Abstract
Background:
Current guidelines favor transabdominal radical resection (RR) over transanal local excision (TAX) followed by adjuvant therapy (TAXa) for pT1N0 rectal tumors with high-risk features. Comparison of oncologic outcomes between these approaches is limited, although the former is associated with increased postoperative morbidity. We hypothesize that such treatment strategies result in equivalent long-term survival.
Methods:
A retrospective cohort study was conducted using the National Cancer Database (2010–2016) to identify patients with pT1N0 rectal adenocarcinoma with high-risk features who underwent TAX or RR for curative intent. The primary outcome was 5-year overall survival (OS), evaluated with log-rank and Cox-proportional hazards testing.
Results:
A total of 1159 patients (age 67.4 ± 12.9 years; 56.6% male; 83.3% White) met study criteria, of which 1009 (87.1%) underwent RR and 150 (12.9%) underwent TAXa. Patients undergoing TAXa had shorter lengths of stay (RR = 6.5 days, TAXa = 2.7 days, p < 0.001). The 5-year OS was equivalent between groups. TAX without adjuvant therapy was associated with an increased risk of mortality (hazard ratio 1.81, 95% confidence interval 1.17–2.78, p = 0.01).
Conclusions:
This is the largest study to demonstrate equivalent 5-year OS between TAXa and RR for T1N0 rectal cancer with high-risk features. These findings may guide the development of prospective, randomized trials and influence changes in practice recommendations for early-stage rectal cancer.
Keywords: adenocarcinoma, digestive system surgical procedures, morbidity, rectal neoplasms
1 |. INTRODUCTION
National Comprehensive Cancer Network (NCCN) 2021 clinical practice guidelines in oncology for rectal cancer recommend transanal local excision (TAX) or transabdominal radical resection (RR) for clinical stage T1N0 rectal cancer.1 Criteria for TAX include tumor size <3 cm, occupying <30% of the circumference of the bowel wall, circumferential resection margins ≥3 mm, absence of lymphovascular or perineural invasion, well to moderate histologic differentiation, and technical feasibility. The literature demonstrates several benefits of TAX over RR including improved quality of life, shorter lengths of stay, and decreased perioperative morbidity.2–4 However, for patients with T2N0 tumors or T1N0 tumors with high-risk features, including positive resection margins, lymphovascular invasion, and poor or undifferentiated tumor histology, further treatment following TAX is recommended given the association of these factors with recurrence, occult lymph node metastases, and mortality.5–7 Recommended adjuvant treatments for tumors with high-risk features following TAX include RR or chemoradiation. NCCN guidelines stipulate that RR is the preferred strategy over adjuvant chemoradiation in acceptable operative candidates.
A recent large-scale analysis of outcomes following TAX with adjuvant chemoradiation (TAXa) or RR for pT2N0 tumors demonstrated equivalent long-term survival between treatment strategies.8 There is a paucity of studies, however, comparing oncologic outcomes in patients with pT1N0 tumors and high-risk features treated by TAXa versus RR. The existing literature is primarily comprised of small, single-institution studies with limited sample sizes that combine T1 and T2 tumors.9 Furthermore, the results of these studies are conflicting. The primary objective of this analysis is to determine differences in long-term survival between patients with pT1N0 rectal tumors with high-risk features undergoing TAXa and RR from a large, nationally representative cohort. Secondary objectives include comparing perioperative and medium-term outcomes and identifying risk factors for mortality in this population. We hypothesized that long-term survival is equivalent between patients with pT1N0 tumors with high-risk features undergoing TAXa or RR.
2 |. MATERIALS AND METHODS
In this retrospective cohort study evaluating outcomes in rectal cancer, we used data from the American College of Surgeons National Cancer Database (NCDB) rectal cancer user file (2010–2016). To understand social determinants of health, these data were combined with the publicly available 2012 American Community Survey data. Institutional Review Board approval was obtained for this study, and this manuscript was prepared in accordance with STROBE guidelines for cohort studies.10
2.1 |. Data source and patient selection
The NCBD captures an estimated 70% of all new cancer diagnoses in the United States and includes pertinent diagnostic, staging, treatment, and pathologic data.11 Patient demographic characteristics include age, sex, race, insurance status, comorbidity burden, median household income, and highest education level. Comorbidity burden was represented by the maximum Charlson–Deyo (CD) Score mapped from International Classification of Diseases (ICD)-9 and −10 codes at the time of diagnosis.12,13 Educational attainment and median income were estimated by matching patients' residential zip codes to 2012 American Community Survey data. Facility and treatment characteristics included facility type and location, distance between the patient's residence and the reporting facility, and time from diagnosis to treatment. Operative approach was defined by primary site-specific surgery codes provided in the NCDB. TAX included “local tumor excision, not otherwise specified (NOS),” “excisional biopsy,” and “polypectomy” codes, as previously described.8 RR included “total proctectomy,” total“ proctectomy, NOS,” proctectomy,“ NOS,” and “wedge or segmental resection; partial proctectomy, NOS,” codes which captured patients undergoing anterior resection, low anterior resection, Hartmann procedure, and proctosigmoidectomy. Tumor characteristics included grade, size, lymphovascular invasion, and margin status. Pathologic stage was determined by American Joint Commission on Cancer (AJCC) staging guidelines.
