Abstract
Phosphorylation regulates connexin43 (Cx43) function from assembly/disassembly to coupling at the plaque. Src is a tyrosine kinase known to both phosphorylate Cx43 (residues Y247 and Y265) and affect gap junction intercellular communication. However, the Cx43 carboxyl-terminal (CT) domain contains additional tyrosine residues and proteomic discovery mass spectrometry data identified Y313 as a potential phosphorylation target. Based upon the study of Lin et al. (2001) J. Cell Biol., which still observed tyrosine phosphorylation by Src when using a Cx43 Y247/Y265F mutant, we addressed the possibility of Y313 phosphorylation (pY313) by Src. In vitro Src phosphorylation of purified Cx43CT followed by mass spectroscopy revealed that Src also phosphorylates Y313. This observation was confirmed by repeating the in vitro phosphorylation using different combinations of Cx43CT Y → F mutants and a general anti-pTyr antibody. Next, a phospho-specific antibody was generated to help characterize the importance of pY313. We established an in cyto experimental system by stably expressing Cx43 WT and mutants (Y247F, Y265F, Y313F, Y247/265F, Y247/313F, Y265/313F, or Y247/265/313F) in Cx43-deficient HeLa cells. Cx43 WT and mutants, in the absence of v-Src, localized to the plasma membrane and formed gap junctions. When v-Src was over-expressed, Cx43 WT localized intracellularly, while all of the single and double mutants remained able to form plaques and transfer dye, albeit variable in number and amount, respectively. Complete Src-resistance was only achieved with the Cx43 Y247/265/313F mutant. Furthermore, Cx43 Y265F inhibited the ability of v-Src to phosphorylate Y247 and Y313 as well as phosphorylation at both Y265 and Y313 was necessary to inhibit the Cx43 interaction with Drebrin. Finally, we observed in diseased cardiac tissue, in which Src is active, an increase in intercalated disc and intracellular localized Cx43 pY313.
Keywords: Gap junctions, Intercellular communication, Connexin43, Src, Drebrin, Phosphorylation
1. Introduction
Gap junctions are integral membrane proteins that enable the direct cytoplasmic exchange of ions and low molecular mass metabolites between adjacent cells [1]. They provide a pathway for propagating and amplifying signal transduction cascades triggered by cytokines, growth factors, and other cell signaling molecules involved in cellular development, growth, and differentiation. In the heart, gap junctions mediate propagation of cardiac action potentials and the maintenance of a regular beating rhythm [2]. Dysfunctional intercellular communication via gap junctions has been implicated in causing many human diseases [3]. Gap junctions are formed by the apposition of connexons from adjacent cells, where each connexon is a hexamer of connexin proteins. Though the 21-connexin isoforms share significant sequence homology, the major divergence in primary structures occurs in the cytoplasmic loop and carboxyl-terminal (CT) domain.
The regulation of gap junction assembly, stability, degradation, as well as channel gating and selectivity properties, all involve CT phosphorylation. Over 20 amino acids in the connexin43 CT (43 kDa isoform, Cx43CT) domain have been identified as phosphorylated and significant progress has been achieved in characterizing the involved kinases [4–7]. The negative charge of the phosphate could affect the permeability of ions to the pore [8], introduce conformational changes distinct from charge alone to alter the CT structure and/or transmembrane α-helices to influence pore size [9], or directly modify the binding affinities of protein partners involved in regulating Cx43 [10–12]. Notably, if phosphorylation alters the kinetics of channel assembly or degradation, cell-to-cell communication will be altered. For example, studies have demonstrated that MAPK phosphorylation of Cx43 residues S279 and S282 increases the binding affinity for the E3 ubiquitin ligase Nedd4, which leads to Cx43 degradation [11,13,14] and Src phosphorylation of Cx43 residue Y265 decreases the binding affinity for the gap junction stabilizing protein Drebrin [15,16]. Other studies have shown that Src phosphorylation of Cx43 residue Y247 inhibits the binding of β-tubulin [10] and Akt phosphorylation of Cx43 residue S373 inhibits the interaction with ZO-1 [12]. In addition, phosphorylation of S373 enables the binding of 14–3-3 leading to gap junction ubiquitination, internalization, and degradation during acute cardiac ischemia [17]. Clearly, our understanding of connexin regulation will not be complete until all kinases, protein-binding partners, and CT sites phosphorylated have been identified, and the impact of such modifications recognized.
We therefore searched the web-based systems biology resource PhosphoSitePlus in an attempt to identify phosphorylation sites that have not been correlated with Cx43 function [18]. Of interest was Y313, which had the greatest number of reported phosphorylation modifications (312 hits) of any Cx43 serine or tyrosine residue (Y247 was next with 253 hits) as observed from proteomic discovery mode mass spectroscopy [18]. Based upon the study of Lin et al. [19], which still observed tyrosine phosphorylation by Src when using a Cx43 Y247/Y265F mutant, we addressed in this study the possibility that Src phosphorylates Cx43 residue Y313. We established an in cyto experimental system by stably expressing Cx43 wild type (WT) and mutants (Y247F, Y265F, Y313F, Y247/265F, Y247/313F, Y265/313F, or Y247/265/313F) in Cx43-deficient HeLa cells. Expression of v-Src causes phosphorylation of Cx43 residue Y313, which contributes to loss of gap junction intercellular communication (GJIC), intracellular localization of Cx43, and the inhibition of the Cx43 interaction with Drebrin. Additionally, an increase in phosphorylation of Cx43 Y313 was observed in diseased human heart.
2. Materials and methods
2.1. Antibodies
Cx43 pY247 and pY265 antibodies were generous gifts from Dr. Paul Lampe (Fred Hutchinson Cancer Research Center). The Cx43 pY313 antibody was produced in rabbit from a peptide designed and chemically synthesized (LifeTein). Additional antibodies used in this study include α-Src (#2123S), α-phospho-Src (Y416) (#6943S), α-rabbit-Alexa488 (#4413), α-mouse-Alexa647 (#4410), Normal Rabbit IgG (#2729), and α-pTyr-1000 Multi-Mab (#8954) purchased from Cell Signaling; α-mouse-HRP (H+L; #12–439) and α-Drebrin (#AB10140) purchased from EMD Millipore; α-Cx43 (#C6219) purchased from Sigma-Aldrich; Texas Red Dextran (3 kDa; #D1863) and Lucifer yellow CH (#L453) purchased from Life Technologies; α-rabbit-HRP (H+L; #20320) purchased from Alpha Diagnostics; α-Cx43 (ab87645 and ab15189) and α-goat-Alexa555 (ab150130), α-rabbit-Alexa647 (ab150079) purchased from Abcam; and DAPI (#157574) purchased from MP Biomedicals.
2.2. Molecular biology
Cx43 WT was PCR amplified from a pGEM-HE (Clontech) plasmid containing the human Cx43 (hCx43) sequence using primers suitable for Gibson assembly into the pD2529 vector (Atum). Purified PCR product and vector were assembled using NEBuilder HIFI DNA Assembly Master Mix (NEB) per manufacturer protocol. All mutant constructs of hCx43 (Y247F, Y265F, Y313F, Y247/265F, Y247/313F, Y265/313F, Y247/265F, Y247/265/313F, and Y313E) were generated using the QuikChange Lightning Site-Directed Mutagenesis Kit (Agilent) and confirmed by DNA sequencing (ACGT, Inc.).
2.3. Cell culture
HeLa and LA-25 cells were generous gifts from Dr. Steve Caplan (University of Nebraska Medical Center) and Dr. Paul Lampe (Fred Hutchinson Cancer Center), respectively. LA-25 is a modified NRK cell line that endogenously expresses Cx43 and contains a temperature sensitive v-Src [20]. All cells were cultured in Dulbecco’s modified Eagle medium (Corning) supplemented with 10% fetal bovine serum (Atlanta biologicals), 2 mM L-glutamine (HyClone), 1% pen-strep (Corning), and 0.2% Normocin (Invivogen) at 37 °C in a humidified 5% CO2 atmosphere.
2.4. Stable clone generation
Approximately 60–70% confluent HeLa cells were transfected with pD2529 SalI linearized hCx43 WT and Y → F mutants using Lipofectamine 2000 in OptiMem. Transfections were carried out under antibiotic free conditions, and cells were cultured 72 h prior to addition of puromycin (2 μg/mL; Tocris) selection media. All Cx43 WT and Y → F mutant cell lines were clonally selected using Whatman paper cloning disks. Western blots and immunofluorescence were used to screen clones.
2.5. Immunofluorescence
Cells grown on coverslips were fixed with 4% Paraformaldehyde for 20 min at room temperature (RT). Coverslips were washed 2 × 5 min in 1× Tris-buffered saline (TBS) and then blocked for 60 min at RT with blocking buffer (1× TBS, 5% Goat Serum, 0.2% Triton X-100). Cells were immunostained with appropriate primary antibodies diluted in blocking buffer overnight at 4 °C. Coverslips were washed 3 × 10 min with 1× TBS, incubated with secondary antibody for 1 h at RT, stained with DAPI (100 ng/mL) (MP Biomedicals) for 10 min, and then washed 3 × 10 min with 1× TBS. Coverslips were mounted on a drop of SlowFade anti-fade (Life Tech), sealed with clear nail polish, and imaged.
