Abstract
Successful hematopoietic cell transplantation (HCT) depends on rapid engraftment of the progenitor and stem cells that will reestablish hematopoiesis. Rap1A and Rap1B are two closely related small GTPases that may affect platelet and neutrophil engraftment during HCT through their roles in cell adhesion and migration. β-adrenergic signaling may regulate the participation of Rap1A and Rap1B in engraftment through their inhibition or activation. We conducted a correlative study of a randomized controlled trial evaluating the effects of the nonselective β-antagonist propranolol on expression and prenylation of Rap1A and Rap1B during neutrophil and platelet engraftment in 25 individuals receiving an autologous HCT for multiple myeloma. Propranolol was administered for 1 week prior to and 4 weeks following HCT. Blood was collected 7 days (baseline) and 2 days (Day -2) before HCT, and 28 days after HCT (Day +28). Circulating polymorphonuclear cells (PMNC) were isolated and analyzed via immunoblotting to determine levels of prenylated and total Rap1A versus Rap1B. Twelve participants were randomized to the intervention and 13 to the control. Rap1A expression significantly correlated with Rap1B expression. Rap1B expression significantly correlated with slower platelet engraftment; however, this association was not observed in the propranolol-treated group. There were no significant associations between neutrophil engraftment and Rap1A or Rap1B expression. Post hoc exploratory analyses did not reveal an association between social health variables and Rap1A or Rap1B expression. This study identifies a greater regulatory role for Rap1B than Rap1A in platelet engraftment and suggests a possible role for β-adrenergic signaling in modulating Rap1B function during HCT.
Keywords: Propranolol, Rap1, Hematopoiesis, Engraftment, β-adrenergic
Introduction
Autologous Hematopoietic cell transplantation (HCT) is used as a therapeutic approach for a variety of malignant and non-malignant blood diseases, including multiple myeloma (MM) [1]. The goal of autologous HCT is to reestablish hematopoiesis and reconstitute the immune system with “self” hematopoietic stem and progenitor cells (HSPCs) after the patient has received irradiation and/or chemotherapy to eliminate malignant cells and all normal hematopoietic elements. Engraftment of platelets and neutrophils is a critical event in HCT, with the time required for successful engraftment usually extending 10-21 days after HCT. Shortening the time required for platelet and neutrophil engraftment allows for shorter recovery times for the hospitalized patient and faster discharge to an outpatient setting.
The small GTPases Rap1A and Rap1B are major regulators of cell adhesion and migration [2-7] and are likely to participate in hematopoietic stem/progenitor cell (HSPC) engraftment [7-9]. These small GTPases promote both integrin-mediated adhesion [10-15] and cadherin-mediated adhesion [16-18], which can have varying effects on cell migration. Rap1A and Rap1B control the adhesion and migration of progenitor cells such as megakaryocytes [10,13,19,20], as well as mature platelets [5,13-15,19-21] and neutrophils [22,23], and are reported to participate in hematopoiesis [7,8]. Interestingly, Rap1B plays a greater role than Rap1A in platelet adhesion [13,14], indicating that platelet engraftment potentially may be regulated more by Rap1B than by Rap1A. Due to these important functions of Rap1A and Rap1B, the signaling events that regulate Rap1A and Rap1B activity during HCT may impact engraftment.
Signaling by β-adrenergic receptors regulates the participation of Rap1A and Rap1B in a variety of cellular responses, including cell adhesion and migration [23-27]. The activities of Rap1A and Rap1B are increased when β-adrenergic receptors transiently activate the guanine nucleotide exchange factor EPAC [22]. The activation of Rap1A and Rap1B by EPAC enhances their participation in cell adhesion and migration [11,12,22,28,29]. In contrast to these transient effects of β-adrenergic signaling, prolonged activation of β-adrenergic receptors can inhibit Rap1A and Rap1B activity due to reduced prenylation, which is a post-translational modification needed for these small GTPases to be active [26,27]. Reduced prenylation diminishes the participation of Rap1A and Rap1B in cell-cell adhesion, which can alter cell migration [26,27,30]. β-adrenergic signaling occurs in platelets and neutrophils [31-35] and has multiple roles in hematopoiesis [36-39]. Based on these properties of β-adrenergic receptors, β-adrenergic signaling may modulate Rap1A and Rap1B functions during HCT, but in currently unclear ways.
Elevated stress activates β-adrenergic receptors due to the release of norepinephrine from sympathetic neurons [40,41], and is often experienced by HCT recipients, which can result in both transient and sustained β-adrenergic signaling in tissues innervated by the sympathetic nervous system (SNS). Sympathetic neurons innervate the HSPC niche in the BM [38,42] and norepinephrine released by sympathetic neurons regulates HSPC trafficking, engraftment, and hematopoiesis [43-45]. Stress-associated social health risk factors are related to both adverse biology [46-50] and increased mortality following HCT [51,52]. Stress-associated adverse gene expression patterns are independently associated with increased relapse and decreased disease-free survival following allogeneic HCT [46,49]. The impact of SNS activity on hematopoietic stem cell biology in patients undergoing HCT may be particularly detrimental in the peri-transplantation period [53,54]. We previously reported that blockade of β-adrenergic receptors with propranolol improves engraftment in MM patients undergoing autologous HCT [50], but the roles of Rap1A and Rap1B in this response have not been examined.
The primary objective of this study was to compare the participation of Rap1A and Rap1B in platelet and neutrophil engraftment in MM patients undergoing autologous HCT and to examine potential changes in the small GTPases induced by propranolol treatment. Secondary objectives included evaluating whether stress-associated social health risk factors – including anxiety, depression, lower education, and income – would alter Rap1A and Rap1B expression. The individual contribution of Rap1A versus Rap1B in HCT has not been previously defined, in part because antibodies that specifically detect Rap1A versus Rap1B have not been well characterized. We screened a panel of commercial antibodies to identify specific antibodies that detect only Rap1A or Rap1B. We used these specific antibodies in immunoblots to assess total levels of the small GTPases and their prenylation status in the fraction of platelets and polymorphonuclear cells (collectively referred to as polymorphonuclear cells, PMNC) isolated from patients undergoing HCT, some of whom were treated with propranolol.
Methods
Study Design and Propranolol Treatment
This study was a single site, phase II randomized controlled trial of propranolol administration to individuals undergoing first autologous HCT for MM. The primary aim of the parent trial was to assess whether β-blocker administration to individuals undergoing HCT reduces CTRA gene expression and myeloid lineage bias, thus facilitating hematopoietic recovery and improved long-term outcomes [50]. Propranolol was chosen as the preferred β-blocker given it is cost-effective, is the most studied nonselective β-blocker [55], has a safe side-effect profile, has no significant drug interactions with other drugs used in autologous HCT for MM, and has been shown to prevent tumor progression in vitro as compared to selective β-antagonists [56,57].
