Abstract
Background
A genetic predisposition can lead to the rare disease pulmonary arterial hypertension (PAH). Most mutations have been identified in the gene BMPR2 in heritable PAH. However, as of today 15 further PAH genes have been described. The exact prevalence across these genes particularly in other PAH forms remains uncertain. We present the distribution of mutations across PAH genes identified at the largest German referral centre for genetic diagnostics in PAH over a course of > 3 years.
Methods
Our PAH-specific gene diagnostics panel was used to sequence 325 consecutive PAH patients from March 2017 to October 2020. For the first year the panel contained thirteen PAH genes: ACVRL1, BMPR1B, BMPR2, CAV1, EIF2AK4, ENG, GDF2, KCNA5, KCNK3, KLF2, SMAD4, SMAD9 and TBX4. These were extended by the three genes ATP13A3, AQP1 and SOX17 from March 2018 onwards following the genes’ discovery.
Results
A total of 79 mutations were identified in 74 patients (23%). Of the variants 51 (65%) were located in the gene BMPR2 while the other 28 variants were found in ten further PAH genes. We identified disease-causing variants in the genes AQP1, KCNK3 and SOX17 in families with at least two PAH patients. Mutations were not only detected in patients with heritable and idiopathic but also with associated PAH.
Conclusions
Genetic defects were identified in 23% of the patients in a total of 11 PAH genes. This illustrates the benefit of the specific gene panel containing all known PAH genes.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12931-022-01987-x.
Keywords: Pulmonary arterial hypertension, Genetic testing, Bi-allelic variants, Gene panel diagnostics
Background
Pulmonary arterial hypertension (PAH) is a rare disease characterised by a remodelling, narrowing or even occlusion of the small pulmonary vessels. This leads to an increased pulmonary vascular resistance, right heart failure and death [1]. The abnormal proliferation of mainly the small pulmonary arteries and rarely the venules in PAH patients can be induced by pathogenic variants (mutations) in genes involved in the transforming growth factor beta (TGF-β) pathway. In a large family with heritable PAH (HPAH) we identified linkage of the disease to chromosome 2q31-32 [2]. In this region the bone morphogenetic protein receptor 2 (BMPR2) gene has been discovered and the first mutations have been detected in families with HPAH [3, 4]. Since the year 2000, more than 650 different heterozygous pathogenic variants have been identified in this PAH gene [5]. It has been shown to be the most affected gene in HPAH (76–86%) and non-familial idiopathic PAH (12–14%) [6, 7]. Since the discovery of BMPR2 as a causative PAH gene, genetic defects have been detected in at least 15 further genes, most of them also involved in the BMPR-II signalling pathway [8, 9]. The co-factors of BMPR-II endoglin (ENG) and activin receptor like kinase 1 (ACVRL1) are predominantly altered in hereditary haemorrhagic telangiectasia associated PAH [10, 11]. Further PAH genes belong to the bone morphogenetic proteins such as BMP9 (GDF2), which initiates the BMPR-II signalling cascade or the SMAD proteins such as SMAD8 (SMAD9) and SMAD4 (SMAD4), which convey BMPR-II signalling from the cell membrane to the nucleus [8]. Over the years, it has become increasingly apparent that patients with distinct forms of PAH were shown to have a higher prevalence of pathogenic variants in specific genes. For example, we detected a mutation in the KLF2 gene in one family [9]. Patients with heritable pulmonary veno-occlusive disease (PVOD) carry pathogenic variants in the gene EIF2AK4, while in patients with congenital heart disease associated PAH and some paediatric cases of PAH mutations were identified in the transcription factors SOX17 and TBX4 [7, 12, 13].
Thus, it is crucial in a genetic diagnostic setting to always include also the most recently discovered genes. Initially, only Sanger sequencing of the three first described genes BMPR2, ACVRL1 and ENG had been conducted. Due to technical advances a switch to a PAH-specific gene panel based on next generation sequencing has enabled the analysis of all known PAH genes [14]. The adaptable nature of the panel ensures a comprehensive genetic diagnostic testing which may even assist in the correct diagnosis of PAH patients. In 2017 we began using our recently patented PAH gene panel (EP3507380) in the routine diagnostics setting as the largest German referral centre for genetic testing in PAH. To the initial set of 13 genes three additional genes (AQP1, ATP13A3 and SOX17) were added subsequent to their discovery as PAH causative genes. Since only a limited number of mutations has been published in the newer PAH genes, it is crucial to describe their role in the genetic background of a representative, unbiased set of consecutive PAH patients. Hence, the aim of this study was to demonstrate the value and the relevance for genetic testing in the clinical routine by assessing the actual proportion and range of genetic predisposition across genes and PAH aetiologies.
Methods
Patient cohort
All 325 PAH patients were diagnosed according to current guidelines including right heart catheterisation [1]. Samples from HPAH, idiopathic PAH (IPAH), associated PAH and PVOD patients were sent for genetic diagnostic testing to the Institute of Human Genetics at Heidelberg University between March 2017 and October 2020. Only unrelated PAH patients, who received a complete genetic diagnostic testing, were included in the reported cohort. Further targeted genetic testing was conducted in three HPAH families after the disease-causing familial mutation had been identified. Patients included in this study underwent genetic counselling and signed written informed consent for their data and samples to be used for research purposes. The Ethics Committee at Heidelberg University had no objections against this study (project identification codes 065/2001 and S-426/2017).
Genetic testing
DNA was extracted from 3 to 10 ml of EDTA-blood samples using an automated procedure (Autopure or QIAsymphony, QIAGEN, Germany). The quantity of DNA was measured with a spectrophotometer (Nanodrop and Qubit, Thermo Fisher Scientific, USA). The extracted DNA was diluted according to the manufacturer’s protocol and prepared with a customised SureSelect QXT kit to enrich for the PAH genes of interest (Agilent, Germany). DNA was sequenced by next-generation sequencing (NGS) using a PAH-specific gene panel including the following PAH diagnostic genes: ACVRL1, BMPR1B, BMPR2, CAV1, EIF2AK4, ENG, GDF2, KCNA5, KCNK3, KLF2, SMAD4, SMAD9 and TBX4. Following their discovery as PAH genes in March 2018 the three genes ATP13A3, AQP1 and SOX17 were added to the panel and sequenced in all subsequent patients. The procedure was carried out as previously described [14] and performed on the MiSeq (Illumina, USA). The entire coding region of all PAH genes plus the exon–intron boundaries were analysed. The PAH-specific gene panel received the recognition as European patent in March 2021 (EP3507380).
