Abstract
Background
Prone positioning in combination with the application of low tidal volume and adequate positive end-expiratory pressure (PEEP) improves survival in patients with moderate to severe acute respiratory distress syndrome (ARDS). However, the effects of PEEP on end-expiratory transpulmonary pressure (Ptpexp) during prone positioning require clarification. For this purpose, the effects of three different PEEP titration strategies on Ptpexp, respiratory mechanics, mechanical power, gas exchange, and hemodynamics were evaluated comparing supine and prone positioning.
Methods
In forty consecutive patients with moderate to severe ARDS protective ventilation with PEEP titrated according to three different titration strategies was evaluated during supine and prone positioning: (A) ARDS Network recommendations (PEEPARDSNetwork), (B) the lowest static elastance of the respiratory system (PEEPEstat,RS), and (C) targeting a positive Ptpexp (PEEPPtpexp). The primary endpoint was to analyze whether Ptpexp differed significantly according to PEEP titration strategy during supine and prone positioning.
Results
Ptpexp increased progressively with prone positioning compared with supine positioning as well as with PEEPEstat,RS and PEEPPtpexp compared with PEEPARDSNetwork (positioning effect p < 0.001, PEEP strategy effect p < 0.001). PEEP was lower during prone positioning with PEEPEstat,RS and PEEPPtpexp (positioning effect p < 0.001, PEEP strategy effect p < 0.001). During supine positioning, mechanical power increased progressively with PEEPEstat,RS and PEEPPtpexp compared with PEEPARDSNetwork, and prone positioning attenuated this effect (positioning effect p < 0.001, PEEP strategy effect p < 0.001). Prone compared with supine positioning significantly improved oxygenation (positioning effect p < 0.001, PEEP strategy effect p < 0.001) while hemodynamics remained stable in both positions.
Conclusions
Prone positioning increased transpulmonary pressures while improving oxygenation and hemodynamics in patients with moderate to severe ARDS when PEEP was titrated according to the ARDS Network lower PEEP table. This PEEP titration strategy minimized parameters associated with ventilator-induced lung injury induction, such as transpulmonary driving pressure and mechanical power. We propose that a lower PEEP strategy (PEEPARDSNetwork) in combination with prone positioning may be part of a lung protective ventilation strategy in patients with moderate to severe ARDS.
Trial registration
German Clinical Trials Register (DRKS00017449). Registered June 27, 2019. https://www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00017449
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-022-03956-8.
Keywords: Acute respiratory distress syndrome, Positive end-expiratory pressure, Transpulmonary pressure, Respiratory mechanics, Prone position, Ventilator-induced lung injury, Lung protective ventilation
Background
Severe acute respiratory distress syndrome (ARDS) is a life-threatening pulmonary disease characterized by inhomogeneous distribution of lung injury with alveolar consolidation/atelectasis and increased shunt fraction resulting in hypoxemia [1]. Positive pressure mechanical ventilation is a life-saving intervention but increases the risk of ventilator-induced lung injury (VILI) mediated by stress, strain, and energy transmission to the inflamed lung parenchyma [2]. Positive end-expiratory pressure (PEEP) increases end-expiratory transpulmonary pressure (Ptpexp), and prevent atelectasis, mainly in the dependent lung regions [3]. However, during protective mechanical ventilation, the optimal PEEP titration strategy remains controversial when taking into account the differences in lung recruitability of the individual patient [4]. Several strategies have been proposed to set PEEP [5]: (A) the use of a minimal PEEP level to achieve adequate oxygenation [6] according to the lower PEEP/fraction of inspired oxygen (FiO2) table, which was recommended by the ARDS Network [7]; (B) evaluation of the lowest static elastance of the respiratory system (Estat,RS) [8] aiming to achieve the lowest driving pressure (Pdriv), thus determining a compromise between recruitment and overinflation [9, 10]; and (C) targeting a positive Ptpexp to account for variability in lung and chest wall mechanics and optimize alveolar recruitment [11]. The pleural pressure is increased in dependent lung regions leading to alveolar collapse [12], therefore the application of a matched PEEP should counteract the pleural pressure and thus promote the balance between recruitment and overdistension [13]. In addition, prone positioning is an effective strategy in patients with moderate to severe ARDS and is known to decrease mortality [14]. This decrease is likely due to the more homogeneous distribution of ventilation as a result of reduced vertical pleural pressure gradient [15], which may lead to less lung damage [16, 17] and improved lung mechanics. To date, the interaction between prone positioning and Ptpexp when using different PEEP titration strategies has not been clarified [18, 19]. We hypothesized that Ptpexp differed significantly according to PEEP titration strategy during supine and prone positioning in patients with moderate to severe ARDS. Secondary endpoints were to evaluate the effects of different PEEP titration strategies on respiratory system, lung and chest wall mechanics, mechanical power, gas exchange and hemodynamics during supine and prone positioning.
Methods
This prospective interventional study was conducted from July 2019 to February 2021 with approval from the local ethical committee (Medizinische Ethikkomission II, University Medical Centre Mannheim, Medical Faculty Mannheim of the University of Heidelberg, Mannheim, registration number 2018-609N-MA) and study registration at the German Clinical Trials Register (DRKS00017449) in the intensive care unit (ICU) of the Department of Anesthesiology and Critical Care Medicine, University Medical Centre Mannheim, Medical Faculty Mannheim of the University of Heidelberg in Mannheim, Germany. All mechanically ventilated patients in the ICU were screened for the presence of moderate to severe ARDS (defined by the ratio of arterial oxygen partial pressure divided by the fraction of inspired oxygen [PaO2/FiO2] < 150 mm Hg) [20]. After obtaining written informed consent from each patient or their relatives, 40 consecutive patients with moderate to severe ARDS (defined by the ratio of arterial oxygen partial pressure divided by the fraction of inspired oxygen [PaO2/FiO2] < 150 mm Hg) were studied. Exclusion criteria were age younger than 18 years, pregnancy, end-stage chronic organ failure, inherited cardiac malformations, severe head injury and hemodynamic instability (mean arterial pressure [MAP] < 65 mm Hg, cardiac index [CI] of < 2.0 L/min/m2).
All patients were ventilated with an Engström Carescape™ R860 ventilator (GE Healthcare, Munich, Germany) and had a five-lumen central venous catheter inserted via the internal jugular vein for the measurement of central venous pressure (CVP) and central venous oxygen saturation (ScvO2). A thermodilution catheter (4F or 5F Pulsiocath™, Pulsion Medical Systems, Munich, Germany) was inserted in the femoral artery to allow hemodynamic measurement and fluid management through a Pulse Contour Cardiac Output monitor (PiCCOplus™, Pulsion Medical Systems, Munich, Germany). Esophageal pressure was measured with an esophageal balloon catheter (NutriVent™ nasogastric catheter, Sidam, Mirandola, Italy) filled with 4 mL of air as indicated by the manufacturer. The esophageal balloon catheter was positioned by slow retraction from the stomach until maximal respiratory pressure swings and minimal cardiac oscillation artefacts were obtained. Catheter positioning was confirmed by applying manual compression on the chest during an end-expiratory airway occlusion. Correct positioning was verified by a ratio of change in esophageal pressure to the change in airway pressure of 0.8–1.2 [21]. All patients were sedated with midazolam (5–15 mg/h) and sufentanil (30–40 µg/h) to achieve a Richmond Agitation-Sedation Score of − 5 [22]. Cisatracurium was infused continuously for neuromuscular blockade throughout the study period. Norepinephrine was administered if MAP was below 65 mmHg despite preload optimization. The patients were ventilated in a volume-controlled mode with tidal volumes (VT) of 6 mL/kg predicted body weight and respiratory rates (RR) to achieve a pHa of 7.25. In accordance with the study protocol, PEEP was initially set using the ARDS Network lower PEEP table (PEEPARDSNetwork) [7]. Allowable combinations of FiO2 and PEEP are presented in Additional file 1: Table S1.