The intervention group received 20 mg of propranolol orally twice daily (bid) starting at 7 ± 2 days before transplant (baseline) and continuing until Day +28 post-transplant. Propranolol was started 7 ± 2 days prior to HCT based on mouse model data demonstrating effective blocking of tumor β-adrenergic signaling and progression 8 days prior to exogenous stress exposure [58,59]. Propranolol dosing was chosen by applying human dose-finding studies to the serum propranolol concentrations needed to diminish the adverse effects of β-adrenergic signaling on tumor progression in mice [60,61]. Drug dosing was adjusted after one week based on patient tolerability as assessed by the Principal Investigator, treating HCT physician, and study coordinator. Propranolol dosing was increased to 40 mg bid after one week if participants were tolerating the medication and not experiencing any side effects. If participants were able to tolerate propranolol with noticeable but less severe side effects, they were maintained at 20 mg bid for the remainder of the study. Patients were weaned off propranolol at the time of study completion or prior to completion if they exhibited drug intolerance secondary to side effects and/or new medical symptoms that resulted in a contraindication to β-blocker therapy. Additional details regarding study drug disbursement, monitoring, follow-up study schedule, and assessment time points were described previously [48].
Participant Population and Monitoring
Participants selected for this study were between the ages of 18-75 years old with MM undergoing their first autologous HCT as shown in Knight et al. Table 1 [50]. Further eligibility, randomization, and exclusion criteria are detailed in Knight et al. [48]. The following baseline demographic data was collected prior to study commencement: age, gender, race, income status, and education, detailed in Knight et al. Table 1 [50]. The Hospital Anxiety and Depression Scale (HADS) was collected weekly in coordination with β-blocker assessments to monitor for the possible adverse effect of depression in the setting of propranolol administration. Scores of 8 or above on HADS-A (7 items) and HADS-D (7 items) connoted significant anxiety or depression, respectively; 19 patients scoring in these ranges were contacted by the study PI and offered a referral for further mental health care.
Identification of Specific Rap1A and Rap1B Antibodies
Immunoblotting was used to screen nine commercial antibodies for reactivity with Rap1A and Rap1B, using lysates prepared from cells overexpressing the small GTPases. The lysates were generated by transfecting HEK293T human embryonic kidney cells with the pcDNA3.1 expression vector encoding one of the following: 1) human Rap1A, 2) human Rap1B, 3) myc-Rap1A, which is human Rap1A with an N-terminal myc epitope tag, 4) myc-Rap1B, which is human Rap1B with an N-terminal myc epitope tag, 5) myc-Rap1A-SAAX, which is myc-Rap1A that cannot be prenylated because the cysteine at aa181 is mutated to serine, or 6) myc-Rap1B-SAAX, which is myc-Rap1B that cannot be prenylated because the cysteine at aa181 is mutated to serine. As a negative control, HEK293T cells were transfected only with the pcDNA 3.1 vector. After transfection of the cDNAs using Lipofectamine 2000 (ThermoFisher, Waltham, MA, USA), the cells were cultured for 18 hours in complete medium consisting of DMEM with 10% heat-inactivated fetal bovine serum and antibiotics. In some cases, 15 µM mevastatin (Sigma, St. Louis, MO, USA) was added to the cell cultures 90 minutes after transfection of the cDNAs to prevent the expressed small GTPases from becoming prenylated. The cells were lysed in Triton-X100/SDS lysis buffer, and the cell lysates were subjected to SDS-PAGE, followed by transfer of the proteins to PVDF as previously described [26,27]. The PVDF membranes were immunoblotted using our previously reported methods [26,27] to test the reactivity of the nine commercial Rap1A and Rap1B antibodies described in Figure 2. Among the nine antibodies that were tested, a mouse monoclonal antibody that specifically detects Rap1A (Santa Cruz catalog number sc-398755) and a rabbit monoclonal antibody that specifically detects Rap1B (Cell Signaling catalog number 2326) were used for the analysis of the patients’ samples. Prior testing revealed that these antibodies do not differentiate between the phosphorylated and non-phosphorylated forms of Rap1 [30].
Collection of Patients’ PMNC and Immunoblotting
A clinical research coordinator drew 8 ml of blood from each patient at each of the three time points in the study: 7 ± 2 days before HCT (baseline), 2 days before HCT (Day -2), and 28 days after HCT (Day +28). Among the 12 patients receiving propranolol, treatment with propranolol was initiated immediately after the first blood draw at the baseline collection period. The blood samples were collected in a BD Vacutainer CPT Tube with Sodium Citrate (Fisher Scientific Cat. No. 02-685-125). The tubes were centrifuged according to the manufacturer’s protocol to generate a buffy layer containing mononuclear cells and platelets (referred to as polymorphonuclear cells, PMNC). The PMNC were lysed in Triton-X100/SDS lysis buffer, and protein concentration was determined using the Pierce BCA Assay Kit (Pierce Cat. No 23225). For immunoblotting analysis, 20 µg of protein from each PMNC lysate was run on a 10% SDS-PAGE gel, transferred to PVDF, and blotted with antibodies that specifically detect Rap 1A (Santa Cruz catalog number sc-398755) or Rap 1B (Cell Signaling catalog number 2326). Digital imaging was performed on a GE ImageQuant LAS 4000 digital imager and densitometry determined using GE ImageQuant TL image analysis software.
Rap1 Analysis and Clinical Outcomes
Comparisons were made using the densitometry values of the prenylated and non-prenylated forms of Rap1A and Rap1B detected in the immunoblots of the patients’ PMNC collected at baseline, Day -2, and Day +28. Total Rap1A and Rap1B levels were log-transformed for analyses to better focus on multiplicative effects (which correspond to additive effects on the log scale) and stabilize the variability with varying means. Mixed effects regression analyses were performed to explore the effects of β-blocker therapy (propranolol vs. control groups) on total levels of Rap1A and Rap1B at each time point. The cross-sectional correlation between log-transformed Rap1A and Rap1B was quantified by Pearson’s correlation coefficient. Mixed effects regression models were also used to explore the relationship between Rap1A and Rap1B at these three time points and the patient social health risk factors – including anxiety, depression, lower education, and income. Cox regression models were performed to assess the relationship between log-transformed baseline Rap1A or Rap1B expression and platelet or neutrophil engraftment time. Time to neutrophil and platelet engraftment were defined as the first day of absolute neutrophil count (ANC) > 0.5 x 109/L and platelet count of >20 x 109/L sustained for three consecutive assessments at least one day apart, hereafter referred to as platelet engraftment as per standard clinical nomenclature.
Results
Participant Enrollment, Demographics, and Adverse Events
There were 154 patients who met initial criteria of having a planned first autologous transplant for MM, with a final total enrollment of 25 participants who met eligibility criteria (12 in the propranolol group, 13 in the control group). Please see paper by Knight et al. for full recruitment details and participant characteristics [50]. Of the 25 participants, there were no significant differences in their baseline demographic, disease state, or transplant-related characteristics between the two study groups. There were no serious adverse events experienced by participants in either study arm [50].
