Abstract
In vitro synergy between extended-spectrum cephalosporins and either clavulanic acid or cefoxitin was found for Chryseobacterium meningosepticum isolates during a double-disk assay on an agar plate. An extended-spectrum β-lactamase (ESBL) gene from a C. meningosepticum clinical isolate was cloned and expressed in Escherichia coli DH10B. Its protein conferred resistance to most β-lactams including extended-spectrum cephalosporins but not to cephamycins or to imipenem. Its activity was strongly inhibited by clavulanic acid, sulbactam, and tazobactam, as well as by cephamycins and imipenem. Sequence analysis of the cloned DNA fragment revealed an open reading frame (ORF) of 891 bp with a G+C content of 33.9%, which lies close to the expected range of G+C contents of members of the Chryseobacterium genus. The ORF encoded a precursor protein of 297 amino acids, giving a mature protein with a molecular mass of 31 kDa and a pI value of 9.2 in E. coli. This gene was very likely chromosomally located. Amino acid sequence comparison showed that this β-lactamase, named CME-2 (C. meningosepticum ESBL), is a novel ESBL of the Ambler class A group (Bush functional group 2be), being weakly related to other class A β-lactamases. It shares only 39 and 35% identities with the ESBLs VEB-1 from E. coli MG-1 and CBL-A from Bacteroides uniformis, respectively. The distribution of blaCME-2 among unrelated C. meningosepticum species isolates showed that blaCME-2-like genes were found in the C. meningosepticum strains studied but were absent from strains of other C. meningosepticum-related species. Each C. meningosepticum strain produced at least two β-lactamases, with one of them being a noninducible serine ESBL with variable pIs ranging from 7.0 to 8.5.
Chryseobacterium meningosepticum (formerly classified as Flavobacterium meningosepticum [45]) is a waterborne saprophytic bacterium. Among Chryseobacterium species, C. meningosepticum is most commonly associated with infections in humans. It may cause meningitis in newborns and pneumonia and sepsis in immunocompromised patients, especially those hospitalized in intensive care units (3, 39). C. meningosepticum is naturally resistant to most β-lactams, including extended-spectrum cephalosporins and carbapenems, with only some isolates remaining susceptible to ureidopenicillins (6).
Phenotype analysis of the β-lactam resistance pattern of a C. meningosepticum PINT clinical isolate revealed the presence of a putative extended-spectrum β-lactamase (ESBL) according to the synergy found between clavulanic acid and most extended-spectrum cephalosporins when a double-disk assay was performed on an agar plate (20). Uncommonly, a similar synergy was also found between cephamycins such as cefoxitin or moxalactam and extended-spectrum cephalosporins. Recently, an Ambler class B carbapenem-hydrolyzing β-lactamase has been reported from C. meningosepticum CIP 6058 (36). Although the hydrolysis spectrum of this β-lactamase is broad, its presence cannot be responsible for the extended-spectrum cephalosporin resistance profile observed in C. meningosepticum.
The aim of this work was to analyze both biochemically and genetically the β-lactamase responsible for the observed phenotype and to determine its distribution among nonepidemiologically related C. meningosepticum isolates and other Chryseobacterium species strains.
MATERIALS AND METHODS
Bacterial strains.
The bacterial strains used in this work are listed in Table 1. C. meningosepticum PINT was isolated at the Raymond Poincaré Hospital in Garches, France, a suburb of Paris. C. meningosepticum AMA and GEO were isolated at the Bicêtre Hospital (Le Kremlin-Bicêtre, France), and both were from tracheoalveolar aspirates. Reference strains were from the Pasteur Institute (Paris, France) and Denmark (7). The C. meningosepticum isolates and reference strains were epidemiologically unrelated (data not shown).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or phenotype | Source or reference |
|---|---|---|
| Strains | ||
| E. coli DH10B | F′ mcrA Δ(mrr-hsdRMS-mrcBC) Φ80ΔlacZDM15 ΔlacX74 deoR recA1 endA1 araD139 Δ(ara, leu)7697 galU galK1 rpsL nupG | Gibco BRL |
| E. coli JM109 | endA1 hsdR17 gyrA96 Δ(lac proA) recA1 relA supE44 thi F′ (lacIqlacZΔM15 proAB+ traD36) | 48 |
| Rifampin-resistant E. coli JM109 obtained in vitro | Rifampin-resistant | This study |
| C. meningosepticum PINT | Extended-spectrum cephalosporin and carbapenem resistant | This study |
| C. meningosepticum AMA | Extended-spectrum cephalosporin and carbapenem resistant | This study |
| C. meningosepticum GEO | Extended-spectrum cephalosporin and carbapenem resistant | This study |
| C. meningosepticum CIP 6058 | Extended-spectrum cephalosporin and carbapenem resistant | IPa |
| C. meningosepticum CIP 6059 | Extended-spectrum cephalosporin and carbapenem resistant | IP |
| C. meningosepticum CIP 7830 | Extended-spectrum cephalosporin and carbapenem resistant | IP |
| C. meningosepticum CIP 7905 | Extended-spectrum cephalosporin and carbapenem resistant | IP |
| C. meningosepticum AB1572 | Extended-spectrum cephalosporin and carbapenem resistant | 7 |
| C. meningosepticum H01, J100 | Extended-spectrum cephalosporin and carbapenem resistant | 7 |
| C. indologenes CIP 101026 | Carbapenem resistant | IP |
| C. indologenes PIT | Carbapenem resistant | This study |
| S. multivorum CIP 103686 | Carbapenem resistant | IP |
| M. odoratus CIP 103105 | Carbapenem resistant | IP |
| Plasmids | ||
| pBK-CMV phagemid | Neomycin and kanamycin resistant | Stratagene |
| pBR322 | Recombinant plasmid containing the 560-bp SspI-PstI internal fragment of blaTEM-1 | 42 |
| pHUC37 | Recombinant plasmid containing the 435-bp PstI-NotI internal fragment of blaSHV-3 | 24 |
| pPZ1 | Recombinant plasmid containing the 1.1-kb SnaBI fragment of blaPER-1 | 25 |
| pRLT1 | Recombinant plasmid containing the 329-bp DraI-XmnI internal fragment of blaVEB-1 | 32 |
| pPL1 | Recombinant plasmid containing the 396-bp EcoRI-PvuI internal fragment of blaOXA-18 | 29 |
| pBS1 | 1.9-kb Sau3AI fragment from C. meningosepticum PINT cloned into pBK-CMV | This study |
IP, Institut Pasteur, Paris, France.
Escherichia coli DH10B and nalidixic acid- and rifampin-resistant E. coli JM109 were used for cloning and conjugation assays, respectively (Table 1). The Chryseobacterium sp. strains were identified by standard techniques as described previously (30, 39), and their identities were confirmed with the API 32GN system (bioMérieux, Marcy l'Etoile, France). All strains were stored at −70°C in Trypticase soy (TS) broth (Becton Dickinson, Le Pont de Claix, France) supplemented with 15% glycerol until testing.
Antimicrobial agents and MIC determinations.
The antimicrobial agents used in this study were obtained in the form of standard laboratory powders and were used immediately after their solubilization. The agents and their sources have been described elsewhere (32). Antibiotic disks were used for routine antibiograms (Sanofi-Diagnostics Pasteur, Marnes-La-Coquette, France).