Experimental protocol
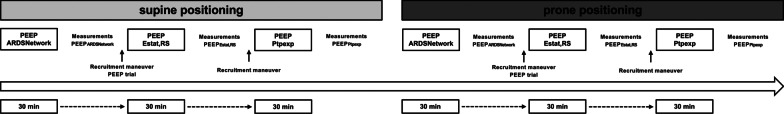
The schematic workflow of the study is presented in Fig. 1. After complete measurement of respiratory mechanics, gas exchange, and hemodynamics at PEEPARDSNetwork, a standardized dynamic recruitment maneuver and a decremental PEEP trial was performed (Additional file 1: Figure S1).
Fig. 1.
Schematic workflow of the study. PEEP positive end-expiratory pressure, PEEPARDSNetwork PEEP titrated according to the ARDS Network lower PEEP table, PEEPEstat,RS PEEP titrated according to the lowest elastance of the respiratory system, PEEPPtpexp PEEP titrated according to end-expiratory transpulmonary pressure
PEEP was slowly increased to 35 cm H2O in a pressure-controlled mode with a Pdriv of 15 cm H2O over a period of 5 min. After 2 min, ventilator mode was switched back to a volume-controlled mode using the initial VT and RR to perform a decremental PEEP trial. Starting with a PEEP of 30 cm H2O, the PEEP was reduced stepwise by 2 cm H2O every 2 min until Estat,RS did not decrease further with a reduction of PEEP. The identified PEEP with the lowest Estat,RS (PEEPEstat,RS) was set after a recruitment maneuver, and a complete measurement of respiratory mechanics, gas exchange, and hemodynamics was performed after a 30-min equilibration period. After a recruitment maneuver as described, PEEP was set to the Ptpexp target according to the empirical table of the EPVent-2 trial [23] (PEEPPtpexp). Allowable combinations of FiO2 and Ptpexp are presented in Additional file 1: Table S2. After a 30-min equilibration period, complete measurement of respiratory mechanics, gas exchange, and hemodynamics was performed. Patients were then moved to the prone position and all the physiological measurements were repeated with PEEPARDSNetwork followed by another titration of PEEPEstat,RS and PEEPPtpexp as described above.
Respiratory mechanics, gas exchange and hemodynamics
Respiratory mechanics, gas exchange and hemodynamics were obtained following the equilibration period for PEEPARDSNetwork, PEEPEstat,RS and PEEPPtpexp in supine and prone position. The mechanics of the respiratory system, lung and chest wall were calculated according to the standard formulas (see Additional file 1). End-inspiratory esophageal pressure (Pesinsp) and end-expiratory esophageal pressure (Pesexp) were recorded during a 5-s inspiratory and 5-s expiratory hold, respectively. The mechanical power, the ratio of physiologic dead space to tidal volume (VD/VT) and the ventilatory ratio were computed according to the conventional equations (see Additional file 1). End-expiratory lung volume (EELV) was measured with the Engström Carescape™ R860 ventilator using the nitrogen wash-in/wash-out technique [24]. All hemodynamic parameters were obtained using the Pulse Contour Cardiac Output monitor after calibration with the transpulmonary thermodilution method using 20 mL iced saline three times. Blood gas analyses were made with a blood gas analyzer (Radiometer ABL 800 Flex, Radiometer, Willich, Germany). For the measurement of intraabdominal pressure (IAP), the transducer was zeroed at the level of the midaxillary line at end-expiration using an instillation volume of 25 mL of saline in the bladder as recommended by the Abdominal Compartment Society [25]. The Simplified Acute Physiology Score II (SAPS II) [26] and Sequential Organ Failure Assessment (SOFA) score [27] were calculated for each patient on admission to the intensive care unit.
Statistical analysis
The primary end point was to analyze Ptpexp when PEEP was set according to three different strategies both in supine and prone positioning in patients with moderate to severe ARDS. Secondary endpoints were to evaluate the effects of different PEEP titration strategies on respiratory system, lung and chest wall mechanics, mechanical power, gas-exchange, and hemodynamics during supine and prone positioning. The number of patients was calculated based on a previous study conducted by our group [28], in which we assumed that the expected value of Ptpexp at PEEP titrated to the lowest Estat,RS is zero with a standard deviation of 4.40 in the supine position. For the prone position, we assumed the same standard deviation but an expected value of Ptpexp equal to 3 cm H2O. We further assumed that the correlation between both measures would be non-negative. Therefore, under these conditions, a sample size of 40 patients showed a power higher than 80% for a two-way repeated measurement ANOVA, with a significance level of 5%.
Statistical analysis was performed using SigmaPlot 12.5 (Systat Software GmbH, Erkrath, Germany). Categorical variables were compared using Fisher’s exact test and presented as frequency and percentages. For continuous variables, the normality of the data and the homogeneity of variances were tested using the Shapiro–Wilk test and Levene’s median test, respectively. As per the study protocol, longitudinal physiologic data were analyzed using two-way repeated measures ANOVA followed by Holm-Sidak’s post hoc test. The results are expressed as means ± standard deviation. The level of significance was set at p < 0.05.
Results
Forty consecutively identified patients with moderate to severe ARDS (PaO2/FiO2 < 150) were included in the analysis. Demographic and clinical characteristics of the patients are shown in Additional file 1, Table S3.
Transpulmonary pressures
Prone compared to supine positioning increased end-expiratory transpulmonary pressure (Ptpexp) when using PEEPARDSNetwork (− 2.4 ± 3.5 versus 1.1 ± 3.4 cm H2O, p < 0.001) and PEEPEstat,RS (− 0.5 ± 2.1 versus 1.1 ± 2.5 cm H2O, p < 0.001) (Fig. 2A). End-inspiratory transpulmonary pressure (Ptpinsp) also increased using PEEPARDSNetwork (3.3 ± 4.2 versus 6.3 ± 4.6 cm H2O, p < 0.001) and PEEPEstat,RS (4.7 ± 3.0 versus 6.1 ± 3.5 cm H2O, p < 0.001) (Fig. 2B). There was a significant effect of positioning and PEEP strategy as well as an interaction between position and PEEP strategy for Ptpexp and Ptpinsp (p < 0.001 each). Prone positioning reduced transpulmonary driving pressure (Ptpdriv) when using PEEPARDSNetwork (5.7 ± 2.2 versus 5.2 ± 2.1 cm H2O, p = 0.031) (Fig. 4B).
Fig. 2.
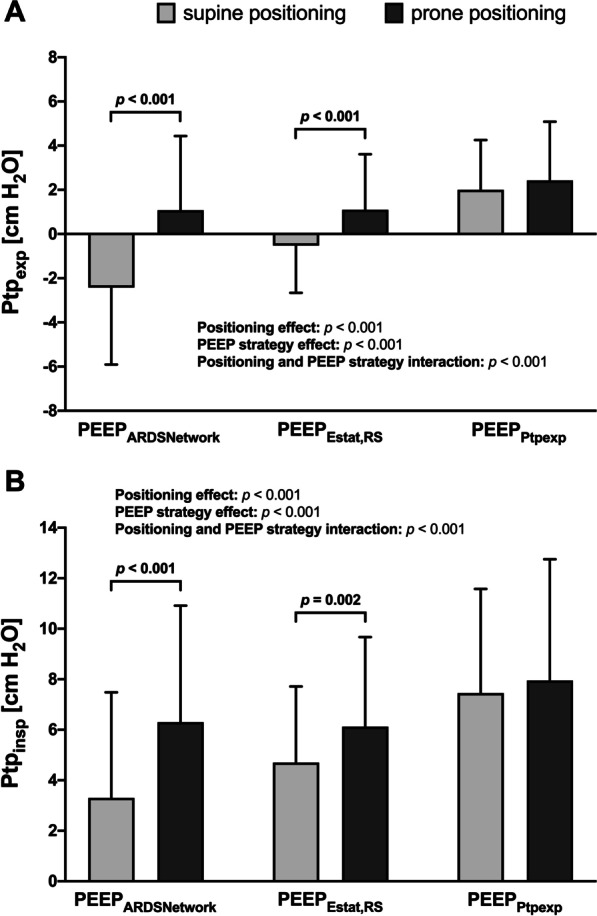
Effects of different PEEP titration strategies on Ptpexp and Ptpinsp during supine and prone positioning. A Ptpexp evaluated according to different PEEP titration strategies during supine and prone positioning. B Ptpinsp evaluated according to different PEEP titration strategies during supine and prone positioning. Bars are means + SD of 40 patients with moderate to severe ARDS. Brackets denote statistically significant differences between PEEP titration strategies, p-values are shown above the brackets. PEEP positive end-expiratory pressure, PEEPARDSNetwork PEEP titrated according to the ARDS Network lower PEEP table, PEEPEstat,RS PEEP titrated according to the lowest elastance of the respiratory system, PEEPPtpexp PEEP titrated according to end-expiratory transpulmonary pressure, Ptpexp end-expiratory transpulmonary pressure, Ptpinsp end-inspiratory transpulmonary pressure
Fig. 4.
