Abstract
Data on antimicrobial resistance (AMR) in zoonotic and indicator bacteria from humans, animals and food are collected annually by the EU Member States (MSs), jointly analysed by the EFSA and the ECDC and reported in a yearly EU Summary Report. The annual monitoring of AMR in animals and food within the EU is targeted at selected animal species corresponding to the reporting year. The 2020 monitoring specifically focussed on poultry and their derived carcases/meat, while the monitoring performed in 2019 specifically focused on fattening pigs and calves under 1 year of age, as well as their derived carcases/meat. Monitoring and reporting of AMR in 2019–2020 included data regarding Salmonella, Campylobacter and indicator E. coli isolates, as well as data obtained from the specific monitoring of presumptive ESBL‐/AmpC‐/carbapenemase‐producing E. coli isolates. Additionally, some MSs reported voluntary data on the occurrence of methicillin‐resistant Staphylococcus aureus in animals and food, with some countries also providing data on antimicrobial susceptibility. This report provides an overview of the main findings of the 2019–2020 harmonised AMR monitoring in the main food‐producing animal populations monitored, in carcase/meat samples and in humans. Where available, monitoring data obtained from pigs, calves, broilers, laying hens and turkeys, as well as from carcase/meat samples and humans were combined and compared at the EU level, with particular emphasis on multidrug resistance, complete susceptibility and combined resistance patterns to critically important antimicrobials, as well as Salmonella and E. coli isolates possessing ESBL‐/AmpC‐/carbapenemase phenotypes. The key outcome indicators for AMR in food‐producing animals, such as complete susceptibility to the harmonised panel of antimicrobials in E. coli and the prevalence of ESBL‐/AmpC‐producing E. coli have been specifically analysed over the period 2014–2020.
Keywords: antimicrobial resistance, zoonotic bacteria, indicator bacteria, ESBL, MRSA
Summary
Data on antimicrobial resistance in zoonotic and indicator bacteria referring to 2019–2020, submitted by 27 EU Member States (MSs), were jointly analysed by the EFSA and the ECDC. Resistance in zoonotic Salmonella and Campylobacter from humans, animals and food, as well as resistance in indicator Escherichia coli and methicillin‐resistant Staphylococcus aureus (MRSA) from animals and food were addressed. ‘Microbiological’ resistance was assessed using epidemiological cut‐off (ECOFF) values; for some countries, qualitative data on human isolates were interpreted in a way which corresponds closely to the ECOFF‐defined ‘microbiological’ resistance.
In Salmonella spp. from human cases in 2019–2020, resistance to ampicillin, sulfonamides and tetracyclines was observed at overall high levels, while resistance to third‐generation cephalosporins in 2020 was noted at overall very low levels of 0.8% for both cefotaxime and ceftazidime, respectively. A decline in resistance to ampicillin and tetracyclines in isolates from humans was observed in 10 and nine countries, respectively, over the period 2016–2020, particularly evident in S. Typhimurium and its monophasic variant, serovars commonly associated with pigs and calves. In Salmonella spp. and indicator E. coli isolates recovered from animals and food during the 2019–2020 routine monitoring, resistance to ampicillin, tetracyclines and sulfonamides was ranging from moderate to very high in isolates in most MSs and resistance to third‐generation cephalosporins was seldom detected; paralleling that observed in Salmonella isolates reported from human cases. Additionally, resistance to (fluoro)/quinolones was high to very high among Salmonella spp. and indicator E. coli isolates recovered from broilers, fattening turkeys and poultry carcases/meat in 2020. In Salmonella spp. isolates from human cases, a moderate occurrence of resistance to ciprofloxacin was observed in 2019–2020, but among S. Kentucky isolates extremely high prevalence of resistance was noted (82.0% in 2020), and in S. Enteritidis increasing trends in resistance were observed in nine countries over the period 2016–2020, both serovars predominantly being associated with poultry.
The monitoring included assessment of the levels of presumptive extended‐spectrum beta‐lactamase (ESBL)‐/AmpC‐/carbapenemase‐producers among Salmonella spp. from human cases, food‐producing animals and animal carcases; as well as among indicator E. coli isolates from food‐producing animals. At the reporting MS group level, the proportion of presumptive ESBL or AmpC producers was very low to low among Salmonella spp. isolates recovered from animals/carcases (broilers, laying hens, fattening turkeys, fattening pigs and carcases of broilers and fattening pigs) and very low in isolates from human cases, although higher in some Salmonella serovars. While the prevalence of ESBL/AmpC‐producing E. coli in food‐producing animals and food (particularly broiler meat) is still high when considering the mean of all MSs (still including the United Kingdom), statistically significant decreasing trends were observed in an important number of countries over the study period.
Within both the routine and specific monitoring (non‐selective and selective media, respectively), varying occurrence/prevalence rates of presumptive ESBL or AmpC producers were observed in different reporting countries. Additionally, in 2020, within the specific monitoring of ESBL/AmpC‐producing E. coli in food‐producing animals, one isolate of E. coli from broilers with a carbapenemase phenotype was reported by Austria. This isolate harboured the metallo‐beta‐lactamase resistance gene bla VIM‐1, as well as the beta‐lactamase genes bla TEM‐1C and bla TEM‐1B. Furthermore, within the voluntary specific monitoring of carbapenemase‐producing microorganisms (using selective media for carbapenemase producers), three isolates from broilers (Romania) and one from fattening turkeys (Spain) were reported. The isolate from fattening turkeys carried the gene bla TEM‐1B but no genes encoding carbapenemases were detected. The isolates reported by Romania are pending genotypic confirmation. No carbapenemase‐producing Salmonella isolates were detected from animals in 2019–2020, while one and two isolates from humans in 2019 and 2020, respectively, were identified as carbapenemase‐producing (two isolates with bla OXA‐48 and one with bla NDM‐1, two from domestically‐acquired infections and one case without information on travel status).
Resistance to colistin was uncommon among Salmonella spp. and E. coli isolates recovered from food‐producing animals (fattening pigs, calves, Gallus gallus and fattening turkeys) and carcases/meat derived from these animals, although moderate resistance was notably observed in certain Salmonella serovars.
Rates of resistance to ciprofloxacin were very high in C. jejuni and C. coli isolates from humans and very high to extremely high in C. jejuni and C. coli isolates from poultry, pigs and calves. Erythromycin resistance was either not detected or detected at very low levels in C. jejuni from humans, poultry and calves, but observed at higher similar levels in C. coli isolates from humans, pigs and poultry. Over the period 2016–2020, ciprofloxacin resistance in C. jejuni from humans increased in nine countries , while erythromycin resistance decreased in five. Similar trends were observed in C. jejuni from broilers over 2009–2020 where resistance to ciprofloxacin increased in 14 countries, and resistance to erythromycin decreased in six countries. Erythromycin resistance also decreased in C. jejuni from turkeys in three countries and in C. coli from pigs in four countries. Combined resistance to both ciprofloxacin and erythromycin, which are considered critically important for treatment of campylobacteriosis, was overall rare to low in C. jejuni from humans, poultry and calves, and low to moderate in C. coli from humans, poultry and pigs. Notably moderate proportions of C. jejuni from poultry, moderate to high proportions of C. coli from poultry and pigs and high proportions of C. coli from humans, were co‐resistant to ciprofloxacin and erythromycin in some countries.
Combined resistance to critically important antimicrobials (cephalosporins and fluoroquinolones) was generally uncommon in E. coli in all animal categories. However, considering resistance to antimicrobial substances separately, median levels of resistance for colistin, azithromycin and third generation cephalosporins (cefotaxime or ceftazidime) ranged between ‘rare’ and ‘low’ in all animal categories, while ciprofloxacin resistance was more common and median levels ranged from ‘low’ in calves and ‘moderate’ in pigs to ‘high’ in turkeys and ‘very high’ in broilers. In Salmonella spp., combined resistance to fluoroquinolones and cephalosporins was very low in isolates from both humans and animals, but higher in certain Salmonella serovars, e.g. S. Kentucky and S. Infantis.
The voluntary monitoring of MRSA from food and healthy animals in 2019–2020 revealed that most MRSA isolates, where typing data were available, were associated with spa‐types assigned to livestock associated (LA‐)MRSA in both reporting years. However, spa‐types associated with community acquired (CA‐) and hospital acquired (HA‐)MRSA were also reported, as well as mecC‐MRSA. The occasional detection of lineages of CA‐ and HA‐MRSA primarily associated with humans is not surprising, since the sporadic interchange of strains between humans and animals may be expected. A significant observation from the 2020 monitoring was the occurrence of a vancomycin‐resistant MRSA t011 isolate in meat from sheep. The isolate lacks the typical vancomycin resistance genes and it is likely that the increased MIC of 4 mg/l was due to multiple point mutations as described in the literature. An important observation from the 2019 monitoring includes the detection of linezolid‐resistant strains harbouring the cfr gene from fattening pigs. Since linezolid is an important compound in human medicine for the treatment of MRSA, further investigating whether linezolid resistance is widespread or more localised in distribution in MRSA in animals is highly relevant.
The key outcome indicators for AMR in food‐producing animals – complete susceptibility (CS) to the harmonised panel of antimicrobials in E. coli and the prevalence of ESBL‐/AmpC‐producing E. coli – have also been specifically analysed over the period 2014–2020. There are marked variations in both key outcome indicators among reporting countries. In seventeen countries (15 MSs and 2 non‐MS including UK), a statistically significant decreasing trend in the key outcome indicator of ESBL‐ and/or AmpC‐producing indicator E. coli (KOIESC) was observed. In six MSs, an increasing trend was registered, and in the remaining six MSs and two non‐MSs, no statistically significant trend was seen. Statistically significant increasing trends in the outcome indicator of complete susceptibility (KOICS) were registered in 14 countries (48.3%) and decreasing trends in four countries (13.8%). The increasing trends in CS and KOICS in indicator E. coli isolates reveals a progress towards lower levels of resistance in several countries and in the MS‐group. An improved situation was most pronounced in poultry. Both key outcome indicators show that encouraging progress has been registered in reducing AMR in food‐producing animals in several EU MSs over the last years.
1. Introduction
Legal basis
Monitoring of AMR in bacteria from food‐producing animals and derived meat
Regulation (EC) 178/2002 1 Article 33 establishes that EFSA is responsible for examining data on AMR collected from the Member States (MSs) in accordance with Directive 2003/99/EC and for preparing the EU Summary Report from the results
Directive 2003/99/EC 2 on the monitoring of zoonoses and zoonotic agents lays down the provisions for monitoring of AMR in zoonotic and indicator bacteria in food‐producing animals and derived meat. The Directive obliges EU MSs to collect relevant and, where applicable, comparable data on zoonoses, zoonotic agents, AMR and food‐borne outbreaks.
Implementing Decision 2013/652/EU 3 on the monitoring and reporting of antimicrobial resistance in zoonotic and commensal bacteria was adopted as part of the 2011–2016 European Commission action plan. It applies from 2014 to 2020 and sets up priorities for the monitoring of AMR from a public health perspective, drafts a list of combinations of bacterial species, food‐producing animal populations and foodstuffs and lays down detailed requirements on the harmonised monitoring and reporting of AMR in food‐producing animals and food.
Monitoring of AMR in bacteria from humans
Decision 2018/945/EU 4 on the communicable diseases and related special health issues to be covered by epidemiological surveillance as well as relevant case definitions came into force in July 2018, repealing Decision 2012/506/EU5. The new decision stipulates mandatory testing and reporting of a representative subset of isolates using methods and criteria specified in the EU protocol for harmonised monitoring of antimicrobial resistance in human Salmonella and Campylobacter isolates (ECDC, 2016).
The data collection on human diseases from MSs is conducted in accordance with Decision 1082/2013/EU 6 on serious cross‐border threats to health.
Terms of Reference.
In accordance with the Zoonoses Directive 2003/99/EC, the EU MSs are required to assess trends and sources of zoonoses, zoonotic agents and AMR, as well as outbreaks in their territory, submitting an annual report each year by the end of May to the European Commission covering the data collected.
In accordance with Article 9 of Directive 2003/99/EC, the EFSA shall examine the submitted national reports of the MSs and publish a summary report on the trends and sources of zoonoses, zoonotic agents and AMR in the EU.
The ECDC has provided data on zoonotic infections in humans, as well as their analyses, for the EU Summary Reports since 2005. Since 2007, data on human cases have been reported from The European Surveillance System (TESSy), maintained by the ECDC.
The antimicrobial agents used in food‐producing animals and in human medicine in Europe are frequently the same or belong to the same classes. The route of administration and the administered quantities of antimicrobials may differ between humans and food‐producing animals and there are important variations between and within food‐producing animal populations, as well as between countries. However, the use of antimicrobials in both, humans and animals, might result in the development of AMR, which results from the continuous positive selection of resistant bacterial clones, whether these are pathogenic, commensal or even environmental bacteria. This will change the population structure of microbial communities with serious consequences for human and animal health.
Antimicrobial resistance.
AMR is defined as the inability or reduced ability of an antimicrobial agent to inhibit the growth of a bacterium, which, in the case of a pathogenic organism, can lead to therapy failure. A bacterial strain can acquire resistance by mutation, by the uptake of exogenous genes by horizontal transfer from other bacterial strains or by the activation/triggering of a genetic cascade, thereby inducing the expression of resistance mechanisms (EMA and EFSA, 2017). Resistance development can be triggered by different factors such as inappropriate use of antimicrobials in human and veterinary medicine, poor hygiene conditions and practices in healthcare settings or in the food chain facilitating the transmission of resistant microorganisms. Over time, this makes antimicrobials less effective and ultimately useless.
Bacterial resistance to antimicrobials occurring in food‐producing animals can spread to humans via food‐borne routes, as has been observed for the zoonotic bacteria Campylobacter, Salmonella and Escherichia coli, by routes such as water or other environmental contamination, as well as through direct animal contact. Infections with antimicrobial resistant bacteria may result in treatment failures or the need of second‐line antimicrobials for therapy. The commensal bacterial flora can also form a reservoir of resistance genes, which may be transferred between bacterial species, including organisms capable of causing disease in both humans and animals (EFSA, 2008).
The European Commission adopted a new Action Plan to tackle AMR on 29 June 2017.7 The Action Plan is underpinned by a One Health approach that addresses resistance in both humans and animals. In particular, EU actions will focus on the areas with the highest added value for MSs, e.g. promoting the prudent use of antimicrobials, enhancing cross‐sectorial work, improving infection prevention and consolidating surveillance of AMR and antimicrobial consumption. AMR monitoring in zoonotic and commensal bacteria in food‐producing animals and their food products entails specific and continuous data collection, analysis and reporting; enables to understand the development and diffusion of resistance, to follow temporal trends in the occurrence and distribution of AMR and the identification of emerging or specific resistance patterns, as well as can provide relevant risk assessment data, and help to evaluate targeted interventions.
This EU Summary Report (EUSR) includes data related to the occurrence of AMR in isolates from animals and foodstuffs and in isolates from human cases, being a collaboration between EFSA and ECDC with the assistance of EFSA’s contractors. The EU MSs, the European C ommissionand the relevant EU Reference Laboratory for antimicrobial resistance (EURL‐AR) are consulted, while preparing the report. The efforts made by the MSs and the other reporting countries are gratefully acknowledged.
Data on AMR collected by the EU MSs and compiled in the EUSR on AMR are also used to perform wider analyses, such as the joint report on consumption of antimicrobial agents (AMC) and AMR in animals, food and humans, produced by ECDC, EFSA and EMA, under a One Health approach on a regular basis (JIACRA I, II and III; ECDC, EFSA and EMA, 2015,2017,2021). This report provides evidence‐based analysis of the possible association between AMC and AMR in humans and food‐producing animals.
Third Joint inter‐agency report on integrated analysis of consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from humans and food‐producing animals in the EU/EEA (JIACRA III)
JIACRA III, is the third joint inter‐agency report on integrated analysis of antimicrobial agent consumption (AMC) and occurrence of antimicrobial resistance in bacteria from humans and food‐producing animals, produced by the European Centre for Disease Prevention and Control (ECDC), the European Food Safety Authority (EFSA) and the European Medicines Agency (EMA) at the request of the European Commission (ECDC, EFSA, EMA, 2021). The data originate from five different surveillance/monitoring networks coordinated by the agencies and cover the EU MSs, two European Economic Area (EEA) countries (Iceland and Norway) and Switzerland (for data on food‐producing animals). Taking a One Health approach and applying univariate and multivariate analysis, this report provides an integrated analysis of possible relationships between AMC in humans and food‐producing animals and the occurrence of AMR in bacteria from humans and food‐producing animals at country and European level. The integrated analyses of data from humans and food‐producing animals presented in the report focused on particular combinations of antimicrobials and bacterial species considered of importance for public health.
The latest report published, using data for 2016–2018, shows that in both food‐producing animals and humans, associations were observed between the consumption of an antimicrobial class and bacterial resistance to the antimicrobials in this class in the same population. The analyses showed that the relative strength of these associations differed markedly depending on antimicrobial class, microorganism and sector. The situation in the EU is diverse and varies significantly by country and by antibiotic class. Aminopenicillins, third‐ and fourth‐generation cephalosporins as well as quinolones (fluoroquinolones and other quinolones) are more used in humans than in food‐producing animals, while polymyxins (colistin) and tetracyclines are used more in food‐producing animals than in humans. In this regard, a significant positive association was found between consumption of carbapenems, third‐ and fourth‐generation cephalosporins and quinolones in humans and resistance to those antimicrobials in invasive E. coli isolates from humans. Polymyxins, almost exclusively used in food‐producing animals, specifically in poultry and pigs, were significantly associated with resistance to polymyxins in E. coli from food‐producing animals.
Resistance in bacteria from humans was also associated with resistance in bacteria from food‐producing animals which, in turn, was related to antimicrobial consumption in animals. The most consistent positive association between AMR in bacteria from food‐producing animals and AMR in bacteria from humans was found for Campylobacter spp., probably due to the fact that Campylobacter spp. is found in food‐producing animals and causes food‐borne infections in humans. Experts found an association between resistance to fluoroquinolones, macrolides and tetracycline in these bacteria in animals and resistance in the same bacteria in humans.
This report also revealed, for the first time since JIACRA was initiated (time series starting in 2011), that overall AMC was lower in food‐producing animals than in humans from 2017 to 2018. The significant fall in antibiotic use in food‐producing animals suggests that the measures taken at country‐level to reduce their use are proving to be effective. However, the high levels of AMC and AMR still being reported in bacterial isolates from both food‐producing animals and humans from several countries show that these interventions should be reinforced. Further interventions to reduce AMC will have a beneficial impact on the occurrence of AMR, to continue promoting prudent use of antimicrobial agents and infection control and prevention in both humans and food‐producing animals.
1.1. Monitoring and reporting of antimicrobial resistance in the EU 8
1.1.1. Monitoring of antimicrobial resistance in animals and food
According to Commission Implementing Decision 2013/652/EU, which applied as of 1 January 2014 until December 2020, monitoring of AMR is mandatory in Salmonella, Campylobacter jejuni and indicator commensal E. coli in the major domestically produced animal populations and their derived meat. Monitoring is performed on a rotating basis, targeting fattening pigs and bovine animals under 1 year of age and meat derived thereof in odd years and poultry populations and derived meat in even years, as specified by the legislation. A specific monitoring of extended‐spectrum β‐lactamase (ESBL)‐, AmpC‐ and carbapenemase‐producing Salmonella and indicator commensal E. coli is also required.
The collection and reporting of data are performed at the isolate level, to enable analyses on the occurrence and traits of multidrug resistance (MDR). Representative random sampling is performed according to the legislation and the technical specifications issued by EFSA in 2014. Monitoring of AMR in food‐producing animals is performed in domestically produced animal populations, corresponding to different production types with the aim of collecting data that could be combined with those on exposure to antimicrobials. MSs may also perform complementary monitoring, such as that of MRSA, on a voluntary basis.
Microdilution methods for testing should be used and results should be interpreted by the application of European Committee on Antimicrobial Susceptibility Testing (EUCAST) epidemiological cut‐off (ECOFF) values9 for the interpretation of ‘microbiological’ resistance. The harmonised panels of antimicrobials used for Salmonella, Campylobacter and indicator E. coli include substances that either are important for human health, such as critically important antimicrobials (CIAs), or can provide clearer insight into the resistance mechanisms involved. The concentration ranges to be used embrace both the ECOFF and the clinical breakpoints (CBPs), as defined by EUCAST, allowing the comparability of results with human data. For Salmonella and E. coli, a supplementary panel of antimicrobials for testing isolates showing resistance to third‐generation cephalosporins or carbapenems in the first panel is also used. MSs may also perform complementary monitoring, such as that of MRSA, on a voluntary basis. The reporting of isolate‐based data also allows in‐depth phenotypic characterisation of certain mechanisms of resistance, e.g. third‐generation cephalosporin resistance and carbapenem resistance can be further characterised.
External quality assurance is provided by the EURL‐AR, which distributes the panels of well‐characterised organisms to all MSs for susceptibility testing, arranges proficiency tests (PTs) trials for the National Reference Laboratories for Antimicrobial Resistance (NRLs‐AR) of the MSs on a yearly basis, and, together with EFSA and the MSs, performs a reference testing exercise that includes re‐testing the antimicrobial susceptibility and whole genome sequencing (WGS) analysis of selected isolates (Appendix F, Materials and methods).The EURL‐AR also provides a source of reference for MSs when there are issues or problems with the susceptibility test methodology.
1.1.2. Monitoring of antimicrobial resistance in humans
Together with its Food‐ and Waterborne Diseases and Zoonoses (FWD) network, ECDC has developed an EU protocol for harmonised monitoring of AMR in human Salmonella and Campylobacter isolates (ECDC, 2016). This document is intended for the National Public Health Reference Laboratories to guide the susceptibility testing required for EU surveillance and reporting to ECDC. Consultation was also sought from EFSA, EUCAST and the EU Reference Laboratory for antimicrobial resistance to facilitate comparison of data between countries and with results from the AMR monitoring performed in isolates from animals and from food products. The protocol is effective from 2014 and supports the implementation of the Commission Action Plan on AMR. One of the recommendations is that, for the purpose of the joint report with EFSA, human data should also be interpreted based on ECOFFs. As this requires quantitative data, ECDC introduced reporting of quantitative antimicrobial susceptibility testing (AST) results in the 2013 data collection and encourages countries to use it. As the EU protocol is not a legal document in itself, it is for each National Public Health Reference Laboratory to decide whether to adapt their practices to the protocol. Since the entry into force of Decision 2018/945/EU in July 2018, however, laboratories are obliged to report their AMR test results to ECDC according to the methods and criteria specified in the EU protocol. In 2019 and 2020, most laboratories had adopted the priority panel of antimicrobials suggested in the protocol with the exception of the last‐line antimicrobials, which were tested by fewer laboratories. The protocol also proposes a testing algorithm for screening and confirmation of ESBL‐producing Salmonella spp., including detection of AmpC. This has been implemented by some laboratories while others use a modification of the algorithm or test suspected isolates directly with PCR or whole genome sequencing. Further testing for ESBL and AmpC was performed in 12 of 15 MSs with third‐generation cephalosporin resistance detected in Salmonella isolated from humans in 2019, and in 10 of 14 MSs in 2020.
External quality assessment to support laboratories in implementing the recommended test methods and antimicrobials and obtaining high‐quality AST results is provided by ECDC via a contract with Statens Serum Institute in Denmark.
1.2. Further harmonised monitoring of antimicrobial resistance
To facilitate the comparability of data, the methodology for AMR surveillance should be harmonised across countries as far as possible. The main issues when comparing AMR data originating from different countries are the use of different laboratory methods and different interpretive criteria of resistance. These issues have been addressed by the development of ECDC’s protocol for harmonised monitoring and reporting of resistance in humans and by the legislation on harmonised monitoring in food‐producing animals and the food produced. To respond effectively to the constantly evolving threat of AMR, further enhancements and specific adaptations will be regularly required on an ongoing basis. Under the new One Health action plan (2017), the European Commission is committed to review this legislation, to consider new scientific developments and data collection needs. EFSA received a mandate from the European Commission to provide recommendations on harmonised randomisation procedures for AMR monitoring. The new technical specifications were published in November 2020 (EFSA, 2020) and provide solid scientific advice to support amendments in the existing legislation (see text box below).
New legislation on the the monitoring and reporting of AMR in animals and food.
Commission Implementing Decision 2013/652/EU lays down rules, for the period 2014‐2020, for the monitoring and reporting of antimicrobial resistance (AMR) in zoonotic and commensal bacteria in so far these bacteria present a threat to public health. Monitoring of AMR is essential to have comprehensive and reliable information on the development and spread of resistant bacteria and resistant determinant and as such, AMR data provide insights to inform decision‐making and facilitate the development of appropriate strategies and actions to manage AMR at the EU level. In its Communication of 29 June 2017 to the Council and the European Parliament — A European One Health Action Plan against AMR, the Commission committed to review EU implementing legislation, namely Decision 2013/652/EU, on monitoring AMR in zoonotic and commensal bacteria in food‐producing animals and food to take into account new scientific developments and data collection needs.
In 2020, based on the new technical specifications issued by EFSA, for implementing updated guidelines for further harmonised monitoring of AMR in food‐producing animals and derived meat in the EU and for ensuring continuity in following up further trends in AMR (EFSA, 2019), the European Commission laid down new technical requirements for AMR monitoring and reporting that are applicable as from 1 January 2021 and to repeal, for the sake of clarity, Commission Implementing Decision 2013/652/EU. The new rules are based on the latest scientific opinions but also on the field experience acquired since 2014 by MSs in Implementing Decision 2013/652/EU. They address known implementation issues while scientifically responding to the constantly evolving threat of AMR and ensuring continuity in assessing future trends in AMR after 2020. As AMR is a global threat that can easily spread across borders, it is important to improve coordination and gain knowledge to help reducing AMR impact globally. Therefore, the new rules also lay down harmonised AMR monitoring requirements for certain fresh meat imported into the EU. Commission Implementing Decision (EU) 2020/1729 of 17 November 2020 lays down specific technical requirements, for the period 2021‐2027, for AMR testing and reporting in representative isolates deriving from randomised sampling in food‐producing animals performed at farm and/or at slaughter and derived meat performed at retail and at border control posts. The new rules apply to monitoring performed in 2021 onwards.
1.3. The 2019–2020 EU Summary Report on AMR
The 2019/2020 EU Summary Report on AMR presents AMR data in zoonotic and indicator bacteria from humans, animals and food collected in 2019 and 2020, jointly analysed by the EFSA and the ECDC. This report includes an introduction section, followed by five main chapters on AMR in Salmonella, Campylobacter, indicator E. coli, ESBL and MRSA. A section on the key findings is included at the beginning of each chapter to provide a summary of the key facts organised as bullet points. Specific appendices providing complementary information are included at the end of the report.
Most data reported by the MSs comprise data collected in accordance with Commission Implementing Decision 2013/652/EU. The antimicrobial susceptibility data reported to EFSA for 2019 and 2020 for Campylobacter, Salmonella and indicator E. coli isolates from animals and food were analysed and all quantitative data were interpreted using ECOFFs. This report also includes results of phenotypic monitoring of resistance to third‐generation cephalosporins and/or carbapenems caused by ESBLs, AmpC β‐lactamases or carbapenemases in Salmonella and indicator E. coli, as well as the investigation at the EU level of the occurrence of complete susceptibility and MDR in data reported at the isolate level. All the information on the methodology applied, list of antimicrobials, criteria, etc. can be found in Appendix F ‘Materials and methods’ available on the EFSA Knowledge Junction community on Zenodo at: https://doi.org/10.5281/zenodo.6257446. Additional information on the human data reported in 2019 can also be found in the European Union Summary Report on Antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food in 2018/2019 (EFSA and ECDC, 2021a).
The report includes resistance in Salmonella and Campylobacter isolates from human cases of salmonellosis and campylobacteriosis, respectively. Results from phenotypic tests were reported by MSs to TESSy either as quantitative or categorical/qualitative data. In addition, two countries reported categorical data from whole genome sequencing, where isolates had been categorised as either predicted wild type or predicted non‐wild type, corresponding to ECOFFs. The quantitative phenotypic data were interpreted using EUCAST ECOFFs, where available. The qualitative phenotypic data had been interpreted using CBPs to guide medical treatment of the patient. The breakpoints for ‘clinical’ resistance are often less sensitive than the ECOFF for a specific bacterium–drug combination resulting in higher levels of ‘microbiological’ resistance than ‘clinical’ resistance. By combining the categories of ‘clinically resistant’ (R) and ‘susceptible with increased exposure (I) into one category, however, close correspondence with the ECOFF was achieved. CBPs enable clinicians to choose the appropriate treatment based on information relevant to the individual patient. ECOFFs recognise that epidemiologists need to be aware of small changes in bacterial susceptibility, which may indicate emerging resistance and allow for appropriate control measures to be considered. ECOFFs, CBPs and related concepts on antimicrobial resistance/susceptibility are presented in detail in Appendix F ‘Materials and methods’.
Effect of the UK withdrawal on the analysis of AMR data at the EU.
As a consequence of the UK withdrawal from the EU on 31 January 2020, the entry into force of the Withdrawal Agreement was ratified by the UK and the EU. The AMR data retrieved until 2019 covered all 28 EU Member States (MSs), which during that period included the UK. From 1 February 2020, the UK withdrew and became a ‘third‐country’ in relation to the EU, with final withdrawal effective after a transition period ending on 31 December 2020. The withdrawal of the UK from the EU has an impact on the AMR data reported at the EU level in 2020. In this 2020 report, data at the EU level are reported in accordance with the membership of the EU, whether before 2020 (EU including the UK) or in 2020 (EU without the UK).
The 2020 EU rates will not include UK data. Still during the transition period, the monitoring of AMR in animals and food was mandatory for the UK. The 2020 UK data have been reported to EFSA and will be presented within the group of “other reporting countries”. For the sake of continuity and comparability with historical data, a new group “EU+UK” has been created in some specific analysis for the purpose.
2. Antimicrobial resistance in Salmonella spp.
Monitoring of non‐typhoidal salmonellas.
Non‐typhoidal salmonellas (NTS) are the focus of this section, which summarises the occurrence and AMR patterns of isolates recovered from various food‐producing animal populations and their derived carcases. Whereas typhoidal salmonellas are human host‐adapted organisms that cause typhoid fever and paratyphoid fever; non‐typhoidal strains may be host generalists, infecting or colonising a broad range of animals, or tend to host‐specificity to particular animal species (Crump et al., 2015). Typhoidal salmonellas refer to Salmonella enterica subsp. enterica serovars Typhi, Paratyphi A, Paratyphi B (d‐tartrate negative) and Paratyphi C, while all other serovars within the subspecies enterica (including the d‐tartrate positive Paratyphi B variant Java) refer to NTS.
The World Health Organisation states that transmission of bacterial infection from non‐human sources to humans, with the ability to cause disease, is more evident in particular bacteria (including non‐typhoidal Salmonella, Campylobacter spp. and E. coli) and comments that the potential for such transmission should be recognised (WHO, 2019). In 2020, salmonellosis was the second most common food‐borne zoonosis in the European Union, with 54,702 confirmed human cases (a significant drop compared to 2019 most likely as a result of the pandemic), as well as the most frequent cause of food‐borne outbreaks accounting for 23% of all food‐borne outbreaks reported in 2020 (EFSA and ECDC, 2021b). A recent review inferred that multidrug resistant NTS infections may have more serious human health implications compared to those of pan‐susceptible strains (Parisi et al., 2018).
2.1. Key findings
Resistance to ampicillin, sulfonamides and tetracyclines was observed at overall high levels in Salmonella spp. isolates from humans in 2019–2020 and ranging from moderate to very high in isolates from animals. A decline in resistance to ampicillin and tetracyclines in isolates from humans was however observed in 10 and nine countries, respectively, over the period 2016–2020, particularly evident in Salmonella Typhimurium and its monophasic variant, serovars commonly associated with pigs and calves.
Resistance to (fluoro)/quinolones was observed at very high/high levels among Salmonella spp. isolates recovered from broilers, fattening turkeys and poultry carcases/meat in 2020. In Salmonella spp. isolates from human cases, moderate levels of resistance to ciprofloxacin were observed in 2019–2020 but among S. Kentucky isolates, extremely high levels of resistance were noted and in S. Enteritidis, increasing trends in resistance were observed in nine countries over the period 2016–2020, both serovars predominantly being associated with poultry.
Resistance to third‐generation cephalosporins was noted at overall very low levels in isolates from humans in 2020 and was seldom detected in animals in 2019–2020, with a few exceptions. Combined resistance to fluoroquinolones and cephalosporins was very low in isolates from both humans and animals, but higher in certain Salmonella serovars, e.g. S. Kentucky and S. Infantis.
At the reporting MS‐group level, the proportion of presumptive ESBL or AmpC producers was very low to low among Salmonella spp. recovered from animals (broilers, laying hens, fattening turkeys, fattening pigs) and carcases of broilers and fattening pigs, and very low in isolates from human cases, although higher in some Salmonella serovars. None carbapenemase‐producing Salmonella isolates were detected in Salmonella spp. from animals in 2019–2020, while one and two isolates from humans in 2019 and 2020, respectively, were identified as carbapenemase‐producing (two isolates with bla OXA‐48 and one with bla NDM‐1, two from domestically acquired infections and one case without information on travel status).
MDR was high overall (25.4%) among Salmonella spp. reported from human cases in the EU, most frequently observed among S. Kentucky (76.6%) and monophasic S. Typhimurium 1,4,[5],12:i:‐ (74.2%). Similarly, MDR was observed at very high to high levels in Salmonella spp. recovered from carcases of broilers, pigs and calves (53.6%, 43.3% and 23.1%, respectively), and at a moderate level in Salmonella isolates recovered from turkey carcases (19.1%).
Overall, complete susceptibility was detected in, respectively, 55.9%, 34.0% and 25.8% of Salmonella spp. isolates from humans, broilers and turkeys.
2.2. Data on AMR in Salmonella spp. addressed
Commission Implementing Decision 2013/652/EU stipulates detailed protocols for the harmonised monitoring and reporting of antimicrobial resistance (AMR) in zoonotic and commensal bacteria. The monitoring of AMR in Salmonella isolates recovered from carcases of broilers and fattening turkeys at slaughter was mandatory in 2020, in accordance with Regulation (EC) No 2073/2005; similarly, the monitoring of AMR in Salmonella isolates recovered from carcase swabs of fattening pigs and bovine animals under 1 year of age at slaughter was mandatory in 2019. Additionally, in 2020, the monitoring of AMR in Salmonella isolates recovered from faecal samples and/or environmental samples (boot swabs or dust) of broiler, laying hen and fattening turkey flocks was mandatory, in accordance with Regulation (EC) No 2160/2003, collected as part of National Control Programmes (NCPs) for Salmonella in poultry. In 2019, some MSs also reported Salmonella AMR data from fattening pigs and calves (under 1 year of age) at slaughter, where in general one representative sample of caecal contents was tested for Salmonella per epidemiological unit (i.e. the holding) to prevent clustering. The reporting of such data was not mandatory but was included for completeness.
The Salmonella spp. data include results for all serovars reported from the different carcase/animal origins, with one isolate per Salmonella serovar from the same epidemiological unit per year being tested for AMR (Decision 2013/652/EU). As the potential for acquiring or occurrence of AMR markedly varies between serovars, the relative contribution of different serovars to the total significantly influences overall resistance levels for Salmonella spp. data. Therefore, results have also been presented for selected serovars because of their importance and/or prevalence. Resistance profiles were also considered when less than 10 isolates were recovered from a given carcase/animal origin in a country, to account for the low prevalence of certain serovars, to prevent exclusion of emerging serovars and to ensure that the analysis included all relevant data. (Some graphical figures within this chapter, however, only present individual MS data where 10 or more Salmonella spp. were reported, although resistance at the MS‐group level includes all reported isolates.) The spread of particular resistant clones and the occurrence of resistance genes within these clones can be exacerbated by the use of antimicrobials in human and animal populations and the associated selective pressure. Other factors, such as foreign travel by humans, international food trade, animal movements, farming systems, animal husbandry and the pyramidal structure of some types of animal primary production, may also influence the spread of resistant Salmonella clones.
Variations in Salmonella prevalence.
It is of note that countries reported Salmonella spp. data from the different origins according to their national situation. Notably, some MSs did not obtain any positive Salmonella isolates from the carcases and animal origins and, therefore, data are not presented for these countries. The number of countries reporting results for pig and broiler carcases was considerably higher than those for calf and turkey carcases, because the size of the veal calf and turkey sectors is relatively small in certain EU MSs, with production levels below the threshold at which mandatory monitoring is required. Additionally, the number of isolates reported by countries varied because of varying Salmonella prevalence, and these factors may introduce a source of variation to results when considering all reporting countries.
In both 2020 and 2019, data for Salmonella spp. from human cases were reported. Section 2.3 presents data for 2020, since 2019 data from humans were published in the EU Summary report for 2018/2019 (EFSA and ECDC, 2021a). The analysis of AMR in Salmonella isolates from human cases includes that of prevalent serovars corresponding to those occurring in animal species.
2.3. Occurrence of antimicrobial resistance in Salmonella from humans
2.3.1. Data reported
For 2020, 20 MSs and two non‐MSs reported data on AMR in Salmonella isolates from human cases of non‐typhoidal salmonellosis. This was four countries less than in 2019. Two countries could not report data due to changes taking place in their reporting systems, the UK did not report data as they were no longer an EU/EEA country and for the fourth country, the reasons are unknown. Sixteen countries provided data as measured values (quantitative data), four as data interpreted with clinical breakpoints and two as predicted phenotypic resistance based on whole genome sequencing. Not all countries reported results for all antimicrobials in the harmonised panel (ECDC, 2016). The reported data represented 15.4% of the confirmed human cases with non‐typhoidal Salmonella reported in the EU/EEA in 2020 which was a decrease from 26.1% in 2019 and most likely an effect of the pandemic.
2.3.2. Occurrence of resistance to commonly used antimicrobials in human and/or veterinary medicine
In 2020, high proportions of human Salmonella isolates were resistant to ampicillin (29.8%), sulfonamides (30.1%) and tetracyclines (31.2%) – see Figure 1 and Annex A, Table 1. By serovar, resistance to these compounds ranged from low (2.8–6.2%) in S. Enteritidis to extremely high (72.3–86.0%) in monophasic S. Typhimurium 1,4,[5],12:i:‐ and S. Kentucky. The variation in the proportion of resistance by country was large. For S. Enteritidis, outliers in terms of high proportion of resistance was observed in Luxembourg for sulfonamides (31.6%) and in Slovakia for tetracycline (24.1% – Annex A, Table 2. For monophasic S. Typhimurium 1,4,[5],12:i:‐), Ireland reported a lower proportion of tetracycline resistance (55.6%) compared to other countries (Annex A, Table 4). For S. Infantis, Italy reported a much higher proportion of resistance (53.3%) to ampicillin than the EU average (17.4%) (Annex A, Table 5). Resistance to gentamicin was overall low (1.6% – see Annex A, Table 1) with the exception of in S. Kentucky where it was high (40.0% – see Annex A, Table 6). Similarly, levels of trimethoprim resistance were overall low among Salmonella spp. (6.1% – see Annex A, Table 1), but moderate (20.4–21.3%) in S. Kentucky and S. Infantis (Annex A, Tables 5 and 6).
Figure 1.
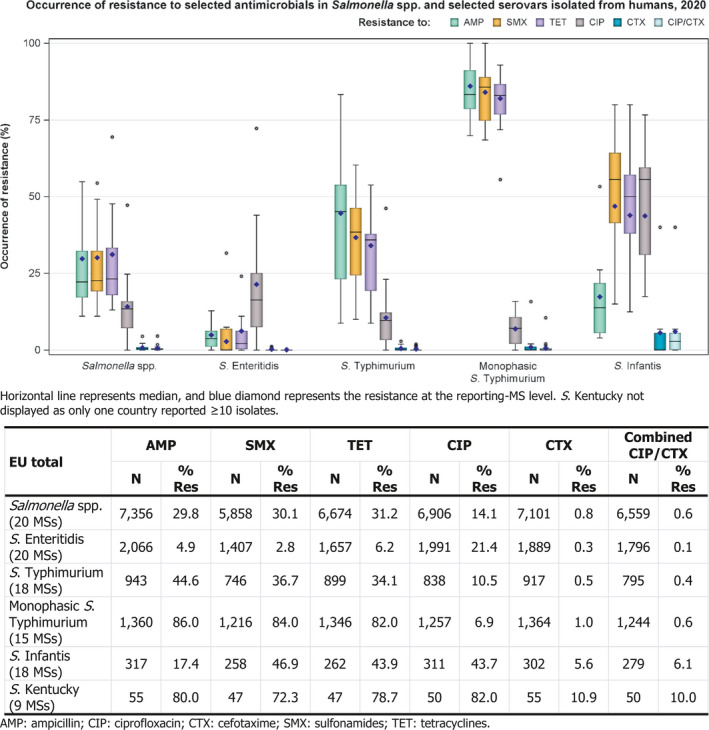
Occurrence of resistance to selected antimicrobials in Salmonella spp. and selected serovars isolated from humans, 2020
2.3.3. Occurrence of resistance to the highest priority ‘critically important antimicrobials’
The proportion of Salmonella isolates resistant to the critically important antimicrobial (CIA) ciprofloxacin was overall 14.1% (see Figure 1 and Annex A, Table 1), with high proportions being resistant in S. Infantis isolates and extremely high proportions in S. Kentucky isolates (43.7% and 82.0%, respectively – see Figure 1 and Annex A, Tables 5–6). Outliers in terms of high ciprofloxacin resistance per serovar were observed in Romania (72.2%) for S. Enteritidis and in Estonia (46.2%) for S. Typhimurium (Annex A, Tables 2 and 3). For the two antimicrobials cefotaxime and ceftazidime, representing third‐generation cephalosporins, another class of critically important antimicrobials for Salmonella, resistance levels were generally very low among Salmonella spp. (0.8% and 0.8%, respectively – see Annex A, Table 1), with higher levels of resistance (5.6–10.9%) noted among S. Infantis and S. Kentucky isolates (see Annex A, Tables 5–6). Outliers for cefotaxime were observed in Sweden (2.9%) regarding S. Typhimurium, Slovenia (15.8%) regarding monophasic S. Typhimurium and Italy (40.0%) regarding S. Infantis (Figure 1 and Annex A, Tables 3–5). Combined resistance to both ciprofloxacin and cefotaxime was overall low in Salmonella spp. (0.6%), but significantly higher in S. Infantis (6.1%) and S. Kentucky (10.0%) with particularly high proportions of combined resistance noted among S. Infantis isolates from Italy (40.0%) – see Figure 2 and Annex A, Tables 7, 11 and 12. In monophasic S. Typhimurium, combined resistance was overall very low (0.6%) but at a higher level in Slovenia (10.5%) – see Figure 2 and Annex A, Table 10.
Figure 2.
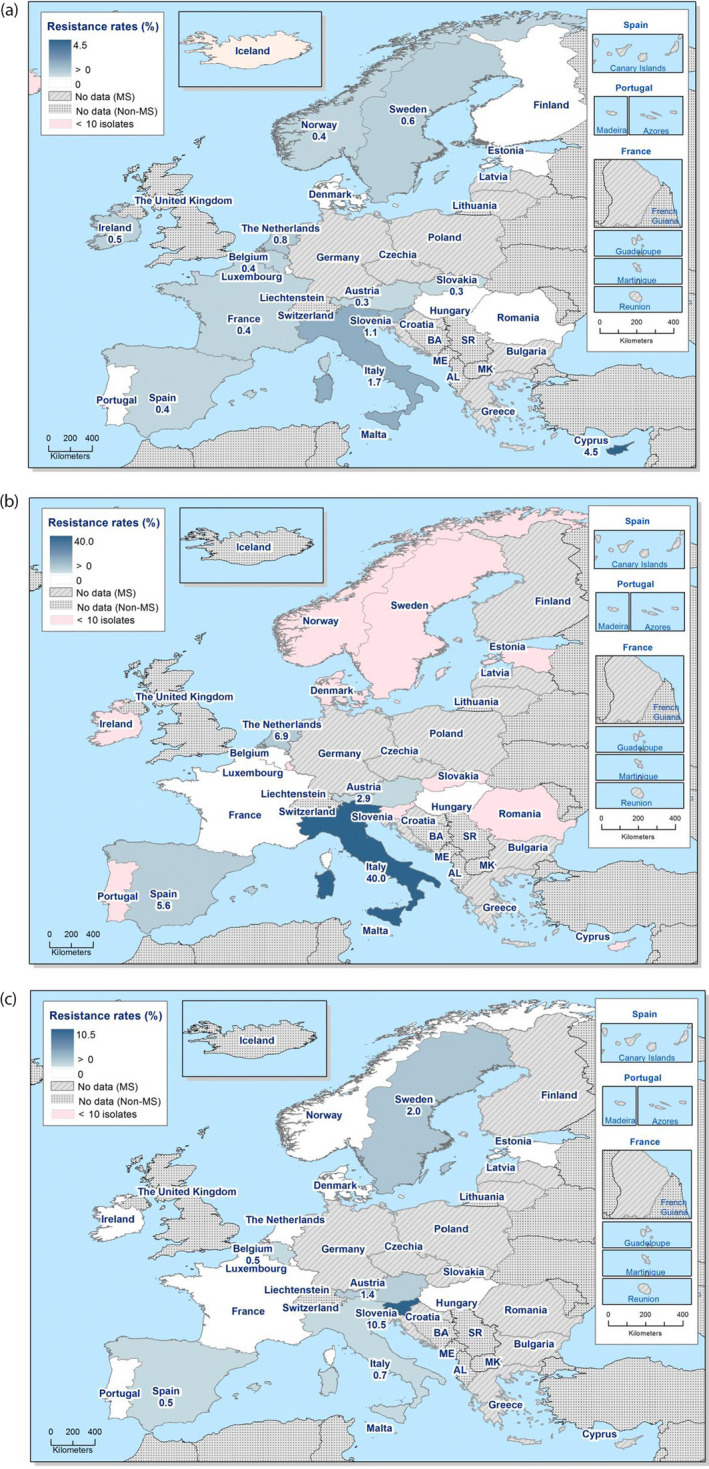
Spatial distribution of combined ‘microbiological’ resistance to ciprofloxacin and cefotaxime among (a) Salmonella spp., (b) S. Infantis and (c) monophasic S. Typhimurium isolated from human cases, 2020 (pink indicates fewer than 10 isolates tested)
Only eight and nine countries, respectively, tested resistance to the last line antimicrobials azithromycin and tigecycline. Resistance was overall low among Salmonella spp. (0.8% and 0.2%, respectively, Annex A, Table 1). Among the individual serovars, the highest proportion of isolates resistant to azithromycin was observed in S. Kentucky (4.9% – see Annex A, Table 7). Resistance to colistin was detected in 7.1% of Salmonella isolates, although 73.2% of these resistant isolates were either S. Enteritidis or S. Dublin; serovars belonging to group D Salmonella which tend to show a higher natural tolerance to colistin (Agersø et al., 2012; Ricci et al., 2020).
2.3.4. ESBL, AmpC and carbapenemase‐producing Salmonella
In 2020, 14 MSs and one non‐MS reported Salmonella isolates resistant/non‐wild type to third‐generation cephalosporins, while all tested Salmonella isolates reported by five MSs and one non‐MS were susceptible/wild‐type. Four countries reporting cephalosporin‐resistant isolates did not provide further details on phenotypic/genotypic characterisation and, therefore, these isolates were excluded from the analysis and Table 1 below. Some countries had only tested further isolates that were clinically resistant, and therefore, results for some isolates are missing in Table 1. In Italy, all isolates resistant to cefotaxime and/or ceftazidime were submitted to the national surveillance system for strict monitoring of ESBL/AmpC resistance, which could have resulted in an overestimation of such findings. Presumptive ESBL‐producing Salmonella were identified in 0.6% of the tested isolates, ranging by MS from 0.3% in Belgium and France to 2.0% in Italy (see Annex A, Table 13). AmpC was less frequent, identified in 0.2% of tested isolates, with the highest occurrence in Malta (0.6%). One isolate (0.02%) was reported as both presumptive AmpC‐ and ESBL‐producing. ESBL was reported in nine different serovars in 2020, most commonly in S. Infantis, S. Kentucky and S. Saintpaul (ranging between 3.0 and 5.0% – see Table 1). Only one isolate of S. Winston was tested and it was ESBL‐producing. Presumptive ESBL production was more frequent in S. Typhimurium and monophasic S. Typhimurium 1,4,[5],12:i:‐ (both 0.6%) than in S. Enteritidis (0.1%). AmpC‐type β‐lactamases were reported in six different serovars, most commonly in S. Bredney, S. Kentucky and S. Thompson (ranging between 2.4% and 6.9%).
Table 1.
ESBL, AmpC and carbapenemase phenotypes and genotypes in Salmonella spp. isolates from humans by serovar, 2020
| Serovar | Tested for CTX and/or CAZ | Res to CTX and/or CAZ | Resistance Phenotype | Genotype | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ESBL | AmpC | AmpC + ESBL | Carbap‐enemase | ||||||||
| N | N | N | % | N | % | N | % | N | % | ||
| S. Bredney | 29 | 2 | 2 | 6.9 | CMY‐2 (2) | ||||||
| S. Derby | 174 | 3 | 3 | 1.7 | CTX‐M‐1 (1), CTX‐M‐65 (1) | ||||||
| S. Dublin | 108 | 1 | 1 | 0.9 | CTX‐M‐15 | ||||||
| S. Enteritidis | 1,508 | 3 | 1 | 0.1 | 1 | 0.1 | DHA (1), OXA‐48 (1) | ||||
| S. Infantis | 278 | 16 | 14 | 5.0 | CTX‐M‐1 (10), CTX‐M‐65 (2), CTX‐M‐9 (1), CTX‐M (1) | ||||||
| S. Kentucky | 54 | 6 | 2 | 3.7 | 2 | 3.7 | 1 | 1.9 | CTX‐M‐14b (1) | ||
| S. Kottbus | 28 | 1 | 1 | 3.6 | NDM‐1 | ||||||
| Monophasic S. Typhimurium 1,4,[5],12:i:‐ | 1,224 | 13 | 7 | 0.6 | 1 | 0.1 | CTX‐M‐1 (4), CTX‐M‐9 (1), DHA‐1 (1), VEB (1), CMY (1) | ||||
| S. Newport | 165 | 1 | 1 | 0.6 | |||||||
| S. Paratyphi B var Java | 35 | 1 | |||||||||
| S. Saintpaul | 33 | 1 | 1 | 3.0 | CTX‐M‐55 | ||||||
| S. Thompson | 41 | 1 | 1 | 2.4 | CMY‐2 | ||||||
| S. Typhimurium | 782 | 7 | 5 | 0.6 | 1 | 0.1 | |||||
| S. Winston | 1 | 1 | 1 | 100.0 | CTX‐M‐1 | ||||||
CTX: cefotaxime; CAZ: ceftazidime; ESBL: extended spectrum beta‐lactamase.
Two Salmonella isolates were reported as resistant to meropenem in 2020. These were an isolate of S. Enteritidis carrying bla OXA‐48 isolated from a case without information on travel in Belgium and an isolate of S. Kottbus carrying bla NDM‐1 from a domestically acquired case in Denmark. In four of 19 reporting MSs, meropenem results were interpreted using the EUCAST clinical breakpoint (CBP), which is substantially higher (+4 dilutions) than the EUCAST ECOFF.
2.3.5. Complete susceptibility (CS) and multidrug resistance (MDR)
MDR was high overall (25.4%) among Salmonella spp. reported from human cases in the EU (Figure 3 and Annex A, Table 14). For the investigated serovars, MDR was most frequently reported among S. Kentucky (76.6%) and monophasic S. Typhimurium 1,4,[5],12:i:‐ (74.2%), followed by S. Infantis (45.3%), S. Typhimurium (30.6%) and lastly S. Enteritidis (2.2%) (Figure 3 and Annex A, Tables 15–19). Eight isolates (three S. Infantis and one each of S. Derby, S. Dublin, monophasic S. Typhimurium, S. Saintpaul and S. Typhimurium) were resistant to eight of the nine tested substances, only susceptible to meropenem.
Figure 3.
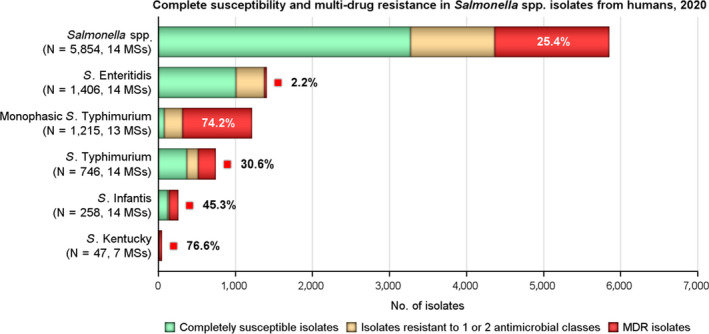
Number of MDR isolates, isolates resistant to 1 and/or 2 antimicrobial classes and completely susceptible Salmonella isolates from humans in 2020
- The MDR analysis of human isolates included the following antimicrobials: ampicillin, cefotaxime/ceftazidime, chloramphenicol, ciprofloxacin/pefloxacin/nalidixic acid, gentamicin, meropenem, sulfonamides/sulfamethoxazole, tetracyclines and trimethoprim/trimethoprim‐sulfamethoxazole (co‐trimoxazole).
The level of complete susceptibility (defined as susceptibility to each of the nine antimicrobial classes tested in the harmonised panel) was 55.9% in Salmonella spp. with the highest proportion in S. Enteritidis (71.5%), followed by S. Typhimurium (49.5%) and S. Infantis (47.7%). The lowest levels of complete susceptibility were observed in S. Kentucky (10.6%) and monophasic S. Typhimurium (6.3%) (Figure 3 and Annex A, Tables 15–19).
2.3.6. Temporal trends
Trends in resistance over the period 2016–2020 were assessed with logistic regression. As the number of isolates reported by country was markedly lower in almost all countries in 2020 compared to previous years as an effect of the pandemic, it reduced the power of the statistical test. Trends varied by country for the different serovars and antimicrobials (Table 2, Annex A, Figures 1–7). For Salmonella spp. overall, 10 and nine countries observed a decrease in resistance to ampicillin and tetracycline, respectively, vs. four and three countries that reported an increase. For cefotaxime and ciprofloxacin, about the same number of countries observed increasing as decreasing trends. By serovar, increasing trends in resistance were more commonly observed for ciprofloxacin/quinolones and tetracycline in S. Enteritidis and for ampicillin in S. Infantis. Nine and three countries, respectively, reported decreasing trends in resistance to ampicillin in S. Typhimurium and monophasic S. Typhimurium and, respectively, 10 and five countries to tetracycline in S. Typhimurium and monophasic S. Typhimurium. Decreasing trends in cefotaxime resistance were observed in S. Enteritidis and monophasic S. Typhimurium and in ciprofloxacin/quinolone resistance in monophasic S. Typhimurium.
Table 2.
Number of countries with statistically significant (p < 0.05) increasing or decreasing trends in resistance to selected antimicrobials for Salmonella spp. and selected serovars in humans in 2016–2020*
| Serovar | Ampicillin | Cefotaxime | Ciprofloxacin/quinolones | Tetracycline | ||||
|---|---|---|---|---|---|---|---|---|
| Incr. | Decr. | Incr. | Decr. | Incr. | Decr. | Incr. | Decr. | |
| Salmonella spp. (24 MSs + 3 non‐MS) | 4 (EL, PL, SI, SK) | 10 (DE, DK, FR, HU, IE, LT, MT, NO, PT, UK) | 4 (DE, FR, SI, UK) | 4 (BE, ES, MT PL) | 6 (AT, BE, EL, HU, LTSK) | 7 (EE, ES, FI, IE, NO, PL, PT) | 3 (EL, IT, SK) | 9 (AT, DE, ES, FR, IE, NO, PT, UK) |
|
S. Enteritidis (24 MSs + 2 non‐MS) |
5 (BE, NL, SI, SK, UK) | 4 (DE, FI, HU, LT) | – | 4 (FI, PL, RO, UK) | 9 (AT, BE, DE, HU, IT, LT, SI, SK, UK) | 3 (ES, FI, PT) | 4 (BE, DE, NL, SK) | 2 (FI, HU) |
| S. Typhimurium (24 MSs + 3 non‐MS) | – | 9 (AT, DE, DK, EE, FR, NO, PT, SK, UK) | 1 (DE) | 2 (BE, SK) | 3 (BE, ES, LT) | 4 (AT, EE, FI, PT) | – | 10 (AT, BE, DE, DK, EE, ES, FR, NO, PT, UK) |
| Monophasic S. Typhimurium (15 MSs + 2 non‐MSs) | – | 3 (AT, FR, PT) | – | 3 (BE, ES, SI) | – | 4 (ES, FR, LU, NO) | – | 5 (AT, ES, FR, IE, PT) |
|
S. Infantis (11 MSs + 1 non‐MS) |
4 (AT, BE, LT, SK) | – | 1 (UK) | – | 2 (BE, ES) | 2 (DE, LT) | 1 (ES) | – |
|
S. Kentucky (6 MSs + 1 non‐MS) |
– | – | – | 1 (UK) | – | – | – | – |
Only countries reporting data for at least 10 isolates for a specific combination and for at least 3 years in the 5‐year period were included.
2.3.7. High ciprofloxacin resistance
In 2020, 1.4% (44/3,076) of Salmonella spp. expressed high‐level resistance to ciprofloxacin (MIC ≥ 4 mg/L – see Table 3). Such isolates were reported from six of the 10 countries reporting MIC values for ciprofloxacin. Among the four serovars reported with MICs of ≥ 4 mg/L, high‐level ciprofloxacin resistance was most frequently observed in S. Kentucky (in 83.7% of tested S. Kentucky).
Table 3.
Occurrence of high‐level resistance to ciprofloxacin (MIC ≥ 4 mg/L) in Salmonella serovars from human cases in 2020, 10 Mss
| Serovar | N | High‐Level Resistance to Ciprofloxacin (MIC ≥ 4 mg/L) | |
|---|---|---|---|
| n | % | ||
| S. Havana | 5 | 1 | NA |
| S. Infantis | 209 | 1 | 0.5 |
| S. Kentucky | 49 | 41 | 83.7 |
| S. Rechovot | 1 | 1 | NA |
| Other | 2,778 | 0 | 0 |
| Total (10 MSs) | 3,043 | 44 | 1.4 |
Additional data on certain resistance traits of Salmonella isolates from humans are provided hereafter and presented in parallel to corresponding data on Salmonella spp. from animals and food.
2.4. Occurrence of antimicrobial resistance in Salmonella from food‐producing animals and meat thereof
2.4.1. Data reported
In 2020, AMR data for Salmonella isolates recovered from the mandatory carcase sampling of broilers and fattening turkeys at slaughter were reported by 18 MSs and two non‐MS for broilers and eight MSs for turkeys; while in 2019, AMR data for Salmonella isolates recovered from the mandatory carcase swabbing of fattening pigs and calves (less than 1 year of age) at slaughter were reported by 26 MSs and one non‐MS for fattening pigs and seven MSs for calves. Additionally, in 2020, 22 MSs and three non‐MSs reported mandatory AMR data for Salmonella isolates recovered from flocks of broilers; 24 MSs and two non‐MSs for laying hens and 16 MSs and one non‐MS for fattening turkeys, in accordance with Regulation (EC) No 2160/2003 and as part of National Control Programmes (NCPs) of Salmonella in poultry. Notably, some MSs did not obtain any positive Salmonella isolates from these carcase/animal origins and, therefore, data are not presented for these countries in corresponding results. In 2019, nine MSs also reported voluntary data on Salmonella isolates recovered from caecal contents of fattening pigs and calves (less than 1 year of age) at slaughter, where in general one representative sample of caecal contents was tested for Salmonella per epidemiological unit (i.e. the holding) to prevent clustering. The reporting of isolate‐based data enable the analysis of MDR patterns, detection of high‐level ciprofloxacin resistance and co‐resistance to ciprofloxacin and cefotaxime; first‐line agents critically important for treating human salmonellosis. Resistance levels were also reported by serovar for the different animal/carcase origins (see Appendix C), which allows detailed analysis and, as required by Decision 2013/652/EU, all MSs included information on serovars and production type. In line with this decision, streptomycin is no longer included in the specified test panels for the monitoring and reporting of AMR in Salmonella, which has an impact on how MDR patterns are interpreted.
Summary data on the occurrence of resistance to commonly used antimicrobials in veterinary medicine (ampicillin, sulfamethoxazole and tetracycline) as well as critically important antimicrobials (CIAs – represented by ciprofloxacin, cefotaxime and combined resistance to these two antimicrobials) are displayed in Figure 4 (a and b) for Salmonella isolates recovered from (a) carcases and (b) faecal samples from food‐producing animals and are described in the forthcoming text. Annex A (available on the EFSA Knowledge Junction community on Zenodo at: https://doi.org/10.5281/zenodo.6257446) presents the occurrence of antimicrobial resistance (%) in Salmonella spp. from carcases of broilers, turkeys, pigs and calves, as well as from broilers, laying hens, turkeys, pigs and calves, at both the MS and MS‐group level.
Figure 4.
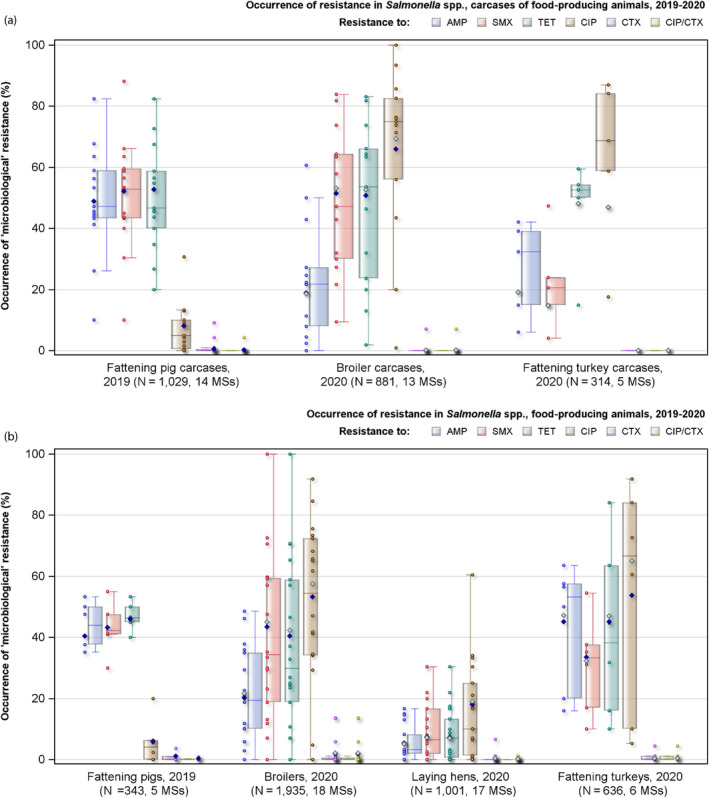
Occurrence of resistance to selected antimicrobials in Salmonella spp. recovered from (a) carcases of broilers, fattening turkeys and fattening pigs, and (b) broilers, laying hens, fattening turkeys and fattening pigs, reporting EU MSs, 2019–2020
- AMP: ampicillin; SMX: sulfamethoxazole; TET: tetracycline; CIP: ciprofloxacin; CTX: cefotaxime; CIP/CTX: combined ‘microbiological’ resistance to ciprofloxacin and cefotaxime; N: total number of Salmonella spp. reported by MSs; blue diamond shows resistance at the reporting‐MS group level. Note: Only MSs reporting data for 10 or more isolates are shown in the graph; however, all isolates are included in the calculation of resistance at the reporting MS group level. As only two MSs reported data on 10 or more Salmonella isolates recovered from calves or their derived carcases, resistance levels for these origins are not presented in Figure 4a,b.
2.4.2. Occurrence of resistance to commonly used antimicrobials in veterinary medicine
Carcases of poultry
Antimicrobials such as ampicillin, sulfamethoxazole and tetracycline have been widely used for many years in veterinary medicine to treat infections in production animals. While ampicillin is categorised as a high priority, 'critically important antimicrobial' (CIA) by the World Health Organisation; sulfamethoxazole and tetracycline are recognised as ‘highly important antimicrobials’ (WHO, 2019). Considering all MSs reporting Salmonella spp. data from poultry carcases in 2020, overall resistance to ampicillin, sulfamethoxazole and tetracycline ranged from moderate to very high10 (Figure 4a). Ampicillin resistance was reported at overall moderate levels in both broiler carcasses and turkey carcases (18.8% and 19.1%, respectively); sulfamethoxazole resistance was noted at a very high level in broiler carcases and a moderate level in turkey carcases (51.0% and 14.7%, respectively); while tetracycline resistance was noted at an overall very high level in broiler carcases and at a high level in turkey carcases (50.3% and 48.1%, respectively). In addition, among Salmonella isolates recovered from turkey carcases, resistance levels were substantially lower than those obtained from broiler carcases for ciprofloxacin (46.9% and 65.3%, respectively); nalidixic acid (32.5% and 61.4%, respectively) as well as for trimethoprim (3.8% and 17.1%, respectively).
Carcases of pigs and calves
Among Salmonella spp. recovered from carcase swabs of pigs and calves in 2019, the highest levels of resistance were noted to ampicillin, sulfamethoxazole and tetracycline considering all reporting MSs. High to extremely high levels of resistance to these antimicrobials were recorded in pig carcases by the majority of the MSs included in the analysis (Figure 4a); while resistance to these compounds generally ranged from high to very high among isolates from calf carcases (overall resistance in pig carcases: 48.9%, 52.1% and 52.7%, respectively; overall resistance in calf carcases: 22%, 31.9% and 41.8%, respectively). Among Salmonella isolates recovered from calf carcases, overall resistance levels were mostly lower than those observed for pig carcases, with the exception of tigecycline and colistin resistance (3.3% and 17.6%, respectively) which were higher than the values registered for pig carcases (0.9% and 1.8%, respectively); however, the total number of isolates from calf carcases (N = 91) was considerably lower than that from pig carcases (N = 1,088).
Food‐producing animals
Among Salmonella spp. recovered from fattening pigs and calves in 2019, as well as flocks of broilers and fattening turkeys in 2020, most MSs reported moderate or high to extremely high resistance to tetracyclines and sulfonamides. Resistance to these two antimicrobials was observed at overall low levels of resistance in Salmonella recovered from flocks of laying hens compared to overall high levels in flocks of broilers and turkeys. Considering reporting MSs, resistance levels to ampicillin were generally observed at similar or slightly lower levels to those of tetracycline and sulfamethoxazole within all food‐producing animal origins; and overall resistance levels to these three antimicrobials were highest in isolates from pigs and turkeys (Figure 4b).
2.4.3. Occurrence of resistance to other ‘critically important antimicrobials’ (CIAs)
Use of ‘critically important antimicrobials’ (CIAs) for the treatment of salmonellosis.
Fluoroquinolones and third‐generation cephalosporins are categorised as highest priority, critically important antimicrobials (CIA) in human medicine (WHO, 2019). Although fluoroquinolones may not be recommended for use in children, these CIAs often constitute first‐line treatment for invasive salmonellosis in humans and as such, the monitoring of resistance to these compounds in zoonotic bacteria, including Salmonella spp., originating from animals is of particular interest. These classes are represented by ciprofloxacin and cefotaxime/ceftazidime, respectively; compounds which are specified in the antimicrobial panels for the monitoring and reporting of AMR in Salmonella spp. The WHO also recognises tigecycline and azithromycin as CIAs. Additionally, colistin is considered as a highest priority CIA for the treatment of serious human infection with some Gram‐negative bacteria (WHO, 2019).
Carcases of poultry, fattening pigs and calves
As (fluoro)quinolones are highest priority, CIAs in human medicine, their use in food‐producing animals is subject to prudent use initiatives. Considering Salmonella spp. recovered from broiler carcases in 2020, resistance to the (fluoro)quinolone antimicrobial agents, ciprofloxacin and nalidixic acid, were reported at very high to extremely high levels by many of the MSs included in the analysis (with overall resistance at 65.3% and 61.4%, respectively). Resistance levels to ciprofloxacin and nalidixic acid in isolates from turkey carcases ranged from low or not detected to extremely high among reporting MSs (overall, 46.9% and 32.5%, respectively). In certain Salmonella serovars recovered from carcases of pigs and poultry, isolates resistant to ciprofloxacin but not to nalidixic acid were observed; possibly indicating the occurrence of plasmid‐mediated quinolone resistance (PMQR) mechanisms – see text box below. This was particularly the case for specific serovars recovered from broilers carcases in 2020, where 15 S. Agona isolates from the United Kingdom and seven S. Hadar isolates from Hungary, displayed ciprofloxacin resistance, yet none showed resistance to nalidixic acid. Similarly, 15/65 monophasic S. Typhimurium isolates reported from pig carcases by Spain displayed ciprofloxacin resistance, yet only 12/65 isolates showed nalidixic acid resistance. Additionally, 3/15 S. Derby isolates and 3/15 S. Brandenburg isolates reported from pig carcases by Croatia and Italy, respectively, displayed ciprofloxacin resistance, without resistance to nalidixic acid.
Quinolone/fluoroquinolone resistance in Salmonella .
Quinolone/fluoroquinolone (i.e. nalidixic acid/ciprofloxacin) resistance in Salmonella usually arises due to point mutations within the DNA gyrase (gyrA and gyrB) and topoisomerase IV (parC and parE) genes, at locations comprising the quinolone resistance‐determining regions (QRDR) of the bacterial chromosome. Additionally, plasmid‐mediated quinolone resistance (PMQR) mechanisms have also been recognised, including the action of efflux pumps (qepA and oqxAB genes), enzymatic modifications (aac(6′)Ib‐cr gene – also conferring resistance to kanamycin) and protection of the DNA gyrase (qnrA, qnrB, qnrC, qnrD, qnrS and qnrVC genes) (Li et al., 2013; Luk‐In et al., 2017).
The clinical breakpoint (CBP) for ciprofloxacin in Salmonella has been lowered by EUCAST from > 1 to > 0.06 mg/L, resulting in the CBP and ECOFF (microbiological breakpoint) for ciprofloxacin applying the same threshold (MIC > 0.064 mg/L). The presence of two‐point mutations in the QRDR will usually confer resistance to ciprofloxacin, with isolates typically exhibiting MICs of > 1 mg/L, as well as conferring resistance to nalidixic acid. In contrast, isolates harbouring only one point mutation in the QRDR will usually still display resistance to ciprofloxacin and nalidixic acid, but the degree of resistance to ciprofloxacin is reduced (MIC > 0.064 mg/L). Salmonella isolates causing systemic infections in humans and displaying MICs of > 0.064 mg/L but < 1 mg/L, have shown a poor response to treatment in some studies. This provides the rationale for setting the CBP at > 0.064 mg/L and it follows that monitoring of low‐level resistance to this compound is therefore indicated.
In the absence of other fluoroquinolone resistance mechanisms, the presence of PMQR determinants (i.e. primarily qnr genes) in a bacterium usually confers resistance to ciprofloxacin, with an MIC of > 0.064 mg/L, but the isolate remains susceptible to nalidixic acid. This contrasts with mutation in the QRDR regions of the bacterial chromosome, which confer resistance to both ciprofloxacin and nalidixic acid.
‘Microbiological’ resistance to third‐generation cephalosporins (cefotaxime and ceftazidime) in Salmonella spp. from carcases of these food‐producing animals was not detected in most of the reporting MSs, with the exception of Italy and Iceland which reported resistance in 1/14 and 1/10, Salmonella spp. from broiler carcases respectively, as well as Romania (N = 3) which reported resistance in 1/3 isolates from pig carcases. No reporting countries detected third‐generation cephalosporin resistance among Salmonella isolates from carcases of calves or turkeys. Section 2.3.6 provides further information on the phenotypic characterisation of third‐generation cephalosporin resistance among Salmonella isolates from pig and broiler carcases.
The Netherlands and Romania were the only countries to report combined ‘microbiological’ resistance to both ciprofloxacin and cefotaxime in single isolates from pig carcases (of serovars Heidelberg and Derby, respectively); while Italy and Iceland were the only countries to report combined ‘microbiological’ resistance to these antimicrobial agents both in single isolates of S. Infantis from broiler carcases. Therefore, considering all reporting MSs, ‘microbiological’ combined resistance to these agents among isolates from pig and broiler carcases were observed at overall very low levels (0.2%) – see Figure 4a. Notably, when clinical breakpoints (CBPs) were applied, no isolates exhibited ‘clinical’ resistance to these compounds.
Resistance to azithromycin (a highest priority CIA) in Salmonella spp. from carcases of pigs, calves and poultry was generally low or not detected, although there were a couple of exceptions: Portugal reported a very high level of resistance to this compound among isolates from pig carcases (64.7%) and Italy reported a high level of resistance among isolates from broiler carcases (21.4%), although data were provided for a low/very low number of isolates (N = 17 and N = 14, respectively).
Considering all reporting MSs, tigecycline resistance was reported generally at very low levels among isolates from pig carcases (0.9%), and at low levels from carcases of broilers, turkeys and calves (1.4%, 2.5% and 3.3%, respectively). Where countries reported resistance to this antimicrobial, generally low/very low levels were observed, there were some exceptions: the Netherlands reported a high level of resistance (21.7%) in 5/23 isolates recovered from pig carcases, whereas Cyprus reported a high level of resistance to tigecycline (25.0%) in 2/8 isolates and Slovenia a moderate level of resistance (14.3%) in 1/7 isolates both recovered from broilers carcases in 2020. However, the small samples size should be considered when interpreting these results. Notably, Hungary reported a moderate level of resistance to tigecycline (13.0%) in 6/46 isolates recovered from turkey carcases. Where tigecycline resistance was reported, multidrug resistance was often a feature; with all resistant isolates recovered from carcases of calves, broilers and turkeys (n = 3, n = 14 and n = 8) and 70% of those from pig carcases (n = 10) exhibiting multiresistance.
Overall, colistin resistance was reported at very low/low levels among isolates from carcases of broilers, turkeys and pigs (1.5%, 1.6% and 1.8%, respectively), while a moderate level was noted among isolates from calf carcases (17.6%). With the exception of calf carcases, where countries reported resistance to this antimicrobial among isolates from the other carcase origins, generally very low or low levels were noted, however, there were a few exceptions. In pig carcases, a moderate level of resistance at 17.6% was noted by Portugal (N = 17), as well as a high level (22.2%) reported by the United Kingdom (N = 9). Additionally, Cyprus (N = 6) and Portugal (N = 1) reported resistance in single isolates recovered from broiler carcases resulting in moderate and extremely high resistance levels as an effect of the very low sample sizes. The contribution of different serovars can influence these outputs, because of the occurrence of a degree of intrinsic resistance to colistin in Group D Salmonella isolates.
Food‐producing animals
Overall, very high/high levels of resistance to ciprofloxacin and nalidixic acid were observed in Salmonella spp. from broilers (52.9% and 49.6%, respectively) and turkeys (53.7% and 32.5%, respectively), compared with moderate levels observed in Salmonella isolates from laying hens (18% and 16.8%, respectively), and moderate/low levels reported in isolates from calves (12.5% and 7.8%, respectively) and pigs (5.8% and 4.5%, respectively) – see Figure 4b. Salmonella isolates exhibiting ciprofloxacin resistance and nalidixic acid susceptibility, most frequently observed in Salmonella isolates turkeys, possibly indicate the occurrence of PMQR mechanisms. This was particularly the case for 13 S. Hadar isolates from Hungary, 46 S. Anatum isolates from Italy and 43 S. Derby isolates from Spain respectively, where all isolates displayed ciprofloxacin resistance, with no resistance to nalidixic acid. Notably, S. Agona isolates exhibiting a similar fluoroquinolone phenotype were reported from the UK in low number in other poultry sources, including turkeys (N = 1), laying hens (N = 4) and broilers (N = 2) which may suggest clonal spread of this variant in poultry in the UK.
Resistance to cefotaxime and ceftazidime in Salmonella isolates from these animal origins was either not discerned or detected at very low/low levels by reporting MSs, although there were a few exceptions. Among isolates from broilers, moderate levels of resistance were reported by Italy (N = 214) and Malta (N = 52). No resistance to third‐generation cephalosporins was detected in calves, consistent with the result obtained for Salmonella spp. from calf carcases; however, only 64 calf isolates were obtained by three MSs in 2019, which was considerably lower than the total number of isolates reported for the other animal sectors. Section 2.3.6 provides further information on the phenotypic characterisation of third‐generation cephalosporin resistance among Salmonella isolates from the animal origins.
Where MSs reported combined ‘microbiological’ resistance to ciprofloxacin and cefotaxime in Salmonella isolates from pigs or broilers, laying hens or turkeys, this was observed at overall very low or low levels, although some exceptions were noted. Among Salmonella isolates from broilers, a moderate level of resistance was reported by Italy (13.6%, N = 214) and Malta (13.5%, N = 52). Nevertheless, when ciprofloxacin and cefotaxime resistance was interpreted using CBPs, only the isolates recovered from broilers from Malta displayed ‘clinical’ resistance; these were all S. Kentucky. Combined ‘clinical’ resistance to these antimicrobials was not observed among isolates from pigs or turkeys.
