Abstract
Background
Findings of observational studies that evaluated the association of serum vitamin D status and high blood pressure were contradictory. This meta-analysis of epidemiologic studies assessed the relation of serum vitamin D levels to hypertension (HTN) and pre-hypertension in adults.
Methods
We conducted a systematic search of all published articles up to March 2021, in four electronic databases (MEDLINE (PubMed), Web of Science (ISI), Embase and Scopus), and Google scholar. Seventy epidemiologic studies (10 prospective cohort, one nested case–control, and 59 cross-sectional investigations) that reported relative risks (RRs), odds ratios (ORs), hazard ratios, or prevalence ratios with 95% CIs for HTN or pre-hypertension in relation to serum vitamin D concentrations in adults were included in the analysis.
Results
In prospective studies, a 16% decrease in risk of hypertension was observed in participants with high levels of serum vitamin D compared to low levels (RR: 0.84; 95%CI: 0.73, 0.96; 12 effect sizes). Dose–response analysis in prospective studies revealed that each 25 nmol/L increase in serum vitamin D concentrations resulted in 5% reduced risk of HTN (RR: 0.95; 95% CI: 0.90, 1.00). Also, a significant nonlinear relationship between serum vitamin D levels and HTN was found (Pnonlinearity < 0.001). In cross-sectional investigations, highest vs. lowest level of serum vitamin D was related to reduced odds of HTN (OR: 0.84; 95%CI: 0.79, 0.90; 66 effect sizes) and pre-hypertension (OR: 0.75; 0.95%CI: 0.68, 0.83; 9 effect sizes). Dose–response analysis in these studies showed that each 25 nmol/L increase in serum vitamin D levels was related to a significant 6% reduction in odds of hypertension in all populations (RR: 0.94; 95%CI: 0.90, 0.99) and 3% in studies with representative populations (RR: 0.97; 95%CI: 0.95, 0.99).
Conclusion
This meta-analysis of epidemiologic studies disclosed that serum vitamin D concentrations were inversely related to the risk of HTN in adults, in a dose–response manner in both prospective cohort and cross-sectional studies.
Systematic Review Registration: http://www.crd.york.ac.uk/Prospero, identifier: CRD42021251513.
Keywords: serum 25-hydroxy vitamin D, hypertension, pre-hypertension, meta-analysis, epidemiologic studies
Introduction
High blood pressure or hypertension (HTN) has a prominent role in cardiovascular disease (CVD). Systolic blood pressure (SBP) ≥ 130 mmHg or diastolic blood pressure (DBP) ≥ 80 mmHg are the threshold values recently proposed to define hypertension (HTN) (1). Hypertension is one of the most important risk factors for global mortality and morbidity and has been associated with non-communicable diseases such as atherosclerosis, cardiomyopathy, and acute myocardial infarction (2). In addition, HTN increases the risk of stroke, heart attack, and kidney failure, which impose a great economic burden on societies (3, 4). The prevalence of HTN varies in different parts of the world and can be influenced by demographic factors such as age, race, gender, and socioeconomic status (5). For example, its prevalence is reported to be 39.1% in Latin America, 29.4% in South Asia, and 22% in Iran (6, 7).
Several modifiable and non-modifiable risk factors, such as age, gender, genetics, high sodium intake, low potassium intake, obesity, lack of physical activity, and unhealthy diet are involved in increasing blood pressure (BP) (8). Previous studies found that serum vitamin D levels could have an inverse association with BP (9). Vitamin D supplementation was also suggested as a critical approach to preventing hypertensive disorders in pregnancy (10). It has been proved that vitamin D insufficiency has a high prevalence worldwide, even in countries that are located in the lower latitude and in industrialized countries where vitamin D fortified foods are easily accessible (11).
Although several epidemiologic studies investigated the association between circulating vitamin D concentrations and hypertension, the findings were contradictory. Some of the research studies documented that lower vitamin D levels significantly increased the risk of hypertension (12, 13), while others did not find a significant association (14, 15). On the other hand, some investigations reported a lower risk of hypertension in vitamin D-deficient individuals; however, these results were not significant (16, 17). In addition, some studies have reported the inverse relationship between vitamin D level and BP only in women (12), or only in male individuals (14). The findings have additionally remained controversial in the case of definitions used to identify hypertensive subjects and vitamin D-deficient/insufficient individuals (14–19). To our knowledge, there is no systematic review and meta-analysis that summarized the relationship between serum vitamin D concentrations and hypertension in observational studies. So, we objected to evaluating the relationship between serum vitamin D concentrations and hypertension/prehypertension in adults and carried out a systematic review and meta-analysis on epidemiologic studies. We also assessed whether serum vitamin D levels could decrease the risk of HTN in a linear or non-linear fashion. We hypothesized that the optimal level of serum vitamin D could be related to a reduced risk of hypertension and prehypertension.
Methods and Materials
Search Strategy
We conducted a systematic search of all published articles up to April, 2021, in the electronic MEDLINE (PubMed), Web of Science (ISI), Scopus databases, and Google scholar, with no limitation in language or time of publication. Applied MeSH and non-MeSH keywords in the systematic search are presented in detail in Supplementary Table 1. Furthermore, we performed a manual search in bibliographies of the relevant investigations to identify additional studies. Gray literature, including conference proceedings, unpublished articles, and theses, was not included in the present review. Duplicate studies were removed. Then, two investigators (EM and ZH) independently carried out the article selection by title and abstract screening, and any disagreement was resolved by discussion with the principal investigator (PS) to reach a consensus. The full text of potentially relevant articles was obtained to extract data. We conformed to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guideline (PRISMA) (20) in the present analysis and the details are presented in Supplementary Table 2. The study protocol was furthermore registered at Prospero (http://www.crd.york.ac.uk/Prospero; no. CRD42021251513).
Inclusion Criteria
Published studies were included in our analysis if they: (1) were population-based epidemiological studies with cross-sectional, cohort, case-control, or nested case–controls design; (2) conducted on adults ( ≥ 18 years old); (3) considered circulating 25-hydroxy vitamin D levels as the exposure; (4) considered hypertension, high BP, or pre-hypertension as the outcome of interest; 5) reported relative risks (RRs), odds ratios (ORs), hazard ratios (HRs), or prevalence ratios (PRs) and corresponding 95% CIs (or sufficient data for calculating these values) for the association between serum vitamin D levels and hypertension or pre-hypertension.
Exclusion Criteria
Details of more relevant studies that were excluded are reported in Supplementary Table 3. Studies were excluded if they: (1) considered vitamin D deficiency as the outcome and hypertension as the exposure; (2) reported OR/RR for pregnancy-induced hypertension; (3) considered BP as a continuous outcome; (4) considered hypertension severity as the outcome; (5) provided standard regression (ß) coefficient for the relationship; (6) reported correlation coefficient for the linkage; (7) reported the relationship in children and adolescents. Moreover, all editorials, letters, comments, theses, case-reports, and review articles were not included in our review. In addition, Barcelo et al. reported ORs for two different definitions of hypertension (defined as ≥ 130/85 mmHg vs. ≥ 140/90 mmHg), to avoid overlapping of populations, we used one of these ORs (provided for BP ≥ 130/85 mmHg) (18). Moreover, for those investigations that provided ORs for both vitamin D quartile categories and vitamin D deficiency vs. sufficiency (19), we included only the estimate for vitamin D deficiency vs. sufficiency in the analysis.
Data Extraction
Based on a pre-designed table, the following data were extracted from each eligible study: the first author's last name, year of publication, study design, duration of follow-up, location, age range or mean age, gender, number of participants, number of hypertensive cases, 25-hydroxyvitamin D [25(OH)D] levels, unit of serum vitamin D, OR, RR, HR or PR, and 95% CI for the association of vitamin D and hypertension or pre-hypertension, methods of serum vitamin D measurement, cut-off points used to define hypertension, the health status of participants, adjustments for potential confounders, and quality scores of studies. Two researchers (EM and ZH) independently extracted data and the principal investigator (PS) supervised the process.
Quality Assessment of Studies
Newcastle–Ottawa quality assessment scale (NOS) (21) (adapted for cross-sectional and cohort studies) was used to assess the quality of eligible investigations. This scale allocates a total score of 9, as the highest quality, to cohort studies: 4 scores for participant selection (representativeness of the exposed cohort, selection of the non-exposed cohort, and ascertainment of exposure and demonstration that hypertension was not present at the start of the study), 2 scores for comparability (considering controls for the most important factors, including season of blood drawn or sun exposure and additional adjustments for age, gender, and BMI) and 3 scores for outcome assessment (using a validated assessment for hypertension as the outcome, enough follow-up duration for hypertension to incidence and adequate follow-up for cohorts). NOS allocates a total score of 10, as the highest quality, to cross-sectional studies: 5 scores for the selection of participants (representativeness of the sample, sample size satisfaction, explanation for non-respondents, and ascertainment of the exposure assessment), 2 scores for comparability (controlling for the most important factors, including season of blood drawn or sun exposure and additional adjustments for age, gender, and BMI), and 3 scores for outcome assessment (using a validated assessment for hypertension and using an appropriate statistical test). Quality assessment for studies is described in detail in Supplementary Table 4. In this meta-analysis, prospective studies with a score 7 or more and cross-sectional studies with a score of 8 or more were classified as high-quality investigations; those with lower scores were deemed to be low-quality studies. Moreover, Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) (22) was used to determine the quality of evidence through GRADEpro (GRADEproGDT, www.gradepro.org) (23). According to this approach, we examined the main factors that could downgrade the study quality including indirectness of evidence, risk of bias, inconsistency of findings, imprecision of findings, and publication bias. The factors upgrading quality were also included: the evaluation of dose–response analysis, large effect, and plausible confounding. Based on the GRADE approach, the certainty of the body of evidence could be rated in one of four categories: high, moderate, low, and very low. Results of GRADE assessment of the current meta-analysis are presented in Supplementary Tables 5 and 6.
Statistical Analysis
Reported RR, OR, HR or PR, and 95% CI for the relationship between vitamin D and hypertension (HTN) or pre-hypertension were used to calculate log OR or RR and its standard error. For those studies that reported the estimate for the lowest vs. the highest level of serum vitamin D, we converted the OR to have the estimate for the highest vs. the lowest level. The overall effect size was calculated by using a fixed-effect model when heterogeneity was low (I2 < 50%) and a random-effect model, that takes between-study variation into account, when heterogeneity was high (I2 > 50%). We evaluated the between-study heterogeneity through the use of Cochran's Q test and I2. In cases of significant between-study heterogeneity, we used subgroup analysis based on confounders/moderators (such as study location [Asian vs. non-Asian countries], developmental status, gender, levels of vitamin D used for comparison, cut-off points used to define hypertension, the health status of participants, adjustment for the season of blood drawn or sun exposure, additional adjustment for age, and gender and BMI, representativeness of the population, and quality of studies) to explore possible sources of heterogeneity. Between-subgroup heterogeneity was examined through a fixed-effect model. Sensitivity analysis was done to examine the extent to which inferences might depend on a particular study. Publication bias was assessed by visual inspection of funnel plots and formal statistical assessment of funnel plot asymmetry was performed by Begg's test and Egger's regression asymmetry test.
For dose–response analysis, a previously described method by Greenland and Longnecker (24) and Orsini et al. (25) was used. The natural logs of the ORs, RRs, HRs or PRs and 95% CIs across categories of serum vitamin D were used to compute study-specific slopes (linear trends) and 95%CIs for 25 nmol/L (or 10 ng/mL) which is the difference between severe deficiency (<25 nmol/L), deficiency (25–50 nmol/L), insufficiency (50–75 nmol/L), and sufficiency levels (>75 nmol/L) of serum vitamin D. In this method, the distribution of individuals with hypertension and the OR/RR/HR/PR with the variance estimates for at least three quantitative categories of exposure for non-linear trends were required. The mean or median level of serum vitamin D in each category was assigned to the corresponding OR/RR/HR/PR for each study. For studies that reported the serum 25(OH) D levels as ranges, we estimated the midpoint in each category by calculating the average of the lower and upper bounds. When the highest category was open-ended, the length of the open-ended interval was assumed to be the same as that of the adjacent interval. When the lowest category was open-ended, the lower boundary for 25(OH) D was set to zero. Restricted cubic splines (3 knots at fixed percentiles of 10, 50, and 90% of the distribution) were used to examine potential non-linear dose–response associations between serum vitamin D and risk of hypertension. Statistical analyses were done with STATA version 14.0 (STATA Corp, College Station, TX, United States). All STATA codes used in the analyses are presented in Supplementary Table 7. P-values < 0.05 were considered statistically significant for all tests including Cochran's Q-test.
Results
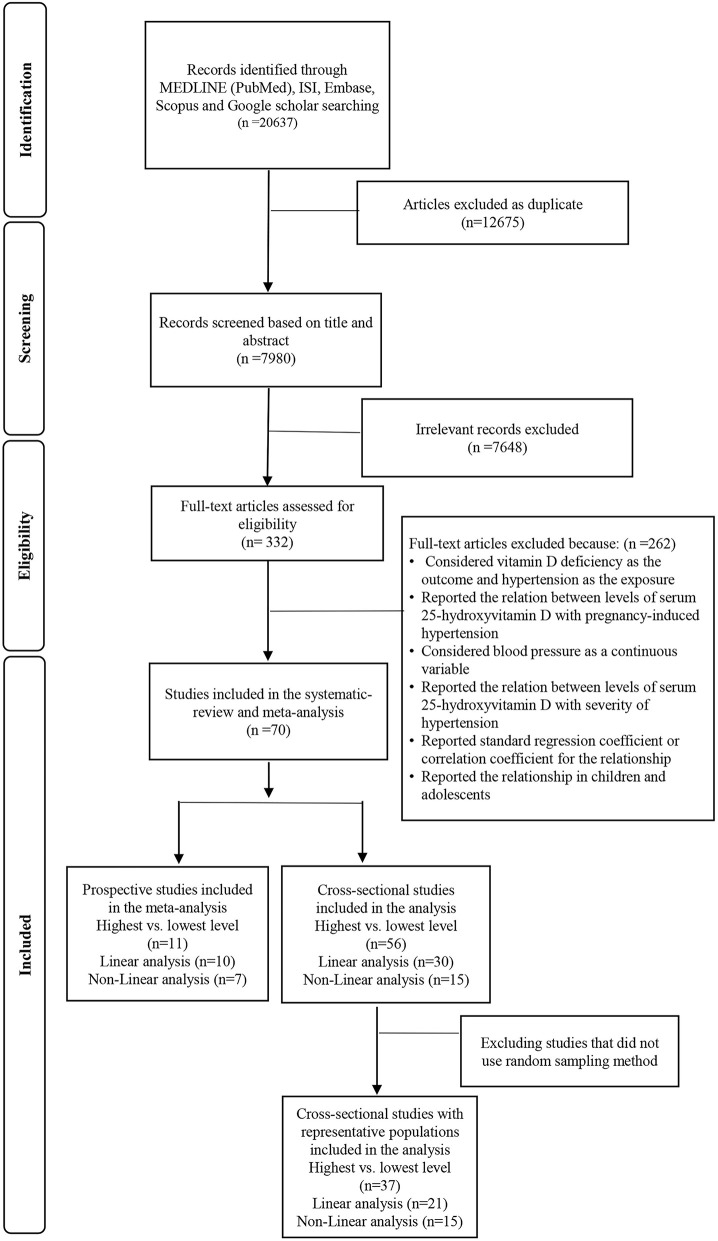
In total, our initial systematic search resulted in 4,255 articles after excluding duplicate studies. In the first round of screening, the titles and abstracts were separately screened. Then, the full text of 102 studies was assessed in the second round. Finally, 70 studies were eligible to be included in the systematic review and meta-analysis. Details of flow diagram search strategy and study selection are presented in Figure 1.
Figure 1.
Flowchart of the study selection process.
