Abstract
Leucocyte recruitment is a critical component of the immune response and is central to our ability to fight infection. Paradoxically, leucocyte recruitment is also a central component of inflammatory‐based diseases such as rheumatoid arthritis, atherosclerosis and cancer. The role of the extracellular matrix, in particular proteoglycans, in this process has been largely overlooked. Proteoglycans consist of protein cores with glycosaminoglycan sugar side chains attached. Proteoglycans have been shown to bind and regulate the function of a number of proteins, for example chemokines, and also play a key structural role in the local tissue environment/niche. Whilst they have been implicated in leucocyte recruitment and inflammatory disease, their mechanistic function has yet to be fully understood, precluding therapeutic targeting. This review summarizes what is currently known about the role of proteoglycans in the different stages of leucocyte recruitment and proposes a number of areas where more research is needed. A better understanding of the mechanistic role of proteoglycans during inflammatory disease will inform the development of next‐generation therapeutics.
Keywords: adhesion, chemokine, glycosaminoglycan, leucocyte, migration, proteoglycan
1. INTRODUCTION
Proteoglycans are extracellular matrix components that play a wide‐ranging role in the immune system and in wider biology. 1 Proteoglycans are key regulators of immune cell (leucocyte) recruitment and positioning during the inflammatory process and associated diseases such as rheumatoid arthritis, atherosclerosis and cancer. 2
Leucocyte recruitment is a key component of the immune response where these cells, produced largely in the bone marrow, are recruited from the circulation and into tissues as required during specific phases of the immune response.3, 4 Once leucocytes have been recruited from the vasculature, they then undergo further migration to achieve distinct positions within the tissue. This process occurs during inflammation to facilitate the removal of invasive pathogens and infected cells by the innate and adaptive immune response. Generally speaking, eosinophils, basophils, neutrophils and monocytes are recruited early in the immune response followed later by T and B cells. Leucocytes (primarily monocytes/macrophages) are also key during the resolution of inflammation and associated tissue damage.
Whilst the process of leucocyte recruitment and positioning via transendothelial migration has been well studied, the role of the extracellular matrix, in particular proteoglycans, is often overlooked. In this review, we will summarize what is known about the mechanistic and functional roles of proteoglycans in the process of leucocyte recruitment and also highlight gaps in our knowledge where further research is needed.
2. PROTEOGLYCAN AND GLYCOSAMINOGLYCAN STRUCTURE
Proteoglycans consist of proteins cores, either embedded in a cell membrane or soluble with sulphated glycosaminoglycan sugar side chains attached (Figure 1).1, 5 Membrane‐embedded protein cores consist of the syndecan and glypican families, whilst the soluble proteoglycans consist of serglycin, agrin, perlecan and collagen XVIII. These proteoglycan structures are found within the extracellular matrix of tissues, on the surface of the majority of mammalian cells, and are particularly prevalent on the surface of the endothelium lining blood vessels as part of the glycocalyx, and also within the basement membrane. 6 We currently do not have much insight into the potential differential function of proteoglycans in these two distinct environments, that is luminal glycocalyx vs basement membrane.
FIGURE 1.
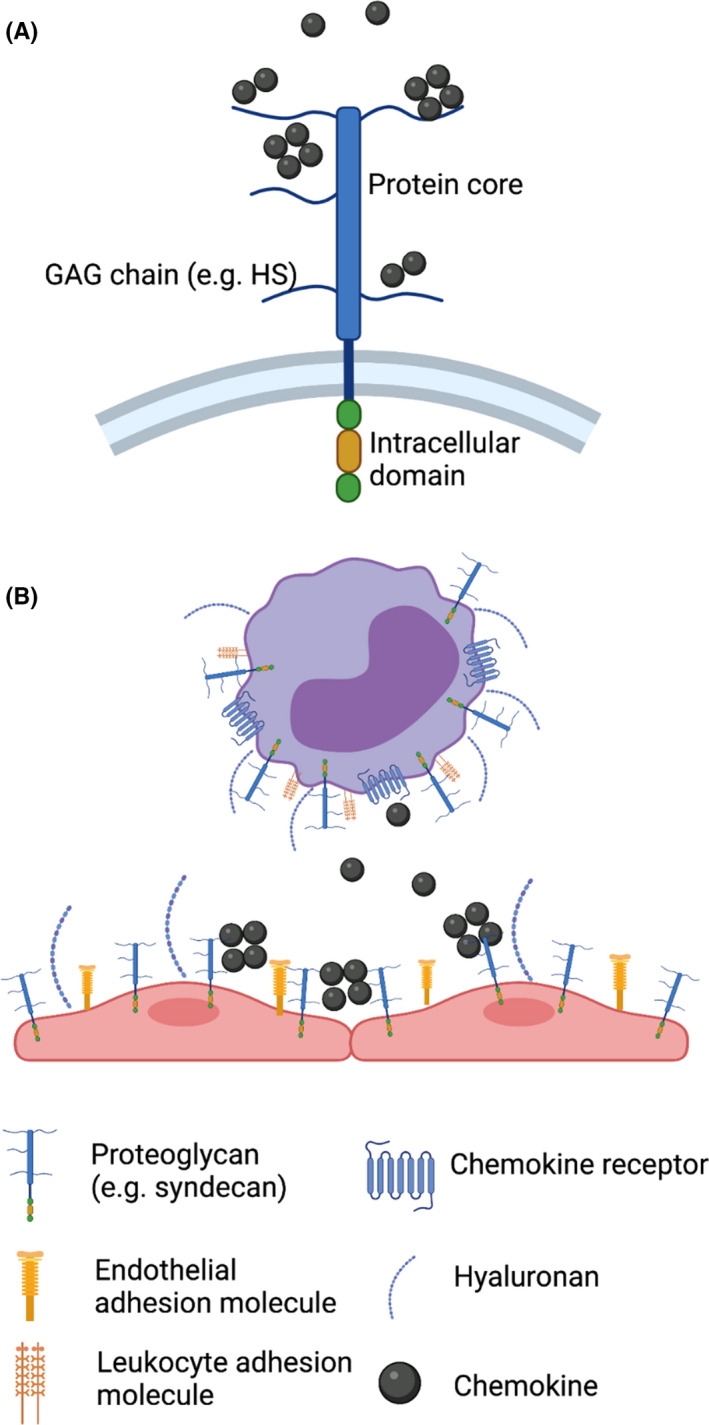
Proteoglycans regulate leucocyte recruitment. (A) Proteoglycans are composed of a protein core, depicted here embedded in a cell membrane as is the case with the syndecan family. Proteoglycans have glycosaminoglycan (GAG) side chains that can bind to a number of proteins, for example chemokines. (B) Proteoglycans on the surface of both endothelial cells and leucocytes regulate interactions between the two by masking adhesion molecules. Created with BioRender.com
The glycosaminoglycan (GAG) sugars that are present as side chains on the protein cores of proteoglycans are made up of repeating disaccharide units that repeat to form long unbranched (linear) chains. 7 Both heparan sulphate (HS) and chondroitin sulphate (CS) GAGs play critical roles in leucocyte recruitment and positioning. 2 Whilst less studied in this context, dermatan sulphate (DS) may also contribute to this process via its ability to bind to relevant proteins, for example chemokines, albeit much weaker when compared to HS. 8 Heparan sulphate is made up of repeating units of glucuronic acid (GlcA) [which can be epimerized to iduronic acid (IdoA) during assembly] and N‐acetylglucosamine (GlcNAc). 7 The CS chain sequence is slightly different and is made up of repeating units of glucuronic acid and N‐acetylgalactosamine (GalNAc). 9 The DS sequence backbone is the same as CS but with some glucuronic acid residues epimerized to become iduronic acid. GAG chains are acidic and are also hydrophilic; thus, when present together they form a hydrated and ‘soft’ gel‐like structure (Figure 1B). 5 This is particularly the case on the endothelial glycocalyx that lines blood vessels and regulates leucocyte recruitment and inflammation by the mechanisms described below.