For a complete genetic testing of PAH patients, multiplex ligation-dependent probe amplification (MLPA) was performed to identify gross deletions and duplications in the genes ACVRL1, BMPR2 and ENG (P093-C2, MRC-Holland, Netherlands). Familial variants were sought by Sanger sequencing in family members (ABI Genetic Analyzer 3130xl, Applied Biosystems, USA). For variants predicted to result in a splice site loss > 2 base pair (bp) away from the canonical splice site, a new EDTA blood was requested. RNA was extracted using standard protocols (RNeasy Mini Kit, Qiagen). Complementary DNA was transcribed with superscript II reverse transcriptase (Life Technologies, USA) with random hexamer primers (Roche Diagnostics Deutschland GmbH, Germany). Polymerase chain reactions were designed with primers located in adjacent exons to the predicted alternative or lost splice site. Sizes of amplified products were measured by agarose gel electrophoresis and Sanger sequenced.
Variant characterisation
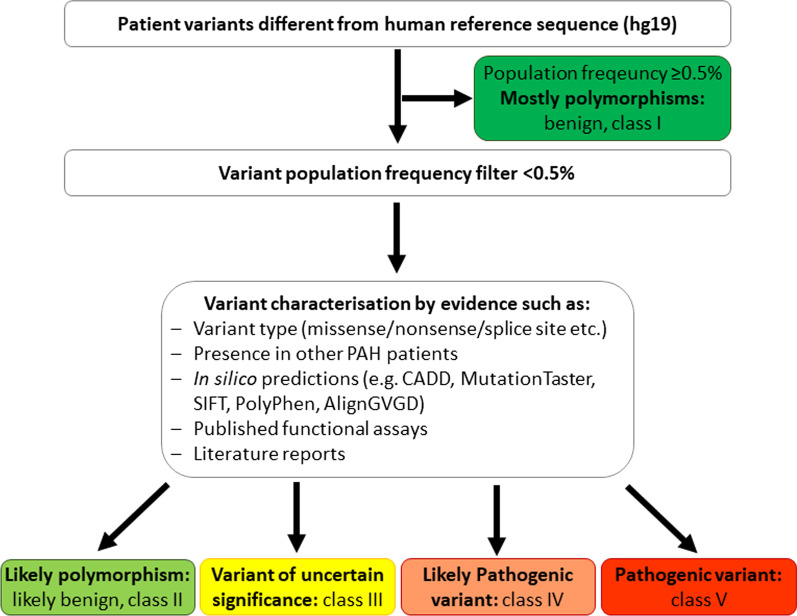
Data quality of each NGS run was confirmed by the amount of generated data, the average base call error rate ≤ 0.01% (≥ Q30) and a cluster density around 1000. The coverage was set to at least 100 reads for each exon and exon–intron boundary. The resulting variants were compared to the human reference genome (GRCh37/hg19). Any variants equal or above 0.5% population frequency in the genome aggregation database (GnomAD) with approximately 130.000 participants were excluded from further analyses. The remaining variants were characterised following the variant interpretation criteria published in the guidelines of the American College of Medical Genetics and Genomics (ACMG) [15]. They were classified as either class V pathogenic variants, class IV likely pathogenic variants, class III variants of uncertain significance or class II likely benign polymorphisms [15, 16]. The variant characterisation process is illustrated in Fig. 1. Pathogenic and likely pathogenic variants were defined for this work as “mutations”. To differentiate between class III, IV and V firstly, in silico prediction programmes (SIFT, Polyphen, AlignGVGD, MutationTaster; embedded in Alamut Visual versions 2.11-2.14) and CADD were employed. The possible impact on splice sites was assessed using SpliceSiteFinder-like, MaxEntScan, NNSPLICE, GeneSplicer and Human Splicing Finder. Finally, the variants were sought in the Human Genome Variation Database (HGMD versions 2017.1-2020.3) and in literature. Evidence was summarised in a medical report returned to the requesting physician to communicate the results to the patient during genetic counselling.
Fig. 1.
Flow chart of variant characterization process. Variants were firstly characterised by their population frequency in the genome aggregation database (GnomAD). Those with a frequency above 0.5% were excluded from further analysis. Secondly, all available evidence for each variant was taken into consideration to obtain a final classification. Likely pathogenic and pathogenic variants were termed “mutations”
Statistical analyses
Statistical analyses were performed by a medical statistician (NB). Descriptives were displayed as mean with standard deviation or frequency and respective percentage. Differences between patients with no mutation, BMPR2 mutation and any other mutation were analysed by analysis of variance (ANOVA) and with post-hoc two-sided t-tests in case of significant global test. P-values ≤ 0.05 were considered as significant. For comparison of categorical variables, chi-square tests were used. All analyses were performed with IBM SPSS V27.0 (Armonk, NY: IBM Corp).
Results
Patient cohort
In the cohort of 325 consecutive PAH patients, 70% were female (n = 227) with an age of onset of 47 ± 17 years (Table 1). The majority (62%) were in WHO functional class III at diagnosis, with severely impaired haemodynamics. Most patients (41%) were treated with a double combination therapy at time of this study, almost a third received triple combination therapy including prostacyclin (28%). Vasoresponsiveness was identified in 6%. Of the 325 patients 62% were classified as idiopathic PAH (IPAH) and 15% as heritable PAH (HPAH), (Table 1). Included were also patients with APAH forms (n = 55), PVOD (n = 16), persistent PH of the newborn (n = 3) and drugs and toxins induced PAH.
Table 1.