Identification of Selective Rap1A and Rap1B Antibodies
To identify an antibody that selectively detects Rap1A, and one that selectively detects Rap1B, lysates of HEK293T cells expressing different forms of myc-tagged or untagged Rap1A or Rap1B were immunoblotted using nine different commercial antibodies reported to react with Rap1A and/or Rap1B (Figure 1). These antibodies were also screened for their ability to detect the prenylated and non-prenylated forms of the GTPases. Prenylated and non-prenylated GTPases can be identified by their migration pattern in immunoblots, because prenylated GTPases migrate faster than non-prenylated GTPases during SDS-PAGE due to the greater solubility of prenylated GTPases in SDS [30]. The ability of an antibody to detect non-prenylated Rap1A or Rap1B was confirmed by the antibody reacting with Rap1A or Rap1B isolated from cells treated with mevastatin, which inhibits prenylation [30], and by the antibody reacting with Rap1A-SAAX or Rap1B-SAAX, which are mutant forms of the GTPases that cannot be prenylated due to serine substitution at C181 [30].
Figure 1.
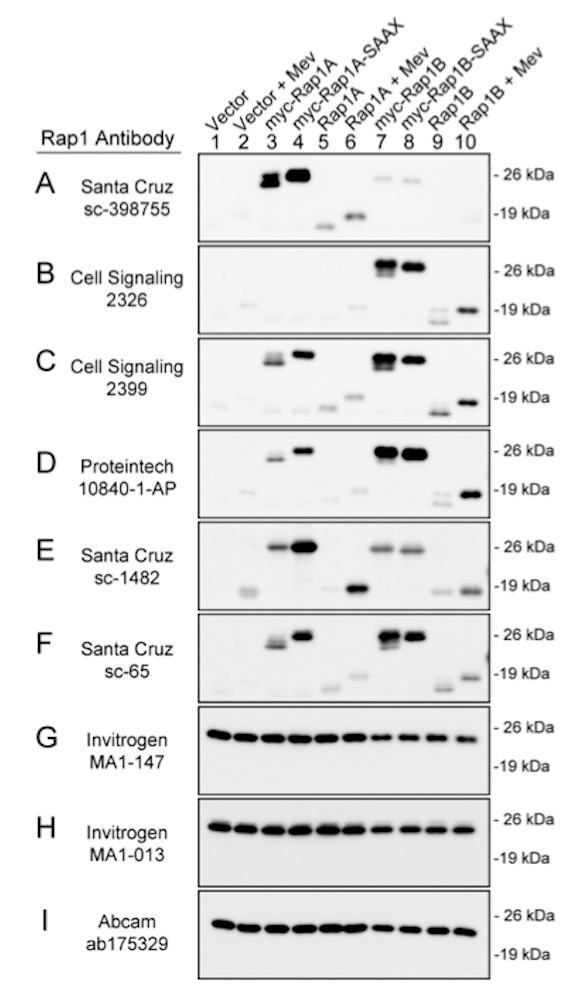
Specificity of commercial antibodies for Rap1A and Rap1B. HEK293T cells were transfected with the indicated cDNAs expressing wildtype or mutant forms of Rap1A or Rap1B. Control cells were transfected with the cDNA vector. The cells were treated with 15 mM mevastatin (Mev, lanes 2, 6, and 10) to inhibit prenylation, or they were untreated (lanes 1, 3-5, and 7-9). Cell lysates were immunoblotted with the indicated commercial antibodies to Rap1. All immunoblots are shown at the same exposure. Results are representative of at least three independent experiments conducted for each antibody.
Among the nine commercial antibodies that were tested, one antibody exhibited greater reactivity with Rap1A than with Rap1B (Figure 1A), whereas another antibody exhibited greater reactivity with Rap1B than with Rap1A (Figure 1B). Four antibodies detected both Rap1A and Rap1B (Figure 1C-1F), and three antibodies reacted with a protein that could not be attributed to Rap1A or Rap1B (Figure 1G-1I). All antibodies that detected prenylated Rap1A and/or Rap1B also detected the non-prenylated forms of the GTPases, as indicated by the antibodies reacting in the immunoblots with mutant Rap1A-SAAX and Rap1B-SAAX (lanes 4 and 8, Figure 1) and with Rap1A and Rap1B that had been expressed in mevastatin-treated cells (lanes 6 and 10, Figure 1).
Two antibodies were chosen for further analysis; a Rap1A-selective antibody (Santa Cruz catalog number sc-398755, Figure 1A) and a Rap1B-selective antibody (Cell Signaling catalog number 2326, Figure 1B). To compare the sensitivities of these antibodies for Rap1A and Rap1B in the prenylated and non-prenylated forms, immunoblotting was conducted using lysates prepared from untransfected HEK293T cells that had been treated with or without mevastatin for 18 hours. Mevastatin was used to inhibit the prenylation of endogenous Rap1A and Rap1B in the cells [30]. As seen in Figure 2, the two antibodies exhibited similar sensitivities for the prenylated and non-prenylated forms of endogenous Rap1A or Rap1B expressed in the cells. Based on these immunoblotting results (Figures 1 and 2), we selected these two antibodies to examine the expression and prenylation of Rap1A and Rap1B in the patients’ PMNC.
Figure 2.
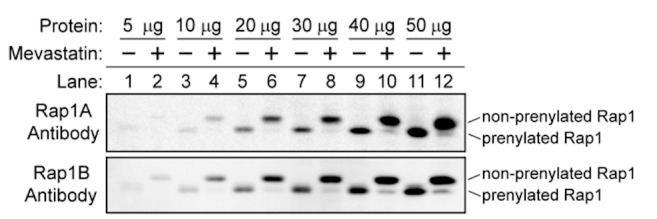
Antibodies that selectively react with either Rap1A or Rap1B exhibit similar abilities to detect the prenylated and non-prenylated forms of the small GTPases. HEK293T cells were treated with 15 mM mevastatin for 18 hours (even numbered lanes) or left untreated (odd numbered lanes). Cell lysates were immunoblotted using the Rap1A-selective antibody (Santa Cruz, sc-3987455; top panel) or the Rap1B-selective antibody (Cell Signaling, 2326; bottom panel). The prenylated and non-prenylated forms of the small GTPases were detected by their differences in migration. Both immunoblots are shown at the same exposure. Results are representative of at least three independent experiments conducted for each antibody.
