Abstract
A better understanding of suicidal ideation (SI), including patterns of SI, may help elucidate links between depression, SI, and suicidal behavior. This study sought to identify trajectories of SI in a large, community-based clinical trial of participants with major depressive disorder (MDD) and to investigate the relationships between these trajectories and predictors of interest, including anxiety and anhedonia. A longitudinal latent class analysis was conducted in 3923 participants enrolled in Level 1 of the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study of citalopram for the treatment of MDD. An unconditional latent class analysis was conducted using SI at study weeks 0, 2, 4, 6, and 9 as the indicators. A multinomial regression was then conducted with SI trajectory as the outcome and anhedonia, severity of depressive symptoms, atypical depression, anxiety, history of suicide attempt, history of substance abuse, history of trauma, and other covariates as the predictors. Four SI trajectories were identified: 1) variable SI; 2) little-to-no SI; 3) persistent SI; and 4) improving SI. Compared to the little-to-no SI trajectory, those with more severe anhedonia were more likely to experience persistent SI, while those with more severe anxiety were more likely to experience improving SI. Factors that distinguish SI trajectories, such as anxiety and anhedonia, may be critical targets for intervention or profiles for prognosis.
Keywords: suicidal ideation, suicide, depression, anxiety, anhedonia, clinical trial
Introduction
In 2019, more than 47,000 individuals died by suicide in the United States, representing a suicide rate of 13.9 per 100,000 individuals (Hedegaard et al., 2021). While both suicidal ideation (SI) and behavior are transdiagnostic in nature, depression remains one of the most commonly reported underlying mental health conditions associated with these phenomena (Bachmann, 2018). Despite a clear association between various risks factors—like depression—with SI and suicidal behavior, it remains difficult for mental health professionals to predict the likelihood of suicide in any particular clinical case.
A better understanding of SI—including patterns of SI such as improvement, persistence, or emergence—may help elucidate the link between depression, SI, and suicidal behavior, and potentially help guide treatment. Previous research on SI trajectories has often focused on large public health datasets, particularly with regard to how suicidal thinking alters over the course of development in adolescent populations. More recent research in adults has explored how shorter-term SI trajectories, such as during a clinical trial or inpatient psychiatric hospitalization, may be related to various risk factors and future suicidal behavior. Such research has suggested that SI trajectories may be distinct from those of non-suicidal depressive symptoms (Batterham et al., 2019), which may help predict future suicide attempts (Lee et al., 2020). The research also suggests that SI trajectories may be associated with certain demographic and clinical characteristics, like previous suicide attempt, help-seeking behavior, and severity of mood symptoms (Kasckow et al., 2016; Kyron et al., 2019; Madsen et al., 2019; Madsen et al., 2016).
This study sought to explore SI trajectories in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study, one of the largest community-based treatment trials for major depressive disorder (MDD) ever conducted in the United States. The STAR*D study followed roughly 4,000 study participants from 41 sites around the country, including specialty care and primary medical care settings. Previous STAR*D studies investigated the time course, risk factors, and predictors of improvement or worsening of SI (Coughlin et al., 2016; Trivedi et al., 2013; Weissman et al., 2021; Zisook et al., 2009), but no latent trajectory analyses for SI have been conducted.
The aims of this analysis were two-fold: (1) to investigate whether specific SI trajectories could be identified in the STAR*D study through latent class analysis, and (2) to explore whether SI trajectories were associated with certain predictors of interest, including previous suicide attempt, severity of depressive symptoms, anxiety, and anhedonia, among other variables. Based on previous research, the first study hypothesis was that five types of SI trajectories would be identified among participants during the first treatment phase (Level 1) of the STAR*D study, in which all participants received the selective serotonin reuptake inhibitor (SSRI) citalopram: 1) individuals with little-to-no SI; 2) individuals with improving SI; 3) individuals with persistent SI; 4) individuals with emerging SI (no SI at baseline, but SI reported during study period); and 5) individuals with variable or fluctuating SI. The second study hypothesis was that predictors of interest would be associated with more severe SI trajectories, particularly persistent SI. Because the latent class analysis does not impose any parametric assumptions about time and uses data from time points across the study period, it can provide a more nuanced description of SI trajectories, including those characterized by high variability, than more commonly-used methods. In addition, applying such an analysis to a large, community-based, and more deeply phenotyped sample population is relatively rare in the suicide literature.
