Abstract
Law enforcement personnel are required to respond to a variety of dangerous, potentially life-threatening high stress scenarios. Virtual reality (VR)-based training has been shown to attenuate stress responses; however, little is known about the acute stress response from VR exposure. This study examined the impact of participating in a VR-based active shooter training drill (ASD) on markers of physiological stress as well as potential differences in men and women. To examine the impact of participation in a ~50 sec VR-based ASD, 29 subjects (n = 29; 17 males, 12 females) participated in a quasi-experimental single group design. Saliva samples were collected and analyzed from 27 of the 29 subjects a total of four times 1) 30-min prior to, 2) 5-min prior to, 3) 5-min after, and 4) 30-min after the ASD and analyzed for α-amylase (AA) activity and concentrations of secretory immunoglobulin-A (SIgA), cortisol (CORT), and uric acid (UA). Participation in the ASD resulted in a significant (p < 0.05) increase in salivary stress markers AA and SIgA. In addition, lower concentrations of CORT and UA were found in women compared to men. These findings have implications for law enforcement and/or military personnel that may seek to implement a VR-based training into their training regimen. Future studies should investigate the impact of longitudinal participation in ASD interventions to determine if this is an effective training method to reduce stress responses to real life active shooter training drills.
Keywords: law enforcement, cortisol, cardiovascular disease, adrenalin, occupational stress
Introduction
Law enforcement personnel are required to respond to a variety of dangerous, potentially life-threatening scenarios on-duty. These high stress scenarios can cause acute physiological or psychological stress, and trigger the “fight or flight” response resulting in activation of the hypothalamic pituitary adrenal [1,2] (HPA) and sympathoadrenal (SA) axes [3]. Frequent exposure to high-stress scenarios and work demands such as high-speed chases, suspect pursuits on foot, and confronting a suspect can increase the secretion of the stress hormone cortisol and neurotransmitters epinephrine and norepinephrine, in addition to other stress-associated biomarkers [4-6]. Repeated exposure to such occupational stressors can place the HPA and SA axes under chronic strain [4] and can accelerate cardiovascular disease progression among various high-stress occupations [4,7]. In fact, law enforcement officers have higher rates of mortality due to cardiovascular disease compared to the general population, which is likely due to chronic stress exposure [8-10]. Moreover, law enforcement officers have been characterized as having high rates of obesity and metabolic syndrome [11], which may augment risk for adverse cardiovascular events when exposed to occupational stressors [4].
Salivary stress markers such as α-amylase (AA), cortisol (CORT), and secretory IgA (SIgA) are commonly used as markers of acute stress following video-based workplace scenarios and active shooter training drills (ASD) involving live actors [6,12,13]. Salivary CORT and AA reflect activation of the HPA [14] and SA axes, [15] respectively, while SIgA is a marker of stress exposure that relates to immunosuppression [16]. Previous work has shown video-based scenarios and ASD scenarios involving professional actors appear to mimic the occupational stress experienced by police officers [6,12,13], resulting in significant increases in salivary markers of stress. Salivary CORT, interleukin-6 (IL-6), AA, and SIgA have been shown to increase significantly in response to video-based law-enforcement scenarios (lasting ~2 to 6 min in duration) involving suspect pursuit via motorcycle or confronting an active shooter suspect within a workplace setting [6]. Moreover, a recent study conducted in participants with no law enforcement experience reported increases in blood epinephrine, salivary SIgA, and AA following a ~50 second real life ASD involving professional actors [13]. These findings highlight an important consideration for training law enforcement personnel with cost effective methods to potentially develop stress resiliency.
Local and federal law enforcement agencies commonly use scenario-based training to prepare for high stress situations. The goal is to improve tactical proficiency and provide a form of stress inoculation training, which has been shown to decrease stress in addition to improving emotional states following exposure to real life scenarios [17]. One method of exposing individuals to stress is virtual reality (VR)-based training. Acute exposure to a VR-based Trier Social Stress Tests (a mock job interview with mental arithmetic) have been shown to produce significant increases in salivary markers of stress, including CORT and AA [18,19]. However, when used as a training intervention, VR-based training has been shown to attenuate stress responses, which may reduce the negative physiological and psychological effects associated with chronic strain and stress exposure in law enforcement [20,21]. VR training is growing in popularity and has been used in the field of medicine [22,23] and military operations [24,25] to improve job performance and reduce occupational-induced stress. The military has used VR training as a form of stress inoculation training to better equip soldiers with the means to mediate the potentially negative physiological effects of combat [20,24,25]. Importantly, the aim of such training when considering military and law enforcement populations would be to improve performance and decision making which may (or may not) be associated with a reduction in the acute response to stress [21,26].