Glycosaminoglycan chains are sulphated at various points, in the case of HS (the dominant proteoglycan GAG chain in leucocyte recruitment), N‐ and 6‐O sulphation on the glucosamine, 2‐O sulphation on the iduronic acid and more rarely 3‐O sulphation on the glucosamine.7, 10 In contrast, CS can be 2‐O‐sulphated on the glucuronic acid and 4‐O‐ and/or 6‐O‐sulphated on the N‐acetylgalactosamine residue. 9 DS is more commonly 2‐O‐sulphated than CS, due to the epimerized iduronic acid residue. These sulphation points are critical to the ability of HS and CS GAG chains to interact with a range of different ligands, many of which are important in the leucocyte migration process (discussed below).
A range of literature has dissected the roles of GAG sulphation points in interactions with different ligands; however, there is still much to learn about the potential specificity of these interactions and the effect this may have during leucocyte recruitment in vivo. New tools to analyse the contribution of specific sulphation points to GAG interactions in a cellular context combined with increasing ability to sequence GAGs purified from biological contexts will significantly develop this field in the coming years.11, 12, 13
3. ENDOTHELIAL PROTEOGLYCANS
The vascular endothelium constitutes a monolayer of endothelial cells, which comprise the innermost cellular lining of blood vessels. These cells serve a variety of important functions, which include the coordination of inflammation and immune responses. 14 The vascular endothelial surface is coated with a carbohydrate‐rich matrix called the glycocalyx, which in the past has been somewhat overlooked, in the context of disease. The endothelial glycocalyx is a negatively charged, membrane‐bound layer of proteoglycans and glycoproteins, which line the luminal surface of blood vessels. This protective structure was first visualized following the invention of transmission electron microscopy in the 1960s. 15
At the luminal surface of the endothelium, syndecans and glypicans (membrane‐bound) and serglycin and agrin (soluble) are thought to be the dominant proteoglycans. 5 The endothelial glycocalyx also contains hyaluronan, which is non‐sulphated and is anchored by the CD44 receptor, and the hyaluronic acid synthase enzyme. Some additional glycocalyx glycoproteins have a sialic acid or a fucose cap, which serve important functions in coagulation (eg, selectins, integrins and immunoglobulins) or act as endothelial adhesion molecules. Additionally, there are both endothelial‐ and plasma‐derived soluble components incorporated into this matrix.
Due to the physical properties of its components, the glycocalyx forms a thick and hydrated barrier. The intact glycocalyx is too thick to allow interaction between adhesion molecules on leucocytes and the endothelium (Figure 1B), a key component of leucocyte migration.16, 17, 18, 19 Even when projected onto endothelial protrusions, the mass of a leucocyte is insufficient to facilitate penetration of the glycocalyx. 20
The inhibition of the first step in leucocyte recruitment by glycocalyx proteoglycans means there is missing mechanistic understanding at the heart of leucocyte recruitment that has yet to be resolved, that is how do leucocytes interact with the endothelium in the context of the glycocalyx?
4. HOW IS THE GLYCOCALYX REMODELLED TO FACILITATE LEUCOCYTE MIGRATION?
A number of studies have demonstrated mechanisms whereby the glycocalyx can be remodelled to facilitate leucocyte:endothelial interactions. 5 For example, injection of the chemoattractant formylmethionyl‐leucyl‐phenylalanine (fMLP) was shown to mediate alterations in glycocalyx structure that promoted leucocyte rolling on the endothelium. 21 Further studies demonstrated that this was by induction of heparinase leading to shedding of HS GAGs from proteoglycans facilitating access to the endothelial adhesion molecules, for example intercellular adhesion molecule 1 (ICAM‐1). 22 More recent studies have demonstrated a similar mechanism whereby the inflammatory cytokine tumour necrosis factor (TNF) can induce heparinase‐mediated glycocalyx shedding during sepsis.18, 23 This was shown to facilitate recruitment of neutrophils and also induce side effects in the brains of sepsis patients by the GAG‐binding protein brain‐derived neurotrophic factor (BDNF). 24
In recent years, the integral role of the glycocalyx in determining cardiovascular health and disease has been established. 25 In chronic conditions such as rheumatoid arthritis, diabetes, sepsis, atherosclerosis and ischaemia/reperfusion injury, leucocyte recruitment can be excessive and detrimental. The presence of the glycocalyx constituents within the blood plasma can act as a biomarker for disease, since glycocalyx shedding occurs in numerous disease pathologies. Specifically shedding of syndecans (major glycocalyx components), for example syndecan‐1 by matrix metalloproteinases, into the circulation has been proposed as a marker of inflammation. 26 Importantly, changes to the resulting composition of the glycocalyx can also drive disease through the exposure of adhesion sites, and thus the facilitation of leucocyte transmigration.
Whilst these studies, and others, have begun to develop our understanding of changes to proteoglycans during inflammation and leucocyte recruitment, a number of outstanding questions remain. For example how does the shedding of the glycocalyx fit with the studies demonstrating that proteoglycans are also essential for leucocyte recruitment (detailed later)? Presumably during inflammation, there is a balance between shedding and retention of proteoglycans that must exist to remove sufficient glycocalyx to not only allow leucocyte:endothelial interaction but also allow the pro‐migratory functions of proteoglycans.
