Abstract
Frailty has traditionally been studied in the elderly population but scarcely in younger individuals. The objective of the present study is to analyze differences according to age in the diagnostic performance of cardiac biomarkers to predict frailty in patients admitted to the hospital for acute heart failure (AHF). A frailty assessment was performed with the SPPB and FRAIL scales (score > 3). We included 201 patients who were divided according to age: those older and younger than 75 years. In the younger group, no biomarker was related to the presence of frailty. This was mainly determined by age and comorbidities. In the elderly group, NT-proBNP was significantly related to the presence of frailty, but none of the baseline characteristics were. The best cut-off point in the elderly group for NT-proBNP was 4000 pg/mL. The area under the curve (AUC) for proBNP for frailty detection was 0.62 in the elderly. Another similar frailty scale, the SPPB, also showed a similar AUC in this group; however, adding the NT-proBNP (one point if NT-proBNP < 4000 pg/mL), it showed a slightly higher yield (AUC 0.65). The addition of biomarkers could improve frailty detection in members of the elderly population who are admitted to the hospital for AHF.
Keywords: biomarkers, heart failure, frailty, elderly, NT-proBNP
1. Introduction
Heart failure (HF) is a growing global health problem and has high incidence and prevalence (1–2% of adults) [1]. The prevalence of HF increases with age, with a prevalence of over 70% in those aged 70 years old or older [2]. In patients with HF, frailty is very common. This condition makes patients more vulnerable to the effects of stressors, independent of age [3], and this usually impacts their management and prognosis. In fact, frailty is associated with a higher risk of hospitalization and a higher risk of death [4]. Therefore, frailty assessment is essential in HF patients.
Frailty is usually focused on physical function, such as in the Fried criteria [5], and on clinical variables, such as in the Rockwood criteria [6], which defines frailty as the result of health deficits. According to these criteria, many scales have been developed to help define frailty, such as the FRAIL scale [7] or the SPPB scale [8], which use clinical and functional variables. However, frailty scales do not consider parameters related to the severity of the underlying disease, such as biomarkers. In fact, it is known that high levels of certain biomarkers, such as ST2 or NT-proBNP, involve a worse prognosis in comorbid frail elderly populations with HF [9]. Other biomarkers, such as Galectin-3, are associated with frailty in elderly patients [10]. Therefore, it would be interesting to evaluate the relation between traditional frailty scales and new biomarkers. Moreover, frailty has traditionally been studied in the elderly population, with this assessment being less frequent in younger individuals.
The purpose of this study was to determine differences in the diagnostic performance of cardiac biomarkers according to age to predict frailty in patients admitted to the hospital for acute HF (AHF).
2. Materials and Methods
2.1. Design and Study Population
We performed a single-center, observational, and cross-sectional study in the University Hospital of Burgos, Spain. We consecutively enrolled all patients with AHF who were admitted to the Cardiology unit of our hospital from July 2020 through May 2021, regardless of age (≥18 years old). AHF was defined according to the European Society of Cardiology guidelines criteria [2]. We excluded those patients with a diagnosis of acute coronary syndrome or stroke in the last 7 days, advanced atrioventricular block at the moment of admission, and systemic disease with a life expectancy of less than 1 year. All of the patients signed written informed consent to participate in the study, and Local Ethics Committee approval was obtained.
2.2. Data Collection and Geriatric Assessment
Data were collected by local investigators during the admission process. Baseline clinical features and echocardiographic and ECG data were studied. A blood sample was collected during the first day of admission, and certain prognostic biomarkers were determined: NT-proBNP, ultrasensitive troponin T, c-reactive protein (CRP), hemoglobin, and estimated glomerural filtration rate (GFR). A geriatric assessment was performed on the last day before discharge using the SPPB and FRAIL scales (Supplementary Figures S1 and S2). We used the FRAIL scale to divide the sample into frail patients (FRAIL score > 3) and non-frail patients.
2.3. Statistical Analysis
We divided the sample into two groups by age according to a cut-off point of 75 years old. We studied the differences in the biomarkers between the frail and non-frail patients in the two age groups using a t-student test. A ROC curve was used to detect the best cut-off point for the biomarkers that showed statistical significance for frailty detection. Finally, we compared the diagnostic performance, which was measured by the area under the curve (AUC), of the SPPB scale to detect frailty in each age group. The results were presented as a number (percentage) for the discrete variables and mean ± standard deviation for the continuous variables. Significant results were deemed statically significant if the p-value < 0.05. Statistical analysis was performed using SPSS Statistics for Windows software, version 20.0 (IBM, Chicago, IL, USA).
