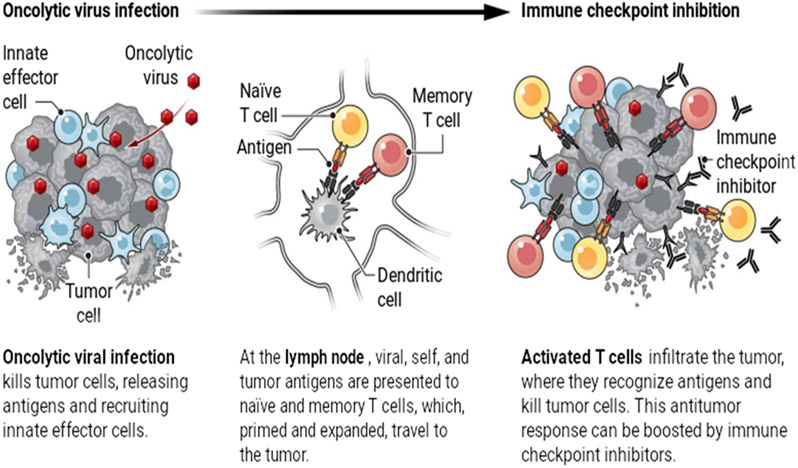
Oncolytic virus (OV) therapy is now something of a misnomer. The initial paradigm was that naturally occurring or genetically engineered viruses would preferentially infect, replicate in, and lyse cancer cells relative to normal cells, leading to selective direct cytotoxicity. The immune system used to be considered detrimental, given its potential to recognize and respond to the virus, prevent its replication, and limit viral spread and tumor cell killing (1). This view has now been largely replaced by a model in which infection of the tumor by the virus makes it more visible to the immune system for recognition and attack. Tumor cell killing by the virus and/or immune effector cells effectively generates an in situ vaccine, with tumor antigens released into a microenvironment modified by viral infection to reverse tumor-induced immune suppression.
By 2020, there were ~100 reported clinical trials using OVs, treating more than 3000 cancer patients. Approvals were granted in different countries for picorna-, adeno-, and herpes viruses, although only one agent was approved by the US Food and Drug Administration (FDA). The growing recognition of the immune basis of oncolytic virotherapy coincided with the success of immunotherapy in the clinic, particularly immune checkpoint inhibitors (ICIs), which block negative regulatory elements that control immune tolerance, specifically the programmed cell death protein 1 (PD-1) axis and cytotoxic T lymphocyte–associated antigen 4 (CTLA-4). These drugs revealed that the immune system can and does respond to cancer, but tumors evolve to escape immune detection through immunoediting (2). However, if the immune system can be reenergized and reactivated, immune control of the tumor can be reestablished.
The number of OVs being tested in preclinical models, with a particular focus on their immunogenicity, has since increased. However, in some ways the field has been struggling with an embarrassment of (potential) riches. It has been unclear how to prioritize the most promising preclinical agents to take forward to clinical testing. Additionally, how to accurately measure the immunogenicity of tumor cell death after OV infection—particularly for viruses that do not readily infect mouse cells (e.g., coxsackie virus, adenovirus, and measles virus) and so cannot be tested reliably in preclinical models—has been a challenge. The variety of OVs covers DNA, single-stranded RNA (ssRNA), and double-stranded RNA (dsRNA) viruses, and the immunobiological consequences after infection of tumor and/or other cells varies, including the signaling pathways activated, such as retinoic acid–inducible gene I (RIG-I)–like receptors for viral RNA and the cyclic GMP–AMP synthase (cGAS)–stimulator of interferon genes (STING) pathway for cytosolic DNA. The degree to which these pathways remain intact within cancer cells, and the relative contribution of the response to infection of nonmalignant cells in the tumor microenvironment (such as cancer-associated fibroblasts, endothelial cells, macrophages, and other immune cell subtypes), adds further complexity to understanding how tumor immunogenicity is generated by OVs.
