Abstract
Background:
Early palliative care consultation to discuss goals-of-care (“PCC”) benefits seriously ill patients. Risk factor profiles associated with the timing of conversations in hospitals, where late conversations most likely occur, are needed.
Objective:
To identify risk factor patient profiles associated with PCC timing before death.
Methods:
Secondary analysis of observational study was conducted at an urban, academic medical center. Patients age 18 years and older admitted to the medical center, who had PCC, and died July 1, 2014 - October 31, 2016 were included. Patients admitted for childbirth or rehabilitation, and patients whose date of death was unknown were excluded. Classification and Regression Tree modeling was employed using demographic and clinical variables.
Results:
Of 1,141 patients, 54% had PCC “close to death” (0–14 days before death); 26% had PCC 15–60 days before death; 21% had PCC >60 days before death (median 13 days before death). Variables associated with receiving PCC close to death included being Hispanic or “Other” race/ethnicity intensive care patients with extreme illness severity (85%), with age <46 or >75 increasing this probability (98%). Intensive care patients with extreme illness severity were also likely to receive PCC close to death (64%) as were 50% of intensive care patients with less than extreme illness severity.
Conclusions:
A majority of patients received PCC close to death. A complex set of variable interactions were associated with PCC timing. A systematic process for engaging patients with PCC earlier in the care continuum, and in intensive care regardless of illness severity, is needed.
Keywords: palliative care, end of life, goals of care, communication, racial disparities, intensive care, timing before death, terminal care
Introduction
As medicine advances and providers are increasingly able to alter the normal dying process with life-extending treatments, patients with serious illness and their families are tasked with making difficult decisions about end-of-life (EOL) care.1 Although less aggressive EOL care is often preferred2–4 and is associated with higher quality of EOL,5–10 evidence suggests almost 20% of critical care patients receive futile treatment (as perceived by their physicians)11 and up to 38% of patients receive non-beneficial treatments near EOL.12 To help providers better understand patient preferences and enable patients to make informed decisions, the National Academy of Medicine recommends healthcare providers engage seriously ill patients in goals-of-care discussions.13 Goals-of-care discussions, including those that occur during palliative care consultation, are associated with less aggressive treatments and lower use of intensive care,6,13–17 lower 30-day readmission rates and hospitalizations,6,13,15,16,18 fewer in-hospital deaths and greater hospice use,6,14–16,19,20 more goal-concordant patient care,6,17,19,21,22 and higher quality EOL care.6,23 Goals-of-care discussions are also associated with lower EOL costs,15,24–29 with one recent study finding an average savings of over $6000 per patient.15
The timing of these conversations matters.30–34 Earlier care planning conversations are associated with higher quality outcomes6,23,35,36 including earlier enrollment in hospice and palliative care,37 whereas discussions closer to death are associated with increased risk of aggressive care, hospital death, and intensive care unit (ICU) admission.6 Each additional day from hospital admission to care planning conversation is associated with a 4% increased risk of aggressive interventions and in-hospital death and 19% greater odds of ICU admission.6 Despite the benefits of having early goals-of-care discussions in hospital settings where late discussions are likely to occur, patient risk factor profiles associated with the timing of these discussions are unknown. The purpose of this study was to identify risk factor profiles associated with patients receiving palliative care consultations to discuss goals-of-care (hereafter called “PCC”) 0–14 days before death (“PCC close to death”), 15–60 days before death (“moderately-timed PCC”), and more than 60 days before death (“early PCC”).
Methods
Study Design
We conducted a secondary analysis of a retrospective cohort study of patients admitted to the Hospital of the University of Pennsylvania, a 776-bed, urban, academic medical center15 that serves a socioeconomically and racially diverse patient area composed of 46% African Americans, 36% Whites, 9% Asians, and 6% Hispanics.38 Its interdisciplinary palliative care team is well-established and predominantly operates as a consultation service. Two-thirds of the team’s consultations involve goals-of-care discussions.15 The parent study (N=41,363) found PCC was associated with significant reductions in future acute care utilization and costs among a propensity-matched cohort of patients with serious illness, but did not explore PCC timing.15 For our study, supplementary data from the center’s electronic records (Medicaid status, days between PCC and patient death) was matched to unique patient identifiers.
Study Sample
Our study included patients 18 and over who were admitted July 1, 2014-October 31, 2016 (study period), received PCC specifically to discuss goals-of-care, and died during the study period. Consistent with palliative care experts’ broad definitions of serious illness,39,40 our study defined serious illness as any condition that carries an elevated risk of mortality or has burdensome symptoms or treatments requiring hospitalization.39 All patients in our sample were hospitalized for acute medical conditions and died within a 2.25 year window, reflecting the serious nature of their illness. Patients were included if they died in the hospital system while on hospice or not on hospice, died outside the hospital while on hospice affiliated with the medical center, or died outside the hospital system but had been seen by a provider affiliated with the medical center who updated the patient’s medical record to reflect their death. Patients who were admitted for childbirth or rehabilitation were excluded, as were patients who died in community hospitals whose deaths were not updated in the medical center’s records.
Traditional statistical power analyses do not apply to CART modeling,41,42 but our sample was sufficient because it was greater than the recommended 100 participant minimum.42 This study was approved by the University of Pennsylvania institutional review board (45 CFR 46.104) and followed strict procedures to ensure patient data privacy, security, and ethics. All data were deidentified before sharing, password protected, and stored on an encrypted network.
Study Measures
The primary outcome of interest was number of days between a patient’s first inpatient PCC during the study period and patient death, categorized into three non-overlapping levels of data: PCC close to death, defined as 0–14 days before death; moderately-timed PCC, defined as 15–60 days before death; and early PCC defined as more than 60 days before death. Timing categories were based on Medicare hospice data, which shows 41% of patients on hospice received 0–14 days of care (28% received seven or fewer days of care, presumably in the last week of life), 26% received 15–60 days of care, and 33% received more than 60 days of hospice care.37 Although the literature suggests early palliative care referral may be defined as more than 90 days before death,33 consultations occurring in that timeframe predominantly occur in outpatient settings.33 Because this study focuses on goals-of-care conversations occurring during inpatient hospitalization, the time parameters are shorter and were defined with other inpatient goals-of-care studies in mind.14,43
Correlates used to identify risk factor profiles included sociodemographic and clinical characteristics of age, gender, self-reported race/ethnicity, Medicaid status, primary diagnosis, All-patient refined diagnosis-related group (APR-DRG) Severity of Illness (the extent of physiologic decomposition), APR-DRG Risk of Mortality (the likelihood a patient will die), use of intensive care, intensive care greater than six says (to indicate high acuity), visitation by Oncology team, source of referral to palliative care, and acute care utilization in the prior 30 days. All variables were recorded at the time of index hospitalization, the hospitalization when PCC first occurred. APR-DRG Severity of Illness and Risk of Mortality have four subcategories (minor, moderate, major, extreme) that are assigned by health system software based on diagnoses and procedures coded during hospitalization, and take into account comorbidities, disease stage, and disease interactions.15,44,45 End-of-life acute care costs (direct acute care costs incurred in the health system during and following index hospitalization to death), discharge to hospice, DNR documentation, and changes in goals-of-care are also described.