Patient characteristics at time of diagnosis stratified by BMPR2 and other mutations
| Parameter | All patients (n = 325) | No mutation (n = 251) | BMPR2 mutation (n = 50) | Other mutation (n = 24) | Overall p-value# | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD or % | n* | Mean ± SD or % | n* | Mean ± SD or % | n* | p-value+ | Mean ± SD or % | n* | p-value+ | ||||||
| Age at diagnosis (years) | 47 | ± 17 | 325 | 48 | ± 18 | 251 | 47 | ± 17 | 50 | 40 | ± 17 | 24 | 0.069 | ||
| Females | 227 | 70% | 325 | 177 | 71% | 251 | 38 | 76% | 50 | 12 | 50% | 24 | 0.066 | ||
| 6 min walking distance (m) | 393 | ± 124 | 249 | 390 | ± 129 | 195 | 412 | ± 96 | 40 | 380 | ± 128 | 15 | 0.529 | ||
| N-terminal pro-brain natriuretic peptide (ng/l) | 1539 | ± 2331 | 225 | 1479 | ± 2428 | 178 | 2062 | ± 1955 | 34 | 996 | ± 1796 | 13 | 0.285 | ||
| Pro-brain natriuretic peptide (pg/ml) | 315 | ± 291 | 19 | 320 | ± 311 | 14 | 235 | 199 | 4 | 11 | - | 1 | 0.602 | ||
| Diagnosis | 325 | 251 | 50 | < 0.001 | 24 | < 0.001 | < 0.001 | ||||||||
| IPAH | 201 | 61.8% | 173 | 68.9% | 20 | 40.0% | 8 | 33.3% | |||||||
| HPAH | 47 | 14.5% | 9 | 3.6% | 29 | 58.0% | 9 | 37.5% | |||||||
| CHD-APAH | 28 | 8.6% | 27 | 10.8% | 0 | – | 1 | 4.2% | |||||||
| CTD-APAH | 23 | 7.1% | 22 | 8.8% | 0 | – | 1 | 4.2% | |||||||
| PVOD | 16 | 4.9% | 12 | 4.8% | 0 | – | 4 | 16.7% | |||||||
| Persistent PH of the newborn | 3 | 0.9% | 3 | 1.2% | 0 | – | 0 | - | |||||||
| Drugs and toxins induced PAH | 3 | 0.9% | 1 | 0.4% | 1 | 2.0% | 1 | 4.2% | |||||||
| Portal hypertension | 2 | 0.6% | 2 | 0.8% | 0 | - | 0 | – | |||||||
| HIV-APAH | 2 | 0.6% | 2 | 0.8% | 0 | - | 0 | - | |||||||
| WHO functional class | 274 | 215 | 42 | 17 | 0.880 | ||||||||||
| WHO functional class I | 3 | 1% | 3 | 1% | 0 | 0% | 0 | 0% | |||||||
| WHO functional class II | 82 | 30% | 67 | 31% | 10 | 24% | 5 | 29% | |||||||
| WHO functional class III | 169 | 62% | 130 | 61% | 29 | 69% | 10 | 59% | |||||||
| WHO functional class IV | 20 | 7% | 15 | 7% | 3 | 7% | 2 | 12% | |||||||
| Current treatment | 281 | 222 | 42 | < 0.001 | 17 | 0.017 | < 0.001 | ||||||||
| Mono-therapy | 71 | 25% | 67 | 30.2% | 1 | 2.4% | 3 | 17.7% | |||||||
| Double combination therapy | 114 | 41% | 94 | 42.3% | 15 | 35.7% | 5 | 29.4% | |||||||
| Triple combination therapy | 80** | 28% | 45 | 20.3% | 26 | 61.9% | 9 | 52.9% | |||||||
| Calcium channel blockers alone | 16 | 6% | 16 | 7.2% | 0 | - | 0 | - | |||||||
| Haemodynamics | |||||||||||||||
| Mean pulmonary artery pressure (mmHg) | 49 | ± 15 | 274 | 47 | ± 15 | 216 | 55 | ± 11 | 42 | 0.011 | 54 | ± 15 | 16 | 0.253 | 0.006 |
| Pulmonary artery wedge pressure (mmHg) | 8.7 | ± 3.8 | 257 | 10.1 | ± 3.8 | 200 | 7.8 | ± 2.9 | 41 | 0.002 | 7.6 | ± 3.4 | 16 | 0.112 | < 0.001 |
| Pulmonary vascular resistance (Wood Units) | 10.8 | ± 6.0 | 249 | 9.9 | ± 5.7 | 195 | 15.5 | ± 5.8 | 38 | < 0.001 | 10.9 | ± 4.7 | 16 | 1.0 | < 0.001 |
| Cardiac output (l/min) | 4.3 | ± 1.7 | 248 | 4.5 | ± 1.7 | 194 | 3.4 | ± 1.1 | 38 | < 0.001 | 4.2 | ± 1.7 | 16 | 1.0 | < 0.001 |
| Cardiac index (l/min/m2) | 2.4 | ± 0.8 | 226 | 2.5 | ± 0.8 | 175 | 1.9 | ± 0.5 | 37 | < 0.001 | 2.4 | ± 0.7 | 14 | 1.0 | < 0.001 |
+Post-hoc t-test comparison with “no mutation” group in case of significant ANOVA
#ANOVA including all groups
*n varies for each parameter, exact numbers are listed in this column
**One patient received imatinib on compassionate use basis in addition to sildenafil, macitentan and treprostinil
APAH associated pulmonary arterial hypertension, CHD congenital heart disease, CTD connective tissue disease, HHT hereditary haemorrhagic telangiectasia, HIV human immunodeficiency virus, HPAH heritable pulmonary arterial hypertension, IPAH idiopathic pulmonary arterial hypertension
Pathogenic variant overview
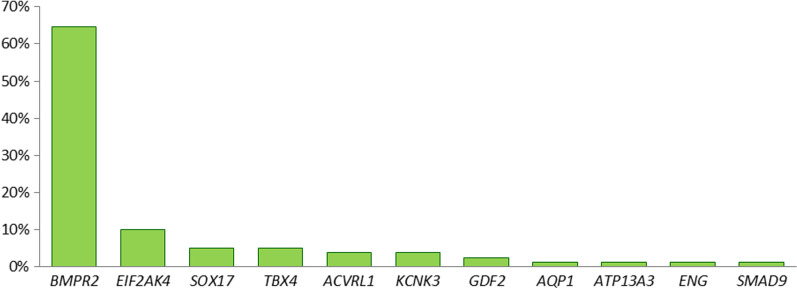
A total of 79 rare, disease-causing variants (mutations) were identified in 74 of the 325 (23%) patients. The majority of these variants (65%) were located in the gene BMPR2 but the remaining 28 (35%) variants were found in the genes 10 other genes (Fig. 2, Table 2). Apart from mutations in BMPR2, familial PAH patients also carried mutations in the genes ACVRL1, AQP1, GDF2, KCNK3, SMAD9 and SOX17 (Table 2 and Fig. 3). In total, the disease-causing variant could be identified in 83% of the HPAH patients. In the group of IPAH patients, 13% had mutations in six different genes (Table 2). Two patients with hereditary haemorrhagic telangiectasia and PAH carried mutations in ACVRL1 and were classified as HPAH. Four patients with PVOD presented with mutations in EIF2AK4. Out of three drugs and toxins induced PAH patients two were mutation carriers (66%). In contrast, in the group of APAH patients with connective tissue disease (n = 23) and congenital heart disease APAH (n = 28) only one patient each had a genetic predisposition. The 28 pathogenic variants in non-BMPR2 genes are listed in Table 3, the 51 pathogenic variants in the gene BMPR2 are provided in Additional file 1: Table S1.
Fig. 2.
Distribution of mutations across genes. The majority of mutations (65%) was identified in the BMPR2 gene. The remaining variants were found in 10 further PAH genes. Six large deletions or duplications in the BMPR2 gene were identified in nine patients using multiplex ligation-dependent amplification (MLPA)
Table 2.
