Abstract
Paracetamol/Acetaminophen was widely used as a first-line antipyretic and analgesic for COVID-19 patients without giving any attention to the potential risk of related toxicities. A survey was conducted on 176 Egyptians using an online survey portal to assess their knowledge, and attitude regarding potential risk of paracetamol toxicities and whether COVID-19 pandemic affected their practices regarding safe use of paracetamol. The self-administered questionnaire was developed by the researchers and was validated by expert opinions. A pilot testing of the questionnaire was done. Alpha Cronbach test used to assess the internal consistency reliability of the survey revealed good reliability. Overall percent-score revealed that only 24.4% of participants had good knowledge about paracetamol and its related potential toxicities. 62.5% of participants considered paracetamol safer than other medications of the same indications. 42.6% of participants could advise others to use paracetamol without prescription. According to the participants' responses, physicians were less concerned to give instructions about possibility of overdosage. Our results also revealed that participants’ administration of paracetamol without physician prescription was more during COVID-19. Practice of paracetamol administration more than the allowed number of tablets/day was significantly more evident during the pandemic. We concluded that the unsupervised use of paracetamol is an alarming sign that should be addressed as this could lead to a high rate of accidental paracetamol toxicity. A lesson learnt from COVID-19 pandemic is the need to implement behavior change measures to mitigate the risk of accidental paracetamol toxicity.
List of abbreviations: ALF, acute liver failure; CFA, confirmatory factor analysis; COVID-19, Coronavirus disease 2019; FDA, Food and Drug Administration; MERS-CoV, Middle East respiratory syndrome coronavirus; NSAIDs, non-steroidal anti-inflammatory drugs; OTC, over the counter; PHC, primary health care; ROS, reactive oxygen species; REC, Research Ethics committee; SARS-CoV, severe acute respiratory syndrome coronavirus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation; WHO, World Health Organization
Keywords: Acetaminophen, Paracetamol, Toxicity, Safe use, COVID-19, Egyptians
Graphical Abstract
Highlights
-
•
Survey on paracetamol safe usage and its potential toxicities among Egyptians.
-
•
Paracetamol usage among participants of this study was more during COVID-19.
-
•
58% had fair knowledge about paracetamol and its related potential toxicities.
-
•
42.6% could advise others to use paracetamol without prescription.
-
•
There is a need to implement measures to mitigate accidental paracetamol toxicity.
1. Introduction
Coronavirus disease 2019 (COVID-19) is outlined as an illness triggered with the aid of a novel coronavirus currently referred to as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. Prior to the COVID-19 outbreak, the twenty-first century had outbreaks produced by two additional extremely pathogenic zoonotic coronaviruses, the severe acute respiratory syndrome coronavirus (SARS-CoV) and the Middle East respiratory syndrome coronavirus (MERS-CoV). Such viruses caused significant human morbidity and mortality in the form of severe respiratory syndrome [2], [3].
Seven distinct coronaviruses have been recognized until recently. Sneezing, coughing, and aerosols spread the virus across the population, producing a deadly lung infection via the nasal cavity or mouth. The seventh coronavirus strain is the highly infectious SARS-CoV-2 which is a positive-sense RNA virus infecting human upper and lower respiratory tracts and thus, resulting in severe respiratory sickness and the present worldwide global pandemic [4], [5].
Infection with SARS-CoV-2 causes oxidative stress via altering redox equilibrium; moreover flavor-enhancing high-lipid diet also directly or indirectly stimulates the severity of the SARS-CoV-2 mediated infection [5], [6], [7].
Fever, fatigue, dry cough, and pulmonary manifestations are COVID-19 common clinical manifestations. The fundamental strategy for treating COVID-19 patients includes early diagnosis, quarantine, and supportive remedies [8], [9]. Accordingly, people's attention may be diverted towards over the counter (OTC) medicines that mitigate fever and aches as non-steroidal anti-inflammatory drugs (NSAIDs), and paracetamol (acetaminophen) [10].
Paracetamol is one of the most effective and most widely used analgesics and antipyretics worldwide. It exists both in the form of a single component or multi-component preparations, also as OTC and prescription formulations [11], [12].
Regardless of various therapeutic benefits for paracetamol, paracetamol toxicity is considered one of the most prevalent forms of poisoning in the globe. Hepatotoxicity can be caused by its repeated supratherapeutic overuse, unintentional misuse, or purposeful intake. Majority of it is transformed to glucuronidated and sulfated conjugates, which are pharmacologically inactive. On the other hand, cytochrome P450 isoforms (CYP2E1, CYP2A6) in liver microsomes convert a small fraction of paracetamol (5–10%) into a reactive metabolite, N-acetyl-para-benzo-quinone imine (NAPQI), which is linked to paracetamol hepatotoxicity. In therapeutic intake, hepatic glutathione quickly conjugates NAPQI. In contrast, in case of paracetamol overdose, glutathione depletion occurs. Accordingly, hazardous levels of NAPQI will accumulate. Excess NAPQI leads to increased oxidative stress, and mitochondrial dysfunction. Another proposed pathway for hepatotoxicity is the creation of peroxynitrite in the mitochondria, thus inducing DNA fragmentation and halting ATP synthesis. Moreover, in therapeutic intake, autophagosomes will engulf damaged mitochondria to eliminate malfunctioning mitochondria via mitophagy, reducing hepatocyte cell death. However, during paracetamol overdose, mitophagy becomes ineffective and hepatocyte cell death is enhanced due to cholesterol accumulaion in the lysosomal membrane [13], [14], [15].
Accordingly, the perception of paracetamol as a safe OTC drug has become very misleading as this has led to a high rate of paracetamol toxicity. Liver failure has been reported even with doses just more than the maximum therapeutic dose [11], [16], [17], [18]. According to the United States Food and Drug Administration (FDA) guidelines, it is safe to consume up to a maximum daily dose of 4000 mg of acetaminophen [19], [20], [21]. It also warns against exceeding the maximum recommended daily dose as this may lead to hepatic injury and may also lead to death [22], [23].
Pregnant patients, chronic alcoholics, hepatic patients, chronic malnutrition, and severe renal-impaired patients are considered risk group for developing paracetamol toxicities [24].
The Poison Control Center at Ain Shams University Hospitals (PCC-ASUH) is Egypt's primary poison control center. According to PPC-ASUH, “paracetamol is one of the top ten most frequently involved exposure substances received in PCC-ASUH during 2019” [25], [26] From March 1 to June 30, 2015, a hospital-based prospective research was conducted on 653 acute poisoning cases admitted to Menoufia University Hospital's Poisoning and Addiction Control Center. Drugs poisoning represented 25.4%, and Paracetamol was 6.7% [27].
