Abstract
High oxidative stress, Th1/Th17 immune response, M1 macrophage inflammation, and cell death are associated with cardiovascular diseases. Controlled oxidative stress, Th2/Treg anti-tumor immune response, M2 macrophage inflammation, and survival are associated with cancer. MiR-21 protects against cardiovascular diseases but may induce tumor growth by retaining the anti-inflammatory M2 macrophage and Treg phenotypes and inhibiting apoptosis. Down-regulation of let-7, miR-1, miR-9, miR-16, miR-20a, miR-22a, miR-23a, miR-24a, miR-26a, miR-29, miR-30a, miR-34a, miR-124, miR-128, miR-130a, miR-133, miR-140, miR-143-145, miR-150, miR-153, miR-181a, miR-378, and miR-383 may aid cancer cells to escape from stresses. Upregulation of miR-146 and miR-223 may reduce anti-tumor immune response together with miR-21 that also protects against apoptosis. MiR-155 and silencing of let-7e, miR-125, and miR-126 increase anti-tumor immune response. MiR expression depends on oxidative stress, cytokines, MYC, and TGF-β, and expression of silencing lncRNAs and circ-RNAs. However, one lncRNA or circ-RNA may have opposite effects by targeting several miRs. For example, PVT1 induces apoptosis by targeting miR-16a and miR-30a but inhibits apoptosis by silencing miR-17. In addition, levels of a non-coding RNA in a cell type depend not only on expression in that cell type but also on an exchange of microvesicles between cell types and tumors. Although we got more insight into the function of a growing number of individual non-coding RNAs, overall, we do not know enough how several of them interact in functional networks and how their expression changes at different stages of disease progression.
Keywords: atherosclerosis, cardiomyopathy, cancer, oxidative stress, inflammation, non-coding RNAs
Introduction
Mitochondrial reactive oxygen (ROS), immune response, inflammation, and apoptosis are associated with cardiovascular diseases and cancer [1-7]. Non-coding RNAs regulate these stress conditions [8-10]. They compass small non-coding RNAs or microRNAs or miRs, circular (circ-) RNAs, and long non-coding (lnc)RNAs [11-14]. We recently gave an overview of the relationship between non-coding RNAs, which are deregulated in association with metabolic diseases, and are related to cardiovascular diseases and cancer [15]. Here, we review non-coding RNAs related to cardiovascular diseases and cancer without taking into account a prior relationship with metabolic diseases, focusing on stress conditions mentioned above. Interestingly, we identified a cluster of miRs related to high oxidative stress, Th1/Th17 immune response, M1 macrophage inflammation, and apoptosis in cardiovascular diseases. Importantly, differential expression of this cluster in tumors allowed cancer cells to escape from oxidative stress, anti-tumor immunity and inflammation, and apoptosis. Their expression depends on oxidative stress, cytokines, MYC, and TGF-β. Differences in miR expressions may be due to differential expression of mainly silencing lncRNAs and circ-RNAs. In addition, we show that many of these lncRNAs and circ-RNAs target several miRs, causing even opposite effects on stress conditions.
Oxidative Stress and Inflammation Within Atherosclerosis
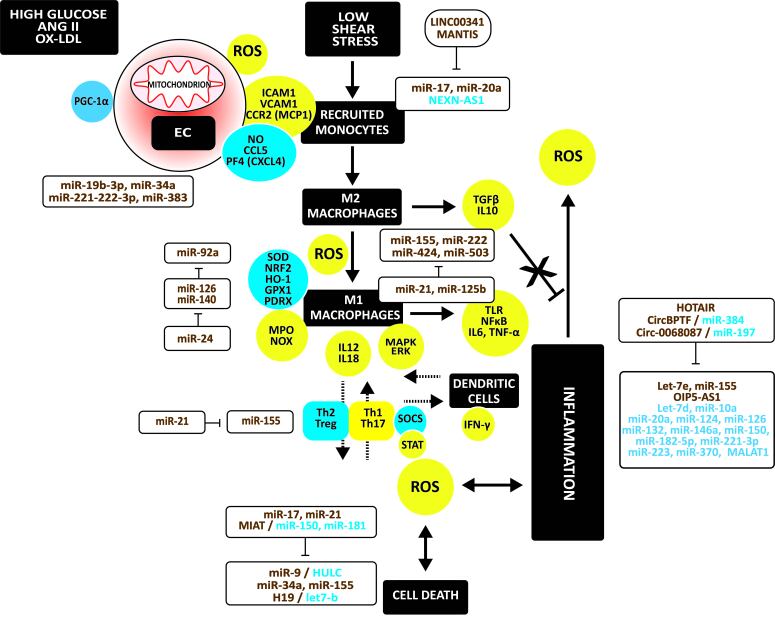
Figure 1 illustrates the mechanisms in atherosclerosis and the involvement of non-coding RNAs in regulating oxidative stress, inflammation, and apoptosis in atherosclerosis. Exposure of endothelial cells (ECs) to high glucose, angiotensinogen (ANG II), oxidized LDL (ox-LDL), and shear stress causes endothelium dysfunction. Dysfunction is due to impaired non-canonical Wnt and phosphatidylinositol 3-kinase (PI3K) / Akt serine/threonine kinase 1 (Akt) / nitric oxide synthase (NOS) signaling [16-18]. Endothelial stress causes adhesion and infiltration of monocytes via vascular cell adhesion molecule 1 (VCAM-1), intercellular adhesion molecule 1 (ICAM-1), and the C-C motif chemokine receptor 2 (CCR2; or MCP-1 receptor). The downregulation of Krüpel-like factor (KLF)-2 and KLF4 induces these adhesion molecules [19]. The binding of monocytes cells to ECs involves C-C motif chemokine ligand 5 (CCL5) and platelet factor-4 (PF4 or CXCL4) [20].
Figure 1.
Oxidative stress, immune response, inflammation, and apoptosis in atherosclerosis. High glucose, ANGII, ox-LDL, and shear stress cause endothelial dysfunction with mitochondrial oxidative stress, releasing ROS. Thus, oxidative stress is due to a shift to more oxidative and less antioxidative factors. Injured endothelium induces adhesion and infiltration of monocytes which differentiate to macrophages. ROS induce M2 to M1 macrophage polarization. In addition, M1 macrophages release proinflammatory cytokines, which induce ROS release and apoptosis in vascular cells. Furthermore, a shift from Th2 and Treg cells to Th1 and Th7 cells occurs, all associated with the activation of DCs. Upregulated regulators are in yellow circles, downregulated ones in blue circles. Upregulated non-coding RNAs are in brown, down-regulated ones in blue.
Usually, infiltrated monocytes differentiate into anti-inflammatory M2 macrophages. They secrete transforming growth factor (TGF)-β and IL10, which counteract vascular inflammation and immune cell activation. However, injured endothelium releases high amounts of ROS, polarizing M2 towards inflammatory M1 macrophages. This polarization involves the activation of toll-like receptors (TLRs) and downstream NFκB and the release of inflammatory cytokines, such as interleukin (IL)-6 and TNF-α [21]. In addition, activated macrophages secrete myeloperoxidase (MPO) and NADPH oxidase (NOX), oxidizing LDL. The disruption of antioxidant defense systems consisting of superoxide dismutases (SODs) [22,23], NRF2 - heme oxygenase (HO)-1 [24], glutathione peroxidase-1 (GPX1) [25], and peroxiredoxin 1 (PRDX1) and PRDX2 [26,27] augment oxidative stress.
The activation of monocytes/macrophages in the vessel wall initiates the innate immune response [28]. Th1 cells exceed the number of Th2 cells in atherosclerotic plaques. Dendritic cells (DCs), activated by cytokines released by M1 macrophages, induce secretion of interferon (IFN)-γ by Th1 cells, no longer counteracted by IL4 produced by Th2 cells. The number of Treg cells producing IL4, IL5, IL10, and IL13, is also lower. Activated DCs release IFN-γ that induces M2 to M1 polarization and secretion of inflammatory cytokines, inducing apoptosis of vascular cells, associated with ROS release.
Non-coding RNAs Regulating Oxidative Stress and Inflammation Within Atherosclerosis
Oxidative Stress
MiR-19b-3p, miR-221-3p, and miR-222-3p repress the proliferator-activated receptor gamma coactivator (PGC)-1α protein expression leading to mitochondrial oxidative stress [29]. MiR-34a and miR-383 mitochondrial biogenesis increase oxidative stress by repressing sirtuin (SIRT)-1, preventing deacetylation of PGC-1α [30,31].
Advanced glycation end products (AGEs) and ox-LDL induce miR-92a, silencing HO-1 [32]. In contrast, miR-126 induces SIRT1 and SOD2 expression, protecting ECs against ROS production and senescence [33]. MiR-140-5p decreased oxidative stress and ROS levels by increasing the protein expression of NRF2 and SIRT2, and HO1 [34]. However, miR-24 may hamper this NRF2 activation [35] (Figure 1).
Inflammation
MiR-17a and miR-20a induce hypoxia-induced infiltration of monocytes and activation of M1 macrophages [36]. In addition, repression of nexilin F-actin binding protein antisense RNA 1 (NEXN-AS1) increases NFκB, monocyte-specific adhesion molecules, and inflammatory cytokines [37]. In contrast, lncRNA LINC00341 and MANTIS repress adhesion molecules [38,39], the latter by targeting KLF2 and KLF4.
MiR-155, miR-222, miR-424, and miR-503 induce M1 macrophage polarization [40]. In contrast, miR-21 and miR-125b retain macrophages in the M2 phenotype [41,42].
Ox-LDL significantly upregulates let-7e that activates NFκB and inflammation. The long intergenic non-protein coding RNA 1826 (LINC01826 or Lnc-MKI67IP-3) may sponge let-7e, suppressing its proinflammatory effects [43]. Ox-LDL-induced miR-155 and the lncRNA Opa-interacting protein five antisense RNA 1 (OIP5-AS1) accelerate ox-LDL-induced EC injury and inflammation via the TLR4/NFκB signaling pathway [44,45]. Furthermore, the silencing of let-7d by lin-28 homolog (LIN28)-b and the decrease of miR-10a, miR-20a, miR-124, miR-126, miR-132, miR-146a, miR-150, miR-182-5p, miR-221-3p, miR-223, and miR-370, and the metastasis-associated lung adenocarcinoma transcript 1 (MALAT) induce inflammation [46-55]. In contrast, lncRNA HOX transcript antisense RNA (lncRNA HOTAIR), the bromodomain PHD finger transcription circular RNA (CircBPTF; or hsa_circ_0000799) targeting miR-384 [56], and the circ-RNA circ_0068087 silencing miR-197 protect against inflammation [56-58].
MiR-21 promotes Treg differentiation [59]. In contrast, miR-155 increased Th17 cells and decreased Th2 and Treg cells [60,61] (Figure 1).
Apoptosis
High miR-9, due to low hepatocellular carcinoma upregulated long non-coding RNA (HULC) [62], miR-34a [63], and miR-155 [64] induce apoptosis. H19 increases apoptosis by silencing let-7b [65]. In contrast, miR-17, miR-21, and MIAT sponging miR-150 and miR-181 protect against apoptosis [66-69] (Figure 1).
Oxidative Stress and Inflammation Within Cardiomyopathy
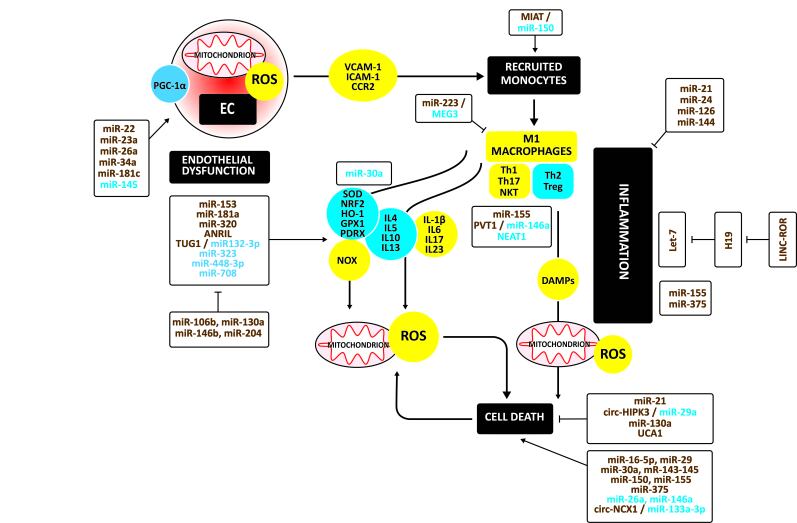
Figure 2 illustrates the mechanisms and the involvement of non-coding RNAs in regulating oxidative stress, inflammation, and apoptosis in the development of cardiomyopathy. Endothelial dysfunction is a hallmark of cardiomyopathy. As in atherosclerotic plaques, mitochondrial dysfunction, due to impaired SIRT1 / PGC-1αsignaling, induces ROS to release. The mitochondrial respiratory chain and oxidases of the NOX family are significant sources of ROS in cardiomyocytes [70]. In addition, low SODs [71], GPX [72], and PRDX [73] and impaired PGC-1α/HO-1 [74] and Keap1-NRF2 signaling [75] increases oxidative stress.
Figure 2.
Oxidative stress, immune response, inflammation, and apoptosis in cardiomyopathy. Endothelial dysfunction is a hallmark of cardiomyopathy. As in atherosclerotic plaques, mitochondrial dysfunction induces ROS to release. Thus, oxidative stress is due to a shift to more oxidative and less antioxidative factors. Again, the initial inflammatory response associated with increased oxidative stress consists of the infiltration of monocytes which differentiate to M1 macrophages secreting inflammatory cytokines. This inflammatory response also augments damage-associated molecular patterns (DAMPs), which trigger inflammation and mitochondrial ROS, inducing cell death. During the later phase of the immune response, T lymphocytes infiltrate. Cardiac T cells undergo a phenotypic change. Th2 and Treg cells decrease whereas Th1 and Th17 cells increase. This shift increases inflammatory cytokines and ROS, inducing cardiac apoptosis. Upregulated regulators are in yellow circles, downregulated ones in blue circles. Upregulated non-coding RNAs are in brown, down-regulated ones in blue.
Again, the initial inflammatory response associated with increased oxidative stress consists of the infiltration of monocytes involving VCAM-1, ICAM1, and CCR2 [76]. The infiltrated monocytes differentiate to M1 macrophages secreting inflammatory cytokines. This inflammatory response also augments damage-associated molecular patterns (DAMPs) [77-81], which trigger inflammation and mitochondrial ROS, inducing cell death [82].
During the later phase of the immune response, T lymphocytes infiltrate. Cardiac T cells undergo a phenotypic change to induce cardiac injury and remodeling [83]. Th1 and Th17 cells increase, while Th2 and Treg cells decrease. This shift increases inflammatory IL-1β, IL6, IL17, IL23, and decreases anti-inflammatory IL4, IL5, IL10, and IL13 [84-87]. Inflammation is associated with cardiac fibrosis and cardiac apoptosis, typically prevented by Treg cells, which are decreased [88].
Non-coding RNAs Regulating Oxidative Stress and Inflammation Within Cardiomyopathy
Oxidative Stress
MiR-22, miR-23a, miR-26a, and miR-34a increase mitochondrial ROS and cell death, the latter by targeting SIRT1 / PGC-1α [89-92]. MiR-181c disturbed the mitochondrial complex IV increasing ROS production [93]. Down-regulation of miR-145 is associated with mitochondrial dysfunction due to lower SIRT1 [94] (Figure 2).
MiR-153 [95] and miR-320 [96], silencing NRF2, and miR-181a [97], silencing GPX1, increased ROS production, disrupted the mitochondrial structure, and activated the mitochondrial apoptotic pathway. The CDKN2B antisense RNA 1 (ANRIL) and downregulation of miR-448-3p increases NOX expression and ROS level [98,99]. The decrease of miR-323-3p and miR-708 is associated with decreased SOD [100,101]. In the oxidative stress-challenged heart, TUG1 sponges miR-132-3p, epigenetically inhibiting antioxidative PRDX2 and heat shock protein Hsp70 [102].
In contrast, miR-106b, miR-130a, miR-148b, and miR-204 may decrease oxidative stress and improve heart function [103,104] (Figure 2).
Inflammation
Silencing miR-150 by myocardial infarction-associated transcript (MIAT) may increase monocytes’ infiltration [105]. ANG II decreases miR-30a inducing ICAM-1 and VCAM by ECs [106]. Down-regulation of maternally expressed three lncRNA (MEG3) decreased M1 and increased M2 macrophage polarization by upregulating miR-223 [107].
MiR-155 induces Th17 cells [108]. PVT1 was associated with higher autophagy in Treg cells by targeting miR-146a [109]. Conversely, deletion of NEAT1 reduces Treg cells [110].
Let-7 induces inflammation. H19 represses let-7, but miR-146a and long intergenic non-protein coding RNA, a regulator of reprogramming (LINC-ROR), compete out this repression [111,112]. MiR-155 and miR-375 induce inflammation and apoptosis [113,114]. In contrast, miR-21 [115], miR-24 [116], miR-126 [117], and miR-144 [118] protect against inflammation [115-118] (Figure 2).
Apoptosis
MiR-16-5p [119], miR-29a [120], miR-30a-5p [121], miR-143-145 [122], miR-150 [123], and miR-155 [124] increase apoptosis. Down-regulation of miR-26a and mi-146a is associated with increased apoptosis [125,126]. In addition, ROS increases the circular RNA derived from solute carrier family eight-member A1 (SLC8A1 or NCX1; CircNCX1) that promotes cardiomyocyte apoptosis by acting as an endogenous miR-133a-3p sponge [127].
In contrast, miR-21 [128], the hypoxia-induced exosomal homeodomain interacting protein kinase three circular RNA (circHIPK3), sponging miR-29a [129], and miR-130a [104] inhibit apoptosis. Urothelial cancer-associated one lncRNA (UCA1) protected from mitochondrial and endoplasmatic reticulum oxidative stress [130] (Figure 2).
Overview of Non-coding RNAs Related to Oxidative Stress and Inflammation Within Cardiovascular Diseases Also Related to Cancer
Oxidative Stress
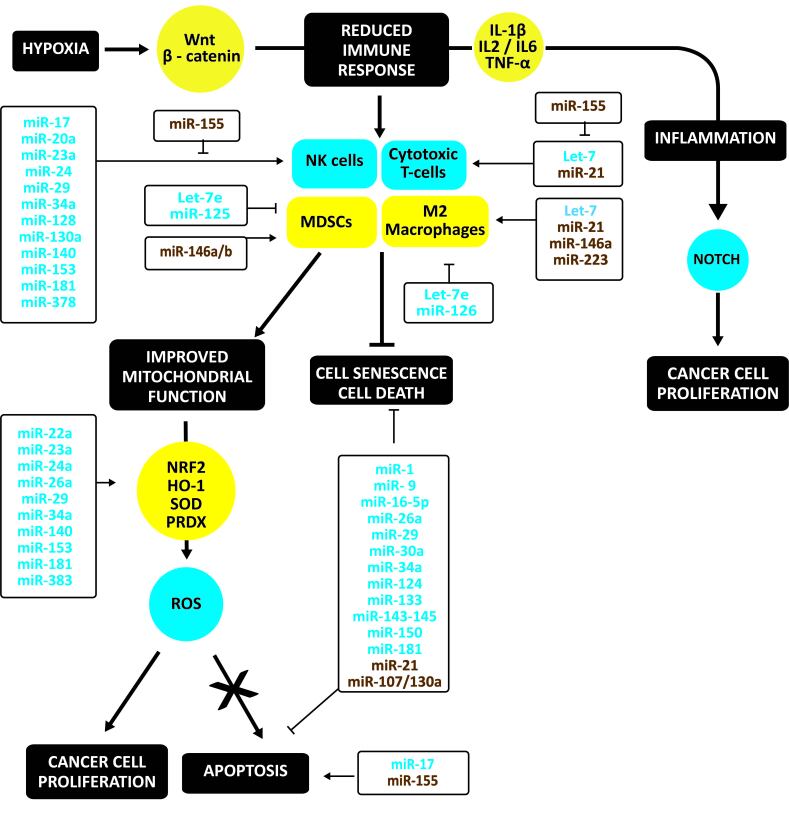
Notably, ROS is increased in cancer cells. However, there is a strict balance of ROS levels in the growing tumor to allow cancer cell proliferation and avoid tumor cell apoptosis. NRF2 regulates the cellular redox status in cancer cells. Besides inducing antioxidant and detoxification genes, NRF2 induces metabolic reprogramming during stress. Increased fumarate inactivates Keap1 and activates NRF2. NRF2 induces antioxidant response genes; for example, HO-1 is essential for retaining colony-forming capacity [131]. In addition, GPX1 is a gatekeeper restraining the oncogenic power of mitochondrial ROS generated by SOD2 [132]. PRDX family is essential in regulating oxidative stress avoiding apoptosis in cancer cells [133,134] (Figure 3).
Figure 3.