Material and Methods
Participants
De-identified data for this secondary data analysis were taken from Level 1 of the STAR*D study. The original study (NCT00021528) was approved by the institutional review boards of the 41 participating study sites, and participants provided written informed consent. The design and details of the STAR*D study have been discussed in detail elsewhere (Rush et al., 2006). Briefly, the study included 4,041 outpatients with unipolar non-psychotic MDD, aged 18–75 years. Participants with current SI were included in the original study if they could be clinically managed in an outpatient setting. All participants in Level 1 received the SSRI citalopram for at least eight weeks and up to a total of 14 weeks.
Measures
Quick Inventory of Depressive Symptomatology–Clinician-Rated (QIDS-C):
The QIDS-C is a clinician-administered, 16-item rating scale of depressive symptoms. The suicide item of the scale (item 12) has the following response categories: “Does not think of suicide or death” (0), “Feels life is empty or not worth living” (1); “Thinks of suicide/death several times a week for several minutes” (2); and “Thinks of suicide/death several times a day in depth, or has made specific plans for or attempted suicide” (3). The rating scale also includes items regarding sleep-onset insomnia (item 1), mid-nocturnal insomnia (item 2), early-morning insomnia (item 3), and anhedonia (item 13), similarly rated on a scale of 0–3, with higher scores representing greater severity of symptoms. A total QIDS-C score of overall depressive symptomology is reported on a scale of 0–27. For this analysis, the suicide item rating was subtracted from the total score to provide a measure of non-suicidal depressive severity. The QIDS-C was administered at baseline (week 0) and at weeks 2, 4, 6, 9, 12, and 14.
Inventory of Depressive Symptomatology–Clinician Rating (IDS):
The IDS is a 30-item, unabbreviated version of the QIDS-C that provides additional information on depressive symptoms, including symptoms of atypical depression. Previous STAR*D findings defined atypical depression based on threshold rating criteria of specific IDS items for mood reactivity, leaden paralysis, weight gain/increased appetite, hypersomnia, and interpersonal sensitivity (Novick et al., 2005; Stewart et al., 2010).
CIRS (Cumulative Illness Rating Scale):
The CIRS is a multi-item, clinician-rated scale of medical disease burden or comorbidity covering 14 areas of health relating to major organ systems or other health aspects, including cardiac, renal, and neurologic systems. These health areas are rated on a 5-point scale (1-no impairment to organ/system; 5-impairment is life threatening). Though originally validated for use in older adults, the CIRS has also been used in general adult populations (Hudon et al., 2005). For this analysis, the rating for psychological health was subtracted from the total score to provide a baseline measure of medical disease burden.
Psychiatric Diagnostic Screening Questionnaire (PDSQ):
The PDSQ is a self-report measure comprising 126 yes-or-no questions covering major Axis 1 psychiatric disorders. The PDSQ was administered at study entry. For this analysis, a sum of the 10 anxiety items of the generalized anxiety disorder subscale was computed as a measure of anxiety (scale=0–10).
STAR*D Screening Forms:
Screening forms were completed at study entry. Age, gender, and history of suicide attempt were collected from the study screening form. History of substance abuse, diagnosis of personality disorder, and history of trauma exposure were extracted from the psychiatric history form. Additional demographic data, including race, ethnicity, and socio-economic status, were not available at the participant level for this de-identified dataset.
Statistical Analysis
A three-step longitudinal latent class analysis was used to identify SI trajectories over the study period as well as the relationships between these trajectories and predictors of interest (Asparouhov and Muthén, 2014). SI was dichotomized (QIDS-C item 12 score of 0 versus score >0) to allow for the best chance of model convergence. Step 1 of the analysis determined a best-fit latent class model using SI at weeks 0, 2, 4, 6, and 9 as the indicators without additional covariates (an unconditional model). Because the original STAR*D study design allowed participants to stop citalopram at study week 8, and because some participants were missing data, this analysis was restricted to the first nine weeks of the study period. Residual missingness was accounted for using a maximum likelihood approach with a missing at random assumption. In Step 2, each participant’s most likely class and associated measurement error were calculated. In Step 3, a multinomial regression was employed using the parameters obtained from Step 2 and with SI trajectories (latent class) as the outcome variable and the following baseline predictor variables: age, gender, sleep-onset insomnia, mid-nocturnal insomnia, early-morning insomnia, anhedonia, severity of depressive symptoms, atypical depression, anxiety, history of suicide attempt, history of substance abuse, history of trauma, and medical disease burden. Covariates were selected a priori based on previous research and hypothesized relationships with SI trajectories (Kasckow et al., 2016; Kyron et al., 2019; Madsen et al., 2019; Madsen et al., 2016). Because handling of missing covariate data is still an active area of research in latent class analyses, a complete case analysis was performed. Two-tailed t-tests and chi-square tests were used to compare complete cases and those with missing covariate data. As an additional sensitivity analysis, the Step 3 regression was rerun including only individuals with at least two SI measurement timepoints. Mplus version 8.6 was used for all analyses (Muthén and Muthén, 2017). Further information regarding the statistical analysis and missing data can be found in the Supplemental Methods.