Data Analysis
Descriptive statistics and measures of central tendency were used to characterize the variables and describe the sample based on PCC timing before death (SAS v. 9.4) (Table 1). Chi-squared tests were performed to examine associations between categorical variables. Kruskal-Wallis tests and analysis of variance (ANOVA) tests were performed for the distributions of continuous measures by PCC timing, as appropriate. Specialist referral data was missing for one patient. This patient was included in the study. No other data were missing.
Table 1.
Description of Study Population: Patients Who Received Palliative Care Consultation to Discuss Goals-of-Care (PCC) Before Death, by Number of Days Between PCC and Death
| Total | PCC 0–14 days before death | PCC 15–60 days before death | PCC > 60 days before death | P value | ||
|---|---|---|---|---|---|---|
| N = 1,141 | N = 612 (53.6%) | N = 292 (25.6%) | N = 237 (20.8%) | |||
| Median number of days between PCC and death (IQR) | 13.0 (4.0–46.0) | 5.0 (2.0–8.0) | 28.0 (20.0–39.5) | 135.0 (89.0–281.0) | <0.0001 | |
| Age (years) | 18–39 | 6.6% | 6.4% | 6.8% | 6.8% | 0.037 |
| 40–45 | 3.7% | 3.6% | 4.4% | 3.0% | ||
| 46–50 | 3.9% | 3.4% | 5.1% | 3.8% | ||
| 51–55 | 9.1% | 7.8% | 9.6% | 11.8% | ||
| 56–60 | 12.9% | 13.2% | 13.0% | 11.8% | ||
| 61–65 | 13.6% | 13.4% | 14.4% | 13.1% | ||
| 66–70 | 16.1% | 15.0% | 20.2% | 13.9% | ||
| 71–75 | 13.1% | 12.6% | 14.7% | 12.2% | ||
| >75 | 21.0% | 24.5% | 11.6% | 23.6% | ||
| Gender | Male | 46.6% | 55.2% | 51.0% | 51.5% | 0.40 |
| Female | 53.4% | 44.8% | 49.0% | 48.5% | ||
| Self-identified Race/Ethnicity | African American/Black | 25.0% | 22.4% | 28.4% | 27.4% | 0.02 |
| Asian | 3.8% | 3.3% | 3.8% | 5.1% | ||
| Hispanic | 2.2% | 2.1% | 2.1% | 2.5% | ||
| White | 59.3% | 60.0% | 59.6% | 57.4% | ||
| Other | 3.2% | 3.1% | 2.7% | 4.2% | ||
| Unknown/Chose not to disclose | 6.5% | 9.2% | 3.4% | 3.4% | ||
| Medicaid | Yes | 10.3% | 9.3% | 10.3% | 13.1% | 0.27 |
| No | 89.7% | 90.7% | 89.7% | 86.9% | ||
| Primary Diagnosis | Cancer | 33.2% | 31.2% | 37.0% | 33.8% | 0.006 |
| Cardiovascular disorder / Heart failure | 20.3% | 22.9% | 16.8% | 18.1% | ||
| Endocrine disorder | 1.9% | 2.0% | 2.7% | 0.8% | ||
| GI disorder | 7.1% | 5.2% | 9.2% | 9.3% | ||
| Gynecologic or urologic disorder | 2.8% | 2.8% | 3.8% | 1.7% | ||
| Infectious disease and Sepsis | 14.0% | 15.4% | 11.6% | 13.5% | ||
| Neurologic disorder | 6.9% | 8.3% | 6.5% | 3.8% | ||
| Respiratory disorder | 7.1% | 7.0% | 6.5% | 8.0% | ||
| Other | 6.6% | 5.2% | 5.8% | 11.0% | ||
| APR-DRG Severity of Illness | Minor | 1.6% | 1.1% | 1.7% | 2.5% | <0.0001 |
| Moderate | 5.5% | 3.6% | 6.2% | 9.7% | ||
| Major | 28.1% | 18.6% | 34.6% | 44.7% | ||
| Extreme | 64.8% | 76.6% | 57.5% | 43.0% | ||
| APR-DRG Risk of Mortality | Minor | 3.0% | 1.8% | 4.5% | 4.2% | <0.0001 |
| Moderate | 8.8% | 4.1% | 11.3% | 17.7% | ||
| Major | 29.8% | 22.7% | 34.2% | 42.6% | ||
| Extreme | 58.5% | 71.4% | 50.0% | 35.4% | ||
| Acute care hospitalization 30 days prior to index hospitalization | Yes | 23.0% | 21.6% | 27.4% | 21.5% | 0.124 |
| No | 77.0% | 78.4% | 72.6% | 78.5% | ||
| ICU care during index hospitalization | Yes | 67.0% | 77.6% | 55.1% | 54.4% | <0.0001 |
| No | 33.0% | 22.4% | 44.9% | 45.6% | ||
| ICU care > 6 days during index hospitalization | Yes | 40.3% | 46.1% | 37.3% | 29.1% | <0.0001 |
| No | 59.7% | 53.9% | 62.7% | 70.9% | ||
| Specialist who referred patient to Palliative Care for PCC (one patient had a missing value) | Cardiology | 10.8% | 10.5% | 13.1% | 8.9% | 0.35 |
| Gynecology/Oncology | 3.9% | 4.4% | 4.1% | 2.1% | ||
| General Medicine/Hospitalist | 13.3% | 13.6% | 14.8% | 11.0% | ||
| Neurology | 7.3% | 6.2% | 7.6% | 9.7% | ||
| Oncology | 34.6% | 34.0% | 31.3% | 40.5% | ||
| Pulmonary | 9.8% | 10.1% | 10.3% | 8.4% | ||
| Surgery | 17.9% | 19.1% | 16.8% | 16.0% | ||
| Other | 2.4% | 2.1% | 2.1% | 3.4% | ||
| Visited by Oncology during index hospitalization | Yes | 30.0% | 27.3% | 36.0% | 29.5% | 0.03 |
| No | 70% | 72.7% | 64% | 70.5% | ||
| Median days spent hospitalized during index hospitalization (IQR) | 11.0 (6–22) | 9.0 (5–17) | 16.0 (7–27.5) | 11.0 (7–24) | <0.0001 | |
| Median ICU days during index hospitalization (IQR) | 3.0 (0–11) | 5.0 (1–11) | 1.5 (0–13) | 1.0 (0–7) | <0.0001 | |
| Median direct acute care costs during index hospitalization (IQR) | $26,005 ($12,908-$59,889) | $28,330 ($13,635-$58,476) | $27,542 ($11,572-$75,175) | $21,397 ($12,357-$47,064) | 0.18 | |
| Median EOL direct acute care costs (index hospitalization plus future acute care costs in health system until death) (IQR) | $31,082 ($14,880-$67,035) | $28,333 ($14,200-$58,476) | $34,113 ($14,484-$77,510) | $37,326 ($18,461-$89,057) | 0.0001 | |
| Changed goals-of-care during PCC index hospitalization (% yes) | 79.7% | 93.0% | 75.7% | 50.2% | <0.0001 | |
| DNR documented during index hospitalization | Yes | 66.9% | 86.3% | 59.2% | 26.2% | <0.0001 |
| No | 33.1% | 13.7% | 40.8% | 73.8% | ||
| Discharged to hospice1 | Yes | 27.1% | 31% | 32.5% | 10.1% | <0.0001 |
| No | 72.9% | 69% | 67.5% | 89.9% | ||
From index hospitalization including PCC. Chi-squared test of association was used to test differences among percentage variables. Kruskal-Wallis tests and analysis of variance (ANOVA) were performed for the distributions of continuous measures by PCC timing, as appropriate.