2.6. Confocal imaging
All cell immunofluorescence images were acquired on a Zeiss LSM 800 Confocal system using appropriate numerical aperture objectives and appropriate filter sets.
2.7. Western blot
Cultured cells were rinsed 2× with cold 1× TBS and then lysed in lysis buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 0.2% SDS, Complete protease inhibitor and PhosSTOP) on ice for 30 min. Protein concentration was quantified by BCA assay (Pierce). A total of 30 μg protein lysate was resolved by SDS-PAGE and transferred to PVDF (EMD Millipore) membrane. Blots were blocked in 5% BSA in TBST (1× TBS with 0.1% Tween-20 plus 5 mM Na3VO4) for 1 h at RT and incubated with indicated primary antibody in blocking buffer overnight at 4 °C. Blots were washed 3 × 10 min with TBST, and then incubated for 1 h at RT with secondary antibody. Blots were then washed again 3 × 10 min with TBST and detected using the Signal West Femto kit (Thermo) per manufacturer protocol and exposed to autoradiography film. Quantifications were done using NIH ImageJ software using a minimum of three independent replicates.
2.8. Co-immunoprecipitation
HeLa cells stably expressing Cx43 were seeded in 10 cm plates, transfected with or without v-Src (Addgene, #14578), and then grown to confluency. Cells were rinsed 2× with 1× TBS + 1 mM EDTA and then scraped up in either 0.5 or 1.0 mL of IP lysis buffer (25 mM Tris, 150 mM NaCl, 0.25% sodium deoxycholate, 0.05% SDS, 1% Triton X-100 plus protease, 10% glycerol, and phosphatase inhibitors). Cells were mechanically lysed (syringe pumping) and then incubated for 30 min at 4 °C with rotation. Lysates were precleared with normal rabbit IgG (2 μg) bound protein G agarose for 1 h at 4 °C and then clarified by centrifugation. Clarified lysates were quantified by BCA (Pierce) and normalized. Input samples were reserved and 2 mg of lysate was immunoprecipitated with 2 μg of either mouse α-Cx43 IF1 (from Dr. Lampe) or rabbit α-Drebrin overnight at 4 °C. Immune-protein complexes were captured by 2 h incubation with protein G agarose (Pierce) at 4 °C, washed 5× with IP wash buffer (IP lysis buffer excluding sodium deoxycholate). Immunoprecipitated proteins were eluted using 35–50 μL of 3× SDS sample buffer. Equal volumes of each sample were analyzed by Western blot.
2.9. In vitro kinase assay
Rat Cx43CT236–382 was expressed and purified as described previously [21]. Purified Cx43CT WT and Y → F mutants were incubated with or without (Control) active Src (Life Technologies) for 16 h at 30 °C [22]. Products from kinase assay were ran on SDS-PAGE gel, stained with Coomassie blue, and Western blotted with an α-pTyr antibody. Abbreviations: Y247/265 = Y267/286/301/313F, Y267 = Y247/265/286/301/313F, Y286 = Y247/265/267/301/313F, Y301 = Y247/265/267/286/313F, and Y313 = Y247/265/267/286/301F.
2.10. Mass spectrometry
Purified Cx43CT236–382 was incubated in vitro with active Src as described above. The reaction was stopped on ice, and 1 nmol of protein was ran on a SDS-PAGE gel and Western blotted using a α-p-Tyr antibody. 10 nmol of protein was run on a SDS-PAGE gel and stained with Coomassie blue. The Cx43CT band was cut out and sent to the Beth Israel Deaconess Medical Center Mass Spectrometry Facility for post-translational mapping.
2.11. NMR
NMR data were acquired at the University of Nebraska Medical Center NMR Facility using a 600 MHz Bruker Avance-III HD NMR Spectrometer outfitted with a cryo-probe. NMR spectra were phased and processed using Bruker TopSpin software and NMRPipe [23], and analyzed using NMRView [24]. Binding isotherms were obtained from gradient-enhanced 15N-heteronuclear single quantum coherence (HSQC) experiments, acquired with 1024 complex points in the direct dimension and 128 complex points in the indirect dimension. Sweep widths were 8000 Hz in the 1H dimension and 1720 Hz in the 15N dimension. 15N-labeled Cx43CT236–382 domain (Y313D or Y265/313D mutant) in 1× PBS (pH 7.5) was maintained constant at 50 μM while adding increasing amount of Drebrin1–300 (purification previously described in [16]). Dissociation constants (KD) were calculated by non-linear fitting of the decrease in signal intensity of at least three residues (KD ± standard deviation of the mean) using GraphPad Prism 5.0 (GraphPad Software).
2.12. Dye-transfer assay
Cells were scrape-loaded as described previously [25]. Briefly, HeLa cells seeded on coverslips were transfected with or without v-Src for 24 h prior to scrape loading at 37 °C. 100% confluent cells were removed from the incubator, washed 1× with 1× PBS, and then overlaid with pre-warmed (at 37 °C) 1× PBS containing 0.25% Lucifer yellow and Texas Red Dextran (3 kDa, 1.5 mg/mL). Cells were scrape-loaded with a fine edged micro-scalpel by two longitudinal scratches and then incubated at RT for 5 min. Cells were rinsed quickly 2× with warm 1× PBS and incubated in cell culture medium at 37 °C for 5 min. After incubation, cells were washed 2× with warm 1× PBS containing 1 mM CaCl2 to stop dye spread and fixed with 4% Paraformaldehyde for 20 min. Auto-fluorescence was quenched by treatment with 0.1 M glycine for 15 min. Coverslips were mounted onto glass slides in a drop of SlowFade anti-fade (Invitrogen). The cells were imaged using confocal microscope with appropriate filters.
2.13. Immunohistochemistry for human tissue array
Tissue arrays of human, left ventricular myocardial hypertrophy tissues were purchased from BioCat and Pantomics Inc. Slides were deparaffinized in xylene and rehydrated in graded ethanol. Slides were then incubated in 0.3% hydrogen peroxide (H2O2) for 30 min in the dark to block endogenous peroxidase activity. After washing with TBS-T (1× TBS, 0.025% Triton), slides were microwaved 25 min for heat-induced epitope retrieval in sodium citrate buffer (10 mM sodium citrate, 0.05% Tween 20, pH 6.0). Slides were washed again with TBS-T, blocked with 2.5% horse serum for 1 h at RT and incubated at 4 °C overnight with the following primary antibodies: α-Cx43 (1:100, ab15189 Abcam) and α-Cx43 pY313 (1:200). The next day, sections were washed in TBS-T and incubated in HRP-conjugated horse anti-rabbit/mouse secondary antibody (Vector Laboratories #MP-7500) for 1 h at RT. Slides were washed in TBS-T and developed using DAB peroxidase substrate kit according to manufacturer instructions (Vector Laboratories #SK4100) and counterstained with haematoxylin. Additional sections were stained with HE for pathologic and histologic assessment. 24 human left heart tissue specimens were reviewed by a cardiovascular pathologist (Dr. Radio) and graded according to staining intensity and percentage positive staining (1 = negative; 2 = weak; 3 = moderate; 4 = strong staining). Immunohistochemistry staining scores were analyzed and compared by one-way ANOVA and an independent t-test. All digital bright field IHC slide scanning were processed by VENTANA iScan HT (Roche) at UNMC tissue Sciences facility. The images were analyzed using Image Viewer.
2.14. Immunofluorescence for human tissue array
Slides were deparaffinized in xylene and rehydrated in graded ethanol. After washing with TBS-T (1× TBS, 0.025% Triton), slides were permeabilized in 50% methanol followed by heat induced epitope retrieval by microwaving for 25 min in sodium citrate buffer (10 mM sodium citrate, 0.05% Tween 20, pH 6.0). Slides were washed again with TBS-T and blocked (1× TBS with 2.5% horse serum and 0.2% Triton) for 1 h at RT and incubated at 4 °C overnight with the following primary antibodies: α-Cx43 (1:50, ab87645 Abcam) and α-Cx43 pY313 (1:100). The next day, sections were washed 3 × 10 min with 1× TBS-T, incubated with secondary antibody (ab150130 and ab150079, Abcam) for 1 h at RT, stained with DAPI (100 ng/mL) (MP Biomedicals) for 10 min, and then washed 3 × 10 min with 1× TBS-T. Coverslips were mounted on a drop of SlowFade anti-fade (Life Tech), sealed with clear nail polish, and imaged.
3. Statistical analysis
All data were analyzed by using GraphPad Prism 5.0 and presented as the mean + s.e.m. Statistical analysis performed in GraphPad Prism 5.0 were either one-way ANOVA with a Neuman-Keuls post-hoc analysis or Student’s T-Test where appropriate. P-values < .05 were considered statistically significant.