A review by Wiederhold & Wiederhold [20] noted VR training was more effective than real-world training in regard to time management, performance efficiency, and overall stress management. Additionally, the benefits of VR-based training and the potential strength of utilizing VR as a means to mitigate physiological markers of occupational stress, while improving overall performance under stressful conditions, were highlighted in a recent review [25]. One of the major benefits of VR-based training is that users are able to engage in dangerous, real-world scenarios in a safe environment. If the VR simulated event is potent enough to cause a significant stress response, it may pose as a more efficacious training modality due to the ability to fully immerse the user in a realistic, fully three-dimensional training environment with low risk of injury and ease of use [27,28]. VR-based training is cost-effective [29] and may require less equipment, space, and personnel (ie, actors for the ASD training scenarios), and may potentially be a more accessible way to train for high stress situations such as confronting and de-escalating an active shooter or high-speed chase. While there are several new VR providers for law enforcement training, the medium is in the early stages of evaluation for long-term training benefits. However, it should be noted that there is potential for adverse effects (eg, added psychological trauma) as a result of long-term training. Furthermore, research has shown that VR can induce temporary changes in static balance, eye fatigue, dizziness, motion sickness, and vertigo for some users [30-32]. Thus, our goal was first to determine the acute stress response to a VR ASD before studying potential effects of a VR ASD training intervention.
While recent research demonstrated ~50 seconds of ASD can significantly increase stress biomarkers [13], little is known about the acute effects of a VR-based ASD on physiological markers of stress. Previous research suggests video-based high stress scenarios cause significant increases in salivary markers of stress [6]. However, to our knowledge, no study has investigated the impact of acute participation in an ASD. Therefore, the primary purpose of this study was to determine the impact of participating in a VR-based ASD on markers of physiological stress. In addition, McAllister and Martaindale [12] reported that while both males and females experienced significant increases in several OS and physiological stress biomarkers, females had lower levels of salivary stress markers when compared to males following a ~50 second real life ASD. Therefore, the secondary purpose of the study was to examine potential differences between men and women in their physiological response to the ASD.
Materials and Methods
Subjects and Experimental Design
To examine the impact of participation in a ~50 sec VR-based ASD, a quasi-experimental single group design was used where measures were collected before and after the ASD. As a secondary analysis, comparisons between male and female subjects were made using two-arm between subject’s design with repeated measures comparisons pre- and post-ASD. Subjects were current university students that provided electronic informed consent prior to participating in the study. Each subject completed a health history questionnaire which included questions regarding age, sex, nicotine use, and physical activity habits. Subjects were required to be: 1) non-cigarette smokers, 2) free from donating blood in the last 30 days, and 3) free from any major stressor in the last 30 days such as a death in the family, new job, etc. All subjects were asked to arrive to testing at least 4 hours fasted, and to avoid nicotine exposure within 24 hours of testing. Testing occurred during the late morning and early afternoon (ie, between ~10:00 – 14:00).
Virtual Reality Study Procedure and Active Shooter Training Drill
Generalized procedures were similar to those done previously [12,13]. Subjects rested in a quiet room for 30-min before being transported approximately 90 m to the VR study room. After collection of 30-min and 5-min pre-ASD stress data, a lab assistant equipped the subject with the VR headset and a firearm. Each subject completed a 1-min VR familiarization exercise to ensure they understood how to operate the equipment. Immediately following this procedure, each subject experienced the ASD. Once complete, the lab assistant removed the VR equipment and transported the subject back to the quiet room for at least 30-min.
The VR-based ASD was purpose-built based on a previous live-action ASD [13]. The research team recorded the live-action ASD using 360-degree cameras and recorded isolated audio from each actor and environmental factor present in the live-action ASD (eg, gunshots, fire alarm, radio traffic). Street Smarts VR (SSVR) (New York, NY, USA) was commissioned to create the ASD. SSVR utilized the 360-degree footage to create an exact replica of the live-action ASD previously studied [13]. During the study, subjects physically walked down a ~10-foot-long virtual hallway where they encountered two wounded victims. One was laying on the ground with traumatic injuries (eg, White female exhibiting eviscerated bowels and gunshot wounds) and the second victim (African American female) “ran” out of the attack room with gunshot wounds to her left arm and leg. Once at the threshold to the attack room, subjects would observe one victim (White female) on the ground with a traumatic head injury and the shooter (White male) firing his handgun at the last victim (African American male). If the subject had not fired his/her weapon yet, the shooter would turn toward the subject to elicit a response. The shooter fell down after being shot. Subjects accessed the ASD through proprietary SSVR software and firearms using HTC VIVE Pro VR equipment (HTC Corp, New Taipei, Taiwan). All subjects experienced the exact same scenario. Furthermore, because the VR lab was larger than the virtual environment (~35 ft x 20 ft) subjects were able to physically move through the entire ASD without interruption.
Saliva Sample Collection and Analysis
Saliva samples were collected using a passive drool method a total of four times: 1) 30-min prior to the ASD, 2) 5-min prior to the ASD, 3) 5-min after the ASD, and 4) 30-min after the ASD. Due to the time required to transport from the data collection building (ie, quiet resting room) to the ASD building (~90 m) saliva collection immediately pre- and post-ASD was not possible. Moreover, these timepoints were selected based on findings from past work involving similar methodology [13]. To provide saliva samples, subjects were asked to tilt their head forward with a mouthpiece adaptor and an attached collection tube until approximately 500uL of saliva was collected. Saliva samples were immediately frozen at -20°C and were transported to the laboratory for storage at -80°C within 6 hours.