5. ENDOTHELIAL PROTEOGLYCANS ACROSS VASCULAR BEDS
One issue that affects our understanding of the role of proteoglycans in leucocyte recruitment is their distribution and heterogeneity within the glycocalyx across different vascular beds, that is arterial vs venous blood vessels.
It has been suggested that glycocalyx thickness increases with vessel diameter, at least in the arterial system where the matrix is more substantial. 27 The estimation of glycocalyx thickness is variable due to its sensitivity to processing and the differential methods used in analysis. 28 However, it seems likely that glycocalyx morphology is different between vessel types, with the thinnest measurements recorded in capillaries and venules, ranging from 0.2 to 0.5 μm. 27 Whereas a glycocalyx of small arteries extends 2–3 μm, a glycocalyx of larger arteries extends up to 4.5 μm. Furthermore, evidence suggests that the glycocalyx differs between the same vessel types of different organs. For instance, capillaries in the brain, heart and lung are all considered continuous with complete glycocalyx covering. However, the glycocalyx of cerebral capillaries is thicker than that of cardiac and pulmonary capillaries, likely due to its important contribution to the blood–brain barrier (BBB). 27
Since the majority of leucocyte recruitment from the vasculature and into tissues is thought to occur within post‐capillary venules, it is possible that the glycocalyx is thinner and thus more permissive to leucocyte:endothelial interactions at this site.3, 5 It seems highly likely that the proteoglycan content and structure of the glycocalyx will differ across vascular beds and also across different tissues. Future studies are needed to specifically define proteoglycan content and structure of the glycocalyx at different vascular beds within different tissues, before and after inflammation and leucocyte recruitment, to comprehensively understand this process.
In addition to overall proteoglycan structure and content of the vascular system, the specific mechanistic role, and geographical location, of GAG sulphation is also likely to be important.7, 29 GAGs can be modified to have the sulphation points described above, and specific patterns likely mediate specific interactions within different ligands involved in leucocyte recruitment (discussed below). Whilst the sulphation of GAGs across tissues and species has been shown to be specific, 30 we still have little information of how this varies across vascular beds and in response to different inflammatory stimuli. Thus, future studies will also need to better address the specifics in changes to GAG sulphation in defined geographical and inflammatory contexts. Such approaches now seem increasingly feasible following recent technological advances in GAG analysis.12, 13
As mentioned above, a number of studies have demonstrated that the application of factors to induce shedding of proteoglycans and their GAG chains has been shown to mediate increased rolling and migration of leucocytes from the vasculature. 5 In contrast, other studies have shown that proteoglycans are required for leucocyte migration, where their removal actually reduces leucocyte recruitment.31, 32 There are likely a number of ways in which proteoglycans promote leucocyte recruitment.
6. PROTEOGLYCAN REGULATION OF CHEMOKINE FUNCTION
One of the most well‐studied functions of proteoglycans during leucocyte recruitment is their ability to interact with chemokines, whose primary function is to facilitate firm adhesion of leucocytes to the endothelium. 33 Chemokine:proteoglycan interactions within the basement membrane are likely also key in leucocyte recruitment and trafficking. Chemokines bind to their receptors on circulating leucocytes to induce signalling events that result in integrin activation and thus firm adhesion to the endothelium. A number of years ago, it was shown that mutation of CCL2, CCL4 and CCL5, so that they could no longer bind to GAGs, ablated their ability to mediate leucocyte recruitment to the peritoneum of mice. 34 Subsequently, a range of studies have demonstrated a similar function for other chemokines and have demonstrated a clear hierarchy in the ability of chemokines to bind to GAGs.35, 36, 37, 38, 39, 40, 41, 42, 43, 44 A number of studies have also explored the potential specificity in binding to different sulphation patterns on GAGs.45, 46 Indeed given the therapeutic potential in targeting chemokines, the chemokine:GAG interaction is an ongoing focus for potential new therapeutics to target inflammatory disease. 47
Despite this range of research, the mechanistic importance of chemokine:GAG interactions has yet to be fully understood. 48 It seems likely that interaction with GAGs within the luminal glycocalyx is important to retain chemokines at inflammatory sites in the presence of blood flow. However, there is very limited evidence for the common assertion that GAGs facilitate formation of chemokine gradients within the vasculature, 49 where gradient formation has more commonly been observed within the lymphatic system or tissues in the absence of blood flow. 50 CCL19 and CCL21 have been shown to have specific functions, by their differential interactions with HS and CS proteoglycans, in trafficking of dendritic cells within the lymphatic system.50, 51, 52, 53 Furthermore, given recent discoveries on the importance of self‐generated gradients there remains the exciting possibility that GAGs are important in this process both within tissues and the vasculature. 54
The other mechanistic role of chemokine:GAG interactions is in protection from proteolysis,55, 56 this again seems likely to be important in chemokine‐mediated leucocyte recruitment. Given that these interactions will result in both bound and non‐bound chemokine at any given time, the cloud hypothesis has recently been proposed.48, 57 This states that GAGs mediate retention of a local cloud of soluble chemokine that is available to bind to circulating leucocytes within the vasculature to facilitate their firm adhesion.
More recently, a number of studies, including our own, have demonstrated that as well as binding to GAGs certain chemokines, for example CXCL4 and CXCL12, can remodel the structure of HS.39, 44 This involved cross‐linking of individual GAG chains to render them less mobile within a biophysical model of the cell membrane lipid bilayer. Cross‐linking also resulted in a reduced thickness of the glycocalyx‐like structures formed by HS GAG chains. Work is now ongoing in our laboratory to determine the biological function of these remodelling events; for example, can chemokines bind and alter the endothelial glycocalyx structure on blood vessels to increase its permeability and enable leucocyte recruitment in vivo?