MICs were determined by an agar dilution technique on Mueller-Hinton (MH) agar (Sanofi-Diagnostics Pasteur) with an inoculum of 104 CFU per spot (8, 23). All drugs were incorporated into MH agar at serial twofold concentrations, and the antimicrobial susceptibilities of all isolates were determined concomitantly. The plates were incubated at 35°C for 18 h. The MICs of β-lactams were determined alone or in combination with a fixed concentration of clavulanic acid (2 μg/ml), tazobactam (4 μg/ml), cefoxitin (0.1 μg/ml), or moxalactam or imipenem (0.05 μg/ml each).
Cloning experiments and analysis of recombinant plasmids.
Genomics DNAs from C. meningosepticum PINT and from other strains were extracted as described previously (25). The XmnI restriction endonuclease was from New England Biolabs (Ozyme, Saint Quentin en Yvelines, France), while all the other enzymes used in the cloning experiments were from Amersham Pharmacia Biotech (Orsay, France). Fragments from genomic DNA partially digested with Sau3AI were ligated into BamHI-restricted phagemid pBK-CMV from Stratagene (Ozyme) (37). Ligation was performed at a 1:2 vector-insert ratio with 200 ng of restricted genomic DNA in a ligation mixture containing 1 U of T4 DNA ligase at 4°C for 18 h. Recombinant plasmids were transformed by electroporation (Bio-Rad Gene Pulser II) into E. coli DH10B electrocompetent cells (Gibco BRL, Life Technologies, Cergy Pontoise, France). Antibiotic-resistant colonies were selected on TS agar plates containing amoxicillin (50 μg/ml) and kanamycin (30 μg/ml).
Recombinant plasmid DNA was obtained from 100-ml TS broth cultures grown overnight in the presence of amoxicillin (100 μg/ml) at 37°C. Plasmid DNAs were recovered by using Qiagen columns (Qiagen, Courtaboeuf, France). Plasmid mapping was performed after double restriction analysis. Fragment sizes were estimated by comparison with the fragment sizes on a 1-kb DNA ladder (Amersham Pharmacia Biotech).
Conjugation assays and plasmid content.
Direct transfer of resistance genes into rifampin-resistant E. coli JM109 obtained in vitro was attempted by liquid and solid conjugation assays at 30 and 37°C. Transconjugants were selected on TS agar plates containing rifampin (200 μg/ml) and amoxicillin (50 μg/ml). Extraction of plasmid DNA from C. meningosepticum PINT was attempted by two different methods (10, 15).
DNA sequencing and protein analysis.
Both strands of the 1.9-kb cloned DNA fragment of recombinant plasmid pBS1 were sequenced with an Applied Biosystems sequencer (ABI 373). The nucleotide sequence and the deduced protein sequence were analyzed with software available over the Internet at the National Center of Biotechnology Information website (http://www.ncbi.nlm.nih.gov) and at Pedro's BioMolecular Research Tools website (http://www.fmi.ch/biology/research_tools.html). Multiple protein sequence alignments were carried out with the program Clustal W, available over the Internet at the University of Cambridge. A dendrogram was derived from the multiple sequence alignment by a parsimony method with the phylogeny package PAUP (Phylogenetic Analysis Using Parsimony), version 3.0 (44). Among the Ambler class A β-lactamases, 11 were compared to CME-2: PER-1 from Pseudomonas aeruginosa RNL-1 (25), VEB-1 from E. coli MG-1 (32), CEP-A from Bacteroides fragilis CS30 (35), CFX-A from Bacteroides vulgatus CLA341 (26), CBL-A from Bacteroides uniformis WAL-7088 (40), TEM-3 from Klebsiella pneumoniae CFF104 (41), SHV-2 from Klebsiella ozaenae (11), MEN-1 from E. coli MEN (2), L-2 from Stenotrophomonas maltophilia 1275 IID (47), TOHO-1 from E. coli TUH12191 (13), and NMC-A from Enterobacter cloacae NOR-1 (22) as a representative of the serine carbapenem-hydrolyzing β-lactamase group (Bush group 2f) (5), which possesses an extended hydrolysis spectrum toward aztreonam.
β-Lactamase preparations.
Cultures of C. meningosepticum clinical isolates and E. coli DH10B harboring recombinant plasmid pBS1 were grown overnight at 37°C in 100 ml of TS broth containing amoxicillin (100 μg/ml) and 4 liters of TS broth, respectively. Bacterial suspensions were pelleted, resuspended in 40 ml of Tris-HCl (50 mM) buffer (pH 8), disrupted by sonification (three times at 50 W for 30 s each time with a Vibra Cell 75022 Phospholyser [Bioblock, Illkirch, France]), and centrifuged at 48,000 × g for 1 h at 4°C. Nucleic acids were precipitated by the addition of spermin (0.2 M; 7% [vol/vol]; Sigma, Saint-Quentin Fallavier, France) for 1 h on ice. This suspension was ultracentrifuged at 100,000 × g for 1 h at 4°C.
Then, the β-lactamase extract from E. coli DH10B(pBS1) was loaded onto a preequilibrated Q-Sepharose column (Amersham Pharmacia Biotech). The β-lactamase was recovered in the flowthrough and was subsequently dialyzed overnight against 100 mM phosphate buffer (pH 7.0). The β-lactamase was loaded onto a preequilibrated S-Sepharose column (Amersham Pharmacia Biotech). The enzyme was eluted with a linear NaCl gradient (0 to 1 M) in phosphate buffer (pH 7). The β-lactamase was eluted with NaCl at a concentration of 320 to 350 mM. The fraction containing the β-lactamase activity was dialyzed overnight against 100 mM phosphate buffer (pH 7.0) prior to a 10-fold concentration with a Centrisart-C30 microcentrifuge filter (Sartorius, Goettingen, Germany).
Isoelectric focusing.
Enzyme preparations from cultures of C. meningosepticum clinical isolates and E. coli DH10B(pBS1) were subjected to analytical isoelectric focusing on a pH 3.5 to 9.5 Ampholine polyacrylamide gel (Ampholine PAG plate; Amersham Pharmacia Biotech) for 90 min at 1,500 V, 50 mA, and 30 W. The focused β-lactamase was detected by overlaying the gel with 1 mM nitrocefin (Oxoid, Paris, France) in 100 mM phosphate buffer (pH 7.0). Since a metalloenzyme has previously been described in C. meningosepticum (36), detection of the pIs of the serine β-lactamases were additionally performed by incubating the enzyme extracts with 100 μM clavulanic acid prior to their loading and focusing on an isoelectric focusing gel, followed by nitrocefin detection (28). The pI values were determined and compared to those of known β-lactamases run on the same gels.
Kinetic measurements.
Purified β-lactamase was used for kinetic measurements (kcat, Km), which were made at 30°C in 100 mM sodium phosphate (pH 7.0) with a Pharmacia ULTROSPEC 2000 spectrophotometer as described previously (17). Various concentrations of clavulanic acid, sulbactam, tazobactam, cefoxitin, imipenem, or moxalactam were preincubated with the enzyme for 3 min at 30°C before testing the rate of benzylpenicillin (100 μM) hydrolysis. The 50% inhibitory concentrations (IC50s) of these inhibitors were determined. Results were expressed in micromolar units.
Induction experiments.
To test the inducibility of the serine β-lactamase in C. meningosepticum clinical isolates, induction experiments were performed as described previously (31) with cefoxitin (10 μg/ml) or imipenem (1 μg/ml) as the inducer. One unit of enzyme activity was defined as that which was required to hydrolyze 1 μmol of aztreonam per min (aztreonam is not hydrolyzed by metalloenzymes). The total protein content was measured with bovine albumin as the standard (Bio-Rad DC Protein assay kit).