Oxidative stress, immune response, inflammation, and apoptosis in cancer. Hypoxia, one of the hallmarks of cancer, reduces the anti-cancer immune responses by activating Wnt/β-catenin. As a result, cytotoxic T cells and NK cells decrease, and immunosuppressive Th2 and Treg cells, myeloid-derived suppressor cells and M2 macrophages increase. This shift augments immunosuppressive cytokines and decreases inflammatory cytokines. Notably, ROS is increased in cancer cells. However, there is a strict balance of ROS levels in the growing tumor to allow cancer cell proliferation and avoid tumor cell apoptosis. This protection is due to a shift from oxidative to antioxidative factors. Upregulated regulators are in yellow circles, downregulated ones in blue circles. Upregulated non-coding RNAs are in brown, down-regulated ones in blue.
Compared to cardiovascular tissues, silencing of miR-22a, miR-23a, miR-24a, miR-29, miR-34a, miR-140, miR-153, miR-181, and miR-383 reduces oxidative stress by de-repressing NRF2, increasing HO-1, SOD, and PDRX [135-146]. Table 1 summarizes candidate silencing lncRNAs and circ-RNAs.
Table 1. Potential Silencing lncRNAs and Circular RNAs.
| MiR | LncRNA | Circular RNA |
| Let-7e | NEAT1 [237], SNHG4 [238] | |
| MiR-9 | CASC2 [239], HULC [240], KCNQ1OT1 [241], NEAT1 [242], TUG1 [243] | FOXO3 [244], MTO1 (hsa_circRNA_0007874, or hsa_circRNA_104135) [245] |
| MiR-16a | PVT1 [246,247] | |
| MiR-17a | MIR17HG [248], BLACAT1 [249], HNF1A-AS1 [250], HOTAIR [251], H19 [252], lincRNAp21 [253], MALAT1 [254], NEAT1 [255], NR2F1-AS1 [256], XIST [257] | ITCH [258], LONP2 [259], MTO1 [260], cSMARCA5 (hsa_circ_0001445) [261], PVT1 [262] |
| MiR-20a | HNF1A-AS1 [263], HOTAIR [264], SNHG16 [265] | PVT1 [266] |
| MiR-22 | MIR22HG [267], HOTAIR [268], H19 [269], LINC00968 [270], MALAT1 [271], MEG3 [272], MIAT [273], NCK1-AS1 [274], PART1 [275] | ITCH [276] |
| MiR-23a | GAS5 [277], MALAT1 [278], MEG3 [279], NEAT1 [280], SNHG5 and SNHG7 [281,282], XIST [283], ZEB1-AS1 [284] | |
| MiR-24 | CASC2 [285], CCAT1 [286], HOXA11-AS [287], NEAT1 [288], SOX21-AS1 [289] | |
| MiR-26a | DLGAP1-AS1 [290], GAN1 [291], GAS5 [292], HCG11 [293], MALAT1 [294], MEG3 [295], MINCR [296], NEAT1 [297], NORAD [298], OIP5-AS1 [299], SNHG5 and SNHG6 [300,301], TUG1 [302], ZNF561-AS1 [303] | Circ-0001146 (derived from miR-26a) [304] |
| MiR-29 | DANCR, GAS5, and SNHG5 [305], H19 [306], MEG3 [307] | |
| MiR-30a | LEF1-AS1 [308,309], NORAD [309] | PVT1 [310] |
| MiR-34a | ARSR [311], CCAT1 [312], FEZF1-AS1 [313], GAS5 [314], HNF1A-AS1 [315], HOTAIR [316], KCNQ1OT1 [317], LINC-ROR [318], MACC1-AS [319], MALAT1 [320], MIAT [321], NEAT1 [322], OIP5-AS1 [323], TUG1 [324], XIST [325] | ANRIL [326], MYLK [327] |
| MiR-128 | MIR4435-2HG [328], HULC [329], MEG3 [330], MIAT [331], OIP5AS1 [332], SNHG3 [333], SNHG16 [334], SNHG22 [335], TUG1 [336], ZNF561-AS1 [303] | PVT1 [337] |
| MiR-140 | CCAT1 [338], H19 [339], MALAT1 [340], MIAT [341], NR2F1-AS1 [342], OIP5-AS1 [343], SNHG16 [344], TUG1 [345] | PVT1 [346] |
| MiR-143 | MIR143HG [347], BLACAT1 [348], CCAT1 [349], HOTAIR [350], H19 [351], MALAT1 [352], NCK1-AS1 [353], OIP5-AS1 [354], SNHG1 [355], SOX2-OT [356], TMPO-AS1 [357], TUG1 [358], UCA1[359], ZEB2-AS1 [360] | FOXM1 [361], FOXO3 [362], PVT1 [363] |
| MiR-150 | BLACAT1 [364], FOXD3-AS1 [365], HULC [366], MIAT [68], NEAT1 [367], PART1 [368], SNHG10 [369], ZFAS1 [370] | PVT1 [371] |
| MiR-153 | FGD5-AS1 [372], HIF1A-AS2 [373], KCNQ1OT1 [374], NEAT1 [375], OIP5-AS1 [376], TTN-AS1 [377], TUG1 [378], XIST [379] | CircPCNXL2 [380] |
| MiR-155 | MIR155HG [381], CCAT1 [382], HOXA11-AS [383], MEG3 [384], MIAT [385], NORAD [386], UCA1 [387], XIST [388] | Circ-CHST15 [389] |
| MiR-181 | CCAT1 [390], MEG3 [391], SNHG6 [183], SNHG7 [392] | |
| MiR-222 | MIR222HG [393], CASC2 [394], DANCR [395], GAS5 [396] | |
| MiR-383 | HOXC13-AS [397], TMPO-AS1 [398] | |
| MiR-424 | MYLK-AS1 [399] | |
| MiR-615 | Circ-ZNF609 [400] |
Inflammation and Anti-Tumor Immunity
Hypoxia, one of the hallmarks of cancer, is caused by an insufficient oxygen supply due to a deficient tumor microcirculation. Hypoxia by activating Wnt/β-catenin reduces the anti-cancer immune responses by (a) reducing survival, the cytolytic and migratory activity of effector cells such as CD4+ cells, CD8+ cytotoxic T cells, natural killer-like T cells, and natural killer (NK) cells, (b) reducing the production and release of effector cytokines, (c) supporting immunosuppressive Treg cells, myeloid-derived suppressor cells and M2 macrophages, (d) increasing the production and release of immunosuppressive cytokines, and (e) inducing the expression of immune checkpoint inhibitors [147]. Wnt ligands stimulate tumor-associated macrophages to produce IL-1β, thus driving systemic inflammation [148]. TAMs are mainly alternatively activated M2 macrophages with immunosuppressive and tumor-promoting capabilities. Hypoxic environment and hypoxia-treated glioma cell supernatants can polarize macrophages toward an M2 phenotype through TGF-β [148,149]. TNF-α derived from M2 tumor-associated macrophages promotes EMT and cancer stemness through the Wnt/β-catenin pathway. Reprogramming TAMs towards classically activated M1 macrophages may thwart tumor-associated immunosuppression and unleash anti-tumor immunity [150].
Suppression of let-7 increased M2 macrophages and abated recruitment of activated cytotoxic T lymphocytes [151]. MiR-21, miR-146a-5p, and miR-223 may promote M2-polarization, but the down-regulation of let-7e and miR-126 increases M1 macrophages [152-158]. MiR-21 decreases, whereas miR-155 stimulates cytotoxic T cells [159-161]. MiR-146a and miR-146b may induce differentiation of monocytes to MDSCs, suppressing the anti-tumor immune response, whereas down-regulation of let-7e and miR-125 increases this response [162].
Effective CD8+ T cells appear to target predominantly tumor-specific neoantigens. To elicit an effective antitumor response, these antigens have to be taken up by dendritic cells (DCs) and cross-presented for CD8+ T cell priming. Then, the antigen must be directly presented for recognition by primed CD8+ T cells and killing [163]. MiR-155 may CD8+ T cell fitness and improve the antitumor activity of adoptively transferred low-affinity tumor-infiltrating lymphocytes, in particular, by rendering them more resistant to the glucose-deprived environment of solid tumors [164].
Downregulation of miR-17 [165], miR-20a [166], miR-23a [167], miR-24 [168], miR-29 [169], miR-34a [170], miR-128 [171], miR-130a [172], miR-140-3p [173], miR-153 [174], miR-181 [175], and miR-378 [176] may suppress NK cytotoxicity. MiR-155 activates NK cells [177].
Apoptosis
Down-regulation of miR-9, miR-16-5p, miR-26a, miR-29, miR-30a, miR-34a, miR-124, miR-133, miR-143-145, miR-150, and miR-181a/b protect tumor cells against apoptosis, whereas upregulation of miR-155 induce apoptosis [178-191]. Specifically, miR-19-3p and miR-200c sensitize cancer cells to apoptosis induced by CD95 (or FAS) [192-194]. However, they are often reduced in tumor cells. The decrease of miR-206, miR-1-3p, and miR-133b upregulates the Fas Apoptotic Inhibitory Molecule (FAIM), which counteracts oxidative stress-induced loss of cell viability [195,196]. As in cardiovascular tissues, high miR-21 [197] and miR-107/miR-130a impede apoptosis in tumors [198]. MiR-21 enriched in exosomes from M2 polarized TAMS can be directly transferred from macrophages to cancer cells to protect them against apoptosis [199]. In contrast, silencing of miR-17 [200] induces apoptosis (Figure 3). Table 1 summarizes potential silencing lncRNAs and circular RNAs.
AGEs stimulate oxidative stress generation through the interaction with a receptor for AGE (RAGE), while oxidative stress promotes AGE’s formation and increases RAGE expression. This crosstalk between the AGE-RAGE system and oxidative stress generation may form a positive feedback loop, thus further increasing the risk for cancers, particularly in patients with diabetes [201,202]. The high-mobility group box 1 protein (HMGB1), a late inflammatory cytokine that signals danger to the immune system through RAGE and TLR, induces the expression of miR-221 and miR-222, associated with higher malignancy scores [203]. MiR-185-5p binds to RAGE, reversing the EMT and migration and invasion of cancer [204,205]. Furthermore, blockage of RAGE with an anti-RAGE antibody suppressed induction of miR-21 [206].
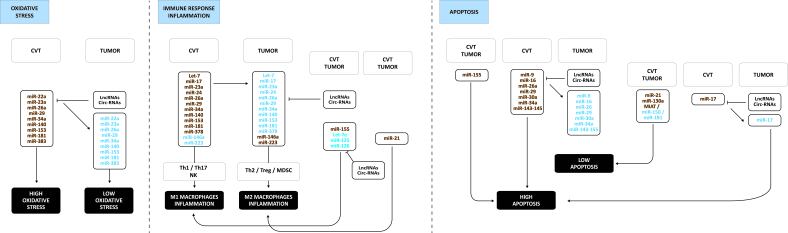
Figure 4 illustrates the main differences in miR expression in tumors compared to cardiovascular tissues explaining the shift from high oxidative stress to low oxidative stress, from Th1/Th2 to Th2/Treg with less activated NK cells, and from M1 macrophage to M2 macrophage inflammation. The downregulation of miRs is possibly due to the overexpression of lncRNAs and circ-RNAs summarized in Table 1.
Figure 4.
Main differences in miR expression in tumors compared to cardiovascular tissues. These changes in miR expression support the shift from high oxidative stress to low oxidative stress, from Th1/Th2 to Th2/Treg with more activated MDSC and less activated NK cells, and from M1 macrophage to M2 macrophage inflammation, ultimately leading to less apoptosis in tumors. The downregulation of miRs is possibly due to the overexpression of lncRNAs and circ-RNAs summarized in Table 1. Upregulated non-coding RNAs are in brown, down-regulated ones in blue.
Discussion
This review focused on miRs related to oxidative stress, immune response related to T cell and MDSC differentiation, inflammation related to M2 or M1 macrophages, and apoptosis. In addition, we identified a cluster of miRs involved in the pathogenesis of cardiometabolic diseases and cancer. This cluster contains: members of let-7 family, miR-1, miR-9, miR-16, miR-17, miR-20a, miR-21, miR-22a, miR-23a, miR-24a, miR-26a, miR-29, miR-30a, miR-34a, miR-128, miR-130a, miR-140, miR-143-145, miR-146a, miR-150, miR-153, miR-155, miR-181 family, miR-221-222, miR-223, miR-378, and miR-383.
MiR-21 protects against cardiovascular diseases by retaining the anti-inflammatory M2 macrophage and Treg phenotypes and inhibiting apoptosis. However, the same effects may induce tumor growth. Transfer of exosomal miR-21 from M2 macrophages to cancer cells may even increase protection against apoptosis. Upregulation of miR-146 and miR-223 may reduce anti-tumor immune response by activating MDSCs and retaining the M2 macrophage phenotype. As in cardiovascular tissues, miR-155 levels are high in tumors. MiR-155 and silencing of let-7e, miR-125, and miR-126 increase anti-tumor immune response.
Most other miRs are downregulated in tumors but upregulated in cardiovascular tissues. Inflammation, oxidative stress, MYC oncogene, and TGF-β regulate miR expression. IL6 increases miR-17, but IFN-γ suppresses miR-17, thereby reverting anti-inflammatory and anti-oxidative action in breast tumors [207-209]. CXCL12 / CXCR4 up-regulate XIST that silences miR-133a-3p, protecting against apoptosis [210]. Mitochondrially encoded COX2 induces methylation of the promoter of let-7, down-regulating let-7 and up-regulating SOX2 [211]. The expression of non-coding RNAs in tumors depends on MYC. MYC induces miR-155 [212] but may silence other miRs by upregulating ANRIL [213], H19, [214-216], and PVT1, enhancing cancer cells’ proliferation. However, PVT1 may induce or inhibit MYC expression [217-220]. MYC directly down-regulates let-7a, let-7d, and let-7g [221], miR-29 [222-226], and miR-34a 4a indirectly by inducing lncRNA-SNHG7 [227,228]. TGF- β reduces miR-29a [229] and miR-34a, thereby up-regulating VEGF and retaining the M2 macrophage phenotype [230,231] or miR-124 [232]. TGF-β1 also decreases miR-133a/b, protecting against apoptosis [233].
These opposite changes in miR expression profiles may be due to differential expression of lncRNAs and circ-RNAs, as discussed above for ANRIL and PVT1. Table 1 shows that other miRs are prone to silencing by lncRNA and circ-RNAs. The same lncRNA and circ-RNAs may obtain the same effect by targeting several miRs. For example, MALAT1 and NEAT1 may reduce oxidative stress and anti-tumor response by targeting miR-23a, miR-24a, miR-26a, and miR-34a. MALAT1 also protects by targeting miR-22 and miR-140, NEAT1 by targeting miR-150 and miR-153.
On the other hand, they may obtain a similar effect by targeting a different miR. For example, NEAT1 may protect against apoptosis by targeting miR-9, MALAT1 by targeting miR-143. In addition, the effects of TUG1 overlap partially with these of MALAT1 and NEAT1 by targeting miR-9, miR-26a, miR-34a, miR-128, miR-140, miR-143, and miR-153. However, the same non-coding RNA may have opposite effects by targeting several miRs. For example, PVT1 may protect against apoptosis by targeting miR-16a and miR-30a but induce apoptosis by targeting miR-17. This non-specificity in targets and function obscures their mechanistic and clinical value. This lack of knowledge is cumbersome because papers identifying a new non-coding RNA are published each month claiming a new function.
Previously, we showed that most of the identified are regulated by adipokines, glucose, insulin, blood pressure, inflammatory cytokines, and ox-LDL related to metabolic diseases, like obesity, type 2 diabetes, and non-alcoholic fatty liver disease. These metabolic diseases increase the overall risk for cardiovascular diseases and cancer [15]. Thus, the identified cluster of miRs will most probably not be specific markers of cardiovascular diseases or cancer. They may, however, be essential to understanding disease mechanisms.
In addition, we have to be aware that levels of non-coding RNAs in a cell type are not only determined by the expression in that cell type but also by exosome-mediated exchange of non-coding RNAs between cell types in a tissue or between tissues [9,15,234,235].
Unfortunately, information about the sequence of changes in expression profiles of non-coding RNAs at different stages of disease progression is lacking. We do not even know which non-coding RNAs are expressed together at the same stages. Indeed, we lack algorithms to determine if non-coding RNAs have any clinical value in addition to phenotypic, therapeutic, behavioral, and social data in a predicting model. Artificial intelligence (AI) or machine-learning methods may be applied to fit vast amounts of expression data combined with phenotypic, therapeutic, behavioral, and social data [236].