4. Results
4.1. Src phosphorylates Cx43 residue Y313
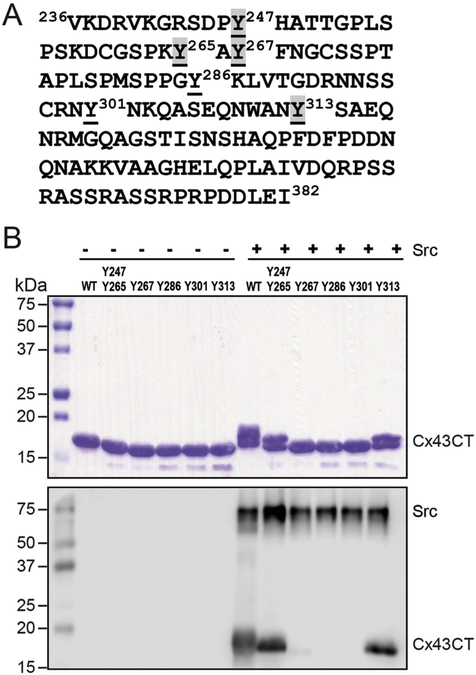
Motivation behind this study was based upon combining two pieces of data: 1) 312 proteomic discovery-mode mass spectroscopy studies showed phosphorylation of Cx43 residue Y313 (PhosphoSitePlus, [18]) and 2) tyrosine phosphorylation remained after Src phosphorylation of Cx43 Y247/265F [19]. To address the potential phosphorylation of Cx43 residue Y313, purified Cx43CT236–382 (Fig. 1A) was incubated in vitro with active Src (Life Technologies) as described in [22,26] and phosphorylation was confirmed using an anti-pTyr antibody (Fig. 1B; WT + Src lane). After trypsin digestion, Tandem MS/MS identified phosphorylation at Y247, Y265, Y267, and Y313 (Table I). To confirm that tyrosine phosphorylation can occur at residues other than Y247 and Y265, the in vitro kinase assay was performed using different Cx43CT236–382 (Y → F) constructs. Phosphorylation was detected by SDS-PAGE and with an anti-pTyr antibody. In the Cx43 WT, Y247/Y265 (all other Y → F), and Y313 (all other Y → F) lanes + Src, a slower mobility band was observed after Coomassie staining (Fig. 1B, top). The altered mobility is likely due to the negative charges on the phosphate group interfering with protein-SDS interactions, thereby disrupting the charge-to-mass ratio and altering the electrophoretic mobility of the protein [9]. Of note, phosphorylated Cx43 results in multiple electrophoretic isoforms: a fast migrating isoform, which includes the non-phosphorylated isoform (P0), and multiple slower migrating isoforms (P1 and P2) [27–29]. The Cx43 WT, Y247/Y265, and Y313 lanes were also the only lanes where a Western blot band was observed with the anti-pTyr antibody (Fig. 1B, bottom). The data indicate that while Src phosphorylation occurs on Y247 and Y265 as previously observed [19,20], Src can also phosphorylate Y313 and Y267 (only detected by MS) in vitro. We moved forward with investigating Y313 because significantly more in vitro phosphorylation and mass-spectroscopy “hits” were observed at this site than Y267 (312 vs. 10).
Fig. 1. Identification of Cx43 Y313 as a novel Src phosphorylation site.
A) Sequence of the Cx43CT domain. Highlighted (grey) are the phosphorylated tyrosine residues identified from mass spectrometry from the in vitro kinase assay. B) The same in vitro kinase assay in (A) was performed using Cx43CT236–382 wild type (WT) or different tyrosine substituted CT constructs (all replaced to a phenylalanine except the labeled tyrosine) as substrate and phosphorylation was detected by Western blot using a general anti-pTyr antibody (top: SDS-PAGE; bottom: Western blot).
Table I.
Phospho-Tyr containing peptides identified from mass spectrometry of the Cx43CT in vitro phosphorylated by Src.
| Peptide sequence | Start-end (residue number) | Average mascot Delta ion score | aNumber of phospho-peptides | Phosphorylation site |
|---|---|---|---|---|
|
| ||||
| GRSDPyHATTGPLSPSK | 242–258 | 16.9 | 4 | Y247 |
| GRSDPyHATTGPLSPSKDCGSPK | 242–264 | 17.0 | 1 | Y247 |
| SDPyHATTGPLSPSK | 244–258 | 19.7 | 8 | Y247 |
| SDPyHATTGPLSPSKDCGSPK | 244–264 | 12.8 | 2 | Y247 |
| yAYFNGCSSPTAPLSPMSPPGYK | 265–287 | 10.8 | 10 | Y265 |
| DRGSPTyAYFNGCSSPTAPLSPMSPPGYK | 259–287 | 11.8 | 2 | Y265 |
| YAyFNGCSSPTAPLSPMSPPGYKLVTGDR | 265–293 | 8.0 | 2 | Y267 |
| yAyFNGCSSPTAPLSPMSPPGYK | 265–287 | 26.9 | 10 | Y265, Y267 |
| QASEQNWANySAEQNR | 304–319 | 16.9 | 8 | Y313 |
Peptides with Mascot Delta Ion Score > 7 were used to obtain the average Mascot Delta Ion Score.
4.2. Cx43 pY313 antibody validation
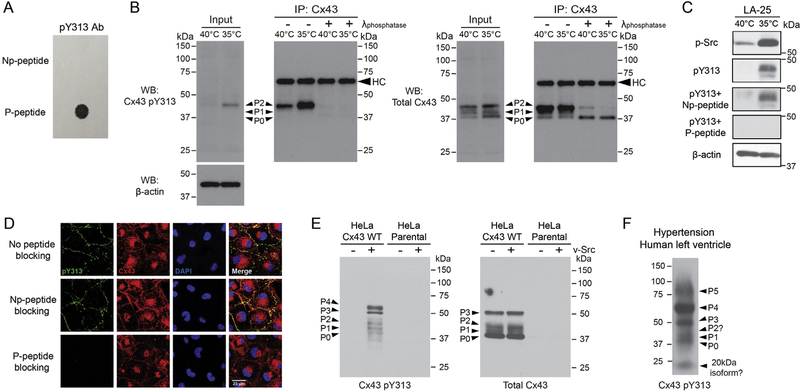
To determine if phosphorylation of Y313 occurs in context of full length Cx43 and in cells, as well as to complement the well-characterized phospho-specific pY247 and pY265 antibodies used in numerous studies to characterize the regulation of Cx43 by Src [20,30], we generated a phospho-specific pY313 antibody. LifeTein produced the antibody and tested specificity using pY313 phospho-(P-peptide; used to create the antibody) and non-phospho-(Np-peptide) peptides (Fig. 2A). We extended the validation process using the LA-25 cell line [20,31]. The presence of active v-Src (35 °C) increased the level of phosphorylation at Cx43 residue Y313 (Fig. 2B, left). The band is specific to phosphorylation of Y313 as the presence of a general tyrosine phosphatase after immunoprecipitation of Cx43 (Fig. 2B, right) or pre-incubation of the antibody with the pY313 peptide (Np-peptide had no effect on the binding of the antibody; Fig. 2C) caused complete loss of the Western blot signal. For comparison, we also present Western blot data using a total Cx43 antibody (Fig. 2B, right). Specificity of the antibody was further confirmed by immunofluorescence in the LA-25 cells. All Cx43 pY313 co-localized with Cx43 (Fig. 2D, top panel). Interestingly, while total Cx43 was distributed between both the plasma membrane and cytoplasm, most Cx43 pY313 signal was at the plasma membrane. Only pre-incubation of the antibody with the P-peptide, not the Np-peptide, caused complete loss of immunofluorescence signal (Fig. 2D, middle and bottom panels).
Fig. 2. Specificity of the Cx43 Y313 phospho-specific antibody.
A) LifeTein performed a dot-blot assay to demonstrate that the pY313 antibody does not recognize non-phosphorylated Y313 peptides (non-phospho-(Np-peptide) and phospho-(P-peptide) peptides). B) Western blot of total cell lysate (input) from LA-25 cells at 40 °C or 35 °C (active v-Src) incubated with the Y313 phospho-specific antibody (left) or a total Cx43 antibody (right). The lysate was used to immunoprecipitate (IP) Cx43, which was subsequently incubated with and without a general tyrosine phosphatase and Western blotted using the same antibodies. C) LA-25 cells at 40 °C or 35 °C (active v-Src) were incubated with the Y313 phospho-specific antibody in the presence of Np-peptide and P-peptide peptides. Data were analyzed by Western blot (antibody used is indicated, left of each panel). D) Immunofluorescence showing co-localization (yellow) of total Cx43 (red) with the Cx43 Y313 phospho-specific antibody (green) at the plasma membrane in LA-25 cells. Preincubation with the P-peptide caused loss of the Y313 phospho-specific antibody signal (green). E) Western blot of total cell lysate from stably Cx43 expressing HeLa cells and parental HeLa cells transiently transfected with v-Src using the Y313 phospho-specific antibody (left) or a total Cx43 antibody (right). F) Western blot of total cell lysate from hypertensive human left ventricle tissue (Novus Biologicals, NB820–59315) using the Y313 phospho-specific antibody.