Much current work is focused on the ability to genetically modify OVs to encode transgenes, such as cytokines, ICIs, tumor-associated antigens, bispecific T cell engagers, and microRNAs, as payloads to boost the antitumor immune response. However, the choice of these encoded transgenes has often been based on limited mechanistic understanding. For example, granulocyte-macrophage colony-stimulating factor (GM-CSF) is expressed in many OVs being clinically tested, even though the evidence supporting the benefit of virally encoded GM-CSF is limited. A further challenge is the inevitable antiviral (alongside antitumor) immune response activated by treatment. Even if viruses to which patients have not been previously exposed are used, neutralizing antiviral antibodies (NAbs) will increase with treatment, potentially compromising repeat administrations, particularly if the route of administration is systemic. Even though intravenously delivered OVs (e.g., adenovirus and vaccinia virus) can infect tumors in patients (3, 4), despite the presence of NAbs in the case of reovirus (5), concerns about sufficient delivery to tumors have led to the administration of many OVs—including talimogene laherparepvec (T-Vec), a clinically approved OV for the treatment of advanced melanoma—being restricted to direct intratumoral injection.
The rapid expansion of ICIs into the clinic has affected the development of OVs and other immunotherapeutics in unexpected ways. Usually, advances in the clinic lag years behind the laboratory discoveries that underpin them, but this has reversed. To improve on the benefit from ICIs, which remains limited to an overall response rate of only 10 to 15% of all cancer patients (although this is higher in certain tumor types and some patient groups), combination strategies have been empirically tested without sufficient understanding of the immunobiology of why they might (or might not) work. Trials of ICI combinations with chemotherapy or radiotherapy are relatively easy to design because the ICI is added to current standard-of-care regimens, and such trials have met with considerable success (6, 7). However, immunotherapy-OV combinations have so far been disappointing. In almost all such studies, the collection and analysis of patient samples has been inadequate and/or unreported, so it remains generally unknown why combinations, which showed promise in preclinical models, have failed to translate into patient benefit. This lack of correlative, translational data particularly applies to negative trials (which are at least as important, in terms of mechanistic understanding, as positive studies), leaving the immune changes that occur in patients after treatment unknown.
There have been few large, randomized controlled trials including OVs so far, and those that have been reported have been discouraging. A study of vocimagene amiretrorepvec (TOCA 511), a replicating retrovirus encoding cytosine deaminase, which converts the prodrug 5-fluorocytosine into 5-fluorouracil, failed in a phase 2/3 study of direct injection into the surgical cavity on first or second resection of high-grade glioma, randomized against standard of care (8). A second phase 2b study involving pexastimogene devacire-pvec (Pexa-Vec), a vaccinia virus encoding GM-CSF, was also unsuccessful when tested in sorafenib-refractory hepatocellular carcinoma (HCC) (9). Both studies have plausible explanations for their lack of success. In the TOCA 511 study, patients had fewer treatment cycles than in earlier phase trials, and for Pexa-Vec, the poor responses of advanced HCC are notoriously difficult to reverse.
However, the trial that best illustrates both the promise and challenges of immunovirotherapy is the testing of T-Vec with the anti-PD1 ICI pembrolizumab (MASTERKEY-265, NCT02263508). T-Vec is a genetically modified type I herpes simplex OV with viral ICP34.5 and ICP47 genes deleted, to enhance tumor tropism and reduce neurovirulence, and encoding GM-CSF. Despite T-Vec being clinically approved, the phase 3 study in advanced melanoma on which its approval was based pre-dated widespread use of ICIs (and BRAF inhibitors) in this disease and had subcutaneous GM-CSF as its control arm. Nevertheless, a randomized study of T-Vec with ipilimumab (an anti-CTLA4 ICI) was promising (10), and clinical analysis of the phase 1b lead-in stage of the randomized pembrolizumab ± T-Vec study was also encouraging, with impressive clinical responses (11).
Alongside clinical data from this early stage of MASTERKEY-265, translational readouts from 21 patients addressed the hypothesis suggested by preclinical data (of this and other ICI-OV combinations) that the virus would turn the immunologically “cold” tumor “hot” and thus prime for ICI efficacy. “Heat” illustrates the variable level of immune activation within a tumor and is described using parameters such as PD-L1 expression, T cell infiltration, and interferon gene signature. However, heat can denote different things, and there remains huge uncertainty around its meaning and importance. Heat could be of the wrong sort, reflecting immune activation directed against the virus rather than immunogenically weaker tumor antigens. Heat also needs to be generated at the right time as well as in the right place. Measuring heat and how its different immunological forms influence antiviral and antitumor immune effectors is a critical area of research that offers the potential to unleash the real value of OV as an immunotherapy (see the figure). However, as a biomarker, immunological heat is currently of limited practical use, with PD-L1 expression being the only manifestation routinely tested and used for clinical decision-making.
