1. INTRODUCTION
Chronic pain remains an area of considerable unmet clinical need. The discovery of novel targets and the development of better analgesics rely primarily on preclinical rodent models of pain; however, there are major challenges to translating discoveries made in animal models to pain management in humans. While many issues limit the predictive validity of preclinical models such as the complicated nature of pain, gender, age, and comorbidities, assessment of pain-like behaviors in rodents remains an important barrier. Historically, most preclinical studies relied on assessing sensory aspects of pain by measuring stimulus-evoked assessment of pain-related behaviors such as mechanical and thermal sensitivity (Percie du Sert and Rice, 2014). While hypersensitivity to thermal/mechanical stimuli are important components of the sensory aspect of chronic pain in humans, the experience of pain is much more complex and encompasses important cognitive and affective aspects also.
In recent years, newer assays of pain-related behaviors in rodent models, such as wheel running, learned avoidance, gait, burrowing and conditioned place preference, have been developed in an effort to improve the translational potential of preclinical findings (Burma, 2017; Deuis, 2017). One of these assays, the voluntary wheel running activity test, has been shown to be reduced in chronic inflammatory and sciatic nerve-injury induced neuropathy rodent models (Stevenson, 2011; Cobos, 2012; Grace, 2014; Whitehead, 2017). In addition, Cobos et al. (2012) validated the use of wheel running test in the mouse complete Freund’s adjuvant (CFA) chronic inflammatory model with well-established analgesic compounds. However, these studies have not identified whether attenuated voluntary running activity is seen across different pain types in the same experimental conditions; some studies record distance traveled in wheel running assay in a 120-minute test session, whereas other studies will record distances over 24 hours (Shenan et al., 2017; Pitzer et al., 2019). These studies also lack efforts to investigate if effects are dependent on genotype or sex. In addition, it is not clear whether the attenuated voluntary running in these chronic pain manipulations is due to a decrease in general activity of the animal.
To assess the role of these factors as determinants of decreased voluntary running, it is important that we compare different pain models under the same conditions to help better understand potential genotypic or sex-dependent differences. We compared voluntary running wheel activity between two different inbred male and female mouse strains, C57BL/6J and DBA/2J, with CFA-induced chronic inflammatory pain, sciatic nerve injury or chemotherapy- induced peripheral neuropathy. C57BL/6J and DBA/2J being two of the most commonly investigated strains in behavior genetics.
2. METHODS AND MATERIALS
2.1. Animals
8-10 week old male and female C57BL/6J and DBA/2J mice were acquired from Jackson Laboratory (Bar Harbor, ME, USA). Animals were kept in an animal care facility approved by the Association for Assessment and Accreditation of Laboratory Animal Care (AALAC) 3 per cage at Virginia Commonwealth University (Richmond, VA) on a regular 12-hour light/dark cycle. Mice were habituated to animal care vivarium for one week before any baseline measures were taken. All experimental testing was performed during the light cycle. Food and water were given ad libitum (7012 Teklad LM-485 Mouse/Rat Sterilizable Diet, Harlan Laboratories Inc., Indianapolis, IN). Animals were euthanized via carbon dioxide gas and followed by cervical dislocation once all testing was completed. All experiments were approved by Institutional Animal Care and Use Committee at Virginia Commonwealth University.
2.2. Drugs and administration
Complete Freund’s Adjuvant (CFA; Sigma-Aldrich, MO, USA) was administered via intraplantar injection in the left hind paw to induce inflammation. C57BL/6J and DBA/2J mice (n=12/genotype/group) of both sexes received 20 μL injections of 100% CFA or mineral oil (Sigma-Aldrich, MO, USA) with a 1710 TLL Hamilton microsyringe (Hamilton Company, NV, USA) while mouse was secured in a restraint tube.
Paclitaxel was purchased from VCU Health Pharmacy (Athenex, NDC 70860-200-50, Richmond, VA, USA) and dissolved in a 1:1:18 mixture of 200 proof ethanol, kolliphor, and distilled water (Sigma-Aldrich, MO, USA). Paclitaxel was administered at a dose of 8 mg/kg intraperitonially every other day; four administrations completed one injection cycle (n=12/genotype/sex/group). Control mice received 1:1:18 at a volume of 10 ml/kg, i.p. and followed the same injection cycle. Animals began behavioral testing 24 hours following the final injection. Diclofenac sodium salt was purchased from Sigma-Aldrich (MO, USA) and was dissolved in saline and injected s.c. to mice.
2.3. Chronic constriction injury (CCI)
C57BL/6J male and female mice (n= 8/group) underwent a surgical procedure to create chronic nerve injury of the sciatic nerve. Animals were put under anesthesia with 4% isoflurane and maintained with 2.5% isoflurane in oxygen using a face mask and vaporizer (VetEquip Inc, Pleasanton, CA). Mice were shaved from their left hip to paw, and an incision was made below the hip bone. The sciatic nerve was exposed at the level proximal to the sciatic trifurcation, and a nerve segment 4 mm long was separated from surrounding connective tissue. Four loose ligatures with 5-0 silk suture were made around the nerve 1 mm apart, causing chronic nerve injury. 8 sham surgeries were also performed; the sciatic nerve was exposed, but no ligation was made. The wound was sutured with silk sutures, and animals were monitored daily following surgery. Behavioral testing began 24 hours following surgery.
2.4. Mechanical sensitivity testing
Mechanical sensitivity was assessed first using Von Frey filaments. Mice were acclimated in the room for 15 minutes in their home cage before being placed in a Plexigas cage where they habituated on a mesh net for one hour prior to Von Frey testing. Withdrawal thresholds were measured by applying a series of calibrated Von Frey filaments from an 18011 Semmes-Weinstein Aesthesiometer Kit (Stoelting, Wood Dale, IL; logarithmically incremental force from 2.83 to 5.88 expressed in dsLog 10 of [10 pound force in milligram]) to each hind paw. Each filament was presented vertically against the paw with enough force causing a slight bend. If paw withdrawal, licking or shaking occurred, the next at weaker stimulus was chosen. In absence of a paw withdrawal response, a thicker filament corresponding to a stronger stimulus was presented. Mechanical sensitivity was always tested blindly by a male experimenter at baseline and before wheel running sessions.
2.5. Paw edema measurement
Immediately following Von Frey testing, paw edema was measured. The thickness of CFA-treated paws was measured before and after injections at the time points indicated above, using a digital caliper (Traceable Calipers, Friendswood, TX). Data were recorded to the nearest ±0.01 mm and expressed as change in paw thickness (ΔPD = difference in the ipsilateral paw diameter before and after injection paw thickness).