Western blots were also performed using stably Cx43 expressing HeLa cells and parental HeLa cells (Cx43 deficient) transiently transfected with v-Src to define the specificity in a different cell line that is commonly used in the gap junction field. For the stably Cx43 expressing HeLa cells, no signal was observed with the Cx43 pY313 antibody in the control lane (no v-Src) (Fig. 2E, left). Conversely, a number of bands were observed in the presence of v-Src. The total Cx43 antibody (Fig. 2E, right) and the Cx43 pY313 antibody had equivalent bands corresponding to the P0, P1, and P2 isoforms as well as a 50 kDa (P3) variant. The Cx43 pY313 antibody also labeled one slower migrating bands at ~60 kDa (P4). The ~60 kDa band was also seen when the pY247 and pY265 antibodies (and not when using a total Cx43 antibody) were initially characterized for specificity and may represent a hyper-phosphorylated isoform [20]. For the parental HeLa cells, no signal observed with the Cx43 pY313 or total Cx43 antibodies in the presence and absence of Src (Fig. 2E, left and right). The data illustrates that the antibody is specific to Cx43. Finally, we tested the feasibility of using the Cx43 pY313 antibody for Western blot using lysate from human tissue (Fig. 2F). The bands from a hypertensive human left ventricle are comparable to the bands observed from the Cx43 expressing HeLa cells + v-Src (P0-P4 isoforms); however, two additional bands at ~20 kDa and ~75 kDa (P5) were observed. We speculate that the ~20 kDa isoform is the same variant as seen in [32,33] and that the ~75 kDa (P5) isoform is a phosphorylated Cx43 dimer.
Unfortunately, the Western blots in the [20] study were cropped around ~70 kDa thus not enabling a comparison with the ~75 kDa (i.e., between our pY313 and their pY247 and pY265 antibodies). Altogether, the data indicates we generated a new tool to characterize Cx43 regulation, a phospho-specific pY313 antibody.
4.3. pY313 contributes to gap junction disassembly
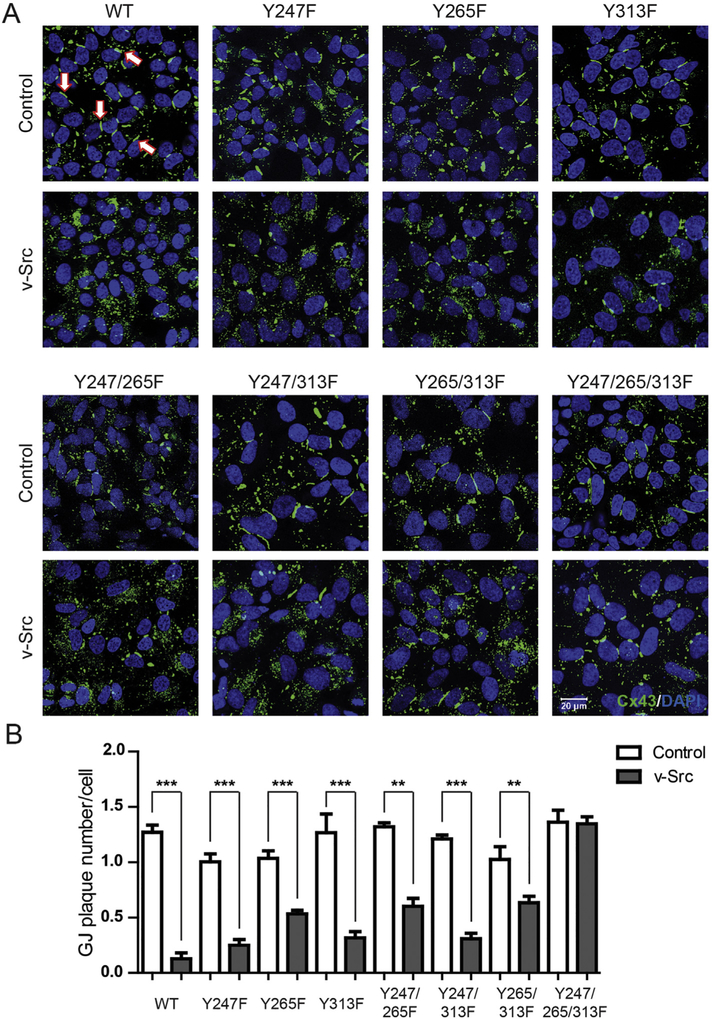
Cx43 residues Y247 and Y265 are well-established phosphorylation sites that contribute to Src mediated down regulation of GJIC (along with MAPK and PKC sites; [19,20,34]). To help determine the contribution of Cx43 Y313 phosphorylation in the regulation imposed by Src, we established an in cyto experimental system by stably expressing Cx43 WT and phosphorylation eliminating mutants (Y247F, Y265F, Y313F, Y247/265F, Y247/313F, Y265/313F, or Y247/265/313F) in Cx43-deficient HeLa cells. We initially evaluated the cellular localization of Cx43 WT and Y → F mutants alone and when expressed with v-Src (Fig. 3). In the absence of v-Src, there was no statistical difference in the number of gap junction plaques formed per cell between Cx43 WT and the Y → F mutants (Supplemental Fig. 1A). In the presence of v-Src, Cx43 WT had the greatest decrease in the number of gap junction plaques formed per cell (90%) (Fig. 3A, B). The single and double Cx43 mutants containing Y265F (Y265F, Y247/265F, Y265/313F) had ~50% decrease in the number of gap junction plaques formed per cell and for all other Cx43 mutants (Y247F, Y313F, Y247/313F) the decrease was ~75%. Interestingly, the most phosphorylation resistant Cx43 mutant Y247/265/313F was the least affected (0.2%). The data suggest that Cx43 residues Y247, Y265, and Y313 all contribute to gap junction disassembly, with phosphorylation of residue Y265 playing a more critical role in the process (i.e. largest decreases observed when this site is not mutated from Y → F).
Fig. 3. Phosphorylation of Cx43 residue Y313 by Src contributes to gap junction disassembly.
Localization of Cx43 in HeLa cells stably expressing Cx43 WT or phosphorylation eliminating mutants (labeled above), and transiently transfected with v-Src. A) Immunofluorescence using an anti-Cx43 antibody (green). The nucleus has been labeled with DAPI (blue). White arrows point to a gap junction plaque. B) Quantification of the number of gap junction (GJ) plaques per cell shows the effect of inhibiting v-Src phosphorylation of different Cx43 tyrosine residues. Results presented as the mean + s.e.m. (n = 3). Statistics used to analyze the data was one-way ANOVA with a Newman-Keuls post-hoc analysis (n = 3, **P < .01; and ***P < .001).
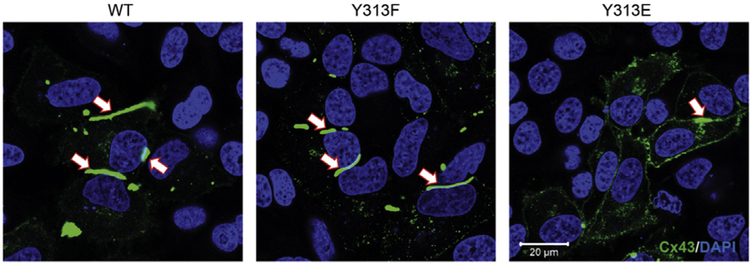
To confirm the direct role of Y313 phosphorylation in gap junction disassembly, we observed the cellular localization of HeLa cells transiently transfected with WT Cx43 or Y313 mutants eliminating (F) or mimicking (E) phosphorylation (Fig. 4). Y313E causes a decrease in the number and size of the gap junction plaques. Diffuse localization throughout the membrane is consistent with a low incidence of cellular coupling and non-functional channels.
Fig. 4. Mimicking phosphorylation of Cx43 residue Y313 decreases the number and size of the gap junction plaques.
Immunofluorescence (anti-Cx43 antibody; green) of HeLa cells transiently transfected with WT Cx43 or Y313 mutants eliminating (F) or mimicking (E) phosphorylation. White arrows point to a gap junction plaque. The nucleus has been labeled with DAPI (blue).
