acid, and vitamin C). There was a total of 120 human patients with 60 patients in each group. Researchers used a VAS from 0 to 10 with higher levels meaning greater reported pain. After 6 and 12 months, the experimental group with vitamin supplementation was found to have lower scores both times (P < .05). In addition, the same group reported better quality of life and less use of painkillers compared with the placebo. 79
Vitamin D
Vitamin D is a fat-soluble vitamin that structurally is a steroid. Vitamin D insufficiency affects approximately 50% of the population worldwide. As the level of melanin increases in an individual, so does the sun exposure required for adequate vitamin D production. 80 Vitamin D is unique in that, naturally, it is made in the skin following exposure to sunlight. The vitamin D from the skin or diet is not active and must be first hydroxylated in the liver where 25(OH)D is formed. It still requires further hydroxylation in the kidneys where the active form of vitamin D, 1,25(OH)2D, is formed. This active form of vitamin D or calcitriol (structure depicted in Figure 4) importantly plays a crucial role in calcium absorption and thus plays an important role in bone health. 80 One study performed a combined analysis on 12 fracture-prevention trials and showed that supplementation with 800 IU of vitamin D daily reduced nonspinal and hip fractures by 20% with no significant benefit when using 400 IU of vitamin D daily. 81
Figure 4.
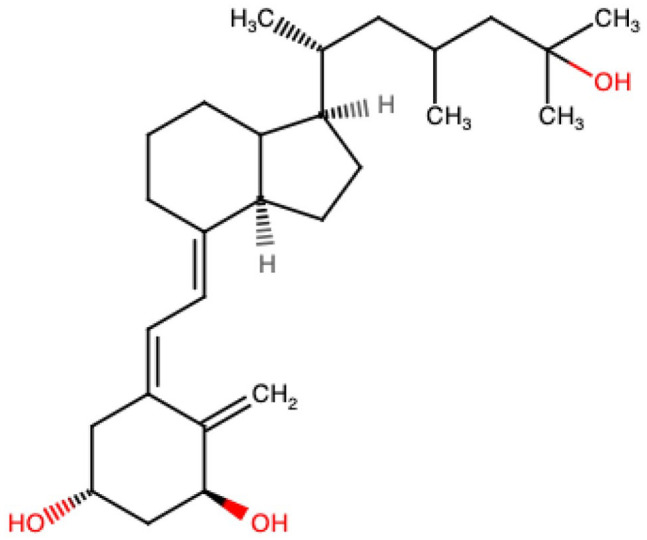
Structure of the active form of vitamin D or calcitriol.a
aThis is an original figure produced using the ChemSPACE website 19 (https://chem-space.com/search).
Vitamin D also plays a role in a wide array of biological functions ranging from inhibiting cellular proliferation to stimulating macrophage cathelicidin production. 80 The use of vitamin D to treat a wide array of illnesses has been in effect for years now and for good reason. Vitamin D deficiency is linked with autoimmune disease, cardiovascular disease, and musculoskeletal decline to just list a few. 82 Vitamin D’s role in cartilage health and OA is unclear, however. Multiple studies have shown that vitamin D receptors are upregulated on damaged cartilage. 83 Vitamin D binding to its receptor activates a signaling cascade. One study showed that chondrocytes will express increased metal metalloproteinases (MMP) 1, 3, and 9 in vivo which ultimately leads to faster bone degradation.83,84 When the joint degenerates, the body’s response is to upregulate vitamin D signaling in the arthritic joint to help spread contact pressures in the joint across new bone formation or osteophytes. It is not clear whether this is a positive or negative response. 85
In terms of OA and vitamin D deficiency, several studies have shown similar results. One prospective study showed that when individuals with similar characteristics and vitamin D deficiencies were assessed radiographically, those individuals had an increased risk of OA of the knee. A cross-sectional study which had controls that were age-matched showed similar results but in patients younger than 60.86,87 Interestingly, some studies found that individuals with OA had significantly decreased serum 25(OH)D levels. Bassiouni et al 88 and Veronese et al 89 similarly showed that this was true when pertaining to knee OA. A cross-sectional study by Jansen and Haddad showed increased prevalence of vitamin D deficiency in elderly patients with advanced knee OA further supporting this point. 90
However, vitamin D supplementation in the prevention of OA has yielded inconclusive results. One study showed that there was an increased risk of hip arthroplasty specifically in men inflicted with OA and increased vitamin D serum concentrations. 90 A 2-year randomized controlled trial including 413 individuals showed, when compared with previously vitamin D–deficient patients, individuals who received monthly vitamin D doses did not have significant clinical or cartilage volume differences. 91 In a separate double-blind, randomized placebo-controlled trial, there was no significant difference in medial joint space narrowing of the knees in individuals that were supplemented with vitamin D versus individuals that were not (placebo). 92
Boswellia
Boswellia serrata is a plant that produces Indian frankincense. The resin of the plant has been used as incense in religious and cultural ceremonies in those areas for thousands of years. It is a moderate-sized tree that grows predominantly in the dry mountainous regions of North Africa, India, and the Middle East. Initially, it is an oleo-gum resin material that is its raw product; special steps are followed that include storage in bamboo to separate the oil content from the resin. 93 The resinous part contains a series of terpenes including monoterpenes, diterpenes, and triterpenes as well as some acids. The major acids contained in the resin are 4 triterpenic acids: β-boswellic acid, acetyl-β-boswellic acid, 11-keto-β-boswellic acid, and acetyl-11-keto-β-boswellic acid. Interestingly, these 4 acids are responsible for inhibiting pro-inflammatory enzymes. In particular, acetyl-11-keto-β-boswellic acid (chemical structure depicted in Figure 5) is an extremely potent inhibitor of 5-lipooxygenase, a key enzyme involved in the inflammatory cascade. 93 This particular acid has shown significant results in several inflammatory conditions such as rheumatoid arthritis, asthma, and inflammatory bowel disease.94,95 Several clinical studies have shown that boswellia is not only effective at treating inflammation and arthritis but also has positive effects in patient-reported outcome measures such as improvements in pain and physical function.96 -99
Figure 5.