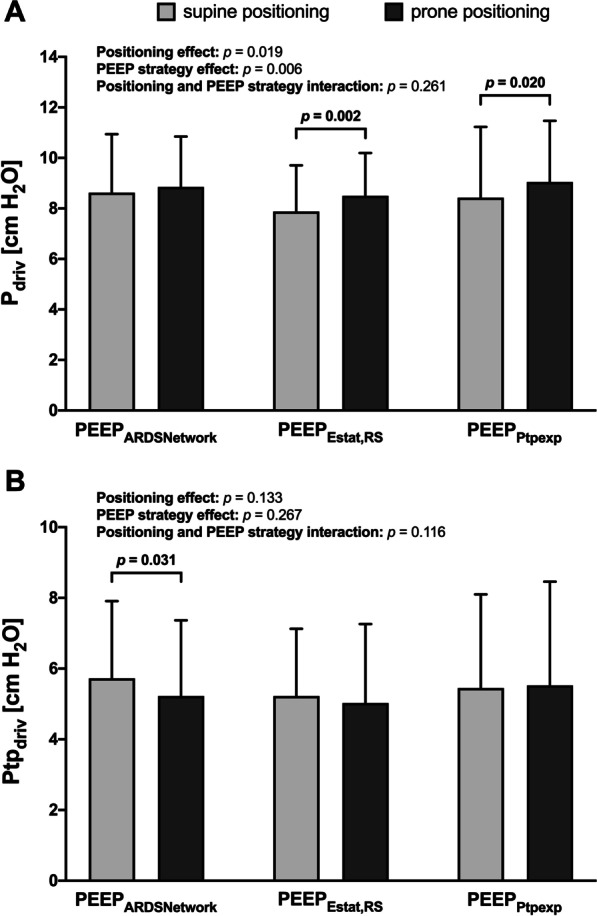
Effects of different PEEP titration strategies on Pdriv and Ptpdriv during supine and prone positioning. A Pdriv evaluated according to different PEEP titration strategies during supine and prone positioning. B Ptpdriv evaluated according to different PEEP titration strategies during supine and prone positioning. Bars are means + SD of 40 patients with moderate to severe ARDS. Brackets denote statistically significant differences between PEEP titration strategies strategies, p-values are shown above the brackets. Pdriv driving pressure, PEEP positive end-expiratory pressure, PEEPARDSNetwork PEEP titrated according to the ARDS Network lower PEEP table, PEEPEstat,RS PEEP titrated according to the lowest elastance of the respiratory system, PEEPPtpexp PEEP titrated according to end-expiratory transpulmonary pressure, Ptpdriv transpulmonary driving pressure pressure
Respiratory mechanics
The titrated PEEP levels were lower in the prone position compared with the supine position using PEEPEstat,RS (11.6 ± 3.9 versus 9.0 ± 3.3 cm H2O, p < 0.001) and PEEPPtpexp (16.1 ± 5.8 versus 11.8 ± 6.3 cm H2O, p < 0.001) but not for PEEPARDSNetwork (Fig. 3A). Similarly, in the prone position, end-inspiratory plateau airway pressure (Pplat) decreased using PEEPEstat,RS (19.5 ± 4.2 versus 17.5 ± 3.3 cm H2O, p = 0.04) and PEEPPtpexp (24.5 ± 7.1 versus 20.9 ± 7.2 cm H2O, p < 0.001) (Fig. 3B). PEEP and Pplat differed significantly according to the position (p < 0.001) and PEEP strategy (p < 0.001). There was an interaction between position and PEEP strategy for PEEP and Pplat (p < 0.001).
Fig. 3.
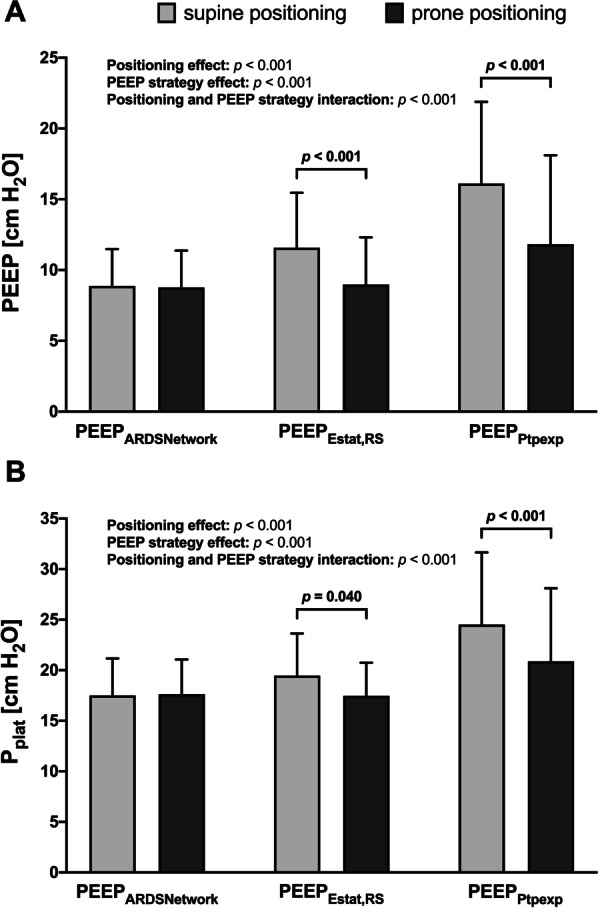
Effects of different PEEP titration strategies on PEEP and Pplat during supine and prone positioning. A PEEP setting according to different PEEP titration strategies during supine and prone positioning. B Pplat evaluated according to different PEEP titration strategies during supine and prone positioning. Bars are means + SD of 40 patients with moderate to severe ARDS. Brackets denote statistically significant differences between PEEP titration strategies strategies, p-values are shown above the brackets. PEEP positive end-expiratory pressure, PEEPARDSNetwork PEEP titrated according to the ARDS Network lower PEEP table, PEEPEstat,RS PEEP titrated according to the lowest elastance of the respiratory system, PEEPPtpexp PEEP titrated according to end-expiratory transpulmonary pressure, Pplat end-inspiratory plateau airway pressure
In the prone position, driving pressure (Pdriv) increased using PEEPEstat,RS (7.9 ± 1.8 versus 8.5 ± 1.7 cm H2O, p = 0.002) and PEEPPtpexp (8.4 ± 2.8 versus 9.1 ± 2.4 cm H2O, p = 0.02) (Fig. 4A). The effects of positioning (p = 0.019) and PEEP strategy (p = 0.006) were significant for Pdriv.