2.6. Voluntary wheel running
Mice were returned to their home cage after paw edema was measured for 30 minutes before voluntary wheel running testing began. Mice were placed in polycarbonate wheels (diameter 21.5 cm; width 5 cm) with a steel rod axle containing an electronic sensor which recorded the number of rotations in a 120-minute session (9:00 am-1:00 pm). The equipment for this assay was built locally (Virginia Commonwealth University, Richmond, VA, USA). Distance traveled was determined by the following formula: distance traveled (m)= (rotations completed) x (wheel circumference, 0.68 cm). Wheels rotated in one direction and came to a stop if the mouse stopped moving. For the CFA inflammatory pain and chronic peripheral neuropathy models, both male and female mice were tested simultaneously but were separated by a foam board which prevented any visual contact between the mice. Testing both sexes at the same time can create covariates such as olfactory and visual ability. The foam board helps prevent visual contact yet does not eliminate olfactory cues. Wheel running test was performed once two days prior to injection or surgery to record baseline distance traveled, and subsequent testing was performed on days 1, 3, 7, and 14 post injection with the exception of CCI model which was conducted until day 21 after surgery.
Two control studies were also performed on C57BL/6J mice. First, we confirmed that a reference analgesic and anti-inflammatory drug reversed the decrease in wheel running observed after CFA injection in mice as reported by Cobos et al., (2012). For that, the effects of diclofenac on CFA-induced decrease in voluntary wheel running was assessed 3 days after CFA injection. Before being placed in the wheel, mice were given a s.c. injection of 5 mg/kg and tested 30 min after. Second, since voluntary wheel running has been reported to reduce pain behaviors in rodents (Grace et al., 2016), we tested if wheel running during the baseline and the first two days after the induction of inflammation could alter the subsequent evaluations. Therefore, separate groups of male and female C57BL/6J mice was evaluated only 3 and 5 days after CFA injection, and compared with those evaluated daily in our experimental procedure.
2.7. Locomotor activity in CFA-treated mice
In a separate group of mice, CFA-treated and vehicle-treated male and female C57BL/6J mice (n=6/sex/group) were placed in room to acclimate for 30 minutes before experimental testing. Mice were individually placed in boxes (20 x 24 × 16.5 cm, Omnitech, Columbus, OH) where locomotor activity was recorded by MED-PC IV software in 10-minute intervals for 120 minutes. Animals were baselined for locomotor activity and paw edema before any injection occurred and testing resumed on days 1, 3, 7, and 14 after injection. Locomotor activity scores were defined as the number of interruptions of the photobeam cells measured for total test period. Data were expressed as mean ± SEM of the number of photocell interruptions.
2.8. Statistical Analysis
All data are expressed as mean ± SEM (Standard Error of the Mean). All data passed normality (Shapiro-Wilk test) and equal variance (Brown-Forsythe) test. Time-course data were analyzed using two-way or three-way repeated measures of variance (ANOVA) followed by post-hoc Sidak multiple comparison tests with the alpha level set at 0.05. To better compare the effect of treatment and time course on wheel running distance, we computed a single value (area under the curve; AUC) to represent each treatment over the entire post-treatment period using the trapezoidal method. All statistical analysis was performed with Graphpad Prism software, version 8 (GraphPad Software, Inc., La Jolla, CA, USA). The probability was considered significant if P<0.05.
3. RESULTS
3.1. Impact of chronic inflammation on voluntary wheel running and locomotion in C57BL/6J and DBA/2J mice
To observe the impact of CFA-induced chronic inflammation in C57BL/6J and DBA/2J mice on voluntary wheel running and locomotor activity, wheel running and locomotion tests were conducted in CFA-treated and non-CFA-treated mice at 1, 3, 5, 7 and 14 days following CFA injections (Figure 1). Mechanical sensitivity and chronic inflammation were respectively measured by von Frey test and paw edema (Figure 1A and 1B). Our data show a significant decrease in mechanical withdrawal threshold in CFA-treated C57BL/6J mice in contrast to vehicle-treated mice (treatment x time [F(5, 110)=16.87], day 1 p<0.001, day 3 p<0.001, day 5 p<0.001, and day 7 p<0.001) reflecting mechanical hypersensitivity (Figure 1A). Additionally, Figure 1B shows an increase in paw edema diameter in mice treated with CFA (treatment [p=0.105, F(4, 88)=3.506]) reflecting paw inflammation. Distance traveled values from vehicle and CFA-treated C57BL/6J mice in wheel running time course were compared to determine potential differences across treatments (Figure 1C). Vehicle-treated mice increased their distance traveled from baseline throughout the duration of the study, while alterations in voluntary running were observed in C57BL/6J mice treated with CFA. Post-hoc analysis revealed significant differences in distance traveled in CFA-treated mice compared to vehicle mice (time x treatment [p=0.0001, F(5, 110)=5.698], D1<0.01, D3<0.001, D5<0.01, D7<0.01). By day 14, there was no difference in distance traveled between treated and non-treated groups. Further analysis was then conducted to examine the potential impact of sex on distance traveled after vehicle or CFA injections. Supplementary Table 1S shows comparison between all groups (3-way ANOVA treatment × sex × time) and an analysis of area under the curve (AUC - 2-way ANOVA treatment × time) between male and female C57BL/6J mice in wheel running assay to see if sex was a factor that impacted distance traveled in wheel running assay. Both analyses revealed that there were no sex differences between male and female C57BL/6J mice (Table S1A). Male and female wheel running results were then examined separately to determine possible treatment difference in each sex (Figure 2). Thus, in male C57BL/6J (Figure 2A), a decrease in wheel running activity was observed on day 5 post CFA-injection, whereas in female C57BL/6J (Figure 2B), attenuation of wheel running was observed on day 3 after CFA-injection.
Figure 1. Impact of chronic inflammation by intraplantar CFA administration in C57BL/6J and DBA/2J mice.