Chemical structure of acetyl-11-keto-β-boswellic acid, a potent inhibitor of 5-lipooxygenase.a,b
aThis is an original figure produced utilizing the ChemSPACE 19 website (https://chem-space.com/search) drawing tool.
bElements from the National Center for Biotechnology Information 100 website (PubChem. Acetyl-11-keto-beta-boswellic acid. Accessed August 7, 2021) were used to produce this figure.
A systematic review by Yu et al explored the effectiveness of boswellia as an alternative form of treatment for OA. They collected data from 7 randomized controlled trials that included a grand total of 545 patients. They showed that compared with control groups, boswellia and its extract improved pain (weighted mean difference of −8.33 on the VAS), improved stiffness (weighted mean difference of −10.04 on WOMAC), and improved joint function (weighted mean difference of −10.75 on WOMAC). All of the data were statistically significant with use of boswellia or its extract for at least 4 weeks. 101 Therefore, it appears boswellia is a more than adequate alternative to treating OA.
Discussion
Osteoarthritis is one of the leading causes of disability universally. As a widespread chronic disease, it affects virtually anyone, correlating with 3% years of living with disability worldwide.102,103 For this reason, several pharmacologic treatments have been developed from intra-articular corticosteroid injections to oral nonsteroidal inflammatory drugs to more natural treatments like capsaicin, which is the active ingredient in different hot peppers.102,103 However, some of these treatments have significant side effects that might sway a clinician from prescribing said drug or administering said medication. For example, NSAIDs are highly effective for reducing inflammation and play a key role in OA treatment but can cause GI bleeds, cardiovascular side effects, and kidney toxicity. 104 This is because the very enzymes they inhibit to reduce inflammation are also involved in other systems of the body. For example, NSAIDs work by inhibiting both cyclooxygenase 1 and 2 (COX-1 and COX-2), enzymes crucial for the inflammatory cascade. However, inhibition of COX-1 reduces prostaglandins responsible for gastric mucosal protection through vasodilation, stimulation, and secretion of mucus and bicarbonate from the gastric and duodenal mucosa. 105 This can leave the mucosa vulnerable to ischemia and can set the stage for possible ulcer formation and bleeding.
Intra-articular injections, although very beneficial at times, also come with their own set of side effects. Designed to be locally acting, intra-articular joint injections can have both local and systemic side effects. A study by Anderson et al included 1708 patients from 3 different regional hospitals from the years 2000 to 2016 that received either ankle or talar joint injections. Of the 1708 patients, 99 patients or 5.8% experienced side effects within 90 days of injection. In all, 78 of the affected patients experienced postinjection flare and 10 of the affected patients experienced a skin rash. There were no postinjection infections seen in the cohort studied. 106 This does not mean more serious side effects do not exist. Some of these more serious complications include but are not limited to subchondral insufficiency fractures, osteonecrosis, rapid joint destruction, and, ironically, accelerated OA progression.106 -109
Often, patients can fail a specific treatment, fail a treatment regimen, or experience side effects as listed above. This has sparked a growing interest in more natural products to help reduce inflammation, something directly applicable to OA. A study by Cameron et al looked at 35 studies evaluating the effectiveness of herbal medicinal products. They found topical capsaicin, avocado-soybean unsaponifiables, and a Chinese herbal mixture called SKI306X provided benefit in alleviating OA-related pain. 110 More recently, a clinical trial study by Sharkey et al investigated Synogesic, a nutritional supplement consisting of vitamin C, vitamin D, rutin, boswellia, turmeric, and ginger root, and its effect on patients with knee OA. A total of 43 individuals were split into 2 groups, one that received the nutritional supplement and one that received safflower oil placebo. After 12 weeks, researchers concluded that OA-related ailments improved based on patient-reported outcome measures. 12
Researchers also found during the study period that 26 pro-inflammatory markers out of 41 decreased for the nutritional supplement group, whereas only 13 pro-inflammatory markers out of 41 decreased for the PG. Interestingly, synovial fluid inflammatory markers overall increased by 6.2% for the group that received nutritional supplement and 54.6% for the group that received placebo. 12 Prior to starting the study, patients were instructed to stop taking all conventional NSAIDs. With the increase in inflammatory markers from baseline in both the experimental group and PG following the conclusion of the study, one may assume that the other inflammatory markers that did not decrease following the study actually increased drastically to account for the increase from baseline. 12 Assuming the 6.2% increase was significant, one may assume NSAIDs are more efficacious when compared with the nutritional supplement in reducing inflammation associated with OA as inflammatory markers were increased from baseline conventional NSAID use. However, the researchers reported that as patients were instructed to stop NSAIDs just prior to starting the trial, the recent use of NSAIDs may have affected baseline inflammatory marker level. Rebound inflammation that could have occurred abruptly after stopping NSAIDs could also be a thought. Nonetheless, the side-effect profile for the nutritional supplement is little to none when compared with conventional NSAIDs.