Results
In total, 3,923 participants (97.1% of all participants enrolled) had at least one QIDS-C measurement reported during the nine-week study interval and were included in Step 1 of the unconditional latent class analysis to determine SI trajectories. Of these, 3,022 (77.0%) had no missing covariate data and were included in the Step 3 regression analysis comparing SI trajectories. Among these participants, mean age was 41.05 years, 62% were female, and 62.4% reported SI at baseline (Table 1). No statistically significant differences in baseline demographic or clinical characteristics were observed between the complete cases and those missing covariate data.
Table 1.
Baseline Demographic and Clinical Characteristics for Complete and Missing Cases (n=3,923)
| Variable | Complete Cases (n=3,022) |
Missing Cases (n=901) |
t | χ2 | df | p | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | mean | SD | n (%) | mean | SD | |||||||
| Age | 41.05 | ± | 13.13 | 40.11 | ± | 13.50 | −1.87 | 3915 | .06 | |||
| Female | 1877 (62.1) | 587 (65.4) | 3.28 | 1 | .07 | |||||||
| Sleep-onset insomnia | 1.75 | ± | 1.25 | 1.72 | ± | 1.28 | −0.55 | 3918 | .58 | |||
| Mid-nocturnal insomnia | 1.13 | ± | 2.01 | 2.05 | ± | 1.12 | 1.00 | 3919 | .32 | |||
| Early-morning insomnia | 1.15 | ± | 1.21 | 1.19 | ± | 1.24 | 0.97 | 3918 | .33 | |||
| Anhedonia | 2.10 | ± | 0.77 | 2.07 | ± | 0.75 | −1.06 | 3919 | .29 | |||
| Severity of depressive symptoms | 15.55 | ± | 3.13 | 15.54 | ± | 3.19 | −0.13 | 3919 | .90 | |||
| Atypical depression | 528 (17.5) | 107 (14.6) | 3.47 | 1 | .06 | |||||||
| Anxiety | 6.69 | ± | 3.04 | 6.63 | ± | 3.13 | −0.49 | 3858 | .63 | |||
| History of suicide attempt | 508 (16.8) | 137 (15.3) | 1.19 | 1 | .28 | |||||||
| History of substance abuse | 237 (7.8) | 17 (9.2) | 0.43 | 1 | .51 | |||||||
| Medical disease burden | 4.20 | ± | 3.70 | 4.15 | ± | 4.15 | −0.46 | 3921 | .65 | |||
| History of trauma | 1451 (48.0) | 387 (45.3) | 1.94 | 1 | .16 | |||||||
| Baseline suicidal ideation | 1886 (62.4) | 587 (65.3) | 2.48 | 1 | .12 | |||||||
Based on goodness-of-fit statistics and other diagnostic criteria, class interpretability, and our theoretical understanding of SI trajectories from prior research, a four-class model was chosen (Supplemental Table S1). The classes were interpreted as follows: Class 1: fluctuating or variable SI trajectory (membership probability=.076); Class 2: little-to-no SI trajectory (membership probability=.460); Class 3: persistent SI trajectory (membership probability=.222); and Class 4: improving SI trajectory (membership probability=.242) (Table 2A, Figure 1, Supplemental Table S2).
Figure 1. Latent Class Trajectories of Suicidal Ideation in Level 1 of the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) Study.