Azithromycin resistance among Salmonella isolates was either not detected or observed at very low to low levels by reporting countries with very low levels of resistance reported in pigs and laying hens (0.3% and 0.5% respectively), while low levels of resistance were observed in broilers and turkeys (1.9% and 1.2%, respectively). Resistance to azithromycin was not detected in Salmonella spp. recovered from calves.
Considering all reporting MSs, tigecycline resistance was reported at low levels among isolates from calves, broilers and turkeys (1.6%, 1.1% and 4.9%, respectively), and at very low levels in isolates from pigs and laying hens (0.3% and 0.2%, respectively). Where countries reported resistance to this antimicrobial among isolates from pigs, calves, broilers and laying hens, very low or low levels were noted. However, among isolates from turkeys, high levels of resistance at 21.2% and 50% were noted by Hungary (N = 170) and Slovenia (N = 2), respectively. For the latter country, the very low number of samples has to be considered when interpreting these results. Where tigecycline‐resistant isolates were detected within the animal origins, the majority of isolates exhibited multidrug resistance (MDR among tigecycline‐resistant isolates were reported at levels of 66.7% in laying hens, 98.1% in broilers and 100% in pigs, calves and turkeys; although the total number of tigecycline‐resistant isolates reported from some origins was extremely low).
Overall, colistin resistance was reported at low levels among isolates from broilers, calves and laying hens (2.4%, 3.1% and 7.5%, respectively); while an overall very low level was noted among isolates from pigs and turkeys (0.8% and 0.7%, respectively). Where countries reported resistance to this antimicrobial within each of the animal origins, often very low or low levels were noted, however, several countries reported moderate to very high levels of resistance in isolates recovered from poultry. Considering broilers, moderate levels of resistance were reported by Germany (23.5%, N = 17) and Cyprus (17.6%, N = 17). In addition, Norway reported their only Salmonella isolate from broilers as colistin resistant. In laying hens, moderate levels of resistance were noted by Austria, Sweden and the United Kingdom (19.2%, 12.5% and 12.5%, respectively), whereas high levels of resistance were reported by Belgium, Cyprus and Germany (22.5%, 34.8% and 26.9%, respectively) and very high levels were noted by Estonia and Latvia (66.7% and 60%, respectively). Importantly, some of these countries provided data for a very low number of isolates; therefore, results may be subject to variation associated with small sample sizes. This was the case for Sweden, where only eight Salmonella isolates were recovered from laying hens. This was also definitely the case for countries reporting very high to extremely high levels of resistance in turkeys: Belgium (33.3%, N = 3), Portugal (50%, N = 2) and Romania (100%, N = 1).
Figure 4a,b summarises the overall resistance to commonly used antimicrobials in veterinary medicine (ampicillin, sulfamethoxazole and tetracycline), as well as critically important antimicrobials (represented by ciprofloxacin, cefotaxime and combined resistance to these two antimicrobials) among Salmonella isolates recovered from (a) carcases of broilers, turkeys and pigs, and (b) broilers, laying hens, fattening turkeys and fattening pigs.
2.4.4. Complete susceptibility (CS) and multidrug resistance (MDR)
Carcases of poultry, fattening pigs and calves
The levels of multidrug resistance (MDR),11 defined as resistance to three or more antimicrobial classes, among Salmonella isolates from carcases of the monitored food‐producing animals are shown in Figure 5. Overall, MDR was observed at very high to high levels in Salmonella spp. recovered from carcases of broilers, pigs and calves (51.2%, 43.3% and 23.1%, respectively), and at a moderate level in Salmonella isolates recovered from turkey carcases (19.1%). Considering only countries reporting data for 10 or more Salmonella spp. (unless otherwise stated), MDR among isolates from broiler carcases ranged from low levels in France and Iceland (9.4% and 10%, respectively) to extremely high levels in Austria, Belgium, Romania and Spain (73.8%, 78.6%, 81.8% and 83.1%, respectively), and among isolates from turkey carcases levels of MDR ranged from 6.8% in France to 43.5% in Hungary. In pig carcases, MDR ranged from low in Malta (10%) to extremely high in Portugal (88.2%). Although seven MSs provided Salmonella spp. data for calf carcases, only two countries reported data on 10 or more isolates; a moderate level of 18.8% was noted by Spain, while a high level of 30.2% was reported by France.
Figure 5.
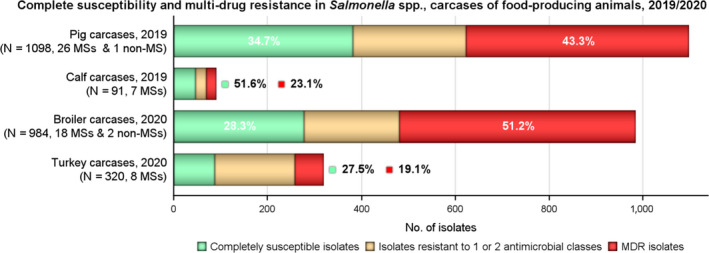
MDR and completely susceptible Salmonella spp. recovered from carcases of broilers, fattening turkeys, fattening pigs and calves (< 1 year of age), for all reporting countries (including two non‐MSs in broiler carcases and one non‐MS in pig carcases) in 2019–2020
-
The MDR analysis of carcase isolates included the following antimicrobials: ampicillin, cefotaxime/ceftazidime, chloramphenicol, ciprofloxacin/nalidixic acid, gentamicin, meropenem, sulfamethoxazole, tetracycline, tigecycline and trimethoprim.MDR and complete susceptibility levels are also expressed as a percentage; N: total number of Salmonella spp. reported by MSs and non‐MSs.
The levels of complete susceptibility (defined as susceptibility to each of the 10 antimicrobial classes tested in the harmonised panel) also varied between reporting countries for most of the carcase origins (Figures 6 and 7). Overall, 51.6%, 34.7%, 28.3% and 27.5% of the isolates reported from calves, pigs, broilers and turkeys, respectively, were completely susceptible. Considering only countries reporting data for 10 or more Salmonella spp. (unless otherwise stated), the proportion of completely susceptible isolates recovered from broiler carcases ranged from not detected in Belgium and Romania to extremely high in France and Iceland (74.5% and 90%, respectively), and for turkey carcases between low proportions in Hungary and Poland (8.7% and 5.3%, respectively) and a very high proportion of complete susceptibility noted in France (39.9%). Among isolates from pig carcases, complete susceptibility ranged from low in Portugal and Czechia (5.9% and 9.1%, respectively) to very high/extremely high in the Netherlands and Malta (52.2% and 80%, respectively). In calf carcases, only two countries reported data on 10 or more Salmonella isolates, with complete susceptibility ranging from high in France (41.9%) to very high in Spain (53.1%). Differences in the prevalence of particular serovars and phage types of Salmonella in different countries and animal populations, and their associated patterns of resistance are likely to explain some of the differences in the levels of MDR and complete susceptibility. The proportions of isolates which were completely susceptible and MDR among particular Salmonella serovars within the carcases origins are presented in Appendix C.
Figure 6.
Spatial distributions of complete susceptibility to the panel of antimicrobials tested among Salmonella spp. from (a) broiler carcases and (b) fattening turkey carcases, using harmonised ECOFFs, 2020
Figure 7.
Spatial distributions of complete susceptibility to the panel of antimicrobials tested among Salmonella spp. from (a) fattening pig carcases and (b) calf carcases (less than 1 year of age), using harmonised ECOFFs, 2019
Food‐producing animals
The levels of MDR and complete susceptibility among Salmonella isolates recovered from the monitored food‐producing animal populations are shown in Figure 8. Overall, MDR was observed at high levels in isolates from turkeys, pigs, broilers and calves (38.2%, 38.4%, 41.8% and 35.9%, respectively), and at a low level in isolates from laying hens (6.3%). Considering only countries reporting data for 10 or more Salmonella spp. (unless otherwise stated), MDR among isolates from broilers ranged from not detected in Latvia to 100% in Cyprus, and among isolates from turkeys between 10% in Austria and 69.4% in Hungary. Generally, MDR among isolates from laying hens spanned much lower levels from not detected in six MSs (Austria, Croatia, Czechia, Greece, Malta and Portugal) to 30.4% in Cyprus. In pigs, MDR ranged from 30% in the Netherlands to 50% in Croatia. Among isolates recovered from calves, MDR ranged from moderate in Spain (16.7%) to extremely high in Italy (75%).
Figure 8.
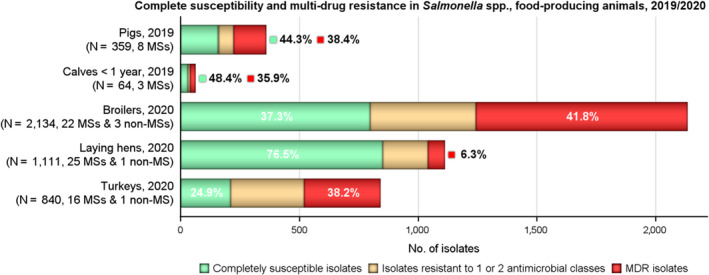
MDR and completely susceptible Salmonella spp. recovered from broilers, laying hens, fattening turkeys, fattening pigs and calves (< 1 year of age), for all reporting countries (including one non‐MS in broilers and laying hens), 2019–2020
-
The MDR analysis of animal isolates included the following antimicrobials: ampicillin, cefotaxime/ceftazidime, chloramphenicol, ciprofloxacin/nalidixic acid, gentamicin, meropenem, sulfamethoxazole, tetracycline, tigecycline and trimethoprim.MDR and complete susceptibility are expressed as percentages; N: total number of Salmonella spp. reported by MSs and non‐MSs.
Considering the proportions of isolates exhibiting susceptibility to each of the 10 antimicrobial classes tested in the harmonised panel, there was a wide variation in the levels of complete susceptibility among the animal origins. Overall, 76.5%, 48.4%, 44.3%, 37.3% and 24.9% of the isolates reported from laying hens, calves, pigs, broilers and turkeys, respectively, were completely susceptible (Figure 8). Furthermore, the levels of complete susceptibility varied widely between reporting countries, particularly within the poultry populations, but this may reflect the greater number of countries reporting data from poultry in comparison to those reporting data from the 2019 monitoring of pigs and calves. Considering only countries reporting data for 10 or more Salmonella spp. (unless otherwise stated), complete susceptibility among isolates recovered from pigs ranged from 35% in Croatia to 46.8% in Italy; and for isolates recovered from calves, from high in Italy (25%) to very high in Spain (58.3%). Among isolates recovered from poultry (Figure 9), the proportion of completely susceptible isolates from broilers ranged from not detected in Cyprus (N = 17) to 100% in Latvia (N = 10), and for turkeys between 8.2% in Hungary (N = 170) and 80% in Austria (N = 10). Generally, complete susceptibility spanned higher levels among isolates from laying hens; ranging from 36.6% in Italy to 100% in Portugal. However, as mentioned previously, the prevalence of particular serovars in different countries and animal populations, and their associated patterns of resistance, may account for the differences in the levels of MDR and complete susceptibility among Salmonella spp. data. Notably in laying hens, S. Enteritidis predominated (accounting for 25% of Salmonella isolates recovered from this poultry origin) with 79.5% of isolates exhibiting complete susceptibility reported from EU MSs and 92% from non‐EU MSs (including the Republic of North Macedonia and the United Kingdom), respectively. The proportions of isolates which were completely susceptible and MDR among particular Salmonella serovars within the animal origins are presented in Appendix C.
Figure 9.
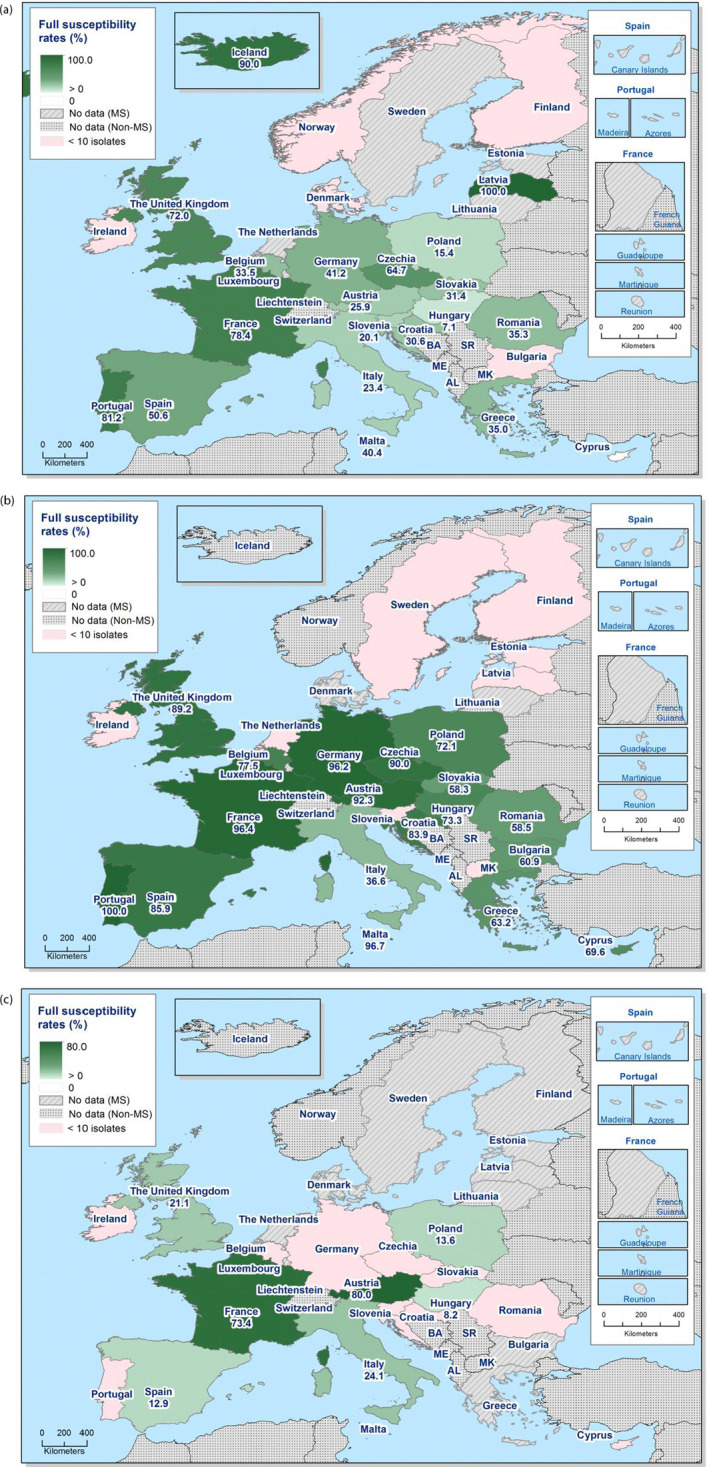
Spatial distributions of complete susceptibility to the panel of antimicrobials tested among Salmonella spp. from (a) broilers, (b) laying hens and (c) fattening turkeys, using harmonised ECOFFs, 2020
2.4.5. High‐level resistance to ciprofloxacin (CIP) in Salmonella spp.
High‐level resistance to ciprofloxacin [minimum inhibitory concentration (MIC) ≥ 4 mg/L] was not observed in Salmonella spp. from pigs or calves, or calf carcases, and only one isolate recovered from a pig carcase displayed an MIC of ≥ 4 mg/L. Considering the total number of Salmonella isolates monitored from the different types of poultry by MSs in 2020, as well as pig carcases in 2019, the highest proportions of isolates displaying ciprofloxacin MICs of ≥ 4 mg/L were noted in turkeys and turkey carcases; with levels of 0.1% (1/1,088), 0.3% (3/1,030), 2.4% (22/905), 2.6% (51/1,955), 3.8% (12/320) and 4.3% (29/674) reported from pig carcases, laying hens, broiler carcases, broilers, turkey carcases and turkeys, respectively.
Among Salmonella isolates displaying ciprofloxacin resistance, 22/643 (3.4%) isolates from broiler carcases, 12/150 (8%) isolates from turkey carcases and 1/88 (1.1%) isolates from pig carcases exhibited MICs of ≥ 4 mg/L. Considering the total number of CIP‐resistant isolates reported by MSs from flocks of broilers, laying hens and turkeys, the highest proportions of Salmonella isolates displaying high‐level ciprofloxacin resistance originated from broilers and turkeys with 4.5% (51/1,129) and 6.4% (29/451), respectively. A lower proportion of CIP‐resistant isolates displayed MICs of ≥ 4 mg/L from laying hens (3/200, 1.5%).
The distribution of CIP‐resistant isolates displaying levels of ‘microbiological’ resistance or ‘clinical’ resistance or high‐level resistance to ciprofloxacin within each of the animal/carcase categories is illustrated in Figure 10. Notably, the distribution of MICs is provided only for CIP‐resistant isolates; the total number of Salmonella isolates monitored is provided in the legend.
Figure 10.
Distribution of MIC levels among ciprofloxacin‐resistant Salmonella spp. from carcases of broilers, turkeys, pigs and calves (< 1 year of age), as well as broilers, laying hens, fattening turkeys, fattening pigs and calves (< 1 year of age), for all reporting EU MSs, 2019–2020
-
n: Total number of Salmonella spp. exhibiting CIP resistance (MSs only); N: total number of Salmonella spp. reported by MSs.(1): In accordance with breakpoints stated in Decision 2013/652/EU.The proportion of isolates showing high‐level resistance is not included with those exhibiting ‘clinical’ or ‘microbiological’ resistance; similarly, the proportion of isolates showing ‘clinical’ resistance is not included with those displaying ‘microbiological’ resistance. Figure 10 excludes one isolate reported from laying hens (by the Republic of North Macedonia), which was ‘microbiologically’ resistant to ciprofloxacin; as well as one isolate reported from pigs (by Switzerland), which showed ‘clinical’ resistance to ciprofloxacin.
The serovars which displayed high‐level resistance to fluoroquinolones are of interest from both epidemiological and public/animal health perspectives. A detailed analysis on the high‐level resistance to ciprofloxacin in S . Kentucky and other Salmonella serovars is presented in Appendix A.
2.4.6. Tigecycline and colistin resistance in Salmonella serovars
Tigecycline resistance in Salmonella serovars
Mechanisms of tigecycline resistance.
The World Health Organisation also recognises tigecycline as a CIA (WHO, 2019). Although tigecycline is not recommended for use in pregnant women or children, this CIA may be considered as a last resort for the treatment of serious infection in adults caused by MDR bacteria.
Several mechanisms of resistance to tigecycline in Salmonella and other members of the family Enterobacteriaceae have previously been described: increased activity of efflux pumps (AcrAB), mutation of the ribosomal protein S10 and modification of the Mla system involved in phospholipid transport in cell membranes (He et al., 2016).
Particular serovars displayed ‘microbiological’ resistance to tigecycline (MIC > 1 mg/L – see Annex A, ‘Materials and methods’), which may suggest clonal expansion of microbiologically resistant strains belonging to these serovars. Figure 11 shows the number of tigecycline‐resistant isolates where detected from the animal/carcase origins by reporting MSs, and the predominant serovars accounting for this resistance. All of the tigecycline‐resistant isolates recovered from calf carcases were S . Derby (n = 3), while S . Typhimurium accounted for 40% of those recovered from pig carcases. Serovar Infantis accounted for the majority (60.9%) of the resistant isolates recovered from broilers and for all tigecycline resistance in isolates of their derived carcases, while S . Bredeney accounted for most (71.8%) of the tigecycline‐resistant isolates recovered from turkeys.
Figure 11.
Breakdown of the number of tigecycline‐resistant isolates by serovar, where detected among the animal/carcase origins by reporting MSs in 2019–2020
-
n: Total number of tigecycline‐resistant isolates reported by the MSs; predominant serovars are also expressed as a percentage;*: monophasic S. Typhimurium includes antigenic formulas;†: serovar unspecified;Salmonellas in the legend are listed according to their predominance within all the animal/carcase origins.
Where tigecycline resistance was reported among certain serovars within the carcase/animal origins, multidrug resistance was often a feature. For instance, among broilers and their derived carcases, all tigecycline‐resistant S. Infantis isolates (n = 14 and n = 14, respectively) were multiresistant, with ciprofloxacin, nalidixic acid, sulfamethoxazole and tetracycline resistance being a feature of all MDR isolates; a pattern typical of recent MDR broiler clones of S. Infantis (Nógrády et al., 2012). Among turkeys, all tigecycline‐resistant S. Bredeney isolates (n = 28) were multiresistant, with ampicillin, nalidixic acid and tetracycline resistance being a feature of all MDR isolates. Similarly, among pig carcases, all tigecycline‐resistant S. Typhimurium isolates (n = 4) were multidrug resistant, showing resistance to ampicillin, sulfamethoxazole, trimethoprim and tetracycline; and among calf carcases, all tigecycline‐resistant S. Derby (n = 3) displayed resistance to chloramphenicol, sulfamethoxazole and tetracycline.
Considering individual countries reporting tigecycline resistance, certain features relating to resistance were also evident. For example, the Netherlands reported five of the 10 resistant isolates recovered from pig carcases, while France reported all three of the resistant isolates recovered from calf carcases. Additionally, 21.2% tigecycline‐resistant isolates recovered from turkeys were reported by Hungary. Notably, where tigecycline‐resistant isolates were detected among the carcase/animal origins, most displayed MICs just above the epidemiological cut‐off (ECOFF) of > 1 mg/L, with only a small proportion of isolates displaying ‘clinical’ resistance (MIC > 2 mg/L). Considering serovars in which markedly elevated tigecycline MICs were observed, a single S. Typhimurium isolate exhibiting a tigecycline MIC of 8 mg/L was reported from a pig carcase by the Netherlands in 2019.
Recent discovery of transferable plasmid‐mediated tigecycline resistance genes, tet(X3) and tet(X4), within Enterobacteriaceae .
Tigecycline is structurally related to the tetracycline class of antibiotics and is active against Gram‐positive and Gram‐negative bacteria, as well as tetracycline‐resistant bacteria and some anaerobes (WHO, 2006). In a recent study, two transferable plasmid‐mediated tigecycline resistance genes, tet(X3) and tet(X4), were reported in numerous Enterobacteriaceae that were isolated from animals and meat (chicken and pork) in China, as well as from hospital patients from different cities around the country (He et al., 2019). Both genes were reported to confer the high levels of tigecycline resistance, with isolates displaying MICs of ≥ 32 mg/L. Furthermore, in a subsequent investigation carried out by Bai et al. (2019), seven tet(X4)‐positive E. coli isolates were identified from retail pork samples in China (Bai et al., 2019). These isolates were all MDR and displayed tigecycline MICs ranging from 16 to 32 mg/L. The tet(X4) gene conferring such resistance in these isolates was located on various conjugative plasmids of diverse replicon types, indicating that the gene may be captured by a range of mobile genetic elements circulating among bacterial strains. The authors also comment that the occurrence of tet(X3) and tet(X4) in food‐producing animals could potentially lead to an increased risk of infection by strains harbouring these genes and treatment failure in humans (Bai et al., 2019).
The potential for other bacteria within the Enterobacteriaceae family (such as Salmonella) to acquire such transferable tigecycline resistance genes is therefore highlighted, and the importance of monitoring tigecycline resistance through determination of MICs or by molecular investigation such as WGS is further underlined.
Colistin resistance in Salmonella spp.
Mechanisms of colistin resistance.
Colistin is an antimicrobial compound belonging to the polymyxin class, considered as a highest priority CIA and last resort for the treatment of serious human infection with some Gram‐negative bacteria (WHO, 2019). Although not frequently used in human medicine due to its nephrotoxic effects, colistin has been widely used in veterinary medicine for prophylactic/metaphylactic treatment (Kieffer et al., 2017). Various mechanisms of polymyxin resistance in Gram‐negative bacteria have been described (lipopolysaccharide modifications, efflux pumps, capsule formation and overexpression of membrane protein – Olaitan et al., 2014); and transferable mobile colistin resistance (mcr) genes have also been detected in Salmonella isolates (Campos et al., 2016; Carnevali et al., 2016; Skov and Monnet, 2016).
Among Salmonella isolates recovered from poultry in 2020, ‘microbiological/clinical’ resistance to colistin (MIC > 2 mg/L) was generally observed in S . Enteritidis isolates; this serovar accounting for 33.3%, 52% and 60.2% of the colistin‐resistant isolates recovered from broiler carcases, broilers and laying hens, respectively. A single colistin‐resistant S. Enteritidis isolate was also reported from turkeys. Considering the monitoring performed in 2019, both of the colistin‐resistant isolates reported from calves, as well as 6/16 colistin‐resistant isolates from calf carcases were serotyped as S . Dublin. A single colistin‐resistant S. Dublin isolate was also reported from pig carcases. Notably, both S. Enteritidis and S. Dublin are group D salmonellas (serogroup O9). Salmonella belonging to group D tend to show decreased susceptibility to colistin without having any known acquired or mutational colistin resistance mechanisms (Agersø et al., 2012; Ricci et al., 2020). This is exemplified by the proportion of colistin‐resistant isolates belonging to S. Enteritidis and S. Dublin in 2020 and 2019, respectively. Figure 12 presents the number of colistin‐resistant isolates where detected from the animal/carcase origins by reporting MSs, and the predominant serovars accounting for this resistance. Serovars Kapemba, Napoli and Panama are also group D salmonellas; the other serovars listed in Figure 12 do not belong to group D (serogroup O9). A colistin‐resistant S. Kapemba isolate was reported from a pig and a S. Panama isolate displaying colistin resistance was reported from a pig carcase. Additionally, a single colistin‐resistant S. Napoli isolate was recovered from a flock of laying hens.
Figure 12.
Breakdown of the number of colistin‐resistant isolates by serovar, where detected among the animal/carcase origins by reporting MSs in 2019–2020
-
n: Total number of colistin‐resistant isolates reported by the MSs; predominant serovars are expressed as a percentage;*: Monophasic S. Typhimurium includes antigenic formulas.†: Serovar unspecified; salmonellas in the legend are listed according to their predominance within all the animal/carcase origins.
S. Newport accounted for 40% and 33.3% of the colistin‐resistant isolates from broilers (n = 6) and turkeys (n = 4), respectively; while monophasic S . Typhimurium accounted for half of the colistin‐resistant isolates from pig carcases (n = 20). Among Salmonella isolates recovered from pigs, only three colistin‐resistant isolates were reported, of which S. Typhimurium and its monophasic variant accounted for 66.7% of these. A variety of different serovars from pig and calf carcases displayed colistin resistance (Bovismorbificans, Brandenburg, Bredeney, London, Montevideo, Paratyphi B var Java, Typhimurium and Welikade), including three S. Derby isolates from pig carcases. Additionally, colistin resistance was reported in a diverse range of serovars from poultry, including serovars Brandenburg, Bredeney, Chester, Coeln, Hadar, Heidelberg, Infantis, Manhattan, Minnesota, Montevideo, Mishmarhaemek, Montevideo, Newport, Paratyphi B. var Java, Telaviv, Typhimurium and its monophasic variant.
Considering serovars in which markedly elevated colistin MICs were observed, a single S. Bredeney isolate exhibiting a colistin MIC of 16 mg/L was reported from turkeys by France in 2018. This strain, which since then has been phenotypically confirmed and sequenced, harboured a mutation in pmrB gene.
2.4.7. Phenotypic characterisation of third‐generation cephalosporin and carbapenem resistance in Salmonella spp.
Further phenotypic characterisation of those Salmonella isolates that exhibited resistance to third‐generation cephalosporins within each of the animal categories and for Salmonella isolates from humans (Appendix B) was performed in 2019–2020 (Table B.1). Notably, no Salmonella isolates recovered from calves or their derived carcases, or turkey carcases exhibited resistance to third‐generation cephalosporins.
Table B.1.
Occurrence of resistance to third‐generation cephalosporins and fluoroquinolones in non‐typhoidal Salmonella spp. from food‐producing animals, animal carcases and humans, reported by MSs in 2019–2020
| Human/animal category | No. of MSs | N | Cefotaxime | Ceftazidime | Ciprofloxacin/Pefloxacin | |||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |||
| Humans – 2020* | See footnote below | – | 0.8( a ) | – | 0.8%( b ) | – | 14.1%( c ) | |
| Humans – 2019* | – | 1.8%( d ) | – | 1.2%( e ) | – | 13.5%( f ) | ||
| Broiler carcases – 2020 | 18 MSs | 905 | 3 | 0.1% | 1 | 0.1% | 627 | 69.3% |
| Turkey carcases – 2020 | 8 MSs | 320 | 0 | 0% | 0 | 0% | 150 | 46.9% |
| Pig carcases – 2019 | 26 MSs | 1,088 | 5 | 0.5% | 5 | 0.5% | 88 | 8.1% |
| Calf carcases (< 1 year) – 2019 | 7 MSs | 91 | 0 | 0 | 0 | 0 | 2 | 2.2% |
| Broilers – 2020 | 22 MSs | 1,955 | 41 | 2.1% | 42 | 2.2% | 1,125 | 57.5% |
| Laying hens – 2020 | 24 MSs | 1,030 | 4 | 0.4% | 4 | 0.4% | 196 | 19.1% |
| Fattening turkeys – 2020 | 16 MSs | 674 | 3 | 0.5% | 3 | 0.5% | 438 | 64.9% |
| Fattening pigs – 2019 | 8 MSs | 359 | 4 | 1.1% | 4 | 1.1% | 21 | 5.8% |
| Calves (< 1 year) – 2019 | 3 MSs | 64 | 0 | 0 | 0 | 0 | 8 | 12.5% |
N: Total number of isolates tested/reported by MSs; n: Total number of isolates resistant; MSs: Member states.
*: In several countries, ciprofloxacin has been replaced by pefloxacin for screening for fluoroquinolone resistance with disk diffusion, as recommended by EUCAST.
N = 7,101, 190 MSs.
N = 6,000, 16 MSs.
N = 6,906, 20 MSs.
N = 16,414, 24 MSs.
N = 14,356, 20 MSs.
N = 18,397, 24 MSs.
Salmonella spp. from food‐producing animals and derived carcases
Considering only isolates from the animal sector, a low percentage (77/6,916, 1.1% of all Salmonella recovered from all animals/carcases in 2019–2020) demonstrated third‐generation cephalosporin resistance and were subjected to further phenotypic characterisation. The proportions of isolates exhibiting ESBL, AmpC or ESBL+AmpC phenotypes within the different animal species and production types are presented in Table 4. Given the total number of Salmonella isolates reported by the MSs within the animal categories, the percentage of presumptive ESBL, AmpC or ESBL+AmpC producers was similar, with the ESBL phenotype more frequently detected than the AmpC phenotype among pig carcases, broilers and turkeys. Considering the individual MSs reporting cephalosporin‐resistant isolates from pigs and poultry and related matrices, where presumptive ESBL, AmpC or ESBL+AmpC‐producers were identified, they were observed at very low or low levels, although there were a few exceptions. Italy reported the highest number of isolates from both broilers and turkeys, with 30 isolates from broilers and 13 isolates from turkeys exhibiting the ESBL phenotype (24.8% and 26.5% of all isolates tested by Italy, respectively). Although only single isolates were reported to exhibit an ESBL phenotype in broilers by the Netherlands and an ESBL+AmpC phenotype in broiler carcases by Portugal, these translate to moderate levels at the MS level (14.3% and 16.7%, respectively) due to a low number of isolates tested (N = 7 and N = 6, respectively). Similarly, only three Salmonella isolates were reported in total from pig carcases by Romania, with one identified as a presumptive ESBL producer (33.3% of all isolates tested by this MS).
Table 4.
Summary of phenotypic characterisation of third‐generation cephalosporin resistance in non‐typhoidal Salmonella spp. from food‐producing animals, animal carcases and humans, reported in 2019–2020
| Matrix |
Presumptive ESBL and/or AmpC‐ producers( a ) n (%R) |
Presumptive ESBL‐producers( b ) n (%R) |
Presumptive AmpC‐producers( c ) n (%R) |
Presumptive ESBL + AmpC‐producers( d ) n (%R) |
|---|---|---|---|---|
| Humans – 2019 (N = 14,389, 21 MSs) | 128 (0.9) | 111 (0.8) | 17 (0.1) | 5 (0.03) |
| Humans – 2020 (N = 5,948, 15 MSs) | 45 (0.8) | 35 (0.6) | 10 (0.2) | 1 (0.02) |
| Broiler carcases (N = 905, 18 MSs) | 3 (0.3) | 2 (0.2) | 1 (0.1) | 0 (0) |
| Pig carcases (N = 1,088, 26 MSs) | 5 (0.5) | 3 (0.3) | 2 (0.2) | 0 (0) |
| Broilers (N = 1,955, 22 MSs) | 42 (2.1) | 37 (1.9) | 4 (0.2) | 1 (< 0.1) |
| Laying hens (N = 1,184, 24 MSs) | 2 (0.2) | 1 (0.1) | 1 (0.1) | 0 (0) |
| Fattening turkeys (N = 674, 16 MSs) | 3 (0.4) | 3 (0.4) | 0 | 0 |
| Fattening pigs (N = 359, 8 MSs) | 3 (0.8) | 1 (0.3) | 2 (0.6) | 0 (0) |
N: Total number of isolates reported by the MSs; n: number of the isolates resistant; %R: percentage of resistant isolates; ESBL: extended‐spectrum β‐lactamase.
Isolates exhibiting only ESBL‐ and/or only AmpC‐ and/or combined ESBL+AmpC phenotype.
Isolates exhibiting an ESBL‐ and/or combined ESBL+AmpC‐phenotype.
Isolates exhibiting an AmpC and/or combined ESBL+AmpC‐phenotype.
Isolates exhibiting a combined ESBL+AmpC phenotype.
Salmonella serovars from food producing animals and carcases
When assessing the 2019 data by serovar, the ESBL or AmpC phenotype was detected in four serovars among porcine isolates, these being: S. Derby, S. Heidelberg, S. Rissen and monophasic S. Typhimurium. Among Salmonella isolates from pig carcases, three displayed an ESBL phenotype (one S. Derby from Romania and two monophasic S. Typhimurium from Italy) and two displayed an AmpC phenotype (a monophasic S. Typhimurium from Czechia and S. Heidelberg from the Netherlands). In pigs, a single Salmonella isolate displayed an ESBL phenotype (monophasic S. Typhimurium from Italy) and two displayed an AmpC phenotype (single isolates of S. Rissen and monophasic S. Typhimurium from Italy).
Considering the 2020 data on poultry, the ESBL or AmpC phenotype was associated with multiple isolates belonging to certain serovars, suggesting the possible clonal expansion of particular strains: namely, S. Infantis, S. Kentucky, S. Bareilly and S. Bredeney. Among both broilers and turkeys, presumptive ESBL‐producing Salmonella were identified more frequently than presumptive AmpC‐producing Salmonella and encompassed a greater number of serovars. The ESBL phenotype was identified in four different serovars from broilers (Infantis, Kentucky, Livingstone and Rissen) and six different serovars from turkeys (Agona, Bareilly, Bredeney, Derby, Infantis and Typhimurium), while the AmpC phenotype was identified in only two different serovars from these origins (Infantis and Orion in broilers; Infantis and Derby in turkeys). Six of the AmpC‐carrying S. Infantis from broilers and two from turkeys, as well as the AmpC‐carrying S. Derby from turkeys, also expressed an ESBL phenotype. Where presumptive ESBL, AmpC or ESBL+AmpC‐producers were identified from broilers (43/2,084 isolates), most were attributed to S. Infantis (30 isolates reported by Italy and four by Hungary) and S. Kentucky (four isolates reported by Malta and one by the Netherlands). All 30 S. Infantis isolates reported by Italy displayed an ESBL phenotype, as well as an AmpC phenotype in six of these; while of the four S. Infantis isolates reported by Hungary, two presented with an ESBL phenotype and two with an AmpC phenotype. Conversely, only the ESBL phenotype was expressed in the five S. Kentucky isolates. Where presumptive ESBL, AmpC or ESBL+AmpC‐producers were identified from turkeys (21/815 isolates), most were attributed to S. Infantis (seven isolates reported by Italy), S. Bareilly (six isolates reported by Italy). All seven S. Infantis isolates reported by Italy displayed an ESBL phenotype, as well as an AmpC phenotype in two of these; while the six S. Bareilly (reported by Italy) presented with an ESBL phenotype only. Among laying hens, a single S. Infantis isolate reported by Italy was also identified as a presumptive ESBL producer, and a single S. Kentucky isolate reported by Hungary was identified as a presumptive AmpC producer. Additionally, both the ESBL and AmpC phenotype were detected in a single S. Paratyphi B var. Java isolate reported from a broiler carcase by Portugal.
2.4.8. Carbapenem resistance in Salmonella spp. from food‐producing animals and carcases
Carbapenems are recognised as CIAs (WHO, 2019) and include meropenem, a compound which is specified in the antimicrobial panels for the monitoring and reporting of AMR in Salmonella spp. (as stipulated by Decision 2013/652/EU). This class of antimicrobials are not therapeutically used in food‐producing animals but are reserved for use in humans.
In 2020 and 2019, none of the Salmonella isolates recovered from any of the animal or carcase origins exhibited ‘microbiological’ resistance to meropenem.
2.4.9. Resistance exhibited by dominant Salmonella serovars
The detailed reporting of results at the serovar level clearly demonstrated the major contribution of a few serovars to the observed overall occurrence of resistance when considering aggregated data for Salmonella spp. The patterns of resistance associated with these different serovars have a marked influence on the overall resistance levels in Salmonella spp., as the proportion of completely susceptible and MDR isolates may vary significantly among particular serovars recovered from each of the carcase origins/food‐producing animal populations studied. The analysis of antimicrobial resistance at the serovar level is presented in Appendix C.
2.5. Comparison of resistance data in Salmonella from human and food‐producing animals
A further comparison of human Salmonella data by serovar to that in food‐producing animals for the years 2019–2020 was performed and is detailed in Appendix D. Comparable AMR data are presented for serovars S. Typhimurium and its monophasic variant, S. Derby, S. Infantis, S. Enteritidis and S. Kentucky, and are discussed in this corresponding Appendix. The prevalence of particular Salmonella serovars within countries and animal populations, and their associated patterns of resistance, may explain some of the observed differences in the occurrence of antimicrobial resistance and multidrug resistance. The spread of resistant clones and the presence of resistance genes within these clones can be exacerbated by the use of antimicrobials in human and animal populations and the associated selective pressure. However, it should be noted that relating the occurrence of AMR in human Salmonella isolates to that in isolates from food/food‐producing animals is complicated because other sources of Salmonella occur; such evaluations should be performed and interpreted considering the complex epidemiology of salmonellosis.
2.6. Discussion
In 2020, information on AMR in Salmonella isolates from human clinical cases was reported by 20 MSs and two non‐MSs. This compares to 24 MSs and two non‐Ms in 2019. Three countries were not able to report data for 2020 due to the impact of the pandemic (affecting laboratory resources) and the UK did not report data as they were no longer an EU/EEA country. The pandemic also affected on the number of isolates reported by countries, as isolates sent for referral from primary laboratories to the national reference centres were much reduced. For all countries but three (Cyprus, Italy and Malta), the number of reported isolates dropped in 2020, ranging by country from 10% to 80% fewer isolates compared to 2019. Sixteen countries in 2020 provided data as measured values (quantitative data), four as data interpreted with clinical breakpoints and two as predicted phenotypic resistance based on whole genome sequencing.
In 2020, 25 MSs and 4 non‐MSs reported AMR data on Salmonella isolates recovered from carcases of broilers and fattening turkeys, as well as data obtained from National Control Plan samples of broiler, laying hen and fattening turkey flocks. In 2019, 26 MSs and one non‐MS reported AMR data on Salmonella from carcases of pigs (fatteners) and calves (under 1 year of age), in some cases with additional data obtained from the monitoring of caecal contents of pigs and calves. Compared with 2018 and 2017, the numbers of MSs reporting data in 2020 and 2019 increased for broilers, laying hens, turkeys and their derived carcases, as well as carcases of pigs while an equal number of MSs reported data on isolates from broiler carcases, pigs and calf carcases. Considering data on calves, the number of reporting MSs decreased from seven MSs in 2017 to three MSs in 2019. MSs which have a very low prevalence or zero prevalence of Salmonella in certain sectors may of course only contribute in years when Salmonella is detected in those sectors and this may result in fluctuations to the numbers of contributing MSs.
Occurrence of resistance to commonly used antimicrobials in veterinary medicine
Moderate to very high levels of resistance to ampicillin, sulfamethoxazole and tetracycline were generally reported by MSs among Salmonella isolates from all animal/carcase origins except laying hens. Overall, high levels were also noted in isolates from humans. In 2019, highest resistance to these antimicrobials was recorded in isolates recovered from pigs and their derived carcases. The lowest resistance was reported in isolates from calf carcases. Similarly, high levels of resistance were reported in 2020 in Salmonella isolates from broilers and turkeys and their derived carcases compared to laying hens. Among isolates from pigs, calves, broilers and turkeys as well as their derived carcases, resistance to ampicillin was generally observed at similar or slightly lower levels to those of tetracycline and sulfamethoxazole. This may be related to the occurrence of underlying genetic structures responsible for resistance and the proportion of Salmonella spp. carrying genetically linked resistance genes to these agents. Considering individual serovars, monophasic S. Typhimurium generally showed the highest resistance to these compounds across most of the animal/carcase origins. The same observation was also noted among isolates from human cases, where overall extremely high levels of resistance to these antimicrobials were reported in monophasic S. Typhimurium and S. Kentucky in both 2019 and 2020.
Occurrence of resistance to other ‘critically important antimicrobials’ (CIAs)
From the monitoring of poultry in 2020, the highest levels of resistance were generally noted to ciprofloxacin/nalidixic acid, as well as to sulfamethoxazole and tetracycline. Considering individual serovars, S. Infantis and S. Kentucky generally showed the highest resistance to ciprofloxacin and nalidixic acid across the poultry origins. This likely reflects the spread of resistant clones belonging to these serovars. From human data reported in 2020, S. Infantis and S. Kentucky also showed the highest resistance to these substances. Resistance to ciprofloxacin/nalidixic acid, sulfamethoxazole and tetracycline is typical of a clone of S. Infantis which is prevalent in Europe in broilers (Nógrády et al., 2012) and S. Infantis is a serovar commonly reported in the monitoring by some countries. Ciprofloxacin resistance was observed at equal high levels among isolates from broilers, turkeys and their derived carcases, and was generally very similar to nalidixic acid resistance. However, Salmonella isolates exhibiting ciprofloxacin resistance and nalidixic acid susceptibility were mostly evident in turkeys and their derived carcases, indicating the occurrence of plasmid‐mediated quinolone resistance (PMQR) mechanisms.
Resistance to third‐generation cephalosporins, cefotaxime and ceftazidime, in Salmonella isolates recovered from the carcases and animal populations monitored was either not discerned, or was generally detected at very low/low levels in most of the reporting MSs. Considering the total number of Salmonella isolates recovered from all carcase/animal origins, a low percentage demonstrated third‐generation cephalosporin resistance and were subjected to supplementary testing with a further panel of antimicrobials. Notably, no Salmonella isolates recovered from turkey carcases, or calves or their derived carcases, exhibited resistance to third‐generation cephalosporins.
Third‐generation cephalosporins and fluoroquinolones are highest priority CIAs for the treatment of human invasive salmonellosis (WHO, 2019). This sets the rationale for monitoring combined resistance to these antimicrobial classes within food‐producing animals. No Salmonella isolates recovered from turkey carcases, or calves and their derived carcases, displayed combined resistance to cefotaxime and ciprofloxacin, while, in the other animal/carcase origins, combined resistance to these antimicrobial classes was detected at overall very low or low levels. Notably, where cefotaxime and ciprofloxacin MICs were interpreted using CBPs, none of the isolates exhibited ‘clinical’ resistance to these compounds. From human cases in 2019, combined resistance to cefotaxime and ciprofloxacin was very low overall but significantly higher in S. Infantis and S. Kentucky with particularly high proportions among S. Infantis isolates from Italy and S. Kentucky isolates from the Netherlands (Malta reported data on less than 10 S. Kentucky isolates; therefore, the proportion of combined resistance was not calculated). The same observation was made in 2020, with Italy again reporting the highest proportion of combined resistance in S. Infantis.
From the monitoring of poultry in 2020, colistin resistance was generally observed in S. Enteritidis isolates. This serovar accounted for 33.3%, 52% and 60.2% of the colistin‐resistant isolates recovered from broiler carcases, broilers and laying hens, respectively. Considering the monitoring performed in 2019, all of the colistin‐resistant isolates reported from calves (n = 2) and six of the colistin‐resistant isolates from calf carcases (n = 16) were serotyped as S. Dublin. Both S. Enteritidis and S. Dublin are group D salmonellas (serogroup O9). This group tends to show decreased susceptibility to colistin without having any known acquired or mutational colistin resistance mechanisms and, therefore, show a degree of intrinsic resistance to colistin (Agersø et al., 2012; Ricci et al., 2020). Considering other serovars, colistin resistance was most frequently reported among S. Newport in turkeys (33.3%) and monophasic S. Typhimurium in pig carcases (50%). Only three colistin‐resistant isolates were reported from pigs of which one was S. Typhimurium and one its monophasic variant. A variety of different serovars from pig and calf carcases displayed colistin resistance, including three S. Derby isolates from pig carcases. In an Italian study, Carnevali et al. (2016) detected mcr‐1 in a number of Salmonella serovars, of which monophasic S. Typhimurium was the most frequent (isolates from pigs, pork and man) and S. Derby was the second most frequently found (isolates from pigs). Further molecular characterisation of colistin‐resistant isolates obtained from the EU AMR monitoring, to determine the underlying genetic mechanisms, would assist in identifying the emergence and dissemination of colistin‐resistant Salmonella clones and also identify colistin resistance plasmids occurring in Salmonella associated with livestock.
Where tigecycline resistance was reported among the carcase/animal origins, most isolates displayed MICs just above the epidemiological cut‐off, with only a small proportion of isolates displaying ‘clinical’ resistance. Certain serovars displayed ‘microbiological’ resistance to this antimicrobial, which may suggest clonal expansion of microbiologically resistant strains belonging to these serovars. Considering poultry, S. Infantis accounted for most of the resistant isolates recovered from broilers (60.9%) and all isolates from their derived carcases, as well as five (12.8%) tigecycline‐resistant isolates reported from turkeys. S. Bredeney accounted for most (71.8%) of the tigecycline‐resistant isolates recovered from turkeys. A low number of tigecycline‐resistant isolates were reported from calf carcases (n = 3, all S. Derby), while S. Typhimurium accounted for 40% of those recovered from pig carcases (n = 10). Multidrug resistance was often a feature of tigecycline‐resistant isolates.
Determining the susceptibility to tigecycline is not straightforward as this compound can be inactivated by oxidation and exposure to light, which may lead to falsely elevated MIC values. Upregulation of normal cell pathways or processes may also contribute to the occurrence of elevated tigecycline MIC values at levels above the ECOFF in Enterobacteriaceae (He et al., 2016). Two transferable plasmid‐mediated tigecycline resistance genes, tet(X3) and tet(X4), conferring higher levels of tigecycline resistance (MICs of ≥ 16 mg/L), have recently been reported in numerous Enterobacteriaceae from animals and meat (chicken and pork) in China (He et al., 2019; Bai et al., 2019). The potential for Salmonella to acquire such transferable tigecycline resistance genes is therefore highlighted, and the importance of monitoring tigecycline resistance through determination of MICs or by molecular investigation such as WGS is further underlined. Considering serovars in which markedly elevated tigecycline MICs were observed from the monitoring of food‐producing animals and their derived carcases, a single S. Typhimurium isolate exhibiting a tigecycline MIC of 8 mg/L was reported from a pig carcase by the Netherlands in 2019.
High‐level resistance to ciprofloxacin
High‐level resistance to ciprofloxacin (MIC ≥ 4 mg/L) was not detected among Salmonella isolates from pigs or calves, or calf carcases, but was observed among isolates from poultry and in a single isolate from a pig carcase (see Appendix A). Considering the total number of Salmonella isolates monitored from the different types of poultry by MSs in 2020, as well as pig carcases in 2019, high‐level resistance to this compound ranged from 0.1% in pig carcases to 13.9% in turkeys. While many serovars (including Bredeney, Derby, Infantis and Newport) were noted to exhibit resistance by this definition among poultry, S . Kentucky accounted for most of the Salmonella isolates recovered from the poultry origins and pig carcases which exhibited ciprofloxacin MICs of ≥ 4 mg/L. The same finding was also noted among isolates from human cases in 2020, where high‐level ciprofloxacin resistance was most commonly found in S. Kentucky (in 83.7% of S. Kentucky isolates). S. Kentucky isolates exhibiting high‐level ciprofloxacin resistance are likely to belong to the multilocus sequence type (ST) 198 clone, which has shown epidemic spread in North Africa and the Middle East (Le Hello et al., 2011,2013). Notably in 2020, the occurrence of this serovar exhibiting high‐level resistance was observed by eight MSs from most parts of Europe, suggesting further clonal expansion (S. Kentucky ST198‐X1) within poultry populations. Furthermore, a very high proportion of the poultry/pig S. Kentucky isolates displaying ciprofloxacin MICs of ≥ 4 mg/L were also multiresistant, primarily showing resistance to ampicillin, gentamicin, nalidixic acid, sulfamethoxazole and tetracycline. The same observation was also noted among S. Kentucky isolates from human cases in 2018 and 2020.
Phenotypic characterisation of third‐generation cephalosporin and carbapenemase resistance
The supplementary testing revealed the presence of isolates with an ESBL, AmpC or combined ESBL+AmpC phenotype. Particularly among poultry, the ESBL or AmpC phenotype was associated with multiple isolates belonging to certain serovars, suggesting the possible clonal expansion of particular strains: namely, S . Infantis, S . Kentucky, S . Bareilly and S . Bredeney. Among both broilers and turkeys, presumptive ESBL‐producing Salmonella were identified more frequently than presumptive AmpC‐producing Salmonella and encompassed a greater number of serovars. With the exception of one MS, presumptive ESBL, AmpC or ESBL+AmpC‐producers were identified at very low or low levels. Italy, however, reported the ESBL phenotype in 24.8% of Salmonella spp. from broilers and 26.5% of Salmonella spp. from turkeys; of which S. Infantis accounted for all of those from broilers (n = 30) and more than half of those from turkeys (7/13). Additionally, some of these isolates also possessed an AmpC phenotype. The proportion of presumptive ESBL/AmpC‐producers attributed to this serovar within broilers and turkeys in Italy suggests clonal expansion and spread among these animal populations in this country. The findings in poultry are interesting because there are no authorised products for use in the poultry sector in the EU which include third‐generation cephalosporins and off‐label use of third‐generation cephalosporins in poultry is not permitted (Franco et al., 2015).
Considering the monitoring performed in 2019, the ESBL or AmpC phenotype was detected in four serovars among porcine isolates: namely, S . Derby, S . Heidelberg, S . Rissen and monophasic S . Typhimurium. Among isolates from pig carcases, three displayed an ESBL phenotype (one S. Derby from Romania and two monophasic S. Typhimurium from Italy) and two displayed an AmpC phenotype (a monophasic S. Typhimurium from Czechia and S. Heidelberg from the Netherlands; the latter genotypically confirmed to possess bla CMY‐2 and displaying ‘microbiological’ resistance to fluoroquinolones). Interestingly, a recent Dutch study detected bla CMY‐2, as well as ciprofloxacin resistance, among isolates of S. Heidelberg originating from imported broiler meat from South America (van den Berg et al., 2019). bla CMY‐2 is also the dominating beta‐lactamase gene in S. Heidelberg in Canada (Carson et al., 2019). In pigs, a single isolate displayed an ESBL phenotype (a monophasic S. Typhimurium from Italy reported with an SHV family enzyme, type not specified) and two displayed an AmpC phenotype (single isolates of S. Rissen and monophasic S. Typhimurium from Italy).
In humans in 2019, presumptive ESBL‐producing Salmonella were identified in 0.8% of the tested isolates with the highest occurrence in Malta (2.7%). AmpC was less frequent (0.1%) and only five isolates were reported to be both AmpC‐ and ESBL‐producing. Of the 23 serovars identified with an ESBL phenotype from humans in 2019, it was most commonly found in S. Anatum, S. Infantis, S. Haifa, S. Kentucky, S. Schwarzengrund and S. Uganda (ranging between 4.5% and 8.3%). The proportion of S. Kentucky with an ESBL phenotype decreased from 20.3% in 2017 to 5.6% in 2019, with four countries (Germany, France, Malta and the Netherlands) reporting presumptive ESBL‐producing S. Kentucky in 2019. The introduction and spread of S. Kentucky with bla CTX‐M‐14b in Europe has recently been described by Coipan et al. (2020). AmpC‐type β‐lactamases were reported in 10 different serovars, most commonly in S. Bardo, S. Chincol and S. Heidelberg (ranging between 6.3% and 100%), although the high proportions observed were most likely an effect of the few isolates tested (N = 1–16). In 2020, presumptive ESBL‐producing Salmonella were identified in 0.6% of the not tested human isolates and AmpC in 0.2%. ESBL was reported in nine different serovars, most commonly in S. Infantis, S. Kentucky and S. Saintpaul (ranging between 3.0% and 5.0%). AmpC‐type β‐lactamases were reported in six different serovars, most commonly in S. Bredney, S. Kentucky and S. Thompson (ranging from 2.4% to 6.9%).
In both 2020 and 2019, no Salmonella spp. recovered from any of the carcase/animal origins were ‘microbiologically’ resistant to meropenem. In humans, however, meropenem resistance was detected via phenotypic screening in a single Salmonella isolate in 2019 and in two isolates in 2020. Two of the infections were domestically acquired and one was missing information on travel status. The carbapenemase genes identified were bla OXA‐48 (two isolates) and bla NDM‐1. The latter finding is suggested to have occurred via interspecies plasmid transfer in an immunocompromised host as the bla NDM‐1 gene was also identified in E. coli and C. freundii on the same 43 kb IncN2 plasmid, isolated from the same patient (Nielsen et al, 2020).
Multidrug resistance (MDR)
Multidrug resistance (MDR) varied between reporting countries and among the animal/carcase origins, with overall levels ranging from 6.3% in laying hens to 51.2% in broiler carcases. Considering all reporting countries (including non‐MSs), overall levels were higher in isolates from turkeys and broilers compared to that in isolates from laying hens. While an overall very high level of MDR was reported among isolates from broiler carcases, an overall moderate level was noted among isolates from turkey carcases. Similarly, MDR was higher among Salmonella spp. from pigs and their derived carcases to that noted among isolates from calves and their derived carcases. It should be noted, however, that the countries reporting Salmonella spp. data from these origins differed and the number of isolates reported by countries varied because of varying Salmonella prevalence; these factors may introduce a source of variation to results when considering all reporting countries. Furthermore, resistance levels varied among serovars which may exhibit particular MDR patterns, so the relative contribution of individual serovars within the different animal origins and between countries should be considered when comparing the situation between reporting countries. For example, the overall lower level of MDR among isolates from laying hens in comparison to those reported from broilers and turkeys most likely reflects in part the predominance of S. Enteritidis, which accounted for 30.6% of Salmonella isolates recovered from laying hens and where 83.3% of S. Enteritidis isolates exhibited complete susceptibility. Additionally, only a limited number of antimicrobial compounds are authorised for the treatment of laying hens in many EU countries, and this factor may also be reflected in overall AMR levels in Salmonella isolates from this sector.
Prevalence of particular Salmonella serovars and associated MDR patterns.
In summary, the prevalence of particular Salmonella serovars within countries and animal populations, and their associated patterns of resistance, are likely to explain many of the observed differences in the overall levels of antimicrobial resistance and multidrug resistance. The spread of resistant clones and the occurrence of resistance genes within these clones can be exacerbated by the use of antimicrobials in human and animal populations and the associated selective pressure. A detailed analysis of antimicrobial resistance at the serovar level, including possible underlying genetic structures responsible for resistance, is presented in Appendix C. Within a given MS, any attempt to relate the occurrence of AMR in human Salmonella isolates to that in isolates from food/food‐producing animals is complicated (see Appendix D), as much of the food consumed in an MS may have originated from other MSs or non‐member countries. Salmonella infections can also be associated with foreign travel, other types of animal contact (such as pets, including reptiles) or the environment. Additionally, some human infections may result from human to human transmission. To improve investigation of these relationships, human isolates from cases notified as having been acquired during travel outside of the reporting country were excluded from the analysis.
3. Antimicrobial resistance in Campylobacter spp.
3.1. Key findings
For 2020, 16 MSs and two non‐MS reported data on AMR in Campylobacter isolates from human cases of campylobacteriosis.
Regarding C. jejuni, the antimicrobial susceptibility of 3,382 isolates from broilers and 1,066 from turkeys in 2020 and 498 from calves in 2019 was reported by, respectively, 27, nine and four MSs
For C. coli, 388,467 and 1,174 isolates from broilers (seven MSs) and turkeys (three MSs) in 2020 and pigs (8 MSs) in 2019 were studied.
Resistance rates differed greatly between MSs.
Rates of resistance to ciprofloxacin were very high in C. jejuni and C. coli isolates from humans and very high to extremely high in C. jejuni and C. coli isolates from poultry, pigs and calves.
Resistance to erythromycin was either not detected or detected at very low levels in C. jejuni from humans, poultry and calves, but observed at higher levels in C. coli isolates from humans, pigs and poultry.
Overall, for C. jejuni, complete susceptibility (i.e. susceptibility to ciprofloxacin, erythromycin, gentamicin and tetracycline) was detected in, respectively, 31.3%, 23.0%, 16.8% and 24.7% of humans, broiler, turkey and calve isolates. For C. coli, the percentages of complete susceptibility were, respectively, 12.5%, 13.7%, 4.4% and 21.2% for humans, broiler, turkey and pig isolates.
Over 2016–2020, resistance to ciprofloxacin in C. jejuni from humans increased in nine countries, and resistance to erythromycin decreased in five countries. Similar trends were observed in C. jejuni from broilers over 2009–2020 where resistance to ciprofloxacin increased in 14 countries, and resistance to erythromycin decreased in six countries. Erythromycin resistance also decreased in C. jejuni from turkeys in three countries and in C. coli from pigs in four countries. Although the period assessed for the trend and the countries providing data differed for the two sectors, similar increasing and decreasing trends were often observed within a country in both humans and poultry, particularly regarding trends in increasing ciprofloxacin resistance.
Combined resistance to both ciprofloxacin and erythromycin, which are considered critically important for treatment of campylobacteriosis, was generally rare to low in C. jejuni from humans, poultry and calves, and low to moderate in C. coli from humans, poultry and pigs. Notably, moderate proportions of C. jejuni from poultry, moderate to high proportions of C. coli from poultry and pigs and in 2020, high proportions of C. coli from humans, were co‐resistant to ciprofloxacin and erythromycin in some countries.
3.2. Data on AMR in Campylobacter spp. addressed
In the framework of Commission Implementing Decision 2013/652/EU, the monitoring of AMR in Campylobacter spp. from food‐producing animals and food is focused on the species C. jejuni and C. coli.12 While the biennial monitoring and reporting of AMR in C. jejuni isolates recovered from caecal samples of broilers and fattening turkeys is mandatory (even years from 2014 to 2020), the monitoring of AMR in C. coli isolates recovered from food‐producing animals is performed on a voluntary basis. The main species responsible for human infections is C. jejuni, which is usually predominant in poultry, followed by C. coli (Jehanne et al., 2020) which is also frequently found in poultry, sometimes at higher rates than C. jejuni (Pergola et al., 2017), and in pigs. C. coli is more often resistant than C. jejuni to several important antimicrobials and may contain and transfer resistance genes to C. jejuni. Thus, MSs are encouraged to monitor AMR levels in C. coli.
Food‐producing animals are considered to be a major source of human campylobacteriosis through contamination of food products. However, wild birds, pets and environmental water may also play a role as potential modes of transmission (Moré et al., 2017; Szczepanska et al., 2017; EFSA, 2019).
While the monitoring of AMR in C. jejuni isolates recovered from caecal samples of broilers and fattening turkeys at slaughter was mandatory in 2020, the monitoring of AMR in Campylobacter isolates recovered from caecal samples of fattening pigs and calves (bovines under 1 year of age) at slaughter was performed on a voluntary basis during 2019. However, too few C. jejuni from pigs were reported, confirming the low prevalence of this species in pigs (Rossler et al., 2019), so they will not be included in this report. In addition, the voluntary monitoring of AMR in Campylobacter isolates recovered from meat samples (of broilers, turkeys, bovine and pigs) at retail, as well as C. coli isolates recovered from caecal samples of broilers and turkeys was performed by some MSs in 2019 and 2020.
In both 2019 and 2020, data for C. jejuni and C. coli from human cases were also reported. Only data for 2020 from humans are presented below as the 2019 data have been presented in the EU Summary report for 2018/2019 (EFSA and ECDC, 2021a).
3.3. Occurrence of antimicrobial resistance in Campylobacter from humans
3.3.1. Data reported
For 2020, 16 MSs and two non‐MS reported data on AMR in Campylobacter isolates from human cases of campylobacteriosis. This was three countries less than for 2019. Three countries were not able to report any result due to the impact of the COVID‐19 pandemic on referral of isolates and laboratory capacity, and the UK did not report data as they were no longer an EU/EEA country while Sweden reported Campylobacter AMR data for the first time. Eleven countries provided data as measured values (quantitative data), five as data interpreted with clinical breakpoints and one as resistance predicted from whole genome sequencing (WGS). Not all countries reported results for all antimicrobials in the harmonised panel (ECDC, 2016). The reported data represented 18.9% and 19.3% of the confirmed human cases with Campylobacter jejuni and Campylobacter coli, respectively, reported in the EU/EEA in 2020.
3.3.2. Occurrence of resistance
In 2020, very high to extremely high resistance levels to ciprofloxacin were reported in human Campylobacter jejuni isolates from all reporting countries with the exception of the Nordic countries where Denmark and Finland reported high levels and Iceland, Norway and Sweden reported moderate levels of resistance (Figure 13; Annex B, Section B.1: Table 1). For C. coli, 6 out of 11 countries reporting more than 10 isolates had levels of ciprofloxacin resistance of > 70–100% (Annex B, Section B.1: Table 2). The ciprofloxacin resistance at EU level was 61.2% and 65.8% for C. jejuni and C. coli, respectively. The proportion of human C. jejuni isolates resistant to erythromycin was very low overall at 0.7% except in one MS where it was moderate (Poland) but markedly higher in C. coli (10.0%), with high proportions (20.4–43.0%) of C. coli being resistant in three MSs (Finland, the Netherlands and Portugal). High (43.7%) and very high (74.0%) proportions of resistance to tetracycline were observed in C. jejuni (except in Norway where it was low) and C. coli, respectively. Countries reported low resistance levels to gentamicin. Similarly, low levels of resistance were observed to co‐amoxiclav except in one MS for C. coli where it was moderate (Luxembourg) (Annex B, Section B.1: Tables 1 and 2).
Figure 13.
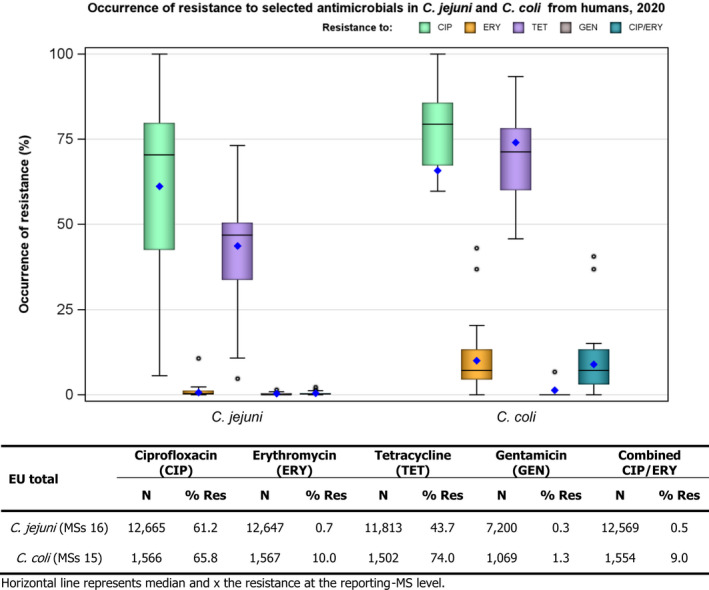
Occurrence of resistance to selected antimicrobials in C. jejuni and C. coli isolates from humans, 2020
3.3.3. Combined resistance to ciprofloxacin and erythromycin
Combined resistance to both ciprofloxacin and erythromycin, which are considered critically important for treatment of campylobacteriosis, was generally very low (0.5%) in C. jejuni and low (8.9%) in C. coli for 2020 (Figure 13). The highest levels of combined resistance were observed for C. coli, where two MSs (Finland and Portugal) reported high levels (36.8–40.6%) of combined resistance (Figure 14; Annex B, Section B.1: Tables 3 and 4).
Figure 14.
Combined resistance to the critically‐important antimicrobials ciprofloxacin and erythromycin in (a) C. jejuni and (b) C. coli isolates from humans
- Note: For Finland, travel information was missing from the AMR data while from the case surveillance data, travel‐associated cases were known to account for 49% of Finnish Campylobacter infections in 2020.
3.3.4. Complete susceptibility and multidrug resistance
Multidrug resistance (MDR) in isolates tested for four antimicrobial classes (fluoroquinolones, macrolides, tetracyclines and aminoglycosides) was overall very low in C. jejuni (0.3%) and low in C. coli (7.5%) (Figure 15, Annex B, Section B.1: Tables 5 and 6). Complete susceptibility to the four antimicrobial classes was 31.3% in C. jejuni and 12.5% in C. coli (Figure 15, Annex B, Section B.1: Tables 5 and 6). The most common resistance pattern in both C. jejuni and C. coli was resistance to both ciprofloxacin and tetracycline, observed in 38.1% of C. jejuni isolates and 45.9% of C. coli isolates. The second most common pattern in C. jejuni (in 31.3% of isolates) was complete susceptibility to the four antimicrobial classes in the harmonised panel while in C. coli it was tetracycline resistance alone (24.3%).
Figure 15.
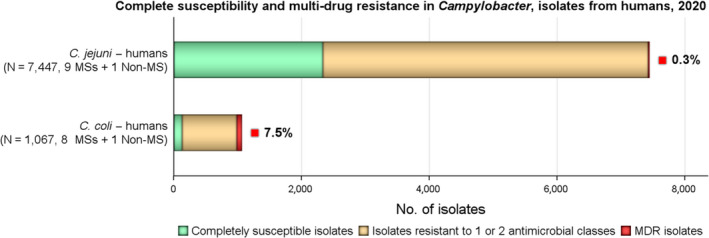
Number of MDR isolates, isolates resistant to 1 and/or 2 antimicrobials and completely susceptible Campylobacter isolates from humans in 2020
- N: Total number of isolates reported.
3.3.5. Temporal trends
Temporal trends were analysed for countries reporting data for at least 3 years over the 5‐year period 2016–2020. As the number of isolates reported by country was markedly lower in almost all countries in 2020 compared to previous years as an effect of the pandemic, it reduced the power of the statistical test. Statistically significant (p < 0.05) increasing trends of fluoroquinolone resistance were observed in C. jejuni in eight MSs and one non‐MS and in C. coli in one, while a decreasing trend was observed in three MSs and one non‐MS in both C. jejuni and C. coli (Table 5). Tetracycline resistance increased significantly in three MSs and one non‐MS for C. jejuni while five MSs and one non‐MS observed a decrease in the same period. Erythromycin resistance in C. jejuni decreased in four MSs and one non‐MS while for C. coli, two MSs observed an increasing trend while two other MSs observed a decreasing trend. For country specific trend graphs, please see Annex B, Section B.1: Figures 1 and 2.
Table 5.
Number of countries with significantly increasing or decreasing trends in resistance to selected antimicrobials for Campylobacter jejuni and C. coli in humans, 2016–2020
| Species | Ciprofloxacin | Erythromycin | Tetracycline | |||
|---|---|---|---|---|---|---|
| Incr. | Decr. | Incr. | Decr. | Incr. | Decr. | |
| C. jejuni (16 MS + 3 non‐MS) | 9 (DK, ES, FR, LT, MT, NL, SI, SK, UK) | 4 (EE, FI, NO, PT) | – | 5 (FI, LT, MT, NO, UK) | 4 (DK, NL, SK, UK) | 6 (CY, EE, FI, LT, NO, PT) |
| C. coli (14 MSs + 1 non‐MS) | 1 (SI) | 4 (FR, LU, PT, UK) | 2 (FI, NL) | 2 (EE, SK) | – | – |
3.3.6. High‐level resistance to erythromycin
High‐level resistance to erythromycin (MIC > 128 mg/L) was assessed as a possible indication for transferrable erythromycin resistance due to the presence of the erm(B) gene. In C. jejuni, 0.1% of the isolates (N = 1,302, six MSs + one non‐MS) had MIC > 128 mg/L while in C. coli this proportion was higher, 1.9% (N = 106, four MSs + one non‐MS) (Figure 16). This was significantly lower than compared to 2019 (1.0% for C. jejuni and 9.1% for C. coli). Similarly, in 0.4% (N = 2,628, six MSs) of C. jejuni and 23.9% (N = 230, six MSs) of C. coli tested with disk diffusion, no inhibition zone could be observed (6 mm zone equals the disk size), which corresponds to an MIC of ≥ 128 mg/L for C. jejuni and 64–≥ 128 mg/L for C. coli (EUCAST, 2020).
Figure 16.
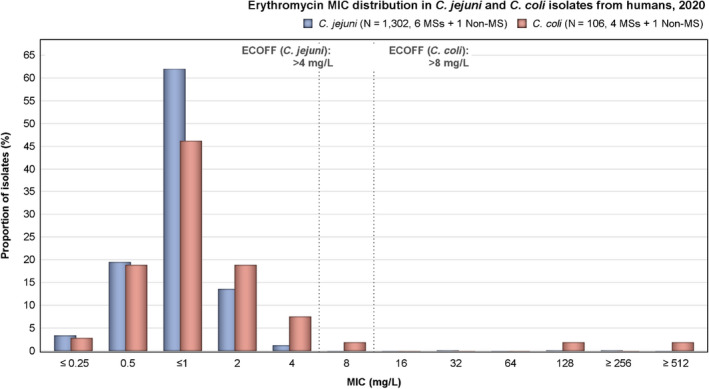
Erythromycin MIC distribution in C. jejuni and C. coli isolates from humans, 2020
- Note: Not visible in the graph due to a very small proportion are three isolates of C. jejuni at MIC 32, 128 and ≥ 256 mg/L.
3.4. Occurrence of antimicrobial resistance in Campylobacter from food‐producing animals and meat thereof
3.4.1. Data reported
In the present report, the 2020 resistance data on Campylobacter from poultry are considered for the comparison with the AMR data on Campylobacter isolates from pigs and calves reported in 2019.
In 2020, all 27 MSs and three non‐MSs (Norway, Switzerland and the United Kingdom) reported mandatory data on C. jejuni isolates recovered from caecal samples of broilers (N = 3,382 and N = 441, respectively), and nine MS (Austria, France, Germany, Hungary, Italy, Poland, Portugal, Romania and Spain) and two non‐MS (Norway and the United Kingdom) reported data on C. jejuni isolates recovered from caecal samples of fattening turkeys (N = 1,066 and N = 174, respectively) (Annex B, Section B.2: Table 1). Additionally, seven MSs (Czechia, France, Ireland, Latvia, Luxembourg, the Netherlands and Slovenia) and one non‐MS (Switzerland) voluntary reported data on C. coli isolates recovered from caecal samples of broilers (N = 388 and N = 68, respectively), and three MSs (France, Germany and Spain) reported data on C. coli isolates recovered from caecal samples of fattening turkeys (N = 567). Several countries voluntarily reported data derived from various categories of samples, including mainly data on C. jejuni recovered from meat from broiler carcases (4 MS: Croatia, Germany, the Netherlands and Romania, N = 361; and Iceland, N = 4) (Annex B, section B.2, Table 1) or fresh broiler meat (5 MS: Austria, Belgium, Germany, Luxembourg and the Netherlands, N = 343; and Switzerland, N = 112) and C. coli isolated from meat from broiler carcases (four MS: Germany, Luxembourg, the Netherlands and Romania, N = 76), and fresh meat from broilers (five MS: Austria, Belgium, Germany, Luxembourg and the Netherlands, N = 99; and Switzerland, N = 16).
In 2019, eight MS (Czechia, Estonia, Germany, Ireland, Luxembourg, Slovenia, Spain and Sweden) and three non‐MS (Norway, Republic of North Macedonia, Switzerland) voluntarily reported AMR monitoring data on C. coli isolates recovered from caecal samples of fattening pigs (N = 1,655), and two MS (Germany and Spain) reported other AMR monitoring data on C. coli from caecal samples of calves (bovines under 1 year of age) (N = 67). Four MSs (Denmark, Germany, Italia and Spain) reported other AMR monitoring data on C. jejuni from caecal samples of calves (N = 498). In addition, 14 isolates of C. jejuni from pigs were reported by three MS (Czechia, Germany and Spain), but the number was too low and not considered in the analysis.
The overview of the data reported in 2019 and 2020 is presented in Annex B (Section B.2: Tables 1 and 2). In addition, some countries voluntary reported data on isolates from non‐legislative categories including animals, milk, meat and vegetables (Annex B, Section B.2: Tables 3 and 4).
Resistance data concerning food‐producing animals and meat thereof reported in 2019 and 2020 are presented in the following sections when more than 50 isolates originating from at least two MSs had been reported (unless differently stated in the text, i.e. data used for trend analysis). Additional tables on Campylobacter in food and food‐producing animals have been included in Annex B that is available as supporting documentation in zenodo (https://doi.org/10.5281/zenodo.6257446).
3.4.2. Occurrence of resistance in meat samples of broilers
In 2019–2020, the highest levels of resistance in broiler meat were noted for ciprofloxacin and nalidixic acid (overall percentages: 64.2–90.0% in 2019, 71–84.8% in 2020), and for tetracycline (overall percentages: 42.9–52.8% in 2019, 57.6–67.7% in 2020) regarding the 1,400 isolates (1225 C. jejuni and 175 C. coli) from all nine reporting MSs. Generally, most MSs reported high to extremely high levels of resistance to these antimicrobials in Campylobacter isolates. Resistance to gentamicin in C. jejuni isolates recovered from poultry meat was not observed and resistance to erythromycin was detected in only three C. jejuni isolates from fresh meat. Resistance to streptomycin was globally observed at low to high levels (Table 6).
Table 6.
Occurrence of resistance (%) to selected antimicrobials in Campylobacter jejuni and C. coli from carcases, fresh meat and meat preparation from broilers, using harmonised ECOFFs, in 2019 and 2020 (only MSs)
| Species | Categories | Year | Number of isolate | Reporting countries (N) | GEN | STR | CIP | ERY | TET | CIP/ERY | CS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| C. jejuni | Broiler carcases | 2019 | 70 | HR, LU, NL, PT, RO (5) | 0.0 | 7.2 | 90.0 | 0.0 | 42.9 | 0.0 | 8.6 |
| 2020 | 361 | HR, DE, NL, RO (4) | 0.0 | 23.8 | 82.8 | 0.5 | 57.6 | 0.3 | 13.85 | ||
| Fresh broiler meat | 2019 | 398 | AT, BE, DK, LU, NL, PT (6) | 0.0 | 8.3 | 68.6 | 0.25 | 44.1 | 0.25 | 30.10 | |
| 2020 | 343 | AT, BE, DE, LU, NL (5) | 0.0 | 22.2 | 74.9 | 0.0 | 58.6 | 0.0 | 21.9 | ||
| Broiler meat preparations | 2019 | 53 | BE, LU, NL (3) | 0.0 | 15.1 | 64.2 | 0.0 | 52.8 | 0.0 | 28.3 | |
| C. coli | Fresh broiler meat | 2020 | 99 | AT, BE, DE, LU, NL (5) | 1.0 | 16.2 | 84.8 | 10.1 | 67.7 | 10.1 | 5.05 |
| Broiler carcases | 2020 | 76 | DE, LU, NL, RO (4) | 2.6 | 7.9 | 71.0 | 10.5 | 57.9 | 10.5 | 21.05 |
GEN: gentamicin, STR: streptomycin, NAL: nalidixic acid, CIP: ciprofloxacin, ERY: erythromycin, TET: tetracycline, CIP/ERY: combined ‘microbiological’ resistance to ciprofloxacin and erythromycin, CS: complete susceptibility to the four antimicrobial classes (ciprofloxacin/nalidixic acid, erythromycin, tetracycline and gentamicin/streptomycin), N: Total number of reporting Member States (MSs).