Patients in the Synogesic study still reported improvement across all patient-reported outcome measures. This makes sense as most of the ingredients that make up this product are anti-inflammatory in nature. The slight increase from baseline in synovial fluid pro-inflammatory markers raises some questions; this could be due to recent NSAID use prior to the study as the authors said or it could be that it is less efficacious (assuming the 6.2% increase was significant). If it be the latter, it still has been shown to be quite effective when compared with using no useful intervention as suggested by Sharkey et al 12 and their use of safflower as a placebo. Also, the nutritional supplement includes some ingredients that can slow progressive degenerative changes. Both facts justify its use in patients. For example, as mentioned above, turmeric’s active ingredient is curcumin which has been shown to inhibit early degenerative changes. It does this by inhibiting IL-1β and antagonizing the suppression of collagen type II along with β1-integrin synthesis. By doing this it has shown to exert anti-apoptotic and anticatabolic effects on chondrocytes, preventing articular degeneration by OA. 25 At this time, the use of the nutritional supplement can be in conjunction with NSAIDs producing a synergistic effect in reducing inflammation and treating OA. Perhaps, it can also decrease the dose of NSAIDs patients use which could ultimately decrease adverse side effects seen in patients with chronic or increased use.
Conclusions
Overall, Synogesic and NSAIDs appear to be effective at treating inflammation associated with OA. The patient-reported outcome measures in the Synogesic trial were all positive. This along with the significantly smaller increase in pro-inflammatory markers as compared with the placebo used in the study should warrant its use if not by itself then definitely as an adjunct to NSAIDs. The question still remains whether NSAIDs are superior to the nutritional supplement or vice versa as there were some limitations to the study by Sharkey et al. At this time, there is a need for basic scientific evidence for the mechanism of action and effects for this supplement in OA models (laboratory or animals). In the future, larger studies specifically looking at pro-inflammatory markers along with further research comparing Synogesic and conventional NSAIDs head on in both animals and a laboratory setting could provide more evidence for additional claims.
Footnotes
Article Classification: Biological Science—Medical Sciences
Author Contributions: KTB and NSM conducted the narrative review and drafted the manuscript, with support from RK. JG, JL, SB, and RK contributed to revising the manuscript. RK also provided feedback, guidance, and supervision.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Krish Tejas Bharat  https://orcid.org/0000-0002-8983-6909
https://orcid.org/0000-0002-8983-6909
References
- 1. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States, part II. Arthritis Rheum. 2008;58:26-35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shane Anderson A, Loeser RF. Why is osteoarthritis an age-related disease? Best Pract Res Clin Rheumatol. 2010;24:15-26. doi: 10.1016/j.berh.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. DiPiro JT, ed. Pharmacotherapy: A Pathophysiologic Approach. 7th ed. New York, NY: McGraw-Hill Medical; 2008. [Google Scholar]
- 4. Buckwalter JA, Mankin HJ. Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr Course Lect. 1998;47:487-504. [PubMed] [Google Scholar]
- 5. Garnero P, Delmas PD. Biomarkers in osteoarthritis. Curr Opin Rheumatol. 2003;15:641-646. doi: 10.1097/00002281-200309000-00020. [DOI] [PubMed] [Google Scholar]
- 6. Felson DT. Risk factors for osteoarthritis: understanding joint vulnerability. Clin Orthop Relat Res. 2004:S16-S21. doi: 10.1097/01.blo.0000144971.12731.a2. [DOI] [PubMed] [Google Scholar]
- 7. Singhal S, Hasan N, Nirmal K, et al. Bioavailable turmeric extract for knee osteoarthritis: a randomized, non-inferiority trial versus paracetamol. Trials. 2021;22:105. doi: 10.1186/s13063-021-05053-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mobasheri A, Henrotin Y, Biesalski H-K, Shakibaei M. Scientific evidence and rationale for the development of curcumin and resveratrol as nutraceutricals for joint health. Int J Mol Sci. 2012;13:4202-4232. doi: 10.3390/ijms13044202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. American Pain Society. Guideline for Management of Pain in Osteoarthritis, Rheumatoid Arthritis, and Juvenile Chronic Arthritis. 2nd ed. Glenview, IL: American Pain Society; 2002. [Google Scholar]
- 10. Zhang W, Jones A, Doherty M. Does paracetamol (acetaminophen) reduce the pain of osteoarthritis? a meta-analysis of randomised controlled trials. Ann Rheum Dis. 2004;63:901-907. doi: 10.1136/ard.2003.018531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64:1697-1707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sharkey P, Shah Z, Gross M, Meade T, Davidoff S, Parvizi J. The effect of a natural oral nutritional supplement on the level of intra-articular inflammatory mediators in patients with osteoarthritis of the knee. J Orthop Exp Innov. https://journaloei.scholasticahq.com/article/22282-the-effect-of-a-natural-oral-nutritional-supplement-on-the-level-of-intra-articular-inflammatory-mediators-in-patients-with-osteoarthritis-of-the-knee. Updated 2021.