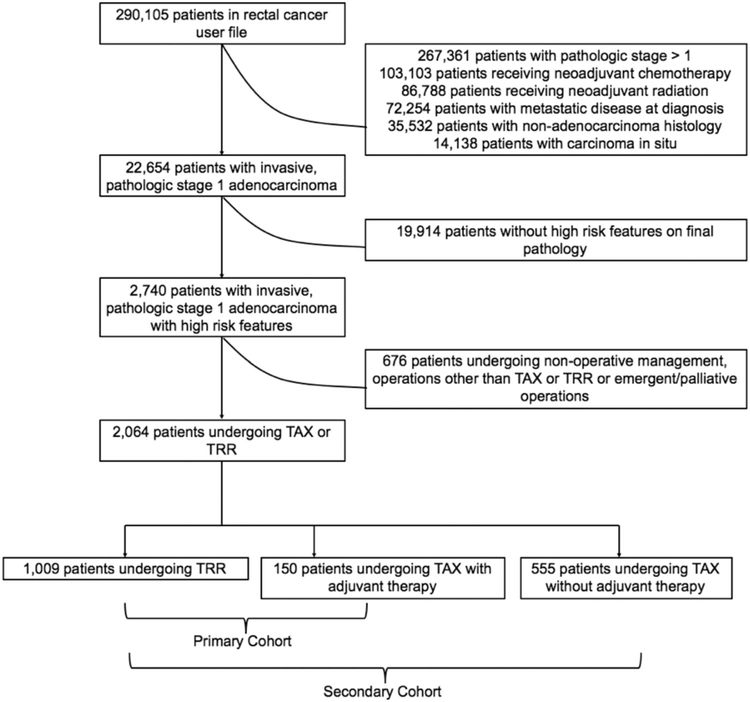
The rectal cancer user file was queried to identify patients with pathologically confirmed, T1N0 rectal adenocarcinoma with high-risk features, as defined by the NCCN, including positive resection margins, poor or undifferentiated tumor histology, or lymphovascular invasion. The rectal cancer user file excludes all patients with colon or rectosigmoid junction tumors. Additional exclusion criteria included metastatic disease at diagnosis, receipt of neoadjuvant therapy, missing pathologic staging information, carcinoma in situ, nonadenocarcinoma histology, palliative or emergent surgery, and procedures other than TAX or RR (Figure 1). Our primary cohort of interest included patients with T1N0 rectal cancer undergoing elective, curative TAX followed by adjuvant therapy or RR. To evaluate the importance of adjuvant therapy following TAX, our secondary analysis cohort included patients with T1N0 rectal cancer undergoing elective, curative TAX without adjuvant chemoradiation or RR.
FIGURE 1.
Flowchart illustrating cohort accrual
2.2 |. Outcomes and statistical analysis
Baseline patient, tumor, and treatment characteristics were compared between cohorts using Wilcoxon signed-rank or Studenťs t tests for continuous variables and X2 or Fisher's exact tests for categorical variables, as appropriate. The primary outcome, 5-year overall survival (OS), was estimated and compared between cohorts using the Kaplan–Meier method and log-rank tests. Noninferiority testing was performed to compare approaches to tumor resection using a noninferiority margin of 8.0%, as previously described.14 The primary outcome was compared between treatment groups in both the primary and secondary cohorts. To understand the effect of patient, tumor, and treatment characteristics on the primary outcomes, we evaluated subgroups of patients by individual high-risk features and adjuvant therapy type. Secondary outcomes included postoperative lengths of stay, 30-day readmission rates, 30- and 90-day mortality, and cumulative 1- and 3-year OS.
Univariate cox proportional hazards regression was performed to identify associations between any patient, tumor, or treatment factors and mortality. A multivariate cox proportional hazards regression model, clustered by facility identifier, was created adjusting for variables found to be significantly associated with mortality on univariate analysis or deemed clinically important. The number of variables included in multivariate analysis was restricted by the number of events to avoid overfitting the model. Variables that were significant on univariate analysis or deemed clinically crucial were included in the multivariate model. The proportional hazards assumption for these models was tested using Schoenfeld residuals, which confirmed the hazards were proportional for variables included in the model. Statistical analyses were performed using Stata version 16.0 (StataCorp). Using the two one-sided test (TOST) procedure, 90% confidence intervals (90% CI) for the difference in 5-year mortality between treatments was constructed and 5-year OS was considered noninferior if this interval did not fall below the lower limit for the defined noninferiority margin.15 For all other tests, statistical significance was defined as a two-tailed p value <0.05.