Study Characteristics
Details of 70 eligible studies that were included in this systematic review and meta-analysis are summarized in Table 1. These investigations were published between 2005 and 2020. A total of 59 of the investigations had a cross-sectional design (13–19, 26–38, 41–43, 45, 47–50, 52–56, 60–75, 77–86), one investigation was a nested case–control (12), and 10 others were cohorts (39, 40, 44, 46, 51, 57–59, 76, 87). One of these cohorts (46) had reported the association in both baseline and follow-up; so, it was included in the analysis of both cross-sectional and cohort studies. Overall 66,757 and 260,944 participants were, respectively, included in cohort and cross-sectional studies. Among cross-sectional articles, fourteen studies were carried out in the United States (16, 29, 32, 36, 38, 53, 60, 63, 64, 67, 68, 77, 78, 82), twelve in South Korea (13, 26, 28, 33, 47, 48, 55, 70, 83–86), nine in China (15, 41, 50, 52, 54, 72–75), four in Iran (30, 37, 45, 65), two in the Netherlands (17, 27), Australia (42, 71), Thailand (62, 69), Mexico (19, 66) and the remaining in Brazil (31), India (34), Japan (35), Spain (18), Sweden (14), United Kingdom (43), Canada (49), Israel (56), Jordon (61), France (79), and Italy (81); the last study was a multi-country investigation (80). In the case of cohort studies, five cohorts were carried out in the United States (39, 40, 51, 58, 59) and the remaining in Norway (44), Finland (46), Netherlands (57), Australia (87), and Denmark (76). Also, the only nested case–control study was done in the United States (12). Four studies were performed on men, 10 investigations on women, eleven others on men and women separately, and the last forty-five publications on both genders. Different methods were used to measure serum vitamin D concentrations including radioimmunoassay (RIA) (n = 22 studies), chemiluminescence immunoassay (CLIA) (n = 19), electrochemiluminescence immunoassay (ECLIA) (n = 6), ELISA (n = 6), enzyme immunoassay (EIA) (n = 4), competitive protein binding assay (CPBA) (n = 2), high-performance liquid chromatography (HPLC) (n = 2), and other assays (n = 6); while three other studies did not mention a particular method for vitamin D measurement. In the case of outcomes of interest, four studies reported the association between vitamin D levels and pre-hypertension (defined as BP: 120–139/80–89 mmHg) (63, 64, 77, 82). Thirty-two other studies assessed the association with hypertension (defined as BP ≥ 140/90 mmHg) and thirty-one others used the cut-off point of 130/85 mmHg to define hypertension and three other investigations evaluated the association with both hypertension ( ≥ 140/90 mmHg) and pre-hypertension in their population (15, 67, 68). While most of the investigations were conducted on healthy participants (n = 59), the participants of 10 other research studies were done on populations with prostate, lung, colon, and ovarian cancers (38), colorectal neoplasia (32), sleep apnea (18), hemodialysis (37), systemic lupus erythematous (19, 49), peritoneal dialysis (73), and obesity (80, 81) and the last study was done on elderly inpatients (79). Also, one study (65) reported ORs for both metabolically healthy obese individuals and metabolically unhealthy obese individuals. The most adjusted confounders in the studies were age (n = 64), gender (n = 53), BMI (n = 45), physical activity (n = 42), smoking status (n = 42), alcohol (n = 36), and season of blood drown (n = 28). It is worth noting that none of the cohort studies had controlled the baseline vitamin D levels in their analysis. Among cohorts, the NOS scores were between 6 and 9. Eight studies were of high quality, while 2 others were classified as of low quality. Also, the only nested case–control had low quality. Four prospective studies (36%) were judged to have low quality, due to the non-representativeness of the exposed cohort. Two prospective studies (18%) were judged to have low quality arising from the demonstration that the outcome of interest was not present at the start of the study. Two prospective studies (18%) were judged to have low quality because of not having adequate follow-up. Among cross-sectional investigations, the NOS scores ranged between 4 and 10; 33 studies had high quality and 26 others had low quality. Nineteen cross-sectional studies (32%) were judged to have low quality due to non-representativeness of the sample. Twenty-eight cross-sectional studies (47%) were judged to have low quality arising from small sample size. Fourteen cross-sectional studies (24%) were judged to have low quality because they did not report details of non-respondents. Sixteen cross-sectional studies (27%) were judged to have low quality due to no appropriate comparability of subjects in different outcome groups (Supplementary Table 4).
Table 1.
Main characteristics of included cohort and cross-sectional studies examined the association between serum vitamin D levels and high blood pressure in adults.
| First author (year) |
Study design/ name study |
Country Latitude, °N |
Age range/ mean age |
Gender | No. of Participants | Hypertensive Cases |
25 (OH)D Levels, unit |
OR/RR (95% CI) |
Method (Exposure) | Definition (Outcome) | Subject | Adjustmenta | Quality of studies | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Lee et al. (26) | Cross-sectional (Baseline Elderly cohort) | Korea | 65 ≤ | Both | 2,936 | CLIA | SBP≥ 130 | Elderly | 1, 5, 6, 7,10, 11, 18 | 8 | |||
| Men | 987 | Q1 (4.20–14.19) ng/ml | 1.07 (0.69, 1.67) | DBP≥ 85 mmHg | ||||||||||
| Q2 (14.20–18.99) | 1.12 (0.73, 1.72) | |||||||||||||
| Q3 (19.00–24.19) | 1.05 (0.69, 1.59) | |||||||||||||
| Q4 (24.20–51.90) | 1.00 (ref.) | |||||||||||||
| Women | 1,949 | |||||||||||||
| Q1 (4.10–11.19) | 1.24 (0.92, 1.66) | |||||||||||||
| Q2 (11.20–15.59) | 1.24 (0.93, 1.64) | |||||||||||||
| Q3 (15.60–21.59) | 1.24 (0.93, 1.64) | |||||||||||||
| Q4 (21.60–54.90) | 1.00 (ref.) | |||||||||||||
| 2 | Vitezova et al. (27) | Cross-sectional (Baseline Cohort | Chile | 55 ≤ | Both | 3,240 | ECLIA | SBP≥ 130 DBP≥ 85 mmHg | Middle-aged and elderly adults | 1, 2, 5, 7, 8, 9, 11, 16, 33, 37, 41, 42 | 9 | |||
| Rotterdam Study1989–1993) | Rotterdam, Netherlands | 1,833 | T1 (<50) nmol/l | 1.00 (ref.) | ||||||||||
| 874 | T2 (50–75) | 1.04 (0.85, 1.27) | ||||||||||||
| 533 | T3 (≥75) | 0.89 (0.70, 1.12) | ||||||||||||
| 3 | Chon et al. (28) | Cross-sectional (Baseline Cohort | South Korea | ≥18 | Women | 4,364 | ECLIA | SBP≥130 | Post-menopausal | 1, 5, 6, 7, 8, 11, 14, 59 | 9 | |||
| KNHANE) | 1,445 | T1 (3.07–14.89) ng/ml | 1.00 (ref.) | DBP≥ 85 mmHg | women | |||||||||
| 1,445 | T2 (14.9–20.96) | 0.95 (0.81, 1.12) | ||||||||||||
| 1,446 | T3 (20.97–66.96) | 0.83 (0.71, 0.98) | ||||||||||||
| 4 | Maki et al. (29) | Cross-sectional (Baseline Cohort | U.S | 20 ≤ | Both | 3,529 | RIA | SBP≥130 | Non- institutionalized civilian U.S. population |
1, 2, 3, 5, 6, 7, 8, 16, 20, 32, 40, 43 | 9 | |||
| (NHANES) | Q1 (7.5–44.9) nmol/l | 1.00 (ref.) | DBP≥ 85 mmHg | |||||||||||
| (45–59.9) Q2 | 0.77 (0.58, 1.01) | or taking anti | ||||||||||||
| (60–74.9) Q3 | 0.74 (0.55, 1.00) | hypertensive | ||||||||||||
| (75–215) Q4 | 0.71 (0.49, 1.02) | medication | ||||||||||||
| 5 | Mansouri et al. (30) | Cross-sectional | Tehran, Iran | 35 ≤ | Both | 352 | ELISA | SBP≥130 | High educated population | 1, 2, 5, 7, 12, 31, 34 | 7 | |||
| 89 | Q1 | 1.00 (ref.) | DBP≥ 85 mmHg | |||||||||||
| 90 | Q2 | 0.42 (0.18, 1.01) | ||||||||||||
| 85 | Q3 | 0.38 (0.16, 0.92) | ||||||||||||
| 88 | Q4 | 0.45 (0.19, 1.03) | ||||||||||||
| 6 | Schmitt et al. (31) | Cross-sectional cohort study | São Paulo, Brazil | 45–75 | Women | 463 | CLIA | SBP≥130 | Post-menopausal women | 1, 4, 5, 7, 60 | 6 | |||
| 219 | <30 ng/ml | 1.23 (0.79, 2.85) | DBP≥ 85 mmHg | |||||||||||
| 98 | ≥30 | 1.00 (ref.) | ||||||||||||
| 7 | Bea et al. (32) | Cross-sectional | USA | 65.7 ± 8.75 | Both | 2,096 | CLIA | SBP≥ 130 | Colorectal neoplasia patients after surgery | 1, 2, 3, 22, 61 | 7 | |||
| Men | 117 | <20 ng/ml | 1.00 (ref.) | DBP≥ 85 | ||||||||||
| 315 | 20– 30 | 0.72 (0.51, 1.03) | mmHg | |||||||||||
| 334 | ≥30 | 0.72 (0.51, 1.03) | ||||||||||||
| Women | 116 | <20 ng/ml | 1.00 (ref.) | |||||||||||
| 136 | 20– 30 | 1.02 (0.70, 1.49) | ||||||||||||
| 72 | ≥30 | 1.13 (0.71, 1.78) | ||||||||||||
| 8 | Kim and Kim (33) | Cross-sectional | South Korea | ≥19 | Both | 5,559 | RIA or EIA | SBP≥130 and/or | Adults | 1, 2, 4, 5, 6, 7, 8, 9, 11, 15, 21 | 10 | |||
| latitude 33–38N | 1,112 | Q1 (2.65–12.87) ng/ml | 1.00 (ref.) | DBP≥ 85mmHg | . | |||||||||
| 1,112 | Q2 (12.88–16.87) | 0.93 (0.72, 1.33) | or currently undergoing treatment for | |||||||||||
| 1,112 | Q3 (16.88–20.8) | 1.18 (0.92, 1.53) | hypertension | |||||||||||
| 1,112 | (20.82–26.13) Q4 | 0.80 (0.61, 1.03) | ||||||||||||
| 1,111 | Q5 (26.14–58.66) | 1.05 (0.79,1.42) | ||||||||||||
| 9 | Majumdar et al. (34) | Cross-sectional | India | 18–75/ | Both | 441 | EIA | SBP≥130 | Adults | 1, 4, 5, 16 | 5 | |||
| 39 ± 12.8 | Men | 237 | Q1 (<28.2) nmol/l | 1.00 (ref.) | DBP≥ 85 mmHg | |||||||||
| Q2 (2.82–38) | 1.40 (0.50, 3.30) | |||||||||||||
| Q3 (38.1–47) | 1.10 (0.50, 2.80) | |||||||||||||
| Q4 (47.1–57.8) | 0.90 (0.30, 2.10) | |||||||||||||
| Q5 (>57.8) | 0.50 (0.20, 1.30) | |||||||||||||
| women | 204 | Q1 (<25.2) | 1.00 (ref.) | |||||||||||
| Q2 (25.2–34.2) | 2.30 (0.70, 7.20) | |||||||||||||
| Q3 (34.3–42.9) | 2.00 (0.60, 6.30) | |||||||||||||
| Q4 (43–53.5) | 1.50 (0.40, 5.10) | |||||||||||||
| Q5 (>53.5) | 2.20 (0.70, 7.10) | |||||||||||||
| 10 | Akter et al. (35) | Cross-sectional | Japan | 18–69 | Both | 1,790 | CPBA | SBP≥130 | Workers | 1, 2, 4, 5, 6, 7, 22,62 | 7 | |||
| 730 | <20 ng/ml | 1.00 (ref.) | DBP≥ 85 mmHg | |||||||||||
| 921 | 20–30 | 1.04 (0.83, 1.32) | ||||||||||||
| 139 | 30 ≤ | 0.92 (0.60, 1.40) | ||||||||||||
| 11 | Barceló et al. (18) | Cross-sectional | Bunyola, Spain | 52.25 ± 12.4 | Both | 826 | EIA* | SBP≥130 or | Newly diagnosed with obstructive sleep apnea | 1, 2, 11, 16 | 4 | |||
| 2013 | 105 | ≥15 ng/ml | 1.00 (ref.) | DBP≥ 85 mmHg | ||||||||||
| 377 | 16–30 | 0.97 (0.51, 1.85) | ||||||||||||
| 344 | >30 | 0.76 (0.39, 1.49) | ||||||||||||
| 12 | Mitri et al. (36) | Cross-sectional | USA | 25 ≤ | Both | 2,000 | Liquid chromato- graphy, tandem mass spectrometry |
SBP≥130 and/or | Diabetes high-risk population | 1, 2, 3, 4, 5, 6, 7, 11, 19, 43, 63, 64 | 9 | |||
| 666 | T1 12.1 (9.7–14.3) ng/ml | 1.00 (ref.) | DBP≥ 85 mmHg or | |||||||||||
| 667 | T2 20.3 (18.3–22.7) | 1.01 (0.88, 1.16) | current antihypertensive drug treatment | |||||||||||
| 667 | T3 30.6 (27.5–34.9) | 1.03 (0.76, 1.41) | ||||||||||||
| 13 | Ahmadi et al. (37) | Cross-sectional | Tehran, Iran | 24 −94/ | Both | 145 | EIA | SBP≥130 | Hemodialysis patients | 1, 2 | 5 | |||
| 58. ± 16.0 | 27 | ≤ 15 ng/ml | 1.05 (0.44, 2.49) | or | ||||||||||
| 76 | 16–30 | 1.11 (0.48, 2.57) | DBP≥ 85 mmHg or treatment of previously | |||||||||||
| 42 | >30 | 1.00 (ref.) | diagnosed hypertension | |||||||||||