Glossary
- AGEs
advanced glycation end products
- Akt
Akt serine/threonine kinase 1
- AMPK
AMP-activated protein kinase
- ANG
angiotensin
- ANRIL
CDKN2B anisense RNA 1
- ARSR
DNA-binding transcriptional repressor ArsR
- ATP
adenosine triphosphate
- BLACAT1
bladder cancerassociated transcript 1
- BMP
bone morphogenetic proteins
- BPTF
bromodomain PHD finger transcription factor
- CASC2
cancer susceptibility 2
- CCAT1
colon cancer-associated transcript 1
- CCL5
C-C motif chemokine ligand 5
- CCL2 (or MCP1)
C-C motif chemokine ligand 2
- CCR2 (or MCP-1 receptor)
C-C motif chemokine receptor 2
- CHST15
carbohydrate sulfotransferase 15
- circ
circular
- CM
cardiomyocyte
- DAMP
damage-associated molecular patterns
- DANCR
differentiation antagonizing non-protein coding RNA
- DC
dendritic cell
- DLGAP1
DLG associated protein 1
- ECs
endothelial cells
- ERK
extracellular-signal-regulated kinase
- FEZF1
FEZ family zinc finger 1
- FGD5
FYVE, RhoGEF and PH domain containing 5
- FOXD3
forkhead box D3
- FOXM1
forkhead box M1
- FOXO
forkhead box O
- GAN1
gigaxonin
- GAS5
growth arrest specific 5
- GPX
glutathione peroxidase
- HCG11
HLA complex group 11
- HIF
hypoxia-inducible factor
- HIPK3
homeodomain interacting protein kinase 3
- HMGB1
high-mobility group box 1 protein
- HNF1A
HNF1 homeobox A
- HO
heme oxygenase
- HOTAIR
homeobox transcript antisense lncRNA
- HOXA11
homeobox 11
- HULC
hepatocellular carcinoma upregulatedlong non-coding RNA
- H19
H19 imprinted maternally expressed transcript
- ICAM-1
intercellular adhesion molecule 1
- IFN
interferon
- IL
interleukin
- IRS
insulin substrate receptor
- ITCH
itchy E3 ubiquitin protein ligase
- KCNQ1OT1
KCNQ1 opposite strand/antisense transcript 1
- KLF
Krüppel-like factor
- LEF1
lymphoid enhancer-binding factor 1
- LIN-28
lin-28 homolog
- lnc-RNA
long-non-coding RNA
- LncRNA-ATB
long non-coding RNA activated by TGF-β
- LINC-ROR
long intergenic non-protein coding RNA regulator of reprogramming
- LONP2
lon peptidase 2
- MACC1
MACC1MET transcriptional regulator
- MALAT1
metastasis-associated lung adenocarcinoma transcript 1
- MAPK
mitogen-activated protein kinase
- MDSC
myeloid-derived suppressor cell
- MEG3
maternally expressed 3 lncRNA
- MIAT
myocardial infarction-associated transcript
- MINCR
MYC-induced long non-coding RNA
- MIRT1
myocardial infarction associated with transcript 1
- MIR22HG
MIR22 host gene
- MTO1
mitochondrial translation optimization 1 homologue
- MPO
myeloperoxidase
- MYLK
myosin light chain kinase
- NCK1
noncatalytic region of tyrosine kinase adaptor protein 1
- NEAT1
nuclear paraspeckle assembly transcript 1
- NEXN-AS1
nexilin F-actin binding protein antisense RNA 1
- NFκB
nuclear factor kappa B
- NK
natural killer
- NLRP3
NLR family pyrin domain containing 3
- NORAD
noncoding RNA activated by DNA damage
- NOS
nitric oxide synthase
- NOX
NADPH oxidase
- NRF2
NF-E2-related factor 2
- NR2F1
nuclear receptor subfamily 2 group F member 1
- OIP5-AS1
Opa-interacting protein five antisense RNA 1
- Ox-LDL
oxidized LDL
- OXPHOS
oxidative phosphorylation
- PART1
prostate androgen regulated transcript 1
- PCNXL2
pecanex 2
- PF4 (or CXCL4)
platelet factor-4
- PPARγ
peroxisome proliferator activated receptor gamma
- PGC-1α
proliferator-activated receptor gamma coactivator-1α
- PI3K
phosphatidylinositol 3-kinase
- PlGF
placental growth factor
- PRDX
peroxiredoxin
- PTEN
phosphatase and tensin homolog
- PVT1
Pvt1 oncogene circular RNA
- ROS
reactive oxygen species
- SIRT
sirtuin
- SLC2A4 (or GLUT4)
glucose transporter solute carrier family two-member four
- SLC8A1 (or NCX1)
solute carrier family eight-member A1
- SMARCA5
SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily a, member 5
- SNHG
small nucleolar RNA host gene
- SOCS
suppressor of cytokine signaling
- SOD
Superoxide dismutases
- SOX
SRY-box transcription factor
- SOX2-OT
SOX2 overlapping transcript
- SPRY4-IT1
Sprouty4-Intron 1
- STAT
signal transducers and activators of transcription
- TF
tissue factor
- TGF
transforming growth factor
- TLR
toll-like receptor
- TMPO
thymopoietin
- TNFα
tumor necrosis factor α
- Treg
regulatory T cell
- TTN
titin
- TUG1
taurine upregulated 1
- UCA1
urothelial cancer-associated one lnc-RNA
- VCAM-1
vascular cell adhesion molecule 1
- VEGF
vascular endothelial growth factor
- XIST
X inactive specific transcript
- ZEB
zinc finger E-box binding homeobox
- ZNF
zinc finger protein
- ZFAS1
ZNFX1 antisense RNA 1
References
- Kattoor AJ, Pothineni NV, Palagiri D, Mehta JL. Oxidative Stress in Atherosclerosis. Curr Atheroscler Rep. 2017. Sep;19(11):42. 10.1007/s11883-017-0678-6 [DOI] [PubMed] [Google Scholar]
- Bugger H, Pfeil K. Mitochondrial ROS in myocardial ischemia reperfusion and remodeling. Biochim Biophys Acta Mol Basis Dis. 2020. Jul;1866(7):165768. 10.1016/j.bbadis.2020.165768 [DOI] [PubMed] [Google Scholar]
- Hayes JD, Dinkova-Kostova AT, Tew KD. Oxidative Stress in Cancer. Cancer Cell. 2020. Aug;38(2):167–97. 10.1016/j.ccell.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017. Jan;541(7637):321–30. 10.1038/nature21349 [DOI] [PubMed] [Google Scholar]
- Rahman MS, Woollard K. Atherosclerosis. Adv Exp Med Biol. 2017;1003:121–44. 10.1007/978-3-319-57613-8_7 [DOI] [PubMed] [Google Scholar]
- El Assar M, Angulo J, Rodríguez-Mañas L. Oxidative stress and vascular inflammation in aging. Free Radic Biol Med. 2013. Dec;65:380–401. 10.1016/j.freeradbiomed.2013.07.003 [DOI] [PubMed] [Google Scholar]
- Greten FR, Grivennikov SI. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity. 2019. Jul;51(1):27–41. 10.1016/j.immuni.2019.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasiadou E, Jacob LS, Slack FJ. Non-coding RNA networks in cancer. Nat Rev Cancer. 2018. Jan;18(1):5–18. 10.1038/nrc.2017.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsmans M, Holvoet P. MicroRNA-containing microvesicles regulating inflammation in association with atherosclerotic disease. Cardiovasc Res. 2013. Oct;100(1):7–18. 10.1093/cvr/cvt161 [DOI] [PubMed] [Google Scholar]
- Bei Y, Yang T, Wang L, Holvoet P, Das S, Sluijter JP, et al. Circular RNAs as Potential Theranostics in the Cardiovascular System. Mol Ther Nucleic Acids. 2018. Dec;13:407–18. 10.1016/j.omtn.2018.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004. Jan;116(2):281–97. 10.1016/S0092-8674(04)00045-5 [DOI] [PubMed] [Google Scholar]
- Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013. Mar;495(7441):333–8. 10.1038/nature11928 [DOI] [PubMed] [Google Scholar]
- Kowalczyk MS, Higgs DR, Gingeras TR. Molecular biology: RNA discrimination. Nature. 2012. Feb;482(7385):310–1. 10.1038/482310a [DOI] [PubMed] [Google Scholar]
- Navickas R, Gal D, Laucevičius A, Taparauskaitė A, Zdanytė M, Holvoet P. Identifying circulating microRNAs as biomarkers of cardiovascular disease: a systematic review. Cardiovasc Res. 2016. Sep;111(4):322–37. 10.1093/cvr/cvw174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holvoet P. Non-coding RNAs at the Cross-Road of Cardiometabolic Diseases and Cancer. Springer International Publishing; 2021. 265 pp. 10.1007/978-3-030-68844-8 [DOI] [Google Scholar]
- Franco CA, Jones ML, Bernabeu MO, Vion AC, Barbacena P, Fan J, et al. Non-canonical Wnt signalling modulates the endothelial shear stress flow sensor in vascular remodelling. eLife. 2016. Feb;5:e07727. 10.7554/eLife.07727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souilhol C, Serbanovic-Canic J, Fragiadaki M, Chico TJ, Ridger V, Roddie H, et al. Endothelial responses to shear stress in atherosclerosis: a novel role for developmental genes. Nat Rev Cardiol. 2020. Jan;17(1):52–63. 10.1038/s41569-019-0239-5 [DOI] [PubMed] [Google Scholar]
- Gimbrone MA Jr, García-Cardeña G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ Res. 2016. Feb;118(4):620–36. 10.1161/CIRCRESAHA.115.306301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Tang Y, Chen C, Qiu D, Cao Y. PEGylated gold nanorods are not cytotoxic to human endothelial cells but affect kruppel-like factor signaling pathway. Toxicol Appl Pharmacol. 2019. Nov;382:114758. 10.1016/j.taap.2019.114758 [DOI] [PubMed] [Google Scholar]
- Domschke G, Gleissner CA. CXCL4-induced macrophages in human atherosclerosis. Cytokine. 2019. Oct;122:154141. 10.1016/j.cyto.2017.08.021 [DOI] [PubMed] [Google Scholar]
- Hulsmans M, Geeraert B, De Keyzer D, Mertens A, Lannoo M, Vanaudenaerde B, et al. Interleukin-1 receptor-associated kinase-3 is a key inhibitor of inflammation in obesity and metabolic syndrome. PLoS One. 2012;7(1):e30414. 10.1371/journal.pone.0030414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukai T, Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal. 2011. Sep;15(6):1583–606. 10.1089/ars.2011.3999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Li X, Jia H, An G, Ni J. The m6A methyltransferase METTL3 modifies PGC-1α mRNA promoting mitochondrial dysfunction and oxLDL-induced inflammation in monocytes. J Biol Chem. 2021. Sep;297(3):101058. 10.1016/j.jbc.2021.101058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis RC, Quimby KR, Greenidge AR. M1/M2 Macrophages in Diabetic Nephropathy: Nrf2/HO-1 as Therapeutic Targets. Curr Pharm Des. 2018;24(20):2241–9. 10.2174/1381612824666180716163845 [DOI] [PubMed] [Google Scholar]
- Cheng F, Torzewski M, Degreif A, Rossmann H, Canisius A, Lackner KJ. Impact of glutathione peroxidase-1 deficiency on macrophage foam cell formation and proliferation: implications for atherogenesis. PLoS One. 2013. Aug;8(8):e72063. 10.1371/journal.pone.0072063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisucka J, Chauhan AK, Patten IS, Yesilaltay A, Neumann C, Van Etten RA, et al. Peroxiredoxin1 prevents excessive endothelial activation and early atherosclerosis. Circ Res. 2008. Sep;103(6):598–605. 10.1161/CIRCRESAHA.108.174870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JG, Yoo JY, Jeong SJ, Choi JH, Lee MR, Lee MN, et al. Peroxiredoxin 2 deficiency exacerbates atherosclerosis in apolipoprotein E-deficient mice. Circ Res. 2011. Sep;109(7):739–49. 10.1161/CIRCRESAHA.111.245530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf D, Ley K. Immunity and Inflammation in Atherosclerosis. Circ Res. 2019. Jan;124(2):315–27. 10.1161/CIRCRESAHA.118.313591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Wei Z, Ding H, Wang Q, Zhou Z, Zheng S, et al. MicroRNA-19b/221/222 induces endothelial cell dysfunction via suppression of PGC-1α in the progression of atherosclerosis. Atherosclerosis. 2015. Aug;241(2):671–81. 10.1016/j.atherosclerosis.2015.06.031 [DOI] [PubMed] [Google Scholar]
- Wu J, Liang W, Tian Y, Ma F, Huang W, Jia Y, et al. Inhibition of P53/miR-34a improves diabetic endothelial dysfunction via activation of SIRT1. J Cell Mol Med. 2019. May;23(5):3538–48. 10.1111/jcmm.14253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Gong Z, Bi Z. Inhibition of miR-383 suppresses oxidative stress and improves endothelial function by increasing sirtuin 1. Braz J Med Biol Res. 2020. Jan;53(2):e8616. 10.1590/1414-431X20198616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gou L, Zhao L, Song W, Wang L, Liu J, Zhang H, et al. Inhibition of miR-92a Suppresses Oxidative Stress and Improves Endothelial Function by Upregulating Heme Oxygenase-1 in db/db Mice. Antioxid Redox Signal. 2018. Feb;28(5):358–70. 10.1089/ars.2017.7005 [DOI] [PubMed] [Google Scholar]
- Togliatto G, Trombetta A, Dentelli P, Gallo S, Rosso A, Cotogni P, et al. Unacylated ghrelin induces oxidative stress resistance in a glucose intolerance and peripheral artery disease mouse model by restoring endothelial cell miR-126 expression. Diabetes. 2015. Apr;64(4):1370–82. 10.2337/db14-0991 [DOI] [PubMed] [Google Scholar]
- Liu QQ, Ren K, Liu SH, Li WM, Huang CJ, Yang XH. MicroRNA-140-5p aggravates hypertension and oxidative stress of atherosclerosis via targeting Nrf2 and Sirt2. Int J Mol Med. 2019. Feb;43(2):839–49. 10.3892/ijmm.2018.3996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Cai W, Fan Z, Yang C, Wang W, Xiong M, et al. MicroRNA-24 inhibits the oxidative stress induced by vascular injury by activating the Nrf2/Ho-1 signaling pathway. Atherosclerosis. 2019. Nov;290:9–18. 10.1016/j.atherosclerosis.2019.08.023 [DOI] [PubMed] [Google Scholar]
- Poitz DM, Augstein A, Gradehand C, Ende G, Schmeisser A, Strasser RH. Regulation of the Hif-system by micro-RNA 17 and 20a - role during monocyte-to-macrophage differentiation. Mol Immunol. 2013. Dec;56(4):442–51. 10.1016/j.molimm.2013.06.014 [DOI] [PubMed] [Google Scholar]
- Hu YW, Guo FX, Xu YJ, Li P, Lu ZF, McVey DG, et al. Long noncoding RNA NEXN-AS1 mitigates atherosclerosis by regulating the actin-binding protein NEXN. J Clin Invest. 2019. Mar;129(3):1115–28. 10.1172/JCI98230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TS, Wang KC, Quon S, Nguyen P, Chang TY, Chen Z, et al. LINC00341 exerts an anti-inflammatory effect on endothelial cells by repressing VCAM1. Physiol Genomics. 2017. Jul;49(7):339–45. 10.1152/physiolgenomics.00132.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisegang MS, Bibli SI, Günther S, Pflüger-Müller B, Oo JA, Höper C, et al. Pleiotropic effects of laminar flow and statins depend on the Krüppel-like factor-induced lncRNA MANTIS. Eur Heart J. 2019. Aug;40(30):2523–33. 10.1093/eurheartj/ehz393 [DOI] [PubMed] [Google Scholar]
- Muñoz-Pacheco P, Ortega-Hernández A, Miana M, Cachofeiro V, Fernández-Cruz A, Gómez-Garre D. Ezetimibe inhibits PMA-induced monocyte/macrophage differentiation by altering microRNA expression: a novel anti-atherosclerotic mechanism. Pharmacol Res. 2012. Dec;66(6):536–43. 10.1016/j.phrs.2012.09.005 [DOI] [PubMed] [Google Scholar]
- Yao M, Cui B, Zhang W, Ma W, Zhao G, Xing L. Exosomal miR-21 secreted by IL-1beta-primed-mesenchymal stem cells induces macrophage M2 polarization and ameliorates sepsis. Life Sci. 2020;118658: 10.1016/j.lfs.2020.118658 [DOI] [PubMed] [Google Scholar]
- Shen Y, Xu H, Pan X, Wu W, Wang H, Yan L, et al. miR-34a and miR-125b are upregulated in peripheral blood mononuclear cells from patients with type 2 diabetes mellitus. Exp Ther Med. 2017. Dec;14(6):5589–96. 10.3892/etm.2017.5254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Ge J, Wang Z, Ren J, Wang X, Xiong H, et al. Let-7e modulates the inflammatory response in vascular endothelial cells through ceRNA crosstalk. Sci Rep. 2017. Feb;7(1):42498. 10.1038/srep42498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Ye J, Guo R, Liang X, Yang L. TRIF Regulates BIC/miR-155 via the ERK Signaling Pathway to Control the ox-LDL-Induced Macrophage Inflammatory Response. J Immunol Res. 2018. Jun;2018:6249085. 10.1155/2018/6249085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Zhang G, Liang X, Li T. LncRNA OIP5-AS1 facilitates ox-LDL-induced endothelial cell injury through the miR-98-5p/HMGB1 axis. Mol Cell Biochem. 2021. Jan;476(1):443–55. 10.1007/s11010-020-03921-5 [DOI] [PubMed] [Google Scholar]
- Wang N, Zhou Y, Jiang L, Li D, Yang J, Zhang CY, et al. Urinary microRNA-10a and microRNA-30d serve as novel, sensitive and specific biomarkers for kidney injury. PLoS One. 2012;7(12):e51140. 10.1371/journal.pone.0051140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TA, Yamakuchi M, Ferlito M, Mendell JT, Lowenstein CJ. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc Natl Acad Sci USA. 2008. Feb;105(5):1516–21. 10.1073/pnas.0707493105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaked I, Meerson A, Wolf Y, Avni R, Greenberg D, Gilboa-Geffen A, et al. MicroRNA-132 potentiates cholinergic anti-inflammatory signaling by targeting acetylcholinesterase. Immunity. 2009. Dec;31(6):965–73. 10.1016/j.immuni.2009.09.019 [DOI] [PubMed] [Google Scholar]
- Qin SB, Peng DY, Lu JM, Ke ZP. MiR-182-5p inhibited oxidative stress and apoptosis triggered by oxidized low-density lipoprotein via targeting toll-like receptor 4. J Cell Physiol. 2018. Oct;233(10):6630–7. 10.1002/jcp.26389 [DOI] [PubMed] [Google Scholar]
- Tian D, Sha Y, Lu JM, Du XJ. MiR-370 inhibits vascular inflammation and oxidative stress triggered by oxidized low-density lipoprotein through targeting TLR4. J Cell Biochem. 2018. Jul;119(7):6231–7. 10.1002/jcb.26851 [DOI] [PubMed] [Google Scholar]
- Manoharan P, Basford JE, Pilcher-Roberts R, Neumann J, Hui DY, Lingrel JB. Reduced levels of microRNAs miR-124a and miR-150 are associated with increased proinflammatory mediator expression in Krüppel-like factor 2 (KLF2)-deficient macrophages. J Biol Chem. 2014. Nov;289(45):31638–46. 10.1074/jbc.M114.579763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Wang H, Liu Y, Song Y, Lai L, Han Q, et al. Inducible microRNA-223 down-regulation promotes TLR-triggered IL-6 and IL-1β production in macrophages by targeting STAT3. PLoS One. 2012;7(8):e42971. 10.1371/journal.pone.0042971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GD, Ng HP, Patel N, Mahabeleshwar GH. Kruppel-like factor 6 and miR-223 signaling axis regulates macrophage-mediated inflammation. FASEB J. 2019. Oct;33(10):10902–15. 10.1096/fj.201900867RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Ching D, Luk FS, Raffai RL. Apolipoprotein E enhances microRNA-146a in monocytes and macrophages to suppress nuclear factor-κB-driven inflammation and atherosclerosis. Circ Res. 2015. Jun;117(1):e1–11. 10.1161/CIRCRESAHA.117.305844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gast M, Rauch BH, Nakagawa S, Haghikia A, Jasina A, Haas J, et al. Immune system-mediated atherosclerosis caused by deficiency of long non-coding RNA MALAT1 in ApoE-/-mice. Cardiovasc Res. 2019. Feb;115(2):302–14. 10.1093/cvr/cvy202 [DOI] [PubMed] [Google Scholar]
- Zhang W, Sui Y. CircBPTF knockdown ameliorates high glucose-induced inflammatory injuries and oxidative stress by targeting the miR-384/LIN28B axis in human umbilical vein endothelial cells. Mol Cell Biochem. 2020. Aug;471(1-2):101–11. 10.1007/s11010-020-03770-2 [DOI] [PubMed] [Google Scholar]
- Cheng J, Liu Q, Hu N, Zheng F, Zhang X, Ni Y, et al. Downregulation of hsa_circ_0068087 ameliorates TLR4/NF-κB/NLRP3 inflammasome-mediated inflammation and endothelial cell dysfunction in high glucose conditioned by sponging miR-197. Gene. 2019. Aug;709:1–7. 10.1016/j.gene.2019.05.012 [DOI] [PubMed] [Google Scholar]
- Pang JL, Wang JW, Hu PY, Jiang JS, Yu C. HOTAIR alleviates ox-LDL-induced inflammatory response in Raw264.7 cells via inhibiting NF-κB pathway. Eur Rev Med Pharmacol Sci. 2018. Oct;22(20):6991–8. 10.26355/eurrev_201810_16170 [DOI] [PubMed] [Google Scholar]