3 |. RESULTS
3.1 |. Patient, tumor, and treatment characteristics
Among 290 015 patients in the NCDB rectal cancer user file, 1159 met study criteria and were included in the primary cohort. An additional 555 patients undergoing TAX without adjuvant therapy were identified for inclusion in the secondary analysis cohort (Figure 1). Among the primary cohort, 1009 (87.1%) and 150 (12.9%) underwent RR and TAXa, respectively. Of patients undergoing TAXa, 11 (7.3%) received adjuvant chemotherapy, 54 (36.0%) received adjuvant radiation, and 85 (56.7%) received combination chemoradiation. There were no differences in age, sex, comorbidity burden, insurance status, or educational attainment between cohorts (Table 1). A greater proportion of patient's receiving TAXa, however, were Black (RR = 7.0%, TAXa = 18.7%, p < 0.001) and resided in a geographic area with a median annual income < $47 999 (RR = 36.6%, TAXa = 46.0%, p = 0.026). There were no differences in facility or tumor characteristics between cohorts including facility type, facility location, average distance from patients' residence to the reporting facility, tumor size, or rate of lymphovascular invasion. For patients receiving RR, mean time from diagnosis to definitive surgical therapy was longer (RR = 48.1 days, TAXa = 22.3 days, p < 0.001). Additionally, rates of R0 resection (RR = 88.7%, TAXa = 70.7%, p < 0.001) and tumor grade (percent poor/undifferentiated tumors, RR = 55.7%, TAXa = 38.0%, p < 0.001) were higher in RR.
TABLE 1.
Baseline patient clinicopathologic and treatment characteristics
| Variable | TAXa (N = 150) | RR (N =1009) | p Value |
|---|---|---|---|
| Demographic characteristics | |||
| Age (years), mean (SD) | 64.0 (12.0) | 62.5 (12.6) | 0.17 |
| Sex, n (%) | 0.50 | ||
| Male | 86 (57.3) | 608 (60.3) | |
| Female | 64 (42.7) | 401 (30.7) | |
| Race, n (%) | <0.001 | ||
| White | 118 (78.7) | 886 (87.8) | |
| Black | 28 (18.7) | 71 (7.0) | |
| Asian | 3 (2.0) | 40 (4.0) | |
| Other | 1 (0.7) | 12 (1.2) | |
| Charlson-Deyo score | 0.57 | ||
| 0 | 109 (72.7) | 754 (74.7) | |
| 1 | 28 (18.7) | 197 (19.5) | |
| 2 | 10 (6.7) | 38 (3.8) | |
| 3 | 3 (2.0) | 20 (2.0) | |
| Insurance status, n (%) | 0.12 | ||
| Private | 60 (40.0) | 524 (51.9) | |
| Medicaid | 9 (6.0) | 37 (3.7) | |
| Medicare | 74 (49.3) | 412 (40.8) | |
| Other government | 2 (1.3) | 7 (0.7) | |
| Unknown | 2 (1.3) | 15 (1.5) | |
| Median income, n (%) | 0.03 | ||
| <$48 000 | 69 (46.0) | 369 (36.6) | |
| ≥$48 000 | 81 (54.0) | 640 (63.4) | |
| Proportion without a high school degree, n (%) | 0.24 | ||
| <12.9% | 81 (54.0) | 596 (59.1) | |
| >13.0% | 69 (46.0) | 413 (40.9) | |
| Facility characteristics | |||
| Facility type, n (%) | 0.35 | ||
| Academic | 49 (33.3) | 369 (38.1) | |
| Comprehensive CP | 20 (13.6) | 59 (6.1) | |
| Comprehensive community CP | 53 (36.1) | 411 (42.4) | |
| Integrated network CP | 25 (17.0) | 130 (13.4) | |
| Facility location, n (%) | 0.95 | ||
| East | 64 (42.7) | 419 (41.5) | |
| Central | 62 (41.3) | 419 (41.5) | |
| West | 24 (16.0) | 171 (16.9) | |
| Distance from patient’s residence to hospital, miles (SD) | 27.5 (65.1) | 39.6 (113.6) | 0.20 |
| Tumor characteristics | |||
| Grade, n (%) | 0.002 | ||
| Well differentiated | 12 (8.0) | 66 (6.5) | |
| Moderately differentiated | 74 (49.3) | 349 (34.6) | |
| Poorly differentiated | 54 (36.0) | 509 (50.4) | |
| Undifferentiated | 3 (2.0) | 53 (5.3) | |
| Unknown | 7 (4.7) | 32 (3.2) | |
| Lymphovascular invasion, n (%) | 56 (40.0) | 380 (39.9) | 0.98 |
| Margin status, n (%) | <0.001 | ||
| R0 | 106 (70.7) | 895 (88.7) | |
| R1 | 42 (28.0) | 109 (10.8) | |
| R2 | 2 (1.3) | 5 (0.5) | |
| Tumor size, cm (SD) | 0.68 | ||
| <1 | 5 (3.3%) | 23 (2.3%) | |
| 1–1.99 | 145 (96.7%) | 985 (97.6%) | |
| 2–2.99 | 0 (0.0%) | 1 (0.1%) |
Abbreviations: CP, cancer program; RR, radical resection; TAXa, transanal excision with adjuvant therapy.