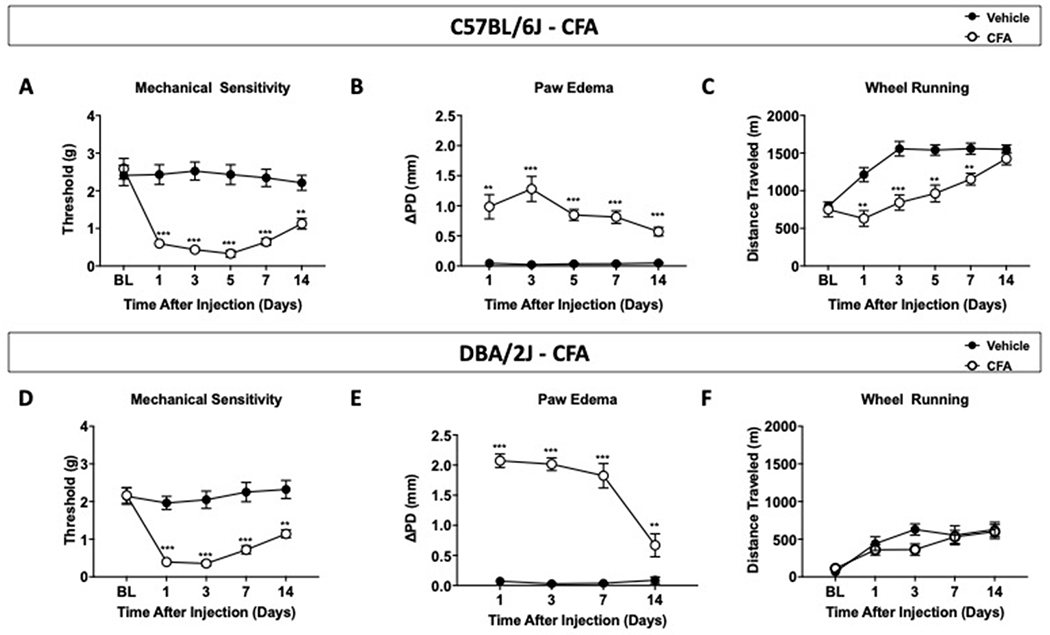
(A) Mechanical sensitivity by Von Frey in C57BL/6J mice that received mineral oil or 100% CFA in left hind paw. (B) Paw diameter relative to before and after injection. (C) Time-course of voluntary wheel running upon CFA administration in 120-minute session. (D) Time course of mechanical sensitivity in DBA/2J mice that received vehicle or CFA. (E) Change in paw edema diameter of ipsilateral paw pre- and post CFA administration. (F) Distance traveled by voluntary wheel running following CFA. Data are expressed as mean ± S.E.M. of n=12/group. **p<0.01, ***p<0.001 vs. vehicle.
Figure 2. Impact of CFA-induced inflammation on distance traveled in male and female C57BL/6J and DBA/2J mice.
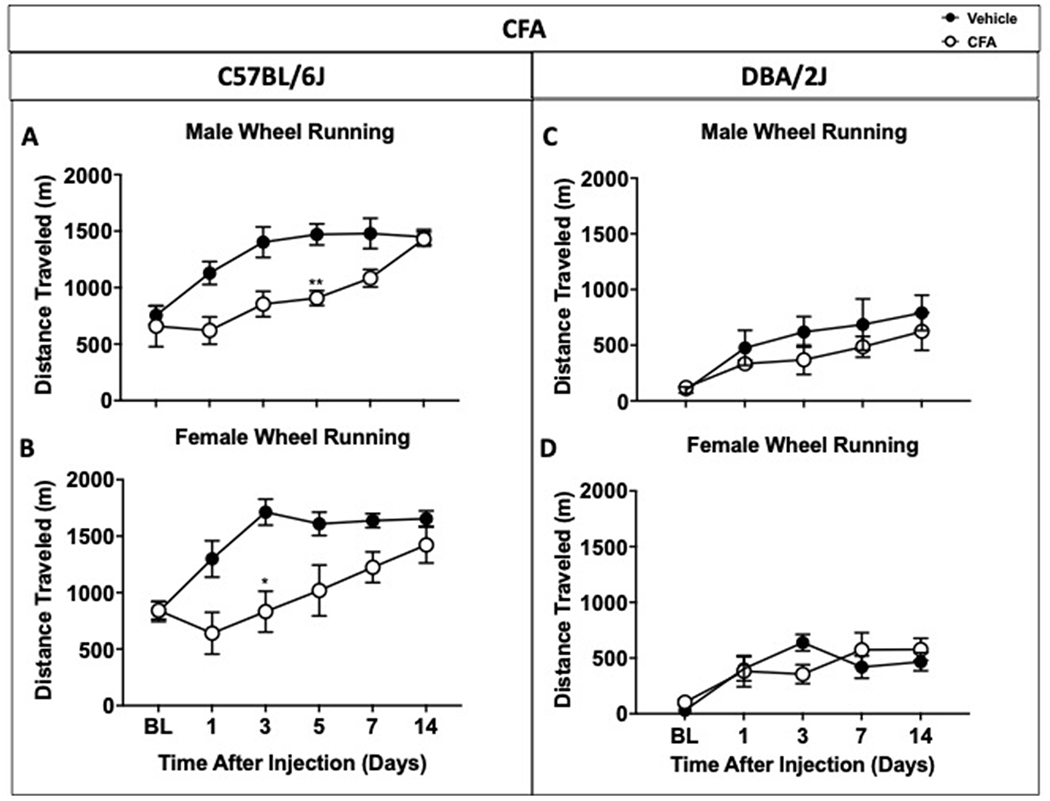
Male (A) and female (B) C57BL/6J mice show differences in distance traveled between treatments, whereas male (C) and female (D) DBA/2J mice did not show any differences in wheel running activity when sexes were separated. Data are expressed as mean ± S.E.M. of n=6/group. *p<0.05, **p<0.01 vs. vehicle.
In addition, separate cohort of C57BL/6J male and female mice were submitted to voluntary wheel running only on days 3 and 5 after CFA injection to determinate if repeated exercise influences the effects of CFA on voluntary WR. We did not find significant differences in the distance traveled between mice evaluated daily after CFA injection and those evaluated 3 and 5 days after CFA administration indicating that repeated exercise did not influence the effects of CFA on voluntary wheel running (Figure S1A; p=0.0013, F(2, 28)=8.500). Consistent with Cobos et al, (2012) results, we found that diclofenac at the dose of 5 mg/kg completely reversed CFA-induced reduction of voluntary wheel running at day 3 after induction of the inflammation in male and female mice C57BL/6J (Supplementary Figure S1B; p<0.0001, F(3, 44)= 13.83). Due to the results obtained from wheel running assay, a separate cohort of mice were tested for locomotion to confirm if the decrease in voluntary running was due to motor impairment or lack of motivation (Figure S1C). No differences were seen between vehicle and CFA-treated mice locomotor activity for 120 min duration (time [p=0.0001, F(2.054, 36.96)=25.05]).
Considering the alterations in mechanical sensitivity and voluntary running were observed in C57BL/6J mice with chronic inflammation, another inbred mouse strain (DBA/2J) was tested. DBA/2J mice given vehicle or CFA were assessed for mechanical sensitivity and voluntary running at baseline and on the following days after administration: 1, 3, 7, and 14. Figure 1D shows that DBA/2J mice treated with CFA displayed a lower mechanical withdrawal threshold compared to the vehicle group (time x treatment [p<0.0001, F(4, 88)=12.64], day 1<0.001, day 3<0.001, day 7<0.001, and day 14<0.01). Figure 1E shows significant increase in paw edema diameter in CFA-treated mice in comparison to control mice (time x treatment [p<0.0001, F(3, 66)=18.05], day 1<0.001, day 3<0.001, day 7<0.001, and day 14<0.01). However, between the vehicle and CFA group, there were no differences in the distance ran in voluntary wheel running (time [p<0.0001, F(2.9, 63.8)=15.31], Figure 1F). Further analysis was then conducted to determine possible sex differences in DBA/2J mice. A comparison between all groups (3-way ANOVA) and AUC analyses (2-way ANOVA) revealed no sex differences in these mice (Table S1A). Similarly, when male and female wheel running results were compared separately, no differences in distance traveled were observed in DBA/2J female mice or DBA/2J male mice (Figure 2C and 2D) between treatments. Since no significant differences in distance traveled between the CFA and control groups were observed, no locomotion tests were performed.