There is a continual increase of the concerns about paracetamol safety. These concerns have been growing more and more during COVID-19 pandemic, as paracetamol has been considered, based on the WHO pain ladder, to be the first-line antipyretic and analgesic for COVID-19 patients [28]. On March 17, 2020, the WHO recommended people with COVID-19 symptoms to avoid using ibuprofen and use paracetamol instead resulting in a disproportionately high purchase of paracetamol medications [29].
The reasons behind such recommendations may be attributed to Leal et al., 2021 declaration that paracetamol may have a protective effect against SARS-CoV-2 infection as it reduced angiotensin-converting enzyme 2 (ACE2) protein levels in cultured cells. Previous findings showed that ACE2 has been shown to be a functional receptor for SARS-CoV. Similarly but with higher affinity is SARS-CoV-2. Paradoxically but interestingly, multiple organs, including the lungs, rely on the ACE2 enzyme as protective. SARS-CoV infection has been found in several investigations to downregulate ACE2 expression on cells, interrupting its protective physiological actions and causing severe organ destruction [29], [30], [31], [32], [33], [34].
Another recent article raised the question of whether paracetamol is the preferable alternative, based on new evidence and hypotheses about the likely significance of limited glutathione (GSH) levels in the worsening of COVID-19. Moreover, During COVID-19, using medications with the illness might exacerbate the liver even more. In this setting, it is critical to not only combat the virus, but also to protect the organ integrity by suitable and focused therapy. Paracetamol was one of the medications that have been utilized for the treatment of COVID-19 in a domiciliary manner without undertaking any safety considerations [35], [36].
Therefore, we aim at assessing the knowledge, and attitude of a sample of Egyptian population regarding paracetamol safe usage and its related potential toxicities as well as their practices regarding the use of paracetamol before and during COVID-19 pandemic.
2. Methods
A cross-sectional survey was conducted in July 2020 on 176 adult Egyptians using an online survey portal, digital google form, that was disseminated on social networking packages characterizing a convenience sampling and snowball recruitment method starting from known professional and social media groups and individuals and then was distributed on various internet platforms. The self-administered questionnaire was developed by the researchers after a review of the previously published studies. The survey questionnaire was designed in the native language in Egypt; Arabic, and it covered basic demographic characteristics, including (age, gender, residence, socio-economic level, occupation), knowledge regarding paracetamol, and its potential toxicities, attitudes towards paracetamol, and practices towards the safe use of paracetamol before and during COVID-19 pandemic. An English translation of the questionnaire is provided in appendix A(Supplementary material).
Content validity of the questionnaire was assessed by four experts in, public health and toxicology medicine. After reviewing the experts’ comments, the authors modified words and questions that were not clear, confusing, or may not be accepted by the participants. The questionnaire was then evaluated for face validity through a pilot study, in which the researchers interviewed 15 participants to ensure the clearness, simplicity and appropriateness of the questions and answer choices. Feedback from the pilot study showed that the participants could understand the questionnaire and no further modifications were needed. Confirmatory factor analysis (CFA) was conducted to assess construct validity. The results of CFA showed a statistically significant correlation between the questionnaire items. The questionnaire was then made available for assessing the internal consistency reliability using Alpha Cronbach test which showed good reliability (Cronbach's Alpha was 0.74)”.
Informed consent to participate in this study was obtained from all study participants. Confidentiality was ensured by keeping the questionnaire anonymous and avoiding mentioning any identifying features of the participants. Total time spent for questionnaire completion was about 15–20 min.
2.1. Statistical analysis
Participants' knowledge was assessed using yes/no and multiple-choice questions. Participants' attitude was assessed with three-point Likert scale (agree, neutral, disagree), while participants' practice was assessed by yes/no questions. A total score was calculated by adding-up the correct choices for knowledge questions and then converted into a percent-score (by division with the maximum score and multiplied by 100). Knowledge percent score was then categorized into poor knowledge (score of 0–25), fair knowledge (score of 26–50), and good knowledge (51−100).
Data were coded, entered, and manipulated using Microsoft Excel (2016 version). Data analyses were performed using IBM SPSS software (version 22). Demographic characteristics, knowledge, attitude, and practices were described using descriptive statistics, including percentages (%), frequencies, mean, and standard deviation (SD). A Chi-square test was used for testing the statistical significance of categorical data. Fisher's exact test was used whenever Chi-square test assumptions were violated (i.e., when more than 20% of the expected values were less than 5 or any of cell values equal 0). P-value < 0.05 was considered statistically significant. The relationship between different demographic characteristics and knowledge level was assessed. The association between participants’ practices of paracetamol administration before and during COVID-19 and their knowledge was also assessed. P-value of (< 0.05) was considered statistically significant.
3. Results
3.1. Sociodemographic characteristics
A total of 176 participated in this study. The mean age (SD) of participants was 36 (9) years and ranged from 19 to 68 years. Participants of age 30-< 50 years constituted 73.3% of all participants. 20.5% were of age 18-< 30 years. 6.3% were ≥ 50 years of age. About 39.2% were males, and 60.8% were females. The study included participants from all levels of education; 5.1% with high school, 72.2% with bachelor and 22.7% with post-graduate degree. The majority (89.8%) of the participants were from urban, while 10.2% were from rural residence. Majority of the participants were of moderate level of socioeconomic status, while 13.6% were high and only 0.6% of low level.
3.2. Participants' knowledge about paracetamol
Many of the participants were not knowledgeable about acetaminophen as a synonym of paracetamol, cautious administration of paracetamol with other drugs, warning signs of overdose, toxicity and possibility of death as an adverse event with paracetamol administration. Only 9.7% knew that acetaminophen is a synonym of paracetamol. 25.6% and 31.8% considered the importance of cautious administration of paracetamol when used with other drugs containing paracetamol in their ingredients and with other non-paracetamol drugs respectively.
Possibility of toxicity with paracetamol administration and existence of warning signs of paracetamol overdose were correctly identified by 36.4% and 18.2% of the study participants respectively. Stomach pain, diarrhea and vomiting were considered the warning signs of paracetamol overdose as identified by the study participants (10.8%, 2.8%, 10.8%). Almost third of the participants (34.7%) knew that liver is the most affected organ with paracetamol overdose. Majority of the study participants (67%) didn’t know about possibility of death with paracetamol overdose (Table 1).
Table 1.