Determination of the β-lactamase relative molecular mass.
The relative molecular mass of the β-lactamase from E. coli DH10B harboring pBS1 was estimated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis analysis. Enzyme extracts and marker proteins were boiled for 10 min in a 1% SDS–3% β-mercaptoethanol solution and were then subjected to electrophoresis on a 12% polyacrylamide gel (25 mA, 4 h) (16). Renaturation of the β-lactamase activity after denaturing electrophoresis and visualization of the β-lactamase on a benzylpenicillin-containing agar gel were performed as described previously (18).
Hybridization experiments.
Southern hybridizations were performed with an ECL nonradioactive hybridization kit as described by the manufacturer (Amersham Pharmacia Biotech). Genomic DNA from C. meningosepticum PINT was hybridized with the following DNA probes corresponding to some β-lactamase genes: the 1.1-kb SnaBI fragment from recombinant plasmid pPZ1 for blaPER-1, the 450-bp PstI-NotI fragment from recombinant plasmid pHUC37 for blaSHV-3, the 560-bp SspI-PstI fragment from plasmid pBR322 for blaTEM-1, the 329-bp DraI-XmnI fragment from recombinant plasmid pRLT1 for blaVEB-1, the 396-bp EcoRI-PvuI from recombinant plasmid pPL1 for blaOXA-18, or the 811-bp PCR-amplified fragment internal to blaCARB-2 (12). Two micrograms of denatured DNA was put onto a nylon membrane (Hybond N+; Amersham Pharmacia Biotech) and cross-linked with UV light for 2 min with a UV Stratalinker 2400 instrument (Stratagene). Membrane was incubated for 1 h at 60°C in a prehybridization buffer containing 100 μg of salmon sperm DNA per ml, 0.1% SDS, 5× SSC (0.75 M sodium chloride and 0.075 M sodium citrate), 5% dextran sulfate, and a 20-fold dilution of liquid block solution (supplied). Hybridizations were performed overnight at 60°C. Then, two washes were performed successively in the following solutions: 1× SSC–0.1% SDS for 15 min at 60°C and 0.5× SSC–0.1% SDS for 15 min at 60°C. The membrane was blocked and was then incubated with anti-fluorescein-horseradish peroxidase conjugate and finally washed. The signal was generated with a luminol solution and was detected with an autoradiographic film after 15 min of exposure.
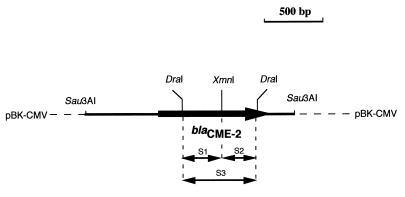
Once the ESBL gene was identified, a set of primers was designed (primer 1, [5′-GTA GCT GTT TCT GTT TTG GGG-3′] and primer 2, [5′-CGA GAC CTG GGC AAT GAT TC-3′] at positions 438 to 458 and 1162 to 1181, respectively; (see Fig. 2) in order to attempt PCR amplification of this ESBL gene from the genomic DNAs of the C. meningosepticum isolates studied and from isolates of the related species studied (Table 1). In order to analyze the genetic organization of an ESBL-like gene in several C. meningosepticum strains, their corresponding genomic DNAs were digested with XmnI and run on a 1% agarose gel. This gel was submitted to Southern hybridization as described above. Since XmnI cut only once, in the middle of the ESBL gene (see below), DraI-XmnI and DraI restriction digests gave three internal fragments for the ESBL gene used as probes (fragments S1, S2, and S3; see Fig. 1).
FIG. 2.
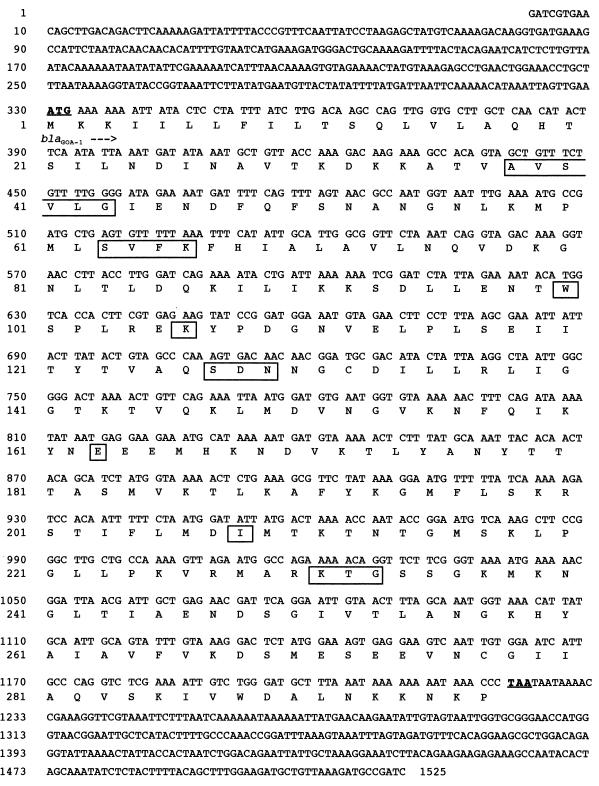
Nucleotide sequence of the 1,525-bp fragment of pBS1 containing the 891-bp coding region for blaCME-2. The deduced amino acid sequence is designated in single-letter code below the nucleotide sequence. The seven conserved boxes described by Joris et al. (14) and the conserved SDN loop are boxed. The start and stop codons of the gene are in boldface type and are underlined.
FIG. 1.
Schematic representation of recombinant plasmid pBS1, which encodes the CME-2 β-lactamase from C. meningosepticum PINT. The thin line represents the cloned insert from C. meningosepticum PINT containing the CME-2 β-lactamase gene, with the arrow indicating its translational orientation (thick line), and the dotted lines indicate the vector pBK-CMV. The three internal probes (probes S1, S2, and S3) used for hybridization experiments are also represented by double-headed arrows.
Nucleotide sequence accession number.
The nucleotide sequence data reported in this paper will appear in the GenBank nucleotide database under the accession no. AF033200.
RESULTS
Preliminary hybridization experiments and cloning of the ESBL gene.
Genomic DNA from C. meningosepticum PINT failed to hybridize with several probes corresponding to class A β-lactamase genes (blaSHV-3, blaPER-1, blaVEB-1, blaTEM-1, and blaCARB-2) or with a probe for the gene of the clavulanic acid-inhibited extended-spectrum oxacillinase OXA-18. Genomic DNA from C. meningosepticum PINT that had been partially digested with Sau3AI was cloned into the BamHI site of pBK-CMV. Sixteen recombinant E. coli DH10B clones harboring plasmids with inserts that varied in size (1.9 to 12 kb) were obtained after selection on amoxicillin- and kanamycin-containing TS agar plates. A schematic representation was generated for one of them, pBS1, which possesses the smallest insert (Fig. 1).
β-Lactam resistance phenotype.