To show how independent variables, or risk factors, interact to create associations with the outcome of PCC timing,46 classification and regression tree (CART) modeling using SAS JMP software was applied with variables of interest. This novel method is increasingly used in health research.47–49 The software randomly divided the dataset into training (N = 571), validation (N = 285), and test (N = 285) sets. Due to random division, these sets were similar and did not significantly differ across variables (Table 2). In the first step, training the decision tree, CART software recursively divided the training data one variable at a time to generate a series of splits that best identified the probability of PCC timing.50 The software chooses where to split variables based on where the division most accurately homogenizes the outcome variable while minimizing the model’s misclassification rate, a process that results in subgroups more homogenous in the outcome of PCC timing than the original sample.46,51 The software provides a tree for the training set but does not provide separate trees for the test set or the validation set. In the second step, the software used the validation set to identify the tree that optimally balanced complexity and prediction accuracy, evidenced by the highest generalized R-squared value. Because Risk of Mortality increases as patient death nears and is a known correlate of PCC timing, it dominated the most optimal model, leading us to exclude it from the model.52 After rerunning both steps without this variable, we then selected a tree with a prediction accuracy similar to the most optimal tree and clinically meaningful patient profiles that could help us achieve our primary aim of describing patients most likely to receive PCC close to death. Finally, we used the test set to further evaluate performance of the decision tree. Additional goodness of fit testing does not apply to CART modeling. The prediction accuracy rate was 58.8% for the training set, 54.4% for the validation set, and 54.7% for the test set. For descriptive purposes, EOL costs defined as all direct acute care costs incurred during index hospitalization to time of death, as coded for billing, were also assessed.
Table 2.
Comparison of Patients Randomly Assigned to Classification and Regression Tree (CART) Training, Validation, and Test Sets (Patients Who Received Palliative Care Consultation to Discuss Goals-of-Care Before Death)
| Total | Patients in CART Training Set | Patients in CART Validation Set | Patients in CART Test Set | P value | ||
|---|---|---|---|---|---|---|
| N = 1,141 | N = 571 (50%) | N = 285 (25%) | N = 285 (25%) | |||
| Age (years) | 18–39 | 6.6% | 6.1% | 8.8% | 5.3% | 0.263 |
| 40–45 | 3.7% | 4.6% | 2.8% | 2.8% | ||
| 46–50 | 3.9% | 4.0% | 5.3% | 2.5% | ||
| 51–55 | 9.1% | 9.1% | 9.1% | 9.1% | ||
| 56–60 | 12.9% | 12.6% | 11.9% | 14.4% | ||
| 61–65 | 13.6% | 14.0% | 13.3% | 13.0% | ||
| 66–70 | 16.1% | 13.7% | 17.9% | 19.3% | ||
| 71–75 | 13.1% | 13.0% | 14.7% | 11.6% | ||
| >75 | 21.0% | 22.9% | 16.1% | 22.1% | ||
| Gender | Male | 46.6% | 44.8% | 47.7% | 49.1% | 0.452 |
| Female | 53.4% | 55.2% | 52.3% | 50.9% | ||
| Self-identified Race/Ethnicity | African American/Black | 25.0% | 24.2% | 27.4% | 24.2% | 0.497 |
| Asian | 3.8% | 4.2% | 2.1% | 4.6% | ||
| Hispanic | 2.2% | 2.3% | 2.5% | 1.8% | ||
| White | 59.3% | 60.6% | 57.5% | 58.6% | ||
| Other | 3.2% | 2.5% | 3.2% | 4.9% | ||
| Unknown/Chose not to disclose | 6.5% | 6.3% | 7.4% | 6.0% | ||
| Medicaid | Yes | 10.3% | 11.0% | 10.5% | 8.8% | 0.588 |
| No | 89.7% | 89.0% | 89.5% | 91.2% | ||
| Primary Diagnosis | Cancer | 33.2% | 31.9% | 32.6% | 36.5% | 0.382 |
| Cardiovascular disorder / Heart failure | 20.3% | 22.2% | 18.9% | 17.9% | ||
| Endocrine disorder | 1.9% | 2.1% | 2.1% | 1.4% | ||
| GI disorder | 7.1% | 6.3% | 6.3% | 9.5% | ||
| Gynecologic or urologic disorder | 2.8% | 2.5% | 2.5% | 3.9% | ||
| Infectious disease and Sepsis | 14.0% | 14.0% | 17.5% | 10.5% | ||
| Neurologic disorder | 6.9% | 6.8% | 7.0% | 7.0% | ||
| Respiratory disorder | 7.1% | 6.8% | 7.4% | 7.4% | ||
| Other | 6.6% | 7.4% | 5.6% | 6.0% | ||
| APR-DRG Severity of Illness | Minor | 1.6% | 1.2% | 1.1% | 2.8% | 0.42 |
| Moderate | 5.5% | 6.1% | 3.9% | 6.0% | ||
| Major | 28.1% | 28.2% | 29.5% | 26.7% | ||
| Extreme | 64.8% | 64.4% | 65.6% | 64.6% | ||
| APR-DRG Risk of Mortality | Minor | 3.0% | 3.3% | 1.8% | 3.5% | 0.238 |
| Moderate | 8.8% | 9.3% | 6.3% | 10.2% | ||
| Major | 29.8% | 28.0% | 34.7% | 28.4% | ||
| Extreme | 58.5% | 59.4% | 57.2% | 57.9% | ||
| Acute care hospitalization 30 days prior to index hospitalization | Yes | 23.0% | 24.7% | 22.8% | 20.0% | 0.305 |
| No | 77.0% | 75.3% | 77.2% | 80.0% | ||
| ICU care during index hospitalization | Yes | 67.0% | 67.4% | 66.0% | 67.4% | 0.904 |
| No | 33.0% | 32.6% | 34.0% | 32.6% | ||
| ICU care > 6 days during index | Yes | 40.3% | 41.0% | 39.3% | 40.0% | 0.887 |
| hospitalization | No | 59.7% | 59.0% | 60.7% | 60.0% | |
| Specialist who referred patient to Palliative Care for PCC (one patient had a missing value) | Cardiology | 10.8% | 11.4% | 10.9% | 9.5% | 0.336 |
| Gynecology/Oncology | 3.9% | 3.9% | 2.8% | 4.9% | ||
| General Medicine/Hospitalist | 13.3% | 11.9% | 17.6% | 11.9% | ||
| Neurology | 7.3% | 7.2% | 6.3% | 8.4% | ||
| Oncology | 34.6% | 35.2% | 35.9% | 32.3% | ||
| Pulmonary | 9.8% | 9.8% | 8.8% | 10.2% | ||
| Surgery | 17.9% | 18.7% | 13.7% | 20.4% | ||
| Other | 2.4% | 3.0% | 2.5% | 1.1% | ||
| Visited by Oncology during index hospitalization | Yes | 30.0% | 28.7% | 32.3% | 30.2% | 0.561 |
| No | 70% | 71.3% | 67.7% | 69.8% | ||
From index hospitalization including PCC.