General Knowledge regarding paracetamol among the studied participants (n = 176).
| General Knowledge regarding paracetamol | Freq. (%) | ||
|---|---|---|---|
| Acetaminophen is different from paracetamol | Yes | 17(9.7) | |
| Noa | 19(10.8) | ||
| Don’t know | 140(79.5) | ||
| Importance of cautious administration of Paracetamol when used with other drugs | Another paracetamol drug | Yesa | 45(25.6) |
| No | 25(14.2) | ||
| Don’t know | 106(60.2) | ||
| Non-paracetamol drug | Yesa | 56(31.8) | |
| No | 27(15.3) | ||
| Don’t know | 93(52.8) | ||
| Possibility of toxicity with Paracetamol administration | Yesa | 64(36.4) | |
| No | 12(6.8) | ||
| Don’t know | 100(56.8) | ||
| Existence of warning signs of Paracetamol overdose | Yesa | 32(18.2) | |
| No | 15(8.5) | ||
| Don’t know | 129(73.3) | ||
| Warning signs of overdose(if present) | Stomach paina | 19(10.8) | |
| Diarrheaa | 5(2.8) | ||
| Vomitinga | 19(10.8) | ||
| Jaundice | 5(2.8) | ||
| Change of urine color | 9(5.1) | ||
| Chest pain | 3(1.7) | ||
| Most affected organ with Paracetamol overdose | No organ | 67(38) | |
| Livera | 61(34.7) | ||
| Stomach | 14(8) | ||
| Kidney | 33(18.8) | ||
| Nerves | 1(0.6) | ||
| Possibility of death with Paracetamol overdose | Yesa | 40(22.7) | |
| No | 18(10.2) | ||
| Don’t know | 118(67) | ||
Correct answers were written in bold and italic.
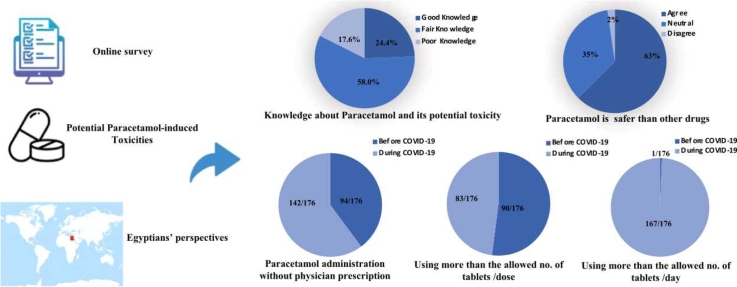
Less than half of the participants were knowledgeable about the maximum number of tablets (500 mg tablet) per dose (48.3%). However, less percentage of the participants were knowledgeable about the maximum number of tablets (500 mg tablet) per day (16.5%), and only 17% were knowledgeable about the minimum time space between doses. On a percent scale, the mean overall knowledge score was 36 ± 15. The overall percent-score revealed that 24.4% of participants had good knowledge, 58% had fair knowledge, and 17.6% had poor knowledge (Table 2).
Table 2.
Knowledge regarding Paracetamol instructions of administration and total Knowledge % Score among the studied participants (n = 176).
| General Knowledge regarding Paracetamol instructions of administration | Freq. (%) | |
|---|---|---|
| Maximum no. of tablets (500 mg tablet) per dosea | Don’t know | 44(25) |
| Right no.b | 85(48.3) | |
| More than the allowed no. | 35(19.9) | |
| Less than the allowed no. | 12(6.8) | |
| Minimum time space between doses (hours)c | Don’t know | 52(29.5) |
| Right time spaceb | 30(17) | |
| More than the allowed time space | 90(51.1) | |
| Less than the allowed time space | 4(2.3) | |
| Maximum no. of tablets (500 mg tablet) per dayd | Don’t know | 37(21) |
| Right no.b | 29(16.5) | |
| More than the allowed no. | 5(2.8) | |
| Less than the allowed no. | 105(59.7) | |
| Knowledge (%) Score | ||
| Total knowledge score | Mean± SD | 36 ± 15 |
| Median (min-max) | 31.2(18.7–81.2) | |
|
Total Knowledge Level Freq. (%) |
Poor | 31(17.6) |
| Fair | 102(58) | |
| Good | 43(24.4) | |
Maximum number of tablets (500 mg tablet) per dose equivalent to 2 tablets.
Correct answers were written in bold and italic.
Minimum time space between doses (hours) equivalent to 4 hrs.
Maximum number of tablets (500 mg tablet) per day equivalent to 8 tablets.
3.3. Participants' attitude regarding paracetamol
About two-thirds of the participants considered paracetamol safer than other medications of the same indications (62.5%). Almost one-third of the participants considered that paracetamol administration is safe without prescription for pregnant, elderly and alcoholics (30.7%, 39.8%, 30.7%), but less percentage considered that for Children (17%). 42.6% could advise others to use paracetamol without prescription (Table 3).
Table 3.
General attitude regarding paracetamol among the studied participants (n = 176).
| General attitude regarding paracetamol | Agree | Neutral | Disagree | |
|---|---|---|---|---|
| Paracetamol is safer than other medications of same indications | 110 (62.5%) | 62(35.2%) | 4(2.3%) | |
| Paracetamol administration is safe without prescription for | Children | 30(17%) | 51(29%) | 95(54%) |
| Pregnant | 54(30.7%) | 53(30.1%) | 69(39.2%) | |
| Advising others to use Paracetamol without prescription | 75(42.6%) | 25(14.2%) | 76(43.2%) | |
3.4. Comparison of participants’ practice regarding paracetamol administration before and during COVID-19 pandemic
Participants’ practice of paracetamol administration without physician prescription was more during the pandemic (P-value <0.05). For those participants who were using paracetamol with physician prescription, communication with the physician for prescription through medical facilities (primary health care (PHC), clinic or hospital) was less during the pandemic (P-value <0.05). Physician instructions included mainly the dose, times/day, and duration, on the other hand, they were less concerned to give instructions about side effects or possibility of overdosage. Commitment to physician instructions was less during the pandemic (P-value <0.05). Practice of paracetamol administration more than the allowed number of tablets/dose and more than the minimum allowed time space between doses was less evident during the pandemic when compared to before the pandemic (P-value <0.05). However, practice of paracetamol administration more than the allowed number of tablets/day was more evident during the pandemic than before the pandemic (P-value <0.05) (Table 4).
Table 4.