Analysis of C. meningosepticum PINT by the conventional disk susceptibility assay suggested that its β-lactam resistance phenotype was partially due to the presence of an ESBL. A similar ESBL phenotype was found for E. coli DH10B harboring recombinant plasmid pBS1 (data not shown). This ESBL phenotype was peculiar since synergy was found not only between ceftazidime or cefotaxime and clavulanic acid or tazobactam but also with cefoxitin, moxalactam, or imipenem (data not shown). The MICs of β-lactams for C. meningosepticum PINT indicated that it was resistant to all tested β-lactams with the exception of ureidopenicillins such as piperacillin (Table 2). Similar MICs of β-lactams were obtained for all the C. meningosepticum isolates tested (data not shown). E. coli DH10B harboring recombinant plasmid pBS1 was resistant to the β-lactams tested but remained susceptible to cefepime, cephamycins, and carbapenems, therefore ruling out the role of the β-lactamase gene cloned into pBS1 in the carbapenem resistance of C. meningosepticum PINT. The MICs of the β-lactams were significantly lowered not only in the presence of clavulanic acid or tazobactam, as reported for a classical ESBL phenotype, but also in the presence of cefoxitin at concentrations as low as 0.1 μg/ml or of moxalactam and imipenem, both at 0.05 μg/ml.
TABLE 2.
MICs of β-lactams for C. meningosepticum PINT, E. coli DH10B harboring recombinant plasmid pBS1, and reference strain E. coli DH10B
| β-Lactama | MIC (μg/ml)
|
||
|---|---|---|---|
| C. meningosepticum PINT | E. coli DH10B(pBS1) | E. coli DH10B | |
| Amoxicillin | 256 | 512 | 4 |
| Amoxicillin + CLA | 64 | 4 | 4 |
| Amoxicillin + TZB | 64 | 4 | 2 |
| Amoxicillin + FOX | 128 | 8 | 4 |
| Amoxicillin + MOX | 64 | 16 | 4 |
| Amoxicillin + IPM | 64 | 2 | 4 |
| Ticarcillin | 256 | 512 | 4 |
| Ticarcillin + CLA | 64 | 8 | 4 |
| Ticarcillin + TZB | 128 | 4 | 4 |
| Ticarcillin + FOX | 128 | 32 | 4 |
| Ticarcillin + MOX | 128 | 64 | 4 |
| Ticarcillin + IPM | 128 | 4 | 4 |
| Piperacillin | 32 | 8 | 1 |
| Piperacillin + CLA | 16 | 2 | 1 |
| Piperacillin + TZB | 16 | 2 | 1 |
| Piperacillin + FOX | 16 | 2 | 1 |
| Piperacillin + MOX | 32 | 2 | 1 |
| Piperacillin + IPM | 32 | 2 | 1 |
| Cephalothin | 512 | 256 | 2 |
| Cefoxitin | 32 | 4 | 1 |
| Ceftazidime | 256 | 32 | 0.5 |
| Ceftazidime + CLA | 16 | 0.5 | 0.5 |
| Ceftazidime + TZB | 16 | 0.25 | 0.5 |
| Ceftazidime + FOX | 16 | 1 | 0.5 |
| Ceftazidime + MOX | 64 | 2 | 0.5 |
| Ceftazidime + IPM | 64 | 0.5 | 0.5 |
| Cefotaxime | 64 | 4 | 0.12 |
| Cefepime | 32 | 0.5 | 0.03 |
| Cefepime + CLA | 4 | 0.03 | 0.03 |
| Cefepime + TZB | 4 | 0.03 | 0.03 |
| Cefepime + FOX | 8 | 0.06 | 0.03 |
| Cefepime + MOX | 16 | 0.06 | 0.03 |
| Cefepime + IPM | 16 | 0.06 | 0.03 |
| Aztreonam | >512 | 16 | 0.25 |
| Aztreonam + CLA | >512 | 0.12 | 0.25 |
| Aztreonam + TZB | >512 | 0.25 | 0.25 |
| Aztreonam + FOX | >512 | 0.25 | 0.25 |
| Aztreonam + MOX | >512 | 0.5 | 0.25 |
| Aztreonam + IPM | >512 | 0.25 | 0.25 |
| Moxalactam | 64 | 0.25 | 0.12 |
| Imipenem | 32 | 0.25 | 0.12 |
CLA, clavulanic acid at a fixed concentration of 2 μg/ml; TZB, tazobactam at a fixed concentration of 4 μg/ml; FOX, cefoxitin at a fixed concentration of 0.1 μg/ml; IPM, imipenem at a fixed concentration of 0.5 μg/ml; MOX, moxalactam at a fixed concentration of 0.05 μg/ml.
Genetic and protein sequence analyses.
Conjugation experiments failed to transfer any β-lactam resistance marker from C. meningosepticum PINT to rifampin-resistant E. coli JM109. However, no control was used in the assays for mating out from C. meningosepticum to E. coli. No plasmid was detected in C. meningosepticum PINT.
DNA sequence analysis of the 1,525-bp region downstream from the 1.9-kb insert of pBS1 revealed an open reading frame (ORF) of 891 bp encoding a 297-amino-acid preprotein (Fig. 2). No sequence typical of a class 1 integron was found within the insert. No ORF likely coding for a regulatory protein was found upstream of the β-lactamase gene.
The overall G+C content of the ORF was 33.9%, which lies close to the expected range of the G+C ratio of members of the Chryseobacterium (Flavobacterium) genus (37%) (34).
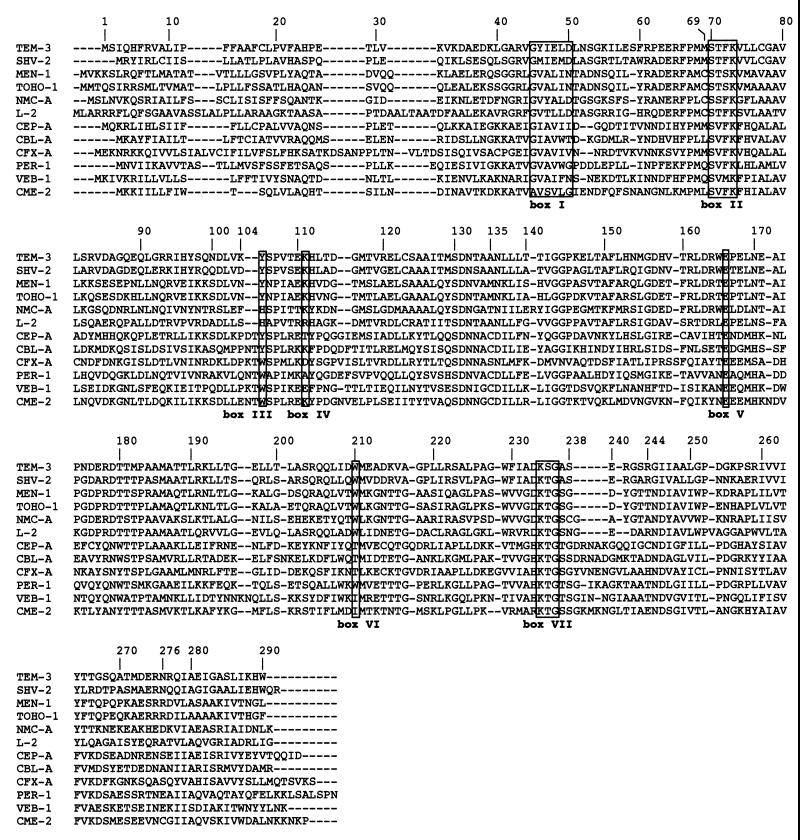
The mature protein (named CME-2 for C. meningosepticum ESBL) expressed in E. coli DH10B had a molecular mass of 31 kDa (data not shown). Within this protein sequence, seven boxes characteristic of β-lactamases possessing a serine-active site were found (14): box I, positions ABL 45 to 50; box II, positions ABL 70 to 73; box III, position ABL 105; box IV, position ABL 111; box V, position ABL 166; box VI, position ABL 210; and box VII, positions ABL 234 to 236 (Fig. 3). In addition, another class A conserved motif, serine-aspartic acid-asparagine (SDN), was found at positions 130 to 132 (Fig. 3).