| 14 | Brock et al. (38) | Cross-sectional | USA | 63 ± 5 | Both | 2,465 | <37 nmol/l | 1.00 (ref.) | Radio-iodinated tracer assay | Self-reported | Middle-aged Caucasian from Prostate, Lung Colon and Ovarian Cancer Screening Trial | 2, 4, 5, 7, 8, 11, 19, 20, 22, 66, 67, 68 | 7 | |
| 37–50 | 0.90 (0.60, 1.20) | |||||||||||||
| 50–80 | 0.90 (0.70, 1.20) | |||||||||||||
| 80≥ | 1.00 (0.70, 1.40) | |||||||||||||
| 15 | Burgaz et al. (14) | Cross-sectional | Uppsala, central Sweden | 0.6 ± 71 | Men | 35 | <37.5 nmol/l | 3.3 (1.0, 11.0) | HPLC | SBP>130 | Elderly men | 4, 6, 7, 11 | 8 | |
| (Uppsala Longitudinal Study of Adult Men (ULSAM) | 798 | ≥ 37.5 | 1.00 (ref.) | and/or DBP>85 mmHg using 24–h BP measurements | ||||||||||
| 16 | Dorjgochoo et al. (15) | Cross-sectional | Shanghai, China | 40– 74 | Both | 1,460 | CLIA | HTN: SBP ≥140 | Adults | 1, 2, 4, 5, 6, 7, 8, 11, 14, 19, 20, 30, 36 | 9 | |||
| (from two large, population-based, prospective cohort studies | Men | 405 | 193 | Deficient (37.5> nmol/l) | 1·00 (ref.) | or | ||||||||
| Women's Health Study (SWHS) | Insufficient (37.5– 74.9) | 0·56 (0·26–, 1·21) | DBP ≥90mmHg | |||||||||||
| And | Sufficient ( ≤ 75.0) | 0·42 (0·12, 1·43) | ||||||||||||
| Men's Health Study (SMHS)) | ||||||||||||||
| Women | 1,055 | 354 | Deficient (37.5>) | 1·00 (ref.) | ||||||||||
| Insufficient (37.5– 74.9) | 1·09 (0·73, 1·63) | |||||||||||||
| Sufficient (≥75.0) | 1·07 (0·31, 3·72) | |||||||||||||
| Pre-HTN: | ||||||||||||||
| Men | 405 | 155 | Deficient (37.5>) | 1·00 (ref.) | SBP: 120–139 mmHg and/or DBP: 80–89 mmHg | |||||||||
| Insufficient (37.5– 74.9) | 0.57 (0.26, 1.22) | |||||||||||||
| Sufficient ( ≤ 75.0) | 0.46 (0.14, 1.56) | |||||||||||||
| Women | 1,055 | 452 | Deficient (37.5>) | 1·00 (ref.) | ||||||||||
| Insufficient (37.5– 74.9) | 1.11 (0.77, 1.60) | |||||||||||||
| Sufficient (≥75.0) | 1.51 (0.49, 4.60) | |||||||||||||
| 17 | Forman et al. (12) | Nested case-control study (NHS2) | USA | 43 (40– 46) | Women | 1,484 | 1.47 (1.10, 1.97) | EIA | Self-reported | Adults | 1, 3, 4, 7, 11, 18, 36, 44, 46, 51, 69, 70, 71, 72 | 7 | ||
| /year of follow-up: 8–10 years) | 975 | <30.0 ng/mL | 1.00 (ref.) | |||||||||||
| 509 | ≥ 30.0 | |||||||||||||
| 18 | Anderson et al. (39) | Cohort | Salt Lake City, Utah, USA | 21 ± 55 | Both | 41,504 | HR | CLIA | NR | Adults | 1, 2, 58, 89, 131 | 6 | ||
| 1.3 years (maximum 9.3) | 6,909 | (deficient) ≤ 15 ng/ml | 1.62 (1.38, 1.89) | |||||||||||
| 19,474 | (insufficient) 16–30 | 1.18 (1.05, 1.33) | ||||||||||||
| 15,121 | normal >30 | 1.00 (ref.) | ||||||||||||
| 19 | Forman et al. (40) | Cohort | Boston, | 64.5 | Both | RIA | SBP> | Adults | 1, 3, 4, 7, 73 | 7 | ||||
| (4–8 y) | Men | 613 | <15 ng/ml | 6.13 (1.00, 37.8) | 140 and DBP> 90 mm Hg | . | ||||||||
| (Based on NHS,HPFS) | 15– 29 | 1.12 (0.51, 2.48) | ||||||||||||
| ≥ 30 | 1.00 (ref.) | |||||||||||||
| Women | 1,198 | <15 | 2.67 (1.05, 6.79) | |||||||||||
| 15–29 | 0.85 (0.53, 1.34) | |||||||||||||
| ≥ 30 | 1.00 (ref.) | |||||||||||||
| 20 | García-Carrasco et al. (19) | Cross-sectional | Puebla, México | 43.3 ± 11.8 | Women | 160 | <20 ng/ml | 1.00 (ref.) | CLIA | SBP≥ 135 or | Non-diabetic SLE women | 1, 4, 5 | 6 | |
| 20–30 | 0.40 (0.10, 1.20) | DBP≥ 85 mm Hg or taking | ||||||||||||
| ≥ 30 | 0.43 (0.10, 3.80) | medication for hypertension; | ||||||||||||
| 21 | Hidru et al. (41) | Cross-sectional | Dalian, China | 62.02 ± 5.73 | Both | 2,624 | ECLIA | SBP>140 | Middle-aged and Elderly Chinese Population | 1, 2, 4, 5, 6, 7, 44, 53, 54,55, 56, 58 | 7 | |||
| (PCSRFHFEP prospective cohort) | Men | 1,105 | ≤ 12.04 nmol/l | 1.00 (ref.) | DBP >90 mmHg | |||||||||
| 12.05–16.50 | 1.09 (0.76, 1.56) | Or self-reported history of hypertension with the current use of blood pressure-reducing medication | ||||||||||||
| 16.51–22.69 | 1.40 (0.97, 2.02) | |||||||||||||
| ≥22.70 | 1.07 (0.74, 1.54) | |||||||||||||
| Women | 1,519 | ≤ 8.60 | 1.00 (ref.) | |||||||||||
| 8.61–12.30 | 0.84 (0.62, 1.14) | |||||||||||||
| 12.31–16.83 | 0.76 (0.56, 1.03) | |||||||||||||
| ≥16.84 | 0.86 (0.63, 1.17) | |||||||||||||
| 22 | Hirani et al. (42) | Cross-sectional | Sydney, Australia | 70 ≤ | Men | 1,659 | SBP ≥140 mmHg (reference <140 mmHg) | RIA | SBP≥ 140 mmHg | Community-dwelling men aged 70 and | Not adjusted because not associated with 25 (OH)D. | 7 | ||
| <50.0 nmol/L | older | |||||||||||||
| 50.0– 74.9 | 0.81 (0.60, 1.09) | DBP ≥ 90 mmHg | ||||||||||||
| ≥75.0 | 1.03 (0.76, 1.39) | |||||||||||||
| 1.00 (ref.) | ||||||||||||||
| DBP ≥90 mmHg (reference <90 mmHg) | ||||||||||||||
| <50.0 | ||||||||||||||
| 50.0– 74.9 | ||||||||||||||
| ≥75.0 | 1.00 (0.69, 1.44) | |||||||||||||
| 1.07 (0.74, 1.55) | ||||||||||||||
| 1.00 (ref.) | ||||||||||||||
| 23 | Hypponen et al. (43) | Cross-sectional | London, U.K | 45 (44–46) | Both | 6,293 | 9–45 nmol/l | 1.00 (ref.) | automated IDS OCTEIA ELISA | SBP>140 | Adults | 2, 4, 5, 6, 7, 11, 17, 74, 75, 76 | 10 | |
| Based on 1958 | 46–67 | 0.80 (0.68, 0.94) | DBP >90 mmHg or use of antihypertensive medication | |||||||||||
| British Birth cohort | 68–231 | 0.72 (0.61, 0.86) | ||||||||||||
| 24 | Jorde et al. (44) | Longitudinal. | Tromsø, Norway | 56.2 ± 9.3 | Both | 2,385 | ECLIA | SBP≥ 140 and/or DBP≥ 90 mm Hg | Adults | 1, 2, 4, 7 | 7 | |||
| (1994–2008) | 532 | <41.4 nmol/l | 1.01 (0.78, 1.32) | |||||||||||
| 599 | 41.4–51.5 | 1.06 (0.83, 1.37) | ||||||||||||
| 625 | 51.6–62.6 | 1.12 (0.87, 1.43) | ||||||||||||
| 629 | >62.6 | 1.00 (ref.) | ||||||||||||
| 25 | Joukar et al. (45) | Cross-sectional (The PERSIAN Guilan Cohort Study (PGCS)) | Guilan | 70–35 | Both | 9,520 | ECLIA | SBP≥140 and/or | Adults | 1, 2, 4, 5, 6, 7, 8, 15,17, 20, 44, 77 | 9 | |||
| Iran | 1,957 | <30 nmol/l | 1.00 (ref.) | DBP≥ 90 mmHg and/or taking antihypertensive drugs | ||||||||||
| 2,762 | 30 −50 | 1.10 (0.94, 1.27) | ||||||||||||
| 4,801 | ≥50 | 1.09 (0.94, 1.25) | ||||||||||||
| 26 | Ke et al. (46) | Cross-sectional | Finland | 50–69 | Men | 2,271 | RIA , with ELISA and CLIA in a sub-set of study | SBP≥ 140 or DBP | Male smoker | 1, 4, 6, 8, 11, 19, 22, 78, 79, 80 | 6 | |||
| 505 | ≤ 25 nmol/l | 1.00 (ref.) | ≥ 90 mm Hg | |||||||||||
| 517 | 25–37 | 1.00 (0.80, 1.10) | ||||||||||||
| 541 | 37–50 | 0.80 (0.60, 1.00) | ||||||||||||
| 560 | 50–80 | 0.80 (0.60, 1.00) | ||||||||||||
| 148 | ≥80 | 0.90 (0.60, 1.10) | ||||||||||||
| 27 | Ke et al. (46) | Cohort/4 years of followup | Finland | 50–69 | Men | 1,957 | RIA , with ELISA and CLIA in a sub-set of study | SBP≥ 140 or DBP | Male smoker | 1, 4, 6, 8, 11, 19, 22, 78, 79, 80 | 8 | |||
| ≤ 25 nmol/l | 1.00 (ref.) | ≥ 90 mm Hg | ||||||||||||
| 25–37 | 1.20 (0.90, 1.60) | |||||||||||||
| 37–50 | 1.00 (0.70, 1.30) | |||||||||||||
| 50–80 | 0.90 (0.70, 1.20) | |||||||||||||
| ≥80 | 1.00 (0.60, 1.50) | |||||||||||||
| 28 | Kim and Kim (33) | Cross-sectional | South Korea | 19 ≤ | Both | 20,440 | RIA | SBP ≥ 140 | Adults | 1, 4, 5, 6, 7, 8,15, 22, 23, 24, 81 | 10 | |||
| (based on the fourth and fifth KNHANES) | Men | or | ||||||||||||
| (19–64) | 1,557 | Q1 (7.5–35.6 nmol/l) | 1.00 (ref.) | DBP≥ 90mm Hg, or the current use of anti-hypertensive medication | ||||||||||
| 1,558 | Q2 (35.7–44.6) | 0.82 (0.66, 1.02) | ||||||||||||
| 1,553 | Q3 (44.6–55.0) | 0.90 (0.73, 1.10) | ||||||||||||
| 1,554 | Q4 (55.1–150.8) | 0.87 (0.70, 1.08) | ||||||||||||
| (≥65) | 519 | Q1 (10.8–38.7) | 1.00 (ref.) | |||||||||||
| 520 | Q2 (38.8–49.4) | 1.21 (0.87, 1.69) | ||||||||||||
| 516 | Q3 (49.4–61.9) | 1.08 (0.77, 1.51) | ||||||||||||
| 518 | Q4 (62.0–134.0) | 0.89 (0.64, 1.22) | ||||||||||||
| (19–64) | Women | 2,363 | Q1 (7.4–30.8) | 1.00 (ref.) | ||||||||||
| 2,360 | Q2 (30.8–38.6) | 0.93 (0.75, 1.15) | ||||||||||||
| 2,368 | Q3 (38.7–48.3) | 0.83 (0.65, 1.06) | ||||||||||||
| 2,355 | Q4 (48.3–137.3) | 0.73 (0.58, 0.91) | ||||||||||||
| (≥65) | 676 | Q1 (10.3–33.6) | 1.00 (ref.) | |||||||||||
| 674 | Q2 (33.7–43.7) | 0.77 (0.58, 1.03) | ||||||||||||
| 676 | Q3 (43.7–55.8) | 0.88 (0.65, 1.18) | ||||||||||||
| 673 | Q4 (55.8–167.1) | 0.80 (0.61, 1.06) | ||||||||||||
| 29 | Kim and Kim (33) | Cross-sectional | South Korea | ≥50 | Both | 5,260 | RIA | SBP≥ 140 | Middled-aged and Older Korean Adults | 1, 2, 4, 5, 6, 7, 8, 15, 18, 22, 23, 24, 45 | 10 | |||
| (based on the fifth KNHANES (V−1,2)) | 1,315 | Q1 (10.3–35.5 nmol/l) | 1.00 (ref.) | DBP ≥ 90 mm Hg, or current use of antihypertensive medication | ||||||||||
| 1,316 | Q2 (35.5–45.2) | 0.83 (0.67, 1.03) | ||||||||||||
| 1,313 | Q3 (45.2–57.3) | 0.85 (0.68, 1.05) | ||||||||||||
| 1,316 | Q4 (57.3–133.6) | 0.86 (0.70, 1.07) | ||||||||||||
| 30 | Kim (47) | Cross-sectional | South Korea | ≥50 | Both | 2,624 | RIA | SBP≥ 135 | Middled-aged and Older Korean Adults | 1, 2, 4, 5, 6 ,7, 8, 9, 15 | 10 | |||
| (Based on fifth KNHANES (V−1)) | 656 | Q1 (10.3–35.6 nmol/l) | 1.00 (ref.) | or DBP≥ 85 mm Hg or daily use of antihypertensive medication | ||||||||||
| 654 | Q2 (35.6–45.9) | 0.91 (0.71, 1.16) | ||||||||||||
| 657 | Q3 (45.9–59.2) | 0.92 (0.72, 1.18) | ||||||||||||
| 657 | Q4 (59.2–122.6) | 0.76 (0.59, 0.97) | ||||||||||||
| 31 | Kim (47) | Cross-sectional | Seoul | ≥30 | Both | 379 | RIA | SBP≥ 135 | North Korean refugee health in South Korea | 1, 2, 4, 5, 7, 11, 82 | 7 | |||
| ( the longitudinal | South Korea | 36 | <10 ng/mL | 2.69 (0.58, 12.60) | or DBP≥ 85 mm Hg or treatment for hypertension | |||||||||
| Cohort NORNS) | 294 | 20–Oct | 2.94 (0.88, 9.88) | |||||||||||
| 2008–2012 | 49 | 20–30 | 1.00 (ref.) | |||||||||||
| 32 | Kim et al. (13) | Cross-sectional | Chungju, Korea | ≥40 | Both | 324 | Q1 (10.0–29.7 nmol/l) | 1.00 (ref.) | Chemilumine scence assays |
SBP≥ 135 | Middled-aged and Older Korean Adults | 1, 2, 4, 5, 6, 7, 11, 18, 19, 22, 24 ,46 | 10 | |
| (median age 65.8 years) | Q2 (30.0–39.2) | 0.62 (0.38, 1.01) | or DBP≥ 85 mmHg or on antihypertensive drug treatment | |||||||||||
| Q3 (39.4–49.4) | 0.94 (0.55, 1.61) | |||||||||||||
| Q4 (49.7–61.2) | 0.84 (0.48, 1.48) | |||||||||||||
| Q5 (61.4–116.8) | 0.47 (0.27, 0.82) | |||||||||||||
| 33 | Kwak et al. (48) | Cross-sectional | Seoul, Republic of Korea | 35.7 ± 0.32 | Women | <30 nmol/l | Ultra-high performance |
SBP≥ 140 | 2,098 premenopausal | 1, 3, 5, 6, 7, 8, 11, 19, 22,23,24,25 | 9 | |||
| (NHANES 2007–2010) | 62.3 ± 0.21 | Premeno- pausal |
238 | 30–49.99 | 1.00 (ref.) | liquid chromato graphy-tandem mass spectrometry |
DBP ≥ 90 mm Hg, or the use of antihypertensive medication or physician diagnosis | |||||||
| 586 | ≥ 50 | 1.23 (0·79, 1·92) | 2,298 postmenopausal |
|||||||||||
| 1,274 | 0.64 (0·39, 1·02) | |||||||||||||
| Postmenopausal | <30 nmol/l | |||||||||||||
| 195 | 30–49.99 | 1.00 (ref.) | ||||||||||||
| 505 | ≥ 50 | 0.68 (0·44, 1·04) | ||||||||||||
| 1,598 | 0.71 (0·47, 1·09) | |||||||||||||
| 34 | Lertratanakul et al. (49) | Cross-sectional | Toronto | 39.3 ± 13.5 | Both | 873 | Q1 (4–13 ng/ml) | 1.00 (ref.) | NR | SBP≥ 140 DBP≥ 90 mm Hg, or taking current treatment for hypertension | Patients With Systemic Lupus Erythematous | 1, 2, 3, 4, 11, 83 | 7 | |