A comparison between all groups (3-way ANOVA) was also performed to observe potential strain differences in wheel running assay which revealed no differences of distance traveled between C57BL/6J and DBA/2J mice. AUC analyze (2-way ANOVA) was calculated for both strains. AUC analyze yielded significant strain differences in total distance traveled between C57BL/6J and DBA/2J mice in both sex (Table S1B).
3.2. Impact of chemotherapy-induced neuropathy on voluntary wheel running in C57BL/6J and DBA/2J mice
To determine the impact of paclitaxel-induced neuropathic pain in C57BL/6J and DBA/2J mice, reflexive and nonreflexive outcome measures has been tested in paclitaxel-treated and control mice on days 1, 3, 7, 10, and 14 (Figure 3). In mechanical sensitivity assay, vehicle mice maintained stable mechanical withdrawal thresholds throughout the duration of the study (Figure 3A). Statistical analysis shows a significant and long-lasting decrease in mechanical withdrawal threshold in paclitaxel-treated C57BL/6J mice when compared to vehicle (time x treatment [p<0.0001. F(5, 230)=34.35]. Mice were also tested in wheel running assay. Figure 3B shows there was no difference in the distance traveled between treated and non-treated C57BL/6J mice combined. A comparison between all groups (3-way ANOVA) and AUC analyses (2-way ANOVA) showed no sex-dependent differences on wheel running behavior (Table S1A). Then, male and female wheel running results were examined separately to observe differences intra-group. In male C57BL/6J mice, no differences in distance traveled were observed despite paclitaxel-treatment (Figure 4A). This may have been due to an abnormal increase in wheel running activity in paclitaxel-treated group on day 3. Wheel running activity in paclitaxel-treated group was low on days 1 and 7 compared to vehicle-treated group but failed to reach statistical significance due to the increased distance traveled on day 3. In contrast, statistical analysis revealed that paclitaxel-treated female mice showed reduction in distance traveled compared to vehicle female mice on day 1 (p<0.05) post paclitaxel treatment in Figure 4B (time x treatment [p<0.05, F(5, 50) =3.956]).
Figure 3. Time course of mechanical sensitivity and voluntary wheel running by paclitaxel-induced peripheral neuropathy in C57BL/6J and DBA/2J mice.
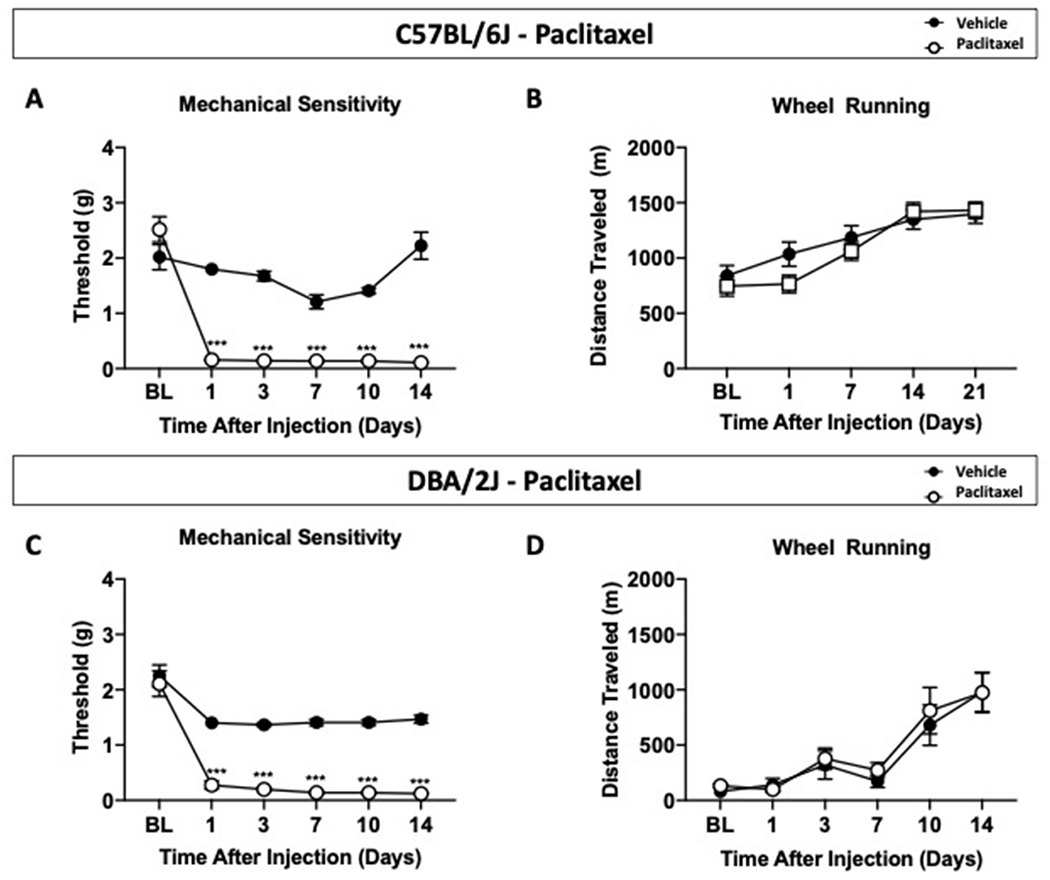
(A) Nociception assay assessed mechanical sensitivity in C57BL/6J mice that received vehicle or 8 mg/kg paclitaxel, i.p. (n=12/group). (B) Distance traveled in wheel running assay by male and female C57BL/6J mice given vehicle or treatment. (C) Mechanical withdrawal thresholds via Von Frey test in DBA/2J mice that received vehicle or 8 mg/kg paclitaxel, i.p. (D) Paclitaxel’s effects on distance traveled by male and female DBA/2J mice in wheel running assay. Data are expressed as mean ± S.E.M. of n=12/group. ***p<0.001 vs. vehicle.
Figure 4. Paclitaxel’s effects on wheel running activity in male and female C57BL/6J and DBA/2J mice.