- Rouas R, Fayyad-Kazan H, El Zein N, Lewalle P, Rothé F, Simion A, et al. Human natural Treg microRNA signature: role of microRNA-31 and microRNA-21 in FOXP3 expression. Eur J Immunol. 2009. Jun;39(6):1608–18. 10.1002/eji.200838509 [DOI] [PubMed] [Google Scholar]
- Zheng J, Wang W, Hong T, Yang S, Shen J, Liu C. Suppression of microRNA-155 exerts an anti-inflammatory effect on CD4+ T cell-mediated inflammatory response in the pathogenesis of atherosclerosis. Acta Biochim Biophys Sin (Shanghai). 2020. Jun;52(6):654–64. 10.1093/abbs/gmaa040 [DOI] [PubMed] [Google Scholar]
- Yao R, Ma Y, Du Y, Liao M, Li H, Liang W, et al. The altered expression of inflammation-related microRNAs with microRNA-155 expression correlates with Th17 differentiation in patients with acute coronary syndrome. Cell Mol Immunol. 2011. Nov;8(6):486–95. 10.1038/cmi.2011.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Huang D, Yang F, Tian M, Wang Y, Shen D, et al. Long Noncoding RNA Highly Upregulated in Liver Cancer Regulates the Tumor Necrosis Factor-α-Induced Apoptosis in Human Vascular Endothelial Cells. DNA Cell Biol. 2016. Jun;35(6):296–300. 10.1089/dna.2015.3203 [DOI] [PubMed] [Google Scholar]
- Su G, Sun G, Liu H, Shu L, Liang Z. Downregulation of miR-34a promotes endothelial cell growth and suppresses apoptosis in atherosclerosis by regulating Bcl-2. Heart Vessels. 2018. Oct;33(10):1185–94. 10.1007/s00380-018-1169-6 [DOI] [PubMed] [Google Scholar]
- Zheng B, Yin WN, Suzuki T, Zhang XH, Zhang Y, Song LL, et al. Exosome-Mediated miR-155 Transfer from Smooth Muscle Cells to Endothelial Cells Induces Endothelial Injury and Promotes Atherosclerosis. Mol Ther. 2017. Jun;25(6):1279–94. 10.1016/j.ymthe.2017.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Pan JX. LncRNA H19 promotes atherosclerosis by regulating MAPK and NF-kB signaling pathway. Eur Rev Med Pharmacol Sci. 2017. Jan;21(2):322–8. [PubMed] [Google Scholar]
- Weber M, Baker MB, Moore JP, Searles CD. MiR-21 is induced in endothelial cells by shear stress and modulates apoptosis and eNOS activity. Biochem Biophys Res Commun. 2010. Mar;393(4):643–8. 10.1016/j.bbrc.2010.02.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheedy FJ, Palsson-McDermott E, Hennessy EJ, Martin C, O’Leary JJ, Ruan Q, et al. Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat Immunol. 2010. Feb;11(2):141–7. 10.1038/ni.1828 [DOI] [PubMed] [Google Scholar]
- Yan B, Yao J, Liu JY, Li XM, Wang XQ, Li YJ, et al. lncRNA-MIAT regulates microvascular dysfunction by functioning as a competing endogenous RNA. Circ Res. 2015. Mar;116(7):1143–56. 10.1161/CIRCRESAHA.116.305510 [DOI] [PubMed] [Google Scholar]
- Yu X, Ruan Y, Shen T, Qiu Q, Yan M, Sun S, et al. Dexrazoxane Protects Cardiomyocyte from Doxorubicin-Induced Apoptosis by Modulating miR-17-5p. BioMed Res Int. 2020. Mar;2020:5107193. 10.1155/2020/5107193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadenas S. ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radic Biol Med. 2018. Mar;117:76–89. 10.1016/j.freeradbiomed.2018.01.024 [DOI] [PubMed] [Google Scholar]
- Koyama H, Nojiri H, Kawakami S, Sunagawa T, Shirasawa T, Shimizu T. Antioxidants improve the phenotypes of dilated cardiomyopathy and muscle fatigue in mitochondrial superoxide dismutase-deficient mice. Molecules. 2013. Jan;18(2):1383–93. 10.3390/molecules18021383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhar M. Roles of mammalian glutathione peroxidase and thioredoxin reductase enzymes in the cellular response to nitrosative stress. Free Radic Biol Med. 2018. Nov;127:160–4. 10.1016/j.freeradbiomed.2018.01.028 [DOI] [PubMed] [Google Scholar]
- Lee YJ. Knockout Mouse Models for Peroxiredoxins. Antioxidants. 2020. Feb;9(2):E182. 10.3390/antiox9020182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman M, Arad M, Abraham NG, Hochhauser E. The Peroxisome Proliferator-Activated Receptor-Gamma Coactivator-1α-Heme Oxygenase 1 Axis, a Powerful Antioxidative Pathway with Potential to Attenuate Diabetic Cardiomyopathy. Antioxid Redox Signal. 2020. Jun;32(17):1273–90. 10.1089/ars.2019.7989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barančík M, Grešová L, Barteková M, Dovinová I. Nrf2 as a key player of redox regulation in cardiovascular diseases. Physiol Res. 2016. Sep;65 Suppl 1:S1–10. 10.33549/physiolres.933403 [DOI] [PubMed] [Google Scholar]
- Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, et al. CCL2/Monocyte Chemoattractant Protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res. 2005. Apr;96(8):881–9. 10.1161/01.RES.0000163017.13772.3a [DOI] [PubMed] [Google Scholar]
- Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res. 2004. Jun;94(12):1543–53. 10.1161/01.RES.0000130526.20854.fa [DOI] [PubMed] [Google Scholar]
- Timmers L, Pasterkamp G, de Hoog VC, Arslan F, Appelman Y, de Kleijn DP. The innate immune response in reperfused myocardium. Cardiovasc Res. 2012. May;94(2):276–83. 10.1093/cvr/cvs018 [DOI] [PubMed] [Google Scholar]
- Ghigo A, Franco I, Morello F, Hirsch E. Myocyte signalling in leucocyte recruitment to the heart. Cardiovasc Res. 2014. May;102(2):270–80. 10.1093/cvr/cvu030 [DOI] [PubMed] [Google Scholar]
- Andrassy M, Volz HC, Igwe JC, Funke B, Eichberger SN, Kaya Z, et al. High-mobility group box-1 in ischemia-reperfusion injury of the heart. Circulation. 2008. Jun;117(25):3216–26. 10.1161/CIRCULATIONAHA.108.769331 [DOI] [PubMed] [Google Scholar]
- Yamauchi-Takihara K, Ihara Y, Ogata A, Yoshizaki K, Azuma J, Kishimoto T. Hypoxic stress induces cardiac myocyte-derived interleukin-6. Circulation. 1995. Mar;91(5):1520–4. 10.1161/01.CIR.91.5.1520 [DOI] [PubMed] [Google Scholar]
- Arslan F, de Kleijn DP, Pasterkamp G. Innate immune signaling in cardiac ischemia. Nat Rev Cardiol. 2011. May;8(5):292–300. 10.1038/nrcardio.2011.38 [DOI] [PubMed] [Google Scholar]
- Laroumanie F, Douin-Echinard V, Pozzo J, Lairez O, Tortosa F, Vinel C, et al. CD4+ T cells promote the transition from hypertrophy to heart failure during chronic pressure overload. Circulation. 2014. May;129(21):2111–24. 10.1161/CIRCULATIONAHA.113.007101 [DOI] [PubMed] [Google Scholar]
- Cheng X, Liao YH, Zhang J, Li B, Ge H, Yuan J, et al. Effects of Atorvastatin on Th polarization in patients with acute myocardial infarction. Eur J Heart Fail. 2005. Dec;7(7):1099–104. 10.1016/j.ejheart.2005.01.020 [DOI] [PubMed] [Google Scholar]
- Cheng X, Liao YH, Ge H, Li B, Zhang J, Yuan J, et al. TH1/TH2 functional imbalance after acute myocardial infarction: coronary arterial inflammation or myocardial inflammation. J Clin Immunol. 2005. May;25(3):246–53. 10.1007/s10875-005-4088-0 [DOI] [PubMed] [Google Scholar]
- Zhang J, Liao Y, Cheng X, Chen J, Chen P, Gao X, et al. Myosin specific-T lymphocytes mediated myocardial inflammation in adoptive transferred rats. Cell Mol Immunol. 2006. Dec;3(6):445–51. 10.1038/cmi.2011.31 [DOI] [PubMed] [Google Scholar]
- Cheng X, Yu X, Ding YJ, Fu QQ, Xie JJ, Tang TT, et al. The Th17/Treg imbalance in patients with acute coronary syndrome. Clin Immunol. 2008. Apr;127(1):89–97. 10.1016/j.clim.2008.01.009 [DOI] [PubMed] [Google Scholar]
- Tang TT, Yuan J, Zhu ZF, Zhang WC, Xiao H, Xia N, et al. Regulatory T cells ameliorate cardiac remodeling after myocardial infarction. Basic Res Cardiol. 2012. Jan;107(1):232. 10.1007/s00395-011-0232-6 [DOI] [PubMed] [Google Scholar]
- Du J, Hang P, Pan Y, Feng B, Zheng Y, Chen T, et al. Inhibition of miR-23a attenuates doxorubicin-induced mitochondria-dependent cardiomyocyte apoptosis by targeting the PGC-1α/Drp1 pathway. Toxicol Appl Pharmacol. 2019. Apr;369:73–81. 10.1016/j.taap.2019.02.016 [DOI] [PubMed] [Google Scholar]
- Suh JH, Choi E, Cha MJ, Song BW, Ham O, Lee SY, et al. Up-regulation of miR-26a promotes apoptosis of hypoxic rat neonatal cardiomyocytes by repressing GSK-3β protein expression. Biochem Biophys Res Commun. 2012. Jun;423(2):404–10. 10.1016/j.bbrc.2012.05.138 [DOI] [PubMed] [Google Scholar]
- Yang B, Ma S, Wang YB, Xu B, Zhao H, He YY, et al. Resveratrol exerts protective effects on anoxia/reoxygenation injury in cardiomyocytes via miR-34a/Sirt1 signaling pathway. Eur Rev Med Pharmacol Sci. 2016. Jun;20(12):2734–41. [PubMed] [Google Scholar]
- Du JK, Cong BH, Yu Q, Wang H, Wang L, Wang CN, et al. Upregulation of microRNA-22 contributes to myocardial ischemia-reperfusion injury by interfering with the mitochondrial function. Free Radic Biol Med. 2016. Jul;96:406–17. 10.1016/j.freeradbiomed.2016.05.006 [DOI] [PubMed] [Google Scholar]
- Das S, Bedja D, Campbell N, Dunkerly B, Chenna V, Maitra A, et al. miR-181c regulates the mitochondrial genome, bioenergetics, and propensity for heart failure in vivo. PLoS One. 2014. May;9(5):e96820. 10.1371/journal.pone.0096820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin KH, Kumar VB, Shanmugam T, Shibu MA, Chen RJ, Kuo CH, et al. miR-145-5p targets paxillin to attenuate angiotensin II-induced pathological cardiac hypertrophy via downregulation of Rac 1, pJNK, p-c-Jun, NFATc3, ANP and by Sirt-1 upregulation. Mol Cell Biochem. 2021. Sep;476(9):3253–60. 10.1007/s11010-021-04100-w [DOI] [PubMed] [Google Scholar]
- Zhu X, Zhao Y, Hou W, Guo L. MiR-153 regulates cardiomyocyte apoptosis by targeting Nrf2/HO-1 signaling. Chromosome Res. 2019. Sep;27(3):167–78. 10.1007/s10577-019-09608-y [DOI] [PubMed] [Google Scholar]
- Zhu XA, Gao LF, Zhang ZG, Xiang DK. Down-regulation of miR-320 exerts protective effects on myocardial I-R injury via facilitating Nrf2 expression. Eur Rev Med Pharmacol Sci. 2019. Feb;23(4):1730–41. 10.26355/eurrev_201902_17135 [DOI] [PubMed] [Google Scholar]
- Wang L, Huang H, Fan Y, Kong B, Hu H, Hu K, et al. Effects of downregulation of microRNA-181a on H2O2-induced H9c2 cell apoptosis via the mitochondrial apoptotic pathway. Oxid Med Cell Longev. 2014;2014:960362. 10.1155/2014/960362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrychenko S, Kyrychenko V, Badr MA, Ikeda Y, Sadoshima J, Shirokova N. Pivotal role of miR-448 in the development of ROS-induced cardiomyopathy. Cardiovasc Res. 2015. Dec;108(3):324–34. 10.1093/cvr/cvv238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Ge S, Gong W, Xu J, Guo Z, Liu Z, et al. LncRNA ANRIL acts as a modular scaffold of WDR5 and HDAC3 complexes and promotes alteration of the vascular smooth muscle cell phenotype. Cell Death Dis. 2020. Jun;11(6):435. 10.1038/s41419-020-2645-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi CC, Pan LY, Zhao YQ, Li Q, Li JG. MicroRNA-323-3p inhibits oxidative stress and apoptosis after myocardial infarction by targeting TGF-β2/JNK pathway. Eur Rev Med Pharmacol Sci. 2020. Jun;24(12):6961–70. 10.26355/eurrev_202006_21688 [DOI] [PubMed] [Google Scholar]
- Zhang S, Wang Y, Wang P, Xuan J. miR-708 affords protective efficacy in anoxia/reoxygenation-stimulated cardiomyocytes by blocking the TLR4 signaling via targeting HMGB1. Mol Cell Probes. 2020. Dec;54:101653. 10.1016/j.mcp.2020.101653 [DOI] [PubMed] [Google Scholar]
- Su Q, Liu Y, Lv XW, Dai RX, Yang XH, Kong BH. LncRNA TUG1 mediates ischemic myocardial injury by targeting miR-132-3p/HDAC3 axis. Am J Physiol Heart Circ Physiol. 2020. Feb;318(2):H332–44. 10.1152/ajpheart.00444.2019 [DOI] [PubMed] [Google Scholar]
- Yang J, Brown ME, Zhang H, Martinez M, Zhao Z, Bhutani S, et al. High-throughput screening identifies microRNAs that target Nox2 and improve function after acute myocardial infarction. Am J Physiol Heart Circ Physiol. 2017. May;312(5):H1002–12. 10.1152/ajpheart.00685.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhang H, Li Z, Yan X, Li Y, Liu S. microRNA-130a-5p suppresses myocardial ischemia reperfusion injury by downregulating the HMGB2/NF-κB axis. BMC Cardiovasc Disord. 2021. Mar;21(1):121. 10.1186/s12872-020-01742-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Liu Y, Zhang Y, Zhu X, Zhang R, Guan L, et al. MicroRNA-150 Protects Against Pressure Overload-Induced Cardiac Hypertrophy. J Cell Biochem. 2015. Oct;116(10):2166–76. 10.1002/jcb.25057 [DOI] [PubMed] [Google Scholar]
- Demolli S, Doebele C, Doddaballapur A, Lang V, Fisslthaler B, Chavakis E, et al. MicroRNA-30 mediates anti-inflammatory effects of shear stress and KLF2 via repression of angiopoietin 2. J Mol Cell Cardiol. 2015. Nov;88:111–9. 10.1016/j.yjmcc.2015.10.009 [DOI] [PubMed] [Google Scholar]
- Xue YL, Zhang SX, Zheng CF, Li YF, Zhang LH, Su QY, et al. Long non-coding RNA MEG3 inhibits M2 macrophage polarization by activating TRAF6 via microRNA-223 down-regulation in viral myocarditis. J Cell Mol Med. 2020. Nov;24(21):12341–54. 10.1111/jcmm.15720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar TM, Kanellopoulou C, Kugler DG, Kilaru G, Nguyen CK, Nagarajan V, et al. miR-155 activates cytokine gene expression in Th17 cells by regulating the DNA-binding protein Jarid2 to relieve polycomb-mediated repression. Immunity. 2014. Jun;40(6):865–79. 10.1016/j.immuni.2014.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Wang X, Zhang B, Li P, Du X, Qi F. The lncRNA PVT1 regulates autophagy in regulatory T cells to suppress heart transplant rejection in mice by targeting miR-146a. Cell Immunol. 2021. Sep;367:104400. 10.1016/j.cellimm.2021.104400 [DOI] [PubMed] [Google Scholar]
- Gast M, Rauch BH, Haghikia A, Nakagawa S, Haas J, Stroux A, et al. Long noncoding RNA NEAT1 modulates immune cell functions and is suppressed in early onset myocardial infarction patients. Cardiovasc Res. 2019. Nov;115(13):1886–906. 10.1093/cvr/cvz085 [DOI] [PubMed] [Google Scholar]
- Fu Z, Li G, Li Z, Wang Y, Zhao Y, Zheng S, et al. Endogenous miRNA Sponge LincRNA-ROR promotes proliferation, invasion and stem cell-like phenotype of pancreatic cancer cells. Cell Death Discov. 2017. May;3(1):17004. 10.1038/cddiscovery.2017.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng F, Li TT, Wang KL, Xiao GQ, Wang JH, Zhao HD, et al. H19/let-7/LIN28 reciprocal negative regulatory circuit promotes breast cancer stem cell maintenance. Cell Death Dis. 2017. Jan;8(1):e2569. 10.1038/cddis.2016.438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garikipati VN, Verma SK, Jolardarashi D, Cheng Z, Ibetti J, Cimini M, et al. Therapeutic inhibition of miR-375 attenuates post-myocardial infarction inflammatory response and left ventricular dysfunction via PDK-1-AKT signalling axis. Cardiovasc Res. 2017. Jul;113(8):938–49. 10.1093/cvr/cvx052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Zhang C, Liu L, A X, Chen B, Li Y, et al. Macrophage-Derived mir-155-Containing Exosomes Suppress Fibroblast Proliferation and Promote Fibroblast Inflammation during Cardiac Injury. Mol Ther. 2017. Jan;25(1):192–204. 10.1016/j.ymthe.2016.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Wang B, Zhou Q, Wang Y, Liu X, Liu Z, et al. MicroRNA-21 prevents excessive inflammation and cardiac dysfunction after myocardial infarction through targeting KBTBD7. Cell Death Dis. 2018. Jul;9(7):769. 10.1038/s41419-018-0805-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maegdefessel L, Spin JM, Raaz U, Eken SM, Toh R, Azuma J, et al. miR-24 limits aortic vascular inflammation and murine abdominal aneurysm development. Nat Commun. 2014. Oct;5(1):5214. 10.1038/ncomms6214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Cui C, Yang X, Xu J, Venkat P, Zacharek A, et al. MiR-126 Affects Brain-Heart Interaction after Cerebral Ischemic Stroke. Transl Stroke Res. 2017. Aug;8(4):374–85. 10.1007/s12975-017-0520-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Cai SX, He Q, Zhang H, Friedberg D, Wang F, et al. Intravenous miR-144 reduces left ventricular remodeling after myocardial infarction. Basic Res Cardiol. 2018. Aug;113(5):36. 10.1007/s00395-018-0694-x [DOI] [PubMed] [Google Scholar]
- Wang X, Shang Y, Dai S, Wu W, Yi F, Cheng L. MicroRNA-16-5p Aggravates Myocardial Infarction Injury by Targeting the Expression of Insulin Receptor Substrates 1 and Mediating Myocardial Apoptosis and Angiogenesis. Curr Neurovasc Res. 2020;17(1):11–7. 10.2174/1567202617666191223142743 [DOI] [PubMed] [Google Scholar]
- Ye Y, Hu Z, Lin Y, Zhang C, Perez-Polo JR. Downregulation of microRNA-29 by antisense inhibitors and a PPAR-gamma agonist protects against myocardial ischaemia-reperfusion injury. Cardiovasc Res. 2010. Aug;87(3):535–44. 10.1093/cvr/cvq053 [DOI] [PubMed] [Google Scholar]
- Wang JJ, Bie ZD, Sun CF. Long noncoding RNA AK088388 regulates autophagy through miR-30a to affect cardiomyocyte injury. J Cell Biochem. 2019. Jun;120(6):10155–63. 10.1002/jcb.28300 [DOI] [PubMed] [Google Scholar]
- Li XQ, Liu YK, Yi J, Dong JS, Zhang PP, Wan L, et al. MicroRNA-143 Increases Oxidative Stress and Myocardial Cell Apoptosis in a Mouse Model of Doxorubicin-Induced Cardiac Toxicity. Med Sci Monit. 2020. Mar;26:e920394. 10.12659/MSM.920394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Kong M, Jiang D, Qian J, Duan Q, Dong A. MicroRNA-150 aggravates H2O2-induced cardiac myocyte injury by down-regulating c-myb gene. Acta Biochim Biophys Sin (Shanghai). 2013. Sep;45(9):734–41. 10.1093/abbs/gmt067 [DOI] [PubMed] [Google Scholar]
- Li Y, Duan JZ, He Q, Wang CQ. miR‑155 modulates high glucose‑induced cardiac fibrosis via the Nrf2/HO‑1 signaling pathway. Mol Med Rep. 2020. Nov;22(5):4003–16. 10.3892/mmr.2020.11495 [DOI] [PubMed] [Google Scholar]
- Chiang MH, Liang CJ, Lin LC, Yang YF, Huang CC, Chen YH, et al. miR-26a attenuates cardiac apoptosis and fibrosis by targeting ataxia-telangiectasia mutated in myocardial infarction. J Cell Physiol. 2020. Sep;235(9):6085–102. 10.1002/jcp.29537 [DOI] [PubMed] [Google Scholar]
- Pan JA, Tang Y, Yu JY, Zhang H, Zhang JF, Wang CQ, et al. miR-146a attenuates apoptosis and modulates autophagy by targeting TAF9b/P53 pathway in doxorubicin-induced cardiotoxicity. Cell Death Dis. 2019. Sep;10(9):668. 10.1038/s41419-019-1901-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Ding W, Tariq MA, Chang W, Zhang X, Xu W, et al. A circular transcript of ncx1 gene mediates ischemic myocardial injury by targeting miR-133a-3p. Theranostics. 2018. Nov;8(21):5855–69. 10.7150/thno.27285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C, Li L, Kim IK, Sun P, Gupta S. NF-κB mediated miR-21 regulation in cardiomyocytes apoptosis under oxidative stress. Free Radic Res. 2014. Mar;48(3):282–91. 10.3109/10715762.2013.865839 [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhao R, Liu W, Wang Z, Rong J, Long X, et al. Exosomal circHIPK3 Released from Hypoxia-Pretreated Cardiomyocytes Regulates Oxidative Damage in Cardiac Microvascular Endothelial Cells via the miR-29a/IGF-1 Pathway. Oxid Med Cell Longev. 2019. Dec;2019:7954657. 10.1155/2019/7954657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Hu Q, Zhang BF, Liu XP, Yang S, Jiang H. Long noncoding RNA UCA1 inhibits ischaemia/reperfusion injury induced cardiomyocytes apoptosis via suppression of endoplasmic reticulum stress. Genes Genomics. 2019. Jul;41(7):803–10. 10.1007/s13258-019-00806-w [DOI] [PubMed] [Google Scholar]