Chi-squared test of association was used to test differences among percentage variables.
Results
The sample included 1,141 patients who received PCC before dying during the study period (Table 1). Over half (54%) received PCC within 14 days of dying (n=612), 26% received PCC 15–60 days before death (n=292), and only 21% received PCC more than 60 days before death (n=237). Patients received PCC a median 13 days before death. Of the 40.6% of patients who died during index admission, 89.4% changed their goals-of-care during or following PCC.
Similar to the predictive error in the test data (53.7%), CART demonstrated 54.7% accuracy identifying the timing outcome (CI 48.8%, 60.6%) (Table 3), likely because the model only accurately identified patients who received PCC close to death (sensitivity, 88.2%). Although the model did not effectively identify patients who had early PCC or moderately-timed PCC (sensitivity 1.7% and 27.4%, respectively), it effectively identified which patients did not receive early or moderately-timed PCC (specificity 99.1% and 82.1%, respectively), which is a clinically important population. Unmeasured variables and smaller sample sizes for early and moderately-timed PCC groups may have contributed to these findings.
Table 3.
Classification and Regression Tree (CART) model performance for identifying profiles associated with the timing of palliative care consultation for goals-of-care (PCC) before death.
| Sample | Statistic | Value |
|---|---|---|
| PCC patients who died during the study period | Root node error | 0.537 |
| Accuracy (95% Confidence Interval) | 0.547 (0.488, 0.606) | |
| P-Value [Acc >NIR] | 0.384 | |
| Patients with PCC 0–14 days before death | Sensitivity | 0.882 |
| Specificity | 0.326 | |
| Positive predictive value | 0.603 | |
| Negative predictive value | 0.705 | |
| Patients with PCC 15–60 days before death | Sensitivity | 0.274 |
| Specificity | 0.821 | |
| Positive predictive value | 0.345 | |
| Negative predictive value | 0.767 | |
| Patients with PCC > 60 days before death | Sensitivity | 0.017 |
| Specificity | 0.991 | |
| Positive predictive value | 0.333 | |
| Negative predictive value | 0.794 |
The P-value represents the probability that model accuracy is higher than the no information rate (NIR). Sensitivity represents the proportion of patients correctly identified in the model as having had PCC in the associated timeframe. Specificity represents the proportion of patients that did not have PCC in the associated timeframe and were correctly identified in the model. Positive predictive value is the proportion of patients who actually received PCC in the associated timeframe out of all patients identified in the model as having received PCC in that timeframe. Negative predictive value is the proportion of patients who actually did not receive PCC in the associated timeframe out of all those identified in the model as having not received PCC in that timeframe.
Risk factor profiles associated with patients likely to receive PCC close to death are illustrated in Figure 1. There were multiple terminal risk factor profiles associated with high probability of PCC close to death (65–98% probability). African American and White ICU patients with extreme illness severity were more likely to have PCC close to death (66%). Intensive care patients with extreme illness severity who self-identified as Hispanic, “Other” or Unknown race/ethnicity were most likely to have PCC close to death (85%), a probability that increased to 98% among young and advanced-age patients (ages 18–45, 56–70, >75 years old). Patients with extreme illness severity who did not receive ICU care; had a primary diagnosis other than heart failure, respiratory disorder, or “Other” condition and were not hospitalized 30 days prior; and were young or advanced-age (<40 or >75 years old) had 96% probability of PCC close to death. On the other hand, patients with less than extreme illness severity who did not receive ICU care were least likely to receive PCC close to death (24%). Patients with extreme illness severity who did not receive ICU care, with a primary diagnosis other than heart failure, respiratory disorder, or “Other” condition, and were hospitalized 30 days prior were also less likely than average to have PCC close to death (43%). It is unknown if these findings represent a case of reverse causation or if other factors are involved. Finally, it is important to note that 67% of ICU patients with extreme illness severity and 50% of ICU patients with less than extreme illness severity received PCC within 14 days of dying.
Figure 1.
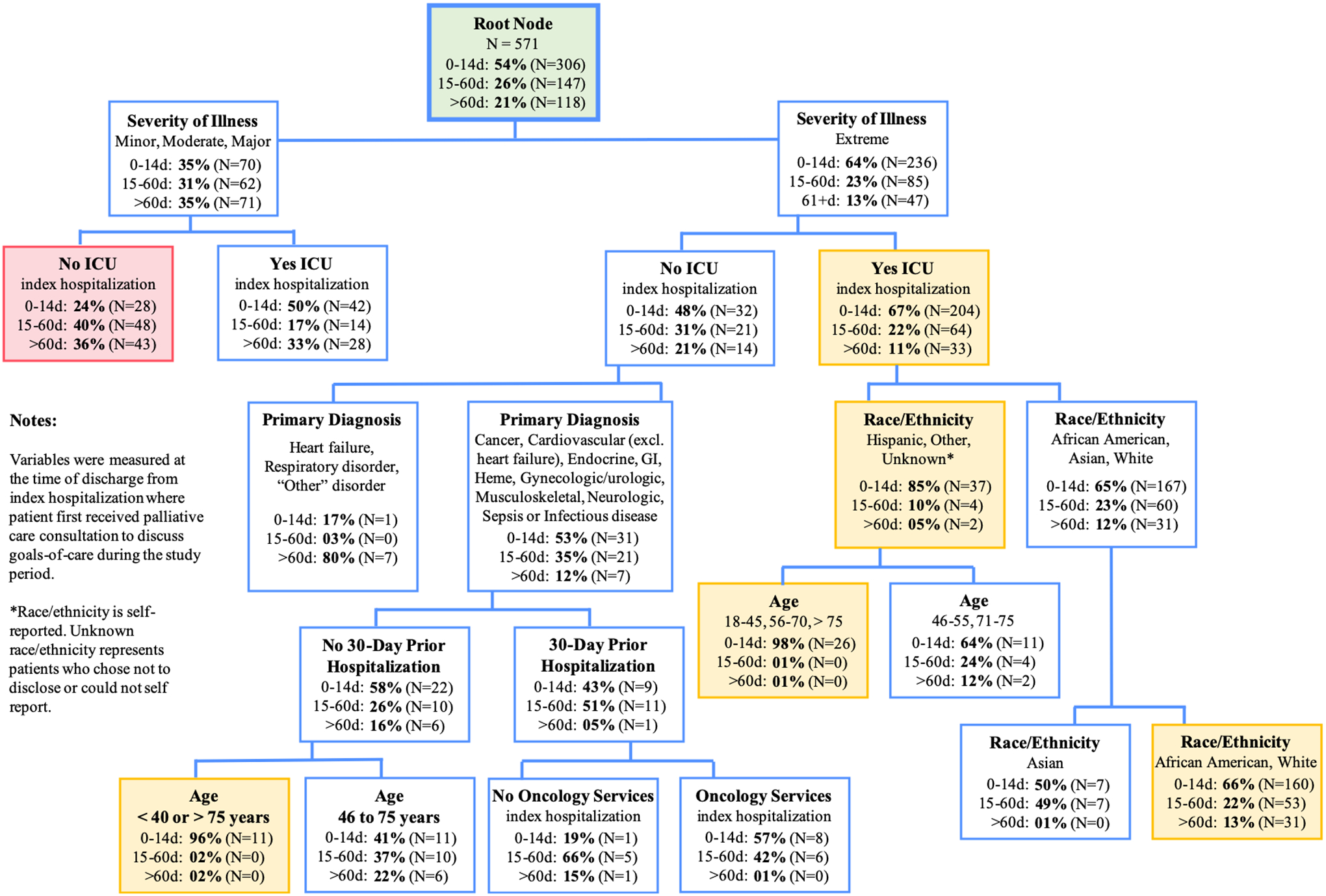
Classification and Regression Tree Showing Risk Factor Profiles of Patients Likely to Have Palliative Care Consultation to Discuss Goals-of-Care Within 14 days of Death
During index hospitalization, patients spent a median 11 days hospitalized (IQR 6–22 days, P<0.0001), 3 days in the ICU (IQR 0–11 days, P<0.0001), and incurred mean direct costs of $51,000 (SD $93,302; P=0.02). Index hospitalization utilization and costs were generally highest among patients with PCC close to death. Timing groups differed in age (P=0.04), race/ethnicity (P=0.02), primary diagnosis (P=0.006), Severity of Illness (P<0.0001), Risk of Mortality (P < 0.0001), ICU during index hospitalization (P<0.0001), ICU >6 days during index hospitalization (P<0.0001), and Oncology services during index hospitalization (P=0.03). Eighty-percent of PCC patients changed their goals-of-care during PCC, with rates increasing closer to death (P<0.0001). Do-not-resuscitate (DNR) documentation during index hospitalization (timing before or after PCC unknown) also increased closer to death (early PCC 26%, PCC 15–60 days 59%, PCC close to death 86%, P<0.0001). Discharge to hospice was lowest for patients with early PCC (10.1%) and higher for patients with moderately-timed PCC (32.5%) and PCC close to death (31%) (P<0.0001), but did not account for patients who died in the hospital.