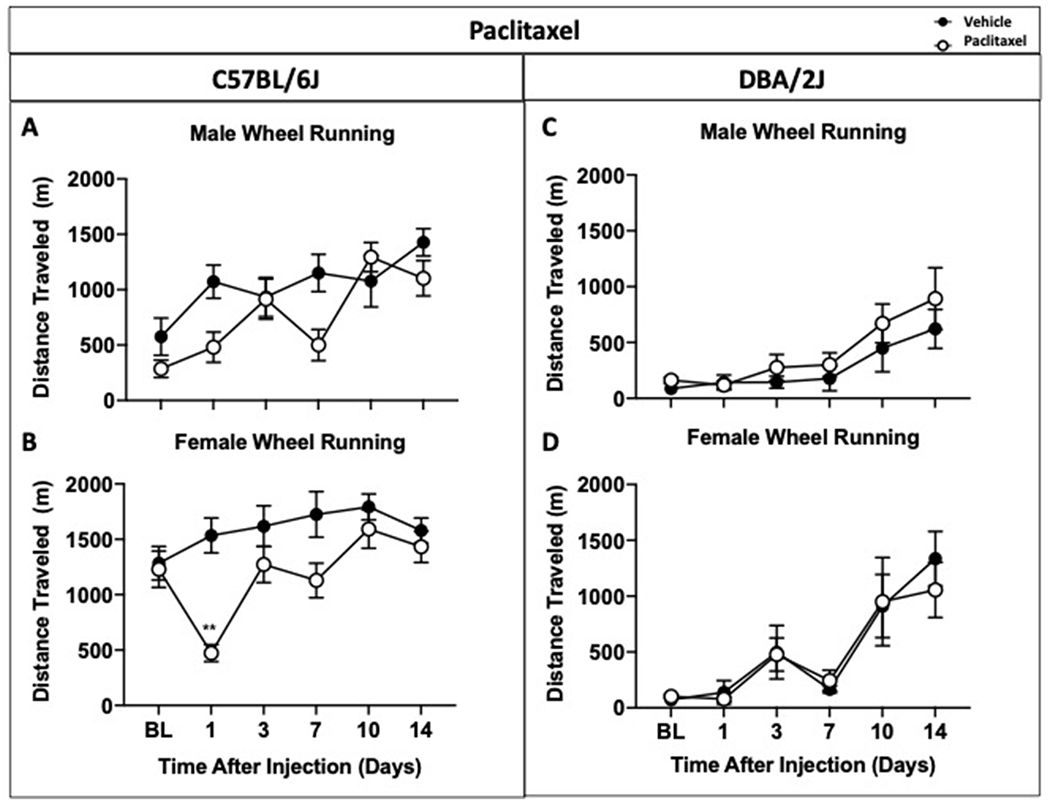
Male (A) and female (B) C57BL/6J mice were separated to observe differences between treated and non-treated groups in a specific sex. DBA/2J male (C) showed no differences between groups in distance traveled whereas paclitaxel-treated female DBA2/J (D) did show attenuation of wheel running on day 1 as opposed to the vehicle-treated group. Data are expressed as mean ± S.E.M. of n=6/group. *p<0.05 vs. vehicle.
We then assessed paclitaxel’s effects on voluntary wheel running in male and female DBA/2J mice. Figure 3C shows significant and prolonged decrease in mechanical withdrawal threshold in paclitaxel-treated DBA/2J mice in contrast to vehicle-treated mice (time x treatment [p<0.001, F(5, 110)=10.58, D1-D14<0.001]). Similar to what was observed in DBA/2J mice after CFA treatment, two-way ANOVA analysis revealed no differences in distance traveled between in DBA/2J mice after paclitaxel treatment in the wheel running test (Figure 3D). Further analysis was then conducted to see the potential impact of sex in DBA/2J mice after paclitaxel. A comparison between all groups (3-way ANOVA) and AUC analyses (2-way ANOVA) revealed no sex differences in terms of distance traveled (Table S1A). Upon separating male and female DBA/2J wheel running results, no significant differences in distance traveled were observed (Figure 4C and 4D).
Lastly, both strains were compared to see if strain impacted wheel running activity. While comparison between all groups (3-way ANOVA) revealed no significant differences in distance traveled, analysis of the calculated AUC, a 2-way ANOVA revealed strain-dependent differences in total distance traveled when comparing C57BL/6J and DBA/2J across treatments.
3.3. Effect of chronic neuropathic injury on voluntary wheel running in C57BL/6J mice
In order to determine the impact of chronic neuropathic injury on wheel running activity, unilateral chronic constriction nerve injury or sham surgeries on male and female C57BL/6J mice were performed. Starting 24 hours after the procedure, mice were assessed for mechanical sensitivity and voluntary wheel running on days 1, 3, 7, 14, and 21 post procedure (Figure 5). Shown in Figure 5A, sham mice demonstrated no changes in mechanical withdrawal threshold, whereas CCI mice showed a significant and gradual decrease in mechanical withdrawal threshold (time x treatment [p<0.0001, F(5, 70)=11.93], D1<0.05, D3<0.01, D7<0.001, D14<0.001, and D21<0.001). Mice were later assessed for their running wheel activity; Figure 5B shows that sham mice gradually increased their distance traveled throughout the duration of study. Contrary to sham mice, a significant attenuation of distance traveled was observed in CCI mice on day 7 after surgery (time x treatment [p=0.006, F(5, 70)=3.590], D7<0.05). Then, male and female wheel running results were analyzed separately to determine a possible treatment effect in each sex. No differences in distance traveled were observed in C57BL/6J male mice but a significant reduction of distance traveled was observed in female CCI mice on day 7 after surgery (Figure 6A and 6B).
Figure 5. Effects of chronic constriction nerve injury in C57BL/6J mice on mechanical sensitivity and voluntary wheel running.
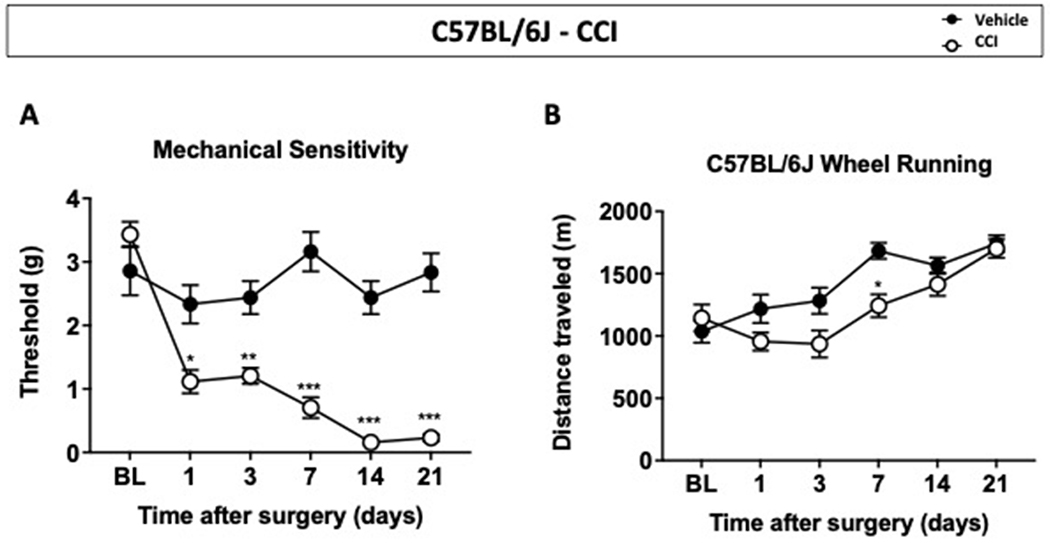
(A) Mechanical sensitivity assay revealed mechanical withdrawal thresholds in male and female C57BL/6J mice with sham or CCI (B) Distance traveled from wheel running session. Data are expressed as mean ± S.E.M. of n=8/group. *p<0.05, **p<0.01, ***p<0.001 vs. vehicle.