Comparison of practice of paracetamol administration before and during COVID-19 pandemic among the studied participants (n = 176).
| Practice of paracetamol administrationFreq. (%) | Before | During | P-value | |
|---|---|---|---|---|
| Physician prescription | With | 82(46.6) | 34(19.3) | 0.001* |
| Without | 94(53.4) | 142(80.7) | ||
| Method of communication with the physician for prescription | PHC, clinic, hospital | 65(79.3) | 15(44.1) | 0.001* |
| Telephone | 13(15.9) | 14(41.2) | ||
| Social media | 4(4.8) | 5(14.7) | ||
| Physician instructions with prescription | Dose | 73(89) | 30(88.2) | 0.001* |
| Times/day | 66(80.5) | 31(91.2) | 0.001* | |
| Duration | 47(57.3) | 16(47) | 0.001* | |
| Side effects | 7(8.5) | 3(8.8) | 1 | |
| Other as possibility of toxicity | 6(7.3) | 5(14.7) | 0.764 | |
| Commitment to Physician instructions with prescription | Yes | 66(80.5) | 27(79.4) | 0.001* |
| No | 16(19.5) | 7(20.6) | ||
| Maximum no. of tablets per dosea | Within or less than the allowed no. | 86(48.9) | 93(52.8) | 0.001* |
| More than the allowed no. | 90(51.1) | 83(47.2) | ||
| Minimum time space between doses (hours)b | Within or less than the allowed time | 103(58.5) | 155(88.1) | 0.001* |
| More than the allowed time | 73(41.5) | 21(11.9) | ||
| Maximum. no. of tablets per dayc | Within or less than the allowed no. | 175(99.4) | 9(5.1) | 0.001* |
| More than the allowed no. | 1(0.6) | 167(94.9) | ||
*P-value is statistically significant.
Maximum number of tablets (500 mg tablet) per dose equivalent to 2 tablets.
Minimum time space between doses (hours) equivalent to 4 hrs.
Maximum number of tablets (500 mg tablet) per day equivalent to 8 tablets.
3.5. Association between participants' knowledge level and their demographic characteristics and practices of paracetamol administration
Participants' overall knowledge was not significantly associated with their age, gender, education, residence, and the socioeconomic status (Table 5).
Table 5.
The relation between participants’ demographic characteristics and their knowledge (N = 176).
| Demographic characteristics |
Knowledge Level |
P-value | |||
|---|---|---|---|---|---|
| Poor | Fair | Good | |||
| Age | 18- | 4(12.9) | 23(22.5) | 9(20.9) | 0.579 |
| 30- | 25(80.6) | 71(69.6) | 33(76.7) | ||
| ≥ 50 | 2(6.5) | 8(7.8) | 1(2.3) | ||
| Gender | Male | 11(35.5) | 38(37.3) | 20(46.5) | 0.539 |
| Female | 20(64.5) | 64(62.7) | 23(53.5) | ||
| Education | High school | 1(6) | 6(5.9) | 2(4.7) | 0.27 |
| Bachelor | 21(67.7) | 74(72.5) | 32(74.4) | ||
| Post-graduate | 9(29) | 22(21.6) | 9(20.9) | ||
| Residence | Urban | 26(83.9) | 95(93.1) | 37(86) | 0.172 |
| Rural | 5(16.1) | 7(6.9) | 6(14) | ||
| Socioeconomic status | Low | 0(0) | 1(1) | 0(0) | 0.733 |
| Moderate | 25(80.6) | 89(87.3) | 37(86) | ||
| High | 6(19.4) | 12(11.8) | 6(14.3) | ||
3.6. Association between participants' knowledge level and their practices of paracetamol administration
The participants' overall knowledge was not significantly associated with their practices of paracetamol administration both before and during the pandemic (Table 6).
Table 6.
Association between participants’ practices of paracetamol administration before and after COVID-19 Pandemic and their knowledge (N = 176).
| Practice of paracetamol administration | Before COVID-19 Pandemic |
After COVID-19 Pandemic |
|||||||
|---|---|---|---|---|---|---|---|---|---|
|
Knowledge Level |
P-value |
Knowledge Level |
P-value | ||||||
| Poor | Fair | Good | Poor | Fair | Good | ||||
| Max. no. of tablets per dose | Within or less than the allowed no. | 29 (93.5) |
88 (86.3) |
38 (88.4) |
0.655 | 17 (54.8) |
46 (45.1) |
20 (46.5) |
0.651 |
| More than the allowed no. | 2 (6.5) |
14 (13.7) |
5 (11.6) |
14 (45.2) |
56 (54.9) |
23 (53.5) |
|||
| Time space between doses (hours) | Within or less than the allowed no. | 21 (67.7) |
45 (44.1) |
20 (46.5) |
0.069 | 1 (3.2) |
3 (2.9) |
5 (11.6) |
0.091 |
| More than the allowed no. | 10 (32.3) |
57 (55.9) |
23 (53.5) |
30 (96.8) |
99 (97.1) |
38 (88.4) |
|||
| Max. no. of tablets per day | Within or less than the allowed no. | 31 (100) |
101 (99) |
43 (100) |
1 | 13 (41.9) |
63 (61.8) |
27 (62.8) |
0.13 |
| More than the allowed no. | 0 (0) |
1 (1) |
0 (0) |
18 (58.1) |
39 (38.2) |
16 (37.2) |
|||
4. Discussion
Challenges encountered during COVID-19 pandemic shouldn't be exacerbated by inappropriate usage of drugs, which in turn leads to increased risk of drug-induced toxicities and shortage of drugs [37].
Our results showed that many of the participants were not knowledgeable about Acetaminophen as a synonym of paracetamol. This disagrees with Hornsby et al. [38] study at Alabama assessing patient knowledge about acetaminophen which showed that 46% knew that “acetaminophen” and “Tylenol” were synonym [38]. Thus, our result is considered an alarming sign as unaware patients of the alternative names of acetaminophen and the different over-the-counter and prescription medications containing paracetamol/acetaminophen are potentially at risk of developing paracetamol-induced toxicities [12], [15], [39], [40]. This explanation also agrees with our results that many of our participants were also not knowledgeable about the cautious administration of paracetamol with other drugs. Such results agree with Ejeikwu and Folashade (2019) study on undergraduate students at University of Jos, Plateau state, Nigeria about the risk perception of paracetamol use where only 39.5% considered intake of paracetamol with “Procold” harmful [41]. Such results indicate that patients aren't always interested in identifying the active ingredients of drugs. Accordingly, they become more subjected to accidental poisoning [42].
Many of the participants were not knowledgeable about the possibility of toxicity with paracetamol administration. These results agree with Salih et al. [43] who conducted an observational cross-sectional study about the knowledge, attitude, and practice of 440 female students at Jazan University on OTC oral analgesics. They concluded that only 12.3% were knowledgeable about the toxic dose and adverse effects of paracetamol [43]. Our results also agree with Ejeikwu and Folashade (2019) study who declared that 53.6% of the studied undergraduate students at the University of Jos, Plateau state, Nigeria do not consider paracetamol self-administration harmful [41]. Another study on 400 consumers at Malaysia showed that 69.1% consider paracetamol a safe drug [44]. This could be explained by the easy availability of paracetamol as OTC drug, thus, lay people underestimate its potentially-induced toxicities [45].