- 13. Krinsky DL, Ferreri SP, Hemstreet B, et al. Handbook of Nonprescription Drugs: An Interactive Approach to Self-Care. 19th ed. Washington, DC: The American Pharmacists Association; 2017. doi: 10.21019/9781582122656. [DOI] [Google Scholar]
- 14. Theiler R, Spielberger J, Bischoff HA, Bellamy N, Huber J, Kroesen S. Clinical evaluation of the WOMAC 3.0 OA Index in numeric rating scale format using a computerized touch screen version. Osteoarthritis Cartilage. 2002;10:479-481. doi: 10.1053/joca.2002.0807. [DOI] [PubMed] [Google Scholar]
- 15. Volpe SL. Turmeric and osteoarthritis. Acsms Health Fit J. 2018;22:31-32. doi: 10.1249/FIT.0000000000000380. [DOI] [Google Scholar]
- 16. McClatchey W. Traditional use of Curcuma longa (Zingiberaceae) in Rotuma. Econ Bot USA. https://agris.fao.org/agris-search/search.do?recordID=US9423282. Updated 1993. Accessed August 6, 2021.
- 17. Turmeric. NCCIH. https://www.nccih.nih.gov/health/turmeric. Accessed August 6, 2021.
- 18. PubChem. Curcumin. https://pubchem.ncbi.nlm.nih.gov/compound/969516. Accessed August 6, 2021.
- 19. Chemical search—draw chemical structure. Chemspace. https://chem-space.com/search. Accessed August 25, 2021.
- 20. Amalraj A, Pius A, Gopi S, Gopi S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—a review. J Tradit Complement Med. 2017;7:205-233. doi: 10.1016/j.jtcme.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Paultre K, Cade W, Hernandez D, Reynolds J, Greif D, Best TM. Therapeutic effects of turmeric or curcumin extract on pain and function for individuals with knee osteoarthritis: a systematic review. BMJ Open Sport Exerc Med. 2021;7:e000935. doi: 10.1136/bmjsem-2020-000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Plummer SM, Holloway KA, Manson MM, et al. Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF-kappaB activation via the NIK/IKK signalling complex. Oncogene. 1999;18:6013-6020. doi: 10.1038/sj.onc.1202980. [DOI] [PubMed] [Google Scholar]
- 23. Onodera S, Kaneda K, Mizue Y, Koyama Y, Fujinaga M, Nishihira J. Macrophage migration inhibitory factor up-regulates expression of matrix metalloproteinases in synovial fibroblasts of rheumatoid arthritis. J Biol Chem. 2000;275:444-450. doi: 10.1074/jbc.275.1.444. [DOI] [PubMed] [Google Scholar]
- 24. Liacini A, Sylvester J, Li WQ, Zafarullah M. Inhibition of interleukin-1-stimulated MAP kinases, activating protein-1 (AP-1) and nuclear factor kappa B (NF-kappa B) transcription factors down-regulates matrix metalloproteinase gene expression in articular chondrocytes. Matrix Biol. 2002;21:251-262. doi: 10.1016/s0945-053x(02)00007-0. [DOI] [PubMed] [Google Scholar]
- 25. Shakibaei M, Schulze-Tanzil G, John T, Mobasheri A. Curcumin protects human chondrocytes from IL-l1beta-induced inhibition of collagen type II and beta1-integrin expression and activation of caspase-3: an immunomorphological study. Ann Anat. 2005;187:487-497. doi: 10.1016/j.aanat.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 26. Cho M-L, Jung YO, Moon Y-M, et al. Interleukin-18 induces the production of vascular endothelial growth factor (VEGF) in rheumatoid arthritis synovial fibroblasts via AP-1-dependent pathways. Immunol Lett. 2006;103:159-166. doi: 10.1016/j.imlet.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 27. Ross S. Turmeric (Curcuma longa): effects of Curcuma longa extracts compared with ibuprofen for reduction of pain and functional improvement in patients with knee osteoarthritis. Holist Nurs Pract. 2016;30:183-186. doi: 10.1097/HNP.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 28. Panahi Y, Rahimnia A-R, Sharafi M, Alishiri G, Saburi A, Sahebkar A. Curcuminoid treatment for knee osteoarthritis: a randomized double-blind placebo-controlled trial. Phytother Res. 2014;28:1625-1631. doi: 10.1002/ptr.5174. [DOI] [PubMed] [Google Scholar]
- 29. Bharti AK, Singh A, Singh SK, Katiyar D, Sachan AK. A comparison of efficacy of tocotrienol and turmeric (Curcuma longa) in knee osteoarthritis. Int J Health Clin Res. 2021;4:128-131. [Google Scholar]
- 30. Calderón-Pérez L, Llauradó E, Companys J, et al. Acute effects of turmeric extracts on knee joint pain: a pilot, randomized controlled trial. J Med Food. 2021;24:436-440. doi: 10.1089/jmf.2020.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Babu PVA, Liu D. Chapter 18—flavonoids and cardiovascular health. In: Watson RR, ed. Complementary and Alternative Therapies and the Aging Population. Amsterdam, The Netherlands: Academic Press; 2009:371-392. doi: 10.1016/B978-0-12-374228-5.00018-4. [DOI] [Google Scholar]
- 32. Gulpİnar AR, Orhan IE, Kan A, Senol FS, Celİk SA, Kartal M. Estimation of in vitro neuroprotective properties and quantification of rutin and fatty acids in buckwheat (Fagopyrum esculentum Moench) cultivated in Turkey. Food Res Int. 2012;46:536-543. [Google Scholar]