3. Results
3.1. Sample Description
We included 201 patients with a mean age of 73 ± 12 years. Of them, 78 (38.6%) were women. In the whole population, 68 (33.7%) of the patients presented frailty defined by a FRAIL score > 3, and the mean FRAIL score was 1.88 ± 1.48. Comorbidities were frequent in our sample, especially hypertension, which was detected in 140 (69.3%) patients, and diabetes, which was detected in 64 (31.7%) patients. There were 30 (14.9%) patients with GFR < 60 mL/min/1.73 m2 and 22 (10.9%) patients with chronic obstructive pulmonary disease. According to the type of HF, 89 (44.1%) patients had HF with a preserved ejection fraction, 27 (13.7%) had HF with a mildly reduced ejection fraction, and 86 (42.6%) had HF with a reduced ejection fraction. HF therapy prescribed before admission is shown in Supplementary Table S1.
The sample comprised 99 patients (49%) who were older than 75 years old and 102 patients who were younger than 75 years old. Frailty according to the FRAIL score criteria was more common in patients over 75 years old than it was in younger patients (2.43 ± 1.48 vs. 1.34 ± 1.28; p < 0.001).
3.2. Analysis of Frailty Determinants in Each Group
In the group of young patients, NT-proBNP, ST2, and CRP were numerically higher in frailty patients, but no biomarker was significantly related to frailty (Table 1). Furthermore, the frailty patients presented a lower value of hemoglobin and worse GFR, no significant differences were observed.
Table 1.
Baseline characteristics in the group of young patients.
| Variable | Frail Patients (n: 17; 16.67%) |
Non-Frail Patients (n: 85; 83.33%) |
p |
|---|---|---|---|
| Age (years old) | 68 ± 5 | 62 ± 9 | <0.001 |
| Women | 6 (35.3%) | 25 (29.4%) | 0.630 |
| Hypertension | 14 (82.4%) | 48 (56.5%) | 0.046 |
| Diabetes | 10 (58.8%) | 21 (24.7%) | 0.005 |
| Atrial fibrillation | 8 (47.1%) | 31 (36.5%) | 0.412 |
| LVEF < 40% | 7 (41.2%) | 41 (48.2%) | 0.595 |
| ST2 (ng/mL) | 128.11 ± 162.54 | 81.92 ± 83.01 | 0.300 |
| Troponin T us (ng/L) | 98.62 ± 246.36 | 73.66 ± 170.82 | 0.620 |
| CRP (mg/L) | 25.03 ± 21.21 | 21.72 ± 34.70 | 0.707 |
| NT-proBNP (pg/mL) | 8704.94 ± 8090.29 | 6028.73 ± 915.02 | 0.161 |
| GFR (mL/min/1.73 m2) | 56.56 ± 23.23 | 63.87 ± 23.74 | 0.142 |
| Hemoglobin (g/dL) | 12.93 ± 2.50 | 13.43 ± 2.32 | 0.417 |
LVEF: left ventricle ejection fraction. Us: ultrasensitive. CRP: C-reactive protein. GFR: glomerular filtration rate.
In this group, frailty was mostly determined by clinical variables (age, hypertension, and diabetes). Frail patients were older (68 ± 5 vs. 62 ± 9 years old), and presented with hypertension more frequently (14 (82.5%) patients vs. 48 (56.5%) patients; p = 0.046) and diabetes (10 (58.8%) patients vs. 21 (24.7%) patients; p = 0.005).
In the group of patients older than 75 years old, only NT-proBNP was related to frailty (12,297.61 ± 13,710.20 pg/mL vs. 7709.40 ± 8374.26 pg/mL; p = 0.046) (Table 2). The rest of the biomarkers presented a non-significant increase in frail patients. No clinical baseline variable had a significant effect on frailty in this group, but frail patients had non-significantly more comorbidities. The best cut-off point for detecting frailty using proBNP was 4000 pg/mL (sensitivity: 78.4%; specificity: 45.8%). The AUC for NT-proBNP was 0.62.
Table 2.