4.4. pY313 contributes to a decrease in GJIC
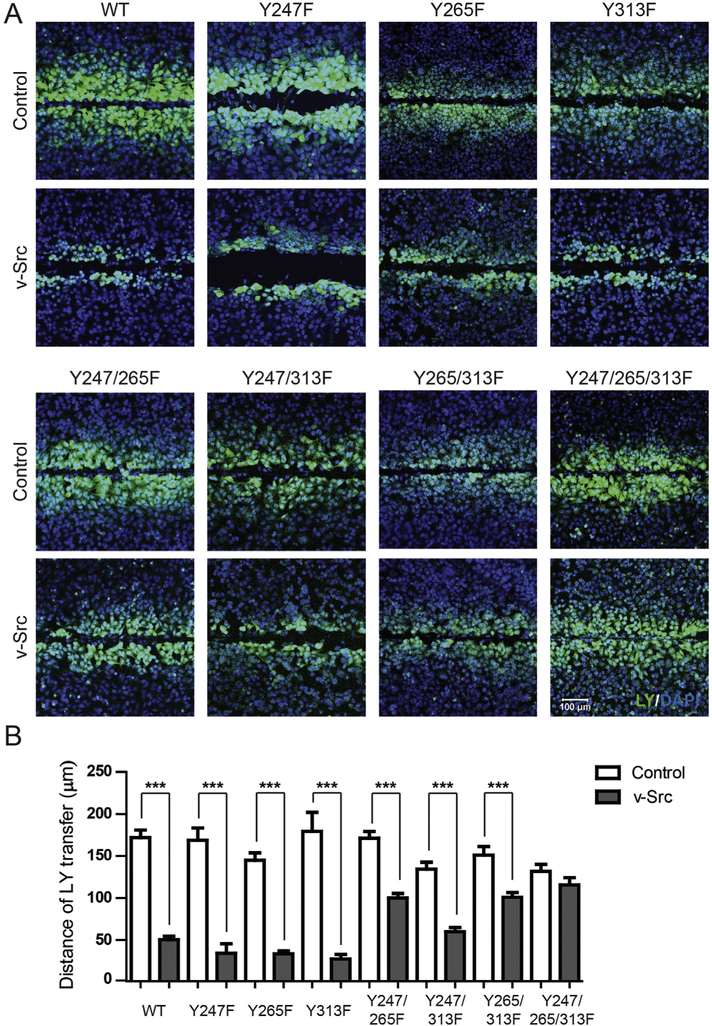
To determine whether phosphorylation of Y313 affects cell-to-cell communication, junctional transfer of the tracer Lucifer Yellow (anionic) was measured in a scrape-loading assay using the stably expressing Cx43 WT and phosphorylation eliminating mutant HeLa cells (Fig. 5). Texas Red Dextran (3 kDa; junction impermeable) marked the loaded cells (data not shown). Similar to previously published studies, Cx43 WT expressing cells (no v-Src) were extensively coupled with respect to Lucifer Yellow demonstrating greater than a fifth order dye transfer (i.e., cell layers past scrape) (Fig. 5A) [35]. The Cx43 phosphorylation eliminating mutants (no v-Src) were similar to WT with respect to being permeable to Lucifer Yellow (Supplemental Fig. 1B). In the presence of v-Src, only the Cx43 Y247/265/313F mutant had intercellular communication similar to itself (v-Src vs. no v-Src). While all the other Cx43 mutants had reduced dye transfer, the decrease observed for the double Cx43 mutants containing Y265F (Y247/265F and Y265/313F) was < 50%. The reduced junctional dye transfer for the single and double mutants is consistent with the altered size and number of junctional channels (Fig. 3).
Fig. 5. Phosphorylation of Cx43 residue Y313 contributes to reduced gap junction intercellular communication (GJIC).
The level of GJIC in HeLa cells stably expressing Cx43 WT or phosphorylation eliminating mutants (labeled above) transiently transfected with v-Src was determined using the scrape loading transfer assay. A) Representative immunofluorescence images of HeLa cells stably expressing Cx43 WT or phosphorylation eliminating mutants (labeled above) transiently transfected with v-Src (Lucifer yellow, green; DAPI, Blue). B) Quantification of the distance of Lucifer Yellow transfer shows the effect of inhibiting v-Src phosphorylation of different Cx43 tyrosine residues. Results presented as the mean + s.e.m. (n = 3). Statistics used to analyze the data was one-way ANOVA with a Newman-Keuls post-hoc analysis (n = 3, *P < .05, ***P < .001).
4.5. Determining the sequential order of Cx43 residues phosphorylated by Src
A suggested mechanism for Src phosphorylation of Cx43 includes the following: Src SH3 domain initially interacts with Cx43 residues A276-S282, Src kinase domain phosphorylates Y265, Src SH2 domain binds pY265, and then the Src kinase domain phosphorylates Y247 [19,36,37]. Here, we determined the role of Y313 in this sequential order of Cx43 tyrosine phosphorylation by Src. HeLa cells stably expressing Cx43 WT or phosphorylation eliminating mutants transiently transfected with v-Src were analyzed by Western blot using total Src, active Src (p-Src), total Cx43, and the phospho-specific antibodies pY247, pY265, and pY313 (Fig. 6 and Supplemental Fig. 2, different orientation to compare the banding patterns of the same construct with the different phospho-specific antibodies). In the absence of v-Src, no phosphorylation was observed on Y247, Y265, and Y313 for Cx43 WT and the phosphorylation eliminating mutants. In the presence of v-Src, Cx43 WT had the greatest increase in phosphorylation of Y247, Y265, and Y313 while the Cx43 mutant Y247/265/Y313F was resistant to phosphorylation. A comparison of the phosphorylation level between all the single and double mutants clearly indicates that while phosphorylation can occur on any tyrosine residue, phosphorylation of Y247 and Y313 is significantly enhanced by Y265 phosphorylation. In addition, numerous studies have shown that differentially phosphorylated Cx43 results in multiple electrophoretic isoforms: a fast migrating isoform, which includes the non-phosphorylated isoform (P0), and multiple slower migrating isoforms (P1 and P2) [27–29]. Interestingly, Cx43 mutants with the Y265F mutation retained the P2 isoform as well as any mutant with the Y247F mutation retained the P1 isoform, the former is consistent with open gap junction channels [38].
Fig. 6. Phosphorylation of Cx43 residue Y265 facilitates Y247 and Y313 phosphorylation.
Western blot analysis using HeLa cells stably expressing Cx43 WT or phosphorylation eliminating mutants (labeled above) transiently transfected with v-Src. Antibodies used are labeled on the left of each panel. Cx43 P0, P1, and P2 bands as well as molecular weight markers are indicated.
4.6. Phosphorylation of Y313 inhibits Cx43 binding with Drebrin
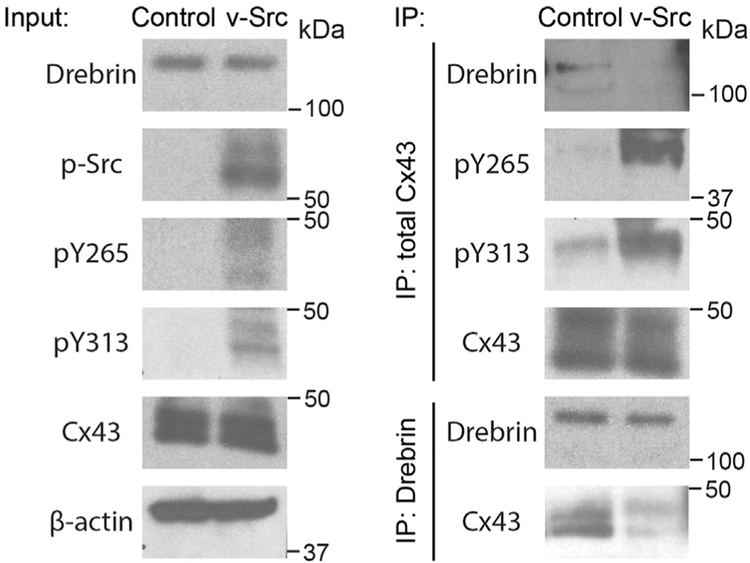
Our lab has previously published the Cx43CT 15N-HSQC assignment [9] and identified that three Cx43CT domains (Area 1, K264-T275; Area 2, S282-T290; and Area 3, R299-G321) were affected by the interaction with Drebrin (Area 1 contains Y265 and Area 3 contains Y313) and that phosphorylation of Y265 partially inhibits this interaction [16]. With the knowledge that Src also phosphorylates Y313, we addressed if both sites need to be phosphorylated for the complete dissociation of Cx43 from Drebrin. NMR titration experiments were performed using purified soluble 15N-labeled Cx43CT phospho-mimetics (residues 236–382; Y313D and Y265/Y313D) and different concentrations of unlabeled Drebrin (residues 1–300). Of note, the use of Asp substitution to study biological effects of phosphorylation is a widely used tool in the gap junction field [9,39,40]. The 15N-HSQC spectrum of each control (no Drebrin, black) has been overlaid with Drebrin (red) at a 1:14 molar ratio (Fig. 7A, B). For the Cx43CT Y313D mutant, significantly fewer residues were affected by the addition of Drebrin when compared to the Cx43CT WT [16]. The Y313D mutation completely abolished the interaction over Area 3, while Areas 1 and 2 combined showed a weaker interaction with Drebrin (2-fold less, [16]). More importantly, when both sites were mutated to mimic Src phosphorylation, the Cx43CT interaction with Drebrin was completely inhibited (Fig. 7B). Altogether, these results implicate three areas in the Cx43CT domain as critical for the interaction with the N-terminal portion of Drebrin, as well as a strong potential for a regulation of this interaction by Src. To confirm that Cx43CT phosphorylation by Src does inhibit the binding of Drebrin, a reciprocal immunoprecipitation assay was performed using HeLa cells stably expressing Cx43 WT transfected with v-Src (Fig. 8). The presence of v-Src led to phosphorylation on Cx43 residues Y265 and Y313 as well as inhibited the Cx43 interaction with Drebrin; a result consistent with the NMR data (Fig. 7).