Distribution of 79 (likely) pathogenic variants in 74 PAH patients
| IPAH (n = 201) | HPAH (n = 47) |
CHD-APAH (n = 28) | CTD-APAH (n = 23) | Drugs and toxins induced PAH (n = 3) | PVOD (n = 16) |
|
|---|---|---|---|---|---|---|
| BMPR2 | 20 | 30 | – | – | 1 | – |
| ACVRL1 | – | 3 | – | – | – | – |
| AQP1 | – | 1 | – | – | – | – |
| ATP13A3 | – | – | – | – | 1 | – |
| EIF2AK4 | – | – | – | 2 | – | 6 |
| ENG | 1 | – | – | – | – | – |
| GDF2 | 1 | 1 | – | – | – | – |
| KCNK3 | 1 | 2 | – | – | – | – |
| SMAD9 | – | 1 | – | – | – | – |
| SOX17 | 2 | 1 | 1 | – | – | – |
| TBX4 | 4 | – | – | – | – | – |
| Total | 29 in 27 patients | 39 in 39 patients | 1 in 1 patient | 2 in 1 patient | 2 in 2 patients | 6 in 4 patients |
APAH associated pulmonary arterial hypertension, CHD congenital heart disease, CTD connective tissue disease, HHT hereditary haemorrhagic telangiectasia, HPAH heritable pulmonary arterial hypertension, IPAH idiopathic pulmonary arterial hypertension, PVOD pulmonary veno-occlusive disease
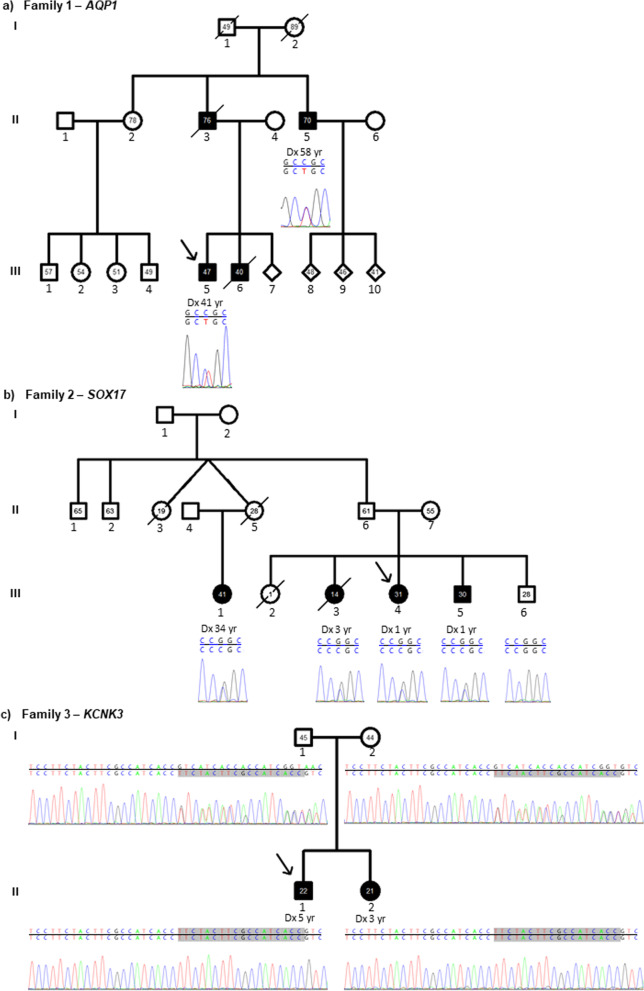
Fig. 3.
Pedigrees of three non-BMPR2 families. a Pedigree of AQP1 family. The index patient and his uncle carried the heterozygous missense mutation in exon 1 of the gene AQP1 X1 c.376C > T p.(Arg126Cys). The brother and father of the index patient had died of PAH. No further samples were available from the family. b Pedigree of SOX17 family. The familial heterozygous missense mutation was located in exon 2 of the gene SOX17 c.413G > C p.(Arg138Pro). It was prevalent in all three siblings and cousin of the index with PAH, while being absent in the only healthy sibling of the index patient. c Pedigree of KCNK3 family. Both parents were heterozygous for the 18 bp duplication in exon 1 c.250_267dup p.(Val84_Thr89dup) displayed in grey. Downstream to the left are the same 18 bp present in the wild-type reference sequence. Both children developed PAH and were homozygous for the duplication. Filled symbol: PAH, empty symbol: healthy family member; the number within the symbols is current age or age at death, Dx: age at diagnosis; upper sequence: wild type reference sequence, bottom sequence: sequence of family member; grey: duplicated sequence not present in the wild-type reference sequence
Table 3.
(Likely) pathogenic variants in non-BMPR2 genes
| Gene | Exon | DNA | Protein | CADD score | GnomAD count | Diagnosis |
|---|---|---|---|---|---|---|
| ACVRL1 | 3 | c.205T > C | p.(Cys69Arg) | 25 | 0 | HPAH (HHT) |
| ACVRL1 | 5 | c.578T > C | p.(Leu193Pro) | 31 | 0 | HPAH (HHT) |
| ACVRL1 | 10 | c.1450C > T | p(Arg484Trp) | 26 | 0 | HPAH |
| AQP1 | 1 | c.376C > T | p.(Arg126Cys) | 26 | 0 | HPAH |
| ATP13A3 | 14 | c.1540C > T | p.(Gln514*) | NA (Nonsense) | 0 | Drugs and toxins induced PAH [17] |
| EIF2AK4 | 1 | c.1A > T | p.(Met1?)b | NA (Start loss) | 0 | PVOD |
| EIF2AK4 | 6 | c.595-1G > A | intronic b | 35 | 0 | PVOD |
| EIF2AK4 | 6 | c.641delA | p.(Lys241Argfs*21)a | NA (Deletion) | 0 | PVOD |
| EIF2AK4 | 9 | c.1362C > A | p.(Cys454*)b | NA (Nonsense) | 0 | CTD-APAH |
| EIF2AK4 | 21 | c.2965C > T | p.(Arg989Trp)b | 31 | 5 | PVOD |
| EIF2AK4 | 24 | c.3380C > T | p.(Ala1127Val)b | 26 | 0 | PVOD |
| EIF2AK4 | 25 | c.3443T > C | p.(Leu1148Ser)b | 31 | 0 | CTD-APAH |
| EIF2AK4 | 31 | c.4260G > A | p.(Trp1420*)a | NA (Nonsense) | 0 | PVOD |
| ENG | 12 | c.1646G > A | p.(Cys549Tyr) | 26 | 0 | IPAH |
| GDF2 | 2 | c.857dupA | p.(Leu287Alafs*11) | NA (Duplication) | 0 | IPAH |
| GDF2 | 1 | c.329G > A | p.(Arg110Gln) | 32 | 0 | HPAH |
| KCNK3 | 2 | c.641T > C | p.(Leu214Pro) | 27 | 0 | IPAH |
| KCNK3 | 2 | c.340G > A | p.(Ala114Thr) | 26 | 0 | HPAH |
| KCNK3 | 1 | c.250_267dup TTCTACTTCGCCATCACC | p.(Val84_Thr89dup)a | NA (Duplication) | 0 | HPAH |
| SMAD9 | 2 | c.29T > C | p.(Leu10Pro) | 27 | 0 | HPAH |
| SOX17 | 1 | c.273_277delAGACC | p.(Asp92Alafs*68) | NA (Deletion) | 0 | IPAH |
| SOX17 | 2 | c.413G > C | p.(Arg138Pro) | 33 | 0 | HPAH |
| SOX17 | 2 | c.1245A > G | p.(*415Trp) | Stop loss | 0 | CHD-APAH |
| SOX17 | 2 | c.499_520del | p.(Leu167Trpfs*213) | NA (Deletion) | 0 | IPAH |
| TBX4 | 2 | c.278G > A | p.(Gly93Asp) | 33 | 0 | IPAH |
| TBX4 | 3 | c.400delT | p.(Trp134Glyfs*38) | NA (Deletion) | 0 | IPAH |
| TBX4 | 6 | c.709_710ins56bp | p.(Gln237Profs*18)b | NA (Insertion) | 0 | IPAH |
| TBX4 | 8 | c.1543G > A | p.(Glu515Lys)b | 24 | 0 | IPAH |
aHomozygous variant
bTogether with 2nd variant in same gene
APAH associated pulmonary arterial hypertension; CADD score Combined Annotation Dependent Depletion score, summation score based on different in silico prediction programmes, CHD congenital heart disease, CTD connective tissue disease, GnomAD Genome Aggregation Consortium Database with n = 125,748 samples, HHT hereditary haemorrhagic telangiectasia, HPAH heritable pulmonary arterial hypertension, IPAH idiopathic pulmonary arterial hypertension, PVOD pulmonary veno-occlusive disease. Used Ensembl transcripts and respective reference sequences: ACVRL1: ENST00000267008.3, NM_000020; AQP1: ENST00000311813.11, NM_198098.3; ATP13A3: ENST00000256031.4, NM_024524.3; EIF2AK4: ENST00000263791.1 NM_001013703; ENG: ENST00000373203.2, NM_001114753; GDF2: ENST00000249598, NM_016204.2; KCNK3: ENST00000302909, NM_002246.2; SMAD9: ENST00000379826.1, NM_001127217; SOX17: ENST00000297316, NM_022454.3; TBX4: ENST00000240335.1, NM_018488.3
Clinical characteristics of variant carriers