FIG. 3.
Amino acid sequence alignment of CME-2 compared with those of selected class A β-lactamases. The numbering is according to Ambler (1). Roman numerals designate the boxes described by Joris et al. (14): box I, positions ABL 45 to 50; box II, positions ABL 70 to 73; box III, position ABL 105; box IV, position ABL 111; box V, position ABL 166; box VI, position ABL 210; box VII, positions ABL 234 to 236. The β-lactamases included in the alignment are TEM-3 from K. pneumoniae CFF104, SHV-2 from K. ozaenae, MEN-1 from E. coli MEN, TOHO-1 from E. coli TUH12191, NMC-A from E. cloacae NOR-1, L-2 from S. maltophilia 1275IID, CEP-A from B. fragilis CS30, CFX-A from B. vulgatus CLA341, VEB-1 from E. coli MG-1, CBL-A from B. uniformis WAL-7088, and PER-1 from P. aeruginosa RNL-1. Dashes indicate gaps within the alignment.
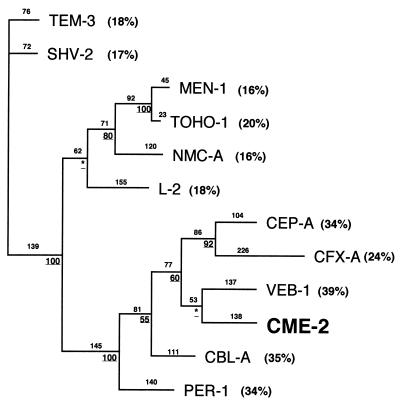
The comparison of CME-2 with other class A β-lactamases revealed weak identity (Fig. 3). The highest degrees of homology were with VEB-1 from E. coli MG-1, CBL-A from B. uniformis, and PER-1 from P. aeruginosa RNL-1 (39, 35, and 34% identities, respectively). A dendrogram was constructed in order to relate CME-2 to representative Ambler class A ESBLs. CME-2 clustered with VEB-1, CFX-A, and CEP-A (Fig. 4).
FIG. 4.
Dendrograms obtained for 12 Ambler class A ESBLs by the parsimony method (44). Branch lengths are drawn to scale and are proportional to the number of amino acid changes. The percentages at the branching points (underlined) refer to the number of times that a particular node was found in 100 bootstrap replications (the asterisks indicate uncertainty for nodes with bootstrap values of less than 50%). The distance along the vertical axis has no significance. Abbreviations for β-lactamases are given in the legend to Fig. 3. Percent amino acid identities to CME-2 are indicated in parentheses.
Biochemical properties of CME-2 β-lactamase.
Analytic isoelectric focusing revealed that E. coli DH10B harboring recombinant plasmid pBS1 produced only one β-lactamase with activity, and it had a pI of 9.2. In the original C. meningosepticum PINT isolate, β-lactamases with pI values of 7.6 and 8.3 were found. Only the β-lactamase with a pI of 7.6 corresponded to a serine β-lactamase.
After purification of the CME-2 β-lactamase from E. coli DH10B(pBS1), the specific activity of 22 mU · mg−1 of protein was determined with 100 μM benzylpenicillin as the substrate. The overall recovery of CME-2 was 41%, with a 120-fold β-lactamase purification. According to visual inspection of the SDS-polyacrylamide gel, the CME-2 β-lactamase gene product was weakly expressed from pBS1 in E. coli.
The kinetic parameters of the purified CME-2 β-lactamase revealed strong activity against cephalosporins, including extended-spectrum cephalosporins (Table 3), and its activity against aztreonam was also noticeable. CME-2 β-lactamase has no detectable activity against piperacillin, cephamycins, and imipenem (Table 3). IC50 results with benzylpenicillin as the substrate showed that CME-2 activity was strongly inhibited by clavulanic acid (0.05 μM), tazobactam (1.5 μM), and sulbactam (0.3 μM). In addition, unlike other class A ESBLs, CME-2 was inhibited more strongly by cefoxitin (0.004 μM), moxalactam (0.002 μM), and imipenem (0.015 μM).
TABLE 3.
Kinetic parameters of the purified β-lactamase CME-2
| Drug | kcat (s−1) | Km (μM) | kcat/Km (μM−1 · s−1) |
|---|---|---|---|
| Cephaloridine | 30.6 | 23.4 | 1.31 |
| Benzylpenicilllin | 12.3 | 7.4 | 1.66 |
| Amoxicillin | 6.7 | 26.5 | 0.26 |
| Ticarcillin | 1.8 | 5.8 | 0.31 |
| Piperacillin | 0.3 | <1 | —a |
| Cephalothin | 60.6 | 41.5 | 1.46 |
| Cefoxitin | <0.01 | — | — |
| Cefuroxime | 57.3 | 81.6 | 0.70 |
| Cefoperazone | 2.6 | 3.8 | 0.68 |
| Cefotaxime | 12.1 | 20.8 | 0.58 |
| Ceftazidime | 23.6 | 125 | 0.19 |
| Aztreonam | 3.2 | 10.5 | 0.31 |
| Imipenem | <0.01 | — | — |
—, not detectable.
Distribution of blaCME-2 in Chryseobacterium sp. isolates.
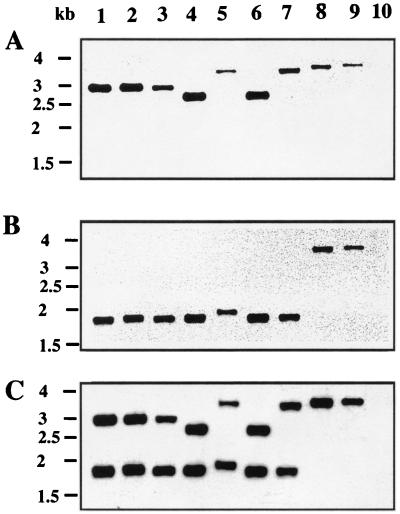
Using a set of primers designed to amplify a 743-bp internal fragment of blaCME-2 by PCR, positive results were obtained for only five of nine C. meningosepticum strains (C. meningosepticum PINT, CIP 6058, AMA, GEO, and CIP 6059). Negative PCR results were obtained not only for four of nine C. meningosepticum isolates but also for all strains of the C. meningosepticum-related species (Chryseobacterium indologenes, Sphingobacterium multivorum, and Myroides odoratus) (data not shown). However, hybridization experiments with a blaCME-2-specific internal probe gave positive results for the nine C. meningosepticum isolates and negative results for the strains of the C. meningosepticum-related species. In order to evaluate the distribution of a blaCME-2-like gene within C. meningosepticum isolates, their DNAs were restricted with XmnI and hybridized with three probes, probes S1 and S2, which are located upstream and downstream from the XmnI site (XmnI cuts in the middle of the blaCME-2 gene), respectively, and S3, a DraI internal fragment, giving a probe specific for the entire blaCME-2 gene (Fig. 1). Six hybridization patterns were obtained for the nine C. meningosepticum isolates (Fig. 5). By using the S1 and S2 probes, C. meningosepticum PINT, CIP 6058, and AMA (pattern I) gave 3- and 1.8-kb fragments, respectively; C. meningosepticum GEO and CIP 6059 (pattern II) gave 2.7- and 1.8-kb fragments, respectively; C. meningosepticum CIP 7830 (pattern III) gave 3.5- and 1.9-kb fragments, respectively; C. meningosepticum CIP 7905 (pattern IV) gave 3.5- and 1.8-kb fragments, respectively; C. meningosepticum AB 1572 (pattern V) gave 3.7-kb fragments with both probes; and C. meningosepticum H01J100 (pattern VI) gave 3.8-kb fragments with both probes. S3 probe hybridizations gave patterns corresponding to the sum of the results obtained after the S1 and S2 probe hybridizations. Only patterns I and II gave positive results after blaCME-2 PCR amplification.