- Frezza C, Zheng L, Folger O, Rajagopalan KN, MacKenzie ED, Jerby L, et al. Haem oxygenase is synthetically lethal with the tumour suppressor fumarate hydratase. Nature. 2011. Aug;477(7363):225–8. 10.1038/nature10363 [DOI] [PubMed] [Google Scholar]
- Ekoue DN, He C, Diamond AM, Bonini MG. Manganese superoxide dismutase and glutathione peroxidase-1 contribute to the rise and fall of mitochondrial reactive oxygen species which drive oncogenesis. Biochim Biophys Acta Bioenerg. 2017. Aug;1858(8):628–32. 10.1016/j.bbabio.2017.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker HC, Patel D, Howat WJ, Warren AY, Kay JD, Sangan T, et al. Peroxiredoxin-3 is overexpressed in prostate cancer and promotes cancer cell survival by protecting cells from oxidative stress. Br J Cancer. 2013. Aug;109(4):983–93. 10.1038/bjc.2013.396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Bogner PN, Baek SH, Ramnath N, Liang P, Kim HR, et al. Up-regulation of peroxiredoxin 1 in lung cancer and its implication as a prognostic and therapeutic target. Clin Cancer Res. 2008. Apr;14(8):2326–33. 10.1158/1078-0432.CCR-07-4457 [DOI] [PubMed] [Google Scholar]
- Zimta AA, Cenariu D, Irimie A, Magdo L, Nabavi SM, Atanasov AG, et al. The Role of Nrf2 Activity in Cancer Development and Progression. Cancers (Basel). 2019. Nov;11(11):E1755. 10.3390/cancers11111755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Qi Y, Xu L, Tao X, Han X, Yin L, et al. MicroRNA-140-5p aggravates doxorubicin-induced cardiotoxicity by promoting myocardial oxidative stress via targeting Nrf2 and Sirt2. Redox Biol. 2018. May;15:284–96. 10.1016/j.redox.2017.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheleschi S, Tenti S, Mondanelli N, Corallo C, Barbarino M, Giannotti S, et al. MicroRNA-34a and MicroRNA-181a Mediate Visfatin-Induced Apoptosis and Oxidative Stress via NF-κB Pathway in Human Osteoarthritic Chondrocytes. Cells. 2019. Aug;8(8):E874. 10.3390/cells8080874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y, Xu Y, Jiang Y, Yu D, Jiang X, Zhao L. Macrophage-derived extracellular vesicles regulates USP5-mediated HDAC2/NRF2 axis to ameliorate inflammatory pain. FASEB J. 2021. Sep;35(9):e21332. 10.1096/fj.202001185RR [DOI] [PubMed] [Google Scholar]
- Khan AU, Rathore MG, Allende-Vega N, Vo DN, Belkhala S, Orecchioni S, et al. Human Leukemic Cells performing Oxidative Phosphorylation (OXPHOS) Generate an Antioxidant Response Independently of Reactive Oxygen species (ROS) Production. EBioMedicine. 2015. Nov;3:43–53. 10.1016/j.ebiom.2015.11.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pant K, Yadav AK, Gupta P, Islam R, Saraya A, Venugopal SK. Butyrate induces ROS-mediated apoptosis by modulating miR-22/SIRT-1 pathway in hepatic cancer cells. Redox Biol. 2017. Aug;12:340–9. 10.1016/j.redox.2017.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace L, Aikhionbare K, Banerjee S, Peagler K, Pitts M, Yao X, et al. Differential Expression Profiles of Mitogenome Associated MicroRNAs Among Colorectal Adenomatous Polyps. Cancer Res J (N Y N Y). 2021. Mar;9(1):23–33. 10.11648/j.crj.20210901.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu L, Wang M, Hu S, Ru X, Ren Y, Zhang Z, et al. Oncogenic Activation of Nrf2, Though as a Master Antioxidant Transcription Factor, Liberated by Specific Knockout of the Full-Length Nrf1α that Acts as a Dominant Tumor Repressor. Cancers (Basel). 2018. Dec;10(12):E520. 10.3390/cancers10120520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Wang N, Wei H, Li C, Wu J, Yang G. miR-24-3p Regulates Progression of Gastric Mucosal Lesions and Suppresses Proliferation and Invasiveness of N87 Via Peroxiredoxin 6. Dig Dis Sci. 2016. Dec;61(12):3486–97. 10.1007/s10620-016-4309-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Chen Q, Zeng X, Li M, Liao J. lnc-NLC1-C inhibits migration, invasion and autophagy of glioma cells by targeting miR-383 and regulating PRDX-3 expression. Oncol Lett. 2021. Sep;22(3):640. 10.3892/ol.2021.12901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He HW, Wang NN, Yi XM, Tang CP, Wang D. Low-level serum miR-24-2 is associated with the progression of colorectal cancer. Cancer Biomark. 2018. Feb;21(2):261–7. 10.3233/CBM-170321 [DOI] [PubMed] [Google Scholar]
- Zhu H, Vishwamitra D, Curry CV, Manshouri R, Diao L, Khan A, et al. NPM-ALK up-regulates iNOS expression through a STAT3/microRNA-26a-dependent mechanism. J Pathol. 2013. May;230(1):82–94. 10.1002/path.4171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair S, Dhodapkar MV. Natural Killer T Cells in Cancer Immunotherapy. Front Immunol. 2017. Sep;8:1178. 10.3389/fimmu.2017.01178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellenstein MD, Coffelt SB, Duits DE, van Miltenburg MH, Slagter M, de Rink I, et al. Loss of p53 triggers WNT-dependent systemic inflammation to drive breast cancer metastasis. Nature. 2019. Aug;572(7770):538–42. 10.1038/s41586-019-1450-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Xue H, Shao Q, Wang J, Guo X, Chen X, et al. Hypoxia promotes glioma-associated macrophage infiltration via periostin and subsequent M2 polarization by upregulating TGF-beta and M-CSFR. Oncotarget. 2016. Dec;7(49):80521–42. 10.18632/oncotarget.11825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Wen H, Zhou C, Su Q, Lin Y, Xie Y, et al. TNF-α derived from M2 tumor-associated macrophages promotes epithelial-mesenchymal transition and cancer stemness through the Wnt/β-catenin pathway in SMMC-7721 hepatocellular carcinoma cells. Exp Cell Res. 2019. May;378(1):41–50. 10.1016/j.yexcr.2019.03.005 [DOI] [PubMed] [Google Scholar]
- Baer C, Squadrito ML, Laoui D, Thompson D, Hansen SK, Kiialainen A, et al. Suppression of microRNA activity amplifies IFN-γ-induced macrophage activation and promotes anti-tumour immunity. Nat Cell Biol. 2016. Jul;18(7):790–802. 10.1038/ncb3371 [DOI] [PubMed] [Google Scholar]
- Yin C, Han Q, Xu D, Zheng B, Zhao X, Zhang J. SALL4-mediated upregulation of exosomal miR-146a-5p drives T-cell exhaustion by M2 tumor-associated macrophages in HCC. OncoImmunology. 2019. Apr;8(7):1601479. 10.1080/2162402X.2019.1601479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Tong L, LiuAnwei Liu F, Liu A, Zeng S, Xiong Q, et al. Tumor‑infiltrating M2 macrophages driven by specific genomic alterations are associated with prognosis in bladder cancer. Oncol Rep. 2019. Aug;42(2):581–94. 10.3892/or.2019.7196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Chen J, Su F, Yu B, Su F, Lin L, et al. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol Cancer. 2011. Sep;10(1):117. 10.1186/1476-4598-10-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontis F, Roz L, Mensah M, Segale M, Moro M, Bertolini G, et al. Circulating extracellular vesicles from individuals at high-risk of lung cancer induce pro-tumorigenic conversion of stromal cells through transfer of miR-126 and miR-320. J Exp Clin Cancer Res. 2021. Jul;40(1):237. 10.1186/s13046-021-02040-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Androulidaki A, Iliopoulos D, Arranz A, Doxaki C, Schworer S, Zacharioudaki V, et al. The kinase Akt1 controls macrophage response to lipopolysaccharide by regulating microRNAs. Immunity. 2009. Aug;31(2):220–31. 10.1016/j.immuni.2009.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga T, Akiyoshi T, Yamamoto N, Taguchi S, Mori S, Nagasaki T, et al. Clinical significance of soluble programmed cell death-1 and soluble programmed cell death-ligand 1 in patients with locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy. PLoS One. 2019. Feb;14(2):e0212978. 10.1371/journal.pone.0212978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Liao B, Xiang X, Ke S. miR-21-5p promotes cell proliferation and G1/S transition in melanoma by targeting CDKN2C. FEBS Open Bio. 2020. May;10(5):752–60. 10.1002/2211-5463.12819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi M, Altomare E, Botta C, Gallo Cantafio ME, Sarvide S, Caracciolo D, et al. miR-21 antagonism abrogates Th17 tumor promoting functions in multiple myeloma. Leukemia. 2021. Mar;35(3):823–34. 10.1038/s41375-020-0947-1 [DOI] [PubMed] [Google Scholar]
- Qiu C, Ma J, Wang ML, Zhang Q, Li YB. MicroRNA-155 deficiency in CD8+ T cells inhibits its anti-glioma immunity by regulating FoxO3a. Eur Rev Med Pharmacol Sci. 2019. Mar;23(6):2486–96. 10.26355/eurrev_201903_17396 [DOI] [PubMed] [Google Scholar]
- Ji Y, Fioravanti J, Zhu W, Wang H, Wu T, Hu J, et al. miR-155 harnesses Phf19 to potentiate cancer immunotherapy through epigenetic reprogramming of CD8+ T cell fate. Nat Commun. 2019. May;10(1):2157. 10.1038/s41467-019-09882-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber V, Vallacchi V, Fleming V, Hu X, Cova A, Dugo M, et al. Tumor-derived microRNAs induce myeloid suppressor cells and predict immunotherapy resistance in melanoma. J Clin Invest. 2018. Dec;128(12):5505–16. 10.1172/JCI98060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhunjhunwala S, Hammer C, Delamarre L. Antigen presentation in cancer: insights into tumour immunogenicity and immune evasion. Nat Rev Cancer. 2021. May;21(5):298–312. 10.1038/s41568-021-00339-z [DOI] [PubMed] [Google Scholar]
- Monnot GC, Martinez-Usatorre A, Lanitis E, Lopes SF, Cheng WC, Ho PC, et al. miR-155 Overexpression in OT-1 CD8+ T Cells Improves Anti-Tumor Activity against Low-Affinity Tumor Antigen. Mol Ther Oncolytics. 2019. Dec;16:111–23. 10.1016/j.omto.2019.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Yu D, Liu J, Yang K, Wu G, Liu H. Antitumor activity of SAHA, a novel histone deacetylase inhibitor, against murine B cell lymphoma A20 cells in vitro and in vivo. Tumour Biol. 2015. Jul;36(7):5051–61. 10.1007/s13277-015-3156-1 [DOI] [PubMed] [Google Scholar]
- Zhu SY, Wu QY, Zhang CX, Wang Q, Ling J, Huang XT, et al. miR-20a inhibits the killing effect of natural killer cells to cervical cancer cells by downregulating RUNX1. Biochem Biophys Res Commun. 2018. Oct;505(1):309–16. 10.1016/j.bbrc.2018.09.102 [DOI] [PubMed] [Google Scholar]
- Sanchez-Martínez D, Krzywinska E, Rathore MG, Saumet A, Cornillon A, Lopez-Royuela N, et al. All-trans retinoic acid (ATRA) induces miR-23a expression, decreases CTSC expression and granzyme B activity leading to impaired NK cell cytotoxicity. Int J Biochem Cell Biol. 2014. Apr;49:42–52. 10.1016/j.biocel.2014.01.003 [DOI] [PubMed] [Google Scholar]
- Zhang LL, Zhang LF, Shi YB. miR-24 inhibited the killing effect of natural killer cells to colorectal cancer cells by downregulating Paxillin. Biomed Pharmacother. 2018. May;101:257–63. 10.1016/j.biopha.2018.02.024 [DOI] [PubMed] [Google Scholar]
- Lee CC, Ho KH, Huang TW, Shih CM, Hsu SY, Liu AJ, et al. A regulatory loop among CD276, miR-29c-3p, and Myc exists in cancer cells against natural killer cell cytotoxicity. Life Sci. 2021. Jul;277:119438. 10.1016/j.lfs.2021.119438 [DOI] [PubMed] [Google Scholar]
- Heinemann A, Zhao F, Pechlivanis S, Eberle J, Steinle A, Diederichs S, et al. Tumor suppressive microRNAs miR-34a/c control cancer cell expression of ULBP2, a stress-induced ligand of the natural killer cell receptor NKG2D. Cancer Res. 2012. Jan;72(2):460–71. 10.1158/0008-5472.CAN-11-1977 [DOI] [PubMed] [Google Scholar]
- Xi Q, Chen Y, Yang GZ, Zhang JY, Zhang LJ, Guo XD, et al. miR-128 Regulates Tumor Cell CD47 Expression and Promotes Anti-tumor Immunity in Pancreatic Cancer. Front Immunol. 2020. May;11:890. 10.3389/fimmu.2020.00890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Liu S, Liu J, Zhang Z, Mao X, Zhou H. MicroRNA-130a enhances the killing ability of natural killer cells against non-small cell lung cancer cells by targeting signal transducers and activators of transcription 3. Biochem Biophys Res Commun. 2020. Mar;523(2):481–6. 10.1016/j.bbrc.2019.11.099 [DOI] [PubMed] [Google Scholar]
- Wang J, Zhu M, Zhou X, Wang T, Xi Y, Jing Z, et al. MiR-140-3p inhibits natural killer cytotoxicity to human ovarian cancer via targeting MAPK1. J Biosci. 2020;45(1):66. 10.1007/s12038-020-00036-3 [DOI] [PubMed] [Google Scholar]
- Ou ZL, Luo Z, Wei W, Liang S, Gao TL, Lu YB. Hypoxia-induced shedding of MICA and HIF1A-mediated immune escape of pancreatic cancer cells from NK cells: role of circ_0000977/miR-153 axis. RNA Biol. 2019. Nov;16(11):1592–603. 10.1080/15476286.2019.1649585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichocki F, Felices M, McCullar V, Presnell SR, Al-Attar A, Lutz CT, et al. Cutting edge: microRNA-181 promotes human NK cell development by regulating Notch signaling. J Immunol. 2011. Dec;187(12):6171–5. 10.4049/jimmunol.1100835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briand J, Garnier D, Nadaradjane A, Clément-Colmou K, Potiron V, Supiot S, et al. Radiotherapy-induced overexpression of exosomal miRNA-378a-3p in cancer cells limits natural killer cells cytotoxicity. Epigenomics. 2020. Mar;12(5):397–408. 10.2217/epi-2019-0193 [DOI] [PubMed] [Google Scholar]
- Trotta R, Chen L, Costinean S, Josyula S, Mundy-Bosse BL, Ciarlariello D, et al. Overexpression of miR-155 causes expansion, arrest in terminal differentiation and functional activation of mouse natural killer cells. Blood. 2013. Apr;121(16):3126–34. 10.1182/blood-2012-12-467597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L, Zhang Z, Qin X, Gao Y, Zhao P, Liu J, et al. Long noncoding RNA ZFAS1 promotes tumorigenesis through regulation of miR-150-5p/RAB9A in melanoma. Melanoma Res. 2019. Dec;29(6):569–81. 10.1097/CMR.0000000000000595 [DOI] [PubMed] [Google Scholar]
- Zhang X, Wu N, Wang J, Li Z. LncRNA MEG3 inhibits cell proliferation and induces apoptosis in laryngeal cancer via miR-23a/APAF-1 axis. J Cell Mol Med. 2019. Oct;23(10):6708–19. 10.1111/jcmm.14549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng K, Liu Y, Xu LJ, Zhao LF, Jia CW, Xu MY. Long noncoding RNA PVT1 enhances the viability and invasion of papillary thyroid carcinoma cells by functioning as ceRNA of microRNA-30a through mediating expression of insulin like growth factor 1 receptor. Biomed Pharmacother. 2018. Aug;104:686–98. 10.1016/j.biopha.2018.05.078 [DOI] [PubMed] [Google Scholar]
- Li J, Xu X, Wei C, Liu L, Wang T. Long noncoding RNA NORAD regulates lung cancer cell proliferation, apoptosis, migration, and invasion by the miR-30a-5p/ADAM19 axis. Int J Clin Exp Pathol. 2020. Jan;13(1):1–13. [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Zhang Y, Liu W, Huang Y, Shen X, Jing R, et al. LncRNA H19 overexpression induces bortezomib resistance in multiple myeloma by targeting MCL-1 via miR-29b-3p. Cell Death Dis. 2019. Feb;10(2):106. 10.1038/s41419-018-1219-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai F, Deng W, Fu C, Wu P, Cao M, Tan S. Long non-coding RNA SNHG6 increases JAK2 expression by targeting the miR-181 family to promote colorectal cancer cell proliferation. J Gene Med. 2020. Dec;22(12):e3262. 10.1002/jgm.3262 [DOI] [PubMed] [Google Scholar]
- Shi L, Cheng Z, Zhang J, Li R, Zhao P, Fu Z, et al. hsa-mir-181a and hsa-mir-181b function as tumor suppressors in human glioma cells. Brain Res. 2008. Oct;1236:185–93. 10.1016/j.brainres.2008.07.085 [DOI] [PubMed] [Google Scholar]
- Huang P, Ye B, Yang Y, Shi J, Zhao H. MicroRNA-181 functions as a tumor suppressor in non-small cell lung cancer (NSCLC) by targeting Bcl-2. Tumour Biol. 2015. May;36(5):3381–7. 10.1007/s13277-014-2972-z [DOI] [PubMed] [Google Scholar]
- Liu L, Ren W, Chen K. MiR-34a Promotes Apoptosis and Inhibits Autophagy by Targeting HMGB1 in Acute Myeloid Leukemia Cells. Cell Physiol Biochem. 2017;41(5):1981–92. 10.1159/000475277 [DOI] [PubMed] [Google Scholar]
- Chen W, Huang L, Hao C, Zeng W, Luo X, Li X, et al. MicroRNA-155 promotes apoptosis in SKOV3, A2780, and primary cultured ovarian cancer cells. Tumour Biol. 2016. Jul;37(7):9289–99. 10.1007/s13277-016-4804-9 [DOI] [PubMed] [Google Scholar]
- Zhuang S, Liu F, Wu P. Upregulation of long noncoding RNA TUG1 contributes to the development of laryngocarcinoma by targeting miR-145-5p/ROCK1 axis. J Cell Biochem. 2019. Aug;120(8):13392–402. 10.1002/jcb.28614 [DOI] [PubMed] [Google Scholar]
- Li Y, Wang P, Wu LL, Yan J, Pang XY, Liu SJ. miR-26a-5p Inhibit Gastric Cancer Cell Proliferation and Invasion Through Mediated Wnt5a. OncoTargets Ther. 2020. Mar;13:2537–50. 10.2147/OTT.S241199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He MQ, Wan JF, Zeng HF, Tang YY, He MQ. miR-133a-5p suppresses gastric cancer through TCF4 down-regulation. J Gastrointest Oncol. 2021. Jun;12(3):1007–19. 10.21037/jgo-20-418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Li S, Zhou Q, Wang D, Zou D, Shu J, et al. MiR-124 inhibits invasion and induces apoptosis of ovarian cancer cells by targeting programmed cell death 6. Oncol Lett. 2017. Dec;14(6):7311–7. 10.3892/ol.2017.7157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceppi P, Hadji A, Kohlhapp FJ, Pattanayak A, Hau A, Liu X, et al. CD95 and CD95L promote and protect cancer stem cells. Nat Commun. 2014. Nov;5(1):5238. 10.1038/ncomms6238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schickel R, Park SM, Murmann AE, Peter ME. miR-200c regulates induction of apoptosis through CD95 by targeting FAP-1. Mol Cell. 2010. Jun;38(6):908–15. 10.1016/j.molcel.2010.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su YF, Zang YF, Wang YH, Ding YL. MiR-19-3p Induces Tumor Cell Apoptosis via Targeting FAS in Rectal Cancer Cells. Technol Cancer Res Treat. 2020. Jan-Dec;19:1533033820917978. 10.1177/1533033820917978 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kaku H, Rothstein TL. FAIM Is a Non-redundant Defender of Cellular Viability in the Face of Heat and Oxidative Stress and Interferes With Accumulation of Stress-Induced Protein Aggregates. Front Mol Biosci. 2020. Feb;7:32. 10.3389/fmolb.2020.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia E, Masanas M, López-Soriano J, Segura MF, Comella JX, Pérez-García MJ. FAIM Is Regulated by MiR-206, MiR-1-3p and MiR-133b. Front Cell Dev Biol. 2020. Dec;8:584606. 10.3389/fcell.2020.584606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Wang D, Sun G, Mei F, Cui Y, Xu H. Effect of miR-21 on Apoptosis in Lung Cancer Cell Through Inhibiting the PI3K/ Akt/NF-κB Signaling Pathway in Vitro and in Vivo. Cell Physiol Biochem. 2018;46(3):999–1008. 10.1159/000488831 [DOI] [PubMed] [Google Scholar]