Discussion
The model effectively identified which patients received PCC close to death and which patients did not receive more optimally-timed PCC, enabling clinicians to identify and target patients who are not getting more timely consultations and develop interventions that better support such patients’ needs. The model was not able to accurately identify which patients received early or moderately-timed PCC, possibly because relatively few patients received early PCC and because already small sample sizes for these groups diminished in the context of variable interactions. Variables not included in our model may have improved the model’s ability to identify patients likely to receive moderately-timed or early PCC. Future studies should include other potential correlates of PCC timing, such as health literacy, religiosity, immigrant status, primary language, and family dynamics.53–55 Research is also needed to better understand which patients receive PCC early and why.
Patients in our study received PCC a median 13 days before dying, which is less than the national median length of hospice care (24 days).56 This timing is problematic because shorter hospice enrollment is associated with lower quality EOL care, inadequate pain control, and unmet patient and family needs.57–59 To give patients and families adequate time to consider EOL care preferences, our findings suggest clinicians need to engage patients with serious illness in goals-of-care discussions earlier in the illness trajectory. In our study, over half the patients who had early PCC changed their goals-of-care, indicating earlier conversations are relevant and appropriate. Research illuminating when and why patients changed their goals is needed.
In our study, 50% of ICU patients with less than extreme illness severity and 67% of ICU patients with extreme illness severity received PCC close to death, suggesting coordination of PCC is needed in the ICU regardless of illness severity. This finding supports evidence-based recommendations to involve PCC in the ICU.13,60,61 One recent study found patients in the neuro-ICU who received PCC were more likely to change goals-of-care to less invasive care, receive fewer procedures in the last 48 hours of life, and receive better symptom management.62 Another study found involving PCC in the ICU increased advance care planning and decreased use of aggressive interventions.63 Consulting palliative care earlier in the ICU stay gives patients and families more time to benefit from goals-of-care discussions and the improvements in symptom and care management that follow. Research into systematic processes for involving PCC early in the ICU admission, such as those developed using evidence-based triggers and machine learning, are recommended.60,64,65 Early evidence suggests systematic processes increase PCC in the ICU,64 possibly reducing disparities that result from referral-driven care.
The interaction of illness severity, ICU care, and race/ethnicity reveals a need for systematic integration of PCC and more culturally effective care for patients with diverse backgrounds. It is unknown why ICU patients who self-identified as Hispanic or “Other” racial/ethnic minority demonstrated such a high probability for receiving PCC close to death. Hispanics and other racial/ethnic minorities endure disparities in access to care and experiences throughout the care continuum that may influence when they first receive PCC.53,54,66–70 For example, providers are less likely to have EOL discussions71 and less likely to discuss prognosis72 with Hispanics and other racial/ethnic minorities, possibly influencing their health literacy and receptivity to PCC.53 Other factors such as religiosity, family dynamics, and socio-cultural preferences may also contribute to PCC timing.53–55 More research is needed to better understand barriers to early PCC with severely ill Hispanics and other racial/ethnic minorities, and how clinicians can overcome such barriers to better support racial/ethnic minorities with serious illness.66–68 Improved communication and earlier involvement with PCC may help reduce well-known disparities in EOL care among Hispanic patients and other minorities.16,55,66,73,74
Our study employed a broad definition of serious illness, which allowed analysis of a wider population of patients who can benefit from PCC. We included patients with established serious illnesses like cancer, heart failure, and organ failure but also included patients hospitalized with acute complications from other diseases such as cardiovascular disease or chronic conditions such as diabetes or frailty, and patients with brain injury or other medically complex conditions. At the time of index admission when PCC first occurred, 93% of patients in our study had major or extreme APR-DRG Severity of Illness and 88% had major or extreme APR-DRG Risk of Mortality, underscoring the appropriateness of PCC in a wide population of acutely ill hospitalized patients.75–80 Evidenced by the high proportion of patients in our study who changed their goals-of-care during index admission (79.7%), PCC benefits myriad patient types. Although goal changes were especially high close to death (93%), half of patients who died more than 60 days after PCC also changed their goals during index admission. These findings imply PCC is useful earlier in illness trajectories. Research is needed to understand how early PCC influences care decision-making among different types of hospitalized patients, specifically patients with understudied conditions, chronic or multiple comorbidities, or a history of frequent hospitalization.
Finally, this medical center treats a high number of severely ill patients transferred from other regional hospitals, which may have contributed to the majority of patients receiving PCC close to death. Although timing improvements can be made in acute care settings using systematic triggers for inpatient PCC among eligible patients,64,65,81–83 poor access to PCC in the community likely contributes to late timing. Increased access to community-based palliative care models would improve the time between PCC and death and help meet patient before inpatient hospitalization.84,85
Limitations
As a secondary analysis, we were unable to assess relevant socioeconomic, religious, and cultural variables not in the dataset. Some unavailable variables may be salient in risk factor profiles associated with the timing of PCC or be important confounding factors, influencing results. The study was, however, able to assess Medicaid use, which can be considered a proxy for socioeconomic variables because its eligibility is based on income. In addition, the index PCC may have occurred before some of the correlates, such as ICU admission or the assignment of severity of illness or risk of mortality, making conclusions about chronology unfeasible but still resulting in relevant insights.