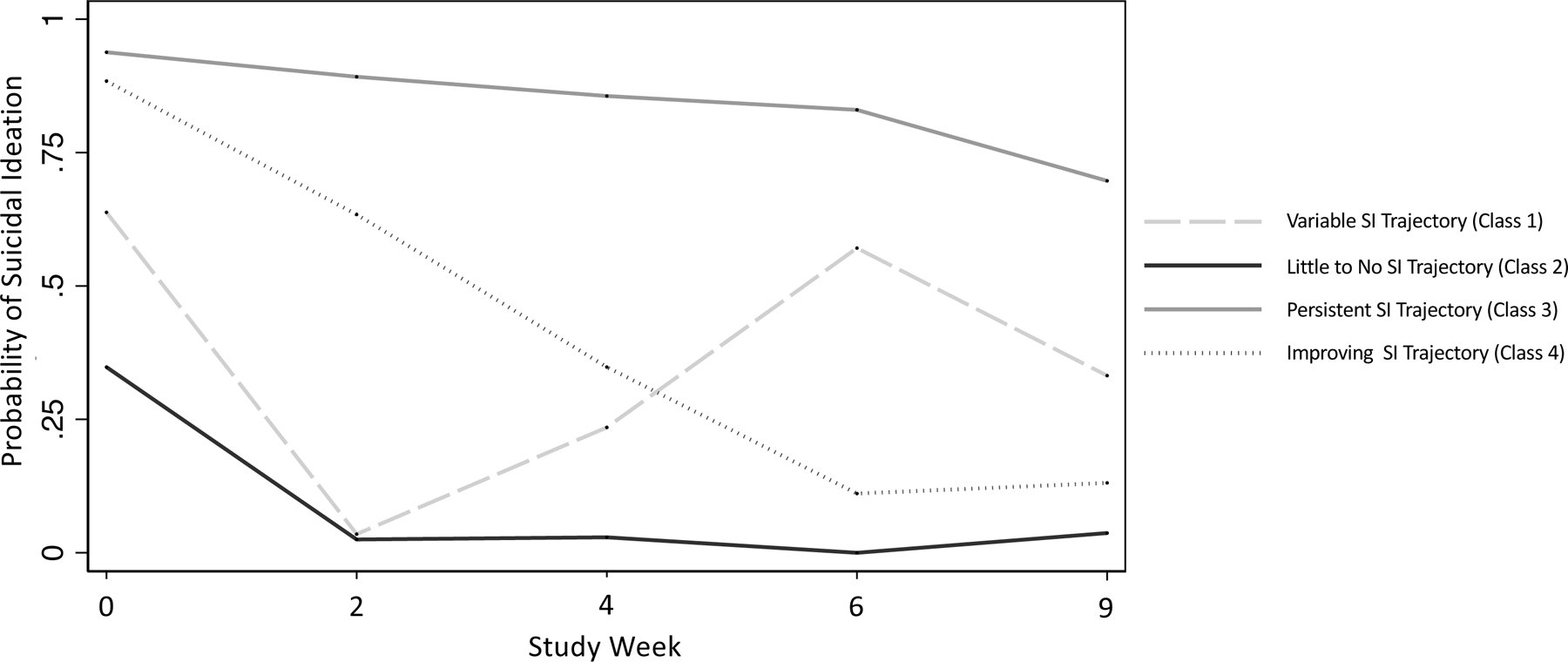
Probability of suicidal ideation (SI) (Quick Inventory of Depressive Symptomatology—Clinician-administered (QIDS-C) item 12 score > 0) by study week (0, 2, 4, 6, and 9) for each latent class (class 1: variable SI trajectory; class 2: little-to-no SI trajectory; class 3: persistent SI trajectory; class 4: improving SI trajectory)
Multinomial regression results comparing predictors of interest across classes are listed in Table 2B; the little-to-no SI trajectory (which had the largest number of participants) served as the reference group. No statistically significant differences were observed between the little-to-no SI trajectory and the fluctuating/variable SI trajectory.
Table 2B.