- Liu T, Liu S, Xu Y, Shu R, Wang F, Chen C, et al. Circular RNA-ZFR Inhibited Cell Proliferation and Promoted Apoptosis in Gastric Cancer by Sponging miR-130a/miR-107 and Modulating PTEN. Cancer Res Treat. 2018. Oct;50(4):1396–417. 10.4143/crt.2017.537 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zheng P, Chen L, Yuan X, Luo Q, Liu Y, Xie G, et al. Exosomal transfer of tumor-associated macrophage-derived miR-21 confers cisplatin resistance in gastric cancer cells. J Exp Clin Cancer Res. 2017. Apr;36(1):53. 10.1186/s13046-017-0528-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi YP, Liu GL, Li S, Liu XL. miR-17-5p knockdown inhibits proliferation, autophagy and promotes apoptosis in thyroid cancer via targeting PTEN. Neoplasma. 2020. Mar;67(2):249–58. 10.4149/neo_2019_190110N29 [DOI] [PubMed] [Google Scholar]
- Abe R, Yamagishi S. AGE-RAGE system and carcinogenesis. Curr Pharm Des. 2008;14(10):940–5. 10.2174/138161208784139765 [DOI] [PubMed] [Google Scholar]
- Haque E, Kamil M, Hasan A, Irfan S, Sheikh S, Khatoon A, et al. Advanced glycation end products (AGEs), protein aggregation and their cross talk: new insight in tumorigenesis. Glycobiology. 2019. Dec;30(1):49–57. 10.1093/glycob/cwz073 [DOI] [PubMed] [Google Scholar]
- Mardente S, Mari E, Consorti F, Di Gioia C, Negri R, Etna M, et al. HMGB1 induces the overexpression of miR-222 and miR-221 and increases growth and motility in papillary thyroid cancer cells. Oncol Rep. 2012. Dec;28(6):2285–9. 10.3892/or.2012.2058 [DOI] [PubMed] [Google Scholar]
- Yin C, Zhang G, Sun R, Pan X, Wang X, Li H, et al. miR‑185‑5p inhibits F‑actin polymerization and reverses epithelial mesenchymal transition of human breast cancer cells by modulating RAGE. Mol Med Rep. 2018. Sep;18(3):2621–30. 10.3892/mmr.2018.9294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing R, Chen W, Wang H, Ju S, Cong H, Sun B, et al. Plasma miR-185 is decreased in patients with esophageal squamous cell carcinoma and might suppress tumor migration and invasion by targeting RAGE. Am J Physiol Gastrointest Liver Physiol. 2015. Nov;309(9):G719–29. 10.1152/ajpgi.00078.2015 [DOI] [PubMed] [Google Scholar]
- Mercado-Pimentel ME, Onyeagucha BC, Li Q, Pimentel AC, Jandova J, Nelson MA. The S100P/RAGE signaling pathway regulates expression of microRNA-21 in colon cancer cells. FEBS Lett. 2015. Aug;589(18):2388–93. 10.1016/j.febslet.2015.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong K, Xu G, Grayson TB, Shalev A. Cytokines Regulate β-Cell Thioredoxin-interacting Protein (TXNIP) via Distinct Mechanisms and Pathways. J Biol Chem. 2016. Apr;291(16):8428–39. 10.1074/jbc.M115.698365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Shi Y, McCaw L, Li YJ, Zhu F, Gorczynski R, et al. Microenvironmental interleukin-6 suppresses toll-like receptor signaling in human leukemia cells through miR-17/19A. Blood. 2015. Aug;126(6):766–78. 10.1182/blood-2014-12-618678 [DOI] [PubMed] [Google Scholar]
- Peña-Cano MI, Saucedo R, Morales-Avila E, Valencia J, Zavala-Moha JA, López A. Deregulated microRNAs and Adiponectin in Postmenopausal Women with Breast Cancer. Gynecol Obstet Invest. 2019;84(4):369–77. 10.1159/000496340 [DOI] [PubMed] [Google Scholar]
- Yu X, Wang D, Wang X, Sun S, Zhang Y, Wang S, et al. CXCL12/CXCR4 promotes inflammation-driven colorectal cancer progression through activation of RhoA signaling by sponging miR-133a-3p. J Exp Clin Cancer Res. 2019. Jan;38(1):32. 10.1186/s13046-018-1014-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooki A, Del Carmen Rodriguez Pena M, Marchionni L, Dinalankara W, Begum A, Hahn NM, et al. YAP1 and COX2 Coordinately Regulate Urothelial Cancer Stem-like Cells. Cancer Res. 2018. Jan;78(1):168–81. 10.1158/0008-5472.CAN-17-0836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Wang W, Gao Z, Peng X, Chen X, Chen W, et al. MicroRNA-155 promotes glioma cell proliferation via the regulation of MXI1. PLoS One. 2013. Dec;8(12):e83055. 10.1371/journal.pone.0083055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Zhou X, Xu L, Rong C, Shen C, Bian W. Long noncoding RNA ANRIL could be transactivated by c-Myc and promote tumor progression of non-small-cell lung cancer. OncoTargets Ther. 2016. May;9:3077–84. 10.2147/OTT.S102658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Mo J, Luo M, Yu Q, Zhou S, Li T, et al. c-Myc-activated long non-coding RNA H19 downregulates miR-107 and promotes cell cycle progression of non-small cell lung cancer. Int J Clin Exp Pathol. 2015. Oct;8(10):12400–9. [PMC free article] [PubMed] [Google Scholar]
- Zhang EB, Han L, Yin DD, Kong R, De W, Chen J. c-Myc-induced, long, noncoding H19 affects cell proliferation and predicts a poor prognosis in patients with gastric cancer. Med Oncol. 2014. May;31(5):914. 10.1007/s12032-014-0914-7 [DOI] [PubMed] [Google Scholar]
- Barsyte-Lovejoy D, Lau SK, Boutros PC, Khosravi F, Jurisica I, Andrulis IL, et al. The c-Myc oncogene directly induces the H19 noncoding RNA by allele-specific binding to potentiate tumorigenesis. Cancer Res. 2006. May;66(10):5330–7. 10.1158/0008-5472.CAN-06-0037 [DOI] [PubMed] [Google Scholar]
- Cho SW, Xu J, Sun R, Mumbach MR, Carter AC, Chen YG, et al. Promoter of lncRNA Gene PVT1 Is a Tumor-Suppressor DNA Boundary Element. Cell. 2018. May;173(6):1398–1412.e22. 10.1016/j.cell.2018.03.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Zou H, Chen A, Yang H, Yu X, Yu X, et al. C-Myc-activated long non-coding RNA PVT1 enhances the proliferation of cervical cancer cells by sponging miR-486-3p. J Biochem. 2020. Jun;167(6):565–75. 10.1093/jb/mvaa005 [DOI] [PubMed] [Google Scholar]
- Tseng YY, Moriarity BS, Gong W, Akiyama R, Tiwari A, Kawakami H, et al. PVT1 dependence in cancer with MYC copy-number increase. Nature. 2014. Aug;512(7512):82–6. 10.1038/nature13311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carramusa L, Contino F, Ferro A, Minafra L, Perconti G, Giallongo A, et al. The PVT-1 oncogene is a Myc protein target that is overexpressed in transformed cells. J Cell Physiol. 2007. Nov;213(2):511–8. 10.1002/jcp.21133 [DOI] [PubMed] [Google Scholar]
- Wang Z, Lin S, Li JJ, Xu Z, Yao H, Zhu X, et al. MYC protein inhibits transcription of the microRNA cluster MC-let-7a-1~let-7d via noncanonical E-box. J Biol Chem. 2011. Nov;286(46):39703–14. 10.1074/jbc.M111.293126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander S, Bullinger L, Klapproth K, Fiedler K, Kestler HA, Barth TF, et al. MYC stimulates EZH2 expression by repression of its negative regulator miR-26a. Blood. 2008. Nov;112(10):4202–12. 10.1182/blood-2008-03-147645 [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhao X, Fiskus W, Lin J, Lwin T, Rao R, et al. Coordinated silencing of MYC-mediated miR-29 by HDAC3 and EZH2 as a therapeutic target of histone modification in aggressive B-Cell lymphomas. Cancer Cell. 2012. Oct;22(4):506–23. 10.1016/j.ccr.2012.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Salvatori B, Iosue I, Djodji Damas N, Mangiavacchi A, Chiaretti S, Messina M, et al. Critical Role of c-Myc in Acute Myeloid Leukemia Involving Direct Regulation of miR-26a and Histone Methyltransferase EZH2. Genes Cancer. 2011. May;2(5):585–92. 10.1177/1947601911416357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey S, Kwon JJ, Liu S, Hodge GA, Taleb S, Zimmers TA, et al. miR-29a Is Repressed by MYC in Pancreatic Cancer and Its Restoration Drives Tumor-Suppressive Effects via Downregulation of LOXL2. Mol Cancer Res. 2020. Feb;18(2):311–23. 10.1158/1541-7786.MCR-19-0594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peta E, Sinigaglia A, Masi G, Di Camillo B, Grassi A, Trevisan M, et al. HPV16 E6 and E7 upregulate the histone lysine demethylase KDM2B through the c-MYC/miR-146a-5p axys. Oncogene. 2018. Mar;37(12):1654–68. 10.1038/s41388-017-0083-1 [DOI] [PubMed] [Google Scholar]
- Chen PC, Yu CC, Huang WY, Huang WH, Chuang YM, Lin RI, et al. c-Myc Acts as a Competing Endogenous RNA to Sponge miR-34a, in the Upregulation of CD44, in Urothelial Carcinoma. Cancers (Basel). 2019. Sep;11(10):E1457. 10.3390/cancers11101457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Fu Y, Guo H. c-Myc-Induced Long Non-Coding RNA Small Nucleolar RNA Host Gene 7 Regulates Glycolysis in Breast Cancer. J Breast Cancer. 2019. Nov;22(4):533–47. 10.4048/jbc.2019.22.e54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Li C, Jian Z, Ou Y, Ou J. TGF-β1 Reduces miR-29a Expression to Promote Tumorigenicity and Metastasis of Cholangiocarcinoma by Targeting HDAC4. PLoS One. 2015. Oct;10(10):e0136703. 10.1371/journal.pone.0136703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Qiu X, Li J, Zheng S, Li L, Zhao H. TGF-β secreted by tumor-associated macrophages promotes proliferation and invasion of colorectal cancer via miR-34a-VEGF axis. Cell Cycle. 2018;17(24):2766–78. 10.1080/15384101.2018.1556064 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Pan Y, Hui X, Hoo RL, Ye D, Chan CY, Feng T, et al. Adipocyte-secreted exosomal microRNA-34a inhibits M2 macrophage polarization to promote obesity-induced adipose inflammation. J Clin Invest. 2019. Feb;129(2):834–49. 10.1172/JCI123069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veremeyko T, Siddiqui S, Sotnikov I, Yung A, Ponomarev ED. IL-4/IL-13-dependent and independent expression of miR-124 and its contribution to M2 phenotype of monocytic cells in normal conditions and during allergic inflammation. PLoS One. 2013. Dec;8(12):e81774. 10.1371/journal.pone.0081774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan LJ, Qi J, Kong XJ, Huang T, Qian XQ, Xu D, et al. MiR-133 modulates TGF-β1-induced bladder smooth muscle cell hypertrophic and fibrotic response: implication for a role of microRNA in bladder wall remodeling caused by bladder outlet obstruction. Cell Signal. 2015. Feb;27(2):215–27. 10.1016/j.cellsig.2014.11.001 [DOI] [PubMed] [Google Scholar]
- Vanhaverbeke M, Gal D, Holvoet P. Functional Role of Cardiovascular Exosomes in Myocardial Injury and Atherosclerosis. Adv Exp Med Biol. 2017;998:45–58. 10.1007/978-981-10-4397-0_3 [DOI] [PubMed] [Google Scholar]
- Huber HJ, Holvoet P. Exosomes: emerging roles in communication between blood cells and vascular tissues during atherosclerosis. Curr Opin Lipidol. 2015. Oct;26(5):412–9. 10.1097/MOL.0000000000000214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrabi F, Ammenwerth E, McNair JB, De Keizer NF, Hyppönen H, Nykänen P, et al. Artificial Intelligence in Clinical Decision Support: Challenges for Evaluating AI and Practical Implications. Yearb Med Inform. 2019. Aug;28(1):128–34. 10.1055/s-0039-1677903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong W, Zheng J, Liu X, Ma J, Liu Y, Xue Y. Knockdown of NEAT1 restrained the malignant progression of glioma stem cells by activating microRNA let-7e. Oncotarget. 2016. Sep;7(38):62208–23. 10.18632/oncotarget.11403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Quan Q. The long non-coding RNA SNHG4/microRNA-let-7e/KDM3A/p21 pathway is involved in the development of non-small cell lung cancer. Mol Ther Oncolytics. 2020. Dec;20:634–45. 10.1016/j.omto.2020.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Dai B, Ni X. Long non-coding RNA cancer susceptibility candidate 2 (CASC2) alleviates the high glucose-induced injury of CIHP-1 cells via regulating miR-9-5p/PPARγ axis in diabetes nephropathy. Diabetol Metab Syndr. 2020. Aug;12(1):68. 10.1186/s13098-020-00574-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M, Xiao Z, Wang Y, Zheng M, Song T, Cai X, et al. Long noncoding RNA HULC modulates abnormal lipid metabolism in hepatoma cells through an miR-9-mediated RXRA signaling pathway. Cancer Res. 2015. Mar;75(5):846–57. 10.1158/0008-5472.CAN-14-1192 [DOI] [PubMed] [Google Scholar]
- Feng L, Li H, Li F, Bei S, Zhang X. LncRNA KCNQ1OT1 regulates microRNA-9-LMX1A expression and inhibits gastric cancer cell progression. Aging (Albany NY). 2020. Jan;12(1):707–17. 10.18632/aging.102651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q, Lin S, Zheng M, Cai Q, Tu Y. Long noncoding RNA NEAT1 promotes the growth of cervical cancer cells via sponging miR-9-5p. Biochem Cell Biol. 2019. Apr;97(2):100–8. 10.1139/bcb-2018-0111 [DOI] [PubMed] [Google Scholar]
- Wang S, Cheng M, Zheng X, Zheng L, Liu H, Lu J, et al. Interactions Between lncRNA TUG1 and miR-9-5p Modulate the Resistance of Breast Cancer Cells to Doxorubicin by Regulating eIF5A2. OncoTargets Ther. 2020. Dec;13:13159–70. 10.2147/OTT.S255113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Qiao L, Zang Y, Ni W, Xu Z. Circular RNA FOXO3 Suppresses Bladder Cancer Progression and Metastasis by Regulating MiR-9-5p/TGFBR2. Cancer Manag Res. 2020. Jun;12:5049–56. 10.2147/CMAR.S253412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D, Li J, Wang H, Su X, Hou J, Gu Y, et al. Circular RNA circMTO1 acts as the sponge of microRNA-9 to suppress hepatocellular carcinoma progression. Hepatology. 2017. Oct;66(4):1151–64. 10.1002/hep.29270 [DOI] [PubMed] [Google Scholar]
- Pidíkova P, Reis R, Herichova I. miRNA Clusters with Down-Regulated Expression in Human Colorectal Cancer and Their Regulation. Int J Mol Sci. 2020. Jun;21(13):E4633. 10.3390/ijms21134633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Wei M, Jiang X, Tan J, Xu W, Fan X, et al. lncRNA PVT1 Promotes Tumorigenesis of Colorectal Cancer by Stabilizing miR-16-5p and Interacting with the VEGFA/VEGFR1/AKT Axis. Mol Ther Nucleic Acids. 2020. Jun;20:438–50. 10.1016/j.omtn.2020.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Meng Q, Li X, Yang H, Xu J, Gao N, et al. Long Noncoding RNA MIR17HG Promotes Colorectal Cancer Progression via miR-17-5p. Cancer Res. 2019. Oct;79(19):4882–95. 10.1158/0008-5472.CAN-18-3880 [DOI] [PubMed] [Google Scholar]
- Huang FX, Chen HJ, Zheng FX, Gao ZY, Sun PF, Peng Q, et al. LncRNA BLACAT1 is involved in chemoresistance of non‑small cell lung cancer cells by regulating autophagy. Int J Oncol. 2019. Jan;54(1):339–47. 10.3892/ijo.2018.4614 [DOI] [PubMed] [Google Scholar]
- Zhang G, An X, Zhao H, Zhang Q, Zhao H. Long non-coding RNA HNF1A-AS1 promotes cell proliferation and invasion via regulating miR-17-5p in non-small cell lung cancer. Biomed Pharmacother. 2018. Feb;98:594–9. 10.1016/j.biopha.2017.12.080 [DOI] [PubMed] [Google Scholar]
- Liu X, Liu G, Lu Y, Shi Y. Long non-coding RNA HOTAIR promotes cell viability, migration and invasion in thyroid cancer cells by sponging miR-17-5p. Neoplasma. 2020. Mar;67(2):229–37. 10.4149/neo_2019_190310N208 [DOI] [PubMed] [Google Scholar]
- Jia J, Zhang X, Zhan D, Li J, Li Z, Li H, et al. LncRNA H19 interacted with miR-130a-3p and miR-17-5p to modify radio-resistance and chemo-sensitivity of cardiac carcinoma cells. Cancer Med. 2019. Apr;8(4):1604–18. 10.1002/cam4.1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ao X, Jiang M, Zhou J, Liang H, Xia H, Chen G. lincRNA‑p21 inhibits the progression of non‑small cell lung cancer via targeting miR‑17‑5p. Oncol Rep. 2019. Feb;41(2):789–800. 10.3892/or.2018.6900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S, Wang K, Huang X, Tang W, Zhao Z, Zhao Z. Knockdown of the lncRNA MALAT1 alleviates lipopolysaccharide‑induced A549 cell injury by targeting the miR‑17‑5p/FOXA1 axis. Mol Med Rep. 2019. Aug;20(2):2021–9. 10.3892/mmr.2019.10392 [DOI] [PubMed] [Google Scholar]
- Wang CL, Wang D, Yan BZ, Fu JW, Qin L. Long non-coding RNA NEAT1 promotes viability and migration of gastric cancer cell lines through up-regulation of microRNA-17. Eur Rev Med Pharmacol Sci. 2018. Jul;22(13):4128–37. 10.26355/eurrev_201807_15405 [DOI] [PubMed] [Google Scholar]
- Peng J, Hou F, Zhu W, Li J, Teng Z. lncRNA NR2F1-AS1 Regulates miR-17/SIK1 Axis to Suppress the Invasion and Migration of Cervical Squamous Cell Carcinoma Cells. Reprod Sci. 2020. Jul;27(7):1534–9. 10.1007/s43032-020-00149-y [DOI] [PubMed] [Google Scholar]
- Sun W, Zu Y, Fu X, Deng Y. Knockdown of lncRNA-XIST enhances the chemosensitivity of NSCLC cells via suppression of autophagy. Oncol Rep. 2017. Dec;38(6):3347–54. 10.3892/or.2017.6056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Yuan W, Yang X, Li P, Wang J, Han J, et al. Circular RNA circ-ITCH inhibits bladder cancer progression by sponging miR-17/miR-224 and regulating p21, PTEN expression. Mol Cancer. 2018. Jan;17(1):19. 10.1186/s12943-018-0771-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han K, Wang FW, Cao CH, Ling H, Chen JW, Chen RX, et al. CircLONP2 enhances colorectal carcinoma invasion and metastasis through modulating the maturation and exosomal dissemination of microRNA-17. Mol Cancer. 2020. Mar;19(1):60. 10.1186/s12943-020-01184-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Guo B. Circ-MTO1 correlates with favorable prognosis and inhibits cell proliferation, invasion as well as miR-17-5p expression in prostate cancer. J Clin Lab Anal. 2020. Mar;34(3):e23086. 10.1002/jcla.23086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Xu QG, Wang ZG, Yang Y, Zhang L, Ma JZ, et al. Circular RNA cSMARCA5 inhibits growth and metastasis in hepatocellular carcinoma. J Hepatol. 2018. Jun;68(6):1214–27. 10.1016/j.jhep.2018.01.012 [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhang Q, Sun Y, Shao F. Long Non-Coding RNA PVT1 Regulates BAMBI To Promote Tumor Progression In Non-Small Cell Lung Cancer By Sponging miR-17-5p. OncoTargets Ther. 2020. Jan;13:131–42. 10.2147/OTT.S217335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Q, Wang L, Lv Y, Wu J, Shi W. Deletion of HNF1A-AS1 Suppresses the Malignant Phenotypes of Breast Cancer Cells In Vitro and In Vivo Through Targeting miRNA-20a-5p/TRIM32 Axis. Cancer Biother Radiopharm. 2021. Feb;36(1):23–35. 10.1089/cbr.2019.3168 [DOI] [PubMed] [Google Scholar]