Second, our study does not include patients whose date of death is not recorded in the system database. Patients who received PCC but whose death information was not available may have had shorter or longer durations between consultation and death. The variables measured may be distributed differently across the groups and may be associated with timing differently. We were unable to differentiate between patients who died in the health system and those who died outside the system but whose death information was updated by affiliated providers; and were unable to assess any differences in demographic or clinical factors between patients whose deaths occurred in-system versus those whose deaths occurred outside the system. If there were differences in these sub-populations, we were unable to account for them. Given most care stays within this large, integrated health system and 64% of patients with PCC in the parent study were known to have died (not all PCC patients likely died during the study), we believe our sample included most patients who died during the study period.
Finally, our study examined a single academic medical center with high acuity and a well-established palliative care team, possibly limiting generalizability of results. Willingness to engage in goals-of-care conversations and referral patterns to PCC may differ in other systems. Despite these limitations, our study increases understanding of risk factor profiles associated with PCC timing.
Conclusions
Identifying patients at risk for goals-of-care consultation close to death is a public health priority. All patients should have time to adequately understand prognosis and consider care options, but our research found most patients receive PCC within 14 days of dying. A complex set of factors was associated with PCC timing before death. Understanding risk factor profiles associated with PCC timing may help clinicians initiate these discussions earlier with patients at-risk for late conversations and enable patients to make informed decisions consistent with preferences. Our results suggest PCC can benefit a wide population of seriously ill hospitalized patients and that systematic integration of PCC in the ICU and earlier communication with extremely ill Hispanic and Other racial/ethnic minority patients is needed to reduce disparities that may result from referral-driven care.
Contributor Information
Lauren T. Starr, University of Pennsylvania School of Nursing, Center for Bioethics, University of Pennsylvania Perelman School of Medicine.
Connie M. Ulrich, University of Pennsylvania School of Nursing, University of Pennsylvania Perelman School of Medicine.
Paul Junker, Program for Clinical Effectiveness and Quality Improvement, University of Pennsylvania Health System.
Liming Huang, BECCA Lab, University of Pennsylvania School of Nursing.
Nina R. O’Connor, University of Pennsylvania Perelman School of Medicine.
Salimah H. Meghani, University of Pennsylvania School of Nursing.
References
- 1.Gieniusz M, Nunes R, Saha V, Renson A, Schubert FD, Carey J. Earlier Goals of Care Discussions in Hospitalized Terminally Ill Patients and the Quality of End-of-Life Care: A Retrospective Study. The American journal of hospice & palliative care. 2018;35(1):21–27. [DOI] [PubMed] [Google Scholar]
- 2.Higginson IJ, Daveson BA, Morrison RS, et al. Social and clinical determinants of preferences and their achievement at the end of life: prospective cohort study of older adults receiving palliative care in three countries. BMC Geriatr. 2017;17(1):271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yun YH, You CH, Lee JS, et al. Understanding disparities in aggressive care preferences between patients with terminal illness and their family members. J Pain Symptom Manage. 2006;31(6):513–521. [DOI] [PubMed] [Google Scholar]
- 4.Heyland DK, Heyland R, Dodek P, et al. Discordance between patients’ stated values and treatment preferences for end-of-life care: results of a multicentre survey. BMJ Supportive & Palliative Care. 2017;7(1):292–299. [DOI] [PubMed] [Google Scholar]
- 5.Bischoff KE, Sudore R, Miao Y, Boscardin WJ, Smith AK. Advance care planning and the quality of end-of-life care in older adults. J Am Geriatr Soc. 2013;61(2):209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gieniusz M, Nunes R, Saha V, Renson A, Schubert F, Carey J. Earlier Goals of Care Discussions in Hospitalized Terminally Ill Patients and the Quality of End-of-Life Care: A Retrospective Study. Am J Hosp Palliat Care. 2018;35(1):21–27. [DOI] [PubMed] [Google Scholar]
- 7.Mack JW, Weeks JC, Wright AA, Block SD, Prigerson HG. End-of-life discussions, goal attainment, and distress at the end of life: predictors and outcomes of receipt of care consistent with preferences. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(7):1203–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith-Howell ER, Hickman SE, Meghani SH, Perkins SM, Rawl SM. End-of-Life Decision Making and Communication of Bereaved Family Members of African Americans with Serious Illness. Journal of Palliative Medicine. 2016;19(2):174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright AA, Keating NL, Ayanian JZ, et al. Family Perspectives on Aggressive Cancer Care Near the End of Life. JAMA. 2016;315(3):284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang B, Nilsson ME, Prigerson HG. Factors important to patients’ quality of life at the end of life. Arch Intern Med. 2012;172(15):1133–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huynh TN, Kleerup EC, Wiley JF, et al. The frequency and cost of treatment perceived to be futile in critical care. JAMA Intern Med. 2013;173(20):1887–1894. [DOI] [PubMed] [Google Scholar]
- 12.Cardona-Morrell M, Kim J, Turner RM, Anstey M, Mitchell IA, Hillman K. Non-beneficial treatments in hospital at the end of life: a systematic review on extent of the problem. International journal for quality in health care : journal of the International Society for Quality in Health Care. 2016;28(4):456–469. [DOI] [PubMed] [Google Scholar]
- 13.Institute of Medicine (IOM). Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington, DC: IOM; Mar 19 2015. [DOI] [PubMed] [Google Scholar]
- 14.Mack JW, Cronin A, Keating NL, et al. Associations between end-of-life discussion characteristics and care received near death: a prospective cohort study. Journal of Clinical Oncology. 2012;30(35):4387–4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Connor NR, Junker P, Appel SM, Stetson RL, Rohrbach J, Meghani SH. Palliative Care Consultation for Goals of Care and Future Acute Care Costs: A Propensity-Matched Study. Am J Hosp Palliat Care. 2017:1049909117743475. [DOI] [PubMed] [Google Scholar]
- 16.Zhang B, Wright AA, Huskamp HA, et al. Health care costs in the last week of life: associations with end-of-life conversations. Arch Intern Med. 2009;169(5):480–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Detering KM, Hancock AD, Reade MC, Silvester W. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ. 2010;340:c1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Connor NR, Moyer ME, Behta M, Casarett DJ. The Impact of Inpatient Palliative Care Consultations on 30-Day Hospital Readmissions. J Palliat Med. 2015;18(11):956–961. [DOI] [PubMed] [Google Scholar]