| Q2 (14–21) | 0.83 (0.55, 1.25) | |||||||||||||
| Q3 (22–30) | 0.69 (0.44, 1.06) | |||||||||||||
| Q4 (31–91) | 0.49 (0.31, 0.77) | |||||||||||||
| 35 | Liu et al. (50) | Cross-sectional | China | 65–112 | Both | 2,493 | ELISA | BP≥ 130/85 mmHg or known treatment for hypertension | Elderly Chinese Individuals | 1, 2, 3, 4, 5, 6, 7, 8, 12, 16 | 7 | |||
| Evidence from CLHLS | 1,029 | <20 ng/ml | 1.00 (ref.) | |||||||||||
| 890 | 20–30 | 1.23 (0.87, 1.75) | ||||||||||||
| 574 | ≥30 | 1.49 (0.97, 2.29) | ||||||||||||
| 36 | Margolis et al. (51) | Cohort (7 years of followup) | Minneapolis | 50–79 | Women | 2,153 | 891 | Q1 (<34.4 nmol/L) | 1.00 (ref.) | CLIA | SBP≥ 140 DBP≥ 90 mm Hg, or self-report of medication prescribed for hypertension | Postmenopausal women | 1, 3, 4, 5, 6, 7, 8, 11, 20, 37, 38, 84 85, 86 | 7 |
| Women's Health Initiative 1993–1998 | USA | Q2 (34.4– <47.7) | 0.91 (0.62, 1.32) | |||||||||||
| Q3 (47.7–64.7) | 0.66 (0.46, 0.96) | |||||||||||||
| Q4 (≥ 64.7) | 0.86 (0.60, 1.23) | |||||||||||||
| 37 | Peng et al. (52) | Cross-sectional | Tangshan City | 49.9 ± 12.5 | Both | 3,788 | ELISA | SBP≥ 140 or | Coal mine workers | 1, 4, 5, 6 , 7, 26, 58, 87, 88, 89 | 7 | |||
| (Kailuan cohort study) | China | 717 | <25nmol/L | 1.39 (0.97, 1.99) | DBP≥ 90 mmHg on at least 3 different visits to the hospital taking antihypertensive agents | |||||||||
| 296 | 25–50 | 1.44 (0.99, 2.11) | ||||||||||||
| 54 | ≥50 | 1.00 (ref.) | ||||||||||||
| 38 | Reis et al. (53) | Cross-sectional | California, San Diego | 44–96 | Both | 1,070 | (CBP) recognition and | SBP≥ 130, DBP≥ 85 mmHg, or use of antihypertensive medication | Community-dwelling older adults | 1, 5, 6, 7, 11, 16, 90 | 8 | |||
| (Rancho Bernardo Study cohort) | Men | 410 | Q1 (<87.5 nmol/l) | 1.00 (ref.) | CLIA | |||||||||
| Q2 (87.5– 97.4) | 0.92 (0.41, 2.07) | |||||||||||||
| Q3 (97.5–110.0) | 0.90 (0.41, 1.96) | |||||||||||||
| Q4 (110.1–126.2) | 0.88 (0.42, 1.84) | |||||||||||||
| Q5 ( ≥126.3) | 1.28 (0.58, 2.81) | |||||||||||||
| Women | 660 | Q1 (<77.5) | 1.00 (ref.) | |||||||||||
| Q2 (77.5–92.4) | 0.77 (0.40, 1.46) | |||||||||||||
| Q3 (92.5– 103.7) | 0.80 (0.44, 1.47) | |||||||||||||
| Q4 (103.8–119.9) | 0.81 (0.43, 1.53) | |||||||||||||
| Q5 (≥ 120.0) | 1.01 (0.53, 1.93) | |||||||||||||
| 39 | Shen et al. (54) | Cross-sectional | Henan, China | 18–93 | Both | 1,539 | ELISA | SBP≥ 140 DBP≥ 90 mmHg, or | Adults | 1, 2, 5, 6, 7, 36 | 6 | |||
| 68 | <10 ng/ml | 1.00 (ref.) | Use of antihypertensive medication | |||||||||||
| 722 | 20–Oct | 0.48 (0.28, 0.85) | ||||||||||||
| 387 | 20–30 | 0.37 (0.20, 0.66) | ||||||||||||
| 362 | ≥30 | 0.48 (0.27, 0.87) | ||||||||||||
| 40 | Snijder et al. (17) | Cross-sectional | Amsterdam | 55–85 | Both | 1,205 | CPBA | SBP> 140 and/or DBP> 90 mmHg and/or taking antihypertensive medication | Older men and women | 1, 2, 5, 6, 7, 10, 11, 17 | 8 | |||
| LASA) | 126 | Q1 (<25.0 nmol/l) | 0.89 (0.47, 1.69) | |||||||||||
| 442 | Q2 (25–50) | 0.79 (0.50, 1.25) | ||||||||||||
| 410 | Q3 (50–75) | 0.75 (0.49, 1.15) | ||||||||||||
| 227 | Q4 (>75) | 1.00 (ref.) | ||||||||||||
| 41 | Song et al. (55) | Cross-sectional | Republic of Korea | 58.0 ± 7.0 | Women | 778 | CLIA | SBP≥ 130 or DBP≥ 85 mmHg, or taking medication | apparently healthy | 1, 6, 7, 11 | 8 | |||
| 193 | Q1 (4.2–9.7 ng/ml) | 1.81 (1.15, 2.85) | Post-menopausal women | |||||||||||
| 199 | Q2 (9.8–14.1) | 1.91 (1.24, 2.94) | ||||||||||||
| 192 | Q3 (14.2–19.8) | 1.55 (1.02, 2.37) | ||||||||||||
| 194 | Q4 (19.9–55.9) | 1.00 (ref.) | ||||||||||||
| 42 | Steinvil et al. (56) | Cross-sectional | Israel | ≥18 | Both | 34,874 | RIA | ICD nine criteria | Adults | 1 | 7 | |||
| (Health care maintenance organization 2001–2008) | Men | 1,662 | <15 ng/ml | 1.11 (0.95, 1.30) | ||||||||||
| 4,672 | 15–30 | 1.06 (0.93, 1.20) | ||||||||||||
| 1,841 | >30 | 1.00 (ref.) | ||||||||||||
| Women | 5,816 | <15 ng/ml | 1.19 (1.09, 1.31) | |||||||||||
| 15,341 | 15–30 | 1.07 (0.99, 1.15) | ||||||||||||
| 5,542 | >30 | 1.00 (ref.) | ||||||||||||
| 43 | van Ballegooijen et al. (57) | Cohort | Amsterdam, The Netherlands | 28–75 | Both | 5,066 | HR | Liquid chromato- graphy-tandem mass spectrometry |
SBP≥ 140 DBP≥ 90 mmHg or taking medication | Adults | 1, 2, 4, 5, 6, 8, 11, 36, 45, 92 | 9 | ||
| /median follow-up of 6.4 years (2.3–9.0) | 1,264 | Q1 (6.7–40.7 nmol/l) | 1.16 (0.95, 1.41) | |||||||||||
| 1,270 | Q2 (40.8–56.7) | 1.07 (0.89, 1.27) | ||||||||||||
| 1,266 | Q3 (56.8–73.7) | 0.91 (0.75, 1.08) | ||||||||||||
| 1,266 | Q4 (73.7–181.3) | 1.00 (ref.) | ||||||||||||
| 44 | van Ballegooijen et al. (58) | Community–based prospective Cohort (MESA)/ 9 years follow-up | USA | 45–84 | Both | 3,002 | liquid chromato- graphy-mass spectroscopy |
SBP≥ 140, DBP≥ 90 mmHg, or taking antihypertensive medication | Adults | 1, 2, 3, 4, 5, 7, 8, 9, 45, 55, 93, 94, 95, 96, 97 | 8 | |||
| 922 | <20 ng/ml | 1.13 (0.96, 1.33) | ||||||||||||
| 1,028 | 20–30 | 1.14 (0.98, 1.31) | ||||||||||||
| 1,052 | >30 | 1.00 (ref.) | ||||||||||||
| 45 | Wang et al. (59) | Prospective Cohort/ 15.3–year follow-up | Boston | NR | Men | 660 | HR | Radio- immunosorbant assay |
SBP≥ 140, | Adults | 1 ,3 , 4, 5, 6, 7, 11, 21, 38, 39 | 6 | ||
| US | 136 | <50 nmol/l | 1.00 (ref.) | DBP≥ 90 mmHg, or use of anti-hypertensive medication | ||||||||||
| 244 | 50– <75 | 1.03 (0.75, 1.42) | ||||||||||||
| 178 | 75– <100 | 0.79 (0.56, 1.11) | ||||||||||||
| 102 | 100 ≤ | 0.94 (0.62, 1.40) | ||||||||||||
| 46 | Martins et al. (60) | Cross-sectional | United States | 20 ≤ | Both | 15,088 | <21 ng/ml | 1.30 (1.13, 1.49) | RIA | SBP≥ 140, | Adults | 1, 2, 3 | 8 | |
| (based on NHANES III) | NR | NR | DBP≥ 90 mmHg | |||||||||||
| NR | NR | |||||||||||||
| ≥37 | 1.00 (ref.) | |||||||||||||
| 47 | Khader et al. (61) | Cross-sectional | Jordan | 19 −90 | Both | 3,234 | RIA | SBP≥ 130 or DBP≥ 85 mm Hg, or treatment of previously diagnosed hypertension | Adults | 1, 2, 4, 7, 8, 10, 12, 18, 44, 46, 49 | 8 | |||
| Men | 776 | <30 ng/ml | 1.008 (0.83, 1.22) | |||||||||||
| Women | 2,458 | ≥30 | 1.00 (ref.) | |||||||||||
| 48 | Jeenduang et al. (62) | Cross-sectional | Southern | 62.6 ± 9.76 | Women | 340 | ECLIA | SBP≥ 130 | Postmenopausal women | 1, 4, 5, 6, 7, 8, 13, 20, 21, 27,35 | 8 | |||
| Thailand | 194 | <30 ng/ml | 1.092 (0.663, 1.798) | and/or DBP | ||||||||||
| 146 | 30 ≤ | 1.00 (ref.) | ≥ 85 mmHg | |||||||||||
| 49 | Gupta et al. (63) | Cross-sectional | USA | 20 ≤ | Both | 461 | ≤ 45.4 nmol/l | 1.21 (0.76, 1.92) | RIA | Pre-HTN (resting SBP: 120–139 mm Hg and/or DBP: | Healthy adult | 1, 2 ,4 | 8 | |
| (based on NHANES 2001–2006) | 160 | >45.4 | 1.00 (ref.) | 80–89 mm Hg) | ||||||||||
| 50 | Gupta et al. (63) | Cross-sectional | LA | 20 ≤ | Both | 591 | ≤ 60.4 nmol/l | 1.20 (0.60, 2.39) | RIA | Pre-HTN | Healthy Mexican Americans | 1, 2,4 | 8 | |
| (based on NHANES 2001–2006) | USA | 197 | >60.4 | 1.00 (ref.) | (resting | |||||||||
| SBP: 120–139 mm Hg and/or DBP: 80–89 mm Hg) | ||||||||||||||
| 51 | Gupta et al. (64) | Cross-sectional | LA | 20 ≤ | Both | 1,272 | ≤ 76.3 nmol/l | 1.61 (1.23, 2.10) | NR | Pre-HTN (SBP: 120–139 mmHg and/or DBP: 80–89 mmHg) | Healthy disease-free Caucasians | 1, 2,4 | 6 | |
| (based on NHANES 2001–2006) | USA | 439 | >76.3 | 1.00 (ref.) | ||||||||||
| 52 | Esteghamati et al. (65) | Cross-sectional | Tehran | >18 | Both | 4,391 | <20 ng/ml | 1.34 (0.74, 2.41) | RIA | SBP≥ 130 or DBP≥ 85 mmHg, or a history of antihypertensive-drug use | Metabolically healthy obesity | 1, 2,4 | 9 | |
| Iran | ≥20 | 1.00 (ref.) | ||||||||||||
| Metabolically unhealthy obesity | ||||||||||||||
| 1.66 (1.50, 1.82) | ||||||||||||||
| 1.00 (ref.) | ||||||||||||||
| 53 | Contreras–Manzano et al. (66) | Cross-sectional | Mexico | 20– 49 | women | 3,260 | <50 nmol/l | 1.24 (0.84– 1.81) | Chemilumi nescence microparticle immunoassay |
SBP> 140 and/or a DBP> 90 mmHg or previous diagnosis by a physician of hypertension | Mexican Women of Reproductive Age | 3, 10, 58, 100, 101, 102, 103,104, 105, 106, 107, 108, 109, 110 | 7 | |
| National Health and Nutrition Survey (ENSANUT 2012) | ≥50 | 1.00 (ref.) | ||||||||||||
| 54 | Caro et al. (67) | Cross-sectional | San Juan, Puerto Rico | 21–51 | Both | 219 | <30 ng/ml | 1.11 (0.35, 3.51) | EIA | HTN (SBP≥ 140 or DBP ≥ 90mmHg or antihypertensive medication) | Adults | 1, 2, 4, 20,111 | 8 | |
| 41.5 ± 13.9 | ≥30 ng/ml | 1.00 (ref.) | Pre-HTN (SBP 120–139, DBP 80–89) | |||||||||||
| <30 ng/ml | 0.88 (0.49, 1.60) | |||||||||||||
| ≥30 ng/ml | 1.00 (ref.) | |||||||||||||
| 55 | Zhao et al. et al. (68) | Cross-sectional | Atlanta | 20 ≤ | Both | 7,228 | (PR) | RIA | SBP≥ 140 or DBP≥ 90mmHg | Non-institutionalized civilian United States population | 1, 2, 3, 4, 6, 7, 8, 9, 12, 18, 40, 43, 45, 46, 47, 48, 53, 94, 97, 112,113 | 9 | ||
| (based on NHANES)2003–2006 | USA | 1,665 | <15 ng/ml | 1.00 (ref.) | or a history of antihypertensive-drug use | |||||||||
| 1,420 | 15– <20 | 0.89 (0.80, 0.99) | ||||||||||||
| 1,536 | 20– <25 | 0.89 (0.79, 1.00) | ||||||||||||
| 1,250 | 25– <30 | 0.89 (0.81, 0.98) | Pre-HTN (SBP 120–139, DBP 80–89) | |||||||||||
| 1,357 | ≥30 | 0.82 (0.73, 0.91) | ||||||||||||
| 1,665 | <15 ng/ml | 1.00 (ref.) | ||||||||||||
| 1,420 | 15– <20 | 0.83 (0.72, 0.96) | ||||||||||||
| 1,536 | 20– <25 | 0.88 (0.74, 1.07) | ||||||||||||
| 1,250 | 25– <30 | 0.87 (0.73, 1.03) | ||||||||||||
| 1,357 | ≥30 | 0.80 (0.69, 0.92) | ||||||||||||
| 56 | Sumriddetchka- jorn et al. (69) |
Cross-sectional | Bangkok, Thailand | 35–54 | Both | 274 | 137 | ≤ 28ng/ml | 0.56 (0.33, 0.96) | NR | SBP≥ 140 and/or | 137 Hypertensive cases and 137 normotensive controls | 1, 2, 4, 54, 56 | 4 |
| >28 | 1.00 (ref.) | DBP ≥ 90mmHg | ||||||||||||
| 57 | Shin et al. (70) | Cross-sectional | Seoul, Korea | 50– 79 | Women | 4,107 | <15 (ng/ml) | 1.28 (1.02, 1.61) | RIA | SBP ≥ 140, or DBP ≥ 90mmHg or taking antihypertensive medication | Postmenopausal .women | 1, 4, 5, 6, 7, 8, 9, 11, 15, 17, 45 | 9 | |
| (based on KNHANES V 2010–2012) | ≥15 | 1.00 (ref.) | ||||||||||||
| 58 | Pannu et al. (71) | Cross-sectional | Melbourne | 18–75 | Both | 3,387 | Per 10 nmol/l increase | 1.02 (0.97, 1.07) | Automated direct competitive chemiluminescent immunoassay | SBP≥ 130 | Adults | 1, 2, 3, 4, 5, 6, 7, 8, 9, 11, 16, 19, 25, 28, 29 | 9 | |
| based on Victorian Health Monitor (VHM) survey | Victoria | |||||||||||||
| Australia | 0.97 (0.92, 1.01) | DBP≥ 85 | ||||||||||||
| mmHg | ||||||||||||||
| or on antihypertensive medications | ||||||||||||||
| 59 | Li et al. (72) | Cross-sectional | Yunnan Province, China | 20–83 | Both | 1,206 | Per 1 ng/ml increase | RIA | SBP ≥140 or DBP ≥ 90mmHg, taking antihypertensive medication | Participants without antihypertensive treatment | 1, 2, 4, 5, 6, 36, 45 | 7 | ||
| Men | 728 | in baseline | 1.00 (0.98, 1.02) | |||||||||||
| Women | 478 | 1.00 (0.97, 1.04) | ||||||||||||