Baseline characteristics in the group of old patients.
| Variable | Frail Patients (n: 51; 51.51%) |
Non-Frail Patients (n: 48; 48.48%) |
p |
|---|---|---|---|
| Age (years old) | 84 ± 5 | 82 ± 5 | 0.069 |
| Women | 22 (43.1%) | 25 (52.1%) | 0.373 |
| Hypertension | 44 (83.3%) | 34 (70.8%) | 0.060 |
| Diabetes | 18 (35.3%) | 15 (31.2%) | 0.670 |
| Atrial fibrillation | 31 (60.8%) | 20 (41.7%) | 0.057 |
| LVEF < 40% | 20 (39.2%) | 17 (35.4%) | 0.696 |
| ST2 (ng/mL) | 108.17 ± 84.16 | 85.49 ± 72.69 | 0.180 |
| Troponin T us (ng/L) | 149.55 ± 401.09 | 130.31 ± 245.94 | 0.776 |
| CRP (mg/L) | 26.30 ± 49.44 | 22.15 ± 35.07 | 0.636 |
| NT-proBNP (pg/mL) | 12,297.61 ± 13,710.20 | 7709.40 ± 8374.26 | 0.046 |
| GFR (mL/min/1.73 m2) | 46.24 ± 18.85 | 51.84 ± 17.23 | 0.127 |
| Hemoglobin (g/dL) | 12.74 ± 2.09 | 12.46 ± 1.85 | 0.480 |
LVEF: left ventricle ejection fraction. Us: ultrasensitive. CRP: C-reactive protein. GFR: glomerular filtration rate.
3.3. Inclusion of Biomarkers in SPPB Scale
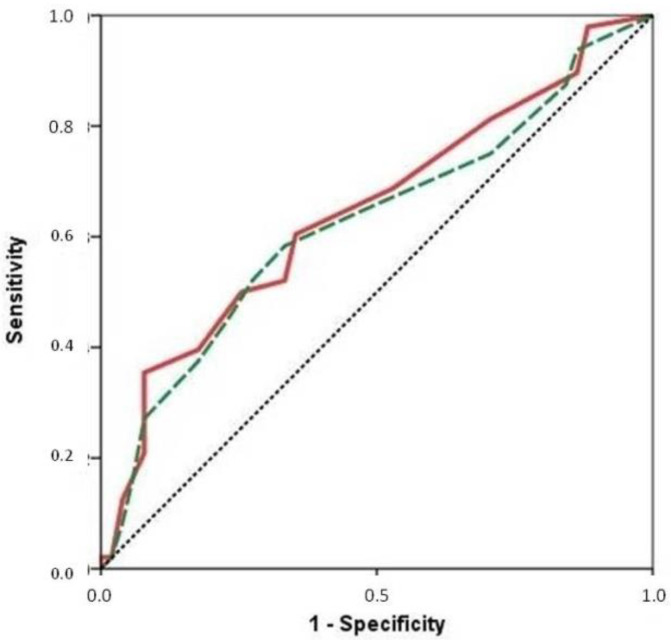
The SPPB scale was significantly related to frailty as measured by the FRAIL test in the young patients (6.59 ± 4.34 points vs. 9.88 ± 2.32 points; p = 0.007) and in the elderly patients (4.10 ± 3.00 points vs. 5.61 ± 3.51 points; p = 0.018). In the young patients, this scale showed an AUC of 0.72 (Supplementary Figure S3), and in the elderly patients, the AUC for frailty detection was 0.63 (FRAIL score > 3). If we add NT-proBNP to the SPPB scale in the older group by adding one point if the patients had an NT-proBNP lower than 4000 pg/mL, then the diagnostic performance of the SPPB-modified scale was slightly better (AUC: 0.65) (Figure 1).
Figure 1.
ROC curve showing the diagnostic performance of the SPPB (dashed green line) and the modified-SPPB (continuous red line) scale to detect frailty defined as FRAIL score > 3 in the older group of patients.
4. Discussion
Frailty is a syndrome that has multiple definitions and a complex underlying pathophysiology. It is a reflection of biological rather than chronological age that contributes to the heterogeneity in clinical outcomes within the elderly patient population [11]. Frailty is particularly relevant in HF patients because it has been demonstrated that functional status, comorbidities, and cognitive function are associated with increased mortality risk in older patients with HF [12]. HF is a leading cause of hospitalization and is associated with poor prognosis in older patients [13]. In fact, frailty associated with HF has mostly been studied in the elderly, and evidence about its implication in young patients is lacking.