- 19.Bernacki R, Block S. American College of Physicians High Value Care Task Force. Communication about serious illness care goals: a review and synthesis of best practices. JAMA Intern Med. 2014;174(12):1994–2003. [DOI] [PubMed] [Google Scholar]
- 20.Patel MI, Sundaram V, Desai M, et al. Effect of a Lay Health Worker Intervention on Goals-of-Care Documentation and on Health Care Use, Costs, and Satisfaction Among Patients With Cancer: A Randomized Clinical Trial. JAMA Oncol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mack JW, Cronin A, Taback N, et al. End-of-life care discussions among patients with advanced cancer: a cohort study. Ann Intern Med. 2012;156(3):204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Emiloju OE, Djibo DAM, Ford JG. Association Between the Timing of Goals-of-Care Discussion and Hospitalization Outcomes in Patients With Metastatic Cancer. Am J Hosp Palliat Care. 2019:1049909119882891. [DOI] [PubMed] [Google Scholar]
- 23.Leung JM, Udris EM, Uman J, Au DH. The effect of end-of-life discussions on perceived quality of care and health status among patients with COPD. Chest. 2012;142(1):128–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanson LC, Usher B, Spragens L, Bernard S. Clinical and economic impact of palliative care consultation. J Pain Symptom Manage. 2008;35(4):340–346. [DOI] [PubMed] [Google Scholar]
- 25.Khandelwal N, Benkeser DC, Coe NB, Curtis JR. Potential Influence of Advance Care Planning and Palliative Care Consultation on ICU Costs for Patients With Chronic and Serious Illness. Crit Care Med. 2016;44(8):1474–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.May P, Normand C, Morrison RS. Economic impact of hospital inpatient palliative care consultation: review of current evidence and directions for future research. J Palliat Med. 2014;17(9):1054–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morrison RS, Penrod JD, Cassel JB, et al. Cost savings associated with US hospital palliative care consultation programs. Arch Intern Med. 2008;168(16):1783–1790. [DOI] [PubMed] [Google Scholar]
- 28.Penrod JD, Deb P, Dellenbaugh C, et al. Hospital-based palliative care consultation: effects on hospital cost. J Palliat Med. 2010;13(8):973–979. [DOI] [PubMed] [Google Scholar]
- 29.Whitford K, Shah ND, Moriarty J, Branda M, Thorsteinsdottir B. Impact of a palliative care consult service. Am J Hosp Palliat Care. 2014;31(2):175–182. [DOI] [PubMed] [Google Scholar]
- 30.Gaertner J, Siemens W, Meerpohl JJ, et al. Effect of specialist palliative care services on quality of life in adults with advanced incurable illness in hospital, hospice, or community settings: systematic review and meta-analysis. BMJ. 2017;357:j2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greer JA, Pirl WF, Jackson VA, et al. Effect of early palliative care on chemotherapy use and end-of-life care in patients with metastatic non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(4):394–400. [DOI] [PubMed] [Google Scholar]
- 32.Kozlov E, Carpenter BD, Thorsten M, Heiland M, Agarwal A. Timing of Palliative Care Consultations and Recommendations: Understanding the Variability. Am J Hosp Palliat Care. 2015;32(7):772–775. [DOI] [PubMed] [Google Scholar]
- 33.Scibetta C, Kerr K, McGuire J, Rabow MW. The Costs of Waiting: Implications of the Timing of Palliative Care Consultation among a Cohort of Decedents at a Comprehensive Cancer Center. J Palliat Med. 2016;19(1):69–75. [DOI] [PubMed] [Google Scholar]
- 34.Robbins SG, Hackstadt AJ, Martin S, Shinall MC Jr. Implications of Palliative Care Consultation Timing among a Cohort of Hospice Decedents. J Palliat Med. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steinhauser KE, Christakis NA, Clipp EC, McNeilly M, McIntyre L, Tulsky JA. Factors considered important at the end of life by patients, family, physicians, and other care providers. JAMA. 2000;284(19):2476–2482. [DOI] [PubMed] [Google Scholar]
- 36.Hjorth NE, Haugen DF, Schaufel MA. Advance care planning in life-threatening pulmonary disease: a focus group study. ERJ Open Res. 2018;4(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eues SK. End-of-life care: improving quality of life at the end of life. Prof Case Manag. 2007;12(6):339–344. [DOI] [PubMed] [Google Scholar]
- 38.Corporation PHM. University of Pennsylvania Health System 2016 Community Needs Assessment. 2016; https://www.pennmedicine.org/~/media/documents%20and%20audio/annual%20reports/community/community_health_needs_assessment_uphs_chna_2016_1.ashx?la=en, 2018.
- 39.Kelley AS. Defining “serious illness”. J Palliat Med. 2014;17(9):985. [DOI] [PubMed] [Google Scholar]
- 40.Kelley AS, Covinsky KE, Gorges RJ, et al. Identifying Older Adults with Serious Illness: A Critical Step toward Improving the Value of Health Care. Health Serv Res. 2017;52(1):113–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andriyashin A, Timofeev R. Classification and Regression Tree Analysis (CART). Zuberlin: Center for Applied Statistics and Economics, Humboldt Universitat Zuberlin; 2005. [Google Scholar]
- 42.Steinberg D. Rules of Thumb When Working With Small Data Samples: CART. In. Dan Steinberg’s Blog. Vol 2018: Salford Systems; 2018. [Google Scholar]
- 43.Starr L, Ulrich CM, Meghani SH. Associations Between End-of-Life Care Planning Communications, Treatments, and Costs at the End-of-Life in Persons with Cancer: An Integrative Review with Ethical Considerations. In: University of Pennsylvania, NewCourtland Center for Transitions and Health; 2018. [Google Scholar]
- 44.Baram D, Daroowalla F, Garcia R, et al. Use of the All Patient Refined-Diagnosis Related Group (APR-DRG) Risk of Mortality Score as a Severity Adjustor in the Medical ICU. Clin Med Circ Respirat Pulm Med. 2008;2:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Merlino JI, Kestranek C, Bokar D, Sun Z, Nissen SE, Longworth DL. HCAHPS Survey Results: Impact of Severity of Illness on Hospitals’ Performance on HCAHPS Survey Results. Journal of patient experience. 2014;1(2):16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neville PG. Decision Trees for Predictive Modeling. SAS Institute Inc. 4 August 1999. 1999. [Google Scholar]
- 47.Cheng Z, Nakatsugawa M, Hu C, et al. Evaluation of classification and regression tree (CART) model in weight loss prediction following head and neck cancer radiation therapy. Adv Radiat Oncol. 2018;3(3):346–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuhn L, Page K, Ward J, Worrall-Carter L. The process and utility of classification and regression tree methodology in nursing research. J Adv Nurs. 2014;70(6):1276–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zimmerman RK, Balasubramani GK, Nowalk MP, et al. Classification and Regression Tree (CART) analysis to predict influenza in primary care patients. BMC Infect Dis. 2016;16(1):503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Greene MZ, Hughes TL, Hanlon A, Huang L, Sommers MS, Meghani SH. Predicting cervical cancer screening among sexual minority women using Classification and Regression Tree analysis. Prev Med Rep. 2019;13:153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lewis R. An Introduction to Classification and Regression Tree (CART) Analysis. 2000 Annual Meeting of the Society for Academic Emergency Medicine; 2000; San Francisco, CA. [Google Scholar]