From the 24 non-BMPR2 mutation carriers 50% were male, in contrast to the approximately 25% male patients in the BMPR2-mutation carrier and no mutation group (Table 1). This difference was not statistically significant likely due to the small sample size in the non-BMPR2 group. BMPR2-mutation carriers showed worse haemodynamic parameters at diagnosis compared to PAH patients without a genetic predisposition. The mean pulmonary artery pressure (p = 0.011) and pulmonary vascular resistance (p < 0.001) were higher while cardiac output and cardiac index were lower (both p < 0.001). Carriers of pathogenic variants in other genes were haemodynamically not statistically different to non-carriers.
Patients with any pathogenic variant more often received a double or triple therapy compared to those patients without a genetic predisposition (p < 0.001). Of the 67 patients receiving mono-therapy, 94% had no pathogenic variant (Table 1). None of the 16 vasoresponders exclusively receiving calcium channel blockers carried a pathogenic variant. The only vasoresponder with a genetic predisposition received triple therapy in addition to a calcium channel blocker.
HPAH families with mono-allelic pathogenic variants
Pathogenic variants were identified in seven genes in 39 HPAH families (Table 2). Around one third had further family members tested for the familial variant (data not shown). In the following, we focused on those families with at least two PAH patients tested for the familial variant in a non-BMPR2 gene.
The first family had a heterozygous missense mutation in the gene AQP1. The mutation c.376C > T p.(Arg126Cys) led to the exchange of the amino acid arginine by cytosine (Fig. 3A). It was absent in the genome aggregation database and had been previously characterised as disease causing in another IPAH patient [7]. In this family, the missense mutation was present in the index patient and his uncle, who had been diagnosed with PAH aged 58 years. Two additional family members had already died of PAH. The grandfather had died of heart failure aged 49 years, while dyspnoea was unknown at the time of death.
A second HPAH family showed co-segregation of PAH with the heterozygous missense mutation c.413G > C p.(Arg138Pro) in the gene SOX17. Four family members of the youngest generation (III) had HPAH (Fig. 3B). Both female twins of the second generation had died before reaching the age of 30, however the cause of death was unclear. The twin II:5 died shortly post-partum while being an obligate carrier of the SOX17 mutation. An additional sister of the index patient had died aged 13 months of an unknown cause. The only healthy sibling (III:6) of the index patient was a homozygous carrier of the SOX17 wild-type allele (Fig. 3B). Since the father of the index patient (II:6) was also an obligate carrier a reduced penetrance has to be assumed. This is one of the largest SOX17 families described up to date.
In the KCNK3 family both children suffered from PAH (Fig. 3C) as described in detail in the next section.
Two mutations in the same gene
Heritable PVOD, in contrast to PAH, is autosomal recessively inherited. In four PVOD patients bi-allelic variants in the gene EIF2AK4 were identified (Table 3). Two were homozygous mutations while two were most likely compound heterozygotes. A co-segregation analysis to show the inheritance of each variant from a different parent has not been performed but the clinical diagnosis of the patients supported the presence of the pathogenic variants on two different alleles. One patient diagnosed with connective tissue disease APAH also carried two most likely bi-allelic pathogenic variants in the EIF2AK4 gene (Table 3). The patient suffered from systemic lupus erythematosus, was treated with targeted PAH medication and had a reduced single-breath diffusion capacity of carbon monoxide (DLCO) of 15%.
Only in very rare cases homozygous variants have been identified in PAH patients so far. In this study, one young HPAH patient aged 5 years at diagnosis carried a homozygous 18 bp duplication in the gene KCNK3 (Fig. 3C). The in-frame duplication was located in a repeat-free region of exon 1. Subsequently, the homozygous duplication was also identified in his affected little sister (Fig. 3C). She was diagnosed with PAH at three years of age and additionally presented with seizures. Both healthy parents were heterozygous carriers of the same duplication.
Two mutations were found in the same IPAH patient in the gene TBX4 (Table 3). The first variant was a 56 bp insertion in exon 6 leading to a premature stop codon and thus, most likely resulted in nonsense mediated decay of the transcribed messenger RNA. The second variant was a missense mutation located in exon 8 of TBX4 gene. Since no RNA was available from the patient, the respective messenger RNA could not be sequenced to identify whether both variants were present on the same allele (in cis) or on two different alleles (in trans).
Finally, two heterozygous BMPR2 mutations were identified in the same IPAH patient. Both variants were located in cis in exon 8. The first variant was a missense mutation and the second variant was a deletion of 7 bp followed by the insertion of three base pairs leading to a frame shift and premature stop codon (see Additional file 1: Table S1). Due to the physical proximity of the two variants, their joined presence or absence could be verified in the 150 bp next generation sequencing reads, thus confirming their mono-allelic presence within the same inherited haplotype.