3.2 |. Follow-up and survival
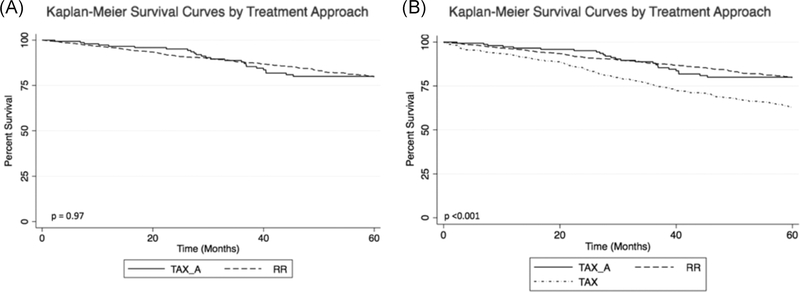
Among all included patients, median follow-up was 51.9 (interquartile range 15.6–74.1) months and was equivalent between groups (RR = 50.6, TAXa = 54.1, p = 0.10). Observed 5-year OS for patients undergoing TAXa was noninferior to that of patients undergoing RR (RR = 85.3%, TAXa = 83.3%, p = 0.97) with a TOST-generated 90% confidence interval for the difference in 5-year OS of −7.0% to 3.0%, contained within the noninferiority margin (−8.0% to 8.0%) (Figure 2A). Five-year OS remained noninferior following TAXa after excluding patients who died within 30 (RR = 86.2%, TAXa = 83.3%, p = 0.76) and 90 days of surgery (RR = 87.1%, TAXa = 84.0%, p = 0.15). Patients undergoing TAX without adjuvant therapy were found to have inferior 5-year OS (TAX = 71.6%, p < 0.001) compared to patients undergoing TAXa (90% CI for difference in survival −0.17 to −0.10) and RR (90% CI for difference in OS −0.18 to −0.05) (Figure 2B).
FIGURE 2.
(A) Five-year overall survival for patients in the primary cohort undergoing RR versus TAX_A. (B) Five-year overall survival for patients in the secondary cohort undergoing TAX_A, RR, or TAX. RR, radical resection; TAX_A, transanal excision without adjuvant therapy
In terms of secondary outcomes, patients undergoing TAXa had shorter lengths of stay (RR = 6.5 days, TAXa = 2.7 days, p < 0.001) and similar 30-day readmissions (RR = 6.2%, TAXa = 3.3%, p = 0.16). Additionally, short-term mortality was equivalent between cohorts (30-day mortality, RR = 0.9% TAXa = 0.0%, p = 0.20; 90-day mortality, RR = 1.8%, TAXa = 0.7%, p = 0.06). Medium-term oncologic outcomes, as assessed by 1 and 3-year cumulative survival were also equivalent (Table 2).
TABLE 2.
Primary and secondary outcomes by treatment strategy
| Cumulative mortality | TAXa (N = 150) | RR (N = 1009) | p Value |
|---|---|---|---|
| Length of stay (days) (SD) | 2.7 (13.6) | 6.5 (8.1) | <0.001 |
| 30-day unplanned readmission, n (%) | 5 (3.3) | 62 (6.2) | 0.16 |
| 30-day mortality, n (%) | 0 (0.0) | 9 (0.9) | 0.20 |
| 90-day mortality, n (%) | 1 (0.7) | 18 (1.8) | 0.06 |
| 1-year overall survival, n (%) | 146 (97.3) | 970 (96.1) | 0.44 |
| 3-year overall survival, n (%) | 135 (90.0) | 906 (89.8) | 0.68 |
| 5-year overall survival, n (%) | 125 (83.3) | 861 (85.3) | 0.97 |
Abbreviations: RR, radical resection; TAXa, transanal excision with adjuvant therapy.
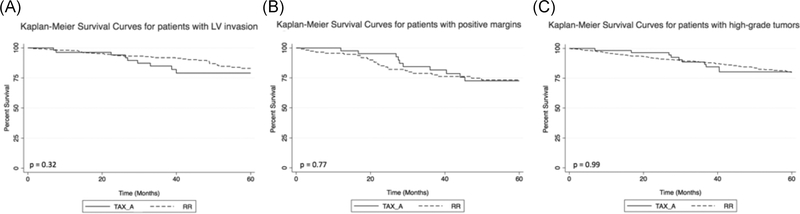
Patients were included in the analysis if they possessed any one of the high-risk features identified by the NCCN. A subgroup analysis was performed to assess for a potential association between treatment approach and survival in patients with specific high-risk features and revealed no difference in cumulative 5-year OS between cohorts in patients with lymphovascular invasion (RR = 90.0%, TAXa = 83.9%, p = 0.32), positive resection margins (RR = 77.2%, TAXa = 70.0%, p = 0.77), or poorly/undifferentiated tumors (RR = 84.5%, TAXa = 82.5%, p = 0.99) (Figure 3). A subgroup analysis of patients undergoing TAXa by adjuvant therapy type showed no difference in 5-year OS by subgroups (RR = 85.3%, TAX + chemotherapy = 90.9%, TAX + radiation = 83.3%, TAX + chemoradiation = 82.4%, p = 0.93).
FIGURE 3.