Conditional Odds Ratios of Predictors of Suicidal Ideation Trajectories (n=3,022)
| Covariate | Variable SI Trajectory (Class 1) | Persistent SI Trajectory (Class 3) | Improving SI Trajectory (Class 4) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| COR | 95% CI | COR | 95% CI | COR | 95% CI | |
| Sleep-onset insomnia | 1.056 | (0.807,1.381) | 0.995 | (0.890,1.113) | 0.917 | (0.781,1.076) |
| Mid-nocturnal insomnia | 0.991 | (0.744,1.318) | 0.921 | (0.813,1.043) | 0.815 | (0.688,0.965) |
| Early-morning insomnia | 1.192 | (0.920,1.545) | 0.979 | (0.878,1.091) | 1.040 | (0.884,1.223) |
| Anhedonia | 0.900 | (0.589,1.376) | 1.454 | (1.164,1.816) | 0.871 | (0.644,1.177) |
| Severity of depressive symptoms | 1.000 | (0.861,1.161) | 1.165 | (1.101,1.232) | 1.168 | (1.070,1.275) |
| History of substance abuse | 0.585 | (0.124,2.759) | 0.992 | (0.644,1.529) | 0.762 | (0.338,1.718) |
| History of suicide attempt | 1.121 | (0.440,2.856) | 3.153 | (2.279,4.362) | 1.340 | (0.773,2.322) |
| Age | 0.977 | (0.949,1.006) | 1.018 | (1.005,1.030) | 0.982 | (0.965,0.999) |
| Female | 0.570 | (0.303,1.071) | 0.442 | (0.340,0.574) | 0.703 | (0.469,1.054) |
| Atypical depression | 0.863 | (0.351,2.119) | 1.026 | (0.753,1.397) | 0.917 | (0.563,1.494) |
| History of trauma | 1.558 | (0.822,2.952) | 1.205 | (0.932,1.558) | 0.996 | (0.678,1.463) |
| Anxiety | 0.947 | (0.850,1.056) | 1.042 | (0.997,1.088) | 1.075 | (1.004,1.151) |
| Medical disease burden | 1.042 | (0.964,1.126) | 1.023 | (0.987,1.061) | 1.063 | (1.003,1.127) |
Note: Estimates are in reference to the little-to-no SI trajectory (Class 2). Abbreviations: Pr (Probability), SE (Standard Error), COR (Conditional Odds Ratio), SI (Suicidal Ideation).
After controlling for other covariates, a 45% increase was observed in the odds of a persistent SI trajectory per one point increase on the four-point anhedonia scale of the QIDS-C (conditional odds ratio (COR)=1.454, 95%CI=1.164–1.816) compared to the little-to-no SI trajectory group. In addition, the odds of a persistent SI trajectory was lower for females (COR=0.442, 95%CI=0.340–0.574) but greater for older participants (COR=1.018, 95%CI=1.005–1.030), those with more severe depressive symptoms (COR=1.16, 95%CI=1.101–1.232), and those with a history of suicide attempt (COR=3.153, 95%CI=2.279–4.362).
After controlling for other covariates, an 8% increase was observed in the odds of an improving SI trajectory per one point increase on the 10-point anxiety scale of the PDSQ (COR=1.075, 95%CI=1.004–1.151) compared to the little-to-no SI trajectory. In addition, the odds of an improving SI trajectory was greater for those with more severe depressive symptoms (COR=1.168, 95%CI=1.070–1.275) and a higher medical disease burden (COR=1.063, 95%CI=1.003–1.127) but lower for older participants (COR=0.982, 95%CI=0.965–0.999) and those with more severe mid-nocturnal insomnia (COR=0.815, 95%CI=0.688–0.965).
Substantially similar results were obtained for the additional sensitivity analysis, which only included participants with at least two SI measurement timepoints in the Step 3 regression (Supplemental Table S3).
Discussion
This secondary analysis of Level 1 data from the STAR*D treatment trial identified four SI trajectories over the nine-week interval studied: a variable SI trajectory, a little-to-no SI trajectory, a persistent SI trajectory, and an improving SI trajectory. Compared to those with a little-to-no SI trajectory, those with more severe anhedonia were more likely to experience persistent SI, and those with more severe anxiety were more likely to experience SI improvement.
Regarding the first hypothesis, the SI trajectories identified by this analysis are similar to those found in other studies and were close to those hypothesized, including the finding of a variable SI trajectory (Batterham et al., 2019; Kasckow et al., 2016; Kyron et al., 2019; Lee et al., 2020; Madsen et al., 2019). Notably, no emerging SI trajectory was observed, as hypothesized and as previously identified in a STAR*D analysis (Zisook et al., 2009). However, our analytic technique purposefully imposed no assumptions regarding the relationship between SI and time so that a variability group could be detected if it existed. Thus, it is possible that those with emerging SI were misclassified into other SI trajectories in this analysis or, conversely, that previous studies misclassified or over-estimated the proportion of those with emerging SI due to parametric or other analytic assumptions.