Fig. 7. Mimicking phosphorylation of Cx43 residues Y265 and Y313 inhibits the interaction with Drebrin in vitro.
NMR titrations were performed using soluble 15N-Cx43CT236–382 phospho-mimetics and Drebrin1–300. 15N-HSQC spectrum of (A) Cx43CT236–382 Y313D or (B) Cx43CT236–382 Y265/313D alone (black) has been over laid with the spectrum obtained in the presence of Drebrin1–300 at a 1:14 M ratio (red). Cx43CT residues affected by Drebrin have been labeled. KD for the Cx43CT236–382 Y313D/Drebrin1–300 interaction was ~600 μM (Cx43CT WT/Drebrin1–300 was ~300 μM [16]).
Fig. 8. Phosphorylation of Cx43 residues Y265 and Y313 inhibits the interaction with Drebrin in cyto.
HeLa cells stably expressing Cx43 WT were transiently transfected with v-Src. Cx43 and Drebrin were reciprocally co-immunoprecipitated and analyzed by Western blot with Cx43, Drebrin, Cx43 pY265, and Cx43 pY313 antibodies.
4.7. Phosphorylation of Cx43 residue Y313 is increased in diseased human heart tissue
To determine if Cx43 Y313 phosphorylation occurs in pathological conditions where Src is activated (e.g. [37,41,42]), we stained left ventricular tissue from 24 patients for total Cx43 and Cx43 pY313. Two patients served as normal controls with no underlying cardiac pathology, 11 patients had left ventricular hypertrophy (LVH) without dilatation and 11 patients had LVH with dilatation (LVH was assessed by gross pathologic analysis, left ventricular wall thickness, and overall heart weight while ventricular dilatation was noted in the pathological analysis provided by tissue supplier). Histologically, there was an increase in the thickness of myocyte fibrils in samples with LVH compared with normal control samples; this finding is consistent with a clinical diagnosis of ventricular hypertrophy. Fig. 9 presents one control sample (transverse plane) and each of the LVH (with and without dilatation; transverse and longitudinal planes) samples; all remaining samples are provided in Supplemental Figs. 3 and 4. Samples were scored on a scale from 1 to 4 with 1 representing nearly absent staining (see Materials and Methods section for details on how scoring was calculated). In Cx43 stained samples, a distinct decrease in staining intensity was observed in patients with LVH compared to normal controls (Fig. 9A,C; Median score: 4 to 3). For patients with LVH, a similar staining intensity and distribution of Cx43 was found with and without dilatation (Median score: 3 and 3). In contrast, we observed near absent staining for Cx43 pY313 in normal controls (Fig. 9B,D; Median score: 1) compared to LVH samples (Median score: 2.5). Further, among patients with LVH, samples with ventricular dilatation stained more intensely than samples from patients without dilatation (Median score: 3 and 2, respectively). In the longitudinal plane (Fig. 9E; left - LVH without dilation, right LVH with dilation), where the intercalated disc can be observed, Cx43 pY313 signal was present at both the disc and inside the cell. The Cx43 pY313 antibody specificity was confirmed by peptide blocking prior to immunohistochemistry (Fig. 9F). Next, a double-labeled immunofluorescence experiment was performed to determine if Cx43 pY313 is a subset of the total Cx43 staining (Fig. 10). The data show that all Cx43 pY313 staining colocalized with total Cx43 (not vice versa) and the cellular localization (at intercalated disc and intracellularly) was consistent with the immunohistochemistry. These data suggest that patients with LVH have an increase in phosphorylation of Cx43 residue Y313.
Fig. 9. Phosphorylation of Cx43 residue Y313 is increased in cardiac left ventricle hypertrophy (LVH) samples.
Paired (A) Cx43 and (B) Cx43 pY313 immunohistochemistry staining in normal and LVH samples cut in transverse section. Green and red arrows indicate staining for total Cx43 and Cx43 pY313, respectively. Boxplots depicting distribution of (C) Cx43 and (D) Cx43 pY313 staining scores when combining each sample in normal (n = 2), LVH without dilatation (n = 11), and LVH with dilatation (n = 11) groups. The field of view for each sample was 40×. E) Cx43 pY313 staining in LVH samples cut in the longitudinal section left - LVH without dilation, right LVH with dilation. Red arrows indicate staining of Cx43 pY313 at the intercalated disc. Green arrows indicate intracellular staining of Cx43 pY313. F) Cx43 pY313 antibody was pre-incubated with excess phospho-peptide 12 h before being applied to the human tissue array slide. The same immunohistochemistry staining protocol was used as in (A) and (B).
Fig. 10. Cx43 pY313 colocalized with total Cx43 in cardiac left ventricle hypertrophy (LVH) samples.
Representative immunofluorescence images of double stained total Cx43 and Cx43 pY313 antibodies on a human tissue array slide (total Cx43, green; Cx43 pY313, red; DAPI, blue; and merge, yellow). The blue arrow highlights co-staining of Cx43 pY313 and total Cx43 at the intercalated disc in the transverse section. White arrows indicate intracellular co-staining in both longitudinal and transverse sections.
5. Discussion
Numerous studies have documented that Src-induced phosphorylation of Cx43 leads to down regulation of GJIC and gap junction disassembly [19,31,34,43–47]. Studies support a “ball-and-chain” mechanism for channel closure triggered by Src, similar to that proposed for pH gating of Cx43 channels [31,48,49] which results in decreased electrical coupling by reducing the opening of channels and altering selectivity [43]. Src activation can also lead to channel closure, indirectly, through serine phosphorylation by activation of MAPK and PKC [7,20,31,50,51]. A working model for Src phosphorylation of Cx43 includes the: 1) association initiates between Cx43 residues A276-S282 and the Src SH3 domain [19,37,52]. SH3 binding causes Cx43CT residues T275-P284 to adopt a left-handed type II helix [37]; 2) Src kinase domain phosphorylates Cx43 residue Y265 [19,20]; 3) Src SH2 domain binds pY265, which stabilizes the Src-Cx43 interaction, promotes phosphorylation at Y247 [19,52,53], and based upon the work presented herein, promotes phosphorylation at Y313 as well.
Lin et al. 2001 [19] performed a similar study that focused on v-Src phosphorylation; however, only Cx43 residues Y247 and Y265 were mutated. In comparison between the data, both conclude that phosphorylation of Y247 and Y265 are involved in disrupting GJIC, tyrosine phosphorylation remains in the Cx43 Y247/265F mutant, and phosphorylation of Y247 depends on Y265 phosphorylation. In addition, neither study (nor others, [34,47,53]) found evidence for a role of MAPK in mediating the disruption of GJIC by v-Src. On the other hand, one study did observe the opposite effect in that activation of Src led to rapid down regulation of GJIC with the Cx43 Y247/265F mutant via MAPK [31]. Potential reasons for these differences include the cell systems used and experimental design which may have identified distinct roles for acute vs. chronic effects of Src mediated inhibition of GJIC [7]. In addition, a factor not appreciated at the time was that Src-mediated phosphorylation of a third Cx43 tyrosine residue is important for regulation. Our data also differ from Lin et al. 2001 [19] in that gap junction plaques from their Cx43 Y247F, Y265F, and Y247/265F mutants were not largely affected by v-Src. We observed ~50–60% decrease in the number of plaques. Another difference is that Lin et al. 2001 [19] found that Y247F or Y265F alone could render Cx43 resistant to the disruption of GJIC, in our experiments these mutants were significantly affected (Y247F, 75% decrease; Y265F, 50% decrease). Src-resistance was only achieved in this study with the Cx43 Y247/265/313F mutant. A reasonable explanation for these differences is that Lin et al. 2001 [19] used knockout Cx43 fibroblasts cells that were infected with the v-Src retrovirus as opposed to HeLa cells that were transiently transfected with v-Src.