3.4.3. Occurrence of resistance in poultry, pigs and calves
Comparison of resistance levels between bacterial and animal species should be interpreted cautiously because of the dispersion of resistance rates between countries and because numbers of isolates and reporting countries vary, particularly for voluntary reporting.
Data on the occurrence of resistance in Campylobacter species from poultry (2020), pigs (2019) and cattle (2019) are presented in Table 7 and Figure 17 (the detailed country‐level information on the occurrence of resistance is presented in Annex B, section B.2: Tables 5–10).
Table 7.
Occurrence of resistance (%) to selected antimicrobials in Campylobacter coli and C. jejuni from broilers, fattening turkeys, from cattle and pigs, using harmonised ECOFFs, 27 EU MSs, 2019 and 2020
| Species | Category | Year | N. of. isolates | Reporting countries (N) | GEN | STR | NAL | CIP | ERY | TET | CIP/ERY | CS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
C. jejuni |
Broilers | 2020 | 3,382 | AT, BE, BG, CY, CZ, DE, DK, EE, ES, FI, FR, EL, HR, HU, IE, IT, LT, LU, LV, MT, NL, PL, PT, RO, SE, SI, SK (27) | 0.1 | 15.6 | 69.2 | 72.8 | 0.8 | 52.7 | 0.7 | 23.0 |
| Fattening turkeys | 2020 | 1,066 | AT, DE, FR, HU, IT, PL, PT, RO, ES (9) | 0.1 | 11.4 | 71.0 | 77.9 | 0.8 | 58.5 | 0.75 | 16.8 | |
| Cattle | 2019 | 498 | DK, DE, IT, ES (4) | 2.2 | 14.1 | 56.0 | 56.8 | 0.0 | 65.3 | 0.0 | 24.7 | |
| C. coli | Broilers | 2020 | 388 | CZ, FR, IE, LV, LU, NL, SL (7) | 0.0 | 16.5 | 61.1 | 61.9 | 4.4 | 67.3 | 4.1 | 13.7 |
| Fattening turkeys | 2020 | 567 | DE, ES, FR (3) | 0.2 | 11.5 | 80.2 | 80.4 | 21.5 | 89.2 | 21.2 | 4.4 | |
| Cattle | 2019 | 67 | DE, ES (2) | 10.5 | 65.7 | 80.6 | 80.6 | 23.9 | 94.0 | 22.4 | 1.5 | |
| Pigs | 2019 | 1,174 | CZ, DE, EE, ES, IE, LU, SI, SE (8) | 1.8 | 70.0 | 51.9 | 52.4 | 11.2 | 62.8 | 8.0 | 21.2 |
GEN: gentamicin, STR: streptomycin, NAL: nalidixic acid, CIP: ciprofloxacin, ERY: erythromycin, TET: tetracycline, CIP/ERY: combined ‘microbiological’ resistance to ciprofloxacin and erythromycin, CS: complete susceptibility to the four antimicrobial classes (ciprofloxacin/nalidixic acid, erythromycin, tetracycline and gentamicin/streptomycin), N: Total number of reporting Member States (MSs).
Figure 17.
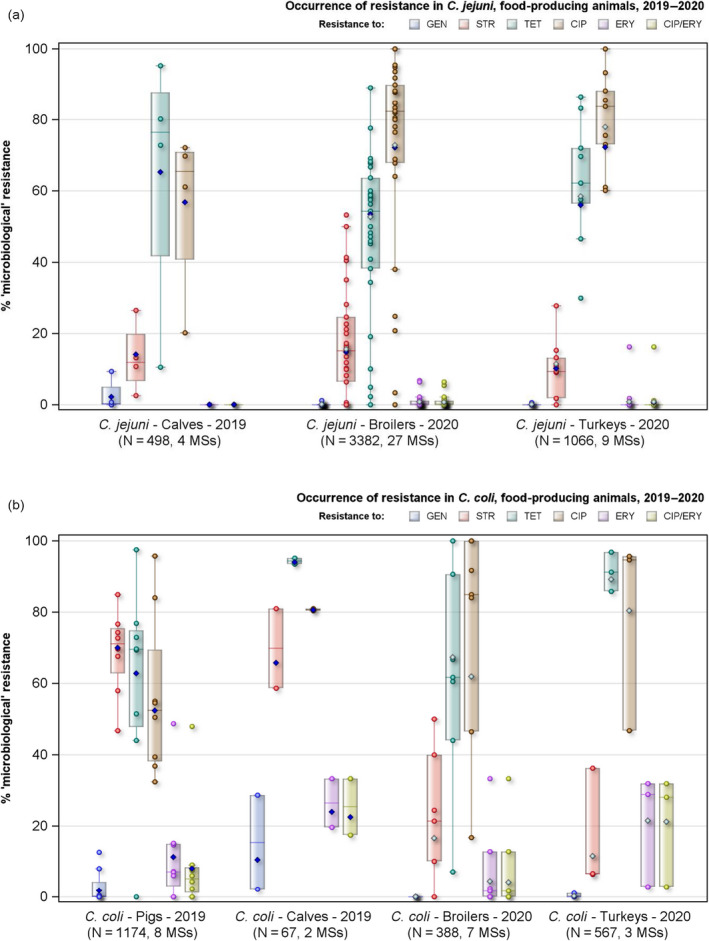
Occurrence of resistance to selected antimicrobials in (a) C. jejuni isolates from calves, broilers and fattening turkeys and (b) C. coli from fattening pigs, calves, broilers and fattening turkeys in reporting EU MSs, 2019–2020
- GEN: gentamicin; STR: streptomycin; TET: tetracycline; CIP: ciprofloxacin; ERY: erythromycin; CIP/ERY: combined ‘microbiological’ resistance to ciprofloxacin and erythromycin; N: Total number of isolates reported by all Member States (MSs); Horizontal line represents median; Blue diamond: resistance at reporting MS group level (including UK); Green diamond: resistance at reporting MS group level (excluding UK, 2020).
Generally, tetracycline resistance ranged from very high to extremely high in each of the animal species considered; overall, the highest level of resistance was noted in C. coli isolates recovered from cattle in 2019 (94.0%, for two MSs only) and fattening turkeys in 2020 (89.2%, for three MSs), the overall levels of the other categories (C. jejuni from poultry in 2020 and calves in 2019 and C. coli from broilers in 2020 and pigs in 2019) ranged from 52.7% to 67.3%. The highest levels of resistance to streptomycin were noted in C. coli isolates recovered from fattening pigs and calves in 2019 (overall, 70.0% and 65.7%, respectively), whereas moderate overall levels (11.4–16.5%) were obtained for C. coli and C. jejuni from broilers and fattening turkeys in 2020 and C. jejuni from calves in 2019.
In 2020, resistance to gentamicin in C. jejuni and C. coli isolates from broilers and fattening turkeys was detected at very low levels in (0.0–0.2%). In 2019, the overall levels were around 2% for C. jejuni from cattle and C. coli from pigs, and reached 10.5% for C. coli from cattle in the two MSs reporting for this category.
Overall resistance to ciprofloxacin and nalidixic acid was very high to extremely high in C. jejuni and C. coli isolates from poultry in 2020, and pigs and calves in 2019. The highest rates (close to 80%) were recorded for C. coli from fattening turkeys and cattle and C. jejuni isolated from fattening turkeys, those for C. jejuni and C. coli isolates obtained from broilers ranged from 61.1% to 72.8%. Resistance levels to these antimicrobials were generally lower in C. jejuni from calves (overall, 56.8% for ciprofloxacin and 56.0% for nalidixic acid) and C. coli isolates recovered from fattening pigs (52.4% for ciprofloxacin and 51.9% for nalidixic acid).
Among C. jejuni from poultry in 2020 and calves in 2019, erythromycin resistance was either not discerned (C. jejuni from cattle, four MSs) or detected at very low levels by most reporting MSs (overall 0.8% for broilers and fattening turkeys). Generally, erythromycin resistance was observed at higher levels in C. coli isolates recovered from calves (overall, 23.9%), followed by fattening turkeys (overall, 21.5%), fattening pigs (overall, 11.2%) and broilers (overall, 4.4%).
3.4.4. Combined resistance to ciprofloxacin and erythromycin
The occurrence of Campylobacter isolates displaying combined resistance to ciprofloxacin and erythromycin is of great importance to public health, since both compounds are recognised as critically important antimicrobials (CIA) for the treatment of Campylobacter infections in humans (WHO, 2019).
Overall combined resistance to these antimicrobials was detected in 22.4% of C. coli recovered from calves (15/67), 21.2% of C. coli isolates from fattening turkeys (120/567), 8.0% of C. coli from pigs (94/1174), 4.1% of C. coli isolates from broilers (16/388), 0.7% of C. jejuni isolates from broilers (24/3,382), 0.7% of C. jejuni isolates from fattening turkeys (8/1,066) and 0% in C. jejuni isolates from calves (0/498) (Table 7).
Combined resistance to both ciprofloxacin and erythromycin in C. jejuni from broilers was detected in 7 out of 27 reporting MSs in 2020. Among those countries recording combined resistance to ciprofloxacin and erythromycin in C. jejuni from broilers, the percentages of combined resistance ranged from 1.1% to 6.4% (Figure 18a). For C. coli from broilers, four out of seven MSs reported isolates resistant to both erythromycin and ciprofloxacin, at levels of 1.7–33.3% (Figure 19a).
Figure 18.
Spatial distribution of combined resistance to ciprofloxacin and erythromycin in Campylobacter jejuni isolates from (a) broilers (27 EU MS and 3 non‐MS, 2020); (b) fattening turkeys (nine EU MSs and two non‐MS, 2020)
Figure 19.
Spatial distribution of combined resistance to ciprofloxacin and erythromycin in Campylobacter coli isolates from (a) broilers (7 EU MS and 1 non MS, 2020); (b) fattening pigs 8 EU MS and 3 non MS, 2019)
Out of nine reporting MSs in 2020, combined resistance to both ciprofloxacin and erythromycin in C. jejuni from fattening turkeys was detected in Italy and Portugal with resistance in 1.2%, and 16.2% of the isolates tested, respectively (Figure 18b). Combined resistance was detected in the three MSs reporting data on C. coli from fattening turkeys (Figure 19b).
Considering the four MS reporting data for a total of 498 C. jejuni from calves in 2019, no C. jejuni isolate was found to be resistant to the two important therapeutic compounds, erythromycin and ciprofloxacin. However, for C. coli isolated from calves, eight isolates from Germany (8/46, 17.4%) and seven isolates from Spain (7/21, 33.3%) were resistant to ciprofloxacin and erythromycin, Germany and Spain being the only two MSs reporting on C. coli from calves.
Considering the eight MSs reporting data on C. coli from pigs in 2019, 94 out of 1174 isolates (8.0%) had combined resistance to both ciprofloxacin and erythromycin. The highest rate was found in Spain (57/119, 47.1%) (Figure 19b).
Maps are presented only if data are reported by at least five countries (Figures 18 and 19).
Detailed data on combined resistance to ciprofloxacin and erythromycin in C. jejuni and C. coli isolates from food‐producing animals are presented in Annex B (Section B.2, Tables 11–14).
3.4.5. Complete susceptibility and multidrug resistance
Overall complete susceptibility to the four antimicrobial classes (ciprofloxacin/nalidixic acid, erythromycin, tetracycline and gentamicin), was around 20–25% for C. jejuni recovered from broilers (23.0%) and calves (24.7%) and C. coli recovered from pigs (21.2%). The proportions were lower for C. jejuni isolated from fattening turkeys (16.8%) and C. coli isolated from broilers (13.7%). Only 4.4% of C. coli from fattening turkeys and 1.5% of C. coli from calves were susceptible to the four antimicrobial classes. Marked differences could be detected between countries with, e.g. percentages of complete susceptibility in C. jejuni isolated from broilers ranging from < 5% in Greece, Latvia, Poland and Portugal to > 75% in Finland, Sweden and Norway.
The complete susceptibility to the targeted antimicrobial classes and the levels of MDR, defined as resistance to three or more antimicrobial classes of the harmonised panel tested, among Campylobacter isolates recovered from these food‐producing animals by MSs are shown in Figure 20.
Figure 20.
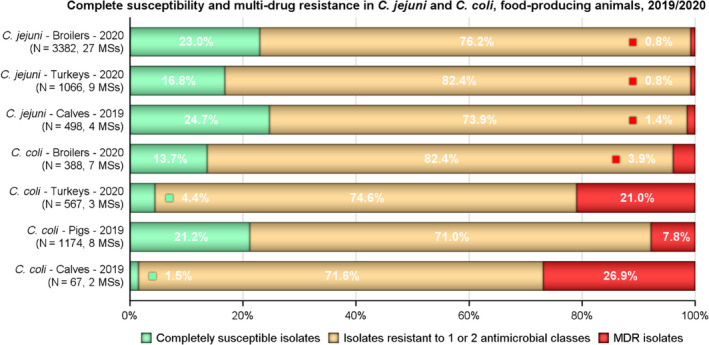
Proportions of isolates completely susceptible, resistant to one or two antimicrobial classes and MDR in C. jejuni and/or C. coli from broilers, fattening turkeys, fattening pigs and calves, in reporting EU MSs, 2019–2020
-
N: Total number of isolates reported by the EU MSs.Complete susceptibility is defined as susceptibility to ciprofloxacin/nalidixic acid, erythromycin, gentamicin and tetracycline. MDR is defined as resistance to at least three antimicrobial classes (panel of antimicrobial tested: ciprofloxacin, nalidixic acid, erythromycin, gentamicin, tetracycline).
Overall, MDR was observed at low levels in C. jejuni isolates recovered from broilers (0.8%), fattening turkeys (0.8%) and calves (1.4%). MDR was detected in 3.9% and 7.8% of the C. coli isolates recovered from broilers and fattening pigs, respectively. The highest levels of MDR were observed in C. coli recovered from fattening turkeys (21.0%) and calves (26.9%).
Detailed data on complete susceptibility and MDR in C. jejuni and C. coli isolates from food‐producing animals are presented in Annex B (section B.2, Tables 11–14).
3.4.6. Temporal trends
Temporal trends in resistance in C. jejuni and C. coli from broilers
Temporal trends in resistance in C. jejuni from broilers over the period 2009–2020 was analysed for 24 reporting EU MSs and 4 non‐MS (see Figure 21 and Table 8; see also Annex B, section B.2, Table 15). Evaluation of temporal trends in resistance was performed only for countries who reported data for at least 3 years (three data points). Countries that reported only data for years before 2015 were not considered in the analysis because the evaluation of trend was not considered informative of the recent situation. A significant increase in resistance to ciprofloxacin was reported in 12 MSs (Austria, Croatia, Cyprus, Czechia, Denmark, Finland, France, Germany, the Netherlands, Romania, Slovakia and Sweden) and two non‐MS (Switzerland and the United Kingdom, whereas a significant decrease was detected in Spain. An increase in resistance was detected for streptomycin in 13 MSs and one non‐MS, and for tetracycline in nine MSs and two non‐MSs. A decrease in resistance was detected in erythromycin, streptomycin and tetracycline in six, six and seven MSs, respectively.
Figure 21.
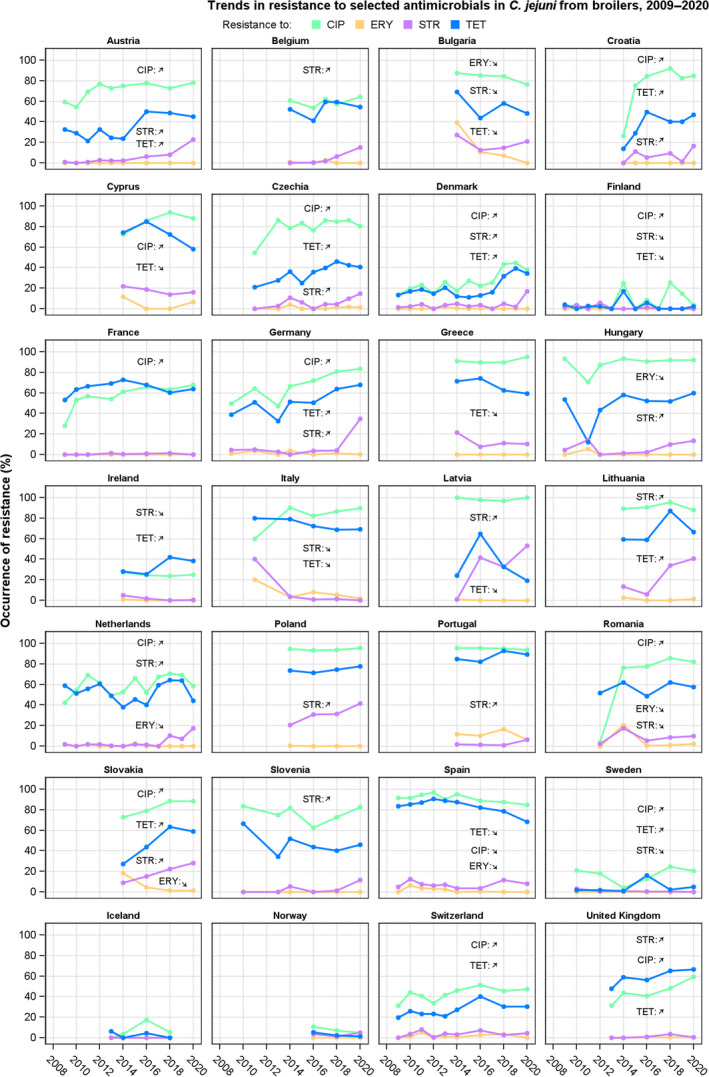
Trends in ciprofloxacin (CIP), erythromycin (ERY), streptomycin (STR) and tetracycline (TET) resistance in C. jejuni from broilers, 2009–2020
- CIP: ciprofloxacin; ERY: erythromycin; STR: streptomycin; TET: tetracycline. Arrows indicate significant increasing (up) or decreasing (down) significant trend over the entire period. Please note that between‐year fluctuation in the occurrence resistance (%) may not be captured in the overall evaluation of the trend for the entire period (2009–2020).
Table 8.
Number of countries with significantly increasing or decreasing trends in resistance to selected antimicrobials for Campylobacter jejuni and C. coli from humans, 2016–2020, broilers, 2009–2020, fattening turkeys 2014–2020 and pigs, 2009–2020
| Species | Ciprofloxacin | Erythromycin | Tetracycline | ||||
|---|---|---|---|---|---|---|---|
| Incr. | Decr. | Incr. | Decr. | Incr. | Decr. | ||
| Humans | C. jejuni (16 MS + 3 non‐MS) | 9 (DK, ES, FR, LT, MT, NL, SI, SK, UK) | 4 (EE, FI, NO, PT) | – | 5 (FI, LT, MT, NO, UK) | 4 (DK, NL, SK, UK) | 6 (CY, EE, FI, LT, NO, PT) |
| C. coli (14 MSs+1 non‐MS) | 1 (SI) | 4 (FR, LU, PT, UK) | 2 (FI, NL) | 2 (EE, SK) | – | – | |
| Broilers |
C. jejuni (24 MS + 4 non‐MS) |
14 (AT, DE, HR, CY, CZ, DK, FI, FR, NL, RO, SE, SK, CH, UK) |
1 (ES) |
– |
6 (BG, HU, NL, RO, SK, ES) |
11 (AT, HR, CZ, DK, DE, IE, LT, SK, SE, CH, UK) |
7 (BG, CY, FI, EL, IT, LV, ES) |
|
C. coli (8 MS, 1 non‐MS) |
3 (HR, DE, NL) |
1 (FR) |
1 (CZ) |
3 (AT, FR, NL) |
4 (CZ, DE, HR, CH) |
– | |
| Turkeys |
C. jejuni (8 MS, 1 non‐MS) |
2 (DE, PL) |
2 (HU, IT) |
3 (DE, PL, ES) |
1 (HU) |
4 (DE, ES, FR, UK) |
|
| Pigs |
C. coli (7 MS + 2 non‐MS) |
3 (DE, NO, CH) | 4 (HR, ES, NL, CH) | 1 (CH) | 1 (HR) | ||
Trends in C. coli from broilers were evaluated in only eight MSs (Austria, Croatia, Czechia, France, Germany, the Netherlands, Slovenia, Spain) and one non‐MS (Switzerland) (Figure 22 and Table 4 see also Annex B (section B.2): Table 15). Increases of resistance were observed for ciprofloxacin (three MSs), erythromycin (one MS), streptomycin (one MS) and tetracycline (three MSs, one non‐MS), whereas decreases were observed for ciprofloxacin (one MS), erythromycin (three MSs) and streptomycin (two MSs).
Figure 22.
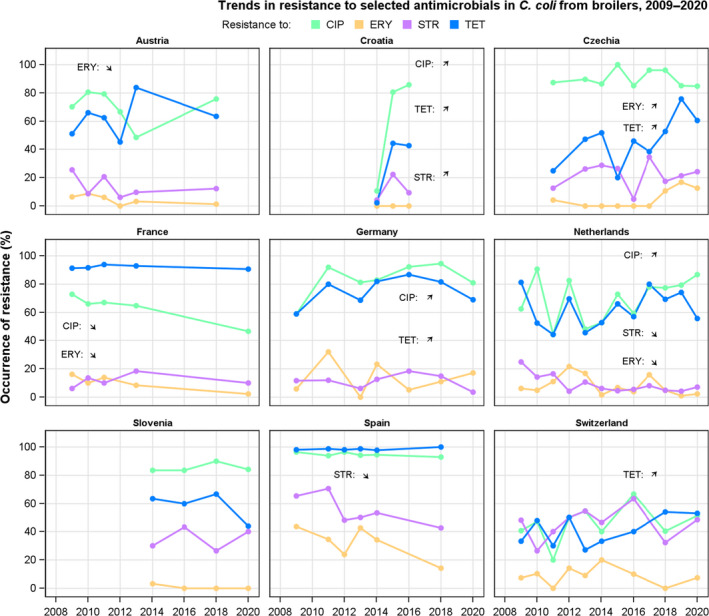
Trends in ciprofloxacin (CIP), erythromycin (ERY), streptomycin (STR) and tetracycline (TET) resistance in C. coli from broilers, 2009–2020
It is important to note that between‐year fluctuations in the occurrence resistance (%) may not be captured in the evaluation of the trend for the entire time period (2009–2020) and that very recent decreasing or increasing trends may therefore be masked by the overall trend.
Temporal trends in resistance in C. jejuni from turkeys
The comparison of resistance in C. jejuni isolates from fattening turkeys referred to the period of 2014–2020 is shown in Figure 23 and Table 8, see also Annex B: section B.2, Table 15). Significant increasing trends in resistance to ciprofloxacin between 2014 and 2020 were detected in Germany, and Poland, whereas a significant decreasing trend was recorded in Hungary and Italy. For resistance to tetracycline, significant decreasing trends were observed in France, Germany, Spain and the United Kingdom, and an increasing trend was detected in Hungary. Resistance to erythromycin showed a decreasing trend in Germany, Poland and Spain, and resistance to streptomycin displayed an increasing trend in Austria, Germany, Hungary, Poland and Portugal. These trends are based only on 3‐year data points, with data only on even years, and thus, for a more robust evaluation, need further follow‐up in the future.
Figure 23.
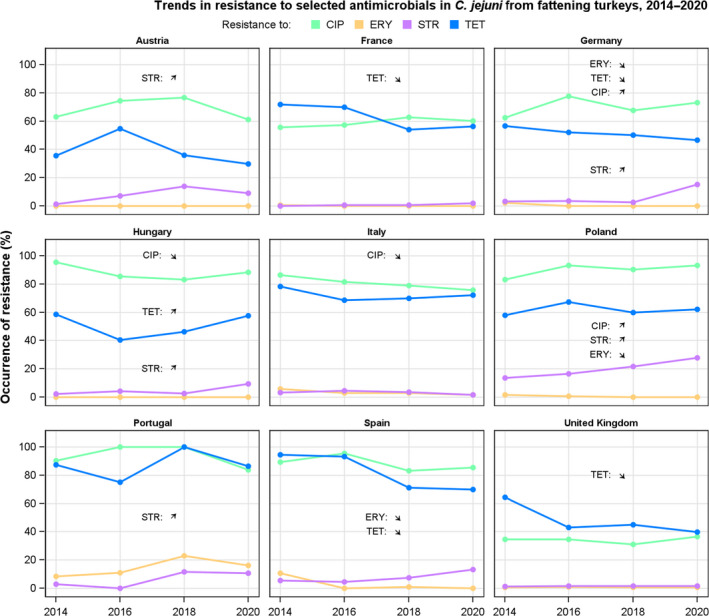
Trends in streptomycin (STR), ciprofloxacin (CIP), erythromycin (ERY), and tetracycline (TET) resistance in C. jejuni from fattening turkeys, 2014–2020
Temporal trends in resistance in C. coli from pigs
Trends in C. coli from pigs were evaluated for seven MSs and two non‐MSs who reported at least three data points (minimum 10 isolates per year). Only the Netherlands reported data for C. coli isolated from pigs in 2020 (Table 8; see also Annex B, Section B.2: Figure 3 and Table 15). Countries that reported only data for years before 2015 were not considered in the analysis because the evaluation of trend was not considered informative of the recent situation. Increases of resistance were observed for ciprofloxacin (Germany, Norway and Switzerland), streptomycin (Croatia, Norway and Switzerland) and tetracycline (Switzerland), whereas decreases were observed for erythromycin (Croatia, the Netherlands, Spain and Switzerland), tetracycline (Croatia) and streptomycin (Czechia and Sweden).
3.4.7. High‐level resistance to erythromycin
While erythromycin resistance was reported overall at very low, low and moderate levels in Campylobacter spp. recovered from caecal samples of the food‐producing animals, isolates displaying MICs > 128 mg/L were detected (Figure 24). Notably, an erythromycin MIC of > 128 mg/L exceeds the highest concentration tested, in accordance with the harmonised method set out in Decision 2013/652/EU. Figure 25 illustrates the proportion of isolates reported by MSs and non‐MSs solely displaying ‘microbiological/clinical’ erythromycin resistance (C. jejuni: MIC > 4 mg/L; C. coli: MIC > 8 mg/L) in comparison to those displaying high‐level erythromycin resistance (MICs > 128 mg/L) within each of the animal categories. Six of 10 (60.0%) and 14 of 27 (51.9%) erythromycin‐resistant C. jejuni isolates from fattening turkeys and broilers, respectively, exhibited an MIC of > 128 mg/L in 2020. Regarding C. coli isolated from fattening turkeys, pigs and broilers, the percentages of erythromycin high level‐resistant isolates among the erythromycin‐resistant isolates were, respectively, 93.4% (114/122), 79.3% (111/140) and 40.9% (9/22). Most high‐level resistant C. jejuni isolates from broilers were detected in Romania (seven isolates) and Cyprus (four isolates). Four of the six erythromycin high‐level resistant C. jejuni isolates from fattening turkeys were isolated in Portugal, and the nine high‐level erythromycin resistant C. coli isolates from broilers were reported by France, Switzerland and the Netherlands. In pigs, 48.7% of the high‐level erythromycin‐resistant C. coli isolates were reported by Spain. A high level of erythromycin resistance (MIC > 128 mg/L) was also reported in 15 C. coli isolates from calves out of 16 isolates exhibiting erythromycin resistance reported by Germany and Spain.
Figure 24.
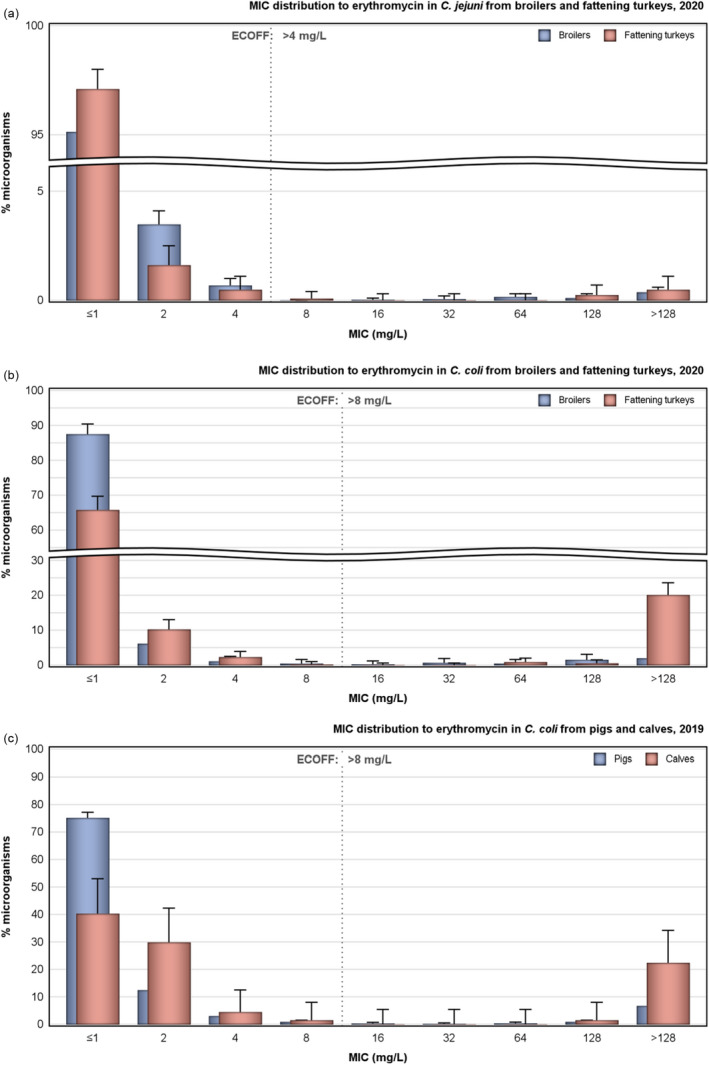
MICs of Campylobacter jejuni isolates exhibiting erythromycin resistance in (a) broilers and fattening turkeys, and C. coli in (b) broilers and fattening turkeys and (c) fattening pigs and calves, in reporting EU MSs and non‐EU MSs, 2020 and 2019
Figure 25.
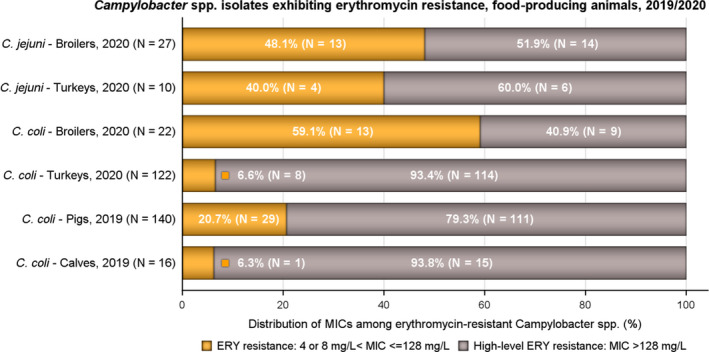
MICs of Campylobacter spp. isolates exhibiting erythromycin resistance in broilers, fattening turkeys, pigs and calves in reporting EU MSs and non‐EU MSs, 2019–2020
-
N: Total number of C. jejuni or C. coli isolates exhibiting erythromycin resistance. ERY: erythromycin.ERY resistance in C. jejuni isolates: 4 mg/L < MIC < 128 mg/L. ERY resistance in C. coli isolates: 8 mg/L < MIC < 128 mg/L.
A high‐level of resistance to macrolides, lincosamides and/or streptogramin B antibiotics in Campylobacter may be associated with the erm(B) gene encoding an rRNA methylase, as described first in a C. coli isolated from a swine in China (Qin et al., 2014). Erm(B) is detected more frequently in C. coli than in C. jejuni but it may also be found on C. jejuni from poultry resistant to all clinically important antimicrobial agents (Liu et al., 2019). In Europe, erm(B) has been reported in C. coli from broilers and turkeys in Spain and from a broiler isolate in Belgium (Florez‐Cuadrado et al., 2017; Elhadidy et al., 2019; Mourkas et al., 2019). It was also detected in Australia, in faecal samples from two travellers returning from Southeast Asia (Wallace et al., 2020) and in patients in France (Jehanne et al., 2021). Importantly, in Campylobacter, the erm(B) gene is carried on plasmids, or more frequently on multidrug resistance islands (MDRI) which often contain genes coding for resistances to other families such as tetracycline and aminoglycosides (Florez‐Cuadrado et al., 2017). The erm(B) gene can be transferred by natural transformation between strains of Campylobacter (Wang et al., 2014). Another methylase, Erm(N) was recently described in human C. coli strains isolated in Canada (Greninger et al., 2020) and in France (Jehanne et al., 2021). The presence of transferable resistance genes, on either plasmids or MDRI in Campylobacter, represents a main hazard, as acquisition of such genetic structures will confer multidrug resistance to isolates, which can be co‐selected and lead to therapeutic issues. Thus, to better detect the emergence of erm(B) in Campylobacter, and because erm(B) is more frequently reported among C. coli rather than C. jejuni in many published studies, C. coli was included in the future monitoring programs (EFSA, 2019, Decision 2020/1729/EU13). An increase of the tested concentrations of erythromycin (up to 512 mg/L instead of 128 mg/L) is also foreseen (EFSA, 2019, Decision 20/1729); this modification should lead to a better screening of isolates which may carry erm(B), as it will enable to differentiate isolates with an MIC < 128 mg/L, which have probably only mutations or alterations in target genes (23S rRNA, L4, L22 CmeABC, CmeR genes and the binding site of CmeR) from those with a higher MIC (≥ 512 mg/L) and therefore have an erythromycin resistance phenotype consistent with either possession of transferable erm(B) or mutational resistance (Wang et al., 2014).
3.5. Comparison of human and animal data on Campylobacter spp.
In 2019–2020, quantitative human data were interpreted using EUCAST ECOFF values, where available, in the same way as for the animal and food data. Figure 26 presents the CBPs and ECOFFs used to interpret the MIC data reported for Campylobacter spp. from humans, animals or food. In the absence of CBPs from EUCAST (i.e. gentamicin), CBPs from the French Society for Microbiology (SFM) were applied (CASFM/EUCAST, 2020). Notably, there is concordance across interpretive categories, with the exception of the EUCAST CBP for tetracycline in C. jejuni which is one dilution above the EUCAST ECOFF and for gentamicin where EUCAST in 2020 lowered the ECOFF with one dilution compared to the ECOFF value listed in the legislation 2013/652/EU.
Figure 26.
Comparison of clinical breakpoints (CBPs) and epidemiological cut‐off values (ECOFFs) used to interpret MIC data reported for Campylobacter spp. from humans, animals or food
Considering all data submitted from MSs, resistance to ciprofloxacin among C. jejuni isolates reported in 2019–2020 was detected in 61.2% (2020, 16 MSs) and 61.5% (2019, 19 MSs) of human isolates, 72.8% of isolates from broilers (2020, 27 MSs), 77.9% of isolates from fattening turkeys (2020, nine MSs) and 56.8% of isolates from calves (2019, four MSs). Overall resistance to erythromycin was reported at 0.7% (2020) and 1.5% (2019) in isolates from humans, 0.8% in isolates from broilers, 0.8% in isolates from fattening turkeys and not detected in isolates from calves. Combined resistance to ciprofloxacin and erythromycin was reported at 0.5% (2020) and 1.0% (2019) in isolates from humans, and 0.7%, 0.7% and 0% in isolates from broilers, fattening turkeys and calves, respectively. Considering MSs’ reported information on the four antimicrobials (ciprofloxacin/nalidixic acid, erythromycin, tetracycline and gentamicin), complete susceptibility was reported at levels of 31.3% (2020, including Norway) and 30.7% (2019, including Norway) in isolates from humans, and 23.3%, 16.8% and 24.7% in isolates from broilers, fattening turkeys and calves, respectively. However, it must be noted that all countries used EUCAST ECOFFs (MIC > 1 mg/L) to determine resistance to tetracycline in C. jejuni isolates from animals, whereas some countries used clinical breakpoints (MIC > 2 mg/L) to determine resistance to tetracycline in C. jejuni isolates from humans, and the gentamicin ECOFF was 1 mg/L for human isolates, and 2 mg/L for animal ones.
Assessing C. jejuni AMR data for 2020 (humans, broilers and fattening turkeys) or 2019 (calves) at the country level did not reveal significant differences in most countries, except those mentioned below. There was a significantly higher occurrence of ciprofloxacin resistance in humans than for broilers in Finland and Spain (among 16 countries reporting both), and higher resistance in humans than for fattening turkeys in Austria (among six countries reporting both) and for cattle in Denmark and Spain (among three countries reporting both). Lower ciprofloxacin resistance percentages were observed for humans compared to broilers in Italy and Slovakia. For erythromycin, significant differences were noted in Poland (higher erythromycin resistance percentages of isolates from humans compared to broilers and fattening turkeys) and the Netherlands (higher erythromycin resistance percentages of isolates from humans compared to broilers) and in Portugal (lower resistance percentages of isolates from humans compared to fattening turkeys). Notably, in Portugal, surprisingly high occurrences of resistance to erythromycin were observed in human (2.2%), broiler (6.4%) and fattening turkey isolates (16.2%).
Combined resistance to ciprofloxacin and erythromycin among C. jejuni isolates was significantly more frequent in turkey isolates compared to human isolates in Portugal. More isolates of human origin were found susceptible to the four classes of antimicrobials compared to isolates from broilers in France, and cattle in Italy but the percentage of complete susceptibility was lower in isolates from humans compared to turkeys in Austria and cattle in Denmark.
Considering all reports from MSs, resistance to ciprofloxacin among C. coli isolates was reported in 65.8% (2020, 15 MSs) and 61.2% (2019, 18 MSs) of isolates from humans, 61.9% of isolates from broilers (2020, seven MSs), 80.4% of isolates from fattening turkeys (2020, three MSs) and 52.4% of isolates from fattening pigs (2019, eight MSs). Overall, resistance to erythromycin was reported in 10.0% (2020) and 12.9% (2019) of isolates from humans, and 4.4%, 21.5% and 11.2% of isolates from broilers, fattening turkeys and fattening pigs, respectively, were resistant. Combined resistance to ciprofloxacin and erythromycin was reported overall at 8.9% (2020) and 10.4% (2019) in isolates from humans and at 4.1%, 21.2% and 8.0% in isolates from broilers, fattening turkeys and fattening pigs, respectively. In view of the reporting MSs, complete susceptibility to the four antimicrobial classes was reported at levels of 12.5% (2020, including Norway) and 12.6% (2019, including Norway) for humans and 13.7%, 4.4% and 21.2%, in isolates from broilers, fattening turkeys and fattening pigs, respectively.
Considering the countries reporting information on C. coli isolates originating from humans (2020) and broilers (2020, four countries reporting both), or humans (2020) and pigs (2019, three countries reporting both), rates of resistance were usually not different, except in the following instances. Significant differences in ciprofloxacin resistance were noted in France with significantly higher percentages of resistance in isolates from humans compared to isolates from broilers and fattening turkeys, and in Estonia with significantly higher percentages of resistance in isolates from humans compared to isolates from pigs. A lower percentage of ciprofloxacin resistance in human isolates compared to broiler isolates was observed in the Netherlands. For erythromycin, the percentages of resistance were also significantly higher for human isolates compared to broiler isolates in France and the Netherlands. Combined resistance to ciprofloxacin and erythromycin was also significantly more frequent in isolates from humans compared to broilers isolates in the Netherlands and in France. In France, isolates from humans were significantly more often susceptible to the four antimicrobial classes, compared to broiler ones.
Comparison of trends in resistance to ciprofloxacin, erythromycin and tetracyclines for isolates from humans (2016–2020) and broilers (2009–2020) were possible for 13 MSs and two non‐MS regarding C. jejuni and five MSs regarding C. coli (Table 8). The results show various situations. For example, similar increasing trends for resistance to ciprofloxacin were observed for C. jejuni from humans and from broilers in Denmark, France, the Netherlands, Slovakia and the United Kingdom but a decreasing trend was observed in France for C. coli isolates from humans and broilers. Resistance to tetracycline showed an increasing trend in C. jejuni isolates of humans and broilers in Denmark, Slovakia and the United Kingdom and a decreasing trend in C. jejuni isolates of humans and broilers in Cyprus and Finland. Differences between countries in the time periods studied and antibiotic usage may explain these discrepancies.
3.6. Discussion
Campylobacter is a major food‐borne zoonotic agent. In the frame of Commission Implementing Decision 2013/652/EU, the monitoring of AMR in Campylobacter of animal origins provides comparable data on the occurrence and enables to assess the trends of AMR in this bacterial genus in animal productions. However, it must be underlined that the number of reporting MSs (e.g. for C. jejuni: 16 for human in 2020, 27 for broilers in 2020, nine for fattening turkeys in 2020 and four for calves in 2019), differed and the numbers of isolates reported per MS showed various ranges (e.g. for C. jejuni: 5–6,530 for human, 2–322 for broilers, 6–189 for fattening turkeys and 106–147 for calves isolates).
In 2020, information on AMR in Campylobacter isolates from human clinical cases was reported by 16 MSs and two non‐MS. This was three countries less than for 2019. Three countries were not able to report any result due to the impact of the COVID‐19 pandemic on referral of isolates and laboratory capacity, and the UK did not report data as they were no longer an EU/EEA country while Sweden reported Campylobacter AMR data for the first time. The pandemic also affected on the number of isolates reported by countries, as isolates sent for referral from primary laboratories to the national reference centres were much reduced. For all countries but two (Denmark and France), the number of reported isolates dropped in 2020, ranging by country from 11% to 91% fewer isolates compared to 2019.
Globally, the data obtained from C. jejuni and C. coli from human and animal origins in 2019–2020, showed very high to extremely high levels of resistance to fluoroquinolones, which are critically important antimicrobials (CIA) for the treatment of Campylobacter infections in humans. Resistance to quinolones and fluoroquinolones is most usually due to the C257T mutation on the gyrA gene (DTU, 2018). Modifications in the expression of the efflux pump CmeABC may also result in higher MICs of various antimicrobials including ciprofloxacin. Recently highly resistant isolates bearing a transferrable ‘super’ efflux pump variant of CmeABC (RE‐CmeABC) were described in China (Yao et al, 2016), and their MICs of ciprofloxacin, and also of florfenicol, chloramphenicol, erythromycin and tetracycline, were increased.
Regarding C. jejuni resistance to ciprofloxacin in the different reporting countries, increasing trends were more frequently observed than decreasing trends in humans (nine increases vs four decreases) and in broilers (14 increases vs one decrease). For isolates from other origins or for C. coli, the MS trends were variable. Noteworthy, the time periods were different for humans or animals, with a longer period studied for animals which may partly mask the very recent trend. Indeed, it is worth mentioning that, for broilers commensal E. coli, at the MS‐group level, resistance to ciprofloxacin has decreased with statistical significance over the period 2009–2019, and a statistically significant decreasing trend were also registered for commensal E. coli from turkeys over the period 2014–2018 (EFSA and ECDC, 2021a). The contrasting effects of changes of antibiotic use in animal productions on resistance in the diverse bacteria may be explained by co‐selection or fitness of resistant strains (Perrin‐Guyomard et al., 2020).
Macrolides are another important family of antibiotics for treatment of human campylobacteriosis. Resistance to erythromycin was detected at low levels in C. jejuni from humans and animals. C. coli isolates displayed higher rates of resistance to erythromycin, with large dispersion between reporting MSs in both humans (range of 0.0–43.0%) and animals (e.g. range of 0.0–33.3% for C. coli from broilers, and range of 0.0–48.7% for C. coli from pigs). Mutations in one or several copies of the ribosomal RNA genes, such as A2074G, A2074C and A2075G, or in the ribosomal proteins L4 and L22 are common mechanisms of resistance to macrolides (Luangtongkum et al., 2009). Additionally, the transferable erm(B) gene encoding an rRNA methylase, usually present on multidrug resistance islands (MDRGI) or plasmids, may confer a high‐level of resistance to macrolides, lincosamides and/or streptogramin B antibiotics (Wang et al., 2014). Initially described in Asia, this emerging resistance mechanism has now also been detected in patients and in animal isolates in Europe (Florez‐Cuadrado et al., 2017; Elhadidy et al., 2019; Jehanne et al., 2021). [see text box]. An interesting observation is that the resistance to erythromycin in both C. jejuni and C. coli from humans decreased markedly from 2019 to 2020. This could possibly be an effect of the COVID‐19 pandemic where travel restrictions have reduced the number of travel‐associated campylobacteriosis infections in 2020 significantly. While cases with known travel are always excluded in the analyses for this report, in six of 16 MSs, > 80% of cases were missing information on whether the case had travelled or not. Among these, the proportion of cases with travel may be smaller in 2020 than in other years.
An increasing trend of resistance of C. jejuni to erythromycin has not been detected in humans or animals and for C. coli, increasing trends were only registered in two MSs for humans and one MS for broilers. Decreasing trends were observed in, respectively, five, six and six countries for C. jejuni from humans, broilers and fattening turkeys, and in two, three and four countries for C. coli from humans, broilers and pigs.
The similar trends of resistance for isolates of humans and animals isolated in the same countries were previously stated, but on several occasions, discrepancies could be detected either between species (e.g. in France, increasing trend for ciprofloxacin resistance in C. jejuni for both human and broiler isolates but decreasing trends in C. coli for both humans and broilers) or between human and animal isolates (e.g. in the Netherlands, increasing trend of resistance to erythromycin in C. coli from humans but decreasing trends for C. coli from broilers and pigs). The observed differences of rates or trends between countries for animal isolates are probably mainly associated with differences in the use of antimicrobials (ECDC, EFSA and EMA, 2021). For human isolates, the differences observed between countries may also partly result from the origins of reported data, according to local medical and diagnostic practices, which may result in the reporting of various clinical or regional subsets of isolates or of proportions of travel‐associated cases or outbreaks. Within a given MS, significant differences between rates and trends of resistance in isolates of humans and animals may be explained by the fact that much of the food consumed in an MS may have originated from other MSs or third countries. Moreover, recent source attribution studies also pointed to various proportions of contributions of poultry, cattle or environmental sources in human contaminations; the proportions may differ between countries according to eating, leisure or hygiene habits (Jehanne et al., 2020; Rosner et al., 2017; Thepault et al., 2017; Kovac et al., 2018; Maësaar et al., 2020). As of 2021, with the implementation of the Decision 2020/1729, the harmonisation of Campylobacter isolation methods and the monitoring in 2021–2027 of the resistance levels of Campylobacter jejuni and C. coli, not only in poultry, but also in pigs (C. coli) and ruminants (C. jejuni and C. coli), will bring a better knowledge of the prevalence and occurrence of antimicrobial resistance of these two zoonotic Campylobacter species in the main animal productions of the different MSs.
Moreover, the modifications of the panel of antimicrobials and concentrations tested (EFSA, 2021a) will enable a better detection of the emerging and threatening resistance mechanisms already mentioned (RE‐CmeABC, erm(B)), and other ones such as the cfr(C) gene, borne on a conjugative plasmid and conferring resistance to phenicols, lincosamides, pleuromutilins and oxazolidinones (Tang et al., 2017). These mechanisms (efflux pumps) and/or their genetic support (plasmids, MDRGI) confer resistance to one or several families of antimicrobials of major importance for therapy (macrolides, fluoroquinolones or aminoglycosides) and could favour co‐selection of resistant clones or plasmids. To this end, the enlargement of the range of concentrations tested for erythromycin and ciprofloxacin and evaluation of the susceptibilities of additional molecules (chloramphenicol, ertapenem) is also foreseen (Decision 2020/1729).
Finally, WGS of isolates, particularly those with multidrug resistance, high‐level resistance to erythromycin or ciprofloxacin, or resistance to gentamicin or ertapenem is strongly encouraged to evidence the involved antimicrobial resistance genes, their genetic localisation (chromosome, plasmid, transposon, integrative and conjugative element, genomic islands) and their potential of horizontal transmission (Mourkas et al., 2019), detect prevalent resistant lineages or subtypes (Webb et al., 2018; Mouftah et al., 2021) in the different sources and compare animal to human isolates.
4. Antimicrobial resistance in indicator E. coli 14
4.1. Key findings
Resistance to ampicillin, sulfamethoxazole, trimethoprim or tetracycline was common and reported by most MSs at ‘high’ or ‘very high’ levels in all animal categories.
Resistance to quinolones was common in poultry and ‘very high’ or ‘extremely high’ levels were reported by several MSs. Resistance to other antimicrobials was less common and notably, meropenem resistance was not detected in any isolate.
Large differences in the levels of resistance were observed between countries and generally lower levels were reported in northern Europe, although countries in southern and central Europe also reported low levels of resistance in isolates from bovines under 1 year of age (calves).
Complete susceptibility (CS) was more common in isolates from fattening pigs and calves than in those from broilers and fattening turkeys (turkeys). Conversely, multidrug resistance (MDR) was more common in isolates from broilers and turkeys than in those from pigs and calves.
Marked differences in the levels of CS and MDR were observed between countries. The antimicrobials most often represented in the patterns of MDR‐isolates were tetracycline, ampicillin, sulfamethoxazole and trimethoprim and, additionally, quinolones in poultry.
The weighted summary key outcome indicator of complete susceptibility (KOICS), accounting for differences in relative size of food animal populations in a country, varied widely between countries ranging from < 20% to > 80%. Lower KOICS were generally observed in countries in eastern and southern Europe and the highest in countries in the northern part.
Resistance to highly prioritised critically important antimicrobials (HPCIA) was uncommon for colistin, azithromycin and third‐generation cephalosporins (cefotaxime or ceftazidime) and median levels ranged between ‘rare’ and ‘low’ in all animal categories. Ciprofloxacin resistance was more common and median levels were ‘very high’ in broilers, ‘high’ in turkeys, ‘low’ in calves and ‘moderate’ in pigs. Combined resistance to third‐generation cephalosporins and fluoroquinolones was generally uncommon in all animal categories.
Statistically significantly decreasing temporal trends in resistance to ampicillin, ciprofloxacin, cefotaxime, tetracycline and colistin, as well as increasing trends in CS and KOICS reveal a progress towards lower levels of resistance in several countries and in the MS‐group. An improvement of the situation was most pronounced in poultry.
4.2. Data on AMR in indicator E. coli addressed
Throughout 2019 and 2020, AMR was monitored in ‘indicator’ E. coli isolates obtained from caecal content sampled at slaughter from the most relevant food‐producing animals in accordance with Commission Implementing Decision 2013/652/EU. In 2019, the mandatory monitoring covered isolates from fattening pigs (pigs) and bovines under 1 year of age (calves) and in 2020, isolates from broilers and fattening turkeys (turkeys). The specific monitoring of ESBL‐/AmpC‐/carbapenemase‐producing E. coli recovered from caecal content of pigs, calves, broilers and turkeys, as well as from fresh meat samples of broilers, pigs, bovines and broilers, was also mandatory over these reporting years.
Studying phenotypic AMR of commensal ‘indicator’ E. coli from the intestinal flora of healthy food‐producing animals and from food thereof provides information on the reservoirs of resistant bacteria that could potentially be transferred between animal populations and between animals and humans. It also provides indirect information on the reservoirs in animals and food of resistance genes that could be transferred to bacteria that are pathogenic for humans and/or animals. Such monitoring, therefore, has relevance to both public and animal health. The occurrence of resistance in indicator E. coli likely depends on several factors including: the selective pressure exerted by the use of antimicrobials in food‐producing animal populations; clonal spread of resistant organisms; dissemination of genetic elements, such as resistance plasmids; and the effects of co‐selection in bacteria exhibiting multi drug resistance (MDR).
4.3. Antimicrobial resistance in poultry, porcine and bovine populations
4.3.1. Data reported
In 2019, 28 MSs and four non‐MSs reported data on ‘indicator’ E. coli isolates from fattening pigs (pigs) and nine MSs and three non‐MSs data on isolates from bovines under 1 year of age (calves). In 2020, 27 MSs and five non‐MSs reported data on isolates from broilers and 11 MSs and two non‐MSs data on isolates from fattening turkeys (turkeys).
4.3.2. Occurrence of resistance
Resistance to ampicillin, sulfamethoxazole, trimethoprim and tetracycline was the most common resistance traits observed. Considering all reporting countries, median levels of resistance to those antimicrobials were ‘high’ or ‘very high’ in all four animal categories except for calves for which the median level of trimethoprim resistance was ‘moderate’ (Figure 27 and Annex C). There were however large differences between countries and whereas some countries reported ‘extremely high’ levels in one or more animal category, others reported ‘moderate’ or even ‘very low’ levels. In poultry, also resistance to ciprofloxacin and nalidixic acid was common, with ‘very high’ median levels in broilers and ‘high’ median levels in turkeys (Figure 27 and Annex C). In contrast, in pigs and calves median resistance levels to both these antimicrobials were ‘low’. For both ciprofloxacin and nalidixic acid resistance, there were large differences between reporting countries. Most countries reported nalidixic acid resistance at lower levels than ciprofloxacin resistance. This was most notable for pigs and calves and in the MS‐group the level of nalidixic acid resistance was only about of half that for ciprofloxacin for both these animal categories. The median levels of chloramphenicol resistance for all reporting countries were ‘moderate’ in pigs, calves and broilers and ‘high’ in turkeys, although ‘high’, ‘very high’, and for turkeys even ‘extremely high’ levels, were recorded in some countries (Annex C). Median levels of gentamicin resistance were ‘low’ in all four animal categories, but individual countries reported up to ‘high’ levels, mainly for broilers. Resistance to cefotaxime, ceftazidime, colistin and azithromycin were ‘rare’, ‘very low’ or ‘low’ in all four animal categories (Annex C). Meropenem resistance was not detected in any isolate of indicator E. coli and tigecycline resistance in only three isolates from pigs (Malta) and in one isolate from turkeys (Portugal).
Figure 27.
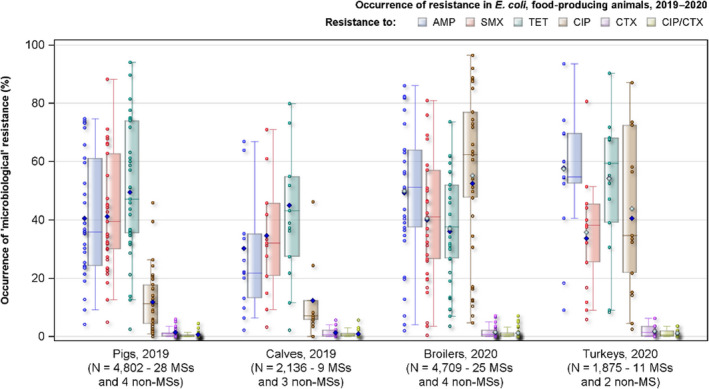
Distribution of occurrence of resistance to selected antimicrobials in indicator E. coli isolates recovered from fattening pigs (pigs) and bovines under 1 year of age (calves) in 2019 and from broilers and fattening turkeys (turkeys) in 2020. MSs and non‐MSs
- N: Total number of isolates reported by Member States (MSs) and non‐Member States (non‐MSs); AMP: ampicillin, SMX sulfamethoxazole, TET: tetracycline, CIP: ciprofloxacin, CTX: cefotaxime, CIP/CTX: combined ‘microbiological’ resistance to ciprofloxacin and cefotaxime; blue diamond: EU level of resistance; horizontal line in the box plot: the median
Resistance to ‘highest priority critically important antimicrobials’
Among the antimicrobials tested in the mandatory monitoring, ciprofloxacin (fluoroquinolones), cefotaxime and ceftazidime (third‐generation cephalosporins), colistin (polymyxins) and azithromycin (macrolides) are categorised by the WHO as highest priority critically important antimicrobials (HPCIA) and among substances of the highest priority (WHO, 2019).
Considering all reporting countries, median levels of colistin resistance were ‘rare’ in pigs, calves and broilers and ‘very low’ in turkeys. For azithromycin, median levels were ‘very low’ in pigs, calves and broilers and ‘low’ in turkeys. In many countries, resistance to these two antimicrobials was not observed but levels up to ‘high’ were reported by some countries (Annex C). In contrast, ‘very high’ levels of resistance to fluoroquinolones/quinolones were recorded in isolates from broilers (median: ciprofloxacin 60.7%; nalidixic acid 51.8%), and ‘high’ levels in isolates from turkeys (median ciprofloxacin 33.5%; median nalidixic acid 22.9%) (Annex C). Resistance to ciprofloxacin and nalidixic acid were reported at much lower levels in isolates from pigs (median 6.1% and 4.7%, respectively) and calves (median 6.1% and 4.7%, respectively). Large variations were however registered between reporting countries for each of the animal populations and in some countries resistance to fluoroquinolones/quinolones was either not detected or found at ‘moderate’, ‘low’ or ‘very low’ levels in all four animal populations (Figure 28 and Annex C).
Figure 28.
Spatial distribution of combined ‘microbiological’ resistance to cefotaxime and ciprofloxacin in indicator Escherichia coli. (a) fattening pigs (pigs) 2019, (b) bovines under 1 year of age (calves) 2019, (c) broilers 2020 and (d) fattening turkeys (turkeys) 2020
In all animal populations monitored, resistance to third‐generation cephalosporins (cefotaxime or ceftazidime) was either not observed or detected at ‘low’ to ‘very low’ levels (Figure 29 and Annex C). Considering all reporting countries, median levels of resistance to cefotaxime and ceftazidime were similar in all four animal populations, at 0.7% vs. 0.7% in isolates from pigs, 0.8% vs. 0.8% in isolates from calves, 0.5% vs. 0.4% in isolates from broilers and 1.4% vs. 1.0% in isolates from turkeys.
Figure 29.
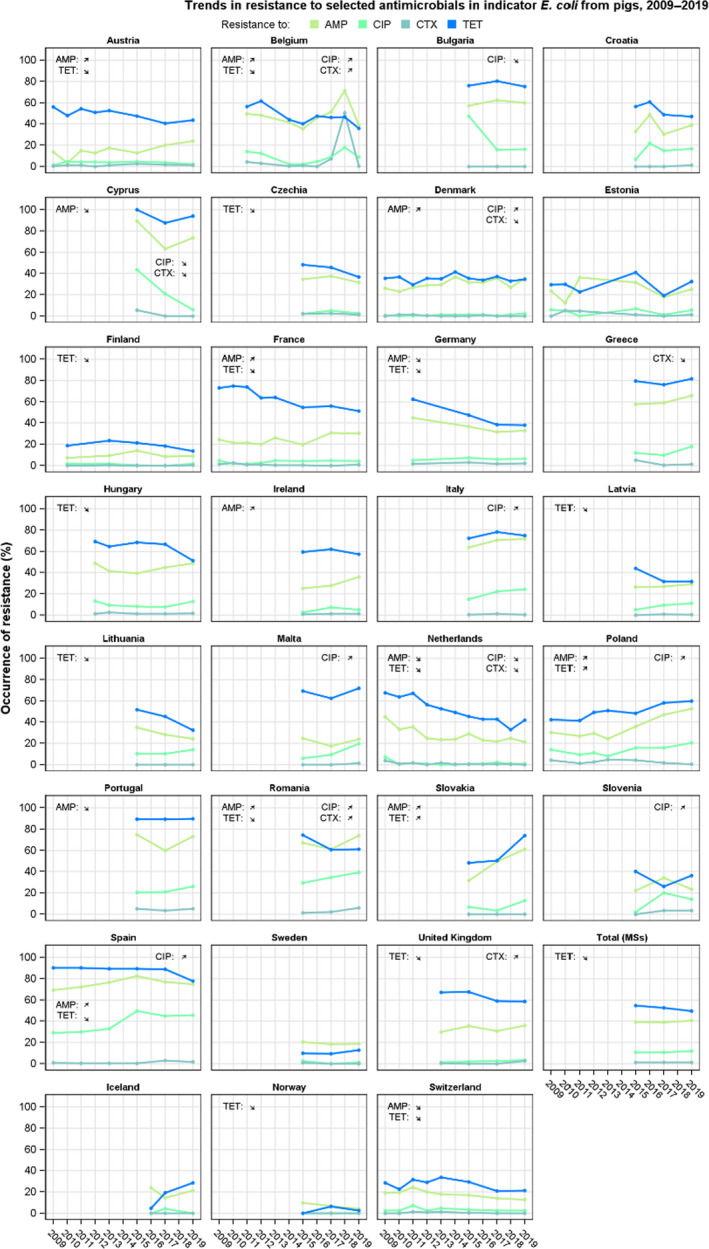
Trends in resistance to ampicillin (AMP), cefotaxime (CTX), ciprofloxacin (CIP) and tetracyclines (TET) in indicator E. coli from fattening pigs (pigs), MSs and non‐MSs
- (↓)(↑): indicates statistically significant trends over the period 2015–2019.
Combined resistance to ciprofloxacin and cefotaxime
In most reporting countries, ‘microbiological’ combined resistance to ciprofloxacin and cefotaxime was either not observed or detected at ‘low’ or ‘very low’ levels in all four animal categories monitored (Figure 28c, Annex C). Isolates with ‘clinical’ combined resistance was even more uncommon and generally not observed or detected at ‘very low’ or ‘low’ levels in all four animal populations monitored (Annex C). Overall, 107 isolates with ‘microbiological’ combined resistance and 42 with ‘clinical’ combined resistance were detected (Table 9). Isolates with combined resistance were more common in poultry than in pigs and calves.
Table 9.
Combined resistance to ciprofloxacin and cefotaxime in isolates of E. coli from fattening pigs (pigs), bovines under 1 year of age (calves), broilers and fattening turkeys (turkeys) applying ECOFFs and clinical breakpoints, as issued by EUCAST. MSs and non‐MSs
| Food‐producing animal population | ‘Microbiological’ combined resistance to CIP & CTX (using ECOFFs) | ‘Clinical’ combined resistance to CIP & CTX (using clinical breakpoints) | ||||
|---|---|---|---|---|---|---|
| No. of isolate | % Resistance | 95% CI | No. of isolates | % Resistance | 95% CI | |
|
Pigs (2019, N = 4,802, 28 MSs, 4 non‐MSs) |
25 | 0.5% | 0.4–0.8 | 6 | 0.1% | 0.1–0.3 |
|
Calves (2019, N = 2,136, 9 MSs, 3 non‐MSs) |
14 | 0.6% | 0.3–1.0 | 4 | 0.2% | 0.0–0.5 |
|
Broilers (2020, N = 4,716, 27 MSs, 5 non‐MSs) |
49 | 1.0% | 0.7–1.35 | 24 | 0.5% | 0.3–0.7 |
|
Turkeys (2020, N = 1,875, 11 MSs, 2 non‐MSs) |
19 | 1.0% | 0.6–1.6 | 8 | 0.4% | 0.2–0.8 |
N: total number of E. coli isolates reported by MSs and non‐MSs; CIP: ciprofloxacin; CTX; cefotaxime.
4.3.3. Temporal trends in resistance among indicator E. coli
Temporal trends in resistance to ampicillin, ciprofloxacin, cefotaxime and tetracycline in indicator E. coli from pigs, calves, broilers and turkeys were evaluated for countries having provided data for 3 years or more over the period 2009–2020 and also for the MS group. Ampicillin and tetracycline resistance are considered because those antimicrobials have been the most used in food‐producing animals in Europe (EMA, 2021) and decreasing trends in resistance are believed to primarily reflect changes in usage. Resistance trends in the HPCIAs ciprofloxacin and cefotaxime have also been addressed as resistance in food‐producing animals might impact human health care. The statistical significance (p ≤ 0.05) of trends was tested by logistic regression (see Appendix F for details on methodology).
Sufficient data for analysis of temporal trends were available from 30 countries for pigs, 11 for calves, 30 for broilers and 12 for turkeys. Thus, 83 different data sets were available and analysed for trends in resistance to ampicillin, ciprofloxacin, cefotaxime and tetracycline. In the 332 separate analyses performed, there were 129 decreasing trends, 55 increasing trends and 148 data sets without statistically significant changes (Table 10). Thus, there were more decreasing than increasing trends and most noticeable was that a decrease in tetracycline resistance was observed in half of the data sets evaluated (50.6%, 42/83). Still, most data sets revealed no temporal change in resistance, but it should be noted that in several countries, levels of resistance were stable at low levels and major changes cannot be expected.
Table 10.
Summary of trends in occurrence of resistance to ampicillin, ciprofloxacin, cefotaxime and tetracyclines in indicator E. coli from fattening pigs (pigs), bovines under 1 year of age (calves), broilers and fattening turkeys (turkeys) in the period 2014–2020. Number of countries with significant decreasing trend (↓), significant increasing trend (↑) and a stable occurrence of resistance (↔)
| Animal category | Ampicillin | Ciprofloxacin | Cefotaxime | Tetracycline | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ↓ | ↑ | ↔ | ↓ | ↑ | ↔ | ↓ | ↑ | ↔ | ↓ | ↑ | ↔ | ↓ | ↑ | ↔ | |
|
Pigs (2019, 27 MSs, 3 non‐MSs) |
5 | 9 | 16 | 3 | 8 | 19 | 4 | 3 | 23 | 15 | 2 | 13 | 27 | 22 | 71 |
|
Calves (2019, 9 MSs, 2 non‐MSs) |
3 | 4 | 4 | 3 | 3 | 5 | 3 | 1 | 7 | 4 | 2 | 5 | 13 | 10 | 21 |
|
Broilers (2020, 25 MSs, 3 non‐MSs) |
15 | 6 | 9 | 18 | 10 | 2 | 20 | 2 | 8 | 15 | 2 | 13 | 68 | 20 | 32 |
|
Turkeys (2020, 10 MSs, 1 non‐MSs) |
7 | 1 | 4 | 4 | 1 | 7 | 2 | 1 | 9 | 8 | 4 | 21 | 3 | 24 | |
| Total | 30 | 20 | 33 | 28 | 22 | 31 | 29 | 7 | 47 | 42 | 6 | 35 | 129 | 55 | 148 |
Fattening pigs (pigs)
In the 30 countries reporting data for isolates from pigs there were 27 decreasing and 22 increasing trends (Table 10, Figure 29). In 13 countries, there were only decreasing trends, notably in the Netherlands for four and in Cyprus for three antimicrobials. In contrast, in six countries there were only increasing trends, in Belgium, Poland and Romania for three antimicrobials. Most notable was that tetracycline resistance has decreased in 15 countries and increased in only two. Also, in the MS group, there was a statistically significant decrease in resistance to tetracycline.
Bovine animals under 1 year of age (calves)
In the 11 countries reporting data on isolates from calves, 13 decreasing and 10 increasing trends were observed (Table 10, Figure 30). In four countries, only decreasing trends were recorded and notably in France, Germany and the Netherlands, resistance to all four antimicrobials has decreased. In contrast, in four countries, there were only increasing trends, in Belgium to all four antimicrobials. In the MS group, there were no statistically significant trends in resistance to any antimicrobial.
Figure 30.
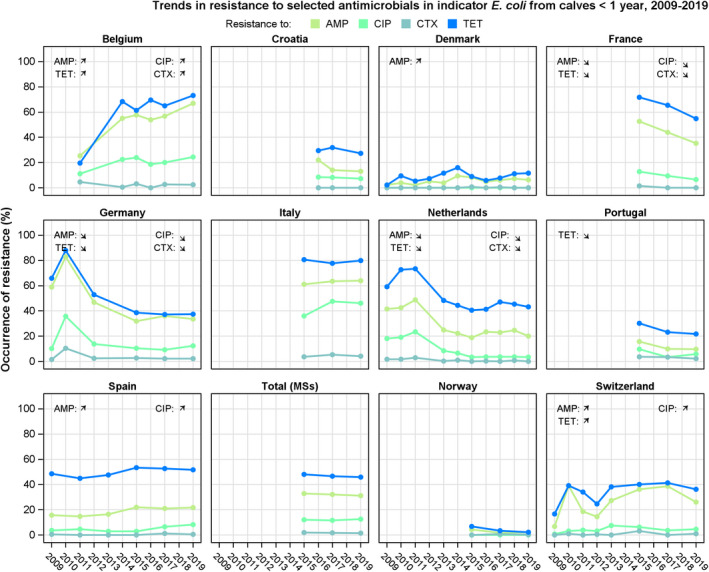
Trends in resistance to ampicillin (AMP), cefotaxime (CTX), ciprofloxacin (CIP) and tetracyclines (TET) in indicator E. coli from bovines under 1 year of age (calves), MSs and non‐MSs 2009–2019
- (↓)(↑): indicates statistically significant trends over the period 2014–2019
Broilers
In the 30 countries reporting data for isolates from broilers, there were 68 decreasing and 20 increasing trends (Table 10, Figure 31). In 16 countries, there were only decreasing trends. Notably, in Ireland, Italy, Latvia, the Netherlands and Spain, resistance has decreased for all four antimicrobials and in France and Portugal for three antimicrobials. In contrast, in five countries, only increasing trends were observed. In the MS group, including also United Kingdom, resistance to all four antimicrobials has decreased with statistical significance.
Figure 31.
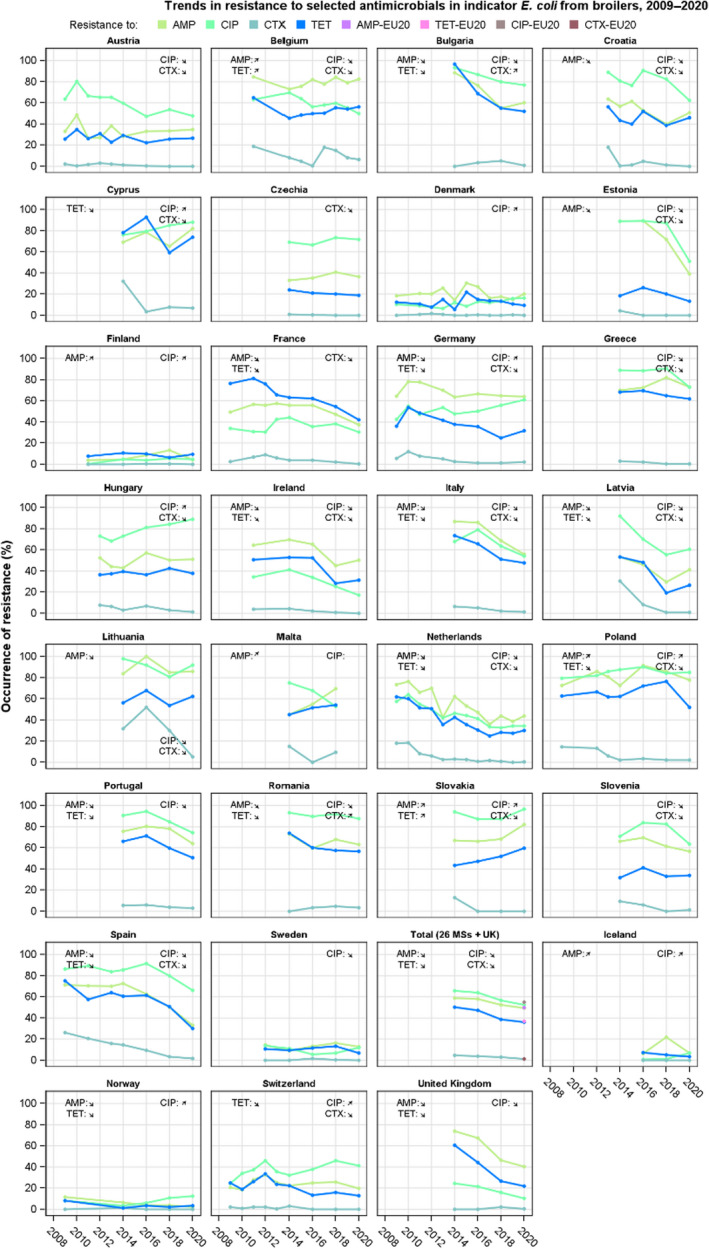
Trends in resistance to ampicillin (AMP), cefotaxime (CTX), ciprofloxacin (CIP) and tetracyclines (TET) in indicator E. coli from bovines under 1 year of age (calves), MSs and non‐MSs 2009–2019
- (↓)(↑): indicates statistically significant trends over the period 2014–2019.
Fattening turkeys (turkeys)
In the 12 countries reporting data for isolates from turkeys, there were 21 decreasing and three increasing trends (Table 10, Figure 32). Seven countries reported only decreasing trends, in Spain for all four antimicrobials and in France, Italy and Portugal for three antimicrobials. In the MSs group, including also United Kingdom, resistance to all four antimicrobials has decreased with statistical significance.
Figure 32.
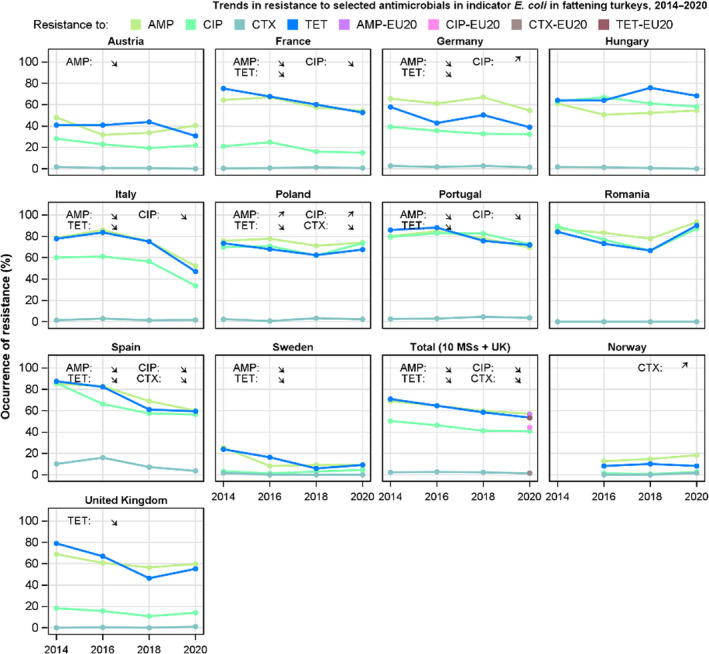
Trends in resistance to ampicillin (AMP), cefotaxime (CTX), ciprofloxacin (CIP) and tetracyclines (TET) in indicator E. coli from broilers, MSs and non‐MSs 2009–2020
- (↓)(↑): indicates statistically significant trends over the period 2015–2020.
4.3.4. Phenotypic characterisation of third‐generation cephalosporin and carbapenem resistance in indicator E. coli from caecal samples
A low number of indicator E. coli isolates from caecal samples from pigs and calves in 2019 and from broilers and turkeys in 2020 were phenotypically resistant to third‐generation cephalosporins (cefotaxime and/or ceftazidime) on initial testing on panel 1 (Table 11). Further phenotypic characterisation of these isolates for presumptive production of ESBL‐ and/or AmpC‐enzymes on panel 2 showed that the total number of presumptive ESBL‐ and/or AmpC‐producers was ‘low’ in all four animal categories (Table 12).
Table 11.
Occurrence of resistance to third‐generation cephalosporins in indicator E. coli isolates from fattening pigs (pigs), bovines under 1 year of age (calves), broilers and fattening turkeys (turkeys). EU MSs and non‐MSs, 2019–2020
| Animal category | No. of MSs/non‐MSs | N | Cefotaxime | Ceftazidime | ||
|---|---|---|---|---|---|---|
| N | % | n | % | |||
| Pigs, 2019 | 28/4 | 4,802 | 61 | 1.3% | 57 | 1.2% |
| Calves, 2019 | 9/3 | 2,136 | 25 | 1.2% | 23 | 1.1% |
| Broilers, 2020 | 27/5 | 4,716 | 58 | 1.2% | 54 | 1.1% |
| Turkeys, 2020 | 11/2 | 1,875 | 32 | 1.7% | 31 | 1.7% |
N: Total number of isolates tested by MSs and non‐MSs; n: Total number of isolates resistant.
Table 12.
Phenotypes of presumptive ESBL‐, AmpC‐ or CP‐ producing indicator E. coli subjected to supplementary testing (panel 2). EU MSs and non‐MSs, 2019–2020
| Animal category |
ESBL and/or AmpC n (% R) |
ESBL( a ) n (% R) |
AmpC( b ) n (% R) |
ESBL + AmpC( c ) n (% R) |
CP( d ) n (%R) |
|---|---|---|---|---|---|
| Pigs, 2019 (N = 4,802) | 58 (1.2) | 44 (0.8) | 14 (0.3) | 0 | 0 |
| Calves, 2019 (N = 2,136) | 22 (1.0) | 20 (0.9) | 2 (0.1) | 0 | 0 |
| Broilers, 2020 (N = 4,716) | 54 (1.1) | 45 (1.0) | 16 (0.3) | 7 (0.1) | 0 |
| Turkeys, 2020 (N = 1,875) | 24 (1.3) | 19 (1.0) | 8 (0.4) | 3 (0.2) | 0 |
ESBL: extended‐spectrum β‐lactamase; CP: carbapenemase; N: Total number of isolates reported by MSs and non‐MSs; n: number of isolates with this phenotype; % R: percentage of isolates from the total tested; ESBL; extended‐spectrum β‐lactamase.
All isolates showing clavulanate synergy with cefotaxime, ceftazidime or with both compounds, suggesting the presence of an ESBL (independently of the presence of other mechanisms).
Isolates with microbiological resistance to cefoxitin, suggesting the presence of an AmpC enzyme (independently of the presence of other mechanisms).