| 60 | Kwak et al. (48) | Cross-sectional | Republic of Korea | ≥20 | Both | 2,591 | Per 1 ng/ml increase in baseline | 0.97 (0.94, 0.99) | RIA | SBP ≥140 | Adults | 1, 2, 4, 5, 6, 7, 8, 77 | 9 | |
| (KNHANES) 2011–2012 | DBP ≥ 90mmHg or on antihypertensive medication | |||||||||||||
| 61 | Gagnon | Cohort | Australia | ≥25 | Both | 4,164 | Per 10–ng/ml decrease in baseline | 1.03 (0.94, 1.14) | CLIA | SBP≥ 130 | Adults | 1, 2, 3, 5, 7, 8, 11 16, 17, 38, 45, 83 | 8 | |
| 2012 | 5 years of follow-up: | DBP≥ 85 | ||||||||||||
| (The Australian Diabetes, Obesity and Lifestyle study, | mmHg | |||||||||||||
| AusDiab) | or on antihypertensive medications | |||||||||||||
| 62 | Dong et al. (73) | Cross-sectional | China | ≥18 | Both | 837 | Per 10 nmol/l increase in baseline | 0.66 (0.38, 1.16) | ELISA | SBP≥ 130 | Peritoneal dialysis patients | 1, 2, 20, 43, 46, 50, 51, 114, 115, 116, 117 | 6 | |
| DBP≥ 85 | ||||||||||||||
| mmHg (or drug treatment) | ||||||||||||||
| 63 | Chen et al. (74) | Cross-sectional | Beijing, China | 60_102 | Both | 1,245 | Per 1 ng/ml increase in baseline | 0.98 (0.97, 0.99) | Chemilumi- nescence assay |
SBP ≥140 | Elderly Chinese Population | 1, 2, 4, 5, 6, 18, 43, 45, 46, 58, 89, 115, 118 | 9 | |
| DBP ≥ 90mmHg or a taking antihypertensive drug | ||||||||||||||
| 64 | Chen et al. (75) | Cross-sectional | China | 54.9 | Both | 10,655 | Per 10 nmol/l increase in baseline | 1.043 (1.004, 1.084) | CLIA | SBP≥ 130 | Adults | 1, 2, 5, 9, 15, 17, 54, 56, 58 | 8 | |
| DBP≥ 85 | ||||||||||||||
| mmHg (or drug treatment) | ||||||||||||||
| 65 | Skaaby et al. (76) | Cohort | Denmark | 29.7–61.2 | Both | 2,571 | 403 | per 10 nmol/l increase in baseline | 1.01 (0.97, 1.05) | HPLC | SBP> 140, DBP> 90 mm Hg or treatment of previously diagnosed | Adults | 1, 2, 4, 5, 6, 7, 8, 11, 17, 18, 97, ,119, 120, 121 | 9 |
| 5 years of followup | hypertension | |||||||||||||
| 66 | Sabanayagam et al. (77) | Cross-sectional | USA | >20 | Both | RIA | Prehypertension: SBP 120–139 mm Hg or DBP 80–89 mm Hg | Adults | 1, 3, 4, 5, 6, 7, 38, 43, 45, 122 | 9 | ||||
| (NHANES III) | Men | 800 | Q1 ( ≤ 17.7 ng/ml) | 1.53 (1.13, 2.07) | ||||||||||
| 1,040 | Q2 (17.8– 24.6) | 1.28 (0.98, 1.66) | ||||||||||||
| 1,207 | Q3 (24.7– 32.4) | 1.07 (0.80, 1.44) | ||||||||||||
| 1,242 | Q4 (>32.4) | 1.00 (ref.) | ||||||||||||
| Women | 1,482 | Q1 ( ≤ 17.7 ng/ml) | 1.44 (1.03, 2.00) | |||||||||||
| 1,278 | Q2 (17.8– 24.6) | 1.23 (0.93, 1.62) | ||||||||||||
| 1,099 | Q3 (24.7– 32.4) | 1.19 (0.89, 1.61) | ||||||||||||
| 1,067 | Q4 (>32.4) | 1.00 (ref.) | ||||||||||||
| 67 | Vacek et al. (78) | Cross-sectional | Kansas | 58.3 ± 14.9 | Both | 10,899 | <30 ng/ml | 1.40 (1.285, 1.536) | CLIA | SBP> 140, DBP> 90 mm Hg | Adults | NR | 8 | |
| USA | ≥30 | 1.00 (ref.) | ||||||||||||
| 68 | Mateus-Hamdan et al. (79) | Cross-sectional | France | 85.87 ± 5.90 | Both | 284 | Per 1 nmol/l increase in baseline | 1.01 (0.99, 1.03) | RIA | SBP> 140, DBP> 90 mm Hg | Elderly inpatients | 1, 2, 11, 20, 22, 50, 77, 126, 127, 128, 129, 130 | 7 | |
| 69 | Ford et al. (16) | Cross-sectional | Boston | 20 ≤ | Both | 8,421 | ≤ 48.4 nmol/l | 1.00 (ref.) | RIA | SBP≥ 130 | Adults | 1, 2, 3, 5, 6, 8, 11, 16, 21, 31, 40, 43, 53, | 7 | |
| (based on NHANES III, 1988–1994) | 48.5– 63.4 | 1.17 ( 0.95, 1.44) | DBP≥ 85 | |||||||||||
| 63.5– 78.1 | 1.00 (0.77, 1.30) | mmHg | ||||||||||||
| 78.2– 96.3 | 1.16 (0.85, 1.59) | |||||||||||||
| ≥96.4 | 1.07 (0.77, 1.50) | |||||||||||||
| 70 | Muldowney et al. (80) | Cross-sectional | Multi Country | 20–40 | Both | 195 | T1 ( ≤ 42.5nmol/l) | 0.87 (0.35, 2.20) | ELISA | SBP≥ 130 mmHg | Participants in a weight loss dietary intervention study | 1, 2, 5, 10, 16, 17, 18, 20, 22, 111 | 7 | |
| Iceland (64°N;), Ireland (51°N;) and Spain (42°N;) | T2 (42.51–63.0) | NR | ||||||||||||
| T3 (>63.0) | 1.00 (ref.) | |||||||||||||
| 1.21 (0.16, 8.87) | ||||||||||||||
| NR | DBP≥ 85 | |||||||||||||
| 1.00 (ref.) | mmHg | |||||||||||||
| 71 | Piantanida et al. (81) | Cross-sectional | Italy | 51 ± 13 | Both | 196 | <10 ng/ml | 1.65 (0.7, 4.0) | CLIA | SBP≥ 130 | Caucasian obese adults | 4 | 6 | |
| 20–Oct | 3.2 (1.5, 7.0) | DBP≥ 85 | ||||||||||||
| ≥20 | 1.00 (ref.) | mmHg |
Adjustments: 1, Age or age range; 2, Gender; 3, Race or ethnicity/ country of birth; 4, BMI or BMI category; 5, Smoking (status); 6, Alcohol; 7,Physical activity or exercise; 8, Education; 9,Income; 10, Region; 11,Time of blood drown(season/month/week); 12, Marital status; 13, Religion; 14, Occupation; 15, Residency/ residential district; 16, The rest of the individual components of the MetS; 17, Waist Circumference/ abdominal obesity; 18, Parathyroid hormone; 19, Total energy intake; 20, Total vitamin D intake/ supplementation; 21, Multivitamin supplementation; 22, Calcium intakes/ supplementation; 23, Potassium intakes; 24, Sodium intakes; 25, Magnesium intakes;26, Salt intake (low, medium, high); 27, Fish oil intake; 28, Zinc intake; 29, Fiber intake; 30, Eggs intake;31, Consumption of fruits, vegetables, dairy, red meat, whole grains and refined sugar; 32, Sum of total fruit and vegetable Healthy Eating Index scores; 33, Diet quality score; 34, Sleeping pattern; 35, The use of sunscreen; 36, Family history of hypertension; 37, (family) History of cardio-metabolic diseases; 38, History of diabetes; 39, History of hypercholesterolemia; 40, Serum cotinine; 41, Baseline cardio-metabolic diseases; 42, Year of blood draw ;43, Serum C-reactive protein; 44, Serum creatinine; 45, eGFR; 46, Serum calcium; 47, Serum sodium; 48, Serum potassium; 49, Serum magnesium levels; 50, Serum albumin; 51, Serum phosphorus; 52, Serum iron; 53, Serum total cholesterol; 54, HDL-C; 55, LDL-C; 56, Triglycerides; 57, HbA1c ranges; 58, Diabetes Mellitus; 59, HT; 60, Time of menopause; 61, Waist to Hip Ratio; 62, Work related physical activity; 63, Recruitment location; 64, Ultraviolet radiation index at participants recruitment location; 65, RI (defined as eGFR <60 mL/min/1.73 m2); 66, 1,25 (OH)2D; 67, Study center; 68, Different casecontrol study vitamin D analysis; 69, Day of menstrual cycle if premenopausal; 70, Hour of the blood collection; 71, Oral contraceptive use; 72, Serum uric acid; 73, Menopausal status; 74,25(OH)D/IGF-1 as relevant; 75, Birth; 76, Adult social class; 77, Antihypertensive medication; 78, Laboratory of 25(OH)D analysis; 79, Number of years smoked; 80, Number of cigarettes smoked per day; 81, The presence of diseases; 82, Length of residence in South Korea; 83, Country (Korea, UK, US, or other); 84, Clinical center; 85, Calcium/vitamin D trial assignment; 86, Blood pressure at enrollment; 87, Work type (mental work, physical work); 88, Work environment (surface or underground); 89, Hyperlipidemia; 90, In women, hormone therapy; 91, %Fat mass; 92, 24-h urinary albumin excretion; 93, Clinic site; 94, Diabetes status; 95, Non-steroidal anti-inflammatory drug use; 96, Cyclooxygenase-2 inhibitor use; 97, Albumin creatinine ratio; 98, Diastolic blood pressure; 99, Low HDL-cholesterol; 100, Area (urban/rurality); 101, Wellbeing index tertiles; 102, HDL-C (<50 mg/dL); 103, TG (<150 mg/dL); 104, TC (<200 mg/dL); 105, CRP (<5 mg/dL); 106, Hcy (<10 umol/L); 107, Sedentarism; 108, Overweightobesity; 109, insulin resistance; 110, Acute myocardial infarction; 111, Sun exposure; 112, Coronary heart disease; 113, Dietary supplement use; 114, Dialysis duration; 115, Serum hemoglobin; 116, Total Kt/V urea; 117, Residual renal function; 118, Fasting glucose; 119, Randomization status; 120, Diet; 121, 5-year changes in BMI; 122, Total to high-density lipoprotein cholesterol ratio; 123, Ratio of non-HDL to HDL cholesterol; 124, Stroke in parents; 125, Atrial fibrillation; 126, The number of chronic diseases (i.e. diseases lasting at least 3 months or running a course with minimal change); 127, Drugs taken per day; 128, Corticosteroid drugs; 129, TSH; mUI/L; 130, Creatinine clearance (mL/min); 131, Peripheral vascular disease.
CLIA, Chemiluminescent immunoassay; RIA, Radioimmunoassay; ELISA, Enzyme-linked immunosorbent assay; ECLIA, electrochemiluminescence immunoassay; EIA, Enzyme immunoassay; CPBA, Competitive protein binding ; DEIA, Direct enzyme immunoassay; HPLC, High-performance liquid chromatography; SBP, Systolic blood pressure; DBP, Diastolic blood pressure; HTN, Hypertension, SLE, Systematic lupus erithematous.
Meta-Analysis of Highest vs. Lowest Vitamin D Level in Relation to Hypertension in Prospective Studies
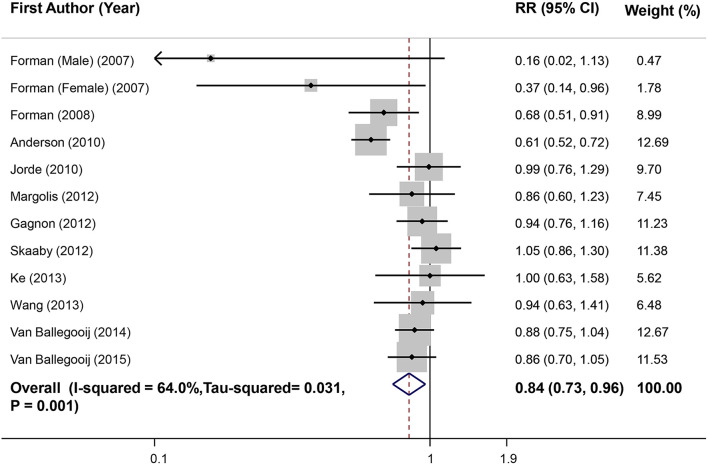
Combination of 12 effect sizes from 11 studies (n = 66,757) led to an overall effect of 0.84 (0.73, 0.96) that showed a 16% decrease in risk of hypertension in participants who had a high level of serum vitamin D compared with those with low level (Figure 2). Heterogeneity was moderate (I2 = 64%, Tau2 = 0.031, P = 0.001). Subgroup analyses were conducted to find the source of heterogeneity and the findings are reported in Table 2. None of the covariates could completely explain the observed heterogeneity. Then, excluding one study of Anderson et al. (39) removed the observed heterogeneity (I2 = 24.3%, P = 0.21), without significant change in the overall estimate (RR = 0.89; 95%CI: 0.81, 0.99). Sensitivity analysis showed that excluding each investigation had no significant effect on pooled RR. Also, there was no asymmetry in the funnel plot and no evidence for publication bias (Begg's test = 0.49, Egger's test = 0.64) (Supplementary Figure 1A).
Figure 2.
Forest plot of prospective studies that examined the association between highest vs. lowest level of serum vitamin D and risk of hypertension (HTN).
Table 2.
Results of subgroup-analysis for circulating vitamin D levels and risk of hypertension in Cohort studies.
| No. of effect sizes | RR (95% CI) | I2 (%) | P withina | P betweenb | |
|---|---|---|---|---|---|
| Overall | 12 | 0.84 (0.73, 0.96) | 64 | 0.001 | |
| Gender | 0.332 | ||||
| Male | 3 | 0.89 (0.58, 1.37) | 37.7 | 0.201 | |
| Female | 3 | 0.71 (0.53, 0.95) | 32.1 | 0.229 | |
| Both | 6 | 0.87 (0.73, 1.03) | 77.5 | <0.001 | |
| Comparison vitamin D levels | <0.001 | ||||
| Q5 vs. Q1 | 1 | 1.00 (0.63, 1.58) | – | – | |
| Q4 vs. Q1 | 4 | 0.90 (0.79, 1.04) | 00.0 | 0.853 | |
| T3 vs. T1 | 5 | 0.90 (0.74, 1.09) | 52.9 | 0.075 | |
| SUF vs. DIF | 2 | 0.63 (0.54, 0.72) | 00.0 | 0.526 | |
| Time of Blood draw adjustment | 0.016 | ||||
| Adjusted | 7 | 0.90 (0.82, 1.00) | 5.1 | 0.388 | |
| Not adjusted | 5 | 0.73 (0.54, 0.98) | 78.3 | 0.001 | |
| Age, gender, BMI adjustment | 0.007 | ||||
| Adjusted | 6 | 0.90 (0.77, 1.06) | 45.5 | 0.103 | |
| Not adjusted | 6 | 0.80 (0.66, 0.97) | 64.4 | 0.015 | |
| Representative | 0.861 | ||||
| Representative | 8 | 0.84 (0.70, 1.00) | 74.8 | <0.001 | |
| Not representative | 4 | 0.82 (0.68, 0.98) | 00.0 | 0.427 | |
| Quality statusc | 0.002 | ||||
| High quality | 9 | 0.88 (0.78, 0.99) | 38.1 | 0.115 | |
| Low quality | 3 | 0.79 (0.55, 1.13) | 70.8 | 0.001 |
P for heterogeneity, within subgroup.
P for heterogeneity, between subgroups.
Quality scores were according to: Newcastle-Ottawa Scale.