In addition, frailty scales consider comorbidities and physical parameters, and both are more frequent in elderly people than they are in younger people. This might result in lower scores on these scales in younger patients than in older patients. In this sense, the FRAIL scale, which was used to divide our sample into two age groups, is used to screen for frailty. It is a validated five-item questionnaire that asks questions about fatigue, resistance, ambulation, comorbidities, and weight loss. It predicts functional decline, mortality, and healthcare utilization [14]. Furthermore, the SPPB assesses the physical functional status in the elderly using balance, speed, and strength measurements. It has demonstrated its capability to predict dependency, institutionalization, hospitalization, and mortality [15].
On the other hand, frailty is sometimes caused by HF itself. Because of this, frailty regression has been demonstrated after a prescription for HF treatment [16] and after valvulopathy correction [17]. Biomarkers are objective measurements of the severity of cardiac disease, mainly HF, and are related to the clinical course of the disease. As such along with GFR, CRP, and hemoglobin, we also considered other biomarkers that are involved in cardiac damage, fibrosis, and stretch, such as troponin T ultrsensible, ST2, and NT-proBNP, respectively. For these reasons, we hypothesize that biomarkers might modulate the effect of chronological age on frailty scales scores.
In our sample of patients admitted to hospital for AHF, the prevalence of frailty measured by the FRAIL test was 33.7%, which is slightly lower than previous evidence [18]. The mean age was 73 ± 12 years old, but about half of patients were younger than 75 years old, similar to other studies [19]. This fact makes it especially important to set up new tools to improve the connection between frailty scores and HF results in both young and old patients.
In the younger group of patients, frailty was mainly determined by age and comorbidities. As we mentioned above, age is related to frailty in HF patients, which is probably due to a higher prevalence of comorbidities and functional disability [20]. The association between hypertension and diabetes with frailty has been studied in depth in previous research. The underlying mechanisms are diverse, but in a few words, it seems that some of the drugs that are used in these patients are involved in muscle weakness [21], and insulin resistance has a role in skeletal muscle function [22].
In the group of older patients, none of the baseline characteristics were related to frailty. This fact could be explained by a more homogenous distribution of comorbidities among older people. Furthermore, the age range in this group was narrower, and frailty might be more influenced by functional status and cognitive impairment than by age.
In younger patients experiencing heart failure, we did not find any association between the biomarkers and frailty, which was probably due to these young patients presenting with a shorter clinical course of HF, and it is likely that the disease has not yet affected physical performance. Cardiac cachexia and functional disability are phenomena that typically affect patients experienced end-stage HF [23]. This also explains the significant increase in NT-proBNP in the frail older patients in our sample. The best cut-off point for detecting frailty using NT-proBNP was 4000 pg/mL. Higher NT-proBNP cut-off values than those that are usually used for HF diagnosis could be more suitable for increasing the sensitivity to better discriminate frailty in these patients.
In the older group of patients, only NT-proBNP was related to frailty. The plasmatic levels of this biomarker increase with the HF stage and predict mortality and readmission among patients aged ≥65 years who have been hospitalized for HF [24]. Previous data have shown a relationship between NT-proBNP with muscle weakness [25] and functional disability [26]. In addition, BNP has been related to grip strength and gait speed [27]. The underling mechanisms that explain this connection have not been analyzed in deoth. First, BNP and NT-proBNP are secreted in response to cardiac stretch, which is increased in cases of muscle mass loss [28]. Second, the release of these biomarkers provokes energy dissipation and oxidative stress on muscular tissues [29]. Finally, BNP stimulates the excessive production of free fatty acids, which impair insulin sensitivity and muscle lipotoxicity [29].
In our study, the SPPB scale was significantly related to the frailty measured by the FRAIL test in both age groups. If we add NT-proBNP to SPPB scale by adding one point if the patient had a NT-proBNP lower than 4000 pg/mL, then the diagnostic performance of the SPPB-modified scale was slightly better. This fact might reflect that using biomarkers in addition to the usual geriatric scales could be useful for determining frailty more accurately in older HF inpatients. This does not mean that the multidimensional nature of frailty syndrome should be reduced to the measurements of some biomarkers, but they might complement geriatric assessment in HF patients.