Mechanical power decreased using PEEPEstat,RS (19.2 ± 5.9 versus 17.5 ± 5.8 J/min, p < 0.001) and PEEPPtpexp (24.4 ± 9.8 versus 21.0 ± 9.6 J/min, p < 0.001) in the prone position. Mechanical power differed significantly according to the position (p < 0.001), PEEP strategy (p < 0.001) and interaction between positioning and PEEP strategy (p < 0.001) (Table 1).
Table 1.
Respiratory mechanics using three different PEEP titration strategies during supine and prone positioning
| PEEPARDSNetwork | PEEPEstat,RS | PEEPPtpexp | p values | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Supine | Prone | Supine | Prone | Supine | Prone | Positioning effect | PEEP strategy effect | Positioning and PEEP strategy interaction | |
| RR (breaths/min) | 22.3 ± 1.9 | 22.3 ± 1.9 | 22.3 ± 1.9 | 22.3 ± 1.9 | 22.3 ± 1.9 | 22.3 ± 1.9 | 1.000 | 1.000 | 1.000 |
| VT (mL/kg PBW) | 6.2 ± 0.3 | 6.2 ± 0.3 | 6.2 ± 0.3 | 6.2 ± 0.3 | 6.2 ± 0.3 | 6.2 ± 0.3 | 1.000 | 1.000 | 1.000 |
| Ppeak,RS (cm H2O) | 23.3 ± 5.1 | 23.5 ± 4.5 | 25.0 ± 5.1 | 23.3 ± 4.5* | 30.6 ± 8.8 | 27.2 ± 8.8* | < 0.001 | < 0.001 | < 0.001 |
| Pmean,RS (cm H2O) | 13.5 ± 2.9 | 13.5 ± 2.7 | 15.8 ± 4.0 | 13.6 ± 3.4* | 20.5 ± 6.1 | 16.5 ± 6.5* | < 0.001 | < 0.001 | < 0.001 |
| Pesinsp (cm H2O) | 14.2 ± 3.5 | 11.4 ± 3.7* | 14.8 ± 4.4 | 11.4 ± 4.6* | 17.1 ± 5.7 | 13.0 ± 6.0* | < 0.001 | < 0.001 | 0.007 |
| Pesexp (cm H2O) | 11.3 ± 3.1 | 7.7 ± 3.2* | 12.1 ± 4.0 | 7.9 ± 4.0* | 13.4 ± 5.4 | 9.4 ± 5.4* | < 0.001 | < 0.001 | 0.572 |
| ∆Pes (cm H2O) | 2.9 ± 1.6 | 3.6 ± 1.9* | 2.7 ± 1.5 | 3.5 ± 1.9* | 3.0 ± 1.8 | 3.5 ± 2.0* | < 0.001 | 0.141 | 0.339 |
| Estat,RS (cm H2O/L) | 21.3 ± 6.5 | 21.7 ± 6.0 | 19.5 ± 5.8 | 20.8 ± 5.2* | 20.7 ± 8.0 | 22.1 ± 6.4* | 0.032 | 0.023 | 0.255 |
| Estat,CW (cm H2O/L) | 7.1 ± 4.2 | 9.0 ± 5.0* | 6.6 ± 4.2 | 8.6 ± 4.9* | 7.3 ± 4.8 | 8.8 ± 5.2* | < 0.001 | 0.132 | 0.397 |
| Estat,L (cm H2O/L) | 14.0 ± 5.5 | 13.0 ± 5.3 | 12.8 ± 4.8 | 12.6 ± 5.4 | 13.4 ± 6.9 | 13.9 ± 6.8 | 0.565 | 0.093 | 0.087 |
| Mechanical power (J/min) | 17.5 ± 5.7 | 17.6 ± 5.5 | 19.2 ± 5.9 | 17.5 ± 5.8* | 24.4 ± 9.8 | 21.0 ± 9.6* | < 0.001 | < 0.001 | < 0.001 |
| IAP (cm H2O) | 8.3 ± 2.8 | 11.1 ± 3.0* | 8.7 ± 2.9 | 11.1 ± 3.5* | 9.9 ± 3.5 | 12.1 ± 3.8* | < 0.001 | < 0.001 | 0.238 |
| VD/VT (%) | 28.5 ± 10.9 | 25.2 ± 9.0* | 27.6 ± 10.8 | 24.7 ± 9.5* | 28.4 ± 10.5 | 25.8 ± 9.5* | < 0.001 | 0.224 | 0.739 |
| Ventilatory rate | 2.09 ± 0.5 | 2.11 ± 0.5 | 2.12 ± 0.5 | 2.13 ± 0.5 | 2.13 ± 0.5 | 2.14 ± 0.5 | 0.308 | 0.176 | 0.345 |
| EELV (mL) | 1630 ± 552 | 1972 ± 693* | 1920 ± 556 | 1993 ± 627 | 2140 ± 615 | 2108 ± 736 | 0.011 | < 0.001 | < 0.001 |
Values are means ± standard deviation of 40 patients with moderate to severe ARDS. Two-way repeated measures ANOVA was used to compare the effects of different PEEP titration strategies on respiratory mechanics during supine and prone positioning (p < 0.05)
Bold numbers represent statistically significant differences between groups
∆Pes difference between esophageal pressure at plateau airway pressure and positive end-expiratory pressure, EELV end-expiratory lung volume, Estat,CW static elastance of the chest wall, Estat,L static elastance of the lung, Estat,RS static elastance of the respiratory system, IAP intraabdominal pressure, PBW predicted body weight, PEEP positive end-expiratory pressure, PEEPARDSNetwork PEEP titrated according to the ARDS Network lower PEEP table, PEEPEstat,RS PEEP titrated according to the lowest elastance of the respiratory system, PEEPPtpexp PEEP titrated according to end-expiratory transpulmonary pressure, Pesexp esophageal pressure at positive end-expiratory pressure, Pesinsp esophageal pressure at plateau airway pressure, Pmean,RS mean airway pressure of the respiratory system, Ppeak,RS peak airway pressure of the respiratory system, RR respiratory rate, VD/VT ratio of physiologic dead space to tidal volume, VT tidal volume
*Significant differences at each PEEP titration strategy between supine and prone positioning
End-expiratory lung volume (EELV) increased using PEEPARDSNetwork (1630 ± 552 versus 1972 ± 693 mL, p = 0.026) in the prone compared to the supine position. EELV differed significantly according to the position (p = 0.011), PEEP strategy (p < 0.001), and interaction between positioning and PEEP strategy (p < 0.001) (Table 1).
Gas exchange and hemodynamics
Prone compared to supine positioning improved oxygenation regardless of the PEEP strategy (PaO2/FiO2: 136 ± 36 versus 228 ± 86 mm Hg in PEEPARDSNetwork, p < 0.001; 170 ± 72 versus 237 ± 91 mm Hg in PEEPEstat,RS, p = 0.002 and 192 ± 76 versus 240 ± 100 mm Hg in PEEPPtpexp, p = 0.002) (Table 2). Mean arterial pressure was higher in the prone position compared with the supine position independent of the PEEP strategy (83.0 ± 10.8 versus 87.1 ± 11.2 mm Hg in PEEPARDSNetwork, p = 0.005; 82.6 ± 9.8 versus 89.5 ± 11.9 in PEEPEstat,RS, p < 0.001 and 79.2 ± 11.3 versus 87.6 ± 11.4 in PEEPPtpexp, p < 0.001) (Table 2). Cardiac index was increased using PEEPEstat,RS (3.5 ± 0.9 versus 3.7 ± 1.0 L/min/m2, p = 0.021) and PEEPPtpexp (3.2 ± 0.7 versus 3.6 ± 0.8 L/min/m2, p < 0.001) during prone positioning (Table 2).
Table 2.