- Zhao W, Geng D, Li S, Chen Z, Sun M. LncRNA HOTAIR influences cell growth, migration, invasion, and apoptosis via the miR-20a-5p/HMGA2 axis in breast cancer. Cancer Med. 2018. Mar;7(3):842–55. 10.1002/cam4.1353 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yang BY, Meng Q, Sun Y, Gao L, Yang JX. Long non-coding RNA SNHG16 contributes to glioma malignancy by competitively binding miR-20a-5p with E2F1. J Biol Regul Homeost Agents. 2018. Mar-Apr;32(2):251–61. [PubMed] [Google Scholar]
- Huang F, Chen W, Peng J, Li Y, Zhuang Y, Zhu Z, et al. LncRNA PVT1 triggers Cyto-protective autophagy and promotes pancreatic ductal adenocarcinoma development via the miR-20a-5p/ULK1 Axis. Mol Cancer. 2018. Jul;17(1):98. 10.1186/s12943-018-0845-6 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Han M, Wang S, Fritah S, Wang X, Zhou W, Yang N, et al. Interfering with long non-coding RNA MIR22HG processing inhibits glioblastoma progression through suppression of Wnt/β-catenin signalling. Brain. 2020. Feb;143(2):512–30. 10.1093/brain/awz406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma Saha S, Roy Chowdhury R, Mondal NR, Chakravarty B, Chatterjee T, Roy S, et al. Identification of genetic variation in the lncRNA HOTAIR associated with HPV16-related cervical cancer pathogenesis. Cell Oncol (Dordr). 2016. Dec;39(6):559–72. 10.1007/s13402-016-0298-0 [DOI] [PubMed] [Google Scholar]
- Gan L, Lv L, Liao S. Long non‑coding RNA H19 regulates cell growth and metastasis via the miR‑22‑3p/Snail1 axis in gastric cancer. Int J Oncol. 2019. Jun;54(6):2157–68. 10.3892/ijo.2019.4773 [DOI] [PubMed] [Google Scholar]
- Li DY, Chen WJ, Luo L, Wang YK, Shang J, Zhang Y, et al. Prospective lncRNA-miRNA-mRNA regulatory network of long non-coding RNA LINC00968 in non-small cell lung cancer A549 cells: A miRNA microarray and bioinformatics investigation. Int J Mol Med. 2017. Dec;40(6):1895–906. 10.3892/ijmm.2017.3187 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Li M, Zhang Z. lncRNA MALAT1 modulates oxaliplatin resistance of gastric cancer via sponging miR-22-3p. OncoTargets Ther. 2020. Feb;13:1343–54. 10.2147/OTT.S196619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Duan M, Lin L, Wu C, Fu X, Wang H, et al. TET2 and MEG3 promoter methylation is associated with acute myeloid leukemia in a Hainan population. Oncotarget. 2017. Mar;8(11):18337–47. 10.18632/oncotarget.15440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Hu K, Cao J, Wang P, Li J, Zeng K, et al. lncRNA miat functions as a ceRNA to upregulate sirt1 by sponging miR-22-3p in HCC cellular senescence. Aging (Albany NY). 2019. Sep;11(17):7098–122. 10.18632/aging.102240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan B, Ma J, Yang Z, Yu F, Yao J. LncRNA NCK1-AS1 exerts oncogenic property in gastric cancer by targeting the miR-22-3p/BCL9 axis to activate the Wnt/β-catenin signaling. Environ Toxicol. 2021. Aug;36(8):1640–53. 10.1002/tox.23160 [DOI] [PubMed] [Google Scholar]
- Cruickshank BM, Wasson MD, Brown JM, Fernando W, Venkatesh J, Walker OL, et al. LncRNA PART1 Promotes Proliferation and Migration, Is Associated with Cancer Stem Cells, and Alters the miRNA Landscape in Triple-Negative Breast Cancer. Cancers (Basel). 2021. May;13(11):2644. 10.3390/cancers13112644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren C, Liu J, Zheng B, Yan P, Sun Y, Yue B. The circular RNA circ-ITCH acts as a tumour suppressor in osteosarcoma via regulating miR-22. Artif Cells Nanomed Biotechnol. 2019. Dec;47(1):3359–67. 10.1080/21691401.2019.1649273 [DOI] [PubMed] [Google Scholar]
- Liu J, Chen M, Ma L, Dang X, Du G. LncRNA GAS5 Suppresses the Proliferation and Invasion of Osteosarcoma Cells via the miR-23a-3p/PTEN/PI3K/AKT Pathway. Cell Transplant. 2020. Jan-Dec;29:963689720953093. 10.1177/0963689720953093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Hu L, Fu G, Lu J, Zheng Y, Li Y, et al. LncRNA MALAT1 Promotes the Proliferation, Migration, and Invasion of Melanoma Cells by Downregulating miR-23a. Cancer Manag Res. 2020. Jul;12:6553–62. 10.2147/CMAR.S249348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wu N, Wang J, Li Z. LncRNA MEG3 inhibits cell proliferation and induces apoptosis in laryngeal cancer via miR-23a/APAF-1 axis. J Cell Mol Med. 2019. Oct;23(10):6708–19. 10.1111/jcmm.14549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Wang S, Zhao Y, Du F, Wang W, Lv P, et al. Long noncoding RNA NEAT1 modulates cell proliferation and apoptosis by regulating miR-23a-3p/SMC1A in acute myeloid leukemia. J Cell Physiol. 2019. May;234(5):6161–72. 10.1002/jcp.27393 [DOI] [PubMed] [Google Scholar]
- Lin H, Shen L, Lin Q, Dong C, Maswela B, Illahi GS, et al. SNHG5 enhances Paclitaxel sensitivity of ovarian cancer cells through sponging miR-23a. Biomed Pharmacother. 2020. Mar;123:109711. 10.1016/j.biopha.2019.109711 [DOI] [PubMed] [Google Scholar]
- Liu Y, Li Q, Tang D, Li M, Zhao P, Yang W, et al. SNHG17 promotes the proliferation and migration of colorectal adenocarcinoma cells by modulating CXCL12-mediated angiogenesis. Cancer Cell Int. 2020. Nov;20(1):566. 10.1186/s12935-020-01621-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao YQ, Liu KW, Zhang X, Kang SX, Zhang K, Han W, et al. GINS2 was regulated by lncRNA XIST/miR-23a-3p to mediate proliferation and apoptosis in A375 cells. Mol Cell Biochem. 2021. Mar;476(3):1455–65. 10.1007/s11010-020-04007-y [DOI] [PubMed] [Google Scholar]
- Wang X, Guo Y, Wang C, Wang Q, Yan G. Long Noncoding RNA ZEB1-AS1 Downregulates miR-23a, Promotes Tumor Progression, and Predicts the Survival of Oral Squamous Cell Carcinoma Patients. OncoTargets Ther. 2021. Apr;14:2699–710. 10.2147/OTT.S297209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Sun J, Lou L, Fan X, Zhang W, Li Q. Overexpression of lncRNA CASC2 inhibits the tumorigenesis of thyroid cancer via sponging miR-24-3p. Am J Transl Res. 2020. Oct;12(10):6314–24. [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Wang W, Lin J, Xiao J, Tian Y. lncRNA CCAT1 promotes bladder cancer cell proliferation, migration and invasion. Int Braz J Urol. 2019. May-Jun;45(3):549–59. 10.1590/S1677-5538.IBJU.2018.0450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Xiong HY, Li YM, Zuo HR, Liu Y, Liao GL. LncRNA HOXA11-AS promotes cell growth by sponging miR-24-3p to regulate JPT1 in prostate cancer. Eur Rev Med Pharmacol Sci. 2021. Jul;25(14):4668–77. 10.26355/eurrev_202107_26377 [DOI] [PubMed] [Google Scholar]
- Luan L, Hu Q, Wang Y, Lu L, Ling J. Knockdown of lncRNA NEAT1 expression inhibits cell migration, invasion and EMT by regulating the miR-24-3p/LRG1 axis in retinoblastoma cells. Exp Ther Med. 2021. Apr;21(4):367. 10.3892/etm.2021.9798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Gu T, Chen Y, Chen Y, Xiong D, Zhu Y. Long non-coding RNA SOX21-AS1 modulates lung cancer progress upon microRNA miR-24-3p/PIM2 axis. Bioengineered. 2021. Dec;12(1):6724–37. 10.1080/21655979.2021.1955578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Jian Z, Jin H, Wei X, Zou X, Guan R, et al. Long non-coding RNA DLGAP1-AS1 facilitates tumorigenesis and epithelial-mesenchymal transition in hepatocellular carcinoma via the feedback loop of miR-26a/b-5p/IL-6/JAK2/STAT3 and Wnt/β-catenin pathway. Cell Death Dis. 2020. Jan;11(1):34. 10.1038/s41419-019-2188-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RQ, Long XR, Zhou NN, Chen DN, Zhang MY, Wen ZS, et al. Lnc-GAN1 expression is associated with good survival and suppresses tumor progression by sponging mir-26a-5p to activate PTEN signaling in non-small cell lung cancer. J Exp Clin Cancer Res. 2021. Jan;40(1):9. 10.1186/s13046-020-01819-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhu Y, Ni S, Liu S. LncRNA GAS5 Suppressed Proliferation and Promoted Apoptosis in Laryngeal Squamous Cell Carcinoma by Targeting MiR-26a-5p and Modifying ULK2. Cancer Manag Res. 2021. Jan;13:871–87. 10.2147/CMAR.S250778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ML, Zhang Y, Ma LT. LncRNA HCG11 accelerates the progression of hepatocellular carcinoma via miR-26a-5p/ATG12 axis. Eur Rev Med Pharmacol Sci. 2019. Dec;23(24):10708–20. 10.26355/eurrev_201912_19771 [DOI] [PubMed] [Google Scholar]
- Xu J, Xiao Y, Liu B, Pan S, Liu Q, Shan Y, et al. Exosomal MALAT1 sponges miR-26a/26b to promote the invasion and metastasis of colorectal cancer via FUT4 enhanced fucosylation and PI3K/Akt pathway. J Exp Clin Cancer Res. 2020. Mar;39(1):54. 10.1186/s13046-020-01562-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia LF, Wei SB, Gan YH, Guo Y, Gong K, Mitchelson K, et al. Expression, regulation and roles of miR-26a and MEG3 in tongue squamous cell carcinoma. Int J Cancer. 2014. Nov;135(10):2282–93. 10.1002/ijc.28667 [DOI] [PubMed] [Google Scholar]
- Wang SH, Yang Y, Wu XC, Zhang MD, Weng MZ, Zhou D, et al. Long non-coding RNA MINCR promotes gallbladder cancer progression through stimulating EZH2 expression. Cancer Lett. 2016. Sep;380(1):122–33. 10.1016/j.canlet.2016.06.019 [DOI] [PubMed] [Google Scholar]
- Fan JT, Zhou ZY, Luo YL, Luo Q, Chen SB, Zhao JC, et al. Exosomal lncRNA NEAT1 from cancer-associated fibroblasts facilitates endometrial cancer progression via miR-26a/b-5p-mediated STAT3/YKL-40 signaling pathway. Neoplasia. 2021. Jul;23(7):692–703. 10.1016/j.neo.2021.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Zhao Y, Su L, Wu Z, Jiang W, Jiang X, et al. Silencing the lncRNA NORAD inhibits EMT of head and neck squamous cell carcinoma stem cells via miR‑26a‑5p. Mol Med Rep. 2021. Nov;24(5):743. 10.3892/mmr.2021.12383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YS, Chu KJ, Ling CC, Wu TM, Zhu XC, Liu JB, et al. Long Noncoding RNA OIP5-AS1 Promotes the Progression of Liver Hepatocellular Carcinoma via Regulating the hsa-miR-26a-3p/EPHA2 Axis. Mol Ther Nucleic Acids. 2020. Sep;21:229–41. 10.1016/j.omtn.2020.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Xu M, Chen X, Lin K, Zeng K, Liu X, Xu X, et al. lncRNA SNHG6 regulates EZH2 expression by sponging miR-26a/b and miR-214 in colorectal cancer. J Hematol Oncol. 2019. Jan;12(1):3. 10.1186/s13045-018-0690-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Zeng K, Liu Y, Gao L, Liu L. LncRNA SNHG5 promotes growth and invasion in melanoma by regulating the miR-26a-5p/TRPC3 pathway. OncoTargets Ther. 2018. Dec;12:169–79. 10.2147/OTT.S184078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Zhao ZF, Xie L, Zhu JP. Taurine up-regulated 1 accelerates tumorigenesis of colon cancer by regulating miR-26a-5p/MMP14/p38 MAPK/Hsp27 axis in vitro and in vivo. Life Sci. 2019. Dec;239:117035. 10.1016/j.lfs.2019.117035 [DOI] [PubMed] [Google Scholar]
- Si Z, Yu L, Jing H, Wu L, Wang X. Oncogenic lncRNA ZNF561-AS1 is essential for colorectal cancer proliferation and survival through regulation of miR-26a-3p/miR-128-5p-SRSF6 axis. J Exp Clin Cancer Res. 2021. Feb;40(1):78. 10.1186/s13046-021-01882-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ni J, Song D, Ding M, Huang J, Li W, et al. The regulatory effect of has-circ-0001146/miR-26a-5p/MNAT1 network on the proliferation and invasion of osteosarcoma. Biosci Rep. 2020. Jun;40(6):BSR20201232. 10.1042/BSR20201232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muluhngwi P, Klinge CM. Identification and Roles of miR-29b-1-3p and miR29a-3p-Regulated and Non-Regulated lncRNAs in Endocrine-Sensitive and Resistant Breast Cancer Cells. Cancers (Basel). 2021. Jul;13(14):3530. 10.3390/cancers13143530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv M, Zhong Z, Huang M, Tian Q, Jiang R, Chen J. lncRNA H19 regulates epithelial-mesenchymal transition and metastasis of bladder cancer by miR-29b-3p as competing endogenous RNA. Biochim Biophys Acta Mol Cell Res. 2017. Oct;1864(10):1887–99. 10.1016/j.bbamcr.2017.08.001 [DOI] [PubMed] [Google Scholar]
- Braconi C, Kogure T, Valeri N, Huang N, Nuovo G, Costinean S, et al. microRNA-29 can regulate expression of the long non-coding RNA gene MEG3 in hepatocellular cancer. Oncogene. 2011. Nov;30(47):4750–6. 10.1038/onc.2011.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T, Liu Z, Zhang R, Ma S, Lin T, Li Y, et al. Long Non-Coding RNA LEF1-AS1 Promotes Migration, Invasion and Metastasis of Colon Cancer Cells Through miR-30-5p/SOX9 Axis. OncoTargets Ther. 2020. Apr;13:2957–72. 10.2147/OTT.S232839 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhang Y, Li Y. Long non-coding RNA NORAD contributes to the proliferation, invasion and EMT progression of prostate cancer via the miR-30a-5p/RAB11A/WNT/β-catenin pathway. Cancer Cell Int. 2020. Nov;20(1):571. 10.1186/s12935-020-01665-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Lv X, Zeng L, Li W, Zhong Y, Yuan J, et al. CircPVT1 promotes proliferation of lung squamous cell carcinoma by binding to miR-30d/e. J Exp Clin Cancer Res. 2021. Jun;40(1):193. 10.1186/s13046-021-01976-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Zhu K, Liu L, Gu J, Niu H, Guo J. lncARSR sponges miR-34a-5p to promote colorectal cancer invasion and metastasis via hexokinase-1-mediated glycolysis. Cancer Sci. 2020. Oct;111(10):3938–52. 10.1111/cas.14617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F, Jiang C, Bu G, Fu Y, Yu Y. Silencing long noncoding RNA colon cancer-associated transcript-1 upregulates microRNA-34a-5p to promote proliferation and differentiation of osteoblasts in osteoporosis. Cancer Gene Ther. 2021. Nov;28(10-11):1150–61. 10.1038/s41417-020-00264-7 [DOI] [PubMed] [Google Scholar]
- Huang S, Li C, Huang J, Luo P, Mo D, Wang H. LncRNA FEZF1-AS1 promotes non-small lung cancer cell migration and invasion through the up-regulation of NOTCH1 by serving as a sponge of miR-34a. BMC Pulm Med. 2020. Apr;20(1):110. 10.1186/s12890-020-1154-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HG, Wang FJ, Wang Y, Zhao ZX, Qiao PF. lncRNA GAS5 inhibits malignant progression by regulating macroautophagy and forms a negative feedback regulatory loop with the miR‑34a/mTOR/SIRT1 pathway in colorectal cancer. Oncol Rep. 2021. Jan;45(1):202–16. 10.3892/or.2020.7825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang C, Qiu S, Sun F, Li W, Wang Z, Yue B, et al. Long non-coding RNA HNF1A-AS1 mediated repression of miR-34a/SIRT1/p53 feedback loop promotes the metastatic progression of colon cancer by functioning as a competing endogenous RNA. Cancer Lett. 2017. Dec;410:50–62. 10.1016/j.canlet.2017.09.012 [DOI] [PubMed] [Google Scholar]
- Zheng F, Li J, Ma C, Tang X, Tang Q, Wu J, et al. Novel regulation of miR-34a-5p and HOTAIR by the combination of berberine and gefitinib leading to inhibition of EMT in human lung cancer. J Cell Mol Med. 2020. May;24(10):5578–92. 10.1111/jcmm.15214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li C, Li D, Yang L, Jin J, Zhang B. lncRNA KCNQ1OT1 enhances the chemoresistance of oxaliplatin in colon cancer by targeting the miR-34a/ATG4B pathway. OncoTargets Ther. 2019. Apr;12:2649–60. 10.2147/OTT.S188054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Li Y, Zhi S, Li J, Miao J, Ding Z, et al. LncRNA-ROR/microRNA-185-3p/YAP1 axis exerts function in biological characteristics of osteosarcoma cells. Genomics. 2021. Jan;113(1 Pt 2):450–61. 10.1016/j.ygeno.2020.09.009 [DOI] [PubMed] [Google Scholar]
- Jin J, Chen X, Chen J, Geng X. Long noncoding RNA MACC1-AS1 is a potential sponge of microRNA-34a in cervical squamous cell carcinoma and upregulates cyclin-dependent kinase 6. Oncol Lett. 2020. Mar;19(3):2339–45. 10.3892/ol.2020.11346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Zhang T, Chen B. Long Non-Coding RNA Metastasis-Associated Lung Adenocarcinoma Transcript 1 (MALAT1) Promotes Proliferation and Metastasis of Osteosarcoma Cells by Targeting c-Met and SOX4 via miR-34a/c-5p and miR-449a/b. Med Sci Monit. 2019. Feb;25:1410–22. 10.12659/MSM.912703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Li C, Luo Y, Li L, Liu J, Gui R. Silencing of Long Non-coding RNA MIAT Sensitizes Lung Cancer Cells to Gefitinib by Epigenetically Regulating miR-34a. Front Pharmacol. 2018. Feb;9:82. 10.3389/fphar.2018.00082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Guo S, Zhang Y, Zhao Y, Li X, Jia Y, et al. LncRNA NEAT1 promotes docetaxel resistance in prostate cancer by regulating ACSL4 via sponging miR-34a-5p and miR-204-5p. Cell Signal. 2020. Jan;65:109422. 10.1016/j.cellsig.2019.109422 [DOI] [PubMed] [Google Scholar]
- Jiang X, Ye Z, Jiang Y, Yu W, Fang Q. LncRNA OIP5-AS1 upregulates snail expression by sponging miR-34a to promote ovarian carcinoma cell invasion and migration. Biol Res. 2020. Oct;53(1):49. 10.1186/s40659-020-00315-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Chen X, Zhang Y, Hu Y, Shen X, Zhu W. Long non-coding RNA TUG1 promotes endometrial cancer development via inhibiting miR-299 and miR-34a-5p. Oncotarget. 2017. May;8(19):31386–94. 10.18632/oncotarget.15607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Deng H, Zhao Y, Li C, Liang Y. LncRNA XIST/miR-34a axis modulates the cell proliferation and tumor growth of thyroid cancer through MET-PI3K-AKT signaling. J Exp Clin Cancer Res. 2018. Nov;37(1):279. 10.1186/s13046-018-0950-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CH, Li QY, Nie L, Ma J, Yao CJ, Chen FP. LncRNA ANRIL promotes cell proliferation, migration and invasion during acute myeloid leukemia pathogenesis via negatively regulating miR-34a. Int J Biochem Cell Biol. 2020. Feb;119:105666. 10.1016/j.biocel.2019.105666 [DOI] [PubMed] [Google Scholar]
- Ye W, Chen L, Feng C, Liang T. CircMYLK promotes the growth, migration, invasion, and survival of bladder cancer cells by upregulating CCND3 level via competitively binding to miR-34a. Drug Dev Res. 2021. Dec;82(8):1206–16. 10.1002/ddr.21835 [DOI] [PubMed] [Google Scholar]
- Zhu L, Wang A, Gao M, Duan X, Li Z. LncRNA MIR4435-2HG triggers ovarian cancer progression by regulating miR-128-3p/CKD14 axis. Cancer Cell Int. 2020. May;20(1):145. 10.1186/s12935-020-01227-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Luo X, Liu Y, Xiong J, Xia H, Liu Y. Potential role of lncRNA HULC/miR‑128‑3p/RAC1 axis in the inflammatory response during LPS‑induced sepsis in HMEC‑1 cells. Mol Med Rep. 2020. Dec;22(6):5095–104. 10.3892/mmr.2020.11601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Y, Li YJ, Li DJ, Li P, Wang JY, Diao YP, et al. Long noncoding RNA MEG3 prevents vascular endothelial cell senescence by impairing miR-128-dependent Girdin downregulation. Am J Physiol Cell Physiol. 2019. Jun;316(6):C830–43. 10.1152/ajpcell.00262.2018 [DOI] [PubMed] [Google Scholar]