Analysis of Rap1A and Rap1B in Patient Samples and Correlation with Platelet and Neutrophil Engraftment
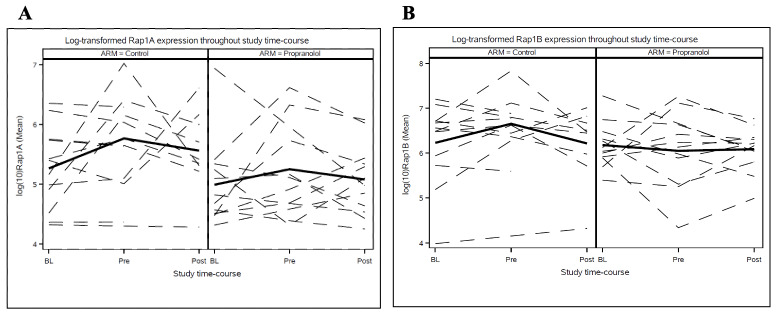
Immunoblotting of the patients’ PMNC collected at baseline, Day -2, and Day +28 indicated that expression of Rap1A and Rap1B varied extensively between different collection times and between different patients (Figure 3). Despite this variability, there was a strong positive correlation between expression of Rap1A and expression of Rap1B in the PMNC samples collected at all time points during the study. This positive correlation between Rap1A and Rap1B expression occurred in PMNC collected at baseline prior to propranolol treatment (r = 0.716, p < 0.0001 for all 25 patients), and in PMNC collected at Day -2 and Day +28 after initiation of propranolol treatment (r = 0.842, p = 0.0043 for the control group, and r = 0.812, p = 0.0013 for the propranolol-treated group). Rap1A and Rap1B were prenylated in the vast majority of the patients’ samples; the slower migrating form of Rap1A and Rap1B that indicates lack of prenylation was detected in only one PMNC sample, collected from Patient 3 at baseline (lane 7, Figure 3). Relative to the control group, propranolol-treated patients did not show significantly altered levels of prenylated Rap1A (F=0.20, p=0.82), prenylated Rap1B (F=0.90, p=0.41), or total levels of Rap1A or Rap1B at any time points (Figure 4).
Figure 3.
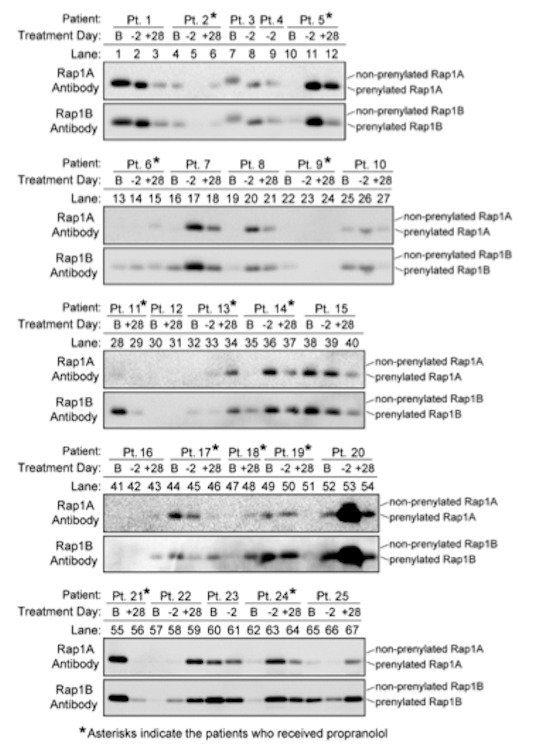
Expression of Rap1A and Rap1B in PMNC differs between different patients and different collection times. Blood samples were collected from patients at baseline at 7 ± 2 days before HCT (B), 2 days before HCT (-2), and 28 days after HCT (+28). Asterisks indicate the patients who received propranolol, which was started immediately after the first blood draw at the baseline collection period. PMNC were isolated from the blood samples, and 20 mg of protein from each PMNC lysate was immunoblotted using the Rap1A-specific antibody (Santa Cruz, sc-3987455; top panel) or the Rap1B-specific antibody (Cell Signaling, 2326; bottom panel). The prenylated and non-prenylated forms of the small GTPases were detected by their differences in migration. Results are representative of at least three independent experiments conducted for each antibody.
Figure 4.
Log-transformed Rap1A (A) and Rap1B (B) values in both control and propranolol treatment groups throughout the study time-course: Baseline (BL), Pre-HCT (Pre), and Post-HCT (Post). Relative to the control group, propranolol-treated patients did not show significantly altered levels of prenylated Rap1A (F=0.20, p=0.82) (A), prenylated Rap1B (F=0.90, p=0.41) (B), or total levels of Rap1A or Rap1B at any time points.
There was a strong correlation between higher Rap1B expression at baseline and more days required for platelet engraftment (Figure 5). This association was statistically significant when including the results from all participants (both untreated and propranolol-treated) in the analysis (p=0.019). Each 10-fold increase in Rap1B at baseline was associated with a 0.4-fold decrease in the hazard of platelet engraftment (HR=0.41; 95% CI: 0.19, 0.86; p=0.019). The correlation between higher Rap1B expression and more days for platelet engraftment was also statistically significant when only the results from the 13 patients who did not receive propranolol were analyzed (HR=0.38; 95% CI: 0.15, 0.93; p=0.034). However, this association did not reach statistical significance when the analysis was restricted only to the results from the 12 patients who were treated with propranolol (HR=0.78; 95% CI: 0.21, 2.9; p=0.707).
Figure 5.
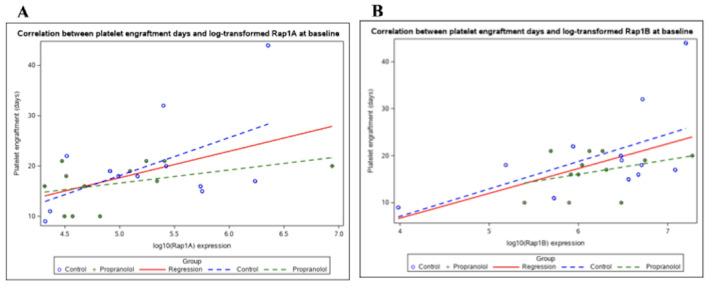
Correlation between log-transformed Rap1A (A) or log-transformed Rap1B (B) and days to platelet engraftment. There was a strong correlation between higher Rap1B (B) expression at baseline in all participants and more days required for platelet engraftment (HR=0.41, 95% CI: 0.19, 0.86; p=0.019). There was no significant correlation between baseline Rap1A (A) expression and the days required for platelet engraftment for all participants (HR=0.55, 95% CI: 0.27, 1.10, p=0.091).
In contrast to Rap1B, there was no significant correlation between baseline Rap1A expression and the days required for platelet engraftment (HR=0.55, 95% CI: 0.27, 1.10, p=0.091) for all patients (Figure 5). Additionally, the time required for neutrophil expression did not significantly correlate with either Rap1A or Rap1B expression.