Isolates showing synergy with cefotaxime or ceftazidime and with microbiological resistance to cefoxitin, suggesting the presence of ESBL and AmpC enzymes in the same isolate. These isolates are also included in the ESBL and AmpC columns.
Isolates with microbiological meropenem resistance.
In countries reporting presumptive ESBL‐ and/or AmpC‐producing isolates, occurrence was generally ‘very low’ or ‘low’, ranging from 0.6% to 5.9% in isolates from pigs, from 0.3% to 5.6% in isolates from calves, from 0.6% to 7.1% in isolates from broilers and from 0.6% to 6.3% in isolates from turkeys. Presumptive ESBL‐producers were more common than AmpC‐producers in all animal categories and isolates with a combined phenotype (ESBL+AmpC) were uncommon (Table 12). No isolate of indicator E. coli recovered from caecal samples by MSs and non‐MSs from the four animal categories in 2018–2019 showed microbiological resistance to carbapenems (meropenem) on initial testing on panel 1 (Table 12).
4.3.5. Multidrug resistance and complete susceptibility in indicator E. coli
Multidrug resistance
Multidrug resistance (MDR), defined as ‘microbiological’ resistance to three or more antimicrobial classes of the harmonised panel tested, was observed in 34.2% of indicator E. coli isolates from pigs, in 26.8% from calves, in 38.7% from broilers and in 41.0% from turkeys. Large variations between reporting countries were observed and MDR ranged 2.8–85.3% in pigs, 0.3–73.4% in calves, 0.4–86.0% in broilers and 4.5–90.3% in turkeys (Annex C).
MDR patterns
A wide variety of resistance patterns were observed in MDR isolates. The antimicrobials most often represented in the patterns of isolates from pigs and calves were tetracycline, ampicillin, sulfamethoxazole and trimethoprim. Considering all reporting countries, about half of the MDR isolates from pigs (52.3%) and calves (48.3%) were resistant to all these four antimicrobials and often also to other substances. These antimicrobials, alone or in combination with other substances, were also common in resistance patterns of MDR isolates from broilers (41.5%) and turkeys (42.2%). MDR patterns of isolates from poultry included quinolones at 75.9% for broilers and 73.8% for turkeys. In contrast, quinolones were less often included in the patterns of MDR isolates from pigs (27.3%) and calves (39.5%).
Colistin was uncommon in the patterns of MDR isolates, at 1.5% in pigs, 1.6% in calves and 2.7% in broilers but more common in isolates from turkeys at 9.5%. Also, resistance to third‐generation cephalosporins was uncommon at 3.0% in pigs, 3.7% in calves, 3.0% in broilers and 3.8% in turkeys.
Completely susceptible isolates
The occurrence of resistance can also be addressed by considering the proportion of indicator E. coli isolates exhibiting susceptibility to all the 14 antimicrobials tested, using epidemiological cut‐off values for interpretation. Considering all reporting countries, 39.8% of isolates from pigs, 57.6% from calves, 30.3% from broilers and 29.2% from turkeys exhibited complete susceptibility (Annex C). For all animal populations, complete susceptibility varied widely between countries and ranged between 0 and 91.2% in pigs, 14.8–93.6% in calves, 1.0–82.9% in broilers and 3.2–79.5% in turkeys (Annex C, Figure 33). Typically, the highest levels of complete susceptibility in all four animal populations were observed in isolates from the Nordic countries, with levels generally decreasing in a north to south gradient and to a lesser extent, in a west to east gradient.
Figure 33.
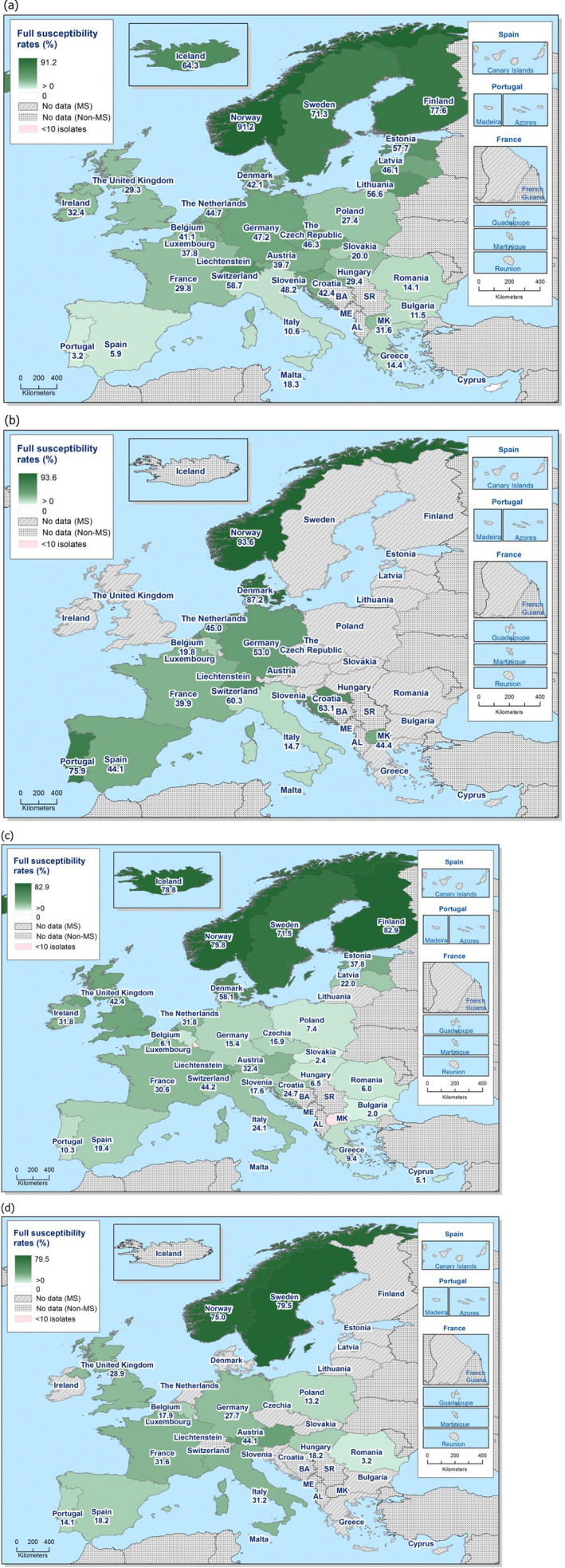
Spatial distribution of complete susceptibility to the antimicrobials tested in indicator E. coli. (a) fattening pigs (pigs) 2019; (b) bovines under 1 year of age (calves) 2019; (c) broilers 2020; (d) fattening turkeys (turkeys) 2020
Changes in complete susceptibility
For pigs, no statistically significant difference in the level of complete susceptibility at the MS group level was observed between 2015 and 2019 (Figure 34). In seven countries (Estonia, Finland, Germany, Lithuania, Spain, Norway and Switzerland), the level of completely susceptible isolates has however increased significantly, whereas it has decreased in two countries (Poland and Slovakia). For calves, the level of complete susceptibility has also remained stable at the MS group level, but an increase was observed in three countries (France, Portugal, Switzerland) (Figure 33).
Figure 34.
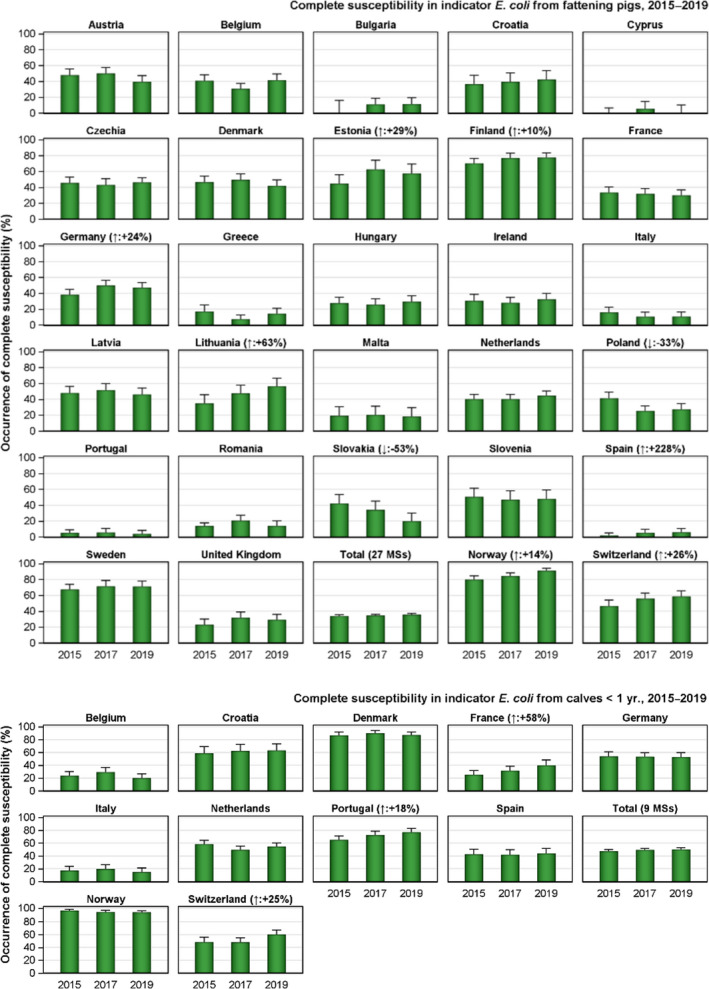
Changes in the occurrence of complete susceptibility to the panel of antimicrobials tested in indicator E. coli isolates from (a) fattening pigs (pigs) and (b) bovines under 1 year of age (calves) in the years 2015, 2017 and 2019
-
(↓)(↑): indicates statistically significant trends over the period 2015–2019.The upper bounds of the 95% confidence interval of the occurrence of complete susceptibility are also indicated.
For broilers, complete susceptibility has increased between 2014 and 2020 at the MS‐group level (including UK) as well as in 14 individual countries (Austria, Croatia, Estonia, France, Greece, Ireland, Italy, Latvia, Netherlands, Portugal, Romania, Slovenia, Spain, the United Kingdom) and decreased in four countries (Belgium, Denmark, Germany, Hungary) (Figure 36). Also, for turkeys the level of complete susceptibility has increased significantly at the MS‐group level (including UK) as well as in eight individual countries (Austria, France, Hungary, Italy, Portugal, Spain, Sweden, the United Kingdom) (Figure 35).
Figure 36.
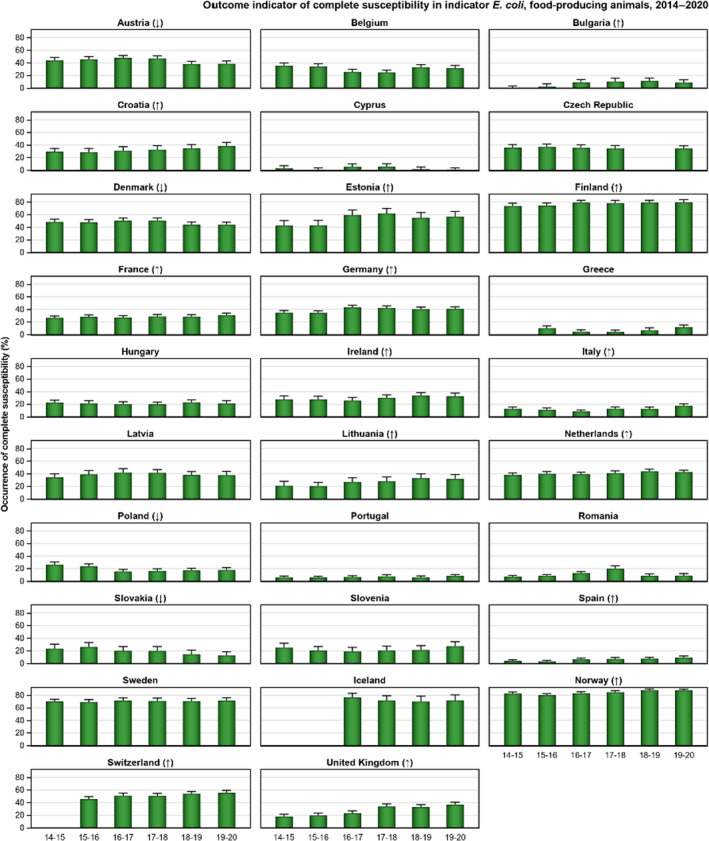
Changes in weighted key outcome indicator of complete susceptibility (KOICS) in 25 EU MSs and four non‐MSs
- (↓)(↑): indicates statistically significant decreasing/increasing trends over the 2018–2014 period. The upper bounds of the 95% confidence interval of the KOICS are also indicated. Rates of change are given for statistically significant trends.
Figure 35.
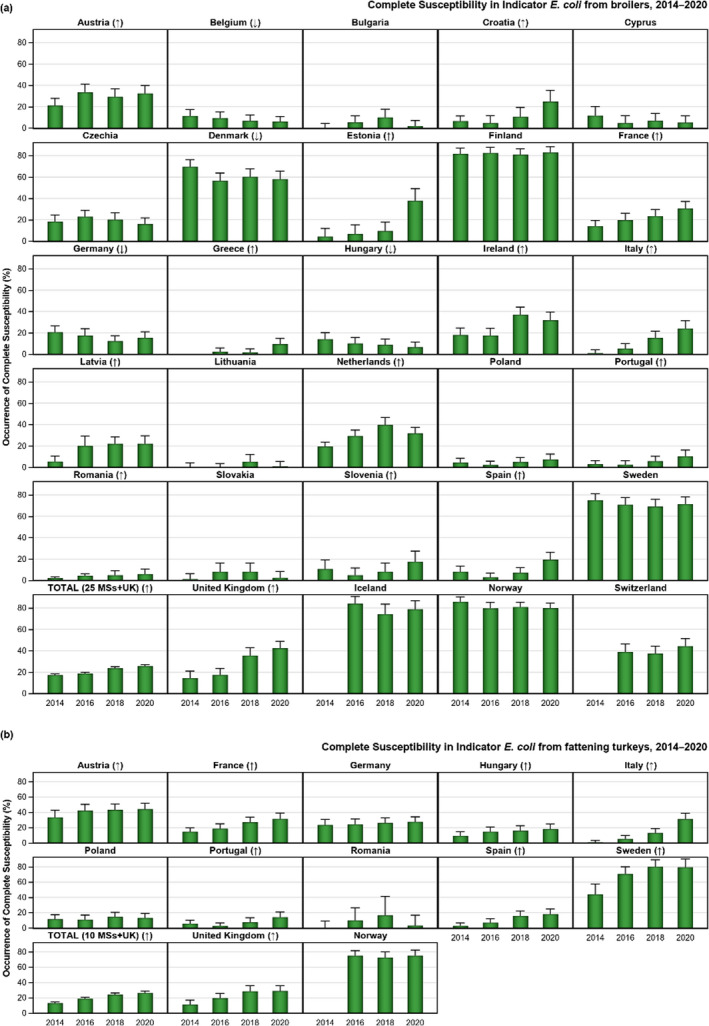
Changes in the occurrence of complete susceptibility to the panel of antimicrobials tested in indicator E. coli isolates from (a) broilers and (b) fattening turkeys (turkeys) in the years 2014, 2016, 2018 and 2020
- (↓)(↑): indicates statistically significant trends over the period 2014–2020. The upper bounds of the 95% CI are also shown.
Summary outcome indicator of complete susceptibility (KOICS).
The proportion of fully susceptible indicator E. coli isolates, weighted by the size of the populations of the most important production animals (broilers, fattening turkeys, fattening pigs, calves) in a country is used as a key outcome indicator (KOICS) for the overall AMR situation in food‐producing animals. As KOICS accounts for differences in the relative size of food animal populations, it is relevant for evaluation of risks related to AMR in food‐producing animals.
AMR genes located on transmissible genetic elements can be transferred from commensal bacteria in the agricultural sector to bacteria of concern in human health care (Florez‐Cuadrado et al., 2018). An abundant commensal bacterial species, such as E. coli was, therefore selected as reporting organism because it is considered more relevant in representing the overall AMR situation, including transmissible AMR genes, than less abundant zoonotic species.
The KOICS can be used to assess the development of AMR in relation to the total use of antimicrobials in food‐producing animals (Queenan et al., 2016; ECDC, EFSA and EMA, 2021). The assumption underlying the choice of this specific indicator is that only E. coli that is rarely, if ever, exposed to antimicrobials will be fully susceptible (Martinez, 2014). Therefore, it is to be expected that a reduction of the use of antimicrobials in food‐producing animals would result in a noticeable improvement of this indicator.
The populations of food‐producing animals differ in size between European countries. The relative size of those varying populations may influence resistance issues related to the overall food animal production at the country level as well as on the European level. This makes it difficult to evaluate overall trends and to assess the overall magnitude of resistance within and between countries. To account for differences in the relative size of food animal populations in a country, the KOICS was calculated as the weighted mean of the proportions of completely susceptible indicator E. coli isolates in each of the four animal populations monitored (fattening pigs, bovines under 1 year of age, broilers, fattening turkeys). For calculation of the KOICS, the value for each population was weighted in relation to the relative size of the populations within a country using the ‘population correction unit’ (PCU). Regarding bovines, only animals under 1 year of age were included in calculation of the PCU. PCU is a technical unit of measurement used as an indicator of animal population size and was developed by the EMA, primarily to estimate sales of antimicrobials corrected by the animal population in individual countries. The data sources and methodology for the calculation of PCU are comprehensively described in EMA’s report ‘Sales of veterinary antimicrobial agents in 31 European countries in 2019 and 2020’ (EMA, 2021).
For each country, KOICS was calculated using data reported for two consecutive years. Thus, values for 2014–2015 were calculated from data for broilers and fattening turkeys reported in 2014 and on data for fattening pigs (pigs) and bovines under 1 year of age (calves) reported in 2015. Likewise, the values for 2015–2016 were calculated from data reported for pigs and calves in 2015 and on data for broilers and fattening turkeys (turkeys) reported in 2016, and so on. For each value of KOICS calculated for a single country, data for broilers and pigs were included. However, since not all countries have reported data for calves and turkeys regularly, calculations for all individual countries do not include data for these two animal categories.
KOICS for the 29 countries reporting data on resistance in 2014–2020 are presented in Figure 37. There were marked variations in KOICS between countries and in 2019–2020 levels of KOICS were < 20% in nine countries, 20–40% in 12 countries, 40–60% in four countries, 60–80% in three countries and > 80% in one country (Norway). The lowest KOICS were generally observed in countries in eastern and southern Europe and the highest in countries in the northern part. KOICS has been stable at a high level over the period in some countries and in others at a low level. Statistically significant increasing trends were registered in 14 countries and decreasing trends in four countries.
Notably, the relative contribution of data on complete susceptibility (CS) on KOICS seems to be greater for pigs than for the other animal categories. In nine of the 18 countries with a positive or negative trend in KOICS, this was concurrent with a similar trend in levels of CS of isolates from pigs (Figures 34 and 35). Conversely, in all nine countries with a positive or negative trend in CS in isolates from pigs, this was reflected in a similar trend in KOICS. However, e.g. in Germany, a negative trend in CS in isolates from broilers was outweighed by a positive trend in pigs, resulting in a positive trend in KOICS. Conversely, in Austria, with a negative trend in KOICS, positive trends in CS in isolates from broilers and turkeys were masked by the lack of significant changes in CS in isolates from pigs. Likewise, in Portugal positive trends in CS for calves, broilers and turkeys are not reflected in KOICS because there is no change CS in pigs. Thus, if KOICS is used as sole indicator of the AMR situation in a country, positive or negative trends in one animal category of small relative size within the country may go unnoticed. To fully appreciate the situation within a country data on resistance/complete susceptibility should therefore also be evaluated at the level of each animal population.
Figure 37.
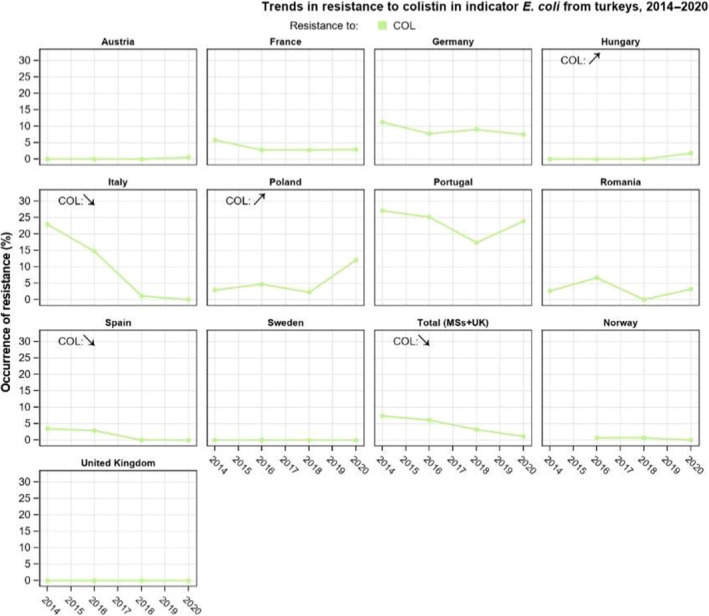
Temporal trends in resistance to colistin in indicator E. coli from fattening turkeys (turkeys), 2014–2020 (10 MSs, two non‐MS). Statistically significant increase (↑) or decrease (↓) indicated (p ≤ 0.05)
4.3.6. Colistin resistance in indicator E. coli
Colistin (polymyxin E) is an antimicrobial of the polymyxin group that has been used extensively in farm animals all over the world, including Europe. In human medicine, use of colistin has historically been limited but an increased usage has been recorded to account for the need of last resort antimicrobials to treat infections caused by multidrug‐resistant Gram‐negative bacteria. Consequently, polymyxins are now among the five antimicrobials listed by WHO as critically important and of highest priority for human medicine. The discovery of transferable genetic elements (e.g. mcr‐ genes) conferring resistance to colistin, further underlines the importance of monitoring such resistance in food‐producing animals. However, the mandatory monitoring according to Decision 2013/652/EU is based on phenotypic susceptibility and molecular testing is required for inference regarding the underlying mechanisms of resistance and the possible presence of mcr‐ genes.
Colistin resistance was generally uncommon and altogether only 165 of the 13,529 isolates of indicator E. coli tested in 2019 and 2020 showed phenotypic resistance to this antimicrobial. Most countries did not detect resistance in any isolate but about half of the countries reported resistant isolates from calves or turkeys and about one fifth from pigs or broilers. In the MS‐group (including UK), median levels of colistin resistance were ‘rare’ in isolates from pigs (0%) and broilers (0%), ‘very low’ in isolates from calves (0.5%) and ‘low’ in isolates and turkeys (1.2%) (Annex C). Much higher levels were however reported in individual countries, up to 23.9% for turkeys, 11.1% for broilers, 8.3% for pigs and 2.3% for calves (Annex C).
Trends in colistin resistance was analysed by logistic regression for countries reporting data for 3 years or more in the period 2014–2020 (see Appendix E for details). For pigs, the level of resistance has decreased in one MS (Germany) and increased in four MSs (France, Greece, Malta, Portugal) (Appendix E). For calves, resistance has increased in one MS (Portugal) and for broilers resistance has decreased in one MS (Romania) (Appendix E). In isolates from turkeys, colistin resistance has decreased in two MSs (Italy, Spain) and increased in two MSs (Hungary, Poland) (Figure 37). In the MS‐group, there were decreasing trends in isolates from broilers and turkeys, an increasing trend in isolates from pigs and no trend in isolates from calves (Appendix E and Figure 37).
4.4. Discussion
Studying AMR in commensal ‘indicator’ E. coli from caecal content of healthy food‐producing animals provides information on the reservoirs of resistant bacteria that could potentially be transferred between animals and between animals and humans. Such monitoring is therefore relevant for both animal and public health. AMR exhibited by indicator E. coli is likely to depend on several factors, such as the selective pressure from the use of antimicrobials in food‐producing animals, co‐selection of bacteria with multiple resistance, clonal spread of resistant bacteria and dissemination of genetic elements, such as plasmids, between bacteria.
Representative monitoring
The data on AMR in indicator E.coli used in the present report were collected over the years 2014–2020 in accordance with Commission Implementing. Decision 2013/652/EU. Those data are therefore harmonised with respect to sampling design, laboratory methodology, reporting and interpretation of resistance. Data collected previously may, however, be impacted by certain slight differences in methodology. Between 2014 and 2020, AMR data on indicator E. coli from fattening pigs (pigs) and broilers were reported by nearly all MSs and can therefore be considered representative at the EU level. Over the same period, only nine MSs reported data for bovine animals under 1 year of age (calves). Likewise, only 11 MSs and United Kingdom reported data for fattening turkeys (turkeys). As the MSs which are the main producers of meat derived from calves and turkeys in the EU are among the reporting MSs, those data can still be considered representative at the MS‐group level.
General observations
In the MS group (including UK), resistance to ampicillin, sulfamethoxazole, trimethoprim and tetracycline was common in indicator E. coli and reported by most MSs at ‘high’ or ‘very high’ levels in pigs and calves in 2019 and in broilers and turkeys in 2020. In poultry, resistance to ciprofloxacin and nalidixic acid was also common and several MSs reported ‘very high’ or ‘extremely high’ levels in both broilers and turkeys. The frequent occurrence of resistance to those substances probably reflects a widespread past and present use of these antimicrobials in food‐producing animals in several MSs.
There were notable spatial differences in the levels of resistance to most antimicrobials as well as in occurrence of MDR, complete susceptibility and for the summary index KOICS. Regarding pigs and broilers, the situation was generally more favourable in northern than in southern and eastern Europe. The limited number of countries reporting data for calves and turkeys precludes valid conclusions on spatial differences, but the available data for turkeys indicate a similar spatial distribution as for pigs and broilers. For calves, the picture was more complex and although the most favourable situation was reported by the Nordic countries (Norway, Denmark), countries in southern (Portugal, Croatia) and central Europe (Switzerland) also reported favourable situations in comparison to neighbouring countries in these regions.
Overall, in several countries, there appear to be trends towards reduction of resistance in indicator E. coli, notably in broilers and turkeys. For several antimicrobials there are statistically significant associations between use of antimicrobials in food‐producing animals and occurrence of resistance in intestinal E. coli from these animals (ECDC, EFSA and EMA, 2021). The positive trends in several countries are therefore possibly due to the overall decline in sales of antimicrobials for use in animals since 2011, as documented in the ESVAC report (EMA, 2021). It should however be noted that in some countries, levels of resistance to individual antimicrobials, complete susceptibility and KOICS are consistently stable at low levels and major changes cannot be expected.
Of the 13,529 isolates of indicator E. coli from pigs, calves, broilers and turkeys phenotypically tested in 2019 and 2020, resistance to carbapenems (meropenem) was not detected. This is a strong indication that carbapenem resistance is infrequent in commensal E. coli from these food‐producing animals in Europe. Further information on carbapenem resistance is found in Section 5 ESBL.
Comparison of resistance in fattening pigs (pigs), bovines under 1 year of age (calves), broilers and fattening turkeys (turkeys) at the EU MS‐group level
At the EU MS‐group level (including UK), there were no major differences in levels of resistance to gentamicin, cefotaxime, ceftazidime, meropenem, tigecycline, azithromycin and colistin between the four animal categories. Median levels were ‘rare’, ‘very low’ or ‘low’ in all four categories, although individual countries reported considerably higher levels for some antimicrobials in some animal categories. Also, for sulfamethoxazole and trimethoprim were there no major differences and median levels were ‘high’ in all four animal categories except for calves where the median level of trimethoprim resistance was ‘moderate’.
In contrast, levels of resistance to some antimicrobials were higher in poultry than in pigs and calves. Thus, median levels of ampicillin resistance were ‘very high' in broilers and turkeys but ‘high’ in pigs and calves. Likewise, median levels of ciprofloxacin and nalidixic acid resistance were ‘very high’ in broilers and ‘high’ in turkeys but ‘low’ or ‘moderate’ in pigs and calves. Additionally, median levels of chloramphenicol resistance were ‘high’ in turkeys, ‘moderate’ in pigs and calves and ‘low’ in broilers. and median levels of tetracycline resistance were ‘very high’ in turkeys and ‘high’ in pigs, broilers and calves. The data on occurrence of MDR and completely susceptible isolates also indicate that resistance in general was more common in isolates from poultry than in isolates from pigs and calves (see below).
The observed differences between animal species likely reflect a difference in the quantity of antimicrobials used in the different animal categories, but possibly also the mode of administration. In poultry, flock treatment is almost exclusively practised, whereas pigs and calves in some countries mainly are treated individually.
Complete susceptibility and multidrug resistance in all reporting countries
Considering all reporting countries, median levels of E. coli isolates susceptible to all antimicrobial classes tested was lower in broilers (22.0%) and turkeys (27.7%) than in pigs (38.8%) and calves (53.9%). Conversely, MDR isolates were more common in broilers (median 38.0%) and turkeys (median 47.6%) than in pigs (median 30.6%) and calves (median 23.2%). In all four animal populations, there were marked differences in levels of complete susceptibility, as well as in MDR, between countries. Generally, completely susceptible isolates from pigs, broilers and turkeys were more common in northern than in southern and eastern Europe whereas the converse situation was observed for MDR. For calves, there was no obvious spatial pattern, and a favourable situation was reported from the Nordic countries (Norway, Denmark) as well as in countries in southern Europe (Portugal, Croatia).
Tetracycline, ampicillin, sulfamethoxazole and trimethoprim were the antimicrobials most often represented in the patterns of MDR isolates, often in combination with other substances. About half of the MDR isolates from pigs (52.3%) and calves (48.3%) were resistant to all these antimicrobials and they were common also in MDR isolates from broilers (41.5% and turkeys (42.2%). Additionally, quinolone resistance was common in MDR isolates from broilers (75.9%) and turkeys (73.8%) but less common in isolates from pigs (27.3%) and calves (39.5%). The frequent occurrence of these substances as a core component of MDR patterns presumably reflects an extensive usage in several countries over many years and that the genes conferring resistance to these substances often are linked on mobile genetic elements, resulting in co‐selection.
Key outcome indicator of complete susceptibility
The key outcome indicator KOICS accounts for differences in the relative size of food animal populations in a country and is relevant in the evaluation of risks related to AMR in food‐producing animals. There were marked variations in KOICS from < 20% in nine MSs to > 80% in Norway. Lower KOICS were generally observed in countries in eastern and southern Europe and the highest in countries in the northern part. It is to be noted that if KOICS is used as sole indicator of the AMR situation in a country, positive or negative trends in one animal category of small relative size may go unnoticed. To fully appreciate the situation within a country, data on resistance/complete susceptibility should therefore also be evaluated at the level of each animal population monitored.
Temporal trends in resistance
Statistically significant decreasing trend in resistance to ampicillin, ciprofloxacin, cefotaxime, tetracycline and colistin as well as trends towards higher levels of complete susceptibility and KOICS reveal a progress towards reduced resistance in several countries and also in the EU MS‐group. It should however be noted that in some countries, levels of resistance to individual antimicrobials, complete susceptibility and KOICS are consistently stable at low levels and major changes cannot be expected.
Considering all animal categories and countries evaluated, there were 129 decreasing and 56 increasing trends in resistance to ampicillin, ciprofloxacin, cefotaxime and tetracycline in the period 2009–2020. In addition, there were seven decreasing and six increasing trends for colistin resistance in the same period. An improved situation was most pronounced in poultry and in the MS‐group (including UK) there were significant decreases in resistance to ampicillin, ciprofloxacin, cefotaxime, tetracycline and colistin in both broilers and turkeys. In contrast, for pigs the only significant decrease in the MS‐group was for tetracycline resistance, whereas colistin resistance had increased significantly. For calves, there were in the MS group no significant trends in resistance to any of the antimicrobials.
For levels of complete susceptibility and considering all animal categories and countries evaluated, there were altogether 32 increasing and six decreasing trends in the period 2014–2020. As for resistance to individual antimicrobials, the improved situation was most pronounced in poultry, and in the MS group (including UK), there were increasing trends in complete susceptibility for both broilers and turkeys but no change for pigs and calves.
There were significant increases of KOICS in 14 of the 29 reporting countries and trends towards decreasing values in four countries. Trends in complete susceptibility of isolates from pigs were usually reflected in KOICS, whereas trends in isolates from calves, broilers and turkeys had smaller impact and were not always mirrored in the summary index.
Resistance to highest priority critically important antimicrobials (HPCIA)
Of the antimicrobials tested in the mandatory monitoring of indicator E. coli from caecal content, ciprofloxacin (fluoroquinolones), cefotaxime and ceftazidime (third‐generation cephalosporins), colistin (polymyxin E) and azithromycin (macrolides) are categorised by the WHO as highest priority critically important antimicrobials (HPCIA) (WHO, 2019). Bacteria resistant to these antimicrobials in food‐producing animals is therefore of particular interest due to the risk of spread to humans along the food chain.
Median levels of phenotypic resistance to third‐generation cephalosporins (cefotaxime and ceftazidime) at the MS‐group level were overall ‘low’ in pigs, broilers and turkeys and ‘very low’ in calves. For each animal category, about two thirds of the countries reported isolates at levels up to at most 5.9% in pigs, 5.6% in calves, 7.1% in broilers and 6.4% in turkeys. Within the mandatory monitoring, samples of caecal content are also cultured on selective media to specifically detect the presence of E. coli resistant to third‐generation cephalosporins. The results of these analyses are presented in Section 5 ESBL.
Ciprofloxacin and nalidixic acid resistance in isolates from pigs and calves were recorded at ‘low’ or ‘moderate’ median levels at the MS‐group level. In contrast, median levels of ciprofloxacin and nalidixic acid resistance were ‘very high’ in broilers and ‘high’ in turkeys. At the MS‐group level (including UK), there were decreasing trends in resistance to ciprofloxacin in E. coli isolates from broilers and turkeys. Notably, a substantial proportion of isolates from all animal categories were resistant to ciprofloxacin but not to nalidixic acid which indicates the presence of transmissible genes mediating quinolone resistance.
‘Microbiological’ resistance to both ciprofloxacin and third‐generation cephalosporins was observed in only 107 of the 13,529 E. coli isolates tested in 2019 and 2020, 42 of these isolates also showed ‘clinical’ resistance to both substances. The levels of ‘microbiological’ co‐resistance were slightly higher in broilers (1.0%) and turkeys (1.0%) than in pigs (0.5%) and calves (0.6%).
Median levels of azithromycin resistance in MSs (including UK) were ‘very low’ in calves and turkeys and ‘low’ in pigs and broilers. Most countries reported no azithromycin resistance or single isolates only, but a few countries reported higher levels, up to 11.3% for broilers, 14.7% for pigs and 22.6% for turkeys. Azithromycin is an azalide antimicrobial, a subgroup of the macrolides, not used in animals. Possibly, selection pressure exerted by use of other macrolides, e.g. tylosin, in food‐producing animals may have favoured emergence of azithromycin resistance.
Altogether only 165 of the 13,529 isolates tested in 2019 and 2020 showed phenotypic resistance to colistin. Median levels of colistin resistance in the MS‐group (including UK) were ‘rare’ for pigs and broilers, ‘very low’ in calves and ‘low’ in turkeys although much higher levels were however reported in individual countries. As documented in the recent JIACRA III report (ECDC, EFSA and EMA, 2021), there is a statistically significant association between consumption of polymyxins (colistin) in food‐producing animals and occurrence of colistin resistance in intestinal E. coli from these animals. The high occurrence of resistance in some animal categories in individual countries indicates large differences in the usage of colistin in Europe, which also was documented in the ESVAC report (EMA, 2021). However, in the MS‐group (including UK) there were statistically significant decreasing trends in colistin resistance in isolates from broilers and turkeys whereas the levels in pigs have increased. The decrease in resistance for poultry agrees with the 69.8% decrease in sales of polymyxins for food‐producing animals in EU in the period 2011–2018, as reported in the ESVAC report (EMA, 2021). This shows that measures taken in individual countries to reduce the use of colistin, and thereby occurrence of resistance, are effective.
5. Extended‐spectrum β‐lactamase (ESBL)‐, AmpC‐ and/or carbapenemase (CP)‐producing Salmonella and Escherichia coli
All ESBL‐, AmpC‐ or CP‐producers prevalence and occurrence Tables from the 2019 and 2020 monitoring, as well as the tables on occurrence of resistance (panel 1 and panel 2) mentioned in this chapter can be found in the Annex D and the Salmonella spp., E. coli or ESBL Microsoft Excel® documents available on Zenodo at: https://doi.org/10.5281/zenodo.6257446. Materials and methods are included in the Appendix F.
5.1. Key findings
The occurrence of presumptive ESBL, AmpC or ESBL+AmpC‐producing Salmonella spp. was generally low in 2020. The situation in specific animal population can however be largely affected by particular circumstances in different countries, as both occurrence of Salmonella spp. and proportion of presumptive ESBL, AmpC or ESBL+AmpC‐producing isolates may vary among countries.
In 2020, five isolates of E. coli with a carbapenemase phenotype were detected in the harmonised monitoring. One isolate from broilers detected through the specific monitoring of ESBL/AmpC‐producing E. coli in food‐producing animals was reported in one Member State (Austria). This isolate harboured the metallo‐β‐lactamase resistance gene bla VIM‐1, as well as the β‐lactamase genes bla TEM‐1C and bla TEM‐1B. Furthermore, within the voluntary specific monitoring of carbapenemase‐producing microorganisms using selective media for carbapenemase producers, three isolates from broilers (Romania) and one from fattening turkeys (Spain) were also reported. The isolate from fattening turkeys carried the gene bla TEM‐1B but no genes encoding carbapenemases was detected. The isolates reported by Romania are pending confirmation.
The occurrence of ESBL/AmpC‐producing E. coli in food‐producing animals is still high, when considering the group of all Member States (+ the United Kingdom). Statistically significant decreasing trends are however observed in many countries for several animal categories, as well as at the level of the reporting MS‐group (+ the United Kingdom) for broilers and turkeys.
The occurrence of ESBL/AmpC‐producing E. coli on broiler meat is still high, but also here a statistically significant decreasing trend has been observed over the study period.
At the reporting MS‐group level (plus the United Kingdom), the key outcome indicator of ESBL‐ and/or AmpC‐producing E. coli (KOIESC) has decreased by 16% since 2015–2016 in a statistically significant manner.
In more than half of the Member States (and the other reporting countries), a statistically significant decreasing trend in the key outcome indicator of ESBL‐ and/or AmpC‐producing E. coli (KOIESC) is observed. In one quarter of the reporting countries, an increasing trend is registered, and in the remaining quarter, no statistically significant trend is seen.
5.2. Context
The occurrence of ESBL, AmpC or CP‐producing bacteria in the intestinal flora of animals is undesirable, as it might lead to dissemination of resistant bacteria from animals and food to humans. Bacteria from animals with such resistance should also be considered as a reservoir of resistance genes which may be transferable to other bacteria including food‐borne zoonoses, such as Salmonella spp., further adding to the potential public health consequences. No statistically significant associations between resistance to third‐generation cephalosporins among indicator E. coli from animals and invasive E. coli from humans were observed in the latest JIACRA report (ECDC/EFSA/EMA, 2021). Nevertheless, the epidemiology of ESBL‐, AmpC‐ and CP‐producing E. coli is complex and the performance of a harmonised monitoring to specifically investigate their prevalence in healthy animals and food derived thereof provides additional information to the data already available in different countries.
The specific monitoring of ESBL‐/AmpC‐/CP‐producing E. coli in caecal samples of fattening pigs and bovine animals under 1 year of age, as well as pig meat and bovine meat gathered at retail was mandatory in 2019, whereas the specific monitoring in caecal samples of broilers, fattening turkeys and fresh broiler meat sampled at retail was mandatory in 2020. In 2019, the specific monitoring was carried out by 28 MSs and four non‐MSs for meat from bovine animals, by 28 MSs and three non‐MSs for fattening pigs and meat from pigs, and by nine MSs and two non‐MSs for bovine animals under 1 year. In 2020, the monitoring was performed by 27 MSs and four non‐MSs for broilers and broiler meat, and 11 MSs and two non‐MS for fattening turkeys.
The categorisation of the isolates as ESBL‐, AmpC‐ or CP‐producers is based on the phenotype exhibited by the isolates and is performed in accordance with criteria described in Materials and Methods, Appendix F. Most, but not all, isolates resistant to extended spectrum cephalosporins (ESC) are therefore classified into those categories. However, not all classified isolates, in particular those exhibiting an AmpC phenotype, do necessarily carry any transferable genes.
5.3. Routine antimicrobial resistance monitoring in food‐producing animals and derived meat: presumptive ESBL/AmpC/CP producers
In 2019 and 2020, third‐generation cephalosporin resistance was identified in Salmonella spp. from broilers, fattening turkeys and laying hens, and from carcases (meat) of broilers, pigs and bovine animals under 1 year of age, as well as in indicator E. coli isolates from broilers, fattening turkeys, fattening pigs and bovine animals under 1 year of age, within the framework of the routine monitoring.15
ESC resistance and ESBL/AmpC/CP phenotypes in Salmonella spp.
The proportion of ESC resistant Salmonella spp. isolates (tested with panel 2) collected within the routine monitoring was generally low in 2019 and 2020 (ranging between 0% and 2.1% of the investigated isolates, depending on the animal population, Annex C.2 and C.3). The occurrence of ESC‐resistant Salmonella isolates in a specific animal population can be greatly affected by the particular situation in certain countries regarding the prevalence of different Salmonella serovars. As an example, considering all reported isolates, about two thirds of the Salmonella isolates from broilers derived from one single MS in 2020 (see chapter 2, Salmonella for further details). At the reporting MS‐group level, the occurrence of presumptive ESBL, AmpC or ESBL+AmpC‐producing Salmonella spp. was 2.1% in broilers, 0.4% in turkeys, 0.2% in laying hens, 0.4% in fattening pigs and 0% in bovine animals under 1 year of age (Table 13).
Table 13.
Summary of presumptive ESBL‐/AmpC‐/CP‐producing Salmonella spp. from animals and meat (carcases) and indicator E. coli from caecal samples collected within the routine monitoring, EU MSs 2019–2020
| Matrix |
Presumptive ESBL and/or AmpC producers( a ) n (%R) |
Presumptive ESBL n (%R) |
Presumptive AmpC n (%R) |
Presumptive ESBL+AmpC n (%R) |
Presumptive CP producers( e ) n (%R) |
|---|---|---|---|---|---|
| Salmonella | |||||
|
Broiler meat (N = 905, 18 MSs) |
3 (0.3) | 2 (0.2) | 1 (0.1) | 0 | 0 |
|
Broilers (N = 1,955, 22 MSs) |
41 (2.1) | 38 (1.9) | 3 (0.2) | 0 | 0 |
| Fattening turkeys (N = 674, 16 MSs) | 3 (0.4) | 3 (0.4) | 0 | 0 | 0 |
|
Laying hens (N = 1,184, 24 MSs) |
4 (0.3) | 2 (0.2) | 2 (0.2) | 0 | 0 |
|
Pig meat (N = 1,088, 26 MSs) |
4 (0.4) | 2 (0.2) | 1 (0.1) | 0 | 0 |
|
Bovine meat (N = 91, 7 MS) |
0 | 0 | 0 | 0 | 0 |
| E. coli | |||||
|
Broilers (N = 3,923, 27 MSs) |
53 (1.4) | 44 (1.1) | 16 (0.4) | 7 (0.2) | 0 |
| Fattening turkeys (N = 1,557, 11 MSs) | 26 (1.7) | 23 (1.5) | 6 (0.4) | 3 (0.2) | 0 |
|
Fattening pigs (N = 4,296, 28 MSs) |
57 (1.3) | 43 (1.0) | 15 (0.3) | 1 (< 0.1) | 0 |
|
Bovines, < 1 year (N = 1,605, 9 MSs) |
19 (1.2) | 17 (1.1) | 2 (0.1) | 0 | 0 |
N: total of isolates reported for this monitoring by the MSs; n: number of the isolates resistant; %R: percentage of resistant isolates; ESBL: extended‐ spectrum b‐lactamase; MSs: EU Member States.
According to EUCAST Guidelines (EUCAST, 2019), only isolates showing an MIC > 1 mg/L for cefotaxime and/or ceftazidime (screening breakpoint) were considered (see Appendix F, Materials and methods).
All isolates showing clavulanate synergy with cefotaxime, ceftazidime or with both compounds, suggesting the presence of an ESBL (independently of the presence of other mechanisms).
Isolates with microbiological resistance to cefoxitin, suggesting the presence of an AmpC enzyme (independently of the presence of other mechanisms).
Isolates showing synergy with cefotaxime or ceftazidime and with microbiological resistance to cefoxitin, suggesting the presence of ESBL and AmpC enzymes in the same isolate. These isolates are also included in the ESBL and AmpC columns.
Isolates with microbiological meropenem resistance.
Detailed data per country and matrix can be found in Annex D.2 and D.3.
ESC resistance and ESBL/AmpC/CP phenotypes in indicator E. coli
The proportion of ESC‐resistant indicator E. coli isolates (tested with panel 2) collected within the routine monitoring was generally low in 2019 and 2020 (ranging between 1.2% and 1.7% of the investigated isolates, depending on the animal population (Annex C). Among the reporting MSs, the occurrence of ESC resistance varied from 0% to 5.9% in fattening pigs, from 0% to 4.1% in bovines under 1 year of age, from 0% to 7.1% in broilers and from 0% to 6.4% in fattening turkeys (see chapter 4, E. coli, for further details). When only considering the isolates of indicator E. coli with ESBL/AmpC‐production, similar variations in occurrences are observed. At the MS‐group level, the occurrence of presumptive ESBL, AmpC or ESBL+AmpC‐producers in indicator E. coli was 1.4% in broilers, 1.7% in turkeys, 1.3% in fattening pigs and 1.2% in bovine animals under 1 year of age (0). For all matrices, the ESBL phenotype was more frequent than the AmpC phenotype. Detailed data per matrix and country can be found in Annex D.2 and D.3.
5.4. Specific monitoring of ESBL/AmpC‐producing E. coli in food‐producing animals and derived meat
5.4.1. Prevalence and occurrence of presumptive ESBL/AmpC/CP producers
The specific monitoring16 of ESBL and AmpC producers employs culture of samples on selective media (containing cefotaxime at 1 mg/L, as recommended by EUCAST), which can detect very low numbers of resistant isolates present within a sample. The occurrence and prevalence of E. coli exhibiting ESBL, AmpC or ESBL+AmpC phenotypes from the food‐producing animal populations and derived meat, assessed at the reporting MS‐group level, are presented in 5.4.2. The prevalence of presumptive ESBL or AmpC‐producing E. coli for all matrices tested in 2019 and 2020 are shown in Figure 38. Detailed prevalence and occurrence data per country and matrix can be found in Annex D.2 and D.3.
Table 14. Summary of presumptive ESBL‐/AmpC‐/CP‐producing E. coli from food‐producing animals and derived meat, specific monitoring, EU MSs, 2019–2020
| Matrix |
Presumptive ESBL and/or AmpC producers( a ) |
Presumptive ESBL producers( b ) |
Presumptive AmpC producers( c ) |
Presumptive ESBL and AmpC producers |
Presumptive CP producers |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N |
Prev % |
N | Occ % |
Prev % |
n | Occ % | Prev % | n | Occ % | Prev % | n | Occ % | Prev % | |
|
Pig meat – 2019 (28 MSs, Ns = 6,487, N = 444) |
443 | 6.7 | 361 | 81.3 | 5.5 | 97 | 21.8 | 1.5 | 15 | 3.4 | 0.2 | 0 | ||
|
Bovine meat – 2019 (28 MSs, Ns = 6,308, N = 292) |
292 | 4.9 | 255 | 87.3 | 4.3 | 43 | 14.7 | 0.7 | 6 | 2.1 | 0.1 | 0 | ||
|
Broiler meat – 2020 (27 MSs, Ns = 6,242, N = 1,977) |
1,956 | 31.5 | 1,457 | 73.7 | 23.4 | 578 | 29.2 | 9.3 | 79 | 4.0 | 1.3 | 0 | ||
|
Pigs – 2019 (28 MSs, Ns = 6,792, N = 2,923) |
2,899 | 42.7 | 2,318 | 79.3 | 34.1 |
6 61 |
22.6 | 9.7 | 79 | 2.7 | 1.2 | 0 | ||
|
Bovines, < 1 year – 2019 (9 MSs, Ns = 2,688, N = 1,215) |
1,205 | 36.4 | 1,123 | 92.4 | 33.9 | 120 | 9.9 | 3.6 | 38 | 3.1 | 1.1 | |||
|
Broilers – 2020 (27 MSs, Ns = 7,537, N = 2,914) |
2,881 | 39.6 | 2,159 | 74.1 | 29.7 | 825 | 28.3 | 11.3 | 103 | 3.5 | 1.4 | 1 | < 0.1 | < 0.1 |
|
Turkeys – 2020 (11 MSs, Ns = 2,757, N = 924) |
921 | 34.2 | 827 | 89.5 | 30.7 | 143 | 15.5 | 5.3 | 49 | 5.3 | 1.8 | |||
Ns: Number of animal/meat samples; N: Number of isolates tested; n: Number of resistant isolates; % Occ: Percentage of cephalosporin‐resistant isolates presenting a presumptive phenotype; % Prev: Percentage of samples harbouring a presumptive ESBL/AmpC‐producing E. coli; ESBL; extended‐spectrum β‐lactamase.
Isolates exhibiting only ESBL‐ and/or only AmpC‐ and/or ESBL+AmpC‐phenotype.
Isolates exhibiting an ESBL‐ and/or ESBL+AmpC‐phenotype.
Isolates exhibiting an AmpC and/or ESBL+AmpC‐phenotype.
Figure 38.
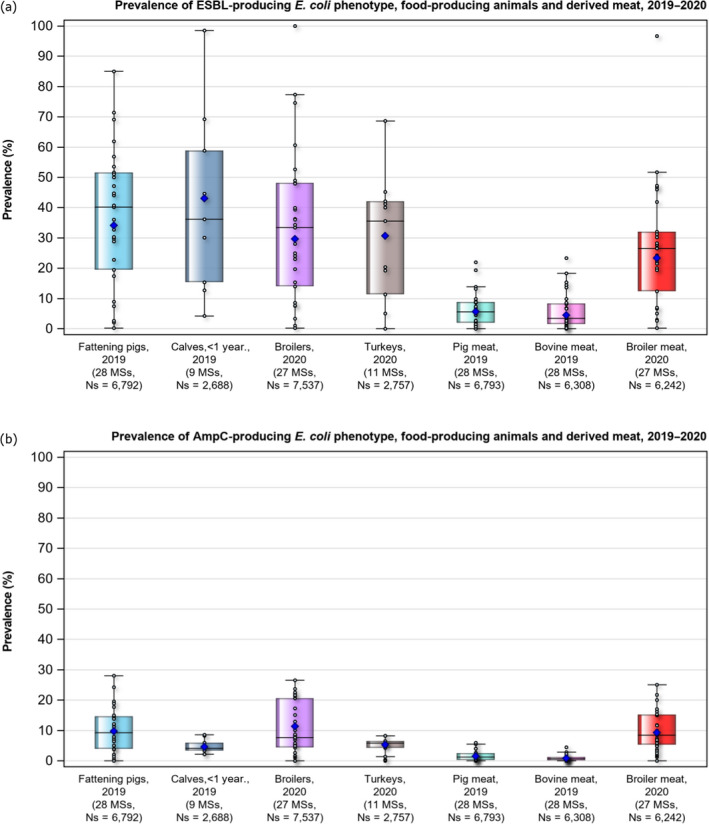
Prevalence of presumptive ESBL‐producing (a) and AmpC‐producing (b) E. coli from the specific monitoring of ESBL/AmpC‐producing E. coli, 2019–2020
Regarding food‐producing animals, marked variations in the prevalence of presumptive E. coli ESBL and/or AmpC‐producers17 were observed between the MSs, as it ranged from 2.1% (Cyprus) to 99.2% (Italy) in fattening pigs, from 1.1% (Italy) to 70.8% (Germany) in bovine animals under 1 year of age, from 0.3% (Finland) to 98.6% (Slovakia) in broilers and from 0% (Sweden) to 70.4% (Spain) in fattening turkeys (Figures 37 and 39, Annex D.2 and D.3). Important differences among the reporting countries were also evident when assessing the prevalence of ESBL or AmpC producers separately (Annex D.2 and D.3). Notably, one Ms (Austria) detected one isolate with a CP phenotype from broilers. This isolate harboured the metallo‐β‐lactamase resistance gene bla VIM‐1, as well as the β‐lactamase genes bla TEM‐1C and bla TEM‐1B.
Figure 39.
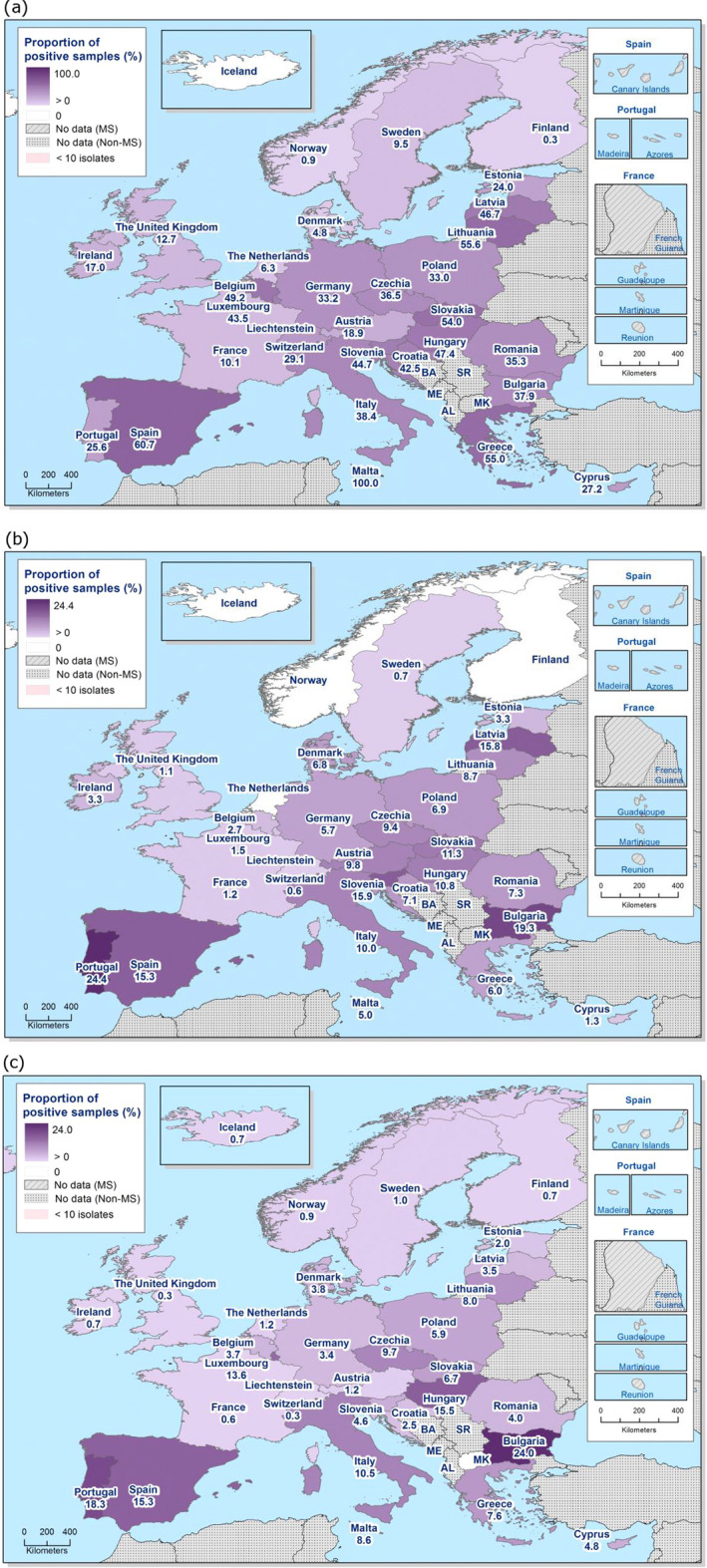
Spatial distribution of the prevalence of presumptive ESBL and/or AmpC‐producing E. coli from (a) meat from broilers in 2020, (b) meat from pigs in 2019 and c) meat from bovines in 2019, EU MSs and non‐MSs, 2019–2020
Regarding meat, the prevalence of presumptive E. coli ESBL and/or AmpC‐producers3 in meat from broilers varied markedly between the MSs, ranging from 0.3% (Finland) to 100% (Malta), whereas the prevalence recorded in meat from pigs and bovine animals was less variable, ranging from 0% (Finland and the Netherlands) to 24.4% (Portugal) for pig meat and from 0.3% (the United Kingdom) to 24.0% (Bulgaria) for bovine meat (Figures 37 and 38, Annex D.2 and D.3). The differences among reporting countries were also evident when the prevalence of ESBL or AmpC producers was considered separately (Annex D.2 and D.3).
5.4.2. Relative abundance (occurrence) of presumptive ESBL/AmpC producers
As only one isolate per sample is further investigated, the relative abundance of E. coli with an ESBL or AmpC phenotype when both are present in the sample influences the probability of detecting either phenotype. In the animal populations/food matrices monitored, at the reporting MS‐group level and in the majority of the countries, the detection of presumptive ESBL‐producing E. coli exceeded that of presumptive AmpC‐producing E. coli (Table 2, Figure 39, 40, 41, 42–43 and Annex D). Nevertheless, the occurrence of the different phenotypes varied considerably among the reporting MSs for certain matrices. After excluding the MSs with less than 10 presumptive isolates tested, the occurrence of the ESBL phenotype ranged from 20.5% (Sweden) to 97.5% (Bulgaria) in fattening pigs; from 29.4% (Sweden) to 98.5% (Portugal) in broilers; and from 27.6% (Sweden) to 96.7% (Malta) in meat from broilers (Annex D.2 and D.3). For the other matrices, the difference in occurrence of the ESBL phenotype was less pronounced and ranged between 52% (Denmark) and 98.5% (Italy) in bovines under 1 year of age, between 66.7% (Hungary) and 100% (Romania) in fattening turkeys; between 41.7% (Denmark) and 100% (Bulgaria and Greece) in meat from pigs and between 66.7% (Denmark and Poland) and 100% (Portugal, Slovakia and Spain) in meat from bovine animals. Bovine animals under 1 year of age and fattening turkeys are, however, only investigated by some Member States, as it is not mandatory for those with a small production. Furthermore, the overall prevalence of presumptive ESBL/AmpC producers in samples of meat from pigs and bovines is low.
Figure 40.
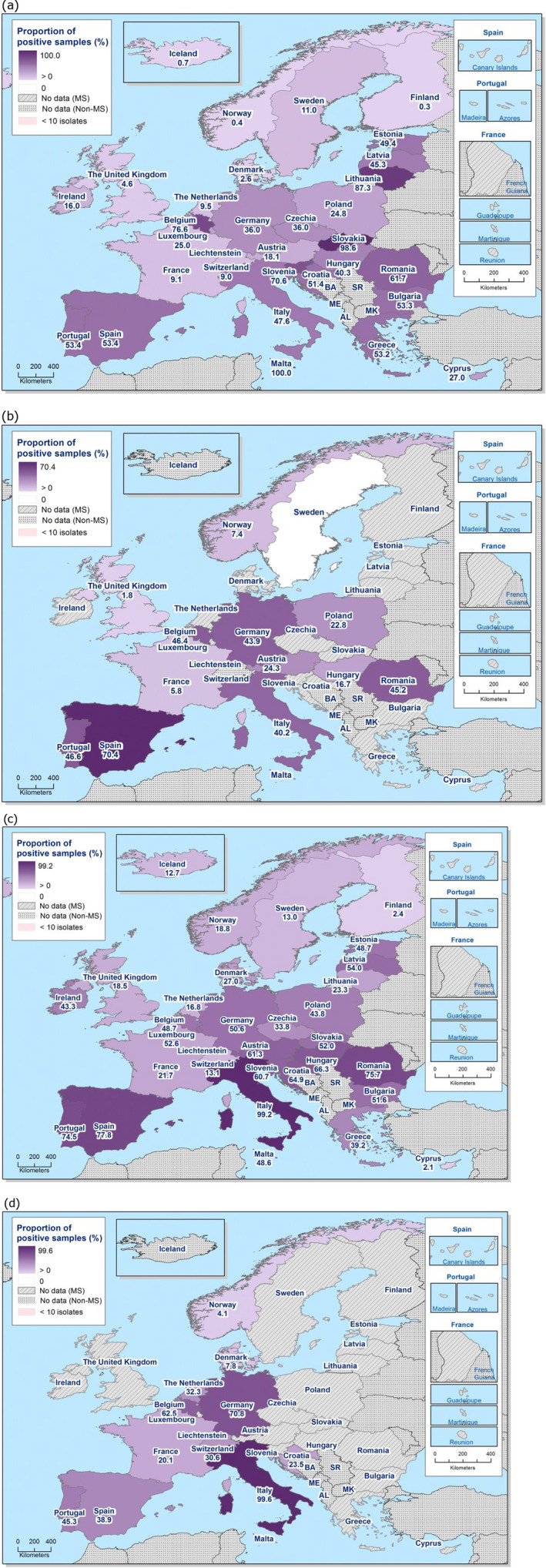
Spatial distribution of the prevalence of presumptive ESBL and/or AmpC‐producing E. coli from (a) broilers in 2020, (b) fattening turkeys in 2020, (c) fattening pigs in 2019 and (d) bovines under 1 year of age in 2019, EU MSs and non‐MSs, 2019–2020
Figure 41.
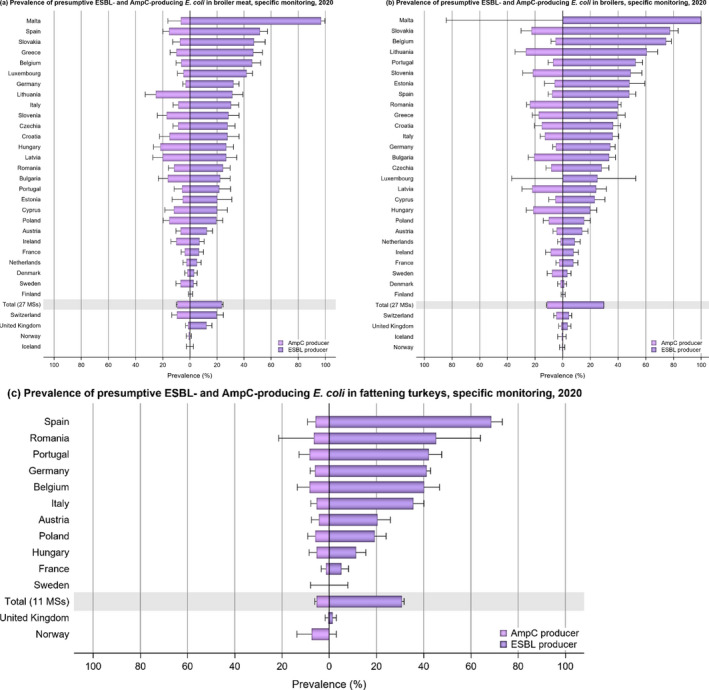
Prevalence of presumptive ESBL‐producing vs. AmpC‐producing E. coli from (a) meat from broilers, (b) broilers and (c) fattening turkeys, EU MSs and non‐EU MSs, 2020
- The upper bounds of the 95% confidence interval of the prevalence of ESBL- and/or AmpC-producing E. coli are also indicated.
Figure 42.
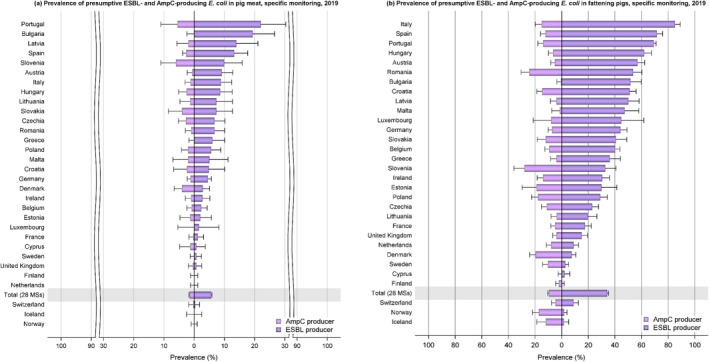
Prevalence of presumptive ESBL‐producing vs. AmpC‐producing E. coli from (a) meat from pigs and (b) fattening pigs, EU MSs and non‐EU MSs, 2019
- The upper bounds of the 95% confidence interval of the prevalence of ESBL‐ and/or AmpC‐producing E. coli are also indicated. Please note the different scales used for the x‐axis in the sub‐figures to improve the visibility of the variations among countries.
Figure 43.
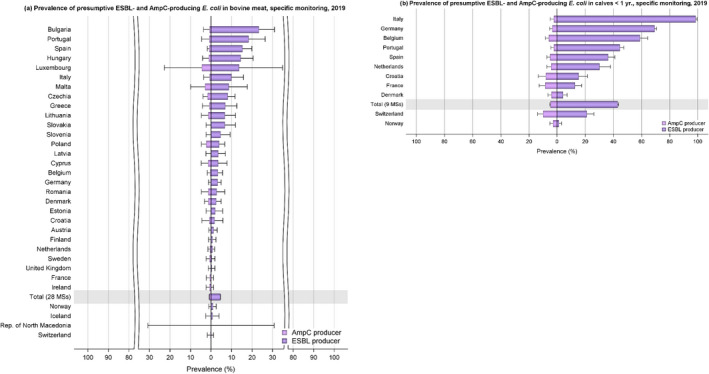
Prevalence of presumptive ESBL‐producing vs. AmpC‐producing E. coli from (a) meat from pigs and (b) fattening pigs, EU MSs and non‐EU MSs, 2019
- The upper bounds of the 95% confidence interval of the prevalence of ESBL‐ and/or AmpC‐producing E. coli are also indicated. Please note the different scales used for the x‐axis in the sub‐figures to improve the visibility of the variations among countries.
5.4.3. Temporal trends in prevalence of presumptive ESBL/AmpC/CP producers
The temporal trend in the prevalence of presumptive ESBL and AmpC‐producing E. coli in each separate animal population and meat category since the start of the harmonised mandatory monitoring is presented at both reporting country and MS‐group levels in Figures 44 and 45.
Figure 44.
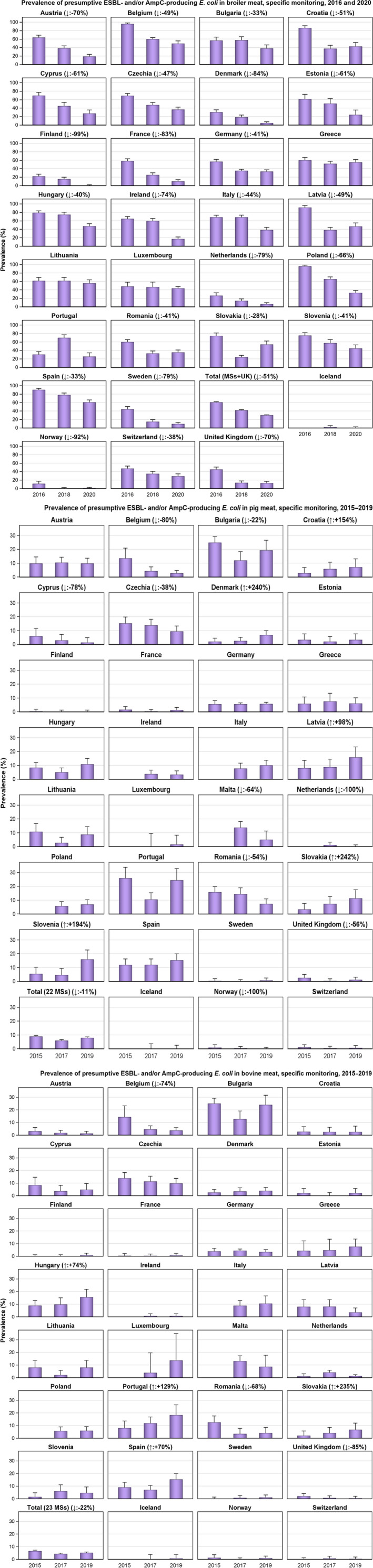
Trends on the prevalence of presumptive ESBL and/or AmpC‐producing E. coli in (a) meat from broilers, (b) meat from pigs and (c) bovine meat over the period 2015–2020, EU MSs and non‐MSs
- The upper bounds of the 95% confidence interval of the prevalence of ESBL‐ and/or AmpC‐producing E. coli are also indicated. Please note the different scales used for the x‐axis in the sub‐figures to improve the visibility of the variations among countries (a, 0–100%; b–c, 0–30%).
Figure 45.
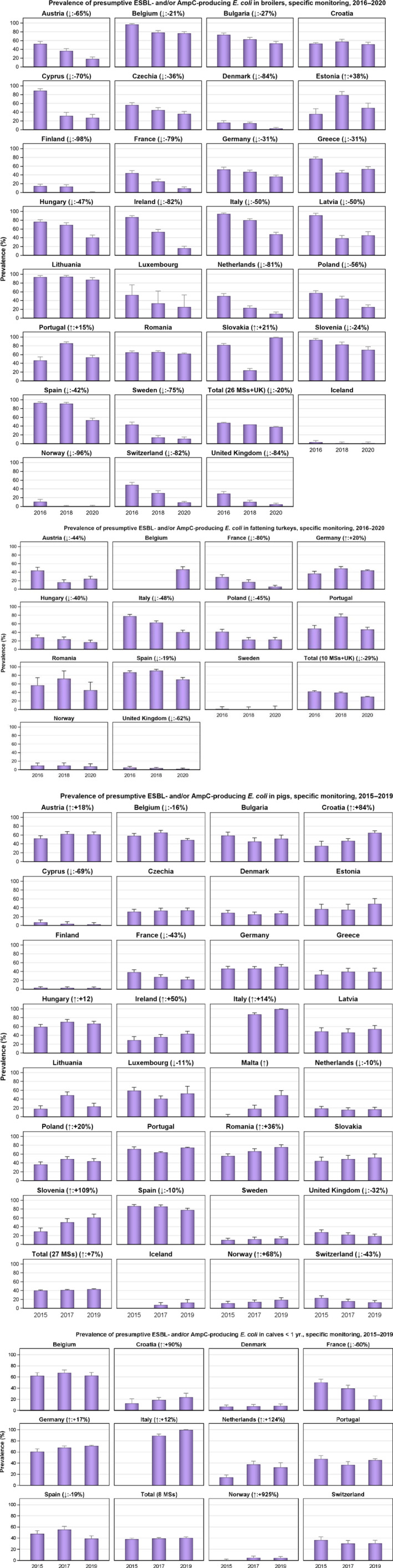
Trends on the prevalence of presumptive ESBL and/or AmpC‐producing E. coli in (a) broilers, (b) fattening turkeys, (c) fattening pigs and (d) bovines under 1 year of age, over the period 2015–2020, EU MSs and non‐MSs
- The upper bounds of the 95% confidence interval of the prevalence of ESBL‐ and/or AmpC‐producing E. coli are also indicated.
The prevalence of presumptive ESBL‐, AmpC‐ or ESBL+AmpC‐producing E. coli at the MS‐group level in broilers and meat from broilers has gradually decreased from around 60% in both sample types in 2016, to 38.0% (broilers) and 30.6% (meat from broilers) in 2020. Likewise, the prevalence in fattening turkeys has decreased from around 40% in 2016 to 30.7% in 2020. These decreasing trends are statistically significant and generally paralleled with statistically significant decreases observed in most reporting countries.
The prevalence of presumptive ESBL‐, AmpC‐ or ESBL+AmpC‐producing E. coli at the MS‐group level assessed in meat from pigs (6.7%) and meat from bovine animals (4.9%) in 2019 is comparable with that assessed in the same meat categories in the previous years. However, not all MSs have reported data for all these years and when only those who have done so are included in the analyses, statistically significant decreasing trends are demonstrated for these matrices.
Likewise, the prevalence of presumptive ESBL‐, AmpC‐ or ESBL+AmpC‐producing E. coli assessed in fattening pigs (42.7%) and bovine animals under 1 year of age (36.4%) in 2019 is comparable with that assessed in the same animal populations in the previous years. However, not all MSs have reported data for all these years and when only those who have done so are included in the analyses, statistically significant decreasing trends are demonstrated for fattening pigs. For bovine animals under 1 year of age, no statistically significant trend is discerned.
A decreasing trend in prevalence is observed in many of the reporting countries, and certain MSs report a considerable improvement over the study period. This however not uniform and some MSs have reported consistently high or very high prevalence over the study period. Detailed data on the prevalence assessed by country, animal population and meat category for 2019 and 2020 monitoring are presented in Figures 45 and 46 as well as in Annex D. For the 2015–2018 period, complementary data can be found in previous published reports.
Figure 46.
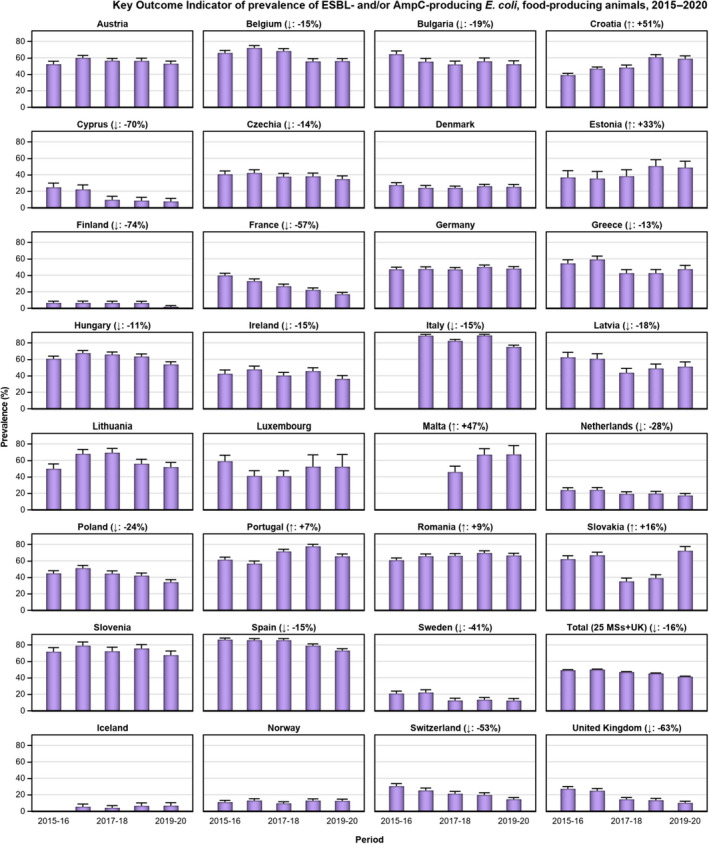
Changes in Outcome Indicator of ESBL‐ and/or AmpC producing E. coli (OIESC), 27 EU MSs and four non‐MSs, 2015–2020
-
(↓)(↑): indicates statistically significant decreasing/increasing trends over the 2015–2020 period.Rates of change are shown for the statistically significant decreasing/increasing trends observed.
5.4.4. Key Outcome Indicator of prevalence of ESBL and/or AmpC producers
The proportion of samples from broilers, fattening turkeys, fattening pigs and bovines under 1 year, weighted by PCU, that are identified as positive for presumptive ESBL and/or AmpC‐producing E. coli in the framework of the specific monitoring for ESBL‐/AmpC‐/CP‐producing E. coli according to Commission Implementing Decision 2013/652/EU has been retained as a summary indicator.
One of the most medically relevant forms of AMR is mediated by plasmid‐encoded ESBL genes (EFSA BIOHAZ Panel, 2011; Maslikowska et al., 2016). In contrast, the AmpC β‐lactamases in E. coli can be due to both upregulation and thus overexpression of chromosomally encoded existing ampC genes or to enzyme production by plasmid‐encoded transferable genes. There are many different enzymes that can destroy the β‐lactam ring (Pimenta et al., 2014), with a corresponding variety of genes and plasmids (Chong et al., 2011). The observation that ESBL‐carrying isolates from humans are often more related to chicken isolates than are susceptible isolates indicates that a proportion of ESBL and/or AmpC‐encoding isolates from agricultural settings may be of importance in human health care situations (Torneke et al., 2015). Plasmids carrying ESBL encoding genes can be transferred rapidly between E. coli strains (Handel et al., 2015) and selection can be driven by the use of many β‐lactam antimicrobials (Cavaco et al., 2008).
To account for differences in the relative size of food animal populations in a country, a weighted Key Outcome Indicator of the prevalence of ESBL‐ and/or AmpC‐producing E. coli (KOIESC) was calculated. The indicator is the weighted mean of the prevalence of ESBL‐ and/or AmpC‐producing E. coli in each of the four animal populations monitored. For the calculation of the mean, the value for each population was weighted in relation to the relative size of the populations within a country using the ‘population correction unit’ (PCU). PCU is a technical unit of measurement used as an indicator of animal population size and was developed by the EMA, primarily to estimate sales of antimicrobials corrected by the animal population in individual countries. The data sources and methodology for the calculation of PCU are comprehensively described in EMA’s report ‘Sales of veterinary antimicrobial agents in 31 European countries in 2019 and 2020’ (EMA, 2021). For each country, KOIESC was calculated using data reported for two consecutive years. Thus, values for 2015–2016 were calculated from data reported for fattening pigs and bovines under 1 year of age in 2015 and on data for broilers and fattening turkeys reported in 2016. Likewise, values for 2016–2017 were calculated from data for broilers and fattening turkeys reported in 2016 and on data for fattening pigs and bovines under 1 year of age reported in 2017, and so on.