FIG. 5.
Autoradiogram after Southern hybridizations of C. meningosepticum genomic DNAs. The DNAs were restricted with XmnI, and the probe consisted of the 372-bp DraI-XmnI fragment (S1) of pBS1 (A), the 306-bp XmnI-DraI fragment (S2) of pBS1 (B), or the 667-bp DraI fragment (S3) of pBS1 (C) (see Fig. 1 for the locations of S1, S2, and S3). Lanes: 1, C. meningosepticum PINT; 2, C. meningosepticum CIP 6058; 3, C. meningosepticum AMA; 4, C. meningosepticum GEO; 5, C. meningosepticum CIP 7830; 6, C. meningosepticum CIP 6059; 7, C. meningosepticum CIP 7905; 8, C. meningosepticum AB 1572; 9, C. meningosepticum H01J100; 10, E. coli DH10B (negative control). The sizes of the DNA fragments may be deduced from the scale (1.5 to 4 kb) shown on the left sides of the gels.
Finally, induction studies with imipenem or cefoxitin as the inducer did not show that these ESBLs from C. meningosepticum would be inducible. As assessed by isoelectric focusing analysis, each isolate produced at least two β-lactamases. However, preincubation of the enzyme extracts with clavulanic acid showed that each C. meningosepticum isolate produced only one serine β-lactamase with variable pIs ranging from 7.0 to 8.5, with no correlation of the pIs with the hybridization patterns.
DISCUSSION
The starting point of this study was the observation of a synergy between ceftazidime and cephamycins in a conventional antibiogram disk susceptibility assay performed with a C. meningosepticum clinical isolate. The overall β-lactam susceptibility pattern of this C. meningosepticum isolate corresponded to that previously described in these bacterial species characterized by resistance to all β-lactams tested, including extended-spectrum cephalosporins, monobactams, and carbapenems, and a moderated susceptibility to piperacillin (6, 20). This profile of resistance to broad-spectrum β-lactams may be explained for all β-lactams except cefepime and imipenem by the hydrolytic properties of the ESBL CME-2. For cefepime and imipenem the resistance is most likely mediated by the class B carbapenem-hydrolyzing β-lactamase recently identified in C. meningosepticum CIP 6058, a strain included in this study (36). The biochemical properties of CME-2 are similar to those of an unsequenced ESBL identified from another C. meningosepticum isolate (9). Both enzymes may be classified within Bush group 2be (5). This group includes several β-lactamases which are weakly related to CME-2, i.e., MEN-1, TOHO-1, and L-2. CME-2 activity not only was inhibited by clavulanic acid but was also strongly inhibited by cephamycins and imipenem. This inhibition profile is found in two other class A β-lactamases, VEB-1 from E. coli and CEP-A from B. fragilis (35). Comparison of their amino acid sequences did not provide evidence of any particular amino acid identity which may explain this cephamycin inhibition property. This property may help to differentiate C. meningosepticum from other Chryseobacterium sp. isolates for which a synergy between cephamycins and ceftazidime is not observed.
CME-2 is only distantly related to other class A ESBLs. However, it belongs to a subgroup of class A β-lactamases, of which VEB-1 from E. coli MG-1 is the most closely related to CME-2 (39% amino acid identity).
Although isolated from C. meningosepticum, the CME-2 β-lactamase does not exhibit features common to class A β-lactamases isolated from some gram-negative species, such as Cys77 and Cys123, which are thought to form a disulfide bridge (33, 38, 46). No cysteine residue is found at these positions in CME-2; instead, an alanine (as for VEB-1, PER-1, CFX-A, CBL-A, CEP-A, L-2, NMC-A, and TOHO-1) and an isoleucine (a leucine in VEB-1, PER-1, CFX-A, and CEP-A), respectively, are found (Fig. 3). Surprisingly, CME-2 possesses a cysteine residue at position 135, close to the SDN motif, as in VEB-1, PER-1, CBL-A, and CEP-A. It is interesting that within the non-TEM and non-SHV class A β-lactamases, cysteine residues are found close to either the SVFK or the SDN conserved motifs at a limited number of positions; position 69 (MEN-1, TOHO-1, NMC-A, and L-2), position 81 (CFX-A), position 123 (L-2), or position 135 (CEPA, CBL-A, PER-1, VEB-1, and CME-2). In CME-2, a disulfide bridge may be formed between cysteine 135 and cysteine 276, as evidenced for NMC-A between cysteine 69 and cysteine 238 (43).
The omega loop which goes from positions 169 to 179 is a structural element of class A enzymes. This loop is present in CME-2 but is totally different from those found in TEM or SHV derivatives. In this respect, CME-2 along with VEB-1, PER-1, CBL-A, and CEP-A possesses a histidine in place of an asparagine at position 170 (Fig. 3). This asparagine together with the highly conserved Glu166 and Ser70 is involved in the positioning of the active-site water molecule (19). It is tempting to speculate that this histidine may play a similar role in the catalytic properties of CME-2, but this needs to be confirmed by site-directed mutagenesis.
Many ESBLs are derived from the parental TEM-1, TEM-2, or SHV-1 enzymes by a few amino acid substitutions at position 104, 164, 238, or 240, leading to increased catalytic activities for cefotaxime, ceftazidime, and aztreonam. In the CME-2 β-lactamase, two additional amino acids are found at position 104 compared to those found in TEM-1, TEM-2, or SHV-1 derivatives. The Arg104Lys substitution in TEM-3 may correspond to either a glutamic acid, an asparagine, or a threonine in CME-2 (Fig. 3). In the PER-1 β-lactamase, additional amino acids (glutamine, asparagine, and threonine) are also found at position 104. PER-1 is the only non-TEM and non-SHV ESBL for which site-directed mutagenesis has so far been performed, but the roles of these amino acids at this position remain unclear (4).
At position 164, some TEM derivatives that possess ESBL properties have a serine or a histidine in place of an arginine. PER-1 possesses an alanine and CME-2 possesses a tyrosine. Since the Ala164Arg substitution in PER-1 results in a mutant with no detectable activity (4), it would be interesting to study the effect of the Tyr164Arg substitution in CME-2. At positions 238 and 240, TEM-1, TEM-2, and SHV-1 possess glycine and glutamine residues, respectively. CME-2, like PER-1, possesses a serine at position 238, as found for some TEM and SHV ESBLs, and a glycine at position 240.