(A) Five-year overall survival for patients with lymphovascular invasion undergoing RR versus TAX_A. (B). Five-year overall survival for patients with positive resection margins undergoing RR versus TAX_A. (C) Five-year overall survival for patients with poorly differentiated or undifferentiated tumors undergoing RR versus TAX_A. RR, radical resection; TAX_A, transanal excision without adjuvant therapy
3.3 |. Risk factors for mortality
On univariate analysis, only age (hazard ratio [HR] 1.04, 95% confidence interval [CI] 1.04–1.08, p < 0.001), CD score (score = 2, HR 2.53, 95% CI 1.4–4.54, p = 0.002; score = 3, HR 2.14, 95% CI 1.22–5.15, p < 0.003), and positive gross or microscopic resection margins (HR 3.85, 95% CI 1.50–9.91, p = 0.005) were associated with 5-year mortality (Table 3). On multivariate analysis, controlling for age, sex, race, comorbidity burden, income level, tumor size, margin status, lymphovascular invasion, and tumor grade, there was no significant association between TAXa and mortality (Table 4). Multivariate analysis including patients receiving TAX without adjuvant therapy, however, illustrated an increased risk of mortality in patients treated solely with TAX (HR 1.81, 95% CI 1.17–2.78, p = 0.01). Age, comorbidity burden, and margin status remained associated with mortality on multivariate analysis.
TABLE 3.
Univariate analysis for predictors of 5-year mortality
| Variable | HR | 95% CI | p Value |
|---|---|---|---|
| Treatment approach (ref = TAXa) | 0.99 | 0.66–1.48 | 0.97 |
| Age | 1.06 | 1.04–1.08 | <0.001 |
| Sex (ref = male) | 0.74 | 0.54–1.03 | 0.07 |
| Race (ref = White) | |||
| Black | 1.59 | 0.99–2.57 | 0.06 |
| Asian | 0.69 | 0.26–1.82 | 0.46 |
| Charlson-Deyo score (ref = 0) | |||
| 1 | 1.43 | 0.99–2.08 | 0.06 |
| 2 | 2.53 | 1.41–4.54 | 0.002 |
| 3 | 2.14 | 1.22–5.15 | 0.03 |
| Insurance status (ref = uninsured) | |||
| Private | 0.45 | 0.13–1.54 | 0.20 |
| Medicaid | 1.76 | 0.47–6.56 | 0.40 |
| Medicare | 1.16 | 0.35–3.91 | 0.81 |
| Median Income (ref = >$48 000) | 1.47 | 1.09–1.97 | 0.01 |
| Percent no high school degree (ref < 12.9%) | 1.16 | 0.87–1.55 | 0.32 |
| Facility type (ref = not academic) | 0.86 | 0.62–1.18 | 0.35 |
| Tumor size (ref < 1.00 cm) | |||
| Size ≥ 1.00 cm | 0.83 | 0.38–1.82 | 0.643 |
| Grade (ref = well-differentiated) | |||
| Poorly differentiated | 1.03 | 0.53–2.02 | 0.92 |
| Undifferentiated | 1.12 | 0.46–2.74 | 0.26 |
| Lymphovascular invasion (ref = no) | 1.04 | 0.55–1.99 | 0.89 |
| Margin status (ref = R0) | |||
| R1 | 1.51 | 0.99–2.29 | 0.05 |
| R2 | 3.85 | 1.50–9.91 | 0.005 |
Abbreviations: CI, confidence interval; HR, hazard ratio; TAXa, transanal excision with adjuvant therapy.
TABLE 4.
Multivariate Cox proportional hazards analysis for 5-year mortality
| Variable | HR | 95% CI | p Value |
|---|---|---|---|
| Treatment approach (ref = TAXa) | 0.99 | 0.66–1.48 | 0.97 |
| Age | 1.06 | 1.04–1.08 | <0.001 |
| Sex (ref = male) | 0.74 | 0.54–1.03 | 0.07 |
| Race (ref = White) | |||
| Black | 1.59 | 0.99–2.57 | 0.06 |
| Asian | 0.69 | 0.26–1.82 | 0.46 |
| Charlson-Deyo score (ref = 0) | |||
| 1 | 1.43 | 0.99–2.08 | 0.06 |
| 2 | 2.53 | 1.41–4.54 | 0.002 |
| 3 | 2.14 | 0.82–5.54 | 0.11 |
| Income (ref = >$48 000) | 1.47 | 1.09–1.97 | 0.01 |
| Tumor size | |||
| Grade (ref = well differentiated) | |||
| Poorly differentiated | 1.03 | 0.53–2.02 | 0.92 |
| Undifferentiated | 1.12 | 0.46–2.74 | 0.26 |
| Lymphovascular invasion (ref = no) | 1.04 | 0.55–1.99 | 0.89 |
| Margin status (ref = R0) | |||
| R1 | 1.51 | 0.99–2.29 | 0.05 |
| R2 | 3.85 | 1.50–9.91 | 0.005 |
Abbreviations: CI, confidence interval; HR, hazard ratio; TAXa, transanal excision with adjuvant therapy.