These considerations about paracetamol -as a safe medication and the decreased possibility of causing toxicity- disagree with existence of paracetamol toxicity cases in different settings; according to PPC-ASUH in Egypt, “paracetamol is one of the top ten most frequently involved exposure substances received in PCC-ASUH during 2019” [25], [26], Over a year (1 August 2008–31 July 2009), a survey was conducted based on acute poisoning admissions to investigate patterns in admissions and the initial management of hospital admissions for acute poisoning at Tygerberg Academic Hospital (TAH) and hospital-based poisoning consultations with the Tygerberg Poison Information Centre (TPIC) in Parow, Cape Town, South Africa. There were 662 TAH hospitalizations and 2459 hospital-based TPIC consults. The most prevalent exposures in both were paracetamol and cholinesterase inhibitors [46], during the one-year period (1 January to 31 December 2017), a retrospective analysis of poisoning calls received by the Ministry of Health Saudi Poisons Control Centers was conducted to determine the most prevalent toxins among Saudi Arabians. Paracetamol was the most often overdosed analgesic drug (57% of all analgesic poisoning) [47].
Many of the participants in our study were not knowledgeable about the existence of warning signs of overdose. This agrees with Chong et al. [44] study in which they concluded that 57.6% of their research participants were not aware of the signs of paracetamol overdosage [44]. This unrecognition of early signs of paracetamol could hinder the effective timely treatment. Accordingly, diverting the patients' attention towards the existence of warning signs could be helpful to prevent significant morbidity and mortality [12], [15], [48].
Almost third of our participants (34.7%) knew that liver is the most affected organ with paracetamol overdose. Majority of the study participants (67%) didn’t know about possibility of death with paracetamol overdose. These results agree with Boudjemai et al. [42] survey conducted in a French hospital on emergency patients where 38% thought that a paracetamol overdose couldn't cause death [42]. On the other hand, Hornsby et al. (2010) study concluded that one-half of their research participants recognized liver damage as the primary toxicity [38]. Ejeikwu and Folashade (2019) study results also showed that most of the studied undergraduate students at the University of Jos, Plateau state, Nigeria (60.8%) answered appropriately as to whether paracetamol overdose can cause acute liver failure [41]. Our results could be explained by the misbelief that paracetamol is harmless. Moreover, lay people may be uninterested in reading the drug's leaflet which declares the risk of liver damage in cases of overdosage[15], [41].
Less than half of the participants in our study were knowledgeable about the maximum number of tablets per dose, the maximum number of tablets per day, and the right time space between doses. These results disagree with Boudjemai et al. (2013) survey among emergency department patients in a French hospital where 30% knew the maximum daily dose to be 4 g and 48% considered the minimum time between two doses to be 4 h [42]. On the other hand, about 86% of the studied consumers in Chong et al. [44] study knew the proper paracetamol dosage in adults and 50% reported correctly the time space between doses [44]. The lack of such knowledge about paracetamol is an alarming sign as this may place patients at risk of toxicity by taking supratherapeutic doses [42].
The overall percent-score revealed that only 24.4% of participants had good knowledge about paracetamol and its related potential toxicities. These results coincide with different studies as the retrospective study of the medical records of 177 adult patients intoxicated with paracetamol at National University Hospital, Singapore which denoted that most of the unintentional overdoses was related to the lack of knowledge about the proper recommended doses. Patients received supratherapeutic doses to get the maximum therapeutic benefit of the drug [49]. Our results also agree with those of a cross-sectional study on a random sample of 237 Saudi people in Rafha and Riyadh where the participants showed poor knowledge, attitudes and practices about usage of analgesic [50]. Moreover, Dorji et al. [51] study among 441 out‑patients in a Bhutanese district hospital bordering India showed poor knowledge about safe use of paracetamol [51]. The poor knowledge on paracetamol safe usage and its related toxicities could result from the easy availability of paracetamol as an OTC drug, thus the public underestimate its potential toxicities [41].
Our studied participants considered that paracetamol administration isn't safe without prescription for pregnant, and children (39.2%, 54% respectively). For pregnant, our results disagree with those of Chong et al. (2020) who concluded that only 19.5% of the consumers were aware of the potential acetaminophen toxicity for pregnant in the form of fetal death and spontaneous abortion [44]. Accordingly, careful monitoring of paracetamol use during pregnancy is mandatory [20]. The results concerning children could be explained to some extent by the inability to understand the children dosage requirements properly [52]. Moreover, children are more liable to be subjected to potential paracetamol toxicities either by unsupervised single supratherapeutic dose or chronic exposure to excessive doses [15]. Accordingly, cautious administration of paracetamol by vulnerable groups should be considered[19].
Our study showed that participants’ practice of paracetamol administration without physician prescription was more during COVID-19 pandemic. This could be explained by considering symptomatic treatment at the start of treatment COVID-19 patients who suffer from fever or pain, including paracetamol and NSAIDs [53]. However, paracetamol is preferred due to the concerns raised on NSAIDs[35], [37]. Moreover, during COVID-19, there was an increased practice of online searching for information about medications. Accordingly, the practice of self-medication has also increased. Lay people encourage their relatives, neighbors, and friends to practice self-medication [54].
For our participants who were using paracetamol with physician prescription, communication with the physician for prescription through medical facilities was less during COVID-19 pandemic. This agrees with the situation of lockdowns occurring during COVID-19 pandemic and the implementation of telemedicine services during pandemics.
In our study, based on the participants' experiences, physicians were less concerned to give instructions about side effects or possibility of overdosage of paracetamol to the patients. This could be explained by that physicians may be under time pressure, thus may miss the opportunity of proper explanation of the safe usage of paracetamol and its potential toxicities [51]. Moreover, they assume that patients are fully knowledgeable about it [42]. Our findings disagrees with Tan and Sklar (2017) findings who suggest that healthcare providers should provide patients with all the needed information about paracetamol to lessen the possibilities of overdosage [49].
Practice of paracetamol administration more than the allowed number of tablets/day was significantly more evident during the pandemic. This could be explained by Piotrowska et al. [19] retrospective observational study of paracetamol-intoxicated patients presenting to Bern University Hospital Emergency Department, as 87% reported that they believed that increasing the doses of paracetamol could relieve the unrelieved pain. Such practice could be a major contribution to the increased rate of paracetamol-induced toxicities[19], [38].