Regarding the second hypothesis, well-established suicide risk factors included in the analysis—for instance, history of suicide attempt and greater severity of depressive symptoms—were associated with more severe SI trajectories, as expected. Interestingly, higher medical disease burden was associated with an improving—but not persistent—SI trajectory. However, the effect was modest, and research related to antidepressant treatment response in those with medical comorbidities is mixed (Iosifescu et al., 2003; Papakostas et al., 2003; Small et al., 1996). Regarding the findings for anxiety, it should be noted that all participants in this study received citalopram; it is thus possible that the anxiolytic effects of this medication led to improvements in SI in participants with higher baseline anxiety levels. Previous population-based research observed a link between anxiety and SI, as well as suicidal behavior, independent of the effects of depression or other psychiatric conditions (Capron et al., 2012; Sareen et al., 2005). Similarly, pharmacologic and non-pharmacologic clinical trials have demonstrated that anxiety may be an effective target for reducing SI, thus suggesting that decreasing high levels of anxiety can have subsequent anti-suicidal effects (Ballard et al., 2014; Schmidt et al., 2017). In contrast, few effective treatment options exist for anhedonia, which has also been identified as a feature of treatment-resistant depression and a risk factor for suicidal behavior; in this context, anhedonia may be a target for newer agents, such as ketamine and deep brain stimulation, or for anhedonia-focused psychotherapy interventions (Craske et al., 2016; Fawcett et al., 1990; Lally et al., 2014; Schlaepfer et al., 2008).
As regards predictors of interest, no statistically significant differences were noted between participants with a variable SI trajectory and those with a little-to-no SI trajectory. Interestingly, previous studies suggested that SI variability may better predict history of suicidal behavior than SI intensity or duration (Witte et al., 2005), and a large, recent, community-based analysis of the association between SI variability and future suicidal behavior produced similar results (Bloomfield-Clagett et al., submitted). The absence of findings for the variable SI trajectory in this analysis may be due to the relatively smaller size of this sample in the STAR*D dataset. Further studies are warranted to investigate how those with SI variability, a potential indicator of suicidal behavior, may differ from those with other SI trajectories.
This analysis had several limitations. First, although the original study included a large sample size and real-world conditions, it was not designed to answer the specific research questions presented here; thus, the findings should be considered preliminary and interpreted with caution. Second, SI outcome was dichotomized in order to complete the analysis; consequently, it was not possible to identify severe to moderate or mild trajectories of worsening SI or SI improvement. Third, no agreed-upon threshold exists for entropy in a latent class analysis. However, the entropy for all possible models was relatively low, suggesting significant uncertainty in class membership for some participants and classes. Although the regression analysis returned significant results even after taking this uncertainty into account, this may be another reason for the lack of findings in the variable SI group. Finally, the sample comprised adults with MDD enrolled in an SSRI treatment trial, and the findings may not be generalizable beyond this group. However, a substantial proportion of individuals with depression and SI entering treatment would likely receive an SSRI first.
In summary, this secondary analysis of a large, community-based SSRI treatment trial of participants with MDD found multiple classes of SI trajectories that differed based on well-established risk factors, such as history of suicide attempt and severity of depressive symptoms, as well as more novel factors, such as anxiety and anhedonia. All these variables may present targets for intervention or profiles for prognosis. Further studies are warranted to reproduce the above findings and to investigate associations between SI trajectories and more distal outcomes, such as suicidal behavior.
Supplementary Material
Table 2.
Latent Class Membership Probability for Suicidal Ideation Trajectories (n=3,923)
| Class 1 | Class 2 | Class 3 | Class 4 | |
|---|---|---|---|---|
| Pr | 0.076 | 0.460 | 0.222 | 0.242 |
Acknowledgments
The authors thank the 7SE research unit and staff for their support. Ioline Henter (NIMH) provided invaluable editorial assistance.
Funding and Role of Funding Source
Funding for this work was provided in part by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH; ZIAMH002927). The NIMH had no further role in study design; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
Dr. Zarate is listed as a co-inventor on a patent for the use of ketamine in major depression and suicidal ideation; as a co-inventor on a patent for the use of (2R,6R)-hydroxynorketamine, (S)-dehydronorketamine, and other stereoisomeric dehydroxylated and hydroxylated metabolites of (R,S)-ketamine metabolites in the treatment of depression and neuropathic pain; and as a co-inventor on a patent application for the use of (2R,6R)-hydroxynorketamine and (2S,6S)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation, and post-traumatic stress disorders. He has assigned his patent rights to the U.S. government but will share a percentage of any royalties that may be received by the government. The remaining authors have no conflict of interest to disclose, financial or otherwise.