Discussion
This study presents a large cohort of consecutive PAH patients who have been genetically assessed using most recent methods including all known PAH genes. Genetic defects were identified in 23% of the patients in a total of 11 PAH genes. This illustrates the benefit of the PAH-specific gene panel containing all known PAH genes. While 65% of mutations were identified in the main PAH gene BMPR2, 35% of disease-causing variants were located in ten other PAH genes. A genetic predisposition could be identified in six different forms of PAH. In three families co-segregating pathogenic variants in the genes AQP1, KCNK3 and SOX17 were revealed. Finally, bi-allelic variants were identified not only in EIF2AK4, but also in KCNK3 and potentially TBX4.
Value of PAH-specific panel diagnostics
The PAH-specific gene panel diagnostics developed in Heidelberg, Germany (European patent EP3507380) proved to be a valuable tool to identify genetic predisposition in PAH patients. The technique allows to sequence all known PAH genes in several patients at the same time [14]. Thus, it constitutes a time- and cost-efficient technique which can be continuously updated following the discovery of novel PAH genes. After starting with 13 PAH genes in 2017, the three genes AQP1, ATP13A3 and SOX17 were added after their description in 2018. In total, 24 pathogenic variants located in eight genes other than the traditionally Saner sequenced PAH/HHT genes BMPR2, ACVRL1 and ENG were detected. As an alternative to panel sequencing whole exome or whole genome sequencing could have been applied. However, by focusing on genes with an established gene-disease relationship for PAH and by using our PAH-specific gene panel faster results could be obtained, with less extensive data and fewer variants of uncertain significance in genes likely not involved in the disease pathogenesis. The high coverage of at least × 100 for each base pair moreover guaranteed the detection of any potential mutations. In contrast, a continuous high coverage for whole exome sequencing is currently still hard to achieve. Thus, the PAH gene panel may be the method of choice for a targeted high-quality approach.
Familial predisposition
The results of the PAH-specific gene panel are not only relevant for index patients but also for healthy family members. In particular BMPR2 mutation carriers show a younger age of onset, worse haemodynamics and a faster disease progression [18] in comparison to patients without a predisposing variant. Thus, a closer supervision of these patients and potentially a faster therapy escalation should be considered. Family members, who currently show no symptoms of PAH but carry a familial mutation, should be regularly seen for a clinical screening at an expert center for pulmonary hypertension to identify a potential disease onset as early as possible [19, 20].
While in most HPAH families BMPR2 is the causal gene, we showed that 23% of families carried mutations in six other PAH genes. This is the second study to identify a HPAH family with a pathogenic missense variant in the water channel gene aquaporin 1 (AQP1). Three PAH families with mutations in AQP1 have been published previously in a single study [7]. Of these families, two shared the familial missense variant, which was distinct from the one in our study. Thus, we describe the third familial pathogenic AQP1 variant so far.
Mutations in the SOX17 transcription factor gene are also very new findings in HPAH. At present, only two other HPAH families have been reported [7, 21]. Variants in this gene are increasingly identified in young PAH patients and patients with congenital heart disease APAH [13, 22]. While we observed a very early onset of PAH in three of four familial SOX17 PAH patients with diagnosis at ages 1–3 years, no congenital heart disease was known.
In a third family, two children carried a homozygous duplication in the gene KCNK3 and presented with a very early age of onset. Both heterozygous parents in our family were unaffected. In the only other previously described KCNK3 family with a homozygous mutation PAH manifested in the homozygous 2 months old child and, in addition, post-partum in the heterozygous mother [23]. Apart from PAH, the sister of the index patient in our KCNK3 family presented with seizures. For KCNK3, which encodes the two-pore potassium channel TASK1, this phenotype has never been described before. A pathogenetic link is likely as seizures are known to be caused by pathogenic variants in other potassium channels [24].
Prevalence of mutations in PAH subgroups
The prevalence of disease-causing variants was the highest for HPAH patients, followed by drugs and toxins induced PAH, PVOD and IPAH patients. In these four groups we could identify mutations in 13–83% patients. In contrast, connective tissue disease APAH patients and congenital heart disease APAH patients both showed a 4% mutation prevalence. This frequency was in line with previous reports of around 3–5% [22]. Interestingly, while connective tissue disease can be a risk factor for PVOD development [25], characteristic bi-allelic variants in the EIF2AK4 gene addition in a connective tissue disease APAH patient have only been described in two other patients before [22]. The congenital heart disease APAH patient carried a mutation in the SOX17 gene. This is in concert with the data published by Zhu and colleagues who described a 3.2% mutation rate for SOX17 in a cohort of congenital heart disease APAH patients [13]. No disease-causing variant could be identified in the two portal hypertension patients, two HIV-APAH patients nor the three patients with persistent PH of the newborn. However, all of these subgroups were too small to draw conclusions.
BMPR2 mutation carriers presented with worse haemodynamics at diagnosis compared to patients without a genetic predisposition. Interestingly, this was not the case for patients with pathogenic variants in other genes than BMPR2. Nevertheless, not only BMPR2-muation carriers but also mutation carriers in the other genes received more often triple therapy at the time of this study than patients with no pathogenic variant. Whether this was due to the genetic variants leading to a more severe disease phenotype or the genetic diagnostic report prompted a faster combination therapy remains to be determined.
Limitations
Patients in this study were analysed with the PAH-specific gene panel. While this targeted approach is the most cost and time effective method, there may be also pathogenic variants in other genes which could have been overlooked. However, since all established PAH genes were included on the panel, variants in other genes without a clear gene-disease relationship would have required further research and would have not been part of an official genetic diagnostic report. Similarly, variants which were located more than 20 base pairs into the introns were not analysed. For these variants extensive functional analyses would have been required to evaluate their potential effect on gene transcription. In addition, a detailed clinical characterisation of mutation carriers in genes other than BMPR2 would have been interesting for each gene. However, the respective numbers were too low to permit such analysis.
Conclusions
Of the 325 consecutive PAH patients referred for genetic testing in this study approximately each fifth patient had a genetic background leading to PAH. Pathogenic variants were not only identified in the BMPR2 gene but also in ten further PAH genes. Genetic predisposition was not restricted to HPAH, IPAH and PVOD but could also be identified in some patients with APAH. HPAH families with mutations in the genes AQP1, SOX17 or KCNK3 underline the broad spectrum of mutations. The PAH-specific gene panel based on next generation sequencing proofed to be readily extendable for newly discovered genes to include all known PAH genes at any time point. Hence, our approach provided a valuable, timely, high-quality tool for the clinical diagnostic work-up of PAH patients.
Supplementary Information
Additional file 1: Table S1. (Likely) pathogenic BMPR2 variants identified in the cohort.
Acknowledgements
Not applicable.