At the reporting MS‐group level, the KOIESC was assessed at 41.3% in 2019–2020 and has decreased by 16% since 2015–2016 (Figure 46 and Annex D.5). The decreasing trend is statistically significant and generally reflected by decreases in many reporting countries.
The KOIESC differs greatly among countries and ranged from 3.1% in Finland to 78.0% in Malta. Furthermore, although nine countries (five MSs and four non‐MSs including UK) have an KOIESC on a moderate or lower level, eighteen countries (all MSs) have a KOIESC on a very high or extremely high level. A positive development manifested by a decreasing trend in the KOIESC is seen in seventeen countries (15 MSs and two non‐MS including UK). Still, in some of these countries, decreases are observed starting from very high or extremely high levels. For six MSs and two non‐MS, the KOIESC has remained stable, with no statistically significant trend discerned in any direction, over the period of study. Still, six MSs show an increasing trend in their KOIESC and all of these on at least a high level.
5.4.5. Discussion
As the classification of the isolates as being ESBL‐, AmpC‐ or ESBL+AmpC‐ producers is based on the phenotype observed, not all classified isolates, in particular those exhibiting an AmpC phenotype, do necessarily carry any transferable genes. Molecular investigations would be needed to show whether the isolates carry any transferable genes encoding resistance to ESC. Such investigations are however not mandatory at this stage.
Overall, the specific monitoring highlighted that presumptive ESBL‐, AmpC‐ or ESBL+AmpC‐producing E. coli were frequently observed in the caecal samples representative of all investigated animal populations monitored. However, a statistically significant decrease in the prevalence of ESBL and/or AmpC‐producing E. coli has been observed in both broilers and turkeys over the period 2016–2020. Notably, since 2012, the off‐label use of extended spectrum cephalosporins in poultry has been discontinued (EMA/CVMP, 2012). This has likely contributed to the decreased occurrence of resistance in these species.
In all monitored animal populations and meat food categories, the ESBL‐producing phenotype were more common than the AmpC‐producing phenotype at the reporting MS‐group level and in the majority of the reporting countries. The occurrence of the different phenotypes did, however, vary considerably among the MSs, and in some countries, the AmpC phenotype was dominant. As only one isolate per sample is to be further investigated, the relative abundance of bacteria with an ESBL and/or AmpC phenotype present in the sample will influence the probability of detecting either phenotype.
Similarly to the caecal samples of healthy broilers collected at slaughter, a statistically significantly decreased prevalence of presumptive ESBL‐, AmpC‐ or ESBL+AmpC‐producing E. coli in broiler meat has been observed. The prevalence assessed in meat was statistically lower, but still comparable to, that observed in broilers. Conversely, the prevalence of ESBL‐, AmpC‐ or ESBL+AmpC‐producing E. coli in meat of pigs and bovine animals was much lower than that assessed in fattening pigs and bovine animals under 1 year of age at slaughter. The range of the prevalence of these phenotypes in pig and bovine meat among the Member States also tended to be narrower than that observed in these animals at slaughter. The findings suggest that many of these animals are carrying ESBL‐, AmpC‐ or ESBL+AmpC‐producing E. coli in their intestinal content, but that the bacteria do not contaminate the carcases during the slaughter process; alternatively, that the bacteria do contaminate the carcases but are somehow removed later in the process. This may be impacted by the differences in the slaughter process applied to broilers compared with those applied to pigs and bovine animals.
5.5. Monitoring of carbapenemase (CP)‐producing E. coli
5.5.1. Mandatory E. coli ESBL/AmpcC/CP‐producers monitoring
The specific monitoring of ESBL/AmpC‐producing E. coli on selective media (including cefotaxime) also enables the detection of isolates with some mechanisms of carbapenem resistance. In 2019, one isolate with CP‐phenotype from a caecal sample collected at slaughter from pigs (Spain) and one isolate from meat from bovines (Republic of North Macedonia) were detected. The isolate reported by Spain has been confirmed to carry the genes bla TEM‐1B and bla CTX‐M‐1, but no genes encoding carbapenemases have been detected. The isolate reported by the Republic of North Macedonia is pending confirmation. Isolates with CP‐phenotype and producing VIM‐1 from pig caecal samples were reported by Germany in both 2015 and 2017. Those isolates belonged to different genetic types which indicates that the occurrence of the bla VIM‐1 gene is not restricted to a specific type of E. coli (EFSA and ECDC, 2019a,b).
Within the 2020 mandatory ESBL/AmpC monitoring, one isolate with CP‐phenotype from a caecal sample collected at slaughter from broilers (Austria) was detected. This isolate harboured the metallo‐β‐lactamase resistance gene bla VIM‐1, as well as the β‐lactamase genes bla TEM‐1C and bla TEM‐1B. In 2018, no isolates of carbapenem‐resistant E. coli were detected. In 2016, isolates of suspected carbapenem‐resistant E. coli were detected in broiler meat samples from Cyprus, but they were not confirmed in further analysis.
5.5.2. Voluntary specific CP producers monitoring
In 2019 and 2020, specific monitoring of CP‐producing microorganisms using selective media for CP‐producers, in accordance with a protocol developed by the EURL on AMR18 , 19, was performed on a voluntary basis by a number of countries (Annex D.4.; see Appendix F, materials and methods). Together during the 2 years, 25 countries (23 MSs and two non‐MSs) investigated 5,713 samples from fattening pigs; 1,778 samples from bovines under 1 year of age; 5,597 samples from broilers; 2,260 samples from fattening turkeys, 4,020 samples of meat from pigs, 2,888 samples of meat from bovines and 5,214 samples of meat from broilers giving a grand total of 27,470 samples. Among those, suspected CP‐producing E. coli was detected in three samples from broilers (Romania), one from fattening turkey (Spain), two samples from fattening pigs (one from Italy and one from Romania) and one sample of meat from pigs (Germany; Annex D.4). In addition, using samples collected on farms Germany detected two additional suspected CP‐producing E. coli from fattening pigs. The three isolates reported from Germany have been confirmed to carry bla VIM‐1 (pig meat), bla OXA‐48 (fattening pig) and bla GES‐5 (fattening pig), respectively (Irrgang et al., 2020a,b; Pauly et al., 2021). The isolate from fattening turkey reported by Spain has been confirmed to carry the gene bla TEM‐1B, but no genes encoding carbapenemases have been detected. The remaining isolates are pending confirmation.
In the years preceding 2019, a total of 6,751 (2015), 11,935 (2016), 16,296 (2017) and 13,202 (2018) samples, respectively, were investigated and nine of these samples generated suspected CP‐producing E. coli. Three of these, two from broilers and one from meat from broilers, reported in 2016 by Romania have been confirmed as bla OXA‐162 (bla oxa‐48‐like) carriers. The other two isolates, one isolate from broiler meat and one from broiler, reported in 2016 by Cyprus, were not confirmed as CP‐producers.
For 2020, the Netherlands also reported data on additional specific monitoring of CP‐producing E. coli in broilers, fattening pigs, bovines under 1 year, laying hens and dairy cows using a different isolation protocol. All these samples (n = 1,408) were negative.
5.5.3. Discussion
Among all samples and isolates investigated within the harmonised monitoring in 2019 and 2020, 11 E. coli with elevated MIC to meropenem were detected. Of these, four (one from broilers, one from broiler meat, one from fattening pigs and one from meat from bovines) where detected within the specific monitoring of ESBL/AmpC‐producing E. coli on selective media and seven (three from broilers, one from fattening turkey, two from fattening pigs and one from pig meat) within the specific voluntary monitoring of CP‐producing microorganisms using selective media for CP producers. In addition, two isolates from fattening pigs sampled on farms but detected by the same culturing methods were reported.
As the total number of suspected CP‐producing E. coli isolated within the monitoring is still low, it is difficult to determine whether the higher number of isolates collected in 2019 and 2020 compared with the previous years is the beginning of an increasing trend or just a coincidence. Regardless, the occurrence of CP‐producing E. coli among farm animals and meat derived thereof in several countries is of concern. Due to the public health importance of CP‐producing E. coli and/or Salmonella, both as pathogens and as vectors for resistance mechanisms, there is a need to follow up possible further developments in this area for farm animals and food derived thereof. In this sense, it is also important that the monitoring of CP‐producing E. coli as by 2021 has been strengthened in the way that this monitoring is now mandatory within the harmonised monitoring (as outlined in Commission Implementing Decision 2020/1729/EU). However, as many of the reporting countries already perform the monitoring on a voluntary basis, it remains to see how much difference in the number of investigated samples the change will make.
Apart from isolates detected within the harmonised monitoring in EU, CP‐producing Enterobacterales has also been reported, not only in companion animals, farm animals and food derived thereof but also from vegetables from many parts of the world including Europe (Zurfluh et al., 2015; Touati et al., 2017; Brouwer et al., 2018; Köck et al., 2018; Liu et al., 2018; Irrgang et al., 2019,2020a). In addition, a number of closely related but not identical plasmids carrying the carbapenem resistance gene bla VIM‐1 have been found in isolates from livestock in Germany (Pauly et al., 2021). Consequently, the concern of animals becoming a reservoir for CP‐producing Enterobacterales has been raised (Taggar et al., 2020) The reasons for CP‐producing bacteria occurring among animals are not known. As carbapenems have not been authorised for use in animals in the EU, one could speculate that, as suggested by Irrgang et al (2020a), the origin is actually the spill‐over of resistant genes and/or bacteria from public health. Furthermore, as CP‐producing Enterobacterales are probably still rare among the investigated animal species in Europe potential actions to preserve this situation can hopefully still be effective, ensuring that farm animals do not become an important source of such bacteria for humans.
6. Antimicrobial resistance in methicillin‐resistant Staphylococcus aureus (MRSA)
6.1. Key findings
The monitoring of MRSA in 2019 and 2020 provided useful information on the occurrence of MRSA in livestock and food. The situation continues to develop and evolve and there is a clear requirement for the continued monitoring and appropriate molecular characterisation of MRSA isolates recovered from livestock and food.
Molecular characterisation is necessary to fully evaluate the significance of MRSA isolates. There are limitations to the analyses which can be performed when spa‐typing is used as the only technique to characterise isolates. Isolates from the same spa‐type may have very divergent sources and characteristics. For the assessment of the risk for humans, determination of the production of the PVL toxin seems relevant as well as the presence of the immune evasion cluster.
Where typing data were available, most MRSA isolates were associated with spa‐types that are assigned to livestock associated (LA‐)MRSA in both reporting years. However, spa‐types associated with community acquired (CA‐) and hospital acquired (HA‐)MRSA were also reported, as well as mecC‐MRSA. The occasional detection of lineages of CA‐ and HA‐MRSA primarily associated with humans is not surprising, since the sporadic interchange of strains between humans and animals may be expected.
All spa‐types of clonal complex 398 and other clonal complexes reported in 2019–2020 in animals and food are provided in Tables 15 and 16 of the present chapter. Changes in the reported occurrence of different MRSA lineages may be assessed against this list in the future.
Isolates that could not be assigned to the clonal complex 398 were more frequent in isolates from food than in isolates from animals. This may point to contamination at slaughter or during processing with strains of non‐animal origin. Many of the observed types have however repeatedly been reported in animals making it difficult to assign them unequivocally to human sources.
A significant observation from the 2020 monitoring was the occurrence of a vancomycin‐resistant t011 isolate in meat from sheep. The isolate lacks the typical vancomycin resistance genes and it is likely, that the increased MIC of 4 mg/L was due to multiple point mutations as described in the literature.
A significant observation from the 2019 monitoring was the detection of linezolid‐resistant strains harbouring the cfr gene from pigs. Since linezolid is an important compound in human medicine for the treatment of MRSA, establishing whether linezolid resistance is widespread or more localised in distribution in MRSA in animals is highly relevant. The cfr gene may confer linezolid MICs of 4 mg/L along with resistance to other antimicrobials. It is therefore recommended that all isolates displaying linezolid MICs of ≥ 4 mg/L and exhibiting resistance to the other compounds typically conferred by cfr, are screened for this gene.
The observation of three mupirocin‐resistant isolates from pigs in 2019 likewise points to a substantial health risk should theses isolates be transmitted to humans.
Table 15.
spa‐types of CC398 and their detection in animals and food 2019–2020
| Spa‐type | Year | Animals (number of isolates) | Tot | Food (number of isolates) | Tot |
|---|---|---|---|---|---|
| t011 | 2019 | Pigs (137), calves (1), horses (4) | 142 | Pig meat (25), bovine meat (2) | 27 |
| 2020 | Broilers (2), turkeys (2), | 4 | Broiler meat (4), meat from sheep (1) | 5 | |
| t034 | 2019 | Pigs (87), calves (8), horses (6) | 101 | Pig meat (13) | 13 |
| 2020 | Broiler meat (4), meat from sheep (2) | 6 | |||
| t108 | 2019 | Pigs (2) | 2 | ||
| t779 | 20 | Pigs (1), calves (1) | 2 | ||
| t899* | 2019 | Pig meat (5) | 5 | ||
| t1451 | 2019 | Pigs (2), horses (1) | 3 | Pig meat (2) | 2 |
| 2020 | Meat from sheep (1) | 1 | |||
| t1456 | 2019 | Pig meat (1) | 1 | ||
| t1457 | 2019 | Pigs (1), calves (1) | 2 | ||
| t1580 | 2019 | Calves (1) | 1 | ||
| t1928 | 2019 | Pigs (1) | 1 | ||
| t2346 | 2019 | Pigs (2) | 2 | Bovine meat (1) | 1 |
| t2370 | 2019 | Pigs (2) | 2 | ||
| t2383 | 2019 | Pigs (1) | 1 | ||
| t2576 | 2020 | Meat from sheep (1) | 1 | ||
| t2582 | 2019 | Pigs (1) | 1 | ||
| t2922 | 2019 | Pigs (1) | 1 | ||
| t3041 | 2019 | Pigs (1) | 1 | ||
| t3119 | 2019 | Pigs (1) | 1 | ||
| t4652 | 2019 | Pigs (1) | 1 | ||
| t19979 | 2020 | Meat from sheep (1) | 1 |
*: t899 is sometimes also attributed to CC9 as it is a hybrid strain.
Table 16.
spa‐types of other clonal complexes and their occurrence in animals and food in 2019–2020
| spa‐type | Clonal complex (sequence type) | Year | Animals | Food | ||
|---|---|---|---|---|---|---|
| Population (number of isolates) | Tot | Matrix (number of isolates) | Tot | |||
| t002 | 5 | 2019 | 0 | Pig meat (1) | 1 | |
| t003 | 5 (ST3944) | 2019 | 0 | Pig meat (1) | 1 | |
| t008 | 8 (ST8) | 2019 | 0 | Pig meat (1), bovine meat (1) | 2 | |
| t012 | 30 | 2019 | 0 | Pig meat (1) | 1 | |
| t127 | 1 | 2019 | Dairy cows (1) | 1 | Pig meat (2), bovine meat (2) | 4 |
| t223 | 22 | 2020 | 0 | Meat from sheep (2) | 2 | |
| t267 | 97 | 2020 | 0 | Meat from sheep (1) | 1 | |
| t321 | 1 (ST5050) | 2019 | 0 | Pig meat (1) | 1 | |
| t843 | 130 | 2019 | Dairy cows (1), horses (1) | 2 | Pig meat (1) | 1 |
| t1154 | 5 | 2020 | 0 | Meat from sheep (1) | 1 | |
| t3256 | 130 | 2019 | Horses (1) | 1 | 0 | |
| t15010 | 97 | 2020 | 0 | Meat from sheep (1) | 1 | |
6.2. Context
Monitoring of methicillin‐resistant Staphylococcus aureus (MRSA) in food‐producing animals and food carried out periodically allows to identify trends in the diffusion and evolution of potentially zoonotic MRSA in food producing and other animals. In conjunction with systematic surveillance of MRSA in humans, transmission of MRSA from food‐producing animals to the human population may by analysed (EFSA, 2009a,b,2012a,b). To this end, in addition to determining the prevalence of MRSA in the different food chains, representative isolates should optimally be characterised with respect to their type and AMR. The monitoring of MRSA in animals and food in Europe is voluntary and only a limited number of countries reported MRSA occurrence data in 2019 and 2020. Some countries additionally reported data on spa‐ and/or sequence types and/or antimicrobial susceptibility.
MRSA is a serious cause of infections in humans. Strains of MRSA can be divided into three broad categories: community‐associated (CA‐), healthcare‐associated (HA‐) and livestock‐associated (LA‐) MRSA. These categories differ in their epidemiology, although the separation between the types is not strict. HA‐MRSA and CA‐MRSA comprise strains that predominantly affect humans, and these strains are much less frequently reported from food‐producing animals. LA‐MRSA has been detected in most farm animal species including those covered by the resistance monitoring according to CID 2013/652/EU. Among humans, LA‐MRSA are predominantly carried by people with repeated occupational contact with colonised livestock.
Although frequently detected in food, MRSA is generally not considered to be transmitted by food‐borne routes to humans, and detection often involves selective culture techniques, which may detect very low levels of contamination (Pauly et al., 2019).
Isolates of LA‐MRSA in Europe predominantly belong to clonal complex (CC) 398, although other livestock‐associated clonal lineages have been reported.
The severity of LA‐MRSA infection in humans has been shown to be generally similar to that of other MRSA strains. However, transmission of LA‐MRSA in the hospital environment has been characterised as less likely than with HA‐MRSA strains although public health surveillance in the Netherlands (2003–2014) and Denmark (1999–2011) detected distinct LA‐MRSA strains disseminating into the community (the Netherlands) or capable of transmission in the community in the absence of livestock contact (Kinross et al., 2017).
A variant of the methicillin resistance gene mecA, termed mecC, was first identified in 2011 in MRSA from humans and cattle in Europe (Garcia‐Alvarez et al., 2011; Shore et al., 2011). It has subsequently been detected in ruminants, pigs and companion animals, with reports also from wild animals (Paterson et al., 2014; Bengtsson et al., 2017). In hindsight, mecC‐MRSA isolates have been found in isolates dating back to the pre‐antibiotic era (Larsen et al., 2022). The mecC gene shares 70% identity with mecA at the DNA level (Garcia‐Alvarez et al., 2011). Risk factors for mecC MRSA carriage in humans include contact to animals and the presence of an underlying disease (Lozano et al., 2020). mecC‐MRSA strains have been negative for Panton‐Valentine leukocidin (PVL) toxin – a virulence feature typically associated with CA‐MRSA. However, some isolates have carried genes belonging to the immune evasion cluster (Lozano et al., 2020). Carriage of these IEC genes is considered an adaptation to enable S. aureus colonisation and infection of humans, and is not usually a feature of animal S. aureus strains (Cuny et al., 2015).
Antimicrobial susceptibility in European invasive Staphylococcus aureus isolates from humans is reported by 29 MSs to the European Antimicrobial Resistance Surveillance Network (EARS‐Net) hosted by ECDC. MRSA typing data are not reported and, therefore, when there may be possible links to the animal reservoir of LA‐MRSA, these cannot easily be detected with current monitoring procedures, at least at the European level. The EU/EEA (excluding the United Kingdom) population‐weighted mean proportion of MRSA among invasive S. aureus infections reported to EARS‐Net decreased significantly from 19.3% in 2016 to 16.7% in 2020, with significantly decreasing trends reported by 10 of the individual reporting EU/EEA countries (excluding the United Kingdom). Nevertheless, MRSA remains an important human pathogen in the EU/EEA, as the levels of MRSA were still high in several countries and combined resistance to another antimicrobial group was common (WHO/ECDC, 2022).
6.3. MRSA in food and animals
This section summarises the occurrence of MRSA and its susceptibility to antimicrobials in various food categories, in food‐producing animals (including horses) and during clinical investigations in food‐producing and other animals in 2019–2020. Additional tables on MRSA in food and food‐producing animals have been included in Annex E that is available as supporting documentation in zenodo (https://doi.org/10.5281/zenodo.6257446).
In 2020, data were reported by six MS (Austria, Belgium, Germany, Denmark, the Netherlands and Slovakia) and one non‐MS (Norway). In 2019, seven MS and two non‐MS had provided data. The methods for the isolation of MRSA from food and animals are not fully harmonised at the EU level and, therefore, the methods used by reporting countries and in different investigations may differ in sensitivity. Similarly, the sampling strategies used by reporting countries and the types of samples collected are not harmonised at the EU level and these may also influence the results obtained.
6.3.1. Monitoring of MRSA in food
In both 2019 and 2020, a low number of countries reported data on the occurrence of MRSA in food (N = 4 and N = 4, respectively). In 2020, Austria, Germany, the Netherlands and Slovakia provided data (see Table 1a in Annex E). MRSA was investigated in meat from broilers (AT, NL), meat from pigs (SK, NL) by two MS each. Moreover, data on meat from bovines and from turkeys were reported by the Netherlands, and data on meat from sheep by Germany. The Netherlands reported few and negative samples from unspecified meat and meat from deer. Germany reported the investigation of soft cheese. In 2019, data had been provided by Austria, Germany, the Netherlands and the non‐MS Switzerland (see Table 1b in Annex E). MRSA was reported in meat from cattle and pigs by three countries (Austria, the Netherlands and Switzerland). Moreover, data on MRSA in poultry meat were reported by the Netherlands and data on raw cows’ milk by Germany (Figure 47).
Figure 47.
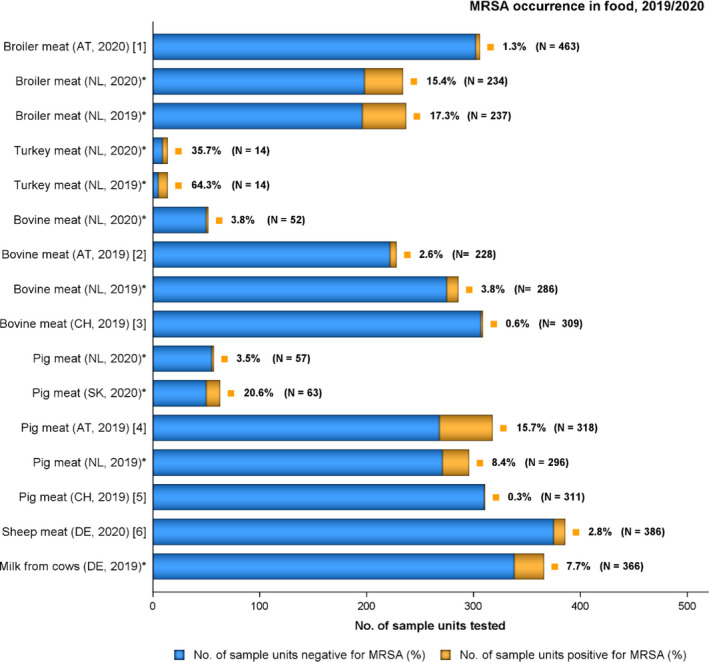
MRSA occurrence in food, 2019–2020
-
Only food origins where positive isolates were obtained are presented.N: Total number of sample units tested; AT: Austria; DE: Germany; NL: the Netherlands; SK: Slovakia; CH: Switzerland.[1] Broiler meat (AT, 2020): spa‐types: t011 (four isolates) t034 (4).[2] Bovine meat (AT, 2019): spa‐types: t008 ST8 (one isolate), t011 (2), t127 ST1 (2), t2346 (1). The t008 isolate was PVL‐positive; the two t127 isolates were PVL‐negative.[3] Bovine meat (CH) : spa‐types were not reported; however, both isolates were confirmed to belong to CC398 using the sau1‐hsdS1 CC398 PCR reaction (Stegger et al., 2011).[4] Pig meat (AT, 2019) : spa‐types : t002 ST5 (1 isolate), t003 ST3944 (1), t008 ST8 (1), t011 (22), t011 ST398 (1), t034 (12), t127 ST1 (2), t321 ST5050 (1), t843 ST130 (1), t899 (5), t1451 (2), t1456 (1). The t002 and t008 isolates were PVL‐positive. The two t127 isolates, as well as the single t003 and t321 isolates were PVL‐negative. The t843 isolate was reported to carry the mecC gene.[5] Pig meat (CH, 2019) spa‐type was not reported; however, the isolate was confirmed to belong to CC398 using the sau1‐hsdS1 CC398 PCR reaction (Stegger et al., 2011).[6] Sheep meat (DE, 2020) spa‐type: t011 (2 isolates), t034 (1), t1451 (1), t2576 (1), t19979 ST 398 (1), t223 (2), t267 (1), t1154, ST5 (1) t15010 ST97 (1).*: spa‐types not reported.
The highest occurrence of MRSA was observed in meat from turkey. However, here only 14 samples were tested in each year. Of the total of 28 samples 50% were positive. In meat from pigs and broiler meat, prevalence differed between countries with prevalence in pig meat ranging from 0.3% in Switzerland in 2019 to 20.6% in Slovakia (2020). In broiler meat, the proportion of positive samples ranged from 2.3% in Austria (2020) to 17.3% in the Netherlands (2019). Occurrence of MRSA was overall lower in bovine meat with a minimum of 0.6% (Switzerland, 2019) and a maximum of 3.8% in the Netherlands (2019 and 2020). Meat from sheep (2.8% Germany, 2020) and raw bovine milk (7.7%, Germany 2019) were also found positive for MRSA.
6.3.2. Monitoring of MRSA in animals
Monitoring of MRSA in healthy animals
MRSA occurrence data from animals were reported by five MS (Belgium, Germany, Finland, the Netherlands and Slovakia) and Norway in 2020 (see Table 2a in Annex E) and by seven countries, five MS (Belgium, Germany, Denmark, the Netherlands and Portugal) and two non‐MS (Norway, Switzerland) in 2019 (see Table 2b in Annex E). The data originated from different frameworks, including a voluntary monitoring, surveillance, control programmes and specific surveys, the latter being restricted to fur animals in Finland. The voluntary monitoring performed over 2019–2020 examined turkeys, laying hens, broilers, fattening pigs, breeding pigs, wild boars and wild freshwater fish. Fur animals surveyed included foxes, mink and racoon dogs.
In 2019 and 2020, MRSA were most frequently detected in pigs (Figure 48). Among the different populations studied at farm were multiplier herds in Denmark in 2019 (94.5% positive), herds of fattening pigs in the Netherlands 2020 (79.0%) and Belgium 2019 (58.3%), and herds of breeding pigs in Belgium in 2019 (46.4%). At slaughter 18.0% of pigs were positive in Slovakia 2020, while all tested batches had been positive in Portugal in 2019. In contrast, MRSA was only detected in one of 722 samples in the framework of the national control and eradication program in pigs in Norway in 2019 and not detected at all in 643 samples in 2020 in that program (see Tables 2a and 2b in Annex E). High detection rates for MRSA in pigs except for Norway confirm the results of previous years.
Figure 48.
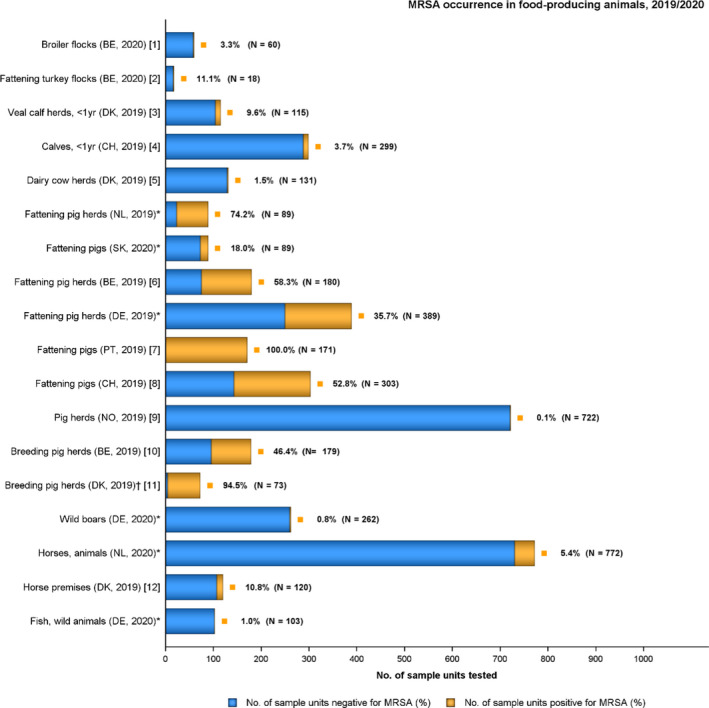
-
Only animal origins where positive isolates were obtained are presented.N: Total number of sample units tested; BE: Belgium; CH: Switzerland; DE: Germany; DK: Denmark; NL: the Netherlands; NO: Norway; PT: Portugal.[1] Broiler flocks, BE 2020: spa‐types: t011 CC398 (2 isolates).[2] Fattening turkey flocks, BE 2020: spa‐types: t011 CC398 (2 isolates).[3] Veal calf herds, DK 2019: spa‐types : t011 CC398 (1 isolate), t034 CC398 (8), t779 CC398 (1), t1580 CC398 (1).[4] Calves < 1 year, CH2019: spa‐types were not reported; however, all 11 isolates were confirmed to belong to CC398 using the sau1‐hsdS1 CC398 PCR reaction (Stegger et al., 2011).[5] Dairy cow herds, DK 2019 : spa‐types : t127 CC1 (one isolate), t843 CC130 (1). The t127 isolate was PVL‐negative, as well as negative for the human IEC gene scn. Spa‐type t843 was confirmed to carry the mecC gene.[6] Fattening pig herds (BE 2019) : spa‐types: t011 CC398 (67 isolates), t034 CC398 (11), t1451 CC398 (2), t1457 CC398 (1), t2346 CC398 (1), t2370 CC398 (2), t2383 CC398 (1), t3041 CC398 (1), t3119 CC398 (1), unspecified (18).[7] Fattening pigs, slaughter batches (PT 2019) : spa‐types : t011 CC398 (3), unspecified (168).[8] Fattening pigs, (CH 2019) : spa‐types were not reported; however, 159/160 isolates were confirmed to belong to CC398 using the sau1‐hsdS1 CC398 PCR reaction (Stegger et al., 2011). The remaining isolate did not survive cryo‐conservation, therefore typing could not be performed.[9] Pig herds (NO 2019) : spa‐type : t034 CC398 (1 isolate).[10] Breeding pig herds (BE 2019) : spa‐types : t011 CC398 (57 isolates), t034 CC398 (18), t108 CC398 (2), t779 CC398 (1), t2346 CC398 (1), t2582 CC398 (1), t2922 CC398 (1), t3119 CC398 (2).[11] Breeding pig herds (DK 2019) : spa‐types: t011 CC398 (10 isolates), t034 CC398 (57), t1928 CC398 (1), t4652 CC398 (1). † These comprised multiplier herds.[12] Horse premises (DK 2019) : spa‐types: t011 CC398 (4 isolates), t034 CC398 (6), t1451 CC398 (1), t843 CC130 (1), t3256 CC130 (1). Spa‐types t843 and t3256 were confirmed to carry the mecC gene.*: spa‐types not reported.
MRSA were detected in turkeys (11.1%) and broilers (3.3%) in Belgium in 2020, but not in broilers in Denmark in 2019 and in laying hens in Belgium in 2020. Low detection rates in broiler flocks and laying hen flocks have been observed frequently. In broilers, this contrasts with higher detection rates in broiler meat indicating that cross contamination at slaughter my play a relevant role here. Turkeys were more frequently positive on farm than broilers, in 2019–2020 as well as in previous years.
Very few MRSA were found in wild boars and wild freshwater fish in Germany (0.8% and 1.0%) (Figure 49; see also Table 2a in Annex E). No MRSA were detected in farms with racoon dogs (1 sample), from farms of mink (15 samples) and farms of foxes (11 samples) in Finland. Absence of MRSA in farmed mink in Finland is in contrast to a considerable proportion of positive samples in Denmark in 2018 (EFSA and ECDC, 2020)
Figure 49.
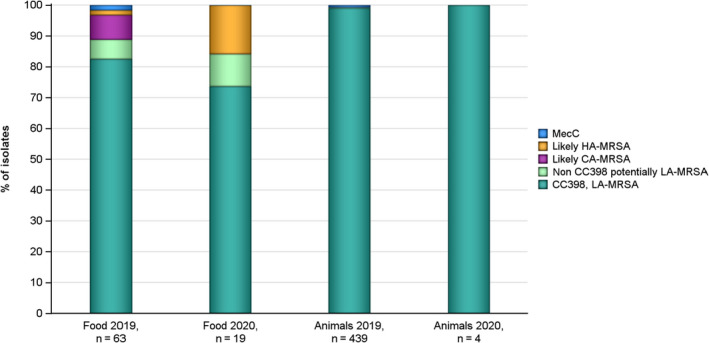
-
N = number of reported isolates with typing data, mecC: MRSA harbouring the mecC gene, HA‐MRSA: hospital acquired MRSA, CA‐MRSA: community acquired MRSA, LA‐MRSA: livestock associated MRSA, CC: clonal complex.Inferred MRSA types in 2019 were recovered from pigs (243 isolates), cattle (13 isolates) and horses (13 isolates) at the herd/slaughter batch/stable level, as well as individual fattening pigs (159 isolates) and calves at slaughter (11 isolates). Isolates from animals in 2020 were derived from broiler flocks (2 isolates) and fattening turkey flocks (2 isolates).Inferred MRSA types in food in 2019 were recovered from cattle meat (8 isolates) and pig meat (55 isolates). Isolates from food in 2020 were recovered from broiler meat (8 isolates) and meat from sheep (11).
In 2020, no cattle were tested for MRSA. However, in 2019, 1.5% of dairy herds and 9.6% of veal calf herds in Denmark, were found positive for MRSA. In Germany, 28 of 366 raw milk samples (7.7%) were positive for MRSA at farm, indicating a higher rate of positive farms than in Denmark. In cattle, a higher occurrence of MRSA in calves than in dairy cows has been reported previously (Graveland et al., 2010; Tenhagen et al., 2014; Schnitt et al., 2020).
Monitoring of MRSA in animals following clinical investigations
Typically, clinical investigations differ from monitoring studies in food‐producing animals: selective culture methods are typically not used and the sample involves a biased sample. These data therefore do not allow prevalence to be inferred and cannot be extrapolated at the population level. However, it is still considered relevant to report the range of animal species/populations which were affected, and the lineages of MRSA detected. In 2019 and 2020, two countries (SK, NL) reported on clinical animal data on MRSA (see Table 3b in Annex E).
In 2020, Slovakia reported data following clinical investigations for MRSA in 46 animals of various species with no positive finding (see Tables 3a and 4a in Annex E). These included dogs (23 samples), cats (9), dairy cows (5), goats (2), horses (2), rabbits (2), guinea pigs, squirrels and falcons (1). The Netherlands reported data, predominantly on cats, dogs and horses in 2020. The highest rate of MRSA findings was in horses (5.4%) followed by cats (0.8%) and dogs (0.4%). One individual finding of MRSA in a caged bird was reported by the Netherlands. The bird species was not reported. No molecular data were provided by the two countries.
In 2019, Slovakia reported data on clinical isolates in 70 bovines, predominantly dairy cows (65 samples, see table 3b in Annex E). They also reported on investigations in 21 sheep and eight goats as well as two broilers. However, all samples were negative. The Netherlands reported on clinical investigations in cats (0.5%), dogs (0.6%) and horses (12.2%, see Table 4b in Annex E).
6.3.3. Results of molecular typing of isolates from animals and food
In 2019, molecular characterisation was reported for 63 of 177 MRSA isolates recovered from food. Among those, 60 originated from MS, and three from Switzerland (all CC398). Among the 439 isolates from animals, 171 originated from Switzerland (170) and Norway (1). Again, all of those were CC398 (see Table 9b in Annex E). In 2020, typing data were only reported for 19 isolates from food and for four isolates from animals, all of which originated from MS. Over both years, most MRSA isolates from food were assignable to the clonal complex 398 (67 of 82, 81.7%). Isolates from animals were almost exclusively assignable to the clonal complex 398 (439/443, 99.1%). Results of molecular typing of isolates from animals and food are presented in Figure 49 and Tables 15 and 16.
Among the CC398 isolates, most isolates with spa‐typing data in both years were from spa‐types t011 and t034. In 2019 this applied for 40/49 (81.6%) isolates from food and for 243/265 isolates from animals (91.4%). No spa‐type was available for isolates from Switzerland. This included two isolates from bovine meat and a single isolate from pig meat, 160 isolates from pigs and 11 isolates from calves < 1year of age. In 2020, t011 and t034 accounted for 11 of 14 CC398 isolates from food (78.6%) and for all four CC398 isolates from animals. All isolates from animals reported in 2020 were from t011.
In 2019, spa‐types that were assignable to CC398 but were not t011 and t034 included five isolates from pig meat that were spa‐type t899. Spa‐type t899 can be associated with different clonal lineages, including CC398 and CC9. LA‐MRSA CC9/CC398 displaying spa‐type t899 is a mosaic strain, consisting of a CC398 chromosomal backbone having acquired the CC9 region containing the staphylococcal protein A gene (Guardabassi et al., 2009; Larsen et al., 2016; Tegegne et al., 2021b). Other CC398 associated spa‐types in food in 2019 included two isolates of t1451 and one t1456 from pig meat, and one t2346 from bovine meat.
In 2020, three isolates from sheep meat reported by Germany were from less frequently observed spa‐types (t1451, t2576 and t19979) of CC398. Among those, t1451 and t2576 have repeatedly been observed in animals, predominantly pigs and food. T1451 had also been observed in two isolates from pig meat in 2019. T19979 is a comparatively new variant but was confirmed as an ST398 by Germany.
In 2019 and 2020, most spa‐types of isolates from animals and food that were assignable to CC398 have already been observed in the EU‐baseline in breeding pigs in 2008 (EFSA, 2009b) demonstrating a remarkable persistence in the food‐producing animal population. This applies for t011, t034, t108, t899, t1451, t1456, t1457, t2346, t2370 and t2922. Most other spa‐types assigned to this clonal complex have likewise been observed previously as exemplified below:
Spa type t779 was identified in one isolate from calves and one from breeding pigs in 2019. It has previously been identified in pigs in Germany (Buntenkoetter et al., 2014).
Spa‐type t1580 was isolated from a calf and has previously been observed in pigs (EFSA and ECDC, 2018) and humans in a hospital in an area with intensive animal husbandry in Germany (Köck et al., 2013)
Spa‐type t1928 has been described in pigs in Germany in companion animals in Austria (Alt et al., 2011; Loncaric et al., 2019).
Spa‐types t2383 and t2582, reported in pigs in 2019, have likewise been described in pigs in Germany previously (Alt et al., 2011).
t3041 had been isolated from a veal calf farmer in Belgium (Vandendriessche et al., 2013) t3119 has been isolated from chicken meat in 2009 (de Boer et al., 2009).
Spa‐type t4652, reported in pigs in 2019, has previously been described in workers in a broiler slaughterhouse (Wendlandt et al., 2013), on turkey carcases and in meat from turkeys (Vossenkuhl et al., 2014).
While this list does not claim full coverage, it clearly indicates that these spa‐types persist in animal production (including slaughter and meat).
Spa‐type t19979 is a recent strain as indicated by the high spa‐type number and has not been reported in animals, food or farmers before.
In 2019, Austria was the only country to report spa‐type for 56 isolates recovered from pig and bovine meat and four isolates with spa‐type information from additional ad hoc sampling of pig meat. Switzerland provided information on clonal complex for three MRSA isolates from pig and bovine meat but no spa‐type data. In 2020, molecular typing data were reported for eight isolates from broiler meat from Austria and 11 isolates from sheep meat from Germany, i.e. 19 of 77 reported isolates.
Isolates that were not assigned to the clonal complex 398 included a range of spa‐types that could be or were assigned to a number of different CC (see Tables 9a and 9b in Annex E). The highest proportion of such isolates was observed in meat from sheep in 2020 (5 of 11, 45.5%) and bovine meat in 2019 (3 of 8, 37.5%). The highest number of isolates not assigned to the clonal complex 398 originated from pig meat in 2019 (8 isolates). However, those were only 14.8% from all 54 pig meat isolates with a reported spa‐type.
In 2019, the following isolates were from spa‐types not assignable to CC398 (see also Table 16).
In 2019, four isolates from bovine and pig meat and one isolate from dairy cows were identified as spa‐type t127 and ST1. This combination has frequently been identified in isolates from animals and food (Alba et al., 2015) and might be considered livestock associated although it is not related to the clonal complex 398. In the Italian study carried out by Alba et al. (2015), the genetic relatedness of bovine, porcine and human t127‐ST1 isolates was investigated. A high similarity was found among bovine and human t127‐ST1 strains, with all isolates carrying SCCmec type Iva and often possessing the human IEC genes, sak and scn; suggesting establishment of the human t127‐ST1 strain within Italian cattle populations. In line with that, according to whole genome sequence data, the isolates harboured the SCCmec‐type Iva that is frequently observed in livestock‐derived strains. Additionally, these isolates were reported to be PVL‐negative. Denmark reported that the t127 isolate from dairy cows was negative for the human immune evasion cluster gene scn. The isolate from dairy cows was therefore considered most likely to represent an LA‐MRSA. In contrast, the isolates from bovine and pig meat in Austria harboured the human immune evasion cluster (IEC) genes, sak and scn. In summary, the origin of these isolates cannot be ascertained.
spa‐type t002 was reported from pig meat by Austria in 2019. This spa‐type has repeatedly been observed in turkey meat and in turkeys (Richter et al., 2012; Vossenkuhl et al., 2014; Tegegne et al., 2021a) The isolate from Austria was confirmed to belong to ST5 and contain SCCmec type Ivc (2B) from WGS data. As the isolate was reported to be PVL‐positive, it was considered most likely to represent a community‐associated lineage, as CA‐MRSA frequently possess the PVL toxin. Additionally, the isolate was reported to harbour the IEC genes, sak and scn. With the exception of b‐lactams (penicillin and cefoxitin; oxacillin not tested), the t002 isolate was susceptible to all other antimicrobials tested, which is not surprising since CA‐MRSA strains are typically susceptible to non‐β‐lactam antimicrobials.
spa‐type t003 was recovered from pig meat by Austria in 2019; the isolate was confirmed to belong to ST3944 (CC5) and contain SCCmec type II (2A) from WGS data. The isolate was considered most likely to represent a HA‐MRSA due to its PVL‐negative status, as well as SCCmec element type. Additionally, the isolate was reported to harbour the IEC genes, sak and scn.
Austria reported spa‐type t008 from a batch of pig meat, as well as a batch of bovine meat. Both isolates were confirmed to belong to ST8 (CC8) and were SCCmec type Iva (2B) from WGS data. This spa‐type and sequence type combination is seen in isolates of the globally significant CA‐MRSA USA300 strain, which is PVL‐positive and frequently possesses the arginine catabolic mobile element (ACME). Austria confirmed that these isolates were PVL‐positive and the ACME was detected. Additionally, these isolates were reported to harbour the IEC genes, sak and scn.
spa‐type t012, detected in additional samples of pig meat in Austria in 2019, can be assigned to CC30, one of the globally occurring clonal complexes of MRSA in humans. Austria confirmed that the t012 isolate was PVL‐negative, belonged to ST30 (CC30) and was SCCmec type Iva (2B) from WGS data. With the exception of b‐lactams (penicillin and cefoxitin; oxacillin not tested), the t012 isolate was susceptible to all other antimicrobials tested. Additionally, the isolate was reported to harbour the IEC genes, sak and scn, as well as the tst gene encoding for toxic shock syndrome toxin 1 (TSST‐1). Although the PVL status of this isolate would suggest a healthcare‐associated lineage, as well as the detection of spa‐type t012 from an Austrian hospital study (Mayerhofer et al., 2015), PVL‐negative t012‐ST30‐IV isolates have previously been described as CA‐MRSA (Karapsias et al., 2008). Furthermore, (Boswihi et al., 2018) describe the ST30‐MRSA‐IV clone as a CA‐MRSA; the t012 isolate recovered from additional ad hoc sampling by Austria was therefore considered most likely to represent a CA‐MRSA.
spa‐type t321 was recovered from pig meat by Austria in 2019. The isolate was confirmed to belong to ST5050 (CC1) and contain SCCmec type Iva (2B) from WGS data. While the t321 isolate was reported to be PVL‐negative, the t321‐CC1 genotype is predominantly regarded as a CA‐MRSA (Huang et al., 2014; Boswihi et al., 2016).
mecC‐MRSA spa‐type t843 was reported by Austria from pig meat. The isolate was confirmed to belong to ST130 (CC130), contain the SCCmec element type XI (8E) and carry the mecC gene. It was also reported from horses (1 isolate) and dairy cows (1) in Denmark. The association of this spa‐type with CC130 has previously been observed in cattle (Mališová et al., 2020; Cui et al., 2021; Silva et al., 2021).
mecC‐MRSA spa‐type t3256, also CC130 was reported by Denmark in one isolate from horses. Considering the two spa‐types of CC130 (t843 and t3256), both have previously been observed in humans (Petersen et al., 2013; Paterson et al., 2014) and possible transmission between humans and animals is well documented (Harrison et al., 2013; Petersen et al., 2013; Angen et al., 2017). Angen et al. (2017) identified the first case of mecC‐MRSA in domesticated pigs and findings strongly indicated transmission between farmers and pigs. Additionally, other studies support the hypothesis that wildlife may constitute a reservoir of mecC‐MRSA (Bengtsson et al., 2017; Rasmussen et al., 2019).
In 2020 five isolates, all from sheep meat reported by Germany, were from spa‐types not assignable to CC398.
Two isolates were spa‐type t223. This spa‐type is frequently assigned to the MLST type ST22, one of the most frequent hospital acquired MLST‐types in Germany [4].
One isolate (t15010) was identified as MLST‐type ST97. This MLST type is frequently observed among S. aureus from cattle and has also been observed in pigs, e.g. in Italy. Despite not being related to the CC398 it is considered as a livestock associated strain by some authors (Feltrin et al., 2016).
A second spa‐type that frequently occurs in ST97 is t267. This spa‐type has been found in bovine milk as well as a PVL positive strain in humans (Käppeli et al., 2019; McManus et al., 2021) underlining that it could be both, of animal and human origin.
One other isolate (t1154) was identified as MLST type ST5. ST5 isolates are frequently observed in humans but have also been reported from turkeys (Richter et al., 2012; Vossenkuhl et al., 2014), although with different spa‐types.
6.4. Temporal trends
Isolation of MRSA from food‐producing animals and the farm environment.
In 2018, the European Union Reference Laboratory‐Antimicrobial Resistance (EURL‐AR) published revised recommendations for the isolation of MRSA from food‐producing animals and the farm environment (known as 1‐step (1‐S) method), which omit the use of a second enrichment step with cefoxitin and aztreonam (EURL (European Union Reference Laboratory for Antimicrobial Resistance), 2018). Prior to this, the recommended method for the detection of MRSA comprised a pre‐enrichment step and a selective enrichment step (known as the 2‐step (2‐S) method).
The revised recommendations followed a study of Danish and Norwegian pig herds which evaluated the sensitivity of the 2‐S method by comparison with an alternative 1‐S method (whereby the selective enrichment step was bypassed), with results confirming that the 1‐S method resulted in a lower proportion of false‐negative results than the 2‐S method (Larsen et al., 2017). The authors urged caution in extrapolating the results to animals other than pigs and commented that previous studies in Belgium in poultry and cattle did not find significant differences between the performance of the two methods. For broiler meat, it has been shown that the 1‐S method may improve sensitivity. However, this goes along with a decrease in specificity, i.e. resulting isolates always need to be confirmed by further typing methods, which puts an additional burden on routine laboratory testing (Pauly et al., 2019).
Notably, changes to the recommended method of isolation may impact longitudinal studies, since direct comparison of the data obtained using the different protocols should be performed with caution.
In 2019, occurrence data were obtained using the 1‐S method by 5/7 reporting countries in food‐producing animals (Austria, Denmark, the Netherlands, Norway, Switzerland) and 3/4 reporting countries in food (Austria, the Netherlands, Switzerland). The remaining countries used the 2‐S method in 2019, i.e. Germany in animals and food and Belgium in animals. In 2020, the 2‐S method was used by Belgium (animals) and Germany (animals and food), while Austria, the Netherlands and Norway used the 1‐S method.
Considering the monitoring performed in 2018/2019 and 2020 and for previous years, comparable longitudinal data were available for the animal species and food items, as presented in the following sections.
Based on the limited number of available data, no statistical trend analyses were carried out. Therefore, changes in occurrence are reported here only descriptively.
6.4.1. Temporal trends of MRSA prevalence in various types of meat
Four countries reported comparable data over time on MRSA from broiler meat (Figure 50). Over the last 3 years, the Netherlands provided data on broiler meat repeatedly. The proportion of positive samples decreased slightly over time from 20.2% in 2018 (no data before that date) to 15.4% in 2020. Austria provided similar data in 2018 (1.0%) and 2020 (2.6%). Both countries used the 1‐S method. Germany and Switzerland used the 2‐S method. Germany last provided data in 2018 (16.4%), which was higher in 2016 (13.0%) but lower than 2013 and before, when detection rates were between 22% and 26%. Samples of broiler meat in Switzerland rarely contained MRSA in 2016 (3.0%) and 2018 (1.3%).
Figure 50.
Temporal trends of MRSA prevalence in various types of meat, 2011–2020
-
AT: Austria; CH: Switzerland; DE: Germany; NL: the Netherlands.The 2‐S method of isolation was used by CH and DE from 2011 to 2018; while the 1‐S method was used by the NL from 2018 to 2019, as well as CH in 2019.*: spa‐types not reported.[1] In 2019, spa‐types were not reported; however, both isolates were confirmed to belong to CC398 using the sau1‐hsdS1 CC398 PCR reaction (Stegger et al., 2011).[2] In 2017, spa‐type: t011 (1 isolate), t002 (1). PVL status of the t002 isolate was not reported. In 2019, spa‐type was not reported; however, the isolate was confirmed to belong to CC398 using the sau1‐hsdS1 CC398 PCR reaction (Stegger et al., 2011).[3] In 2018, spa‐types: t011 (2 isolates), t034 (1 isolate). In 2020, spa‐types: t011 (4 isolates), t034 (4 isolates)[4] In 2016, spa‐types: t034 (3 isolates), t153 (1), t1430 (3), t2123 (2). PVL status of the t153 isolate was not reported.In 2018, spa‐types: t034 CC398 (1 isolate), t1430 (1), t571 CC398 (1), t13177 (1).
The Netherlands provided MRSA data from turkey meat in 2018, 2019 and 2020. However, the number of samples was very low (31 over the 3 years) not allowing a meaningful analysis of trends. Germany last reported on MRSA in turkey meat in 2018 with 42.7% positive samples. This was in the range of results reported between 2009 and 2017 for fresh turkey meat from Germany (39.9–44.5%). While the Netherlands used the 1‐S method throughout, Germany used the 2‐S method.
The Netherlands reported data on pig meat in 2018–2020, with results ranging from 3.5 (2020) to 8.4% (2018) and 2015 in between (5.9%), i.e. without a clear trend. Slovakia reported on different kinds of pig meat over the years precluding straightforward comparison of data. Switzerland reported data in 2017 and 2019, with very low detection rates (0.7% and 0.3%). While the Netherlands used the 1‐S method throughout, Switzerland changed from 2‐S to 1‐S in 2019.
The Netherlands reported on bovine meat in 2018–2020. While in 2019 and 2020 3.8% of samples were positive, the number of positive samples was slightly lower in 2018 (2.1%). In Switzerland, reporting in 2017 and 2019, MRSA were only detected in two samples of fresh bovine meat in 2019 (0.6%). As in pig meat, the Netherlands used the 1‐S method throughout, while Switzerland changed from 2‐S to 1‐S in 2019.
6.4.2. Temporal trends of MRSA prevalence in various types of animals
As monitoring of MRSA is not mandatory and methodology is not harmonised, comparable data over time are only available for some populations in few reporting countries. These are presented in Figures 51 and 52 (as well as in Annex E: Figure 1 and Table 6).
Figure 51.
Temporal trends of MRSA prevalence in poultry, 2011–2020
-
BE: Belgium; DE: Germany.[1] Broiler flocks in 2014: spa‐types t011 CC398 (one isolate), t1985 CC398 (1)Broiler flocks in 2017 and 2020: spa‐type t011 (two isolates each)[2] Laying hens in 2014: spa‐types t011 CC398 (one isolate), t037 (5)Laying hens in 2017: spa‐types t011 (two isolates), t037 ST239 (1)*: No spa‐types reported
Figure 52.
Temporal trends of MRSA prevalence in pigs, 2010–2020
-
BE: Belgium; CH: Switzerland; DE: Germany; DK: Denmark; NO: Norway.Note: The 2‐S method of isolation was used by Belgium and Germany from 2016 to 2019, as well as by Denmark in 2016, in Switzerland from 2010 to 2017 and in Norway from 2014 to 2017. The 1‐S method was used by Switzerland since 2019 and by Denmark and Norway since 2018.*: spa‐types not reported.†: Prevalence data for fattening pig herds (not raised under controlled housing conditions) from 2018 are not included.[1] In 2010, spa‐types: t034 ST398 (17 isolates), t011 ST398 (1), t208 ST49 (5). In 2011, spa‐types: t034 ST398 (19 isolates), t011 ST398 (1), t208 ST49 (1), t2279 ST1 (1). In 2012, spa‐types: t034 CC398 (61 isolates), t011 CC398 (9), t208 ST49 (2). In 2013, spa‐types: t034 (63 isolates), t011 (10). In 2014, spa‐types: t034 (57 isolates), t011 (19), t208 (1), t899 (1), t2741 (1). In 2015, spa‐types: t034 (48 isolates), t011 (23), t032 (1), t571 (1), t899 (1), t1145 (1), t1250 (1), t4475 (1). In 2017, spa‐types: t034 (63 isolates), t011 (61), t899 (2), t1451 (3), t2330 (1), t2876 (1). In 2019, spa‐types were not reported; however, 159/160 isolates were confirmed to belong to CC398 using the sau1‐hsdS1 CC398 PCR reaction (Stegger et al., 2011). The remaining isolate did not survive cryo‐conservation, therefore typing could not be performed.[2] In 2016, spa‐types: t011 CC398 (71 isolates), t1451 (1), t1456 (1), t1456 CC398 (1), t1580 (5), t1985 (8), t1985 CC398 (3), t034 (7), t034 CC398 (2), t037 (1), t898 (1), unspecified (11). In 2019, spa‐types: t011 CC398 (67 isolates), t034 CC398 (11), t1451 CC398 (2), t1457 CC398 (1), t2346 CC398 (1), t2370 CC398 (2), t2383 CC398 (1), t3041 CC398 (1), t3119 CC398 (1), unspecified (18).[3] In 2016, spa‐types not reported. In 2018, spa‐types: t011 CC398 (22 isolates), t034 CC398 (85), t571 CC398 (3), t898 CC398 (1), t2383 CC398 (1), t2974 CC398 (1), t3423 CC398 (1), t4652 CC398 (1), t9266 CC398 (1).[4] In 2016, spa‐types: t011 CC398 (55 isolates), t1451 (2), t1456 (1), t1456 CC398 (3), t1580 (1), t1985 (5), t1985 CC398 (1), t034 (1), t034 CC398 (4), t4659 CC398 (1), unspecified (17). In 2019, spa‐types: t011 CC398 (57 isolates), t034 CC398 (18), t108 CC398 (2), t779 CC398 (1), t2346 CC398 (1), t2582 CC398 (1), t2922 CC398 (1), t3119 CC398 (2).[5] In 2016, spa‐types not reported. In 2018, spa‐types: t011 CC398 (6 isolates), t034 CC398 (24), t1250 CC398 (2), t1793 CC398 (1), t3171 CC398 (1). In 2019, spa‐types t011 CC398 (10), t034 CC398 (57), t1928 CC398 (1), t4652 CC398 (1) were identified in isolates from multiplier pig herds.[6] In 2014, spa‐type: t011 CC398 (1). In 2015, spa‐type: t034 CC398 (2), t177 CC1 (2). In 2016, spa‐type: t034 CC398 (1).In 2017, spa‐types: t091 CC7 (1 isolate), t843 CC130 (1), t6292 CC425 (1). The t091 isolate was PVL‐negative, spa‐types t843 and t6292 were confirmed to carry the mecC gene. In 2019, spa‐type: t034 CC398 (1).
Belgium provided data on MRSA in broiler flocks using the 2‐S method in 2014, 2017 and 2020 (Figure 51). While sampling details changed between the years, the proportion of positive holdings or flocks was very low in all 3 years with only two herds positive in 2014 (2.5%), 2017 (2.5%) and 2020 (3.3%). Spa‐type was t011 of CC398 in five positive samples and t1985 (likewise CC398) in one isolate in 2014.
Flocks/holdings of laying hens investigated in the same years in Belgium using the 2‐S method were also rarely positive for MRSA (Figure 51). Six herds (2.4%) were positive in 2014, three (1.3%) in 2017 and none in 2020. Among the nine isolates collected in 2014 and 2017, three were t011 CC398 and six were spa type t037 (ST239).
Investigations in turkey flocks were repeatedly reported from Germany (2012, 2014 and 2018). In contrast to chicken flocks, these were more frequently positive with 12.8, 21.9 and 17.2% positive over the years (Figure 51). Investigations were carried out using the 2‐S method.
Switzerland reported on bovines < 1 year of age at slaughter in 2015, 2017 and 2019, with results ranging between 3.7% (2019) and 8.1% (2017) without a clear trend (Annex E: Figure 1). In contrast to 2015 and 2017, Switzerland used the 1‐S method in 2019. In Belgium, MRSA in herds of calves under 1 year of age was investigated in 2012, 2015 and 2018 using the 2‐S method. Results varied widely with 78.9% in 2015 and 47.1% in 2012. The last investigation in 2018 resulted in a prevalence in between the two (54.5%).
Data on MRSA in dairy cows using the 2‐S method were provided by Belgium in 2012, 2015 and 2018 (Annex E: Figure 1) with values ranging between 9.9% (2012) and 14.0% (2018). Denmark reported on dairy cows in 2018 and 2019 using the 1‐S method, with prevalence seemingly higher in 2018 (6.1%) than in 2019 (1.6%). Germany investigated bulk tank milk from dairy herds using the 2‐S method in 2009, 2010 and 2019 (data not included in the trend graphs). Between 2009 and 2019, there was an increase from 4.1% to 7.7%.
Meat production cattle herds were reported by Belgium in 2012, 2015 and 2018 using the 2‐S method with values ranging between 8.7% (2018) and 15.4% (2015) (Annex E: Figure 1).
Considering the monitoring of pigs (Figure 52), MRSA prevalence data for Swiss fattening pigs at slaughter were reported from 2010 to 2015, as well as in 2017 and 2019. Generally, prevalence has increased annually, rising from 5.9% in 2010 to 52.8% in 2019; and from 2015 to 2017, a marked increase was observed from 25.7% to 44.0%, respectively. Notably, spa‐types associated with CC398 have shown a steady increase in prevalence; and where molecular typing data were available, all isolates in 2017 and 2019 were those associated with CC398 (with most belonging to spa‐types t011 and t034 in 2017, only information on clonal complex were provided for isolates recovered in 2019). It should be noted, however, that from 2010 to 2017, the 2‐S method of isolation was used in comparison to the 1‐S method in 2019.
MRSA prevalence data from Belgian fattening pig herds using the 2‐S method was reported in 2016 (63.3%) and 2019 (58.3%) with a similar number of herds tested in both years. Belgium also reported MRSA prevalence data from breeding pig herds in 2016 (59.5%) and 2019 (46.4%. In both populations, the prevalence tended to be lower in 2019 than in 2016. All MRSA isolates recovered from these production types over these years were spa‐types associated with CC398 (LA‐MRSA), with the exception of a single isolate of spa‐type t037 reported from fattening pigs in 2016 (categorised as HA‐MRSA) in Belgium.
Denmark reported results from a national survey on the prevalence of MRSA in breeding pig herds in 2016, 2018 and 2019. However, the number of tested herds in 2016 (n = 6) was too low for a meaningful comparison of the results between the years and populations studied differed slightly between years with multiplier herds sampled in 2019 and a census on breeding pig herds carried out in 2018. Spa‐typing data were reported in 2018 and 2019. All reported spa‐types were those associated with CC398 (LA‐MRSA). While in 2016 Denmark used the 2‐S method, they changed to 1‐S in 2018.
Norway is the only reporting country with a systematic surveillance and control programme that is run since 2013. From 2014 to 2017, similar very low levels of prevalence were recorded (0.1%, 0.5%, 0.1% and 0.4%, respectively), with a very low level also reported in 2019 (0.1%) and no pig herds testing positive for MRSA in 2018 and 2020 although the number of tested herds was high (641 in 2020). Since 2018, Norway used the 1‐S method of isolation instead of the 2‐S method that was used until 2017.
MRSA prevalence data obtained from horse premises in Denmark were reported in 2018 and 2019 using the 1‐S method, with similar levels in both years (8.1% and 10.8%). In both years, most isolates were spa‐types associated with LA‐MRSA, except for t843 in 2018/2019 and t3256 in 2019; all three isolates were confirmed to belong to CC130 and carry the mecC gene.
6.5. Summary data on the occurrence and susceptibility of MRSA
Determination of the susceptibility of MRSA isolates to antimicrobials, including those of particular medical importance, such as linezolid and vancomycin, provides valuable information on the MRSA situation in animals and food. The importance of monitoring AMR patterns among different lineages is underlined by the potential for multiple resistance genes harboured by less virulent strains to spread to other S. aureus strains (Sahibzada et al., 2017).
In 2019, data on the antimicrobial susceptibility of MRSA isolates were reported by Austria, Belgium, Portugal and Switzerland. In 2020, such data were provided by Austria, Belgium and Germany (see Tables 7a and 7b, Annex E). All countries used a broth dilution method and applied EUCAST epidemiological cut‐offs (ECOFFs) to determine the susceptibility of isolates, and as expected, all MRSA isolates were resistant to penicillin and cefoxitin. In 2019, Austria also reported antimicrobial susceptibility and molecular typing data for four isolates (spa‐types t011, t012 and t034) recovered from additional ad hoc sampling of some batches of pig meat. Results of testing of a minimum of 10 isolates per origin are depicted in Figures 53 and 54.
Figure 53.
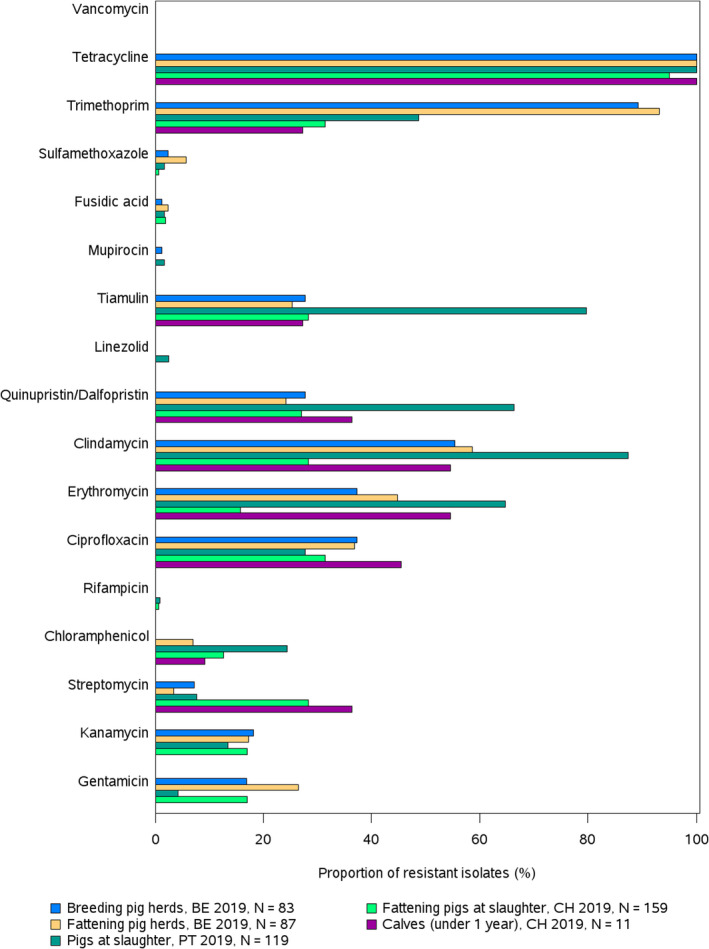
Antimicrobial resistance in MRSA from animals, in 2019
-
BE: Belgium; CH: Switzerland; PT: Portugal. N: number of tested isolates. All isolates were resistant to Penicillin and Cefoxitin.Portugal: susceptibility data for a further 52 isolates recovered from batches of fattening pigs at slaughter were not reported. Switzerland: susceptibility data for one isolate recovered from a fattening pig was not available; the isolate did not survive cryo‐conservation.
Figure 54.
Antimicrobial resistance in MRSA from food in 2019 and 2020
- AT: Austria; DE: Germany. N: number of tested isolates. All isolates were resistant to Penicillin and Cefoxitin.Austria: susceptibility data are also included for four isolates recovered from additional ad hoc sampling.Only food matrices with 10 or more reported isolates per country.
6.5.1. Susceptibility data of MRSA isolates obtained from meat and food‐producing animals (excluding clinical investigations)
In 2019–2020, tetracycline resistance was at 100% in MRSA isolates from most reported animal populations. Only few isolates (8/159) from pigs reported by Switzerland in 2019 were not resistant to tetracycline. This is in line with an extremely high proportion of isolates from animals that can be assigned to the CC398. These are typically resistant to tetracycline (Crombe et al., 2012).
Resistance to other antimicrobials was more heterogenous among the animal populations. High heterogeneity was observed in the three sets of isolates from fattening pigs from Belgium, Portugal and Switzerland in 2019 (see Figure 53, see also Table 7b in Annex E). Whether the observed differences can be attributed to differences in antimicrobial use in pigs in the countries is not clear as no animal population level data on antimicrobial use are available on the European level. Comparatively small differences were detected between MRSA isolates from breeding pigs and fattening pigs in Belgium in 2019. The difference in the proportion of resistant isolates did not exceed 10% for any antimicrobial and minor differences were seen in both directions.
An extremely high level of resistance to trimethoprim was noted among MRSA isolates recovered from Belgian fattening and breeding pig herds in 2019 (93.1% and 89.2%, respectively), as well as an extremely high level of tiamulin resistance in isolates from batches of Portuguese fattening pigs at slaughter in 2019 (79.8%). Lincosamide and macrolide resistance were reported at very high (58.6%) and high levels (44.8%) among MRSA isolates from Belgian fattening pig herds in 2019. This pattern was also noted among Belgian data on breeding pig herds in 2019, where clindamycin and erythromycin resistance were reported at levels of 55.4% and 37.3%, respectively, and Portuguese data on fattening pigs at slaughter: clindamycin resistance was also observed at a higher level when compared to erythromycin (87.4% and 64.7%, respectively). This finding was also apparent among Swiss fattening pigs in 2019, albeit at a lower level (clindamycin: 28.3%; erythromycin: 15.7%).
Among the MRSA isolates reported from Swiss calves in 2019, lincosamide and macrolide resistance was reported at an equal very high level (54.6%). However, susceptibility data were only reported on 11 isolates.
Figures 53 and 54 (see also Tables 7a and 7b in Annex E) present the overall resistance to selected antimicrobials within food‐producing animal and the meat origins in 2019 and 2020 including only those where data on 10 or more isolates were reported.
Remarkably, three isolates from pigs in Portugal in 2019 were resistant to linezolid. These three isolates were reported as spa‐type t011, CC398. Two of these isolates showed an identical resistance pattern to the panel of antimicrobials tested, displaying resistance to chloramphenicol, clindamycin, erythromycin, quinupristin/dalfopristin, tetracycline, tiamulin and trimethoprim, and the third isolate showed a similar resistance pattern to that noted of the other two isolates, with the exception of erythromycin and trimethoprim susceptibility. Notably, the pattern of resistance to linezolid, tiamulin, clindamycin and chloramphenicol is typical of possession of the cfr gene, whose presence was confirmed in all three isolates by Portugal.
In 2019, few isolates from pigs were also resistant to other antimicrobials of specific importance to the control of MRSA in humans. Two were resistant to rifampicin, and another three to mupirocin (Figures 53 and 54 see also Table 7b in Annex E), a drug that is frequently used for decolonisation therapy in humans preceding surgery. This should be closely followed.
Among the 11 isolates from calves < 1 year of age at slaughter in Switzerland in 2019, all were resistant to tetracycline and none was resistant to the drugs of specific medical importance mentioned above (Figure 53, see also Table 7b in Annex E). However, interpretation of results from 11 isolates needs to be cautious on account of the small sample size. This limitation is even more valid for the two isolates reported by Belgium from broilers and from turkeys in 2020.
Two countries reported data on more than 10 isolates for a specific food commodity. Austria reported on 54 isolates from pig meat in 2019 and Germany reported on 11 isolates from meat from sheep in 2020 (Figure 54, see also Tables 7a and 7b in Annex E). Isolates from pig meat in Austria showed a lower frequency of resistance to gentamicin, kanamycin, trimethoprim and tetracycline than those from fattening pigs.
Resistance data on eight isolates from broiler meat were reported by Austria in 2020 (Table 7a, Annex E). All were resistant to penicillin, cefoxitin and tetracycline being in line with them being from CC398. Five of those isolates were also resistant to erythromycin and clindamycin. However, interpretation of results from eight isolates needs to be cautious on account of the small sample size.
In 2019, Switzerland had reported data on two isolates from bovine meat and one isolate from pig meat (see Table 7b in Annex E). Interestingly, the latter was only resistant to penicillin, cefoxitin, trimethoprim and ciprofloxacin. Austria in the same year reported six isolates from bovine meat.
Only six of the 11 isolates from meat from sheep in 2020 were resistant to tetracycline (Figure 54, see also Table 7a in Annex E). This is in line with the high proportion of non‐CC398 strains among these isolates. All non‐CC398 strains were susceptible to tetracycline. Resistance in more than one of the isolates was only observed to three antimicrobials, i.e. erythromycin (five isolates), clindamycin (two) and trimethoprim (three). However, interpretation of the results needs caution on account of the limited number of isolates. Interestingly, one of the isolates from meat from sheep was resistant to vancomycin, an antimicrobial of utmost importance for the treatment of MRSA infections in the medical field. Whole genome sequencing revealed that the isolate did not carry a specific vancomycin‐resistance gene. This is in accordance with the limited MIC value of 4mg/L, i.e. one dilution step above the cut off.
6.5.2. Multidrug resistance in MRSA isolates obtained from meat and food‐producing animals (excluding clinical investigations)
MRSA are typically resistant to penicillins and cephalosporins. Moreover, most isolates also show resistance to more substances and can be considered multidrug resistant. Overall, MDR patterns displayed substantial variability (Table 9, Annex E). In 2020, most of the 23 MDR isolates combined resistance to beta‐lactams with resistance to tetracycline (18/23), with only five isolates from sheep meat not resistant to this substance. The latter were the non‐CC398 isolates that were identified in sheep meat. Among the four isolates from animals with this pattern, three also included trimethoprim in the MDR‐pattern, two of those also in combination with erythromycin and clindamycin.
Among the isolates from food in 2020, 10 of the 17 MDR‐isolates were resistant to erythromycin. Six of those were likewise resistant to clindamycin. Resistance to trimethoprim was included in the MDR pattern of seven isolates.
MRSA isolates from slaughter batches of fattening pigs reported by Portugal in 2019 showed resistance in one or more isolates to all antimicrobials tested (except for vancomycin) (Table 7b, Annex E).
6.6. Discussion
The monitoring of MRSA in animals and food was voluntary in 2019 and 2020 and only a limited number of countries reported data on the occurrence of MRSA. Moreover, sampling and laboratory techniques are not fully harmonised across countries. Therefore, comparisons across countries should be done with prudence. Moreover, data on clinical investigations that were reported by some countries per se do not allow for prevalence estimations in the populations as the clinical conditions lead to bias in the sampled animals.
6.6.1. Occurrence of MRSA in food
The monitoring of MRSA in various food products performed by MSs in 2019–2020 indicates that MRSA can be detected in different types of food. However, occurrence varies between types of food and countries. While the numbers of investigated samples differed, MRSA were detected in all reported meat categories.
It should be underlined that the laboratory techniques used to detect MRSA employ selective bacterial culture and therefore, very low levels of contamination in food can be detected (Pauly et al., 2019). Although a previous report has cautiously suggested that some strains of LA‐MRSA may be adapted to colonise and infect humans and reported poultry meat as a possible source of MRSA for humans (Larsen et al., 2016), food is not generally considered to be a significant source of MRSA infection or colonisation of humans (EFSA, 2009a; FSA, 2017).
6.6.2. Occurrence of MRSA in animals
In 2019–2020, MRSA were reported in animals in the framework of monitoring programmes, surveys and as a result of clinical investigations. While monitoring results and surveys will typically allow for an estimation of the prevalence of MRSA in the investigated animal population, this is not valid for clinical investigations on account of the sampling bias associated with the clinical conditions. The comparison of the occurrence of MRSA in the animal populations is always hampered by the lack of precision and harmonisation of the definition of the animal populations. For example, herds of breeding pigs have been tested by Belgium in 2019 and Denmark in 2018 and 2019, but Denmark tested multiplier herds in 2019 while Belgium in 2019 and Denmark in 2018 did not further elaborate on the type of breeding pigs investigated. As AMR tends to differ between different animal populations of the same animal species, these differences need to be taken into account, when comparing the results from different reporting countries (Mesa Varona et al., 2020).
Another potential confounder is the sampling strategy. Norway has been investigating pig herds in the framework of the control program for several years. The investigations may be considered a census which increases the likelihood to detect positive herds even if they are rare.
A third aspect to be considered are differences in the types and numbers of samples collected. Sampling of nasal swabs is a frequent approach, but the numbers of samples tested may differ between countries with a likely effect on the prevalence estimate. Frequently, for the convenience of sample collection, dust from the housing environment is sampled despite concerns over the sensitivity of testing these samples (EFSA, 2012a,b).
The sampling location also has an effect. Batches of pigs at slaughter are more frequently positive than pigs at farm, potentially due to contamination of pigs during transport (Broens et al., 2011). Therefore, prevalence estimates should not be directly compared for the two sampling locations.
Occurrence of MRSA in flocks of broilers at farm in Belgium was low in 2020, as it had been in 2017 and 2014 (Figure 52; see also Table 6 in Annex E). It was absent in laying hens from Belgium, where it had been low in 2014 and 2017. This is in line with findings from other countries such as Denmark in 2019 or Germany in 2009 and 2013. Likewise, the Netherlands did not find MRSA in chickens at farm (EFSA and ECDC, 2013). The reason for this rare occurrence is not known yet and it contrasts with the regular detection of MRSA in broiler meat (Figure 50).
In turkey flocks, MRSA were more regularly observed in Germany (Vossenkuhl et al., 2014). Which agrees with the frequent detection of MRSA on carcases and meat from turkeys (Vossenkuhl et al., 2014).
In 2020, MRSA was not reported in bovine animals and only two countries reported data on MRSA in pigs. This is in line with the focus of monitoring programs on poultry in even years as prescribed by CID 2013/652/EU and the new CID 2020/1729/EU. Occurrence of MRSA in pigs was again frequent confirming the results of previous years.
6.6.3. Typing results of MRSA from animals and food
Some countries additionally reported data on spa‐type/clonal complex and antimicrobial susceptibility of the MRSA isolates from animals and food. The number of reported isolates was higher in 2019 than in 2020.
Among the isolates from animals with available typing results in 2019–2020, only four isolates reported in 2019 could not be assigned to CC398. This underlines that the CC398 widely dominates the MRSA from animals in the reporting countries. Two of the non‐CC398 isolates originated from horses another two from dairy cows. Remarkably, all isolates were from one country and three of the four isolates were assigned to CC130 and harboured the mecC gene. Isolates of CC130 harbouring the mecC gene have been observed in horses, cattle and sheep before (Paterson et al., 2014; Haenni et al., 2015; Giacinti et al., 2017; Islam et al., 2017). However, they are also a medical concern (Lozano et al., 2020). The other non‐CC398 isolate was an ST1 of spa‐type t127, that is frequently reported in cattle (Albert et al., 2020), sheep (Giacinti et al., 2017), but also in humans (Karampatakis et al., 2021) and dogs (Schmitt et al., 2020). The origin of such isolates is debatable. On the one hand, they have repeatedly been observed in animals and it is possible that they have been established in the food‐producing animal populations. On the other hand, they might originate from people handling the animals as exchange of bacterial strains between animals and humans is likely for strains that can colonise several species.