The G+C ratio of 33.9%, typical of Chryseobacterium species genes, together with negative conjugation and negative plasmid research results, indicated a likely chromosomal location of the blaCME-2 gene. No sequence typical of E. coli or P. aeruginosa promoters was identified upstream of the ATG initiation site. Interestingly, the pI value for CME-2 in E. coli DH10B (9.2) did not correspond to the pI value of the serine β-lactamase (7.6) found in C. meningosepticum PINT, thus indicating a putative difference in the cleavage site of the peptide leader. No regulatory protein gene was found immediately upstream of the blaCME-2 gene. Therefore, CME-2 expression may be either not regulated at all or not regulated like other chromosomally located class A ESBLs such as NMC-A and SME-1 (21, 22). In that respect, induction experiments failed to identify any inducible serine ESBL in C. meningosepticum isolates, whereas inducible L-2 enzymes are found in S. maltophilia (27). However, as found in S. maltophilia (28), several β-lactamases of Ambler class A and class B were identified in each C. meningosepticum isolate (data partially shown), and these may together account for the naturally occurring β-lactam resistance profiles of the C. meningosepticum isolates.
The results of PCR with nondegenerated primers for blaCME-2 detection showed that this technique may be too specific for the identification of blaCME-2-like genes. However, hybridization experiments identified in each C. meningosepticum isolate a blaCME-2-like gene that was present in only one copy. Moreover, they revealed that blaCME-2 gene variants are identified in all C. meningosepticum isolates tested, although their β-lactam resistance phenotypes were identical. The presence of a blaCME-2-like gene in C. meningosepticum isolates makes it an identification marker for this bacterial species among Chryseobacterium species. Finally, the identification of CME-2 β-lactamase gives an additional clue that class A ESBLs may be a means for naturally occurring β-lactam resistance.
ACKNOWLEDGMENTS
This work was financed by a grant from the Ministère de l'Education Nationale et de la Recherche (grant UPRES-JE 2227), Université Paris XI, Paris, France.
We thank Esthel Ronco and Brita Bruun for providing us some C. meningosepticum clinical isolates and D. Aubert for help in determining kinetic constants.
ADDENDUM
After the work was submitted, we noticed a report from Rossolini et al. (36) showing a very similar enzyme, CME-1, from C. meningosepticum CCUG4310. CME-1 displays five amino acid changes with respect to CME-2: valine in CME-1 to isoleucine in CME-2 at position 157, isoleucine to methionine at position 278, and asparagine to lysine at position 295 and deletion of the last two amino acids, lysine and proline, of CME-1 in CME-2. This report explains why the name CME-2 was retained. None of the amino acid differences may be critical to the extended hydrolysis spectrum profile, which was found to be similar in both enzymes.
REFERENCES
- 1.Ambler R P. The structure of beta-lactamases. Philos Trans R Soc Lond B Biol Sci. 1980;289:321–331. doi: 10.1098/rstb.1980.0049. [DOI] [PubMed] [Google Scholar]
- 2.Barthélémy M, Péduzzi J, Bernard H, Tancrède C, Labia R. Close amino-acid sequence relationship between the new plasmid-mediated extended-spectrum beta-lactamase MEN-1 and chromosomally encoded enzymes of Klebsiella oxytoca. Biochim Biophys Acta. 1992;1122:15–22. doi: 10.1016/0167-4838(92)90121-s. [DOI] [PubMed] [Google Scholar]
- 3.Bloch K C, Nadarajah R, Jacobs R. Chryseobacterium meningosepticum: an emerging pathogen among immunocompromised adults. Report of 6 cases and literature review. Medicine (Baltimore) 1997;76:30–41. doi: 10.1097/00005792-199701000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Bouthors A T, Dagoneau-Blanchard N, Naas T, Nordmann P, Jarlier V, Sougakoff W. Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 beta-lactamase hydrolysing third-generation cephalosporins. Biochem J. 1998;330:1443–1449. doi: 10.1042/bj3301443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bush K, Jacoby G A, Medeiros A A. A functional classification scheme for beta-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39:1211–1233. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang J C, Hsueh P R, Wu J J, Ho S W, Hsieh W C, Luh K T. Antimicrobial susceptibility of flavobacteria as determined by agar dilution and disk diffusion methods. Antimicrob Agents Chemother. 1997;41:1301–1306. doi: 10.1128/aac.41.6.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colding H, Bangsborg J, Fiehn N E, Bennekov T, Bruun B. Ribotyping for differentiating Flavobacterium meningosepticum isolates from clinical and environmental sources. J Clin Microbiol. 1994;32:501–505. doi: 10.1128/jcm.32.2.501-505.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fraser S L, Jorgensen J H. Reappraisal of the antimicrobial susceptibilities of Chryseobacterium and Flavobacterium species and methods for reliable susceptibility testing. Antimicrob Agents Chemother. 1997;41:2738–2741. doi: 10.1128/aac.41.12.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujii T, Sato K, Yokota E, Maejima T, Inoue M, Mitsuhashi S. Properties of a broad spectrum beta-lactamase isolated from Flavobacterium meningosepticum GN14059. J Antibiot. 1988;41:81–85. doi: 10.7164/antibiotics.41.81. [DOI] [PubMed] [Google Scholar]
- 10.Hansen J B, Olsen R H. Isolation of large bacterial plasmids and characterization of the P2 incompatibility group plasmids pMG1 and pMG5. J Bacteriol. 1978;135:227–238. doi: 10.1128/jb.135.1.227-238.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huletsky A, Couture F, Levesque R C. Nucleotide sequence and phylogeny of SHV-2 beta-lactamase. Antimicrob Agents Chemother. 1990;34:1725–1732. doi: 10.1128/aac.34.9.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huovinen P, Jacoby G A. Sequence of the PSE-1 beta-lactamase gene. Antimicrob Agents Chemother. 1991;35:2428–2430. doi: 10.1128/aac.35.11.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishii Y, Ohno A, Taguchi H, Imajo S, Ishiguro M, Matsuzawa H. Cloning and sequence of the gene encoding a cefotaxime-hydrolyzing class A beta-lactamase isolated from Escherichia coli. Antimicrob Agents Chemother. 1995;39:2269–2275. doi: 10.1128/aac.39.10.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joris B, Ledent P, Dideberg O, Fonze E, Lamotte-Brasseur J, Kelly J A, Ghuysen J M, Frère J M. Comparison of the sequences of class A beta-lactamases and of the secondary structure elements of penicillin-recognizing proteins. Antimicrob Agents Chemother. 1991;35:2294–2301. doi: 10.1128/aac.35.11.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kado C I, Liu S T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981;145:1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laemmli U K, Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973;80:575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- 17.Laurent F, Poirel L, Naas T, Chaibi E B, Labia R, Boiron P, Nordmann P. Biochemical-genetic analysis and distributions of FAR-1, a class A β-lactamase from Nocardia farcinica. Antimicrob Agents Chemother. 1999;43:1643–1650. doi: 10.1128/aac.43.7.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Massida O, Rossolini G M, Satta G. The Aeromonas hydrophila cphA gene: molecular heterogeneity among class B metallo-β-lactamases. J Bacteriol. 1991;173:4611–4617. doi: 10.1128/jb.173.15.4611-4617.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Medeiros A A. Evolution and dissemination of beta-lactamases accelerated by generations of beta-lactam antibiotics. Clin Infect Dis. 1997;24:S19–S45. doi: 10.1093/clinids/24.supplement_1.s19. [DOI] [PubMed] [Google Scholar]