In our study, participants' overall knowledge was not significantly associated with their age, gender, education, residence, and the socioeconomic status. This agrees with the results of a cross sectional survey among 1037 residents of Al Ahsa city- Saudi Arabia which showed that age, gender, and education do not show any association (>0.05) with questions concerned with paracetamol [55].
Moreover, our participants’ practices of paracetamol administration both before and during the pandemic were not significantly associated with the participants' overall knowledge. This result disagrees with Salih et al. (2019) who conducted an observational cross-sectional study on 440 female students in Jazan University to estimate their knowledge, attitude, and practice on OTC oral analgesics. Based on their findings, there was a strong association between the awareness level and usage of OTC analgesics [43]. Our result could be explained by that such risky practices are based on deep-seated firm beliefs that paracetamol is a harmless drug. Eliminating such risky behaviors isn't that easy. Accordingly, increasing the patients' awareness is one of the means but other means should be considered to achieve such a belief change.
We found that 42.6% of the participants could advise others to use paracetamol without prescription. Encouraging others to use paracetamol without considering the concerns regarding its potential toxicities is an alarming sign. That’s why, the suggestion of drug legalization to be prescription only or at least a pharmacy-only sale should be considered [45], [56], [57].
People should be educated to follow a "poison prevention" lifestyle by giving them knowledge and increasing their awareness of preventive concerns, empowering people to make their own well-informed decisions and taking responsibility for their own health. Potential health concerns must be informed to the general public, and policymakers at all levels. They need to be persuaded that issues exist and that proper measures are both doable and useful [58].
The present study shows some limitations. First, the use of a self-report questionnaire might have resulted in social desirability and recall bias. Second, we were more concerned with the potential risk of paracetamol-induced toxicity, therefore, we did not clarify the specific reasons for paracetamol consumption. We believed that whatever the cause of paracetamol intake, how participants administer it is an alarming sign in itself. Third, we used a convenience sampling approach, which resulted in a non-probability sample, limiting the generalizability of our findings. Although generalizability of our findings cannot be assured, major concerns on risk perception regarding paracetamol potential toxicities among Egyptians were revealed. Fourth, there were deficient similar studies in developing countries to compare with. Therefore, we recommend future studies to be conducted in developing countries to deeply explore this issue in order to implement culturally oriented strategies aiming at mitigating future paracetamol-induced toxicity in developing countries.
5. Conclusion
We concluded that the unsupervised use of paracetamol is an alarming sign that should be addressed as this could lead to a high rate of accidental paracetamol toxicity. A lesson learnt from COVID-19 pandemic is the need to implement behavior change measures to mitigate the risk of accidental paracetamol toxicity.
Declarations
Ethics approval and consent to participate.
The questionnaire and methodology for this study were approved by the institutional Research Ethics committee; Research Ethics committee at Faculty of Medicine Suez Canal University (Reference number: 4211). This study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments and those of CIOMS, 2019.
Informed consent was obtained from all individual participants included in the study. A detailed information sheet explaining the purpose of the research, potential benefits, risks, ensuring that participation is voluntary and that the participant has the right to refuse participation or to withdraw without any reasons and without any negative consequences was at the beginning of the online survey. Participants have to agree to the following statement “ Continuing with the the survey is considered giving your informed consent to participate in the study” before proceeding to the survey items. Completion of the online survey denoted participants’ consent to participate in the study.
Confidentiality was ensured by keeping the questionnaire anonymous and avoiding mentioning any identifying features of the participants.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Enas M. A. Mostafa, Ayat M. Tawfik, Khadiga M. Abd-Elrahman: Conceptualization, Writing − original draft preparation, Writing − review & editing, Visualization. Enas M. A. Mostafa, Khadiga M. Abd-Elrahman: Methodology, Investigation. Ayat M. Tawfik: Formal analysis. All authors read and approved the final manuscript.
Competing interests.
The authors declare that they have no competing interests.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.toxrep.2022.03.035.
Appendix A. Supplementary material
Supplementary material
.
Availability of data and material
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Kannan S., Shaik Syed Ali P., Sheeza A., Hemalatha K. COVID-19 (Novel Coronavirus 2019) - recent trends. Eur. Rev. Med. Pharmacol. Sci. 2020;24:2006–2011. doi: 10.26355/eurrev_202002_20378. [DOI] [PubMed] [Google Scholar]