Prior to analysis, samples were thawed and centrifuged at 4°C for 15-min at 1500 x g. Samples were analyzed in duplicate for α-amylase (AA) activity and concentrations of secretory immunoglobulin-A (SIgA), cortisol (CORT), and uric acid (UA) using commercially available kits (Salimetrics, PA, USA). Note, AA is a kinetic assay, which required multiple absorbance measures and a 37°C incubation. Salivary UA also required incubation, for which a standard laboratory incubator was used (Thermo Fisher Scientific, Waltham, MA, USA). Absorbance was determined using a colorimetric plate reader (BioTek, Winooski, VT, USA). For assays that required washing, an automated washer was used (BioTek, Winooski, VT, USA). The intra-assay and inter-assay %CV for these assays in our laboratory is typically between 4.5% and 7.2% [13].
State-Anxiety Inventory Assessment
To assess subjective stress, a short (6-item) version of the state-trait anxiety inventory (SAI) was used a total of four times (immediately after each saliva sample was collected). The SAI has six statements such as “I feel calm” and “I am tense” and the values are scored on a scale of 1 to 4 and subsequent composite scores for each scale are used in analysis. The validity and reliability of this scale has been previously established [33,34].
Statistical Analysis
All statistical procedures were conducted using SAS 9.4 (Cary, NC, USA). To assess potential changes across time before and after the ASD, one way analysis of variance (ANOVA) was conducted on all outcome measures with repeated measures using a proc glm procedure. To compare potential differences in the response to the ASD between men and women, 2 x 4 (sex x time) repeated measures ANOVA were conducted. In the instance of a significant main effect or interaction, Fisher’s Least Significant Difference test was conducted to compare means. Effect sizes were calculated and reported as partial eta squared (ηp²).
Results
Twenty-nine subjects completed the experimental testing (n = 29; 17 males, 12 females); however, two subjects did not provide usable saliva samples. Therefore, only data from 27 subjects were used (n = 27; 16 males, 11 females) for saliva analysis. A post hoc power analysis using G*Power 3.1.9.7 for a repeated measures ANOVA (RMANOVA) revealed that a sample size of 27 resulted in a reported power of 0.88 to detect a large effect (f = 0.5) with alpha at .05. Data for all 29 subjects were available for SAI results. Additionally, while adverse effects of VR (eg, cyber sickness) are a concern, no subjects reported feeling adverse effects from the experiment.
Impact of ASD
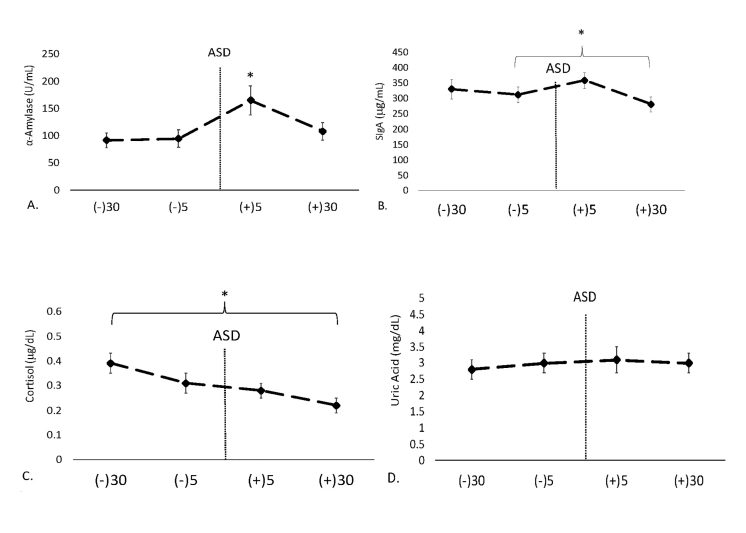
Regarding mean AA activity, a significant time effect was noted (F = 9.24, p < 0.001, ηp² = 0.28). Post hoc analysis indicated 5-min post-ASD was significantly higher than 30-min pre-ASD (p < 0.001), 5-min pre-ASD (p < 0.001), and 30-min post-ASD (p < 0.001). Mean AA activity is shown in Figure 1A.
Figure 1.
Data are shown as mean ± SE. -30 = 30-min pre-ASD, -5 = 5-min pre-ASD, +5 = 5-min post-ASD, +30 = 30-min post-ASD. A. Changes in salivary α-amylase (AA) activity over time. * Denotes significantly (p < 0.05) higher than all other timepoints. B. Changes in concentrations of secretory immunoglobulin-A (SIgA) over time. * Denotes significantly higher concentration at 5-min post-ASD compared to 5-min pre- and 30-min post-ASD. C. Changes in cortisol (CORT) concentrations over time. * Denotes a significant (p < 0.05) decrease in concentrations at each timepoint. D. Mean uric acid (UA) concentrations over time.