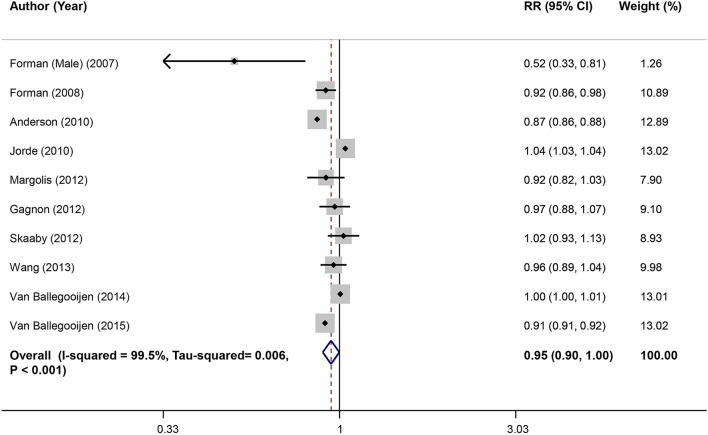
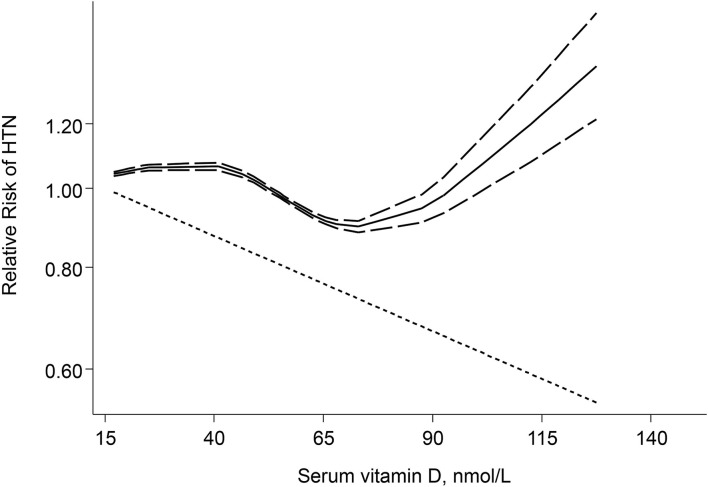
Dose–Response Meta-Analysis of Serum Vitamin D and Risk of Hypertension in Prospective Studies
Combining effect sizes of 10 studies involving a total of 63,602 individuals and 25,019 cases of hypertension showed that each 25 nmol/L increase in serum vitamin D level resulted in a 5% reduction in risk of hypertension (RR: 0.95; 95% CI: 0.90, 1.00) (Figure 3). Also, a significant non-linear association between serum vitamin D levels and hypertension was observed (Pnon−linearity <0.001). A reduction trend in risk of hypertension was observed for serum vitamin D levels between 45 and 70 nmol/L, although for higher vitamin D levels the risk did not decrease anymore and eventually started increasing (Figure 4).
Figure 3.
Linear dose–response meta-analysis of serum vitamin D and risk of HTN in prospective studies.
Figure 4.
Non-Linear dose–response meta-analysis of serum vitamin D and risk of HTN in prospective studies.
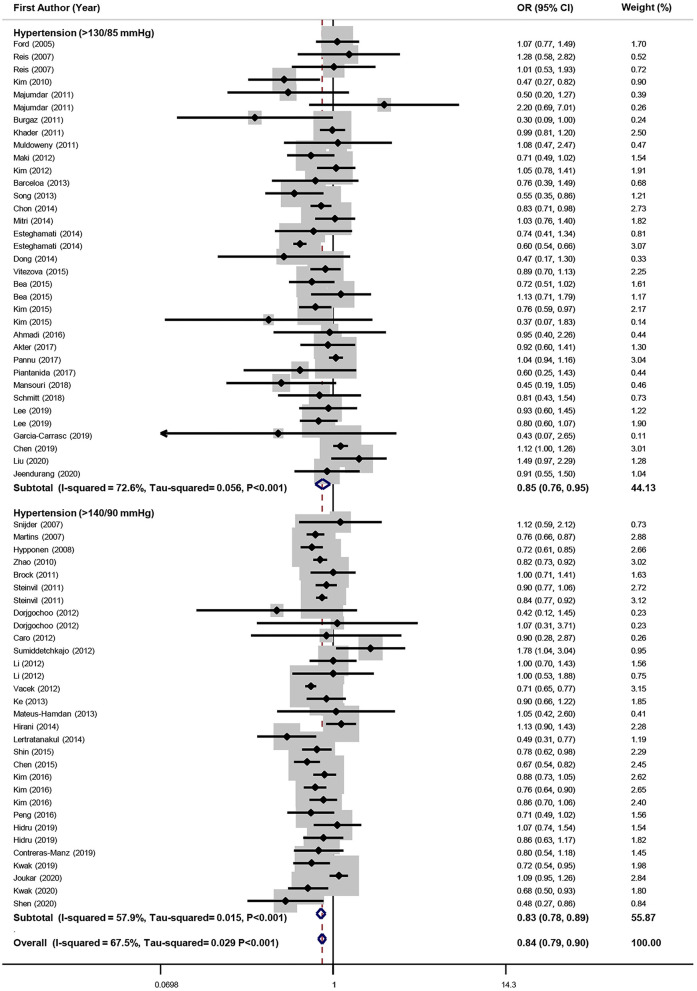
Meta-Analysis of Highest vs. Lowest Vitamin D Level in Relation to Hypertension in Cross-Sectional Studies
The association between serum vitamin D and odds of hypertension was examined in 56 investigations; 66 effect sizes were included in this analysis (n = 248,657). Meta-analysis determined that highest level of vitamin D in comparison to the lowest level was associated with a 16% significant decrease in odds of hypertension in cross-sectional studies (95%CI: 0.79, 0.90). Heterogeneity was significant (I2 = 67.5%, Tau2 = 0.029, P < 0.001). Subgroup analyses were conducted according to different confounders and the findings are presented in Table 3. The definition of hypertension (BP ≥ 140/90 vs. ≥ 130/85 mmHg) had no effect on results (for BP ≥ 140/90: OR = 0.83; 95%CI: 0.78, 0.89 and for BP ≥ 135/80 OR = 0.85; 95%CI: 0.76, 0.95) (Figure 5). Also, in both representative and not representative studies, a significant reduction in the odds of hypertension was observed (for representative studies: OR = 0.86; 95%CI: 0.80, 0.92 and for not representative studies: OR = 0.78; 95%CI: 0.69, 0.88). Although in most of the subgroups significant associations between vitamin D level and hypertension were found, none of the confounders could fully explain the observed heterogeneity. Sensitivity analysis determined that the exclusion of each study did not significantly affect the overall estimate. No significant publication bias was observed (Begg's test = 0.44, Egger's test = 0.84) (Supplementary Figure 1B).
Table 3.
Results of subgroup-analysis for circulating vitamin D levels and odds of hypertension in cross-sectional studies.
| No. of effect sizes | OR (95% CI) | I2 (%) | P withina | P betweenb | |
|---|---|---|---|---|---|
| Overall | 66 | 0.84 (0.79, 0.90) | 67.5 | <0.001 | |
| Asian vs. Non-Asian | 0.716 | ||||
| Asian | 39 | 0.84 (0.77, 0.91) | 72.2 | <0.001 | |
| Non-Asian | 27 | 0.85 (0.78, 0.94) | 58.7 | <0.001 | |
| Development status | 0.337 | ||||
| Developed | 41 | 0.83 (0.79, 0.88) | 49.7 | <0.001 | |
| Developing | 25 | 0.87 (0.74, 1.01) | 79.9 | <0.001 | |
| Gender | 0.072 | ||||
| Male | 12 | 0.92 (0.82, 1.02) | 17.8 | 0.269 | |
| Female | 17 | 0.82 (0.77, 0.87) | 00.0 | 0.735 | |
| Both | 37 | 0.84 (0.76, 0.92) | 78.7 | <0.001 | |
| Comparison Vitamin D levels | <0.001 | ||||
| Q5 vs. Q1 | 9 | 0.90 (0.76, 1.07) | 42.1 | 0.087 | |
| Q4 vs. Q1 | 16 | 0.79 (0.73, 0.86) | 26.6 | 0.156 | |
| T3 vs. T1 | 26 | 0.89 (0.82, 0.97) | 59.2 | <0.001 | |
| SUF vs. DIF | 15 | 0.82 (0.69, 0.96) | 79.8 | <0.001 | |
| Outcome definition | 0.034 | ||||
| Blood Pressure ≥ 140/90 | 31 | 0.83 (0.78, 0.89) | 57.9 | <0.001 | |
| Blood Pressure ≥ 130/85 | 35 | 0.85 (0.76, 0.95) | 72.6 | <0.001 | |
| Health status | <0.001 | ||||
| Healthy | 54 | 0.86 (0.81, 0.91) | 62.9 | <0.001 | |
| Unhealthy | 12 | 0.74 (0.61, 0.90) | 44.4 | 0.048 | |
| Time of Blood draw adjustment | 0.003 | ||||
| Adjusted | 35 | 0.85 (0.79, 0.91) | 50.6 | <0.001 | |
| Not adjusted | 31 | 0.85 (0.77, 0.93) | 75.5 | <0.001 | |
| Age, gender, BMI adjustment | 0.003 | ||||
| Adjusted | 30 | 0.86 (0.77, 0.95) | 76.5 | <0.001 | |
| Not adjusted | 36 | 0.84 (0.78, 0.90) | 54.3 | <0.001 | |
| Representative | 0.136 | ||||
| Representative | 45 | 0.86 (0.80, 0.92) | 74.7 | <0.001 | |
| Not Representative | 21 | 0.78 (0.69, 0.88) | 14.9 | 0.265 | |
| Quality Statusc | 0.030 | ||||
| High quality | 36 | 0.82 (0.76, 0.89) | 76.4 | <0.001 | |
| Low quality | 30 | 0.89 (0.81, 0.99) | 38.3 | 0.018 |
P for heterogeneity, within subgroup.
P for heterogeneity, between subgroups.
Quality scores were according to: Newcastle–Ottawa Scale.
Figure 5.
Forest plot of cross-sectional studies that examined the association between highest vs. lowest level of serum vitamin D and odds of HTN.
Dose–Response Meta-Analysis of Serum Vitamin D and Risk of Hypertension in Cross-Sectional Studies
Combing effect sizes of 30 studies involving a total of 139,685 individuals and 40,178 cases of hypertension showed that each 25 nmol/L increase in serum vitamin D level resulted in a 6% reduction in risk of hypertension (OR = 0.94; 95% CI: 0.90, 0.99) (Supplementary Figure 2). Also, a significant non-linear association between serum vitamin D levels and hypertension was seen (Pnon−linearity <0.001); such that a reduction trend in odds of hypertension for serum vitamin D levels was observed between levels of 40–75 nmol/L, higher vitamin D levels did not reduce odds of HTN (Supplementary Figure 3). When the analysis was restricted to 21 cross-sectional studies with representative populations (133.497 general adult population with 37,341 cases of hypertension), we found that each 25 nmol/L increase in circulating vitamin D concentration reduced the risk of HTN by 3% (RR: 0.97; 95%CI: 0.95, 0.99) (Supplementary Figure 4). Also, a significant non-linear association between serum vitamin D levels and hypertension was observed (Pnon−linearity <0.001). As shown in Supplementary Figure 5, a U-shaped relationship was found.
Meta-Analysis of Highest vs. Lowest Vitamin D Level in Relation to Pre-hypertension in Cross-Sectional Studies
Combining 9 effect sizes from 7 studies (n = 21,242) revealed that the highest level of vitamin D was associated with a 25% significant reduction in odds of pre-hypertension, compared to the lowest level (0.95%CI: 0.68, 0.83), without heterogeneity between studies (I2 = 0.0%, Tau2 = 0.000, P = 0.44) (Supplementary Figure 6). Sensitivity analysis was carried out and excluding each investigation had no significant effect on the overall estimate. No evidence for publication bias was seen (Begg's test = 0.40, Egger's test = 0.82) (Supplementary Figure 1C).
Quality of the Evidence
GRADE evidence profile for serum vitamin D concentration in relation to hypertension and pre-hypertension are presented in Supplementary Tables 5 and 6. The certainty of the evidence was rated as “high quality” for both cohort and cross-sectional studies for serum vitamin D concentration in relation to hypertension, and “moderate quality” for cross-sectional studies that investigated serum vitamin D concentration in relation to pre-hypertension. For serum vitamin D–hypertension relation, the endpoint for both cohort and cross-sectional studies was upgraded for domains of “risk of bias” and “inconsistency.” For cohort studies, 95% CI of overall effect contained a minimal value of 0.75; so, the certainty of the evidence was downgraded for “imprecision.” Both cohort and cross-sectional studies had also reported essential data for dose–response analysis; so, the endpoint of these investigations was upgraded for “other considerations.“ For pre-hypertension, the endpoint for cross-sectional studies was upgraded for the ”risk of bias “domain. Also, 95% CI of overall effect contained a minimal value of 0.75; so, the certainty of the evidence was downgraded for ”imprecision.“ Included studies in the analysis of prehypertension did not provide enough data for dose–response analysis and the endpoint for these studies was downgraded for “other considerations.”
Discussion
In this meta-analysis, we found an inverse significant association between serum vitamin D concentrations and risk of hypertension in the adult population, when we compared the highest level of serum vitamin D vs. the lowest level, in both prospective cohort and cross-sectional studies. This inverse association was independent of hypertension definition (BP ≥ 140/90 vs. ≥ 130/85 mmHg). Also, dose–response analysis showed a significant linear and non-linear relationship between serum vitamin D and risk of hypertension.
Hypertension is one of the most prevalent chronic diseases all over the world and imposes a great economic burden on health care systems (88, 89). BP control rates are far from satisfactory worldwide, while hypertension is a main preventable cause of CVD and all-cause mortality (88). We demonstrated that normal levels of serum vitamin D concentrations were associated with a lower risk of hypertension, but the lowering risk did not continue after increasing serum vitamin D from normal levels. This finding could be particularly important for vitamin D-deficient adults to have a successful treatment, while they should avoid receiving extra vitamin D supplements.
In line with our meta-analysis, several previous systematic reviews and meta-analyses have evaluated the association between serum vitamin D levels and the risk of different non-communicable diseases. In 2013, a dose–response meta-analysis on 5 prospective cohorts revealed that each 10 ng/mL (or 25 nmol/L) increment in serum vitamin D levels was associated with a 12% decreased risk of future hypertension (90). However, almost all of the included studies were conducted in United States which made it impossible to generalize the finding to other populations and the number of included individuals (n = 6,716) and cases of HTN (n = 2,371) were limited (90). A recent dose–response analysis indicated that each 25 nmol/L increment in serum vitamin D concentration was related to 8% reduced risk of abdominal obesity (91). Furthermore, another dose–response analysis of prospective studies reported that a decrease of 10 nmol/L vitamin D was associated with a 7% increment in the risk of CVD mortality in older adults (92), although the small overall sample size of the study (21,079 participants) might have a certain impact on the estimated results in the mentioned analysis (92). Another meta-analysis on prospective cohorts showed that decreased vitamin D levels were associated with a 54% increment in risk of CVD mortality with no significant results among gender subgroups (93). Some studies revealed that the CVD mortality was higher in vitamin D-deficient men (94) and some others confirmed a lower mortality rate in vitamin D-deficient women than men (95). In the current meta-analysis, an inverse significant association was found in women in both cohort and cross-sectional studies, while no significant relation was observed in the male population. However, it should be considered that a few number of effect sizes were available from the male population and almost half of the included studies did not provide separate reports for men and women. Taken these findings together, future studies should provide gender-stratified analysis to shed a light on the gender-specific relations.
In a meta-analysis of randomized controlled trials, a small reduction in DBP was seen in response to vitamin D supplementation in hypertensive patients but had no significant effect on normotensive individuals (96). Another meta-analysis on 8 trials with 917 participants indicated that vitamin D supplementation had a moderate SBP-lowering effect (-1.964 mmHg) without significant effect of DBP. So, this study suggested that vitamin D supplementation could not be used as an antihypertensive agent. While interpreting the results of the mentioned meta-analysis, it should be taken into account that the included trials were performed on both hypertensive and normotensive individuals without considering their baseline 25(OH)D status. Also, the number of included studies was limited and ethnicity and latitude, as 2 effective factors on baseline vitamin D concentrations, had not been considered (97). Another meta-analysis of cohort studies and randomized controlled trials suggested a 7% decrease in risk of hypertension per 25 nmol/L increment in serum vitamin D levels, meanwhile did not find any significant evidence of blood pressure reduction by vitamin D supplementation. Considering that the included randomized controlled trials had small sample sizes and a short duration of follow-up, vitamin D supplementation might have positive effects on blood pressure control in the long term, especially in vitamin D-deficient individuals (98).
Serum vitamin D status might be linked to blood pressure through several mechanisms. The first possible mechanism for the association between a low concentration of 25(OH)D and HTN might be through the activation of the rennin–angiotensin system (RAS). It has been proved that the transcription renin gene could be inversely regulated by 1,25(OH)2D through a vitamin D receptor-mediated mechanism (99). As a result, vitamin D might play the role of a negative regulator to prevent the over-stimulation of the RAS. In fact, 1,25(OH)2D activates the vitamin D receptor which binds the cyclic adenosine monophosphate (CAMP)-response element-binding protein and blocks the renin gene promoter activity, thereby resulting in a decrease in renin secretion (100). Second, vitamin D might affect the cells of the vessel wall such as endothelial and vascular smooth muscle cells; all of these cells could express the vitamin D receptor as well as 1α-hydroxylase (101). Third, lower levels of 25(OH)D concentrations are associated with insulin resistance, and vitamin D supplementation may improve insulin production and insulin sensitivity (102). Insulin resistance has been suggested to be involved in the pathogenesis of hypertension (103). Fourth, vitamin D is indirectly associated with blood pressure due to the role of 25(OH)D in the regulation of calcium absorption (104) and in the maintenance of calcium homeostasis due to the interaction with parathyroid hormone (105). Fifth, 25(OH)D is proposed to have a role in reduction of free radicals local production, with positive effects on vascular health (106).