Oncolytic viruses are multifaceted tumor killers.
Oncolytic viruses delivered to the tumor not only kill tumor cells directly, but also potentiate antitumor immune responses by releasing antigens and activating inflammatory responses. This may allow immunologically “cold” tumors to become “hot.”
In the early stages of the MASTERKEY-265 trial, detailed patient sample analysis showed that OV injection turned cold tumors hotter (11). Unfortunately, however, the later randomized phase 3 trial was stopped because of clinical futility. Given the encouraging early clinical data, this setback requires explanation. It may, again, be down to the clinical context, in that a larger group of melanoma patients, with limited metastatic disease, did too well with single-agent pembrolizumab (with an expected 5-year survival rate approaching 50%) for the addition of T-Vec to make a significant difference. More provocatively, there were subtle differences in the protocols between the phase 1b and 3 stages of the trial, with T-Vec injections starting 5 weeks earlier in phase 1b, before pembrolizumab, whereas the two treatments began at the same time in phase 3. Hence, in the phase 3 component, the virus may not have had time to heat up the tumor before ICI treatment began. Whatever the reason, this study illustrates, for OVs in particular and immunotherapy more generally, how informative immune analysis can be and that the testing of a sufficient number of patients within a consistent, clinically appropriate trial setting is crucial.
Looking at immunotherapy more broadly, the mechanistic basis of successful treatments is incompletely understood and targets only a small fraction of the pathways that are increasingly studied. For example, stimulatory antibodies targeting positive immune checkpoints, such as CD40 and 4-1BB, have yet to show substantial clinical activity despite preclinical promise, and this is also true for small molecules targeting other inhibitory immune targets, such as indoleamine 2,3-dioxygenase (IDO) (12). Hence, OVs are not alone in struggling to make the transition from early- to late-stage trial success and clinical adoption, although encouraging single-agent phase 1 studies of OVs have recently been reported (13, 14). These trials involved clinically challenging methods of delivery, namely of a herpes simplex OV through intratumoral catheters (13) and neural stem cell delivery of an adenovirus injected into the surgical resection cavity (14), both in high-grade glioma. Limited, correlative immune data were included in these studies, but the challenge of therapy and immune analyses in larger trials (in terms of practicality and outcome) remains.
The progression of a drug from promising early-phase clinical and translational data to more widespread application in patients is always challenging. For immunotherapy, this has been achieved for ICI and chimeric antigen receptor (CAR)–T cells but not yet for OVs, where the need for combination strategies to improve outcomes brings complications. What is required is an imaginative and collaborative approach to early-then-late–phase trials, perhaps focusing on less immunotherapy-responsive diseases than melanoma, such as microsatellite stable colorectal and ovarian cancer, or brain tumors. Studies should maximize translational immune analysis to improve scientific insights, which can then support larger trials to determine true efficacy as well as reverse translation back to the laboratory for truly iterative basic and clinical research. Multiarm trials, which combine experimental arms in the same study, may be one way to cover more ground faster, to fully realize the potential of OVs as powerful and adaptable candidates to turn cold tumors hot, thus improving the benefits of current immunotherapy for more cancer patients.
REFERENCES AND NOTES
- 1.Filley AC, Dey M, Front. Oncol 7, 106 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunn GP et al. , Annu. Rev. Immunol 22, 329 (2004). [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Carbonero R et al. , J. Immunother. Cancer 5, 71 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breitbach CJ et al. , Nature 477, 99 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Samson A et al. , Sci. Transl. Med 10, 422 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paz-Ares L et al. N. Engl. J. Med 379, 2040 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Antonia SJ et al. , N. Engl. J. Med 379, 2342 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Cloughesy TF et al. , JAMA Oncol. 6, 1939 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moehler M et al. , OncoImmunology 8, 1615817 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chesney J et al. , J. Clin. Oncol 36, 1658 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ribas A et al. , Cell 170, 1109 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Long GV et al. , Lancet Oncol. 20, 1083 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Friedman GK et al. , N. Engl. J. Med 384, 1613 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fares J et al. , Lancet Oncol. 22, 1103 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]