- 2.Cui J., Li F., Shi Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu J., Zheng X., Tong Q., Li W., Wang B., Sutter K., Trilling M., Lu M., Dittmer U., Yang D. Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS‐CoV, MERS‐CoV, and 2019–nCoV. J. Med. Virol. 2020;92:491–494. doi: 10.1002/jmv.25709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lotfi M., Rezaei N. CRISPR/Cas13: A potential therapeutic option of COVID-19. Biomed. Pharmacother. 2020;131 doi: 10.1016/j.biopha.2020.110738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banerjee A., Mukherjee S., Maji B.K. Manipulation of genes could inhibit SARS-CoV-2 infection that causes COVID-19 pandemics. Exp. Biol. Med. 2021;246:1643–1649. doi: 10.1177/15353702211008106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banerjee A., Das D., Paul R., Roy S., Das U., Saha S., Dey S., Adhikary A., Mukherjee S., Maji B.K. Mechanistic study of attenuation of monosodium glutamate mixed high lipid diet induced systemic damage in rats by Coccinia grandis. Sci. Rep. 2020;10:15443. doi: 10.1038/s41598-020-72076-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banerjee A., Mukherjee S., Maji B.K. Worldwide flavor enhancer monosodium glutamate combined with high lipid diet provokes metabolic alterations and systemic anomalies: An overview. Toxicol. Rep. 2021;8:938–961. doi: 10.1016/j.toxrep.2021.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi Y., Wang G., Cai X., Deng J., Zheng L., Zhu H., Zheng M., Yang B., Chen Z. An overview of COVID-19. J. Zhejiang Univ. -Sci. B. 2020;21:343–360. doi: 10.1631/jzus.B2000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhai P., Ding Y., Wu X., Long J., Zhong Y., Li Y. The epidemiology, diagnosis and treatment of COVID-19. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lauder S.N., Taylor P.R., Clark S.R., Evans R.L., Hindley J.P., Smart K., Leach H., Kidd E.J., Broadley K.J., Jones S.A., Wise M.P., Godkin A.J., O’Donnell V., Gallimore A.M. Paracetamol reduces influenza-induced immunopathology in a mouse model of infection without compromising virus clearance or the generation of protective immunity. Thorax. 2011;66:368–374. doi: 10.1136/thx.2010.150318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jóźwiak-Bebenista M., Nowak J.Z. Paracetamol: Mechanism of action, applications and safety concern. Acta Pol Pharm. 2014;71:11–23. [PubMed] [Google Scholar]
- 12.Saccomano S.J. Acute acetaminophen toxicity in adults. Nurs. Crit. Care. 2019;14:10–17. doi: 10.1097/01.CCN.0000578816.14164.9f. [DOI] [PubMed] [Google Scholar]
- 13.Essawy A.E., Alkhuriji A.F., Soffar A.A. Paracetamol Overdose Induces Physiological and Pathological Aberrations in Rat Brain. J. Appl. Pharm. Sci. 2017;7:185–190. doi: 10.7324/JAPS.2017.70925. [DOI] [Google Scholar]
- 14.Moles A., Torres S., Baulies A., Garcia-Ruiz C., Fernandez-Checa J.C. Mitochondrial–Lysosomal Axis in Acetaminophen Hepatotoxicity. Front. Pharmacol. 2018;9:453. doi: 10.3389/fphar.2018.00453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tittarelli R., Pellegrini M., Scarpellini M.G., Marinelli E., Bruti V., Luca N.M.D., Busardò F.P., Zaami S. Hepatotoxicity of paracetamol and related fatalities. Eur. Rev. Med. Pharmacol. Sci. 2017;21:95–101. [PubMed] [Google Scholar]
- 16.S. Agrawal, B. Khazaeni, Acetaminophen Toxicity, StatPearls Publishing [Internet], 2018. [PubMed]
- 17.Heubi J.E., Barbacci M.B., Zimmerman H.J. Therapeutic misadventures with acetaminophen: Hepatoxicity after multiple doses in children. J. Pediatr. 1998;132:22–27. doi: 10.1016/s0022-3476(98)70479-2. https://doi.org/doi: 10.1016/s0022-3476(98)70479-2. [DOI] [PubMed] [Google Scholar]
- 18.Lancaster E.M., Hiatt J.R., Zarrinpar A. Acetaminophen hepatotoxicity: an updated review. Arch. Toxicol. 2015;89:193–199. doi: 10.1007/s00204-014-1432-2. [DOI] [PubMed] [Google Scholar]
- 19.Piotrowska N., Klukowska-Rötzler J., Lehmann B., Krummrey G., Haschke M., Exadaktylos A.K., Liakoni E. Presentations Related to Acute Paracetamol Intoxication in an Urban Emergency Department in Switzerland. Emerg. Med. Int. 2019;2019:1–7. doi: 10.1155/2019/3130843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rotundo L., Pyrsopoulos N. Liver injury induced by paracetamol and challenges associated with intentional and unintentional use. World J. Hepatol. 2020;12:125–136. doi: 10.4254/wjh.v12.i4.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoon E., Babar A., Choudhary M., Kutner M., Pyrsopoulos N. Acetaminophen-Induced Hepatotoxicity: a Comprehensive Update. J. Clin. Transl. Hepatol. 2016;4 doi: 10.14218/JCTH.2015.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Food and Drug Administration [FDA], 2015. 〈https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/204767s000lbl.pdf〉 (accessed January 2, 2021).
- 23.Food and Drug Administration [FDA], 2018. 〈https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-prescription-acetaminophen-products-be-limited-325-mg-dosage-unit〉 (accessed 2 January 2021).
- 24.Dart R.C., Erdman A.R., Olson K.R., Christianson G., Manoguerra A.S., Chyka P.A., Martin Caravati E., Wax P.M., Keyes D.C., Woolf A.D., Scharman E.J., Booze L.L., Troutman W.G. Acetaminophen poisoning: an evidence-based consensus guideline for out-of-hospital management. Clin. Toxicol. 2006;44:1–18. doi: 10.1080/15563650500394571. [DOI] [PubMed] [Google Scholar]
- 25.Tawfik H., Khalifa E. Evaluation of Poisoning and Drug Overdose among Cases Presented to Poison Control Centre, Ain Shams University Hospital during the Year 2015. Ain Shams J. Forensic Med. Clin. Toxicol. 2017;29:100–112. doi: 10.21608/ajfm.2017.41227. [DOI] [Google Scholar]
- 26.Abdelhamid W. Evaluation of Severity of Poisoning Exposures among Patients Presented to Poison Control Center, Ain Shams University Hospitals, Egypt during 2019. Ain Shams J. Forensic Med. Clin. Toxicol. 2021;36:106–122. doi: 10.21608/ajfm.2021.139281. [DOI] [Google Scholar]
- 27.Hegazy M., Elfiky A. Pattern of Acute Poisoning Cases Admitted to Menoufia Poisoning and Addiction Control Center: A Prospective Study. Ain Shams J. Forensic Med. Clin. Toxicol. 2016;26:35–43. doi: 10.21608/ajfm.2016.18536. [DOI] [Google Scholar]
- 28.Tan S.H.S., Hong C.C., Saha S., Murphy D., Hui J.H. Medications in COVID-19 patients: summarizing the current literature from an orthopaedic perspective. Int Orthop. 2020;44:1599–1603. doi: 10.1007/s00264-020-04643-5. https://doi.org/doi: 10.1007/s00264-020-04643-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Enners S., Gradl G., Kieble M., Böhm M., Laufs U., Schulz M. Utilization of drugs with reports on potential efficacy or harm on COVID ‐19 before, during, and after the first pandemic wave. Pharmacoepidemiol. Drug Saf. 2021;30:1493–1503. doi: 10.1002/pds.5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leal N.S., Yu Y., Chen Y., Fedele G., Martins L.M. Paracetamol is associated with a lower risk of COVID-19 infection and decreased ACE2 protein expression: a retrospective analysis. COVID. 2021:218–229. doi: 10.3390/covid1010018. [DOI] [Google Scholar]