Post Hoc Analyses of Social Health Risk Factors
Since stress promotes β-adrenergic signaling in patients undergoing HCT50, it is possible that socially-mediated stressors would accentuate β-adrenergic effects on Rap1A and Rap1B in these patients. To investigate this possibility, we compared social health risk factors with Rap1A and Rap1B expression detected in patient samples. There was no significant association between the participants’ income before diagnosis and the baseline expression of Rap1A (r=-0.37, p=0.090) or Rap1B (r=-0.23, p=0.30), nor between the participant’s highest level of education and the baseline expression of Rap1A (p=0.76) or Rap1B (r=.030, p=0.89). The participants’ reported anxiety pre-transplant and post-transplant, respectively, was not associated with Rap1A expression (r=0.28, p=0.23/r=0.030, p=0.89) or Rap1B expression (r=0.31, p=0.19/r=-0.032, p=0.89). There was also no significant association between the participant’s reported depression pre-transplant or post-transplant, respectively, and the expression of Rap1A (r=0.36, p=0.12/r=0.21, p=0.37) or Rap1B (r=0.42, p=0.07/r=0.18, p=0.44).
Discussion
This study identifies different potential roles of Rap1A and Rap1B in platelet engraftment after HCT. We observed that high expression of Rap1B, but not Rap1A, is associated with more days required for platelet engraftment. This observation supports a greater regulatory role for Rap1B than for Rap1A in platelet engraftment after HCT, consistent with recent reports that platelet adhesion is regulated more by Rap1B than by Rap1A [5,14]. High Rap1B expression may be a negative regulator of platelet engraftment in patients undergoing autologous HCT. Interestingly, this association between Rap1B and longer days to engraftment was not observed in the propranolol-treated group, suggestive of a possible role for β-adrenergic signaling to modulate Rap1B function during HCT.
We observed unexpected variability in the expression of Rap1A and Rap1B in the samples collected from individual patients at different times during the study. The reasons for this variability are unclear. All samples were collected using identical procedures, and 20 µg of protein from each patient’s PMNC sample was routinely analyzed for immunoblotting. Thus, it seems unlikely that technical issues were responsible for this variability. The PMNC samples that we analyzed consisted of a mixed population of lymphocytes, granulocytes, neutrophils, and platelets obtained from the unfractionated buffy coat. Unexplained physiologic or pathophysiologic events occurring in this mixed population in the PMNC samples may have affected the expression levels of Rap1B and Rap1A over the course of the study. Even though the events that caused this variability remain unexplained, these events affected Rap1A and Rap1B in similar ways, as indicated by the strong correlation between Rap1A expression and Rap1B expression throughout the study.
The prenylated forms of Rap1A and Rap1B predominated in almost all PMNC samples; the non-prenylated forms of the small GTPases were detected in only one sample, which was collected from Patient 3 at baseline (lane 7, Figure 2). We previously described several signaling events that suppress the prenylation of Rap1A and Rap1B [26,27,30]. It is possible that these signaling events were active in the PMNC collected from Patient 3 at baseline, suppressing the prenylation of both Rap1A and Rap1B in this sample. The lack of prior studies examining the prenylation status of Rap1 in leukocytes provided the rationale to investigate this question, as well as to determine if Rap1 prenylation is altered by propranolol. However, since we observed that that nearly all Rap1 was prenylated in the patients’ PMNC samples, future testing of the impact of propranolol on Rap1 prenylation is likely not warranted.
The signals that regulate Rap1A expression and prenylation are expected to similarly regulate Rap1B expression and prenylation, since these two small GTPases are nearly identical and share the same activators and effectors [5]. Due to their similarities, relatively few studies have defined differences in Rap1A versus Rap1B, though they do exist. Rap1B generally controls dynamic changes in cell-cell adhesion in response to environmental cues, whereas Rap1A resists these cues and instead promotes steady-state, basal cell-cell adhesion [62,63]. Consistent with the responsive nature of Rap1B, we previously reported that Rap1B responds more than Rap1A to signaling cascades initiated by adenosine receptors and β-adrenergic receptors [27]. Furthermore, platelet adhesion was recently found to be regulated more by Rap1B than by Rap1A [5,14]. Due to these unique characteristics of Rap1B, it is reasonable that Rap1B participates more than Rap1A in platelet engraftment after HCT. The current study is the first to identify this in human samples. Further, it is noteworthy this relationship persists in the setting of HCT where the normal hematopoietic milieu is significantly altered.
We found that high expression of Rap1B correlated with a longer time required for platelet engraftment in the MM patients undergoing HCT. This finding indicates that Rap1B negatively regulates platelet engraftment in these patients. It is possible the signals that elevate Rap1B expression in the patients’ PMNC at baseline continue to impact Rap1B during engraftment, promoting Rap1B-mediated events that slow engraftment. Multiple events undoubtedly contribute to the negative regulation of platelet engraftment by Rap1B, most likely involving the Rap1B-mediated regulation of cell adhesion and migration. Increased expression and/or activity of Rap1B can enhance cell-cell adhesion [16-18], which might negatively affect specific steps during platelet engraftment that require cell migration.
In patients undergoing HCT, elevated stress and the norepinephrine-mediated activation of β-adrenergic receptors may regulate Rap1B activity during HPSC migration and engraftment. β-adrenergic signaling can have opposing effects on Rap1B activity, depending on the duration of the signaling event. Transient β-adrenergic signaling activates Rap1B through EPAC [11,12,22,28,29], while sustained β-adrenergic signaling inhibits Rap1B by suppressing its prenylation [26,27]. It is unlikely that Rap1B was inhibited by β-adrenergic signaling in this study, since Rap1B was prenylated in the majority of the patients’ samples. Instead, β-adrenergic signaling most likely activated Rap1B, which may have increased the ability of Rap1B to promote cell-cell adhesion and slow engraftment. It is through inhibition of this β-adrenergic signaling that propranolol may disassociate the negative effect of Rap1B on platelet engraftment. This is consistent with other clinical studies demonstrating that adrenergic input may worsen MM outcomes [64,65], while β-blockade may improve them [50]. Given the integral role of stress in this pathway, future studies utilizing catecholamines and/or cortisol as physiologic measurements of stress could provide a useful complementary approach to its assessment.
Our observation that high Rap1B expression significantly correlates with slower platelet engraftment for untreated patients, but not for propranolol-treated patients, is suggestive of the potential utility of propranolol for use in aiding engraftment. However, interpretation of these findings is limited due to small sample size and subsequent lack of statistical comparison between the two groups. As such, these findings do not definitively prove that Rap1B mediates any effect of propranolol on this process. Nevertheless, these findings support the development of future studies using a larger sample size to determine if antagonism of β-adrenergic signaling may inhibit Rap1B activity and subsequently hasten engraftment. Evidence to continue this area of investigation is further corroborated by studies demonstrating the clinically meaningful impact of propranolol on earlier platelet engraftment [50] and would help identify candidate components of affected signaling pathways.