4 |. DISCUSSION
To our knowledge, this is the largest comparison of perioperative and oncologic outcomes between patients undergoing transabdominal radical resection versus local transanal excision for pathologically confirmed T1N0 rectal tumors with high-risk features to date. We demonstrate noninferior survival for patients undergoing TAXa and did not identify the operative approach as a risk factor for mortality on univariate or multivariate analyses. Furthermore, we delineated the importance of adjuvant therapy among those undergoing TAX by demonstrating lower 5-year OS for patients in this population receiving TAX without further treatment.
While TAXa and RR are both acceptable treatment approaches for T1N0 tumors with high-risk features, RR is considered the preferred approach under NCCN guidelines.1 Evidence for this recommendation is rooted in the results of a 2009 study of 282 patients from a single institution with clinical stage T1 disease who underwent RR versus TAX with or without adjuvant therapy and reported increased recurrence and disease-specific mortality in patients undergoing local resection.16 Radiographic staging methods such as endorectal ultrasound or pelvic magnetic resonance imaging (MRI) were not utilized in this study, and 20% of patients in the RR cohort were found to have lymph node metastases on final pathology. This suggests that a substantial proportion of patients were incorrectly staged and not appropriate candidates for upfront TAX. Consequently, these results may not accurately reflect the natural history of T1N0 disease and are not specific to the subset of patients with pT1 disease and high-risk features. Overall, the literature aimed at comparing these two treatment approaches is comprised of retrospective studies featuring small, heterogeneous cohorts.
The greatest risk in performing TAX for this subset of tumors is the possibility of occult lymph node metastases resulting in under staging, under treatment, and local recurrence if not followed by RR. The estimated incidence of nodal disease in T1 tumors is approximately 13%.17 Prior studies have identified invasion into the lower third of the submucosa, lymphovascular invasion, and unfavorable histologic grade as independent risk factors for occult nodal disease and have demonstrated inferior outcomes following TAX in tumors with these features.18–20 Clearly, the evidence substantiates an increased risk of advanced or aggressive disease in tumors with any of these characteristics and supports the recommendation for adjuvant therapy or secondary RR.
Our analysis of national oncologic outcomes corroborates these findings and demonstrates inferior survival following TAX without adjuvant therapy as compared to RR. The physiologic stress of RR when compared to TAX results in an increased postoperative length of stay and a trend towards increased early postoperative mortality. With the addition of adjuvant therapy, however, 5-year overall survival between operative approaches was equivalent, thus treating any persistent microscopic disease in the resection bed or locoregional lymph nodes. We are unable to comment on local recurrence rates, due to database limitations, but prior small-scale, single-center studies have demonstrated promising results. A study of 16 patients with high-risk pT1 tumors undergoing transanal endoscopic microsurgery and R0 resection demonstrated a 6.7% reduction in local recurrence in patients treated with adjuvant chemoradiation.21 A separate study reported 100% 3-year disease-free survival among 20 patients at high risk for recurrence following TAX treated with chemoradiation and close MRI surveillance.22 Long-term follow-up of 52 patients with high-risk T1 tumors treated with TAX demonstrated reduced local recurrence with 5-year local control rates of 72%, 90%, and 96% following TAX alone, adjuvant radiation, and adjuvant chemoradiation, respectively. Commensurate with our findings, this study reported poorly differentiated histology and lymphovascular invasion were only associated with local failure in patients receiving TAX without adjuvant therapy.23
Despite the encouraging results of our analysis, practitioners may display warranted caution in pursuing TAXa without concrete evidence of equivalent local control and disease-free survival, particularly in young, healthy patients who tolerate the physiologic stress of radical surgery and are at low operative risk. Indeed, we demonstrated that patients undergoing TAXa were older with more medical comorbidities signifying increased consideration of this approach in patients whose operative risk may outweigh long-term oncologic considerations. Low anterior resection with coloanal anastomosis carries an associated 35%–45% risk of complications and 15%–20% risk of reoperation or intensive care unit admission.24–26 More concerning, there is evidence to suggest postoperative complications are associated with inferior recurrence-free and overall survival.27 Risk factors for poor perioperative outcomes include smoking, high comorbidity burden, poor nutrition, and immunosuppression.25,26 A thorough informed consent process and serious consideration of a TAX is warranted in patients with these risk factors with early-stage disease.
Interestingly, we also found a greater relative proportion of Black and low-income patients who underwent TAXa. Further studies are needed to determine if this finding is primarily patient or provider-driven. Low socioeconomic status was associated with mortality on adjusted analysis, as has been reported previously.28–30 The need for closer surveillance after TAXa should be considered in the decisionmaking to ensure understanding and capability for more frequent surveillance.
Most importantly, these data may help identify patients at high risk for poor outcomes under either approach. In the present study, we identified positive resection margins as a treatment-related risk factor for mortality. Positive margins are a well-described risk factor for inferior outcomes.31–34 Despite a higher incidence of positive resection margins after TAX, treatment approach was not associated with mortality on univariate or multivariate analyses. This finding is likely explained by disparate rates of adjuvant radiation between operative approaches. All patients with positive margins in the TAXa group received adjuvant radiation and 56.8% also received chemotherapy compared to only 16.7% and 11.4% in the RR cohort. A multicenter trial of 120 patients with Stage I–III disease and positive resection margins, however, demonstrated no difference in local recurrence rates between patients who did or did not receive postoperative pelvic irradiation.35 The results of this and prior analyses highlight the need for prospective, randomized trial data to guide patient selection and decisionmaking in this clinical scenario. Ideally, these data would allow for direct comparison of local and distant treatment failure rates, survival, perioperative morbidity, quality of life, and costs.