The current meta-analysis has some strengths and weaknesses. Our analysis included a large population of adults in both cohort and cross-sectional studies. The effect of several confounders was considered via a subgroup analysis. Dose–response analysis was also conducted. In addition, most eligible studies made adjustments for potential confounders including age, gender, BMI, and sampling time (season/month) or sun exposure. GRADE approach provided the certainty that serum vitamin D concentration is related to reduced odds of HTN and pre-HTN and may have a role in decreasing the risk of HTN. However, some limitations should be considered. The number of eligible studies that separately reported the relationship between blood vitamin D levels and hypertension in men and women was limited, so we could not provide appropriate estimates for males and females. More gender-specific studies are needed to obtain the relation between vitamin D and hypertension in males and females separately. In addition, studies were conducted on different age groups of adults and it could lead to heterogeneity because individuals with different age groups had different sun-exposure times and various rates of vitamin D synthesis due to differences in the capacity of the skin to synthesize vitamin D. Moreover, none of the cohort studies made an adjustment for the baseline vitamin D levels in their analyses.
In conclusion, this meta-analysis of epidemiologic studies disclosed that serum vitamin D concentration was inversely associated with risk of hypertension in adults, in a dose–response manner in both cohort and cross-sectional studies. The same association was found for pre-hypertension.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
EM, ZH, and PS contributed in conception, design, statistical analyses, data interpretation, and manuscript drafting. All authors approved the final manuscript for submission.
Funding
The financial support for this study comes from Isfahan University of Medical Sciences, Isfahan, Iran (no. 1400161). Isfahan University of Medical Sciences had no role in the design/conduct of the study, collection/analysis/interpretation of the data, and preparation/review/approval of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.829307/full#supplementary-material
References
- 1.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task force on clinical practice guidelines. J Am Coll Cardiol. (2018) 71:e127–248. 10.1016/j.jacc.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 2.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. Heart disease and stroke statistics−2021 update: a report from the American Heart Association. Circulation. (2021) 143:e254–743. 10.1161/CIR.0000000000000950 [DOI] [PubMed] [Google Scholar]
- 3.Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, Marczak L, et al. Global Burden of Hypertension and Systolic Blood Pressure of at Least 110 to 115 mm Hg, 1990–2015. Jama. (2017) 317:165–82. 10.1001/jama.2016.19043 [DOI] [PubMed] [Google Scholar]
- 4.Sheeran P, Gollwitzer PM, Bargh JA. Nonconscious processes and health. Health Psychol. (2013) 32:460–73. 10.1037/a0029203 [DOI] [PubMed] [Google Scholar]
- 5.Oori MJ, Mohammadi F, Norozi K, Fallahi-Khoshknab M, Ebadi A, Gheshlagh RG. Prevalence of HTN in Iran: meta-analysis of published studies in 2004-2018. Curr Hypertens Rev. (2019) 15:113–22. 10.2174/1573402115666190118142818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mirzaei M, Moayedallaie S, Jabbari L, Mohammadi M. Prevalence of hypertension in Iran 1980-2012: a systematic review. J Tehran Heart Cent. (2016) 11:159−67. [PMC free article] [PubMed] [Google Scholar]
- 7.Sarki AM, Nduka CU, Stranges S, Kandala NB, Uthman OA. Prevalence of hypertension in low- and middle-income countries: a systematic review and meta-analysis. Medicine. (2015) 94:e1959. 10.1097/MD.0000000000001959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. (2020) 16:223–37. 10.1038/s41581-019-0244-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pilz S, Tomaschitz A, Ritz E, Pieber TR. Vitamin D status and arterial hypertension: a systematic review. Nat Rev Cardiol. (2009) 6:621–30. 10.1038/nrcardio.2009.135 [DOI] [PubMed] [Google Scholar]
- 10.Fogacci S, Fogacci F, Cicero AF. Nutraceuticals and hypertensive disorders in pregnancy: the available clinical evidence. Nutrients. (2020) 12:378. 10.3390/nu12020378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bandeira F, Griz L, Dreyer P, Eufrazino C, Bandeira C, Freese E. Vitamin D deficiency: a global perspective. Arq Bras Endocrinol Metabol. (2006) 50:640–6. 10.1590/S0004-27302006000400009 [DOI] [PubMed] [Google Scholar]
- 12.Forman JP, Curhan GC, Taylor EN. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension among young women. Hypertension. (2008) 52:828–32. 10.1161/HYPERTENSIONAHA.108.117630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim MK, Il Kang M, Won Oh K, Kwon HS, Lee JH, Lee WC, et al. The association of serum vitamin D level with presence of metabolic syndrome and hypertension in middle-aged Korean subjects. Clin Endocrinol. (2010) 73:330–8. 10.1111/j.1365-2265.2010.03798.x [DOI] [PubMed] [Google Scholar]
- 14.Burgaz A, Byberg L, Rautiainen S, Orsini N, Hakansson N, Arnlov J, et al. Confirmed hypertension and plasma 25(OH)D concentrations amongst elderly men. J Intern Med. (2011) 269:211–8. 10.1111/j.1365-2796.2010.02309.x [DOI] [PubMed] [Google Scholar]
- 15.Dorjgochoo T, Ou Shu X, Xiang YB, Yang G, Cai Q, Li H, et al. Circulating 25-hydroxyvitamin D levels in relation to blood pressure parameters and hypertension in the Shanghai Women's and Men's Health Studies. Br J Nutr. (2012) 108:449–58. 10.1017/S0007114511005745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ford ES, Ajani UA, McGuire LC. Liu S. Concentrations of serum vitamin D and the metabolic syndrome among US adults. Diabetes care. (2005) 28:1228–30. 10.2337/diacare.28.5.1228 [DOI] [PubMed] [Google Scholar]
- 17.Snijder MB, Lips P, Seidell JC, Visser M, Deeg DJ, Dekker JM, et al. Vitamin D status and parathyroid hormone levels in relation to blood pressure: a population-based study in older men and women. J Intern Med. (2007) 261:558–65. 10.1111/j.1365-2796.2007.01778.x [DOI] [PubMed] [Google Scholar]
- 18.Barcelo A, Esquinas C, Pierola J., De la Pena M, Sanchez-de-la-Torre M, Montserrat JM, et al. Vitamin D status and parathyroid hormone levels in patients with obstructive sleep apnea respiration. Int Rev Thoracic Dis. (2013) 86:295–301. 10.1159/000342748 [DOI] [PubMed] [Google Scholar]
- 19.García-Carrasco M, Mendoza-Pinto C, Cabrera-Jiménez M, Munguía-Realpozo P, Méndez-Martínez S, Etchegaray-Morales I, et al. 25-Hydroxyvitamin D concentrations and risk of metabolic syndrome in systemic lupus erythematosus women. Int J Rheum Dis. (2019) 22:2067–72. 10.1111/1756-185X.13715 [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wells GA TP, O'Connell D, Welch V, Peterson J, Shea B, Losos M. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-analyses. (2015). Available online at:: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. (2015).
- 22.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clinic Epidemiol. (2011) 64:383–94. 10.1016/j.jclinepi.2010.04.026 [DOI] [PubMed] [Google Scholar]
- 23.GRADEpro G. GRADEpro Guideline Development Tool [Software]. McMaster University, (2015). (developed by Evidence Prime, Inc.). (2016). [Google Scholar]
- 24.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. (1992) 135:1301–9. 10.1093/oxfordjournals.aje.a116237 [DOI] [PubMed] [Google Scholar]
- 25.Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose–response data. Stata J. (2006) 6:40–57. 10.1177/1536867X0600600103 [DOI] [Google Scholar]
- 26.Lee SJ, Lee EY, Lee JH, Kim JE, Kim KJ, Rhee Y, et al. Associations of serum 25-hydroxyvitamin D with metabolic syndrome and its components in elderly men and women: the Korean Urban Rural Elderly cohort study. BMC Geriatr. (2019) 19:102. 10.1186/s12877-019-1118-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vitezova A, Zillikens MC, van Herpt TT, Sijbrands EJ, Hofman A, Uitterlinden AG, et al. Vitamin D status and metabolic syndrome in the elderly: the Rotterdam Study. Euro J Endocrinol. (2015) 172:327–35. 10.1530/EJE-14-0580 [DOI] [PubMed] [Google Scholar]
- 28.Chon SJ, Yun BH, Jung YS, Cho SH, Choi YS, Kim SY, et al. Association between vitamin D status and risk of metabolic syndrome among Korean postmenopausal women. PLoS One. (2014) 9:e89721. 10.1371/journal.pone.0089721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maki KC, Fulgoni VL, Keast DR, Rains TM, Park KM, Rubin MR. Vitamin D intake and status are associated with lower prevalence of metabolic syndrome in U.S. Adults: National health and nutrition examination surveys 2003–2006. Metabolic Syndrome Relat Disord. (2012) 10:363–72. 10.1089/met.2012.0020 [DOI] [PubMed] [Google Scholar]
- 30.Mansouri M, Abasi R, Nasiri M, Sharifi F, Vesaly S, Sadeghi O, et al. Association of vitamin D status with metabolic syndrome and its components: a cross-sectional study in a population of high educated Iranian adults. Diabetes Metab Syndr. (2018) 12:393–8. 10.1016/j.dsx.2018.01.007 [DOI] [PubMed] [Google Scholar]
- 31.Schmitt EB, Nahas-Neto J, Bueloni-Dias F, Poloni PF, Orsatti CL, Petri Nahas EA. Vitamin D deficiency is associated with metabolic syndrome in postmenopausal women. Maturitas. (2018) 107:97–102. 10.1016/j.maturitas.2017.10.011 [DOI] [PubMed] [Google Scholar]
- 32.Bea JW, Jurutka PW, Hibler EA, Lance P, Martinez ME, Roe DJ, et al. Concentrations of the vitamin D metabolite 1,25(OH)2D and odds of metabolic syndrome and its components. Metabolism. (2015) 64:447–59. 10.1016/j.metabol.2014.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim D, Kim J. Age and sex differences in the relationship between serum 25-hydroxyvitamin D and hypertension in the general Korean population. Eur J Clin Nutr. (2012) 70:326–32. 10.1038/ejcn.2015.115 [DOI] [PubMed] [Google Scholar]
- 34.Majumdar V, Nagaraja D, Christopher R. Vitamin D status and metabolic syndrome in Asian Indians. Int J obesity. (2005). 35:1131–4. 10.1038/ijo.2010.232 [DOI] [PubMed] [Google Scholar]
- 35.Akter S, Eguchi M, Kurotani K, Kochi T, Kashino I, Ito R, et al. Serum 25-hydroxyvitamin D and metabolic syndrome in a Japanese working population: the furukawa nutrition and health study. Nutrition. (2017) 36:26–32. 10.1016/j.nut.2016.02.024 [DOI] [PubMed] [Google Scholar]
- 36.Mitri J, Nelson J, Ruthazer R, Garganta C, Nathan DM, Hu FB, et al. Plasma 25-hydroxyvitamin D and risk of metabolic syndrome: an ancillary analysis in the Diabetes Prevention Program. Eur J Clin Nutr. (2014) 68:376–83. 10.1038/ejcn.2013.293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahmadi F, Damghani S, Lessan-Pezeshki M, Razeghi E, Maziar S, Mahdavi-Mazdeh M. Association of low vitamin D levels with metabolic syndrome in hemodialysis patients. Hemodial Int Sympos Home Hemodial. (2016) 20:261–9. 10.1111/hdi.12316 [DOI] [PubMed] [Google Scholar]
- 38.Brock KE, Huang WY, Fraser DR, Ke L, Tseng M, Mason RS, et al. Diabetes prevalence is associated with serum 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D in US middle-aged Caucasian men and women: a cross-sectional analysis within the prostate, lung, colorectal and ovarian cancer screening trial. Br J Nutr. (2011) 106:339–44. 10.1017/S0007114511001590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson JL, May HT, Horne BD, Bair TL, Hall NL, Carlquist JF, et al. Relation of Vitamin D Deficiency to cardiovascular risk factors, disease status, and incident events in a general healthcare population. Am J Cardiol. (2010) 106:963–8. 10.1016/j.amjcard.2010.05.027 [DOI] [PubMed] [Google Scholar]
- 40.Forman JP, Giovannucci E, Holmes MD, Bischoff-Ferrari HA, Tworoger SS, Willett WC, et al. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. (2007) 49:1063–9. 10.1161/HYPERTENSIONAHA.107.087288 [DOI] [PubMed] [Google Scholar]
- 41.Hidru TH, Yang X, Xia Y, Ma L, Li HH. The relationship between Plasma Markers and essential hypertension in middle-aged and elderly Chinese population: a community based cross-sectional study. Sci Rep. (2019) 9:6813. 10.1038/s41598-019-43278-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hirani V, Cumming RG, Naganathan V, Blyth F, Le Couteur DG, Handelsman DJ, et al. Associations between serum 25-hydroxyvitamin D concentrations and multiple health conditions, physical performance measures, disability, and all-cause mortality: The concord health and ageing in men project. J Am Geriatr Soc. (2014) 62:417–25. 10.1111/jgs.12693 [DOI] [PubMed] [Google Scholar]
- 43.Hypponen E, Boucher BJ, Berry DJ, Power C. 25-hydroxyvitamin D, IGF-1, and metabolic syndrome at 45 years of age: a cross-sectional study in the (1958). British Birth Cohort Diabetes. (2008) 57:298–305. 10.2337/db07-1122 [DOI] [PubMed] [Google Scholar]
- 44.Jorde R, Figenschau Y, Emaus N, Hutchinson M, Grimnes G. Serum 25-hydroxyvitamin D levels are strongly related to systolic blood pressure but do not predict future hypertension. Hypertension. (2010) 55:792–8. 10.1161/HYPERTENSIONAHA.109.143990 [DOI] [PubMed] [Google Scholar]
- 45.Joukar F, Naghipour M, Hassanipour S, Salari A, Alizadeh A, Saeidi-Saedi H, et al. Association of Serum Levels of Vitamin D with blood pressure status in Northern Iranian Population: The PERSIAN guilan cohort study (PGCS). Int J Gen Med. (2020) 13:99–104. 10.2147/IJGM.S244472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ke L, Graubard BI, Albanes D, Fraser DR, Weinstein SJ, Virtamo J, et al. Hypertension, pulse, and other cardiovascular risk factors and vitamin D status in Finnish men. Am J Hypertens. (2013) 26:951–6. 10.1093/ajh/hpt051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim J. Association between serum vitamin D, parathyroid hormone and metabolic syndrome in middle-aged and older Korean adults. Eur J Clin Nutr. (2015) 69:425–30. 10.1038/ejcn.2014.192 [DOI] [PubMed] [Google Scholar]
- 48.Kwak JH, Hong YC, Choi YH. Serum 25-hydroxyvitamin D and hypertension in premenopausal and postmenopausal women: National Health and Nutrition Examination Surveys 2007–2010. Public Health Nutri. (2020) 2020:1–11. 10.1017/S1368980019003665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lertratanakul A, Wu P, Dyer A, Urowitz M, Gladman D, Fortin P, et al. 25-hydroxyvitamin D and cardiovascular disease in patients with systemic lupus erythematosus: data from a large international inception cohort. Arthritis Care Res. (2014) 66:1167–76. 10.1002/acr.22291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu L, Cao ZJ, Lu F, Liu YC, Lv YB, Qu YL, et al. Vitamin D deficiency and metabolic syndrome in elderly Chinese individuals: evidence from CLHLS. Nutri Metabol. (2020). 17:1. 10.1186/s12986-020-00479-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Margolis KL, Martin LW, Ray RM, Kerby TJ, Allison MA, Curb JD, et al. A prospective study of serum 25-hydroxyvitamin D levels, blood pressure, and incident hypertension in postmenopausal women. Am J Epidemiol. (2012) 175:22–32. 10.1093/aje/kwr274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peng M, Chen S, Jiang X, Zhang W, Wang Y, Wu S, et al. Dissociation between Low Vitamin D level and hypertension in coal mine workers: evidence from the kailuan study. Intern Med. (2016) 55:1255–60. 10.2169/internalmedicine.55.5898 [DOI] [PubMed] [Google Scholar]