We previously reported that inhibiting β-adrenergic signaling using propranolol improves engraftment in MM patients undergoing HCT [50]. By studying Rap1A and Rap1B, we have identified Rap1B as a potential mechanistic molecule regulating platelet engraftment after HCT. Future studies are needed to further define Rap1B as a participant in the β-adrenergic-mediated signaling cascade that diminishes successful HCT.
Acknowledgments
This work was funded in part by the National Cancer Institute (NCI) Contract No. HHSN261200800001E and the NCI Network on Biobehavioral Pathways in Cancer; the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), through Grant Numbers UL1TR001436, KL2TR001438 and R03 HL155174; Medical College of Wisconsin Institutional Research Grant #86-004-26 from the American Cancer Society; and the Laura Gralton Philanthropic Fund. These studies were also supported by funding from NCI R01CA238562 (JMK), NCI R01 BA188871 (CLW), and NCI R01 CA204231 (SR).
Glossary
- HCT
Hematopoietic cell transplantation
- MM
multiple myeloma
- HSPCs
hematopoietic stem and progenitor cells
- SNS
sympathetic nervous system
- PMNC
polymorphonuclear cells
- HADS
Hospital Anxiety and Depression Scale
Author Contributions
Designed research: JMK, CLW; Performed Research: JMK, AKJ, CLW, ELL; Contributed vital new reagents or analytical tools: CLW; Collected Data: JMK, CLW, NNS, AD, SC, MH, BD, PH; Analyzed and interpreted data: AKJ, JMK, CLW, ELL, SR, KC; Performed statistical analysis: AS, RW; Manuscript writing: AKJ, JMK, CLW, AS, NNS, AD, SC, MH, BD, PH, SR, KC.
References
- Kanate AS, Majhail NS, Savani BN, Bredeson C, Champlin RE, Crawford S, et al. Indications for Hematopoietic Cell Transplantation and Immune Effector Cell Therapy: Guidelines from the American Society for Transplantation and Cellular Therapy. Biol Blood Marrow Transplant. 2020. Jul;26(7):1247–56. 10.1016/j.bbmt.2020.03.002 [DOI] [PubMed] [Google Scholar]
- Bos JL, de Bruyn K, Enserink J, Kuiperij B, Rangarajan S, Rehmann H, et al. The role of Rap1 in integrin-mediated cell adhesion. Biochem Soc Trans. 2003. Feb;31(Pt 1):83–6. 10.1042/bst0310083 [DOI] [PubMed] [Google Scholar]
- Kinashi T, Katagiri K. Regulation of lymphocyte adhesion and migration by the small GTPase Rap1 and its effector molecule, RAPL. Immunol Lett. 2004. Apr;93(1):1–5. 10.1016/j.imlet.2004.02.008 [DOI] [PubMed] [Google Scholar]
- Bos JL. Linking Rap to cell adhesion. Curr Opin Cell Biol. 2005. Apr;17(2):123–8. 10.1016/j.ceb.2005.02.00 [DOI] [PubMed] [Google Scholar]
- Stefanini L, Bergmeier W. RAP GTPases and platelet integrin signaling. Platelets. 2019;30(1):41–7. 10.1080/09537104.2018.1476681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagarrigue F, Kim C, Ginsberg MH. The Rap1-RIAM-talin axis of integrin activation and blood cell function. Blood. 2016. Jul;128(4):479–87. 10.1182/blood-2015-12-638700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stork PJ, Dillon TJ. Multiple roles of Rap1 in hematopoietic cells: complementary versus antagonistic functions. Blood. 2005. Nov;106(9):2952–61. 10.1182/blood-2005-03-1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minato N, Kometani K, Hattori M. Regulation of immune responses and hematopoiesis by the Rap1 signal. Adv Immunol. 2007;93:229–64. 10.1016/S0065-2776(06)93006-5 [DOI] [PubMed] [Google Scholar]
- Imai T, Tanaka H, Hamazaki Y, Minato N. Rap1 signal modulators control the maintenance of hematopoietic progenitors in bone marrow and adult long-term hematopoiesis. Cancer Sci. 2019. Apr;110(4):1317–30. 10.1111/cas.13974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruyn KM, Zwartkruis FJ, de Rooij J, Akkerman JW, Bos JL. The small GTPase Rap1 is activated by turbulence and is involved in integrin [alpha]IIb[beta]3-mediated cell adhesion in human megakaryocytes. J Biol Chem. 2003. Jun;278(25):22412–7. 10.1074/jbc.M212036200 [DOI] [PubMed] [Google Scholar]
- Rangarajan S, Enserink JM, Kuiperij HB, de Rooij J, Price LS, Schwede F, et al. Cyclic AMP induces integrin-mediated cell adhesion through Epac and Rap1 upon stimulation of the beta 2-adrenergic receptor. J Cell Biol. 2003. Feb;160(4):487–93. 10.1083/jcb.200209105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enserink JM, Price LS, Methi T, Mahic M, Sonnenberg A, Bos JL, et al. The cAMP-Epac-Rap1 pathway regulates cell spreading and cell adhesion to laminin-5 through the alpha3beta1 integrin but not the alpha6beta4 integrin. J Biol Chem. 2004. Oct;279(43):44889–96. 10.1074/jbc.M404599200 [DOI] [PubMed] [Google Scholar]
- Stefanini L, Lee RH, Paul DS, O’Shaughnessy EC, Ghalloussi D, Jones CI, et al. Functional redundancy between RAP1 isoforms in murine platelet production and function. Blood. 2018. Nov;132(18):1951–62. 10.1182/blood-2018-03-838714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieman MT. RAPid signaling in platelets. Blood. 2018. Nov;132(18):1864–5. 10.1182/blood-2018-09-872093 [DOI] [PubMed] [Google Scholar]
- Bromberger T, Klapproth S, Rohwedder I, Zhu L, Mittmann L, Reichel CA, et al. Direct Rap1/Talin1 interaction regulates platelet and neutrophil integrin activity in mice. Blood. 2018. Dec;132(26):2754–62. 10.1182/blood-2018-04-846766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price LS, Hajdo-Milasinovic A, Zhao J, Zwartkruis FJ, Collard JG, Bos JL. Rap1 regulates E-cadherin-mediated cell-cell adhesion. J Biol Chem. 2004. Aug;279(34):35127–32. 10.1074/jbc.M404917200 [DOI] [PubMed] [Google Scholar]
- Hogan C, Serpente N, Cogram P, Hosking CR, Bialucha CU, Feller SM, et al. Rap1 regulates the formation of E-cadherin-based cell-cell contacts. Mol Cell Biol. 2004. Aug;24(15):6690–700. 10.1128/MCB.24.15.6690-6700.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooistra MR, Dubé N, Bos JL. Rap1: a key regulator in cell-cell junction formation. J Cell Sci. 2007. Jan;120(Pt 1):17–22. 10.1242/jcs.03306 [DOI] [PubMed] [Google Scholar]