Figure 6.
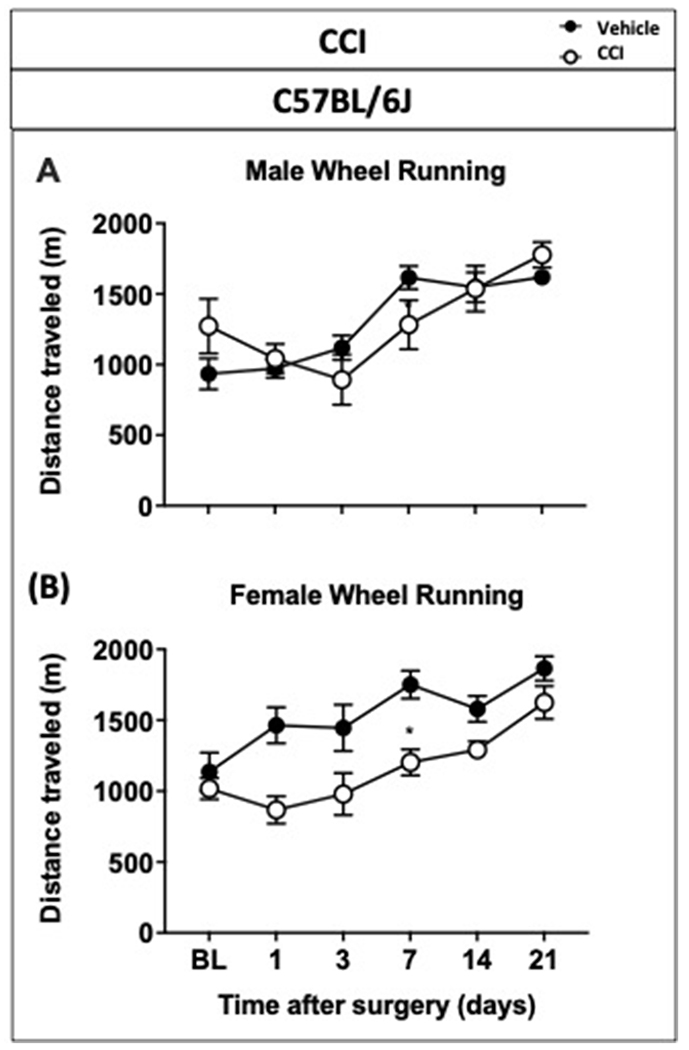
Impact of chronic constriction nerve injury on distance traveled in male and female C57BL/6J mice. Distance traveled in voluntary wheel running test (time-course) in 120-minute session in male (A) and female (B) C57BL/6J mice with sham or CCI. Data are expressed as mean ± S.E.M. of n = 4/group. *p < 0.05 vs. vehicle.
4. DISCUSSION
Recently, several studies have suggested that the paucity of behavioral tests used to measure nociception in animals contributes to the poor translational results of basic pain research (Sheahan, 2017). The International Association for the Study of Pain describes pain in human as an “unpleasant sensory and emotional experience associated with actual or potential tissue damage,” (IASP, 2020). Thus, the sole use of evoked “pain-like” behavioral tests such as the von Frey filament test, acetone test and hot/cold plate seems to show its limitations in terms of clinical relevance (Sheahan, 2017). Thus, recent studies have investigated the effect of chronic inflammation in rodents via the voluntary wheel running test (Lightfoot, 2004; Cobos, 2012; Merritt, 2015; Walker, 2018; Slivicki, 2019). However, there is limited data examining the impact of different chronic pain models such as chronic peripheral neuropathy caused by sciatic nerve injury and chemotherapy on wheel running activity. In addition, most studies in mice have been conducted in C57BL/6J male mice and did not assess motor impairment in these animals. As the equipment and conditions differed between laboratories (Walker, 2018), it seemed important to carry out in the same experimental conditions a comparative study involving different models of chronic peripheral neuropathic pain and in different strains of mice.
Expanding on Cobos et al., 2012 study, we examined the impact of chronic inflammation (with the CFA model), chemotherapy-induced peripheral neuropathy (with paclitaxel) and chronic neuropathic injury (using the CCI model) on reflexive and non-reflexive outcome measures in mice. In addition, we conducted this study in two different strains of inbred mice, C57BL/6J and DBA/2J in the CFA and CIPN pain models specifically to explore the impact of genetic background on these measures. Finally, possible sex differences were also studied.
Our results showed that a unilateral intraplantar administration of CFA to C57BL/6J mice caused significant mechanical hypersensitivity and decrease in distance traveled in wheel running assay. This decrease in voluntary running after CFA injection occurred without a significant alteration of general locomotor activity, suggesting a change in motivational-like state in mice. Indeed, voluntary wheel running has been shown to be biologically distinct from general activity and is associated with neuronal systems allocated to mood and reward that it may reflect the motivational and emotional state of animals (Novak, 2012). Assessing locomotor activity in CFA- treated C57BL/6J mice further supports this claim and confirms that there was is no muscle damage causing immobility, but possibly a decrease in motivation-like behavior. In contrast, CFA-treated DBA/2J mice only showed a decrease in mechanical hypersensitivity, but not a significant reduction in voluntary wheel running upon CFA administration. Increase in edema diameter to a similar extent from baseline was also seen in both mouse strains.
In order to confirm that the decrease in wheel running activity was indeed due to pain- induced loss of activity, we performed two control tests on male and female C56BL6J mice (presented in Supplementary Figure). As reported by Cobos et al (2012), we confirmed that a reference analgesic and anti-inflammatory drug, diclofenac (5 mg/kg), reversed the decrease in wheel running activity observed in chronic inflammation model induce by CFA in C57BL/6J male and female mice. In addition, as it has been reported that voluntary wheel running can reduce pain behaviors in rodents (Grace et al., 2016), we tested if repeated wheel running activity during our testing after CFA injection would influence the results. Under our experimental conditions, no difference in the distance traveled between the mice evaluated daily and those evaluated 3 and 5 days after the injection of CFA was observed. This indicates that, under our conditions, the repeated exercise did not influence the effects of the CFA on wheel running activity.