- 53.Reis JP, von Muhlen D, Kritz-Silverstein D, Wingard DL, Barrett-Connor E. Vitamin D, parathyroid hormone levels, and the prevalence of metabolic syndrome in community-dwelling older adults. Diabetes Care. (2007) 30:1549–55. 10.2337/dc06-2438 [DOI] [PubMed] [Google Scholar]
- 54.Shen F, Guo C, Wang Y, Yu F, Zhang D, Liu X, et al. Low serum 25-hydroxyvitamin D levels may increase the detrimental effect of VDR variants on the risk of essential hypertension. Eur J Clin Nutr. (2020) 74:1091–9. 10.1038/s41430-019-0543-5 [DOI] [PubMed] [Google Scholar]
- 55.Song HR, Park CH. Low serum vitamin D level is associated with high risk of metabolic syndrome in post-menopausal women. J Endocrinol Invest. (2013) 36:791–6. 10.1007/BF03346758 [DOI] [PubMed] [Google Scholar]
- 56.Steinvil A, Leshem-Rubinow E, Berliner S, Justo D, Finn T., Ish-shalom M, et al. Vitamin D deficiency prevalence and cardiovascular risk in Israel. Euro J Clinic Investigat. (2011) 41:263–8. 10.1111/j.1365-2362.2010.02403.x [DOI] [PubMed] [Google Scholar]
- 57.van Ballegooijen AJ, Gansevoort RT, Lambers-Heerspink HJ, de Zeeuw D, Visser M, Brouwer IA, et al. Plasma 1,25-Dihydroxyvitamin D and the risk of developing hypertension: the prevention of renal and vascular end-stage disease study. Hypertension. (2015) 66:563–70. 10.1161/HYPERTENSIONAHA.115.05837 [DOI] [PubMed] [Google Scholar]
- 58.van Ballegooijen AJ, Kestenbaum B, Sachs MC, de Boer IH, Siscovick DS, Hoofnagle AN, et al. Association of 25-hydroxyvitamin D and parathyroid hormone with incident hypertension: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. (2014) 63:1214–22. 10.1016/j.jacc.2014.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang L, Ma J, Manson JE, Buring JE, Gaziano JM, Sesso HD, et al. prospective study of plasma vitamin D metabolites, vitamin D receptor gene polymorphisms, and risk of hypertension in men. Eur J Nutr. (2013) 52:1771–9. 10.1007/s00394-012-0480-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martins D, Wolf M, Pan D, Zadshir A, Tareen N, Thadhani R, et al. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. (2007) 167:1159–65. 10.1001/archinte.167.11.1159 [DOI] [PubMed] [Google Scholar]
- 61.Khader YS, Batieha A, Jaddou H, Batieha Z, El-Khateeb M, Ajlouni K. Relationship between 25-hydroxyvitamin D and metabolic syndrome among Jordanian adults. Nutr Res Pract. (2011) 5:132–9. 10.4162/nrp.2011.5.2.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jeenduang N, Plyduang T, Horpet D. Association of 25-hydroxyvitamin D levels and metabolic syndrome in Thai postmenopausal women. Diabetes and Metabolic Syndrome: Clinical Research and Reviews. (2020) 14:1585–90. 10.1016/j.dsx.2020.08.018 [DOI] [PubMed] [Google Scholar]
- 63.Gupta AK, Brashear MM, Johnson WD. Predisease conditions and serum vitamin D levels in healthy Mexican American adults. Postgrad Med. (2012) 124:136–42. 10.3810/pgm.2012.09.2599 [DOI] [PubMed] [Google Scholar]
- 64.Gupta AK, Brashear MM, Johnson WD. Prediabetes and prehypertension in healthy adults are associated with low vitamin D levels. Diabetes Care. (2011) 34:658–60. 10.2337/dc10-1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Esteghamati A, Aryan Z, Esteghamati A, Nakhjavani M. Differences in vitamin D concentration between metabolically healthy and unhealthy obese adults: associations with inflammatory and cardiometabolic markers in 4391 subjects. Diabetes Metab. (2014) 40:347–55. 10.1016/j.diabet.2014.02.007 [DOI] [PubMed] [Google Scholar]
- 66.Contreras-Manzano A, Villalpando S, García-Díaz C, Flores-Aldana M. Cardiovascular risk factors and their association with vitamin d deficiency in mexican women of reproductive age. Nutrients. (2019). 11:6. 10.3390/nu11061211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Caro Y, Negron V, Palacios C. Association between vitamin D levels and blood pressure in a group of Puerto Ricans. P R Health Sci J. (2012) 31:123–9. [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao G, Ford ES Li C, Kris-Etherton PM, Etherton TD, Balluz LS. Independent associations of serum concentrations of 25-hydroxyvitamin D and parathyroid hormone with blood pressure among US adults. J Hypertens. (2010) 28:1821–8. 10.1097/HJH.0b013e32833bc5b4 [DOI] [PubMed] [Google Scholar]
- 69.Sumriddetchkajorn K, Chailurkit L, Thakkinstian A, Sritara P. Hypertension is statistically associated with higher body mass index but not with vitamin D level in a Thai population. Eur J Clin Nutr. (2012) 66:405–7. 10.1038/ejcn.2011.156 [DOI] [PubMed] [Google Scholar]
- 70.Shin JH, Lee HT, Lim YH, Park HC, Shin J, Kim KS, et al. Defining Vitamin D deficiency and its relationship to hypertension in postmenopausal korean women. J Women's Health. (2002) 24:1021–9. 10.1089/jwh.2015.5358 [DOI] [PubMed] [Google Scholar]
- 71.Pannu PK, Soares MJ, Piers LS, Zhao Y, Ansari Z. The association of vitamin D status and dietary calcium intake with individual components of the metabolic syndrome: a population-based study in Victoria, Australia. Cardiovascul Endocrinol. (2017) 6:136–44. 10.1097/XCE.0000000000000133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li L, Yin X, Yao C, Zhu X, Wu X. Vitamin D, parathyroid hormone and their associations with hypertension in a Chinese population. PLoS One. (2012) 7:e43344. 10.1371/journal.pone.0043344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dong J, Wang Q, Chen MH, Zhao HP, Zhu TY, Tian N, et al. Associations between serum-intact parathyroid hormone, serum 25-hydroxyvitamin D, oral vitamin D analogs and metabolic syndrome in peritoneal dialysis patients: A multi-center cross-sectional study. Peritoneal Dial Int. (2014) 34:447–55. 10.3747/pdi.2013.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen WR, Chen YD, Shi Y, Yin DW, Wang H, Sha Y. Vitamin D, parathyroid hormone and risk factors for coronary artery disease in an elderly Chinese population. J Cardiovascul Med. (2015) 16:59–68. 10.2459/JCM.0000000000000094 [DOI] [PubMed] [Google Scholar]
- 75.Chen C, Chen Y, Weng P, Xia FZ, Li Q, Zhai HL, et al. Association of 25-hydroxyvitamin D with cardiometabolic risk factors and metabolic syndrome: a mendelian randomization study. Nutri J. (2019). 18:7. 10.1186/s12937-019-0494-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Skaaby T, Husemoen LL, Pisinger C, Jørgensen T, Thuesen BH, Fenger M, et al. Vitamin D status and changes in cardiovascular risk factors: a prospective study of a general population. Cardiology. (2012) 123:62–70. 10.1159/000341277 [DOI] [PubMed] [Google Scholar]
- 77.Sabanayagam C, Shankar A, Somasundaram S. Serum vitamin D level and prehypertension among subjects free of hypertension. Kidney Blood Press Res. (2012) 35:106–13. 10.1159/000330716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vacek JL, Vanga SR, Good M, Lai SM, Lakkireddy D, Howard PA. Vitamin D deficiency and supplementation and relation to cardiovascular health. Am J Cardiol. (2012) 109:359–63. 10.1016/j.amjcard.2011.09.020 [DOI] [PubMed] [Google Scholar]
- 79.Mateus-Hamdan L, Beauchet O, Bouvard B, Legrand E, Fantino B, Annweiler C. High parathyroid hormone, but not low vitamin D concentrations, expose elderly inpatients to hypertension. Geriatr Gerontol Int. (2013) 13:783–91. 10.1111/j.1447-0594.2012.00945.x [DOI] [PubMed] [Google Scholar]
- 80.Muldowney S, Lucey AJ, Paschos G, Martinez JA, Bandarra N, Thorsdottir I, et al. Relationships between vitamin D status and cardio-metabolic risk factors in young European adults. Ann Nutr Metab. (2011) 58:85–93. 10.1159/000324600 [DOI] [PubMed] [Google Scholar]
- 81.Piantanida E, Gallo D, Veronesi G, Dozio E, Trotti E, Lai A, et al. Cardiometabolic healthy and unhealthy obesity: does vitamin D play a role? Endocrine connections. (2017) 6:943–51. 10.1530/EC-17-0304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gupta AK, Brashear MM, Johnson WD. Low vitamin D levels, prediabetes and prehypertension in healthy African American adults. Nutr Metab Cardiovasc Dis. (2012) 22:877–82. 10.1016/j.numecd.2012.01.006 [DOI] [PubMed] [Google Scholar]
- 83.Kim D, Kim J. Association of Serum 25-Hydroxyvitamin D and parathyroid hormone with hypertension in middle-Aged and Older Korean adults. Am J Hypertens. (2016) 29:96–103. 10.1093/ajh/hpv059 [DOI] [PubMed] [Google Scholar]
- 84.Kim KJ, Kim YJ, Kim SH, An JH, Yoo HJ, Kim HY, et al. Vitamin D status and associated metabolic risk factors among North Korean refugees in South Korea: a cross-sectional study. BMJ open. (2015). 5:11. 10.1136/bmjopen-2015-009140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim S, Lim J, Kye S, Joung H. Association between vitamin D status and metabolic syndrome risk among Korean population: based on the Korean national health and nutrition examination survey IV-2, (2008). Diabetes Res Clin Pract. (2012) 96:230–6. 10.1016/j.diabres.2012.01.001 [DOI] [PubMed] [Google Scholar]
- 86.Kwak SY, Cho Y, Oh H, Shin MJ. Association of circulating 25-hydroxyvitamin D levels with hypertension and blood pressure values in Korean adults: a Mendelian randomization study on a subset of the Korea National Health and Nutrition Survey 2011–2012 population. Nutr Res Pract. (2019) 13:498–508. 10.4162/nrp.2019.13.6.498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gagnon C, Lu ZX, Magliano DJ, Dunstan DW, Shaw JE, Zimmet PZ, et al. Low serum 25-hydroxyvitamin D is associated with increased risk of the development of the metabolic syndrome at 5 years: results from a national, population-based prospective study (The Australian Diabetes, Obesity and Lifestyle Study: AusDiab). J Clin Endocrinol Metab. (2012) 97:1953–61. 10.1210/jc.2011-3187 [DOI] [PubMed] [Google Scholar]
- 88.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet (London, England). (2005) 365:217–23. 10.1016/S0140-6736(05)17741-1 [DOI] [PubMed] [Google Scholar]
- 89.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics−2016 update: a report from the American Heart Association. Circulation. (2016) 133:e38–e360. [DOI] [PubMed] [Google Scholar]
- 90.Kunutsor SK, Apekey TA, Steur M. Vitamin D and risk of future hypertension: meta-analysis of 283,537 participants. Eur J Epidemiol. (2013) 28:205–21. 10.1007/s10654-013-9790-2 [DOI] [PubMed] [Google Scholar]
- 91.Hajhashemy Z, Shahdadian F, Ziaei R, Saneei P. Serum vitamin D levels in relation to abdominal obesity: a systematic review and dose-response meta-analysis of epidemiologic studies. Obes Rev. (2021) 22:e13134. 10.1111/obr.13134 [DOI] [PubMed] [Google Scholar]
- 92.Yang J, Ou-Yang J, Huang J. Low serum vitamin D levels increase the mortality of cardiovascular disease in older adults: A dose-response meta-analysis of prospective studies. Medicine. (2019) 98:e16733. 10.1097/MD.0000000000016733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gholami F, Moradi G, Zareei B, Rasouli MA, Nikkhoo B, Roshani D, et al. The association between circulating 25-hydroxyvitamin D and cardiovascular diseases: a meta-analysis of prospective cohort studies. BMC Cardiovasc Disord. (2019) 19:248. 10.1186/s12872-019-1236-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fiscella K, Franks P. Vitamin D, race, and cardiovascular mortality: findings from a national US sample. Ann Fam Med. (2010) 8:11–8. 10.1370/afm.1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ginde AA, Scragg R, Schwartz RS, Camargo CA. Prospective study of serum 25-hydroxyvitamin D level, cardiovascular disease mortality, and all-cause mortality in older US adults. J Am Geriatrics Society. (2009) 57:1595–603. 10.1111/j.1532-5415.2009.02359.x [DOI] [PubMed] [Google Scholar]
- 96.Witham MD, Nadir MA, Struthers AD. Effect of vitamin D on blood pressure: a systematic review and meta-analysis. J Hypertens. (2009) 27:1948–54. 10.1097/HJH.0b013e32832f075b [DOI] [PubMed] [Google Scholar]
- 97.Qi D, Nie X, Cai J. The effect of vitamin D supplementation on hypertension in non-CKD populations: A systemic review and meta-analysis. Int J Cardiol. (2017) 227:177–86. 10.1016/j.ijcard.2016.11.040 [DOI] [PubMed] [Google Scholar]
- 98.Zhang D, Cheng C, Wang Y, Sun H, Yu S, Xue Y, et al. Effect of vitamin D on blood pressure and hypertension in the general population: an update meta-analysis of cohort studies and randomized controlled trials. Prev Chronic Dis. (2020) 17:E03. 10.5888/pcd17.190307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li YC. Vitamin D regulation of the renin-angiotensin system. J Cell Biochem. (2003) 88:327–31. 10.1002/jcb.10343 [DOI] [PubMed] [Google Scholar]
- 100.Li YC, Qiao G, Uskokovic M, Xiang W, Zheng W, Kong J. Vitamin D: a negative endocrine regulator of the renin-angiotensin system and blood pressure. J Steroid Biochemistr Mol Biol. (2004) 89:387–92. 10.1016/j.jsbmb.2004.03.004 [DOI] [PubMed] [Google Scholar]
- 101.Sugden JA, Davies JI, Witham MD, Morris AD, Struthers AD. Vitamin D improves endothelial function in patients with Type 2 diabetes mellitus and low vitamin D levels. Diabet Med. (2008) 25:320–5. 10.1111/j.1464-5491.2007.02360.x [DOI] [PubMed] [Google Scholar]
- 102.Borissova AM, Tankova T, Kirilov G, Dakovska L, Kovacheva R. The effect of vitamin D3 on insulin secretion and peripheral insulin sensitivity in type 2 diabetic patients. Int J Clin Pract. (2003) 57:258−61. [PubMed] [Google Scholar]
- 103.Soleimani M. Insulin resistance and hypertension: new insights. Kidney Int. (2015) 87:497–9. 10.1038/ki.2014.392 [DOI] [PubMed] [Google Scholar]
- 104.Wu L, Sun D. Effects of calcium plus vitamin D supplementation on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J Hum Hypertens. (2017) 31:547–54. 10.1038/jhh.2017.12 [DOI] [PubMed] [Google Scholar]
- 105.Vimaleswaran KS, Cavadino A, Berry DJ, Jorde R, Dieffenbach AK, Lu C, et al. Association of vitamin D status with arterial blood pressure and hypertension risk: a mendelian randomisation study. Lancet Diabetes Endocrinol. (2014) 2:719–29. 10.1016/S2213-8587(14)70113-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Valcheva P, Cardus A, Panizo S, Parisi E, Bozic M, Lopez Novoa JM, et al. Lack of vitamin D receptor causes stress-induced premature senescence in vascular smooth muscle cells through enhanced local angiotensin-II signals. Atherosclerosis. (2014) 235:247–55. 10.1016/j.atherosclerosis.2014.05.911 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.