- 33. International Symposium on Buckwheat, Matano T, Ujihara A. Shinshu University IBRA (IBRA). Current Advances in Buckwheat Research: Proceedings of the 6th International Symposium on Buckwheat in Shinshu, August 24-29, 1995. Nagano, Japan: Shinshu University Press; 1995. [Google Scholar]
- 34. Chua LS. A review on plant-based rutin extraction methods and its pharmacological activities. J Ethnopharmacol. 2013;150:805-817. doi: 10.1016/j.jep.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 35. van der Watt E, Pretorius JC. Purification and identification of active antibacterial components in Carpobrotus edulis L. J Ethnopharmacol. 2001;76:87-91. doi: 10.1016/s0378-8741(01)00197-0. [DOI] [PubMed] [Google Scholar]
- 36. Soares MS, da Silva DF, Forim MR, et al. Quantification and localization of hesperidin and rutin in Citrus sinensis grafted on C. limonia after Xylella fastidiosa infection by HPLC-UV and MALDI imaging mass spectrometry. Phytochemistry. 2015;115:161-170. doi: 10.1016/j.phytochem.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 37. Hollman PC, van Trijp JM, Mengelers MJ, de Vries JH, Katan MB. Bioavailability of the dietary antioxidant flavonol quercetin in man. Cancer Lett. 1997;114:139-140. doi: 10.1016/s0304-3835(97)04644-2. [DOI] [PubMed] [Google Scholar]
- 38. Sakanashi Y, Oyama K, Matsui H, et al. Possible use of quercetin, an antioxidant, for protection of cells suffering from overload of intracellular Ca2+: a model experiment. Life Sci. 2008;83:164-169. doi: 10.1016/j.lfs.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 39. Djelili H, Arrar L, Naline E, Devillier P. Relaxant effects of quercetin and rutin on human isolated bronchus. 2012;2012. doi: 10.4236/cm.2012.32015. [DOI] [Google Scholar]
- 40. Magalingam KB, Radhakrishnan A, Haleagrahara N. Rutin, a bioflavonoid antioxidant protects rat pheochromocytoma (PC-12) cells against 6-hydroxydopamine (6-OHDA)-induced neurotoxicity. Int J Mol Med. 2013;32:235-240. doi: 10.3892/ijmm.2013.1375. [DOI] [PubMed] [Google Scholar]
- 41. Song K, Kim S, Na J-Y, et al. Rutin attenuates ethanol-induced neurotoxicity in hippocampal neuronal cells by increasing aldehyde dehydrogenase 2. Food Chem Toxicol. 2014;72:228-233. doi: 10.1016/j.fct.2014.07.028. [DOI] [PubMed] [Google Scholar]
- 42. Picot D, Loll PJ, Garavito RM. The X-ray crystal structure of the membrane protein prostaglandin H2 synthase-1. Nature. 1994;367:243-249. doi: 10.1038/367243a0. [DOI] [PubMed] [Google Scholar]
- 43. Guardia T, Rotelli AE, Juarez AO, Pelzer LE. Anti-inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Farmaco. 2001;56:683-687. doi: 10.1016/s0014-827x(01)01111-9. [DOI] [PubMed] [Google Scholar]
- 44. Han Y. Rutin has therapeutic effect on septic arthritis caused by Candida albicans. Int Immunopharmacol. 2009;9:207-211. doi: 10.1016/j.intimp.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 45. Blanco FJ, Ochs RL, Schwarz H, Lotz M. Chondrocyte apoptosis induced by nitric oxide. Am J Pathol. 1995;146:75-85. [PMC free article] [PubMed] [Google Scholar]
- 46. Kauss T, Moynet D, Rambert J, et al. Rutoside decreases human macrophage-derived inflammatory mediators and improves clinical signs in adjuvant-induced arthritis. Arthritis Res Ther. 2008;10:R19. doi: 10.1186/ar2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Horcajada M-N, Sanchez C, Membrez Scalfo F, et al. Oleuropein or rutin consumption decreases the spontaneous development of osteoarthritis in the Hartley guinea pig. Osteoarthritis Cartilage. 2015;23:94-102. doi: 10.1016/j.joca.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 48. Na J-Y, Song K, Kim S, Kwon J. Rutin protects rat articular chondrocytes against oxidative stress induced by hydrogen peroxide through SIRT1 activation. Biochem Biophys Res Commun. 2016;473:1301-1308. doi: 10.1016/j.bbrc.2016.04.064. [DOI] [PubMed] [Google Scholar]
- 49. Klein G, Kullich W, Schnitker J, Schwann H. Efficacy and tolerance of an oral enzyme combination in painful osteoarthritis of the hip. A double-blind, randomised study comparing oral enzymes with non-steroidal anti-inflammatory drugs. Clin Exp Rheumatol. 2006;24:25-30. [PubMed] [Google Scholar]
- 50. Therkleson T. Ginger and osteoarthritis [published online ahead of print January 1, 2012]. ECU Publ. doi: 10.5772/26274. [DOI] [Google Scholar]
- 51. Lai J-N, Chen H-J, Chen C-C, Lin J-H, Hwang J-S, Wang J-D. Duhuo Jisheng Tang for treating osteoarthritis of the knee: a prospective clinical observation. Chin Med. 2007;2:4. doi: 10.1186/1749-8546-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. The complete German Commission E Monographs: therapeutic guide to herbal medicines. Ann Inter Med. https://www.acpjournals.org/doi/10.7326/0003-4819-130-5-199903020-00024. Accessed August 7, 2021.