As with the other areas of proteoglycan function in leucocyte recruitment, we are only at the beginning of our understanding of this complex biological problem. Exciting technological developments will be at the heart of future studies to understand the mechanistic function of chemokine:GAG interactions. For example why do chemokines exhibit such a wide range of affinities for GAGs, why can certain chemokines modify GAG structure and what is the role of specific GAG sulphation patterns in chemokine function? 48
7. GAGs AND ADHESION MOLECULES
Numerous studies have highlighted the importance of sialyl‐Lewis X (SLex) as a selectin ligand in the interactions between leucocytes and endothelial cells during leucocyte migration.58, 59, 60 Another direct function of proteoglycans, by their GAG side chains, in facilitating leucocyte recruitment is their interaction with leucocyte adhesion molecules, for example selectins. The ability of L (leucocyte)‐ and P (platelet)‐selectins but not E (endothelial)‐selectins, to bind GAGs, has been demonstrated. 58 P‐selectin has also been shown to bind to CS, in a model of metastatic breast cancer, where it is involved in facilitating tumour cell adhesion to platelet and endothelial cells, promoting tumour metastasis. 59 This interaction has been shown to play a role in selectin‐dependent cell adhesion, for example neutrophil and monocyte rolling on the endothelium.60, 61, 62, 63, 64
8. PROTEOGLYCAN:CYTOKINE INTERACTIONS
Whilst much less appreciated than specific chemokine interactions, there is a wide range of literature demonstrating that proteoglycans, via GAGs, can also bind to, and modulate the function of, cytokines more generally. Given that many of these cytokines are pro‐inflammatory, these interactions again play an important, if less direct, role in leucocyte recruitment.
Glycosaminoglycans (GAGs) have been shown to bind to interferon‐γ (IFN‐γ) with high affinity, comparable to higher affinity chemokine:GAG interactions. 65 IFN‐γ is a cytokine that plays a key role in the complex immune response to infection, in particular by viruses, and as such plays an important role in leucocyte recruitment, for example by inducing production of the chemokines CXCL9, CXCL10 and CXCL11. 66 The IFN‐γ:GAG interaction has been shown to reduce signalling of this cytokine through its receptor, 65 suggesting overlapping binding sites on IFN‐γ for its receptor and GAGs. Thus, it seems likely the function of this interaction is independent of signalling, in contrast to the fibroblast growth factor (FGF) system. 65 However, GAGs can also promote IFN‐γ‐mediated outcomes, suggesting that this interaction may facilitate the function of this cytokine through a currently undefined mechanism. Furthermore, a number of cytokines may bind to GAGs, for example IL‐2, IL‐5, IL‐6, IL‐7, IL‐12 and IL‐27, within the tissue extracellular matrix. 2
Various members of the transforming growth factor‐β (TGF‐β) cytokine superfamily have been shown to contain heparin binding sites; for example, TGF‐β1 and TGF‐β2 are described to bind HS PGs. 67 Although the effect of HS:TGF‐β interactions on cytokine activity has not been fully elucidated, it could be speculated that as these cytokines are comparatively small, HS binding may interfere with TGF‐β receptor signalling. 67
The function of proteoglycan:cytokine interactions remains unclear; however, it seems likely that they are important in cytokine localization, in protection from proteolysis and in regulation of signalling through their receptors. The effects of these interactions on leucocyte recruitment are indirect in that these cytokines are involved in the inflammatory process that results eventually in recruitment of leucocytes. This again highlights that further mechanistic work is needed to understand the role of proteoglycans in regulation of cytokine function and immunology more widely.
9. GAGs AND TLR SIGNALLING
Toll‐like receptors (TLRs) are transmembrane receptors with a critical role in the activation of the innate immune response through recognition of pathogen‐associated molecular patterns (PAMPs) and damage‐associated molecular patterns (DAMPs). 68 This process is key in signalling to the immune system to recruit leucocytes to sites of infection to fight pathogenic agents. HS and CS GAGs have been shown to act as DAMPs by signalling through TLR4.69, 70
Heparan sulphate can activate TLR4 on dendritic cells, in vitro, producing dendritic cell (DC) activation and alloreactive T‐cell responses. 71 TLR4‐dependent HS signalling has also been shown to mediate recruitment of neutrophils to the pancreas. 72 Given the presence of HS GAGs on the endothelial surface and within the tissue extracellular matrix, it would make sense that their shedding during disease would be an important signal to the immune system that pathogens are present within the vasculature and surrounding tissues. There are still a number of questions around the role of proteoglycans as DAMPs and in facilitating leucocyte recruitment that can now be explored using the advancing tools in the area.
10. LEUCOCYTE PROTEOGLYCANS
Whilst the majority of the research understanding the role of proteoglycans in leucocyte recruitment is within the context of the endothelial luminal glycocalyx, we are now beginning to understand the function of proteoglycans on leucocytes themselves. A number of studies have demonstrated the presence of proteoglycans, either directly or indirectly, on the surface of neutrophils, monocytes, macrophages, mast cells and T cells. Indeed, surface proteoglycans are required for entry of viruses into cells such as leucocytes, including SARS‐CoV‐2.73, 74, 75
The proteoglycan Syndecan‐1 has been found to be expressed at higher levels on leucocytes during inflammation and disease. For instance, neutrophils and plasma cells from patients with type 2 diabetes or systemic lupus erythematosus (SLE) have been shown to enhance their expression of syndecan‐1, relative to healthy controls.76, 77 Genetic ablation of the proteoglycan syndecan‐1 in monocytes and neutrophils reduces their ability to adhere to endothelial cells in vitro. 78
An early study demonstrated that acidic mucopolysaccharides, resembling chondroitin sulphate, could be isolated from human leucocytes. 79 Further studies have then gone on to report that human leucocytes can indeed synthesize and secrete glycosaminoglycans, 80 with chondroitin 4‐sulphate being thought to represent the major component. 81 Furthermore, there is indirect evidence for proteoglycans on the surface of T cells; the entry of human T‐cell leukaemia virus (HTLV) into CD4+ T cells 74 or herpesvirus 8 (HHV‐8) into B cells 73 all requires heparan sulphate.
Serglycin has been demonstrated to facilitate storage granule formation in mast cells 82 and T cells. 83 Proteoglycans have also been detected on B cells where they go through structural changes during development and play a role in the survival of long‐lived plasma cells.84, 85, 86 Eosinophils have been shown to have cell surface proteoglycans that change in response to cytokine stimulation. 87
CXCL8, which can bind to GAGs, has been shown to bind to the surface of neutrophils, where GAG‐mediated inhibition of this interaction reduced in vitro chemotaxis of neutrophils and reactive oxygen species (ROS) production. 88 Additionally, enzymatic removal of GAGs has also been shown to ablate migration of neutrophils, in vitro. 31 Whilst the mechanism underlying these observations is unclear, the authors propose that interactions between the chemokines CXCL8 and proteoglycans on the leucocyte surface promotes locomotion by creating local stores of ligand. This mechanism would provide a currently overlooked understanding of chemokine function in addition to binding to proteoglycans on the endothelial surface.