- 20.Moulin V, Freney J, Hansen W, Philippon A. Comportement phénotypique des Flavobacterium vis-à-vis de 39 antibiotiques. Med Mal Infect. 1992;22:902–908. [Google Scholar]
- 21.Naas, T., D. M. Livermore, and P. Nordmann. Characterization of an LysR family protein, SmeR from Serratia marcescens S6, its effect on expression of the carbapenem-hydrolyzing beta-lactamase Sme-1, and comparison of this regulator with other beta-lactamase regulators. 39:629–637. [DOI] [PMC free article] [PubMed]
- 22.Naas T, Nordmann P. Analysis of a carbapenem-hydrolyzing class A beta-lactamase from Enterobacter cloacae and of its Lys-R type regulatory protein. Proc Natl Acad Sci USA. 1994;91:7693–7697. doi: 10.1073/pnas.91.16.7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A3. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1993. [Google Scholar]
- 24.Nicolas M H, Jarlier V, Honoré N, Philippon A, Cole S T. Molecular characterization of the gene encoding SHV-3 beta-lactamase responsible for transferable cefotaxime resistance in clinical isolates of Klebsiella pneumoniae. Antimicrob Agents Chemother. 1989;33:2096–2100. doi: 10.1128/aac.33.12.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nordmann P, Naas T. Sequence analysis of PER-1 extended-spectrum beta-lactamase from Pseudomonas aeruginosa and comparison with class A beta-lactamases. Antimicrob Agents Chemother. 1994;38:104–114. doi: 10.1128/aac.38.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parker A C, Smith C J. Genetic and biochemical analysis of a novel Ambler class A beta-lactamase responsible for cefoxitin resistance in Bacteroides species. Antimicrob Agents Chemother. 1993;37:1028–1036. doi: 10.1128/aac.37.5.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patton R, Miles R S, Amyes S G. Biochemical properties of inducible β-lactamases produced from Xanthomonas maltophilia. Antimicrob Agents Chemother. 1994;38:2143–2149. doi: 10.1128/aac.38.9.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Payne D J, Cramp R, Bateson J H, Neal J, Knowles D. Rapid identification of metallo- and serine β-lactamases. Antimicrob Agents Chemother. 1994;38:991–996. doi: 10.1128/aac.38.5.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phillipon L N, Naas T, Bouthors A T, Barakett V, Nordmann P. OXA-18, a class D clavulanic acid-inhibited extended-spectrum β-lactamase from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1997;41:2188–2195. doi: 10.1128/aac.41.10.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pickett M J. Methods for identification of flavobacteria. J Clin Microbiol. 1989;27:2309–2315. doi: 10.1128/jcm.27.10.2309-2315.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poirel L, Guibert M, Girlich D, Naas T, Nordmann P. Cloning, sequence analyses, expression and distribution of ampC-ampR from Morganella morganii clinical isolates. Antimicrob Agents Chemother. 1999;43:769–776. doi: 10.1128/aac.43.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poirel L, Naas T, Guibert M, Chaibi E B, Labia R, Nordmann P. Molecular and biochemical characterization of VEB-1, a novel class A extended-spectrum β-lactamase encoded by an Escherichia coli integron gene. Antimicrob Agents Chemother. 1999;43:573–581. doi: 10.1128/aac.43.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pollitt S, Zalkin H. Role of primary structure and disulfide bond formation in β-lactamase secretion. J Bacteriol. 1983;153:27–32. doi: 10.1128/jb.153.1.27-32.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richard C, Monteil H. Isolement, identification, signification clinique du genre Flavobacterium. Ann Biol Clin. 1983;4:187–198. [PubMed] [Google Scholar]
- 35.Rogers M B, Parker A C, Smith C J. Cloning and characterization of the endogenous cephalosporinase gene, cepA, from Bacteroides fragilis reveals a new subgroup of Ambler class A β-lactamases. Antimicrob Agents Chemother. 1993;37:2391–2400. doi: 10.1128/aac.37.11.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rossolini G M, Franceschini N, Riccio M L, Mercuri P S, Perilli M, Galleni M, Frère J M, Amicosante G. Characterization and sequence of the Chryseobacterium (Flavobacterium) meningosepticum carbapenemase: a new molecular class B β-lactamase showing a broad substrate profile. Biochem J. 1998;332:145–152. doi: 10.1042/bj3320145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 38.Schultz S C, Dabadie-McFarkand G, Neitzel J J, Richards J H. Stability of wild-type and mutant RTEM-1 β lactamase: effect of the disulfide bond. Proteins. 1987;2:290–297. doi: 10.1002/prot.340020405. [DOI] [PubMed] [Google Scholar]
- 39.Siegman-Igra Y, Schwartz D, Soferman G, Konforti N. Flavobacterium group IIb bacteremia: report of a case and review of Flavobacterium infections. Med Microbiol Immunol. 1987;176:103–111. doi: 10.1007/BF00200682. [DOI] [PubMed] [Google Scholar]
- 40.Smith C J, Bennett T K, Parker A C. Molecular and genetic analysis of the Bacteroides uniformis cephalosporinase gene, cblA, encoding the species-specific beta-lactamase. Antimicrob Agents Chemother. 1994;38:1711–1715. doi: 10.1128/aac.38.8.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sougakoff W, Goussard S, Courvalin P. The TEM-3 β-lactamase, which hydrolyses broad-spectrum cephalosporins, is derived from the TEM-2 penicillinase by two amino-acid substitutions. FEMS Microbiol Lett. 1988;56:343–348. [Google Scholar]
- 42.Sutcliffe J G. Nucleotide sequence of the ampicillin resistance gene of Escherichia coli plasmid pBR322. Proc Natl Acad Sci USA. 1978;75:3737–3741. doi: 10.1073/pnas.75.8.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swaren P, Maveyraud L, Raquet X, Cabantous S, Duez C, Pedelacq J D, Mariotte-Boyer S, Mourey L, Labia R, Nicolas-Chanoine M H, Nordmann P, Frère J M, Samama J P. X-ray analysis of the NMC-A β-lactamase at 1.64 Å resolution, a class A carbapenemase with broad substrate activity. J Biol Chem. 1998;41:26714–26721. doi: 10.1074/jbc.273.41.26714. [DOI] [PubMed] [Google Scholar]
- 44.Swofford D L. PAUP (version 3.0): phylogenetic analysis using parsimony. Champaign: Illinois Natural History Survey; 1989. [Google Scholar]
- 45.Vandamme P, Bernardet J F, Segers P, Kersters K, Holmes B. New perspectives in the classification of the flavobacteria: description of Chryseobacterium gen. nov., Bergeyella gen. nov., and Empedobacter nom. rev. Int J Syst Bacteriol. 1994;44:827–831. [Google Scholar]
- 46.Vanhove M, Guillaume G, Ledent P, Richards J H, Pain R H, Frère J M. Kinetic and thermodynamic consequences of the removal of the cys-77-cys-123 disulphide bond for the folding of TEM-1 β-lactamase. Biochem J. 1997;321:413–417. doi: 10.1042/bj3210413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walsh T R, MacGowan A P, Bennett P M. Sequence analysis and enzyme kinetics of the L2 serine beta-lactamase from Stenotrophomonas maltophilia. Antimicrob Agents Chemother. 1997;41:1460–1464. doi: 10.1128/aac.41.7.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and hosts strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]