- 53. Castleman M. The New Healing Herbs. Penguin Random House. https://www.penguinrandomhouse.com/books/598147/the-new-healing-herbs-by-michael-castleman/. Accessed August 7, 2021. [Google Scholar]
- 54. Ferry-Swainson K, Brown D. Ginger (The Herb Library Series). 1st ed. Clarendon, VA: Turtle Pub. [Google Scholar]
- 55. West C, Kim E. A computational study for optimizing a synthetic pathway to a difluorinated gingerol compound. https://www.methodist.edu/wp-content/uploads/2018/09/mr2017_west2.pdf. Updated 2018.
- 56. Lee HY, Park SH, Lee M, et al. 1-Dehydro-[10]-gingerdione from ginger inhibits IKKβ activity for NF-κB activation and suppresses NF-κB-regulated expression of inflammatory genes. Br J Pharmacol. 2012;167:128-140. doi: 10.1111/j.1476-5381.2012.01980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Naderi Z, Mozaffari-Khosravi H, Dehghan A, Nadjarzadeh A, Huseini HF. Effect of ginger powder supplementation on nitric oxide and C-reactive protein in elderly knee osteoarthritis patients: a 12-week double-blind randomized placebo-controlled clinical trial. J Tradit Complement Med. 2015;6:199-203. doi: 10.1016/j.jtcme.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mozaffari-Khosravi H, Naderi Z, Dehghan A, Nadjarzadeh A, Fallah Huseini H. Effect of ginger supplementation on proinflammatory cytokines in older patients with osteoarthritis: outcomes of a randomized controlled clinical trial. J Nutr Gerontol Geriatr. 2016;35:209-218. doi: 10.1080/21551197.2016.1206762. [DOI] [PubMed] [Google Scholar]
- 59. Dingle JT. Cartilage maintenance in osteoarthritis: interaction of cytokines, NSAID and prostaglandins in articular cartilage damage and repair. J Rheumatol Suppl. 1991;28:30-37. [PubMed] [Google Scholar]
- 60. Mustafa T, Srivastava KC, Jensen KB. Drug development report. 9. Pharmacology of ginger, zingiber-officinale. J Drug Dev;6:25-39. [Google Scholar]
- 61. Paramdeep G. Efficacy and tolerability of ginger (Zingiber officinale) in patients of osteoarthritis of knee. Indian J Physiol Pharmacol. 2013;57:177-183. [PubMed] [Google Scholar]
- 62. Tjendraputra E, Tran VH, Liu-Brennan D, Roufogalis BD, Duke CC. Effect of ginger constituents and synthetic analogues on cyclooxygenase-2 enzyme in intact cells. Bioorg Chem. 2001;29:156-163. doi: 10.1006/bioo.2001.1208. [DOI] [PubMed] [Google Scholar]
- 63. Grzanna R, Lindmark L, Frondoza CG. Ginger—an herbal medicinal product with broad anti-inflammatory actions. J Med Food. 2005;8:125-132. doi: 10.1089/jmf.2005.8.125. [DOI] [PubMed] [Google Scholar]
- 64. Sertie J, Basile A, Oshiro T, Silva F, Mazella A. Preventive anti-ulcer activity of the rhizome extract of Zingiber officinale. Fitoterapia. 1992;63:55-59. [Google Scholar]
- 65. Rayati F, Hajmanouchehri F, Najafi E. Comparison of anti-inflammatory and analgesic effects of Ginger powder and Ibuprofen in postsurgical pain model: a randomized, double-blind, case–control clinical trial. Dent Res J (Isfahan). 2017;14:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tosun B, Unal N, Yigit D, Can N, Aslan O, Tunay S. Effects of self-knee massage with ginger oil in patients with osteoarthritis: an experimental study. Res Theory Nurs Pract. 2017;31:379-392. doi: 10.1891/1541-6577.31.4.379. [DOI] [PubMed] [Google Scholar]
- 67. Padayatty SJ, Levine M. Vitamin C: the known and the unknown and Goldilocks. Oral Dis. 2016;22:463-493. doi: 10.1111/odi.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chen Q, Espey MG, Sun AY, et al. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc Natl Acad Sci USA. 2007;104:8749-8754. doi: 10.1073/pnas.0702854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Englard S, Seifter S. The biochemical functions of ascorbic acid. Annu Rev Nutr. 1986;6:365-406. doi: 10.1146/annurev.nu.06.070186.002053. [DOI] [PubMed] [Google Scholar]
- 70. Draper MH. Lind’s treatise on scurvy. A bicentenary volume containing a reprint of the First Edition of “A Treatise of the Scurvy” by James Lind, M.D., with Additional Notes. Edited by Stewart C. P., Guthrie Douglas. Edinburgh: University Press. 1953. Pp. 440. With a portrait, three plates, and a genealogy. Q J Exp Physiol Cogn Med Sci. 1953;38:201-202. doi: 10.1113/expphysiol.1953.sp001028. [DOI] [Google Scholar]
- 71. Svirbely JL, Szent-Györgyi A. The chemical nature of vitamin C. Biochem J. 1932;26:865-870. doi: 10.1042/bj0260865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Boyera N, Galey I, Bernard BA. Effect of vitamin C and its derivatives on collagen synthesis and cross-linking by normal human fibroblasts. Int J Cosmet Sci. 1998;20:151-158. doi: 10.1046/j.1467-2494.1998.171747.x. [DOI] [PubMed] [Google Scholar]