Indirect evidence from a number of papers has suggested that proteoglycans are on the surface of monocytes and may be functional. The GAG‐binding chemokines CXCL4 and CCL5 can both bind to the monocyte surface. 89
The wider presence and function of proteoglycans on the leucocyte surface is an exciting research topic with huge potential for a better understanding of leucocyte recruitment and potential therapeutic targeting of inflammatory‐based disease. It seems highly likely that proteoglycans on the leucocyte surface have a key role in fighting infection, given that the glycocalyx represents the first part of a cell that any intracellular pathogens encounter. The proteoglycans, within the glycocalyx, play an important role during inflammation and represent a potential therapeutic target during disease.
11. CONCLUSION
Together, these studies emphasize that proteoglycans are key modulators of leucocyte recruitment and positioning, as well as the wider immune response. However, this review also highlights that much more research is needed to improve our current understanding regarding the architecture, expression patterns and functional role of proteoglycans in inflammation and disease. Greater understanding will facilitate better targeted therapeutic interventions, such as GAG mimetics, in inflammatory diseases such as rheumatoid arthritis. We are now at an exciting time in this field where development of new technologies, particularly GAG analytics, will facilitate new and exciting discoveries in the near future.
12. ACKNOWLEDGEMENT
We would like to acknowledge the British Society for Matrix Biology and the estate of Professor John Scott for the BSMB Early Career Research Award to DPD.
Gray AL, Pun N, Ridley AJL, Dyer DP. Role of extracellular matrix proteoglycans in immune cell recruitment. Int J Exp Path. 2022;103:34–43. doi: 10.1111/iep.12428
Anna L. Gray, Nabina Pun and Amanda J. L. Ridley: Contributed equally.
Funding information
This work was supported by a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (Grant Number 218570/Z/19/Z to DPD).
REFERENCES
- 1. Couchman JR, Pataki CA. An introduction to proteoglycans and their localization. J Histochem Cytochem. 2012;60:885‐897. doi: 10.1369/0022155412464638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Collins LE, Troeberg L. Heparan sulfate as a regulator of inflammation and immunity. J Leukoc Biol. 2019;105:81‐92. doi: 10.1002/jlb.3ru0618-246r [DOI] [PubMed] [Google Scholar]
- 3. Nourshargh S, Alon R. Leukocyte migration into inflamed tissues. Immunity. 2014;41:694‐707. doi: 10.1016/j.immuni.2014.10.008 [DOI] [PubMed] [Google Scholar]
- 4. Sadik CD, Kim ND, Luster AD. Neutrophils cascading their way to inflammation. Trends Immunol. 2011;32:452‐460. doi: 10.1016/j.it.2011.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marki A, Esko JD, Pries AR, Ley K. Role of the endothelial surface layer in neutrophil recruitment. J Leukoc Biol. 2015;98(4):503‐515. doi: 10.1189/jlb.3mr0115-011r [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Randles MJ, Humphries MJ, Lennon R. Proteomic definitions of basement membrane composition in health and disease. Matrix Biol. 2017;57:12‐28. doi: 10.1016/j.matbio.2016.08.006 [DOI] [PubMed] [Google Scholar]
- 7. Xu D, Esko JD. Demystifying heparan sulfate‐protein interactions. Annu Rev Biochem. 2014;83:129‐157. doi: 10.1146/annurev-biochem-060713-035314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mizumoto S, Fongmoon D, Sugahara K. Interaction of chondroitin sulfate and dermatan sulfate from various biological sources with heparin‐binding growth factors and cytokines. Glycoconj J. 2012;30:619‐632. doi: 10.1007/s10719-012-9463-5 [DOI] [PubMed] [Google Scholar]
- 9. Mikami T, Kitagawa H. Biosynthesis and function of chondroitin sulfate. Biochim Biophys Acta. 2013;1830:4719‐4733. doi: 10.1016/j.bbagen.2013.06.006 [DOI] [PubMed] [Google Scholar]
- 10. Thacker BE, Xu D, Lawrence R, Esko JD. Heparan sulfate 3‐O‐sulfation: a rare modification in search of a function. Matrix Biol. 2014;35:60‐72. doi: 10.1016/j.matbio.2013.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen Y‐H, Narimatsu Y, Clausen TM, et al. The GAGOme: a cell‐based library of displayed glycosaminoglycans. Nat Methods. 2018;15:881‐888. doi: 10.1038/s41592-018-0086-z [DOI] [PubMed] [Google Scholar]
- 12. Miller RL, Guimond SE, Schwörer R, et al. Shotgun ion mobility mass spectrometry sequencing of heparan sulfate saccharides. Nat Commun. 2020;11:1481‐1512. doi: 10.1038/s41467-020-15284-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Karlsson R, Chopra P, Joshi A, et al. Dissecting structure‐function of 3‐O‐sulfated heparin and engineered heparan sulfates. Sci Adv. 2021;7(52):eabl6026. doi: 10.1126/sciadv.abl6026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Krüger‐Genge A, Blocki A, Franke R‐P, Jung F. Vascular endothelial cell biology: an update. Int J Mol Sci. 2019;20:4411. doi: 10.3390/ijms20184411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Luft JH. Fine structures of capillary and endocapillary layer as revealed by ruthenium red. Fed Proc. 1966;25:1773‐1783. [PubMed] [Google Scholar]
- 16. Lipowsky HH. Role of the glycocalyx as a barrier to leukocyte‐endothelium adhesion. Adv Exp Med Biol. 2018;1097:51‐68. doi: 10.1007/978-3-319-96445-4_3 [DOI] [PubMed] [Google Scholar]
- 17. Lipowsky HH. The endothelial glycocalyx as a barrier to leukocyte adhesion and its mediation by extracellular proteases. Ann Biomed Eng. 2012;40:840‐848. doi: 10.1007/s10439-011-0427-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schmidt EP, Yang Y, Janssen WJ, et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med. 2012;18:1217‐1223. doi: 10.1038/nm.2843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Constantinescu AA, Vink H, Spaan JAE. Endothelial cell glycocalyx modulates immobilization of leukocytes at the endothelial surface. Arterioscler Thromb Vasc Biol. 2003;23:1541‐1547. doi: 10.1161/01.atv.0000085630.24353.3d [DOI] [PubMed] [Google Scholar]