In 2020, typing information on isolates from animals was only provided for four isolates (two isolates from broiler flocks and two from turkey flocks). These were all from the predominant spa‐type t011.
The livestock associated lineage of CC398 was also by far the most prevalent MRSA type detected in food with 82.5% of 63 typed isolates assignable to that CC in 2019 and 73.7% of 19 isolates in 2020 assignable to that CC. Remarkably, the proportion of non‐CC398 isolates in food is higher than in animals, which may indicate contamination at or post harvest from other than animal sources. This is in line with the literature and requires further targeted investigations (Tenhagen et al., 2014; Vossenkuhl et al., 2014). Isolates from broiler meat in Austria in 2020 were, however, always assignable to the CC398.
Isolates of other CCs (CC5, CC97) and of spa‐types assignable to other CCs were most frequently observed in meat from sheep in 2020 (5/11 isolates). CC97 isolates have previously been associated with bovines (Käppeli et al., 2019) and pigs in Italy (Feltrin et al., 2015), but they have also been observed in human cases unrelated to animal production (Monecke et al., 2011; Boswihi et al., 2020). Therefore, their origin in food is doubtful. Isolates of CC5 have likewise previously been observed in animals (Vossenkuhl et al., 2014). However, they are also well established among isolates from humans, including CA‐MRSA strains that are PVL positive (Monecke et al., 2011). Their observation in meat from sheep might therefore also indicate contamination during slaughter and processing of meat as they have not frequently been found in sheep previously.
For some isolates, the spa‐type was determined, but information on the sequence type or CC was not provided. However, spa‐type t223, identified in isolates from meat from sheep is frequently assigned to ST22 one of the predominant sequence types among isolates from humans in Germany (Layer et al., 2019). This too may indicate a contamination of meat during processing. This has previously been pointed out while most isolates on meat probably originate from primary production. Overall, the variability of spa‐ and sequence types is higher in isolates from meat than in isolates from animals indicating potential contamination from other sources, including humans (Vossenkuhl et al., 2014). spa‐type t223 has, however, also been reported from cattle in Belgium in 2018 (see Figure 1 and Table 6 in Annex E) pointing to a potential animal origin of the isolates. On the other hand, CC22 isolates have also been observed in people handling sheep in Italy (Mascaro et al., 2019). Hence the origin and transmission are difficult to discern and more data on this are needed for non‐CC398 isolates. These findings highlight the importance of routine typing of MRSA to avoid assignment errors due to a lack of available data. In 2019, isolates of other CCes were likewise reported for meat. These have been dealt with extensively in the previous EU‐SR (EFSA and ECDC, 2020).
Further typing data would in many cases provide extremely useful additional information to aid classification and help assess the origin and significance of the MRSA isolates. For example, possession of the IEC genes (chp, sak and scn) is considered an adaptation facilitating colonisation and infection of humans and is not usually a feature of animal strains (Cuny et al., 2015; Larsen et al., 2016). Similarly, the presence of the PVL toxin is a virulence feature typically associated with most CA‐MRSA strains; other genetic factors can be associated with particular strains or may suggest a particular host preference (e.g. lukM has been associated with certain animal strains, particularly those affecting ruminants).
6.6.4. Antimicrobial resistance in MRSA from animals and food
Antimicrobial resistance of MRSA differed substantially between the different origins of the bacteria. In line with the literature, MRSA of CC398 were mostly resistant to tetracyclin and lower resistance rates to tetracycline were only observed if MRSA of other CCes were involved, e.g. in meat from sheep in Germany in 2020. Here, all five non‐CC398 isolates were susceptible to tetracycline.
Resistance to important medical drugs for the treatment and control of MRSA was overall extremely low. However, resistance to linezolid was observed in three isolates of spa‐type t011 from batches of fattening pigs at slaughter in Portugal in 2019 (EFSA and ECDC, 2020), and resistance to vancomycin was observed in a t011 isolate from sheep meat in Germany in 2020 (Figures 53 and 54; see also Table 7a in Annex E).
The detection of cfr in MRSA spa‐type t011 from fattening pigs is significant because of the importance of linezolid in treating highly resistant MRSA infections in man. The number of countries which have reported susceptibility data on MRSA in animals is low; more widespread testing would indicate whether the occurrence of cfr is a localised phenomenon or the gene occurs more widely in LA‐MRSA in the animal population. The presence of the cfr gene may confer linezolid MICs of 4mg/L determined by broth microdilution (Li et al., 2015) (wild‐type by the EUCAST linezolid ECOFF of ≤ 4 mg/L), although resistance to the other compounds typically conferred by cfr (CLI‐CHL‐TIA) was consistently noted in both the EFSA monitoring and by Li et al. (2015). For optimal detection of the cfr gene therefore, it is recommended that all isolates exhibiting resistance to CLI‐CHL‐TIA and displaying linezolid MICs of ≥ 4mg/L, are screened for the cfr gene.
The occurrence of these types of resistance is worrisome as they concern drugs of last choice in the medical setting (Maraolo et al., 2021). Interestingly the vancomycin resistant isolate did not harbour one of the typical vancomycin resistance genes, which is in line with the limited increase in the MIC (4 mg/L). These moderate increases are typically caused by multiple point mutations rather than by specific vancomycin resistance genes like vanA or vanB (McGuinness et al., 2017). Resistance to other medically important drugs for the control of MRSA such as mupirocin was also observed in individual cases. Mupirocin is an important component of the decolonisation therapy used prior to surgery in MRSA positive patients.
6.6.5. Temporal trends of MRSA prevalence in various types of meat and food‐producing animals
In 2018, the recommended method for the isolation of MRSA was revised by the EURL‐AR from the 2‐S method (which comprised a pre‐enrichment step and a selective enrichment step) to the 1‐S method (whereby the selective enrichment step is omitted). Therefore, and because of the limited number of reported samples direct comparisons of longitudinal data obtained using different protocols should be performed with caution. In line with these limitations, we did not perform statistical trend analyses but only presented changes over time descriptively.
Considering MRSA prevalence data obtained from fattening pigs at slaughter reported by Switzerland, generally prevalence has increased annually from 2010 to 2019, with a more marked increase noted from 2015 to 2017. This increase most likely represents the dissemination of particular livestock‐associated spa‐types in Swiss fattening pig populations; and where molecular typing data were available, all isolates in 2017 and 2019 were those associated with CC398, with most belonging to spa‐types t011 and t034 in 2017. A longitudinal study carried out by Kraemer et al. (2017) also supports these trends, in which MRSA prevalence of pig farms in Western Switzerland were reported to increase from 7.3% in 2008 to 31% in 2015. The complete epidemiological data should however be considered when evaluating trends apparent in this chapter, because the summary data reported to EFSA may not include full details of any methodological or other changes to monitoring procedures. A detailed longitudinal study illustrated that pigs are intermittently and repeatedly colonised, and that colonisation may also occur during transportation and while in the lairage (Bangerter et al., 2016). The detection of intermittent, repeated colonisation suggests that the number of animals sampled as part of a batch, including whether individual animals are sampled to represent a herd or batch, is likely to influence the batch or herd prevalence obtained. These factors should therefore be taken into consideration when comparing trends, as the Swiss annual MRSA monitoring examines a single pig from a herd at slaughter.
Abbreviations
- %
percentage
- % f
percentage frequency of isolates tested
- % Res
percentage of resistant isolates
- AMR
antimicrobial resistance
- CASFM
Comité de l'Antibiogramme de la Société Française de Microbiologie
- CBP
clinical breakpoints
- CC
clonal complex
- CLSI
Clinical and Laboratory Standards Institute
- CS
Complete Susceptibility
- CP
carbapenemase producer
- CTX‐M
cefotaxime
- DD
disc diffusion method
- DL
dilution/dilution method
- DLG
dilution with gradient step
- EARS‐Net
European Antimicrobial Resistance Surveillance Network
- ECDC
European Centre for Disease Prevention and Control
- ECOFF
epidemiological cut‐off value
- EEA
European Economic Area
- ESBL
extended spectrum beta‐lactamase
- EUCAST
European Committee on Antimicrobial Susceptibility Testing
- EURL‐AR
EU Reference Laboratory for Antimicrobial Resistance (www.crl‐ar.eu)
- FWD
food‐ and waterborne diseases and zoonoses
- I
Intermediate
- MRSA
meticillin‐resistant Staphylococcus aureus
- MS
Member State
- NA
not applicable/not available
- NCP
National Control Programme
- NRL
National Reference Laboratory
- PMQR
plasmid‐mediated quinolone resistance
- Q
Quantitative
- QRDR
quinolone resistance‐determining regions
- R
Resistant
- res1–res9
resistance to one antimicrobial substance/resistance to nine antimicrobial substances of the common set for Salmonella
- S
Susceptible
- SIR
susceptible, intermediate, resistant
- ST
sequence type
- TESSy
The European Surveillance System
- WGS
whole genome sequencing
- WHO
World Health Organization
Antimicrobial substances
| AMC | amoxicillin/clavulanate |
| AMP | ampicillin |
| AZM | azithromycin |
| CAZ | ceftazidime |
| CHL | chloramphenicol |
| CIP | ciprofloxacin |
| CLA | clavulanate |
| CLI | clindamycin |
| CST | colistin |
| CTX | cefotaxime |
| ERY | erythromycin |
| FUS | fusidic acid |
| GEN | gentamicin |
| KAN | kanamycin |
| LZD | linezolid |
| MEM/MER | meropenem |
| MUP | mupirocin |
| NAL | nalidixic acid |
| QD | quinupristin/dalfopristin |
| RIF | rifampicin |
| SUL | sulfonamides |
| STR | streptomycin |
| SXT | sulfamethoxazole |
| TGC | tigecycline |
| TIA | tiamulin |
| TET | tetracycline |
| TMP | trimethoprim |
MSs of the EU and other reporting countries
| Austria | AT |
| Belgium | BE |
| Bulgaria | BG |
| Croatia | HR |
| Cyprus | CY |
| Czechia | CZ |
| Denmark | DK |
| Estonia | EE |
| Finland | FI |
| France | FR |
| Germany | DE |
| Greece | EL |
| Hungary | HU |
| Ireland | IE |
| Italy | IT |
| Latvia | LV |
| Lithuania | LT |
| Luxembourg | LU |
| Malta | MT |
| Netherlands | NL |
| Poland | PL |
| Portugal | PT |
| Romania | RO |
| Slovakia | SK |
| Slovenia | SI |
| Spain | ES |
| Sweden | SE |
Non‐MSs reporting
| Albania | AL |
| Iceland | IS |
| Republic of North Macedonia | MK |
| Norway | NO |
| Switzerland | CH |
| United Kingdom | UK |
Definitions
| ‘Antimicrobial‐resistant isolate’ |
In the case of quantitative data, an isolate was defined as ‘resistant’ to a selected antimicrobial when its minimum inhibitory concentration (MIC) value (in mg/L) was above the cut‐off value or the disc diffusion diameter (in mm) was below the cut‐off value. The cut‐off values, used to interpret MIC distributions (mg/L) for bacteria from animals and food, are shown in Appendix F ‘Material and methods’, Tables F.5–F.7. In the case of qualitative data, an isolate was regarded as resistant when the country reported it as resistant using its own cut‐off value or break point |
| ‘Level of antimicrobial resistance’ | The percentage of resistant isolates among the tested isolates |
| ‘Reporting MS group’ | Member States (MSs) that provided data and were included in the relevant table for antimicrobial resistance data for the bacteria–food/animal category–antimicrobial combination |
| Terms used to describe the antimicrobial resistance levels |
Rare: < 0.1% Very low: 0.1–1.0% Low: > 1.0–10.0% Moderate: > 10.0–20.0% High: > 20.0–50.0% Very high: > 50.0–70.0% Extremely high: > 70.0% |
Table F.5.
Panel of antimicrobial substances included in AMR monitoring, EUCAST ECOFFs and concentration ranges tested in Salmonella spp. and indicator commensal E. coli (first panel) as laid down in Commission Implementing Decision 2013/652/EU
| Antimicrobial |
Salmonella EUCAST ECOFF( a ) |
E. coli EUCAST ECOFF( a ) |
Concentration range, mg/L (no. of wells) |
|---|---|---|---|
| Ampicillin | > 8 | > 8 | 1–64 (7) |
| Cefotaxime | > 0.5 | > 0.25 | 0.25–4 (5) |
| Ceftazidime | > 2 | > 0.5 | 0.5–8 (5) |
| Meropenem | > 0.125 | > 0.125 | 0.03–16 (10) |
| Nalidixic acid | > 16 | > 16 | 4–128 (6) |
| Ciprofloxacin | > 0.064 | > 0.064 | 0.015–8 (10) |
| Tetracycline | > 8 | > 8 | 2–64 (6) |
| Colistin | > 2 | > 2 | 1–16 (5) |
| Gentamicin | > 2 | > 2 | 0.5–32 (7) |
| Trimethoprim | > 2 | > 2 | 0.25–32 (8) |
| Sulfamethoxazole | NA( b ) | > 64 | 8–1,024 (8) |
| Chloramphenicol | > 16 | > 16 | 8–128 (5) |
| Azithromycin | NA( c ) | NA( c ) | 2–64 (6) |
| Tigecycline | > 1 | > 1 | 0.25–8 (6) |
AMR: antimicrobial resistance; ECOFFs: epidemiological cut‐off values; EUCAST: European Committee on Antimicrobial Susceptibility Testing; NA: not available.
EUCAST epidemiological cut‐off values available in Decision 2013/652/EU was drafted (2013). ‘>’ than the ECOFF, criteria used for determing microbiological resistance.
> 256 mg/L was used.
> 16 mg/L was used.
Table F.7.
Panel of antimicrobial substances, EUCAST ECOFFs and concentration ranges used for testing only Salmonella spp. and indicator commensal E. coli isolates resistant to cefotaxime, ceftazidime or meropenem (second panel)
| Antimicrobial |
Salmonella EUCAST ECOFF( a ) |
E. coli EUCAST ECOFF( a ) |
Concentration range, mg/L (no. of wells) |
|---|---|---|---|
| Cefoxitin | > 8 | > 8 | 0.5–64 (8) |
| Cefepime | NA( b ) | > 0.125 | 0.06–32 (10) |
| Cefotaxime + clavulanic acid | NA | NA | 0.06–64 (11) |
| Ceftazidime + clavulanic acid | NA | NA | 0.125–128 (11) |
| Meropenem | > 0.125 | > 0.125 | 0.03–16 (10) |
| Temocillin | NA( d ) | NA( d ) | 0.5–64 (8) |
| Imipenem | > 1 | > 0.5 | 0.12–16 (8) |
| Ertapenem | > 0.06 | > 0.06 | 0.015–2 (8) |
| Cefotaxime | > 0.5 | > 0.25 | 0.25–64 (9) |
| Ceftazidime | > 2 | > 0.5 | 0.25–128 (10) |
ECOFFs: epidemiological cut‐off values; EUCAST: European Committee on Antimicrobial Susceptibility Testing; NA: not available.
EUCAST epidemiological cut‐off values available as the Decision 2013/652/EU was drafted (2013). For some antimicrobials, these values have been updated (see below). ‘>’ than the ECOFF, criteria used for determining microbiological resistance.
> 0.125 mg/L was used.
Current ECOFFs 0.25 and 0.5 mg/L, respectively.
For temocillin, the cut‐off value used in the analysis was > 32 mg/L.
Appendix A – High‐level resistance to ciprofloxacin among certain Salmonella serovars recovered from the poultry origins, as well as a pig carcase
High‐level resistance to ciprofloxacin in S. Kentucky
Considering individual serovars, S. Kentucky accounted for most of the Salmonella isolates recovered from the poultry origins and pig carcases which exhibited MICs to ciprofloxacin of ≥ 4 mg/L. Within each of the poultry origins, the highest number of Salmonella isolates exhibiting high‐level resistance to this antimicrobial were attributed to S. Kentucky; this serovar accounting for 91%, 92%, 76.5%, 100% and 93% of the total number of isolates displaying MICs of ≥ 4 mg/L from broiler carcases, turkey carcases, broilers, laying hens and turkeys, respectively. Additionally, a single isolate recovered from a pig carcase also displayed high‐level ciprofloxacin resistance and was serotyped as S. Kentucky. S. Kentucky isolates exhibiting high‐level ciprofloxacin resistance are likely to belong to the multilocus sequence type (ST) 198 clone, which has shown epidemic spread across Africa first, then to the Middle East, Asia and Europe (Le Hello et al., 2011,2013; Hawkey et al., 2019). Notably in 2018, the occurrence of this serovar exhibiting high‐level resistance was observed by many MSs from most parts of Europe, suggesting further clonal expansion (S. Kentucky ST198‐X1) within poultry populations. In view of reported MIC values, most of the S. Kentucky isolates exhibiting high‐level ciprofloxacin resistance in 2019–2020 displayed MICs of ≥ 8 mg/L (only one S. Kentucky isolate from a broiler flock displayed an MIC of 4 mg/L). Additionally, a very high proportion of the poultry/pig S. Kentucky isolates displaying ciprofloxacin MICs of ≥ 4 mg/L were also multiresistant, primarily showing resistance to ampicillin, gentamicin, nalidixic acid, sulfamethoxazole and tetracycline (AMP‐CIP‐GEN‐NAL‐SMX‐TET). Figure A.1 presents the overall AMR levels among MDR S. Kentucky isolates from poultry which exhibited high‐level ciprofloxacin resistance.
Figure A.1.
Resistance levels among MDR S. Kentucky isolates exhibiting high‐level ciprofloxacin resistance from poultry, reported by MSs in 2020
-
n: Total number of MDR S. Kentucky isolates exhibiting high‐level ciprofloxacin resistance.NB: The single S. Kentucky isolate recovered from a pig carcase in 2019, which also displayed high‐level ciprofloxacin resistance, additionally showed resistance to AMP‐GEN‐NAL‐SMX‐TET. None of the S. Kentucky isolates reported exhibited either azithromycin or colistin resistance.
In 2020, S. Kentucky was the sixteenth most commonly reported serovar in humans, with 158 cases reported by EU/EEA countries. From the monitoring of human cases in 2020, high to extremely high levels of resistance were noted to gentamicin (40.0%), sulfonamides (72.3%), ampicillin (80.0%), tetracyclines (78.7%), ciprofloxacin (82.0%) and nalidixic acid (81.0%); consistent with the multiresistance patterns observed in isolates from the monitoring of poultry in 2020, and the possible dissemination of the S. Kentucky ST198 strain within Europe. Furthermore, of 3,042 Salmonella isolates from humans where ciprofloxacin MIC data were available, 44 of these (1.4%) exhibited MICs of ≥ 4 mg/L, of which S. Kentucky accounted for 41 (93.2%).
Hawkey et al. (2019) recently documented that MDR S. Kentucky ST198 is a globally disseminated clone, capable of rapid spread and accumulation of resistance determinants to last‐line antimicrobials. Acquisition of Salmonella genomic island 1 (SGI1) and plasmids, as well as mutations in the Quinolone Resistance‐determining regions (QRDR), were the only genetic features found during this study to explain the global epidemiological success of the MDR S. Kentucky ST198 lineage which is highly resistant to ciprofloxacin. Coipan et al. (2020) describe how a clone of this lineage, with an ESBL‐gene (bla CTX‐M‐14b) incorporated on the chromosome, has emerged in Europe. In contrast to plasmid‐mediated resistance, chromosomal‐mediated resistance is most likely to be maintained also without antibiotic pressure, especially if it incurs no fitness cost for the bacteria.
High‐level resistance to ciprofloxacin among other Salmonella serovars
While S. Kentucky generally accounted for an important number of the Salmonella isolates exhibiting high‐level resistance to ciprofloxacin, there was also a significant contribution from S. Infantis in broilers and broiler carcases, many other serovars exhibiting resistance by this definition were noted among the poultry origins (namely S. Newport, S. Derby, S. Reading and S. Bredeney). Figure A.2 shows the number of isolates exhibiting high‐level resistance to ciprofloxacin by serovar within each of the poultry origins.
Figure A.2.
Number of isolates displaying high‐level ciprofloxacin resistance by serovar, reported from the different poultry origins by MSs in 2020
- n: Total number of Salmonella isolates exhibiting high‐level ciprofloxacin resistance; ns: number of isolates by serovar exhibiting high‐level ciprofloxacin resistance; * serovar unspecified; salmonellas in the legend are listed according to their predominance within all the animal/carcase origins; in addition, a single S. Kentucky isolate displaying high‐level ciprofloxacin resistance was recovered from a pig carcase in 2019.
Considering ciprofloxacin MICs among the serovars presented in Figure A.2 (excluding S. Kentucky), MICs of 4 mg/L were reported in S. Derby (from turkeys), S. Infantis and S. Newport (from broiler carcases), S. Bredeney (from turkey carcases), S. Infantis and S. Newport (from broilers).
Appendix B – Cefotaxime, ceftazidime and ciprofloxacin resistance in Salmonella spp. recovered from each of the animal/carcase origins and humans considering all reporting MSs in 2019–2020
Table B.1 summarises cefotaxime, ceftazidime and ciprofloxacin resistance in Salmonella spp. recovered from each of the animal/carcase origins and humans considering all reporting MSs in 2019–2020.
Appendix C – Occurrence of resistance at the Salmonella serovar level
In carcases of food‐producing animals
Breakdown of the most prevalent serovars
The detailed reporting of results at the serovar level clearly demonstrated the major contribution of a few serovars to the observed occurrence of resistance in Salmonella spp. Figure C.1 illustrates the relative contribution of some of the most dominant serovars recovered from each of the carcase origins considering all reporting countries (including non‐MSs). In pig carcases, six serovars (monophasic Typhimurium, Derby, Typhimurium, Rissen, Infantis and Brandenburg) accounted for 83.3% of Salmonella spp.; while in calf carcases, serovars monophasic Typhimurium, Derby, Anatum, Dublin, London and Ohio accounted for 57.1% of the total Salmonella spp. isolated from this origin. Additionally, in broiler carcases, six serovars (Infantis, Enteritidis, Agona, Montevideo, Indiana and Chester) accounted for 76.3% of Salmonella isolates; while in turkey carcases, Bredeney, Hadar, Indiana, monophasic Typhimurium and Kentucky accounted for 57.5% of Salmonella spp. isolated from this origin.
Figure C.1.
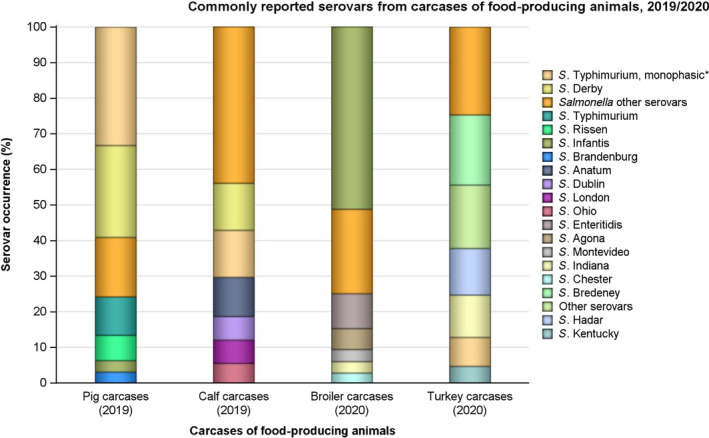
The six most commonly reported serovars from carcases of broilers, fattening turkeys, fattening pigs and calves (< 1 year of age), for all reporting countries (including two non‐MSs in broiler carcases and one non‐MS in pig carcases) in 2019–2020
- *: Monophasic S. Typhimurium includes antigenic formulas; serovars in the legend are listed according to their predominance within all the carcase origins. From calf carcases, S. Dublin and S. London were joint fourth most frequently reported.
Complete susceptibility and multidrug resistance
Patterns of resistance associated with these different serovars have a marked influence on the overall resistance levels in Salmonella spp., and Figure C.2 summarises the proportion of completely susceptible and MDR isolates among particular serovars recovered from each of these carcase origins considering all reporting countries (including non‐MSs). Large contributions of a few resistant serovars to the overall level of MDR among Salmonella spp. were evident within some of the carcase origins; notably S. Infantis in broiler carcases and S. Typhimurium and its monophasic variant in pig carcases.
Figure C.2.
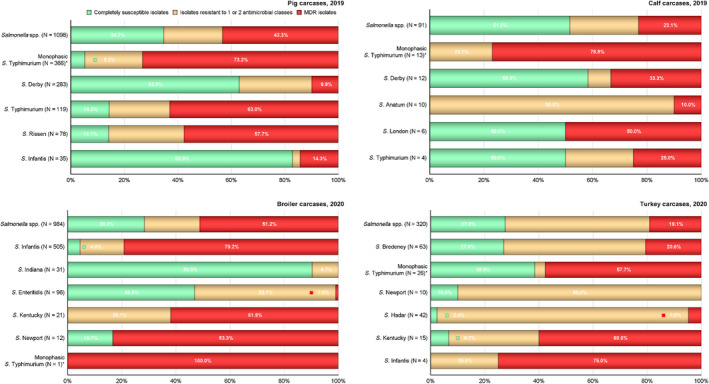
Proportions of isolates completely susceptible and MDR in Salmonella spp. and particular Salmonella serovars from carcases of fattening pigs, calves (< 1 year of age), broilers and fattening turkeys, for all reporting countries (including one non‐MS in pig carcases and two non‐MSs in broiler carcases) in 2019–2020
-
N: Total number of Salmonella spp. or total number of particular serovars recovered from the carcase monitoring.*: Monophasic S. Typhimurium includes antigenic formulas.
In food‐producing animal populations
Breakdown of the most prevalent serovars
Considering all reporting countries (including non‐MSs), the relative contribution of some of the most dominant serovars recovered from each of the food‐producing animal populations is illustrated in Figure C.3. In pigs, six serovars (Derby, monophasic Typhimurium, Typhimurium, Rissen, Brandenburg and Goldcoast) accounted for 83% of Salmonella spp.; while in calves, serovars monophasic Typhimurium, Typhimurium, Anatum, Meleagridis, Enteritidis and Mbandaka accounted for 64.1% of the total Salmonella spp. isolated from this origin. Additionally, in broilers, six serovars (Infantis, Enteritidis, Kedougou, Montevideo, Mbandaka and Newport) accounted for 59.8% of Salmonella isolates, while in laying hens five serovars (Enteritidis, Kentucky, Infantis, Typhimurium and Mbandaka) accounted for 55.4% of isolates; and in turkeys, serovars Derby, Anatum, Infantis, Bredeney, Kedougou and Senftenberg accounted for 56.5% of Salmonella isolates.
Figure C.3.
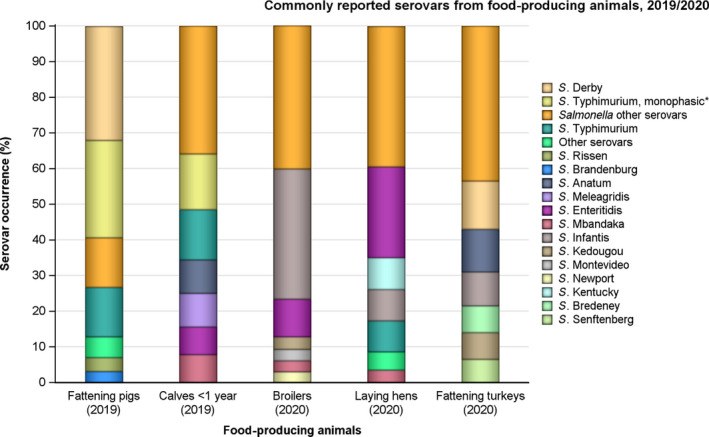
The six most commonly reported serovars recovered from broilers, laying hens, fattening turkeys, fattening pigs and calves (< 1 year of age), for all reporting countries (including one non‐MS in broilers and laying hens), 2019–2020
- *: Monophasic S. Typhimurium includes antigenic formulas; serovars in the legend are listed according to their predominance within all the animal origins. From calves, S. Anatum and S. Meleagridis were the joint third most frequently reported; S. Enteritidis and S. Mbandaka were the joint fourth most frequently reported.
Complete susceptibility and multidrug resistance
The patterns of resistance associated with these different serovars influenced the overall resistance levels in Salmonella isolates, and Figure C.4 summarises the proportion of completely susceptible and MDR isolates among particular serovars recovered from each of these food‐producing animal populations considering all reporting countries (including non‐MSs).
Figure C.4.
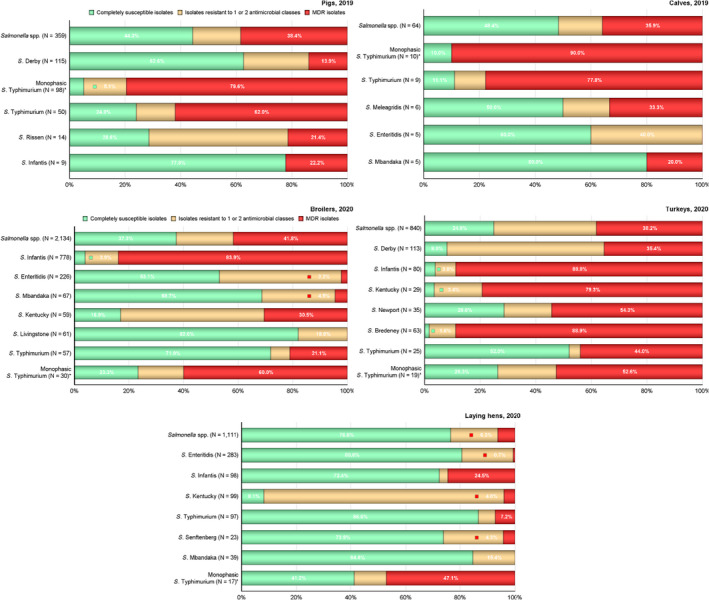
Proportions of isolates completely susceptible and MDR in Salmonella spp. and certain serovars recovered from fattening pigs, calves (< 1 year of age), broilers, laying hens and fattening turkeys, for all reporting countries, 2019–2020
-
N: Total number of Salmonella spp. or total number of particular serovars recovered from the monitoring of animals.*: Monophasic S. Typhimurium includes antigenic formulas.
Resistance exhibited by particular serovars
S. Derby was the most common serovar detected in fattening pigs and turkeys, as well as the second most frequently recovered from pig and calf carcases, accounting for 32%, 25.2%, 25.8% and 13.2% of Salmonella isolates recovered from these animal/carcase origins, respectively (see Figures C.1 and C.3). While multidrug resistance was not frequently observed among S. Derby isolates from pigs and their derived carcases (13.9% and 9.9%, respectively), it was detected at high levels in isolates from calf carcases (33.3%) and turkeys (43.1%); with 96.77% (90/93) of turkey isolates showing resistance to one or more antimicrobials. Notably, the MDR analysis performed on the carcase/animal isolates comprised 10 antimicrobial classes and included the glycylcycline class.
Among S. Derby isolates recovered from these animal/carcase origins, different core resistance patterns predominated. In pigs, where 16/115 (13.9%) isolates were MDR, the most common resistance pattern was to ampicillin, sulfamethoxazole and trimethoprim (4/16 MDR S. Derby reported by Denmark); while in pig carcases, where 28/283 (9.9%) were MDR, the predominant core resistance pattern was to sulfamethoxazole, trimethoprim and tetracycline (9/28 MDR S. Derby reported by Croatia, Denmark, France and Spain). This latter core pattern with the addition of ampicillin, CIP and NAL RESISTANCE (AMP‐NAL‐CIP‐SMX‐TMP‐TET) was most frequently noted among MDR isolates from turkeys (32/93, 34.4%). Among MDR isolates recovered from calf carcases (4/12, 33.3%), the most frequent core pattern was resistance to chloramphenicol, tigecycline, sulfamethoxazole and tetracycline.
Recent studies on S. Derby originating from the pork and poultry sectors in France identified four major host‐specific lineages, corresponding to multilocus sequence typing (MLST) profiles ST39, ST40, ST71 and ST682 (Sévellec et al., 2018, 2020). The lineages ST39, ST40 and ST682 were determined to be associated with pork, and ST71 with poultry. While ST71 and ST682 isolates were generally devoid of resistance genes, ST40 isolates commonly harboured several resistance genes, with Clade 2 of this lineage characterised by SGI1 and the pattern of resistance to streptomycin, sulfonamides and tetracycline. Additionally, the presence of a resistance gene for fosfomycin was detected in all genomes from ST39 (Sévellec et al., 2020). Results obtained from the EFSA monitoring of turkeys at the European level in 2018 contrast with the findings of Sévellec et al., with a proportion of S. Derby from turkeys demonstrating resistance in the EFSA monitoring. The results may therefore illustrate geographical differences in the distribution of S. Derby lineages and associated resistance patterns in the different reporting European countries. Notably, five out of six MSs reported MDR among S. Derby isolates recovered from turkeys, with the most frequent core MDR pattern (AMP‐SMX‐TMP‐TET) noted among isolates from Spain and the United Kingdom (38.3% of all MDR S. Derby isolates). Multidrug resistance was not observed among three isolates recovered from turkeys by France, however, one‐third of these isolates were resistant to both sulfamethoxazole and tetracycline.
Resistance to five antimicrobial classes was observed in single isolates recovered from calf carcases and pigs, as well as two isolates recovered from pig carcases and 39 isolates from turkeys. Additionally, a single isolate recovered from a pig carcase was resistant to six antimicrobial classes. Ciprofloxacin/nalidixic acid resistance among MDR isolates was reported in single isolates recovered from calf carcases and pigs, three isolates from pig carcases and 33 isolates from turkeys. Tigecycline resistance was only observed in a single MDR isolate recovered from a calf carcase.
Resistance to third‐generation cephalosporins was not detected in S. Derby isolates from pigs, calves, or calf carcases, and only single isolates reported from pig carcases by Romania (N = 1) and turkeys by Poland (N = 1) were determined to be resistant to this antimicrobial class. Both S. Derby isolates exhibited an ESBL phenotype, with the isolate from turkeys. Where third‐generation cephalosporin resistance was reported in these two isolates, only the isolate recovered from a pig carcase also displayed ‘microbiological’ resistance to ciprofloxacin (MIC > 0.064 mg/L). Nevertheless, when ciprofloxacin and cefotaxime resistance were interpreted using clinical breakpoints (CBPs), this isolate did not display combined ‘clinical’ resistance.
Monophasic S . Typhimurium commonly exhibited resistance, and was the most dominant serovar recovered from pig carcases, calf carcases and calves, as well as the second most dominant serovar recovered from turkey carcases and pigs; accounting for 33.3%, 14.3%, 15.6%, 12.6% and 27.3% of Salmonella isolates recovered from these animal/carcase origins, respectively (see Figures C.1 and C.3). Notably, the proportion of all Salmonella isolates showing MDR in calves, calf carcases, pig carcases and pigs was greatly influenced by the occurrence of multiresistant monophasic S. Typhimurium, which accounted for 39.1% (9/23), 47.6% (10/21), 56.4% (268/475) and 56.5% (78/138) of the MDR Salmonella isolates recovered from these carcase/animal origins, respectively (see Figure C.5). Similarly, this serovar contributed the highest level of multiresistance (13%, 7/54) to overall MDR levels among Salmonella isolates recovered from turkey carcases, as did S. Infantis.
Figure C.5.
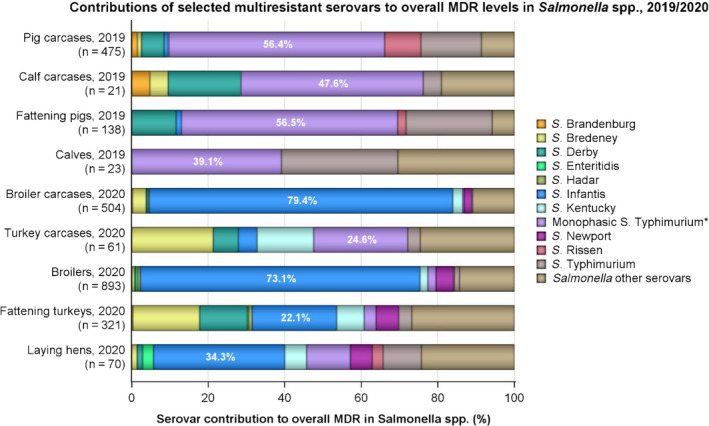
Proportions of certain serovars exhibiting multiresistance to overall MDR levels in Salmonella spp. recovered from each of the food‐producing animal populations and derived carcases, for all reporting countries in 2019–2020
- n: Total number of Salmonella isolates exhibiting MDR; serovars contributing the highest levels of MDR to overall MDR levels in Salmonella spp. are illustrated with a percentage; * monophasic S. Typhimurium includes antigenic formulas; serovars in the legend are listed according to their predominance within all the animal/carcase origins.
Although a greater number of monophasic S. Typhimurium isolates were recovered from pigs and their derived carcases, this serovar exhibited MDR among all carcase/animal origins, with the most frequent pattern of resistance to ampicillin, sulfamethoxazole and tetracycline. This was followed in pig carcases by the same pattern with the addition of chloramphenicol (AMP‐CHL‐SMX‐TET), while in pigs, two core MDR patterns were next most frequently observed: resistance to ampicillin, sulfamethoxazole, trimethoprim and tetracycline, as well as the same pattern with the addition of chloramphenicol (AMP‐CHL‐SMX‐TMP‐TET). Notably among the MDR isolates recovered from pigs and calves, as well as their derived carcases, sulfamethoxazole resistance was observed at levels of 98.7%, 77.8%, 98.5% and 100% from these origins, respectively. Among the multiresistant monophasic S. Typhimurium isolates recovered from poultry, all isolates displayed resistance to sulfamethoxazole.
Monophasic S. Typhimurium has spread widely among European pig populations. Particular MDR patterns are associated with monophasic S. Typhimurium and because this serovar is prevalent in many countries, these patterns greatly influenced the overall resistance figures. This is exemplified by resistance to ampicillin, sulfamethoxazole and tetracycline which occurred as an MDR pattern without additional resistances in 179/366 (48.9%) monophasic S. Typhimurium isolates from pig carcases and in 42/98 (42.9%) monophasic S. Typhimurium isolates from pigs. This resistance pattern (together with resistance to streptomycin) is typical of the European clone of monophasic S. Typhimurium (Hopkins et al., 2010). The genes conferring resistance to these antimicrobials are commonly found in association together with IS26 mobile genetic elements, responsible for their integration at different chromosomal locations, in recently described European strains of monophasic S. Typhimurium (Sun et al., 2019). It is noteworthy that multidrug resistance in the European clone of monophasic S. Typhimurium appears to have originated from integration of MDR plasmids into the chromosome, facilitated by the presence of these IS26 mobile genetic elements (Sun et al., 2019).
Resistance to five antimicrobial classes was observed among isolates from pigs, calves and broilers, as well as pig carcases; resistance to six antimicrobial classes was noted in nine isolates from pig carcases and two isolates from pigs, as well as single isolates from calves and broilers. Single isolates originating from pig carcases, pigs and calves also exhibited resistance to seven antimicrobial classes; a single isolate recovered from a pig additionally showed resistance to eight antimicrobial classes. Ciprofloxacin/nalidixic acid resistance among MDR isolates from broilers, pigs and calves, as well as carcases of pigs, calves and turkeys were observed at levels of 5.6.1%, 9%, 22.2%, 6.3%, 0% and 28.6%, respectively. Tigecycline resistance was reported in two MDR isolates from pig carcases.
In 2019, monophasic S. Typhimurium was the third most frequent serovar causing human infection in Europe, with 6,493 cases reported by EU/EEA countries. While extremely high levels of MDR (73.8%) were observed among 1,594 isolates from human cases in 2019 (those tested against the panel of nine antimicrobial classes; the MDR analysis of human isolates did not include the glycylcycline class), combined ‘microbiological’ resistance to ciprofloxacin and cefotaxime was very low (0.4%) among 1,819 tested isolates from human cases. Notably, only one monophasic S. Typhimurium isolate recovered from a pig by Italy displayed combined ‘microbiological’ resistance to these two highest priority CIAs; combined ‘microbiological’ resistance to ciprofloxacin and cefotaxime was not detected among monophasic S. Typhimurium isolates recovered from the other carcase/animal origins. Considering resistance to third‐generation cephalosporins among the carcase/animal origins, only six monophasic S. Typhimurium isolates recovered from pigs and their derived carcases displayed resistance to this antimicrobial class. Italy reported resistance to cefotaxime/ceftazidime in 2/59 monophasic S. Typhimurium isolates from pig carcases (both with an ESBL phenotype), as well as 3/31 isolates from pigs (one with an ESBL phenotype and one with an AmpC phenotype; the third isolate, although resistant to cefotaxime/ceftazidime on supplementary testing, was not characterised as possessing either an ESBL or AmpC phenotype due to borderline but negative synergy testing, as well as cefoxitin susceptibility); while Czechia reported resistance to this class in 1/3 monophasic S. Typhimurium isolates from pig carcases (AmpC phenotype). No monophasic S. Typhimurium isolates recovered from calves or poultry, or their derived carcases displayed resistance to third‐generation cephalosporins. From the monitoring of human monophasic S. Typhimurium cases reported to ECDC in 2019, out of 1,805 isolates for which data were available, eight had an ESBL phenotype and six had an AmpC phenotype, with genes encoding different types of CTX‐M enzymes as well as CMY‐2, SHV‐12 and AmpC1 enzymes detected.
S . Typhimurium was the second most frequently reported serovar from calves, as well as the third most commonly reported serovar from pigs and pig carcases; accounting for 14.1%, 13.9% and 10.8% of Salmonella isolates recovered from these origins, respectively (see Figures C.1 and C.3). Among S. Typhimurium isolates recovered from calves, pigs and pig carcases, multidrug resistance was also frequently observed: 77.8%, 62% and 63%, respectively. Notably, the proportion of all Salmonella isolates showing MDR in calves was greatly influenced by the occurrence of multiresistant S. Typhimurium, which accounted for 30.4% (7/23) of the MDR Salmonella isolates recovered from this animal population (see Figure C.5).
Although a greater number of S. Typhimurium isolates were recovered from pigs, laying hens and pig carcases, this serovar exhibited MDR among all carcase/animal origins. A wide range of different MDR patterns were reported among S. Typhimurium isolates from pigs and their derived carcases. The most frequent MDR core pattern among isolates from pigs, as well as calf carcases, was resistance to ampicillin, sulfamethoxazole and tetracycline; although only one S. Typhimurium isolate exhibited MDR from calf carcases. Among isolates from pig carcases, the predominant MDR pattern was resistance to ampicillin, chloramphenicol, sulfamethoxazole and tetracycline. Although genotypic data were not reported, mobile genetic elements which could account for this resistance pattern in S. Typhimurium isolates have previously been described. Salmonella genomic island 1 (SGI1), known to contain a multidrug resistance region located on a complex class 1 integron designated In104, confers pentavalent resistance (the ampicillin, chloramphenicol, streptomycin, sulfamethoxazole, tetracycline resistance phenotype – ACSSuT) and has widely been documented in a range of Salmonella serovars.
Of the seven MDR S. Typhimurium isolates recovered from calves, six different resistance patterns were noted, with resistance to ampicillin, sulfamethoxazole, trimethoprim and tetracycline observed in two of these isolates. In laying hens, the most frequent MDR core pattern was to gentamicin, sulfamethoxazole and tetracycline; while in broiler carcases, the pattern ampicillin, sulfamethoxazole and tetracycline was most commonly reported. Of only four MDR S. Typhimurium isolates recovered from turkey carcases, four different combinations were noted. Notably, all MDR S. Typhimurium isolates recovered from calves and turkeys, as well as their derived carcases exhibited resistance to ampicillin (100%); while resistance to this antimicrobial was noted in most of the MDR isolates from pigs and broilers and their derived carcases.
Resistance to five antimicrobial classes was observed among isolates from pigs and their derived carcases, as well as a couple of isolates from calves, turkeys and turkey carcases. Among a few isolates from pigs, broilers and pig carcases, resistance to six antimicrobial classes was noted. Ciprofloxacin/nalidixic acid resistance among MDR isolates from laying hens, pig carcases, turkey carcases, calves, broilers, pigs and turkeys were observed at levels of 10%, 14.7%, 25%, 28.6%, 33.3%, 0% and 45.5%, respectively. Tigecycline resistance among multiresistant S. Typhimurium isolates was reported in four MDR isolates from pig carcases, and single MDR isolates from pigs, calves and broiler carcases.
No S. Typhimurium isolates recovered from the animal or carcase origins displayed resistance to third‐generation cephalosporins. In view of human cases of S. Typhimurium, this serovar was identified as the second most common in 2019, with 9,100 cases reported by EU/EEA countries. While multidrug resistance among human isolates was observed at a lower level (30.9% of 834 isolates) to that noted among its monophasic variant (73.8%), combined 'microbiological' resistance to ciprofloxacin and cefotaxime was observed at the same level (0.4% of 2,429 tested S. Typhimurium isolates and 0.4% of 1,819 tested monophasic S. Typhimurium isolates). Additionally of 2,375 human S. Typhimurium isolates for which data were available to ECDC in 2019, 21 isolates exhibited an ESBL phenotype and 3 isolates exhibited an AmpC phenotype; genes encoding different types of CTX‐M enzymes, as well as CMY‐2, OXA‐1 and OXA‐48 were detected.
Interestingly, S . Rissen isolates recovered from pig carcases displayed similar levels of MDR to those of S. Typhimurium isolates (recovered from pigs and their derived carcases), where 57.7% (45/78) of S. Rissen isolates were multiresistant. Although the proportion of MDR Salmonella isolates in pig carcases was mostly influenced by the occurrence of multiresistant S. Typhimurium and its monophasic variant (72.2%, 343/475), S. Rissen accounted for 9.5% of the MDR Salmonella isolates recovered from this carcase origin (see Figure C.5).
Multiresistant S. Rissen isolates were recovered from pigs, broilers and laying hens, as well as carcases of pigs and broilers. While MDR was not frequently observed among S. Rissen isolates from pigs (3/14, 21.4%), a very high proportion of isolates exhibited MDR from pig carcases (45/78, 57.7%), with a wide range of different resistance patterns noted. The most frequent MDR pattern among isolates from pig carcases was resistance to ampicillin, chloramphenicol, sulfamethoxazole, trimethoprim and tetracycline (26.7%). García‐Fierro et al. (2016) previously identified a dominant S. Rissen clone in pigs, pork and humans in Spain, which was shown to carry genes conferring resistance to ampicillin, chloramphenicol, streptomycin, sulfonamides, tetracycline and trimethoprim at varying frequencies, mostly on integrons. S. Rissen is also a common serovar in pigs, chicken, pork and man in some parts of Asia. Pornsukarom et al. (2015) demonstrated that S. Rissen isolates originating from Thai pig farms were frequently multidrug resistant to most of the antimicrobials listed above.
A single S. Rissen isolate recovered from broiler carcases also exhibited resistance to ampicillin, chloramphenicol, sulfamethoxazole, trimethoprim and tetracycline. Of only three MDR isolates recovered from pigs, three different combinations were noted, of which resistance to ampicillin, trimethoprim and tetracycline was a feature of all three. In broilers, where 2/7 (28.8%) S. Rissen isolates exhibited MDR, four different combinations were noted (the most common being resistance to ampicillin, cefotaxime, chloramphenicol, ciprofloxacin, gentamicin, sulfamethoxazole, trimethoprim and tetracycline). Resistance to six antimicrobial classes was also observed among isolates from pig carcases and broilers, as well as resistance to seven antimicrobial classes among isolates from pigs and their derived carcases. The core pattern of resistance to ampicillin, sulfamethoxazole, trimethoprim and tetracycline was noted in the only two MDR S. Rissen isolates recovered from laying hens (N = 12). Tigecycline resistance was not a feature of any of the MDR S. Rissen isolates recovered from these animal/carcase origins.
Resistance to third generation cephalosporins was detected in a single isolate recovered from pigs by Italy (AmpC phenotype).
Considering S . Infantis, this serovar was most frequently recovered from broilers and their derived carcases, accounting for 36.4% and 50.81% of Salmonella isolates recovered from these origins, respectively (see Figures C.1 and C.3). Additionally, this serovar was the second most frequently reported in turkeys (9.5%), and the third most frequently reported in laying hens (8.8%) as well as the fourth most common among pig carcases (3.6%). While multidrug resistance was common among S. Infantis isolates from broilers and their derived carcases, as well as turkeys and their derived carcases (84.0%, 79.8%, 88.8% and 75.0%, respectively), isolates recovered from laying hens (N = 98) were less frequently MDR (24.5%). This was also apparent in S. Infantis isolates recovered from pig carcases (MDR: 0%), although the total number of isolates available for analysis was relatively low (N = 6). Notably, the proportion of all Salmonella isolates showing MDR in broilers and their derived carcases was greatly influenced by the occurrence of multiresistant S. Infantis, which accounted for 84% (653/777) and 79.8% (399/500) of the MDR Salmonella isolates from these origins, respectively (see Figure C.5). Similarly, this serovar contributed the highest levels of multiresistance to overall MDR among Salmonella isolates recovered from laying hens, turkeys and turkey carcases (as did monophasic S. Typhimurium in turkey carcases).
Although a wide range of different MDR patterns were reported among S. Infantis isolates from poultry, the most frequent core pattern of resistance was to AMP, ciprofloxacin, nalidixic acid, sulfamethoxazole and tetracycline. This resistance pattern is typical of a major European clone of S. Infantis which is prevalent among broilers (Nógrády et al., 2012). Where MDR was detected, this resistance profile (resistance to only AMP, ciprofloxacin, nalidixic acid, sulfamethoxazole and tetracycline) accounted for 26.8, 95.6%, 95.5%, 83.7%, 81.9% Although genotypic data were not reported, previous scientific publications in Europe highlight the involvement of plasmids, which appear to be responsible for resistance in many European MDR S. Infantis isolates (Franco et al., 2015; Nógrády et al. 2012). In Australia, an S. Infantis strain harbouring a SGI1 homologue with an integron related to In104 and conferring resistance to streptomycin, sulfamethoxazole and trimethoprim was identified (Levings et al., 2005).
Resistance to five antimicrobial classes was noted among isolates from all poultry origins, with the exception of turkey carcases where all isolates displayed the core resistance pattern as described above (CIP‐NAL‐SMX‐TET). Resistance to six antimicrobial classes was noted among isolates from broilers and turkeys, as well as a single isolate from laying hens; while four isolates from broilers also displayed resistance to seven antimicrobial classes. Additionally, tigecycline resistance was observed among some MDR isolates from poultry, with the exception once more of turkey carcases. Multiresistant S. Infantis was also reported from pig carcases; of only five MDR isolates, four different combinations were noted, of which resistance to sulfamethoxazole and tetracycline was a feature of all five isolates.
Resistance to third‐generation cephalosporins was detected in 34 S. Infantis isolates recovered from broilers, 30 originating from Italy (all displaying an ESBL phenotype, with 6/30 also exhibiting an AmpC phenotype) and 4 from Hungary (2 exhibiting an ESBL phenotype and 2 exhibiting an AmpC phenotype). Additionally, Italy reported resistance to this antimicrobial class in 7/12 S. Infantis isolates from turkeys and in 1/11 isolates from laying hens. An ESBL phenotype was identified in the isolate from laying hens and seven isolates from turkeys, as well as an AmpC phenotype in two of the seven isolates from turkeys. For information on ESBL‐carrying S. Infantis, please see the specific text box within the discussion. Where third‐generation cephalosporin resistance was reported, 32/34 isolates from broilers and all seven isolates from turkeys, as well as the single isolate from laying hens, displayed ‘microbiological’ resistance to ciprofloxacin (MIC > 0.064 mg/L). Nevertheless, when ciprofloxacin and cefotaxime resistance were interpreted using clinical breakpoints (CBPs), no isolates displayed combined ‘clinical’ resistance. While high‐level resistance to ciprofloxacin (MIC ≥ 4 mg/L) was mostly observed among S. Kentucky isolates from poultry, 19.8% (50/252) of isolates displaying resistance by this definition were attributed to S. Infantis. Particular MDR patterns are associated with S. Infantis and because this serovar was prevalent in many countries, these patterns greatly influenced the overall resistance figures. Moreover, resistance to third‐generation cephalosporins, as well as high‐level resistance to ciprofloxacin, further underline the significance of this multiresistant serovar.
Considering human cases of S. Infantis, this serovar was identified as the fourth most common in 2020, with 1,069 cases reported by EU/EEA countries. While high levels of MDR (45.3%) were observed among 258 isolates from human cases in 2020, combined ‘microbiological’ resistance to ciprofloxacin and cefotaxime was low (6.1%) among 279 tested isolates from human cases. Additionally of 278 human S. Infantis isolates for which data were available to ECDC in 2020, 14 isolates (5.0%) exhibited an ESBL phenotype; genes encoding different types of CTX‐M enzymes were detected.
MDR and ESBL‐producing S . Infantis .
In 2020, the proportion of all Salmonella isolates showing MDR in broilers and their derived carcases was greatly influenced by the occurrence of multiresistant S. Infantis, which accounted for 73% and 79.4% of the MDR Salmonella isolates from these origins, respectively. Additionally, this serovar accounted for 22% and 5% of the MDR isolates in turkeys and their derived carcases, respectively. In human cases from 2019 and 2020, 35.7% and 45.3% of S. Infantis were MDR (those tested against the panel of nine antimicrobial classes; the MDR analysis of human isolates did not include the glycylcycline class), with 10 and four countries reporting S. Infantis with an ESBL phenotype, respectively, and most frequently reported by Italy. All ESBL‐carrying S. Infantis in 2020 were also ciprofloxacin‐resistant.
Over the last decade, multiresistant S. Infantis has increasingly been reported from food‐producing animals and humans in Italy. Subsequently, an S. Infantis clone harbouring a pESI‐like megaplasmid and carrying the ESBL gene bla CTX‐M‐1 (mediating cefotaxime resistance), as well as the resistance genes tet(A), sul1, dfrA1 and dfrA14 (conferring resistance to tetracycline, sulfamethoxazole and trimethoprim, respectively), was identified from food‐producing animals and humans in Italy (Franco et al., 2015). This MDR clone was mainly detected among the Italian broiler chicken industry, where it is thought to have disseminated through the food chain to humans (Franco et al., 2015). A proportion of the Italian isolates of MDR S. Infantis also possessed the streptomycin resistance gene aadA1.
The pESI megaplasmid (pESI = ‘plasmid for emerging S. Infantis;’ Tate et al., 2017) was first reported among S. Infantis isolates from Israel; and while these isolates were susceptible to extended‐spectrum cephalosporins, this megaplasmid also conferred resistance to tetracycline, sulfamethoxazole and trimethoprim (Aviv et al., 2014).
Additionally, a S. Infantis clone harbouring the pESI‐like megaplasmid, but carrying the ESBL gene bla CTX‐M‐65, has been reported in the United States (Tate et al., 2017), as well as Switzerland (Hindermann et al., 2017). In the US, this genotype has been associated with travel to South America (Tate et al., 2017; Brown et al., 2018). In 2019, three countries (Denmark, Germany and Spain) together reported 11 domestically‐acquired cases of S. Infantis with bla CTX‐M‐65 (the majority of cases in Germany) and in 2020, two countries (Spain and the Netherlands) reported one case each. The majority of the bla CTX‐M‐65 isolates were resistant to ciprofloxacin, nalidixic acid, chloramphenicol, gentamicin, sulfamethoxazole, tetracycline and trimethoprim, in addition of being ESBL‐producing.
In contrast, S . Enteritidis isolates exhibited much lower multiresistance. This serovar was the most frequently reported in laying hens, the second most commonly reported in broilers and the second most frequently reported in broiler carcases, accounting for 25.5%, 10.6% and 9.8% of Salmonella spp. recovered from these poultry origins, respectively (see Figures C.1 and C.3). While complete susceptibility to the harmonised panel of antimicrobials was observed at 46.9% in S. Enteritidis isolates from broiler carcases; in isolates recovered from broilers and laying hens, the majority of isolates exhibited complete susceptibility (53.1% and 92%, respectively). Notably, a greater number of countries reported S. Enteritidis data from broilers and laying hens in comparison to those reporting data from broiler carcases, which may reflect the overall reported levels of complete susceptibility. S. Enteritidis belongs to group D Salmonella (serogroup O9) which tend to show elevated colistin MICs, a phenomenon considered to reflect slightly decreased intrinsic susceptibility of wild‐type isolates belonging to Group D (Agersø et al., 2012; Ricci et al., 2020). This is exemplified by the proportion of colistin‐resistant isolates attributed to S. Enteritidis (from laying hens, broilers and broiler carcases) in comparison to other serovars belonging to different serogroups.
Considering the monitoring of human Salmonella cases, S. Enteritidis was the most dominant serovar identified in 2020, with 24,240 cases reported by EU/EEA countries. As was observed among poultry S. Enteritidis isolates, this serovar exhibited much lower multiresistance (31/1,406, 2.2%), and combined ‘microbiological’ resistance to ciprofloxacin and cefotaxime was noted at very low levels (0.1%) among 1,796 tested isolates from human cases. Additionally of 1,508 human S. Enteritidis isolates for which data were available to ECDC in 2020, one isolate (0.1%) exhibited an ESBL phenotype and one isolate exhibited carbapenem resistance, carrying bla OXA‐48.
Among S. Enteritidis isolates from human cases in 2020, moderate to high levels of resistance to the fluoroquinolone ciprofloxacin were reported by 12/18 MSs and extremely high levels in one MS. Considering the EFSA monitoring of poultry in 2020, a low number of countries reported data on 10 or more S. Enteritidis isolates from broilers and their derived carcases (N = 3 and N = 2, respectively), with (fluoro)quinolone resistance noted at moderate levels by Czechia and very high/extremely high levels by Poland. Among S. Enteritidis isolates from laying hens, only eight MSs reported data on 10 or more isolates, with (fluoro)quinolone resistance ranging from not detected in Austria to moderate/high in Poland. From an EU baseline survey in 2005–2006, all S. Enteritidis phage type 1 (PT1) isolates recovered from broiler flocks (N = 7) exhibited resistance to nalidixic acid; however, other phage types (e.g. PT4, 7 and 21) also recovered from the monitoring of broilers demonstrated resistance to this antimicrobial, although less frequently (EFSA, 2007). Additionally, phage type 8 frequently showed susceptibility to the panel of antimicrobials tested in EFSA’s baseline survey of broilers, although the method used in the EFSA 2007 baseline survey differed in certain respects from that currently performed (EFSA, 2007). A recent Spanish study screened human clinical isolates of S. Enteritidis (N = 491) for antimicrobial drug resistance, with phage type determined for a significant number of these isolates (García et al., 2019). PT1, PT14b, PT56, PT6, PT4 and PT8 predominated (accounting in total for 82% of isolates), with 46.4% and 6.1% of all isolates resistant to nalidixic acid and ampicillin, respectively. Notably, nalidixic acid resistance was statistically associated with PT1 and PT14b, while ampicillin resistance with PT6/PT6a. Findings were considered to support clonal expansion being primarily responsible for the occurrence of nalidixic acid resistance in S. Enteritidis. All ampicillin‐resistant isolates were determined to carry a plasmid‐encoded bla TEM‐1 resistance gene (García et al., 2019).
S. Kentucky was the second most commonly reported serovar in laying hens and the tenth in turkeys, as well as the eighth most frequently reported in broilers, accounting for 8.9%, 3.5% and 2.8% of Salmonella spp. recovered from these poultry origins, respectively (see Figure C.3). While multidrug resistance was observed at an extremely high level in S. Kentucky isolates from turkeys (79.3%), isolates recovered from broilers and laying hens were less frequently MDR (30.5% and 4%, respectively). MDR was also frequent among S. Kentucky isolates recovered from carcases of turkeys and broilers (60% and 61.9%, respectively), although the total number of isolates available for analysis from these carcase origins was relatively low (N = 15 and N = 21, respectively).
A wide range of different MDR patterns were reported among S. Kentucky isolates from broilers, laying hens and turkeys. Among all poultry origins (including carcases of broilers and turkeys), the most frequent core pattern of resistance was to ampicillin, ciprofloxacin, nalidixic acid, gentamicin, sulfamethoxazole and tetracycline. Where MDR was detected, this resistance profile (resistance to ampicillin, ciprofloxacin, nalidixic acid, gentamicin, sulfamethoxazole and tetracycline) accounted for 64.9%, 75.6%, 80% and 83.3% of the multiresistant S. Kentucky isolates recovered from broilers, turkeys, broiler carcases and turkey carcases, respectively. The same resistance pattern was also found in 47.2% of MDR S. Kentucky isolates from humans in 2020, as well as in a single MDR S. Kentucky isolate recovered from a pig carcase in 2019.
Considering isolates exhibiting high‐level resistance to ciprofloxacin (MIC ≥ 4 mg/L), S. Kentucky accounted for most of the Salmonella isolates recovered from poultry which exhibited resistance by this definition (180/252). Additionally, a single S. Kentucky isolate recovered from a pig carcase displayed high‐level resistance to ciprofloxacin. Resistance to third‐generation cephalosporins was detected in five S. Kentucky isolates recovered from broilers by Malta (four isolates) and the Netherlands (one isolate), as well as an isolate recovered from laying hens by Hungary. An ESBL phenotype was reported in the five isolates from broilers, while an AmpC phenotype was reported in the single isolate from laying hens. Where third‐generation cephalosporin resistance was reported in these S. Kentucky isolates, ‘microbiological’ resistance to ciprofloxacin was also observed. The detection of third‐generation cephalosporin resistance and high‐level resistance to ciprofloxacin, underline the significance of this serovar; and notably, when cefotaxime and ciprofloxacin resistance were interpreted using CBPs, the five isolates from broilers as well as the single isolate from laying hens displayed combined ‘clinical’ resistance to these compounds. Coipan et al. (2020) have recently described the introduction and spread of a highly ciprofloxacin‐resistant S. Kentucky with the ESBL‐gene bla CTX‐M‐14b among humans in Europe. Cases were identified in eight countries in the period 2013–2018. While the clone seems to have originated in Egypt, the study pointed to a potential establishment of the clone in Malta. Germany and the Netherlands reported human cases of S. Kentucky bla CTX‐M‐14b to TESSy in 2019 and the Netherlands also in 2020. Additionally, in 2019 and 2020, Malta reported two respectively one S. Kentucky isolate, one with an ESBL phenotype and two with a combined ESBL+AmpC phenotype; genotyping was not performed. Medialdea Carrera et al. (2020) were able to confirm a link between Maltese isolates of ESBL‐producing S. Kentucky from poultry and at least one human case in 2019.
In view of the monitoring of human Salmonella cases, S. Kentucky was the sixteenth (dropped from seventh place in 2019) most commonly reported serovar in 2020, with 158 cases reported by EU/EEA countries. While extremely high levels of MDR (76.6%) were observed among 47 isolates from human cases in 2020, combined ‘microbiological’ resistance to ciprofloxacin and cefotaxime was low (10.0%) among 50 tested isolates from human cases, but still the highest among the investigated serovars. Additionally, of 54 human S. Kentucky isolates for which data were available to ECDC in 2020, two isolates exhibited an ESBL phenotype, two other isolates exhibited an AmpC phenotype and one isolate both ESBL and AmpC; only one of these were genotyped, carrying the bla CTX‐M‐14b.
S. Newport isolates recovered from turkeys in 2020 displayed very high levels of MDR, where 58.8% (30/51) of isolates were multiresistant. Notably, the level of MDR among turkeys was greatly influenced by one MS, with Hungary (N = 39) reporting 30 multiresistant isolates. While a relatively low number of S. Newport isolates were available for analysis from broiler and turkey carcases (N = 26 and N = 27, respectively), a greater proportion of isolates from broiler carcases were multiresistant in comparison to those from turkey carcases (84.6% and 18.5%, respectively). Once more however, the level of MDR among broiler carcases was greatly influenced by one MS, with Poland (N = 22) reporting 22 multiresistant isolates. In 2020, out of 165 S. Newport isolates from humans, one had a presumptive AmpC phenotype.
Among MDR S. Newport isolates recovered from turkeys and their derived carcases, the most frequent pattern of resistance was to ampicillin, ciprofloxacin, nalidixic acid and tetracycline; followed by the same pattern but without nalidixic acid resistance. In broiler carcases, the combination ampicillin, ciprofloxacin, nalidixic acid, sulfamethoxazole and tetracycline predominated. This pattern was also the second most frequently reported in broilers, although the combination chloramphenicol, ciprofloxacin, nalidixic acid, sulfamethoxazole and tetracycline was most frequently noted.
Interestingly, multidrug resistance was observed at an extremely high level among S . Bredeney isolates from turkeys in 2020 (82%); while a low level was noted among S. Bredeney isolates from turkey carcases (4.7%). Notably, among isolates reported from turkeys (N = 50), the level of MDR was greatly influenced by one MS, with Hungary (N = 31) reporting 31 multiresistant isolates. In 2020, out of 29 S. Bredeney isolates from humans, two AmpC‐producers were identified, both by Spain and carrying bla CMY‐2.
Among MDR S. Bredeney isolates recovered from turkeys, the most frequent pattern of resistance was to ampicillin, ciprofloxacin, nalidixic acid, tigecycline and tetracycline (34.1%); followed by the same pattern but with the addition of trimethoprim (24.4%). This second core pattern was also the most commonly noted among turkey carcases (40%). While a wide range of different resistance patterns were noted among S. Bredeney isolates from turkeys, tigecycline resistance among MDR isolates from this animal origin was observed at 68.3% (all MDR isolates displaying tigecycline‐resistance originated from Hungary).
Third‐generation cephalosporin resistance was detected in a single S . Heidelberg isolate recovered from a pig carcase in the Netherlands (AmpC phenotype, with bla CMY‐2 detected) in 2019. Additionally, this isolate displayed ‘microbiological’ resistance to ciprofloxacin; although when CBPs were applied, combined ‘clinical’ resistance to cefotaxime and ciprofloxacin was not observed. The isolate also exhibited resistance to ampicillin, gentamicin, sulfamethoxazole and tetracycline. In 2019, among the 16 isolates of S. Heidelberg from humans, one AmpC‐producer with bla CMY‐2 was identified.
Multiresistant serovars
Considering all reporting countries (including non‐MSs), the contributions of particular multiresistant serovars to overall MDR levels in Salmonella spp. from each of the animal/carcase categories are illustrated in Figure C.5.
Appendix D – Comparison of human Salmonella data by serovar to that in food‐producing animals
In 2019–2020, the quantitative human data were interpreted using EUCAST ECOFF values (categorised into wild‐type and non‐wild type), when available, in the same way as for the animal and food data. For 2020 data, the revised EUCAST ECOFFs for ampicillin and nalidixic acid were used, resulting in one dilution difference compared to Decision 2013/652/EU. Where ECOFFs do not exist, EUCAST or Clinical and Laboratory Standards Institute (CLSI) CBPs were applied. Notably, for sulfamethoxazole/sulfonamides, there is no EUCAST interpretative criterion for Salmonella and therefore a threshold of > 256 mg/L was applied to both the human and animal data. For qualitative data interpreted with clinical breakpoints (S = susceptible, I = susceptible with increased exposure* and R = resistant), I + R results were combined into one category, with the exception of tetracycline where only R was used. When aligning susceptible isolates with wild‐type isolates and I + R isolates with non‐wild‐type isolates, there is generally close concordance across categories (Figure D.1). An exception is meropenem where the EUCAST CBP is substantially higher (+4 dilutions) than the ECOFF.
Figure D.1.
- *: EUCAST has changed the definitions of SIR from 2019 (EUCAST, 2019 – http://www.eucast.org/newsiandr/). For I, the new definition ‘susceptible, increased exposure’ is used when there is a high likelihood of therapeutic success because exposure to the agent is increased by adjusting the dosing regimen or by its concentration at the site of infection. ? Only R category included.
1.
It is of note that the countries reporting data on particular Salmonella serovars from human cases are not always the same as those reporting corresponding serovar data within the animal categories. Additionally, the number of isolates reported from human cases and from the animal origins varied, both at the MS and MS‐group level. These factors may introduce a source of variation to results when comparing overall percentage resistance to particular antimicrobials and MDR levels among human and animal isolates.
Notably, the panel of nine antimicrobial classes comprising the MDR analysis of human isolates included the following agents – ampicillin, cefotaxime/ceftazidime, chloramphenicol, ciprofloxacin/pefloxacin/nalidixic acid, gentamicin, meropenem, sulfonamides/sulfamethoxazole, tetracyclines and trimethoprim/trimethoprim‐sulfamethoxazole (co‐trimoxazole) – and did not include tigecycline. For animal isolates, the MDR analysis included the same nine antimicrobial classes, as well as the glycylcycline class. Although tigecycline was not included in the MDR analysis of human isolates, either inclusion or exclusion of tigecycline from the MDR analysis of animal isolates has very limited effect on MDR outputs and therefore, negligible effect on human and animal comparisons.
Comparison of 2020 human data to that in poultry and their derived carcases
S. Infantis was the fourth most common serovar identified in human cases in 2020, with 1,069 cases reported in the EU/EEA. Considering all reporting MSs, the highest levels of resistance in S. Infantis from humans were noted to ciprofloxacin/pefloxacin (43.7%), nalidixic acid (41.2%), sulfonamides (46.9%) and tetracyclines (43.9%), although levels varied markedly between reporting countries. At the reporting MS‐group level for S. Infantis from poultry, generally very high or extremely high resistance to ciprofloxacin, nalidixic acid, sulfamethoxazole and tetracycline was reported, with the exception of laying hens where much lower resistance levels to these antimicrobials were noted. Figure D.2 presents the resistance levels to these four antimicrobials considering all reporting MSs.
Figure D.2.
Occurrence of resistance to selected antimicrobials in S. Infantis from humans, poultry and poultry carcases, reported by MSs in 2020
- N: Total number of isolates reported by MSs.
With the exception of laying hens, MDR among S. Infantis was reported at higher levels in isolates from poultry compared to those from humans considering all reporting countries. In human isolates, overall MDR was observed at 45.3% (14 MSs); while 79.8%, 88.8%, 84% and 75% of isolates from broiler carcases (13 MSs and one non‐MS), turkeys (six MSs), broilers (16 MSs) and turkey carcases (one MS) were MDR, respectively. At the reporting MS‐group level for S. Infantis isolates recovered from laying hens, MDR was noted at a much lower level of 24.5% (16 MSs). Notably, only four S. Infantis isolates were reported by one MS in turkey carcases and only 80 isolates were reported by six MSs in turkeys; comparative assessment of AMR data to that in humans at the country level was therefore not considered for these categories. Comparative data for nalidixic acid resistance among isolates from both broilers and humans was available for six MSs. Considering only the four MSs reporting a similar number of S. Infantis isolates from both broilers and humans and where more than 10 isolates were reported, a higher occurrence of nalidixic acid resistance as well as ciprofloxacin/pefloxacin resistance was noted in isolates from broilers in comparison to those from humans by Austria, Belgium, Italy and Spain. Also concerning sulfamethoxazole resistance and tetracycline resistance, Austria, Belgium, Italy and Spain registered a higher occurrence of resistance in isolates from broilers compared to isolates from humans. When applying the same considerations to AMR data for S. Infantis from both broiler carcases and humans (i.e. where a similar number and 10 or more isolates were reported), apparent differences in the levels of nalidixic acid resistance were noted by Austria, Belgium and Spain; with a higher occurrence of resistance in isolates from broiler carcases compared to those from humans. This was also the case for resistance to ciprofloxacin/pefloxacin, sulfamethoxazole and tetracycline. Although comparable AMR data for S. Infantis from both laying hens and humans was available, a much lower number of isolates were reported from laying hens, with only three MSs (Italy, Poland and Spain) reporting data on 10 or more isolates from this poultry origin. Italy and Spain reported a lower occurrence of resistance to nalidixic acid, ciprofloxacin, sulfamethoxazole and tetracyclines among isolates from laying hens in comparison to isolates from humans.
S. Enteritidis was the most common Salmonella serovar identified in human cases in 2020, with 24,159 cases reported in the EU/EEA. While MDR was uncommon among S. Enteritidis isolates (from both humans and poultry), the highest levels of resistance in S. Enteritidis from humans were noted to ciprofloxacin/pefloxacin (21.4%), nalidixic acid (22.5%) and colistin (20.5%). Colistin resistance among S. Enteritidis is not uncommon, since this serovar belongs to group D salmonellas (serogroup O9) which tend to show decreased intrinsic susceptibility to colistin (Agersø et al., 2012; Ricci et al., 2020). Figure D.3 presents the resistance levels to these antimicrobials considering all reporting MSs.
Figure D.3.
Occurrence of resistance to selected antimicrobials in S. Enteritidis from humans, poultry and broiler carcases, reported by MSs in 2020
-
N: Total number of isolates reported by MSs.NB. S. Enteritidis was not reported from turkey carcases.
Only 22 S. Enteritidis isolates were reported by six MSs in turkeys; comparative assessment of AMR data to that in humans at the country level was therefore not considered for this category. Considering data available for broiler carcases, Poland and Slovakia were the only countries to report on 10 or more S. Enteritidis isolates from this poultry origin. A much higher occurrence of nalidixic acid and ciprofloxacin/pefloxacin resistance was reported among isolates from broiler carcases in Slovakia in comparison to those from humans; however, for ciprofloxacin/pefloxacin, a considerably lower number of isolates were available from broiler carcases than from humans (N = 81 and N = 343, respectively). Similarly, France, Poland, Romania and Slovakia were the only countries to report on 10 or more S. Enteritidis isolates from broilers in 2020, while also reporting data from humans in 2019–2020. While nalidixic acid and ciprofloxacin/pefloxacin resistance was not detected among broiler isolates from France and low levels of resistance to these antimicrobials were reported among human isolates (7.4% and 7.4%, respectively), a much lower number of isolates were available from broilers in comparison to humans (N = 18 and N = 94, respectively). In Poland, a much higher occurrence of nalidixic acid and ciprofloxacin/pefloxacin resistance was reported among broiler isolates in 2020 (77,0% and 76,0%, respectively) than in isolates collected from humans in 2019 (25.0% and 20.9%, respectively); however, for ciprofloxacin/pefloxacin, a considerably lower number of isolates were available from broilers in comparison to those from humans (N = 91 and N = 402, respectively). Considering data available in laying hens, Belgium, Cyprus, France, Hungary, Italy, Poland and Spain reported on 10 or more S. Enteritidis isolates from both poultry in 2020 and humans in 2019–2020. In four of those Member States (Belgium, Cyprus, France and Hungary), the ciprofloxacin/pefloxacin resistance among laying hen isolates, which ranged from none detected to low or moderate, was lower than that observed in isolates from humans. Conversely, in Italy and Poland, ciprofloxacin/pefloxacin resistance in laying hen isolates was greater than that reported in human isolates (41.7% and 32.8% vs. 13.8% and 20.9% , respectively). In Spain, the resistance levels were similar in isolates from laying hens and humans (16.0% vs. 12.2%). In isolates from human cases from all reporting MSs, resistance to ciprofloxacin/pefloxacin was reported at 21.4% (N = 1,991) and to nalidixic acid at 22.5% (N = 1,070); while in laying hens (N = 258), ciprofloxacin and nalidixic acid resistance were reported at levels of 17.4% and 17.1%, respectively.
Considering S . Kentucky, the 16th most commonly reported serovar from human cases in 2020, with 158 cases reported in the EU/EEA, the highest levels of resistance in human isolates were noted to ampicillin (80.0%), ciprofloxacin/pefloxacin (82.0%), gentamicin (40.0%), nalidixic acid (81.0%), sulfonamides (72.3%) and tetracyclines (78.7%). Figure D.4 presents the resistance levels to these antimicrobials in human and poultry isolates considering all reporting MSs.
Figure D.4.
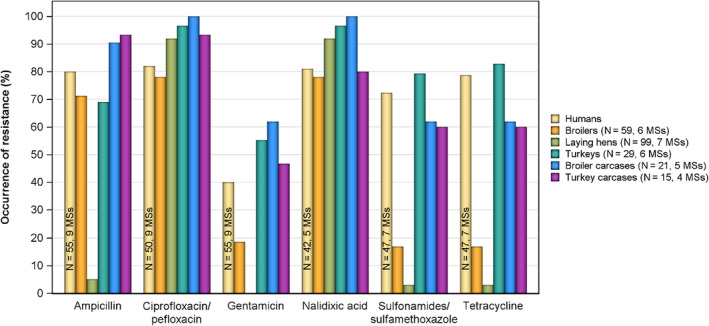
- N: Total number of isolates reported by MSs.
Considering all reporting countries, extremely high levels of MDR were reported among S. Kentucky isolates from humans, turkeys and turkey carcases (76.6%, 79.3% and 60%, respectively), although the number of isolates recovered from turkeys and their derived carcases was relatively low (N = 29 and N = 15, respectively). While an overall high and very high level of MDR was noted among isolates from broilers and their derived carcases (30.5% and 61.9%, respectively), an overall low level was reported among isolates from laying hens (4.0%). Only 21 S. Kentucky isolates were reported by five MSs in broiler carcases and only 15 isolates were reported by four MSs in turkey carcases; comparative assessment of AMR data to that in humans at the country level was therefore not considered for these categories. Although comparable AMR data for S. Kentucky from both laying hens and humans were available, the only country having reporting data for 10 or more isolates from laying hens in 2020 was Italy, which reported less than 10 isolates from humans (N = 86 and N = 1, respectively): comparative assessment of AMR data at the country level was therefore not considered for that category. Similarly, Hungary, Malta and Romania were the only countries to report AMR data for 10 or more isolates from both broilers (N = 15, 23 and 11, respectively), but comparable data on isolates from humans were not available in 2020 (nor in 2019). Neither was comparable AMR data for S. Kentucky from both turkeys and humans available.