References
- Asparouhov T, Muthén B, 2014. Auxiliary variables in mixture modeling: three-step approaches using Mplus. Struct Equ Modelling 21, 329–341. [Google Scholar]
- Bachmann S, 2018. Epidemiology of suicide and the psychiatric perspective. Int J Environ Res Public Health 15, 1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard ED, Ionescu DF, Vande Voort JL, Niciu MJ, Richards EM, Luckenbaugh DA, Brutsché N, Ameli R, Furey ML, Zarate CA Jr., 2014. Improvement in suicidal ideation after ketamine infusion: relationship to reductions in depression and anxiety. J Psychiatr Res 58, 161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batterham PJ, van Spijker B, Mackinnon AJ, Calear AL, Wong Q, Christensen H, 2019. Consistency of trajectories of suicidal ideation and depression symptoms: evidence from a randomized controlled trial. Depress Anxiety 36, 321–329. [DOI] [PubMed] [Google Scholar]
- Capron DW, Cougle JR, Ribiero JD, Joiner TE, Schmidt NB, 2012. An interactive model of anxiety sensitivity relevant to suicide attempt history and future suicidal ideation. J Psychiatr Res 46, 174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin CG, Jakubovski E, Bloch MH, 2016. Time course and predictors of suicidal ideation during citalopram treatment in the STAR*D trial. J Clin Psychiatry 2016, e1262–e1269. [DOI] [PubMed] [Google Scholar]
- Craske MG, Meuret AE, Ritz T, Treanor M, Dour HJ, 2016. Treatment for anhedonia: a neuroscience driven approach. Depress Anxiety 33, 927–938. [DOI] [PubMed] [Google Scholar]
- Fawcett J, Scheftner WA, Fogg L, Clark DC, Young MA, Hedeker D, Gibbons R, 1990. Time-related predictors of suicide in major affective disorder. Am J Psychiatry 147(9), 1189–1194. [DOI] [PubMed] [Google Scholar]
- Hedegaard H, Curtin MA, Warner M, 2021. Suicide mortality in the United States, 1999–2019. NCHS Data Brief No. 398, February 2021 Available at: https://www.cdc.gov/nchs/data/databriefs/db398-H.pdf. [PubMed]
- Hudon C, Fortin M, Vanasse A, 2005. Cumulative illness rating scale was a reliable and valid index in a family practice context. J Clin Epidemiol 58, 603–608. [DOI] [PubMed] [Google Scholar]
- Iosifescu DV, Nierenberg AA, Alpert JE, Smith M, Bitran S, Dording C, Fava M, 2003. The impact of medical comorbidity on acute treatment in major depressive disorder. Am J Psychiatry 160, 2122–2127. [DOI] [PubMed] [Google Scholar]
- Kasckow J, Youk A, Anderson SJ, Dew MA, Butters MA, Marron MM, Begley AE, Szanto K, Dombrovski AY, Mulsant BH, Lenze EJ, Reynolds CF 3rd, 2016. Trajectories of suicidal ideation in depressed older adults undergoing antidepressant treatment. J Psychiatr Res 73, 96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyron MJ, Hooke GR, Page AC, 2019. Assessing interpersonal and mood factors to predict trajectories of suicidal ideation within an inpatient setting. J Affect Disord 252, 315–324. [DOI] [PubMed] [Google Scholar]
- Lally N, Nugent AC, Luckenbaugh DA, Ameli R, Roiser JP, Zarate CA, 2014. Anti-anhedonic effect of ketamine and its neural correlates in treatment-resistant bipolar depression. Transl Psychiatry 4, e469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DJ, Bryan CJ, Rudd MD, 2020. Longitudinal sucide ideation trajectories in a clinical trial of brief CBT for US military personnel recently discharged from psychiatric hospitalization. Psychiatry Res 293, 113335. [DOI] [PubMed] [Google Scholar]
- Madsen T, Buttenschøn HN, Uher R, Behrendt-Møller I, Perroud N, Maier W, Hauser J, Dernovsek MZ, Henigsberg N, Souery D, Rietschel M, McGuffin P, Aitchison KJ, Mors O, Köhler-Forsberg O, 2019. Trajectories of suicidal ideation during 12 weeks of escitalopram or nortriptyline antidepressant treatment among 811 patients with major depressive disorder. J Clin Psychiatry 80, 18m12575. [DOI] [PubMed] [Google Scholar]