- 20. Sundd P, Pospieszalska MK, Cheung LSL, Konstantopoulos K, Ley K. Biomechanics of leukocyte rolling. Biorheology. 2011;48:1‐35. doi: 10.3233/bir-2011-0579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mulivor AW, Lipowsky HH. Inflammation‐ and ischemia‐induced shedding of venular glycocalyx. Am J Physiol Heart Circ Physiol. 2004;286:H1672‐H1680. doi: 10.1152/ajpheart.00832.2003 [DOI] [PubMed] [Google Scholar]
- 22. Mulivor AW, Lipowsky HH. Role of glycocalyx in leukocyte‐endothelial cell adhesion. Am J Physiol Heart Circ Physiol. 2002;283:H1282‐H1291. doi: 10.1152/ajpheart.00117.2002 [DOI] [PubMed] [Google Scholar]
- 23. Kataoka H, Ushiyama A, Akimoto Y, Matsubara S, Kawakami H, Iijima T. Structural behavior of the endothelial glycocalyx is associated with pathophysiologic status in septic mice: an integrated approach to analyzing the behavior and function of the glycocalyx using both electron and fluorescence intravital microscopy. Anesth Analg. 2017;125:874‐883. doi: 10.1213/ane.0000000000002057 [DOI] [PubMed] [Google Scholar]
- 24. Hippensteel JA, Anderson BJ, Orfila JE, et al. Circulating heparan sulfate fragments mediate septic cognitive dysfunction. J Clin Invest. 2019;129(4):1779‐1784. doi: 10.1172/jci124485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Villalba N, Baby S, Yuan SY. The endothelial glycocalyx as a double‐edged sword in microvascular homeostasis and pathogenesis. Front Cell Dev Biol. 2021;9:711003. doi: 10.3389/fcell.2021.711003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gopal S. Syndecans in inflammation at a glance. Front Immunol. 2020;11:227. doi: 10.3389/fimmu.2020.00227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ando Y, Okada H, Takemura G, et al. Brain‐specific ultrastructure of capillary endothelial glycocalyx and its possible contribution for blood brain barrier. Sci Rep. 2018;8:17523. doi: 10.1038/s41598-018-35976-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reitsma S, Slaaf DW, Vink H, van Zandvoort MAMJ, oude Egbrink MGA. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. 2007;454:345‐359. doi: 10.1007/s00424-007-0212-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dyer DP. Understanding the mechanisms that facilitate specificity, not redundancy, of chemokine mediated leukocyte recruitment. Immunology. 2020;290:21292. doi: 10.1111/imm.13200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Warda M, Toida T, Zhang F, et al. Isolation and characterization of heparan sulfate from various murine tissues. Glycoconj J. 2006;23:555‐563. doi: 10.1007/s10719-006-7668-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Goldblatt J, Lawrenson RA, Muir L, et al. A requirement for neutrophil glycosaminoglycans in chemokine: receptor interactions is revealed by the streptococcal protease SpyCEP. J Immunol. 2019;202:3246‐3255. doi: 10.4049/jimmunol.1801688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sarris M, Masson J‐B, Maurin D, et al. Inflammatory chemokines direct and restrict leukocyte migration within live tissues as glycan‐bound gradients. Curr Biol. 2012;22:2375‐2382. doi: 10.1016/j.cub.2012.11.018 [DOI] [PubMed] [Google Scholar]
- 33. Hughes CE, Nibbs RJB. A guide to chemokines and their receptors. FEBS J. 2018;285(16):2944‐2971. doi: 10.1111/febs.14466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Proudfoot AEI, Handel TM, Johnson Z, et al. Glycosaminoglycan binding and oligomerization are essential for the in vivo activity of certain chemokines. Proc Natl Acad Sci USA. 2003;100:1885‐1890. doi: 10.1073/pnas.0334864100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ali S, Robertson H, Wain JH, Isaacs JD, Malik G, Kirby JA. A non‐glycosaminoglycan‐binding variant of CC chemokine ligand 7 (monocyte chemoattractant protein‐3) antagonizes chemokine‐mediated inflammation. J Immunol. 2005;175:1257‐1266. doi: 10.4049/jimmunol.175.2.1257 [DOI] [PubMed] [Google Scholar]
- 36. Peterson FC, Elgin ES, Nelson TJ, et al. Identification and characterization of a glycosaminoglycan recognition element of the C chemokine lymphotactin. J Biol Chem. 2004;279:12598‐12604. doi: 10.1074/jbc.m311633200 [DOI] [PubMed] [Google Scholar]
- 37. Gangavarapu P, Rajagopalan L, Kolli D, Guerrero‐Plata A, Garofalo RP, Rajarathnam K. The monomer‐dimer equilibrium and glycosaminoglycan interactions of chemokine CXCL8 regulate tissue‐specific neutrophil recruitment. J Leukoc Biol. 2012;91:259‐265. doi: 10.1189/jlb.0511239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Campanella GSV, Grimm J, Manice LA, et al. Oligomerization of CXCL10 is necessary for endothelial cell presentation and in vivo activity. J Immunol. 2006;177:6991‐6998. doi: 10.4049/jimmunol.177.10.6991 [DOI] [PubMed] [Google Scholar]
- 39. Dyer DP, Migliorini E, Salanga CL, Thakar D, Handel TM, Richter RP. Differential structural remodelling of heparan sulfate by chemokines: the role of chemokine oligomerization. Open Biol. 2017;7(1):160286. doi: 10.1098/rsob.160286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Salanga CL, Dyer DP, Kiselar JG, Gupta S, Chance MR, Handel TM. Multiple glycosaminoglycan‐binding epitopes of monocyte chemoattractant protein‐3/CCL7 enable it to function as a non‐oligomerizing chemokine. J Biol Chem. 2014;289:14896‐14912. doi: 10.1074/jbc.m114.547737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dyer DP, Salanga CL, Johns SC, et al. The anti‐inflammatory protein TSG‐6 regulates chemokine function by inhibiting chemokine/glycosaminoglycan interactions. J Biol Chem. 2016;291:12627‐12640. doi: 10.1074/jbc.m116.720953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dyer DP, Salanga CL, Volkman BF, Kawamura T, Handel TM. The dependence of chemokine‐glycosaminoglycan interactions on chemokine oligomerization. Glycobiology. 2016;26:312‐326. doi: 10.1093/glycob/cwv100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dyer DP, Thomson JM, Hermant A, et al. TSG‐6 inhibits neutrophil migration via direct interaction with the chemokine CXCL8. J Immunol. 2014;192:2177‐2185. doi: 10.4049/jimmunol.1300194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Migliorini E, Thakar D, Kühnle J, et al. Cytokines and growth factors cross‐link heparan sulfate. Open Biol. 2015;5(8):150046. doi: 10.1098/rsob.150046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Miller RL, Dykstra AB, Wei W, Holsclaw C, Turnbull JE, Leary JA. Enrichment of two isomeric heparin oligosaccharides exhibiting different affinities toward monocyte chemoattractant protein‐1. Anal Chem. 2016;88:11551‐11558. doi: 10.1021/acs.analchem.6b02803 [DOI] [PubMed] [Google Scholar]