The main strength of our study was that it analyzed the different performance of frailty scales in young and old patients admitted to the hospital for HF. Similar to us, Woo et al. showed a better relationship between frailty and HF severity in patients older than 75 years old with HF (in this case, only with preserved ejection fraction) than in younger patients [30]. They also connected NT-proBNP and high degrees of frailty, suggesting a possible role for this biomarker in the geriatric assessment of these patients. This association seems to be mediated by higher diastolic dysfunction in older frail patients, so, as mentioned above, HF itself might have a role in the prevalence of frailty.
Our article presents several limitations. First, we used a cross-sectional design in a relatively small sample, so we did not analyze variations in the biomarker levels or frailty scores. Second, we studied the biomarkers during admission for AHF, so the plasmatic levels might have been influenced by the acute severity of the episode. Third, some data such as the time from the HF diagnosis were not available. Fourth, the NT-proBNP assessment was performed at admission, and the geriatric evaluation was performed just before discharge; this might have an implication in clinical practice. Finally, most of the biomarkers are also considered biological markers of aging that could also be associated with the aging process independent of the presence of frailty [31,32]. Nevertheless, we believe that our study reflects the usefulness and feasibility of systematic assessment of the biomarkers in the geriatric evaluation of patients admitted for HF.
5. Conclusions
In younger populations, frailty seems to be more determined by age and comorbidities; however, in the elderly population, it seems that the situation of myocardial and remodeling stress marked by NT-proBNP has an impact on frailty. The addition of biomarkers to traditional geriatric assessments could improve t frailty detection in the members of the elderly population who are admitted to the hospital for AHF.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/biom12020245/s1, Figure S1: SPPB scale, Figure S2: FRAIL scale, Figure S3: ROC-curve showing the diagnostic performance of the SPPB scale to detect frailty defined as FRAIL score > 3 in the younger group of patients, Table S1: HF treatment before admission.
Author Contributions
Conceptualization, J.-A.P.-R.; methodology, J.-A.P.-R. and L.A.-I.; investigation, L.A.-I., E.S.-C., M.-J.G.-S., and I.S.-S.; resources, J.-A.P.-R. and A.M.-M.; data curation, L.A.-I., E.S.-C., M.-J.G.-S.; I.S.-S., and A.M.-M.; laboratory analysis R.S.-M.; writing—original draft preparation, L.A.-I. and A.M.-M.; writing—review and editing, J.-A.P.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Local Ethics Committee of University Hospital of Burgos (protocol code CEIm 2364).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Roger V.L. Epidemiology of Heart Failure. Circ. Res. 2013;113:646–659. doi: 10.1161/CIRCRESAHA.113.300268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McDonagh T.A., Metra M., Adamo M., Gardner R.S., Baumbach A., Böhm M., Burri H., Butler J., Čelutkienė J., Chioncel O., et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 3.Vitale C., Jankowska E., Hill L., Piepoli M., Doehner W., Anker S.D., Lainscak M., Jaarsma T., Ponikowski P., Rosano G.M.C., et al. Heart Failure Association of the European Society of Cardiology Position Paper on Frailty in Patients with Heart Failure. Eur. J. Heart Fail. 2019;21:1299–1305. doi: 10.1002/ejhf.1611. [DOI] [PubMed] [Google Scholar]
- 4.Vidán M.T., Blaya-Novakova V., Sánchez E., Ortiz J., Serra-Rexach J.A., Bueno H. Prevalence and Prognostic Impact of Frailty and Its Components in Non-Dependent Elderly Patients with Heart Failure. Eur. J. Heart Fail. 2016;18:869–875. doi: 10.1002/ejhf.518. [DOI] [PubMed] [Google Scholar]