Abbreviations
- ACMG
American College of Medical Genetics and Genomics
- ACVRL1
Activin receptor like kinase 1
- APAH
Associated pulmonary arterial hypertension
- AQP1
Aquaporin 1
- BMPR2
Bone morphogenetic protein receptor 2
- DLCO
Diffusion capacity of carbon monoxide
- ENG
Endoglin
- HPAH
Heritable pulmonary arterial hypertension
- HIV
Human immunodeficiency virus
- IPAH
Idiopathic pulmonary arterial hypertension
- MLPA
Multiplex ligation-dependent probe amplification
- NGS
Next generation sequencing
- PAH
Pulmonary arterial hypertension
- PVOD
Pulmonary veno-occlusive disease
- TGF-β
Transforming growth factor beta
Authors' contributions
Conceptualization, CAE, KH and EG; methodology, CAE, ZS and KH; formal analysis, CAE and ZS; investigation, CAE, ZS, MS, DC, PX, HG, NS, HAG, HJS, MLe, MH, JK, NB, SH, BE, KM, SR, RE, HK, MMH, KMO, MLa, TJL, KH and EG; resources, KH and EG; data curation, CAE and ZS; writing—original draft preparation, CAE, KH and EG; writing—review and editing, ZS, MS, DC, PX, HG, NS, HAG, HJS, MLe, MH, JK, NB, SH, BE, KM, SR, RE, HK, MMH, KMO, MLa and TJL; visualization, CAE and ZS; supervision, KH and EG; project administration, CAE; All authors have read and agreed to the published version of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. For the publication fee we acknowledge financial support by Deutsche Forschungsgemeinschaft (DFG) within the funding programme “Open Access Publikationskosten” as well as by Heidelberg University. NS and HAG were supported by Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) project number 268555672 – SFB A1213, Project A06 and A10; SH was supported by M. Osler Foundation Germany. MLa received institutional funding from the German Federal Ministry of Education and Research (BMBF 01EO1503) and project funding from BRAHMS – Thermo Fisher Scientific.
Availability of data and materials
The dataset supporting the conclusions of this article is included within the article (and its additional file).
Declarations
Ethics approval and consent to participate
Patients included in this study underwent genetic counselling and signed written informed consent for their data and samples to be used for research purposes. The Ethics Committee at Heidelberg University had no objections against this study (project identification codes 065/2001 and S-426/2017).
Consent for publication
Not applicable.
Competing interests
E.G., C.A.E. and K.H. report an issued patent “Gene panel specific for pulmonary hypertension and its uses” European Patent ID: EP3507380. Z.S., M.S., D.C., M.Le. and J.K. declare that they have no competing interests related to this study. P.X. has received personal fees from MSD and OMT outside the submitted work. N.B. received speaker fees from Actelion pharmaceuticals / Janssen Medical, Bayer HealthCare and MSD, outside the submitted work. BE received travel fees, con-sulting fees, speaking fees, and/or honoraria from Actelion, MSD, Bayer and OMT, outside the submitted work. H.A.G. received consulting fees and or payment or honoraria for lectures from Actelion, Janssen, Bayer, MSD, Acceleron, Gossamer Bio, Novartis, Pfizer outside the submitted work. H.K. reports grants from GSK and Actelion and personal fees from Pfizer, GSK, Bayer, United Therapeutics, Actelion and Jansen outside the submitted work. H.G. reports personal fees from Actelion, AstraZeneca, Bayer, BMS, GSK, Janssen-Cilag, Lilly, MSD, Novartis, OMT, Pfizer, and United Therapeutics outside the submitted work. H.J.S. reports consulting fees from Actelion/Janssen and speaker fees for Actelion, Bayer, GSK, Janssen and MSD outside the submitted work. K.M. reports personal speaker honoraria from Janssen, MSD, GSK outside the submitted work. K.M.O. received fees for lectures and/or consultations from Acceleron, Actelion, Bayer, GSK, Janssen, MSD, Pfizer and United Therapeutics outside the submitted work. M.La. received con-sultancy or lecture honoraria from Actelion, Bayer, BRAHMS – Thermo Fisher Scientific, Daiichi-Sankyo, MSD and Bristol-Myers Squibb-Pfizer outside the submitted work. M.H. has re-ceived personal fees for consultations and lectures from Acceleron, Actelion, AstraZeneca, Bayer, BerlinChemie, GSK, Janssen-Cilag, MSD, and Novartis, outside the publication. M.M.H. has re-ceived fees for consultations and/or lecturers from Acceleron, Actelion, Bayer, GSK, Janssen, MSD and Pfizer, all outside the submitted work. N.S. received honoraria for presentations from Actelion outside the submitted work. R.E. received honoraria for lectures from GSK, United Therapeutics, OMT, Janssen, outside the submitted work. S.H. received honoraria for lectures, consultancy, or both from Actelion, AOP, Bayer/MSD, GSK, Novartis, OMT, Pfizer, and United Therapeutics out-side the submitted work. S.R. has received honoraria for lectures, consultancy, or both from Ac-tavis, Actelion, Bayer, GSK, Lilly, Novartis, Pfizer, and United Therapeutics outside the submitted work. T.L. received consulting fees from Acceleron Pharma, Actelion/Janssen-Cilag, Bayer, GSK, MSD and personal honoraria for lectures from Actelion/Janssen-Cilag, BMS, MSD, Pfizer outside the submitted work. EG has received grants and personal fees from Actelion, Bayer AG, and MSD; grants from GSK, Novartis, and United Therapeutics and personal fees from SCOPE, OrPha Swiss GmbH, and Zurich Heart House, outside the submitted work. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Heart J. 2016;37:67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 2.Grünig E, Janssen B, Mereles D, Barth U, Borst MM, Vogt IR, Fischer C, Olschewski H, Kuecherer HF, Kübler W. Abnormal pulmonary artery pressure response in asymptomatic carriers of primary pulmonary hypertension gene. Circulation. 2000;102:1145–1150. doi: 10.1161/01.CIR.102.10.1145. [DOI] [PubMed] [Google Scholar]
- 3.Deng Z, Morse JH, Slager SL, Cuervo N, Moore KJ, Venetos G, Kalachikov S, Cayanis E, Fischer SG, Barst RJ, et al. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am J Hum Genet. 2000;67:737–744. doi: 10.1086/303059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA, 3rd, Loyd JE, Nichols WC, Trembath RC. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. Nat Genet. 2000;26:81–84. doi: 10.1038/79226. [DOI] [PubMed] [Google Scholar]