- 46. Jayson GC, Hansen SU, Miller GJ, et al. Synthetic heparan sulfate dodecasaccharides reveal single sulfation site interconverts CXCL8 and CXCL12 chemokine biology. Chem Commun. 2015;51(72):13846‐13849. doi: 10.1039/c5cc05222j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Crijns H, Vanheule V, Proost P. Targeting chemokine—glycosaminoglycan Interactions to Inhibit Inflammation. Front Immunol. 2020;11:907. doi: 10.3389/fimmu.2020.00483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Handel TM, Dyer DP. Perspectives on the biological role of chemokine: glycosaminoglycan interactions. J Histochem Cytochem. 2020;69(2):87‐91. doi: 10.1369/0022155420977971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Massena S, Christoffersson G, Hjertström E, et al. A chemotactic gradient sequestered on endothelial heparan sulfate induces directional intraluminal crawling of neutrophils. Blood. 2010;116:1924‐1931. doi: 10.1182/blood-2010-01-266072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Weber M, Hauschild R, Schwarz J, et al. Interstitial dendritic cell guidance by haptotactic chemokine gradients. Science. 2013;339:328‐332. doi: 10.1126/science.1228456 [DOI] [PubMed] [Google Scholar]
- 51. Schumann K, Lämmermann T, Bruckner M, et al. Immobilized chemokine fields and soluble chemokine gradients cooperatively shape migration patterns of dendritic cells. Immunity. 2010;32:703‐713. doi: 10.1016/j.immuni.2010.04.017 [DOI] [PubMed] [Google Scholar]
- 52. Stoler‐Barak L, Moussion C, Shezen E, Hatzav M, Sixt M, Alon R. Blood vessels pattern heparan sulfate gradients between their apical and basolateral aspects. PLoS One. 2014;9:e85699. doi: 10.1371/journal.pone.0085699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vaahtomeri K, Moussion C, Hauschild R, Sixt M. Shape and function of interstitial chemokine CCL21 gradients are independent of heparan sulfates produced by lymphatic endothelium. Front Immunol. 2021;12:630002. doi: 10.3389/fimmu.2021.630002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tweedy L, Thomason PA, Paschke PI, et al. Seeing around corners: cells solve mazes and respond at a distance using attractant breakdown. Science. 2020;369:eaay9792. doi: 10.1126/science.aay9792 [DOI] [PubMed] [Google Scholar]
- 55. Liang WG, Ren M, Zhao F, Tang W‐J. Structures of human CCL18, CCL3, and CCL4 reveal molecular determinants for quaternary structures and sensitivity to insulin degrading enzyme. J Mol Biol. 2015;427:1345‐1358. doi: 10.1016/j.jmb.2015.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Metzemaekers M, Mortier A, Janssens R, et al. Glycosaminoglycans regulate CXCR3 ligands at distinct levels: protection against processing by dipeptidyl peptidase IV/CD26 and interference with receptor signaling. Int J Mol Sci. 2017;18:1513. doi: 10.3390/ijms18071513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Graham GJ, Handel TM, Proudfoot AEI. Leukocyte adhesion: reconceptualizing chemokine presentation by glycosaminoglycans. Trends Immunol. 2019;40(6):472‐481. doi: 10.1016/j.it.2019.03.009 [DOI] [PubMed] [Google Scholar]
- 58. Koenig A, Norgard‐Sumnicht K, Linhardt R, Varki A. Differential interactions of heparin and heparan sulfate glycosaminoglycans with the selectins. Implications for the use of unfractionated and low molecular weight heparins as therapeutic agents. J Clin Invest. 1998;101:877‐889. doi: 10.1172/jci1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Monzavi‐Karbassi B, Stanley JS, Hennings L, et al. Chondroitin sulfate glycosaminoglycans as major P‐selectin ligands on metastatic breast cancer cell lines. Int J Cancer. 2007;120:1179‐1191. doi: 10.1002/ijc.22424 [DOI] [PubMed] [Google Scholar]
- 60. Nelson R, Cecconi O, Roberts W, Aruffo A, Linhardt R, Bevilacqua M. Heparin oligosaccharides bind L‐ and P‐selectin and inhibit acute inflammation. Blood. 1993;82:3253‐3258. doi: 10.1182/blood.v82.11.3253.3253 [DOI] [PubMed] [Google Scholar]
- 61. Wang L, Fuster M, Sriramarao P, Esko JD. Endothelial heparan sulfate deficiency impairs L‐selectin‐ and chemokine‐mediated neutrophil trafficking during inflammatory responses. Nat Immunol. 2005;6:902‐910. doi: 10.1038/ni1233 [DOI] [PubMed] [Google Scholar]
- 62. Rops AL, Jacobs CW, Linssen PC, et al. Heparan sulfate on activated glomerular endothelial cells and exogenous heparinoids influence the rolling and adhesion of leucocytes. Nephrol Dial Transplant. 2007;22:1070‐1077. doi: 10.1093/ndt/gfl801 [DOI] [PubMed] [Google Scholar]
- 63. Giuffrè L, Cordey AS, Monai N, Tardy Y, Schapira M, Spertini O. Monocyte adhesion to activated aortic endothelium: role of L‐selectin and heparan sulfate proteoglycans. J Cell Biol. 1997;136:945‐956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Stoler‐Barak L, Barzilai S, Zauberman A, Alon R. Transendothelial migration of effector T cells across inflamed endothelial barriers does not require heparan sulfate proteoglycans. Int Immunol. 2014;26:315‐324. doi: 10.1093/intimm/dxt076 [DOI] [PubMed] [Google Scholar]