Since the CFA-induced chronic inflammation revealed differences in wheel running activity between C57BL/6J and DBA/2J mouse strains, we tested reflexive and non-reflexive outcome measures in a chemotherapy-induced chronic peripheral neuropathic pain model. Previously, we have shown that locomotor activity was not affected by the administration of paclitaxel in mice (Toma, 2017). Both mouse strains showed a long-lasting mechanical paw hypersensitivity. Paclitaxel-treated male C57BL/6J mice showed no difference in distance traveled in the wheel running compared to male vehicle mice. However, paclitaxel-treated female C57BL/6J mice ran less distance in wheel running assay one day following the injection regimen. These data are consistent with a previous study in which paclitaxel was shown to induce no change in wheel running activity in male C57BL/6J mice (Slivicki, 2019). Thus, we demonstrate in this study, the presence of a sex difference in wheel running activity in paclitaxel-induced neuropathic pain in mice. Paclitaxel-treated and non-treated-DBA/2J male and female mice ran similar distances in wheel running test throughout the duration of the study. These data in the paclitaxel- induced pain model show, in this model also, strain differences in non-reflexive measures.
Similar to CFA-induced chronic inflammation, chronic constriction nerve injury in C57BL/6J mice showed a time-dependent mechanical hypersensitivity that lasted for the time- course of our study (21 days) compared to sham mice. However, the reduction in voluntary wheel running test in mice with CCI was shown only at day 7 after surgery.
Our study shows for the first time in two chronic pain models a difference between DBA/2J and C57BL/6J in a non- reflexive test, the wheel running test. Indeed, chronic inflammation or neuropathy does not seem to affect wheel running activity in DBA/2J mice. At the same time, similar hypersensitive withdrawal responses in the von Frey test were observed in these two mouse strains mice. The difference of nociception-induced changes in activity observed in the wheel running test between two strains of mice could be explained in part by differences in baseline activity between the two strains. Similar to previous reports (Lightfoot, 2004; Merritt, 2015), our results show that DBA/2J mice run less on average in the wheel running test than C57BL/6J mice. In addition, neurobiological changes after chronic painful stimulus may differ between the two mouse strains. Overall, our results suggest that the C57BL/6J mouse strain would be a better choice to utilize for future pharmacological studies since they run significantly more than DBA/2J mice. Any drastic differences in wheel running activity by induction of pain or drug administration would be more easily detected in this strain as opposed to DBA/2J. In fact, Neddenriep et al., 2019 utilized CFA-treated male and female C57BL/6J mice to assess ethanol’s impact on voluntary wheel running activity. Ethanol (1.25 g/kg, p.o.) reversed the attenuation of wheel running activity as seen in mice treated with CFA alone.
We also observed in the chronic inflammatory and peripheral neuropathic pain models that wheel running activity decreased transiently relative to the long duration of hypersensitive withdrawal responses to mechanical stimuli. The rapidity with which wheel running behavior returns to normal compared to the reflexive test can be explained by a faster central adaptive response of the animals, using central nervous system structures and complex pathways such as the spinal cord, hippocampus and prefrontal cortex (Wilkerson, 2018). On the contrary, the persistence of the hypersensitivity observed in the reflexivity test use a different anatomic pathway (i.e. spinothalamic tract) and can be explained notably by disorganized skin reinnervation after injury, by a local release of neurotrophin or by the presence of an inflammatory soup, due to CCI, CFA and paclitaxel injections (Wilkerson, 2018; Lebonvallet, 2018).
The limitations of our study lie notably in the fact that we do not use free access to the wheel, but a constrained duration of 2 hours for the animal (Sheahan, 2017). We chose this short duration to have an identical test time between animals in order to be able to compare them. However, the time of day chosen to perform the test may not be optimal for all animals by conscripting circadian cycle differences (Lightfoot, 2004). In other studies, the choice was made to carry out their studies over 24 hours with free access to the wheel (Merritt, 2015). This allows the mice, whose metabolism is fast, to have access to food and drink during the test. Finally, it is important to note that some studies use bi-lateral injections of CFA in mice and showed more impaired wheel running activity in animals with bilateral CFA injections. (Cobos, 2012). We, however, chose the unilateral injection because it seemed more relevant and allowed us to compare with the contralateral leg, the animal being thus its own control.
5. CONCLUSIONS
Taken together, our results tend to demonstrate the value of using spontaneous behavioral outcome measures, such as wheel running test, in addition to evoked pain tests in nociception study in rodents. In addition, the choice of mouse strains and sex is a crucial element to consider in these studies. The impact of pain on mobility, anxiety, physical and social activities observed in humans seems to be more limited in mice (Sheahan, 2017). Thus, the choice of behavioral tests is therefore a function of the type of pain model studied. Nevertheless, the wheel running test seems to be a non-reflexive outcome test of measuring the pain component in mice in different neuropathic pain models. The use of this test, which has the advantage of being independent of experimenter, could therefore be more commonly used in the pharmacological studies of pain, as we have recently shown in two models of neuropathic pain with alcohol and formalin (Neddenriep, 2019; Ulker, 2020). Although our analysis did not reveal sex differences for the time course after various treatments, area under the curve 2-way ANOVA analysis revealed differences in total wheel running activity between both strains C57BL/6J and DBA/2J in CFA and paclitaxel models. In conclusion, the development of new behavioral tests in animals, to complement evoked pain tests, should be further investigated.
Supplementary Material
Figure S1. Effects of diclofenac in vehicle and CFA-treated C57BL/6J mice on wheel running. (A) Pretreatment of diclofenac eliminated attenuation of distance traveled in voluntary wheel running assay. (B) Diclofenac administration reversed decrease in wheel running activity in CFA-treated mice on days 3 and 5 after CFA injection. (C) Number of beam interrupts in locomotion assay in a 120-minute test session. Data are expressed as mean ± S.E.M. of n=12/group. **p<0.01, ***p<0.001 vs. vehicle.
Table S1. Multiple statistical comparison of distance traveled in wheel running test between male and female, and between C57BL/6J and DBA 2/J mice after CFA and paclitaxel treatment. S1A: 3-way and 2-way ANOVA by area under the curve revealed no sex differences in distance traveled in voluntary wheel running assay. Data are expressed as mean ± S.E.M. of n=6/group. *p<0.05, **p<0.01, ***p<0.001 vs. vehicle. S1B: 3-way revealed no strain differences in distance traveled in voluntary wheel running assay. Data are expressed as mean ± S.E.M. of n=6/group. *p<0.05, **p<0.01, ***p<0.001 vs. vehicle.
6. ACKNOWLEDGEMENTS
We would like to acknowledge Ed Dimen at Virginia Commonwealth University for constructing the equipment used for the wheel running assay.
7. FUNDING INFORMATION
This work was funded by National Institute of Health grant R01CA221260 and R01CA219637 to MID.
ABBREVIATIONS
- CFA
Complete Freund’s Adjuvant
- CCI
Chronic Constructive Injury
- CIPN
Chemotherapy-induced peripheral neuropathy
- WR
Wheel Running
Footnotes
8. CONFLICT OF INTEREST DISCLOSURES
No conflict of interest was declared.