- Madsen T, van Spijker B, Karstoft KI, Nordentoft M, Kerkhof AJ, 2016. Trajectories of suicidal ideation in people seeking web-based help for suicidality: secondary analysis of a Dutch randomized controlled trial. J Med Internet Res 18, e178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO, 2017. Mplus User’s Guide, 8th Edition. Muthén & Muthén, CA. [Google Scholar]
- Novick JS, Stewart JW, Wisniewski SR, Cook IA, Manev R, Nierenberg AA, Rosenbaum JF, Shores-Wilson K, Balasubramani GK, Biggs MM, Zisook S, Rush AJ, STAR*D Investigators, 2005. Clinical and demographic features of atypical depression in outpatients with major depressive disorder: preliminary findings from STAR*D. J Clin Psychiatry 66, 1002–1011. [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Petersen T, Iosifescu DV, Roffi PA, Alpert JE, Rosenbaum JF, Fava M, Nierenberg AA, 2003. Axis III disorders in treatment-resistant major depressive disorder. Psychiatry Res 118, 183–188. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M, 2006. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 163, 1905–1917. [DOI] [PubMed] [Google Scholar]
- Sareen J, Cox BJ, Afifi TO, de Graaf R, Asmundson GJ, ten Have M, Stein MB, 2005. Anxiety disorders and risk for suicidal ideation and suicide attempts: a population-based longitudinal study of adults. Arch Gen Psychiatry 62(11), 1249–1257. [DOI] [PubMed] [Google Scholar]
- Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, Joe AY, Kreft M, Lenartz D, Sturm V, 2008. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology 33(2), 368–377. [DOI] [PubMed] [Google Scholar]
- Schmidt NB, Norr AM, Allan NP, Raines AM, Capron DW, 2017. A randomized clinical trial targeting anxiety sensitivity for patients with suicidal ideation. J Consult Clin Psychology 85, 596–610. [DOI] [PubMed] [Google Scholar]
- Small GW, Birkett M, Meyers BS, Koran LM, Bystritsky A, Nemeroff CB, Fluoxetine Collaborative Study Group, 1996. Impact of physical illness on quality of life and antidepressant response in geriatric major depression. J Am Geriatr Soc 44, 1220–1225. [DOI] [PubMed] [Google Scholar]
- Stewart JW, McGrath PJ, Fava M, Wisniewski SR, Zisook S, Cook I, Nierenberg AA, Trivedi MH, Balasubramani GK, Warden D, Lesser I, Rush AJ, 2010. Do atypical features affect outcome in depressed outpatients treated with citalopram? Int J Neuropsychopharmacol 13, 15–30. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Morris DW, Wisniewski SR, Nierenberg AA, Gaynes BN, Kurian BT, Warden D, Stegman D, Shores-Wilson K, Rush AJ, 2013. Clinical and sociodemographic characteristics associated with suicidal ideation in depressed outpatients. Can J Psychiatry 58(2), 113–122. [DOI] [PubMed] [Google Scholar]
- Weissman CR, Hadas I, Yu D, Jones B, Kong D, Mulsant BH, Blumberger DM, Daskalakis ZJ, 2021. Predictors of change in suicidal ideation across treatment phases of major depressive disorder: analysis of the STAR*D data. Neuropsychopharmacology 46, 1293–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte TK, Fitzpatrick KK, Joiner TEJ, Schmidt NB, 2005. Variability in suicidal ideation: a better predictor of suicide attempts than intensity or duration of ideation? J Affect Disord 88, 131–136. [DOI] [PubMed] [Google Scholar]
- Zisook S, Trivedi MH, Warden D, Lebowitz B, Thase ME, Stewart JW, Moutier C, Fava M, Wisniewski SR, Luther J, Rush AJ, 2009. Clinical correlates of the worsening or emergence of suicidal ideation during SSRI treatment of depression: An examination of citalopram in the STARD study. J Affect Disord 117, 63–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