- 52.Romano PS, Chan BK. Risk-adjusting acute myocardial infarction mortality: are APR-DRGs the right tool? Health Serv Res. 2000;34(7):1469–1489. [PMC free article] [PubMed] [Google Scholar]
- 53.Smith AK, Sudore R, Perez-Stable E. Palliative Care for Latino Patients and Their Families: “Whenever We Prayed, She Wep. JAMA. 2009;301(10):1047–E1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vega WA, Rodriguez MA, Gruskin E. Health disparities in the Latino population. Epidemiol Rev. 2009;31:99–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Volandes AE, Paasche-Orlow M, Gillick MR, et al. Health literacy not race predicts end-of-life care preferences. J Palliat Med. 2008;11(5):754–762. [DOI] [PubMed] [Google Scholar]
- 56.National Hospice and Palliative Care Organization (NHPCO). Facts and Figures: Hospice Care in America. Alexandria, VA: 2017. 2018. [Google Scholar]
- 57.Rickerson E, Harrold J, Kapo J, Carroll JT, Casarett D. Timing of hospice referral and families’ perceptions of services: are earlier hospice referrals better? J Am Geriatr Soc. 2005;53(5):819–823. [DOI] [PubMed] [Google Scholar]
- 58.Schockett ER, Teno JM, Miller SC, Stuart B. Late referral to hospice and bereaved family member perception of quality of end-of-life care. J Pain Symptom Manage. 2005;30(5):400–407. [DOI] [PubMed] [Google Scholar]
- 59.Wachterman MW, Hailpern SM, Keating NL, Kurella Tamura M, O’Hare AM. Association Between Hospice Length of Stay, Health Care Utilization, and Medicare Costs at the End of Life Among Patients Who Received Maintenance Hemodialysis. JAMA Intern Med. 2018;178(6):792–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mathews KS, Nelson JE. Palliative care in the ICU of 2050: past is prologue. Intensive Care Med. 2017;43(12):1850–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Turnbull AE, Bosslet GT, Kross EK. Aligning use of intensive care with patient values in the USA: past, present, and future. Lancet Respir Med. 2019;7(7):626–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tabibian BE, Salehani AA, Kuhn EN, Davis MC, Shank CD, Fisher Iii WS. Transitioning the Treatment Paradigm: How Early Palliative Care Service Involvement Affects the End-of-Life Course for Critically Ill Patients in the Neuro-Intensive Care Unit. J Palliat Med. 2018. [DOI] [PubMed] [Google Scholar]
- 63.O’Mahony S, McHenry J, Blank A, et al. Preliminary report of the integration of a palliative care team into an intensive care unit. Palliative medicine. 2010;24(2):154–165. [DOI] [PubMed] [Google Scholar]
- 64.Jones BW, Bernstein C. Palliative Care Triggers in the Intensive Care Unit: A Pilot Success Story. Dimens Crit Care Nurs. 2017;36(2):106–109. [DOI] [PubMed] [Google Scholar]
- 65.McCarroll CM. Increasing Access to Palliative Care Services in the Intensive Care Unit. Dimens Crit Care Nurs. 2018;37(3):180–192. [DOI] [PubMed] [Google Scholar]
- 66.LoPresti MA, Dement F, Gold HT. End-of-Life Care for People With Cancer From Ethnic Minority Groups: A Systematic Review. Am J Hosp Palliat Care. 2016;33(3):291–305. [DOI] [PubMed] [Google Scholar]
- 67.Lorenz KA. Quality of end-of-life care: how far have we come in addressing the needs of multicultural patients? Ann Palliat Med. 2017;6(1):3–5. [DOI] [PubMed] [Google Scholar]
- 68.Periyakoil VS, Neri E, Kraemer H. Patient-Reported Barriers to High-Quality, End-of-Life Care: A Multiethnic, Multilingual, Mixed-Methods Study. J Palliat Med. 2016;19(4):373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barnato AE, Anthony DL, Skinner J, Gallagher PM, Fisher ES. Racial and ethnic differences in preferences for end-of-life treatment. J Gen Intern Med. 2009;24(6):695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Loggers ET, Maciejewski PK, Paulk E, et al. Racial differences in predictors of intensive end-of-life care in patients with advanced cancer. J Clin Oncol. 2009;27(33):5559–5564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mack JW, Cronin A, Keating NL, et al. Associations between end-of-life discussion characteristics and care received near death: a prospective cohort study. J Clin Oncol. 2012;30(35):4387–4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ingersoll LT, Alexander SC, Priest J, et al. Racial/ethnic differences in prognosis communication during initial inpatient palliative care consultations among people with advanced cancer. Patient Educ Couns. 2019. [DOI] [PubMed] [Google Scholar]
- 73.Hanchate A, Kronman AC, Young-Xu Y, Ash AS, Emanuel E. Racial and ethnic differences in end-of-life costs: why do minorities cost more than whites? Arch Intern Med. 2009;169(5):493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mantwill S, Monestel-Umana S, Schulz PJ. The Relationship between Health Literacy and Health Disparities: A Systematic Review. PLoS ONE. 2015;10(12):e0145455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hyatt R, Dean J, O’Neill M, Patel N, Abu-Rabia M, Taylor J. The frailest of the frail? Addressing the palliative care needs of frail older patients. Future Healthc J. 2018;5(1):10–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee YJ, Yoo JW, Hua L, Kim PC, Kim SJ, Shen JJ. Ten-year trends of palliative care utilization associated with multiple sclerosis patients in the United States from 2005 to 2014. J Clin Neurosci. 2018;58:13–19. [DOI] [PubMed] [Google Scholar]
- 77.Kirkpatrick JN, Hauptman PJ, Swetz KM, et al. Palliative Care for Patients With End-Stage Cardiovascular Disease and Devices: A Report From the Palliative Care Working Group of the Geriatrics Section of the American College of Cardiology. JAMA Intern Med. 2016;176(7):1017–1019. [DOI] [PubMed] [Google Scholar]
- 78.Johnston GM, Lethbridge L, Talbot P, et al. Identifying persons with diabetes who could benefit from a palliative approach to care. Can J Diabetes. 2015;39(1):29–35. [DOI] [PubMed] [Google Scholar]
- 79.Hwang F, Pentakota SR, Glass NE, Berlin A, Livingston DH, Mosenthal AC. Older Patients With Severe Traumatic Brain Injury: National Variability in Palliative Care. J Surg Res. 2020;246:224–230. [DOI] [PubMed] [Google Scholar]
- 80.Pollack LR, Goldstein NE, Gonzalez WC, et al. The Frailty Phenotype and Palliative Care Needs of Older Survivors of Critical Illness. J Am Geriatr Soc. 2017;65(6):1168–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chang RS, Poon WS. “Triggers” for referral to neurology palliative care service. Ann Palliat Med. 2018;7(3):289–295. [DOI] [PubMed] [Google Scholar]
- 82.Hussain J, Allgar V, Oliver D. Palliative care triggers in progressive neurodegenerative conditions: An evaluation using a multi-centre retrospective case record review and principal component analysis. Palliative medicine. 2018;32(4):716–725. [DOI] [PubMed] [Google Scholar]
- 83.Psotka MA, McKee KY, Liu AY, Elia G, De Marco T. Palliative Care in Heart Failure: What Triggers Specialist Consultation? Prog Cardiovasc Dis. 2017;60(2):215–225. [DOI] [PubMed] [Google Scholar]
- 84.Block SD, Bernier GM, Crawley LM, et al. Incorporating palliative care into primary care education. National Consensus Conference on Medical Education for Care Near the End of Life. J Gen Intern Med. 1998;13(11):768–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ramanayake RP, Dilanka GV, Premasiri LW. Palliative care; role of family physicians. J Family Med Prim Care. 2016;5(2):234–237. [DOI] [PMC free article] [PubMed] [Google Scholar]