Our study presented here and those of other research groups support an additional mechanism of Src to regulate GJIC, the altering of Cx43 protein partners to enhance degradation. A commonality among the proteins that link Cx43 to the cytoskeleton is that Src can inhibit their interaction. For example, Cx43CT residue Y247 phosphorylated by Src inhibits the binding of tubulin [10]. At the gap junction plaque, this may be a mechanism in the disassembly process; at the trans-Golgi network, this may re-route trafficking to the plasma membrane (e.g., lateral membrane vs. intercalated disc) or inhibit trafficking to the plasma membrane, leading to increased intracellular proteasomal and/or lysosomal degradation [54]. In the case of Cx43CT residue Y265, we previously observed that phosphorylation at this site decreased the binding affinity for the F-actin binding protein Drebrin [16]. Here, we demonstrated that phosphorylation of Y265 and Y313 by Src is necessary to completely inhibit Cx43 binding to Drebrin. For Drebrin, depletion in cells results in impaired cell-cell coupling, internalization of gap junctions, and targeting of Cx43 for degradation [15]. Phosphorylation of Y265 and Y313 also inhibits the Cx43 interaction with β-catenin [55]. Like Drebrin, β-catenin helps Cx43 to indirectly interact with F-actin (through α-catenin) and stabilize gap junctions to favor intercellular communication [56]. Conversely, we speculate they both cannot interact at the same time. Therefore, the available data would suggest that β-catenin binds first and then at some point in the maturation of the gap junction plaque the Cx43CT switches to interact with Drebrin. Finally, while phosphorylation of the Cx43CT by Src does not inhibit ZO-1 binding, we found that active c-Src could compete with Cx43 to directly bind ZO-1 [37]. This would transition Cx43 from the non-junctional plasma membrane into the gap junction plaque, and then through the degradation pathway(s) [57].
In this study, we have also validated a new tool for the gap junction field, a Cx43 pY313 antibody, which is amenable for Western blots, immunofluorescence, and immunohistochemistry. For the LA-25 cells at 35 °C (active v-Src), a majority of Cx43 (total antibody) was intracellular with a diffuse pattern when at the plasma membrane. Interestingly, while all Cx43 pY313 signal colocalized with total Cx43, this mainly occurred at the plasma membrane and all Cx43 pY313 Western blot signal was in the P2 isoform. Similarly, all Cx43 pY313 signal also colocalized with total Cx43 from LVH samples, however, while colocalization was seen at the intercalated disk, the majority occurred inside the cell. In this case, Western blot data observed multiple electrophoretic isoforms (like the HeLa cell data), including at ~20 kDa. This band may be the internally translated isoform that stabilizes actin filaments to aid Cx43 trafficking to the gap junction plaque [32,33]. Of interest for future studies would be in determining if the level and phosphorylation state of the ~20 kDa isoform is altered in LVH.
In summary, our data support that Cx43 residue Y313 is a novel site targeted for phosphorylation by Src. These conclusions were based upon several observations: 1) mass spectroscopy data, 2) Cx43 Y247/265F did not completely eliminate tyrosine phosphorylation by v-Src nor did GJIC resemble WT, 3) v-Src enhanced Y313 phosphorylation as observed by the phospho-specific antibody, and 4) in diseased human left ventricle, a condition where Src is activated, Y313 phosphorylation is increased.
The following are the supplementary data related to this article.
Supplementary Material
Acknowledgments
We thank Janice A. Taylor and James R. Talaska of the Advanced Microscopy Core Facility at the University of Nebraska Medical Center for providing assistance with confocal microscopy.
Sources of funding
This work was supported by NIH Grants GM072631, GM319613, and GM103427 to Paul Sorgen. Support for the UNMC Advanced Microscopy Core Facility was provided by the Nebraska Research Initiative and an Institutional Development Award (IDeA) from the NIGMS of the NIH (P30GM106397). The following NIH SIG funded instruments were used: LSM 800 Zeiss Confocal Microscope (NIH S10RR027301).
Footnotes
Disclosures
The authors declare that they have no conflicts of interest with the contents of this article.
References
- [1].Goodenough DA, Goliger JA, Paul DL, Connexins, connexons, and intercellular communication, Annu. Rev. Biochem. 65 (1996) 475–502. [DOI] [PubMed] [Google Scholar]
- [2].Severs NJ, Bruce AF, Dupont E, Rothery S, Remodelling of gap junctions and connexin expression in diseased myocardium, Cardiovasc. Res. 80 (1) (2008) 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Evans WH, Martin PE, Gap junctions: structure and function (Review), Mol. Membr. Biol. 19 (2) (2002) 121–136. [DOI] [PubMed] [Google Scholar]
- [4].Axelsen LN, Calloe K, Holstein-Rathlou NH, Nielsen MS, Managing the complexity of communication: regulation of gap junctions by post-translational modification, Front. Pharmacol. 4 (2013) 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Solan JL, Lampe PD, Connexin phosphorylation as a regulatory event linked to gap junction channel assembly, Biochim. Biophys. Acta 1711 (2) (2005) 154–163. [DOI] [PubMed] [Google Scholar]
- [6].Lampe PD, Lau AF, The effects of connexin phosphorylation on gap junctional communication, Int. J. Biochem. Cell Biol. 36 (7) (2004) 1171–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Solan JL, Lampe PD, Specific Cx43 phosphorylation events regulate gap junction turnover in vivo, FEBS Lett. 588 (8) (2014) 1423–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ek-Vitorin JF, Burt JM, Structural basis for the selective permeability of channels made of communicating junction proteins, Biochim. Biophys. Acta 1828 (1) (2013) 51–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Grosely R, Kopanic JL, Nabors S, Kieken F, Spagnol G, Al-Mugotir M, Zach S, Sorgen PL, Effects of phosphorylation on the structure and backbone dynamics of the intrinsically disordered connexin43 C-terminal domain, J. Biol. Chem. 288 (34) (2013) 24857–24870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Saidi Brikci-Nigassa A, Clement MJ, Ha-Duong T, Adjadj E, Ziani L, Pastre D, Curmi PA, Savarin P, Phosphorylation controls the interaction of the connexin43 C-terminal domain with tubulin and microtubules, Biochemistry 51 (21) (2012) 4331–4342. [DOI] [PubMed] [Google Scholar]
- [11].Spagnol G, Kieken F, Kopanic JL, Li H, Zach S, Stauch KL, Grosely R, Sorgen PL, Structural studies of the Nedd4 WW domains and their selectivity for the Connexin43 (Cx43) carboxyl terminus, J. Biol. Chem. 291 (14) (2016) 7637–7650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Dunn CA, Lampe PD, Injury-triggered Akt phosphorylation of Cx43: a ZO-1-driven molecular switch that regulates gap junction size, J. Cell Sci. 127 (Pt 2) (2014) 455–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Girao H, Catarino S, Pereira P, Eps15 interacts with ubiquitinated Cx43 and mediates its internalization, Exp. Cell Res. 315 (20) (2009) 3587–3597. [DOI] [PubMed] [Google Scholar]
- [14].Leykauf K, Salek M, Bomke J, Frech M, Lehmann WD, Durst M, Alonso A, Ubiquitin protein ligase Nedd4 binds to connexin43 by a phosphorylation-modulated process, J. Cell Sci. 119 (2006) 3634–3642 Pt 17. [DOI] [PubMed] [Google Scholar]
- [15].Butkevich E, Hulsmann S, Wenzel D, Shirao T, Duden R, Majoul I, Drebrin is a novel connexin-43 binding partner that links gap junctions to the submembrane cytoskeleton, Curr. Biol. 14 (8) (2004) 650–658. [DOI] [PubMed] [Google Scholar]
- [16].Ambrosi C, Ren C, Spagnol G, Cavin G, Cone A, Grintsevich EE, Sosinsky GE, Sorgen PL, Connexin43 forms supramolecular complexes through non-overlapping binding sites for Drebrin, Tubulin, and ZO-1, PLoS One 11 (6) (2016) e0157073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Park DJ, Wallick CJ, Martyn KD, Lau AF, Jin C, Warn-Cramer BJ, Akt phosphorylates Connexin43 on Ser373, a “mode-1” binding site for 14–3-3, Cell Commun. Adhes. 14 (5) (2007) 211–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hornbeck PV, Kornhauser JM, Tkachev S, Zhang B, Skrzypek E, Murray B, Latham V, Sullivan M, PhosphoSitePlus: a comprehensive resource for investigating the structure and function of experimentally determined post-translational modifications in man and mouse, Nucleic Acids Res. 40 (Database issue) (2012) D261–D270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lin R, Warn-Cramer BJ, Kurata WE, Lau AF, V-Src phosphorylation of connexin 43 on Tyr247 and Tyr265 disrupts gap junctional communication, J. Cell Biol. 154 (4) (2001) 815–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Solan JL, Lampe PD, Connexin 43 in LA-25 cells with active v-src is phosphorylated on Y247, Y265, S262, S279/282, and S368 via multiple signaling pathways, Cell Commun. Adhes. 15 (1) (2008) 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Li H, Spagnol G, Naslavsky N, Caplan S, Sorgen PL, TC-PTP directly interacts with connexin43 to regulate gap junction intercellular communication, J. Cell Sci. 127 (2014) 3269–3279 Pt 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cooper CD, Solan JL, Dolejsi MK, Lampe PD, Analysis of connexin phosphorylation sites, Methods 20 (2) (2000) 196–204. [DOI] [PubMed] [Google Scholar]