Gas exchange and hemodynamics using three different PEEP titration strategies during supine and prone positioning
| PEEPARDSNetwork | PEEPEstat,RS | PEEPPtpexp | p values | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Supine | Prone | Supine | Prone | Supine | Prone | Positioning effect | PEEP strategy effect | Positioning and PEEP strategy interaction | |
| PaO2/FiO2 (mm Hg) | 136 ± 36 | 228 ± 86* | 170 ± 72 | 237 ± 91* | 192 ± 76 | 240 ± 100* | < 0.001 | < 0.001 | < 0.001 |
| PaCO2 (mm Hg) | 57.0 ± 10.5 | 57.9 ± 10.5 | 57.6 ± 10.3 | 57.7 ± 10.7 | 57.6 ± 10.4 | 58.0 ± 10.4 | 0.346 | 0.308 | 0.297 |
| pHa | 7.3 ± 0.1 | 7.3 ± 0.1 | 7.3 ± 0.1 | 7.3 ± 0.1 | 7.3 ± 0.1 | 7.3 ± 0.1 | 0.812 | 0.070 | 0.101 |
| HR (beats/min) | 92.6 ± 19.1 | 93.1 ± 21.2 | 92.8 ± 20.5 | 93.2 ± 21.4 | 92.6 ± 19.2 | 92.4 ± 20.6 | 0.903 | 0.603 | 0.711 |
| MAP (mm Hg) | 83.0 ± 10.8 | 87.1 ± 11.2* | 82.6 ± 9.8 | 89.5 ± 11.9* | 79.2 ± 11.3 | 87.6 ± 11.4* | < 0.001 | 0.009 | 0.011 |
| Norepinephrine (µg/kg/min) | 0.2 ± 0.3 | 0.2 ± 0.3 | 0.2 ± 0.3 | 0.2 ± 0.3 | 0.2 ± 0.2 | 0.2 ± 0.2 | 0.182 | 0.458 | 0.756 |
| CVP (mm Hg) | 14.1 ± 6.4 | 16.8 ± 5.6* | 15.1 ± 6.9 | 16.8 ± 6.2 | 16.4 ± 7.4 | 17.5 ± 6.8 | 0.068 | < 0.001 | < 0.001 |
| ScvO2 (%) | 75.3 ± 7.4 | 81.2 ± 6.8 | 77.6 ± 5.2 | 82.8 ± 6.2 | 77.6 ± 6.1 | 81.4 ± 6.3 | < 0.001 | 0.002 | 0.046 |
| CI (L/min/m2) | 3.7 ± 1.0 | 3.7 ± 0.9 | 3.5 ± 0.9 | 3.7 ± 1.0* | 3.2 ± 0.7 | 3.6 ± 0.8* | 0.004 | < 0.001 | < 0.001 |
Values are means ± standard deviation of 40 patients with moderate to severe ARDS. Two-way repeated measures ANOVA was used to compare the effects of different PEEP titration strategies on gas exchange and hemodynamic parameters during supine and prone positioning (p < 0.05)
Bold numbers represent statistically significant differences between groups
CI cardiac index, CVP central venous pressure, HR heart rate, MAP mean arterial pressure, PaCO2 arterial partial pressure of carbon dioxide, PEEP positive end-expiratory pressure, PEEPARDSNetwork PEEP titrated according to the ARDS Network lower PEEP table, PEEPEstat,RS PEEP titrated according to the lowest elastance of the respiratory system, PEEPPtpexp PEEP titrated according to end-expiratory transpulmonary pressure, PaO2/FiO2 arterial oxygen partial pressure divided by the fraction of inspired oxygen, pHa negative logarithm of the molar concentration of dissolved hydronium ions in arterial blood, ScvO2 central venous oxygen saturation
*Significant differences at each PEEP titration strategy between supine and prone positioning
Further details regarding the effects of the three different PEEP titration strategies on respiratory mechanics, gas exchange and hemodynamics during supine and prone positioning are presented in Tables 1, 2, and Additional file 1: Table S4 to S9.
Discussion
In patients with moderate to severe ARDS under protective mechanical ventilation, the interaction between three different PEEP titration strategies, positioning, and the resulting Ptpexp was evaluated. PEEP was titrated according to oxygenation (PEEPARDSNetwork), the lowest static elastance of the respiratory system (Estat,RS) [8] aiming to achieve the lowest Pdriv, thus determining a compromise between recruitment and overinflation [9, 10], and targeting a positive Ptpexp to account for variability in lung and chest wall mechanics and optimize alveolar recruitment [11].
We found that (A) Ptpexp and Ptpinsp increased using PEEPARDSNetwork and PEEPEstat,RS during prone positioning; (B) PEEP and Pplat decreased when PEEP was titrated according to Estat,RS or Ptpexp in the prone position; (C) mechanical power was higher when PEEP was set using PEEPEstat,RS and PEEPPtpexp strategies compared to PEEPARDSNetwork during supine positioning, and prone positioning attenuated this effect. In short, the PEEP titration strategy as well as the positioning significantly affected oxygenation, ventilatory and mechanical variables, as well as hemodynamics in patients with moderate to severe ARDS. PEEPARDSNetwork minimizes parameters associated with VILI while providing adequate gas exchange and preserves hemodynamics in both supine and prone positioning.
Effects of prone positioning on transpulmonary pressure
Prone positioning induces substantial changes in lung mechanics because it reduces the compressive force of the mediastinum on the dependent lung regions, reduces pleural pressure [29], and thus modifies the vertical pleural pressure gradient [15]. From experimental [15, 30] and clinical [31, 32] studies, prone positioning promotes a more homogeneous distribution of regional aeration and compliance between the non-dependent and dependent lung compared with supine positioning. Riad et al. found an increase in the static elastance of the chest wall (Estat,CW) with prone positioning [32], which is in line with the results of this study as well as previous studies [33, 34]. The Estat,CW may be further affected by increased IAP in the prone position [32–35]. Because Estat,CW is increased in the prone position, ventilation and the resulting transpulmonary pressures are distributed more homogenously [36]. This has been shown to induce recruitment by shifting lung aeration more dorsally in patients with ARDS [37] and is associated with an improvement in gas exchange, ventilation/perfusion matching, and reduced shunting [33, 38, 39].
In our study, Ptpexp and Ptpinsp increased in prone positioning compared to the supine position in PEEPARDSNetwork and PEEPEstat,RS (Fig. 2) because of the decreased Pesinsp and Pesexp (Table 1). Correspondingly Ptpdriv decreased in PEEPARDSNetwork following prone positioning (Fig. 4B) while Pdriv remained unchanged. Pdriv has been correlated with mortality in patients with ARDS [40], but it does not reflect the transmural pressure applied to the lung given the effect of prone positioning on chest wall mechanics [41]. Therefore, Ptpdriv might be the most important variable evaluated during PEEP titration in prone positioning as it represents true lung stress independent of chest wall mechanics [42].