In terms of mean SIgA concentrations, a time effect was noted (F = 4.36, p = 0.007, ηp² = 0.14). Mean SIgA concentrations were significantly elevated at 5-min post-ASD compared to 5-min pre-ASD (p = 0.03) and 30-min post-ASD (p < 0.001). Mean SIgA concentrations are shown in Figure 1B.
Regarding CORT concentrations, a significant time effect was found (F = 13.4, p < 0.001, ηp² = 0.34). Post hoc analysis demonstrated a significant decrease in CORT concentrations from 30-min pre-ASD to each subsequent timepoint for collection (p < 0.01). Mean concentrations for CORT are shown in Figure 1C.
There was no time effect for mean UA concentrations (F = 0.71, p = 0.54, ηp² = 0.02). Mean UA concentrations are shown in Figure 1D.
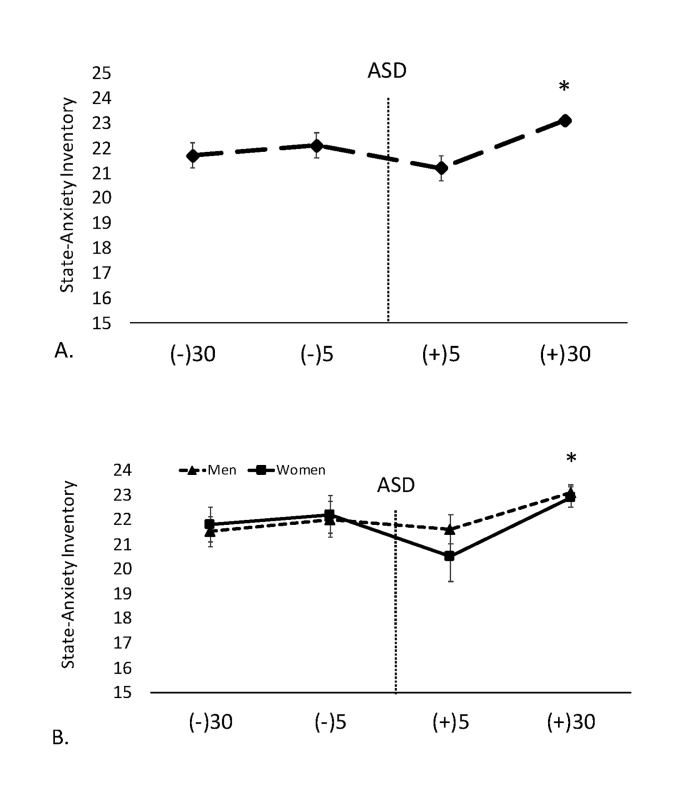
With respect to SAI data, a main effect for time was noted (F = 5.04, p = 0.002, ηp² = 0.15). Post hoc analysis showed SAI levels were significantly higher 30-min post-ASD compared to 30-min pre- and 5-min post-ASD. SAI data are shown in Figure 2A.
Figure 2.
Data are shown as mean ± SE. -30 = 30-min pre-ASD, -5 = 5-min pre-ASD, +5 = 5-min post-ASD, +30 = 30-min post-ASD. A. State anxiety inventory (SAI) in both men and women in response to the ASD. * Denotes significantly (p < 0.05) higher at 30-min post-ASD compared to 30-min pre- and 5-min post-ASD. B. Mean SAI data in men and women. * Denotes significantly higher (p < 0.05) at 30-min post-ASD compared to 30-min pre- and 5-min post-ASD.
Male/Female Comparison
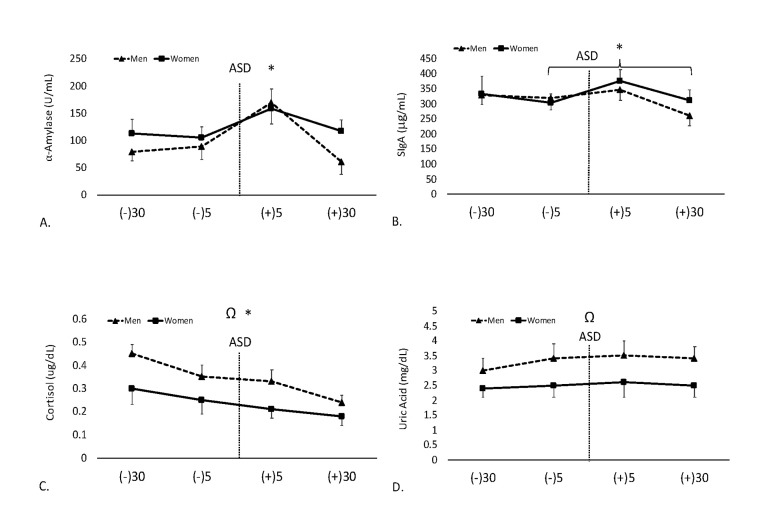
A gender x time interaction was not found for mean AA activity (F = 0.23, p = 0.87). There was no main effect for gender (F = 1.7, p = 0.19). Mean AA activity in men and women are shown in Figure 3A.