The results of this study should be interpreted within the context of its limitations. First, and perhaps most notably, the NCDB does not collect information on the incidence or treatment of recurrences, thus limiting our evaluation of treatments and associated outcomes to survival. Second, the NCDB does not contain information related to postoperative complications that would help describe the difference in potential perioperative morbidity between groups. Furthermore, the NCDB only records the primary operative intervention for each patient. Patients who underwent TAX followed by RR could not be specifically identified and were included in the RR cohort in our study. Finally, given the multicenter nature of the NCDB, our analysis accounted for only the variability within and between institutions; but was unable to account for surgeon-specific outcomes and cannot account for the role of surgeon experience.
5 |. CONCLUSIONS
NCCN guidelines for the management of T1N0 rectal cancer with high-risk features currently favor radical resection over local excision followed by adjuvant chemotherapy and/or radiation. While the latter approach has been employed selectively by surgeons, often among patients for whom radical resection is associated with prohibitive risks, differences in oncologic outcomes between these approaches have not been well-studied. Our study found that among patients included in a large, multicenter, national database, TAXa results in equivalent 5-year OS relative to RR with potentially less surgical stress and shorter postoperative admission.
While further research is needed to determine differences in locoregional or distant recurrence, quality of life, and functional status, the results of this study can be used to guide the development of prospective, randomized trials to validate these findings and potentially change practice recommendations.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon request.
REFERENCES
- 1.National Comprehensive Cancer Network. Rectal cancer (Version 1). 2021. Accessed April 6, 2021. https://www.nccn.org/professinoal/physician_gls/pdf/rectal_blocks.pdf
- 2.Fenech DS, Takahashi T, Liu M, et al. Function and quality of life after transanal excision of rectal polyps and cancers. Dis Colon Rectum. 2007;50(5):598–603. [DOI] [PubMed] [Google Scholar]
- 3.Ma B, Gao P, Song Y, et al. Transanal total mesorectal excision (taTME) for rectal cancer: a systematic review and meta-analysis of oncological and perioperative outcomes compared with laparoscopic total mesorectal excision. BMC Cancer. 2016;16:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ptok H, Marusch F, Meyer F, et al. Oncologic outcome of local vs radical resection of low-risk pT1 rectal cancer. Arch Surg. 2007; 142(7):649–655. [DOI] [PubMed] [Google Scholar]
- 5.Leijssen L, Dinaux AM, Amri R, et al. Perineural invasion is a prognostic but not a predictive factor in nonmetastatic colon cancer. Dis Colon Rectum. 2019;62(10):1212–1221. [DOI] [PubMed] [Google Scholar]
- 6.Willett CG, Badizadegan K, Ancukiewics M, Shellito PC. Prognostic factors in stage T2N0 rectal cancer. Dis Colon Rectum. 1999;42: 167–173. [DOI] [PubMed] [Google Scholar]
- 7.Birbeck KF, Macklin CP, Tiffin NJ, et al. Rates of circumferential resection margin involvement vary between surgeons and predict outcomes in rectal cancer surgery. Ann Surg. 2002;235(4): 449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee L, Kelly J, Nassif GJ, et al. Chemoradiation and local excision for T2N0 rectal cancer offers equivalent overall survival compared to standard resection: a National Cancer Database analysis. J Gastrointest Surg. 2017;21:1666–1674. [DOI] [PubMed] [Google Scholar]
- 9.Borstlap WA, Coeymans TJ, Tanis PJ, et al. Meta-analysis of oncological outcomes after local excision of pT1–2 rectal cancer requiring adjuvant (chemo)radiotherapy or completion surgery. Br J Surg. 2016;103(9):1105–1116. [DOI] [PubMed] [Google Scholar]
- 10.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349. [DOI] [PubMed] [Google Scholar]
- 11.American College of Surgeons. National Cancer Data Base. Accessed April 19, 2021. www.facs.org/quality-programs/cancer/ncdb
- 12.American College of Surgeons. National Cancer Data Base participant user file (PUF). Accessed April 19, 2021. www.facs/org/-/media/files/quality-programs/cancer/ncdb/puf_data_dictionary.ashx
- 13.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 14.Stevenson AR, Solomon MJ, Lumley JW, et al. Effect of laparoscopic-assisted resection vs open resection on pathologic outcomes in rectal cancer: the ALaCaRT randomized clinical trial. JAMA. 2015;314(13):1356–1363. [DOI] [PubMed] [Google Scholar]