- 65. Sadir R, Forest E, Lortat‐Jacob H. The heparan sulfate binding sequence of interferon‐gamma increased the on rate of the interferon‐gamma‐interferon‐gamma receptor complex formation. J Biol Chem. 1998;273:10919‐10925. doi: 10.1074/jbc.273.18.10919 [DOI] [PubMed] [Google Scholar]
- 66. Groom JR, Luster AD. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol Cell Biol. 2011;89:207‐215. doi: 10.1038/icb.2010.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rider CC, Mulloy B. Heparin, heparan sulphate and the TGF‐β cytokine superfamily. Molecules. 2017;22:713. doi: 10.3390/molecules22050713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Goulopoulou S, McCarthy CG, Webb RC. Toll‐like receptors in the vascular system: sensing the dangers within. Pharmacol Rev. 2016;68:142‐167. doi: 10.1124/pr.114.010090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. O'Callaghan P, Zhang X, Li J‐P. Heparan sulfate proteoglycans as relays of neuroinflammation. J Histochem Cytochem. 2018;66:305‐319. doi: 10.1369/0022155417742147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Campo GM, Avenoso A, Campo S, Traina P, D'Ascola A, Calatroni A. Glycosaminoglycans reduced inflammatory response by modulating toll‐like receptor‐4 in LPS‐stimulated chondrocytes. Arch Biochem Biophys. 2009;491:7‐15. doi: 10.1016/j.abb.2009.09.017 [DOI] [PubMed] [Google Scholar]
- 71. Brennan TV, Lin L, Huang X, et al. Heparan sulfate, an endogenous TLR4 agonist, promotes acute GVHD after allogeneic stem cell transplantation. Blood. 2012;120:2899‐2908. doi: 10.1182/blood-2011-07-368720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Akbarshahi H, Axelsson JB, Said K, Malmström A, Fischer H, Andersson R. TLR4 dependent heparan sulphate‐induced pancreatic inflammatory response is IRF3‐mediated. J Transl Med. 2011;9:219. doi: 10.1186/1479-5876-9-219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Akula SM, Wang F‐Z, Vieira J, Chandran B. Human herpesvirus 8 interaction with target cells involves heparan sulfate. Virology. 2001;282:245‐255. doi: 10.1006/viro.2000.0851 [DOI] [PubMed] [Google Scholar]
- 74. Jones KS, Petrow‐Sadowski C, Bertolette DC, Huang Y, Ruscetti FW. Heparan sulfate proteoglycans mediate attachment and entry of human T‐cell leukemia virus type 1 virions into CD4+ T cells. J Virol. 2005;79:12692‐12702. doi: 10.1128/jvi.79.20.12692-12702.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Clausen TM, Sandoval DR, Spliid CB, et al. SARS‐CoV‐2 infection depends on cellular heparan sulfate and ACE2. Cell. 2020;183:1043‐1057.e15. doi: 10.1016/j.cell.2020.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Minowa K, Amano H, Nakano S, et al. Elevated serum level of circulating syndecan‐1 (CD138) in active systemic lupus erythematosus. Autoimmunity. 2011;44:357‐362. doi: 10.3109/08916934.2010.545846 [DOI] [PubMed] [Google Scholar]
- 77. Wang J‐B, Zhang Y‐J, Guan J, et al. Enhanced syndecan‐1 expression on neutrophils in patients with type 2 diabetes mellitus. Acta Diabetol. 2012;49:41‐46. doi: 10.1007/s00592-011-0265-1 [DOI] [PubMed] [Google Scholar]
- 78. Götte M, Bernfield M, Joussen AM. Increased leukocyte‐endothelial interactions in syndecan‐1‐deficient mice involve heparan sulfate‐dependent and ‐independent steps. Curr Eye Res. 2005;30:417‐422. doi: 10.1080/02713680590956289 [DOI] [PubMed] [Google Scholar]
- 79. Kerby GP. The occurrence of acid mucopolysaccharides in human leukocytes and urine. J Clin Invest. 1955;34:1738‐1743. doi: 10.1172/jci103228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hart GW. Biosynthesis of glycosaminoglycans by thymic lymphocytes. Effects of mitogenic activation. Biochemistry. 1982;21:6088‐6096. doi: 10.1021/bi00267a010 [DOI] [PubMed] [Google Scholar]
- 81. Olsson I, Gardell S. Isolation and characterization of glycosaminoglycans from human leukocytes and platelets. Biochim Biophys Acta. 1967;141:348‐357. doi: 10.1016/0304-4165(67)90109-2 [DOI] [PubMed] [Google Scholar]
- 82. Åbrink M, Grujic M, Pejler G. Serglycin is essential for maturation of mast cell secretory granule. J Biol Chem. 2004;279:40897‐40905. doi: 10.1074/jbc.m405856200 [DOI] [PubMed] [Google Scholar]
- 83. Grujic M, Braga T, Lukinius A, et al. Serglycin‐deficient cytotoxic t lymphocytes display defective secretory granule maturation and granzyme B storage. J Biol Chem. 2005;280:33411‐33418. doi: 10.1074/jbc.m501708200 [DOI] [PubMed] [Google Scholar]
- 84. Engelmann S, Ebeling O, Schwartz‐Albiez R. Modulated glycosylation of proteoglycans during differentiation of human B lymphocytes. Biochim Biophys Acta. 1995;1267:6‐14. doi: 10.1016/0167-4889(95)00057-y [DOI] [PubMed] [Google Scholar]
- 85. Reijmers RM, Spaargaren M, Pals ST. Heparan sulfate proteoglycans in the control of B cell development and the pathogenesis of multiple myeloma. FEBS J. 2013;280:2180‐2193. doi: 10.1111/febs.12180 [DOI] [PubMed] [Google Scholar]
- 86. Ren Z, Spaargaren M, Pals ST. Syndecan‐1 and stromal heparan sulfate proteoglycans: key moderators of plasma cell biology and myeloma pathogenesis. Blood. 2021;137:1713‐1718. doi: 10.1182/blood.2020008188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Rothenberg ME, Pomerantz JL, Owen WF, et al. Characterization of a human eosinophil proteoglycan, and augmentation of its biosynthesis and size by interleukin 3, interleukin 5, and granulocyte/macrophage colony stimulating factor. J Biol Chem. 1988;263:13901‐13908. [PubMed] [Google Scholar]
- 88. Schlorke D, Thomas L, Samsonov SA, Huster D, Arnhold J, Pichert A. The influence of glycosaminoglycans on IL‐8‐mediated functions of neutrophils. Carbohydr Res. 2012;356:1‐8. doi: 10.1016/j.carres.2012.02.025 [DOI] [PubMed] [Google Scholar]
- 89. von Hundelshausen P, Koenen RR, Sack M, et al. Heterophilic interactions of platelet factor 4 and RANTES promote monocyte arrest on endothelium. Blood. 2005;105:924‐930. doi: 10.1182/blood-2004-06-2475 [DOI] [PubMed] [Google Scholar]