- 73. Ellulu MS, Rahmat A, Patimah I, Khaza’ai H, Abed Y. Effect of vitamin C on inflammation and metabolic markers in hypertensive and/or diabetic obese adults: a randomized controlled trial. Drug Des Devel Ther. 2015;9:3405-3412. doi: 10.2147/DDDT.S83144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bonham MJ, Abu-Zidan FM, Simovic MO, et al. Early ascorbic acid depletion is related to the severity of acute pancreatitis. Br J Surg. 1999;86:1296-1301. doi: 10.1046/j.1365-2168.1999.01182.x. [DOI] [PubMed] [Google Scholar]
- 75. Jiang Q, Lykkesfeldt J, Shigenaga MK, Shigeno ET, Christen S, Ames BN. Gamma-tocopherol supplementation inhibits protein nitration and ascorbate oxidation in rats with inflammation. Free Radic Biol Med. 2002;33:1534-1542. doi: 10.1016/s0891-5849(02)01091-2. [DOI] [PubMed] [Google Scholar]
- 76. Bakaev VV, Duntau AP. Ascorbic acid in blood serum of patients with pulmonary tuberculosis and pneumonia. Int J Tuberc Lung Dis. 2004;8:263-266. [PubMed] [Google Scholar]
- 77. Carr AC, Rosengrave PC, Bayer S, Chambers S, Mehrtens J, Shaw GM. Hypovitaminosis C and vitamin C deficiency in critically ill patients despite recommended enteral and parenteral intakes. Crit Care Lond Engl. 2017;21:300. doi: 10.1186/s13054-017-1891-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chiu P-R, Hu Y-C, Huang T-C, et al. Vitamin C protects chondrocytes against monosodium iodoacetate-induced osteoarthritis by multiple pathways. Int J Mol Sci. 2016;18:38. doi: 10.3390/ijms18010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ripani U, Manzarbeitia-Arroba P, Guijarro-Leo S, Urrutia-Graña J, De Masi-De Luca A. Vitamin C may help to reduce the knee’s arthritic symptoms. Outcomes assessment of nutriceutical therapy. Med Arch. 2019;73:173-177. doi: 10.5455/medarh.2019.73.173-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Nair R, Maseeh A. Vitamin D: the “sunshine” vitamin. J Pharmacol Pharmacother. 2012;3:118-126. doi: 10.4103/0976-500X.95506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bischoff-Ferrari HA, Willett WC, Wong JB, et al. Prevention of nonvertebral fractures with oral vitamin D and dose dependency: a meta-analysis of randomized controlled trials. Arch Intern Med. 2009;169:551-561. doi: 10.1001/archinternmed.2008.600. [DOI] [PubMed] [Google Scholar]
- 82. Haines ST, Park SK. Vitamin D supplementation: what’s known, what to do, and what’s needed. Pharmacotherapy. 2012;32:354-382. doi: 10.1002/phar.1037. [DOI] [PubMed] [Google Scholar]
- 83. Orfanidou T, Malizos KN, Varitimidis S, Tsezou A. 1,25-Dihydroxyvitamin D(3) and extracellular inorganic phosphate activate mitogen-activated protein kinase pathway through fibroblast growth factor 23 contributing to hypertrophy and mineralization in osteoarthritic chondrocytes. Exp Biol Med (Maywood). 2012;237:241-253. doi: 10.1258/ebm.2011.011301. [DOI] [PubMed] [Google Scholar]
- 84. Mabey T, Honsawek S. Role of vitamin D in osteoarthritis: molecular, cellular, and clinical perspectives. Int J Endocrinol. 2015;2015:383918. doi: 10.1155/2015/383918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Garfinkel RJ, Dilisio MF, Agrawal DK. Vitamin D and its effects on articular cartilage and osteoarthritis. Orthop J Sports Med. 2017;5:1-8. doi: 10.1177/2325967117711376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zhang FF, Driban JB, Lo GH, et al. Vitamin D deficiency is associated with progression of knee osteoarthritis. J Nutr. 2014;144:2002-2008. doi: 10.3945/jn.114.193227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Heidari B, Heidari P, Hajian-Tilaki K. Association between serum vitamin D deficiency and knee osteoarthritis. Int Orthop. 2011;35:1627-1631. doi: 10.1007/s00264-010-1186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bassiouni H, Aly H, Zaky K, Abaza N, Bardin T. Probing the relation between vitamin D deficiency and progression of medial femoro-tibial osteoarthitis of the knee. Curr Rheumatol Rev. 2017;13:65-71. doi: 10.2174/1573397112666160404124532. [DOI] [PubMed] [Google Scholar]
- 89. Veronese N, Maggi S, Noale M, et al. Serum 25-hydroxyvitamin D and osteoarthritis in older people: the Progetto Veneto Anziani study. Rejuvenation Res. 2015;18:543-553. doi: 10.1089/rej.2015.1671. [DOI] [PubMed] [Google Scholar]
- 90. Hussain SM, Daly RM, Wang Y, et al. Association between serum concentration of 25-hydroxyvitamin D and the risk of hip arthroplasty for osteoarthritis: result from a prospective cohort study. Osteoarthritis Cartilage. 2015;23:2134-2140. doi: 10.1016/j.joca.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 91. Jin X, Jones G, Cicuttini F, et al. Effect of vitamin D supplementation on tibial cartilage volume and knee pain among patients with symptomatic knee osteoarthritis: a randomized clinical trial. JAMA. 2016;315:1005-1013. doi: 10.1001/jama.2016.1961. [DOI] [PubMed] [Google Scholar]