Figure 3.
Data are shown as mean ± SE. -30 = 30-min pre-ASD, -5 = 5-min pre-ASD, +5 = 5-min post-ASD, +30 = 30-min post-ASD. A. Changes in salivary α-amylase (AA) activity over time. * Denotes significantly (p < 0.05) higher than all other timepoints. B. Changes in concentrations of secretory immunoglobulin-A (SIgA) over time. * Denotes significantly higher concentration at 5-min post-ASD compared to 5-min pre- and 30-min post-ASD. C. Changes in cortisol (CORT) concentrations over time. * Denotes a significant (p < 0.05) decrease in concentrations at each timepoint. Ω Denotes significantly lower concentrations (p < 0.05) in women compared to men. D. Mean uric acid (UA) concentrations over time. Ω Denotes significantly lower (p < 0.05) concentrations in women compared to men.
In terms of mean SIgA concentration, a gender time interaction was not found (F = 0.80, p = 0.49). There was also no main effect for gender (F = 1.83, p = 0.18). Mean SIgA concentrations in men and women are shown in Figure 3B.
With respect to CORT concentrations, there was no gender time interaction (F = 0.92, p = 0.43). However, there was a main effect for time (F = 11.99, p < 0.001, ηp² = 0.32) and gender (F = 27.93, p < 0.001, ηp² = 0.27). Post hoc analysis demonstrated significantly lower CORT concentrations in women compared to men (p < 0.001) and a decrease in CORT concentrations at each timepoint (p < 0.01). CORT concentrations in men and women are shown in Figure 3C.
In terms of mean UA concentrations, a gender time interaction was not found (F = 0.27, p = 0.84). However, there was a main effect for gender (F = 25.69, p < 0.001, ηp² = 0.25). Post hoc analysis demonstrated significantly lower UA concentrations in women compared to men (p < 0.001). Mean UA concentrations in men and women are shown in Figure 3D.
Finally, there was no gender time interaction or main effect for gender for mean SAI levels (F = 0.44, p = 0.50). SAI data for men and women are shown in Figure 2B.
Discussion
This study suggests the VR-based ASD resulted in significant increases in salivary and subjective stress markers AA, SIgA, and SAI. In addition, lower concentrations of CORT and UA were found in women compared to men. These findings have implications for law enforcement and/or military personnel that may seek to implement a VR-based training into their training regimen. It can be assumed that individuals participating in VR-based scenarios similar to the one utilized in the current study, likely experience a sense of realism during participation.
The ASD platform utilized in this study was based off a real-life active shooter scenario, which involved professional actors playing the role of victims and a gunman [13]. The results of the current study are similar to those previously published [12,13] in that the real-life scenario also elicited significant increases in AA and SIgA. Past work has shown acute stress exposure results in increases in AA and SIgA concentrations which are likely attributed to sympathetic and immune system activation respectively [15,16]. While acute stress exposure has been consistently shown to impact SIgA concentrations [35,36], the validity in relation to blood immunoglobulins may be inconsistent [37] and warrants further study. In addition, the current study did not find changes in salivary UA before or after the ASD. It is possible that the subjects anticipated the real life ASD to be more stressful prior to participation and this might have led to the significant increase in salivary UA prior to participating in the real life ASD [12]. However, without a direct statistical comparison, this is merely speculation.
The present results are similar to a previous study [6] that implemented a video-based scenario involving two law enforcement-based scenarios including an active shooter environment and a motorcycle chase. Groer et al., [6] analyzed salivary stress markers, AA, SIgA, CORT, and interleukin-6 in law enforcement officers exposed to a ~2 min motorcycle chase and a ~6 min active shooter video-based scenario and reported increases in AA and SIgA. It was also reported that salivary CORT increased significantly in response to the active shooter scenario, but not the motorcycle chase scenario [6]. While the findings are similar, there are some apparent differences in the methodology that may explain the discrepancy in CORT reporting. The current study utilized a shorter duration ASD (~50 sec), which may be substantially less physiologically stressful compared to a 6-min active shooter drill. The differences in duration of stress exposure may explain the discrepancy in findings related to salivary CORT. In addition, the Groer et al., [6] study involved a law enforcement sample which may also explain the discrepancies. Moreover, while only speculative, it is possible that the majority of the subjects in the present study did not perceive the VR ASD as being a life threatening or uncontrollable situation, which may be a reason for the lack of a significant increase in CORT [38].
The secondary purpose of this study was to examine potential differences between men and women in their acute physiological response to the ASD. Previous research has shown men and women differ in their physiological response to stress and men may demonstrate greater activation of the HPA and autonomic axes compared to women [39,40]. However, as reported by Kajantie & Phillips [40], these responses may be affected by the phases of the menstrual cycle in women; therefore, caution is warranted when interpreting the current results. However, the findings from the current study showing lower levels of CORT in women compared to men are similar to previous work showing lower levels of AA and CORT in women exposed to an ASD [12]. Regardless, future studies examining differences between men and women in terms of the physiological responses to stress should attempt to standardize or control for the menstrual cycle phases.