Effects of the PEEP strategy in supine and prone positioning
Setting PEEP to meet oxygenation goals (PEEPARDSNetwork) resulted in the least invasive ventilator settings (i.e., Pplat, PEEP, transpulmonary pressures, and mechanical power) (Fig. 3 and Table 1) and provided sufficient gas exchange and hemodynamics (Table 2). On the other hand, in the supine position, mechanical power due to Pplat, PEEP, and transpulmonary pressures progressively increased with PEEPEstat,RS and PEEPPtpexp (Table 1). The resulting Pplat increased similarly and was significantly higher for PEEPPtpexp (Fig. 3B). PEEP titration according to Estat,RS and Ptpexp has been evaluated in different clinical trials. The ART trial including patients with moderate to severe ARDS found increased mortality in patients randomized to PEEPEstat,RS compared with PEEPARDSNetwork. The investigators proposed breath stacking and dynamic overinflation as causative mechanisms [43]. Beitler et al. reported that a PEEP strategy based on Ptpexp compared with PEEPARDSNetwork was not associated with better survival in patients with moderate to severe ARDS [23]. In a post hoc analysis, PEEP titrated to a Ptpexp closer to 0 cm H2O was associated with greater survival than more positive or negative values, implying a reduction in alveolar cycling and hyperinflation [44]. Chiumello et al. evaluating different PEEP titration strategies found that the PEEPARDSNetwork was the only strategy where PEEP correlated with recruitability [45]. A recent meta-analysis found no beneficial effects on outcome when PEEP was set based on oxygenation or lowest Pdriv [46]. In PEEPARDSNetwork, Ptpinsp and Ptpexp was higher with prone positioning (Fig. 2), presumably because of a corresponding decrease in esophageal (pleural) pressure, decreased Ptpdriv (Fig. 4B), and unchanged Pplat, respiratory system, and lung elastance. The increase in EELV is associated with greater parenchymal aeration due to lung recruitment but not overdistension [19]. As highlighted by Gattinoni et al., the homogeneous distribution of transpulmonary pressure with dorsal shift of ventilation following prone positioning may improve recruitment while limiting alveolar overdistension [47]. Therefore, in the current study, the higher transpulmonary pressures during prone positioning might be associated with a favorable shift of ventilation and not overdistension. In fact, when using PEEPARDSNetwork, EELV increased while Pdriv and Pplat remained unchanged and Ptpdriv and mechanical power decreased after prone positioning. This finding of lung recruitment due to prone positioning with a low PEEP strategy is in line with the experimental findings from Scaramuzzo et al. [30] who found a significant recruitment of pulmonary parenchyma utilizing computed tomography and electrical impedance tomography. On the other hand, and albeit with significantly lower PEEP and Pplat in the prone position with the PEEPEstat,RS strategy, Pdriv and Ptpdriv did not change (Fig. 4A, B). Estat,RS increased mainly due to an increased Estat,CW and increased IAP with no further increase in EELV (Table 1). These findings are in line with the results of the experimental study by Katira et al. [15]. Because prone positioning reduced the vertical pleural pressure gradient, the effect of PEEP differs significantly from supine positioning. Lung homogeneity was maintained during a greater range of PEEP, and the level of PEEP to optimize the elastance of the dependent and non-dependent lung was lower compared with supine positioning. There is a ventral-dorsal pressure gradient of up to 10 cm H2O in the pleural space, therefore titrating PEEP to Ptpexp optimizes end-expiratory aeration in the zone between the non-dependent and dependent lung regions [48]. In our study, the PEEPPtpexp strategy resulted in ventilator settings with the highest airway pressures applied and transferred the most energy in the lung in the supine as well as the prone position (Table 1). This is in line with the findings of Beitler et al. who also found a trend to higher Pplat and PEEP levels in the PEEPPtpexp group of patients in the EPVent-2 trial [23]. Notably, EELV was higher compared to PEEPARDSNetwork and PEEPEstat,RS in the supine position but only compared with PEEPARDSNetwork in the prone position (Table 1).
Effect of prone positioning on gas exchange and hemodynamics
PaO2/FiO2 differed between PEEP titration strategies with supine positioning but not prone positioning (Table 2). Prone positioning increased PaO2/FiO2 irrespective of the PEEP titration strategy. Prone positioning has been shown to improve gas exchange by homogenization of the gas/tissue ratio [49] and shape matching of the lungs and chest wall [33, 47]. Correspondingly, we found an increase in Ptpexp, Ptpinsp, and Estat,CW in the prone position, which potentially changed regional ventilation, improved ventilation/perfusion matching, and thus increased oxygenation and decreased dead space ventilation (Table 2). Furthermore, prone positioning significantly increased CI and MAP when using PEEPEstat,RS and PEEPPtpexp (Table 2). Prone positioning has been shown to improve hemodynamics by several mechanisms. In preload-dependent patients, the increased IAP may improve venous return and thus cardiac output [50]. Right ventricular unloading is another beneficial effect of prone positioning because the improved gas exchange may limit hypoxic pulmonary vasoconstriction and permit a protective ventilation strategy with lower airway pressures [50]. The higher PEEP and Pplat when using PEEPEstat,RS and PEEPPtpexp likely negatively affected cardiac pre- and afterload. This was presumably mitigated by the reduced airway pressures and increased IAP in the prone position.
Clinical implications
Our data suggest that prone positioning increases Ptpexp irrespective of the chosen PEEP strategy and thus permits a reduction in PEEP compared with supine positioning, minimizing airway pressures and mechanical power as well as improving oxygenation and hemodynamics. The use of a minimal PEEP level to achieve adequate oxygenation (PEEPARDSNetwork) results in the most pronounced relative increase in Ptpexp in the prone position compared with the other PEEP titration strategies. PEEPARDSNetwork caused the least invasive ventilator settings (i.e., Ptpdriv and mechanical power) without an increase in Pdriv in prone positioning and with sufficient oxygenation and hemodynamics. This approach following the concept of "permissive atelectasis" might be sufficient to further minimize lung injury and VILI in lung protective ventilation [6].
Limitations
Our study has several limitations that should be addressed. The non-randomized sequence of positioning and PEEP titration strategies may influence the results due to the longitudinal design with repeated measurements. To minimize a potential interaction between different PEEP titration strategies and positioning, a 30-min equilibration period between the measurements was permitted [51]. Similarly, a recruitment maneuver was performed to standardize the history of lung volume [52] between each measurement, although frequent recruitment maneuvers are not systematically recommended [5]. Individual recruitability was not assessed before the study to account for differences in lung morphology. This may have contributed to the limited recruitment effect of PEEPEstat,RS and PEEPPtpexp.
Conclusions
Prone positioning increased transpulmonary pressures while improving oxygenation and hemodynamics in patients with moderate to severe ARDS when PEEP was titrated according to the ARDS Network lower PEEP table. This PEEP titration strategy minimized known parameters associated with VILI induction like transpulmonary driving pressure and mechanical power. We propose that a lower PEEP strategy (PEEPARDSNetwork) in combination with prone positioning may be part of a lung protective ventilation strategy in patients with moderate to severe ARDS.
Supplementary Information
Additional file 1. Study details, calculations, and additional analysis of the effects of three different PEEP titration strategies during supine and prone positioning.
Acknowledgements
Not applicable.
Abbreviations
- ARDS
Acute respiratory distress syndrome
- CI
Cardiac index
- CVP
Central venous pressure
- EELV
End-expiratory lung volume
- Estat,RS
Static elastance of the respiratory system
- Estat,CW
Static elastance of the chest wall
- FiO2
Fraction of inspired oxygen
- IAP
Intraabdominal pressure
- ICU
Intensive care unit
- MAP
Mean arterial pressure
- PaO2/FiO2
Ratio of arterial oxygen partial pressure divided by the fraction of inspired oxygen
- Pdriv
Driving pressure
- PEEP
Positive end-expiratory pressure
- PEEPARDSNetwork
PEEP titrated according to the ARDS Network lower PEEP table
- PEEPEstat,RS
PEEP titrated according to the lowest static elastance of the respiratory system
- PEEPPtpexp
PEEP titrated according to the end-expiratory transpulmonary pressure
- Pesexp
End-expiratory esophageal pressure
- Pesinsp
End-inspiratory esophageal pressure
- pHa
Negative logarithm of the molar concentration of dissolved hydronium ions in arterial blood
- Pplat
End-inspiratory plateau airway pressure
- Ptpdriv
Transpulmonary driving pressure
- Ptpexp
End-expiratory transpulmonary pressure
- Ptpinsp
End-inspiratory transpulmonary pressure
- RR
Respiratory rate
- SAPS II
Simplified Acute Physiology Score II
- ScvO2
Central venous oxygen saturation
- SOFA
Sequential Organ Failure Assessment
- VD/VT
Ratio of physiologic dead space to tidal volume
- VILI
Ventilator-induced lung injury
- VT
Tidal volume
Authors' contributions
CB, PTG, PP, TL, and JK participated in the study design. CB, PTG, FS, and JK performed the study. CB and JK processed the data and performed the statistical analysis. CB, MT, PP, PRMR, TL, and JK wrote the manuscript. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was supported by departmental funds.