Comparison of 2019 human data to that in pigs and calves, and their derived carcases
S. Typhimurium was the second most common Salmonella serovar identified in human cases in 2019, with 9,100 cases reported in the EU/EEA. Considering all reporting MSs, the highest levels of resistance in S. Typhimurium from humans were observed for ampicillin (54.3%), sulfonamides (37.2%) and tetracyclines (44.7%); as was the case for isolates from pigs, calves and their derived carcases. Figure D.5 presents the resistance levels to these compounds considering all reporting MSs.
Figure D.5.
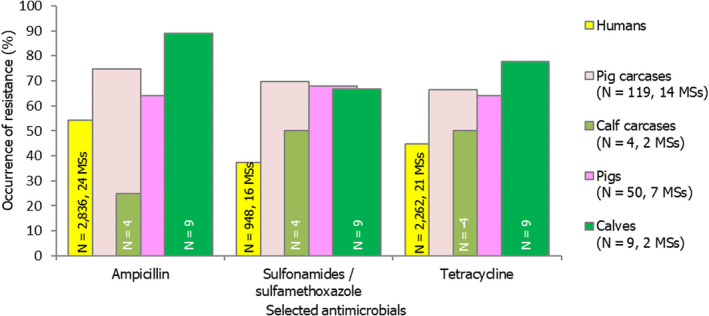
- N: Total number of isolates reported by MSs.
Considering all reporting countries, MDR levels in S. Typhimurium were reported at 30.9%, 63%, 25%, 62% and 77.8% in isolates from humans (14 MSs), pig carcases (14 MSs), calf carcases (2 MSs), pigs (7 MSs) and calves (2 MSs), respectively. While 834 isolates were included in the MDR analysis from humans, a much lower number of isolates were available from animals and their derived carcases. Assessment of human and animal S. Typhimurium AMR data at the country level was not performed, as where comparable data were available, a much lower number of isolates were reported from animals in comparison to humans by given MSs; small sample sizes are subject to high statistical variation.
Monophasic S . Typhimurium was the third most common serovar reported from human cases in 2019, with 6,493 registered cases in the EU/EEA. Considering all reporting MSs, the highest levels of resistance in monophasic S. Typhimurium from humans were observed for ampicillin (87.1%), sulfonamides (83.9%) and tetracyclines (80.5%); as was also the case for isolates from pigs, calves and carcases of pigs and calves. Notably, this resistance pattern (together with resistance to streptomycin) is typical of monophasic S. Typhimurium (Hopkins et al., 2010). Figure D.6 presents resistance levels to these compounds considering all reporting MSs.
Figure D.6.
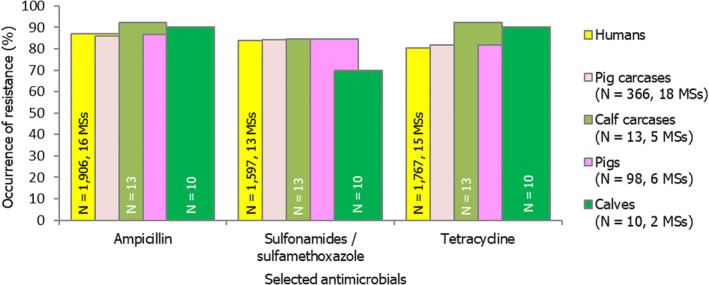
- N: Total number of isolates reported by MSs.
Considering all reporting countries, MDR levels in monophasic S. Typhimurium were reported at 73.8%, 73.2%, 76.9%, 79.6% and 90% in isolates from humans (12 MSs), pig carcases (18 MSs), calf carcases (5 MSs), pigs (6 MSs) and calves (2 MSs), respectively. In total, 1,594 isolates were included in the MDR analysis from humans, while a much lower number of isolates were available from animals and their derived carcases, particularly in calves and their derived carcases (N = 10 and N = 13, respectively). Assessment of human and animal monophasic S. Typhimurium AMR data at the country level was not performed, as where comparable data were available, a much lower number of isolates were reported from animals.
S . Derby was the sixth most common serovar reported from human cases in 2019, with 721 cases registered by EU/EEA countries. While MDR was not as frequently observed among human/animal S. Derby isolates in comparison to S. Typhimurium and its monophasic variant, resistance to sulfonamides and tetracycline was relatively common in S. Derby isolates from human cases (31.8% and 21.7%, respectively). This was also observed among S. Derby isolates from the animal/carcase origins. Figure D.7 presents resistance levels to these compounds considering all reporting MSs. Assessment of human and animal S. Derby AMR data at the country level was not performed due to the low number of isolates reported by MSs from human cases and within the animal categories.
Figure D.7.
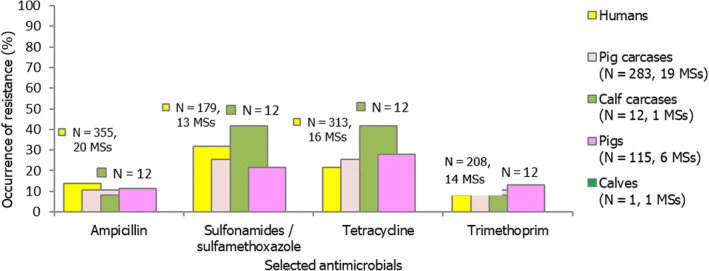
-
N: Total number of isolates reported by MSs.NB: The S. Derby isolate reported from calves was completely susceptible to all of the 14 antimicrobials tested in the harmonised panel.
1.1.
Within a given MS, attempts to relate the occurrence of AMR in human Salmonella isolates to that in isolates from food/food‐producing animals is complicated, as much of the food consumed in an MS may have originated from other MSs or non‐member countries. Salmonella infections can also be associated with foreign travel, other types of animal contact (such as pet reptiles) or the environment. Additionally, some human infections may result from human to human transmission and, although known travel‐associated isolates from human cases were excluded from the analysis, a large proportion of cases lacked information on travel status. Such circumstances may influence the human AMR data at the reporting MS level. Furthermore, the local medical and diagnostic practices and policies for referral to clinical laboratories may vary between countries, which may result in reporting of various clinical or regional subsets of isolates from humans.
Appendix E – Trends in colistin resistance in indicator E. coli from fattening pigs, calves under 1 year of age and broilers
The statistical significance (p ≤ 0.05) of trends in colistin resistance in indicator E. coli from fattening pigs, calves under 1 year of age, broilers and fattening turkeys was tested by logistic regression for countries reporting data for 3 years or more in the in the period 2014–2020 (see Annex A for details on methodology). The data for fattening turkeys are presented in the main report (Section 4. Antimicrobial resistance in indicator E. coli, Figure 38) and the data for fattening pigs, calves under 1 year of age and broilers are presented in Figures E.1, E.2–E.3 below.
Figure E.1.
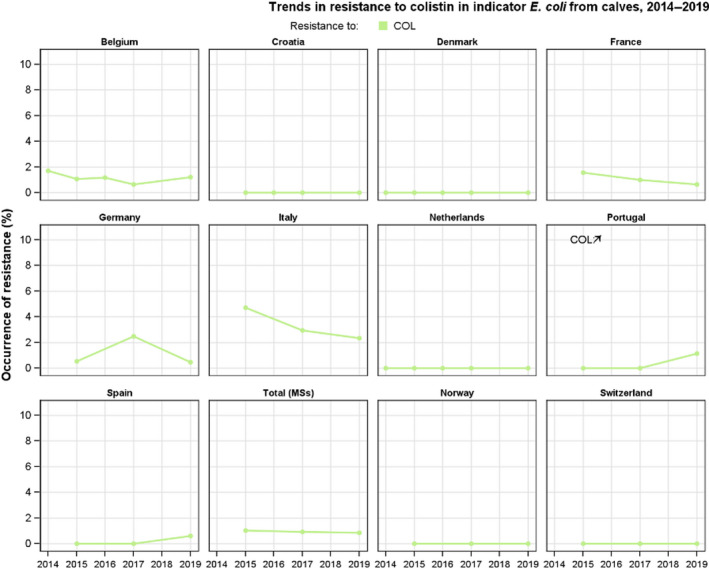
Temporal trends in resistance to colistin in indicator E. coli from calves under 1 year of age, 2014–2019 (nine MSs, two non‐MSs). Statistically significant increase (↑) or decrease (↓) indicated (p ≤ 0.05)
Figure E.2.
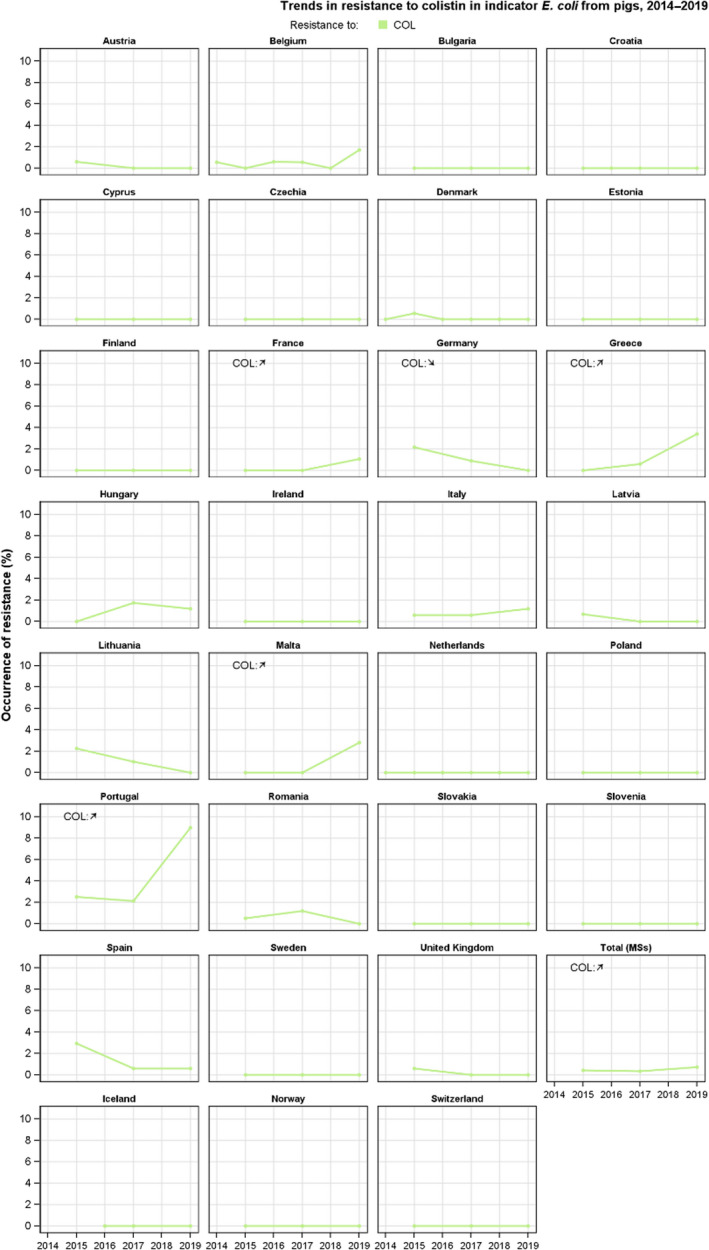
Temporal trends in resistance to colistin in indicator E. coli from fattening pigs, 2014–2019 (27 MSs, four non‐MSs). Statistically significant increase (↑) or decrease (↓) indicated (p ≤ 0.05)
Figure E.3.
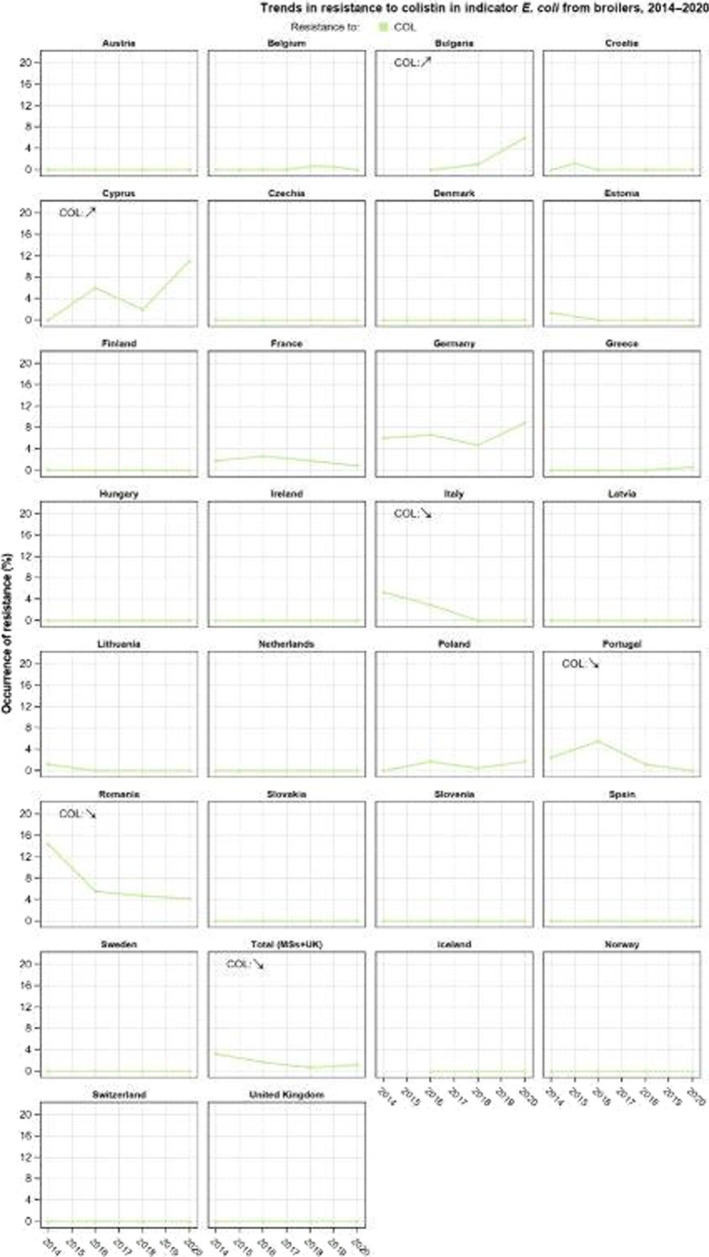
Temporal trends in resistance to colistin in indicator E. coli from broilers, 2014–2020 (25 MSs, four non‐MSs). Statistically significant increase (↑) or decrease (↓) indicated (p ≤ 0.05)
At the MS‐group level, there are decreasing trends in isolates from broilers and turkeys, an increasing trend for pigs and no trend for calves. In individual countries, the level of resistance has decreased for pigs in one MS (Germany) and increased in four MSs (France, Greece, Malta, Portugal). For calves resistance has increased in one MS (Portugal) and for broilers resistance has decreased in three MSs (Italy, Portugal, Romania) and increased in two MSs (Bulgaria, Cyprus). In isolates from turkeys, colistin resistance has decreased in two MSs (Italy, Spain) and increased in two MSs (Hungary, Poland).
Appendix F – Materials and methods
Antimicrobial susceptibility data from humans available in 2020
Data reported to The European Surveillance System (TESSy)
MSs report results from antimicrobial susceptibility testing of Salmonella spp. and Campylobacter spp. isolated from clinical cases to ECDC on an annual basis. Data can be submitted to ECDC and The European Surveillance System (TESSy) either as measured values (inhibition zone diameters or minimum inhibitory concentrations (MIC)) through the isolate‐based reporting in TESSy or as results interpreted with clinical breakpoints via the case‐based reporting of Salmonella and Campylobacter infections. From 2019 it is also possible to submit data as phenotypes predicted from sequencing of the bacterial genome, also via the isolate‐based reporting. The reporting of quantitative data via the isolate‐based reporting is the preferred route, as stipulated in the EU protocol for harmonised monitoring of AMR in human Salmonella and Campylobacter isolates (ECDC, 2016).
Salmonella spp.: For 2020, 20 MSs, plus Iceland and Norway provided data on antimicrobial resistance (AMR) in human Salmonella isolates. Sixteen countries reported measured values and four reported results interpreted as susceptible standard dosing regimen, susceptible increased exposure or resistant (SIR) according to the clinical breakpoints (CBPs) applied. Two countries reported results categorised as predicted wild type or predicted non‐wild type based on analysis of bacterial genomes (Table F.1).
Table F.1.
Antimicrobials reported, methods used, type of data reported and interpretive criteria applied by MSs for human Salmonella AST data in 2020
| Country | Gentamicin | Chloramphenicol | Ampicillin | Cefotaxime | Ceftazidime | Meropenem | Tigecycline | Nalidixic acid | Ciprofloxacin/pefloxacin | Azithromycin | Colistin | Sulfonamides | Trimethoprim | Trimethoprim‐sulfa | Tetracyclines | Method used | Quantitative (Q) or categorical (SIR or PWT/PNWT) | Interpretive criteria |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Austria | ● | ● | ● | ● | ● | ● | ● | ● | ●( a ) | ● | ● | ● | ● | DD | Q | Interpreted by ECDC. EUCAST ECOFFs for all except CLSI CBP for SUL | ||
| Belgium | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | DL | Q | Interpreted by ECDC, as for Austria. EFSA criteria for AZM MIC | |||||
| Cyprus | ● | ● | ● | ● | ● | ● | ● | ● | DL/DLG | Q | Interpreted by ECDC, as for Austria, except for CTX, MEM and SXT where EUCAST CBP were used | |||||||
| Denmark | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | DL | Q | Interpreted by ECDC, as for Austria. EFSA criteria for AZM MIC | |
| Estonia | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | DL | Q | Interpreted by ECDC, as for Austria | ||
| Finland | ● | ● | ● | ● | ● | ● | ●( a ) | ● | ● | DD | Q | Interpreted by ECDC, as for Austria | ||||||
| France | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | DL | Q | Interpreted by ECDC, as for Austria. EFSA criteria for AZM MIC | |
| Hungary | ● | ● | ● | ● | ● | ● | ●( a ) | ● | ● | ● | DD | SIR | EUCAST CBP except CLSI CBP for NAL, SUL and TET | |||||
|
Iceland |
● | ● | ●( a ) | ● | DD | SIR | EUCAST CBP | |||||||||||
| Ireland | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | WGS | PWT/PNWT | Sequencing results interpreted by the laboratory with BioNumerics tools for acquired AMR genes and point mutations | ||||
| Italy | ● | ● | ● | ● | ● | ● | ● | ● | ●( a ) | ● | ● | ● | ● | ● | DD/DL | Q | Interpreted by ECDC, as for Austria | |
| Latvia | ● | ● | ● | DD | SIR | No recent information on guidelines used | ||||||||||||
| Luxembourg | ● | ● | ● | ● | ● | ● | ●( a ) | ● | ● | ● | ● | DD/DL/DLG | Q | Interpreted by ECDC, as for Austria | ||||
| Malta | ● | ● | ● | ● | ● | ● | DL/DLG/DD | Q | Interpreted by ECDC, as for Austria | |||||||||
| Netherlands | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | DL | Q | Interpreted by ECDC, as for Austria. EFSA criteria for AZM MIC | |
| Norway | ● | ● | ● | ● | ● | ●( a ) | ● | DD | Q | Interpreted by ECDC, as for Austria | ||||||||
| Portugal | ● | ● | ● | ● | ● | ● | ● | ● | ●( a ) | ● | ● | ● | ● | DD | Q | Interpreted by ECDC, as for Austria | ||
| Romania | ● | ● | ● | ● | ● | ● | ● | ●( a ) | ● | ● | ● | ● | DD | Q | Interpreted by ECDC, as for Austria | |||
| Slovakia | ● | ● | ● | ● | ● | ● | ● | ● | DD/DL | SIR | EUCAST CBP except CLSI CBP for NAL, SUL and TET | |||||||
| Slovenia | ● | ● | ● | ● | ● | ● | ●( a ) | ● | ● | ● | ● | DD/DLG | Q | Interpreted by ECDC, as for Austria | ||||
| Spain | ● | ● | ● | ● | ● | ● | ● | ●( a ) | ● | ● | ● | DD | Q | Interpreted by ECDC, as for Austria | ||||
| Sweden | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | WGS | PWT/PNWT | Sequencing results interpreted by the laboratory with NCBI AMRFinder and CGE ResFinder and PointFinder |
AST: antimicrobial susceptibility testing; CBP: clinical breakpoint; DD: disk diffusion; DL: dilution; DLG: dilution with gradient strip; WGS: whole genome sequencing; Q: quantitative data; SIR: susceptible standard dosing regimen, susceptible increased exposure, resistant (categorical data); PWT/PNWT: predicted wild type/predicted non‐wild type (categorical); ECDC: European Centre for Disease Prevention and Control; ECOFF: epidemiological cut‐off; CLSI: Clinical and Laboratory Standards Institute; EUCAST: European Committee on Antimicrobial Susceptibility Testing; NCBI: National Center for Biotechnology Information, US; CGE: Center for Genomic Epidemiology, Denmark; MIC: minimum inhibitory concentration.
AZM: azithromycin; CTX: cefotaxime; GEN: gentamicin; MEM: meropenem; NAL: nalidixic acid; SUL: sulfonamides; TET: tetracycline.
Pefloxacin used in disk diffusion.
Campylobacter spp.: 16 MSs, plus Iceland and Norway provided AMR data from human isolates for 2020. Sweden reported Campylobacter AMR data for the first time. Twelve countries reported measured values, five reported results interpreted as susceptible standard dosing regimen, susceptible increased exposure or resistant (SIR) according to the clinical breakpoints (CBPs) applied and one reported result categorised as predicted wild type or predicted non‐wild type based on analysis of bacterial genomes (Table F.2).
Table F.2.
Antimicrobials reported, method used, type of data reported and interpretive criteria applied by MSs for human Campylobacter AST data in 2020
| Country | Gentamicin | Co‐amoxiclav | Ciprofloxacin | Erythromycin | Tetracyclines | Method used | Quantitative (Q) or categorical (SIR) | Interpretive criteria |
|---|---|---|---|---|---|---|---|---|
| Austria | ● | ● | ● | ● | DL | Q | Interpreted by ECDC. EUCAST ECOFF (CIP, ERY, GEN, TET), CASFM CBP 2020 (AMC) | |
| Cyprus | ● | ● | ● | DD | Q | Interpreted by ECDC, as for Austria | ||
| Denmark | ● | ● | ● | ● | DL | Q | Interpreted by ECDC, as for Austria | |
| Estonia | ● | ● | ● | ● | DL | Q | Interpreted by ECDC, as for Austria | |
| Finland | ● | ● | ● | DD/DLG | Q | Interpreted by ECDC, as for Austria | ||
| France | ● | ● | ● | ● | ● | DD | SIR | EUCAST CBP (CIP, ERY, TET), CASFM CBP (AMC, GEN) |
| Iceland | ● | ● | DD/DLG | SIR | EUCAST CBP | |||
| Italy | ● | ● | ● | ● | DD | Q | Interpreted by ECDC, as for Austria. | |
| Luxembourg | ● | ● | ● | ● | ● | DD | Q | Interpreted by ECDC, as for Austria. |
| Malta | ● | ● | ● | ● | ● | DLG/DL/DD | Q | Interpreted by ECDC, as for Austria |
| Netherlands | ● | ● | ● | DD/DL | SIR | EUCAST CBP | ||
| Norway | ● | ● | ● | ● | DLG | Q | Interpreted by ECDC, as for Austria | |
| Poland | ● | ● | ● | No information | SIR | No information provided. | ||
| Portugal | ● | ● | ● | ● | DD | Q | Interpreted by ECDC, as for Austria | |
| Slovakia | ● | ● | ● | ● | No information | SIR | In 2013, CLSI CBP. No update since | |
| Slovenia | ● | ● | ● | DD | Q | Interpreted by ECDC, as for Austria | ||
| Spain | ● | ● | ● | ● | ● | DLG | Q | Interpreted by ECDC, as for Austria |
| Sweden | ● | ● | ● | WGS | PWT/PNWT | Sequencing results interpreted by the laboratory with NCBI AMRFinder and CGE ResFinder and PointFinder |
AST: antimicrobial susceptibility testing; CASFM: French Society for Microbiology; CBP: clinical breakpoint; DD: disk diffusion; DL: dilution; DLG: dilution with gradient strip; ECDC: European Centre for Disease Prevention and Control; ECOFF: epidemiological cut‐off; EUCAST: European Committee on Antimicrobial Susceptibility Testing; Q: quantitative data; SIR: susceptible standard dosing regimen, susceptible increased exposure, resistant (categorical data).
AMC: amoxicillin/clavulanate; CIP: ciprofloxacin; ERY: erythromycin; GEN: gentamicin; TET: tetracycline.
Harmonised testing
Most laboratories follow the ‘EU protocol for harmonised monitoring of antimicrobial resistance in human Salmonella and Campylobacter isolates’ (ECDC, 2016) on the antimicrobial panel to be tested. The antimicrobials tested, the method used (dilution, disk diffusion, gradient strip), the type of data provided and the interpretive criteria applied are presented in Table F.1 for Salmonella and in Table F.2 for Campylobacter. For Salmonella, seven MSs, plus Iceland and Norway used only disk diffusion methods (DDs) for their AST, six MSs used dilution methods (DLs) and another five MSs used a combination of the two. Two countries used sequencing and bioinformatics tools to predict phenotypic resistance from the genome (Table F.1). For Campylobacter, six MSs used only disk diffusion methods (DDs) for their AST, four MSs and Norway used dilution methods (DLs), three MSs and Iceland used a combination of the two, mostly disk diffusion and gradient strip. One country used sequencing and bioinformatics tools to predict phenotypic resistance from the genome. For two MSs, the methodology was not provided (Table F.2). All data on measured MIC or zone mm values were results of AST at the national public health reference laboratories, with the exception of Italy for Salmonella where two regional laboratories also contributed, and Finland for Campylobacter where the quantitative data had been collected from regional laboratories. Data interpreted with clinical breakpoints were normally from local or regional laboratories and reported together with the information on the clinical case. In these cases, AST had primarily been performed with the purpose of treatment of the case rather than AMR monitoring. For this reason, the number of tests per antimicrobial varied.
Salmonella test panel
The national public health laboratories within the Food‐ and Waterborne Diseases and Zoonoses (FWD) network has agreed on a panel of priority antimicrobials and optional antimicrobials to test for and report to ECDC (ECDC, 2016). Compared with earlier recommendations, a second beta‐lactam (ceftazidime) and a carbapenem (meropenem) were added. For 2020, all MS reported results on meropenem and all but four for ceftazidime. Three last‐line antimicrobials – azithromycin, colistin and tigecycline – are also included in the priority list. For colistin, however, the methodology is complicated due to chemical properties of the substance and a joint EUCAST and Clinical and Laboratory Standards Institute (CLSI) subcommittee confirmed that broth microdilution is so far the only valid method for colistin susceptibility testing (CLSI and EUCAST, 2016). Disk diffusion does not work because of poor diffusion of the large colistin molecule in the agar and tested gradient strips also underestimate colistin MIC values, again most likely due to poor diffusion in the agar (Matuschek et al., 2017). Therefore, only countries performing broth microdilution (or those predicting resistance from WGS) should report on colistin resistance. Eight MSs were reporting on azithromycin, nine on tigecycline and nine on colistin for 2020.
Due to the problems in detecting low‐level fluoroquinolone resistance in Salmonella spp. using disk diffusion, nalidixic acid was, for a long time, used as a marker for fluoroquinolone resistance. After the discovery that plasmid‐mediated fluoroquinolone resistance is often not detected using nalidixic acid, EUCAST studied alternative disks and concluded that pefloxacin was an excellent surrogate marker (except for isolates having the aac(6′)‐Ib‐cr gene as the only resistance determinant) (Skov et al., 2015). Since 2014, EUCAST has recommend this agent for screening of low‐level fluoroquinolone resistance in Salmonella with disk diffusion (EUCAST, 2014) and, since June 2016, this is also reflected in the EU protocol. In 2020, all countries reporting measured values for disk diffusion tested with pefloxacin instead of ciprofloxacin. Eight countries reported the combination drug co‐trimoxazole (trimethoprim–sulfamethoxazole) in addition to, or instead of, testing the substances separately, partly because this combination is used for clinical treatment and partly because no EUCAST interpretive criterion exists for sulfamethoxazole for Salmonella.
Campylobacter test panel
The antimicrobials included in the 2020 report followed the panel of antimicrobials from the EU protocol for harmonised monitoring of AMR in human Salmonella and Campylobacter isolates (ECDC, 2016). The priority panel for Campylobacter includes ciprofloxacin, erythromycin, tetracyclines and, since June 2016, gentamicin. Gentamicin is recommended for screening of invasive isolates and was added to the priority panel after a EUCAST ECOFF became available for disk diffusion for C. jejuni. Co‐amoxiclav (combination drug with amoxicillin and clavulanic acid) was included from the list of optional antimicrobials. In 2020, all countries except Iceland tested the three antimicrobials ciprofloxacin, erythromycin and tetracycline (although Malta only tested one isolate for tetracycline), 10 also tested gentamicin and five tested co‐amoxiclav.
Analyses of antimicrobial resistance data
Harmonised interpretation of data with animal and food data
Data reported as measured values were interpreted by ECDC based on the EUCAST ECOFF values, when available. For MIC data, the same criteria were applied as used by EFSA (Tables F.5 and F.6) except for 2020 data where EUCAST had recently changed the ECOFFs, as for ampicillin and nalidixic acid for Salmonella enterica (MIC lowered with 1 dilution) and gentamicin for Campylobacter (MIC lowered with one dilution). Where EUCAST had removed the ECOFF (colistin, tigecycline and trimethoprim‐sulfamethoxazole for Salmonella), the old ECOFF was still applied. For zone diameter data, corresponding EUCAST disk diffusion ECOFF values were applied with a few exceptions (Tables F.1 and F.2). Regarding data reported as SIR values, the categories of ‘susceptible, increased exposure’ (I) and ‘clinically’ resistant (R) were combined into one group, except for tetracycline and Salmonella. Alignment of the susceptible category with the ‘wild type’ category based on epidemiological cut‐off values (ECOFFs) and of the I+R category with the ECOFF‐based ‘non‐wild type’ category provides better comparability and more straightforward interpretation of the data for most antimicrobial agents included (Figure 27 in Section 3.5 and Figure D.1 in Appendix D of the 2019/2020 EUSR‐AMR). For Salmonella, this procedure results in good concordance (±1 dilution) across categories with the exception of meropenem where the MIC for non‐susceptible category is substantially higher (+4 dilutions) than the ECOFF. For Campylobacter, there was full concordance across interpretive categories with this procedure, except for the EUCAST CBP for C. jejuni for tetracyclines, which is one dilution step higher than the EUCAST ECOFF and for gentamicin where the EUCAST ECOFF is one dilution lower than the ECOFF value listed in the Commission Implementing Decision 2013/652/EU.
Table F.6.
Panel of antimicrobial substances included in AMR monitoring, EUCAST ECOFFs and concentration ranges tested in C. jejuni and C. coli
| Antimicrobial |
C. jejuni EUCAST ECOFF( a ) |
C. coli EUCAST ECOFF( a ) |
Concentration range, mg/L (no. of wells) |
|---|---|---|---|
| Erythromycin | > 4 | > 8 | 1–128 (8) |
| Ciprofloxacin | > 0.5 | > 0.5 | 0.12–16 (8) |
| Tetracycline | > 1 | > 2 | 0.5–64 (8) |
| Gentamicin | > 2 | > 2 | 0.12–16 (8) |
| Nalidixic acid | > 16 | > 16 | 1–64 (7) |
| Streptomycin ( b ) | > 4 | > 4 | 0.25–16 (7) |
AMR: antimicrobial resistance; EUCAST: European Committee on Antimicrobial Susceptibility Testing; ECOFFs: epidemiological cut‐off values; NA: not available.
EUCAST epidemiological cut‐off values. ‘>’ than the ECOFF, criteria used for determing microbiological resistance.
On a voluntary basis.
Separation by species or serovar
As resistance levels differ substantially between Salmonella serovars, results are presented separately for selected serovars of importance in humans. The serovars presented in the report are S. Enteritidis, S. Typhimurium, monophasic S. Typhimurium, S. Infantis and S. Kentucky. AMR data on the 10 most common serovars in human cases in the last years are also available in the ECDC Surveillance Atlas for Infectious Diseases (https://atlas.ecdc.europa.eu/public/index.aspx). For Campylobacter, resistance levels differ quite substantially between the two most important Campylobacter species, C. jejuni and C. coli, and data are therefore presented by species. The proportion of resistant isolates is only shown when at least 10 isolates were reported from an MS.
Exclusion of travel‐associated cases
To better assess the impact from food consumed within each reporting country on the AMR levels found in human isolates, cases known to have travelled outside of the country during the incubation period was excluded from the analysis. However, as several countries had not provided any information on travel status of their cases, cases with unknown travel status were also included in addition to domestically acquired cases. The proportions of travel‐associated, domestic and cases with unknown travel status among the tested isolates are presented in Tables 3 and 4.
Temporal trends in resistance
Trends in the proportion of resistant isolates to selected antimicrobials over the 5‐year period 2016–2020 were analysed by country. The statistical significance was assessed with logistic regression in Stata 16.0 and a p‐value of < 0.05 was considered to be significant. Only countries testing at least 10 isolates per year and for at least 3 years in the 5‐year period were included. As the number of isolates reported by country was markedly lower in almost all countries in 2020 compared to previous years as an effect of the pandemic, it effected on the power of the statistical test. For Salmonella, the antimicrobials analysed were ciprofloxacin/pefloxacin/nalidixic acid, cefotaxime, ampicillin and tetracycline. For Campylobacter, the corresponding antimicrobials were ciprofloxacin, erythromycin and tetracycline.
Within the framework of the temporal trend analyses covering data from 2016 to 2020, all EU/EEA countries reporting data during the period have been included. From 1 February 2020, the United Kingdom withdrew and became a ‘third‐ country’ in relation to the EU, with final withdrawal effective after a transition period ending on 31 December 2020. The withdrawal of the UK from the EU has an impact on the AMR data reported at the EU level in 2020. In this report, data at the EU level are reported in accordance with the membership of the EU, whether before 2020 (EU including the UK) or in 2020 (EU without the UK). In relation to trend analysis at country level for the 2020 report, UK data are referred to as from a non‐EU MS.
Maps for critically important antimicrobials resistance
For Salmonella, the proportions of human isolates resistant to both of the critically important antimicrobials for treatment of severe Salmonella infections (WHO, 2019), fluoroquinolones (ciprofloxacin/pefloxacin) and third‐generation cephalosporins (cefotaxime), were presented in blue shaded maps to provide an overview of the geographical distribution of resistance in the EU/EEA. Combined ‘microbiological resistance’ was presented for Salmonella spp and selected serovars (tables with combined resistance are also available in Appendix B). In addition, a map of ciprofloxacin/pefloxacin resistance in S. Kentucky isolated from humans was included in Appendix A – high‐level resistance to ciprofloxacin. For Campylobacter, the proportions of human isolates resistant to both of the critically important antimicrobials for treatment of severe Campylobacter infections (WHO, 2019), fluoroquinolones (ciprofloxacin) and macrolides (erythromycin), were presented in maps to provide an overview of the geographical distribution of resistance in the EU/EEA. Combined ‘microbiological’ resistance (using EUCAST ECOFFs) were presented for C. jejuni and C. coli.
Analysis of multidrug resistance
Multidrug resistance (MDR) of human Salmonella spp. to nine antimicrobial classes was analysed, these classes being harmonised between ECDC and EFSA for better comparison between the two sectors. Multidrug resistance of an isolate was defined as resistance or non‐susceptibility to at least three different antimicrobial classes (Magiorakos et al., 2012). The antimicrobials included were ampicillin, cefotaxime/ceftazidime, chloramphenicol, ciprofloxacin/pefloxacin/nalidixic acid, gentamicin, meropenem, sulfonamides/sulfamethoxazole, tetracyclines and trimethoprim/trimethoprim‐sulfamethoxazole (co‐trimoxazole). Resistance to nalidixic acid, ciprofloxacin and pefloxacin were addressed together, as they belong to the same class of antimicrobials: quinolones. Isolates that were non‐wild type or I+R to any of these antimicrobials were classified as microbiologically resistant to the class of quinolones. The same method was applied to the two third‐generation cephalosporins cefotaxime and ceftazidime. Trimethoprim and co‐trimoxazole were also addressed together, as a few countries had only tested for susceptibility to the combination. This approach was considered appropriate because among the countries that provided data on both trimethoprim alone and the combination co‐trimoxazole, the proportion of resistant or non‐susceptibles corresponded closely between the two. Multidrug resistance of a C. jejuni or C. coli isolate was defined as resistance or non‐susceptibility to at least three different antimicrobial classes (Magiorakos et al., 2012). The antimicrobials in the MDR analysis were harmonised between EFSA and ECDC and included ciprofloxacin, erythromycin, gentamicin and tetracycline.
Analysis of ESBL, AmpC and carbapenemase‐production in Salmonella
All countries reported results from AST of third‐generation cephalosporins in 2020. Those which reported findings of ESBL and/or AmpC or non‐wild type results to third‐generation cephalosporins and ampicillin, were contacted by mail to provide further details on phenotypic and/or genotypic results. Of the 14 MSs and one non‐MS reporting such isolates, all except four could provide further information (Tables F.3–F.7).
Table F.3.
Proportion of tested Salmonella spp. isolates from human cases associated with travel, domestic cases and cases with unknown travel information by country in 2020
| Country | Total Salmonella tested | Travel‐associated | Domestic | Unknown |
|---|---|---|---|---|
| N | % | % | % | |
| Austria | 894 | 0.0 | 0.0 | 100.0 |
| Belgium | 728 | 3.0 | 17.6 | 79.4 |
| Cyprus | 106 | 0.0 | 0.0 | 100.0 |
| Denmark | 317 | 20.5 | 79.5 | 0.0 |
| Estonia | 102 | 6.9 | 62.7 | 30.4 |
| Finland | 103 | 2.9 | 97.1 | 0.0 |
| France | 750 | 4.9 | 18.3 | 76.8 |
| Hungary | 805 | 0.0 | 100.0 | 0.0 |
| Ireland | 203 | 9.9 | 19.2 | 70.9 |
| Italy | 872 | 0.0 | 0.0 | 100.0 |
| Latvia | 18 | 0.0 | 100.0 | 0.0 |
| Luxembourg | 93 | 3.2 | 3.2 | 93.5 |
| Malta | 166 | 1.2 | 0.0 | 98.8 |
| Netherlands | 523 | 5.5 | 0.0 | 94.5 |
| Portugal | 241 | 1.2 | 14.1 | 84.6 |
| Romania | 36 | 0.0 | 0.0 | 100.0 |
| Slovakia | 518 | 0.2 | 99.8 | 0.0 |
| Slovenia | 190 | 2.6 | 29.5 | 67.9 |
| Spain | 768 | 0.0 | 100.0 | 0.0 |
| Sweden | 392 | 12.2 | 84.7 | 3.1 |
| Total (20 MSs) | 7,825 | 3.1 | 41.6 | 55.3 |
| Iceland | 28 | 25.0 | 60.7 | 14.3 |
| Norway | 337 | 31.2 | 44.2 | 24.6 |
MSs: Member States; N: number of isolates tested.
Table F.4 Proportion of tested Campylobacter jejuni and C. coli isolates from human cases associated with travel, domestic cases and cases with unknown travel information by country in 2020
| Country | Total C. jejuni & C. coli tested | Travel‐associated | Domestic | Unknown |
|---|---|---|---|---|
| N | % | % | % | |
| Austria | 437 | 0.0 | 89.2 | 10.8 |
| Cyprus | 23 | 0.0 | 0.0 | 100.0 |
| Denmark | 254 | 13.4 | 86.6 | 0.0 |
| Estonia | 277 | 0.0 | 59.6 | 40.4 |
| Finland | 1,465 | 0.0 | 0.0 | 100.0 |
| France | 7,539 | 0.0 | 0.0 | 100.0 |
| Italy | 93 | 1.1 | 18.3 | 80.6 |
| Luxembourg | 226 | 0.0 | 0.0 | 0.0 |
| Malta | 172 | 0.0 | 0.0 | 100.0 |
| Netherlands | 1,390 | 0.0 | 0.0 | 100.0 |
| Poland | 36 | 0.0 | 97.2 | 2.8 |
| Portugal | 265 | 0.0 | 100.0 | 0.0 |
| Slovakia | 1,026 | 0.0 | 100.0 | 0.0 |
| Slovenia | 811 | 0.9 | 52.0 | 47.1 |
| Spain | 24 | 0.0 | 100.0 | 0.0 |
| Sweden | 370 | 10.8 | 87.8 | 1.4 |
| Total (MSs 16) | 14,408 | 0.6 | 20.1 | 79.4 |
| Iceland | 90 | 15.6 | 74.4 | 10.0 |
| Norway | 327 | 16.5 | 59.6 | 23.9 |
MSs: Member States; N: number of isolates tested.
Antimicrobial susceptibility data from animals and food in 2019–2020
Data reported under Directive 2003/99/EC and Commission Implementing Decision 2013/652/EU
For 2020 MSs reported mandatory data collected from AMR routine monitoring in Salmonella spp. and indicator commensal E. coli, as well as from the E. coli specific extended spectrum β‐lactamase (ESBL)‐/AmpC‐/carbapenemase‐producing monitoring, according to Commission Implementing Decision 2013/652/EU20.
For the routine monitoring of AMR in Salmonella spp., in 2019, 26 MSs and one non‐MS reported data on meat from pigs (carcases) and seven MSs on meat from bovine animals (carcases), eight MSs reported data on fattening pigs, three MSs in calves under 1 year of age and in 2020, 18 MSs and two non‐MSs reported data on meat from broilers and eight MSs on meat from fattening turkeys, 24 MSs and two non‐MS reported data on laying hen flocks, 22 MSs and three non‐MSs on broiler flocks and 16 MSs and one non‐MS on fattening turkey flocks. For the routine monitoring of AMR in indicator commensal E. coli, in 2019, 27 MSs and five non‐MSs reported data on fattening pigs and nine MSs and three non‐MS reported on calves under 1 year, whereas, in 2020, 27 MSs and five non‐MSs reported data on broilers and 11 MSs and two non‐MS reported on fattening turkeys.
In 2020, for the routine monitoring of AMR in Campylobacter jejuni, 27 MSs and three non‐MSs reported data on broilers and nine MSs and two non‐ MS on fattening turkeys. Some data on Campylobacter coli were also reported on a voluntary basis.
For the specific monitoring of ESBL‐/AmpC‐/carbapenemase‐producing E. coli, in 2019, all MSs and three non‐MSs, reported data on fresh meat from pigs and bovines gathered at retail, and fattening pigs, whereas nine MSs and two non‐MS reported data on calves under 1 year of age. In 2020, all 27 MSs as well as four non‐MSs reported data on fresh meat from broilers gathered at retail, whereas all 27 MSs and four non‐MSs reported data on broilers, and 11 MSs and two non‐MSs, on fattening turkeys.
Isolates were sampled through harmonised national schema. Microbroth dilution testing methods were used for susceptibility testing, and quantitative21 isolate‐based data were reported to EFSA and considered for this report. Resistance was interpreted using EUCAST ECOFF values (see following text box for further information). The antimicrobials incorporated in this summary analysis were selected based on their public health relevance and as representatives of different antimicrobial classes.
Data on C. coli in fattening pigs and calves and C. jejuni in calves, as well as data on meticillin‐resistant Staphylococcus aureus (MRSA) and on specific monitoring of carbapenemase‐producing microorganisms were reported on a voluntary basis.
Harmonised representative sampling and monitoring
Representative sampling should be performed according to general provisions of the legislation and to detailed technical specifications issued by EFSA (EFSA, 2014).
Salmonella spp.
In 2019, representative Salmonella isolates for monitoring AMR were collected by MSs from carcases of fattening pigs sampled for testing and verification of compliance, in accordance with point 2.1.4 of Chapter 2 of Annex I to Regulation (EC) No 2073/200522; as well as carcases of bovines under 1 year of age where the production of meat of those bovines in the MSs is more than 10,000 tonnes slaughtered per year sampled for testing and verification of compliance, in accordance with point 2.1.3 of Chapter 2 of Annex I to Regulation (EC) No 2073/2005. MSs sampled carcases of fattening pigs/carcases of bovines under 1 year of age of healthy slaughter at the slaughterhouse. A two‐stage stratified sampling design, with slaughterhouses as primary sampling units and carcases as secondary units, with proportional allocation of the number of samples to the annual throughput of the slaughterhouse, was applied in the reporting countries.
In 2020, representative Salmonella isolates for monitoring AMR were collected by MSs from the populations of laying hens, broilers and fattening turkeys sampled according to the Salmonella National Control Programmes (NCPs), set up in accordance with Article 5(1) of Regulation (EC) No 2160/200323, as well as from carcasses of both broilers and fattening turkeys sampled for testing and verification of compliance, in accordance with point 2.1.5 of Chapter 2 of Annex 1 to Regulation (EC) No 2073/2005.
Not more than one isolate per Salmonella serovar from the same epidemiological unit (herd/holding/flock of birds) per year should be included in the AMR monitoring. In most MSs, the isolates tested for antimicrobial susceptibility constituted a representative subsample of the total Salmonella isolates available at the National Reference Laboratory (NRL) and/or other laboratories involved, obtained in a way that ensured geographical representativeness and even distribution over the year. Conversely, for low prevalence, all the Salmonella isolates available should be tested for susceptibility.
Campylobacter and indicator commensal E. coli 24
Routine monitoring of indicator E. coli
In 2019, MSs collected indicator E. coli isolates as part of their national monitoring programme of AMR according to the provisions of the Decision 2013/652/EU, based on random sampling of caecal samples gathered at slaughter from fattening pigs and calves under 1 year of age where the production of meat of those bovines in the MSs is more than 10,000 tonnes slaughtered per year. Only one representative caecal sample (single or pooled) per epidemiological unit (batch of carcases deriving from the same herd), was gathered to account for clustering. Isolates were recovered from caecal contents samples (single or pooled), in accordance with EFSA’s recommendations (EFSA, 2014). MSs shall test 170 isolates for antimicrobial susceptibility testing for each of animal population listed above. However, in MSs with a production of less than 100,000 tonnes of pig meat slaughtered per year they shall test 85 isolates instead of 170 isolates. The sample collection was approximately evenly distributed over the year 2019.
In 2020, MSs collected Campylobacter jejuni and indicator commensal E. coli isolates as part of their national monitoring programme of AMR according to the provisions of Commission Implementing Decision 2013/652/EU, based on representative random sampling of carcasses of healthy slaughter broilers/fattening turkeys at the slaughterhouse. A two‐stage stratified sampling design, with slaughterhouses as primary sampling units and carcasses as secondary units, with proportional allocation of the number of samples to the annual throughput of the slaughterhouse, was applied in the reporting countries. Only one representative caecal sample (single or pooled) per epidemiological unit (batch of carcasses deriving from the same flock), was gathered to account for clustering. Isolates were recovered from caecal contents samples (single or pooled), in accordance with EFSA’s recommendations (EFSA, 2014). The sample collection was approximately evenly distributed over the year 2020.
Specific monitoring of E. coli ESBL/AmpC/carbapenemase producers
In 2019, caecal samples gathered at slaughter from fattening pigs and bovines under 1 year of age, where the production of meat of those bovines in the MSs is more than 10,000 tonnes slaughtered per year and samples of fresh pig meat and bovine meat gathered at retail were collected. In 2020 caecal samples gathered at slaughter from broilers and from fattening turkeys, in those MSs where the production of turkey meat in the MS is more than 10,000 tonnes slaughtered per year, and samples of fresh meat from broilers gathered at retail were collected. Only one representative caecal sample (single or pooled) per epidemiological unit (batch of carcases deriving from the same herd/flock), was gathered to account for clustering. Isolates were recovered from caecal contents samples (single or pooled), in accordance with EFSA’s recommendations (EFSA, 2014). MSs shall analyse 300 samples of each of the animal population and food category, listed in above. However, in MSs with a production of less than 100,000 tonnes of pig meat slaughtered per year, less than 50,000 tonnes bovine meat slaughtered per year and less than 100,000 tonnes of poultry meat slaughtered per year, the MS shall analyse 150 samples instead of 300 samples for each corresponding specific combination. The sample collection was approximately evenly distributed over the years 2019 and 2020 as described above.
Epidemiological cut‐off values (ECOFFs) and clinical breakpoints (CBPs)
Epidemiological cut‐off values (ECOFFs) and clinical breakpoints (CBPs)
A microorganism is defined as ‘clinically’ resistant when the degree of resistance shown is associated with a high likelihood of therapeutic failure. The microorganism is categorised as resistant by applying the appropriate CBP in a defined phenotypic test system, and this breakpoint may alter with legitimate changes in circumstances (e.g. alterations in dosing regimen, drug formulation, patient factors). A microorganism is defined as wild type for a bacterial species when no acquired or mutational resistance mechanisms are present to the antimicrobial in question. A microorganism is categorised as wild type for a given bacterial species presenting a lower MIC to the antimicrobial in question than the appropriate ECOFF in a defined phenotypic test system. This cut‐off value will not be altered by changing circumstances (such as alterations in frequency of antimicrobial administration). Wild‐type microorganisms may or may not respond clinically to antimicrobial treatment. A microorganism is defined as non‐wild type for a given bacterial species by the presence of an acquired or mutational resistance mechanism to the antimicrobial in question. A microorganism is categorised as non‐wild type for a given bacterial species by applying the appropriate ECOFF value in a defined phenotypic test system; non‐wild‐type organisms are considered to show ‘microbiological’ resistance (as opposed to ‘clinical’ resistance). CBPs and ECOFFs may be the same, although it is often the case that the ECOFF is lower than the CBP. EUCAST has defined CBPs and ECOFFs.
Clinical breakpoints (clinical resistance)
The clinician, or veterinarian, choosing an antimicrobial agent to treat humans or animals with a bacterial infection requires information that the antimicrobial selected is effective against the bacterial pathogen. Such information will be used, together with clinical details such as the site of infection, ability of the antimicrobial to reach the site of infection, formulations available and dosage regimes, when determining an appropriate therapeutic course of action. The in vitro susceptibility of the bacterial pathogen can be determined and CBPs used to ascertain whether the organism is likely to respond to treatment. CBPs will take into account the distribution of the drug in the tissues of the body following administration and assume that a clinical response will be obtained if the drug is given as recommended and there are no other adverse factors which affect the outcome. Conversely, if the CBP indicates resistance, then it is likely that treatment will be unsuccessful. Frequency of dosing is one factor that can affect the antimicrobial concentration achieved at the site of infection. Therefore, different dosing regimens can lead to the development of different CBPs, as occurs in some countries for certain antimicrobials where different therapeutic regimes are in place. Although the rationale for the selection of different CBPs may be clear, their use makes the interpretation of results from different countries in reports of this type problematic, as the results are not directly comparable between those different countries.
Epidemiological cut‐off values (microbiological resistance)
For a given bacterial species, the pattern of the MIC distribution (i.e. the frequency of occurrence of each given MIC plotted against the MIC value) can enable the separation of the wild‐type population of microorganisms from those populations that show a degree of acquired resistance. The wild‐type susceptible population is assumed to have no acquired or mutational resistance and commonly shows a normal distribution. When bacteria acquire resistance by a clearly defined and efficacious mechanism, such as the acquisition of a plasmid bearing a gene which produces an enzyme capable of destroying the antimicrobial, then the MIC commonly shows two major subpopulations, one a fully susceptible normal distribution of isolates and the other a fully resistant population which has acquired the resistance mechanism. Resistance may be achieved by a series of small steps, such as changes in the permeability of the bacterial cell wall to the antimicrobial or other mechanisms which confer a degree of resistance. In this case, there may be populations of organisms which occur lying between the fully susceptible population and more resistant populations. The ECOFF value indicates the MIC or zone diameter above which the pathogen has some detectable reduction in susceptibility. ECOFFs are derived by testing an adequate number of isolates to ensure that the wild‐type population can be confidently identified for a given antimicrobial. The clinical breakpoint, which is set to determine the therapeutic effectiveness of the antimicrobial, may fail to detect emergent resistance. Conversely, the ECOFF detects any deviation in susceptibility from the wild‐type population, although it may not be appropriate for determining the likelihood of success or failure for clinical treatment.
Campylobacter coli
Caecal samples gathered at slaughter from fattening pigs were collected on a voluntary basis. One representative caecal sample (single or pooled) per epidemiological unit (batch of carcases deriving from the same herd), was gathered to account for clustering. Isolates were recovered from caecal contents samples (single or pooled), in accordance with EFSA’s recommendations (EFSA, 2014). The sample collection was approximately evenly distributed over the year 2020.
MRSA
Isolates may have been collected by different monitoring approaches, either by active monitoring of animals and foods or, in some cases, by passive monitoring based on diagnostic submission of samples from clinical cases of disease in animals, or from foods sampled as part of investigatory work.
Harmonised antimicrobial susceptibility testing
Routine monitoring antimicrobial susceptibility
MSs tested antimicrobials and interpreted the results using the epidemiological cut‐off values and concentration ranges shown in Tables F.5 and F.6 to determine the susceptibility of Salmonella spp., C. coli, C. jejuni and indicator commensal E. coli. All E. coli isolates, randomly selected isolates of Salmonella spp. and E. coli that, after testing with the first panel of antimicrobials in accordance with Commission Implementing Decision 2013/652/EU were found to be resistant to cefotaxime, ceftazidime or meropenem, were further tested with a second panel of antimicrobial substances as shown in Table F.7. This panel notably includes cefoxitin, cefepime and clavulanate in combination with cefotaxime and ceftazidime for the detection of presumptive ESBL and AmpC producers, as well as imipenem, meropenem and ertapenem to phenotypically identify presumptive carbapenemase producers.
Specific monitoring of ESBL‐/AmpC‐/carbapenemase‐producing E. coli
For the specific monitoring of ESBL‐/AmpC‐/carbapenemase‐producing E. coli, the isolation method started with a non‐selective pre‐enrichment step, followed by inoculation on MacConkey agar containing a third‐generation cephalosporin in a selective concentration (cefotaxime 1 mg/L), in accordance with the most recent version of the detailed protocol for standardisation of the EU Reference Laboratory for Antimicrobial Resistance (EURL‐AR).25 Using this protocol, also carbapenemase‐producing isolates can be recovered. If available, one presumptive ESBL‐/AmpC‐/carbapenemase‐producing E. coli isolate obtained from each positive caecal sample and meat sample was tested for its antimicrobial susceptibility to the first panel of antimicrobials (Table F.5) to confirm the microbiological resistance to cefotaxime (expected as the antimicrobial is present in the isolation medium at a concentration higher than the ECOFF), and identify possible resistance to ceftazidime and/or ceftazidime and/or meropenem. In a second step, the isolate should be tested using the second panel of antimicrobials (Table F.7) to infer the presumptive ESBL‐/AmpC‐/carbapenemase‐producing phenotype according to the β‐lactam resistance phenotype obtained (Figure F.1).
Figure F.1.
Phenotypes inferred based on the resistance to the β‐lactams included in Panel 2
-
Presumptive ESBL‐producers include isolates exhibiting Phenotype 1 or 3.Presumptive AmpC producers include isolates exhibiting Phenotype 2 or 3.
Specific monitoring of carbapenemase‐producing microorganisms
This monitoring programme was performed and reported on a voluntary basis. For the specific monitoring of carbapenemase‐producing microorganisms, isolation required the use of non‐selective pre‐enrichment and subsequent selective plating on carbapenem‐containing media, in accordance with the most recent version of the detailed protocol of the EURL‐AR. The microbial species was identified using an appropriate method. If available, one presumptive carbapenemase‐producing isolate (primarily E. coli, but also Salmonella) obtained from each positive caecal sample and meat sample should be tested for its antimicrobial susceptibility to the first panel of antimicrobials (Table F.5) to confirm the microbiological resistance to meropenem and identify possible resistance to cefotaxime and/or ceftazidime. In a second step, the isolate should be tested using the second panel of antimicrobials (Table F.7) to infer the presumptive carbapenemase‐producer phenotype according to the β‐lactam resistance phenotype obtained (Figure F.1). The EUCAST epidemiological cut‐off values applied for the antimicrobial susceptibility testing (Tables F.5–F.7) are the ones available during the drafting of the Decision 2013/652/EU. For some antimicrobials, these values have been updated by EUCAST (www.eucast.org, last accessed 9 January 2020). Currently, for Salmonella, there is no ECOFF available anymore for colistin, tigecycline, nor ertapenem; for E. coli, there is no tigecycline nor ertapenem ECOFFs available anymore (additional updates, for E. coli, for temocillin, the current value is 16 mg/L, and for cefotaxime/clavulanic acid and ceftazidime/clavulanic acid, 0.25 and 0.5 mg/L, respectively; for both Salmonella spp. and E. coli, the current ECOFFs for nalidixic acid is 8 mg/L). To allow comparison with the data collected in previous years, the ECOFFs laid down in the legislation are considered.
Data validation
Validation against business rules
The reported data were first checked for usability against a series of ‘business rules’, which were automatically applied in the EFSA data collection system once a file was sent. This automatic data validation process refers to the first validation of incoming data. Quality checks are related to a specific business only. The positive result of the automatic validation process places the file in a valid state and makes it available for further steps of validation performed by EFSA.
Scientific data validation
The scientific validation of the data collected by the MSs/non‐MSs and submitted to EFSA consisted on the revision of data and comparison between data reported for the same antimicrobials when tested by different panels. Special attention was given to carbapenems, colistin, azithromycin, tigecycline and to possible discrepancies between results for antimicrobials present in both panels (i.e. cefotaxime, ceftazidime, meropenem). MSs were contacted by EFSA asking for clarifications. If considered needed, MSs were asked to confirm the MIC results and the species identification of the reported isolates.
Reference testing
To ensure the quality of data submitted, a reference testing exercise was run by the EURL‐AR in close collaboration with the MSs. The exercise consisted in retesting the AST of the isolates received using both Panel 1 and Panel 2 of antimicrobials, as well as whole genome sequencing (WGS) analyses of the isolates (WGS analyses on‐going by the time of drafting the present report). Based on the data submitted to EFSA, a selection of approximately 400 isolates/per year was made. The selection of these isolates was based on different criteria:
The EURL‐AR had reported technical issues when testing azithromycin, tigecycline and colistin during the EURL workshop hold in Lyngby (Denmark) in 2016 (www.eurl‐ar.eu). Resistant isolates from countries with outstanding prevalence for these antimicrobials were asked to provide selected isolates to the EURL‐AR. Most of the E. coli isolates chosen were selected among the ones reported mainly for the specific ESBL/AmpC/carbapenemase monitoring.
There was a discrepancy between MIC values reported for the antimicrobials present in both panels (impacting the categorisation of the isolate as resistant or susceptible).
If according to the criteria applied (2.5.1), the presence of carbapenemase producers was suspected.
Isolates representing the categorisations presumptive ESBLs‐, AmpC and ESBL + AmpC producers.
Isolates with odd phenotypes.
Selected multidrug resistant isolates from specific Salmonella serotypes that could represent widespread clones.
Isolates microbiologically resistant to ciprofloxacin and susceptible to nalidixic acid (presence of plasmid mediated quinolone resistance encoding genes, PMQR, suspected) were included in the selection.
The MSs/non‐MSs sent the selected isolates to the EURL‐AR, where they were retested. EFSA, EURL‐AR and MSs liaised together to address possible discrepancies found.
Analyses of antimicrobial resistance data
Data are reported in separate sections dedicated to each microorganism. Clinical investigation data were not accounted for in this report.
Overview tables of the resistance data reported
Data generated from the antimicrobial susceptibility testing and reported as quantitative at the isolate level by MSs have been described in the overview tables included in the Annexes A–E published on the EFSA Knowledge Junction community on Zenodo (https://doi.org/10.5281/zenodo.6257446). The tables also display complete susceptibility, multidrug resistance and co‐resistance. These analyses are described in Section 2.4.
Minimum inhibitory concentration distributions
For each combination of microorganism, antimicrobial and food category/animal population were tested, MIC distributions were tabulated in frequency tables, giving the number of isolates tested that have a given MIC at each test dilution (mg/L) of the antimicrobial. Isolate‐based dilution results allowed MIC distributions reported:
for Salmonella for ampicillin, azithromycin, cefepime, cefotaxime, cefotaxime and clavulanic acid, ceftazidime, ceftazidime and clavulanic acid, cefoxitin, chloramphenicol, ciprofloxacin, colistin, ertapenem, gentamicin, imipenem, meropenem, nalidixic acid, sulfamethoxazole, temocillin, tetracycline, tigecycline and trimethoprim;
for Campylobacter for ciprofloxacin, erythromycin, gentamicin, nalidixic acid, streptomycin and tetracycline;
for indicator E. coli for ampicillin, azithromycin, cefepime, cefotaxime, cefotaxime and clavulanic acid, ceftazidime, ceftazidime and clavulanic acid, cefoxitin, chloramphenicol, ciprofloxacin, colistin, ertapenem, gentamicin, imipenem, meropenem, nalidixic acid, sulfamethoxazole, temocillin, tetracycline, tigecycline and trimethoprim;
for MRSA for cefoxitin, chloramphenicol, ciprofloxacin, clindamycin, erythromycin, fusidic acid, gentamicin, kanamycin, linezolid, mupirocin, penicillin, quinupristin/dalfopristin, rifampicin, streptomycin, sulfamethoxazole, tetracycline, tiamulin, trimethoprim and vancomycin.
Epidemiological cut‐off values and the occurrence of resistance
ECOFFs, as listed in Decision 2013/652/EC, have been used in this report to interpret the isolate‐based reported MIC data and determine non‐wild‐type organisms also termed ‘microbiologically’ resistant organisms (i.e. displaying a decreased susceptibility), and to ensure that results from different MSs are comparable. From this point onwards in this report, ‘microbiologically’ antimicrobial‐resistant organisms are referred to as ‘resistant’ for brevity. This report also incorporates re‐evaluation of the historical data accounting for the revised EU legislation, which included the revised ECOFFs.
The occurrence of resistance26 to a number of antimicrobials was determined for Salmonella, Campylobacter and indicator commensal E. coli isolates and are tabulated at the production‐type level in this report. The occurrence of resistance (i.e. resistance levels) in reporting MS groups was calculated as totals (the total number of resistant isolates out of the total number of tested isolates across reporting MSs) and in the E. coli chapter, also as weighted means to account for the animal population sizes.
Data description
Throughout the report, level or occurrence of AMR means the percentage of resistant isolates as a proportion of the isolates tested of that microorganism. MSs reporting group means the MSs that provided data and were included in the relevant table of antimicrobial resistance for that bacterium–food or animal category–antimicrobial combination. Terms used to describe the levels or occurrence of antimicrobial resistance are ‘rare’: < 0.1%, ‘very low’: 0.1–1.0%, ‘low’: > 1–10.0%, ‘moderate’: > 10.0–20.0%, ‘high’: > 20.0–50.0%, ‘very high’: > 50.0–70.0%, ‘extremely high’: > 70.0%. Although these terms are applied to all antimicrobials, the significance of a given level of resistance depends on the particular antimicrobial and its importance in human and veterinary medicine.
Temporal trends in resistance
Where the minimum criteria for data inclusion in this report were met, temporal trend graphs were generated to show the resistance to different antimicrobials from 2009 to 2020, by plotting the level of resistance for each year of sampling. Graphs were created for those countries for which resistance data were available for three or more years in the 2009–2020 period. MS‐specific resistance levels trend graphs use a unique scale and countries are shown in alphabetical order. For ampicillin, cefotaxime, ciprofloxacin and tetracyclines (Salmonella and indicator E. coli), ciprofloxacin, erythromycin, streptomycin and tetracycline (Campylobacter), resistance trends over time were visually explored by trend graphs, produced using SAS® Studio.
To assess the statistical significance of temporal trends, the proportions of resistance were modelled against time in a logistic regression. This analysis was carried out using the PROC LOGISTIC of SAS 9.4 for each country reporting at least 10 total tested isolates, where there were 3 years or more of available data to use in the model. The PROC LOGISTIC function uses a logit transformation to model the proportion of prevalence against year and provides estimates for both intercepts and slope. Models where the likelihood ratio test suggested it to be meaningful and resulting in a p‐value associated with slope of < 0.05 were considered to be significant (linear model fit). It is important to note that between‐year fluctuations in the occurrence resistance (%) may not be captured in the evaluation of the trend over the entire time period (2009–2020) and that very recent decreasing or increasing trends may therefore be masked by the overall trend.
The withdrawal of the UK from the EU has an impact on the AMR data reported at the EU level in 2020. In this 2020 report, data at the EU level are reported in accordance with the membership of the EU, whether before 2020 (EU including the UK) or in 2020 (EU without the UK). In order that the data presented in the report are not misleading going forward from 2020 and to provide a comparable output with previous years, two data points have been included for the year 2020 in the temporal trend graphs. One 2020 data point provides the data at the EU level including the UK, which is consistent with data reported at the EU level over the years previous to 2020 and this can be used to assess the significance of trends using the same contributing countries. An additional data point corresponding to the EU level data reported in 2020 (where the UK data are not included) is also shown on the temporal trend graph, providing the EU output for MSs only and representing the beginning of a new time series at the EU level starting in 2020, reflecting the change in contributing MSs.
Spatial analysis of resistance through maps
MS‐specific AMR levels for selected bacterium–food category/animal population combinations were plotted in blue shaded maps for 2019 and 2020, using ArcGIS 9.3. In the maps, resistance levels are presented with colours reflecting the continuous scale of resistance to the antimicrobial of interest among reporting MSs; so, there might be some apparent discrepancies between the colours and resistance levels between maps.
Resistance in Salmonella serovars of public health importance
In this report, AMR in tested Salmonella isolates were aggregated to give a value for Salmonella spp. for each country and food/animal category. In addition, the most prevalent Salmonella serovars were also reported separately for particular food/animal category. Additional tables have been included in this report to describe the occurrence of AMR among selected Salmonella serovars of public health importance or of high prevalence in animals. To present a complete overview of the animal populations and food categories in which specific Salmonella serovars of public health importance have been recovered, all the data reported (derived even from fewer than four reporting countries and less than 10 isolates tested) have been included.
Analysis of multidrug resistance, complete susceptibility and co‐resistance data
The analysis of MDR and co‐resistance data is important in light of the emergence of multiresistant bacteria. The intention is to focus mainly on multi/co‐resistance patterns involving critically important antimicrobials (WHO, 2019), such as cephalosporins, fluoroquinolones and macrolides, and to summarise important information in the EU Summary Report. The occurrence of the isolates of a serotype/resistance pattern of interest is studied both at the MS level and at the EU level (by grouping data for all MSs and where also relevant for MSs and other reporting countries), as the overall picture for all MSs might show a more definite pattern of emergence and spread. In addition, the analysis of data may reveal the existence of new or emerging patterns of MDR, particularly in Salmonella serotypes.
Analysis of MDR and complete susceptibility
For the analysis of MDR and complete susceptibility, a multiresistant isolate is one defined as resistant to at least three of the antimicrobial substances that should be included in the AMR monitoring according to Commission Implementing Decision 2013/652/EU (see Tables F.5 and F.6). In contrast, a completely susceptible isolate is one defined as non‐resistant (MIC < ECOFF) to these antimicrobial substances. Resistance to nalidixic acid and resistance to ciprofloxacin, as well as the resistance to cefotaxime and to ceftazidime are, respectively, addressed together. Due to the presence of resistance to colistin considered as intrinsic in serogroup D of Salmonella spp., colistin was not included in the analysis of MDR and complete susceptibility for Salmonella. MDR and completely susceptibility are visually displayed in ‘traffic light graphs’. Geographical distribution of complete susceptibility is also shown in green shaded maps.
For indicator E. coli, the occurrence of complete susceptibility (OICS) is also displayed in bar charts showing the trends for the years 2015, 2017 and 2019 for porcine and bovine populations and for the years 2014, 2016, 2018 and 2020 for poultry populations, respectively. The statistical significance of the trends was analysed using chi‐squared tests for trends. The rate of change (ROC) (expressed in percent) is shown for significant temporal trends of OICS. It is used to mathematically describe the percentage change in value of OI over a defined period of time. It represents the momentum of the OI. The calculation for ROC takes the last value of OI and divides it by the initial measurement. One is subtracted from this value and the resulting number is multiplied by 100 to give it a percentage representation.
For Campylobacter, an MDR isolate is one defined as resistant to at least three of the antimicrobial substances included in the AMR monitoring according to Commission Implementing Decision 2013/652/EU (see table 2 of this Decision), except for streptomycin. Ciprofloxacin and nalidixic acid are addressed together in the calculation of MDR for Campylobacter. In contrast, a completely susceptible isolate is one defined as non‐resistant (MIC< or equal to ECOFF) to the panel of antimicrobial substances described in the Decision, excluding streptomycin. As streptomycin is not used in humans, it’s exclusion in the analysis of MDR and complete susceptibility for Campylobacter has been agreed by EFSA and ECDC to allow comparability of MDR and CS in humans and food‐producing animals.
In the chapter on Campylobacter, the distributions of the number of susceptible and resistant isolates from humans and animals were compared using a Chi2 test, or a Fisher exact test (when values in the contingency table ≤ 5). The results were considered significant when p < 0.05.
MDR patterns
The frequency and percentage of isolates exhibiting various MDR patterns considering the antimicrobials tested were determined for Salmonella (Salmonella spp. and for certain serovars of interest), Campylobacter species and indicator E. coli for each country and each animal population/food category. Isolates for which no susceptibility data were provided for some of the antimicrobial substances were disregarded.
‘Key Outcome Indicators’
To support EU countries in their progress to reduce use of antimicrobials and AMR a list of key outcome indicators has been jointly published by ECDC, EFSA and EMA (ECDC, EFSA and EMA, 2017). Two of these key outcome indicators (KOI) are included in the report: (1) The key outcome indicator of complete susceptibility (KOICS) in indicator E. coli; and (2) the key outcome indicator of the prevalence of ESBL‐ and/or AmpC‐producing E. coli (KOIESC).
KOICS is the proportion of fully susceptible indicator E. coli isolates, weighted by the size of the populations of the most important production animals (broilers, fattening turkeys, fattening pigs, calves) and is used as an indicator (KOICS) for the overall AMR situation in food‐producing animals. KOIESC is the weighted mean of the prevalence of ESBL‐ and/or AmpC‐producing E. coli in each of the four animal populations monitored. The identification of presumptive ESBL and AmpC producers is described in Section 2.5. The KOICS and KOIESC account for differences in the relative size of food animal populations in a country and are therefore relevant in evaluation of risks related to resistance in food animals.
These KOIs are displayed in bar charts showing changes in KOI over the 2014–2020 period for OICS in indicator E. coli and 2015–2020 period for OIESC. The statistical significance of the trends was analysed using chi‐squared tests for trends. The rate of change (ROC) (expressed in percent) is shown for significant temporal trends of KOICS and KOIESC. It is used to mathematically describe the percentage change in value of KOI over a defined period of time. It represents the momentum of the KOI. The calculation for ROC takes the last value of KOI and divides it by the initial measurement. One is subtracted from this value and the resulting number is multiplied by 100 to give it a percentage representation.
The co‐resistance patterns of interest
The term combined resistance is used in this report to indicate phenotypic resistance to two or more different classes of antimicrobials, exhibited by the same bacterial isolate. In Salmonella and E. coli isolates, co‐resistance to cefotaxime (CTX) and ciprofloxacin (CIP) was estimated, as these two antimicrobials are of particular interest in human medicine. Co‐resistance was addressed using both ECOFFs (CTX > 0.25 mg/L and CIP > 0.064 mg/L) and CBPs (CTX > 2 mg/L and CIP > 1 mg/L) for E. coli. In C. jejuni and C. coli isolates, co‐resistance to ciprofloxacin and erythromycin (ERY) was estimated, as these two antimicrobials are of particular interest in human medicine in the treatment of severe campylobacteriosis. The interpretive ECOFFs used to address co‐resistance to ciprofloxacin and erythromycin were, for C. jejuni, CIP > 0.5 mg/L and ERY > 4 mg/L and, for C. coli, CIP > 0.5 mg/L and ERY > 8 mg/L. These values may be considered as very similar to CBPs.
Identification of presumptive ESBL, AmpC and/or carbapenemase producers
Definition of ESBL, AmpC, ESBL+AmpC, CP‐phenotypes:
The categorisation of isolates resistant to third‐generation cephalosporins and/or carbapenems in presumptive ESBL, AmpC or carbapenemase producers was carried out based on the EUCAST guidelines for detection of resistance mechanisms and specific resistances of clinical and/or epidemiological importance (EUCAST, 2017). In these expert guidelines and, based on other EUCAST and CLSI guidelines to detect ESBL/AmpC producers, a screening breakpoint of > 1 mg/L is recommended for cefotaxime and ceftazidime. This screening breakpoint is higher than the ECOFFs applied for antimicrobial susceptibility of both antimicrobials for E. coli, and to cefotaxime for Salmonella. For this report, a first condition for classifying isolates as presumptive ESBL/AmpC producers related to their MIC for either cefotaxime or ceftazidime, was to apply this screening breakpoint of MICs > 1 mg/L. Only isolates which presented MIC values accomplishing with this requisite (as expected for most of the ESBL/AmpC producers) were further considered. In total, for the third‐generation cephalosporin‐ and/or carbapenem‐resistant isolates, five main categorisations are made: (1) ESBL phenotype; (2) AmpC phenotype; (3) ESBL + AmpC phenotype; (4) CP‐phenotype; and (5) Other phenotypes (Figure F.1).
To detect the production of ESBLs, a synergy test for cefotaxime and ceftazidime, in combination with clavulanic acid was performed. An eightfold reduction in the MIC for the cephalosporin combined with clavulanic acid compared with that obtained for the cephalosporin alone was interpreted as a positive synergy test. In all other cases, the synergy test was considered negative. For the present report, isolates with MICs > 1 mg/L for cefotaxime and/or ceftazidime and a synergy test positive for any of these antimicrobials, together with susceptibility to cefoxitin (≤ 8 mg/L) and meropenem (MEM ≤ 0.125 mg/L see CP phenotype) were classified as ESBL phenotype (Figure F.1).
For the AmpC phenotype, the combination MIC > 8 mg/L (ECOFF) for cefoxitin together with MICs > 1 mg/L for cefotaxime and/or ceftazidime was used as phenotypic criteria to investigate the presence of AmpC production in E. coli. It should be also underlined that there are a few AmpC enzymes that do not confer resistance to cefoxitin (i.e. ACC‐1), and that there are other mechanisms (porin loss, presence of carbapenemases, a few ESBLs like cefotaxime (CTX‐M‐5) that could generate similar MIC values for the different antimicrobials (EFSA, 2012a; EUCAST, 2017). Phenotypic AmpC confirmation tests (i.e. cloxacillin synergy) were not required for the present monitoring. For the present report, isolates with MICs > 1 mg/L for cefotaxime and/or ceftazidime and cefoxitin MIC > 8 mg/L, together with negative synergy test for both cefotaxime and ceftazidime/clavulanic acid, together with susceptibility to meropenem (MEM ≤ 0.125 mg/L) were classified in the AmpC phenotype category. No distinction between acquired AmpC and natural AmpC was made (Figure F.1).
-
For the present report, isolates with MICs > 1 mg/L for cefotaxime and/or ceftazidime, positive synergy tests for any of these antimicrobials with clavulanic acid and cefoxitin MIC > 8 mg/L, together with susceptibility to meropenem (MEM ≤ 0.125 mg/L) were classified under the ESBL + AmpC phenotype category (Figure F.1).
In some isolates, several mechanisms can be present at the same time, making it very difficult to differentiate the phenotypes. Also the high‐level expression of AmpC β‐lactamases can mask the presence of ESBLs. AmpC can also be present in isolates with positive ESBL tests (clavulanic acid synergy). In this case, the cefepime/clavulanic acid synergy test should be used to overturn/confirm the presence of ESBLs in these isolates (EUCAST, 2017) but, unfortunately, the combination cefepime/clavulanic acid was not included among the substances tested for monitoring. The inclusion of resistance to cefepime with an MIC value ≥ 4 mg/L as an additional criterion proposed elsewhere (EFSA, 2012a,b), could be useful to ascertain the presence of an ESBL‐producer.