- 15.Walker E, Nowacki AS. Understanding equivalence and noninferiority testing. J Gen Intern Med. 2011;26(2):192–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nash GM, Weiser MR, Guillem JG, et al. Long-term survival after transanal excision of T1 rectal cancer. Dis Colon Rectum. 2009;52(4): 577–582. [DOI] [PubMed] [Google Scholar]
- 17.Nascimbeni R, Burgart LJ, Nivatvongs S, Larson DR. Risk of lymph node metastasis in T1 carcinoma of the colon and rectum. Dis Colon Rectum. 2002;45(2):200–206. [DOI] [PubMed] [Google Scholar]
- 18.Bach SP, Hill J, Monson JR, et al. A predictive model for local recurrence after transanal endoscopic microsurgery for rectal cancer. Br J Surg. 2009;96:280–290. [DOI] [PubMed] [Google Scholar]
- 19.Yasuda K, Inomata M, Shiromizu A, Shiraishi N, Higashi H, Kitano S. Risk factors for occult lymph node metastasis of colorectal cancer invading the submucosa and indications for endoscopic mucosal resection. Dis Colon Rectum. 2007;50(9):1370–1376. [DOI] [PubMed] [Google Scholar]
- 20.Willett CG, Compton CC, Shellito PC, Efird JT. Selection factors for local excision or abdominoperineal resection of early-stage rectal cancer. Cancer. 1994;73(11):2716–2720. [DOI] [PubMed] [Google Scholar]
- 21.Amann M, Burghardt J, Stratz C, Buess GF, Modabber A. Transanal endoscopic microsurgery in treatment of small rectal T1 high-risk, T2, and T3 carcinomas combined with radiochemotherapy. Eur Surg. 2015;47:226–237. [Google Scholar]
- 22.Balyasnikova S, Read J, Tait D, et al. The results of local excision with or without postoperative adjuvant chemoradiotherapy for early rectal cancer among patients choosing to avoid radical surgery. Colorectal Dis. 2017;19(2):139–147. [DOI] [PubMed] [Google Scholar]
- 23.Chakravarti A, Compton CC, Shellito PC, et al. Long-term follow-up of patients with rectal cancer managed by local excision with and without adjuvant irradiation. Ann Surg. 1999;230(1):49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bennis M, Parc Y, Lefevre JH, Chafai N, Attal E, Tiret E. Morbidity risk factors after low anterior resection with total mesorectal excision and coloanal anastomosis: a retrospective series of 483 patients. Ann Surg. 2012;255(3):504–510. [DOI] [PubMed] [Google Scholar]
- 25.McDermott FD, Heeney A, Kelly ME, Steele RJ, Carlson GL, Winter DC. Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leak. Br J Surg. 2015;102(5):462–479. [DOI] [PubMed] [Google Scholar]
- 26.Kang CY, Halabi WJ, Chaudhry OO, et al. Risk factors for anastomotic leakage after anterior resection for rectal cancer. JAMA Surg. 2013;148(1):65–71. [DOI] [PubMed] [Google Scholar]
- 27.Gamboa AC, Lee RM, Turgeon MK, et al. Impact of postoperative complications on oncologic outcomes after rectal cancer surgery: an analysis of the US Rectal Cancer Consortium. Ann Surg Onc. 2021; 28(3):1712–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nitzkorski JR, Willis AI, Nick D, Zhu F, Farma JM, Sigurdson ER. Association of race and socioeconomic status and outcomes of patients with rectal cancer. Ann Surg Onc. 2013;20:1142–1147. [DOI] [PubMed] [Google Scholar]
- 29.Harris AR, Bowley DM, Stannard A, Kurrimboccus S, Geh JI, Karandikar S. Socioeconomic deprivation adversely affects survival of patients with rectal cancer. Br J Surg. 2009;96(7):763–768. [DOI] [PubMed] [Google Scholar]
- 30.Lin Gomez S, O'Malley CD, Stroup A, Shema SJ, Satariano WA. Longitudinal, population-based study of racial/ethnic differences in colorectal cancer survival: impact of neighborhood socioeconomic status, treatment and comorbidity. BMC Cancer. 2007;7:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wibe A, Rendedal PR, Svensson E, et al. Prognostic significance of the circumferential resection margin following total mesorectal excision for rectal cancer. Br J Surg. 2002;89(3):327–334. [DOI] [PubMed] [Google Scholar]
- 32.Phang PT, MacFarlane JK, Taylor RH, et al. Effects of positive resection margin and tumor distance from anus on rectal cancer treatment outcomes. Am J Surg. 2002;183(5):504–508. [DOI] [PubMed] [Google Scholar]
- 33.Fitzgerald TL, Brinkley J, Zervos EE. Pushing the envelope beyond a centimeter in rectal cancer: oncologic implications of close, but negative margins. J Am Coll Surg. 2011;213(5):589–595. [DOI] [PubMed] [Google Scholar]
- 34.Adam IJ, Mohamdee MO, Martin IG, et al. Role of circumferential margin involvement in the local recurrence of rectal cancer. Lancet. 1994;344(8924):707–11. [DOI] [PubMed] [Google Scholar]
- 35.Marijnen CAM, Nagtegaal ID, Kapiteijn E, et al. Radiotherapy does not compensate for positive resection margins in rectal cancer patients: report of a multicenter randomized trial. Int J Rad Onc Biol Physics. 2003;55(5):1311–1320. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.