Availability of data and materials
The datasets analyzed during this study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved (registration number 2018-609N-MA) by the local ethics committee (Medizinische Ethikkommission II, University Medical Centre Mannheim, Medical Faculty Mannheim of the University of Heidelberg, Mannheim) and registered at the German Clinical Trials Register (DRKS00017449). Written informed consent was acquired from each patient or their relatives.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Christoph Boesing, Email: christoph.boesing@umm.de.
Peter T. Graf, Email: tobias.graf@umm.de
Fabian Schmitt, Email: fabian.schmitt@medma.uni-heidelberg.de.
Manfred Thiel, Email: manfred.thiel@umm.de.
Paolo Pelosi, Email: ppelosi@hotmail.com.
Patricia R. M. Rocco, Email: prmrocco@biof.ufrj.br
Thomas Luecke, Email: thomas.luecke@medma.uni-heidelberg.de.
Joerg Krebs, Email: joerg.krebs@umm.de.
References
- 1.Meyer NJ, Gattinoni L, Calfee CS. Acute respiratory distress syndrome. Lancet (London, England) 2021;398(10300):622–637. doi: 10.1016/S0140-6736(21)00439-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Serpa Neto A, Deliberato RO, Johnson AEW, Bos LD, Amorim P, Pereira SM, et al. Mechanical power of ventilation is associated with mortality in critically ill patients: an analysis of patients in two observational cohorts. Intensive Care Med. 2018;44(11):1914–1922. doi: 10.1007/s00134-018-5375-6. [DOI] [PubMed] [Google Scholar]
- 3.Gattinoni L, Carlesso E, Brazzi L, Caironi P. Positive end-expiratory pressure. Curr Opin Crit Care. 2010;16(1):39–44. doi: 10.1097/MCC.0b013e3283354723. [DOI] [PubMed] [Google Scholar]
- 4.Mauri T. Personalized positive end-expiratory pressure and tidal volume in acute respiratory distress syndrome: bedside physiology-based approach. Crit Care Explor. 2021;3(7):e0486. doi: 10.1097/CCE.0000000000000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pelosi P, Ball L, Barbas CSV, Bellomo R, Burns KEA, Einav S, et al. Personalized mechanical ventilation in acute respiratory distress syndrome. Crit Care (London, England) 2021;25(1):250. doi: 10.1186/s13054-021-03686-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pelosi P, Rocco PRM, Gama de Abreu M. Close down the lungs and keep them resting to minimize ventilator-induced lung injury. Crit Care (London, England). 2018;22(1):72. doi: 10.1186/s13054-018-1991-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Acute Respiratory Distress Syndrome Network. Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–8. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 8.Kacmarek RM, Villar J, Sulemanji D, Montiel R, Ferrando C, Blanco J, et al. Open lung approach for the acute respiratory distress syndrome: a pilot, randomized controlled trial. Crit Care Med. 2016;44(1):32–42. doi: 10.1097/CCM.0000000000001383. [DOI] [PubMed] [Google Scholar]
- 9.Chen L, Jonkman A, Pereira SM, Lu C, Brochard L. Driving pressure monitoring during acute respiratory failure in 2020. Curr Opin Crit Care. 2021;27(3):303–310. doi: 10.1097/MCC.0000000000000827. [DOI] [PubMed] [Google Scholar]
- 10.Zhao Z, Chang MY, Chang MY, Gow CH, Zhang JH, Hsu YL, et al. Positive end-expiratory pressure titration with electrical impedance tomography and pressure-volume curve in severe acute respiratory distress syndrome. Ann Intensive Care. 2019;9(1):7. doi: 10.1186/s13613-019-0484-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Talmor D, Sarge T, Malhotra A, O'Donnell CR, Ritz R, Lisbon A, et al. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med. 2008;359(20):2095–2104. doi: 10.1056/NEJMoa0708638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pelosi P, Goldner M, McKibben A, Adams A, Eccher G, Caironi P, et al. Recruitment and derecruitment during acute respiratory failure: an experimental study. Am J Respir Crit Care Med. 2001;164(1):122–130. doi: 10.1164/ajrccm.164.1.2007010. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida T, Amato MBP, Grieco DL, Chen L, Lima CAS, Roldan R, et al. Esophageal manometry and regional transpulmonary pressure in lung injury. Am J Respir Crit Care Med. 2018;197(8):1018–1026. doi: 10.1164/rccm.201709-1806OC. [DOI] [PubMed] [Google Scholar]
- 14.Guerin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 15.Katira BH, Osada K, Engelberts D, Bastia L, Damiani LF, Li X, et al. Positive end-expiratory pressure, pleural pressure, and regional compliance during pronation: an experimental study. Am J Respir Crit Care Med. 2021;203(10):1266–1274. doi: 10.1164/rccm.202007-2957OC. [DOI] [PubMed] [Google Scholar]
- 16.Broccard A, Shapiro RS, Schmitz LL, Adams AB, Nahum A, Marini JJ. Prone positioning attenuates and redistributes ventilator-induced lung injury in dogs. Crit Care Med. 2000;28(2):295–303. doi: 10.1097/00003246-200002000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Valenza F, Guglielmi M, Maffioletti M, Tedesco C, Maccagni P, Fossali T, et al. Prone position delays the progression of ventilator-induced lung injury in rats: does lung strain distribution play a role? Crit Care Med. 2005;33(2):361–367. doi: 10.1097/01.ccm.0000150660.45376.7c. [DOI] [PubMed] [Google Scholar]
- 18.Beitler JR, Guerin C, Ayzac L, Mancebo J, Bates DM, Malhotra A, et al. PEEP titration during prone positioning for acute respiratory distress syndrome. Crit Care (London, England) 2015;19:436. doi: 10.1186/s13054-015-1153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guerin C, Albert RK, Beitler J, Gattinoni L, Jaber S, Marini JJ, et al. Prone position in ARDS patients: why, when, how and for whom. Intensive Care Med. 2020;46(12):2385–2396. doi: 10.1007/s00134-020-06306-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ARDS Definition Task Force. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–33. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 21.Akoumianaki E, Maggiore SM, Valenza F, Bellani G, Jubran A, Loring SH, et al. The application of esophageal pressure measurement in patients with respiratory failure. Am J Respir Crit Care Med. 2014;189(5):520–531. doi: 10.1164/rccm.201312-2193CI. [DOI] [PubMed] [Google Scholar]
- 22.Ely EW, Truman B, Shintani A, Thomason JW, Wheeler AP, Gordon S, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA. 2003;289(22):2983–2991. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 23.Beitler JR, Sarge T, Banner-Goodspeed VM, Gong MN, Cook D, Novack V, et al. Effect of titrating positive end-expiratory pressure (PEEP) with an esophageal pressure-guided strategy vs an empirical high PEEP-Fio2 strategy on death and days free from mechanical ventilation among patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2019;321(9):846–857. doi: 10.1001/jama.2019.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dellamonica J, Lerolle N, Sargentini C, Beduneau G, Di Marco F, Mercat A, et al. Accuracy and precision of end-expiratory lung-volume measurements by automated nitrogen washout/washin technique in patients with acute respiratory distress syndrome. Crit Care. 2011;15(6):R294. doi: 10.1186/cc10587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirkpatrick AW, Roberts DJ, De Waele J, Jaeschke R, Malbrain ML, De Keulenaer B, et al. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med. 2013;39(7):1190–1206. doi: 10.1007/s00134-013-2906-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270(24):2957–2963. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 27.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–10. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 28.Krebs J, Pelosi P, Rocco PRM, Hagmann M, Luecke T. Positive end-expiratory pressure titrated according to respiratory system mechanics or to ARDSNetwork table did not guarantee positive end-expiratory transpulmonary pressure in acute respiratory distress syndrome. J Crit Care. 2018;48:433–442. doi: 10.1016/j.jcrc.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Albert RK, Hubmayr RD. The prone position eliminates compression of the lungs by the heart. Am J Respir Crit Care Med. 2000;161(5):1660–1665. doi: 10.1164/ajrccm.161.5.9901037. [DOI] [PubMed] [Google Scholar]