The findings regarding SAI changes in response to the ASD were unexpected. Based on findings from a previous study [41], it was expected that SAI values would increase concurrently with salivary AA concentrations. In short, Yuka et al., [41] reported a significant correlation with salivary AA and SAI values in a pilot study involving a mental arithmetic task conducted in ten female participants. However, a more recent study by Szarmach et al., [42] reported no relationship between baseline AA and SAI in patients with major depressive disorder. The present study found higher SAI values at 30-min post-ASD which may be attributed to the sensitivity of the measurement. Briefly, AA is highly sensitive to acute stress [43] and reflects activity from the autonomic nervous system. While only speculation, the subjects involved in the study did not know the details of the VR ASD (eg, the number of victims, etc.) prior to participation; thus, it is possible that the increase in SAI 30-min post-ASD was attributed to an acute state of anxiety that was experienced after completion of the ASD. Perhaps during the 30-min post-ASD waiting time, the subjects spent time reflecting on what was experienced, which could potentially explain the delayed increase in SAI. More studies are needed to further examine this hypothesis.
It should be noted that the current study has some limitations. First, we did not standardize the timing of day for testing for each subject. Since the examined markers can vary based on time of day, this should be viewed as a limitation. In addition, we did not ask subjects to minimize physical activity prior to testing which can impact the stress markers. However, initial arrival to a laboratory or field-based site for participation in potentially stressful research is likely to result in increases in stress hormones such as CORT. Therefore, the current study attempted to minimize this effect by having the subjects rest in a quiet room for 30-min prior to participation in the ASD, which has been similarly done previously [13]. In fact, this is a likely explanation for the significant decreases in CORT found in the present study. It should also be mentioned that both men and women participated in this study, which has similarly been done previously [6,13]. However, since menstrual phases can impact the physiological responses to stress, the lack of control for this variable should be viewed as a limitation. Finally, since the subjects in the study were university students that have presumably not been through police academy training, they may differ from law enforcement officers with respect to baseline levels of stress and thus, the results of the study may be impacted by such differences. Further research is needed in law enforcement officers to elucidate. Future work should also include heart rate assessments, which may be more sensitive to transient changes in autonomic nervous system activity.
The present findings suggest acute exposure to an ASD results in increases in physiological stress markers AA and SIgA. In short, the ~50-second-long VR-based ASD was stressful to subjects. However, no data currently exists detailing physiological response following longitudinal participation in ASD. Therefore, it is unclear if continued exposure to ASD mediates stress responses among subjects. Future research endeavors should examine longitudinal effects of exposure to VR scenarios both in the form of ASD and more general VR environments where stressful scenarios are utilized (eg, police use of force, nursing, military applications).
Glossary
- VR
virtual reality
- ASD
Active shooter training drill
- AA
α-amylase
- SIgA
secretory immunoglobulin-A
- CORT
cortisol
- UA
uric acid
- HPA
hypothalamic pituitary adrenal
- SA
sympathoadrenal
Author Contributions
MJM and MHM conceptualized and designed the study. MJM performed formal sample and statistical analysis. MHM, AEG, MJC performed data collection. All authors contributed to the initial and final write up.
Funding
The authors disclose receipt of partial financial support for the execution of this project: U.S. DOJ – Office of Community Oriented Policing Services (COPS Office) Award # 2019ASWXK001.
References
- Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006;8(4):383–95. 10.31887/DCNS.2006.8.4/ssmith [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens MA, Wand G. Stress and the HPA axis: role of glucocorticoids in alcohol dependence. Alcohol Res. 2012;34(4):468–83. [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS. Adrenal responses to stress. Cell Mol Neurobiol. 2010. Nov;30(8):1433–40. 10.1007/s10571-010-9606-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C-J, Webb HE, Zourdos MC, Acevedo EO. Cardiovascular reactivity, stress, and physical activity. Front Physiol. 2013;4:314-. https://doi.org/ 10.3389/fphys.2013.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giessing L, Frenkel MO, Zinner C, Rummel J, Nieuwenhuys A, Kasperk C, et al. Effects of Coping-Related Traits and Psychophysiological Stress Responses on Police Recruits’ Shooting Behavior in Reality-Based Scenarios. Front Psychol. 2019. Jul;10:1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groer M, Murphy R, Bunnell W, Salomon K, Van Eepoel J, Rankin B, et al. Salivary measures of stress and immunity in police officers engaged in simulated critical incident scenarios. J Occup Environ Med. 2010. Jun;52(6):595–602. 10.1097/JOM.0b013e3181e129da [DOI] [PubMed] [Google Scholar]
- Huang CJ, Webb HE, Evans RK, McCleod KA, Tangsilsat SE, Kamimori GH, et al. Psychological stress during exercise: immunoendocrine and oxidative responses. Exp Biol Med (Maywood). 2010. Dec;235(12):1498–504. 10.1258/ebm.2010.010176 [DOI] [PubMed] [Google Scholar]
- Dubrow R, Burnett CA, Gute DM, Brockert JE. Ischemic heart disease and acute myocardial infarction mortality among police officers. J Occup Med. 1988. Aug;30(8):650–4. 10.1097/00043764-198808000-00011 [DOI] [PubMed] [Google Scholar]
- Franke WD, Collins SA, Hinz PN. Cardiovascular disease morbidity in an Iowa law enforcement cohort, compared with the general Iowa population. J Occup Environ Med. 1998. May;40(5):441–4. 10.1097/00043764-199805000-00006 [DOI] [PubMed] [Google Scholar]
- Calvert GM, Merling JW, Burnett CA. Ischemic heart disease mortality and occupation among 16- to 60-year-old males. J Occup Environ Med. 1999. Nov;41(11):960–6. 10.1097/00043764-199911000-00007 [DOI] [PubMed] [Google Scholar]
- Mumford EA, Liu W, Taylor BG, Ramey S. Profiles of US Law Enforcement Officers’ Diagnosed Health Conditions: Results From a Probability-Based Sample of Officers. J Occup Environ Med. 2021. May;63(5):422–31. 10.1097/JOM.0000000000002162 [DOI] [PubMed] [Google Scholar]
- McAllister MJ, Martaindale MH. Women demonstrate lower markers of stress and oxidative stress during active shooter training drill. Comprehensive Psychoneuroendocrinology. 2021;6:100046. 10.1016/j.cpnec.2021.100046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister MJ, Martaindale MH, Rentería LI. Active Shooter Training Drill Increases Blood and Salivary Markers of Stress. Int J Environ Res Public Health. 2020. Jul;17(14):5042. 10.3390/ijerph17145042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SH, Wand GS, Malhotra S, Kamel I, Horton K. Reliability of hypothalamic-pituitary-adrenal axis assessment methods for use in population-based studies. Eur J Epidemiol. 2011. Jul;26(7):511–25. 10.1007/s10654-011-9585-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrakova L, Doering BK, Vits S, Engler H, Rief W, Schedlowski M, et al. Psychosocial Stress Increases Salivary Alpha-Amylase Activity Independently from Plasma Noradrenaline Levels. PLoS One. 2015. Aug;10(8):e0134561. 10.1371/journal.pone.0134561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivkovic N, Racic M. Biomarkers of Stress in Saliva / Biomarkeri stresa u pljuvački. Acta Facultatis Medicae Naissensis. 2015;32(2):91–9. 10.1515/afmnai-2015-0010 [DOI] [Google Scholar]
- Greco G, Fischetti F. Physical, technical and tactical training and stress management in law enforcement. J Phys Educ Sport. 2018;18(2):555–60. [Google Scholar]
- Zimmer P, Buttlar B, Halbeisen G, Walther E, Domes G. Virtually stressed? A refined virtual reality adaptation of the Trier Social Stress Test (TSST) induces robust endocrine responses. Psychoneuroendocrinology. 2019. Mar;101:186–92. 10.1016/j.psyneuen.2018.11.010 [DOI] [PubMed] [Google Scholar]
- Vatheuer CC, Vehlen A, von Dawans B, Domes G. Gaze behavior is associated with the cortisol response to acute psychosocial stress in the virtual TSST. J Neural Transm (Vienna). 2021. Sep;128(9):1269–78. 10.1007/s00702-021-02344-w [DOI] [PubMed] [Google Scholar]
- Wiederhold MD. A continuum of care: pre-deployment medical and tactical stress inoculation training using virtual reality. International Review of the Armed Forces Medical Services. 2014;(87):3. [Google Scholar]
- Low WR, Sandercock GR, Freeman P, Winter ME, Butt J, Maynard I. Pressure training for performance domains: A meta-analysis. Sport Exerc Perform Psychol. 2021;10(1):149–63. 10.1037/spy0000202 [DOI] [Google Scholar]
- Izard SG, Juanes JA, García Peñalvo FJ, Estella JM, Ledesma MJ, Ruisoto P. Virtual Reality as an Educational and Training Tool for Medicine. J Med Syst. 2018. Feb;42(3):50. 10.1007/s10916-018-0900-2 [DOI] [PubMed] [Google Scholar]