- 31.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jia H.P., Look D.C., Shi L., Hickey M., Pewe L., Netland J., Farzan M., Wohlford-Lenane C., Perlman S., McCray P.B. ACE2 Receptor Expression and Severe Acute Respiratory Syndrome Coronavirus Infection Depend on Differentiation of Human Airway Epithelia. J. Virol. 2005;79:14614–14621. doi: 10.1128/JVI.79.23.14614-14621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ciaglia E., Vecchione C., Puca A.A. COVID-19 Infection and Circulating ACE2 Levels: Protective Role in Women and Children. Front. Pediatr. 2020;8:206. doi: 10.3389/fped.2020.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ni W., Yang X., Yang D., Bao J., Li R., Xiao Y., Hou C., Wang H., Liu J., Yang D., Xu Y., Cao Z., Gao Z. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit. Care. 2020;24:422. doi: 10.1186/s13054-020-03120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sestili P., Fimognari C. Paracetamol-Induced Glutathione Consumption: Is There a Link With Severe COVID-19 Illness? Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.579944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vitiello A., La Porta R., D’Aiuto V., Ferrara F. The risks of liver injury in COVID-19 patients and pharmacological management to reduce or prevent the damage induced. Egypt. Liver J. 2021;11:11. doi: 10.1186/s43066-021-00082-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberts D.M., Bennett A. COVID‑19 and the quality use of medicines: evidence, risks and fads. Aust Prescr. 2020;43:78–80. doi: 10.18773/austprescr.2020.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hornsby L.B., Whitley H.P., Kelly Hester E., Melissa T., Donaldson A. Survey of patient knowledge related to acetaminophen recognition, dosing, and toxicity. J. Am. Pharm. Assoc. 2010;50:485–489. doi: 10.1331/JAPhA.2010.08175. [DOI] [PubMed] [Google Scholar]
- 39.Hosary R.E., Wazzan V.S.E., Hassan E.S. Safety of Splitting Some Paracetamol Tablets in Egyptian Market for Children Administeration: A Quality Control Overview. J. Drug Res. Egypt. 2016;37 [Google Scholar]
- 40.Bari K., Fontana R.J. Acetaminophen overdose: What practitioners need to know: Acetaminophen Overdose. Clin. Liver Dis. 2014;4:17–21. doi: 10.1002/cld.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ejeikwu T.M., Folashade W. Risk Perception of Paracetamol Use among Undergraduate Students of University of Jos. OALib. 2019;06:1–10. doi: 10.4236/oalib.1105810. [DOI] [Google Scholar]
- 42.Boudjemai Y., Mbida P., Potinet-Pagliaroli V., Géffard F., Leboucher G., Brazier J.-L., Allenet B., Charpiat B. Patients’ knowledge about paracetamol (acetaminophen): A study in a French hospital emergency department. Ann. Pharm. Fr. 2013;71:260–267. doi: 10.1016/j.pharma.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 43.Salih S., Madkhali A.A., Al-Hazmi W.M., Al-Khaldy A.Y., Moafa T.A., Al-Gahtani E.Z., Al-Muhib M.O. Knowledge, attitude, and practices on over the counter oral analgesics among female students of Jazan University. Int. J. Med. Dev. Countries. 2019;3:311–315. [Google Scholar]
- 44.Chong C.P., Tan S.F., Chooi W.-T. Exploring consumers’ perceptions and knowledge of acetaminophen (paracetamol): a cross-sectional study from Penang, Malaysia. Int. J. Pharma Sci. Res. (IJPSR) 2020;11 [Google Scholar]
- 45.Ibrahim T., Agnihotri S., Agnihotri A.K. Paracetamol toxicity- an overview. Emergency Med. 2013;03 doi: 10.4172/2165-7548.1000158. [DOI] [Google Scholar]
- 46.Veale D.J.H., Wium C.A., Müller G.J. Toxicovigilance II: A survey of the spectrum of acute poisoning and current practices in the initial management of poisoning cases admitted to South African hospitals. S. Afr. Med. J. 2012;103:298. doi: 10.7196/SAMJ.6648. [DOI] [PubMed] [Google Scholar]
- 47.Al-Mousa F.A., Al-khayyal R.A., Gado A.M., Tammam H.G., Ragab A.R. 2017 Annual Report of Medical Toxicology Consultations/General Directorate of Poison Control Centres-Ministry of Health-Saudi Arabia. J. Clin. Toxicol. 2018;08 doi: 10.4172/2161-0495.1000391. [DOI] [Google Scholar]
- 48.Vidhya Malar H.L., Mary Mettilda Bai S. Beware of paracetamol toxicity. J. Clin. Toxicol. 2012;02 doi: 10.4172/2161-0495.1000142. [DOI] [Google Scholar]
- 49.Tan C., Sklar G. Characterisation and outcomes of adult patients with paracetamol overdose presenting to a tertiary hospital in Singapore. Singapore Med. J. 2017;58:695–702. doi: 10.11622/smedj.2016170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raja M.A.G., Al-Shammari S.S., Al-Otaibi N., Amjad M.W. Public attitude and perception about analgesic and their side effects. J. Pharm. Res. Int. 2020:35–52. doi: 10.9734/jpri/2020/v32i330412. [DOI] [Google Scholar]
- 51.Dorji T., Gyeltshen K., Pongpirul K. Rational use of paracetamol among out-patients in a Bhutanese district hospital bordering India: a cross-sectional study. BMC Res. Notes. 2018;11:660. doi: 10.1186/s13104-018-3764-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bennin F., Rother H.-A. “But it’s just paracetamol”: Caregivers’ ability to administer over-the-counter painkillers to children with the information provided. Patient Educ. Couns. 2015;98:331–337. doi: 10.1016/j.pec.2014.11.025. [DOI] [PubMed] [Google Scholar]
- 53.Tuccori M., Convertino I., Ferraro S., Cappello E., Valdiserra G., Focosi D., Blandizzi C. The Impact of the COVID-19 “Infodemic” on Drug-Utilization Behaviors: Implications for Pharmacovigilance. Drug Saf. 2020;43:699–709. doi: 10.1007/s40264-020-00965-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Malik M., Tahir M.J., Jabbar R., Ahmed A., Hussain R. Self-medication during Covid-19 pandemic: challenges and opportunities. Drugs Ther. Perspect. 2020;36:565–567. doi: 10.1007/s40267-020-00785-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ali S.I., Khawaja R., Khan A., Almulhim A.M. Perception towards appropriate use and adverse effects of paracetamol among the residents of Al Ahsa. Int. J. Healthcare Sci. 2015;3:307–310. [Google Scholar]
- 56.Sheen C.L., Dillon G.F., Bateman D.N., Simpson K.J., Macdonald T.M. Paracetamol toxicity: epidemiology, prevention and costs to the health-care system. Q. J. Med. 2002;95:609–619. doi: 10.1093/qjmed/95.9.609. [DOI] [PubMed] [Google Scholar]
- 57.Ghaffar U.B., Tadvi N.A. Paracetamol toxicity: a review. J. Contemp. Med. Dent. 2014;2:12–15. doi: 10.18049/jcmad/232. [DOI] [Google Scholar]
- 58.World Health Organization in collaboration with the United Nations Environment Programme and the International Labour Organization, Guidelines On the Prevention of Toxic Exposures: Education and public awareness activities/ International Programme on Chemical Safety., (2004).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.