- 30.Scaramuzzo G, Ball L, Pino F, Ricci L, Larsson A, Guerin C, et al. Influence of positive end-expiratory pressure titration on the effects of pronation in acute respiratory distress syndrome: a comprehensive experimental study. Front Physiol. 2020;11:179. doi: 10.3389/fphys.2020.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumaresan A, Gerber R, Mueller A, Loring SH, Talmor D. Effects of prone positioning on transpulmonary pressures and end-expiratory volumes in patients without lung disease. Anesthesiology. 2018;128(6):1187–1192. doi: 10.1097/ALN.0000000000002159. [DOI] [PubMed] [Google Scholar]
- 32.Riad Z, Mezidi M, Subtil F, Louis B, Guerin C. Short-term effects of the prone positioning maneuver on lung and chest wall mechanics in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2018;197(10):1355–1358. doi: 10.1164/rccm.201709-1853LE. [DOI] [PubMed] [Google Scholar]
- 33.Pelosi P, Tubiolo D, Mascheroni D, Vicardi P, Crotti S, Valenza F, et al. Effects of the prone position on respiratory mechanics and gas exchange during acute lung injury. Am J Respir Crit Care Med. 1998;157(2):387–393. doi: 10.1164/ajrccm.157.2.97-04023. [DOI] [PubMed] [Google Scholar]
- 34.Guerin C, Badet M, Rosselli S, Heyer L, Sab JM, Langevin B, et al. Effects of prone position on alveolar recruitment and oxygenation in acute lung injury. Intensive Care Med. 1999;25(11):1222–1230. doi: 10.1007/s001340051050. [DOI] [PubMed] [Google Scholar]
- 35.Mentzelopoulos SD, Roussos C, Zakynthinos SG. Prone position reduces lung stress and strain in severe acute respiratory distress syndrome. Eur Respir J. 2005;25(3):534–544. doi: 10.1183/09031936.05.00105804. [DOI] [PubMed] [Google Scholar]
- 36.Marini JJ, Gattinoni L. Improving lung compliance by external compression of the chest wall. Crit Care (London, England) 2021;25(1):264. doi: 10.1186/s13054-021-03700-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gattinoni L, Marini JJ, Pesenti A, Quintel M, Mancebo J, Brochard L. The, "baby lung" became an adult. Intensive Care Med. 2016;42(5):663–673. doi: 10.1007/s00134-015-4200-8. [DOI] [PubMed] [Google Scholar]
- 38.Lamm WJ, Graham MM, Albert RK. Mechanism by which the prone position improves oxygenation in acute lung injury. Am J Respir Crit Care Med. 1994;150(1):184–193. doi: 10.1164/ajrccm.150.1.8025748. [DOI] [PubMed] [Google Scholar]
- 39.Richter T, Bellani G, Scott Harris R, Vidal Melo MF, Winkler T, Venegas JG, et al. Effect of prone position on regional shunt, aeration, and perfusion in experimental acute lung injury. Am J Respir Crit Care Med. 2005;172(4):480–487. doi: 10.1164/rccm.200501-004OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372(8):747–755. doi: 10.1056/NEJMsa1410639. [DOI] [PubMed] [Google Scholar]
- 41.Grieco DL, Chen L, Brochard L. Transpulmonary pressure: importance and limits. Ann Transl Med. 2017;5(14):285. doi: 10.21037/atm.2017.07.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baedorf Kassis E, Loring SH, Talmor D. Mortality and pulmonary mechanics in relation to respiratory system and transpulmonary driving pressures in ARDS. Intensive Care Med. 2016;42(8):1206–1213. doi: 10.1007/s00134-016-4403-7. [DOI] [PubMed] [Google Scholar]
- 43.Writing Group for the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial I. Cavalcanti AB, Suzumura EA, Laranjeira LN, Paisani DM, Damiani LP, et al. Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs low PEEP on mortality in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2017;318(14):1335–45. doi: 10.1001/jama.2017.14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sarge T, Baedorf-Kassis E, Banner-Goodspeed V, Novack V, Loring SH, Gong MN, et al. Effect of esophageal pressure-guided positive end-expiratory pressure on survival from acute respiratory distress syndrome: a risk-based and mechanistic reanalysis of the EPVent-2 trial. Am J Respir Crit Care Med. 2021;204:1153–1163. doi: 10.1164/rccm.202009-3539OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chiumello D, Cressoni M, Carlesso E, Caspani ML, Marino A, Gallazzi E, et al. Bedside selection of positive end-expiratory pressure in mild, moderate, and severe acute respiratory distress syndrome. Crit Care Med. 2014;42(2):252–264. doi: 10.1097/CCM.0b013e3182a6384f. [DOI] [PubMed] [Google Scholar]
- 46.Ball L, Serpa Neto A, Trifiletti V, Mandelli M, Firpo I, Robba C, et al. Effects of higher PEEP and recruitment manoeuvres on mortality in patients with ARDS: a systematic review, meta-analysis, meta-regression and trial sequential analysis of randomized controlled trials. Intensive Care Med Exp. 2020;8(Suppl 1):39. doi: 10.1186/s40635-020-00322-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gattinoni L, Taccone P, Carlesso E, Marini JJ. Prone position in acute respiratory distress syndrome. Rationale, indications, and limits. Am J Respir Crit Care Med. 2013;188(11):1286–93. doi: 10.1164/rccm.201308-1532CI. [DOI] [PubMed] [Google Scholar]
- 48.Pelosi P, D'Andrea L, Vitale G, Pesenti A, Gattinoni L. Vertical gradient of regional lung inflation in adult respiratory distress syndrome. Am J Respir Crit Care Med. 1994;149(1):8–13. doi: 10.1164/ajrccm.149.1.8111603. [DOI] [PubMed] [Google Scholar]
- 49.Guerin C, Baboi L, Richard JC. Mechanisms of the effects of prone positioning in acute respiratory distress syndrome. Intensive Care Med. 2014;40(11):1634–1642. doi: 10.1007/s00134-014-3500-8. [DOI] [PubMed] [Google Scholar]
- 50.Jozwiak M, Teboul JL, Anguel N, Persichini R, Silva S, Chemla D, et al. Beneficial hemodynamic effects of prone positioning in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2013;188(12):1428–1433. doi: 10.1164/rccm.201303-0593OC. [DOI] [PubMed] [Google Scholar]
- 51.Chiumello D, Coppola S, Froio S, Mietto C, Brazzi L, Carlesso E, et al. Time to reach a new steady state after changes of positive end expiratory pressure. Intensive Care Med. 2013;39(8):1377–1385. doi: 10.1007/s00134-013-2969-x. [DOI] [PubMed] [Google Scholar]
- 52.Nishida T, Suchodolski K, Schettino GP, Sedeek K, Takeuch M, Kacmarek RM. Peak volume history and peak pressure-volume curve pressures independently affect the shape of the pressure-volume curve of the respiratory system. Crit Care Med. 2004;32(6):1358–1364. doi: 10.1097/01.ccm.0000128573.28173.2e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Study details, calculations, and additional analysis of the effects of three different PEEP titration strategies during supine and prone positioning.
Data Availability Statement
The datasets analyzed during this study are available from the corresponding author on reasonable request.