- 5.Fried L.P., Tangen C.M., Walston J., Newman A.B., Hirsch C., Gottdiener J., Seeman T., Tracy R., Kop W.J., Burke G., et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 6.Rockwood K., Andrew M., Mitnitski A. A Comparison of Two Approaches to Measuring Frailty in Elderly People. J. Gerontol. A Biol. Sci. Med. Sci. 2007;62:738–743. doi: 10.1093/gerona/62.7.738. [DOI] [PubMed] [Google Scholar]
- 7.Van Kan G.A., Rolland Y., Bergman H., Morley J.E., Kritchevsky S.B., Vellas B., On Behalf of the Geriatric Advisory Panel The I.A.N.A. Task Force on Frailty Assessment of Older People in Clinical Practice. J. Nutr. Health Aging. 2008;12:29–37. doi: 10.1007/BF02982161. [DOI] [PubMed] [Google Scholar]
- 8.Guralnik J.M., Simonsick E.M., Ferrucci L., Glynn R.J., Berkman L.F., Blazer D.G., Scherr P.A., Wallace R.B. A Short Physical Performance Battery Assessing Lower Extremity Function: Association with Self-Reported Disability and Prediction of Mortality and Nursing Home Admission. J. Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.M85. [DOI] [PubMed] [Google Scholar]
- 9.Pacho C., Domingo M., Núñez R., Lupón J., Núñez J., Barallat J., Moliner P., de Antonio M., Santesmases J., Cediel G., et al. Predictive Biomarkers for Death and Rehospitalization in Comorbid Frail Elderly Heart Failure Patients. BMC Geriatr. 2018;18:109. doi: 10.1186/s12877-018-0807-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komici K., Gnemmi I., Bencivenga L., Vitale D.F., Rengo G., Di Stefano A., Eleuteri E. Impact of Galectin-3 Circulating Levels on Frailty in Elderly Patients with Systolic Heart Failure. J. Clin. Med. 2020;9:2229. doi: 10.3390/jcm9072229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh M., Alexander K., Roger V.L., Rihal C.S., Whitson H.E., Lerman A., Jahangir A., Nair K.S. Frailty and Its Potential Relevance to Cardiovascular Care. Mayo Clin. Proc. 2008;83:1146–1153. doi: 10.4065/83.10.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sánchez E., Vidán M.T., Serra J.A., Fernández-Avilés F., Bueno H. Prevalence of Geriatric Syndromes and Impact on Clinical and Functional Outcomes in Older Patients with Acute Cardiac Diseases. Heart Br. Card. Soc. 2011;97:1602–1606. doi: 10.1136/hrt.2011.227504. [DOI] [PubMed] [Google Scholar]
- 13.Shah R.U., Tsai V., Klein L., Heidenreich P.A. Characteristics and Outcomes of Very Elderly Patients after First Hospitalization for Heart Failure. Circ. Heart Fail. 2011;4:301–307. doi: 10.1161/CIRCHEARTFAILURE.110.959114. [DOI] [PubMed] [Google Scholar]
- 14.Morley J.E., Malmstrom T.K., Miller D.K. A Simple Frailty Questionnaire (FRAIL) Predicts Outcomes in Middle Aged African Americans. J. Nutr. Health Aging. 2012;16:601–608. doi: 10.1007/s12603-012-0084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guralnik J.M., Ferrucci L., Pieper C.F., Leveille S.G., Markides K.S., Ostir G.V., Studenski S., Berkman L.F., Wallace R.B. Lower Extremity Function and Subsequent Disability: Consistency across Studies, Predictive Models, and Value of Gait Speed Alone Compared with the Short Physical Performance Battery. J. Gerontol. A Biol. Sci. Med. Sci. 2000;55:M221–M231. doi: 10.1093/gerona/55.4.M221. [DOI] [PubMed] [Google Scholar]
- 16.Cacciatore F., Amarelli C., Maiello C., Mattucci I., Salerno G., Di Maio M., Palmieri V., Curcio F., Pirozzi F., Mercurio V., et al. Sacubitril/Valsartan in Patients Listed for Heart Transplantation: Effect on Physical Frailty. ESC Heart Fail. 2020;7:757–762. doi: 10.1002/ehf2.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bertschi D., Moser A., Stortecky S., Zwahlen M., Windecker S., Carrel T., Stuck A.E., Schoenenberger A.W. Evolution of Basic Activities of Daily Living Function in Older Patients One Year After Transcatheter Aortic Valve Implantation. J. Am. Geriatr. Soc. 2021;69:500–505. doi: 10.1111/jgs.16927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denfeld Q.E., Winters-Stone K., Mudd J.O., Gelow J.M., Kurdi S., Lee C.S. The Prevalence of Frailty in Heart Failure: A Systematic Review and Meta-Analysis. Int. J. Cardiol. 