For the classification of isolates into the putative carbapenem producers (CPs), a meropenem screening cut‐off of > 0.125 mg/L (which coincides with the harmonised ECOFF) was chosen. It is known that other mechanisms (i.e. hyperproduction or combination of ESBLs and/or AmpC and porin loss) can also affect to the MIC values generated for the different carbapenems, especially for ertapenem. The confirmation of the carbapenemase production recommended by the EUCAST guidelines cannot be inferred from the carbapenem susceptibility testing data reported but needs further phenotypic or molecular testing. Those MSs that reported data suggesting the presence of putative CPs were recommended to validate the results by performing further confirmatory testing, and the EURL‐AR offered to apply WGS of the isolates. For the present report, isolates with MIC > 0.125 mg/L for meropenem would be considered as presumptive CP producers and were classified under the CP phenotype. The presence of other resistance mechanisms (ESBLs, AmpC, etc.) within the isolates placed in this group cannot be ruled out.
In this group, phenotypes not included in the categorisations defined above were included: isolates with an MIC > 0.125 for ertapenem and/or MIC > 1 mg/L for imipenem (EUCAST screening cut‐offs, one dilution step higher than the currently defined ECOFFs) but no resistance to meropenem (MIC < 0.125 mg/L) were classified under the category ‘other phenotype’. Finally, isolates with MICs ≤ 1 mg/L for cefotaxime and ceftazidime would be considered as not ESBL and/or AmpC producers. This implied that some isolates considered as microbiologically resistant (MICs over the ECOFFs) would not be further classified, as probably other mechanisms or technical issues in the MIC testing (i.e. MIC value close to the ECOFF) would be responsible for the MIC values obtained. For the present report, cefotaxime‐ and ceftazidime‐resistant isolates with MICs ≤ 1 mg/L for both antimicrobials were considered as putative non‐ESBL/AmpC producers and were classified under the category ‘other phenotype’.
We are aware that without a further molecular characterisation of the isolates, it will not be possible to know exactly which resistance mechanisms are present. For epidemiological purposes and based on the EUCAST guidelines, the classification of ‘presumptive’ producers for the different mechanism conferring resistance to third‐generation cephalosporins and/or carbapenems was considered. Molecular characterisation of these mechanisms is recommended.
1.
For the occurrence and prevalence tables, as well as the violet shaded maps and graphics shown in Section ‘ESBL/AmpC/CP producers monitoring’, presumptive ESBL producers were considered as those exhibiting an ESBL and/or ESBL + AmpC phenotype and presumptive AmpC producers, those with an AmpC and ESBL + AmpC phenotype (see below).
For the present report, the terms:
‘Presumptive ESBL/AmpC producers’ refers to those isolates who present an ESBL and/or and AmpC and/or an ESBL + AmpC phenotype (presumptive ESBL producers and/or presumptive AmpC producers).
‘Presumptive ESBL producers’ refers to those isolates with MICs > 1 mg/L for cefotaxime and/or ceftazidime and a synergy test positive for any of these antimicrobials and susceptibility to meropenem (MEM ≤ 0.125 mg/L, see CP phenotype). These isolates may also harbour other resistance mechanisms (e.g. AmpC‐encoding genes).
‘Presumptive ESBL‐cefotaximase producers’ refers to those presumptive ESBL producers with MICs > 1 mg/L for cefotaxime and a synergy test positive for cefotaxime only. These isolates may also harbour other resistance mechanisms.
‘Presumptive ESBL‐ceftazidimase producers’ refers to those presumptive ESBL producers with MICs > 1 mg/L for ceftazidime and synergy test positive for ceftazidime only. These isolates may also harbour other resistance mechanisms.
‘Presumptive AmpC producers’ refers to isolates with MICs > 1 mg/L for cefotaxime and/or ceftazidime and cefoxitin MIC > 8 mg/L together with susceptibility to meropenem (MEM ≤ 0.125 mg/L, see CP phenotype). No distinction between acquired AmpC and natural AmpC was made. These isolates may also harbour other resistance mechanisms (e.g. ESBL‐encoding genes).
‘Presumptive ESBL + AmpC producers’ refers to isolates with the ESBL + AmpC phenotype described above.
‘Presumptive carbapenemase‐producers (CP‐producers)’ refers to those isolates with the CP phenotype described above.
Data on meticillin‐resistant Staphylococcus aureus (MRSA)
The occurrence of MRSA and its susceptibility to antimicrobials in various food categories (including meat samples from various species) and food‐producing animals was reported by seven MSs and two non‐MSs in 2019 and by six Mss and one non‐MS in 2020 (excluding clinical investigations). In 2019, Finland and Switzerland were the only countries to report susceptibility data for MRSA isolates from meat samples (both countries also reported molecular typing data); Belgium and Switzerland were the only countries in 2019 to report such data for MRSA isolates from food‐producing animals (both countries also reported molecular typing data, as did Finland, Norway and Spain). In 2020, Austria and Germany were the only countries to report susceptibility data on MRSA isolates from meat samples, with both countries additionally reporting molecular typing data; Belgium was the only country in 2020 to report susceptibility data on isolates from food‐producing animals (and also provided molecular typing data, as did Denmark). MRSA occurrence data reported from clinical investigations of food‐producing and companion animals in 2019–2020 were also reported. Details of the antimicrobials selected are provided in the section on MRSA. For further information on reported MIC distributions and the number of resistant isolates, refer to the submitted and validated MS data published on the EFSA website.
The methods for collecting and testing samples for MRSA are not harmonised between MSs and, as a result, MSs may use differing procedures. Due to the variety of methods employed by MSs, these are explained in detail within the section on MRSA to enable readers to better follow the procedures carried out by individual countries.
Appendix G – Additional information and supporting data
List of Annexes
The annexes are available on the EFSA Knowledge Junction community on Zenodo at: https://doi.org/10.5281/zenodo.6257446
The annexes contain the following information:
Annex A – Data reported on antimicrobial resistance in Salmonella spp.
The annex contains tables on antimicrobial resistance data:
-
–
Antimicrobial resistance in Salmonella spp. from humans, 2020;
-
–
Occurrence of resistance to selected antimicrobials in Salmonella spp. from animal carcases, 2019 and 2020;
-
–
Occurrence of resistance to selected antimicrobials in Salmonella spp. from animals, 2019 and 2020;
-
–
Occurrence of resistance (%) to selected antimicrobials in specific Salmonella serovars.
Annex B – Data reported on antimicrobial resistance in Campylobacter spp.
The annex contains tables and figures showing antimicrobial resistance data:
-
–
Antimicrobial resistance in Campylobacter spp. from humans, 2020 and trends for 2016–2020 period;
-
–
Data reported on antimicrobial resistance and occurrence of resistance to selected antimicrobials in Campylobacter spp. from food‐producing animals and derived meat, for 2019 and 2020 and trends for 2009–2020.
Annex C – Data reported on AMR in indicator Escherichia coli from food‐producing animals and derived meat
The annex contains tables on data reported on AMR in indicator Escherichia coli from food‐producing animals and derived meat.
Annex D – Data on presumptive ESBL‐, AmpC‐ and/or carbapenemase‐producing microorganisms and their resistance occurrence (routine and specific monitorings)
The annex contains the tables (Tables 1–22) with the data reported on presumptive ESBL‐, AmpC‐ and/or carbapenemase‐producing microorganisms for pigs and cattle (2019) and poultry (2020) and meat thereof, and their resistance occurrence (routine and specific monitorings):
-
–
ESBL‐, AmpC‐producers prevalence and occurrence tables – pigs and cattle and meat thereof, 2019;
-
–
ESBL‐, AmpC‐, carbapenemase‐producers prevalence and occurrence tables – poultry 2020;
-
–
Specific carbapenemase‐producing E. coli monitoring 2019–2020;
-
–
Occurrence of antimicrobial resistance in poultry isolates collected in 2020.
Annex E – Data reported on antimicrobial resistance in MRSA from food‐producing animals and derived meat
The annex contains tables on 2019 and 2020 data reported on the prevalence, genetic diversity and antimicrobial resistance of MRSA from food‐producing animals and derived meat.
Supporting data
All tables produced for the European Union Summary Report on Antimicrobial Resistance in Zoonotic and Indicator Bacteria from Humans, Animals and Food in 2019–2020 are available on the EFSA Knowledge Junction community on Zenodo at: https://doi.org/10.5281/zenodo.6257446
The aggregated data set submitted on the negative results for extended‐spectrum β‐lactamase (ESBL) is also available on the Knowledge Junction at: https://doi.org/10.5281/zenodo.6257446
Country Data sets
All country data sets containing the tables on the occurrence of antimicrobial resistance per each country are available on the EFSA Knowledge Junction community on Zenodo – please see below the list and corresponding link to the data sets.
The countries that submitted data sets on the 2020 monitoring data year are the 27 EU Member States, the four non‐EU Member States and Albania and Republic of North Macedonia as pre‐accession countries.
Supporting information
Plain language summary
Suggested citation: EFSA (European Food Safety Authority) and ECDC (European Centre for Disease Prevention and Control) , 2022. The European Union Summary Report on Antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food in 2019–2020. EFSA Journal 2022;20(3):7209, 197 pp. 10.2903/j.efsa.2022.7209
Requestor: European Commission
Question number: EFSA‐Q‐2021‐00768
Declarations of interest: The declarations of interest of all scientific experts active in EFSA’s work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgements: EFSA and ECDC wish to thank the members of the Scientific Network for Zoonoses Monitoring Data (EFSA) and the Food‐ and Waterborne Diseases and Zoonoses Network (ECDC) who provided the data and reviewed the report and the members of the Scientific Network for Zoonoses Monitoring Data, for their endorsement of this scientific output. Also, the contribution of EFSA staff members: Pierre‐Alexandre Beloeil, Giusi Amore, Anca‐Violeta Stoicescu, Kenneth Mulligan, Krisztina Nagy, Gina Cioacata and Raquel Garcia Fierro, the contributions of ECDC staff members: Therese Westrell and Hanna Merck, and the contributions of EFSA’s contractors: Björn Bengtsson, Isabelle Kempf, Oskar Nilsson, Bernd‐Alois Tenhagen, Kees Veldman and Karoline Noerstrud for the support provided to this scientific output. Also, the support from the EURL‐AR, specifically Inge Marianne Hansen, Jette Sejer Kjeldgaard, Anne Mette Seyfarth, Pimlapas Leekitcharoenphon and Rene S. Hendriksen for the confirmatory testing are gratefully acknowledged.
Approved: 1 March 2022
Plain language summary: A plain language summary of this report is available under Supporting Information. It aims to enhance transparency and inform interested parties on EFSA's work on the topic using simplified language. Those interested in the more technical analysis should consult the full report.
The Plain Language Summary of the opinion is available under supporting information section of ON‐7209
Notes
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the EFSA and laying down procedures in matters of food safety. OJ L 31, 1.2.2002, p. 1–24.
Directive 2003/99/EC of the European Parliament and of the Council of 17 November 2003 on the monitoring of zoonoses and zoonotic agents, amending Council Decision 90/424/EEC and repealing Council Directive 92/117/EEC. OJ L325/31, 12.12.2003, p. 31–40.
Commission Implementing Decision 2013/652/EU of 12 November 2013 on the monitoring and reporting of antimicrobial resistance in zoonotic and commensal bacteria. OJ L 303, 14.11.2013, p. 26–39.
Commission Implementing Decision 2018/945/EU of 22 June 2018 on the communicable diseases and related special health issues to be covered by epidemiological surveillance as well as relevant case definitions. OJ L 170, 6.7.2018, p. 1–74.
Commission Decision 2012/506/EU amending Decision 2002/253/EC laying down case definitions for reporting communicable diseases to the Community network under Decision No. 2119/98/EC of the European Parliament and of the Council. OJ L 262, 27.9.2012, p. 1–57.
Decision No 1082/2013/EU of the European Parliament and of the Council of 22 October 2013 on serious cross‐border threats to health and repealing Decision No. 2119/98/EC. OJ L 293, 5.11.2013, p. 1–15.
Additional information on Materials and methods can be found in Appendix F and links to supporting data for this chapter (Annex A–E) are provided in Appendix G.
The epidemiological cut‐off (ECOFF) values separate the naive, susceptible wild‐type bacterial populations from isolates that have developed reduced susceptibility to a given antimicrobial agent (Kahlmeter et al., 2003). The ECOFFs may differ from breakpoints used for clinical purposes, which are set out against a background of clinically relevant data, including therapeutic indication, clinical response data, dosing schedules, pharmacokinetics and pharmacodynamics. The use of harmonised methods and ECOFFs ensures the comparability of data over time at the country level and also facilitates the comparison of resistance between MSs.
See Materials and Methods (Annex A) for terminology used to describe the levels of antimicrobial resistance.
See Materials and Methods (Annex A) for further information regarding the MDR analysis.
As outlined in the Commission Implementing Decision 2013/652/EU of 12 November 2013 on the monitoring and reporting of antimicrobial resistance in zoonotic and commensal bacteria. OJ L 303, 14.11.2013, p. 26–39.
Commission Implementing Decision 2020/1729/EU of 17 November 2020 on the monitoring and reporting of antimicrobial resistance in zoonotic and commensal bacteria and repealing Implementing Decision 2013/652/EU. OJ L 387, 19.11.2020, p. 8–21.
Additional information on Materials and methods can be found in Appendix F and links for supporting data for this chapter (Annex C) are provided in Appendix G.
All Salmonella and indicator E. coli isolates tested with the harmonised panel of antimicrobial substances (Panel 1) and exhibiting microbiological resistance to cefotaxime, ceftazidime or meropenem were subsequently subjected to further testing using a supplementary panel of substances (Panel 2) to obtain more detailed phenotypic characterisation (see Appendix F, materials and methods).
The method is described in Appendix F and protocols are available at: https://www.eurl‐ar.eu/protocols.aspx
E. coli isolates exhibiting an ESBL‐, AmpC‐ or ESBL+AmpC‐producing phenotype
Commission Implementing Decision 2013/652/EU of 12 November 2013 on the monitoring and reporting of antimicrobial resistance in zoonotic and commensal bacteria. OJ L 303, 14.11.2013, p. 26–39.
‘Quantitative data’ derived from dilution methods consisted of the number of isolates having a specific MIC value (measured in mg/L) relative to the total number of isolates tested, for each antimicrobial agent and specific food/animal category.
Commission Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. OJ L 338, 22.12.2005, p. 1–26.
Regulation (EC) No 2160/2003 of the European Parliament and of the Council of 17 November 2003 on the control of salmonella and other specified food‐borne zoonotic agents. OJ L 325, 12.12.2003, p. 1–15.
The same sampling design was used to collect indicator E. coli isolates, whether dedicated to the routine monitoring of AMR or the specific monitoring of ESBL‐/AmpC‐/carbapenemase‐producing E. coli.
Available online: www.eurl‐ar.eu
Giving the percentage of isolates ‘microbiologically’ resistant out of those tested.
Contributor Information
European Food Safety Authority, Email: zoonoses@efsa.europa.eu.
European Centre for Disease Prevention and Control, Email: FWD@ecdc.europa.eu.
References
- Agersø Y, Torpdahl M, Zachariasen C, Seyfarth A, Hammerum AM and Nielsen EM, 2012. Tentative colistin epidemiological cut‐off value for Salmonella spp. Foodborne Pathogens and Disease, 9, 367–369. 10.1089/fpd.2011.1015 [DOI] [PubMed] [Google Scholar]
- Alba P, Feltrin F, Cordaro G, Porrero MC, Kraushaar B, Argudin MA, Nykasenoja S, Monaco M, Stegger M, Aarestrup FM, Butaye P, Franco A and Battisti A, 2015. Livestock‐associated methicillin resistant and methicillin susceptible staphylococcus aureus sequence type (CC)1 in European Farmed Animals: high genetic relatedness of isolates from italian cattle herds and humans. PLoS One, 10, e0137143. 10.1371/journal.pone.0137143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert E, Sipos R, Jánosi S, Kovács P, Kenéz Á, Micsinai A, Noszály Z and Biksi I, 2020. Occurrence and characterisation of methicillin‐resistant Staphylococcus aureus isolated from bovine milk in Hungary. Acta Veterinaria Hungarica, 68, 236–241. 10.1556/004.2020.00040 [DOI] [PubMed] [Google Scholar]
- Alt K, Fetsch A, Schroeter A, Guerra B, Hammerl JA, Hertwig S, Senkov N, Geinets A, Mueller‐Graf C, Braeunig J, Kaesbohrer A, Appel B, Hensel A and Tenhagen BA, 2011. Factors associated with the occurrence of MRSA CC398 in herds of fattening pigs in Germany. BMC Veterinary Research, 7, 7–69. 10.1186/1746-6148-7-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angen Ø, Stegger M, Larsen J, Lilje B, Kaya H, Pedersen KS, Jakobsen A, Petersen A and Larsen AR, 2017. Report of mecC‐carrying MRSA in domestic swine. Journal of Antimicrobial Chemotherapy, 72, 60–63. 10.1093/jac/dkw389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv G, Tsyba K, Steck N, Salmon‐Divon M, Cornelius A, Rahav G, Grassl GA and Gal‐Mor O, 2014. A unique megaplasmid contributes to stress tolerance and pathogenicity of an emergent Salmonella enterica serovar Infantis strain. Environmental Microbiology, 16, 977–994. 10.1111/1462-2920.12351 [DOI] [PubMed] [Google Scholar]
- Bai L, Du P, Du Y, Sun H, Zhang P, Wan Y, Lin Q, Fanning S, Cui S and Wu Y, 2019. Detection of plasmid‐mediated tigecycline‐resistant gene tet(X4) in Escherichia coli from pork, Sichuan and Shandong Provinces, China, February 2019. Euro Surveillance, 24, 4. 10.2807/1560-7917.es.2019.24.25.1900340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangerter PD, Sidler X, Perreten V and Overesch G, 2016. Longitudinal study on the colonisation and transmission of methicillin‐resistant Staphylococcus aureus in pig farms. Veterinary Microbiology, 183, 125–134. 10.1016/j.vetmic.2015.12.007 [DOI] [PubMed] [Google Scholar]
- Bengtsson B, Persson L, Ekström K, Unnerstad HE, Uhlhorn H and Börjesson S, 2017. High occurrence of mecC‐MRSA in wild hedgehogs (Erinaceus europaeus) in Sweden. Veterinary Microbiology, 207, 103–107. 10.1016/j.vetmic.2017.06.004 [DOI] [PubMed] [Google Scholar]
- Boswihi SS, Udo EE and Al‐Sweih N, 2016. Shifts in the Clonal Distribution of Methicillin‐Resistant Staphylococcus aureus in Kuwait Hospitals: 1992–2010. PLoS One, 11, e0162744. 10.1371/journal.pone.0162744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boswihi SS, Udo EE, Monecke S, Mathew B, Noronha B, Verghese T and Tappa SB, 2018. Emerging variants of methicillin‐resistant Staphylococcus aureus genotypes in Kuwait hospitals. PLoS One, 13, e0195933. 10.1371/journal.pone.0195933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boswihi SS, Udo EE and AlFouzan W, 2020. Antibiotic resistance and typing of the methicillin‐resistant Staphylococcus aureus clones in Kuwait hospitals, 2016–2017. BMC Microbiology, 20, 314. 10.1186/s12866-020-02009-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broens EM, Graat EAM, Van der Wolf PJ, Van de Giessen AW and De Jong MCM, 2011. Transmission of methicillin resistant Staphylococcus aureus among pigs during transportation from farm to abattoir. Veterinary Journal, 189, 302–305. [DOI] [PubMed] [Google Scholar]
- Brouwer MSM, Rapallini M, Geurts Y, Harders F, Bossers A, Mevius DJ, Wit B and Veldman KT, 2018. Enterobacter cloacae complex isolated from shrimps from Vietnam encoding blaIMI‐1, resistant to carbapenems but not cephalosporins. Antimicrobial Agents and Chemotherapy, 62. 10.1128/AAC.00398-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AC, Chen JC, Francois Watkins LK, Campbell D, Folster JP, Tate H, Wasilenko J, Van Tubbergen C and Friedman CR, 2018. CTX‐M‐65 extended‐spectrum b‐lactamase–producing Salmonella enterica serotype Infantis, United States. Emerging Infectious Diseases, 24, 2284–2291. 10.3201/eid2412.180500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buntenkoetter V, Blaha T, Tegeler R, Fetsch A, Hartmann M, Kreienbrock L and Meemken D, 2014. Comparison of the phenotypic antimicrobial resistances and spa‐types of methicillin‐resistant Staphylococcus aureus (MRSA) isolates derived from pigs in conventional and in organic husbandry systems. Berliner Und Munchener Tierarztliche Wochenschrift, 127, 135–143. [PubMed] [Google Scholar]
- Campos J, Cristino L, Peixe L and Antunes P, 2016. MCR‐1 in multidrug‐resistant and copper‐tolerant clinically relevant Salmonella 1,4[5],12:i:‐ and S. Rissen clones in Portugal, 2011 to 2015. Euro Surveillance, 21, 5. 10.2807/1560-7917.ES.2016.21.26.30270 [DOI] [PubMed] [Google Scholar]
- Carnevali C, Morganti M, Scaltriti E, Bolzoni L, Pongolini S and Casadei G, 2016. Occurrence of mcr‐1 in colistin resistant Salmonella enterica isolates recovered from humans and animals in Italy, 2012 to 2015. Antimicrobial Agents and Chemotherapy, 60, 7532–7534. 10.1128/AAC.01803-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson C, Li X‐Z, Agunos A, Loest D, Chapman B, Finley R, Mehrotra M, Sherk LM, Gaumond R and Irwin R, 2019. Ceftiofur‐resistant Salmonella enterica serovar Heidelberg of poultry origin – a risk profile using the Codex framework. Epidemiology and Infection, 147, E296. 10.1017/S0950268819001778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASFM/EUCAST , 2020. Comité de l’antibiogramme de la Société Française de Microbiologie [in French]. Recommandations 2020 V.1.1 Avril. Available at: https://www.sfm‐microbiologie.org/wp‐content/uploads/2020/04/CASFM2020_Avril2020_V1.1.pdf
- Cavaco LM, Abatih E, Aarestrup FM and Guardabassi L, 2008. Selection and persistence of CTX‐M‐producing Escherichia coli in the intestinal flora of pigs treated with amoxicillin, ceftiofur, or cefquinome. Antimicrobial Agents and Chemotherapy, 52, 3612–3616. 10.1128/aac.00354-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong Y, Ito Y and Kamimura T, 2011. Genetic evolution and clinical impact in extended‐spectrum beta‐lactamase producing Escherichia coli and Klebsiella pneumoniae . Infection Genetics and Evolution, 11, 1499–1504. 10.1016/j.meegid.2011.06.001 [DOI] [PubMed] [Google Scholar]
- CLSI and EUCAST (Clinical and Laboratory Standards Institute and European Committee on Antimicrobial Susceptibility Testing) , 2016. Recommendations for MIC determination of colistin (polymyxin E) as recommended by the joint CLSI‐EUCAST Polymyxin Breakpoints Working Group. 22 March 2016. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/General_documents/Recommendations_for_MIC_determination_of_colistin_March_2016.pdf
- Coipan CE, Westrell T, van Hoek AHAM, Alm E, Kotila S, Berbers B, de Keersmaecker SCJ, Ceyssens P‐J, Borg ML, Chattaway M, McCormick J, Dallman TJ and Franz E, 2020. Genomic epidemiology of emerging ESBL‐producing Salmonella Kentucky bla CTX‐M‐14b in Europe. Emerging Microbes & Infections, 9, 2124–2135. 10.1080/22221751.2020.1821582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombe F, Willems G, Dispas M, Hallin M, Denis O, Suetens C, Gordts B, Struelens M and Butaye P, 2012. Prevalence and antimicrobial susceptibility of methicillin‐resistant Staphylococcus aureus among pigs in Belgium. Microbiological Drug Resistance, 18, 125–131. [DOI] [PubMed] [Google Scholar]
- Crump JA, Sjölund‐Karlsson M, Gordon MA and Parry CM, 2015. Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of invasive Salmonella infections. Clinical Microbiology Reviews, 28, 901–937. 10.1128/CMR.00002-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui C, Ba X and Holmes MA, 2021. Prevalence and characterization of mecC MRSA in bovine bulk tank milk in Great Britain, 2017‐18. JAC Antimicrob Resist, 3, dlaa125. 10.1093/jacamr/dlaa125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuny C, Wieler LH and Witte W, 2015. Livestock‐associated MRSA: the impact on humans. Antibiotics (Basel), 4, 521–543. 10.3390/antibiotics4040521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer E, Zwartkruis‐Nahuis JT, Wit B, Huijsdens XW, de Neeling AJ, Bosch T, van Oosterom RA, Vila A and Heuvelink AE, 2009. Prevalence of methicillin‐resistant Staphylococcus aureus in meat. International Journal of Food Microbiology, 134, 52–56. 10.1016/j.ijfoodmicro.2008.12.007 [DOI] [PubMed] [Google Scholar]
- ECDC (European Centre for Disease Prevention and Control) , 2016. EU protocol for harmonised monitoring of antimicrobial resistance in human Salmonella and Campylobacter isolates – June 2016. Stockholm: ECDC; 2016. Available at: https://www.ecdc.europa.eu/en/publications-data/eu-protocol-harmonised-monitoring-antimicrobial-resistance-human-salmonella-and-0
- ECDC, EFSA and EMA (European Centre for Disease Prevention and Control, European Food Safety Authority and European Medicines Agency) , 2015. ECDC/EFSA/EMA first joint report on the integrated analysis of the consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from humans and food‐producing animals. Stockholm/Parma/London: ECDC/EFSA/EMA, 2015. EFSA Journal 2015;13(1):4006, 114 pp. 10.2903/j.efsa.2015.4006 [DOI] [Google Scholar]
- ECDC, EFSA and EMA (European Centre for Disease Prevention and Control, European Food Safety Authority and European Medicines Agency) , 2017. ECDC/EFSA/EMA second joint report on the integrated analysis of the consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from humans and food‐producing animals – Joint Interagency Antimicrobial Consumption and Resistance Analysis (JIACRA) Report. EFSA Journal 2017;15(7):4872, 135 pp. 10.2903/j.efsa.2017.4872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECDC, EFSA and EMA (European Centre for Disease Prevention and Control European Food Safety Authority and European Medicines Agency) , 2021. Third joint inter‐agency report on integrated analysis of consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from humans and food‐producing animals in the EU/EEA, JIACRA III. 2016–2018. Stockholm, Parma, Amsterdam: ECDC. EFSA, EMA; 2021. ISBN 978‐92‐9498‐541‐5. 10.2903/j.efsa.2021.6712 [DOI]
- EFSA (European Food Safety Authority) , 2008. Report from the Task Force on Zoonoses Data Collection including guidance for harmonized monitoring and reporting of antimicrobial resistance in commensal Escherichia coli and Enterococcus spp. from food animals. EFSA Journal 2008;6(4):141, 44 pp. 10.2903/j.efsa.2008.141r [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2009a. Scientific Opinion of the panel on Biological Hazards on a request from the European Commission on Assessment of the public health significance of meticillin resistant Staphylococcus aureus (MRSA) in animals and foods. EFSA‐Journal, 2009;7(10):993, 73 pp. 10.2903/j.efsa.2009.993 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2009b. Analysis of the baseline survey on the prevalence of methicillin‐resistant Staphylococcus aureus (MRSA) in holdings with breeding pigs, in the EU, 2008, Part A: MRSA prevalence estimates; on request from the European Commission. EFSA Journal 2009;7(11):1376, 82 pp. 10.2903/j.EFSA.2009.1376 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2012a. Technical specifications on the harmonised monitoring and reporting of antimicrobial resistance in methicillin‐resistant Staphylococcus aureus in food‐producing animals and food. EFSA Journal 2012;10(10):2897, 48 pp. 10.2903/j.EFSA.2012.2897 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2012. Technical specifications for the analysis and reporting of data on antimicrobial resistance (AMR) in the European Union Summary Report. EFSA Journal 2012;10(2):2587, 53 pp. 10.2903/j.efsa.2012.2587 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2014. Technical specifications on randomised sampling for harmonised monitoring of antimicrobial resistance in zoonotic and commensal bacteria. EFSA Journal 2014;12(5):3686, 33 pp. 10.2903/j.efsa.2014.3686 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2020. Technical specifications on a randomisation of sampling for the purpose of antimicrobial resistance monitoring from food‐producing animals and food as from 2021. EFSA Journal 2020;18(12):6364, 31 pp. 10.2903/j.efsa.2020.6364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , Aerts M, Battisti A, Hendriksen R, Kempf I, Teale C, Tenhagen B‐A, Veldman K, Wasyl D, Guerra B, Liébana E, Thomas‐López D and Belœil P‐A, 2019. Scientific report on the technical specifications on harmonised monitoring of antimicrobial resistance in zoonotic and indicator bacteria from food‐producing animals and food. EFSA Journal 2019;17(6):5709, 122 pp. 10.2903/j.efsa.2019.5709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , Amore G, Beloeil P‐A, Garcia Fierro R, Guerra B, Papanikolaou A, Rizzi V and Stoicescu A‐V, 2021a. Manual for reporting 2021 antimicrobial resistance data within the framework of Directive 2003/99/EC and Decision 2020/1729/EU. EFSA supporting publication 2021;EN‐6652, 31 pp. 10.2903/sp.efsa.2021.EN-6652 [DOI]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control) , 2013. The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2011. EFSA Journal 2013;11(5):359, 11 pp. 10.2903/j.efsa.2013.3196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control) , 2018. The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2016. EFSA Journal, 2018;16(2):5182, 45 pp. 10.2903/j.efsa.2018.5182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control) , 2019. Scientific report on the European Union One Health 2018 Zoonoses Report. EFSA Journal 2019;17(12):5926, 276 pp. 10.2903/j.efsa.2019.5926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control) , 2019. The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2017. EFSA Journal 2019;17 (2):5598, 278 pp. 10.2903/j.efsa.2019.5598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control) , 2020. The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2017/2018. EFSA Journal 2020;18(3):6007, 55 pp. 10.2903/j.efsa.2020.6007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control) , 2021a. The European Union Summary Report on Antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food in 2018/2019. EFSA Journal 2021;19(4):6490, 179 pp. 10.2903/j.efsa.2021.6490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control) , 2021b. The European Union One Health 2020 Zoonoses Report. EFSA Journal 2021;19(12):6971, 324 pp. 10.2903/j.efsa.2021.6971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , 2011. Scientific Opinion on the public health risks of bacterial strains producing extended‐spectrum b‐lactamases and/or AmpC b‐lactamases in food and food producing animals. EFSA Journal 2011;9(8):2322, 95 pp. 10.2903/j.efsa.2011.2322/full [DOI] [Google Scholar]
- Elhadidy M, Miller WG, Arguello H, Álvarez‐Ordóñez A, Dierick K and Botteldoorn N, 2019. Molecular epidemiology and antimicrobial resistance mechanisms of Campylobacter coli from diarrhoeal patients and broiler carcasses in Belgium. Transboundary Emerging Disease, 66, 463–475. 10.1111/tbed.13046 [DOI] [PubMed] [Google Scholar]
- EMA/CVMP , 2012. Opinion following an Article 35 referral for all veterinary medicinal products containing systemically administered (parenteral and oral) 3rd and 4th generation cephalosporins intended for use in food producing species. In https://www.ema.europa.eu/medicines/veterinary/referrals/cephalosporins
- EMA (European Medicines Agency, European) Surveillance of Veterinary Antimicrobial Consumption , 2021. ‘Sales of veterinary antimicrobial agents in 31 European countries in 2019 and 2020’. (EMA/58183/2021). Available online: https://www.ema.europa.eu/en/documents/report/sales‐veterinary‐antimicrobial‐agents‐31‐european‐countries‐2019‐2020‐trends‐2010‐2020‐eleventh_en.pdf
- EMA and EFSA (European Medicines Agency and European Food Safety Authority) , 2017. Joint Scientific Opinion on measures to reduce the need to use antimicrobial agents in animal husbandry in the European Union, and the resulting impacts on food safety (RONAFA). EFSA Journal 2017;15(1):4666, 245 pp. 10.2903/j.efsa.2017.4666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EUCAST (European Committee for Antimicrobial Susceptibility Testing) , 2014. Screening for fluoroquinolone resistance in Salmonella spp. with pefloxacin 5 lg. Tentative quality control criteria for users and disk manufacturers. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/QC/Tentative_QC_criteria_for_pefloxacin_5__g.pdf
- EUCAST (European Committee for Antimicrobial Susceptibility Testing) , 2017. The EUCAST guidelines for detection of resistance mechanisms and specific resistances of clinical and/or epidemiological importance. Version 2. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Resistance_mechanisms/EUCAST_detection_of_resistance_mechanisms_170711.pdf
- EUCAST (European Committee for Antimicrobial Susceptibility Testing) , 2020. Campylobacter jejuni and coli ‐ calibration of zone diameter breakpoints to MIC values. Version 3.0, January 2020. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Disk_criteria/Validation_2020/Camplyobacter_v_3.0_January_2020.pdf
- EURL (European Union Reference Laboratory for Antimicrobial Resistance) , 2018. Laboratory Protocol, Isolation of methicillin‐resistant Staphylococcus aureus (MRSA) from food‐producing animals and farm environment. Technical University of Denmark.
- Feltrin F, Alba P, Kraushaar B, Ianzano A, Argudin MA, Di Matteo P, Porrero MC, Aarestrup FM, Butaye P, Franco A and Battisti A, 2015. A livestock‐associated, multidrug‐resistant, methicillin‐resistant Staphylococcus aureus Clonal Complex 97 Lineage Spreading in Dairy Cattle and Pigs in Italy. Applied and Environment Microbiology, 82, 816–821. 10.1128/AEM.02854-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltrin F, Alba P, Kraushaar B, Ianzano A, Argudin MA, Di Matteo P, Porrero MC, Aarestrup FM, Butaye P, Franco A and Battisti A, 2016. A livestock‐associated, multidrug‐resistant, methicillin‐resistant Staphylococcus aureus Clonal Complex 97 Lineage Spreading in Dairy Cattle and Pigs in Italy. Applied and Environment Microbiology, 82, 816–821. 10.1128/AEM.02854-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florez‐Cuadrado D, Ugarte‐Ruiz M, Meric G, Quesada A, Porrero MC, Pascoe B, Saez‐Llorente JL, Orozco GL, Dominguez L and Sheppard SK, 2017. Genome comparison of Erythromycin Resistant Campylobacter from Turkeys Identifies Hosts and Pathways for Horizontal Spread of erm(B) Genes. Frontiers in Microbiology, 8, 2240. 10.3389/fmicb.2017.02240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florez‐Cuadrado D, Moreno MA, Ugarte‐Ruiz M and Dominguez L, 2018. Antimicrobial Resistance in the Food Chain in the European Union. Advances in Food and Nutrition Research, 86, 115–136. 10.1016/bs.afnr.2018.04.004 [DOI] [PubMed] [Google Scholar]
- Franco A, Leekitcharoenphon P, Feltrin F, Alba P, Cordaro G, Iurescia M, Tolli R, D’Incau M, Staffolani M, Di Giannatale E, Hendriksen RS and Battisti A, 2015. Emergence of a clonal lineage of multidrug‐resistant ESBL producing Salmonella Infantis transmitted from broilers and broiler meat to humans in Italy between 2011 and 2014. PLoS One, 10. 10.1371/journal.pone.0144802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FSA (Food Standards Agency) , 2017. Risk Assessment on Meticillin‐Resistant Staphylococcus aureus (MRSA), with a focus on Livestock‐associated MRSA in the UK Food Chain.
- Garcia‐Alvarez L, Holden MT, Lindsay H, Webb CR, Brown DF, Curran MD, Walpole E, Brooks K, Pickard DJ, Teale C, Parkhill J, Bentley SD, Edwards GF, Girvan EK, Kearns AM, Pichon B, Hill RL, Larsen AR, Skov RL, Peacock SJ, Maskell DJ and Holmes MA, 2011. Meticillin‐resistant Staphylococcus aureus with a novel mecA homologue in human and bovine populations in the UK and Denmark: a descriptive study. The Lancet Infectious Diseases, 11, 595–603. 10.1016/S1473-3099(11)70126-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García‐Fierro R, Montero I, Bances M, Gonzalez‐Hevia MA and Rodicio MR, 2016. Antimicrobial drug resistance and molecular typing of Salmonella enterica serovar rissen from different sources. Microbial Drug Resistance, 22, 211–217. [DOI] [PubMed] [Google Scholar]
- García V, Vázquez X, Bances M, Herrera‐León L, Herrera‐León S and Rodicio MR, 2019. Molecular characterization of Salmonella enterica serovar Enteritidis, genetic basis of antimicrobial drug resistance and plasmid diversity in ampicillin‐resistant isolates. Microbial Drug Resistance, 25, 219–226. 10.1089/mdr.2018.0139 [DOI] [PubMed] [Google Scholar]
- Giacinti G, Carfora V, Caprioli A, Sagrafoli D, Marri N, Giangolini G, Amoruso R, Iurescia M, Stravino F, Dottarelli S, Feltrin F, Franco A, Amatiste S and Battisti A, 2017. Prevalence and characterization of methicillin‐resistant Staphylococcus aureus carrying mecA or mecC and methicillin‐susceptible Staphylococcus aureus in dairy sheep farms in central Italy. Journal of Dairy Science, 100, 7857–7863. 10.3168/jds.2017-12940 [DOI] [PubMed] [Google Scholar]
- Graveland H, Wagenaar JA, Heesterbeek H, Mevius D, van Duijkeren E and Heederik D, 2010. Methicillin resistant Staphylococcus aureus ST398 in veal calf farming: human MRSA carriage related with animal antimicrobial usage and farm hygiene. PLoS One, 5, e10990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greninger AL, Addetia A, Starr K, Cybulski RJ, Stewart MK, Salipante SJ, Bryan AB, Cookson B, Gaudreau C, Bekal S and Fang FC, 2020. International spread of multidrug‐resistant Campylobacter coli in men who have sex with men in Washington State and Quebec, 2015–2018. Clinical Infectious Diseases, 71, 1896–1904. 10.1093/cid/ciz1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardabassi L, O'Donoghue M, Moodley A, Ho J and Boost M, 2009. Novel lineage of methicillin‐resistant Staphylococcus aureus, Hong Kong. Emerging Infectious Diseases, 15, 1998–2000. 10.3201/eid1512.090378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenni M, Châtre P, Dupieux C, Métayer V, Maillard K, Bes M, Madec JY and Laurent F, 2015. mecC‐positive MRSA in horses. Journal of Antimicrobial Chemotherapy, 70, 3401–3402. 10.1093/jac/dkv278 [DOI] [PubMed] [Google Scholar]
- Handel N, Otte S, Jonker M, Brul S and ter Kuile BH, 2015. Factors that affect transfer of the IncI1 beta‐lactam resistance plasmid pESBL‐283 between E. coli strains. PLoS One, 10, e0123039. 10.1371/journal.pone.0123039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison EM, Paterson GK, Holden MT, Larsen J, Stegger M, Larsen AR, Petersen A, Skov RL, Christensen JM, Bak Zeuthen A, Heltberg O, Harris SR, Zadoks RN, Parkhill J, Peacock SJ and Holmes MA, 2013. Whole genome sequencing identifies zoonotic transmission of MRSA isolates with the novel mecA homologue mecC. EMBO Molecular Medicine, 5, 509–515. 10.1002/emmm.201202413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkey J, Le Hello S, Doublet B, Granier SA, Hendriksen RS, Fricke WF, Ceyssens P‐J, Gomart C, Billman‐Jacobe H, Holt KE and Weill F‐X, 2019. Global phylogenomics of multidrug‐resistant Salmonella enterica serotype Kentucky ST198. Microbial Genomics, 5. 10.1099/mgen.0.000269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Xu J, Wang J, Chen Q, Hua X, Fu Y and Yu Y, 2016. Decreased susceptibility to tigecycline mediated by a mutation in mlaA in Escherichia coli strains. Antimicrobial Agents and Chemotherapy, 60, 7530–7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He T, Wang R, Liu D, Walsh TR, Zhang R, Lv Y, Ke Y, Ji Q, Wei R, Liu Z, Shen Y, Wang G, Sun L, Lei L, Lv Z, Li Y, Pang M, Wang L, Sun Q, Fu Y, Song H, Hao Y, Shen Z, Wang S, Chen G, Wu C, Shen J and Wang Y, 2019. Emergence of plasmid‐mediated high‐level tigecycline resistance genes in animals and humans. Nature Microbiology, 4, 1450–1456. [DOI] [PubMed] [Google Scholar]
- Hopkins KL, Kirchner M, Guerra B, Granier SA, Lucarelli C, Porrero MC, Jakubczak A, Threlfall EJ and Mevius DJ, 2010. Multiresistant Salmonella enterica serovar 4,[5],12:i:‐ in Europe: a new pandemic strain? Eurosurveillance, 15, pii=19580. [PubMed] [Google Scholar]
- Huang E, Gurzau AE, Hanson BM, Kates AE, Smith TC, Pettigrew MM, Spinu M and Rabinowitz PM, 2014. Detection of livestock‐associated methicillin‐resistant Staphylococcus aureus among swine workers in Romania. Journal of Infect Public Health, 7, 323–332. 10.1016/j.jiph.2014.03.008 [DOI] [PubMed] [Google Scholar]
- Irrgang A, Tenhagen B‐A, Pauly N, Schmoger S, Kaesbohrer A and Hammerl JA, 2019. Characterization of VIM‐1‐ Producing E. coli isolated from a German fattening pig farm by an improved isolation procedure. Frontiers in Microbiology, 10, 2256. 10.3389/fmicb.2019.02256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irrgang A, Pauly N, Tenhagen B‐A, Grobbel M, Kaesbohrer A and Hammerl JA, 2020a. Spill‐Over from Public Health? First Detection of an OXA‐48‐Producing Escherichia coli in a German Pig Farm. Microorganisms, 8, 855. 10.3390/microorganisms8060855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irrgang A, Simon HT, Pauly N, Grobbel M, Kaesbohrer A and Hammerl JA, 2020b. First Detection of GES‐5‐Producing Escherichia coli from Livestock—An Increasing Diversity of Carbapenemases Recognized from German Pig Production. Microorganisms, 8, 1593. 10.3390/microorganisms8101593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MZ, Espinosa‐Gongora C, Damborg P, Sieber RN, Munk R, Husted L, Moodley A, Skov R, Larsen J and Guardabassi L, 2017. Horses in Denmark are a reservoir of diverse clones of methicillin‐resistant and ‐susceptible Staphylococcus aureus. Frontiers in Microbiology, 8. 10.3389/fmicb.2017.00543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehanne Q, Pascoe B, Benejat L, Ducournau A, Buissonniere A, Mourkas E, Megraud F, Bessede E, Sheppard SK and Lehours P, 2020. Genome‐wide identification of host‐segregating single‐nucleotide polymorphisms for source attribution of clinical Campylobacter coli isolates. Applied and Environmental Microbiology, 86. 10.1128/AEM.01787-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehanne Q, Bénéjat L, Ducournau A, Domingues‐Martins C, Cousinou T, Bessède E and Lehours P, 2021. Emergence of Erythromycin Resistance Methyltransferases in Campylobacter coli Strains in France. Antimicrobial Agents and Chemotherapy, 65. 10.1128/AAC.01124-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käppeli N, Morach M, Corti S, Eicher C, Stephan R and Johler S, 2019. Staphylococcus aureus related to bovine mastitis in Switzerland: Clonal diversity, virulence gene profiles, and antimicrobial resistance of isolates collected throughout 2017. Journal of Dairy Science, 102, 3274–3281. 10.3168/jds.2018-15317 [DOI] [PubMed] [Google Scholar]
- Karampatakis T, Papadopoulos P, Tsergouli K, Angelidis AS, Sergelidis D and Papa A, 2021. Genetic characterization of two methicillin‐resistant Staphylococcus aureus spa type t127 strains isolated from workers in the dairy production chain in Greece. Acta Microbiologica Et Immunologica Hungarica, 10.1556/030.2021.01460 [DOI] [PubMed] [Google Scholar]
- Karapsias S, Piperaki ET, Spiliopoulou I, Katsanis G and Tseleni‐Kotsovili A, 2008. Methicillin‐resistant Staphylococcus aureus nasal carriage among healthy employees of the Hellenic Air Force. Eurosurveillance Weekly, 13. 10.2807/ese.13.40.18999-en [DOI] [PubMed] [Google Scholar]
- Kieffer N, Aires‐de‐Sousa M, Nordmann P and Poirel L, 2017. High Rate of MCR‐1–producing Escherichia coli and Klebsiella pneumoniae among Pigs, Portugal. Emerging Infectious Diseases, 23, 2023–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinross P, Petersen A, Skov R, Van Hauwermeiren E, Pantosti A, Laurent F, Voss A, Kluytmans J, Struelens MJ, Heuer O, Monnet DL and group tEhL‐Ms, 2017. Livestock‐associated meticillin‐resistant Staphylococcus aureus (MRSA) among human MRSA isolates, European Union/European Economic Area countries, 2013. Eurosurveillance, 22, 16–00696. 10.2807/1560-7917.ES.2017.22.44.16-00696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köck R, Schaumburg F, Mellmann A, Koksal M, Jurke A, Becker K and Friedrich AW, 2013. Livestock‐associated methicillin‐resistant Staphylococcus aureus (MRSA) as causes of human infection and colonization in Germany. PLoS One, 8, e55040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köck R, Daniels‐Haardt I, Becker K, Mellmann A, Friedrich AW, Mevius D, Schwarz S and Jurke A, 2018. Carbapenem‐resistant Enterobacteriaceae in wildlife, food‐producing, and companion animals: a systematic review. Clinical Microbiology and Infection, 24, 1241–1250. 10.1016/j.cmi.2018.04.004 [DOI] [PubMed] [Google Scholar]
- Kovac J, Stessl B, Čadež N, Gruntar I, Cimerman M, Stingl K, Lušicky M, Ocepek M, Wagner M and Smole Možina S, 2018. Population structure and attribution of human clinical Campylobacter jejuni isolates from central Europe to livestock and environmental sources. Zoonoses and Public Health, 65, 51–58. 10.1111/zph.12366 [DOI] [PubMed] [Google Scholar]
- Kraemer JG, Pires J, Kueffer M, Semaani E, Endimiani A, Hilty M and Oppliger A, 2017. Prevalence of extended‐spectrum β‐lactamase‐producing Enterobacteriaceae and Methicillin‐Resistant Staphylococcus aureus in pig farms in Switzerland. Science of the Total Environment, 603–604, 401–405. 10.1016/j.scitotenv.2017.06.110 [DOI] [PubMed] [Google Scholar]
- Larsen J, Raisen CL, Ba X, Sadgrove NJ, Padilla‐González GF, Simmonds MSJ, Loncaric I, Kerschner H, Apfalter P, Hartl R, Deplano A, Vandendriessche S, Černá Bolfíková B, Hulva P, Arendrup MC, Hare RK, Barnadas C, Stegger M, Sieber RN, Skov RL, Petersen A, Angen Ø, Rasmussen SL, Espinosa‐Gongora C, Aarestrup FM, Lindholm LJ, Nykäsenoja SM, Laurent F, Becker K, Walther B, Kehrenberg C, Cuny C, Layer F, Werner G, Witte W, Stamm I, Moroni P, Jørgensen HJ, de Lencastre H, Cercenado E, García‐Garrote F, Börjesson S, Hæggman S, Perreten V, Teale CJ, Waller AS, Pichon B, Curran MD, Ellington MJ, Welch JJ, Peacock SJ, Seilly DJ, Morgan FJE, Parkhill J, Hadjirin NF, Lindsay JA, Holden MTG, Edwards GF, Foster G, Paterson GK, Didelot X, Holmes MA, Harrison EM and Larsen AR, 2022. Emergence of methicillin resistance predates the clinical use of antibiotics. Nature, 602, 135–141. 10.1038/s41586-021-04265-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen J, Stegger M, Andersen PS, Petersen A, Larsen AR, Westh H, Agerso Y, Fetsch A, Kraushaar B, Kasbohrer A, Febetaler AT, Schwarz S, Cuny C, Witte W, Butaye P, Denis O, Haenni M, Madec JY, Jouy E, Laurent F, Battisti A, Franco A, Alba P, Mammina C, Pantosti A, Monaco M, Wagenaar JA, de Boer E, van Duijkeren E, Heck M, Dominguez L, Torres C, Zarazaga M, Price LB and Skov RL, 2016. Evidence for human adaptation and foodborne transmission of livestock‐associated methicillin‐resistant Staphylococcus aureus. Clinical Infectious Diseases, 63, 1349–1352. 10.1093/cid/ciw532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen J, Sunde M, Islam MZ, Urdahl AM, Barstad AS, Larsen AR, Grøntvedt CA and Angen Ø, 2017. Evaluation of a widely used culture‐based method for detection of livestock‐associated meticillin‐resistant Staphylococcus aureus (MRSA), Denmark and Norway, 2014 to 2016. Eurosurveillance Weekly, 22. 10.2807/1560-7917.Es.2017.22.28.30573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layer F, Strommenger B, Cuny C, Noll I, Klingeberg A and Werner G, 2019. Häufigkeit, Eigenschaften und Verbreitung von MRSA in Deutschland – Zur Situation 2017/2018. Epidemiologisches Bulletin, 2019, 437–442. 10.25646/6320.2 [DOI] [Google Scholar]
- Le Hello S, Hendriksen RS, Doublet B, Fisher I, Nielsen EM, Whichard JM, Bouchrif B, Fashae K, Granier SA, Jourdan‐Da Silva N, Cloeckaert A, Threlfall EJ, Angulo FJ, Aarestrup FM, Wain J and Weill FX, 2011. International spread of an epidemic population of Salmonella enterica serotype Kentucky ST198 resistant to ciprofloxacin. Journal of Infectious Diseases, 204, 675–684. 10.1093/infdis/jir409 [DOI] [PubMed] [Google Scholar]
- Le Hello S, Bekhit A, Granier SA, Barua H, Beutlich J, Zając M, Münch S, Sintchenko V, Bouchrif B, Fashae K, Pinsard J‐L, Sontag L, Fabre L, Garnier M, Guibert V, Howard P, Hendriksen RS, Christensen JP, Biswas PK, Cloeckaert A, Rabsch W, Wasyl D, Doublet B and Weill F‐X, 2013. The global establishment of a highlyfluoroquinolone resistant Salmonella enterica serotype Kentucky ST198 strain. Frontiers in Microbiology, 4, 395. 10.3389/fmicb.2013.00395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levings RS, Lightfoot D, Partridge SR, Hall RM and Djordjevic SP, 2005. The genomic island SGI1, containing the multiple antibiotic resistance region of Salmonella enterica serovar typhimurium DT104 or variants of it, is widely distributed in other S. enterica serovars. Journal of Bacteriology, 187, 4401–4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Liao X, Yang Y, Sun J, Li L, Liu B, Yang S, Ma J, Li X, Zhang Q and Liu Y, 2013. Spread of oqxAB in Salmonella enterica serotype Typhimurium predominantly by IncHI2 plasmids. Journal of Antimicrobial Chemotherapy, 68, 2263–2268. 10.1093/jac/dkt209 [DOI] [PubMed] [Google Scholar]
- Li D, Wu C, Wang Y, Fan R, Schwarz S and Zhang S, 2015. Identification of multiresistance gene cfr in methicillin‐resistant Staphylococcus aureus from pigs: plasmid location and integration into a staphylococcal cassette chromosome mec complex. Antimicrobial Agents and Chemotherapy, 59, 3641–3644. 10.1128/aac.00500-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu BT, Zhang XY, Wan SW, Hao JJ, Jiang RD and Song FJ, 2018. Characteristics of Carbapenem‐resistant Enterobacteriaceae in ready‐to‐eat vegetables in China. Frontiers in Microbiology, 9, 1147. 10.3389/fmicb.2018.01147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Liu W, Lv Z, Xia J, Li X, Hao Y, Zhou Y, Yao H, Liu Z, Wang Y, Shen J, Ke Y and Shen Z, 2019. Emerging erm(B)‐Mediated Macrolide Resistance Associated with Novel Multidrug Resistance Genomic Islands in Campylobacter. Antimicrobial Agents and Chemotherapy, 63. 10.1128/AAC.00153-19 [DOI] [PMC free article] [PubMed]
- Loncaric I, Lepuschitz S, Ruppitsch W, Trstan A, Andreadis T, Bouchlis N, Marbach H, Schauer B, Szostak MP, Feßler AT, Künzel F, Licka T, Springer B, Allerberger F, Monecke S, Ehricht R, Schwarz S and Spergser J, 2019. Increased genetic diversity of methicillin‐resistant Staphylococcus aureus (MRSA) isolated from companion animals. Veterinary Microbiology, 235, 118–126. 10.1016/j.vetmic.2019.06.013 [DOI] [PubMed] [Google Scholar]
- Lozano C, Fernández‐Fernández R, Ruiz‐Ripa L, Gómez P, Zarazaga M and Torres C, 2020. Human mecC‐Carrying MRSA: clinical implications and risk factors. Microorganisms, 8. 10.3390/microorganisms8101615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luangtongkum T, Jeon B, Han J, Plummer P, Logue CM and Zhang Q, 2009. Antibiotic resistance in Campylobacter: emergence, transmission and persistence. Future Microbiology, 4, 189–200. 10.2217/17460913.4.2.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk‐In S, Pulsrikarn C, Bangtrakulnonth A, Chatsuwan T and Kulwichit W, 2017. Occurrence of a novel class 1 integron harboring qnrVC4 in Salmonella Rissen. Diagnostic Microbiology and Infectious Disease, 88, 282–286. [DOI] [PubMed] [Google Scholar]
- Maësaar M, Tedersoo T, Meremaë K and Roasto M, 2020. The source attribution analysis revealed the prevalent role of poultry over cattle and wild birds in human campylobacteriosis cases in the Baltic States. PLoS One, 15. 10.1371/journal.pone.0235841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson‐Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT and Monnet DL, 2012. Multidrug‐resistant, extensively drug‐resistant and pandrug‐resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clinical Microbiology and Infection, 18, 268–281. [DOI] [PubMed] [Google Scholar]
- Mališová L, Jakubů V, Musílek M, Kekláková J and Žemličková H, 2020. Phenotype and Genotype Characteristics of Staphylococcus aureus Resistant to Methicillin/Oxacillin Carrying Gene mecC in the Czech Republic from 2002 to 2017. Microbial Drug Resistance, 26, 918–923. 10.1089/mdr.2019.0319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maraolo AE, Giaccone A, Gentile I, Saracino A and Bavaro DF, 2021. Daptomycin versus Vancomycin for the Treatment of Methicillin‐Resistant Staphylococcus aureus Bloodstream Infection with or without Endocarditis: A Systematic Review and Meta‐Analysis. Antibiotics (Basel), 10. 10.3390/antibiotics10081014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez JL, 2014. General principles of antibiotic resistance in bacteria, Drug Discovery Today. Technologies, 11, 33–39. 10.1016/j.ddtec.2014.02.001 [DOI] [PubMed] [Google Scholar]
- Mascaro V, Squillace L, Nobile CG, Papadopoli R, Bosch T, Schouls LM, Casalinuovo F, Musarella R and Pavia M, 2019. Prevalence of methicillin‐resistant Staphylococcus aureus (MRSA) carriage and pattern of antibiotic resistance among sheep farmers from Southern Italy. Infect Drug Resist, 12, 2561–2571. 10.2147/idr.S211629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslikowska JA, Walker SAN, Elligsen M, Mittmann N, Palmay L, Daneman N and Simor A, 2016. Impact of infection with extended‐spectrum beta‐lactamase‐producing Escherichia coli or Klebsiella species on outcome and hospitalization costs. Journal of Hospital Infection, 92, 33–41. 10.1016/j.jhin.2015.10.001 [DOI] [PubMed] [Google Scholar]
- Matuschek E, Åhman J, Webster C and Kahlmeter G, 2017. Evaluation of five commercial MIC methods for colistin antimicrobial susceptibility testing for Gram‐negative bacteria. Poster session presented at: 27th European Congress of Clinical Microbiology and Infectious Diseases22–25 April 2017, Vienna, Austria. [Google Scholar]
- Mayerhofer B, Stöger A, Pietzka AT, Fernandez HL, Prewein B, Sorschag S, Kunert R, Allerberger F and Ruppitsch W, 2015. Improved protocol for rapid identification of certain spa types using high resolution melting curve analysis. PLoS One, 10, e0116713. 10.1371/journal.pone.0116713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuinness WA, Malachowa N and DeLeo FR, 2017. Vancomycin Resistance in Staphylococcus aureus. Yale Journal of Biology Medicine, 90, 269–281. [PMC free article] [PubMed] [Google Scholar]
- McManus BA, Aloba BK, Earls MR, Brennan GI, O'Connell B, Monecke S, Ehricht R, Shore AC and Coleman DC, 2021. Multiple distinct outbreaks of Panton‐Valentine leucocidin‐positive community‐associated meticillin‐resistant Staphylococcus aureus in Ireland investigated by whole‐genome sequencing. Journal of Hospital Infection, 108, 72–80. 10.1016/j.jhin.2020.11.021 [DOI] [PubMed] [Google Scholar]
- Medialdea Carrera R, Melillo T, Rocco G and Borg M, 2020. Investigation of an outbreak of ESBL‐producing Salmonella Kentucky in humans linked to poultry in Malta, 2013–2020. In: Proceedings of the European Scientific Conference on Applied Infectious Disease Epidemiology (ESCAIDE); 24‐27 November 2020; Online. ECDC, 2020. Available online: https://www.escaide.eu/sites/default/files/documents/Escaide2020‐abstractbook.pdf
- Mesa Varona O, Chaintarli K, Muller‐Pebody B, Anjum MF, Eckmanns T, Norstrom M, Boone I and Tenhagen BA, 2020. Monitoring antimicrobial resistance and drug usage in the human and livestock sector and foodborne antimicrobial resistance in Six European Countries. Infectious Drug Resistance, 13, 957–993. 10.2147/IDR.S237038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monecke S, Coombs G, Shore AC, Coleman DC, Akpaka P, Borg M, Chow H, Ip M, Jatzwauk L, Jonas D, Kadlec K, Kearns A, Laurent F, O'Brien FG, Pearson J, Ruppelt A, Schwarz S, Scicluna E, Slickers P, Tan HL, Weber S and Ehricht R, 2011. A field guide to pandemic, epidemic and sporadic clones of methicillin‐resistant Staphylococcus aureus. PLoS One, 6, e17936. 10.1371/journal.pone.0017936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- More E, Ayats T, Ryan PG, Naicker PR, Keddy KH, Gaglio D, Witteveen M and Cerda‐Cuellar M, 2017. Seabirds (Laridae) as a source of Campylobacter spp., Salmonella spp. and antimicrobial resistance in South Africa. Environmental Microbiology, 10.1111/1462-2920.13874 [DOI] [PubMed] [Google Scholar]
- Mouftah SF, Cobo‐Diaz JF, Alvarez‐Ordonez A, Elserafy M, Saif NA, Sadat A, El‐Shibiny A and Elhadidy M, 2021. High‐throughput sequencing reveals genetic determinants associated with antibiotic resistance in Campylobacter spp. from farm‐to‐fork. PLoS One, 16, e0253797. 10.1371/journal.pone.0253797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourkas E, Florez‐Cuadrado D, Pascoe B, Calland JK, Bayliss SC, Mageiros L, Meric G, Hitchings MD, Quesada A, Porrero C, Ugarte‐Ruiz M, Gutierrez‐Fernandez J, Dominguez L and Sheppard SK, 2019. Gene pool transmission of multidrug resistance among Campylobacter from livestock, sewage and human disease. Environmental Microbiology, 21, 4597–4613. 10.1111/1462-2920.14760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen HL, Thomsen PK, Litrup E, Torpdahl M, Overballe‐Petersen S, Hansen F, Christensen AKS and Hasman H, 2021. A case of blaNDM‐1‐positive Salmonella Kottbus, Denmark, November 2020. Eurosurveillance, 26. 10.2807/1560-7917.ES.2021.26.26.2100569 [DOI] [PMC free article] [PubMed]
- Nógrády N, Király M, Davies R and Nagy B, 2012. Multidrug resistant clones of Salmonella Infantis of broiler origin in Europe. International Journal of Food Microbiology, 157, 108–112. [DOI] [PubMed] [Google Scholar]
- Olaitan AO, Morand S and Rolain J‐M, 2014. Mechanisms of polymyxin resistance: acquired and intrinsic resistance in bacteria. Frontiers in Microbiology, 643, 18. 10.3389/fmicb.2014.00643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi A, Crump JA, Glass K, Howden BP, Furuya‐Kanamori L, Vilkins S, Gray DJ and Kirk MD, 2018. Health outcomes from multidrug‐resistant Salmonella infections in high‐income countries: a systematic review andmeta‐analysis. Foodborne Pathogens and Disease, 15. 10.1089/fpd.2017.2403 [DOI] [PubMed] [Google Scholar]
- Paterson GK, Morgan FJ, Harrison EM, Peacock SJ, Parkhill J, Zadoks RN and Holmes MA, 2014. Prevalence and properties of mecC methicillin‐resistant Staphylococcus aureus (MRSA) in bovine bulk tank milk in Great Britain. Journal of Antimicrobial Chemotherapy, 69, 598–602. 10.1093/jac/dkt417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly N, Wichmann‐Schauer H, Ballhausen B, Torres Reyes N, Fetsch A and Tenhagen BA, 2019. Detection and quantification of methicillin‐resistant Staphylococcus aureus in fresh broiler meat at retail in Germany. International Journal of Food Microbiology, 292, 8–12. 10.1016/j.ijfoodmicro.2018.11.025 [DOI] [PubMed] [Google Scholar]
- Pauly N, Hammerl JA, Schwarz S, Grobbel M, Meemken D, Malorny B, Tenhagen B‐A, Kaesbohrer A and Irrgang A, 2021. Co‐occurrence of the blaVIM‐1 and blaSHV‐12 genes on an IncHI2 plasmid of an Escherichia coli isolate recovered from German livestock. Journal of Antimicrobial Chemotherapy, 76, 531–533. 10.1093/jac/dkaa436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergola S, Franciosini MP, Comitini F, Ciani M, De Luca S, Bellucci S, Menchetti L and Casagrande Proietti P, 2017. Genetic diversity and antimicrobial resistance profiles of Campylobacter coli and Campylobacter jejuni isolated from broiler chicken in farms and at time of slaughter in central Italy. Journal of Applied Microbiology, 122, 1348–1356. 10.1111/jam.13419 [DOI] [PubMed] [Google Scholar]
- Perrin‐Guyomard A, Jouy E, Urban D, Chauvin C, Granier SA, Mourand G, Chevance A, Adam C, Moulin G and Kempf I, 2020. Decrease in fluoroquinolone use in French poultry and pig production and changes in resistance among E. coli and Campylobacter. Veterinary Microbiology, 243. [DOI] [PubMed]
- Petersen A, Stegger M, Heltberg O, Christensen J, Zeuthen A, Knudsen LK, Urth T, Sorum M, Schouls L, Larsen J, Skov R and Larsen AR, 2013. Epidemiology of methicillin‐resistant Staphylococcus aureus carrying the novel mecC gene in Denmark corroborates a zoonotic reservoir with transmission to humans. Clinical Microbiology & Infection, 19, E16–e22. 10.1111/1469-0691.12036 [DOI] [PubMed] [Google Scholar]
- Pimenta AC, Fernandes R and Moreira IS, 2014. Evolution of drug resistance: insight on TEM beta‐lactamases structure and activity and beta‐lactam antibiotics. Mini Reviews in Medicinal Chemistry, 14, 111–122. [DOI] [PubMed] [Google Scholar]
- Pornsukarom S, Patchanee P, Erdman M, Cray PF, Wittum T, Lee J and Gebreyes WA, 2015. Comparative phenotypic and genotypic analyses of Salmonella Rissen that originated from food animals in Thailand and United States. Zoonoses and Public Health, 62, 151–158. [DOI] [PubMed] [Google Scholar]
- Qin S, Wang Y, Zhang Q, Zhang M, Deng F, Shen Z, Wu C, Wang S, Zhang J and Shen J, 2014. Report of ribosomal RNA methylase gene erm(B) in multidrug‐resistant Campylobacter coli . The Journal of Antimicrobial Chemotherapy, 69, 964–968. 10.1093/jac/dkt492 [DOI] [PubMed] [Google Scholar]
- Queenan K, Hasler B and Rushton J, 2016. A one Health approach to antimicrobial resistance surveillance: is there a business case for it? International Journal of Antimicrobial Agents, 48, 422–427. 10.1016/j.ijantimicag.2016.06.014 [DOI] [PubMed] [Google Scholar]
- Rasmussen SL, Larsen J, van Wijk RE, Jones OR, Berg TB, Angen Ø and Larsen AR, 2019. European hedgehogs (Erinaceus europaeus) as a natural reservoir of methicillin‐resistant Staphylococcus aureus carrying mecC in Denmark. PLoS One, 14, e0222031. 10.1371/journal.pone.0222031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci V, Zhang D, Teale C and Piddock LJV, 2020. The O‐Antigen Epitope Governs Susceptibility to Colistin in Salmonella enterica. MBio, 11. e02831–e2919. 10.1128/mBio.02831-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter A, Sting R, Popp C, Rau J, Tenhagen BA, Guerra B, Hafez HM and Fetsch A, 2012. Prevalence of types of methicillin‐resistant Staphylococcus aureus in turkey flocks and personnel attending the animals. Epidemiology and Infection, 140, 2223–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner BM, Schielke A, Didelot X, Kops F, Breidenbach J, Willrich N, Gölz G, Alter T, Stingl K, Josenhans C, Suerbaum S and Stark K, 2017. A combined case‐control and molecular source attribution study of human Campylobacter infections in Germany, 2011–2014 /692/308/174 /692/499 article. Scientific Reports, 7. 10.1038/s41598-017-05227-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossler E, Signorini ML, Romero‐Scharpen A, Soto LP, Berisvil A, Zimmermann JA, Fusari ML, Olivero C, Zbrun MV and Frizzo LS, 2019. Meta‐analysis of the prevalence of thermotolerant Campylobacter in food‐producing animals worldwide. Zoonoses and Public Health, 66, 359–369. 10.1111/zph.12558 [DOI] [PubMed] [Google Scholar]
- Sahibzada S, Abraham S, Coombs GW, Pang S, Hernández‐Jover M, Jordan D and Heller J, 2017. Transmission of highly virulent community‐associated MRSA ST93 and livestock‐associated MRSA ST398 between humans and pigs in Australia. Scientific Reports, 7, 5273. 10.1038/s41598-017-04789-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt S, Stephan R, Huebschke E, Schaefle D, Merz A and Johler S, 2020. DNA microarray‐based characterization and antimicrobial resistance phenotypes of clinical MRSA strains from animal hosts. Journal of Veterinary Science, 21, e54. 10.4142/jvs.2020.21.e54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitt A, Lienen T, Wichmann‐Schauer H, Cuny C and Tenhagen BA, 2020. The occurrence and distribution of livestock‐associated methicillin‐resistant Staphylococcus aureus ST398 on German dairy farms. Journal of Dairy Science, 103, 11806–11819. 10.3168/jds.2020-18958 [DOI] [PubMed] [Google Scholar]
- Sévellec Y, Vignaud ML, Granier SA, Lailler R, Feurer C, Le Hello S, Mistou MY and Cadel-Six S, 2018. Polyphyletic nature of Salmonella enterica serotype Derby and lineage-specific host-association revealed by genome-wide analysis. Frontiers in Microbiology, 9, 891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sévellec Y, Granier SA, Le Hello S, Weill F‐X, Guillier L, Mistou MY and Cadel‐Six S, 2020. Source attribution study of sporadic Salmonella Derby cases in France. Frontiers in Microbiology, 11, 889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore AC, Deasy EC, Slickers P, Brennan G, O'Connell B, Monecke S, Ehricht R and Coleman DC, 2011. Detection of staphylococcal cassette chromosome mec type XI carrying highly divergent mecA, mecI, mecR1, blaZ, and ccr genes in human clinical isolates of clonal complex 130 methicillin‐resistant Staphylococcus aureus. Antimicrobial Agents and Chemotherapy, 55, 3765–3773. 10.1128/aac.00187-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva V, Gabriel SI, Borrego SB, Tejedor‐Junco MT, Manageiro V, Ferreira E, Reis L, Caniça M, Capelo JL, Igrejas G and Poeta P, 2021. Antimicrobial Resistance and Genetic Lineages of Staphylococcus aureus from Wild Rodents: First Report of mecC‐Positive Methicillin‐Resistant S. aureus (MRSA) in Portugal. Animals, 11. 10.3390/ani11061537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skov R, Matuschek E, Sjolund‐Karlsson M, Ahman J, Petersen A, Stegger M, Torpdahl M and Kahlmeter G, 2015. Development of a pefloxacin disk diffusion method for detection of fluoroquinolone‐resistant salmonella enterica. Journal of Clinical Microbiology, 53, 3411–3417. 10.1128/JCM.01287-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skov RL and Monnet DL, 2016. Plasmid‐mediated colistin resistance (mcr‐1 gene): three months later, the story unfolds. Eurosurveillance, 21, pii=30155. Available online: https://www.eurosurveillance.org/images/dynamic/EE/V21N09/art21403.pdf [DOI] [PubMed]
- Sun H, Wan Y, Du P and Bai L, 2019. The epidemiology of monophasic Salmonella Typhimurium. Foodborne Pathogens and Disease, 10.1089/fpd.2019.2676 [DOI] [PubMed] [Google Scholar]
- Szczepanska B, Andrzejewska M, Spica D and Klawe JJ, 2017. Prevalence and antimicrobial resistance of Campylobacter jejuni and Campylobacter coli isolated from children and environmental sources in urban and suburban areas. BMC Microbiology, 17, 80. 10.1186/s12866-017-0991-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taggar G, Attiq Rehman M, Boerlin P and Sory Diarra M, 2020. Molecular Epidemiology of Carbapenemases in Enterobacteriales from Humans, Animals, Food and the Environment. Antibiotics, 9, 693. 10.3390/antibiotics9100693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Dai L, Sahin O, Wu Z, Liu M and Zhang Q, 2017. Emergence of a plasmid‐borne multidrug resistance gene cfr(C) in foodborne pathogen Campylobacter . The Journal of Antimicrobial Chemotherapy, 72, 1581–1588. 10.1093/jac/dkx023 [DOI] [PubMed] [Google Scholar]
- Tate H, Folster JP, Hsu C‐H, Chen J, Hoffmann M, Li C, Morales C, Tyson GH, Mukherjee S, Brown AC, Green A, Wilson W, Dessai U, Abbott J, Joseph L, Haro J, Ayers S, McDermott PF and Zhaoa S, 2017. Comparative analysis of extended‐spectrum‐b‐lactamase CTX‐M‐65‐producing Salmonella enterica serovar Infantis isolates from humans, food animals, and retail chickens in the United States. Antimicrobial Agents and Chemotherapy, 61, e00488–17. 10.1128/aac.00488-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegegne HA, Koláčková I, Florianová M, Gelbíčová T, Madec JY, Haenni M and Karpíšková R, 2021a. Detection and molecular characterisation of methicillin‐resistant Staphylococcus aureus isolated from raw meat in the retail market. J Glob Antimicrob Resist, 26, 233–238. 10.1016/j.jgar.2021.06.012 [DOI] [PubMed] [Google Scholar]
- Tegegne HA, Koláčková I, Florianová M, Wattiau P, Gelbíčová T, Boland C, Madec JY, Haenni M and Karpíšková R, 2021b. Genomic Insights into Methicillin‐Resistant Staphylococcus aureus spa Type t899 Isolates Belonging to Different Sequence Types. Applied and Environment Microbiology, 87. 10.1128/aem.01994-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenhagen BA, Vossenkuhl B, Kasbohrer A, Alt K, Kraushaar B, Guerra B, Schroeter A and Fetsch A, 2014. Methicillin‐resistant Staphylococcus aureus in cattle food chains ‐ prevalence, diversity, and antimicrobial resistance in Germany. Journal of Animal Science, 92, 2741–2751. 10.2527/jas.2014-7665 [DOI] [PubMed] [Google Scholar]
- Thepault A, Meric G, Rivoal K, Pascoe B, Mageiros L, Touzain F, Rose V, Beven V, Chemaly M and Sheppard SK, 2017. Genome‐Wide Identification of Host‐Segregating Epidemiological Markers for Source Attribution in Campylobacter jejuni. Applied and Environmental Microbiology, 83. 10.1128/AEM.03085-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torneke K, Torren‐Edo J, Grave K and Mackay DK, 2015. The management of risk arising from the use of antimicrobial agents in veterinary medicine in EU/EEA countries ‐ a review. Journal of Veterinary Pharmacology and Therapeutics, 38, 519–528. 10.1111/jvp.12226 [DOI] [PubMed] [Google Scholar]
- Touati A, Mairi A, Baloul Y, Lalaoui R, Bakour S, Thighilt L, Gharout A and Rolain J‐M, 2017. First detection of Klebsiella pneumoniae producing OXA‐48 in fresh vegetables from Béjaïa city, Algeria. Journal of Global Antimicrobial Resistance, 9, 17–18. 10.1016/j.jgar.2017.02.006 [DOI] [PubMed] [Google Scholar]
- Van den Berg RR, Dissel S, Rapallini MLBA, van der Weijden CC, Wit B and Heymans R, 2019. Characterization and whole genome sequencing of closely related multidrug‐resistant Salmonella enterica serovar Heidelberg isolates from imported poultry meat in the Netherlands. PLoS One, 14, e0219795. 10.1371/journal.pone.0219795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandendriessche S, Vanderhaeghen W, Soares FV, Hallin M, Catry B, Hermans K, Butaye P, Haesebrouck F, Struelens MJ and Denis O, 2013. Prevalence, risk factors and genetic diversity of methicillin‐resistant Staphylococcus aureus carried by humans and animals across livestock production sectors. Journal of Antimicrobial Chemotherapy, 68, 1510–1516. 10.1093/jac/dkt047 [DOI] [PubMed] [Google Scholar]
- Vossenkuhl B, Brandt J, Fetsch A, Kasbohrer A, Kraushaar B, Alt K and Tenhagen BA 2014. Comparison of spa Types, SCCmec Types and Antimicrobial Resistance Profiles of MRSA Isolated from Turkeys at Farm, Slaughter and from Retail Meat Indicates Transmission along the Production Chain. PLoS One, 9, e96308. 10.1371/journal.pone.0096308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace RL, Bulach D, Valcanis M, Polkinghorne BG, Pingault N, Stylianopoulos A, Givney RC, Glass K and Kirka MD, 2020. Identification of the first erm(B)‐positive Campylobacter jejuni and Campylobacter coli associated with novel multidrug resistance genomic islands in Australia. Journal of Global Antimicrobial Resistance, 23, 311–314. 10.1016/j.jgar.2020.09.009 [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang M, Deng F, Shen Z, Wu C, Zhang J, Zhang Q and Shen J, 2014. Emergence of multidrug‐resistant Campylobacter species isolates with a horizontally acquired rRNA methylase. Antimicrobial Agents and Chemotherapy, 58, 5405–5412. 10.1128/AAC.03039-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb AL, Selinger LB, Taboada EN and Inglis GD, 2018. Subtype‐specific selection for resistance to fluoroquinolones but not to tetracyclines is evident in Campylobacter jejuni isolates from beef cattle in confined feeding operations in Southern Alberta, Canada. Applied and Environment Microbiology, 84. 10.1128/AEM.02713-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendlandt S, Kadlec K, Fessler AT, Monecke S, Ehricht R, van de Giessen AW, Hengeveld PD, Huijsdens X, Schwarz S and van Duijkeren E, 2013. Resistance phenotypes and genotypes of methicillin‐resistant Staphylococcus aureus isolates from broiler chickens at slaughter and abattoir workers. Journal of Antimicrobial Chemotherapy, 68, 2458–2463. 10.1093/jac/dkt239 [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization) , 2006. WHO Drug Information. Available online: https://www.who.int/medicines/publications/druginformation/issues/DrugInfo06vol20_4/en/
- WHO (World Health Organization ‐ Advisory Group on Integrated Surveillance of Antimicrobial Resistance) , 2019. Critically important antimicrobials for human medicine, 6th Revision 2018. 45 pp. Available online: https//www.who.int/foodsafety/publications/antimicrobials‐sixth/en/
- WHO/ECDC (World Health Organization Regional Office for Europe/European Centre for Disease Prevention and Control) , 2022. Antimicrobial resistance surveillance in Europe 2022 – 2020 data.
- Yao H, Shen Z, Wang Y, Deng F, Liu D, Naren G, Dai L, Su CC, Wang B, Wang S, Wu C, Yu EW, Zhang Q and Shen J, 2016. Emergence of a Potent Multidrug Efflux Pump Variant That Enhances Campylobacter Resistance to Multiple Antibiotics. mBio, 7. 10.1128/mBio.01543-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurfluh K, Poirel L, Nordmann P, Klumpp J and Stephan R, 2015. First detection of Klebsiella variicola producing OXA‐181 carbapenemase in fresh vegetable imported from Asia to Switzerland. Antimicrobial Resistance and Infection Control, 4, 38. 10.1186/s13756-015-0080-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Plain language summary