- Molina-Ortiz P, Polizzi S, Ramery E, Gayral S, Delierneux C, Oury C, et al. Rasa3 controls megakaryocyte Rap1 activation, integrin signaling and differentiation into proplatelet. PLoS Genet. 2014. Jun;10(6):e1004420. 10.1371/journal.pgen.1004420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Rivero S, Baquero C, Hernández-Cano L, Roldán-Etcheverry JJ, Gutiérrez-Herrero S, Fernández-Infante C, et al. C3G, through its GEF activity, induces megakaryocytic differentiation and proplatelet formation. Cell Commun Signal. 2018. Dec;16(1):101. 10.1186/s12964-018-0311-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackeng CM, Franke B, Relou IA, Gorter G, Bos JL, van Rijn HJ, et al. Low-density lipoprotein activates the small GTPases Rap1 and Ral in human platelets. Biochem J. 2000. Jul;349(Pt 1):231–8. 10.1042/0264-6021:3490231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash-Koney M, Deevi RK, McFarlane C, Dib K. Exchange protein directly activated by cAMP 1 (Epac1) is expressed in human neutrophils and mediates cAMP-dependent activation of the monomeric GTPase Rap1. J Leukoc Biol. 2011. Oct;90(4):741–9. 10.1189/jlb.0211108 [DOI] [PubMed] [Google Scholar]
- Scott J, Harris GJ, Pinder EM, Macfarlane JG, Hellyer TP, Rostron AJ, et al. Exchange protein directly activated by cyclic AMP (EPAC) activation reverses neutrophil dysfunction induced by β2-agonists, corticosteroids, and critical illness. J Allergy Clin Immunol. 2016. Feb;137(2):535–44. 10.1016/j.jaci.2015.07.036 [DOI] [PubMed] [Google Scholar]
- Bastian P, Balcarek A, Altanis C, Strell C, Niggemann B, Zaenker KS, et al. The inhibitory effect of norepinephrine on the migration of ES-2 ovarian carcinoma cells involves a Rap1-dependent pathway. Cancer Lett. 2009. Feb;274(2):218–24. 10.1016/j.canlet.2008.09.008 [DOI] [PubMed] [Google Scholar]
- Steininger TS, Stutz H, Kerschbaum HH. Beta-adrenergic stimulation suppresses phagocytosis via Epac activation in murine microglial cells. Brain Res. 2011. Aug;1407:1–12. 10.1016/j.brainres.2011.06.050 [DOI] [PubMed] [Google Scholar]
- Wilson JM, Lorimer E, Tyburski MD, Williams CL. β-Adrenergic receptors suppress Rap1B prenylation and promote the metastatic phenotype in breast cancer cells. Cancer Biol Ther. 2015;16(9):1364–74. 10.1080/15384047.2015.1070988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JM, Prokop JW, Lorimer E, Ntantie E, Williams CL. Differences in the Phosphorylation-Dependent Regulation of Prenylation of Rap1A and Rap1B. J Mol Biol. 2016. Dec;428(24 24 Pt B):4929–45. 10.1016/j.jmb.2016.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos JL. Epac proteins: multi-purpose cAMP targets [published correction appears in Trends Biochem Sci. 2007 Jan;32(1):5]. Trends Biochem Sci. 2006. Dec;31(12):680–6. 10.1016/j.tibs.2006.10.002 [DOI] [PubMed] [Google Scholar]
- Holz GG, Kang G, Harbeck M, Roe MW, Chepurny OG. Cell physiology of cAMP sensor Epac. J Physiol. 2006. Nov;577(Pt 1):5–15. 10.1113/jphysiol.2006.119644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntantie E, Gonyo P, Lorimer EL, Hauser AD, Schuld N, McAllister D, et al. An adenosine-mediated signaling pathway suppresses prenylation of the GTPase Rap1B and promotes cell scattering. Sci Signal. 2013. May;6(277):ra39. 10.1126/scisignal.2003374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjemdahl P, Larsson PT, Wallén NH. Effects of stress and beta-blockade on platelet function. Circulation. 1991. Dec;84(6 Suppl):VI44–61. [PubMed] [Google Scholar]
- Larsson PT, Wallén NH, Martinsson A, Egberg N, Hjemdahl P. Significance of platelet beta-adrenoceptors for platelet responses in vivo and in vitro. Thromb Haemost. 1992. Dec;68(6):687–93. 10.1055/s-0038-1646345 [DOI] [PubMed] [Google Scholar]
- Bonten TN, Plaizier CE, Snoep JJ, Stijnen T, Dekkers OM, van der Bom JG. Effect of β-blockers on platelet aggregation: a systematic review and meta-analysis. Br J Clin Pharmacol. 2014. Nov;78(5):940–9. 10.1111/bcp.12404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls AJ, Wen SW, Hall P, Hickey MJ, Wong CH. Activation of the sympathetic nervous system modulates neutrophil function. J Leukoc Biol. 2018. Feb;103(2):295–309. 10.1002/JLB.3MA0517-194RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunskole Hummel I, Reinartz MT, Kälble S, Burhenne H, Schwede F, Buschauer A, et al. Dissociations in the effects of β2-adrenergic receptor agonists on cAMP formation and superoxide production in human neutrophils: support for the concept of functional selectivity. PLoS One. 2013. May;8(5):e64556. 10.1371/journal.pone.0064556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino M, Marino F, Maestroni GJ. Sympathoadrenergic modulation of hematopoiesis: a review of available evidence and of therapeutic perspectives. Front Cell Neurosci. 2015;9:302. Published 2015 Aug 5. doi: 10.3389/fncel.2015.00302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestroni GJ. Adrenergic modulation of hematopoiesis. J Neuroimmune Pharmacol. 2020. Mar;15(1):82–92. 10.1007/s11481-019-09840-7 [DOI] [PubMed] [Google Scholar]
- Ho YH, Del Toro R, Rivera-Torres J, Rak J, Korn C, García-García A, et al. Remodeling of bone marrow hematopoietic stem cell niches promotes myeloid cell expansion during premature or physiological aging. Cell Stem Cell. 2019. Sep;25(3):407–418.e6. 10.1016/j.stem.2019.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raaijmakers MH. Aging of the hematopoietic stem cell niche: an unnerving matter. Cell Stem Cell. 2019. Sep;25(3):301–3. 10.1016/j.stem.2019.08.008 [DOI] [PubMed] [Google Scholar]
- Carrasco GA, Van de Kar LD. Neuroendocrine pharmacology of stress. Eur J Pharmacol. 2003. Feb;463(1-3):235–72. 10.1016/s0014-2999(03)01285-8 [DOI] [PubMed] [Google Scholar]
- Shin KJ, Lee YJ, Yang YR, Park S, Suh PG, Follo MY, et al. Molecular mechanisms underlying psychological stress and cancer. Curr Pharm Des. 2016;22(16):2389–402. 10.2174/1381612822666160226144025 [DOI] [PubMed] [Google Scholar]
- Maryanovich M, Zahalka AH, Pierce H, Pinho S, Nakahara F, Asada N, et al. Adrenergic nerve degeneration in bone marrow drives aging of the hematopoietic stem cell niche [published correction appears in Nat Med. 2019 Apr;25(4):701]. Nat Med. 2018. Jun;24(6):782–91. 10.1038/s41591-018-0030-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006. Jan;124(2):407–21. 10.1016/j.cell.2005.10.041 [DOI] [PubMed] [Google Scholar]