10. REFERENCES
- [1].Percie Du Sert N, Rice ASC, Improving the translation of analgesic drugs to the clinic: Animal models of neuropathic pain, Br. J. Pharmacol 171 (2014) 2951–2963. 10.1111/bph.12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Burma NE, Leduc-Pessah H, Fan CY, Trang T, Animal models of chronic pain: Advances and challenges for clinical translation, J. Neurosci. Res 95 (2017) 1242–1256. 10.1002/jnr.23768. [DOI] [PubMed] [Google Scholar]
- [3].Deuis JR, Dvorakova LS, Vetter I, Methods used to evaluate pain behaviors in rodents, Rontiers Mol. Neurosci 10 (2017). 10.3389/fnmol.2017.00284.\ [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Stevenson GW, Mercer H, Cormier J, Dunbar C, Benoit L, Adams C, Jezierski J, Luginbuhl A, Bilsky EJ, Monosodium iodoacetate-induced osteoarthritis produces pain-depressed wheel running in rats: implications for preclinical behavioral assessment of chronic pain., Pharmacol. Biochem. Behav 98 (2011) 35–42. 10.1016/j.pbb.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cobos EJ, Ghasemlou N, Araldi D, Segal D, Duong K, Woolf CJ, Inflammation-induced decrease in voluntary wheel running in mice: A nonreflexive test for evaluating inflammatory pain and analgesia, Pain. 153 (2012) 876–884. 10.1016/j.pain.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Grace PM, Strand KA, Maier SF, Watkins LR, Suppression of voluntary wheel running in rats is dependent on the site of inflammation: Evidence for voluntary running as a measure of hind paw-evoked pain, J. Pain 15 (2014) 121–128. 10.1016/j.jpain.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Whitehead R, Lam N, Sun M, Sanchez J, Noor S, Vanderwall A, Petersen T, Martin H, Milligan E, Chronic sciatic neuropathy in rat reduces voluntary wheel running activity with concurrent chronic mechanical allodynia, (n.d.). 10.1213/ANE.0000000000001662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sheahan TD, Siuda ER, Bruchas MR, Shepherd AJ, Mohapatra DP, Gereau Iv RW, Golden JP, Inflammation and nerve injury minimally affect mouse voluntary behaviors proposed as indicators of pain, (2017). 10.1016/j.ynpai.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pitzer C, Kuner R, Tappe-Theodor A, Voluntary and evoked behavioral correlates in inflammatory pain conditions under different social housing conditions, PAIN Reports. 1 (2016) e564. 10.1097/pr9.0000000000000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].IASP Terminology - IASP, (n.d.). https://www.iasp-pain.org/Education/Content.aspx?ItemNumber=1698 (accessed March 27, 2020).
- [11].Lightfoot JT, Turner MJ, Daves M, Vordermark A, Kleeberger SR, Genetic influence on daily wheel running activity level, Physiol. Genomics. 19 (2005) 270–276. 10.1152/physiolgenomics.00125.2004. [DOI] [PubMed] [Google Scholar]
- [12].Merritt JR, Rhodes JS, Mouse genetic differences in voluntary wheel running, adult hippocampal neurogenesis and learning on the multi-strain-adapted plus water maze, Behav. Brain Res 280 (2015) 62–71. 10.1016/j.bbr.2014.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Walker M, Mason G, A comparison of two types of running wheel in terms of mouse preference, health, and welfare, Physiol. Behav 191 (2018) 82–90. 10.1016/j.physbeh.2018.04.006. [DOI] [PubMed] [Google Scholar]
- [14].Slivicki RA, Mali SS, Hohmann AG, Voluntary exercise reduces both chemotherapy- induced neuropathic nociception and deficits in hippocampal cellular proliferation in a mouse model of paclitaxel-induced peripheral neuropathy, Neurobiol. Pain 6 (2019) 100035. 10.1016/j.ynpai.2019.100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Novak CM, Burghardt PR, Levine JA, The use of a running wheel to measure activity in rodents: Relationship to energy balance, general activity, and reward, Neurosci. Biobehav. Rev 36 (2012) 1001–1014. 10.1016/j.neubiorev.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Toma W, Kyte SL, Bagdas D, Alkhlaif Y, Alsharari SD, Lichtman AH, Chen ZJ, Del Fabbro E, Bigbee JW, Gewirtz DA, Damaj MI, Effects of paclitaxel on the development of neuropathy and affective behaviors in the mouse, Neuropharmacology. 117 (2017) 305–315. 10.1016/j.neuropharm.2017.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wilkerson JL, Curry ZA, Kinlow PD, Mason BL, Hsu KL, Van Der Stelt M, Cravatt BF, Lichtman AH, Evaluation of different drug classes on transient sciatic nerve injury- depressed marble burying in mice, Pain. 159 (2018) 1155–1165. 10.1097/j.pain.0000000000001199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lebonvallet N, Laverdet B, Misery L, Desmoulière A, Girard D, New insights into the roles of myofibroblasts and innervation during skin healing and innovative therapies to improve scar innervation, Exp. Dermatol 27 (2018) 950–958. 10.1111/exd.13681. [DOI] [PubMed] [Google Scholar]
- [19].Neddenriep B, Bagdas D, Contreras KM, Ditre JW, Wolstenholme JT, Miles MF, Damaj MI, Pharmacological mechanisms of alcohol analgesic-like properties in mouse models of acute and chronic pain, Neuropharmacology. 160 (2019) 107793. 10.1016/j.neuropharm.2019.107793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ulker E, Caillaud M, Patel T, White A, Rashid D, Alqasem M, Lichtman AH, Bryant CD, Damaj MI, C57BL/6 substrain differences in formalin-induced pain-like behavioral responses, Behav. Brain Res 390 (2020). 10.1016/j.bbr.2020.112698. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Effects of diclofenac in vehicle and CFA-treated C57BL/6J mice on wheel running. (A) Pretreatment of diclofenac eliminated attenuation of distance traveled in voluntary wheel running assay. (B) Diclofenac administration reversed decrease in wheel running activity in CFA-treated mice on days 3 and 5 after CFA injection. (C) Number of beam interrupts in locomotion assay in a 120-minute test session. Data are expressed as mean ± S.E.M. of n=12/group. **p<0.01, ***p<0.001 vs. vehicle.
Table S1. Multiple statistical comparison of distance traveled in wheel running test between male and female, and between C57BL/6J and DBA 2/J mice after CFA and paclitaxel treatment. S1A: 3-way and 2-way ANOVA by area under the curve revealed no sex differences in distance traveled in voluntary wheel running assay. Data are expressed as mean ± S.E.M. of n=6/group. *p<0.05, **p<0.01, ***p<0.001 vs. vehicle. S1B: 3-way revealed no strain differences in distance traveled in voluntary wheel running assay. Data are expressed as mean ± S.E.M. of n=6/group. *p<0.05, **p<0.01, ***p<0.001 vs. vehicle.