- Zhang C, Xie L, Liang H, Cui Y. LncRNA MIAT facilitates osteosarcoma progression by regulating mir-128-3p/VEGFC axis. IUBMB Life. 2019. Jul;71(7):845–53. 10.1002/iub.2001 [DOI] [PubMed] [Google Scholar]
- Liu Y, Fu X, Wang X, Liu Y, Song X. Long non‑coding RNA OIP5‑AS1 facilitates the progression of ovarian cancer via the miR‑128‑3p/CCNG1 axis. Mol Med Rep. 2021. May;23(5):388. 10.3892/mmr.2021.12027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang PF, Wang F, Wu J, Wu Y, Huang W, Liu D, et al. LncRNA SNHG3 induces EMT and sorafenib resistance by modulating the miR-128/CD151 pathway in hepatocellular carcinoma. J Cell Physiol. 2019. Mar;234(3):2788–94. 10.1002/jcp.27095 [DOI] [PubMed] [Google Scholar]
- Yang L, Zhang L, Lu L, Wang Y. Long Noncoding RNA SNHG16 Sponges miR-182-5p And miR-128-3p To Promote Retinoblastoma Cell Migration And Invasion By Targeting LASP1. OncoTargets Ther. 2019. Oct;12:8653–62. 10.2147/OTT.S212352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Wang C, Dong X, Zhang Y, Li Y, Zhou H, et al. lncRNA SNHG22 sponges miR‑128‑3p to promote the progression of colorectal cancer by upregulating E2F3. Int J Oncol. 2021. Sep;59(3):71. 10.3892/ijo.2021.5251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao SD, Ma JX, Liu Y, Liu PJ, Qin Y. Long non-coding TUG1 accelerates prostate cancer progression through regulating miR-128-3p/YES1 axis. Eur Rev Med Pharmacol Sci. 2020. Jan;24(2):619–32. 10.26355/eurrev_202001_20038 [DOI] [PubMed] [Google Scholar]
- Yu C, Longfei L, Long W, Feng Z, Chen J, Chao L, et al. LncRNA PVT1 regulates VEGFC through inhibiting miR-128 in bladder cancer cells. J Cell Physiol. 2019. Feb;234(2):1346–53. 10.1002/jcp.26929 [DOI] [PubMed] [Google Scholar]
- Yang F, Peng ZX, Ji WD, Yu JD, Qian C, Liu JD, et al. LncRNA CCAT1 Upregulates ATG5 to Enhance Autophagy and Promote Gastric Cancer Development by Absorbing miR-140-3p. Dig Dis Sci. 2021. Aug;•••: 10.1007/s10620-021-07187-9 [DOI] [PubMed] [Google Scholar]
- Li J, Su T, Zou C, Luo W, Shi G, Chen L, et al. Long Non-coding RNA H19 Regulates Porcine Satellite Cell Differentiation Through miR-140-5p/SOX4 and DBN1. Front Cell Dev Biol. 2020. Nov;8:518724. 10.3389/fcell.2020.518724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou ZH, Xu XW, Fu XY, Zhou LD, Liu SP, Tan DM. Long non-coding RNA MALAT1 promotes angiogenesis and immunosuppressive properties of HCC cells by sponging miR-140. Am J Physiol Cell Physiol. 2020. Mar;318(3):C649–63. 10.1152/ajpcell.00510.2018 [DOI] [PubMed] [Google Scholar]
- He J, Xue Y, Wang Q, Zhou X, Liu L, Zhang T, et al. Long non-coding RNA MIAT regulates blood tumor barrier permeability by functioning as a competing endogenous RNA. Cell Death Dis. 2020. Oct;11(10):936. 10.1038/s41419-020-03134-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Li Y, Bai S, Zhang J, Liu Z, Yang J. NR2F1-AS1/miR-140/HK2 Axis Regulates Hypoxia-Induced Glycolysis and Migration in Hepatocellular Carcinoma. Cancer Manag Res. 2021. Jan;13:427–37. 10.2147/CMAR.S266797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei J, Liu G, Wang W, Xiao P, Yang D, Bai H, et al. OIP5-AS1 modulates epigenetic regulator HDAC7 to enhance non-small cell lung cancer metastasis via miR-140-5p. Oncol Lett. 2020. Oct;20(4):7. 10.3892/ol.2020.11868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Chen J, Song H, Chen LB. SNHG16/miR-140-5p axis promotes esophagus cancer cell proliferation, migration and EMT formation through regulating ZEB1. Oncotarget. 2017. Dec;9(1):1028–40. 10.18632/oncotarget.23178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan JB, Gu L, Chen L, Yin Y, Fan BY. Annexin A8 regulated by lncRNA-TUG1/miR-140-3p axis promotes bladder cancer progression and metastasis. Mol Ther Oncolytics. 2021. Apr;22:36–51. 10.1016/j.omto.2021.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Fang Q, Li Y, Wang Y. Amplification of lncRNA PVT1 promotes ovarian cancer proliferation by binding to miR-140. Mamm Genome. 2019. Aug;30(7-8):217–25. 10.1007/s00335-019-09808-1 [DOI] [PubMed] [Google Scholar]
- Wang P, Bao W, Liu X, Xi W. LncRNA miR143HG inhibits the proliferation of glioblastoma cells by sponging miR-504. Int J Neurosci. 2021. Feb;•••:1–9. 10.1080/00207454.2020.1865950 [DOI] [PubMed] [Google Scholar]
- Cheng H, Tian J, Wang C, Ren L, Wang N. LncRNA BLACAT1 Is Upregulated in Cervical Squamous Cell Carcinoma (CSCC) and Predicts Poor Survival. Reprod Sci. 2020. Feb;27(2):585–91. 10.1007/s43032-019-00058-9 [DOI] [PubMed] [Google Scholar]
- Hu M, Zhang Q, Tian XH, Wang JL, Niu YX, Li G. lncRNA CCAT1 is a biomarker for the proliferation and drug resistance of esophageal cancer via the miR-143/PLK1/BUBR1 axis. Mol Carcinog. 2019. Dec;58(12):2207–17. 10.1002/mc.23109 [DOI] [PubMed] [Google Scholar]
- Liu M, Jia J, Wang X, Liu Y, Wang C, Fan R. Long non-coding RNA HOTAIR promotes cervical cancer progression through regulating BCL2 via targeting miR-143-3p. Cancer Biol Ther. 2018. May;19(5):391–9. 10.1080/15384047.2018.1423921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi D, Wang M, Yu F. Knockdown of lncRNA-H19 inhibits cell viability, migration and invasion while promotes apoptosis via microRNA-143/RUNX2 axis in retinoblastoma. Biomed Pharmacother. 2019. Jan;109:798–805. 10.1016/j.biopha.2018.10.096 [DOI] [PubMed] [Google Scholar]
- Fattahi Dolatabadi N, Dehghani A, Shahand E, Yazdanshenas M, Tabatabaeian H, Zamani A, et al. The interaction between MALAT1 target, miR-143-3p, and RALGAPA2 is affected by functional SNP rs3827693 in breast cancer. Hum Cell. 2020. Oct;33(4):1229–39. 10.1007/s13577-020-00422-x [DOI] [PubMed] [Google Scholar]
- Qiao Z, Dai H, Zhang Y, Li Q, Zhao M, Yue T. LncRNA NCK1-AS1 Promotes Cancer Cell Proliferation and Increase Cell Stemness in Urinary Bladder Cancer Patients by Downregulating miR-143. Cancer Manag Res. 2020. Mar;12:1661–8. 10.2147/CMAR.S223172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zhang H, Luo H. Long Non-Coding RNA OIP5-AS1 Contributes to Gallbladder Cancer Cell Invasion and Migration by miR-143-3p Suppression. Cancer Manag Res. 2020. Dec;12:12983–92. 10.2147/CMAR.S278719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang W, Lyu L, Huang T, Zheng F, Yuan J, Zhang C, et al. The long non-coding RNA SNHG1 promotes bladder cancer progression by interacting with miR-143-3p and EZH2. J Cell Mol Med. 2020. Oct;24(20):11858–73. 10.1111/jcmm.15806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Bi M, Lou M, Yang X, Sun L. Downregulation of SOX2-OT Prevents Hepatocellular Carcinoma Progression Through miR-143-3p/MSI2. Front Oncol. 2021. Jul;11:685912. 10.3389/fonc.2021.685912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gang X, Yuan M, Zhang J. Long Non-Coding RNA TMPO-AS1 Promotes Cervical Cancer Cell Proliferation, Migration, and Invasion by Regulating miR-143-3p/ZEB1 Axis. Cancer Manag Res. 2020. Mar;12:1587–99. 10.2147/CMAR.S226409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue YN, Yan Y, Chen ZZ, Chen J, Tang FJ, Xie HQ, et al. LncRNA TUG1 regulates FGF1 to enhance endothelial differentiation of adipose-derived stem cells by sponging miR-143. J Cell Biochem. 2019. Nov;120(11):19087–97. 10.1002/jcb.29232 [DOI] [PubMed] [Google Scholar]
- Luan Y, Li X, Luan Y, Zhao R, Li Y, Liu L, et al. Circulating lncRNA UCA1 Promotes Malignancy of Colorectal Cancer via the miR-143/MYO6 Axis. Mol Ther Nucleic Acids. 2020. Mar;19:790–803. 10.1016/j.omtn.2019.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, Liu L. Long non-coding RNA ZEB2-AS1 promotes proliferation and inhibits apoptosis of colon cancer cells via miR-143/bcl-2 axis. Am J Transl Res. 2019. Aug;11(8):5240–8. [PMC free article] [PubMed] [Google Scholar]
- Tian S, Han G, Lu L, Meng X. Circ-FOXM1 contributes to cell proliferation, invasion, and glycolysis and represses apoptosis in melanoma by regulating miR-143-3p/FLOT2 axis. World J Surg Oncol. 2020. Mar;18(1):56. 10.1186/s12957-020-01832-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang T, Jiang HS, Zhang BT, Liu G. CircFOXO3 functions as a molecular sponge for miR-143-3p to promote the progression of gastric carcinoma via upregulating USP44. Gene. 2020. Aug;753:144798. 10.1016/j.gene.2020.144798 [DOI] [PubMed] [Google Scholar]
- Chen J, Yu Y, Li H, Hu Q, Chen X, He Y, et al. Long non-coding RNA PVT1 promotes tumor progression by regulating the miR-143/HK2 axis in gallbladder cancer. Mol Cancer. 2019. Mar;18(1):33. 10.1186/s12943-019-0947-9 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hu X, Liu Y, Du Y, Cheng T, Xia W. Long non-coding RNA BLACAT1 promotes breast cancer cell proliferation and metastasis by miR-150-5p/CCR2. Cell Biosci. 2019. Jan;9(1):14. 10.1186/s13578-019-0274-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Lee H, Haspel JA, Jin Y. Long noncoding RNA FOXD3-AS1 regulates oxidative stress-induced apoptosis via sponging microRNA-150. FASEB J. 2017. Oct;31(10):4472–81. 10.1096/fj.201700091R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Ma Z. LncRNA highly upregulated in liver cancer regulates imatinib resistance in chronic myeloid leukemia via the miR-150-5p/MCL1 axis. Anticancer Drugs. 2021. Apr;32(4):427–36. 10.1097/CAD.0000000000001019 [DOI] [PubMed] [Google Scholar]
- Wang X, Jiang G, Ren W, Wang B, Yang C, Li M. LncRNA NEAT1 Regulates 5-Fu Sensitivity, Apoptosis and Invasion in Colorectal Cancer Through the MiR-150-5p/CPSF4 Axis. OncoTargets Ther. 2020. Jul;13:6373–83. 10.2147/OTT.S239432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou T, Ke K, Zhang L, Miao C, Liu Y. LncRNA PART1 facilitates the malignant progression of colorectal cancer via miR-150-5p/LRG1 axis. J Cell Biochem. 2020. Oct;121(10):4271–81. 10.1002/jcb.29635 [DOI] [PubMed] [Google Scholar]
- Lan T, Yuan K, Yan X, Xu L, Liao H, Hao X, et al. LncRNA SNHG10 Facilitates Hepatocarcinogenesis and Metastasis by Modulating Its Homolog SCARNA13 via a Positive Feedback Loop. Cancer Res. 2019. Jul;79(13):3220–34. 10.1158/0008-5472.CAN-18-4044 [DOI] [PubMed] [Google Scholar]
- Chen X, Zeng K, Xu M, Hu X, Liu X, Xu T, et al. SP1-induced lncRNA-ZFAS1 contributes to colorectal cancer progression via the miR-150-5p/VEGFA axis. Cell Death Dis. 2018. Sep;9(10):982. 10.1038/s41419-018-0962-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Ren H. Long noncoding RNA PVT1 promotes tumor cell proliferation, invasion, migration and inhibits apoptosis in oral squamous cell carcinoma by regulating miR‑150‑5p/GLUT‑1. Oncol Rep. 2020. Oct;44(4):1524–38. 10.3892/or.2020.7706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge C, Dong J, Chu Y, Cao S, Zhang J, Wei J. LncRNA FGD5-AS1 promotes tumor growth by regulating MCL1 via sponging miR-153-3p in oral cancer. Aging (Albany NY). 2020. Jul;12(14):14355–64. 10.18632/aging.103476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Liu K, Pei Y, Tan J, Ma J, Zhao J. Long Noncoding RNA HIF1A-AS2 Promotes Non-Small Cell Lung Cancer Progression by the miR-153-5p/S100A14 Axis. OncoTargets Ther. 2020. Aug;13:8715–22. 10.2147/OTT.S262293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang J, Hao H, Luo X. lncRNA KCNQ1OT1 promotes the proliferation, migration and invasion of retinoblastoma cells by upregulating HIF-1α via sponging miR-153-3p. J Investig Med. 2020. Dec;68(8):1349–56. 10.1136/jim-2020-001431 [DOI] [PubMed] [Google Scholar]
- Zhao L, Bi M, Zhang H, Shi M. Downregulation of NEAT1 Suppresses Cell Proliferation, Migration, and Invasion in NSCLC Via Sponging miR-153-3p. Cancer Biother Radiopharm. 2020. Jun;35(5):362–70. 10.1089/cbr.2019.3119 [DOI] [PubMed] [Google Scholar]
- Zhi XH, Jiang K, Ma YY, Zhou LQ. OIP5-AS1 promotes the progression of gastric cancer cells via the miR-153-3p/ZBTB2 axis. Eur Rev Med Pharmacol Sci. 2020. Mar;24(5):2428–41. 10.26355/eurrev_202003_20510 [DOI] [PubMed] [Google Scholar]
- Cui Z, Luo Z, Lin Z, Shi L, Hong Y, Yan C. Long non-coding RNA TTN-AS1 facilitates tumorigenesis of papillary thyroid cancer through modulating the miR-153-3p/ZNRF2 axis. J Gene Med. 2019. May;21(5):e3083. 10.1002/jgm.3083 [DOI] [PubMed] [Google Scholar]
- Shao H, Dong D, Shao F. Long non-coding RNA TUG1-mediated down-regulation of KLF4 contributes to metastasis and the epithelial-to-mesenchymal transition of colorectal cancer by miR-153-1. Cancer Manag Res. 2019. Sep;11:8699–710. 10.2147/CMAR.S208508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen JF, Jiang YQ, Li C, Dai XK, Wu T, Yin WZ. LncRNA-XIST promotes the oxidative stress-induced migration, invasion, and epithelial-to-mesenchymal transition of osteosarcoma cancer cells through miR-153-SNAI1 axis. Cell Biol Int. 2020. Oct;44(10):1991–2001. 10.1002/cbin.11405 [DOI] [PubMed] [Google Scholar]
- Zhou B, Zheng P, Li Z, Li H, Wang X, Shi Z, et al. CircPCNXL2 sponges miR-153 to promote the proliferation and invasion of renal cancer cells through upregulating ZEB2. Cell Cycle. 2018;17(23):2644–54. 10.1080/15384101.2018.1553354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Wang Y, Yu T, Nie E, Hu Q, Wu W, et al. Blocking MIR155HG/miR-155 axis inhibits mesenchymal transition in glioma. Neuro-oncol. 2017. Sep;19(9):1195–205. 10.1093/neuonc/nox017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S, Dong L, Jing X, Gen-Yang C, Zhan-Zheng Z. Abnormal lncRNA CCAT1/microRNA-155/SIRT1 axis promoted inflammatory response and apoptosis of tubular epithelial cells in LPS caused acute kidney injury. Mitochondrion. 2020. Jul;53:76–90. 10.1016/j.mito.2020.03.010 [DOI] [PubMed] [Google Scholar]
- Xu J, Bo Q, Zhang X, Lei D, Wang J, Pan X. lncRNA HOXA11-AS Promotes Proliferation and Migration via Sponging miR-155 in Hypopharyngeal Squamous Cell Carcinoma. Oncol Res. 2020. May;28(3):311–9. 10.3727/096504020X15801233454611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Kou D, Liu B, Huang Y, Li S, Qi Y, et al. LncRNA MEG3 contributes to drug resistance in acute myeloid leukemia by positively regulating ALG9 through sponging miR-155. Int J Lab Hematol. 2020. Aug;42(4):464–72. 10.1111/ijlh.13225 [DOI] [PubMed] [Google Scholar]
- Luan T, Zhang X, Wang S, Song Y, Zhou S, Lin J, et al. Long non-coding RNA MIAT promotes breast cancer progression and functions as ceRNA to regulate DUSP7 expression by sponging miR-155-5p. Oncotarget. 2017. Jul;8(44):76153–64. 10.18632/oncotarget.19190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong L, Ao Y, Zhang H, Wang K, Wang Y, Ma Q. Long noncoding RNA NORAD is upregulated in epithelial ovarian cancer and its downregulation suppressed cancer cell functions by competing with miR-155-5p. Cancer Med. 2019. Aug;8(10):4782–91. 10.1002/cam4.2350 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yang TJ, Wang L, Zhang Y, Zheng JD, Liu L. LncRNA UCA1 regulates cervical cancer survival and EMT occurrence by targeting miR-155. Eur Rev Med Pharmacol Sci. 2020. Oct;24(19):9869–79. 10.26355/eurrev_202010_23197 [DOI] [PubMed] [Google Scholar]
- Zheng R, Lin S, Guan L, Yuan H, Liu K, Liu C, et al. Long non-coding RNA XIST inhibited breast cancer cell growth, migration, and invasion via miR-155/CDX1 axis. Biochem Biophys Res Commun. 2018. Apr;498(4):1002–8. 10.1016/j.bbrc.2018.03.104 [DOI] [PubMed] [Google Scholar]
- Yang J, Jia Y, Wang B, Yang S, Du K, Luo Y, et al. Circular RNA CHST15 Sponges miR-155-5p and miR-194-5p to Promote the Immune Escape of Lung Cancer Cells Mediated by PD-L1. Front Oncol. 2021. Mar;11:595609. 10.3389/fonc.2021.595609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Ma Y, Peng X, Jin H, Liu J. LncRNA CCAT1 promotes autophagy via regulating ATG7 by sponging miR-181 in hepatocellular carcinoma. J Cell Biochem. 2019. Oct;120(10):17975–83. 10.1002/jcb.29064 [DOI] [PubMed] [Google Scholar]
- Peng W, Si S, Zhang Q, Li C, Zhao F, Wang F, et al. Long non-coding RNA MEG3 functions as a competing endogenous RNA to regulate gastric cancer progression. J Exp Clin Cancer Res. 2015. Aug;34(1):79. 10.1186/s13046-015-0197-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei YF, He Y, Hu LZ, Zhou B, Xu HY, Liu XQ. The Crosstalk between lncRNA-SNHG7/miRNA-181/cbx7 Modulates Malignant Character in Lung Adenocarcinoma. Am J Pathol. 2020. Jun;190(6):1343–54. 10.1016/j.ajpath.2020.02.011 [DOI] [PubMed] [Google Scholar]
- Sun Q, Hao Q, Lin YC, Song YJ, Bangru S, Arif W, et al. Antagonism between splicing and microprocessor complex dictates the serum-induced processing of lnc-MIRHG for efficient cell cycle reentry. RNA. 2020. Nov;26(11):1603–20. 10.1261/rna.075309.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Liu Y, Yang C, Gao C, Guo X, Cheng J. LncRNA CASC2 inhibits hypoxia-induced pulmonary artery smooth muscle cell proliferation and migration by regulating the miR-222/ING5 axis. Cell Mol Biol Lett. 2020. Mar;25(1):21. 10.1186/s11658-020-00215-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Cheng ML, Gong Y, Ma WJ, Li B, Jiang YZ. LncRNA DANCR promotes ATG7 expression to accelerate hepatocellular carcinoma cell proliferation and autophagy by sponging miR-222-3p. Eur Rev Med Pharmacol Sci. 2020. Sep;24(17):8778–87. 10.26355/eurrev_202009_22816 [DOI] [PubMed] [Google Scholar]
- Liu L, Wang HJ, Meng T, Lei C, Yang XH, Wang QS, et al. lncRNA GAS5 Inhibits Cell Migration and Invasion and Promotes Autophagy by Targeting miR-222-3p via the GAS5/PTEN-Signaling Pathway in CRC. Mol Ther Nucleic Acids. 2019. Sep;17:644–56. 10.1016/j.omtn.2019.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gao C, Lu W, Lou W, Wang L, Xu Q. Long noncoding RNA HOXC13-AS positively affects cell proliferation and invasion in nasopharyngeal carcinoma via modulating miR-383-3p/HMGA2 axis. J Cell Physiol. 2019. Aug;234(8):12809–20. 10.1002/jcp.27915 [DOI] [PubMed] [Google Scholar]
- Liu G, Yang H, Cao L, Han K, Li G. LncRNA TMPO-AS1 Promotes Proliferation and Invasion by Sponging miR-383-5p in Glioma Cells. Cancer Manag Res. 2020. Nov;12:12001–9. 10.2147/CMAR.S282539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng F, Zhang JX, Chang QM, Wu XB, Tang WG, Wang JF, et al. LncRNA MYLK-AS1 facilitates tumor progression and angiogenesis by targeting miR-424-5p/E2F7 axis and activating VEGFR-2 signaling pathway in hepatocellular carcinoma. J Exp Clin Cancer Res. 2020. Nov;39(1):235. 10.1186/s13046-020-01739-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Yao MD, Li CP, Shan K, Yang H, Wang JJ, et al. Silencing Of Circular RNA-ZNF609 Ameliorates Vascular Endothelial Dysfunction. Theranostics. 2017. Jul;7(11):2863–77. 10.7150/thno.19353 [DOI] [PMC free article] [PubMed] [Google Scholar]