- Méndez-Ferrer S, Battista M, Frenette PS. Cooperation of beta(2)- and beta(3)-adrenergic receptors in hematopoietic progenitor cell mobilization. Ann N Y Acad Sci. 2010. Mar;1192(1):139–44. 10.1111/j.1749-6632.2010.05390.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanoun M, Zhang D, Mizoguchi T, Pinho S, Pierce H, Kunisaki Y, et al. Acute myelogenous leukemia-induced sympathetic neuropathy promotes malignancy in an altered hematopoietic stem cell niche. Cell Stem Cell. 2014. Sep;15(3):365–75. 10.1016/j.stem.2014.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight JM, Syrjala KL, Majhail NS, Martens M, Le-Rademacher J, Logan BR, et al. Patient-Reported Outcomes and Socioeconomic Status as Predictors of Clinical Outcomes after Hematopoietic Stem Cell Transplantation: A Study from the Blood and Marrow Transplant Clinical Trials Network 0902 Trial. Biol Blood Marrow Transplant. 2016. Dec;22(12):2256–63. 10.1016/j.bbmt.2016.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight JM, Rizzo JD, Logan BR, Wang T, Arevalo JM, Ma J, et al. Low Socioeconomic Status, Adverse Gene Expression Profiles, and Clinical Outcomes in Hematopoietic Stem Cell Transplant Recipients. Clin Cancer Res. 2016. Jan;22(1):69–78. 10.1158/1078-0432.CCR-15-1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight JM, Kerswill SA, Hari P, Cole SW, Logan BR, D’Souza A, et al. Repurposing existing medications as cancer therapy: design and feasibility of a randomized pilot investigating propranolol administration in patients receiving hematopoietic cell transplantation. BMC Cancer. 2018. May;18(1):593. 10.1186/s12885-018-4509-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight JM, Rizzo JD, Wang T, He N, Logan BR, Spellman SR, et al. Molecular Correlates of Socioeconomic Status and Clinical Outcomes Following Hematopoietic Cell Transplantation for Leukemia. JNCI Cancer Spectr. 2019. Sep;3(4):pkz073. 10.1093/jncics/pkz073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight JM, Rizzo JD, Hari P, Pasquini MC, Giles KE, D’Souza A, et al. Propranolol inhibits molecular risk markers in HCT recipients: a phase 2 randomized controlled biomarker trial. Blood Adv. 2020. Feb;4(3):467–76. 10.1182/bloodadvances.2019000765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KS, Davies SM, Majhail NS, Hassebroek A, Klein JP, Ballen KK, et al. Race and socioeconomic status influence outcomes of unrelated donor hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2009. Dec;15(12):1543–54. 10.1016/j.bbmt.2009.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silla L, Fischer GB, Paz A, Daudt LE, Mitto I, Katz B, et al. Patient socioeconomic status as a prognostic factor for allo-SCT. Bone Marrow Transplant. 2009. Apr;43(7):571–7. 10.1038/bmt.2008.358 [DOI] [PubMed] [Google Scholar]
- Shih M, Simon PA. Health-related quality of life among adults with serious psychological distress and chronic medical conditions. Qual Life Res. 2008;17(4):521-528. [DOI] [PubMed] [Google Scholar]
- Wang XS, Shi Q, Shah ND, Heijnen CJ, Cohen EN, Reuben JM, et al. Inflammatory markers and development of symptom burden in patients with multiple myeloma during autologous stem cell transplantation. Clin Cancer Res. 2014. Mar;20(5):1366–74. 10.1158/1078-0432.CCR-13-2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong GW, Wright JM. Blood pressure lowering efficacy of nonselective beta-blockers for primary hypertension. Cochrane Database Syst Rev. 2014. Feb;(2):CD007452. 10.1002/14651858.CD007452.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Sood AK. Molecular pathways: beta-adrenergic signaling in cancer. Clin Cancer Res. 2012. Mar;18(5):1201–6. 10.1158/1078-0432.CCR-11-0641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Luo K, Lv Z, Huang J. Beta-adrenoceptor action on pancreatic cancer cell proliferation and tumor growth in mice. Hepatogastroenterology. 2012. Mar-Apr;59(114):584–8. 10.5754/hge11271 [DOI] [PubMed] [Google Scholar]
- Sloan EK, Priceman SJ, Cox BF, Yu S, Pimentel MA, Tangkanangnukul V, et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010. Sep;70(18):7042–52. 10.1158/0008-5472.CAN-10-0522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamkin DM, Sloan EK, Patel AJ, Chiang BS, Pimentel MA, Ma JC, et al. Chronic stress enhances progression of acute lymphoblastic leukemia via β-adrenergic signaling. Brain Behav Immun. 2012. May;26(4):635–41. 10.1016/j.bbi.2012.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Fuchs C, Le CP, Pimentel MA, Shackleford D, Ferrari D, Angst E, et al. Chronic stress accelerates pancreatic cancer growth and invasion: a critical role for beta-adrenergic signaling in the pancreatic microenvironment. Brain Behav Immun. 2014. Aug;40:40–7. 10.1016/j.bbi.2014.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008. Mar;22(3):659–61. 10.1096/fj.07-9574LSF [DOI] [PubMed] [Google Scholar]
- Wittchen ES, Aghajanian A, Burridge K. Isoform-specific differences between Rap1A and Rap1B GTPases in the formation of endothelial cell junctions. Small GTPases. 2011. Mar;2(2):65–76. 10.4161/sgtp.2.2.15735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen ES, Nishimura E, McCloskey M, Wang H, Quilliam LA, Chrzanowska-Wodnicka M, et al. Rap1 GTPase activation and barrier enhancement in rpe inhibits choroidal neovascularization in vivo. PLoS One. 2013. Sep;8(9):e73070. 10.1371/journal.pone.0073070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa YL, Shi Q, Kumar SK, Lacy MQ, Gertz MA, Kapoor P, et al. Beta-blockers improve survival outcomes in patients with multiple myeloma: a retrospective evaluation. Am J Hematol. 2017. Jan;92(1):50–5. 10.1002/ajh.24582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Sun F, D’Souza A, Dhakal B, Pisano M, Chhabra S, et al. Autonomic nervous system control of multiple myeloma. Blood Rev. 2020. Aug;100741: 10.1016/j.blre.2020.100741 [DOI] [PMC free article] [PubMed] [Google Scholar]