2017;236:283–289. doi: 10.1016/j.ijcard.2017.01.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng P.-P., Yao S.-M., Shi J., Wan Y.-H., Guo D., Cui L.-L., Sun N., Wang H., Yang J.-F. Prevalence and Prognostic Significance of Frailty in Gerontal Inpatients with Pre-Clinical Heart Failure: A Subgroup Analysis of a Prospective Observational Cohort Study in China. Front. Cardiovasc. Med. 2020;7:607439. doi: 10.3389/fcvm.2020.607439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murad K., Kitzman D.W. Frailty and Multiple Comorbidities in the Elderly Patient with Heart Failure: Implications for Management. Heart Fail. Rev. 2012;17:581–588. doi: 10.1007/s10741-011-9258-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aprahamian I., Sassaki E., Dos Santos M.F., Izbicki R., Pulgrossi R.C., Biella M.M., Borges A.C.N., Sassaki M.M., Torres L.M., Fernandez Í.S., et al. Hypertension and Frailty in Older Adults. J. Clin. Hypertens. 2018;20:186–192. doi: 10.1111/jch.13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Assar M.E., Laosa O., Rodríguez Mañas L. Diabetes and Frailty. Curr. Opin. Clin. Nutr. Metab. Care. 2019;22:52–57. doi: 10.1097/MCO.0000000000000535. [DOI] [PubMed] [Google Scholar]
- 23.Martínez-Sellés M., Teresa Vidán M., López-Palop R., Rexach L., Sánchez E., Datino T., Cornide M., Carrillo P., Ribera J.M., Díaz-Castro Ó., et al. End-Stage Heart Disease in the Elderly. Rev. Esp. Cardiol. Engl. Ed. 2009;62:409–421. doi: 10.1016/S0300-8932(09)70898-X. [DOI] [PubMed] [Google Scholar]
- 24.Molvin J., Jujic A., Bachus E., Gallo W., Tasevska-Dinevska G., Holm H., Melander O., Fedorowski A., Magnusson M. Cardiovascular Biomarkers Predict Post-Discharge Re-Hospitalization Risk and Mortality among Swedish Heart Failure Patients. ESC Heart Fail. 2019;6:992–999. doi: 10.1002/ehf2.12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikeda M., Honda H., Takahashi K., Shishido K., Shibata T. N-Terminal Pro-B-Type Natriuretic Peptide as a Biomarker for Loss of Muscle Mass in Prevalent Hemodialysis Patients. PLoS ONE. 2016;11:e0166804. doi: 10.1371/journal.pone.0166804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Peet P.G., de Craen A.J.M., Gussekloo J., de Ruijter W. Plasma NT-ProBNP as Predictor of Change in Functional Status, Cardiovascular Morbidity and Mortality in the Oldest Old: The Leiden 85-plus Study. AGE. 2014;36:9660. doi: 10.1007/s11357-014-9660-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graf C.E., Herrmann F.R., Genton L. Relation of Disease with Standardized Phase Angle among Older Patients. J. Nutr. Health Aging. 2018;22:601–607. doi: 10.1007/s12603-018-1034-4. [DOI] [PubMed] [Google Scholar]
- 28.Ohara M., Kohara K., Tabara Y., Ochi M., Nagai T., Igase M., Miki T. Sarcopenic Obesity and Arterial Stiffness, Pressure Wave Reflection and Central Pulse Pressure: The J-SHIPP Study. Int. J. Cardiol. 2014;174:214–217. doi: 10.1016/j.ijcard.2014.03.194. [DOI] [PubMed] [Google Scholar]
- 29.Moro C., Lafontan M. Natriuretic Peptides and CGMP Signaling Control of Energy Homeostasis. Am. J. Physiol.-Heart Circ. Physiol. 2013;304:H358–H368. doi: 10.1152/ajpheart.00704.2012. [DOI] [PubMed] [Google Scholar]
- 30.Woo J., Yang X., Tin Lui L., Li Q., Fai Cheng K., Fan Y., Yau F., Lee A.P.W., Lee J.S.W., Fung E. Utility of the FRAIL Questionnaire in Detecting Heart Failure with Preserved Ejection Fraction. J. Nutr. Health Aging. 2019;23:373–377. doi: 10.1007/s12603-019-1158-1. [DOI] [PubMed] [Google Scholar]
- 31.Cannizzo E.S., Clement C.C., Sahu R., Follo C., Santambrogio L. Oxidative Stress, Inflamm-Aging and Immunosenescence. J. Proteom. 2011;74:2313–2323. doi: 10.1016/j.jprot.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Mitnitski A., Collerton J., Martin-Ruiz C., Jagger C., von Zglinicki T., Rockwood K., Kirkwood T.B.L. Age-Related Frailty and Its Association with Biological Markers of Ageing. BMC Med. 2015;13:161. doi: 10.1186/s12916-015-0400-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.