- 92. Arden NK, Cro S, Sheard S, et al. The effect of vitamin D supplementation on knee osteoarthritis, the VIDEO study: a randomised controlled trial. Osteoarthritis Cartilage. 2016;24:1858-1866. doi: 10.1016/j.joca.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Siddiqui MZ. Boswellia serrata, a potential antiinflammatory agent: an overview. Indian J Pharm Sci. 2011;73:255-261. doi: 10.4103/0250-474X.93507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Singh GB, Atal CK. Pharmacology of an extract of salai guggal ex-Boswellia serrata, a new non-steroidal anti-inflammatory agent. Agents Actions. 1986;18:407-412. doi: 10.1007/BF01965005. [DOI] [PubMed] [Google Scholar]
- 95. Basch E, Boon H, Davies-Heerema T, et al. Boswellia: an evidence-based systematic review by the Natural Standard Research Collaboration. J Herb Pharmacother. 2004;4:63-83. [PubMed] [Google Scholar]
- 96. Kimmatkar N, Thawani V, Hingorani L, Khiyani R. Efficacy and tolerability of Boswellia serrata extract in treatment of osteoarthritis of knee—a randomized double blind placebo controlled trial. Phytomedicine. 2003;10:3-7. doi: 10.1078/094471103321648593. [DOI] [PubMed] [Google Scholar]
- 97. Perera PK, Perera M, Kumarasinghe N. Effect of Sri Lankan traditional medicine and Ayurveda on Sandhigata Vata (osteoarthritis of knee joint). Ayu. 2014;35:411-415. doi: 10.4103/0974-8520.159007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Shah MR, Mehta CS, Shukla VD, Dave AR, Bhatt NN. A Clinical study of Matra Vasti and an ayurvedic indigenous compound drug in the management of Sandhigatavata (Osteoarthritis). Ayu. 2010;31:210-217. doi: 10.4103/0974-8520.72399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Gupta PK, Samarakoon SMS, Chandola HM, Ravishankar B. Clinical evaluation of Boswellia serrata (Shallaki) resin in the management of Sandhivata (osteoarthritis). Ayu. 2011;32:478-482. doi: 10.4103/0974-8520.96119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. PubChem. Acetyl-11-keto-beta-boswellic acid. https://pubchem.ncbi.nlm.nih.gov/compound/6918115. Accessed August 7, 2021.
- 101. Yu G, Xiang W, Zhang T, Zeng L, Yang K, Li J. Effectiveness of Boswellia and Boswellia extract for osteoarthritis patients: a systematic review and meta-analysis. BMC Complement Med Ther. 2020;20:225. doi: 10.1186/s12906-020-02985-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Meccariello L, Fazarano G, Medici A, et al. In the aging knee: which mitigation and intervention strategies do we apply in the Intra-articular Knee Joint Injection? The comparison of the effects of 5 drugs and review of the literature. Open Access. 2015;2:13. [Google Scholar]
- 103. Meccariello L. Intraarticular knee joint injection: hyaluronic acid vs polynucleotides. EuroMediterranean Biomed J. 2013:35-41. doi: 10.3269/1970-5492.2013.8.7. [DOI] [Google Scholar]
- 104. Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018;9:143-150. doi: 10.14336/AD.2017.0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Lanas A, Panés J, Piqué JM. Clinical implications of COX-1 and/or COX-2 inhibition for the distal gastrointestinal tract. Curr Pharm Des. 2003;9:2253-2266. doi: 10.2174/1381612033453992. [DOI] [PubMed] [Google Scholar]
- 106. Anderson SE, Lubberts B, Strong AD, Guss D, Johnson AH, DiGiovanni CW. Adverse events and their risk factors following intra-articular corticosteroid injections of the ankle or subtalar joint. Foot Ankle Int. 2019;40:622-628. doi: 10.1177/1071100719835759. [DOI] [PubMed] [Google Scholar]
- 107. Walker EA, Davis D, Mosher TJ. Rapidly progressive osteoarthritis: biomechanical considerations. Magn Reson Imaging Clin N Am. 2011;19:283-294. doi: 10.1016/j.mric.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 108. Irwin LR, Roberts JA. Rapidly progressive osteoarthrosis of the hip. J Arthroplasty. 1998;13:642-646. doi: 10.1016/s0883-5403(98)80007-7. [DOI] [PubMed] [Google Scholar]
- 109. Lohmander LS, Felson D. Can we identify a “high risk” patient profile to determine who will experience rapid progression of osteoarthritis? 11 supported by the Swedish Research Council (Medicine) and NIH AR47785. Osteoarthritis Cartilage. 2004;12:49-52. doi: 10.1016/j.joca.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 110. Cameron M, Gagnier JJ, Little CV, Parsons TJ, Blümle A, Chrubasik S. Evidence of effectiveness of herbal medicinal products in the treatment of arthritis. Part I: osteoarthritis. Phytother Res. 2009;23:1497-1515. doi: 10.1002/ptr.3007. [DOI] [PubMed] [Google Scholar]