- Bernardo A. Virtual Reality and Simulation in Neurosurgical Training. World Neurosurg. 2017. Oct;106:1015–29. 10.1016/j.wneu.2017.06.140 [DOI] [PubMed] [Google Scholar]
- Lele A. Virtual reality and its military utility. J Ambient Intell Humaniz Comput. 2013;4(1):17–26. 10.1007/s12652-011-0052-4 [DOI] [Google Scholar]
- Pallavicini F, Argenton L, Toniazzi N, Aceti L, Mantovani F. Virtual Reality Applications for Stress Management Training in the Military. Aerosp Med Hum Perform. 2016. Dec;87(12):1021–30. 10.3357/AMHP.4596.2016 [DOI] [PubMed] [Google Scholar]
- Regehr C, LeBlanc V, Jelley RB, Barath I. Acute stress and performance in police recruits. Stress Health. 2008;24(4):295–303. 10.1002/smi.1182 [DOI] [Google Scholar]
- Kamat VR, Martinez JC, Fischer M, Golparvar-Fard M, Peña-Mora F, Savarese S. Research in Visualization Techniques for Field Construction. J Constr Eng Manage. 2011;137(10):853–62. 10.1061/(ASCE)CO.1943-7862.0000262 [DOI] [Google Scholar]
- Shendarkar A, Vasudevan K, Lee S, Son Y. Crowd Simulation for Emergency Response using BDI Agent Based on Virtual Reality. Proceedings of the 2006 Winter Simulation Conference. 2006:545-553, doi: 10.1109/WSC.2006.323128 [DOI]
- Farra SL, Gneuhs M, Hodgson E, Kawosa B, Miller ET, Simon A, et al. Comparative Cost of Virtual Reality Training and Live Exercises for Training Hospital Workers for Evacuation. Comput Inform Nurs. 2019. Sep;37(9):446–54. 10.1097/CIN.0000000000000540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Lee G. Full-immersion virtual reality: adverse effects related to static balance. Neurosci Lett. 2020. Aug;733:134974. 10.1016/j.neulet.2020.134974 [DOI] [PubMed] [Google Scholar]
- Imaizumi LF, Polastri PF, Penedo T, Vieira LH, Simieli L, Navega FR, et al. Virtual reality head-mounted goggles increase the body sway of young adults during standing posture. Neurosci Lett. 2020. Oct;737:135333. 10.1016/j.neulet.2020.135333 [DOI] [PubMed] [Google Scholar]
- Viirre E, Ellisman M. Vertigo in virtual reality with haptics: case report. Cyberpsychol Behav. 2003. Aug;6(4):429–31. 10.1089/109493103322278826 [DOI] [PubMed] [Google Scholar]
- Marteau TM, Bekker H. The development of a six-item short-form of the state scale of the Spielberger State-Trait Anxiety Inventory (STAI). Br J Clin Psychol. 1992. Sep;31(3):301–6. 10.1111/j.2044-8260.1992.tb00997.x [DOI] [PubMed] [Google Scholar]
- Tluczek A, Henriques JB, Brown RL. Support for the reliability and validity of a six-item state anxiety scale derived from the State-Trait Anxiety Inventory. J Nurs Meas. 2009;17(1):19–28. 10.1891/1061-3749.17.1.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos-Gomes N, Katsurayama M, Makimoto FH, Santana LL, Paredes-Garcia E, Becker MA, et al. Psychological stress and its influence on salivary flow rate, total protein concentration and IgA, IgG and IgM titers. Neuroimmunomodulation. 2010;17(6):396–404. 10.1159/000292064 [DOI] [PubMed] [Google Scholar]
- Ring C, Carroll D, Hoving J, Ormerod J, Harrison LK, Drayson M. Effects of competition, exercise, and mental stress on secretory immunity. J Sports Sci. 2005. May;23(5):501–8. 10.1080/02640410410001729955 [DOI] [PubMed] [Google Scholar]
- Plomp R, de Haan N, Bondt A, Murli J, Dotz V, Wuhrer M. Comparative Glycomics of Immunoglobulin A and G From Saliva and Plasma Reveals Biomarker Potential. Front Immunol. 2018. Oct;9:2436. 10.3389/fimmu.2018.02436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004. May;130(3):355–91. 10.1037/0033-2909.130.3.355 [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol Psychol. 2005. Apr;69(1):113–32. 10.1016/j.biopsycho.2004.11.009 [DOI] [PubMed] [Google Scholar]
- Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006. Feb;31(2):151–78. 10.1016/j.psyneuen.2005.07.002 [DOI] [PubMed] [Google Scholar]
- Noto Y, Sato T, Kudo M, Kurata K, Hirota K. The relationship between salivary biomarkers and state-trait anxiety inventory score under mental arithmetic stress: a pilot study. Anesth Analg. 2005. Dec;101(6):1873–6. 10.1213/01.ANE.0000184196.60838.8D [DOI] [PubMed] [Google Scholar]
- Szarmach J, Cubała WJ, Landowski J, Chrzanowska A. No relationship between baseline salivary alpha-amylase and State-Trait Anxiety Inventory Score in drug-naïve patients with short-illness-duration first episode major depressive disorder: an exploratory study. J Clin Exp Dent. 2017. Apr;9(4):e527–30. 10.4317/jced.53631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher S, Kirschbaum C, Fydrich T, Ströhle A. Is salivary alpha-amylase an indicator of autonomic nervous system dysregulations in mental disorders?—a review of preliminary findings and the interactions with cortisol. Psychoneuroendocrinology. 2013. Jun;38(6):729–43. 10.1016/j.psyneuen.2013.02.003 [DOI] [PubMed] [Google Scholar]