- [23].Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A, NMRPipe: a multidimensional spectral processing system based on UNIX pipes, J. Biomol. NMR 6 (3) (1995) 277–293. [DOI] [PubMed] [Google Scholar]
- [24].Johnson BA, Using NMRView to visualize and analyze the NMR spectra of macromolecules, Methods Mol. Biol. 278 (2004) 313–352. [DOI] [PubMed] [Google Scholar]
- [25].Stauch K, Kieken F, Sorgen P, Characterization of the structure and intermolecular interactions between the connexin 32 carboxyl-terminal domain and the protein partners synapse-associated protein 97 and calmodulin, J. Biol. Chem. 287 (33) (2012) 27771–27788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Huang QY, Chen YC, Liu SP, Connexin 43, angiotensin II, endothelin 1, and type III collagen alterations in heart of rats having undergone fatal electrocution, Am J Forensic Med Pathol 33 (3) (2012) 215–221. [DOI] [PubMed] [Google Scholar]
- [27].Solan JL, Lampe PD, Key connexin 43 phosphorylation events regulate the gap junction life cycle, J. Membr. Biol. 217 (1–3) (2007) 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Crow DS, Beyer EC, Paul DL, Kobe SS, Lau AF, Phosphorylation of connexin43 gap junction protein in uninfected and Rous sarcoma virus-transformed mammalian fibroblasts, Mol. Cell. Biol. 10 (4) (1990) 1754–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Musil LS, Cunningham BA, Edelman GM, Goodenough DA, Differential phosphorylation of the gap junction protein connexin43 in junctional communication-competent and -deficient cell lines, J. Cell Biol. 111 (5) (1990) 2077–2088 Pt 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Li H, Spagnol G, Zheng L, Stauch KL, Sorgen PL, Regulation of connexin43 function and expression by Tyrosine kinase 2, J. Biol. Chem. 291 (30) (2016) 15867–15880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zhou L, Kasperek EM, Nicholson BJ, Dissection of the molecular basis of pp60(v-src) induced gating of connexin 43 gap junction channels, J. Cell Biol. 144 (5) (1999) 1033–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Smyth JW, Shaw RM, Autoregulation of connexin43 gap junction formation by internally translated isoforms, Cell Rep. 5 (3) (2013) 611–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Basheer WA, Xiao S, Epifantseva I, Fu Y, Kleber AG, Hong T, Shaw RM, GJA1–20k Arranges Actin to Guide Cx43 delivery to Cardiac Intercalated Discs, Circ. Res. 121 (9) (2017) 1069–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Giepmans BN, Hengeveld T, Postma FR, Moolenaar WH, Interaction of c-Src with gap junction protein connexin-43. Role in the regulation of cell-cell communication, J. Biol. Chem. 276 (11) (2001) 8544–8549. [DOI] [PubMed] [Google Scholar]
- [35].Valiunas V, Beyer EC, Brink PR, Cardiac gap junction channels show quantitative differences in selectivity, Circ. Res. 91 (2) (2002) 104–111. [DOI] [PubMed] [Google Scholar]
- [36].Lau AF, Kurata WE, Kanemitsu MY, Loo LW, Warn-Cramer BJ, Eckhart W, Lampe PD, Regulation of connexin43 function by activated tyrosine protein kinases, J. Bioenerg. Biomembr. 28 (4) (1996) 359–368. [DOI] [PubMed] [Google Scholar]
- [37].Kieken F, Mutsaers N, Dolmatova E, Virgil K, Wit AL, Kellezi A, Hirst-Jensen BJ, Duffy HS, Sorgen PL, Structural and molecular mechanisms of gap junction remodeling in epicardial border zone myocytes following myocardial infarction, Circ. Res. 104 (9) (2009) 1103–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cooper CD, Lampe PD, Casein kinase 1 regulates connexin-43 gap junction assembly, J. Biol. Chem. 277 (47) (2002) 44962–44968. [DOI] [PubMed] [Google Scholar]
- [39].Solan JL, Marquez-Rosado L, Sorgen PL, Thornton PJ, Gafken PR, Lampe PD, Phosphorylation at S365 is a gatekeeper event that changes the structure of Cx43 and prevents down-regulation by PKC, J. Cell Biol. 179 (6) (2007) 1301–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Remo BF, Qu J, Volpicelli FM, Giovannone S, Shin D, Lader J, Liu FY, Zhang J, Lent DS, Morley GE, Fishman GI, Phosphatase-resistant gap junctions inhibit pathological remodeling and prevent arrhythmias, Circ. Res. 108 (12) (2011) 1459–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Sovari AA, Iravanian S, Dolmatova E, Jiao Z, Liu H, Zandieh S, Kumar V, Wang K, Bernstein KE, Bonini MG, Duffy HS, Dudley SC, Inhibition of c-Src tyrosine kinase prevents angiotensin II-mediated connexin-43 remodeling and sudden cardiac death, J. Am. Coll. Cardiol. 58 (22) (2011) 2332–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rutledge CA, Ng FS, Sulkin MS, Greener ID, Sergeyenko AM, Liu H, Gemel J, Beyer EC, Sovari AA, Efimov IR, Dudley SC, C-Src kinase inhibition reduces arrhythmia inducibility and connexin43 dysregulation after myocardial infarction, J. Am. Coll. Cardiol. 63 (9) (2014) 928–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Cottrell GT, Lin R, Warn-Cramer BJ, Lau AF, Burt JM, Mechanism of v-Src-and mitogen-activated protei nkinase-induced reduction of gap junction communication, Am. J. Phys. Cell Phys. 284 (2) (2003) C511–C520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lin R, Warn-Cramer BJ, Kurata WE, Lau AF, V-Src-mediated phosphorylation of connexin43 on tyrosine disrupts gap junctional communication in mammalian cells, Cell Commun. Adhes. 8 (4–6) (2001) 265–269. [DOI] [PubMed] [Google Scholar]
- [45].Toyofuku T, Yabuki M, Otsu K, Kuzuya T, Tada M, Hori M, Functional role of c-Src in gap junctions of the cardiomyopathic heart, Circ. Res. 85 (8) (1999) 672–681. [DOI] [PubMed] [Google Scholar]
- [46].Loo LW, Berestecky JM, Kanemitsu MY, Lau AF, pp60src-mediated phosphorylation of connexin 43, a gap junction protein, J. Biol. Chem. 270 (21) (1995) 12751–12761. [DOI] [PubMed] [Google Scholar]
- [47].Swenson KI, Piwnica-Worms H, McNamee H, Paul DL, Tyrosine phosphorylation of the gap junction protein connexin43 is required for the pp60v-src-induced inhibition of communication, Cell. Regul. 1 (13) (1990) 989–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Homma N, Alvarado JL, Coombs W, Stergiopoulos K, Taffet SM, Lau AF, Delmar M, A particle-receptor model for the insulin-induced closure of connexin43 channels, Circ. Res. 83 (1) (1998) 27–32. [DOI] [PubMed] [Google Scholar]
- [49].Morley GE, Taffet SM, Delmar M, Intramolecular interactions mediate pH regulation of connexin43 channels, Biophys. J. 70 (3) (1996) 1294–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Pahujaa M, Anikin M, Goldberg GS, Phosphorylation of connexin43 induced by Src: regulation of gap junctional communication between transformed cells, Exp. Cell Res. 313 (20) (2007) 4083–4090. [DOI] [PubMed] [Google Scholar]
- [51].Mitra SS, Xu J, Nicholson BJ, Coregulation of multiple signaling mechanisms in pp60v-Src-induced closure of Cx43 gap junction channels, J. Membr. Biol. 245 (8) (2012) 495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kanemitsu MY, Loo LW, Simon S, Lau AF, Eckhart W, Tyrosine phosphorylation of connexin 43 by v-Src is mediated by SH2 and SH3 domain interactions, J. Biol. Chem. 272 (36) (1997) 22824–22831. [DOI] [PubMed] [Google Scholar]
- [53].Toyofuku T, Akamatsu Y, Zhang H, Kuzuya T, Tada M, Hori M, c-Src regulates the interaction between connexin-43 and ZO-1 in cardiac myocytes, J. Biol. Chem. 276 (3) (2001) 1780–1788. [DOI] [PubMed] [Google Scholar]
- [54].Alberts B, Lewis J, Raff MC, Roberts K, Walter P, Transport from the Trans Golgi Network to Lysosomes, Molecular Biology of the Cell, Garland Science, New York, 2002. [Google Scholar]
- [55].Spagnol G, Trease AJ, Zheng L, Gutierrez M, Basu I, Sarmiento C, Moore G, Cervantes M, Sorgen PL, Connexin43 Carboxyl-terminal domain directly interacts with beta-Catenin, Int. J. Mol. Sci. 19 (6) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ai Z, Fischer A, Spray DC, Brown AM, Fishman GI, Wnt-1 regulation of connexin43 in cardiac myocytes, J. Clin. Invest. 105 (2) (2000) 161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Rhett JM, Jourdan J, Gourdie RG, Connexin 43 connexon to gap junction transition is regulated by zonula occludens-1, Mol. Biol. Cell 22 (9) (2011) 1516–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.