- 5.Machado RD, Southgate L, Eichstaedt CA, Aldred MA, Austin ED, Best DH, Chung WK, Benjamin N, Elliott CG, Eyries M, et al. Pulmonary arterial hypertension: a current perspective on established and emerging molecular genetic defects. Hum Mutat. 2015;36:1113–1127. doi: 10.1002/humu.22904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pfarr N, Szamalek-Hoegel J, Fischer C, Hinderhofer K, Nagel C, Ehlken N, Tiede H, Olschewski H, Reichenberger F, Ghofrani AH, et al. Hemodynamic and clinical onset in patients with hereditary pulmonary arterial hypertension and BMPR2 mutations. Respir Res. 2011;12:99. doi: 10.1186/1465-9921-12-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gräf S, Haimel M, Bleda M, Hadinnapola C, Southgate L, Li W, Hodgson J, Liu B, Salmon RM, Southwood M, et al. Identification of rare sequence variation underlying heritable pulmonary arterial hypertension. Nat Commun. 2018;9:1416. doi: 10.1038/s41467-018-03672-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morrell NW, Aldred MA, Chung WK, Elliott CG, Nichols WC, Soubrier F, Trembath RC, Loyd JE. Genetics and genomics of pulmonary arterial hypertension. Eur Respir J. 2019;53:1801899. doi: 10.1183/13993003.01899-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eichstaedt CA, Song J, Rodríguez Viales R, Pan Z, Benjamin N, Fischer C, Hoeper M, Ulrich S, Hinderhofer K, Grünig E. First identification of Krüppel-like factor 2 mutation in heritable pulmonary arterial hypertension. Clin Sci (Lond) 2017;131:689–698. doi: 10.1042/CS20160930. [DOI] [PubMed] [Google Scholar]
- 10.Trembath RC, Thomson JR, Machado RD, Morgan NV, Atkinson C, Winship I, Simonneau G, Galiè N, Loyd JE, Humbert M, et al. Clinical and molecular genetic features of pulmonary hypertension in patients with hereditary hemorrhagic telangiectasia. N Engl J Med. 2001;345:325–334. doi: 10.1056/NEJM200108023450503. [DOI] [PubMed] [Google Scholar]
- 11.Harrison RE, Berger R, Haworth SG, Tulloh R, Mache CJ, Morrell NW, Aldred MA, Trembath RC. Transforming growth factor-beta receptor mutations and pulmonary arterial hypertension in childhood. Circulation. 2005;111:435–441. doi: 10.1161/01.CIR.0000153798.78540.87. [DOI] [PubMed] [Google Scholar]
- 12.Zhu N, Gonzaga-Jauregui C, Welch CL, Ma L, Qi H, King AK, Krishnan U, Rosenzweig EB, Ivy DD, Austin ED, et al. Exome sequencing in children with pulmonary arterial hypertension demonstrates differences compared with adults. Circ Genom Precis Med. 2018;11:e001887. doi: 10.1161/CIRCGEN.117.001887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu N, Welch CL, Wang J, Allen PM, Gonzaga-Jauregui C, Ma L, King AK, Krishnan U, Rosenzweig EB, Ivy DD, et al. Rare variants in SOX17 are associated with pulmonary arterial hypertension with congenital heart disease. Genome Med. 2018;10:56. doi: 10.1186/s13073-018-0566-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song J, Eichstaedt CA, Rodríguez Viales R, Benjamin N, Harutyunova S, Fischer C, Grünig E, Hinderhofer K. Identification of genetic defects in pulmonary arterial hypertension by a new gene panel diagnostic tool. Clin Sci (Lond) 2016;130:2043–2052. doi: 10.1042/CS20160531. [DOI] [PubMed] [Google Scholar]
- 15.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plon SE, Eccles DM, Easton D, Foulkes WD, Genuardi M, Greenblatt MS, Hogervorst FB, Hoogerbrugge N, Spurdle AB, Tavtigian SV. Group IUGVW: sequence variant classification and reporting: recommendations for improving the interpretation of cancer susceptibility genetic test results. Hum Mutat. 2008;29:1282–1291. doi: 10.1002/humu.20880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lerche M, Eichstaedt CA, Hinderhofer K, Grünig E, Tausche K, Ziemssen T, Halank M, Wirtz H, Seyfarth HJ. Mutually reinforcing effects of genetic variants and interferon-beta 1a therapy for pulmonary arterial hypertension development in multiple sclerosis patients. Pulm Circ. 2019;9:2045894019872192. doi: 10.1177/2045894019872192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evans AM, Mahmoud AD, Moral-Sanz J, Hartmann S. The emerging role of AMPK in the regulation of breathing and oxygen supply. Biochem J. 2016;473:2561–2572. doi: 10.1042/BCJ20160002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grünig E, Weissmann S, Ehlken N, Fijalkowska A, Fischer C, Fourme T, Galie N, Ghofrani A, Harrison RE, Huez S, et al. Stress Doppler echocardiography in relatives of patients with idiopathic and familial pulmonary arterial hypertension: results of a multicenter European analysis of pulmonary artery pressure response to exercise and hypoxia. Circulation. 2009;119:1747–1757. doi: 10.1161/CIRCULATIONAHA.108.800938. [DOI] [PubMed] [Google Scholar]
- 20.Montani D, Girerd B, Jais X, Laveneziana P, Lau EMT, Bouchachi A, Hascoet S, Gunther S, Godinas L, Parent F, et al. Screening for pulmonary arterial hypertension in adults carrying a BMPR2 mutation. Eur Respir J. 2021;58:2004229. doi: 10.1183/13993003.04229-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang TM, Wang SS, Xu YJ, Zhao CM, Qiao XH, Yang CX, Liu XY, Yang YQ. sox17 loss-of-function mutation underlying familial pulmonary arterial hypertension. Int Heart J. 2021;62(3):566–574. doi: 10.1536/ihj.20-711. [DOI] [PubMed] [Google Scholar]
- 22.Zhu N, Pauciulo MW, Welch CL, Lutz KA, Coleman AW, Gonzaga-Jauregui C, Wang J, Grimes JM, Martin LJ, He H, et al. Novel risk genes and mechanisms implicated by exome sequencing of 2572 individuals with pulmonary arterial hypertension. Genome Med. 2019;11:69. doi: 10.1186/s13073-019-0685-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Navas Tejedor P, Tenorio Castano J, Palomino Doza J, Arias P, Gordo G, Lopez Meseguer M, Roman Broto A, Lapunzina P, Escribano Subias P. An homozygous mutation in KCNK3 is associated with an aggressive form of hereditary pulmonary arterial hypertension. Clin Genet. 2017;91:453–457. doi: 10.1111/cge.12869. [DOI] [PubMed] [Google Scholar]
- 24.Köhling R, Wolfart J. Potassium channels in epilepsy. Cold Spring Harb Perspect Med. 2016;6:a022871. doi: 10.1101/cshperspect.a022871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montani D, Lau EM, Dorfmüller P, Girerd B, Jais X, Savale L, Perros F, Nossent E, Garcia G, Parent F, et al. Pulmonary veno-occlusive disease. Eur Respir J. 2016;47:1518–1534. doi: 10.1183/13993003.00026-2016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. (Likely) pathogenic BMPR2 variants identified in the cohort.
Data Availability Statement
The dataset supporting the conclusions of this article is included within the article (and its additional file).